CHAPTER 204 Recurrent Respiratory Papillomatosis
Epidemiology
Recurrent respiratory papillomatosis is a disease of viral etiology, caused by human papillomavirus (HPV) types 6 and 11, and is characterized by the proliferation of benign squamous papillomas within the aerodigestive tract.1–3 Although it is a benign disease, RRP has potentially fatal consequences because of its involvement of the airway and because of the risk, albeit low, of malignant conversion.4 In addition to the emotional burden to the patients and their families associated with the need for repeated surgery,5 the economic cost of this relatively rare chronic disease is high, having been estimated at $150 million annually.6
RRP is both the most common benign neoplasm of the larynx among children and the second most frequent cause of childhood hoarseness.7 The disease is often difficult to treat because of its tendency to recur and spread throughout the aerodigestive tract. Although it most often involves the larynx, RRP may involve the entire aerodigestive tract. The course of the disease is variable, with some patients experiencing spontaneous remission, whereas others may suffer from aggressive papillomatous growth and respiratory compromise, requiring multiple surgical procedures through many years.
RRP may have its clinical onset during either childhood or adulthood. It may affect people of any age, with the youngest patient identified at 1 day of life and the oldest at 84 years.6 Two distinct forms of RRP are generally recognized: a juvenile or aggressive form and an adult or less aggressive form. The aggressive form, although most prevalent in children, can also occur in adults. Children whose RRP was diagnosed at younger ages (younger than 3 years) have been found to be 3.6 times more likely to have more than four surgical procedures per year and almost two times more likely to have two or more anatomic sites affected than children whose RRP was diagnosed at older ages (older than 3 years).6 Similarly, children with disease progression are generally diagnosed at younger ages than those who remain stable or become disease-free.8–10 In most pediatric series, the delay in diagnosis from the time of onset of symptoms averages about 1 year.6,11 In 75% of children with RRP, diagnosis was made before the fifth birthday.12
The true incidence and prevalence of RRP are uncertain. It is estimated that between 80 and 1500 new cases of childhood-onset RRP occur in the United States each year.6,13 While the incidence among children in the United States is estimated at 4.3 per 100,000 children, the incidence among adults is 1.8 per 100,000.6,14 These figures are comparable with those found in a Danish survey. In a subpopulation incorporating 50% of the population of that country, the rate among children was 3.62 per 100,000, whereas adult-onset cases occurred at a rate of 3.94 per 100,000.15 The National Registry of children with RRP, composed of the clinical practices at 22 pediatric otolaryngology sites, calculates a mean number of procedures at 19.7 per child with an average of 4.4 procedures per year.6,14 Based on the incidence data, this translates into more than 10,000 surgical procedures annually for children with RRP in the United States.
Virology of Human Papillomavirus
HPV, belonging to the Papovaviridae family, is a small deoxyribonucleic acid (DNA)-containing, nonenveloped, icosahedral (20-sided), capsid virus with a double-stranded circular DNA of 7900 base-pairs long. HPV is epitheliotropic (infects epithelial cells). The HPVs have been grouped on the basis of shared genetic code homology, with viruses that share less than 90% identity in specific regions of the virus being defined as separate types. On this basis, the HPVs are numbered to distinguish them. Nearly 100 different HPV types have been identified. Grouping HPV types based on their DNA homology has allowed us to identify closely related types. Functionally, these groupings correlate with their tissue preference as well as similar pathophysiology.16 The HPVs are members of a large family of papillomaviruses that infect vertebrates ranging from birds to humans, causing epithelial neoplasms that can be benign or malignant. These viruses are designated by their natural host species (e.g., bovine papillomavirus, murine papillomavirus, and human papillomavirus). Each papillomavirus is specific for its host species, and this specificity is thought to be absolute. Within each species, similar types of papillomavirus exhibit specificity for epithelial tissues of different sites (e.g., oral mucosa, genital mucosa, or skin). In humans, this tissue specificity is less absolute, and some HPV types exhibit more of a preference for certain tissues.
Until the 1990s, HPV had been suspected but not confirmed as the causative agent in RRP. This uncertainty resulted from an inability to culture the virus in vitro and from the failure to demonstrate viral particles consistently in papilloma lesions using electron microscopy or HPV antibodies. With the advent of molecular probes, HPV DNA has been identified in virtually every papilloma lesion studied. The most common types identified in the airway are HPV 6 and HPV 11—the same types responsible for more than 90% of genital condylomata. Specific viral subtypes may be correlated with disease severity and clinical course. Children infected with HPV 11 appear to have a more obstructive airway course early in the disease and a greater likelihood of undergoing tracheotomy to maintain a safe airway.10,17
In addition to the HPV group that includes HPV 6 and 11, two other major groups of HPV are associated with mucosal lesions in the aerodigestive and genital tracts. HPV 6 and 11, responsible for the majority of RRP, are members of a group believed to have a low malignant potential compared with some other groups. In contrast, the group that contains HPV 16 and HPV 18 is associated with malignancies in the genital and aerodigestive tracts.16 The group that contains HPV 31 and HPV 33 exhibits malignant potential that lies somewhere in between.
HPV is thought to infect stem cells within the basal layer of mucosa.18,19 After infection of the stem cells, the viral DNA can either be actively expressed or it can exist as a latent infection in mucosa that remains clinically and histologically normal. To produce viral proteins or to replicate the virus, the viral DNA must somehow reactivate the host replication genes. The viral genome consists of three regions: an upstream regulatory region and the two regions named according to the phase of infection in which they are expressed—early (E) and late (L) regions. The E-region genes are involved in the replication of the viral genome, interaction with the host cell intermediate filaments, and transforming activities, and are potential oncogenes, depending on the HPV type. The L-region genes encode the viral structural proteins.20–22
The induction of cellular proliferation is a fundamental property of HPV, although the mechanism of action remains unclear. We are slowly gathering information regarding the interaction of viral gene products with cellular proteins. For example, several of the viral E-region gene products have been shown to bind and inactivate certain cellular tumor-suppressor proteins.21,23 Conversely, HPV has been shown to activate the epidermal growth factor (EGF) receptor pathway, known to be associated with proliferation of epithelial cells.3 Thus there are likely several mechanisms by which HPV induces cellular proliferation in the aerodigestive mucosa.
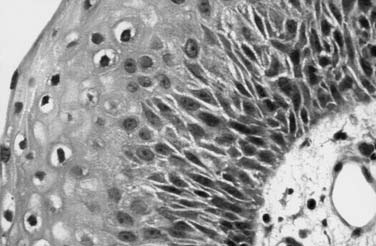
Histologically, this mucosal proliferation results in multiple “fronds” or finger-like projections with a central fibrovascular core, covered by stratified squamous epithelium (Fig. 204-1).18 When papillomas are microscopic, they may assume a superficial-spreading configuration, with a velvety appearance (Fig. 204-2). When they exhibit a more macroscopic or exophytic growth pattern, they appear grossly as “cauliflower” projections (see Fig. 204-2). Papilloma lesions may be sessile or pedunculated and often occur in irregular exophytic clusters. Typically, the lesions are pinkish to white in coloration. Iatrogenic implantation of papilloma may be preventable by avoiding injury to nondiseased squamous or ciliated epithelium adjacent to areas of frank papilloma. Ciliated epithelium undergoes squamous metaplasia when exposed to repeated trauma and is replaced with nonciliated epithelium that creates an iatrogenic squamocellularly junction. This may also explain the observation that RRP flourishes in the presence of uncontrolled gastroesophageal reflux. Most RRPs do not exhibit dysplasia, abnormal mitoses, or hyperkeratosis.18 Without exception, RRP exhibits delayed maturation of the epithelium, resulting in significantly thickened basal cell layer and nucleated cells in the superficial layers.24 This is thought to be in part because of the interaction of HPV gene products with the EGF receptor pathway.3 The sum result of these cellular effects is that, although HPV-infected cells do not rapidly divide, there is a disproportionate increase in the number of dividing basal cells. Thus expansion of the RRP tissue mass may occur very rapidly, because of the large number of dividing cells.19
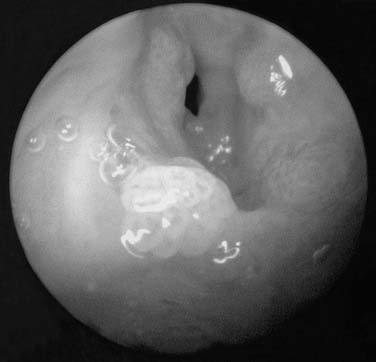
Figure 204-2. Gross appearance of respiratory papillomas during laryngoscopy. Appearance of massive, exophytic papillomatosis of the larynx.
During viral latency there is very little viral RNA expressed. Even so, HPV DNA can be detected in normal-appearing mucosa in RRP patients who have been in remission for years, and unknown stimuli can result in reactivation and clinical recurrence following years of remission.25,26 Thus activation of viral expression can occur any time after the establishment of latent infection. Gene products of early region gene E6, E7, and possibly E5 are required for papilloma induction, but the details of the mechanism of HPV activation are unknown. To “cure” RRP, it is necessary to modulate the host response to the virus and, ideally, eliminate latent infection.
It is likely that the host immune system plays an important role in the pathogenesis of HPV-induced lesions. Both the humoral and the cellular immune responses may be compromised in children with RRP, and the patient’s immunocompetence may be associated with the clinical course of the disease. The role of cytokines, such as interleukin-2, interleukin-4, and interleukin-10, and expression of the major histocompatibility complex (MHC) antigens in the dysfunction of the cell-mediated immune response in children with RRP has been demonstrated.27,28
Transmission
An association between cervical HPV infection in the mother and the incidence of RRP has been well established. However, the precise mode of transmission is still not clear.18 The universality of HPV in the lower genital tract rivals that of any other sexually transmitted disease in humans. It is estimated that at least one million cases of genital papillomas occur per year in the United States.29 These are most commonly manifested as condylomata acuminata involving the cervix, vulva, or other anogenital sites in women or the penis of male sexual partners of affected women. Colposcopic (subclinical) changes are seen in about 4% of women, whereas DNA positive biopsies without a visible lesion are seen in 10% of women. HPV antibody positivity (without DNA or a clinical lesion) is estimated in 60% of women (81 million). HPV has been estimated to be present in the genital tract of as many as 25% of all women of child-bearing age worldwide. One study reported that the incidence of HPV infections in sexually active young college women is highest, with a cumulative incidence of 43% during a 36-month period.30 Clinically apparent HPV infection has been noted in 1.5% to 5% of pregnant women in the United States.31 More than 30% of American women are currently infected with HPV with 7.5 million 14- to 24-year-olds currently infected and one fourth of women younger than 60 years of age infected at any given time.32 Up to 90% of lesions are undetectable clinically at 2 years. The highest prevalence is in women 20 to 24 years of age. More than 50% of women will initially acquire HPV within 4 years of their first sexual intercourse. As in RRP, HPV 6 and HPV 11 are the most common subtypes identified in cervical condylomata.
Vertical transmission occurring during delivery through an infected birth canal is presumed to be the major mode of transmitting the infection in children whereas in utero and transplacental transfer of HPV, sexual abuse, and direct contact are thought to play a minor role. The support for vertical transmission lies in the fact that overt maternal condyloma are seen in more than 50% of mothers who give birth to children with RRP.33 The same subtypes (HPV 6 and 11) are involved, and cesarean delivery of children seems to be preventive to some extent.34
Patients with childhood-onset RRP are more likely to be first born and vaginally delivered than are control patients of similar age.35,36 Kashima and others hypothesized that primigravid mothers are more likely to have a long second stage of labor and that the prolonged exposure to the virus leads to a higher risk of infection in the first-born child. They also suggested that newly acquired genital HPV lesions are more likely to shed virus than longstanding lesions, accounting for the higher incidence of papilloma disease observed among the offspring of young mothers of low socioeconomic status—the same group that is most likely to acquire sexually transmitted diseases such as HPV.35,36
Despite the close association between maternal condylomata and the development of RRP, only a small portion of children exposed to genital condylomata at birth actually go on to development of clinical RRP.37 Although HPV could be recovered from the nasopharyngeal secretions of 30% of infants exposed to HPV in the birth canal, the number of infants expected to manifest evidence of RRP is only a small fraction of this population.37 Clearly, other factors (patient immunity, timing, length and volume of virus exposure, local trauma) must be important determinants in the development of RRP. Even though cesarean section delivery would seem to reduce the risk of transmission of the disease, this procedure is associated with a higher morbidity and mortality for the mother and a much higher economic cost than elective vaginal delivery. Furthermore, reports of neonatal papillomatosis suggest that, in at least some cases, transmission may occur in utero.1 However, with such a high rate of subclinical maternal HPV infection and such a low rate of actual new cases of childhood RRP, elective cesarean delivery as a means of preventing RRP is currently not practical or recommended.1 The risk of a child contracting the disease from a mother who has an active genital condyloma lesion during vaginal delivery is only approximately 1 in 231 to 400.22,34,36 The characteristics that distinguish this one child from the other 230 to 399 remain elusive. In summary, a better understanding of the risk factors associated with RRP is needed before the efficacy of cesarean delivery or other preventive measures can be fully assessed.
Prevention
The new quadrivalent HPV vaccine (Gardasil, Merck and Co., Inc.), is licensed and indicated for the prevention of cervical cancer, adenocarcinoma in situ, and intraepithelial neoplasia grades 1-3; vulvar and vaginal intraepithelial neoplasias grades 2-3; and genital warts associated with HPV 6, 11, 16, and 18. The CDC Advisory Committee on Immunization Practices (ACIP)32 has recommended vaccination for all girls ages 11 to 12, girls and women ages 13 to 26 who have not yet been vaccinated, and girls as young as age 9, in whom the physician believes it would be appropriate. Based upon the pivotal clinical studies, the vaccine is predicted to reduce the incidence, morbidity, and mortality of cervicovaginal HPV disease. An added and often overlooked benefit may be a concomitant decrease in the incidence of RRP and HPV-associated head and neck cancers.
The ability of the quadrivalent vaccine to prevent HPV 6/11/16/18-associated cervical and genital disease was established in the phase 3 FUTURE I and II trials, and their immunogenicity in the target group of vaccinees was established in immunogenicity bridging trials.38 In FUTURE I, the quadrivalent vaccine was 100% effective in preventing cervical intraepithelial neoplasia (CIN) or worse, genital warts, and vulvovaginal neoplasia. In FUTURE II, the vaccine was 100% effective in preventing HPV 16/18-associated CIN. Both Future I and II were conducted in women in the age range at highest risk for HPV acquisition.38,39 However, it appears that the vaccine will be most effective if administered to individuals who have not yet become sexually active. Immunogenicity bridging studies were conducted to assess the immunogenicity of the vaccine in this population, and established that immunogenicity among younger girls was equal to if not superior to the response among 16- to 23-year-old women, suggesting that the quadrivalent HPV vaccine is immunogenic in this population and thus likely to be effective in preventing disease.40 A separate study in 9- to 15-year-olds established that in this younger population immunogenicity lasts at least 18 months.41
There is also a bivalent HPV vaccine currently in phase 3 trials. This vaccine provides protection against HPV 16 and 18, but not 6 and 11.38 Early phase 2 data for this vaccine suggest that it is 100% effective in preventing incident and persistent cervical HPV 16 and 18 infections in the according-to-protocol (ATP) sample, and 93% effective in preventing HPV 16- or 18-related disease in the intention-to-treat analysis; efficacy against disease was not presented for the ATP cohort. This vaccine’s efficacy against HPV 16 and 18 suggests that, like the quadrivalent vaccine, it may reduce the incidence of HPV-associated head and neck cancers. However, because the bivalent vaccine does not protect against HPV 6 and 11, it will not likely affect the vertical transmission of HPV 6 or 11 from mother to child.
Clinical Features
Inasmuch as the vocal fold is usually the first and predominant site of papilloma lesions, hoarseness is the principal presenting symptom in RRP.42 The child’s voice may be described as hoarse or weak from the time of birth. Particularly in very young children, changes in voice may go unnoticed. Stridor is often the second clinical symptom to develop, beginning as an inspiratory noise and becoming biphasic with progression of the disease. Less commonly, chronic cough, recurrent pneumonia, failure to thrive, dyspnea, dysphagia, or acute life-threatening events may be the presenting symptoms. The duration of symptoms before diagnosis varies. Not uncommonly, a mistaken diagnosis of asthma, croup, allergies, vocal nodules, or bronchitis is entertained before a definitive diagnosis is made. However, the initial presentation in infants, whose airway dimensions are small, may be with acute respiratory distress during an otherwise routine upper respiratory tract infection. The natural history of RRP is highly variable. After presentation, the disease may undergo spontaneous remission or persist in a stable state requiring only periodic surgical treatment. At the other extreme, RRP may become extremely aggressive, requiring frequent surgical treatment (every few days to weeks), prompting early institution of medical adjuvant therapy. A waxing-waning clinical course of remissions and exacerbations is common for RRP.
Because of the rarity of RRP and the slowly progressive nature of the disease, some cases may go unrecognized until respiratory distress results from papillomas obstructing the airway. The result is a relatively high need for tracheotomy to be performed in these children. Shapiro and others noted that RRP tracheotomy patients presented at a younger age and with more widespread disease, often involving the distal airway before tracheotomy.43 In their experience with 13 patients, they did not believe that the tracheotomy itself led to spread of disease outside the larynx. In the CDC National RRP Registry, children with tracheotomy were initially diagnosed with RRP at a younger age (2.7 years) than those without a tracheotomy (3.9 years).14 Others have suggested that tracheotomy may activate or contribute to the spread of disease lower in the respiratory tract.44 Cole and colleagues reported that tracheal papillomas developed in half of their tracheotomy patients and that, despite attempts to avoid this procedure, 21% of their patients still required a long-term tracheotomy.45 Prolonged tracheotomy and the presence of subglottic papillomata at the time of tracheotomy have been associated with an increased risk of distal tracheal spread. Most authors agree that tracheotomy is a procedure to be avoided unless absolutely necessary. Interestingly, Boston and colleagues from Cincinnati noted successful laryngotracheal reconstruction in a series of children with subglottic stenosis and RRP.46 When a tracheotomy is unavoidable, decannulation should be considered as soon as the disease is managed effectively with endoscopic techniques. Children with bronchopulmonary dysplasia who require prolonged endotracheal intubation may also be at increased risk for development of RRP.47 Through interruption of the continuous respiratory mucosal surface, an endotracheal tube may have the same role in the mechanical dissemination-implantation of RRP as tracheotomy, as a risk for distal spread of disease. Several authors have noted an association between RRP caused by HPV 11 (as opposed to HPV 6) and distal spread of papilloma.10
Extralaryngeal spread of respiratory papillomata has been identified in approximately 30% of children and in 16% of adults with RRP.6 The most frequent sites of extralaryngeal spread were, in decreasing order of frequency, the oral cavity, trachea, and bronchi (Fig. 204-3).6,42 Pulmonary papilloma lesions begin as asymptomatic noncalcified peripheral nodules.48 These lesions eventually enlarge to undergo central cavitation and central liquefactive necrosis with air-fluid levels on a computed tomography scan (Fig. 204-4). These patients present clinically with recurrent bronchiectasis, pneumonia, and declining pulmonary status. The clinical course of the pulmonary spread of RRP is insidious and may progress through the years, but eventually manifests in respiratory failure because of destruction of lung parenchyma. For this reason, the finding of pulmonary lesions in a patient with RRP is a grave development with no currently available treatment modality that has shown more than anecdotal promise. Furthermore, pulmonary dissemination is anecdotally associated with a higher risk of malignant transformation of RRP.
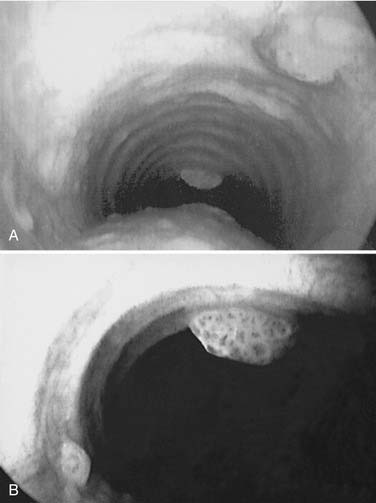
Figure 204-3. Tracheal spread of recurrent respiratory papillomatosis. Note nodular, exophytic lesions in distal trachea.
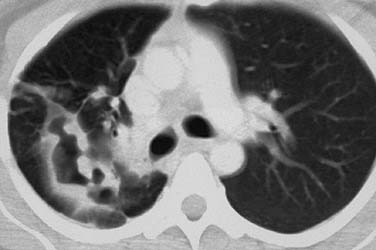
Figure 204-4. Pulmonary spread of recurrent respiratory papillomatosis. Note lytic, cavitary lesions on computed tomography.
Malignant transformation of RRP into squamous cell carcinoma has been documented in several case reports. A total of 26 patients was identified as having progressed to squamous cell carcinoma in the task force survey.6 Dedo and Yu reported malignant transformation in 4 of 244 (1.6%) RRP patients treated during 2 decades.49 When death occurs in a patient with RRP, it is usually as a complication of frequent surgical procedures or caused by respiratory failure because of distal disease progression. RRP presenting in the neonatal period is thought to be a negative prognostic factor, with a greater likelihood for mortality and need for tracheotomy.8,9
Patient Assessment
History
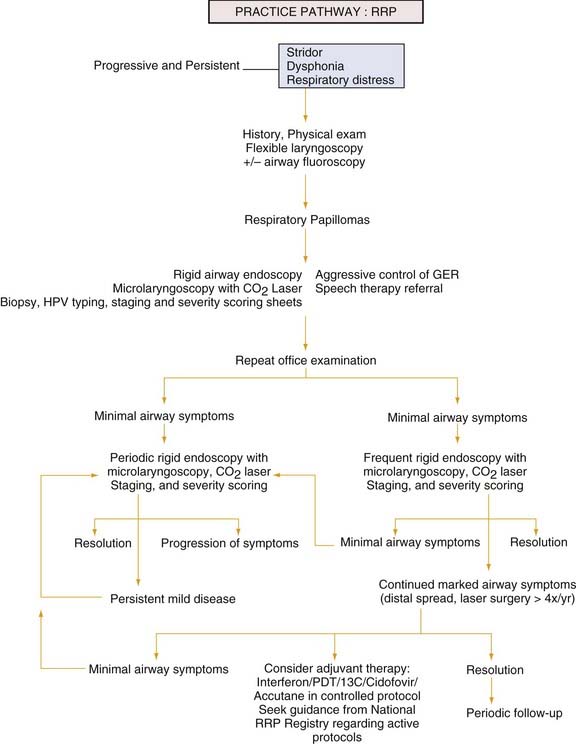
Persistent or progressive stridor and dysphonia, with the possible development of respiratory distress, are the most consistent signs and symptoms of RRP in children (see the Practice Pathway Flow Chart, Fig. 204-5). In the absence of severe respiratory distress, a careful history should be obtained. Information regarding the time of onset of symptoms, possible airway trauma including a history of previous intubation, and characteristics of the cry are obviously important. Hoarseness, although a common and often benign clinical complaint in young children, always indicates some abnormality of structure or function. Because of the precision of laryngeal mechanics, hoarseness may result from a remarkably small lesion and thus be an early sign in the course of a disease process. On the other hand, if the lesion’s origin is remote from the vocal cords, hoarseness may present as a late sign. Although histologically the same lesion, a papilloma that produces hoarseness in one patient may produce stridor and obstruction in another, depending on the size and location of the lesion. The quality of the voice change may give only limited clues to its etiology, whereas other characteristics such as age of onset, rate of progression, associated infection, history of trauma or surgery, and the presence of respiratory or cardiac distress may be of much greater significance. A low-pitched, coarse, fluttering voice suggests a subglottic lesion, whereas a high-pitched, cracking voice; aphonia; or breathy voice suggests a glottic lesion. Associated high-pitched stridor also suggests a glottic or subglottic lesion. Although stridor that has been present since birth is more often associated with laryngomalacia, subglottic stenosis, vocal cord paralysis, or a vascular ring, it should be realized that neonates can also present with papillomatosis. Associated symptoms such as feeding difficulties, allergic symptoms, vocal abuse, and the presence of hereditary congenital anomalies may help distinguish RRP from alternative diagnoses, including vocal fold nodules, vocal fold paralysis, subglottic cysts, subglottic hemangioma, and subglottic stenosis. In the absence of any history suggesting these lesions, review of the perinatal period may reveal a history of maternal or paternal condylomata. If the onset of stridor and dysphonia is gradual and progressive through weeks or months, then neoplastic growth compromising the airway must be considered and investigated.
Certainly not every child with a hoarse voice or cry merits investigation beyond an assessment of symptoms. However, in the presence of hoarseness with respiratory distress, tachypnea, decreased air entry, tachycardia, cyanosis, dysphagia, chronic cough, failure to thrive, recurrent pneumonia, or dysphagia, the larynx must be visualized and a firm diagnosis of the cause of hoarseness must be made. Any child with slowly progressive hoarseness merits investigation and the clinician should not wait until total aphonia or airway problems occur.50
Airway Endoscopy
Most clinicians find that visualization with the flexible nasopharyngoscope is far superior to that obtained with indirect mirror laryngoscopy in young children. Patient cooperation, however, is required even with good topical anesthesia. In infants, this is not a large issue because they can easily be restrained in an upright, sitting position in the parent’s or nurse’s lap for evaluation. Likewise, most children older than 6 or 7 years of age can be “talked into” cooperating for the examination. It is the intermediate age group, between 1 and 6 years of age, who may be the most difficult to examine, taxing the patience and skill of even the most experienced clinicians. Although dynamic evaluation can be appreciated when children are spontaneously breathing, endoscopy in the OR under anesthesia is warranted in any child suspected to have RRP who cannot be fully examined in the outpatient setting.50
Surgical Management
Classic Management
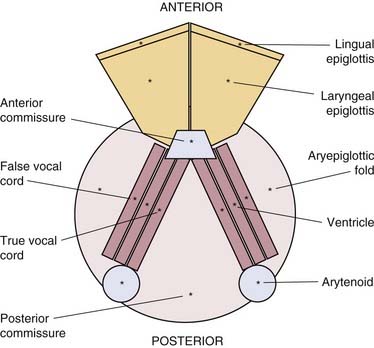
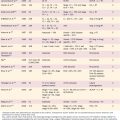
At present, there is no “cure” for RRP, and no single modality has consistently been shown to be effective in eradication of RRP. The current standard of care is surgical therapy with a goal of complete removal of papillomas and preservation of normal structures. In patients in whom anterior or posterior commissure disease or highly aggressive papillomas are present, the goal may be subtotal removal with clearing of the airway. It is advisable to debulk as much disease as possible while preserving normal morphology and anatomy and preventing the complications of subglottic and glottic stenosis, web formation, and resulting airway stenosis. Table 204-1 lists treatment options for RRP.
| Treatment | Number of Patients Reported |
|---|---|
| Laser therapy | Hundreds |
| The standard for papilloma removal. | |
| Microdébrider removal | Hundreds |
| An evolving standard for papilloma removal. | |
| Acyclovir | 8 |
| Inhibits thymidine kinase, an enzyme not encoded by HPV. | |
| 4 patients treated with postoperative acyclovir, with “no recurrence in 3 cases”105 | |
| 4 patients treated with acyclovir as adjuvant therapy to routine surgical/laser treatment. One of the 4 patients exhibited “less aggressive disease during the treatment period.” The authors conclude that acyclovir “is not recommended in the treatment of juvenile respiratory papillomatosis.”106 | |
| Cidofovir | >50 |
| First member of group of antiviral agents known as acyclic phosphonate nucleotide analogs; they inhibit viral DNA polymerase. Cidofovir is directly injected into laryngeal sites of RRP. Large-scale trials are ongoing. | |
| 14 of 17 patients with “severe” RRP who underwent cidofovir injections experienced a “complete response.”71 | |
| 10 of 10 children with RRP who underwent cidofovir injections showed a response with short-term follow-up.72 | |
| 14 of 14 adults with RRP exhibited disease remission after 1 to 6 injections of cidofovir.107 | |
| Similarly, percutaneous injections of cidofovir resulted in clinical improvement in 5 of 5 adults and 5 of 5 children undergoing intralesional injections.108,109 | |
| A larger study of 26 adults and children receiving intralesional injections found that one third exhibited complete remission; two thirds of the patients exhibited reduction in disease; none exhibited worsening of disease.75,76 | |
| A report of a single patient with pulmonary spread of RRP suggests that systemic cidofovir may be effective in some instances. A patient had documented regression of lung RRP lesions in response to systemic cidofovir.78 | |
| Indole-3-carbinol (I-3-C) | 18 |
| I-3-C affects the ratio of estradiol hydroxylation. | |
| 18 patients, Phase I trial: one third (6 patients) exhibited “cessation of their papilloma growth”; one third exhibited “reduced papilloma growth rate”; one third exhibited “no clinical response.”84 | |
| Interferon-alpha | >100 |
| This most widely used adjuvant therapy appears to modulate host immune response to virus. Most common adverse side effects include flulike symptoms, but can include severe central nervous system effects such as coma.67,68 | |
| Mumps vaccine | 49 |
| 11 children with RRP were treated with regular laser excision; 9 of 11 patients (82%) had “remission induced by 1 to 10 injections, with follow-up at 5 to 19 months.” | |
| In subsequent series, remission was induced in 29 of 38 patients (76%) by 4 to 26 injections, with follow-up at 2 to 5 years.95 | |
| Retinoids | ? |
| ANY in RRP trials? | |
| Photodynamic therapy (PDT-DHE) | >100 |
| Photodynamic therapy (PDT-Foscan) | Ongoing Clinical Trials |
| Vaccines | Ongoing Clinical Trials |
| A fusion protein composed of heat shock protein and the E7 gene product of human papillomavirus (HSP-E7) has been used successfully as a novel vaccine in patients with anogenital warts, where 13 of 14 adult patients had resolution of warts at week 24.110 This novel approach had been in clinical trials as potential therapy for RRP; however, the pharmaceutical company responsible was financially insolvent and clinical trials have been halted. The quadrivalent HPV vaccine indicated for the prevention of cervical cancer32 promises to dramatically reduce the morbidity and mortality of cervical cancer, but also has the potential to reduce aerodigestive squamous cell carcinoma as well as to eradicate RRP in future generations.32 | |
Until recently, the CO2 laser has been favored over cold instruments in the treatment of RRP involving the larynx, pharynx, upper trachea, and nasal and oral cavities.6 When coupled to an operating microscope, the laser vaporizes the lesions with precision, causing minimal bleeding. When used with a no-touch technique, it minimizes damage to the vocal cords and limits scarring. Dedo reported on a series of 244 patients with RRP treated with the CO2 laser used every 2 months and achieved “remission” in 37%, “clearance” in 6%, and “cure” in 17%.49 The CO2 laser has an emission wave length of 10,600 nm and converts light to thermal energy. The CO2 laser delivers energy that is absorbed by intracellular water, effectively vaporizing the cells.51 It provides a controlled destruction of tissues with vaporization of water, and it cauterizes tissue surfaces. The smoke plume contains water vapor and destroyed tissue material. Its drawbacks relate to safety of the operating room personnel, patient, and surgeon. The laser may glance off metal used in the suspension of the larynx and injure eyes or skin that it hits. A misfire may also hit areas on the patient that are not protected by a wet towel to absorb the laser energy. In addition, the laser smoke or “plume” has been found to contain active viral DNA, a potential source of infection.52–54 Smoke evacuators are necessary for safety of exposed individuals. Most importantly, since the laser generates heat, ignition of the endotracheal tube may occur with an inadvertent laser strike. In the oxygen-rich environment provided by anesthetic gases, this can lead to explosion or fire in the airway. The surgeon who is not aware of injury to deeper tissue layers with injudicious laser usage may cause unacceptable scarring and subsequent abnormal vocal fold function. Inappropriate and aggressive use of the laser may also cause injury to tissues that are not affected and create an environment suitable for implantation of viral particles. Use of the CO2 laser can also result in delayed local tissue damage, which may be related to the total number of laser surgeries and the severity of RRP disease.
Emerging Technologies
The use of the CO2 laser on the true vocal folds must be judicious given the potential for significant postoperative scar tissue formation from unrecognized heat transfer. To minimize the risk of scar formation in the true vocal folds, cold steel excision can be used successfully following the principles of phono-microsurgery, submucosal dissection, and microinstrumentation. This approach may have treatment advantages over CO2 laser surgery, especially in the adult RRP patient.55–57 In their initial series, Zeitels and Sataloff report recurrence of papilloma in none of the six adults at 2 years follow-up after resection for primary disease. Of those who presented with recurrent papillomatosis, 6 of 16 (38%) continued to recur after their microflap procedure.57
Publications have highlighted the potential benefits of other surgical technologies in managing RRP. Bower and others evaluated the feasibility and safety of the flash pump dye laser in nine children and found good early results.58 McMillan and others published preliminary results regarding their positive experience with the 585-nm pulsed dye laser in three patients.59 Zeitels reported on office-based use of the 532-nm pulsed KTP laser for treatment of recurrent glottal papillomatosis and dysplasias, noting at least 75% regression of disease in two thirds of patients who could tolerate the procedure.60 Rees and Koufman also demonstrated tolerance for in-office pulsed dye laser treatments involving 328 procedures in 131 adult patients, with patients overwhelmingly preferring the in-office pulsed dye laser over surgeries under general anesthesia.61 Zeitels and others currently favor use of a solid-state fiber-based thulium laser that functions similarly to a CO2 laser with the benefit of being delivered through a small glass fiber.62
A number of investigators are now replacing their use of the CO2 laser with the endoscopic microdébrider as a means of quickly debulking laryngeal disease. In a small, randomized study, Pasquale and others observed improved voice quality, less OR time, less mucosal injury, and a cost benefit in direct comparison of the microdébrider and the CO2 laser.63 El-Bitar and Zalzal, and Patel and others, have observed similar improved outcomes with the use of the endoscopic microdébrider.64,65 A web-based survey of members of the American Society of Pediatric Otolaryngologists (ASPO) found the majority of respondents now favoring the use of “shaver” technology.4
Anesthetic Techniques
Adjuvant Treatment Modalities
Although surgical management remains the mainstay therapy for RRP, ultimately as many as 20% of patients with the disease will require some form of adjuvant therapy. The most widely adopted criteria for initiating adjuvant therapy are a requirement for more than four surgical procedures per year, rapid regrowth of papilloma disease with airway compromise, or distal multisite spread of disease. Some of these adjuvant treatment options are summarized in Table 204-1.
Antiviral Modalities
Interferon
The first widely employed adjuvant therapy was alpha-interferon.6,66,67 The exact mechanism by which interferon elicits its response is unknown. It appears to modulate host immune response by increasing production of a protein kinase and endonuclease, which inhibits viral protein synthesis.68 Interferons are a class of proteins that are manufactured by cells in response to a variety of stimuli including viral infection. The enzymes that are produced block the viral replication of RNA and DNA and alter cell membranes to make them less susceptible to viral penetration.
Ribavirin
Ribavirin is an antiviral drug used to treat respiratory syncytial virus pneumonia in infants that has also shown some promise in the treatment of aggressive laryngeal papillomatosis. Ostrow and others at the University of Minnesota have completed a small trial in eight patients using ribavirin in an oral form at 23 mg/kg/day divided four times daily after an initial intravenous loading dose, showing an increase in surgical interval in those treated.69
Acyclovir
Another antiviral treatment that has been advocated in the treatment of RRP is acyclovir. Although the activity of acyclovir is dependent on the presence of virally encoded thymidine kinase, an enzyme that is not known to be encoded by papillomavirus, conflicting clinical results have been obtained in several small series. Theoretically, it would not be expected to have a positive effect. However, it has been postulated that perhaps acyclovir is most effective when there are simultaneous disease factors such as infection with herpes simplex virus. Such viral co-infections with herpes simplex virus 1, cytomegalovirus, and Epstein-Barr virus (HSV-1, CMV, and EBV) have been demonstrated in RRP patients, and these patients with viral co-infections appear to have a more aggressive clinical course.70
Cidofovir
Recent reports have stimulated interest in the intralesional injection of cidofovir (Vistide)—(S)-1-[3-hydroxy-2-(phosphonylmethoxyethyl)-propyl]-cytosine (HPMPC)—a drug approved by the U.S. Food and Drug Administration for use in human immunodeficiency virus (HIV) patients with cytomegalovirus under expedited, fast-track guidelines. Despite a lack of controlled, randomized, blinded clinical trials in children with RRP, cidofovir is currently the most frequently used adjuvant drug.4 Snoeck and others reported a series of 17 patients with severe RRP treated with cidofovir at 2.5 mg/mL injected directly into the papilloma bed after laser surgery experienced a complete response in 14 days.71 Pranksy and coworkets have used this therapy in 10 children with severe RRP with short-term follow-up showing a response in all 10 patients,72 and long-term follow-up has been encouraging.73,74 Similarly encouraging results have been reported by Naiman and others in a small cohort of adults and children treated with a protocol-driven approach using a high dose of cidofovir at 2-week intervals.75,76 Co and Woo have also demonstrated efficacy in a small cohort of adults with RRP treated with serial office-based intralesional injections of cidofovir.77 Finally, there has been one encouraging successful treatment of pulmonary multicystic papillomatosis using systemic cidofovir.78
Based on animal studies that demonstrated a high level of carcinogenicity, and on case reports of progressive dysplasia in patients with RRP,79 the RRP Task Force has published guidelines for clinicians interested in using cidofovir in their RRP patients.80 Despite the anecdotal nature of most of the cidofovir studies and reports, the preliminary results are encouraging enough to consider this a treatment option in severely affected RRP patients.
Photodynamic Therapy
Photodynamic therapy (PDT) applied to the treatment of RRP has been studied extensively at Long Island Jewish Hospital by Shikowitz and associates.81 PDT is based on the transfer of energy to a photosensitive drug. The original drug used was dihematoporphyrin ether (DHE), which has a tendency to concentrate within papillomas more so than in surrounding normal tissue. Patients are typically treated intravenously with 4.25 mg/kg of DHE before photoactivation with an argon pump dye laser. A small but statistically significant decrease in RRP growth, especially in patients whose disease was worse, was seen with the use of PDT and DHE. The drawback of this therapy is that patients become markedly photosensitive for periods lasting 2 to 8 weeks. A new drug, m-tetra(hydroxyphenyl)chlorine (Foscan), has shown efficacy in HPV-induced tumors in rabbits with minimal tissue damage and less photosensitivity. A clinical trial using this drug in a parallel-arm, randomized trial of 23 severely affected patients ages 4 to 60 resulted in improvement in laryngeal disease; however, remissions failed to be maintained over 3 to 5 years and the therapy was poorly tolerated by a quarter of the patients.82
Indole-3-Carbinol
There has been recent interest in the dietary supplement, indole-3-carbinol, a compound that is found in high concentration in cruciferous vegetables such as cabbage, cauliflower, and broccoli. Results in an open label, multicenter study coordinated by Rosen of treating RRP with indole-3-carbinol were encouraging. During the short follow-up of the study (a minimum of 8 months), one third of the patients had a cessation of their papilloma growth and did not require further surgery, one third of the patients exhibited reduced papilloma growth rate, and one third did not show a clinical response.83,84 The role of estrogens on the growth of RRP has been conjectured since the discovery that RRP exhibits increased binding of estrogen.85 Subsequent to that report, inhibition of estrogen metabolism using indole-3-carbinol has been shown to reduce the formation of HPV-induced papilloma tumors in immunocompromised mice by nearly 75%.86 Most recently, the clinical response to indole-3-carbinol in children with RRP was closely correlated with the ratio of hydroxylated estradiol in urine.84 Longer follow-up of a larger, blinded, controlled trial seems warranted before universally recommending this therapy.
Celecoxib
Celecoxib (Celebrex) is a selective inhibitor of the enzyme COX-2, which is overexpressed in RRPs and stimulates their growth.87 Preliminary encouraging results in a small cohort of adults with Celebrex has led to an NIH-funded multicenter trial led by investigators at Long Island Jewish Hospital that is currently active (no longer enrolling) for adults with RRP.
Retinoids
Retinoids, which are metabolites and analogues of vitamin A, are known modulators of cellular proliferation and differentiation of diverse histologic cell types. In the aerodigestive tract, excess vitamin A has been found to suppress squamous differentiation, and may result in mucous metaplasia.88 In contrast, vitamin A deficiency may result in hyperkeratinization and squamous metaplasia.89 However, there are a variety of retinoids with opposing effects on epithelial cell metabolism.90–93 Thus to state that retinoids as a class of agents have a beneficial or deleterious effect on mucosal differentiation would be an oversimplification. With this caveat, 13-cis-retinoic acid (Accutane) has gained some popularity as a systemic treatment for RRP.94 The dosage for retinoic acid is 1 to 2 mg/kg/day. This therapy is maintained for 6 months or until the patient develops side effects he cannot tolerate. Care has to be taken in sexually active patients because Accutane has well-known teratogenic effects.
Mumps Vaccine
Intralesional injection of mumps vaccine and MMR vaccine has been investigated in an open-label single-center trial with moderate success.95 These positive results, however, have not been reproduced by other investigators.96
Antireflux Therapy
The role of gastroesophageal reflux in exacerbation of RRP deserves special mention. Recent case reports have shown that the rate of recurrence of respiratory papillomatosis in children may decrease significantly after antireflux therapy.97,98 The H2-antihistamine Cimetidine (ranitidine) has been shown to have immunomodulatory effects.99,100 This has resulted in several reports of its use against various virally based diseases, including RRP.98,101 A recent small case series from Buffalo demonstrated efficacy in previously resistant children with RRP.102 It is for these reasons that optimal control of extraesophageal reflux disease has been advocated as a means of improving outcomes, along with surgical therapy. The authors routinely place their RRP patients on an antireflux regimen.
Staging Severity
It is helpful when tracking the progression of a child’s disease, communicating with other surgeons, and treating patients in a protocol format to have a surgical scoring system to assess severity and clinical course of RRP disease. Although several scoring and staging systems have been proposed, clinicians and researchers have not yet adopted a uniformly acceptable nomenclature for describing RRP lesions that is simple yet comprehensive. This has created confusion in the RRP literature and in physician-to-physician communications regarding patients’ response to therapies. In addition, the absence of a universally accepted staging system has hampered our abilities to accurately report the results of adjuvant therapies or document the natural course of the disease. We have introduced a severity-staging system for RRP in a format that incorporates the best qualities of the existing systems by numerically grading the extent of papillomatosis at defined aerodigestive subsites, assessing functional parameters, diagrammatically catalogs subsite involvement, and assigning a final numeric score to the patient’s current extent of disease (Figs. 204-5 through 204-7).47,103,104 Using software designed at the University of Washington (Seattle) and licensed to the ASPO, this staging system is now computerized and available to pediatric otolaryngologists and bronchoesophagologists to allow them to objectively and subjectively measure an individual patient’s clinical course and response to therapy through time. Health Insurance Portability and Accountability Act–compliant encryption technology with this software offers promise to allow clinicians from around the world to anonymously share some of their patient data to enhance our knowledge of this disease and promote multi-institutional investigations.
Registry and Task Force Initiatives
It should be stressed that participation in national and regional protocols of adjuvant treatment modalities is essential for the scientific community to learn more about RRP. A national registry of patients with RRP has been formed through the cooperation of the ASPO and the CDC.14 The registry tracked nearly 600 children at 22 sites with data on more than 11,000 surgical procedures. Data from the national registry aided in the identification of patients suitable for enrollment in multi-institutional studies of adjuvant therapies and better defined the risk factors for transmission of HPV and the cofactors that may determine the aggressiveness of RRP. An RRP task force, made up of the principal investigators at each of the registry sites as well as representatives from the adult RRP research community and patient/parent advocacy groups, meets twice yearly to facilitate research initiatives. The RRP task force has completed a set of Practice Guidelines (RRP Task Force, 2000) to help guide the clinician in the diagnosis and management of children with RRP, and has authored public health guidelines regarding RRP and statements on the use of cidofovir. The Task Force is engaged in providing a statement on the value of viral typing and is facilitating an investigation of the genes responsible for aggressive RRP. They have also facilitated the development of similar groups of RRP investigators in Canada and Europe and are currently working with their colleagues outside of the United States to study the benefits of the quadrivalent HPV vaccine for treatment and prevention of RRP.
Other Considerations
Children with newly diagnosed RRP warrant a substantial time commitment on the part of the otolaryngologist to engage the family in a frank and open discussion of the disease and its management. Support groups such as the Recurrent Respiratory Papilloma Foundation (www.rrpf.org) and the International (www.rrpwebsite.org) can be a vital resource for information and support. RRP patients require frequent office visits and endoscopic procedures at the outset to establish the aggressiveness of their disease. The patients are encouraged to return to the office or call as often as necessary while family members and the health care team become familiar with the child’s symptoms and level of distress. While infant home intercom-type monitors are often recommended, apnea-bradycardia monitors and pulse oximetry are generally not necessary. Repeat flexible fiberoptic laryngoscopy may be used in the office, and speech and language therapy is offered early in the course of the disease. Control of other medical factors such as reflux and asthma is also aggressively pursued.
Armstrong LR, Derkay CS, Reeves WC. Initial results from the National Registry for juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 1999;125:743-748.
Dedo HH, Yu KC. CO2 laser treatment in 244 patients with respiratory papillomatosis. Laryngoscope. 2001;111:1639-1644.
Derkay CS, Malis DJ, Zalzal G, et al. A staging system for assessing severity of disease and response to therapy in recurrent respiratory papillomatosis. Laryngoscope. 1998;108:935-937.
Derkay CS. Task force on recurrent respiratory papillomas: a preliminary report. Arch Otolaryngol Head Neck Surg. 1995;121:1386-1391.
Derkay CS, Smith RJ, McClay J, et al. HspE7 treatment of pediatric P: final results of an open-label trial. Ann Otol Rhinol Laryngol. 2005;114:730-737.
Derkay CS. Cidofovir for recurrent respiratory papillomatosis (RRP): a re-assessment of risks. International Journal Pediatric Otolaryngology. 2005;11:1465-1467. Epub 2005 Sep19
Freed G, Derkay CS. HPV vaccines and RRP. International Journal of Pediatric Otorhinolaryngology. 2006;70(November (11)):1-5.
Healy GB, Gelber RD, Trowbridge AL, et al. Treatment of recurrent respiratory papillomatosis with human leukocyte interferon. N Engl J Med. 1988;319:401-407.
Kashima HK, Mounts P, Leventhal B, Hruban RH. Sites of predilection in recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 1993;102:580-583.
Kosko J, Derkay CS. Role of cesarean section in the prevention of recurrent respiratory papillomatosis: is there one? Int J Pediatr Otorhinolaryngol. 1996;1:31-38.
Leventhal BG, Kashima HK, Weck PW, et al. Randomized surgical adjuvant trial of interferon alpha-n1 in recurrent papillomatosis. Arch Otolaryngol Head Neck Surg. 1988;114:1163-1169.
Maloney EM, Unger ER, Reeves RA, et al. Longitudinal measures of HPV 6 and 11 viral loads and antibody response in children with RRP. Arch OTOHNS. 2006;132:711-715.
Mounts P, Shah KV, Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc Natl Acad Sci U S A. 1982;79:5425-5429.
Pasquale K, Wiatrak KB, Wooley A, et al. Microdébrider versus CO2 laser for RRP: a comparative study. Laryngoscope. 2003;113(1):139-143.
Pransky SM, Magit AE, Kearns DB, et al. Intralesional Cidofovir for recurrent respiratory papillomatosis in children. Arch Otolaryngol Head Neck Surg. 1999;125:1143-1148.
Reeves WC, Ruparelia SS, Swanson KI, et al. National registry for juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2003;129(9):976-982.
Rimmel FL, Shoemaker DL, Pou AM, et al. Pediatric respiratory papillomatosis: prognostic role of viral typing and cofactors. Laryngoscope. 1997;107:915-918.
Schraff S, Derkay CS. Survey of ASPO membership regarding management of RRP distal spread and the use of adjuvant therapies. Arch Otolaryngol Head Neck Surg. 2004;130:1039.
Shah K, Kashima HK, Polk BF, et al. Rarity of cesarean delivery in cases of juvenile-onset respiratory papillomatosis. Obstet Gynecol. 1986;68:795-799.
Shah KV, Stern WF, Shah FK, et al. Risk factors for juvenile-onset recurrent respiratory papillomatosis. Pediatr Infect Dis J. 1998;17:372-376.
Shapiro AM, Rimell FL, Shoemaker D, et al. Tracheotomy in children with juvenile-onset recurrent respiratory papillomatosis: the Children’s Hospital of Pittsburgh experience. Ann Otol Rhinol Laryngol. 1996;105:1-5.
Shikowitz MJ, Abramson AL, Steinberg BM, et al. Clinical Trial of photodynamic therapy with meso-tetra (hydroxyphenyl) chlorin for respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2005;131:99-105.
Wiatrak BJ, Wiatrak DW, Broker TR, et al. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope. 2004;114(November (11 Pt 2 Suppl 104)):1-23.
Zeitels SM, Sataloff RT. Phonomicrosurgical resection of glottal papillomatosis. J Voice. 1999;13:123-127.
Zeitels SM, Akst LM, Burns JA, et al. Office-based 532 nm pulsed KTP laser treatment of glottal papillomatosis and dysplasia. Ann Otol Rhinol Laryngol. 2006;115:679-685.
1. Kosko JR, Derkay CS. Role of cesarean section in prevention of recurrent respiratory papillomatosis—is there one? Int J Pediatr Otorhinolaryngol. 1996;35:31.
2. Mounts P, Shah KV, Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc Natl Acad Sci U S A. 1982;79:5425.
3. Vambutas A, Di Lorenzo TP, Steinberg BM. Laryngeal papilloma cells have high levels of epidermal growth factor receptor and respond to epidermal growth factor by a decrease in epithelial differentiation. Cancer Res. 1993;53:910.
4. Schraff S, Derkay CS, Burke B, et al. American Society of Pediatric Otolaryngology members’ experience with recurrent respiratory papillomatosis and the use of adjuvant therapy. Arch Otolaryngol Head Neck Surg. 2004;130:1039.
5. Lindman JP, Lewis LS, Accortt N, et al. Use of the Pediatric Quality of Life Inventory to assess the health-related quality of life in children with recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2005;114:499.
6. Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg. 1995;121:1386.
7. Morgan AH, Zitsch RP. Recurrent respiratory papillomatosis in children: a retrospective study of management and complications. Ear Nose Throat J. 1986;65:19.
8. Reeves WC, Ruparelia SS, Swanson KI, et al. National registry for juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2003;129:976.
9. Ruparelia S, Unger ER, Nisenbaum R, et al. Predictors of remission in juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2003;129:1275.
10. Wiatrak BJ, Wiatrak DW, Broker TR, et al. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope. 2004;114:1.
11. Silverberg MJ, Thorsen P, Lindeberg H, et al. Clinical course of recurrent respiratory papillomatosis in Danish children. Arch Otolaryngol Head Neck Surg. 2004;130:711.
12. Cohn AM, Kos JT2nd, Taber LH, et al. Recurring laryngeal papilloma. Am J Otolaryngol. 1981;2:129.
13. Armstrong LR, Preston EJ, Reichert M, et al. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis. 2000;31:107.
14. Armstrong LR, Derkay CS, Reeves WC. Initial results from the national registry for juvenile-onset recurrent respiratory papillomatosis. RRP Task Force. Arch Otolaryngol Head Neck Surg. 1999;125:743.
15. Lindeberg H, Elbrond O. Laryngeal papillomas: the epidemiology in a Danish subpopulation 1965-1984. Clin Otolaryngol Allied Sci. 1990;15:125.
16. Pfister H, editor. Papillomaviruses and Human Cancer. Boca Raton, Fla: CRC Press, 1990.
17. Rimell FL, Shoemaker DL, Pou AM, et al. Pediatric respiratory papillomatosis: prognostic role of viral typing and cofactors. Laryngoscope. 1997;107:915.
18. Abramson AL, Steinberg BM, Winkler B. Laryngeal papillomatosis: clinical, histopathologic and molecular studies. Laryngoscope. 1987;97:678.
19. Steinberg BM, DiLorenzo TP. A possible role for human papillomaviruses in head and neck cancer. Cancer Metastasis Rev. 1996;15:91.
20. Aaltonen LM, Wahlstrom T, Rihkanen H, et al. A novel method to culture laryngeal human papillomavirus-positive epithelial cells produces papilloma-type cytology on collagen rafts. Eur J Cancer. 1998;34:1111.
21. Swan DC, Vernon SD, Icenogle JP. Cellular proteins involved in papillomavirus-induced transformation. Arch Virol. 1994;138:105.
22. Silverberg MJ, Thorsen P, Lindeberg H, et al. Condyloma in pregnancy is strongly predictive of juvenile-onset recurrent respiratory papillomatosis. Obstet Gynecol. 2003;101:645.
23. Ward P, Coleman DV, Malcolm AD. Regulatory mechanisms of the papillomaviruses. Trends Genet. 1989;5:97.
24. Steinberg BM, Meade R, Kalinowski S, et al. Abnormal differentiation of human papillomavirus-induced laryngeal papillomas. Arch Otolaryngol Head Neck Surg. 1990;116:1167.
25. Smith EM, Pignatari SS, Gray SD, et al. Human papillomavirus infection in papillomas and nondiseased respiratory sites of patients with recurrent respiratory papillomatosis using the polymerase chain reaction. Arch Otolaryngol Head Neck Surg. 1993;119:554.
26. Steinberg BM, Topp WC, Schneider PS, et al. Laryngeal papillomavirus infection during clinical remission. N Engl J Med. 1983;308:1261.
27. Snowden RT, Thompson J, Horwitz E, et al. The predictive value of serum interleukins in recurrent respiratory papillomatosis: a preliminary study. Laryngoscope. 2001;111:404.
28. Stern Y, Felipovich A, Cotton RT, et al. Immunocompetency in children with recurrent respiratory papillomatosis: prospective study. Ann Otol Rhinol Laryngol. 2007;116:169.
29. Koutsky LA, Wolner-Hanssen P. Genital papillomavirus infections: current knowledge and future prospects. Obstet Gynecol Clin North Am. 1989;16:541.
30. Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423.
31. Bennett RS, Powell KR. Human papillomaviruses: associations between laryngeal papillomas and genital warts. Pediatr Infect Dis J. 1987;6:229.
32. CDC. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices. Morbidity Mortality Weekly Review. 2007;56:1-24.
33. Hallden C, Majmudar B. The relationship between juvenile laryngeal papillomatosis and maternal condylomata acuminata. J Reprod Med. 1986;31:804.
34. Shah K, Kashima H, Polk BF, et al. Rarity of cesarean delivery in cases of juvenile-onset respiratory papillomatosis. Obstet Gynecol. 1986;68:795.
35. Kashima HK, Shah F, Lyles A, et al. A comparison of risk factors in juvenile-onset and adult-onset recurrent respiratory papillomatosis. Laryngoscope. 1992;102:9.
36. Shah KV, Stern WF, Shah FK, et al. Risk factors for juvenile onset recurrent respiratory papillomatosis. Pediatr Infect Dis J. 1998;17:372.
37. Tenti P, Zappatore R, Migliora P, et al. Perinatal transmission of human papillomavirus from gravidas with latent infections. Obstet Gynecol. 1999;93:475.
38. Rouzier R, Uzan C, Collinet P. [HPV vaccination: principles, results and future perspectives]. J Gynecol Obstet Biol Reprod (Paris). 2007;36:13.
39. Harper DM. Quadrivalent HPV vaccine prevented cervical neoplasia caused by HPV-16 and HPV-18. ACP J Club. 2007;147:49.
40. Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118:2135.
41. Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26:201.
42. Kashima H, Mounts P, Leventhal B, et al. Sites of predilection in recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 1993;102:580.
43. Shapiro AM, Rimell FL, Shoemaker D, et al. Tracheotomy in children with juvenile-onset recurrent respiratory papillomatosis: the Children’s Hospital of Pittsburgh experience. Ann Otol Rhinol Laryngol. 1996;105:1.
44. Blackledge FA, Anand VK. Tracheobronchial extension of recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2000;109:812.
45. Cole RR, Myer CM3rd, Cotton RT. Tracheotomy in children with recurrent respiratory papillomatosis. Head Neck. 1989;11:226.
46. Boston M, Rutter M, Myer CM3rd, et al. Airway reconstruction in children with recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2006;70:1097.
47. Hester RP, Derkay CS, Burke BL, et al. Reliability of a staging assessment system for recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2003;67:505.
48. Kramer SS, Wehunt WD, Stocker JT, et al. Pulmonary manifestations of juvenile laryngotracheal papillomatosis. AJR Am J Roentgenol. 1985;144:687.
49. Dedo HH, Yu KC. CO2 laser treatment in 244 patients with respiratory papillomas. Laryngoscope. 2001;111:1639.
50. Faust RA. Childhood voice disorders: ambulatory evaluation and operative diagnosis. Clin Pediatr (Phila). 2003;42:1.
51. Cotton RT, Richardson MA. Advances in head and neck surgery in children. Head Neck Surg. 1981;3:424.
52. Hallmo P, Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol. 1991;248:425.
53. Kashima HK, Kessis T, Mounts P, et al. Polymerase chain reaction identification of human papillomavirus DNA in CO2 laser plume from recurrent respiratory papillomatosis. Otolaryngol Head Neck Surg. 1991;104:191.
54. Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41.
55. Dean C, Sataloff RT, Hawkshaw M. Recurrent vocal fold papilloma: resection using cold instruments. Ear Nose Throat J. 1998;77:882.
56. Uloza V. The course of laryngeal papillomatosis treated by endolaryngeal microsurgery. Eur Arch Otorhinolaryngol. 2000;257:498.
57. Zeitels SM, Sataloff RT. Phonomicrosurgical resection of glottal papillomatosis. J Voice. 1999;13:123.
58. Bower CM, Waner M, Flock S, et al. Flash pump dye laser treatment of laryngeal papillomas. Ann Otol Rhinol Laryngol. 1998;107:1001.
59. McMillan K, Shapshay SM, McGilligan JA, et al. A 585-nanometer pulsed dye laser treatment of laryngeal papillomas: preliminary report. Laryngoscope. 1998;108:968.
60. Zeitels SM, Akst LM, Burns JA, et al. Office-based 532-nm pulsed KTP laser treatment of glottal papillomatosis and dysplasia. Ann Otol Rhinol Laryngol. 2006;115:679.
61. Rees CJ, Halum SL, Wijewickrama RC, et al. Patient tolerance of in-office pulsed dye laser treatments to the upper aerodigestive tract. Otolaryngol Head Neck Surg. 2006;134:1023.
62. Zeitels SM, Burns JA, Akst LM, et al. Office-based and microlaryngeal applications of a fiber-based thulium laser. Ann Otol Rhinol Laryngol. 2006;115:891.
63. Pasquale K, Wiatrak B, Woolley A, et al. Microdébrider versus CO2 laser removal of recurrent respiratory papillomas: a prospective analysis. Laryngoscope. 2003;113:139.
64. El-Bitar MA, Zalzal GH. Powered instrumentation in the treatment of recurrent respiratory papillomatosis: an alternative to the carbon dioxide laser. Arch Otolaryngol Head Neck Surg. 2002;128:425.
65. Patel N, Rowe M, Tunkel D. Treatment of recurrent respiratory papillomatosis in children with the microdébrider. Ann Otol Rhinol Laryngol. 2003;112:7.
66. Healy GB, Gelber RD, Trowbridge AL, et al. Treatment of recurrent respiratory papillomatosis with human leukocyte interferon. Results of a multicenter randomized clinical trial. N Engl J Med. 1988;319:401.
67. Leventhal BG, Kashima HK, Weck PW, et al. Randomized surgical adjuvant trial of interferon alfa-n1 in recurrent papillomatosis. Arch Otolaryngol Head Neck Surg. 1988;114:1163.
68. Sen GC. Mechanism of interferon action: progress toward its understanding. Prog Nucleic Acid Res Mol Biol. 1982;27:105.
69. McGlennen RC, Adams GL, Lewis CM, et al. Pilot trial of ribavirin for the treatment of laryngeal papillomatosis. Head Neck. 1993;15:504.
70. Pou AM, Rimell FL, Jordan JA, et al. Adult respiratory papillomatosis: human papillomavirus type and viral coinfections as predictors of prognosis. Ann Otol Rhinol Laryngol. 1995;104:758.
71. Snoeck R, Wellens W, Desloovere C, et al. Treatment of severe laryngeal papillomatosis with intralesional injections of cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine]. J Med Virol. 1998;54:219.
72. Pransky SM, Magit AE, Kearns DB, et al. Intralesional cidofovir for recurrent respiratory papillomatosis in children. Arch Otolaryngol Head Neck Surg. 1999;125:1143.
73. Pransky SM, Albright JT, Magit AE. Long-term follow-up of pediatric recurrent respiratory papillomatosis managed with intralesional cidofovir. Laryngoscope. 2003;113:1583.
74. Pransky SM, Brewster DF, Magit AE, et al. Clinical update on 10 children treated with intralesional cidofovir injections for severe recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2000;126:1239.
75. Naiman AN, Ayari S, Nicollas R, et al. Intermediate-term and long-term results after treatment by cidofovir and excision in juvenile laryngeal papillomatosis. Ann Otol Rhinol Laryngol. 2006;115:667.
76. Naiman AN, Ceruse P, Coulombeau B, et al. Intralesional cidofovir and surgical excision for laryngeal papillomatosis. Laryngoscope. 2003;113:2174.
77. Co J, Woo P. Serial office-based intralesional injection of cidofovir in adult-onset recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2004;113:859.
78. Dancey DR, Chamberlain DW, Krajden M, et al. Successful treatment of juvenile laryngeal papillomatosis-related multicystic lung disease with cidofovir: case report and review of the literature. Chest. 2000;118:1210.
79. Wemer RD, Lee JH, Hoffman HT, et al. Case of progressive dysplasia concomitant with intralesional cidofovir administration for recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2005;114:836.
80. Derkay C. Cidofovir for recurrent respiratory papillomatosis (RRP): a re-assessment of risks. Int J Pediatr Otorhinolaryngol. 2005;69:1465.
81. Shikowitz MJ, Abramson AL, Freeman K, et al. Efficacy of DHE photodynamic therapy for respiratory papillomatosis: immediate and long-term results. Laryngoscope. 1998;108:962.
82. Shikowitz MJ, Abramson AL, Steinberg BM, et al. Clinical trial of photodynamic therapy with meso-tetra (hydroxyphenyl) chlorin for respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2005;131:99-105.
83. Rosen CA, Bryson PC. Indole-3-carbinol for recurrent respiratory papillomatosis: long-term results. J Voice. 2004;18:248.
84. Rosen CA, Woodson GE, Thompson JW, et al. Preliminary results of the use of indole-3-carbinol for recurrent respiratory papillomatosis. Otolaryngol Head Neck Surg. 1998;118:810.
85. Essman EJ, Abramson A. Estrogen binding sites on membranes from human laryngeal papilloma. Int J Cancer. 1984;33:33.
86. Newfield L, Goldsmith A, Bradlow HL, et al. Estrogen metabolism and human papillomavirus-induced tumors of the larynx: chemo-prophylaxis with indole-3-carbinol. Anticancer Res. 1993;13:3371.
87. Wu R, Coniglio SJ, Chan A, et al. Up-regulation of Rac1 by epidermal growth factor mediates COX-2 expression in recurrent respiratory papillomas. Mol Med. 2007;13:143.
88. Lawrence DJ, Bern HA. Mucous metaplasia and mucous gland formation in keratinized adult epithelium in situ treated with vitamin A. Exp Cell Res. 1960;21:443.
89. Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980;605:33.
90. Bollag W, Peck R, Frey JR. Inhibition of proliferation by retinoids, cytokines and their combination in four human transformed epithelial cell lines. Cancer Lett. 1992;62:167.
91. ElAttar TM, Lin HS. Effect of retinoids and carotenoids on prostaglandin formation by oral squamous carcinoma cells. Prostaglandins Leukot Essent Fatty Acids. 1991;43:175.
92. Meyskens FLJr, Gilmartin E, Alberts DS, et al. Activity of isotretinoin against squamous cell cancers and preneoplastic lesions. Cancer Treat Rep. 1982;66:1315.
93. Zou CP, Clifford JL, Xu XC, et al. Modulation by retinoic acid (RA) of squamous cell differentiation, cellular RA-binding proteins, nuclear RA receptors in human head and neck squamous cell carcinoma cell lines. Cancer Res. 1994;54:5479.
94. Bell R, Hong WK, Itri LM, et al. The use of cis-retinoic acid in recurrent respiratory papillomatosis of the larynx: a randomized pilot study. Am J Otolaryngol. 1988;9:161.
95. Pashley NR. Can mumps vaccine induce remission in recurrent respiratory papilloma? Arch Otolaryngol Head Neck Surg. 2002;128:783.
96. Kimberlin DW. Current status of antiviral therapy for juvenile-onset recurrent respiratory papillomatosis. Antiviral Res. 2004;63:141-151.
97. Borkowski G, Sommer P, Stark T, et al. Recurrent respiratory papillomatosis associated with gastroesophageal reflux disease in children. Eur Arch Otorhinolaryngol. 1999;256:370.
98. Harcourt JP, Worley G, Leighton SE. Cimetidine treatment for recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 1999;51:109.
99. Brockmeyer NH, Kreuzfelder E, Chalabi N, et al. The immunomodulatory potency of cimetidine in healthy volunteers. Int J Clin Pharmacol Ther Toxicol. 1989;27:458-462.
100. Ishikura H, Fukui H, Takeyama N, et al. Cimetidine activates interleukin-12, which enhances cellular immunity. Blood. 1999;93:1782.
101. Leman JA, Benton EC. Verrucas. Guidelines for management. Am J Clin Dermatol. 2000;1:143.
102. McKenna M, Brodsky L. Extraesophageal acid reflux and recurrent respiratory papilloma in children. Int J Pediatr Otorhinolaryngol. 2005;69:597.
103. Derkay CS, Hester RP, Burke B, et al. Analysis of a staging assessment system for prediction of surgical interval in recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2004;68:1493.
104. Derkay CS, Malis DJ, Zalzal G, et al. A staging system for assessing severity of disease and response to therapy in recurrent respiratory papillomatosis. Laryngoscope. 1998;108:935.
105. Yadav SP, Gera A, Singh J, et al. Multiple papilloma larynx. Indian J Pediatr. 2000;67:567.
106. Morrison GA, Evans JN. Juvenile respiratory papillomatosis: acyclovir reassessed. Int J Pediatr Otorhinolaryngol. 1993;26:193.
107. Bielamowicz S, Villagomez V, Stager SV, et al. Intralesional cidofovir therapy for laryngeal papilloma in an adult cohort. Laryngoscope. 2002;112:696.
108. Chhetri DK, Blumin JH, Shapiro NL, et al. Office-based treatment of laryngeal papillomatosis with percutaneous injection of cidofovir. Otolaryngol Head Neck Surg. 2002;126:642.
109. Chhetri DK, Shapiro NL. A scheduled protocol for the treatment of juvenile recurrent respiratory papillomatosis with intralesional cidofovir. Arch Otolaryngol Head Neck Surg. 2003;129:1081.
110. Goldstone SE, Palefsky JM, Winnett MT, et al. Activity of HspE7, a novel immunotherapy, in patients with anogenital warts. Dis Colon Rectum. 2002;45:502.