4 Recovery and Rehabilitation
After reading this chapter, you should be able to:
• discuss the physical, psychological and cognitive sequelae present for some survivors of a critical illness
• outline the common functional, psychological and health-related quality of life (HRQOL) instruments used to assess patient outcomes after a critical illness
• describe the benefits and challenges for implementing rehabilitation interventions in-ICU, in hospital after ICU-discharge, and after hospital discharge
Introduction
A critical illness requiring admission to a general intensive care unit (ICU) affects approximately 113,000 adults in Australia and 17,000 in New Zealand per year.1 Although survival rates approximate 89% at hospital discharge,2 functional recovery for individuals is delayed often beyond six months post-discharge.3–5 Physical de-conditioning and neuromuscular dysfunction6,7 as well as psychological sequelae8 are common, adding to the burden of illness for survivors, carers, the health care system and broader society.9
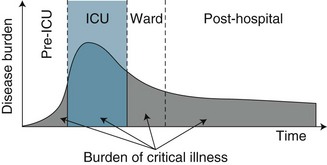
While ICU clinicians have traditionally focused on survival as the principal indicator of patient outcome and unit performance,9 physical and psychological functioning and health-related quality of life (HRQOL) have now emerged as legitimate patient outcomes from both practice and research perspectives.4 With this shifting focus towards long-term health and wellbeing has also come a reconsideration and re-conceptualisation of critical care as only one component in the continuum of care for a critically ill patient. An episode of critical illness is now viewed as a continuum that begins with the onset of acute clinical deterioration, includes the ICU admission, and continues until the patient’s risk of late sequelae has returned to the baseline risk of a similar individual who has not incurred a critical illness9 (see Figure 4.1). Timing of this recovery trajectory is variable, and related to a number of individual, illness and treatment factors.
Reviews of numerous observational studies confirm delayed recovery in HRQOL,e.g.3–5 with both physical and psychological symptoms prevalent:
• weakness: 46%6; 25–60% in patients ventilated >7 days10
The effects of a critical illness on cognitive functioning are now also beginning to be examined and discussed in the literature as an important patient outcome.11,15–19 While significant sequelae therefore exist for a substantial proportion of critical illness survivors, little evidence is currently available to support specific interventions for improving their recovery.9,20
A further and more recent re-conceptualisation of holistic critical care practice promotes a unifying approach for minimising intensive care unit acquired weakness (ICU-AW) and delirium, reflected in the acronym ABCDE,11,21 to minimise physical, psychological and cognitive sequelae:
B Breathing trials (to minimise mechanical ventilation duration)
C Coordination (of daily awakening and spontaneous breathing trials)22
E Exercise/Early mobility (requires a patient to be awake, alert and co-operative).
Further chapters in this book discuss psychological issues including sedation management and delirium monitoring while in ICU (Chapter 7), and breathing trials and weaning from mechanical ventilation (Chapter 15). This chapter discusses common physical and psychological sequelae associated with a critical illness, and how this impacts on a survivor’s HRQOL. Common instruments measuring physical, psychological and HRQOL are described. Physical rehabilitation strategies, commencing with exercise and early mobility in-ICU, post-ICU and post-hospital services are also discussed.
Icu-Acquired Weakness
Critical illness myopathy (CIM), polyneuropathy (CIP) and neuromyopathy (CINM) syndromes23 occur in 46% of ICU survivors.6 More recently, ICU-Acquired Weakness (ICU-AW) has been proposed as a term to encompass these syndromes of muscle wasting and functional weakness in patients with a critical illness who have no other plausible aetiology.24 The three syndromes above form the sub-categories of ICU-AW, with CINM used when both myopathy and axonal polyneuropathy are evident. Development of ICU-AW is associated with a number of risk factors:24–26
• co-existing conditions: chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus
• critical illness: sepsis, systemic inflammatory response syndrome (SIRS)
• treatments: mechanical ventilation, hyperglycaemia, glucocorticoids, sedatives, neuromuscular blocking agents, immobility.
Local and systemic inflammation acts synergistically with bed rest and immobility to alter metabolic and structural function of muscles,27 resulting in muscle atrophy and contractile dysfunction,26 loss of flexibility, CIP, heterotopic ossification and entrapment neuropathy.6 Muscle strength can reduce by 1–1.5% per day with a total loss of 25–50% of body strength possible following immobilisation.28 Patients can lose 2% of muscle mass per day, which contributes to weakness and disability, and a prolonged recovery period.25 These neuromuscular dysfunctions are diagnosed by clinical assessment, diagnostic studies (electrophysiology, ultrasound) or histology of muscle or nerve tissue.24
The syndrome manifests as prolonged weaning time, inability to mobilise and reduced functional capacity. Some groups of ICU survivors report relatively poor HRQOL due to prolonged weakness that may persist for months and years after discharge, particularly for those recovering from Acute Lung Injury/Adult Respiratory Distress Syndrome.29–31 A related factor is nutrition, with one study noting that 39% of patients post-ICU had little or no appetite and 15% were still receiving either a soft diet or tube feeding while in hospital.32
Clinical Assessment
Clinical assessment includes identification of generalised weakness following the onset of a critical illness, exclusion of other diagnoses (e.g. Guillain–Barré syndrome), and measurement of muscle strength. Patients suspected of ICU-AW have diffuse flaccid weakness that is symmetrical and involves both proximal and distal muscles, with relative sparing of cranial nerves and variable deep tendon reflex responses.23
Manual Muscle Testing (MMT) is commonly assessed using the Medical Research Council (MRC) Scale,33 a 0–5 point ordinal scale:
1 = flicker or trace of muscle contraction
2 = active movement with gravity eliminated
3 = reduced power but active movement against gravity
4 = reduced power but active movement against gravity and resistance
• upper limb: deltoid, biceps, wrist extensors
• lower limb: quadriceps, gluteus maximus, ankle dorsiflexion34
Weakness is evident with an MRC total score of <48 (<4 in all testable muscle groups), and re-tested after 24 hours. Weakness (<4 MRC Scale) was associated with an increased hospital mortality.34 Inter-rater reliability following appropriate training using the MRC has been demonstrated.35
Hand-held dynamometry enables measurement of grip strength force using a calibrated device for patients who are conscious and cooperative. Dynamometry was demonstrated to be a reliable, rapid and simple alternative to comprehensive MMT assessment,34 and may be a surrogate measure for global strength.24
Diagnostic Testing
Electrophysiological testing (nerve conduction studies, needle electromyography) may be useful as an adjunct in diagnosing ICU-AW, but differentiating between CIM and CIP is difficult.24 Muscle wasting is a consequence of inflammatory responses (including COPD-associated inflammation).25 Histology for CIP is primarily noted as distal axonal degeneration in both sensory and motor fibres, while the characteristic findings in CIM is patchy loss of myosin (thick filaments), necrosis and fast twitch fibre atrophy.24 Ultrasound is also being examined as a reliable assessment of muscle mass/volume in this cohort, although findings can be confounded by tissue oedema.24
Practice tip
Current clinical recommendations to limit muscle wasting include:
• minimising patient exposure to corticosteroids and neuromuscular blocking agents
• limiting excessive analgesia and sedation
• glycaemic control may also be of value, although further investigations continue
• early nutrition or specific nutritional supplements or components may limit loss of muscle mass or enhance muscle recovery, but also requires further research.25
Patient Outcomes Following a Critical Illness
Examination of patient outcomes beyond survival is an important contemporary topic for critical care practice and research.3–536 Patient outcomes after a critical illness or injury were traditionally measured using a number of objective parameters (e.g. number of organ failure-free days, 28-day status, or 1-year mortality).37 Other measures that examined patient-centred concepts such as functional status and HRQOL38,39 have become more prevalent in the literature.3,4,40–42 As the recovery trajectory from a critical illness may be long and incomplete, mapping this path is a complex process. The range of HRQOL instruments available is large, but can be divided into two groups: generic to all illnesses, or specific to a particular disease state. One limitation of generic instruments is that, while they can be applied to a broad spectrum of populations, they may not be responsive to specific disease characteristics.43 This section discusses the measurement of health outcomes, focusing on HRQOL, and the physical and psychological measures commonly used to assess survivors of a critical illness.
As introduced earlier, reviews of numerous observational studies with survivors of a critical illness have demonstrated a delayed recovery trajectory, highlighting particularly the effect of physical function on an individual’s usual role. Recommendations for future studies noted that patients should be followed for at least six months, have neuropsychological testing as part of their assessment, and be assessed using a HRQOL instrument that enables comparison across countries and languages.3,9,44,45 Common instruments used to assess HRQOL, physical functioning and psychological functioning for cohorts of patients after a critical illness are discussed below.
Measures of Health-Related Quality of Life after a Critical Illness
A generic instrument that measures baseline HRQOL and exhibits responsiveness in a recovering critically ill patient with demonstrated reliability and validity has been elusive, although recent review papers have identified some useful instruments4,9 (see Table 4.1). SF-36 is the most commonly used and validated instrument in the literature, including with a variety of critically ill patient groups (e.g. general ICU, ARDS, trauma and septic shock). A recent comparison of two related instruments demonstrated that the 15D was more sensitive to clinically important differences in health status than EQ-5D in a critical care cohort.46
TABLE 4.1 Summary of health-related quality of life (HRQOL) instruments used for patients following a critical illness
| Instrument | Items | Concepts/domains |
|---|---|---|
| Medical outcomes study (SF-36)162,163 | 36 | physical: functioning, role limitations, pain, general health; mental: vitality, social, role limitations, mental health; health transition; variable response levels (2–5) |
| EuroQol 5D46,164 | 5 | mobility, self-care, usual activities, pain/discomfort, anxiety/depression; 3 response levels; cost-utility index |
| 15D46,165 | 15 | mobility, vision, hearing, breathing, sleeping, eating, speech, elimination, usual activities, mental function, discomfort, distress, depression, vitality, and sexual activity; 5-point ordinal scale (1 = full function; 5 = minimal/no function) |
| Quality of life–Italian (QOL–IT)166 | 5 | physical activity; social life; perceived quality of life; oral communication; functional limitation; varied response levels (4–7) |
| Assessment of Quality of Life (AQOL)167 | 15 | Illness (3 items); independent living (3 items); physical senses (3 items); social relationships (3 items); psychological wellbeing (3 items); 4 response levels; enables cost-utility analysis |
| Quality of life–Spanish (QOL–SP)168 | 15 | basic physiological activities (4 items); normal daily activities (8 items); emotional state (3 items) |
| Sickness impact profile (SIP)169 | 68 | physical: somatic autonomy; mobility control; mobility range psychosocial: psychic autonomy and communication; social behaviour; emotional stability; developed from original 136-item170 |
| Nottingham Health Profile (NHP)171 | 45 | experience: energy, pain, emotional reactions, sleep, social isolation, physical mobility; daily life: employment, household work, relationships, home life, sex, hobbies, holidays |
| Perceived quality of life (PQOL)172 | 11 | satisfaction with: bodily health; ability to think/remember; happiness; contact with family and friends; contribution to the community; activities outside work; whether income meets needs; respect from others; meaning and purpose of life; working/not working/retirement; each scored on 0–100 scale |
Measures of Physical Function Following a Critical Illness
A variety of instruments have been developed to examine the physical capacity of individuals, usually focusing on functional status ranging from independent to dependent. Table 4.2 describes some common instruments used with individuals after an acute or critical illness. Many other instruments exist for specific clinical cohorts, including Katz’s ADL index,47 the Karnofsky performance status,48 and the instrumental activities of daily living,49 but these have not been used commonly with survivors of a critical illness.
TABLE 4.2 Common measures of physical function following a critical illness
| Instrument | Measurement | Score range/comments |
|---|---|---|
| St George’s Respiratory Questionnaire (SGRQ),173 (SGRQ-C)174 | COPD-specific items assessing three domains: symptoms (7 items), activity (2 multi-part items), impacts (5 multi-part items) | Item responses have empirical weights; higher scores indicate poorer health; used with patients with chronic lung disease, including ARDS |
| Six-minute walk test (6MWT)51 | Walk distance, reflects functional capacity in respiratory or cardiac diseases | Assesses walk function in patients with moderate heart failure, ARDS |
| Barthel Index (BI)175–177 | 10 items of functional status (Activities of Daily Living [ADLs]) | Dependence: total = 0–4; severe = 5–12; moderate = 13–18; slight = 19; independent = 20 |
| Functional Independence Measure (FIM)178 | Severity of disability in inpatient rehabilitation settings | 18 activities of daily living in two themes: motor (13 items), cognitive (5 items); 7-point ordinal scales; score range 18–126 (fully dependent–functional independence) |
| Timed Up and Go (TUG)179 | Functional ability to stand from sitting in a chair, walk 3 m at regular pace and return to sit in the chair | ≤10 seconds = normal; ≤20 seconds = good mobility, independent, can go out alone; 21–30 seconds = requires supervision/walk aid |
| Shuttle walk test (SWT)180 | 10 m shuttle walk with pre-recorded audio prompts to complete a shuttle turn | Participant keeps pace with audio sounds; 12 levels of speed (0.5–2.37 m/second) |
ARDS = Adult Respiratory Distress Syndrome
Physical activity associated with cardiac or pulmonary dysfunction may be assessed using perceived breathlessness (dyspnoea) during exercise by the modified Borg scale,50 ranging from 0 (no dyspnoea) to 10+ (maximal). The Borg scale is commonly used with other physical activity instruments, e.g. the six-minute walk test (6MWT).51
Measures of Psychological Function after a Critical Illness
The recovery process and trajectory for survivors of a critical illness remains an important but under-researched area.18,52 Exploration of the impact of the intensive care experience, including ongoing stress53–56 and memories for the patient,16,57–59 is now emerging in the literature as an important area of research and practice. Instruments that assess mental function after a critical illness focus on psychological constructs, including anxiety, avoidance, depression and fear (see Table 4.3). Other instruments are also available to examine post-traumatic stress symptoms.60 Constructs that relate to an individual during a critical illness episode also include agitation, and confusion/delirium14 (discussed further in Chapter 7).
TABLE 4.3 Examples of common measures of psychological function after critical illness
| Instrument | Measurement | Score range |
|---|---|---|
| Impact of event scale (IES);181 IES-R182 | 15-item; assesses levels of post-traumatic distress; two subscales: intrusive thoughts, avoidance behaviours; revised form (IES-R) adds hyper-arousal subscale (7 items)182 | frequency of thoughts over past 7 days; 0 = no thoughts; 5 = often; higher scores indicate greater distress: scores ≥26 (combined intrusion and avoidance) are significant |
| Hospital anxiety and depression scale (HADS)89 | 14 items; 4-point scale; measures mood disorders in non-psychiatric patients; focuses on psychological rather than physical symptoms of anxiety and depression | combined score ≥11 indicates a clinical disorder |
| Center for Epidemiologic Studies–Depression Scale (CES–D)183 | 20-item self-report scale assessing frequency and severity of depressive symptoms experienced in the previous week | score range 0–60; higher scores reflect increased symptoms and severity |
Assessment for ongoing neuro-cognitive dysfunctions17,61,62 is recommended for some survivors, with the beginning of research on cognitive rehabilitation for survivors of a critical illness evident.15,17–19,63 Cognitive executive functioning includes attention, planning, problem-solving and multi-tasking.64
Psychological Recovery
Psychological responses to a critical illness and patients’ memories of experiences during an ICU admission have been explored using quantitative57,65–69 and/or qualitative approaches.70,71 Some survivors reported increased anxiety, including transfer anxiety (discharge from ICU);32 depression;13 post-traumatic stress;14,72–74 hallucinations;58,75,76 and continuing cognitive dysfunction.77 A range of memories and experiences were also noted after ICU transfer78and hospital discharge,58,70,79 including powerlessness, reality–unreality, reactions and acceptance, and comfort–discomfort.
For some patients, recovery from a critical illness results in short- and long-term psychological dysfunction (e.g. anxiety, depression and posttraumatic stress symptoms).8,80 Our understanding of these sequelae has improved over the last decade in part due to increased research activity and evaluations of intensive care follow-up clinics in the UK (discussed in a later section). Importantly, negative psychological consequences of intensive care can result in poorer health status and perceptions of HRQOL.81
Assessment of psychological outcomes has mainly relied on self-report questionnaires administered via either a postal survey or a structured interview format. These screening, rather than diagnostic, strategies enable identification of individuals at risk of developing a significant clinical problem. A number of standardised questionnaires have demonstrated reliability and validity in this patient group, but the use of different questionnaires makes it difficult to generalise findings. Studies that assessed anxiety and depression used the Hospital Anxiety and Depression Scale (HADS),57,82–86 Beck Anxiety Inventory,87 State Trait Anxiety Inventory (STAI),68 and the Beck Depression Inventory.68,87 Posttraumatic stress has been assessed using the Impact of Event Scale (IES),57,68,84,85 Post-Traumatic Stress Syndrome 10-Questions Inventory (PTSS-10),73 Davidson Trauma Scale,74 and the Experience after Treatment in Intensive Care 7 (ETIC-7) item scale.88
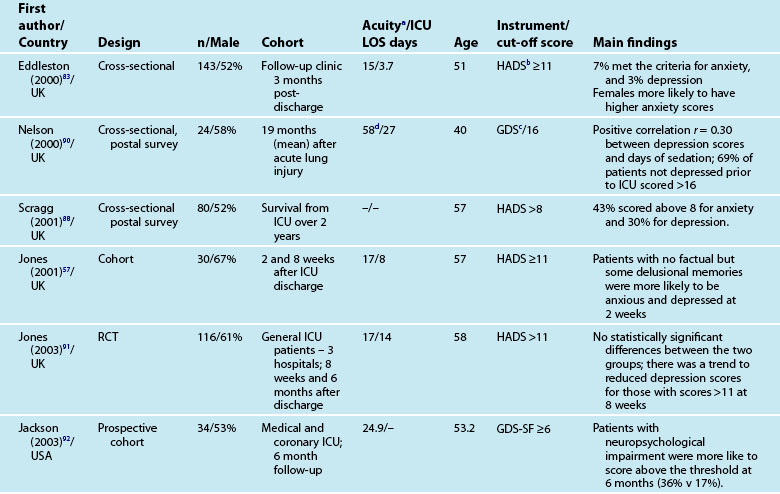
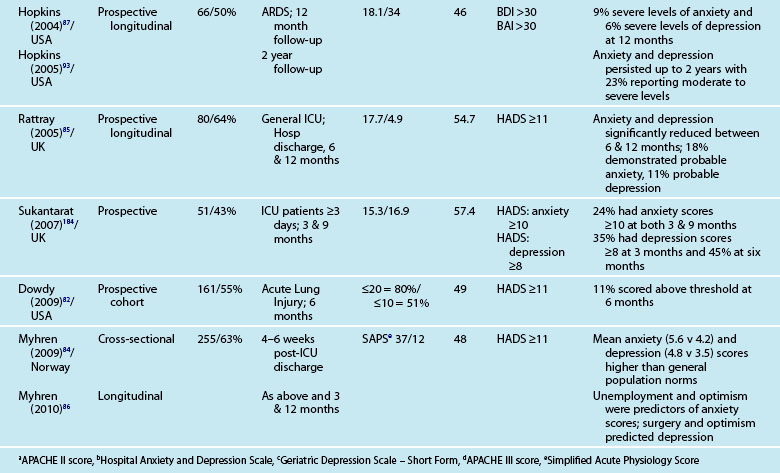
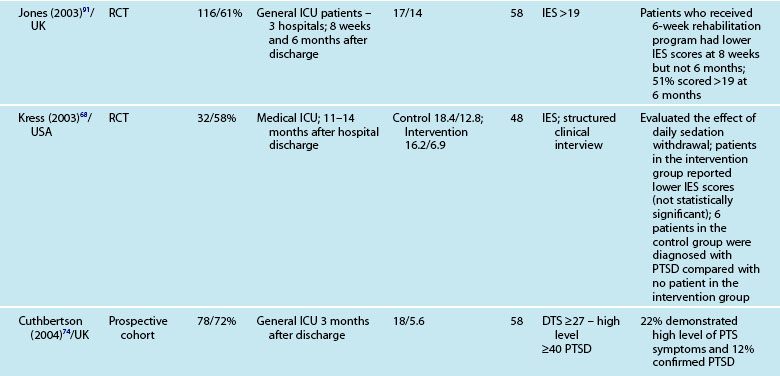
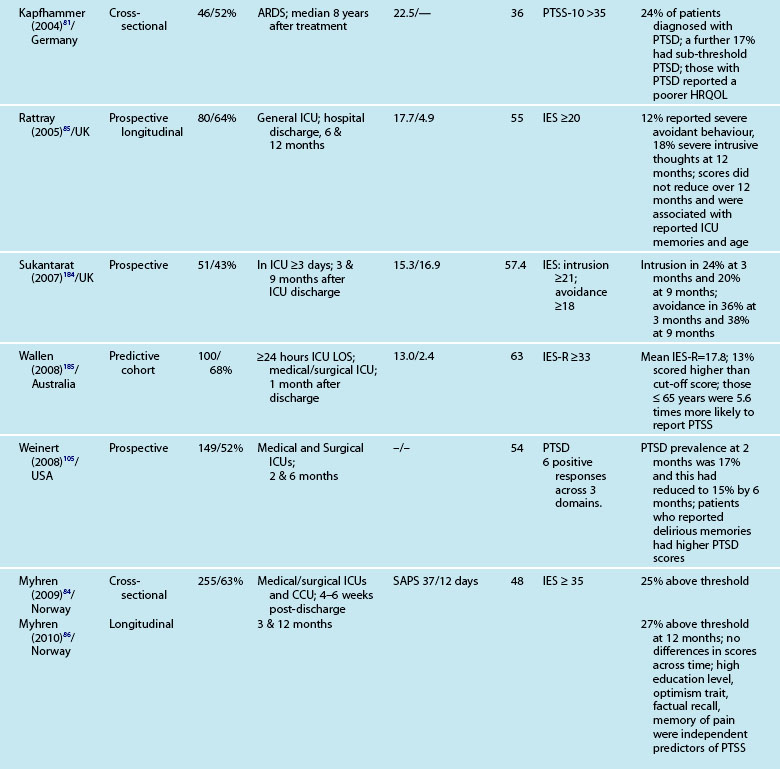
These instruments often include ‘cut-off’ or ‘threshold’ scores that enable screening for the presence or severity of a disorder. For example, a score of 8–10 on either subscale of the HADS indicates possible presence of a disorder, while a score of 11 or above indicates probable presence of such a condition.89 One limitation of these self-report measures is that while sensitivity (ability to correctly identify all patients with the condition) can be high, specificity (ability to correctly identify all patients without the condition) is less easy to determine, and therefore the incidence of psychological distress may be over-stated. This makes estimation difficult and is one of the challenges in establishing the actual magnitude of psychological distress after a critical illness. Other challenges include the recruitment of different cohorts or subgroups of patients (e.g. patients with Adult Respiratory Distress Syndrome87 or Acute Lung Injury90). Variations in the international provision of ICU services also means that differences may exist in case mix in the areas of illness severity, planned or unplanned admissions, ages and reasons for admission. For example, in a sample of studies mean age ranged from 4090–59 years,91 mean APACHE II scores ranged from 1583–24.9,92 and median length of ICU stay from 3.783–3487 days.
Anxiety and Depression
Reported prevalence of anxiety and depression after ICU discharge varies depending upon the questionnaire and ‘cut-off’ scores used, and the research design (see Table 4.4). For example, one study of an intensive care follow-up clinic reported anxiety prevalence of 7% three months after discharge;83 much less than a similar study where anxiety was 18% one year after discharge.85 Both studies used the HADS with scores of ≥11 to indicate an anxiety or depressive problem. Prevalence of depression in these studies was more equivalent, 10%83 and 11%.85 Table 4.4 provides a summary of studies reporting the prevalence of anxiety and depression. These differences may be explained by differences in case mix or timing of assessment.
Patients often exhibit high levels of distress at time of hospital discharge and these tend to reduce in the first year after discharge.85,90 However the episodic timing of assessments may not fully capture patterns of anxiety and depression, and establish whether full resolution is achieved. For example, in patients with ARDS, levels of depression increased from 16% at 1 year after discharge to 23% at 2 years.93 This may reflect prolonged recovery in general for this subgroup of patients, who tend to be among the most critically ill patients, with a mean ICU stay of 34 days noted. A rise in depression scores may therefore be a reflection of that prolonged physical recovery.
What is emerging from the literature is that certain patient demographic and clinical characteristics predict subsequent anxiety and depression, although not consistently. Women tend to be more anxious than men83,85 and younger patients more anxious than older patients.85 Other consequences of being in intensive care such as neuropsychological impairment can also predict significantly higher depression scores.92 Sicker patients, those with a longer length of ICU stay, and also a longer duration of sedation and mechanical ventilation are more likely to have measurable depression.82 This is perhaps not surprising as patients who are in intensive care longer tend to have more prolonged hospital stay and recovery period. What is also evident in the emerging literature is the effect of patients’ subjective intensive care experiences. These experiences tend to be reported as unpleasant memories of being in ICU16,57–59,84,85 and are discussed later in this chapter.
Depression is also associated with other aspects of recovery and in particular HRQOL. Depressed patients tend to rate their HRQOL as poorer than those who are not.85,93 However what is less clear is the direction of this relationship; it could be that patients with a poorer HRQOL tend to be depressed rather than depression leading to perceptions of poorer HRQOL. Patients who have psychological problems prior to intensive care are likely to develop these after discharge. Although assessment of pre-ICU status is difficult, in some cases this information can be obtained from relatives or caregivers.
Posttraumatic Stress
In recent years there has been increasing interest in the development of posttraumatic stress reactions such as Posttraumatic Stress Disorder (PTSD) as a response to critical illness,94,95 and there is increasing recognition of these symptoms as a problem for some intensive care survivors.14,72 Individuals do not perceive or respond to traumatic or life-threatening events in the same way, but there are commonalities96 including that events are often perceived as a threat to life, are uncontrollable and unpredictable97 and that they are beyond the usual human experience.98 Many symptoms of posttraumatic stress that patients experience in the initial days after intensive care discharge may be considered a normal reaction. Therefore practitioners need to clearly separate the normal from the abnormal response; this is achieved by assessing the severity, duration of symptoms, and their effect on an individual’s life. PTSD should not be diagnosed until at least one month after the event, and until the symptoms have been present for one month. Symptoms commonly cause problems in relation to work, social or other important activities;99 this is important to consider when developing critical care follow-up services. Importantly, PTSD symptoms may be reactivated after some time, and being in ICU may serve as a catalyst for some patients, e.g. reliving a war event.e.g.58
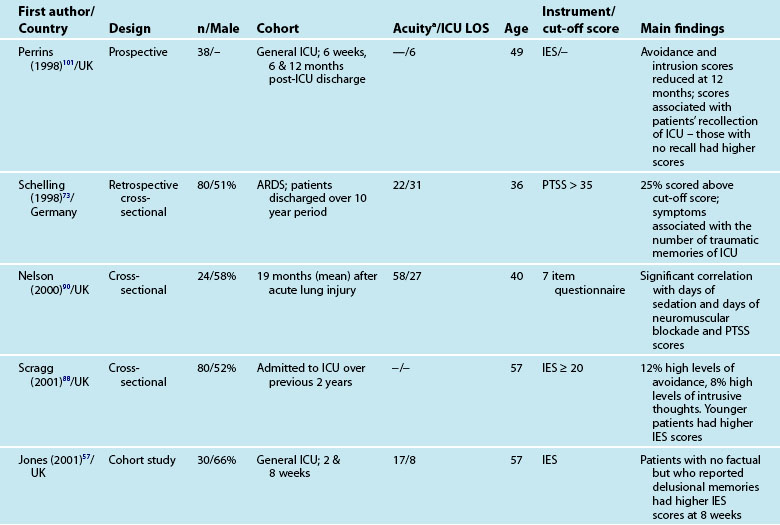
As with other psychological symptoms such as anxiety and depression, it has been difficult to establish the prevalence of PTSD after intensive care because of the use of self-report measures, different research designs, varied patient casemix and international variations in the delivery of intensive care. These variations have resulted in overestimation of the prevalence of PTSD and posttraumatic stress symptoms (PTSS),100 although note that patients with significant PTSS may be less likely to participate in research studies. While PTSD should be diagnosed through a structured clinical interview,12 few studies use this approach. One small study compared the prevalence of PTSD in patients who had daily sedation withdrawal versus those who did not; 6/19 patients who did not receive daily sedation withdrawal were diagnosed with PTSD, while 0/13 were diagnosed from the intervention group.68 The small sample size was a limitation but nonetheless these were important findings.
Patients may have significant PTSS without developing PTSD and it is mainly these symptoms that are assessed using the self-report measures. Reported prevalence of a significant posttraumatic stress reaction or PTSD is 14–27%.74 As for anxiety and depression, there are certain patient and clinical characteristics that can predict likelihood of a posttraumatic stress reaction. Trauma101 and younger patients tend to have higher scores on measures of posttraumatic stress.74,85,88 Aspects of an intensive care experience are associated also with a posttraumatic stress reaction. Patients with a longer ICU stay,85,90 longer duration of sedation and/or neuromuscular blockade,90 and mechanical ventilation74 are more likely to report posttraumatic stress symptoms. Patients who have daily sedative interruption had lower scores on the Impact of Event Scale.68 Importantly, daily sedative interruption or withdrawal, or titration of sedation is becoming more common in practice and therefore requires further research. Certain subgroups of patients appear to have a higher prevalence of PTSD (e.g. ARDS patients81), and PTSD can often endure for many years.
Memories and Perceptions
Interestingly, illness severity does not consistently predict a PTSS reaction,73,85 but rather perceptions of the intensive care experience. This is one of the unique features of being in intensive care; patients have little recall for factual events and often report large gaps where they remember very little about their critical illness. Patients’ accounts often include disturbing recollections with memories of ‘odd perceptual experiences’,54,102 ‘nightmares’ or ‘hallucinations’.57,58 While not all patients experience these, those who do so tend to report memories that are persecutory in nature,103 are often associated with feelings of being elsewhere,102 reliving a previous life event,104 or fighting for survival.102 These memories often seemed ‘real’ and were distressing to patients at the time, and may be recalled in detail some months afterwards.58 Having delusional rather than factual memories is more likely to result in distress;56,57,85,105 and lack of memory for factual events may result in longer-term psychological problems,57 with the important element being the content of the ICU memories rather than the number of memories. Table 4.5 summarises studies exploring posttraumatic stress after ICU.
Interventions to Improve Psychological Recovery
Although there is now strong empirical evidence that some patients experience significant psychological dysfunctions after a critical illness, it is less clear how to treat these symptoms. Systematic follow-up services may offer appropriate assessment support during recovery for individuals identified with psychological disturbances. Intensive care follow-up clinics where patients have the opportunity to discuss their intensive care experiences and receive information about what had happened to them could be a useful intervention, although there are currently no empirical data to support this,53 and further research work is required.
Patient diaries were also thought to be important in providing missing pieces of information that might help a patient make sense of their critical illness experience. A diary approach has been adopted in a number of European ICUs,106,107 and while there has been some variation in how the diaries were compiled and then viewed by a patient, there is emerging evidence that supports their use.108 Note however that not all patients may wish to be reminded of their ICU experience; this is especially the case for patients who demonstrate avoidant behaviours. Others may wish not to be reminded of being critically ill but wish to concentrate on recovery.53 Further research that incorporates these issues during assessment of posttraumatic stress symptoms will further establish the effectiveness of diary use (see Research Vignette later in this chapter).
The recent UK NICE guidelines109 emphasised regular assessment of patient recovery including psychological recovery. Assessment periods include during intensive care, ward-based care, before discharge home or community care and 2–3 months after ICU discharge, with the use of existing referral pathways and stepped care models to treat identified psychological dysfunctions. These services are usually well established and allow patients to be treated by appropriately qualified practitioners. The role of critical care practitioners may therefore be to establish the causes of psychological disturbances associated with critical illness, identifying at-risk patients through systematic and standardised screening activities, closely monitoring identified patients and referring to appropriate specialties where appropriate, to optimise their recovery trajectory while not introducing any further harm.
Rehabilitation and Mobility in Icu
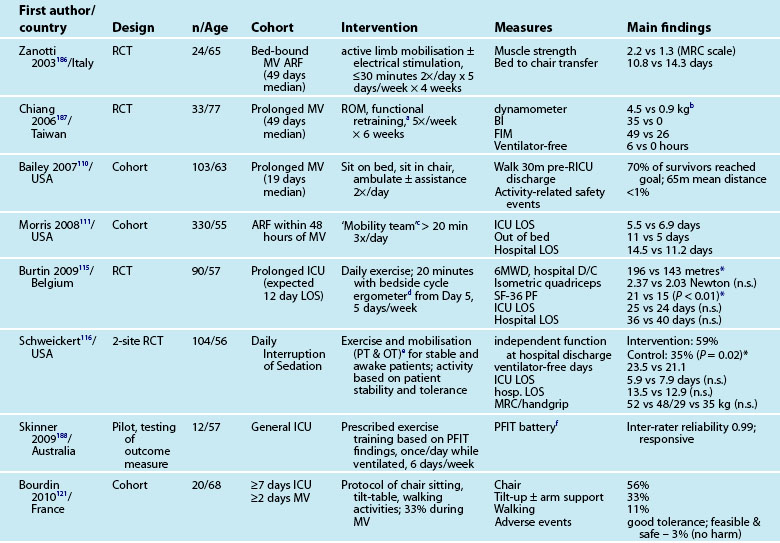
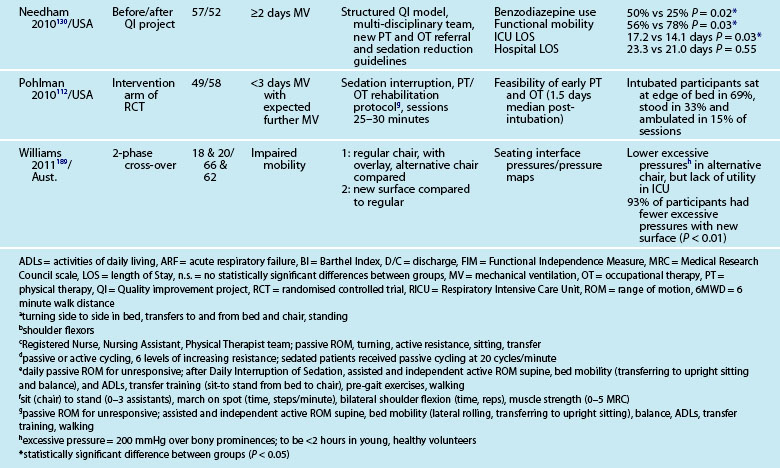
Interventions to minimise ICU-AW, particularly in relation to muscle de-conditioning from disuse (e.g. sedation; bed-rest) have recently focused on active exercises and mobility, even while patients are intubated and ventilated.26 Early studies of in-ICU mobility have demonstrated safe and feasible interventions,110–112 although this focus requires a cultural shift with a multi-disciplinary team approach and changes in care processes.26,113,114 In-ICU rehabilitation has also reduced ICU and hospital lengths of stay and improved physical function at hospital discharge.111,115,116 Table 4.6 outlines recent studies examining physical activity and mobility strategies for patients in ICU.
Mobility and Walking
Testing of ‘early’ activity for ICU patients relates to after clinical stabilisation is evident, and includes those still intubated.110,112 Factors to ensure patient safety during mobilisation have been identified, including confirming that a patient has sufficient cardiovascular and respiratory reserve and cognitive function,117 and subsequently tested.111,118 Potential barriers to mobilisation during mechanical ventilation (e.g. acute lung injury, vasoactive infusions) have also been examined.112
Physiotherapy recommendations for physical de-conditioning include development of ‘exercise prescriptions’ and ‘mobilising plans’.119 Activities range from passive stretching and range of motion exercises for limbs and joints, positioning, resistive muscle training to aerobic training and muscle strengthening and ambulation.119,120 Specific mobility activities include:
• in-bed (range of motion, roll, bridge, sitting on edge of bed)
Patient support for each activity ranges from assistance with 1–2 staff through to independence under supervision. Rehabilitation devices can also include a tilt-table,121 neuromuscular electrical stimulation (NMES), bedside cycle ergometry and adapted walking frames.122 Inspiratory muscle training (IMT) has been used for weakness associated with prolonged mechanical ventilation,123 using resistance and threshold-training devices. There is however no current strong evidence124 supporting an independent benefit of IMT, but it can be used as adjunctive therapy.125,126
A survey of practices in Australian ICUs noted that 94% of physiotherapists prescribed exercise frequently for both ventilated and non-ventilated patients, but practices did vary widely and no validated functional outcome measures were used.127 As noted earlier, a culture of patient wakefulness and early in-ICU activity and mobility is advocated but challenged by the status quo of work practices and health professional role delineations.113,118,128–130 A re-engineering of work processes and practices to promote patient activity is therefore required to ensure optimal outcomes for survivors of a critical illness.
Further development and testing of candidate interventions also remain, particularly in terms of patient selection, when to commence, and the duration, intensity and frequency of the rehabilitation interventions.131 Activities may also be adopted and adapted from other established rehabilitation programs in pulmonary stroke cohorts.128 Technological devices, such as virtual reality rehabilitation132 may also prove to be beneficial in this cohort with further development and testing.
Ward-Based Post-ICU Recovery
Follow-up services for survivors of a critical illness in Australia and New Zealand have occurred sporadically in individual units with interested clinician teams,133 but there is currently no widespread systematic approach to recovery and rehabilitation and the management of physical, psychological or cognitive dysfunctions beyond clinical stability and deterioration with ICU Liaison services134,135 or Medical Emergency Teams (MET).136
Commencement or continuation of rehabilitation activities in the general wards after discharge from ICU highlights a potentially different set of challenges, particularly in terms of physiotherapy resources, involvement of other medical teams, compliance to a prescribed plan. While some cohorts of critically ill patients (e.g. pulmonary, cardiac, stroke, brain injury) have defined rehabilitation pathways,128 patients with other clinical presentations may not be routinely prescribed a rehabilitation plan or be referred to a rehabilitation specialist.
For Australian and New Zealand patients who survive to ICU discharge, approximately 3% will die prior to hospital discharge.137 Some work in Europe on prognosis post-ICU discharge using the 4-point Sabadell Score (0 = good prognosis; 1 = long-term poor prognosis; 2 = short-term poor prognosis; 3 = expected hospital death)138 demonstrated that subjective intensivist assessment was able to predict the risk of patient mortality,139 and conversely those patients potentially suitable for rehabilitation.
Impairment in functional ability can be significant for some patients after ICU-discharge. In a small Dutch observational study (n = 69) of patients who had mechanical ventilation of 48 hours of more, over 75% of the sample were totally or severely dependent for activities of daily living (Barthel Index 0–12) 4 days after ICU-discharge.140 Close monitoring and early rehabilitation during this period was therefore recommended.
Specific ward-based rehabilitation interventions following ICU discharge are beginning to be investigated. Some exploratory work in the UK implemented a generic rehabilitation assistant to support enhanced physiotherapy and nutritional rehabilitation in collaboration with ward-based staff.141,142 Feasibility of the role and process was established, and further work in a larger sample will test the efficacy of the intervention.142
The benefits of physical exercise training for patients with COPD was affirmed in a recent review, with recommendations focused on maintenance of health behaviour change,143 and these guidelines could be applied to some cohorts of critically ill survivors. Identification of the most effective level of intervention however remains elusive. One Australian study of acute medical patients (not after critical illness) noted that individually tailored physical exercise (20–30 minutes twice daily, 5 days per week) in hospital was not sufficient to influence functional activity at discharge.144 Further research is therefore required to test specific interventions during the post-ICU hospital period aimed at improving the recovery trajectory and health outcomes for patients with limited physical function. As noted with in-ICU rehabilitation, the optimal duration, intensity and frequency of interventions is not yet clear.131
Recovery after Hospital Discharge
Of patients who survive their critical illness to hospital discharge, 5% will die within 12 months, and their risk of death is 2.9 times higher than for the general population.2 Functional recovery can be delayed in some individuals for 6–12 months3–5145 or longer.30 In a recent study in Norway, only half of 194 patients had returned to work or study one year after surviving their critical illness.145
There is however only limited research and mixed study findings identifying specific interventions during the post-hospital period that may improve a patient’s recovery trajectory and health outcomes. Most work has involved practice evaluations or studies of outpatient ‘ICU follow-up’ clinics,e.g.91,146,147 while there is some beginning work exploring home-based programs.e.g.148,158
ICU Follow-Up Clinics
Systematic follow-up for survivors of a critical illness after hospital discharge emerged in the UK in the early 1990s, after a number of government reviews on the cost and effectiveness of critical care services highlighted: the need to evaluate longer-term patient outcomes, in particular quality of life;149 and recognised that patients had sequelae that were best understood and managed by ICU clinicians. In 2000, the UK Department of Health published a comprehensive review of critical care services. With emerging albeit limited evidence of the benefits of an ICU follow-up clinic, the review recommended the provision of follow-up services for those patients expected to benefit.150 Importantly, this review also recommended collection of patient recovery and outcome data; this has been facilitated through follow-up clinics. The review did not however indicate how these services should be delivered or funded. The emerging pattern in the UK has therefore been to invite patients to a follow-up clinic.
The first intensive care follow-up clinics were established in the UK in the early 1990s,151 driven by a few interested and committed intensive care clinicians. From this early beginning the number of clinics has increased; a recent survey noted at least 80 follow-up clinics from approximately 300 ICUs throughout the UK.151 A number of decisions are required when implementing a follow-up clinic, as different models have evolved as nurse-led, doctor-led or a combination of both; more than half in the UK are currently nurse-led.151 Some services include input from allied health professionals and psychologists, although this multi-disciplinary approach is less common. This may be a reflection of resource implications but is in keeping with the general development of nurse-led services within the UK. Many clinics restrict patients invited to return to those with an ICU length of stay of at least 3 or 4 days. This decision is often based upon resources rather than evidence, as patients who have a shorter stay may also have subsequent physical and psychological problems.146
Common practice is to invite patients to attend a first clinic appointment approximately 2–3 months after discharge from intensive care or hospital, although timing has to be flexible given the length of hospital stay for some patients. For many, one appointment is sufficient,152 but others have continuing problems and may need to return on a number of occasions. Some clinics routinely offer return appointments up to one year after discharge, determined on an individual patient basis. Attendance can be problematic; only 70–90% in some studies.146,153 Non-attendance can occur because a patient has no identified problems (shorter ICU LOS; less ill); or more importantly because of individual limitations (limited mobility; living a distance away from the clinic, or significant post-traumatic stress symptoms including avoidant behaviours).153
While these services developed in a relatively ad hoc manner, tended to be underfunded and used a variety of models in their delivery, the purposes for such a service are similar (see Box 4.1).
Box 4.1
Purpose of an intensive care follow-up service
• Review and assess patient progress
• Early identification of problems and refer to appropriate specialties where necessary
• Support a rehabilitation program
• Discuss the intensive care experience and offer patient the opportunity to comment on care
• Offer patient opportunity to visit the ICU
Clinic Activities
Patient progress is reviewed for identification of subsequent problems, and timely referral to appropriate services for further treatment. A major advantage of follow-up clinics is the increased understanding of patient recovery, as a range of physical and psychological assessments can be conducted (see an example in Table 4.7). Content of assessment is informed by the understanding and knowledge of the problems patients commonly face during their recovery period. Critical care and rehabilitation staff, however, need to ensure that issues are not ‘problematising’ for aspects of recovery that is not of concern to the patient.
| Subject area | Rationale |
|---|---|
| General health | Assessed on a linear analogue or forced choice response to elicit a patient’s subjective account of how they view their general health and how it has changed since critical illness |
| Medications | Review of medications commenced during the critical illness and continued post-discharge, with advice provided to the patient’s General Practitioner146 |
| Movement and mobility, household management and joints | Assess mobility problems, often due to continuing fatigue and weakness, but also perhaps joint problems;190,191 identify impact on daily activities109,192,193 |
| Breathing and tracheostomy | Breathlessness is common after critical illness192 and there are a number of potential difficulties post-tracheostomy; these can be identified and the patient referred to the appropriate specialist |
| Sleep and eating | Sleep and concentration disturbances are common, and muscle loss and weakness are important contributors to delayed recovery109 |
| Urology/reproduction, skin and senses | Patients may have sexual problems192,194 and skin and nail problems192 |
| Recreation, work and lifestyle change | Patients may experience difficulties reintegrating into society and in particular returning to work192 |
| Intensive care experience | Patients rarely remember factual events of their time in ICU, but their memories are often of unpleasant and disturbing events;54,58,85 offering an opportunity to discuss actual events and sometimes distressing memories can be beneficial152 |
| Quality of life | The ultimate aim of treatment and care is to return a patient to an acceptable and optimal quality of life; it is important to gauge how patients perceive their life quality, and may identify areas for practice improvement53 |
Content of an assessment tool structures the clinic visit and identifies any patient problems. These assessments can include the use of standardised questionnaires of HRQOL and psychological status, and other free-text responses that incorporate patient comments and other issues. Use of standardised questionnaires is however inconsistent151 which limits evaluation and comparisons of clinic outcomes. Common examples of questionnaires were previously listed in Tables 4.1 and 4.3. Cognitive status can also be assessed using a number of neuro-cognitive tests including Ravens Progressive Matrices, Hayling Sentence and the Six Element test.77 The issue of respondent burden must be considered and questionnaire fatigue recognised. This can be managed in part by asking patients to bring completed questionnaires with them to the clinic appointment. Administration, scoring and interpretation of questionnaires must also be managed in accordance with instrument guidelines.
Referral to appropriate specialties using a systematic approach and timely response times are necessary, as other healthcare professionals will not usually be present when patients attend the clinic. Delays in treatment following identification of significant post-traumatic symptomatology can result in PTSD that is enduring and lasts for several years.81 Implementing defined referral criteria and pathways can however be challenging particularly when a clinic is nurse-led.146 While identification of referrals during a follow-up clinic reflect a potential unmet need for these patients, one survey reported that 51% of clinics had no formal referral mechanisms.151 This referral activity also reflects an additional function of the clinic in coordinating patient care after hospital discharge.
Coordination of care for these patients with complex needs often includes multiple out-patient appointments and investigations at a time when they are least able to cope with this complexity. An additional patient benefit of returning to a follow-up clinic is in supporting them to negotiate their way through this complex care, co-ordinate out-patient appointments, and to have someone who they know help them understand and interpret the whole critical illness and recovery experience. This coordinating role was unforeseen in a recent study evaluating the effectiveness of a nurse-led clinic.146
The follow-up clinic can also be a vehicle for supporting and evaluating a rehabilitation program.91 Rehabilitation in the form of a 6-week supported self-help manual with weekly telephone calls and completion of a diary demonstrated an improvement in physical recovery at 8 weeks and 6 months after intensive care discharge.
As noted earlier, a unique element of a patient’s intensive care experience is their limited recall of factual events but a common experience of ‘nightmares’ and ‘hallucinations’ that can be distressing both at the time and during recovery. The benefits of having an opportunity to discuss their experiences with intensive care staff should not be underestimated. Patients value being able to speak to ‘experts’ about their experience, be given information about what happened to them in ICU and also receive reassurances about the length of time that recovery will take and that their distressing memories are common. Clinics also offer patients the opportunity to comment on their care both during and after intensive care.152,153 This is important not just for the patient but to inform care delivery. For ICUs who complete patient diaries, the follow-up clinic is often the place where these are introduced and discussed with the patient.108,154
A follow-up clinic also provides an important forum for relatives. Relatives may have different needs to a patient, and it is common to encourage relatives to attend with the patient. Relatives may not only have short- and long-term consequences for their emotional wellbeing and physical health,155 but also be faced with supporting a patient who has unrealistic expectations about their recovery. Clinic attendance by relatives varies;146 and may be related to them having no identified problems or unanswered questions about the patient’s recovery, or being unable to attend because of work commitments. Some relatives, however, may not attend because of also adopting avoidant strategies if they are experiencing posttraumatic stress symptomatology156 or other health problems.
Clinic Evaluation
Given the development of follow-up clinics and the nature of implementation, formal evaluation is difficult and this is reflected in the paucity of empirical evidence. Anecdotally, nurses who deliver these clinics consider them beneficial and patients seem to value them. Intuitively it is a good idea for intensive care practitioners who have unique insights into patient experiences, to follow their patients after discharge. Three approaches to follow-up clinic evaluation are evident: a service evaluation,153 a qualitative study152 and a pragmatic, randomised controlled trial;146 each providing different insights.
Twenty-five interviews were performed to evaluate one service, with a number of important themes evident: patients valued easy access to the clinic, being well-treated by staff and not having to wait long to be seen. Some patients attended because they simply received the appointment, while others identified the need to have questions answered, and wanted to discuss their distressing dreams and hallucinations.153 While there was an insightful account of the development and initial evaluation, no demonstrable patient benefits were evident.
Four main themes emerged from another study of 34 patients: continuity of care; receiving information; importance of expert reassurance and giving feedback to intensive care staff.152 Continuity of care enabled reassurance to patients that their progress was being monitored and any problems dealt with if referral to other specialties was needed. Opinions varied about the number of clinic appointments and this reflects individual perceptions and needs. Receiving information was invaluable because of the poor memory for factual events. General information about what had happened to them in ICU was also important for gauging the length of time needed for recovery. Patients also found specific information about tracheostomy scars and other specific areas beneficial. While much of this information could be delivered by non-ICU staff, it was noted patients and relatives were specifically reassured from experts familiar with their ICU experiences.152 Being informed that other patients had similar experiences, particularly with problems sleeping or the nightmares and hallucinations, was also comforting to patients. Clinics also offered the patient the opportunity to give feedback to ICU staff, and also allowed patients and relatives to thank staff for the care received.
The PRaCTICaL study randomised eligible patients to a control group of usual care (in-hospital review by a liaison nurse) or intervention group (a physical rehabilitation handbook and a nurse-led intensive care follow-up clinic 2–3 months after discharge and 6 months later).146 Referral pathways were developed with ‘fast-track’ access to psychiatric or psychological services. There was no demonstrated differences between groups for the primary (HRQOL: SF-36) or secondary outcome measures (anxiety and depression: Hospital Anxiety and Depression Scale; post-traumatic stress: Davidson Trauma Scale). There were also no differences between patients who had a short intensive care stay and those with longer stays.146
There is little doubt that patients value intensive care follow-up, but there is no evidence to support any improved patient outcomes.157 There may be a number of reasons for this finding.146 The study intervention was based on existing models of ICU follow-up and our contemporary understanding of patient recovery has evolved since then. For example, no recognition was made of cognitive function and the effects of delirium, and perhaps the timing of the intervention was too late. It may also be that particular subgroups benefited, e.g. those who received psychiatric or psychological referral, but the study was not sufficiently powered to detect this.
Other considerations
It is important to consider that while interventions may not always benefit patients, it also has to be demonstrated that they cause no harm and therefore initiating a new service has governance issues. There are also knowledge and skill-set development issues. Intensive care nurses tend not to have training in managing patients on an outpatient basis and new skills have to be learned. They may also not have knowledge and experience in managing many of the patient problems evident at follow-up, in particular the psychological issues. Other considerations include accommodation, documentation, communication with other healthcare professionals and evaluation processes. Table 4.8 provides an example of how these issues were addressed when setting up a follow-up service in a Scottish teaching hospital; an evolving service that developed from the PRaCTICaL study.146 A more flexible approach is used with different options discussed with the patient regarding delivery, with a telephone consultation, home visit and/or clinic appointment.
TABLE 4.8 Example of considerations in setting up a nurse-led follow-up service
| Consideration | Action |
|---|---|
| Staff preparation |
• Patients’ case notes reviewed prior to the clinic appointment and discussed between nurse and intensivist
• General assessment questionnaire forms the basis of the discussion between the nurse and patient
• Standardised measures include: Short-Form 36, Hospital Anxiety and Depression Scale, and Intensive Care Experience Questionnaire
• Patients are offered a visit to the ICU if they do not ‘trigger’ referral on the Hospital Anxiety and Depression Scale
Home-Based Care
While there are home-based programs to manage ongoing care for some clinical cohorts (e.g. patients with heart failure) no specific follow-up programs currently exist to support survivors of a critical illness. Initial studies in this setting are also yet to identify an optimal intervention to improve recovery. A recent Australian multi-site study demonstrated that an individualised 8-week home-based physical rehabilitation program did not increase the underlying rate of recovery in a sample of 183 patients, with no group differences identified for 6MWT distances or HRQOL at 8 weeks or 6 months.158 The authors recommended further research to improve the effects of the intervention by increasing exercise intensity and frequency, and identifying individuals who would benefit from a home-based rehabilitation intervention. Other research is continuing in this area, but findings are not yet available.e.g.131 Findings from other clinical cohorts may also inform the development of rehabilitation interventions, for example with the use of web-based or mobile technologies.159
Further research is therefore also required in this post-hospital period160 as well as across the continuum of critical illness.e.g.131 With further study, future continuity of care and follow-up services after hospital discharge should enable the development of a series of seamless services that start recovery and rehabilitation activities for a patient while in ICU, is carried through to hospital discharge and continues into the community setting.
Summary
Research vignette
Critique
The findings from this study are encouraging and add to our understanding of the effectiveness of using patient diaries. A smaller UK-based study had also found a reduction in anxiety and depression in patients who had received diaries.161 Both studies evaluated the effectiveness of diaries over a relatively short period of time. PTSD may have late or delayed onset, has been shown not to reduce over time and in fact tends to be enduring. It is therefore important to be confident that any intervention causes no harm to patients and further study that explores the longer-term effect of the diaries would be beneficial.
Learning activities
1. Patients transferred from ICU to the ward may have complex care needs. In your hospital, who assesses these physical, psychological and cognitive needs and ensures that appropriate health professionals become involved in the patient’s care? When does this assessment take place?
2. Review the evidence of PTSD assessment and management for patients after a critical illness and intensive care admission.
3. What are the educational implications for staff in relation to supporting the psychological problems patients experience after ICU?
I-CAN UK (Intensive Care After Care Network). http://www.i-canuk.co.uk/default.aspx.
ICU Steps http://www.icusteps.com
Intensive Care Recovery Manual. St Helens and Knowsley Hospitals NHS Trust. http://www.sthk.nhs.uk/library/documents/icurecoverymanual2009.pdf.
Patient-reported Outcome and Quality of Life Instruments Database (PROQOLID). http://www.proqolid.org.
Oeyen SG, Vandijck DM, Benoit DD, Annemans L, Decruyenaere JM. Quality of life after intensive care: a systematic review of the literature. Crit Care Med. 2010;38(12):2386–2400.
National Institute for Health and Clinical Excellence. Rehabilitation after critical illness. NICE clinical guideline 83. 2009 March:1–91. Available from http://www.nice.org.uk/CG83
1 Drennan K, Hicks P, Hart GK. Intensive care resources and activity: Australia & New Zealand 2007/2008. Melbourne: Australian and New Zealand Intensive Care Society; 2010.
2 Williams TA, Dobb GJ, Finn JC, Knuiman MW, Geelhoed E, et al. Determinants of long-term survival after intensive care. Crit Care Med. 2008;36:1523–1530.
3 Adamson H, Elliott D. Quality of life after a critical illness: a review of general ICU studies 1998–2003. Aust Crit Care. 2005;18:50–60.
4 Dowdy DW, Eid MP, Sedrakyan A, Mendez-Tellez PA, Pronovost PJ, et al. Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med. 2005;31(5):611–620.
5 Oeyen SG, Vandijck DM, Benoit DD, Annemans L, Decruyenaere JM. Quality of life after intensive care: a systematic review of the literature. Crit Care Med. 2010;38(12):2386–2400.
6 Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: A systematic review. Intensive Care Med. 2007;33(11):1876–1891.
7 De Jonghe B, Lacherade JC, Durand MC, Sharshar T. Critical illness neuromuscular syndromes. Neurologic Clinics. 2008;26(2):507–520.
8 Griffiths RD, Jones C. Delirium, cognitive dysfunction and posttraumatic stress disorder. Curr Opin Anesthesiol. 2007;20(2):124–129.
9 Angus DC, Carlet J. Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29(3):368–377.
10 de Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37(10 Suppl):S309–S315.
11 Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU-acquired delirium and weakness – crossing the quality chasm. Chest. 2010;138(5):1224–1233.
12 Griffiths JA, Morgan K, Barber VS, Young JD. Study protocol: The Intensive Care Outcome Network (‘ICON’) study. BMC Hlth Serv Res. 2008;8(1):132.
13 Davydow DS, Gifford JM, Desai SJ, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796–809.
14 Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33(9):1506–1518.
15 Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520.
16 Hopkins RO. Haunted by delusions: Trauma, delusional memories, and intensive care unit morbidity. Crit Care Med. 2010;38(1):300–301.
17 Hopkins RO, Jackson JC. Short- and long-term cognitive outcomes in intensive care unit survivors. Clin Chest Med. 2009;30(1):143.
18 Jackson JC, Mitchell N, Hopkins RO. Cognitive functioning, mental health, and quality of life in ICU survivors: an overview. Crit Care Clinics. 2009;25(3):615–628.
19 Jones C, Griffiths RD, Slater T, Benjamin KS, Wilson S. Significant cognitive dysfunction in non-delirious patients identified during and persisting following critical illness. Intensive Care Med. 2006;32:923–926.
20 Rubenfeld GD, Curtis JR. Health status after critical illness: Beyond descriptive studies. Intensive Care Med. 2003;29(10):1626–1628.
21 Pandharipande P, Banerjee A, McGrane S, Ely EW. Liberation and animation for ventilated ICU patients: the ABCDE bundle for the back-end of critical care. Crit Care. 2010;14(3):157.
22 Girard TD, Kress JP, Fuchs BD, Thomason JWW, Schweickert WD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134.
23 Fan E, Zanni JM, Dennison CR, Lepre SJ, Needham DM. Critical illness neuromyopathy and muscle weakness in patients in the intensive care unit. AACN Adv Crit Care. 2009;20(3):243.
24 Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;27(10 [Suppl.]):S299–S308.
25 Griffiths RD, Hall JB. Intensive care unit-acquired weakness. Crit Care Med. 2010;38(3):779–787.
26 Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit – from pathophysiology to clinical trials. Crit Care. 2009;13:216–224.
27 Winkelman C. Bed rest in health and critical illness. AACN Adv Crit Care. 2009;20(3):254–266.
28 Sliwa JA. Acute weakness syndromes in the critically ill patient. Arch Phys Med Rehabil. 2000;81(3 [Supp. 1]):S45–S54.
29 Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matte A, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Resp Crit Care Med. 2006;174(5):538.
30 Cuthbertson BH, Roughton S, Jenkinson D, MacLennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14(R6):1–12.
31 Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. New Eng J Med. 2003;348(8):683–693.
32 Strahan EH, Brown RJ. A qualitative study of the experiences of patients following transfer from intensive care. Intensive Crit Care Nurs. 2005;21(3):160–171.
33 United Kingdom Medical Research Council. Aids to the examination of the peripheral nervous system. Edinburgh: W.B. Saunders; 2000.
34 Ali NA, O’Brien JM, Hoffmann SP, Phillips G, Garland A, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Resp Crit Care Med. 2008;178(3):261.
35 Fan E, Ciesla ND, Truong AD, Bhoopathi V, Zeger SL, Needham DM. Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med. 2010;36(6):1038–1043.
36 Black NA, Jenkinson C, Hayes JA, Young D, Vella K, et al. Review of outcome measures used in adult critical care. Crit Care Med. 2001;29(11):2119–2124.
37 Ridley S. Critical care outcomes. Anaesthesia. 2001;56(1):1–3.
38 Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health-related quality of life. J Nurs Schol. 2005;37(4):336–342.
39 Hofhuis JGM, van Stel HF, Schrijvers AJP, Rommes JH, Bakker J, Spronk PE. Health-related quality of life in critically ill patients: how to score and what is the clinical impact? Curr Opin Crit Care. 2009;15(5):425–430.
40 Chaboyer W, Elliott D. Health-related quality of life of ICU survivors: review of the literature. Intensive Crit Care Nurs. 2000;16(2):88–97.
41 Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, et al. Quality of life after acute respiratory distress syndrome: A meta-analysis. Intensive Care Med. 2006;32(8):1115–1124.
42 Elliott D. Measuring the health outcomes of general ICU patients: a systematic review of methods and findings. Aust Crit Care. 1999;12(4):132–140.
43 Buckley TA, Cheng AY, Gomersall CD. Quality of life in long-term survivors of intensive care. Annals Acad Med Singapore. 2001;30(3):287–292.
44 Needham DM, Dowdy DW, Mendez-Tellez PA, Herridge MS, Pronovost P. Studying outcomes of intensive care unit survivors: measuring exposures and outcomes. Intensive Care Med. 2005;31:1153–1160.
45 Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, et al. Outcome measures for adult critical care: a systematic review. Health Technol Assess. 2000;4(24):1–111.
46 Vainiola T, Pettila V, Roine RP, Rasanen P, Rissanen AM, Sintonen H. Comparison of two utility instruments, the EQ-5D and the 15D, in the critical care setting. Intensive Care Med. 2010;36:2090–2093.
47 Katz S, Ford A, Moskowitz R. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. J Am Med Assoc. 1963;185:914–919.
48 Karnofsky D, Abelmann W, Craver L, Burchenal J. The use of nitrogen mustards in the palliative treatment of cancer. Cancer. 1948;1:634–656.
49 Lawton MP, Brody BM. Assessment of older people:self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186.
50 Borg GA. Psychophysical bases of perceived exertion. Med & Sci Sports & Exercise. 1982;14:377–381.
51 American Thoracic Society. Guidelines for the six-minute walk test. Am J Resp Crit Care Med. 2002;166:111–117.
52 Jones C, Humphris GM, Griffiths RD. Psychological morbidity following critical illness: the rationale for care after intensive care. Clin Intensive Care. 1998;9:199–205.
53 Rattray JE, Hull AM. Emotional outcome after intensive care: literature review. J Adv Nurs. 2008;64(1):2.
54 Rattray J, Johnston M, Wildsmith JAW. The intensive care experience: development of the ICE questionnaire. J Adv Nurs. 2004;47(1):64–73.
55 Jones C. Aftermath of intensive care: the scale of the problem. Brit J Hosp Med. 2007;68(9):464–466.
56 Jones C, Twigg E, Lurie A, McDougall M, Heslett R, et al. The challenge of diagnosis of stress reactions following intensive care and early intervention: a review. Clin Intensive Care. 2003;14:83–89.
57 Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med. 2001;29(3):573–580.
58 Adamson H, Murgo M, Boyle M, Kerr S, Crawford M, Elliott D. Memories of intensive care and experiences of survivors of a critical illness: an interview study. Intens Crit Care Nurs. 2004;20:257–263.
59 Ringdal M, Plos K, Ortenwall P, Bergbom I. Memories and health-related quality of life after intensive care: a follow-up study. Crit Care Med. 2010;38(1):38–44.
60 Brewin CR. Systematic review of screening instruments for adults at risk of PTSD. J Trauma Stress. 2005;18(1):53–62.
61 Gordon SM, Jackson JC, Ely EW, Burger C, Hopkins RO. Clinical identification of cognitive impairment in ICU survivors: insights for intensivists. Intensive Care Med. 2004;30(11):1997–2008.
62 Jackson JC, Gordon MW, Ely EW, Burger C, Hopkins RO. Research issues in the evaluation of cognitive impairment in intensive care unit survivors. Intensive Care Med. 2004;30(11):2009–2016.
63 Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson LV. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Resp Crit Care Medicine. 1999;160(1):50–56.
64 Chan RC, Shum D, Toulopoulou T, Chen EY. Assessment of executive functions: review of instruments and identification of critical issues. Arch Clin Neuropsychol. 2008;23(2):201–216.
65 Granja C, Gomes E, Amaro A, Ribeiro O, Jones C, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: The early illness amnesia hypothesis. Crit Care Med. 2008;36(10):2801.
66 Granja C, Lopes A, Moreira S, Dias C, Costa-Pereira A, Carneiro A. Patients’ recollections of experiences in the intensive care unit may affect their quality of life. Crit Care. 2005;9:R96–109.
67 Boyle M, Murgo M, Adamson H, Gill J, Elliott D, Crawford M. The effect of chronic pain on health related quality of life amongst intensive care survivors. Aust Crit Care. 2004;17:104–113.
68 Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Resp Crit Care Med. 2003;168(12):1457–1461.
69 Jones C, Humphris G, Griffiths RD. Preliminary validation of the ICUM tool: A tool for assessing memory of the intensive care experience. Clinical Intensive Care. 2000;11(5):251–255.
70 Löf L, Berggren L, Ahlström G. ICU patients’ recall of emotional reactions in the trajectory from falling critically ill to hospital discharge: Follow-ups after 3 and 12 months. Intensive Crit Care Nurs. 2008;24(2):108–121.
71 Stein-Parbury J, McKinley S. Patients’ experiences of being in an intensive care unit: a select literature review. Am J Crit Care. 2000;9(1):20–27.
72 Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: A systematic review. Gen Hosp Psych. 2008;30(5):421–434.
73 Schelling G, Stoll C, Haller M, Briegel J, Manert W, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med. 1998;26(4):651–659.
74 Cuthbertson BH, Hull A, Strachan M, Scott J. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensive Care Med. 2004;30(3):450–455.
75 Rundshagen I, Schnabel K, Wegner C, am Esch S. Incidence of recall, nightmares, and hallucinations during analgosedation in intensive care. Intensive Care Med. 2002;28(1):38–43.
76 Magarey JM, McCutcheon HH. ‘Fishing with the dead’ – Recall of memories from the ICU. Intensive Crit Care Nurs. 2005;21(6):344–354.
77 Sukantarat KT, Burgess PW, Williamson RCN, Brett SJ. Prolonged cognitive dysfunction in survivors of critical illness. Anaesthesia. 2005;60(9):847–853.
78 McKinney AA, Deeny P. Leaving the intensive care unit: a phenomenological study of the patients’ experience. Intensive Crit Care Nurs. 2002;18(6):320–331.
79 Maddox M, Dunn SV, Pretty LE. Psychosocial recovery following ICU: experiences and influences upon discharge to the community. Intensive Crit Care Nurs. 2001;17(1):6–15.
80 de Miranda S, Pochard F, Chaize M, Megarbane B, Cuvelier A, et al. Postintensive care unit psychological burden in patients with chronic obstructive pulmonary disease and informal caregivers: A multicenter study. Crit Care Med. 2011;39(1):1. 12–18
81 Kapfhammer HP, Rothenhäusler HB, Krauseneck T, Stoll C, Schelling G. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004;161(1):45–52.
82 Dowdy DW, Bienvenu OJ, Dinglas VD, Mendez-Tellez PA, Sevransky J, et al. Are intensive care factors associated with depressive symptoms 6 months after acute lung injury? Crit Care Med. 2009;37(5):1702–1707.
83 Eddleston JM, White P, Guthrie E. Survival, morbidity, and quality of life after discharge from intensive care. Crit Care Med. 2000;28(7):2293–2299.
84 Myhren H, Tøien K, Ekeberg O, Karlsson S, Sandvik L, Stokland O. Patients’ memory and psychological distress after ICU stay compared with expectations of the relatives. Intensive Care Med. 2009;35(12):2078–2086.
85 Rattray JE, Johnson M, Wildsmith JAW. Predictors of emotional outcomes of intensive care. Anaesthesia. 2005;60:1085–1092.
86 Myhren H, Ekeberg O, Tøien K, Karlsson S, Stokland O. Posttraumatic stress, anxiety and depression symptoms in patients during the first year post intensive care unit discharge. Crit Care. 2010;14(1):125.
87 Hopkins RO, Weaver LK, Chan KJ, Orme JF. Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc. 2004;10(7):1005–1017.
88 Scragg P, Jones A, Fauvel N. Psychological problems following ICU treatment. Anaesthesia. 2001;56(1):9–14.
89 Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370.
90 Nelson BJ, Weinert CR, Bury CL, Marinelli WA, Gross CR. Intensive care unit drug use and subsequent quality of life in acute lung injury patients. Crit Care Med. 2000;28(11):3626–3630.
91 Jones C, Skirrow P, Griffiths RD, Humphris GH, Ingleby S, et al. Rehabilitation after critical illness: a randomized, controlled trial. Crit Care Med. 2003;31(10):2456–2461.
92 Jackson JC, Hart RP, Gordon SM, Shintani A, Truman B, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31(4):1226–1234.
93 Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF, Jr. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Resp Crit Care Med. 2005;171(4):340.
94 Capuzzo M, Valpondi V, Cingolani E, Gianstefani G, De Luca S, et al. Post-traumatic stress disorder-related symptoms after intensive care. Minerva Anestesiologica. 2005;71(4):167–179.
95 Tedstone JE, Tarrier N. Posttraumatic stress disorder following medical illness and treatment. Clin Psychol Review. 2003;23(3):409–448.
96 O’Donnell ML, Creamer M, Pattison P. Posttraumatic stress disorder and depression following trauma: understanding comorbidity. Am J Psychiatry. 2004;161(8):1390–1396.
97 Foa EB, McNally R, Murdock TB. Anxious mood and memory. Behav Res Ther. 1989;27(2):141–147.
98 Brewin CR, Andrews B, Rose S, Kirk M. Acute stress disorder and posttraumatic stress disorder in victims of violent crime. Am J Psychiatry. 1999;156(3):360–366.
99 American Psychiatric Association. Diagnostic and statistical manual of mental disorders 4th edn, text revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
100 Jackson J, Hart R, Gordon S, Hopkins R, Girard T, Ely EW. Post-traumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Crit Care. 2007;11(1):R27.
101 Perrins J, King N, Collings J. Assessment of long-term psychological well-being following intensive care. Intensive Crit Care Nurs. 1998;14(3):108–116.
102 Granberg A, Engberg IB, Lundberg D. Acute confusion and unreal experiences in intensive care patients in relation to the ICU syndrome. Part II. Intensive Crit Care Nurs. 1999;15(1):19–33.
103 Jones C, Humphris G, Griffiths RD. Preliminary validation of the ICUM tool: a tool for assessing memory of the intensive care unit experience. Clinical Intensive Care. 2000;11:251–255.
104 Russell S. An exploratory study of patients’ perceptions, memories and experiences of an intensive care unit. J Adv Nurs. 1999;29(4):783–791.
105 Weinert CR, Sprenkle M. Post-ICU consequences of patient wakefulness and sedative exposure during mechanical ventilation. Intensive Care Med. 2008;34(1):82–90.
106 Backman CG, Walther SM. Use of a personal diary written on the ICU during critical illness. Intensive Care Med. 2001;27(2):426–429.
107 Bergbom I, Svensson C, Berggren E, Kamsula M. Patients’ and relatives’ opinions and feelings about diaries kept by nurses in an intensive care unit: pilot study. Intensive Crit Care Nurs. 1999;15(4):185–191.
108 Jones C, Bäckman C, Capuzzo M, Egerod I, Flaatten H, et al. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Crit Care. 2010;14(5):R168.
109 National Institute for Health and Clinical Excellence. Rehabilitation after critical illness. NICE clinical guideline 83. 2009: Available from http://www.nice.org.uk/CG83
110 Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145.
111 Morris PE, Goad A, Thompson C, Taylor K, Harry B, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243.
112 Pohlman MC, Schweickert WD, Pohlman AS, Nigos C, Pawlik AJ, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;38(11):2089–2094.
113 Morris PE, Herridge MS. Early intensive care unit mobility: future directions. Crit Care Clinics. 2007;23(1):97–110.
114 Fan E. What it stopping us from early mobility in the intensive care unit? [Editorial]. Crit Care Med. 2010;38(11):2254–2255.
115 Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37(9):2499–2505.
116 Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882.
117 Stiller K, Phillips A. Safety aspects of mobilising acutely ill inpatients. Physio Theory Prac. 2003;19(4):239–257.
118 Bailey PP, Miller RR, Clemmer TP. Culture of early mobility in mechanically ventilated patients. Crit Care Med. 2009;37(10 Suppl):S429–S435.
119 Gosselink R, Bott J, Johnson M, Dean E, Nava S, et al. Physiotherapy for adult patients with critical illness: Recommendations of the European Respiratory Society and European Society of Intensive Care Medicine Task Force on physiotherapy for critically ill patients. Intensive Care Med. 2008;34(7):1188–1199.
120 Clini E, Ambrosino N. Early physiotherapy in the respiratory intensive care unit. Respiratory Medicine. 2005;99(9):1096–1204.
121 Bourdin G, Barbier J, Burle J, Durante G, Passant S, et al. The feasibility of early physical activity in intensive care unit patients: a prospective observational one-center study. Respiratory Care. 2010;55(4):400–407.
122 Needham DM, Truong AD, Fan E. Technology to enhance physical rehabilitation of critically ill patients. Crit Care Med. 2009;37(10 [Suppl.]):S436–S441.
123 De Jonghe B, Lacherade J-C, Durand M-C, Sharshar T. Critical illness neuromuscular syndromes. Crit Care Clinics. 2007;23(1):55–69.
124 Cader SA, Vale RG, Castro JC, Bacelar SC, Biehl C, et al. Inspiratory muscle training improves maximal inspiratory pressure and may assist weaning in older intubated patients: a randomised trial. J Physiother. 2010;56(3):171–177.
125 Choi JY, Tasota FJ, Hoffman LA. Mobility interventions to improve outcomes in patients undergoing prolonged mechanical ventilation: a review of the literature. Biological Res Nurs. 2008;10(1):21.
126 Korupolu R, Gifford JM, Needham DM. Early mobilization of critically ill patients: reducing neuromuscular complications after intensive care. Contemp Critical Care. 2009;6(9):1.
127 Skinner EH, Berney S, Warrillow S, Denehy L. Rehabilitation and exercise prescription in Australian intensive care units. Physiotherapy. 2008;94(3):220–229.
128 Morris PE. Moving our critically ill patients: Mobility barriers and benefits. Crit Care Clinics. 2007;23(1):1–20.
129 Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685.
130 Needham DM, Korupolu R, Zanni JM, Pradhan P, Colantuoni E, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Physical Med & Rehab. 2010;91(4):536–542.
131 Denehy L, Berney S, Skinner E, Edbrooke L, Warrillow S, et al. Evaluation of exercise rehabilitation for survivors of intensive care: protocol for a single blind randomised controlled trial. Open Crit Care Med J. 2008;1(1):39–47.
132 Van de Meent H, Baken BCM, Van Opstal S, Hogendoorn P. Critical illness VR rehabilitation device (X-VR-D): evaluation of the potential use for early clinical rehabilitation. J Electromyography & Kinesiology. 2008;18(3):480–486.
133 Daffurn K, Bishop GF, Hillman KM, Bauman A. Problems following discharge after intensive care. Intensive Crit Care Nurs. 1994;10(4):244–251.
134 Williams TA, Leslie GD, Finn J, Brearley L, Asthifa M, et al. Clinical effectiveness of a critical care nursing outreach service in facilitating discharge from the intensive care unit. Am J Crit Care. 2010;19(5):e63–e72.
135 Eliott SJ, Ernest D, Doric AG, Page KN, Worrall-Carter LJ, et al. The impact of an ICU liaison nurse service on patient outcomes. Crit Care Resusc. 2008;10(4):296–300.
136 Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised trial. Lancet. 2005;365:2091–2097.
137 Moran JL, Bristow P, Solomon PJ, George C, Hart GK. Mortality and length-of-stay outcomes, 1993–2003, in the binational Australian and New Zealand intensive care adult patient database. Crit Care Med. 2008;36(1):46–61.
138 Fernandez R, Baigorri F, Navarro G, Artigas A. A modified McCabe score for stratification of patients after intensive care unit discharge: the Sabadell score. Crit Care. 2006;10(6):R179.
139 Fernandez R, Serrano JM, Umaran I, Abizanda R, Carrillo A, et al. Ward mortality after ICU discharge: a multicenter validation of the Sabadell score. Intensive Care Med. 2010;36(7):1196–1201.
140 van der Schaaf M, Dettling DS, Beelen A, Lucas C, Dongelmans DA, Nollet F. Poor functional status immediately after discharge from an intensive care unit. Disability & Rehabilitation. 2008;30(23):1812–1818.
141 Salisbury LG, Merriweather JL, Walsh TS. Rehabilitation after critical illness: could a ward-based generic rehabilitation assistant promote recovery? Nurs Crit Care. 2010;15(2):57–65.
142 Salisbury LG, Merriweather JL, Walsh TS. The development and feasibility of a ward-based physiotherapy and nutritional rehabilitation package for people experiencing critical illness. Clin Rehabil. 2010;24:489–500.
143 Langer D, Hendriks EJM, Burtin C, Probst V, van der Schans CP, et al. A clinical practice guideline for physiotherapists treating patients with chronic obstructive pulmonary disease based on a systematic review of available evidence. Clin Rehabil. 2009;23(5):445.
144 de Morton NA, Berlowitz J, Jackson B, Lim WK. Additional exercise does not change hospital or patient outcomes in older medical patients: a controlled clinical trial. Aust J Physiother. 2007;53(2):105–111.
145 Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: A 1-year follow-up study. Crit Care Med. 2010;38(7):1554–1561.
146 Cuthbertson BH, Rattray J, Campbell MK, Gager M, Roughton S, et al. The PRaCTICal study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723.
147 McWilliams DJ, Atkinson D, Carter A, Foëx BA, Benington S, Conway DH. Feasibility and impact of a structured, exercise-based rehabilitation programme for intensive care survivors. Physiotherapy Theory & Practice. 2009;25(8):566–571.
148 Elliott D, McKinley S, Alison JA, Aitken LM, King MT. Study protocol: Home-based physical rehabilitation for survivors of a critical illness. Crit Care. 2006;10:R90.
149 UK NHS Audit Commission. Critical to Success. The place of efficient and effective critical care services within the acute hospital. In: Audit Commission. London: Editor; 1999.
150 UK Department of Health. Comprehensive critical care. a review of adult critical Care services. London: Department of Health; 2000.
151 Griffiths JA, Barber VA, Cuthbertson BH, Young JD. A national survey of intensive care follow-up clinics. Anaesthesia. 2006;61:950–955.
152 Prinjha S, Field K, Rowan K. What patients think about ICU follow-up services: a qualitative study. Crit Care. 2009;13(2):R46.
153 Cutler L, Brightmore K, Colqhoun V, Dunstan J, Gay M. Developing and evaluating critical care follow-up. Nurs Crit Care. 2003;8:116–125.
154 Combe D. The use of patient diaries in an intensive care unit. Nurs Crit Care. 2005;10(1):31–34.
155 Paul FBN, Rattray J. Short-and long-term impact of critical illness on relatives: literature review. J Adv Nurs. 2008;62(3):276.
156 Paul F, Hendry C, Cabrelli L. Meeting patient and relatives’ information needs upon transfer from an intensive care unit: the development and evaluation of an information booklet. J Clin Nurs. 2004;13(3):396–405.
157 Williams TA, Leslie GD. Beyond the walls: A review of ICU clinics and their impact on patient outcomes after leaving hospital. Aust Crit Care. 2008;21(1):6–17.
158 Elliott D, McKinley S, Alison JA, Aitken LM, King MT, et al. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care. 2011;15:R142.
159 Sarela A, Korhonen I, Salminen J, Koskinen E, Kirkeby O, Walters D. A home-based care model for outpatient cardiac rehabilitation based on mobile technologies. Pervasive Health. 2009:5970.
160 van der Schaaf M, Beelen A, Dongelmans DA, Vroom MB, Nollet F. Functional status after intensive care: a challenge for rehabilitation professionals to improve outome. J Rehabil Med. 2009;41:360–366.
161 Knowles RE, Tarrier N. Evaluation of the effect of prospective patient diaries on emotional well-being in intensive care unit survivors: a randomized controlled trial. Crit Care Med. 2009;37(1):184–191.
162 Ware JE. SF-36 health survey update. Spine. 2000;25(24):3130–3139.
163 Ware JE, Snow KK, Kosinski M. SF-36 Version 2 Health Survey: Manual and interpretation guide. Lincoln: Quality Metric Incorporated; 2000.
164 Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72.
165 Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Annals of Medicine. 2001;33:328–336.
166 Capuzzo M, Grasselli C, Carrer S, Gritti G, Alvisi R. Validation of two quality of life questionnaires suitable for intensive care patients. Intensive Care Med. 2000;26(9):1296–1303.
167 Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res. 1999;8(3):209–224.
168 Rivera-Fernandez R, Sanchez Cruz SJ, G. VMV. Validation of a quality of life questionnaire for critically ill patients. Intensive Care Med. 1996;22:1034–1042.
169 de Bruin AF, Diederikis JP, de Witte LP, Stevens FC, Philipsen H. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407–418.
170 Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: Development and final revision of a health status measure. Med Care. 1981;19:787–805.
171 Hunt S, McKenna S, McEwan J, Backett E, Williams J, Papp E. Measuring health status: a new tool for clinicians and epidemiologists. J Royal College of Gen Pract. 1985;35:185–188.
172 Patrick DL, Danis M, Southerland LI, Hong G. Quality of life following intensive care. J Gen Intern Med. 1988;3:218–223.
173 Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic arirflow limitation: The St George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327.
174 Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved COPD-specfic version of the St. George Respiratory Questionnaire. Chest. 2007;132:456–463.
175 Mahoney F, Barthek D. Functional evaluation: the Barthel Index. Maryland State Med J. 1965;14:61–65.
176 Novak S, Johnson J, Greenwood R. Barthel revisited: making guidelines work. Clin Rehabil. 1996;10(2):128–134.
177 Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability? Disability and Rehabilitation. 1988;10(2):64–67.
178 Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the Functional Independence Measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil. 1993;74(5):531–536.
179 Podsiadlo D, Richardson S. The Timed Up and Go: a test of basic functional mobility for frail elderly persons. J Am Geriatric Soc. 1991;39(2):142–148.
180 Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. BMJ. 1992;47(12):1019–1024.
181 Horowitz M, Wilner N, Alvarez W. Impact of event scale: a measure of subjective stress. Psychosomatic Medicine. 1979;41(3):209–218.
182 Weiss DS, Marmar C. The impact of event scale – revised. In: Wilson JP, Keane TM. Assessing psychological trauma and PTSD: A practitioner’s handbook. 2nd edn. New York: Guilford Press; 2004:168–189.
183 Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401.
184 Sukantarat K, Greer S, Brett S, Williamson R. Physical and psychological sequelae of critical illness. Brit J Health Psychol. 2007;12(1):65–74.
185 Wallen K, Chaboyer W, Thalib L, Creedy DK. Symptoms of acute postraumatic stress disorder after intensive care. Am J Crit Care. 2008;17(6):534–544.
186 Zanotti E, Felicetti G, Maini M, Fracchia C. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation. Chest. 2003;124(1):292.
187 Chiang LL, Wang LY, Wu CP, Wu HD, Wu YT. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Physical Therapy. 2006;86(9):1271.
188 Skinner EH, Berney S, Warrillow S, Denehy L. Development of a physical function outcome measure (PFIT) and a pilot exercise training protocol for use in intensive care. Crit Care Resusc. 11(2), 2009.
189 Williams TA, Leslie GD, Bingham R, Brearley L. Optimizing seating in the intensive care unit for patients with impaired mobility. Am J Crit Care. 2011;20(1):e19–e27.
190 Griffiths R, Jones C. Seven lessons from 20 years of follow-up of intensive care unit survivors. Curr Opin Crit Care. 2007;13:508–513.
191 Herridge MS. Legacy of intensive care unit-acquired weakness. Crit Care Med. 2009;37(10 Suppl):S457–S461.
192 Broomhead LR, Brett SJ. Intensive care follow-up: what has it told us? Critical Care. 2002;6(5):411–417.
193 Crocker C. A multidisciplinary follow-up clinic after patients’ discharge from ITU. Brit J Nurs. 2003;12(15):910–914.
194 Griffiths J, Gager M, Alder N, Fawcett D, Waldmann C, Quinlan J. A self-report-based study of the incidence and associations of sexual dysfunction in survivors of intensive care treatment. Intensive Care Med. 2006;32(3):445–451.