CHAPTER 58 Recurrence of Meningiomas and Its Management
INTRODUCTION
For benign meningiomas, clinically relevant recurrences are common during the patients’ lifetimes. Seemingly complete removal is achieved in 64% to 97% of operated patients1–5 but is curative only in 68% to 80%.1,3,6,7 After surgery of any tumor there is a risk of recurrence. Common sense holds that the risk of recurrence depends on the extent of removal, but also that biological features such as growth rate influence how rapidly a recurrence occurs. Radical surgery with a large margin can be achieved only rarely in the central nervous system. The risk of a minute residual is relatively high and, subsequently, biological variables have a relatively large influence on whether patients risk a tumor recurrence.
Clinically, the “radicality” of meningioma surgery is either defined by the surgeon’s intraoperative assessment,8 which is clearly subjective, or from a postoperative radiological examination.2 Neither strategy is sufficient to exclude completely the presence of residual tumor cells, and pathology exams are only rarely utilized to evaluate the extent of tumor removal. We recently evaluated a series of parasagittal meningiomas and detected residual microscopic meningioma growth in the dural resection margins in 41% of “radical” Simpson grade 1 classed operations.9 The distinction between a recurrence and progressive growth is not of major importance. We cannot be sure whether we are detecting tumor progression or true recurrences. Circumstantial evidence suggests that most recurrences are actually progressing tumor residuals while de novo recurrences occur more rarely. Typically, we refer to returning tumor growth as a “recurrence” whenever initial surgery included radical tumor removal as assessed intraoperatively (Simpson grades 1–2) and postoperative radiologic examinations failed to reveal a residual tumor. Simpson defined only grades 1–2, which included removal or coagulation of the tumor origin as “radical,” while grade 3 constitutes only total removal without managing the tumor origin.8 The intraoperative assessment is subjective and largely influenced by the surgeon’s experience and surgical tactics. Subsequently, the recurrence rates of different series will reflect biological tumor features, surgical goals, and experience; the boundary between recurrence and progression will never be sharp. It has been suggested that “all meningiomas may recur, if the follow-up is long enough,” hence a thorough understanding of the natural history and sufficient follow-up are of utmost importance when treating patients who are expected to have a long life expectancy after treatment.
Extra-axial metastases are rare but still seen regularly, and 60% occur in histologically benign meningiomas. The most common sites are lung, abdominal viscera, vertebral bodies, and other bone sites (reviewed by Drummond10 and in Chapter 60).
FREQUENCY AND CONSEQUENCES OF RECURRENCES
The recurrence rate 20 years after seemingly radical surgery (Simpson grades 1–2) for benign meningiomas was at least 19% in a Finnish population-based study.6 Mirimanoff1 found a 32% recurrence rate after 15 years; Adgebite and colleagues7 found a 37% to 55% recurrence rate at 20 years and Stafford and colleagues3 quoted a 25% recurrence rate in 10 years. Our 18-year follow-up of cranial base meningiomas revealed a 9% to 14% recurrence rate following Simpson grade 1 surgery.9
Subtotal surgery (Simpson grade 4) led to recurrence/progression rates of 81% to 85% in 15 to 18 years.1,9,11 A true long-term study of parasagittal meningiomas (Mathiesen, unpublished) showed that Simpson grade 4 surgery led to progressive growth in all patients who survived for more than 10 years, that these patients needed additional surgery, and that only approximately 10% were alive and independent after 25 years. We have also evaluated long-term outcomes (mean 18 years) after surgery of cranial-base meningiomas. In this series, 69 patients underwent subtotal resection (Simpson grade 4), with 8% stable at follow-up, 10% retreated for progressive tumor growth, and 82% of whom died. Subtotal surgery thus carries a high risk of clinically relevant tumor progression in long-term perspective.
Recurrences are rarer after radical surgery, but their consequences are similar. Recurrence and progression of incompletely removed tumors is the major reason for excess mortality in patients who have a good condition after surgery.9,12,13 Another figure14 that described the impact of recurrences was the finding that olfactory meningiomas led to recurrences in 10% at 10 years and 25% at 20, and that 20% of the patients actually died from the recurrences.
Recurrences After Radiosurgery
Recurrences after radiosurgery have not been systematically studied, and the literature is ambiguous. The aim of radiosurgery is tumor control, which means that the tumor does not progress past a possible treatment-related swelling during the first year after treatment. A smaller proportion of meningiomas were reported to actually shrink during follow-up and long-term control rates were 90% to 95%, which would translate to the favorably low recurrence/progression rate of 5% to 10%.15–19
A difficulty in interpretation of these figures is the fact that best series include a number of small, circumscribed, sometimes incidental, meningiomas. Their growth rates before treatment were unknown, but it is likely that many of these tumors would have had an extremely low progression rate also without surgery. In this case, the causative role of radiosurgery is not obvious and it is probable that tumors with demonstrated growth would have less favorable responses. We have even seen the daunting progression rate of 50% in a subgroup of meningiomas that recurred or progressed during a 5.9-year follow-up after initial microsurgery.9
Grading and Rationale of Radicality
The relationship between “radicality” of surgery and recurrence rates was first analyzed and a classification scheme provided by Simpson in 1957.8 The Simpson grading differentiates surgery according to whether the tumor was only biopsied (grade 5), subtotally removed (grade 4), totally removed but without removal of the origin (grade 3), totally removed with coagulation of the origin (grade 2), or totally removed with removal of the dural origin and underlying bone (grade 1). The risk of recurrence increases with each grade and already mere coagulation of the dural origin instead of complete dural and bony removal doubles the number of future recurrences from 9% to 19%. The need to deal with the tumor origin was corroborated when the findings and figures were repeated by Chan and colleagues2 and Yamashita and colleagues.20
One rationale of the Simpson differentiation among removal of the bony origin, coagulation, or just removing the tumor is that meningiomas, as derived from the mesenchyme, possess a potential of growing invasively in mesenchymal tissues: bone and muscle. Only a limited number of meningiomas affect musculature. Thus, although muscular invasion may be difficult to remove as the dissection planes are poorly defined, the practical impact is limited. In contrast, a relevant relationship to the cranial bone is frequent. Hyperostosis and osteolytic changes at the bony origin are common and their significance has been debated. The hyperostosis is sometimes argued to be a reactive phenomenon, but frank tumor invasion appears to be more common when the actual histology is assessed.21–23 Osteolytic changes, which are rarer, clearly reflect tumor invasion.24
The fact that a benign tumor could recur in spite of radical removal was disturbing and sparked further investigations of tumor borders. Borovich and colleagues25,26 described isolated meningioma nests that surround the tumor origin and were detectable at distance from the visible tumor bulk. Simpson’s concept of radicality was expanded by Borovich and colleagues25,26 and Al Mefty and colleagues,27 who described a “grade 0 removal” for convexity meningiomas: a dural margin of 2 cm was removed after regular tumor removal; during an albeit short follow-up of 5.6 years grade 0 surgery did not lead to recurrences.
Simpson 4 Gamma
The Simpson classification is inadequate to describe radiosurgery. Pure radiosurgery may not need any specific classification, as the radiation is sufficiently quantified and defined through a dose plan. The situation is different when combining micro- and radiosurgery. The intentional upfront combination of two different meningioma treatments, microsurgery and radiosurgery, is different from subtotal surgery and from radiosurgery. The term “Simpson 4 gamma” was used for such a combination of tailored microsurgery and immediate radiosurgery for the residual in 3 months.28 The classification made sense when applied to meningiomas growing into venous sinuses. In a retrospective analysis of 100 patients with meningiomas that grew into large venous sinuses, Simpson grade 1 surgery led to a recurrence rate of 10%. Patients with deliberate nonradical surgery (Simpson grade 4) had a tumor recurrence rate of 72% while a combined treatment of upfront Gamma Knife ® radiosurgery after a tailored microsurgical resection (Simpson 4 gamma) allowed return to a low recurrence rate of 10%.
Interestingly, we found a major difference between a 90% control rate after upfront radiosurgery for a residual (Simpson 4 gamma) and a 50% control rate for secondary treatment of a demonstrated tumor recurrence. The figures may be influenced by a selection bias, but the actual progression rate for Simpson grade 4 was 73%, indicating that a selection bias could not explain the entire difference. An important factor in combining micro- with radiosurgery is the deliberate tumor tailoring for radiosurgery. Kondziolka and colleagues29 followed a series of patients with parasagittal meningiomas after radiosurgery and detected a 20% complication rate while we found no radiosurgical complications. The difference is that smaller volumes were targeted when radiosurgery was an integral part of a deliberate combined treatment strategy: Microsurgery was tailored to produce small tumor volumes that were suitable for safe radiation. Radiosurgical risk is strongly related to the radiated volumes and patients with larger tumors were included in the series by Kondziolka and colleagues.29
Location
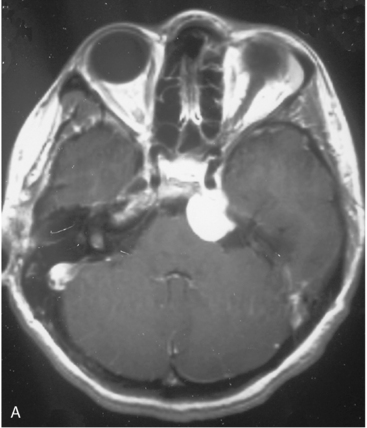
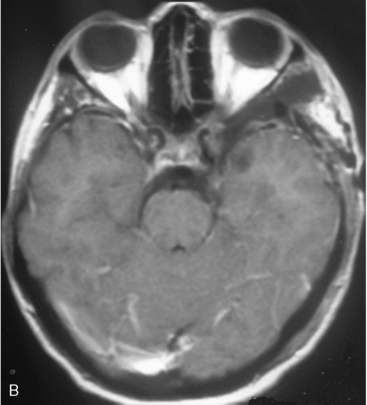
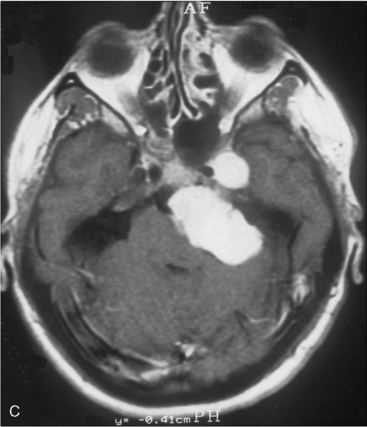
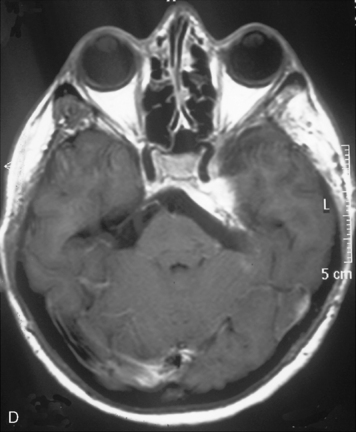
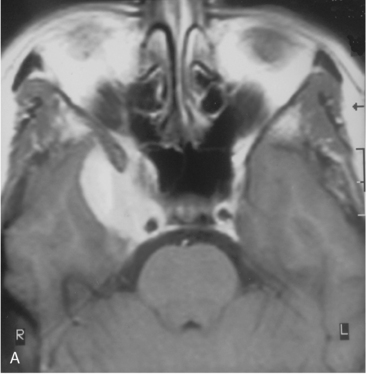
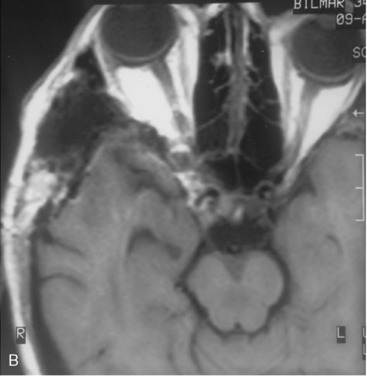
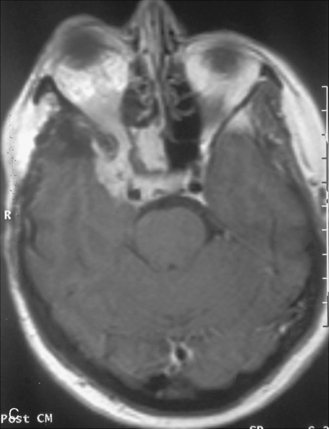
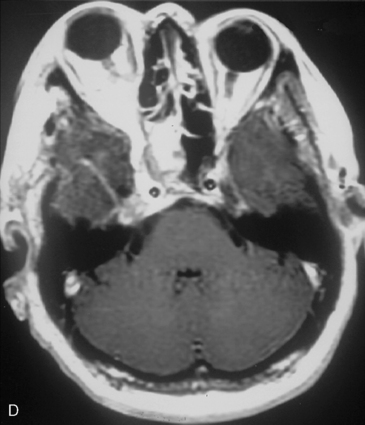
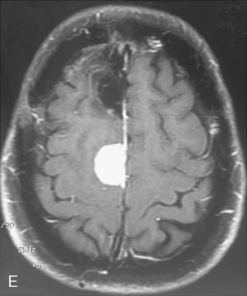
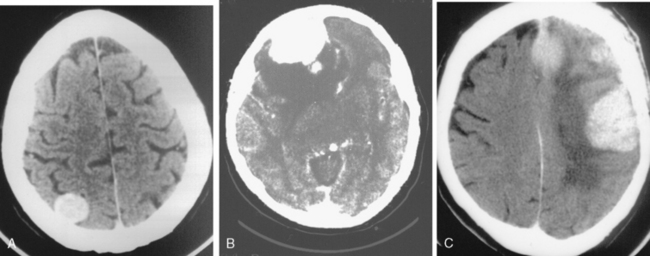
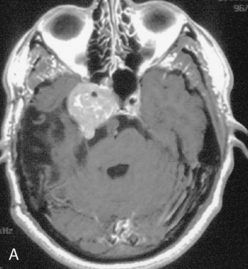
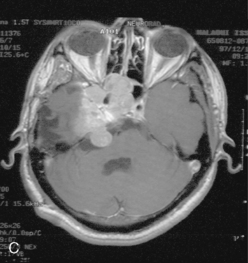
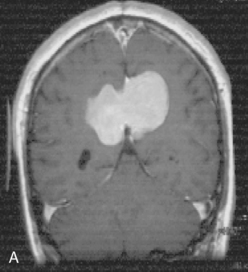
The recurrence rates vary in different meningioma locations. Location affects the risk of recurrence in two ways. First, a readily accessible tumor is easier to remove radically than a tumor with growth onto or into venous sinuses or in the cranial base. A specific location probably represents a certain likelihood of radical surgery and a location is usually not a risk factor that is independent of surgical strategy, tactics, and experience. Typically, deep locations in the cranial base and locations where tumor grows into major vessels could be expected to pose specific problems with radicality and definition of tumor margins. Jääskeläinen and colleagues6 found that recurrence after seemingly complete removal was more likely in the sphenoid ridge and olfactory sites: the 20-year recurrence rates were 7% for suprasellar meningiomas, 18% and 21% for convexity and parasagittal locations, and 29% and 36% for sphenoid wing and olfactory meningiomas, respectively. The latter finding agrees with Philippon30 and Simpson.8 Petroclival locations are rarer, but constitute another cranial-base location with high recurrence rates12 (Fig. 58-1). The high frequency of subtotal surgery explains why tumor recurrence isrelatively common around the medial sphenoid wing and the cavernous sinus12 (Fig. 58-2). Meningiomas in close proximity of venous sinuses had a 10-year recurrence rate after radiologically radical removal of 40% versus 15%.31 Similarly, parasagittal meningiomas also carry a higher risk of recurrence8,12 (Fig. 58-3).
The findings differ slightly depending on whether a tumor is believed to be totally removed or whether a subtotal removal has been deliberately undertaken and on the experience of the surgeon. Typically, the degree of bone work to remove the entire tumor origin and to reach behind corners and into invaded canals affects radicality in the cranial base while bypass surgery and vascular reconstructions affect surgical tactics in tumors that affect large veins and arteries. Typically, sphenoid wing meningiomas that were believed to be radically removed, were found to recur at high rates.6,9 Cranial base approaches provide wide exposures for these cranial base tumors, comparable even to convexity meningiomas, allowing for more complete resections.
Spinal meningiomas were less studied. Elderly patients with spinal meningiomas had recurrence rates of 4% to 5%32,33 while the group younger than 50 years of age had a recurrence rate of 22%.32 Another technical aspect is the membranous, vascular, arachnoid-like extension that frequently surrounds meningiomas. It was described by Simpson8 as “thin, flat, fringe,” and it probably corresponds to the “dural tail” seen on imaging.34 This structure contains meningioma cells in approximately 50% of patients.
Second, the likelihood of aggressive behavior varies with location. The risk of aggressive or malignant features is highest in parasagittal and convexity locations. Interestingly, the risk of malignancy is three times higher in males than females35 and in addition, the sex distribution varies with location. Parasagittally and in the convexity two thirds of tumors are in females; in the cranial base three-fourths are in females and, finally, 9/10 spinal meningiomas are in females (Westerlund et al., unpublished).
Recurrences thus vary with the locations, but the main variable is still surgical radicality. If surgical techniques are chosen with the objective to be radical, which may not always be indicated if surgical risk increases, location loses its prognostic value. In a recent study of only Simpson grade 1 to 2 operations where radicality was carefully controlled, recurrences became independent of location.36
Timing of Recurrence
The median times to recurrence were stated to be 7.5 years for benign, 2.4 years for atypical, and 3.5 for anaplastic meningiomas.6 The time between surgery and recurrence depends on how much tumor was left and how rapidly it regrows, but the time to detection also includes the method and length of follow-up. A true long-term follow-up study is difficult to make and cannot be expected to be prospective. The fact that meningiomas are potentially curable although late recurrences have been described mandates knowledge of long-term outcomes of more than 20 years. The mean time to recurrences seems to be longer in studies with longer follow-up.9,30,37,38 Philippon had two groups of patients with recurrent tumor and either a 5- to 10-year or a 10- to 15-year follow-up30 and found not only that the time to follow-up was longer in the second group, but also that one third of the recurrences occurred after 10 years. In a study of 362 cranial base meningiomas,9 approximately 60% of all recurrences occurred in the interval 1 to 10 years and 40% between 10 and 20 years. Two true long-term series6,9 have a few patients with negative radiologic follow-up between 15 and 20 years, who subsequently developed tumors. A majority of recurrences occur early, with more than half of all diagnoses within 5 years,9,30,39 but the cumulative incidence of recurrence increased steadily up to 20 years without any signs of stabilizing to a plateau in the studies that actually followed the patients over a long term. It must be kept in mind that the group that can be affected by recurrences decreases steadily as occurrences occur or as patients die. It is thus reasonable to keep patients in radiologic follow-up beyond 20 years, although it appears reasonable to increase intervals between scanning.
One recurrence can be followed by additional ones, but the intervals to the next recurrence are not easily predictable. Mirimanoff and Böker found that in a majority of patients with multiple surgeries the time between multiple recurrences kept decreasing.1,40 In our analysis, we found that approximately one third of the patients had longer intervals, one third had similar, and only one third had a shortened interval between diagnoses of new recurrences.9
The other factor that affects timing is the mode of follow-up. Today, magnetic resonance imaging (MRI) is the tool of choice, as it is more sensitive and avoids unnecessary exposure to ionizing radiation. The available long-term studies were, however, initiated before common access to MRI and have initially utilized either pure clinical follow-up or computed tomography (CT) scanning. The sensitivity increases as imaging becomes more refined and subsequently any growing lesion would be detected earlier by more modern technology. MRI is more sensitive than CT, and CT imaging is more sensitive than clinical follow-up. Chan and colleagues2 found a mean time to recurrence of 5.7 years with clinical follow-up but only 2.9 years with CT scanning.
Biological Markers of Increased Recurrence Rates
Regarding age and sex, conflicting data have been published,7,41–44 but younger age and male sex appear to correlate with a higher incidence of recurrences. A younger age confers a relatively higher chance of a rapidly growing lesion, because it has, at least theoretically, had fewer years to grow. A gender effect is likely, as malignant meningiomas are more frequent in men than in women,35 but epidemiologic findings may vary with selection bias of different meningioma subgroups and their definition.
HISTOPATHOLOGY
Traditionally, beginning with Cushing and Eisenhart, the meningiomas were classified into subtypes depending on their microscopic appearance.45 These subtypes, however, had little prognostic value. Instead, the WHO classification has a high correlation to prognosis (Table 58-1). WHO histopathologic classification divides meningiomas into benign (WHO grade I), aggressive (WHO grade II), or anaplastic (malignant; WHO grade III) variants.46 Certain histopathologic criteria (Table 58-2) are associated with a more aggressive behavior. WHO grade II and III tumors are diagnosed when histopathologic features such as brain invasion, sheeting, macronucleoli, hypercellularity, necroses, nuclear atypia, small cells, and a high number of mitoses are detectable.46,47 In addition, some of the rarer histologic subtypes may show aggressive behavior: the rare tumors that are classified as atypical, clear cell, chordoid, rhabdoid, or anaplastic meningiomas are graded as II or III.
TABLE 58-1 Meningiomas with different histopathological characteristics
| Meningiomas with low risk of recurrence and/or aggressive growth | |
| Meningothelial meningioma | WHO grade I |
| Fibrous/fiberblastic meningioma | WHO grade I |
| Transitional (mixed) meningioma | WHO grade I |
| Psammomatous meningioma | WHO grade I |
| Angiomatous meningioma | WHO grade I |
| Macrocystic meningioma | WHO grade I |
| Secretory meningioma | WHO grade I |
| Lymphoplasmacyte-rich meningioma | WHO grade I |
| Metaplastic meningioma | WHO grade I |
| Meningiomas with greater risk of recurrence and/or aggressive growth | |
| Atypical meningioma | WHO grade II |
| Clear cell meningioma (intracranial) | WHO grade II |
| Chordoid meningioma | WHO grade II |
| Rhabdoid meningioma | WHO grade III |
| Papillary meningioma | WHO grade III |
| Anaplastic (malignant) meningioma | WHO grade III |
| Further: Meningiomas of any subtype or grade with high proliferation index and/or brain invasion. | |
TABLE 58-2 WHO criteria for meningioma grading
WHO Grade I
About 80% of all meningiomas are slow-growing tumors of WHO grade I. Any histologic variant is compatible with WHO grade I, except for the chordoid, clear-cell, papillary, and rhabdoid meningiomas, which are consistently associated with more aggressive clinical features and higher WHO grades. The histologic variants most commonly diagnosed in pathology specimens are meningothelial, fibrous, and transitional meningioma. After gross total resection, benign meningiomas are associated with 5-year recurrence rates of only 5%.47 They have similar clinical behavior in spite of the fact that they have a striking difference in their relation to the “meningioma chromosome,” chromosome 22. The most frequent genetic aberration in meningiomas, inactivation of the NF2 gene on chromosome 22, hardly occurs in meningothelial meningiomas.48
WHO Grade II
Atypical meningiomas constitute 15% to 20% of meningiomas. The diagnostic criteria are histopathological (see Table 58-2) and include the subtypes atypical, clear-cell, and chordoid meningiomas. The estimated recurrence rate for totally resected atypical meningiomas is about 40% at 5 years, and this continues to increase with additional follow-up time.46,49,50
WHO Grade III
Anaplastic meningiomas account for 1% to 3% of all meningioma cases. The tumors are anaplastic (see Table 58-2) and include papillary, rhabdoid, and anaplastic subtypes. These tumors have clinical characteristics similar to truly malignant tumors, which can invade neighboring tissues and form metastases. Anaplastic meningiomas are associated with recurrence rates of up to 50% to 80% after surgical resection and median survival is less than 2 years.46,49,50
MARKERS OF CELL GROWTH RATE: PROLIFERATIVE INDEX
Cell death has not been sufficiently studied in this context. Necrosis is not a common feature of meningiomas unless they are aggressive and the fraction of cells with apoptotic features appears to be stable around 1% or less in our experience. Theoretically, a diminished rate of cell death would be expected to correlate with more rapid growth. In agreement, Maiuri and colleagues36 found increased levels of the anti-apoptotic protein Bcl-2 in recurring tumors. Others have instead found signs of increased apoptosis in the more aggressive tumors.51,52 These discrepancies reflect an insufficient understanding of tumor kinetics, as small differences in cell death and proliferation may, when iterated many times, lever to large differences in tumor growth rate. In addition, proliferation and stagnation may be simultaneous in different regions of a tumor.
Hitherto, the study of proliferating cells has provided more robust findings. Markers of proliferating cells are valuable to prognosticate tumor growth and recurrence. The mitotic index, which is cited as a relevant marker for WHO grading (see Table 58-2), is calculated from the visual detection of mitoses. The portion of proliferating cells can also be reflected by specific markers. S-phase fraction, proliferating cell nuclear antigen (PCNA), bromodeoxyuridine (BrdU) uptake, and agyrophilic nucleolar organizer regions (AgNORS) all have a correlation with proliferation and recurrences.53,54 The PCNA is a nuclear protein, also known as Ki-67, that is stained with the antibody MIB-1. This is the most used marker of proliferative activity.55,56 MIB-1/Ki-67 is expressed during all phases of the cell cycle except G0,57,58 with a peak in the G2 and M phases. Subsequently, MIB-1 expression differs between WHO grade I (mean 3.5%, range 0–58), WHO grade II (mean 11.9%, range 3–30), and WHO grade III (mean 18.2%, range 5–30). The proliferative indices by themselves are not necessarily sufficient for prediction of recurrence and their clinical value has been questioned.59 The reality is complex. The findings of Roser are not unique in the overlap of MIB-1 expression ranges between the groups. Some of the highest values were actually found in grade I tumors and many recurring tumors had low MIB-1 indices. The distribution of proliferative indices is skewed, as the majority of patients have very low indices below 2% to 4%. This and the needs to stratify for location or radicality are reasons to why proliferation indices are difficult to evaluate. Regardless of these difficulties, MIB-1 expression is a relevant marker. Vankalakunti and colleagues60 found a mean MIB-index of 4.2 in tumors that recurred compared to 2.7 in nonrecurring WHO grade I tumors. The risk of recurrence was 4-fold higher in tumors with a MIB-1 index >2.6. Hoshino and colleagues61 have stated that a MIB-1 index above 1% predicted recurrence. In malignant subtypes, Ki-67/MIB-1 also correlates with prognosis62 and MIB-1 expression was prognostic for tumor control after Gamma Knife ® radiosurgery of meningioma residuals.63 The MIB-1 index is an independent predictor of recurrence, but all laboratories need to establish their own cutoff values. Tumors with increased indices should be followed closely. It is reasonable to accept a higher surgical risk to achieve a radical removal of tumors with high proliferative potential. It is still possible to cure patients with high proliferative indices, although it is not permissible to consider tumors with indices below the cutoff range as harmless.
HORMONE AND GROWTH FACTOR RECEPTOR EXPRESSION
Tumors are typically influenced by growth factor signaling, and aberrant growth factor receptor expression or growth factor production is typical in tumorigenesis. A number of growth factors and their receptors are frequent in meningiomas as well as in other tumors (for review see Perry44): epidermal growth factor receptor (EGFR), platelet-derived growth factor (PDGF) β receptor, growth hormone (GH) receptors, somatostatin receptors, and fibroblast growth factor (FGF). Although they may have biological significance for tumorigenesis and implications for treatment, and PDGFR activity is increased in aggressive tumors.64,65 EGFR and PDGFR can be activated by EGF and transforming growth factor-α (TGF-α),66 which are present in meningioma cells65,67,68 and cerebrospinal fluid (CSF) from meningioma patients.69,70 The expression of these factors has, however, not shown any prognostic significance.
Insulin-like Growth Factor
The insulin-like growth factor (IGF) system is relevant for meningioma tumorigenesis, but the exact mechanisms are unknown. Both IGF-II and IGFBP2 are expressed in meningiomas, and increased concentrations of IGF-II are associated with invasiveness and malignant progression.71
Progesterone Receptors
Sex hormone receptors were considered important in meningiomas because meningiomas may grow during pregnancy and are predominant in females. Estrogen receptors have, however, little pathogenic or prognostic significance.72 Surprisingly, progesterone receptors are associated with benign tumors and a lower likelihood of tumor recurrence44,73,74 and seem to be lost in tumor progression.
MARKERS OF INVASION AND EDEMA
Vascular Endothelial Growth Factor and Tissue Proteases
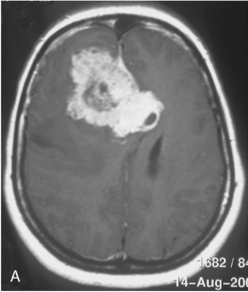
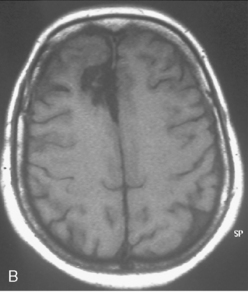
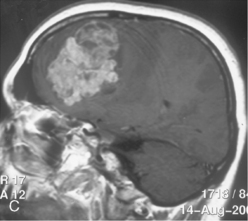
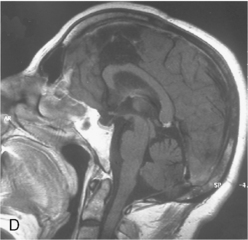
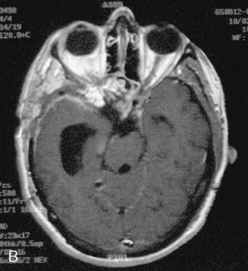
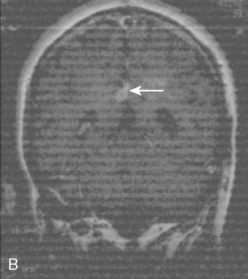
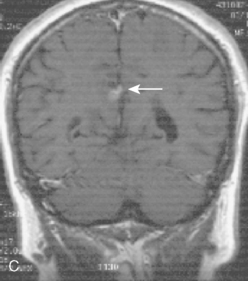
Vascular endothelial growth factor (VEGF) affects the vasculature both as an angiogenic factor and as a factor that can promote fluid transport over the basal membranes. Its expression appeared to correlate with edema75,76 and with increased risk of recurrence.77 Its functions are, however, complex as many aggressive meningiomas were sparsely vascularized and other studies failed to confirm a strong prognostic influence (Fig. 58-4). A similar correlation with invasiveness, edema, and prognosis was detected for the tissue metalloprotease MMP-9.78–80 This field is expanding, and basal membrane degrading mechanisms appear to be relevant for prognosis and recurrences.81 These preliminary findings are hardly surprising, as basal membrane degradation is relevant for tumor invasion and vascularization.
CHROMOSOMAL ABERRATIONS
Several chromosomal aberrations were described in meningiomas, but only a few specific aberrations were prognostic. It is possible that the number of aberrations correlates independently with clinical behavior because atypical and malignant tumors can be expected to have more karyotypic abnormalities than benign. An average of 2.9 genomic aberrations was found in benign, 9.2 in atypical, and 13.5 in malignant meningiomas.82 Typically losses have been described on 1p, 2p, 6q, 10, 14q, and 18q and gains on 20q, 12q, 15q, 1q, and 17q (for review see Drummond83). Losses of chromosomes 1, 9, 10, 14, and 17 are more frequent with higher tumor grades and losses on 1q and 14q appear to be particularly associated progression from benign to more aggressive meningiomas.84–86 Chromosome 9 may also be relevant, as chromosome 9p21 deletions were reported in the malignant progression of meningiomas and the prognosis of anaplastic meningiomas.87 The most common abnormality is a loss of chromosome 22 and decreased synthesis of the NF2 gene product Merlin, which normally inhibits cellular proliferation when binding to CD44. Chromosome 22 aberrations, however, are not indicative of recurrences.
Chromosome 1
1p deletions increase with tumor grade (13%–26% WHO grade I, 40%–76% WHO grade II, 100% WHO III)88,89 and correlate with recurrence.90,91 Both 1p34 and 1p36 have shown prognostic relevance. In addition to structural chromosomal aberrations, epigenetics are of importance. Genetic alterations in the methylation status of p73 or RASSF1A along with 1p36 LOH may result in the malignant transformation of a meningioma.92 Coexistence of –14 and del(1p36) in the ancestral tumor cell clone, together with tumor size, represented the best combination of independent prognostic factors for the identification of those patients with a high risk of an early relapse.93
Telomerase Activity
Telomerases are enzymes that stabilize telomere length and are necessary for unlimited cell proliferation. Telomerase is usually not active in most normal tissues, but reactivated in cancer. Telomerase is much more active grade II and III meningiomas than in grade I, and its activity appears to be an independent prognostic factor that may predict rapid recurrences also in grade I tumors.94
RADIOLOGIC PREDICTORS
With radiologic predictors, we think of imaging parameters that are detectable before surgical data and histopathology are available. The radiologic predictors of recurrence reflect either a location with an increased recurrence rate or features related to histologic and clinical features of aggressive behavior. Typically, the more aggressive tumors are larger and have more edema.75,95 In contrast to the more common lobular shape, a mushrooming growth pattern is also indicative of a more aggressive behavior and frequent recurrences.96–98 Contrast enhancement may be either homo- or heterogeneous; the latter is associated with necrosis and nucleolar prominence99 and a higher recurrence rate.
Bone involvement and its nature may also predict recurrences. Osteolytic changes were related to a more aggressive behavior.24 Hyperostosis, which correlated with recurrences,12 is probably more likely to represent tumor growth in the hyperostotic bone.99 This may or may not dispose to recurrence depending of the radicality of bone work.99 Another feature that correlates with the ease of achieving radicality is the presence of a dural tail. A tail appears to contain actual tumor cells in approximately half of all cases, and may thus predispose to recurrence depending on how radically it was treated.34
RECURRENCE OUTSIDE THE ORIGINAL LOCATION: MULTICENTRICITY AND MENINGIOMATOSIS
Both multicentric meningiomas and meningiomatosis are known phenomena. The latter diagnosis indicates that the individual tumors are not countable. Meningiomas can also metastasize, usually to the lungs or liver, but extremely rarely. Distant metastases are covered in Chapter 60 and are not further addressed here. Sporadic multiple meningiomas were found in 1% to 8%.100 The reasons are twofold. Meningiomas were reported to have multicentric growth with islands of meningioma in a neoplastic field, a 4 cm region of the dura that surrounds the main tumor.25 Even after Simpson grade 1 removal, continued growth of such clusters may explain regional recurrences outside of the initial tumor origin. Similarly, CSF spread has been described, and cannot necessarily be differentiated from other mechanisms of multicentricity.
Ionizing radiation, which is the only known risk factor for meningiomas,101 is also likely to cause multiple hits in exposed tissues. Subsequently, radiation induced meningiomas are frequently multiple102 and have genetic abnormalities that differentiate them from sporadic meningiomas.103
TREATMENT OF RECURRENT MENINGIOMAS
Surgery
The surgical goal can be either curative or symptomatic. Curative surgery needs to consider aggressive drilling to remove osseous tumor in the skull or cranial base. Meningioma tissue may hide behind corners and in cranial canals.9 Further, sufficient exposure is necessary, especially to deal with a recurrence. Depending on the location, venous and arterial sacrifice and subsequent reconstruction may be necessary for radicality. To avoid unwarranted morbidity, small residuals can be treated by radiosurgery after microsurgery. In older patients and in extensive lesions, adjuvant surgery is instead to be preferred. Even repeated decompression and debulking can prolong good quality life for extended periods of time if carefully planned and executed.
Radiosurgery
Radiosurgery refers to a single high-dose targeted treatment of a radiologically defined lesion with the Leksell Gamma Knife ® 104 or a LINAC.105 Single-dose radiosurgery is well established for small lesions and long-term control, equating to cure, is considered possible in a large fraction of the treated patients.104 It has not been studied systematically for recurrent tumors, although tumor controll is to be expected also in recurrent cases.106,107 Technically, small recurrent tumors are good targets for radiosurgery provided they can be clearly defined on radiologic images and are not too close to the surface or deep sensitive structures. Scarring, unclear tumor margins, and metal implants that distort radiologic imaging make radiosurgery difficult to apply. It also appears that tumor control for recurrent tumors is modest in comparison to primary tumors or residuals that have not progressed.9,104 Tumor control is also worse with tumors that show signs of aggressive histology or anaplasia.3,28,108 Malik and colleagues109 reported a 51% and Harris a 83% progression-free survival in 5 years for aggressive meningiomas, the figures are 0 and 72%, respectively, for malignant meningiomas.
Radiosurgery is best indicated when renewed surgery is disadvantageous, when microsurgery is not expected to be radical or as part of a Simpson 4 gamma approach (Figs. 58-5 and 58-6). Individual decision making is necessary: it may be unreasonable to subject a patient to a large procedure for a small recurrence that appears controllable by radiosurgery, and likewise, a series of radiosurgical treatments can be a way of controlling tumors in spite of repeated out-of-field recurrences. On the other hand, subtotal treatment of large lesions or lowering doses below the levels known to be efficient cannot be expected to provide tumor control.
Radiotherapy
The biological impact by fractionated radiotherapy is fundamentally different from single-dose radiosurgery, and data cannot be extrapolated. Radiotherapy is frequently used for incompletely controlled tumors and retrospective data suggest good control rates.110 Kokubo and colleagues111 reported a local control rate of 41% for benign recurrent and 20% for atypical or malignant recurrent meningioma. These studies, however, use actuarial data and the encouraging long-term figures are not completely reliable, as only a small fraction of the patients are actually followed long term. A high projected control rate with actuarial statistics should not be confused with an actual cure rate. Other studies indicate a high incidence of complications and a low likelihood of cure.13 Interestingly, the impact of radiotherapy was modest in Palmas series of aggressive and malignant meningiomas.112 Technological advancements are, however, continuous. Better imaging and refined dose-planning procedures such as by intensity modulation can improve control rates and decrease complications after fractionated radiotherapy.
Another development is fractionated stereotactic radiotherapy. The rationale is to combine the benefit of fractionation, which would allow radiation of sensitive structures such as the optic nerve and brain stem with the steeper dose gradient, which is the hallmark of stereotactic radiosurgery. Fractionated radiotherapy was delivered stereotactically with reports of very good results for even for large inoperable meningiomas,113 but so far we and others have not been able to reproduce these findings; we have seen high complication rates and inferior tumor control in a few selected patients referred for stereotactic fractionated treatment of large tumors, while small tumors around the optic apparatus have responded very well.
Another mode of focusing radiation to the relevant tissues is boron neutron capture therapy (BNCT), which has had benefit in a small number of desolate cases of malignant meningiomas.114
Medical Treatment
A number of different putative chemotherapeutic agents have been tested for meningiomas, but we still lack effective therapies. Several agents that are used for glioma chemotherapy were ineffective in meningiomas, among them temozolomide.115 Several other agents have been tested with disappointing results while others have modest effects in selected patients.
Interferons may exert its effect on vascularization and interferon α-2B provided a few promising responses. Unfortunately, the treatment is not well tolerated and does not lead to a cure; it has not become an established treatment.116
Hydroxyurea is another drug that was claimed to induce apoptosis in meningioma cells and reported to be very effective in a group of selected patients.117,118 Later data, however, showed no cures and little effect in recurrent and anaplastic tumors. Nevertheless, with modest efficacy and low toxicity, hydroxyurea is another treatment that could be considered for progressive tumors that have failed other treatment modalities.117–120
The medical treatments that have been most studied in meningiomas target the hormonal receptors. The antiprogesterone agent mifepristone had limited effect in early human studies,121,122 while a subsequent phase III trial showed no benefit.123 Estrogen receptors are less common, and subsequently the estrogen receptor antagonist tamoxifen had no effect in meningiomas.124
Another approach that offers promise, but has not been clinically tested, is the utilization of existing drugs that target mechanisms that regulate meningioma growth. EGFR and PDGFR are present in meningiomas and their activation with subsequent induction of MAPK/ERK may be relevant in meningioma growth.125 COX-2 is also a possible target, as increased prostaglandin production stimulates growth via the Ras-MAPK pathway and cytosolic arachidonic acid levels are high in meningiomas.126 The COX-2 antagonist Celecoxib inhibits meningioma growth in vitro.127 Likewise, drugs that target the IGF and GH systems are available, but have so far have not been evaluated for meningiomas.
FOLLOW-UP
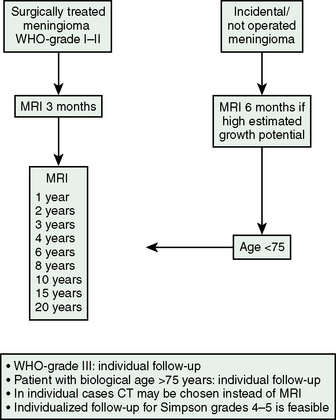
The follow-up data cited in the preceding text indicate that the median times to recurrence are between 5 and 10 years, that more than one third of recurrences occur after 10 years and that the mean time to recurrences increases with the length of follow-up without a cutoff below 20 years. We found two recurrences of skull-base meningiomas after 20 years in patients with normal scans at 15 and 17 years.9 Regular long-term follow-up with imaging is thus indicated. The higher sensitivity and absence of exposure to radiation, which in itself constitutes a risk factor for meningiomas, argue in favor of imaging via MRI. Experience suggests that follow-up should not be terminated for 10 to 15 years and that imaging intervals should be annual initially while longer intervals are sufficient in later stages. The follow-up should probably be tailored according to the surgical and histologic findings. The most important prognostic factors are radicality, WHO grade, and proliferative index. For aggressive or rapidly growing tumors, initial examinations should be at 6-month intervals. We have no access to data that define patients to follow longer or when follow-up could be terminated after a shorter interval and the theoretical considerations are conflicting. The most slowly growing tumors may be followed for the longest intervals, as a potential recurrence will take long to develop. A follow-up algorithm thus risks being counterintuitive and complicated: the most radically operated slow-growing tumors would be the ones that could remain silent for the longest periods before a possible recurrence. We are at present following a follow-up strategy with follow-up until 20 years, but with very long intervals between imaging after 10 years (Fig. 58-7), and suggest individual tailoring depending on the patient, age, and tumor characteristics.
[1] Mirimanoff R.O., Dosoretz D.E., Linggood R.M., et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18-24.
[2] Chan R.C., Thompson G.B. Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. A retrospective study in 257 cases. J Neurosurg. 1984;60:52-60.
[3] Stafford S.L., Perry A., Suman V.J., et al. Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc. 1998;73:936-942.
[4] Giombini S., Solero C.L., Lasio G., Morello G. Immediate and late outcome of operations for parasagittal and falx meningiomas. Report of 342 cases. Surg Neurol. 1984;21:427-435.
[5] Giombini S., Solero C.L., Morello G. Late outcome of operations for supratentorial convexity meningiomas. Report on 207 cases. Surg Neurol. 1984;22:588-594.
[6] Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26:461-469.
[7] Adegbite A.B., Khan M.I., Paine K.W., Tan L.K. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51-56.
[8] Simpson D. Recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
[9] Mathiesen T., Lindquist C., Kihlstrom L., Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39:2-7. discussion 8–9
[10] Drummond K.J., Bittar R.G., Fearnside M.R. Metastatic atypical meningioma: case report and review of the literature. J Clin Neurosci. 2000;7:69-72.
[11] Taylor B.W.Jr, Marcus R.B.Jr, Friedman W.A., et al. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299-304.
[12] Kallio M., Sankila R., Hakulinen T., Jaaskelainen J. Factors affecting operative and excess long-term mortality in 935 patients with intracranial meningioma. Neurosurgery. 1992;31:2-12.
[13] Mathiesen T., Kihlstrom L., Karlsson B., Lindquist C. Potential complications following radiotherapy for meningiomas. Surg Neurol. 2003;60:193-198. discussion 199–200
[14] Mathiesen T., Kihlstrom L. Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression. Neurosurgery. 2006;59:570-576. discussion 570–6
[15] Kreil W., Luggin J., Fuchs I., et al. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76:1425-1430.
[16] Lee J.Y., Kondziolka D., Flickinger J.C., Lunsford L.D. Radiosurgery for intracranial meningiomas. Prog Neurol Surg. 2007;20:142-149.
[17] Mindermann T., de Rougemont O. The significance of tumor location for Gamma Knife treatment of meningiomas. Stereotact Funct Neurosurg. 2004;82:194-195.
[18] Nicolato A., Ferraresi P., Foroni R., et al. Gamma Knife radiosurgery in skull base meningiomas. Preliminary experience with 50 cases. Stereotact Funct Neurosurg. 1996;66(Suppl. 1):112-120.
[19] Nicolato A., Foroni R., Alessandrini F., et al. Radiosurgical treatment of cavernous sinus meningiomas: experience with 122 treated patients. Neurosurgery. 2002;51:1153-1159. discussion 1159–61
[20] Yamashita J., Handa H., Iwaki K., Abe M. Recurrence of intracranial meningiomas, with special reference to radiotherapy. Surg Neurol. 1980;14:33-40.
[21] Bonnal J., Thibaut A., Brotchi J., Born J. Invading meningiomas of the sphenoid ridge. J Neurosurg. 1980;53:587-599.
[22] Pieper D.R., Al-Mefty O., Hanada Y., Buechner D. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 1999;44:742-746.
[23] Pompili A., Derome P.J., Visot A., Guiot G. Hyperostosing meningiomas of the sphenoid ridge–clinical features, surgical therapy, and long-term observations: review of 49 cases. Surg Neurol. 1982;17:411-416.
[24] Olmsted W.W., McGee T.P. Prognosis in meningioma through evaluation of skull bone patterns. Radiology. 1977;123:375-377.
[25] Borovich B., Doron Y. Recurrence of intracranial meningiomas: the role played by regional multicentricity. J Neurosurg. 1986;64:58-63.
[26] Borovich B., Doron Y., Braun J., et al. Recurrence of intracranial meningiomas: the role played by regional multicentricity. Part 2: clinical and radiological aspects. J Neurosurg. 1986;65:168-171.
[27] Kinjo T., al-Mefty O., Kanaan I. Grade zero removal of supratentorial convexity meningiomas. Neurosurgery. 1993;33:394-399. discussion 399
[28] Mathiesen T., Gerlich A., Kihlstrom L., et al. Effects of using combined transpetrosal surgical approaches to treat petroclival meningiomas. Neurosurgery. 2007;60:982-991. discussion 991–2
[29] Kondziolka D., Flickinger J.C., Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43:405-413. discussion 413–4
[30] Philippon J. Les recidives des meningiomes sus-tentoriels. Neurochirurgie (Suppl). 1986;32:6-53.
[31] Ildan F., Erman T., Gocer A.I., et al. Predicting the probability of meningioma recurrence in the preoperative and early postoperative period: a multivariate analysis in the midterm follow-up. Skull Base. 2007;17:157-171.
[32] Cohen-Gadol A.A., Spencer D.D., Krauss W.E. The development of techniques for resection of spinal cord tumors by Harvey W. Cushing. J Neurosurg Spine. 2005;2:92-97.
[33] Gezen F., Kahraman S., Canakci Z., Beduk A. Review of 36 cases of spinal cord meningioma. Spine. 2000;25:727-731.
[34] Nakau H., Miyazawa T., Tamai S., et al. Pathologic significance of meningeal enhancement (“flare sign”) of meningiomas on MRI. Surg Neurol. 1997;48:584-590. discussion 590–1
[35] Salcman M. Malignant meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1995:50-74.
[36] Maiuri F., De Caro Mdel B., Esposito F., et al. Recurrences of meningiomas: predictive value of pathological features and hormonal and growth factors. J Neuro-oncol. 2007;82:63-68.
[37] Gabibov G.A., Kuklina A.S., Martynov V.A., et al. Radiation-induced meningiomas of the brain. Zh Vopr Neirokhir Im N N Burdenko. 1983:13-18.
[38] Gabibov G.A., Sokolova O.N., Aleksandrova A.A., Osman Iu S. Cranio-orbital meningiomas and their surgical treatment. Zh Vopr Neirokhir Im N N Burdenko. 1981:24-32.
[39] Melamed S., Sahar A., Beller A.J. The recurrence of intracranial meningiomas. Neurochirurgia (Stuttg). 1979;22:47-51.
[40] Boker D.K., Meurer H., Gullotta F. Recurring intracranial meningiomas. Evaluation of some factors predisposing for tumor recurrence. J Neurosurg Sci. 1985;29:11-17.
[41] Mahmood A., Qureshi N.H., Malik G.M. Intracranial meningiomas: analysis of recurrence after surgical treatment. Acta Neurochir (Wien). 1994;126:53-58.
[42] Nakasu S., Li D.H., Okabe H., et al. Significance of MIB-1 staining indices in meningiomas: comparison of two counting methods. Am J Surg Pathol. 2001;25:472-478.
[43] Jaaskelainen J., Haltia M., Laasonen E., et al. The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg Neurol. 1985;24:165-172.
[44] Perry A., Gutmann D.H., Reifenberger G. Molecular pathogenesis of meningiomas. J Neurooncol. 2004;70:183-202.
[45] Cushing H., Eisenhardt L. Meningiomas: Their classification, regional behavior, life history and surgical end results. Springfield, IL: Charles C Thomas, 1938.
[46] Louis D.N., Scheithauer B.W., Budka H., et al. Meninigomas. In: Kleihues P., Cavenee W.K., editors. The WHO classification of tumors of the nervous system. Lyon: IARC Press; 2002:176-184.
[47] Perry A., Stafford S.L., Scheithauer B.W., et al. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455-1465.
[48] Sanson M., Richard S., Delattre O., et al. Allelic loss on chromosome 22 correlates with histopathological predictors of recurrence of meningiomas. Int J Cancer. 1992;50:391-394.
[49] Perry A., Jenkins R.B., Dahl R.J., et al. Cytogenetic analysis of aggressive meningiomas: possible diagnostic and prognostic implications. Cancer. 1996;77:2567-2573.
[50] Perry A., Scheithauer B.W., Stafford S.L., et al. Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046-2056.
[51] Konstantinidou A.E., Korkolopoulou P., Mahera H., et al. Hormone receptors in non-malignant meningiomas correlate with apoptosis, cell proliferation and recurrence-free survival. Histopathology. 2003;43:280-290.
[52] Konstantinidou A.E., Korkolopoulou P., Kavantzas N., et al. Mitosin, a novel marker of cell proliferation and early recurrence in intracranial meningiomas. Histol Histopathol. 2003;18:67-74.
[53] Demirtas E., Yilmaz F., Ovul I., Oner K. Recurrence of meningiomas versus proliferating cell nuclear antigen (PCNA) positivity and AgNOR counting. Acta Neurochir (Wien). 1996;138:1456-1463.
[54] Langford L.A., Cooksley C.S., DeMonte F. Comparison of MIB-1 (Ki-67) antigen and bromodeoxyuridine proliferation indices in meningiomas. Hum Pathol. 1996;27:350-354.
[55] Kolles H., Niedermayer I., Schmitt C., et al. Triple approach for diagnosis and grading of meningiomas: histology, morphometry of Ki-67/Feulgen stainings, and cytogenetics. Acta Neurochir (Wien). 1995;137:174-181.
[56] Ho D.M., Hsu C.Y., Ting L.T., Chiang H. Histopathology and MIB-1 labeling index predicted recurrence of meningiomas: a proposal of diagnostic criteria for patients with atypical meningioma. Cancer. 2002;94:1538-1547.
[57] Nakasu S., Nakasu Y., Nakajima M., et al. Preoperative identification of meningiomas that are highly likely to recur. J Neurosurg. 1999;90:455-462.
[58] Perry A., Stafford S.L., Scheithauer B.W., et al. The prognostic significance of MIB-1, p53, and DNA flow cytometry in completely resected primary meningiomas. Cancer. 1998;82:2262-2269.
[59] Roser F., Samii M., Ostertag H., Bellinzona M. The Ki-67 proliferation antigen in meningiomas. Experience in 600 cases. Acta Neurochir (Wien). 2004;146:37-44. discussion 44
[60] Vankalakunti M., Vasishta R.K., Das Radotra B., Khosla V.K. MIB-1 immunolabeling: a valuable marker in prediction of benign recurring meningiomas. Neuropathology. 2007;27:407-412.
[61] Hoshino T., Nagashima T., Murovic J.A., et al. Proliferative potential of human meningiomas of the brain. A cell kinetics study with bromodeoxyuridine. Cancer. 1986;58:1466-1472.
[62] Ko K.W., Nam D.H., Kong D.S., et al. Relationship between malignant subtypes of meningioma and clinical outcome. J Clin Neurosci. 2007;14:747-753.
[63] Mathiesen T., von Holst H., Askensten U., Collins P.V. DNA-determination in the clinical management of patients with meningioma or haemangioblastoma. Br J Neurosurg. 1989;3:575-581.
[64] Wang J.L., Nister M., Hermansson M., et al. Expression of PDGF beta-receptors in human meningioma cells. Int J Cancer. 1990;46:772-778.
[65] Maxwell M., Galanopoulos T., Hedley-Whyte E.T., et al. Human meningiomas co-express platelet-derived growth factor (PDGF) and PDGF-receptor genes and their protein products. Int J Cancer. 1990;46:16-21.
[66] Jones N.R., Rossi M.L., Gregoriou M., Hughes J.T. Epidermal growth factor receptor expression in 72 meningiomas. Cancer. 1990;66:152-155.
[67] Linggood R.M., Hsu D.W., Efird J.T., Pardo F.S. TGF alpha expression in meningioma–tumor progression and therapeutic response. J Neurooncol. 1995;26:45-51.
[68] Hsu D.W., Efird J.T., Hedley-Whyte E.T. MIB-1 (Ki-67) index and transforming growth factor-alpha (TGF alpha) immunoreactivity are significant prognostic predictors for meningiomas. Neuropathol Appl Neurobiol. 1998;24:441-452.
[69] Nister M., Enblad P., Backstrom G., et al. Platelet-derived growth factor (PDGF) in neoplastic and non-neoplastic cystic lesions of the central nervous system and in the cerebrospinal fluid. Br J Cancer. 1994;69:952-956.
[70] Van Setten G.B., Edstrom L., Stibler H., et al. Levels of transforming growth factor alpha (TGF-alpha) in human cerebrospinal fluid. Int J Dev Neurosci. 1999;17:131-134.
[71] Nordqvist A.C., Peyrard M., Pettersson H., et al. A high ratio of insulin-like growth factor II/insulin-like growth factor binding protein 2 messenger RNA as a marker for anaplasia in meningiomas. Cancer Res. 1997;57:2611-2614.
[72] Pravdenkova S., Al-Mefty O., Sawyer J., Husain M. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg. 2006;105:163-173.
[73] Hsu D.W., Efird J.T., Hedley-Whyte E.T. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86:113-120.
[74] Fewings P.E., Battersby R.D., Timperley W.R. Long-term follow up of progesterone receptor status in benign meningioma: a prognostic indicator of recurrence? J Neurosurg. 2000;92:401-405.
[75] Bitzer M., Wockel L., Morgalla M., et al. Peritumoural brain oedema in intracranial meningiomas: influence of tumour size, location and histology. Acta Neurochir (Wien). 1997;139:1136-1142.
[76] Yoshioka H., Hama S., Taniguchi E., et al. Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. 1999;85:936-944.
[77] Yamasaki F., Yoshioka H., Hama S., et al. Recurrence of meningiomas. Cancer. 2000;89:1102-1110.
[78] Nordqvist A.C., Smurawa H., Mathiesen T. Expression of matrix metalloproteinases 2 and 9 in meningiomas associated with different degrees of brain invasiveness and edema. J Neurosurg. 2001;95:839-844.
[79] Paek S.H., Kim C.Y., Kim Y.Y., et al. Correlation of clinical and biological parameters with peritumoral edema in meningioma. J Neurooncol. 2002;60:235-245.
[80] Okada M., Miyake K., Matsumoto Y., et al. Matrix metalloproteinase-2 and matrix metalloproteinase-9 expressions correlate with the recurrence of intracranial meningiomas. J Neurooncol. 2004;66:29-37.
[81] Panagopoulos A.T., Lancellotti C.L., Veiga J.C., et al. Expression of cell adhesion proteins and proteins related to angiogenesis and fatty acid metabolism in benign, atypical, and anaplastic meningiomas. J Neurooncol. 2008;89:73-87. [Epub ahead of print]
[82] Weber R.G., Bostrom J., Wolter M., et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci U S A. 1997;94:14719-14724.
[83] Drummond K.J., Zhu J.J., Black P.M. Meningiomas: updating basic science, management, and outcome. Neurologist. 2004;10:113-130.
[84] Wrobel G., Roerig P., Kokocinski F., et al. Microarray-based gene expression profiling of benign, atypical and anaplastic meningiomas identifies novel genes associated with meningioma progression. Int J Cancer. 2005;114:249-256.
[85] Menon A.G., Rutter J.L., von Sattel J.P., et al. Frequent loss of chromosome 14 in atypical and malignant meningioma: identification of a putative ‘tumor progression’ locus. Oncogene. 1997;14:611-616.
[86] Ishino S., Hashimoto N., Fushiki S., et al. Loss of material from chromosome arm 1p during malignant progression of meningioma revealed by fluorescent in situ hybridization. Cancer. 1998;83:360-366.
[87] Perry A., Banerjee R., Lohse C.M., et al. A role for chromosome 9p21 deletions in the malignant progression of meningiomas and the prognosis of anaplastic meningiomas. Brain Pathol. 2002;12:183-190.
[88] Bello M.J., de Campos J.M., Vaquero J., et al. High-resolution analysis of chromosome arm 1p alterations in meningioma. Cancer Genet Cytogenet. 2000;120:30-36.
[89] Kim Y.J., Ketter R., Henn W., et al. Histopathologic indicators of recurrence in meningiomas: correlation with clinical and genetic parameters. Virchows Arch. 2006;449:529-538.
[90] Buckley P.G., Jarbo C., Menzel U., et al. Comprehensive DNA copy number profiling of meningioma using a chromosome 1 tiling path microarray identifies novel candidate tumor suppressor loci. Cancer Res. 2005;65:2653-2661.
[91] Sulman E.P., White P.S., Brodeur G.M. Genomic annotation of the meningioma tumor suppressor locus on chromosome 1p34. Oncogene. 2004;23:1014-1020.
[92] Nakane Y., Natsume A., Wakabayashi T., et al. Malignant transformation-related genes in meningiomas: allelic loss on 1p36 and methylation status of p73 and RASSF1A. J Neurosurg. 2007;107:398-404.
[93] Maillo A., Orfao A., Espinosa A.B., et al. Early recurrences in histologically benign/grade I meningiomas are associated with large tumors and coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone. Neuro Oncol. 2007;9:438-446.
[94] Langford L.A., Piatyszek M.A., Xu R., et al. Telomerase activity in ordinary meningiomas predicts poor outcome. Hum Pathol. 1997;28:416-420.
[95] Ide M., Jimbo M., Kubo O., et al. Peritumoral brain edema and cortical damage by meningioma. Acta Neurochir Suppl (Wien). 1994;60:369-372.
[96] Alvarez F., Roda J.M., Perez Romero M., et al. Malignant and atypical meningiomas: a reappraisal of clinical, histological, and computed tomographic features. Neurosurgery. 1987;20:688-694.
[97] New P.F., Hesselink J.R., O’Carroll C.P., Kleinman G.M. Malignant meningiomas: CT and histologic criteria, including a new CT sign. AJNR Am J Neuroradiol. 1982;3:267-276.
[98] Jaaskelainen J., Haltia M., Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25:233-242.
[99] Ayerbe J., Lobato R.D., de la Cruz J., et al. Risk factors predicting recurrence in patients operated on for intracranial meningioma. A multivariate analysis. Acta Neurochir (Wien). 1999;141:921-932.
[100] Sheehy J.P., Crockard H.A. Multiple meningiomas: a long-term review. J Neurosurg. 1983;59:1-5.
[101] Ron E., Modan B., Boice J.D.Jr, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033-1039.
[102] Rubinstein A.B., Shalit M.N., Cohen M.L., et al. Radiation-induced cerebral meningioma: a recognizable entity. J Neurosurg. 1984;61:966-971.
[103] Shoshan Y., Chernova O., Juen S.S., et al. Radiation-induced meningioma: a distinct molecular genetic pattern? J Neuropathol Exp Neurol. 2000;59:614-620.
[104] Kondziolka D., Lunsford L.D., Coffey R.J., Flickinger J.C. Gamma knife radiosurgery of meningiomas. Stereotact Funct Neurosurg. 1991;57:11-21.
[105] Villavicencio A.T., Black P.M., Shrieve D.C., et al. Linac radiosurgery for skull base meningiomas. Acta Neurochir (Wien). 2001;143:1141-1152.
[106] Pollock B.E. Stereotactic radiosurgery for intracranial meningiomas: indications and results. Neurosurg Focus. 2003;14:e4.
[107] Pollock B.E., Stafford S.L., Utter A., et al. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55:1000-1005.
[108] Kobayashi T., Kida Y., Mori Y. Long-term results of stereotactic gamma radiosurgery of meningiomas. Surg Neurol. 2001;55:325-331.
[109] Malik I., Rowe J.G., Walton L., et al. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg. 2005;19:13-20.
[110] Goldsmith B.J., Wara W.M., Wilson C.B., Larson D.A. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195-201.
[111] Kokubo M., Shibamoto Y., Takahashi J.A., et al. Efficacy of conventional radiotherapy for recurrent meningioma. J Neurooncol. 2000;48:51-55.
[112] Palma L., Celli P., Franco C., et al. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg. 1997;86:793-800.
[113] Milker-Zabel S., Zabel-du Bois A., Huber P., et al. Intensity-modulated radiotherapy for complex-shaped meningioma of the skull base: long-term experience of a single institution. Int J Radiat Oncol Biol Phys. 2007;68:858-863.
[114] Miyatake S., Tamura Y., Kawabata S., et al. Boron neutron capture therapy for malignant tumors related to meningiomas. Neurosurgery. 2007;61:82-90. discussion 90–1
[115] Chamberlain M.C., Tsao-Wei D.D., Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;62:1210-1212.
[116] Kaba S.E., DeMonte F., Bruner J.M., et al. The treatment of recurrent unresectable and malignant meningiomas with interferon alpha-2B. Neurosurgery. 1997;40:271-275.
[117] Schrell U.M., Rittig M.G., Anders M., et al. Hydroxyurea for treatment of unresectable and recurrent meningiomas. I. Inhibition of primary human meningioma cells in culture and in meningioma transplants by induction of the apoptotic pathway. J Neurosurg. 1997;86:845-852.
[118] Schrell U.M., Rittig M.G., Anders M., et al. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg. 1997;86:840-844.
[119] Mason W.P., Gentili F., Macdonald D.R., et al. Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J Neurosurg. 2002;97:341-346.
[120] Newton H.B., Slivka M.A., Stevens C. Hydroxyurea chemotherapy for unresectable or residual meningioma. J Neurooncol. 2000;49:165-170.
[121] Grunberg S.M., Weiss M.H., Spitz I.M., et al. Treatment of unresectable meningiomas with the antiprogesterone agent mifepristone. J Neurosurg. 1991;74:861-866.
[122] Lamberts S.W., Tanghe H.L., Avezaat C.J., et al. Mifepristone (RU 486) treatment of meningiomas. J Neurol Neurosurg Psychiatry. 1992;55:486-490.
[123] Grunberg S., Rankin C., Townsend J. Phase III double-blind randomized placebo-controlled study of mifepristone for the treatment of unresectable meningioma. Proceedings of the American Society of Clinical Oncology Annual Meeting, 2001. pp Abstract No. 222
[124] Goodwin J.W., Crowley J., Eyre H.J., et al. A phase II evaluation of tamoxifen in unresectable or refractory meningiomas: a Southwest Oncology Group study. J Neurooncol. 1993;15:75-77.
[125] Johnson M.D., Woodard A., Kim P., Frexes-Steed M. Evidence for mitogen-associated protein kinase activation and transduction of mitogenic signals by platelet-derived growth factor in human meningioma cells. J Neurosurg. 2001;94:293-300.
[126] Wang J.L., Zhang Z.J., Hartman M., et al. Detection of TP53 gene mutation in human meningiomas: a study using immunohistochemistry, polymerase chain reaction/single-strand conformation polymorphism and DNA sequencing techniques on paraffin-embedded samples. Int J Cancer. 1995;64:223-228.
[127] Ragel B.T., Jensen R.L., Gillespie D.L., et al. Ubiquitous expression of cyclooxygenase-2 in meningiomas and decrease in cell growth following in vitro treatment with the inhibitor celecoxib: potential therapeutic application. J Neurosurg. 2005;103:508-517.