Reconstruction of the Scalp
Anatomy
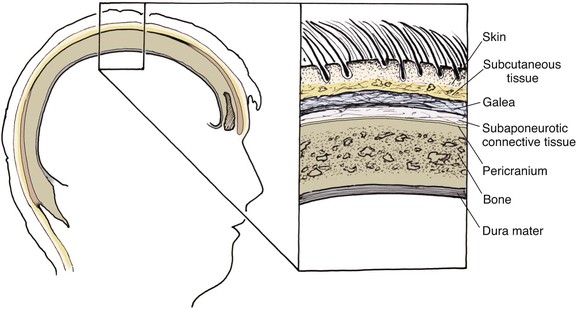
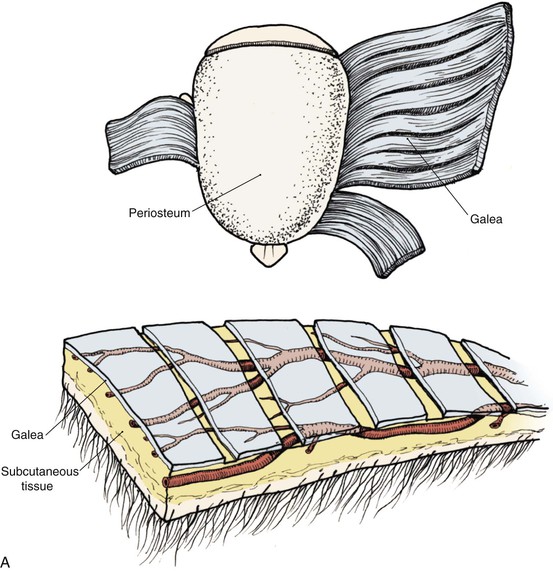
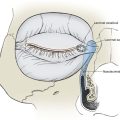
The anatomic layers of the scalp are well described by the convenient acronym SCALP (Fig. 24-1): S, the skin; C, the subcutaneous tissue; A, the galea aponeurosis; L, the loose connective tissue; and P, the periosteum, also known as the pericranium. The scalp has the thickest skin on the body. However, the thickness of scalp skin not only varies across the surface of the head but also may change throughout one’s life. The scalp skin is thickest when hair growth is at its most robust. If the hair thins, as in male pattern baldness or pathologic alopecia, scalp thickness significantly decreases. The subcutaneous fat layer contains hair follicles and integumentary structures such as sweat and sebaceous glands. It provides a tight connection between the skin and the underlying galea. The galea is a dense, inelastic layer that provides a fibrous connection between the frontalis muscle anteriorly and the occipitalis muscle in the nape of the neck. Within and adjacent to the galea run numerous vascular and neural structures. In the forehead region, the galea envelops the frontalis muscle, which has no direct attachment to bone. Posteriorly, the galea encompasses the occipitalis, which inserts in the bone along the nuchal line. The periosteum is usually tightly affixed to the cranium and possesses its own vascular supply.
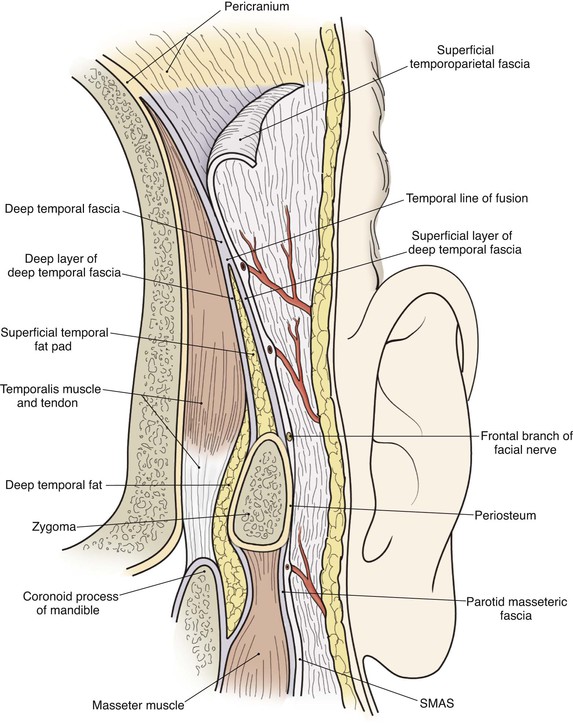
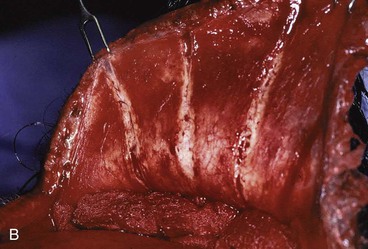
In the region of the temple, the anatomic layers of the scalp are more complex. Medial to the temporal line, which represents the superior aspect of the temporalis muscle, the SCALP acronym applies. However, over the temporalis muscle, the layers of the scalp differ slightly from the remaining scalp (Fig. 24-2). Over the superior aspect of the temporalis muscle, the first three layers of the scalp, the skin, subcutaneous fat, and galea, remain the same. Inferiorly, however, the galeal layer of the temporal region becomes the temporoparietal fascia. The temporoparietal fascia is loosely attached to the subcutaneous fat and is continuous with the fascia of the frontalis muscle in the forehead region. The temporoparietal fascia accounts for the difference between the loose and tight attachment of the scalp to the cranium (Fig. 24-3). The tight region of the scalp has a dense galeal layer, no underlying muscle, and poor distensibility. In the loose regions of the scalp, the galea is thinner and overlies muscle anteriorly as well as posteriorly below the nuchal line and is considerably more distensible. It is important to understand this anatomic difference to be successful in planning scalp flaps. For example, small advancement and transposition flaps are effective for repair of medium-sized (3 cm) defects in the loose regions of the scalp. Such flaps are not as effective for repair of similar-sized defects in the tight regions of the scalp because of the inelastic nature of the tissues in these areas.
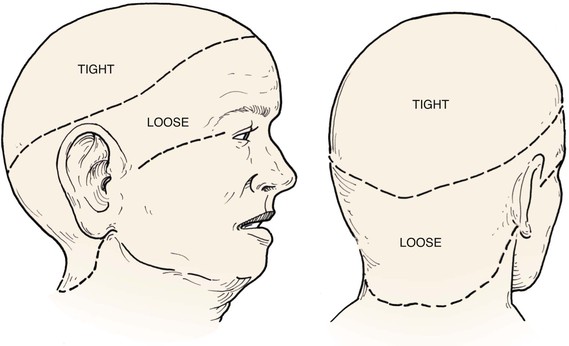
FIGURE 24-3 A, B, Tight and loose regions of scalp. Tight region has dense galeal layer, no underlying muscle, and poor distensibility. In loose region, the galea is the thin fascial layer overlying muscle, and scalp is more distensible.
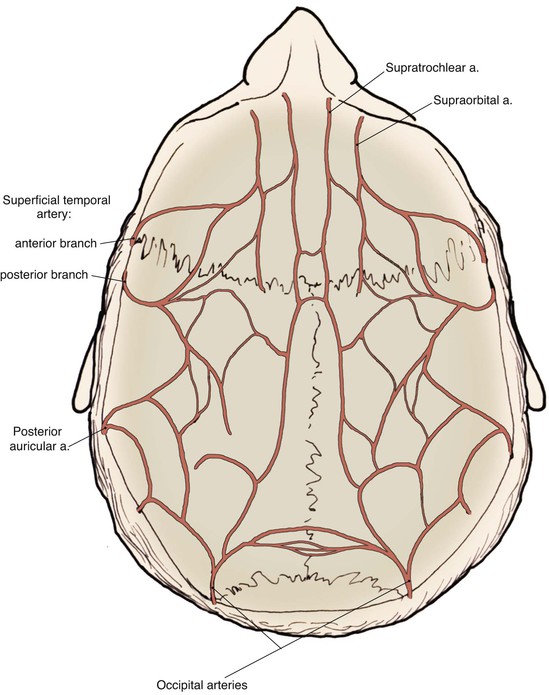
The scalp has a rich and redundant vascular supply (Fig. 24-4). The external carotid artery provides the majority of the blood supply to the scalp through the superficial temporal, posterior auricular, and occipital arteries. The internal carotid system also supplies the scalp anteriorly through its terminal supraorbital and supratrochlear arteries, which course up the forehead after exiting the orbit. The robust vascular network of the scalp will usually support large random patterned local flaps with variable configurations. However, there are limited anastomotic connections across the midline. Thus, flaps that extend a considerable distance across the midline may benefit from delay. This is particularly true in children.1 When designing flaps, the surgeon should be cognizant of the location and typical course of the principal arterial branches that supply the scalp and try to incorporate them in the base of the flap. A Doppler probe may be helpful in outlining the course of these vessels through the thick overlying skin. Many of the larger vascular branches run within the galea and temporoparietal fascia. For preservation of these vessels, scalp flaps are ideally dissected just deep to this layer within the loose connective tissue, creating a fasciocutaneous flap. The galea provides some protection to the vascularity of scalp flaps by limiting the elongation and crimping of vessels. The skin and subcutaneous tissue layers of the scalp also have a dense vascular plexus, but there is a greater risk of ischemia when flaps are dissected in the subcutaneous tissue plane. In addition, elevating a flap in the subcutaneous tissue plane subjects the hair follicles to the risk of injury and subsequent hair loss. The principal disadvantage of flaps that include the galeal layer is that the flaps are inelastic and nondistensible. Hence, scalp flaps must be designed with a large surface area relative to the size of a defect the flap is intended to repair. In addition, wound closure tension is likely to be higher compared with local cutaneous flaps used in other areas of the head and face.
The layers of the scalp provide convenient surgical dissection planes. Dissection beneath the galeal layer is simple, swift, and relatively avascular. Large flaps can be readily dissected in this tissue plane with minimal effort and good reliability. Flaps dissected in the subcutaneous plane compared with subgaleal dissection are considerably more difficult as this is a “nonanatomic” plane that requires sharp surgical dissection. Typically, these types of flaps are elevated just below the hair follicles within the subcutaneous fat. Numerous small vessels are encountered and hemostasis can be difficult. In addition, electrocautery used to secure hemostasis can injure the hair follicles. For these reasons, scalp flaps consisting only of skin and subcutaneous fat are rarely employed. In the area over the temporalis muscle, the temporoparietal fascia can be raised as an independent flap on a pedicle or as a microsurgical free flap and transferred to an adjacent or distant site.2,3 Incisions for the flap must remain posterior to the course of the temporal branch of the facial nerve to avoid denervation of the frontalis and corrugator muscles. The temporoparietal fascia flap is useful in auricular and orbital reconstruction as it provides thin, supple, and highly vascular tissue supplied by the posterior branch of the superficial temporal artery.4,5 Details of this flap are discussed elsewhere in this chapter. Flaps consisting of periosteum can also be developed independent of the overlying scalp tissues.
Healing by Secondary Intention
In some instances, allowing a scalp defect to heal by secondary intention may be a good option.6 However, several criteria should be met before this option is selected for the treatment of defects of the scalp. Most important, there must be a base of viable tissue, such as pericranium, on which granulation tissue can form. On occasion, bare bone may support the formation of granulation tissue on its own, but the process is exceedingly slow and fraught with problems. The surgeon can enhance the formation of granulation tissue by removing the outer table of the cranium to the level of the diploic space, which is quite vascular. This may allow islands of granulation to form, which will then coalesce into a continuous layer that can support re-epithelialization of the wound or a skin graft.
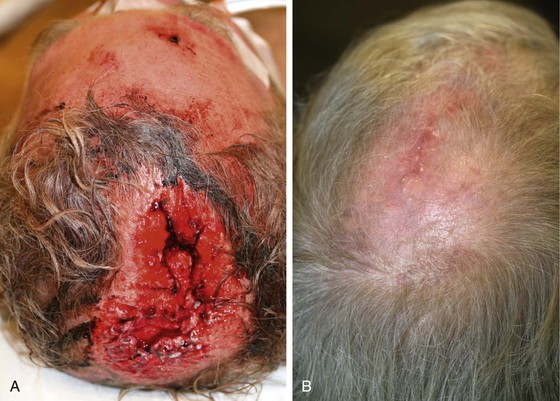
There are several drawbacks to allowing scalp defects to heal by secondary intention. Unlike wounds elsewhere on the head and neck, scalp wounds will minimally contract because of the stiff galea. Thus, it may require many weeks for a wound to heal. Wound care can be laborious and tedious for the patient or the caregiver. When healing has occurred, the area will be devoid of hair, and the regenerated skin covering the area is significantly thinner than the skin of the surrounding scalp. The thin skin has a propensity to break down into an open wound from minimal trauma to the area. In areas that have previously been irradiated or otherwise traumatized, the healing may be incomplete or extremely slow. Nonetheless, in selected patients and in certain areas such as the vertex in a bald patient, the result of wound healing by secondary intention can be acceptable (Fig. 24-5).
Primary Wound Closure
In general, defects of the scalp up to 3 cm in diameter may be repaired with primary wound closure. However, to accomplish this, wide undermining of the scalp in the subgaleal plane is required. This can often be accomplished by blunt finger dissection. Galeal relaxing incisions may also be judiciously employed on the undersurface of the scalp to allow some stretching of the galea, thereby reducing wound closure tension (Fig. 24-6). This technique can also be used in transferring scalp flaps. Galeal relaxing incisions must be performed cautiously to avoid damaging the vascular structures that are immediately superficial to the galea. Some authors have advocated intraoperative tissue expansion to assist flap elevation and to facilitate closure of scalp wounds.7,8 This technique is discussed in more detail later in this chapter. Other tissue-stretching devices have been employed as an adjunct to facilitate primary wound repair.9–12 Once the scalp is sufficiently undermined, the wound edges are advanced and apposed in layered fashion. The galeal layer should bear the majority of the closure tension to minimize the tension on the skin closure line. Wound closure tension placed on the galea and not the skin of the scalp avoids trauma to hair follicles and reduces the risk of postoperative alopecia. It is common for standing cutaneous deformities to form at the borders of the defect because of the inelastic nature of scalp. However, most of these will gradually resolve over time and rarely require secondary revision.
Skin Grafting
Split-thickness skin grafts provide a reliable and simple but suboptimal reconstruction of the scalp.13,14 For the majority of patients, a skin graft is distinctly inferior to a flap repair. Although an intact pericranium will readily support a skin graft, this option has numerous disadvantages. One obvious disadvantage is that skin grafts lack hair follicles. In addition, skin grafts are considerably thinner than normal scalp and as a result are associated with contour deformities. Skin grafts also tend to be unsightly, painful, and susceptible to trauma. Skin grafts may be so fragile that the patient cannot wear a hairpiece over it without pain or injury to the graft (Fig. 24-7).
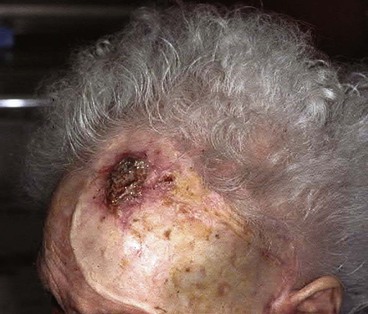
FIGURE 24-7 Repair of large scalp and forehead defect with split-thickness skin graft. Note crusting and ulceration in superior aspect of graft.
In patients who are not appropriate candidates for more extensive procedures and in whom the aesthetic outcome is of less concern, a skin graft may be a reasonable reconstructive option. Skin grafts may be useful to provide temporary immediate coverage of a scalp defect while the patient is being prepared for a more definitive repair with an expanded local flap or microsurgical free flap. Skin grafts may be useful in assisting with the closure of secondary defects, which are frequently located in a less visible area of the scalp, such as the occiput. A skin graft may be indicated when patients have a high risk of local recurrence after removal of a scalp malignant neoplasm. In such instances, repair with a scalp flap is performed at some time in the future after the major risk of recurrence has passed. In most cases, the subsequent removal of the skin graft is accomplished by serial excisions or a single excision followed by a scalp flap with or without prior tissue expansion. Ideally, skin grafts should be placed over viable pericranium, muscle, or fascia. When a sizable area of exposed bone without periosteum is present, an adjacent pericranial, temporoparietal fascial, or muscle flap can be used to cover the exposed bone and serve as a vascularized recipient site for the skin graft. Some skin grafts may even survive over denuded cranium, although certainly this is considerably less reliable and more prone to subsequent traumatic ulceration even when the graft is successful. As with secondary intention healing, there may be some benefit to removing the outer cranial table to allow contact of the skin graft with the diploic space and its vasculature or to allow the wound to granulate before the graft is placed. The best locations for use of a skin graft to repair defects of the scalp are on the vertex in a balding patient and on the forehead. Use of skin grafts should be limited in patients who require postoperative radiation therapy because of the propensity of the graft to break down during or after radiation treatments.
Local Flaps
Scalp flaps can be designed as advancement, pivotal, or interpolated.9 Unlike in other regions of the face, there are no relaxed skin tension lines in the scalp. Thus, incisions can be positioned to maximize vascular supply and tissue recruitment. The principal anatomic landmark that the surgeon should be cognizant of during flap design is the anterior hairline. All incisions in the scalp should be made parallel to the direction of hair growth to minimize hair follicle injury. Electrocautery should be used judiciously for the same reason.
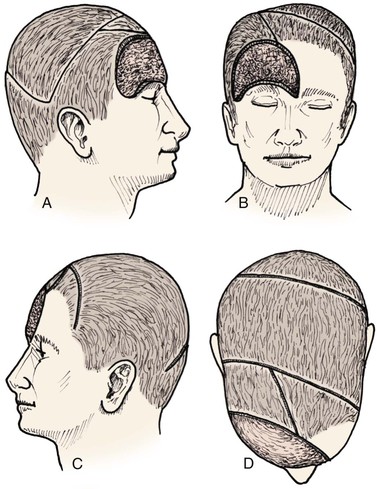
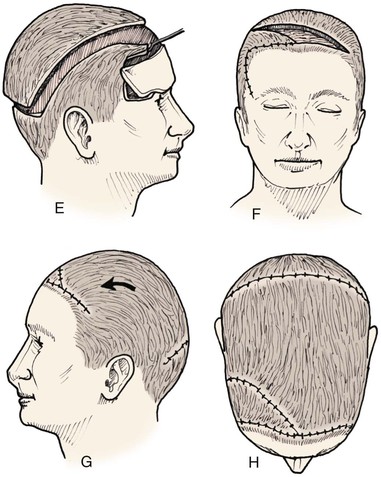
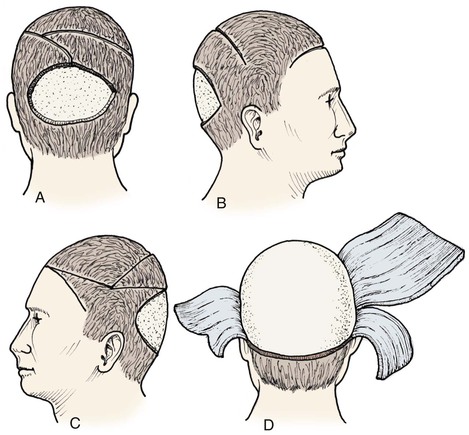
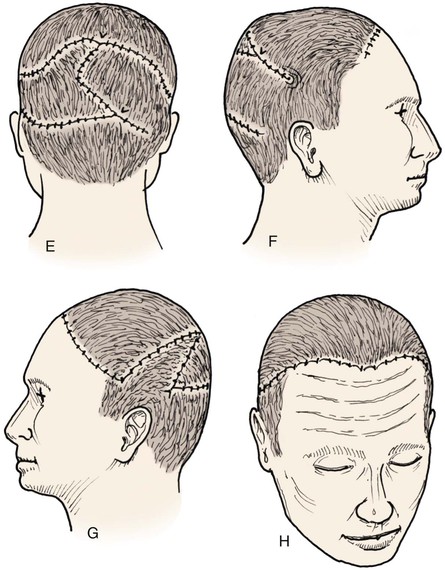
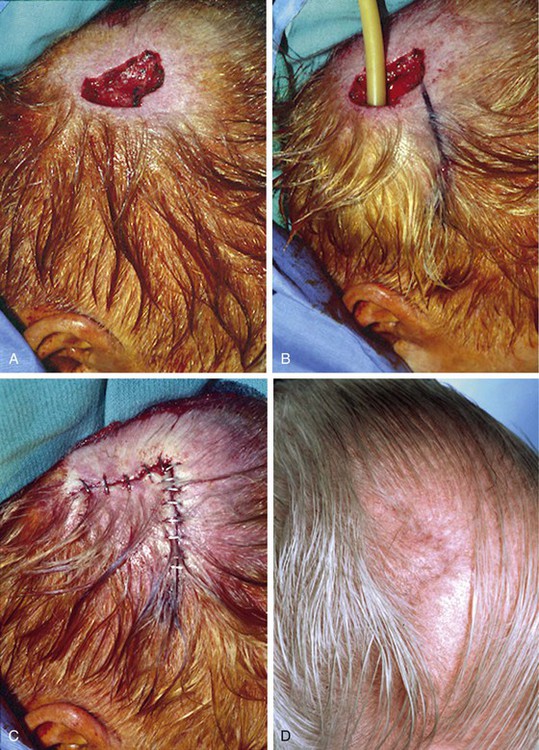
Advancement flaps have limited usefulness for reconstruction of the scalp because of the inelasticity of scalp tissue. Primary closure of a linear wound is in essence two large opposing advancement flaps. Primary wound closure can typically be used to repair small (3 cm or less) scalp defects (Fig. 24-8). The most useful flap employed for the repair of the majority of scalp defects is a rotation flap15–18 (Figs. 24-9 and 24-10). The curvilinear incision necessary to create a rotation flap is well suited to the spherical shape of the cranium. Even relatively small defects require a rather long flap incision. Rotation scalp flaps are designed four to six times as long as the defect is wide. The incision is beveled to parallel the direction of growth of the hair follicles to minimize alopecia along the incision. For defects located in the anterior scalp, the incision is designed to follow the hairline whenever possible (Fig. 24-11). Keeping with the concept of reconstructing different aesthetic regions of the face with independent flaps, defects that involve the forehead and hair-bearing scalp should be repaired with separate flaps. One flap is used to reconstruct the scalp component of the defect, and a separate flap is used to repair the forehead component of the defect. This approach will place the borders of the flaps along the aesthetic boundary of the anterior hairline to maximize scar camouflage. Scalp flaps should be elevated in the subgaleal plane in most cases, and the surrounding scalp must be widely undermined in the same tissue plane to distribute wound closure tension over a wide area. As the flap is pivoted into the defect, tension develops from the tip of the flap to its base over the convex cranium. On occasion, a “relaxing” incision or back cut extending from the base of the flap may be helpful to reduce the tension (see Fig. 24-10). A back cut has the effect of reducing the width of the pedicle of the flap. Thus, care should be taken so that the back cut does not compromise the flap’s vascularity. The surgeon should resist the temptation to excise standing cutaneous deformities that develop with flap movement because doing so has the same effect as a back cut in reducing the width of the base of the flap. Even large standing cutaneous deformities will diminish significantly over time. If necessary, the deformity can be excised at a later date without jeopardizing the vascularity of the flap. Transfer of the flap is typically best accomplished by first pivoting the flap into the defect and suturing the leading border of the flap in place. The curvilinear border of the flap is sutured next, using the principle of halves to distribute the inequity of the lengths of the border of the flap and the peripheral border of the wound. Depending on the size of the flap and the degree of pivoting, a secondary defect of considerable size may develop near the base. This is often in a more inconspicuous site than the primary defect and can heal by secondary intention (see Fig. 24-10) or be covered with a skin graft if closure by advancement of adjacent tissue is not possible. If the skin graft cannot be adequately covered with hair styling, it may be removed at a later date after the flap has healed and the scalp has relaxed to its natural state through the process of tissue creep. If the defect is near or involves the anterior hairline, the rotation flap should be designed so that the hairline is restored as naturally as possible (see Fig. 24-11). It is also helpful to bevel the flap edges bordering the hairline, preserving the underlying follicles. This facilitates the growth of hair through the incision line in a trichophytic fashion that assists with camouflaging the scar line.
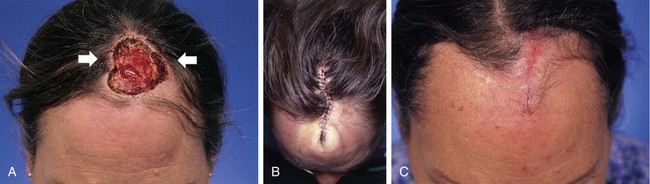
FIGURE 24-8 A, A 3.5 × 5-cm defect of anterior scalp. B, Wound closed primarily by bilateral wide undermining of scalp in subgaleal plane. Standing cutaneous deformity of forehead resulting from wound closure left undisturbed. C, Postoperative result at 3 months. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
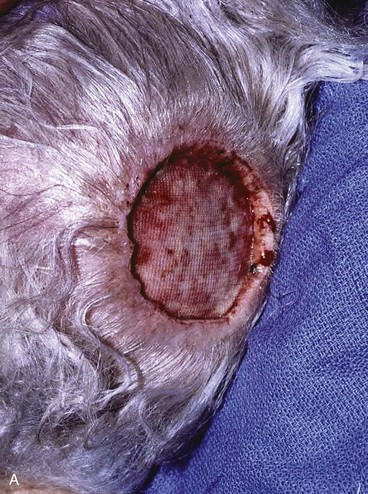
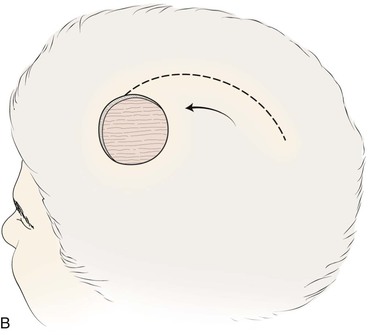
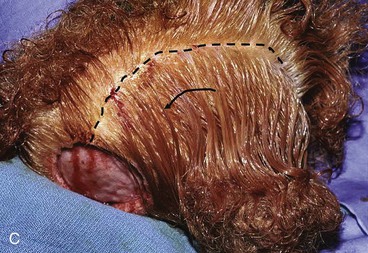
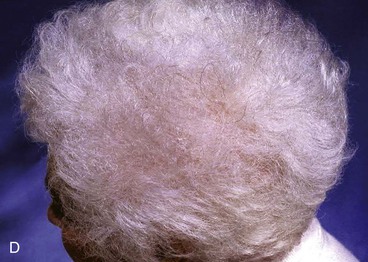
FIGURE 24-9 Length of border of rotation flap is four to six times width of defect. Wide undermining of scalp is required. A, Large defect of parietal scalp after excision of basal cell carcinoma. B, Drawing depicting rotation flap designed for repair. C, Single rotation flap designed for repair of defect. D, Postoperative result.
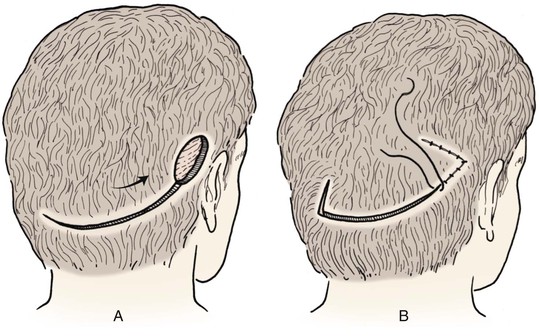
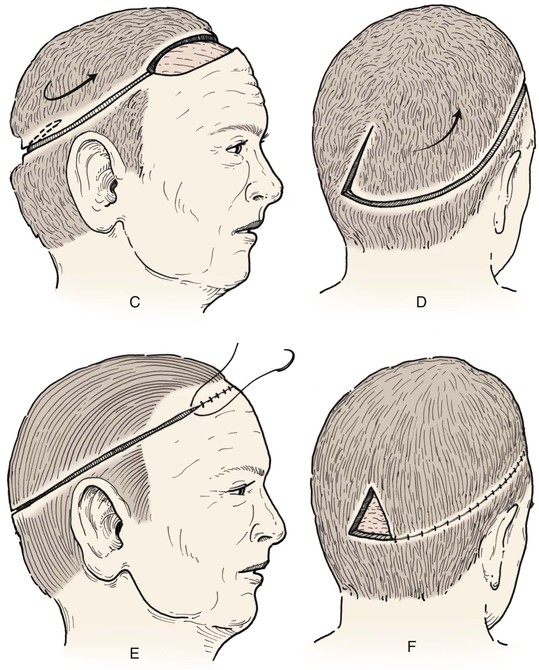
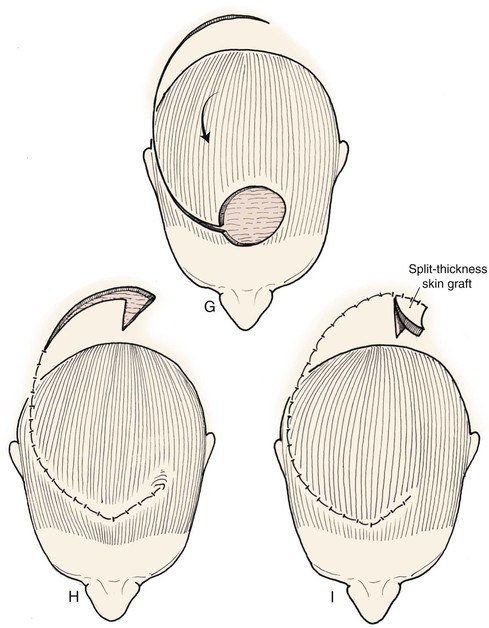
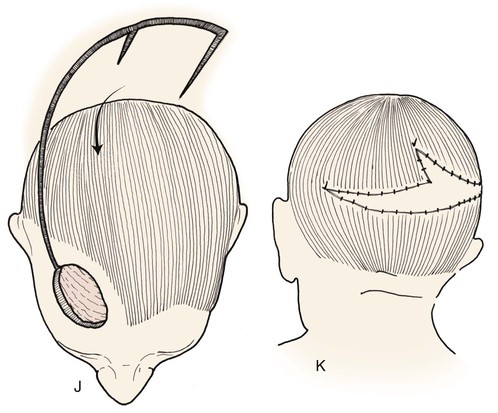
FIGURE 24-10 Border of flaps should be at least four to six times width of defects. Back cuts reduce wound closure tension but narrow base. Secondary defect can be covered by skin graft or may heal by secondary intention.
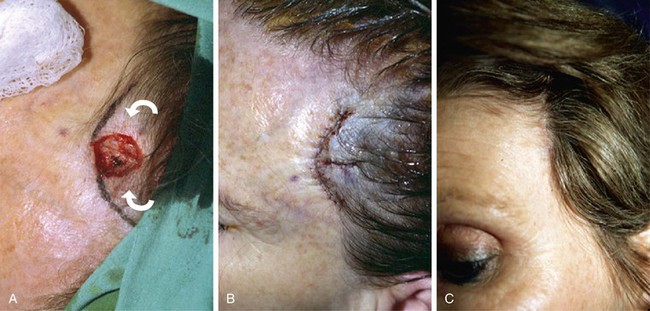
FIGURE 24-11 A, Defect in left hair-bearing temple. Bilateral rotation flaps designed for repair. B, Wound repaired. Incisions designed as trichophytic to allow hair growth through incision scar. C, Postoperative result with well-concealed scar.
Frequently, it may be beneficial to employ multiple rotation flaps simultaneously (Fig. 24-12). This has the advantage of distributing wound closure tension over a larger area of the scalp. It also enables the recruitment of scalp for repair of the wound from multiple donor areas (Fig. 24-13). This in turn allows the use of smaller flaps that do not span a large expanse of convex bone, which has the effect of increasing wound closure tension. The use of multiple flaps also allows the recruitment of tissue from areas of the scalp with more tissue elasticity, such as scalp covering the occiput and the temporalis muscle. The primary disadvantage of using multiple rotation scalp flaps to repair a defect is the greater number of incisions required, each with the potential for development of alopecia. Multiple rotation flaps are especially well suited for repair of scalp defects of the vertex (Fig. 24-14). For centrally located scalp defects, two rotation flaps may be designed to pivot from either opposite directions or the same direction (Fig. 24-15). Three rotation flaps may be used concurrently to repair central defects of the anterior or posterior scalp. All three flaps are designed to pivot in the same direction as in a pinwheel (Fig. 24-16). Whether one or more rotation flaps are used, undermining of the scalp must be extensive, typically extending from ear to ear and from forehead to occiput.
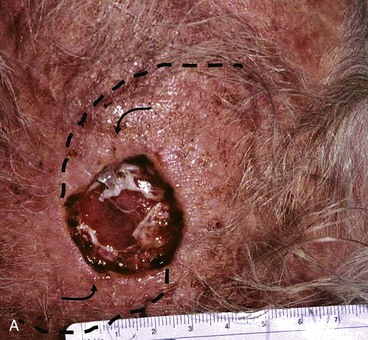
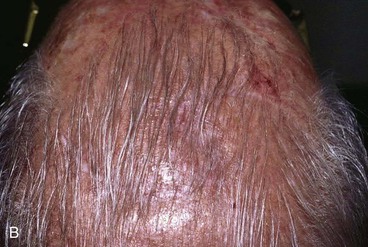
FIGURE 24-12 Two rotation flaps used for O-Z wound repair. A, Defect of scalp vertex. Broken lines represent two opposing rotation scalp flaps, each pivoting in counterclockwise direction. B, Postoperative result.
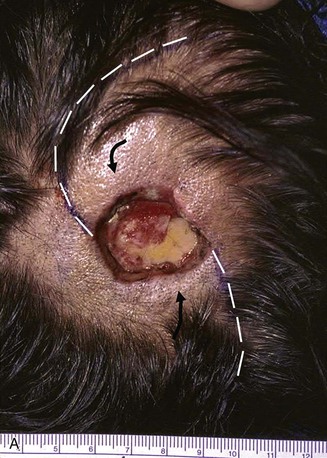
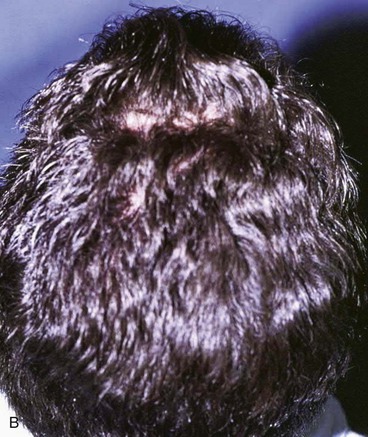
FIGURE 24-13 Multiple rotation flaps for repair of defect of scalp vertex in hair-bearing area. A, A 6-cm scalp defect. Rotation flaps designed for O to Z wound repair. B, Postoperative result.
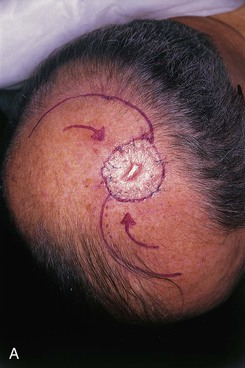
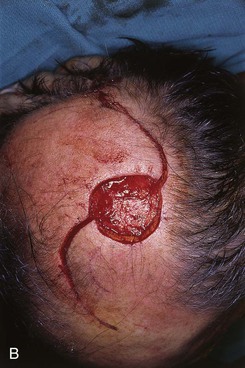
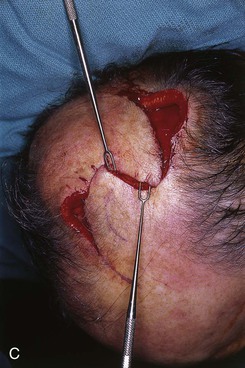
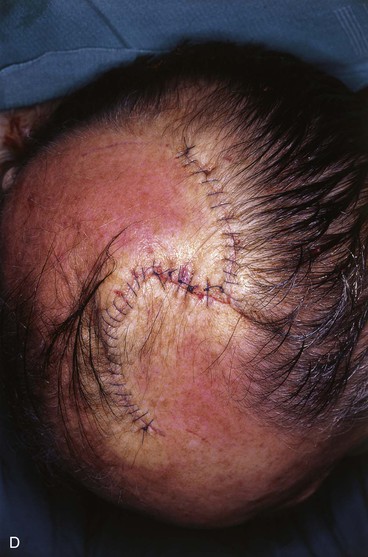
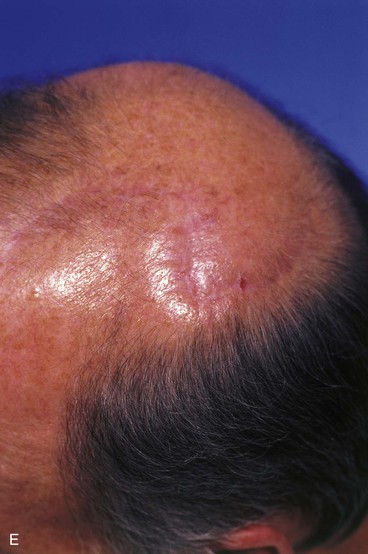
FIGURE 24-14 A, Melanoma of posterior parietal scalp marked by circle. Two rotation flaps pivoting in clockwise direction designed for O to Z wound repair. B, C, Melanoma excised and flaps incised, undermined, and pivoted toward defect. D, Wound repaired. E, Postoperative result at 3 months. (Courtesy of Shan R. Baker, MD.)
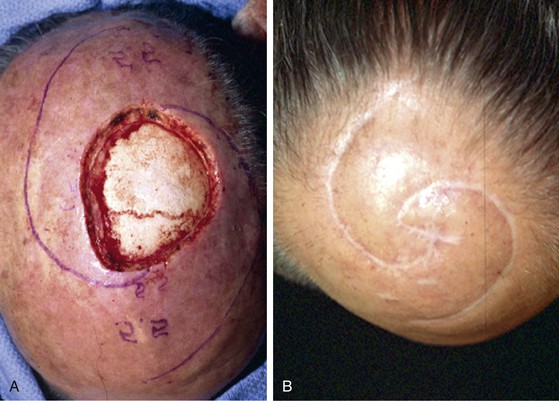
FIGURE 24-15 A, Large full-thickness defect including periosteum of anterior scalp. Two rotation flaps designed for repair. Anterior flap designed to recruit forehead skin. B, Postoperative result.
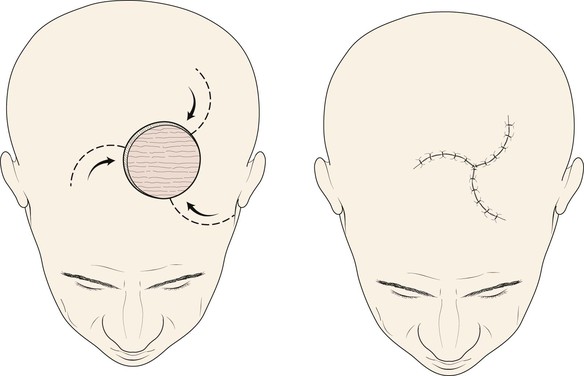
FIGURE 24-16 Three-flap “pinwheel” technique. Three separate rotation flaps, equally positioned around defect, raised and pivoted in same direction. Wound closure is analogous to closing camera lens aperture.
Large defects of the scalp can also be repaired with multiple transposition flaps that essentially involve the entire scalp. First described by Orticochea in 1967,19 these flaps have proved useful in selected patients (Fig. 24-17). More recent reports have shown that these flaps are useful for reconstruction of scalp defects up to 9 × 12 cm.20 The three-flap technique described by Orticochea may be particularly useful in patients who cannot tolerate a prolonged anesthesia required for repair with a microsurgical flap or who desire optimal aesthetic reconstruction with hair-bearing flaps and are unwilling to undergo prolonged tissue expansion of the scalp. The technique of Orticochea can be combined with simultaneous cranial vault reconstruction; however, microsurgical tissue transfer has become so reliable and versatile that many would consider a microsurgical flap to be a better option when significant bone reconstruction is required.21 If multiple scalp flaps are being considered for repair of a defect after an oncologic resection, the surgeon should be confident that the surgical margins are free of persistent tumor because recurrent disease beneath the flaps may be difficult to detect.
The three-flap technique of Orticochea mandates that the entire scalp be undermined in the course of dissection and transfer of the flaps (Fig. 24-18). Multiple transposition flaps are particularly useful for reconstruction of defects that involve the anterior scalp and hairline. The technique typically involves the creation of three or four flaps, but a two-flap technique may occasionally be employed22 (Fig. 24-19). An advantage of transposition flaps as described is that they allow the transfer of hair-bearing scalp to more visible areas in the anterior and temporal regions; the posterior donor sites can be covered with a skin graft. Less visible areas of the posterior scalp that are devoid of hair after flap transfer can sometimes be corrected by hair transplantation. Prolonged controlled tissue expansion can be combined with transposition flaps to facilitate the repair of large scalp defects.23
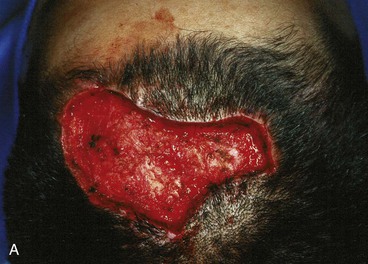
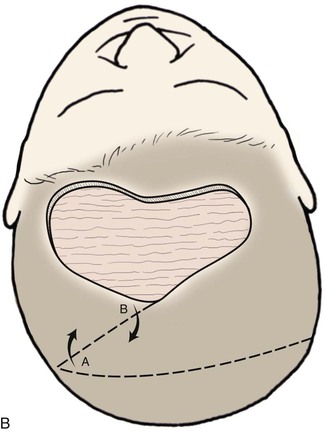
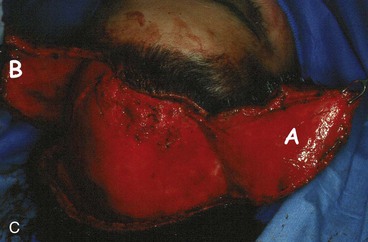
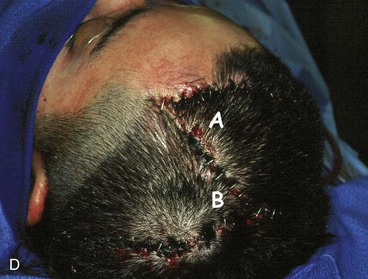
FIGURE 24-19 Two-flap Orticochea technique for traumatic anterior scalp defects. A, Traumatic anterior scalp defect. B, Drawing depicting design of two transposition flaps. C, Flaps raised and surrounding scalp widely undermined. D, Flaps transposed, restoring anterior hairline. (Courtesy of John Frodel, MD.)
Scalp advancement flaps play a limited role in scalp reconstruction principally because the inelastic nature of the scalp limits their usefulness. Advancement flaps can be useful for repair of small (3 cm or less) defects located in the lateral scalp. Because the scalp is loose in this area relative to the more central scalp, advancement flaps can be stretched sufficiently to repair defects (Fig. 24-20). This is especially true if two advancement flaps are combined to reconstruct the defect. Advancement of scalp tissue to repair a relatively linear defect (Fig. 24-21) is also required whenever a scalp wound is closed primarily. Galeal relaxing incisions are used if necessary to achieve wound closure. All wound closing tension should be born by the galeal sutures to minimize trauma to the hair follicles.
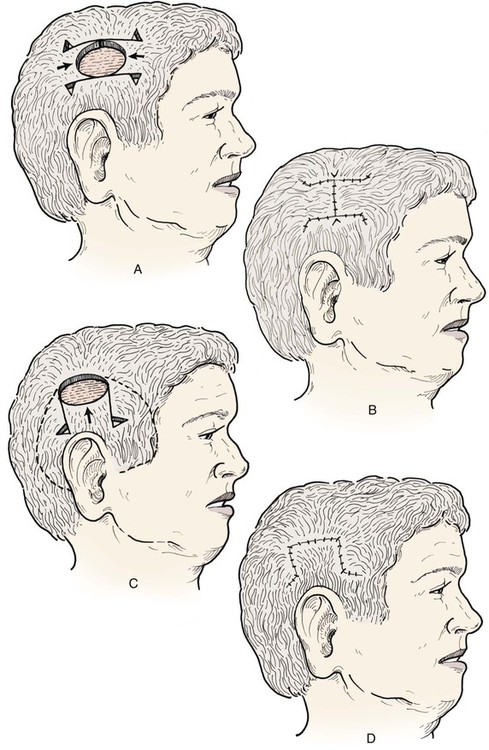
FIGURE 24-20 A-D, Bilateral or unilateral advancement scalp flaps can be effective in repair of small (less than 3 cm) defects in lateral areas of scalp.
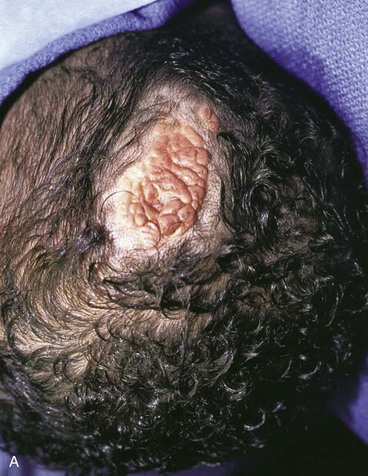
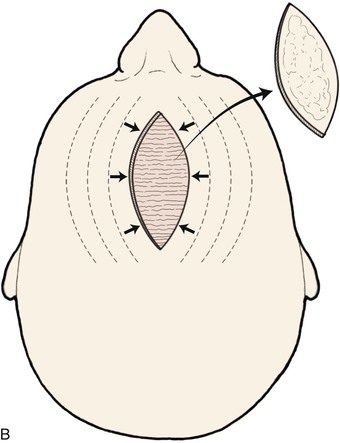
FIGURE 24-21 Longitudinal defects of scalp may be repaired with large, bilateral advancement flaps. Wide undermining is required, and galeal relaxing incisions made parallel to long axis of defect will help reduce wound closure tension. A, A 10-cm-long cerebriform nevus of scalp. B, Drawing depicting primary wound repair with galeotomies (broken lines) to assist in wound closure. C, Nevus excised and wound repaired with bilateral advancement flaps.
Regional Flaps
On occasion, regional pedicled musculocutaneous flaps or muscle flaps may be necessary for reconstruction of extensive scalp defects. These flaps are largely confined to the repair of defects of the inferior occipital or temporal regions of the scalp because of the limited reach of their vascular pedicles. Several authors have reported success with trapezius and latissimus dorsi musculocutaneous flaps for reconstruction of large defects in these areas.24–26 Regional flaps do not provide hair-bearing tissues and they have significant donor site morbidity.
Temporoparietal Fascia Flap
The temporoparietal fascia flap or fasciocutaneous flap is useful for reconstruction of selected scalp and facial defects.2,5,27, 28 This flap is supplied by branches of the superficial temporal artery and vein and can be raised as a pedicled flap measuring as large as 14 × 17 cm. The temporoparietal fascia flap can be designed in many different forms: local pedicled flap, microsurgical flap, or composite flap containing underlying bone or overlying hair-bearing scalp. The course of the temporal artery that supplies the flap is variable and tortuous. Because the vessel must be included in the pedicle of the flap, it should be carefully mapped with a Doppler probe before the flap is designed or a local anesthetic is injected. Flaps can be based on either the anterior or posterior branches of the temporal artery. Most often, a flap that includes the hair-bearing scalp will be based on the posterior branches of the temporal artery so that the donor site is hidden posteriorly and less likely to distort the anterior hairline. Hair-bearing flaps may require controlled tissue expansion of the adjacent scalp to allow closure of the secondary defect created by the flap.27 A temporoparietal fascia flap may also be used as a pure fascial flap that can provide coverage of exposed bone with well-vascularized tissue to support a skin graft.
Microsurgical Tissue Transfer
In recent years, free tissue transfer has become a reliable technique for the repair of large head and neck defects. Microsurgical flaps are now considered by many to be the best option for reconstruction of extensive scalp defects, especially those that require cranial vault reconstruction.21,29–33 Microsurgical techniques enable the transfer of large flaps of soft tissue and skin to the scalp with a high degree of success. Such flaps are resilient during postoperative radiation therapy. The most commonly employed microsurgical flaps for scalp reconstruction are the latissimus dorsi (Figs. 24-22 and 24-23), scapula, rectus abdominis, radial forearm (Fig. 24-24), and anterolateral thigh flaps.34–38 The latissimus flap has enjoyed the most widespread use because of its flexibility, long and large-caliber vascular pedicle, large surface area, and relatively minor donor site morbidity. The anterolateral thigh flap shares similar advantages with the latissimus flap and is gaining use in repair of extensive scalp defects.36,38 Although muscle flaps tend to be bulky initially, the muscle component of the flap atrophies over time, ultimately providing a natural contour and thickness comparable to normal scalp. The greatest disadvantage of microsurgical flaps used for scalp reconstruction is the lack of hair replacement. However, this aesthetic concern is typically secondary in patients with extensive and critical scalp and cranial bone defects. The reliability, flexibility, and durability of microsurgical flaps make them invaluable for repair of extensive scalp wounds.
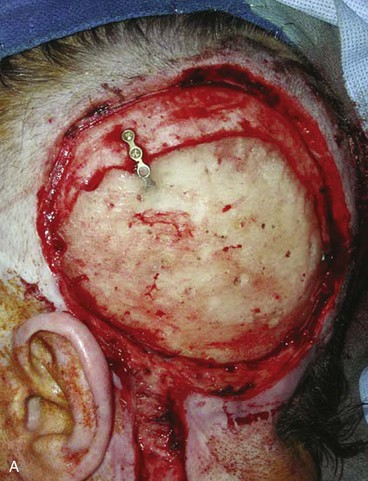
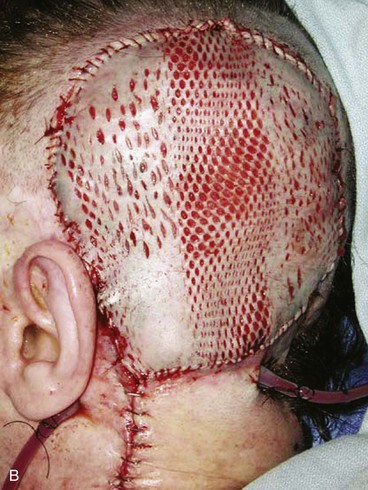
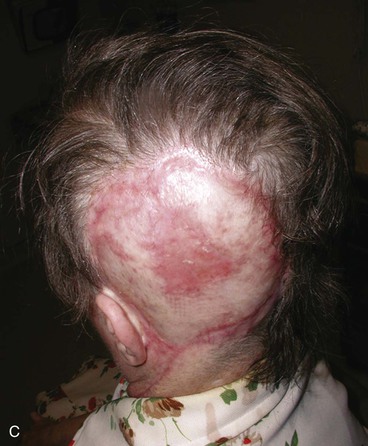
FIGURE 24-22 Microsurgical flap reconstruction. A, Full-thickness defect of parietal and occipital scalp with exposed bone flap. B, Reconstruction with latissimus dorsi microsurgical muscle flap covered with split-thickness skin graft. C, Postoperative result at 9 months. (Courtesy of Neal Futran, MD, DDS.)
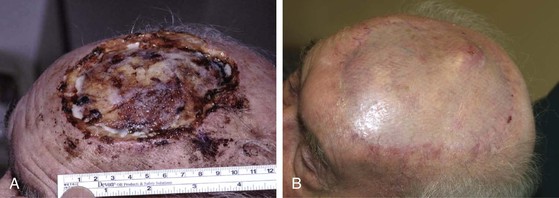
FIGURE 24-23 Microsurgical flap reconstruction of anterior scalp defect. A, Defect with exposed cranial bone. Initial attempts at stimulating granulation tissue in anticipation of using skin graft for repair were unsuccessful. B, Postoperative result at 6 months after repair with latissimus dorsi muscle microsurgical flap and split-thickness skin graft. Note thinness of flap from denervated muscle.
Adjuvant Techniques
Controlled Tissue Expansion
Although the subject of tissue expansion is covered in detail in Chapter 25, the specific application of tissue expansion for scalp reconstruction is important to discuss as part of this chapter. Prolonged controlled tissue expansion has played an important role in the management of large scalp defects for decades.23,39–44 Tissue expansion enables the creation of large, well-vascularized hair-bearing scalp flaps.45,46 Although microsurgical tissue transfer can provide large robust flaps, these flaps are less desirable than scalp flaps because they do not possess hair-bearing tissue. Prolonged tissue expansion induces profound metabolic and physical changes in the expanded scalp. Tissue expansion is a process that takes weeks or months to complete, and considerable mechanical stress in the form of tension is placed on wound margins and tissues surrounding the defect. Therefore, tissue expansion is applicable only to stable, healed wounds or as a preliminary stage before the resection of a scalp lesion. This greatly limits the applications in acute traumatic wounds or in many oncologic cases, in which a long delay before resection is inadvisable.
The most significant disadvantages of prolonged controlled tissue expansion of the scalp are the time required for expansion, the discomfort, the temporary deformity of the scalp, and the need for multiple surgeries and frequent office visits. Careful preoperative planning is essential. As multiple scalp flaps are often beneficial in reconstruction of the scalp, multiple expanders are frequently employed.23 An expander is commonly placed on each side of the head so that large bilateral advancement flaps can be created. Expanders should be selected that have a shape similar to the intended flap design. Because the majority of scalp flaps are designed as rotation flaps, a crescent-shaped or oval expander is frequently the most appropriate configuration. An expander with an easily palpable remote injection port is used. The injection port is placed where it is readily palpable through the thick scalp and in a location that will be the most comfortable for the patient. When planning the placement of a tissue expander, the surgeon should keep in mind where flap incisions will be located and use a portion of the planned border of the flap as the access incision for placement of the expander. The expander is most commonly placed under the galea and on top of the periosteum. Two weeks after insertion of the expander, inflation is commenced. Progressive inflation is performed once or twice a week until sufficient tissue expansion has been achieved. At that point, the expander is removed and flaps are created and transferred. In this manner, large scalp defects can be repaired with hair-bearing flaps with a high degree of success. An example of this surgical approach is shown in Figure 24-25. A large anterior traumatic scalp wound was initially managed with a pericranial flap and secondary intention healing. After the wound had healed, two expanders were placed on either side of the defect and expanded during the course of approximately 2 months. Once the expansion was complete, the expanders were removed, the scar tissue was excised, and hair-bearing advancement flaps were developed and used to close the defect.
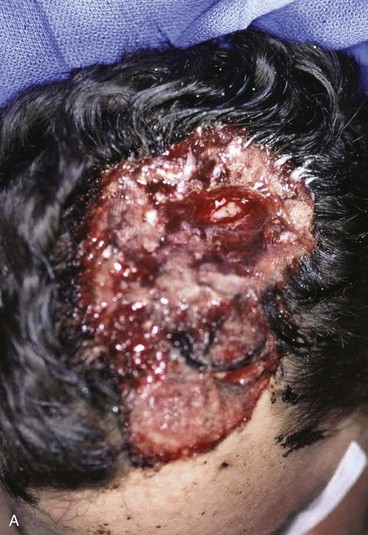
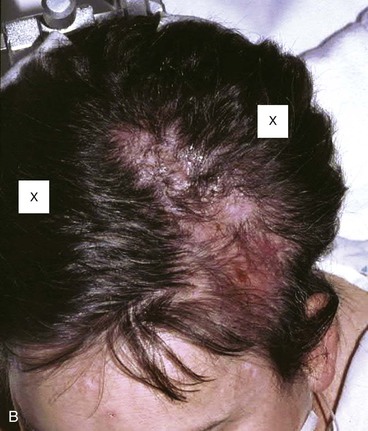
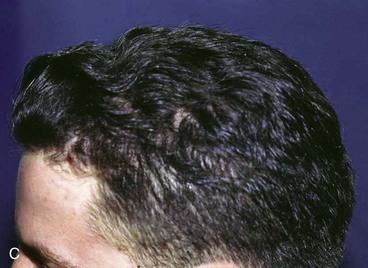
FIGURE 24-25 Large anterior traumatic scalp wound. Wound was initially managed with pericranial flap and secondary intention healing. After healing, tissue expanders were placed on either side of defect and expanded for 2 months. Subsequently, expanders were removed, scar tissue was excised, and hair-bearing advancement flaps were developed and used to close the defect. A, Traumatic defect with exposed bone. B, Tissue expanders in place at conclusion of expansion process. Expander locations marked by X’s. C, Postoperative result at 3 months after reconstruction with expanded scalp advancement flaps.
Intraoperative Tissue Expansion
Intraoperative tissue expansion is a process in which scalp tissue is sequentially and rapidly stretched in one setting for a period of a few minutes immediately before a flap is raised and transferred. Three cycles of expander inflation lasting 3 minutes each are typically employed with a rest period between inflations. The expander is inflated until tissue blanching is observed. In contrast to prolonged controlled tissue expansion, intraoperative rapid expansion causes mechanical changes in the tissue rather than metabolic and physiologic changes. Many have argued that this merely represents a way of facilitating undermining of tissues,47,48 whereas others have championed its use.7,49 Intraoperative tissue expansion can be employed with fresh scalp defects because it does not place the same requirements on the tissues that prolonged controlled expansion does (Fig. 24-26). Whether this technique offers any advantage over well-designed and widely undermined flaps remains uncertain.
Continuous External Tissue Expansion and Wound Closure
A recent innovation in complex wound closure is the introduction of an external wound closure device.12,50 Skin anchors are applied to the perimeter of the wound, and the device is attached to the anchors with heavy nylon threaded radially around the wound (Fig. 24-27). The nylon thread is then tightened; continuous tension on the wound perimeter facilitates gradual wound closure. The device is designed to apply sufficient tension to allow mechanical tissue creep without causing wound necrosis.50 The wound closure process typically takes 1 to 2 weeks (Fig. 24-28). Although it is likely that most of the benefit obtained with this device is mechanical in nature, the time involved in wound closure, specifically 1 to 2 weeks, may allow some physiologic response as well. Any physiologic response that may occur certainly is not to the extent observed with prolonged tissue expansion. These devices may offer some advantages in the closure of defects deemed too large for traditional advancement flaps, although more clinical experience is required.
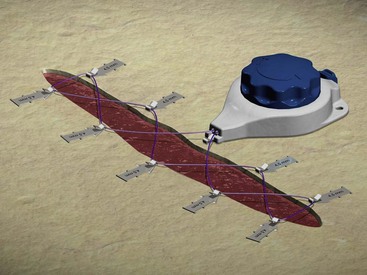
FIGURE 24-27 External wound closure with intermediate tissue expansion device. (Courtesy of Wound Care Technologies, Inc.)
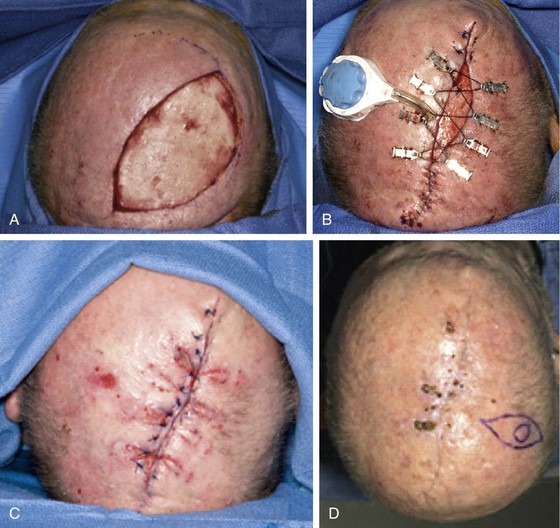
FIGURE 24-28 Wound closure with external tissue expander and wound closure device. A, A 5.0 × 9.5-cm scalp wound after excision of cutaneous malignant neoplasm. B, Application of wound closure device with partial closure. C, Wound closure at conclusion of expansion and removal of device. D, Final result. (Courtesy of Ashley O’Reilly, MD.)
Hair Transplants
Widely accepted in the management of baldness, hair transplants can also be a valuable adjunct in scalp reconstruction.51,52 Successful hair transplantation requires a vascular recipient site, and as such, hair transplants should rarely be used acutely in the management of a scalp defect. Rather, they are planned as a secondary procedure. Wounds that have healed by secondary intention rarely contain hair. However, these wounds will support hair transplants once they are healed and the resulting scar has matured (Fig. 24-29). Similarly, areas of alopecia resulting from incisional scars or from excessive wound closure tension can be managed secondarily with hair transplantation by minigrafts or micrografts. Typical of hair replacement surgery, multiple stages of grafting are required, with each stage separated by several months of healing. With patience, even large areas of alopecia can be restored to an acceptable hair density.
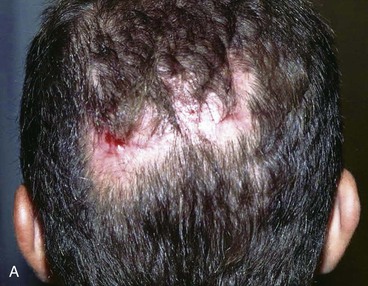
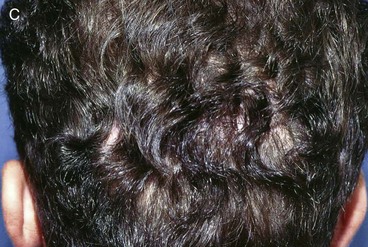
FIGURE 24-29 Traumatic scalp wound initially allowed to heal by secondary intention. After healing, three hair grafting procedures separated by intervals of 3 months were performed in scar area. A, Scar after secondary intention healing. B, Hair grafts. C, Nine months after final hair grafting procedure.
References
1. Jurkiewicz, MJ. Scalp reconstruction with multiple flaps. In: Brent B, ed. The artistry of reconstructive surgery. St. Louis: CV Mosby, 1987.
2. Bekara, F, Yachouch, J, Ziade, M, et al. Reconstruction of anterior scalp defect with V-Y advancement flap pedicled on the temporal fascia superficialis. J Craniofac Surg. 2012; 23:1434.
3. Higgins, KM, Ashford, B, Erovic, BM, et al. Temporoparietal fascia free flap for pharyngeal coverage after salvage total laryngectomy. Laryngoscope. 2012; 122:523.
4. Duvdevani, S, Magnitz, R, Siegert, R. Sulcus construction in microtia repair: a retrospective comparison of different techniques. Arch Facial Plast Surg. 2012; 22:1.
5. Jacquet, Y, Higgins, KM, Enepekides, DJ. The temporoparietal fascia flap: a versatile tool in head and neck reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2011; 19:235.
6. Becker, GD, Adams, LA, Levin, BC. Secondary intention healing of exposed scalp and forehead bone after Mohs surgery. Otolaryngol Head Neck Surg. 1999; 121:751.
7. Mandy, SH. Intraoperative expander-assisted scalp reduction. J Dermatol Surg Oncol. 1993; 19:1110.
8. Ling, EH, Wang, TD. Local flaps in forehead and temporal reconstruction. Facial Plast Surg Clin North Am. 1996; 4:469.
9. Marathe, US, Sniezek, JC. Use of vacuum-assisted closure device in enhancing closure of a massive skull defect. Laryngoscope. 2004; 114:961.
10. Chaouat, M, Lalanne, B, Levan, P, Mimoun, M. Skin expansion and external extension techniques in the treatment of a traumatic scalp defect. Scand J Plast Reconstr Surg Hand Surg. 2002; 36:50.
11. Cox, AJ, Wang, TD, Cook, TA. Closure of a scalp defect. Arch Facial Plast Surg. 1999; 1:212.
12. O’Reilly, AG, Schmitt, WR, Roenigk, RK, et al. Closure of scalp and forehead defects using external tissue expander. Arch Facial Plast Surg. 2012; 14:419.
13. Newman, MI, Hanasono, MM, Disa, JJ, et al. Scalp reconstruction: a 15 year experience. Ann Plast Surg. 2004; 52:501.
14. Seline, PC, Siegle, RJ. Scalp reconstruction. Dermatol Clin. 2005; 23:13.
15. Hoffmann, JF. Management of scalp defects. Otolaryngol Clin North Am. 2001; 34:571.
16. Lesavoy, MA, Dubrow, TJ, Schwartz, RJ, et al. Management of large scalp defects with local pedicle flaps. Plast Reconstr Surg. 1993; 91:783.
17. Ahuja, RB. Geometric consideration in the design of rotational flaps in the scalp and forehead region. Plast Reconstr Surg. 1988; 81:900.
18. Terkonda, RP, Sykes, JM. Concepts in scalp and forehead reconstruction. Otolaryngol Clin North Am. 1997; 30:519.
19. Orticochea, M. Four flap scalp reconstruction technique. Br J Plast Surg. 1967; 20:159.
20. Orticochea, M. Three flap scalp reconstruction technique. Br J Plast Surg. 1971; 24:184.
21. Beasley, NJ, Gilbert, RW, Gullane, PJ, et al. Scalp and forehead reconstruction using free revascularized tissue transfer. Arch Facial Plast Surg. 2004; 6:16.
22. Frodel, JL, Ahlstrom, K. Reconstruction of complex scalp defects. Arch Facial Plast Surg. 2004; 6:54.
23. Bauer, BS, Margulis, A. The expanded transposition flap: shifting paradigms based on experience from two decades of pediatric tissue expansion. Plast Reconstr Surg. 2004; 114:98.
24. Lynch, JR, Hansen, JE, Chaffoo, R, Seyfer, AE. The lower trapezius musculocutaneous flap revisited: versatile coverage for complicated wounds to the posterior cervical and occipital regions based on the deep branch of the transverse cervical artery. Plast Reconstr Surg. 2002; 109:444.
25. Tanaka, Y, Miki, K, Tajima, S, et al. Reconstruction of an extensive scalp defect using the split latissimus dorsi flap in combination with the serratus anterior musculo-osseous flap. Br J Plast Surg. 1998; 51:250.
26. Har-El, G, Bhaya, M, Sundaram, K. Latissimus dorsi myocutaneous flap for secondary head and neck reconstruction. Am J Otolaryngol. 1999; 20:287.
27. Kim, JC, Hadlock, T, Varvares, MA, Cheney, ML. Hair-bearing temporoparietal fascial flap reconstruction of upper lip and scalp defects. Arch Facial Plast Surg. 2001; 3:170.
28. Tellioglu, AT, Tekdemir, I, Erdemli, EA, et al. Temporoparietal fascia: an anatomic and histological reinvestigation with new potential clinical applications. Plast Reconstr Surg. 2000; 105:40.
29. Lipa, JE, Butler, CE. Enhancing the outcome of free latissimus dorsi muscle flap reconstruction of scalp defects. Head Neck. 2004; 26:46.
30. Wax, MK, Burkey, BB, Bascom, D, Rosenthal, EL. The role of free tissue transfer in the reconstruction of massive neglected skin cancers of the head and neck. Arch Facial Plast Surg. 2003; 5:479.
31. Hussussian, CJ, Reece, GP. Microsurgical scalp reconstruction in the patient with cancer. Plast Reconstr Surg. 2002; 109:1828.
32. Anderson, PJ, Ragbir, M, Berry, RB, McLean, NR. Reconstruction of the scalp and cranium using multiple free-tissue transfers following recurrent basal cell carcinoma. J Reconstr Microsurg. 2000; 16:89.
33. Lutz, BS, Wei, FC, Chen, HC, et al. Reconstruction of scalp defects with free flaps in 30 cases. Br J Plast Surg. 1998; 51:186.
34. O’Connell, DA, Teng, MS, Mendez, E, Futran, ND. Microvascular free tissue transfer in the reconstruction of scalp and lateral temporal bone defects. J Craniofacial Surg. 2011; 22:801.
35. Park, CW, Miles, BA. The expanding role of the anterolateral thigh free flap in head and neck reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2011; 19:263.
36. Kwee, MM, Rozen, WM, Ting, JW, et al. Total scalp reconstruction with bilateral anterolateral thigh flaps. Microsurgery. 2012; 32:393.
37. Sweeny, L, Eby, B, Magnuson, JS, et al. Reconstruction of scalp defects with the radial forearm free flap. Head Neck Oncol. 2012; 4:21.
38. Park, JH, Min, KH, Eun, SC, et al. Scalp free flap reconstruction using anterolateral thigh flap pedicle for interposition artery and vein grafts. Arch Plast Surg. 2012; 39:55.
39. Gurlek, A, Alaybeyoglu, N, Demir, CY, et al. Aesthetic reconstruction of large scalp defects by sequential tissue expansion without interval. Aesthetic Plast Surg. 2004; 28:245.
40. Bilkay, U, Kerem, H, Ozek, C, et al. Alopecia treatment with scalp expansion: some fine points and a simple modification to improve the results. J Craniofac Surg. 2004; 15:758.
41. Hudson, DA, Lazarus, D, Silfen, R. The use of serial tissue expansion in pediatric plastic surgery. Ann Plast Surg. 2000; 45:589.
42. Chun, JT, Rohrich, RJ. Versatility of tissue expansion in head and neck burn reconstruction. Ann Plast Surg. 1998; 41:11.
43. Konior, RJ, Kridel, RWH. Tissue expansion in scalp surgery. Facial Plast Surg Clin North Am. 1994; 2:203.
44. Baker, SR, Swanson, NA. Clinical applications of tissue expansion in head and neck surgery. Laryngoscope. 1990; 10:313.
45. Kasper, EM, Ridgway, EB, Rabie, A, et al. Staged soft tissue expansion before delayed allograft cranioplasty: a technical report. Neurosurgery. 2012; 71:15.
46. Maillet-DeClerck, M, Calibre, C, Herbaux, B, Duquennoy-Martinoy, V. Long-term results in scalp tissue expansion in children. Eur J Pediatr Surg. 2012; 22:269.
47. Shapiro, AL, Hochman, M, Thomas, JR, Branham, G. Effects of intraoperative tissue expansion and skin flaps on wound closure tension. Arch Otolaryngol Head Neck Surg. 1996; 122:1107.
48. Siegert, R, Weerda, H, Hoffman, S, et al. Clinical and experimental evaluation of intermittent intraoperative tissue expansion. Plast Reconstr Surg. 1993; 92:248.
49. Sasaki, GH. Tissue expanders and general guidelines for tissue expansion technique. In: Sasaki GH, ed. Tissue expansion in reconstructive and aesthetic surgery. St. Louis: Mosby, 1998.
50. Santiago, GF, Bograd, B, Basile, PL, et al. Soft tissue injury management with a continuous external tissue expander. Ann Plast Surg. 2012; 69:418.
51. Barrera, A. The use of micrografts and minigrafts in the aesthetic reconstruction of the face and scalp. Plast Reconstr Surg. 2003; 112:883.
52. Limmer, BL, Buchwach, KA. Hair transplantation using follicular unit micrografting. Facial Plast Surg Clin North Am. 1999; 7:523.