CHAPTER 101 Reconstruction of the Oropharynx
Oropharyngeal reconstruction has gone through several stages in the past 25 years. Reconstruction of this area before the advent of the pectoralis major flap proved difficult and was commonly complicated by wound infection and breakdown. First described in 1979 by Ariyan1 and Baek,2 the pectoralis major myocutaneous flap revolutionized head and neck reconstruction at that time and added a new dimension to our reconstructive capability. This was soon followed by the introduction of free tissue transfer with the belief that reconstructions that rendered the patient functionally and esthetically superior could now be performed. These effective reconstructions are even more important as the prognosis for advanced oropharyngeal cancer has improved.3
Careful three-dimensional (3D) analysis of the defect is critical at the conclusion of the ablative portion of the case. The size of the primary tumor, specifically the T stage, has been shown to directly correlate with the functional status of the patient in the postoperative period.4,5 The soft tissue and the bony defect should be determined. The purpose of the soft-tissue reconstruction is to re-establish bulk, sensation, and a re-epithelialized passageway for respiration and deglutination. The dimensions of the defect should be measured with a ruler, or a template of the defect can be fabricated with Esmarch (Microtek Medical, The Netherlands). The subsites of the oropharynx involved should be determined. Although this chapter focuses on oropharyngeal reconstruction, these defects do not exist in isolation. Factors such as laryngeal and mandibular arch preservation must be taken into account before determining the reconstruction of choice. For example, a base of tongue defect in combination with a supraglottic laryngectomy may be best reconstructed with a sensate fasciocutaneous free flap, as opposed to a musculocutaneous flap that cannot be reinnervated. Also, surgeon and hospital factors come into play in the decision-making process. A surgeon will decide on a reconstructive technique on the basis of his or her experience and level of training. Hospital factors include the availability of an intensive care unit experienced in monitoring head and neck patients with free flaps, microsurgical instrumentation, and appropriate paramedical staff for rehabilitation.
Cost has also become a factor in recent times. Managed care has affected most American centers, and more cost-effective methods of reconstruction are often stressed elsewhere. The use of free tissue transfer for oropharyngeal reconstruction has been compared with pedicled soft tissue transfer. These studies have demonstrated that the operative costs are higher in the free flap group; however, the overall costs are lower compared with the pectoralis major flap.6,7 A more recent study, however, showed no evidence that cost or morbidity differed in the two groups.8 In other areas where universal health care systems are in place, operative time and long waiting lists may dictate which form of reconstruction is used. All things being equal, however, the most important determining factor should be the resultant quality of life for the patient. The method of reconstruction chosen must give the patient the best chance of re-establishing an oral diet, intelligible speech, and a stable airway without cannulation. It has been shown that quality of life and functional status can be restored at 6 months after microvascular reconstruction for advanced (T3, T4) oropharyngeal cancers, and most have improved post-treatment scores at 1 year.9
Options for Reconstruction
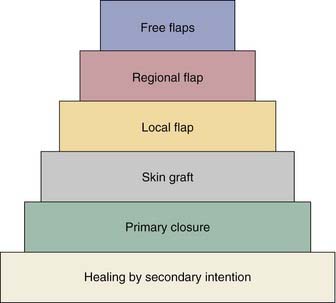
Various reconstructive options are available for defects of the oropharynx. It is helpful for the surgeon to consider the reconstructive ladder and to work through the possible techniques for a given defect (e.g., soft palate versus base of tongue, starting with the simplest and progressing to the more complicated) (Fig. 101-1). The simplest method of reconstruction that achieves the highest level of function should be chosen.
Primary closure is sometimes possible after surgical ablation of an oropharyngeal tumor. It should only be considered when a tension-free closure is obtained without distortion of the normal anatomy of the region. A prospective case-comparison study by McConnel and colleagues challenged the idea of free and pedicled flap reconstruction of the oral cavity and base of tongue. They concluded that speech and swallowing are superior with primary closure for defects of 60% or less of the tongue base.10 Although there are inherent flaws in the study and the defects addressed were small, their findings bring to light the need for objective research on reconstructive efforts of the oropharynx and its effect on speech and swallowing.
Local Flaps
Local flaps have not gained popularity for oropharyngeal reconstruction. Tongue flaps are of historical interest and are mentioned here only to be discouraged. The flap is performed by dividing the anterior tongue and then rotating it posteriorly into the surgical defect. It is clear that the use of this flap leads to restriction in the mobility of the remaining tongue and leads to impaired oral and oropharyngeal function.11,12
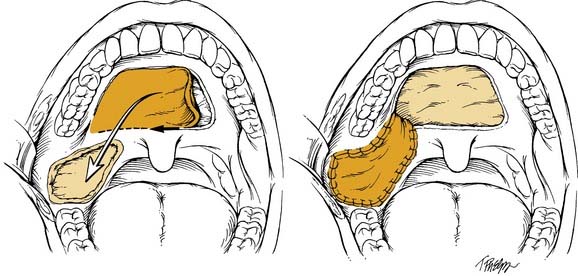
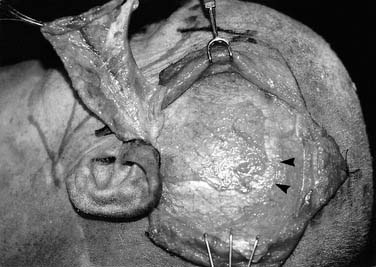
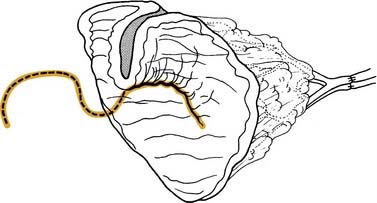
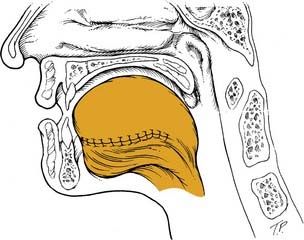
By comparison, the palatal island flap does have a role in oropharyngeal reconstruction.13 The flap is composed of hard palatal mucoperiosteum and is based on a single greater palatine artery (Fig. 101-2). It is performed by sharply incising the mucoperiosteum of the hard palate approximately 1 cm medial to the maxillary alveolus. A periosteal elevator is then used to elevate the flap, and the base is narrowed accordingly. The hard palate is left to remucosalize. The flap provides thin but relatively nonpliable tissue for reconstruction.
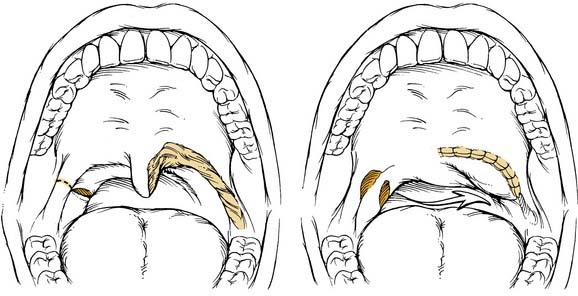
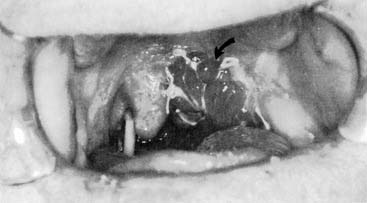
The uvulopalatal flap can be used for limited lateral soft palatal defects.14 Most of the uvula and the contralateral soft palate must be intact for this flap to be used. The uvula is denuded of its mucosa and is then rotated into the defect (Fig. 101-3). A releasing incision is made in the contralateral anterior and posterior tonsillar pillars to increase the arc of rotation. Other local flaps such as inferior and superior pharyngeal flaps, the superior-constrictor advancement-rotation flap (SCARF), and the buccinator myomucosal flap, should also be considered as local flap options for palatal defects,15,16 but all must be measured against the gold standard procedure for through-and-through defects, which is the folded radial forearm flap.
Regional Flaps
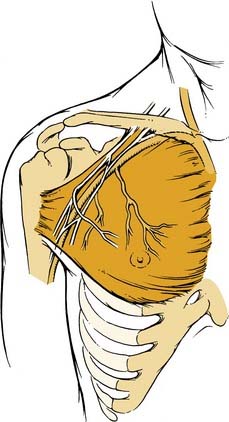
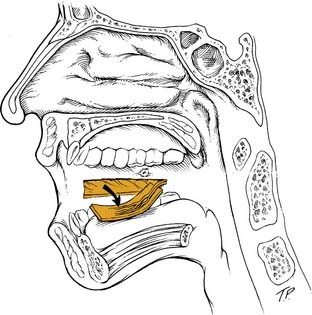
Before the advent of free tissue transfer, oropharyngeal reconstruction was most commonly performed with regional flaps. Currently, these flaps remain an excellent option for selected defects of the oropharynx because they are reliable and relatively easy to harvest. The pectoralis major flap has served as the workhorse of head and neck reconstruction for more than 2 decades (Fig. 101-4). The flap is based on the thoracoacromial artery, which originates from the second part of the axillary artery. It passes medial to the pectoralis minor and courses on the undersurface of the pectoralis major muscle. The lateral thoracic artery may contribute to the blood supply of the flap as well.17 The flap can be used as a myocutaneous or a myofascial transfer.18 The bulk of the flap can make it difficult to fold into a complex oropharyngeal defect, and this flap is therefore not the technique of choice at most centers.
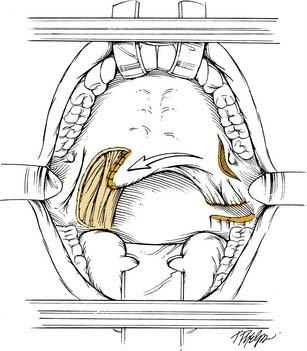
The platysma myocutaneous flap was initially proposed for oropharyngeal reconstruction in 1978.19 The flap has not gained wide acceptance in the literature, and many believe it is an underused technique for head and neck reconstruction (Fig. 101-5). Koch20 reported on 34 patients who had this flap performed for head and neck defects. Postoperative fistula formation was noted in five patients (15%), and flap desquamation was seen in six cases (18%). Donor site complications were rare, with two cases developing distal skin loss at the closure site. Complications were not related to previous radiation. The platysma myocutaneous flap is based on the submental branch of the facial artery. Ligation of the facial artery during neck dissection does not compromise the blood supply to the flap.12,21 The flap can be harvested in 20 to 30 minutes and can be done with or without a neck dissection. It provides a thin, pliable skin paddle that is ideal for oropharyngeal reconstruction. The main disadvantage cited in the literature is the reliability of the flap because flap-related complication rates have been reported to be as high as 40%.22
The temporal area provides two regional flaps for oropharyngeal reconstruction. The temporoparietal fascial flap provides thin, highly vascular tissue for reconstruction.23 Its blood supply is based on the superficial temporal artery and vein. It can be used either as a pedicled or free tissue transfer if this is required to enhance inset of the flap. An outer table calvarial bone graft superior to the temporal line may also be harvested with the flap.24 Identification of the vascular pedicle by Doppler ultrasonography is done at the initiation of the harvest. A preauricular incision is then made and is carried superiorly through the temporal scalp and can be extended to or above the vertex if necessary (Fig. 101-6). The dissection is begun superiorly just deep to the hair follicles. Care must be taken when dissecting the anterior branch of the artery because this is accompanied by the frontal branch of the facial nerve. The fascia is then divided by carrying the dissection to the level of the superficial layer of the deep temporal fascia. It is then narrowed inferiorly; at this point the anterior branch of the artery is divided close to the origin of the posterior branch of the superficial temporal artery, being aware of the facial nerve. The flap is then tunneled into the oropharynx and is sutured into the defect. A skin graft may be used on the flap but is not necessary because the surface of the flap will remucosalize. Similarly, the temporalis muscle can be tunneled into the oropharynx after removal of the zygomatic arch, which is later replaced. It is less commonly used than the temporoparietal flap because of its added bulk, limited arc of rotation, and difficult tunneling into the defect, although in experienced hands and with a fascial extension it has proved useful in soft palate and pharyngeal wall defects.25
The latissimus dorsi musculocutaneous flap is another viable option for reconstructing oropharyngeal defects.26,27 It can be used either as a pedicled or free flap, especially for total glossectomy defects (Fig. 101-7). The flap is based on the thoracodorsal artery, which is a branch of the subscapular system of vessels. The subscapular artery arises from the third part of the axillary artery. The latissimus muscle arises from the thoracolumbar fascia, the lower six thoracic vertebrae, and the iliac crest and inserts onto the medial surface of the humerus. Several musculocutaneous perforators supply the overlying skin and are more abundant in the upper two thirds of the muscle. The flap is harvested by first incising the lateral aspect of the skin paddle and then extending this superiorly following the lateral border of the muscle into an axillary crease. The pedicle is found along the lateral border of the muscle 8 to 10 cm from the midpoint of the axilla. The medial skin paddle incision is next fashioned, and tacking sutures are placed between the skin and underlying muscle. The muscle is then divided distally and medially, and the pedicle is traced into the axilla after identifying and dividing the branch to the serratus anterior. Finally, the superior muscle cut is made protecting the vascular pedicle. If the flap is to be used as a pedicled transfer, a tunnel is made above or below the pectoralis major muscle. We have had success with a tunnel above the muscle in combination with a partial-thickness myotomy of the lateral aspect of the pectoralis major.
Less commonly, the trapezius musculocutaneous flap may be used. It is generally not a good option because trapezius muscle sacrifice leads to significant shoulder dysfunction and chronic pain. Other factors that make it an unpopular choice are the flap’s limited arc of rotation and need for patient repositioning in the lateral decubitus position. By comparison, the longus colli muscle flap can be used to reconstruct defects of the lateral and posterior pharyngeal wall.28 There have been reports on 13 patients who had pharyngeal reconstruction with this regional technique with no postoperative fistulas. The flap is reported to have minimal, if any, donor site complications; however, three patients had postoperative Horner’s syndrome, and there is a question as to whether this was related to the ablation of high pharyngeal wall tumors as opposed to a flap-related problem. Experience with the longus colli flap is limited, and more evidence is necessary before the flap can be championed for oropharyngeal reconstruction.
Free Tissue Transfer
The introduction of free tissue transfer has been the greatest advance in the area of oropharyngeal reconstruction. This technique is available at most tertiary care centers. As stated previously, reconstructive advances have not changed the prognosis for oropharyngeal cancer.3 Quality of life and postoperative function are then paramount when deciding on reconstructive efforts. Free flap reconstruction has been found to be superior from a functional standpoint in both prospective and retrospective studies.7,9 In the following sections, commonly used free flaps from their respective donor tissues are outlined, and details of their use are addressed.
Fasciocutaneous
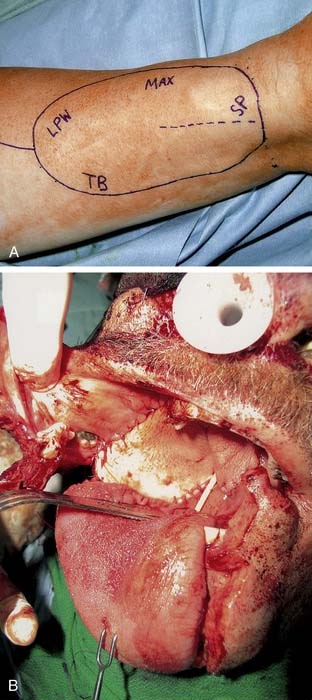
The radial forearm flap is the most commonly used free flap for oropharyngeal reconstruction (Fig. 101-8). The flap was initially described in the Chinese literature. The flap is based on the radial artery and the accompanying paired venae comitantes. The cephalic vein is also routinely incorporated into the flap and serves as the superficial venous drainage system. The lateral antebrachial cutaneous nerve provides sensation to the forearm skin, and the flap can be successfully neurotized to improve pharyngeal sensation.29 Sensory recovery is also present in most patients even when a neural anastomosis is not performed, and more commonly occurs in fasciocutaneous flaps than musculocutaneous flaps.30 This reinnervation is, however, often incomplete and unpredictable, and it is not valid to rely on spontaneous sensory recovery of free flaps in oral and oropharyngeal reconstruction.31 The skin paddle of the forearm flap is thin and pliable and thus makes it ideal for a variety of oropharyngeal defects. In fact, because of its thinness it can be used for reconstruction of large defects after transoral resections, inset through the mouth albeit with greater technical difficulty. It can be elevated either deep or superficial to the investing fascia of the forearm, depending on the bulk required. A preoperative Allen test should be performed on all patients. The flap is elevated under tourniquet control. The skin paddle is incised along its ulnar, distal, and radial aspect. The flap is then elevated at the desired level, preserving the sensory branches of the radial nerve. The level of dissection is taken to the subfascial level as the pedicle is approached. The pedicle is then identified between the medial head of the brachioradialis and the flexor carpi radialis and is then divided. A lazy S-shaped incision is fashioned into the antecubital fossa, and the cephalic vein is incorporated into the flap, leaving a generous cuff of fat and fascia to prevent kinking. An external skin monitor can be incorporated into the flap proximally if the main skin paddle is not visible for evaluation.32 The radial artery is dissected to its origin and is divided distal to the radial recurrent artery. We routinely incorporate the deep and superficial venous systems and generally have two veins for performing venous anastomoses. The donor site is covered with a split-thickness skin graft, and a bolster and cast is left in place for 1 week.
The lateral thigh free flap is another commonly used fasciocutaneous flap for oropharyngeal reconstruction. It was first described by Baek33 in 1983 and has since been used extensively in the head and neck with excellent results. Hayden34 reported a 98% success rate in 43 pharyngeal reconstructions, of which 11 were used in the oropharynx. The flap is usually based on the third perforator of the profunda femoris artery, which terminates in the intermuscular septum between the long head of the biceps femoris and the vastus lateralis. Occasionally, the second or fourth perforator will serve as the dominant blood supply to the flap. The lateral femoral cutaneous nerve provides sensation to the skin of the lateral thigh and may be incorporated into the flap. The flap is typically harvested as an ellipse of skin whose epicenter is based on the midpoint of the intermuscular septum between the greater trochanter and the lateral epicondyle of the femur. The dominant perforator is identified in the subcutaneous plane and is then traced through the biceps femoris to the main pedicle. Release of the adductor magnus from the linea aspera assists dissection of the main pedicle, which is divided distal to the origin of the dominant perforator and is then dissected proximally, where its diameter is usually 2 to 5 mm. Paired venae comitantes serve as the venous drainage of the flap. These veins typically converge before entering the deep femoral vein, and this vein ranges in size from 3 to 5 mm. The advantages of this flap are the ability to close the donor site primarily and the relative separation between the ablative site and the donor site, which tends to assist a simultaneous harvest. The disadvantages of the flap are the prevalence of atherosclerosis in the donor artery and the variability of the donor perforators to the skin paddle.
The anterolateral thigh flap has gained recent popularity in head and neck reconstruction and is now more commonly used than the lateral thigh flap. It was first described by Song and colleagues in 1984 and has been used extensively in Asia ever since.35 The flap is most often supplied by perforating vessels arising from the descending branch of the lateral circumflex femoral artery. This artery courses in the intramuscular space between the vastus lateralis and rectus femoris muscles and provides musculocutaneous and septocutaneous perforators to the overlying skin of the anterolateral thigh. The flap can be harvested as a subcutaneous, fasciocutaneous, myocutaneous (a portion of the vastus lateralis muscle may be incorporated for ease of harvest or to increase bulk), or adipofascial flap, giving this flap the required versatility for reconstructing a variety of head and neck defects.36 The advantages of this flap are its versatility, as mentioned previously, a potentially long vascular pedicle (16 cm) of excellent caliber (2 to 3 mm), low donor site morbidity, and the ability to harvest the flap simultaneously with an ablative team. The main disadvantage relates to the variations in the vascular pedicle, which may exist at the time of harvest.36 Also, with the incidence of obesity in Western countries the flap donor is bulkier; thus the flap has not gained widespread use in North America37 and is usually too thick for base of tongue or palatal reconstruction. Wei and colleagues reported on their experience with 475 anterolateral thigh free flaps used for defects of the head and neck.38 The overall flap survival rate was approximately 95%. The authors concluded that the thigh flap has potential in many Asian patients because it is as thin as the forearm flap and has essentially replaced the forearm flap at their center.
The lateral arm flap was described by Song in 1982 and was specifically applied to head and neck reconstruction by Sullivan 1 decade later.39,40 Civantos41 reported on the use of this flap in 28 head and neck defects, of which 14 encompassed the oropharynx. The flap success rate in this series was 100%. The authors concluded that this flap is ideal for oropharyngeal reconstruction because it incorporates thin skin from the proximal forearm, which is ideal for reconstructing the pharyngeal wall, and thick skin from the upper arm, which can be used in the tongue base where additional bulk is sometimes required. The flap is supplied by the posterior radial collateral artery, which is a terminal branch of the profunda brachi artery. It courses in the lateral intermuscular septum between the triceps posteriorly and the brachialis and brachioradialis anteriorly. Sensation to the flap is provided by the posterior cutaneous nerve of the arm. The flap is harvested from the nondominant arm without the use of a tourniquet. The desired skin paddle is based on the lateral intermuscular septum that topographically is located between the lateral epicondyle and the V-shaped insertion of the deltoid on the humerus. The flap can also be extended distally over the upper forearm to achieve additional pedicle length. The pedicle is identified between the previously described muscles and is divided distally. The radial nerve is identified along the anterior aspect of the pedicle and is preserved, dissecting free the posterior cutaneous nerve of the arm. The deltoid is released, and the radial nerve and pedicle are followed into the spiral groove. The pedicle is then divided at this point. Wide undermining of the skin is performed, allowing for primary closure of the donor site in most cases. The main advantage of this flap is that the feeding vessels are terminal and nonessential for perfusion of the arm. Also, the donor site may be closed primarily and may be more aesthetically pleasing than the forearm flap. The disadvantages of the lateral arm flap are the risk to the radial nerve and the relatively small caliber of the donor vessels.
Visceral Tissue Free Flaps
Jejunum, gastric wall, and colon are sources of free tissue that have been described in oropharyngeal reconstruction. The jejunum and colon segments are split along their antimesenteric borders to form a flat patch. Examples of split jejunum used for palatal reconstruction are seen in Figures 101-9 and 101-10.
Musculocutaneous Flaps
The latissimus dorsi myocutaneous free flap is our flap of choice in this category. The anatomy of the flap and the technique of harvest have been described previously in the regional flap section. The authors have found this flap most useful after total glossectomy. One of the authors (BHH) has described a useful technique of using an innervated latissimus myocutaneous free flap for reconstructing total glossectomy defects.42 The skin paddle is designed at a right angle to the course of the underlying muscle (see Fig. 101-7). The muscle is inset as a transverse sling on the undersurface of the mandible by suturing it to the remnant of the pterygoid, masseter, or superior constrictor, depending on what is available. The thoracodorsal nerve is then anastomosed to a suitable hypoglossal nerve, usually ipsilateral to the microvascular anastomoses. This gives the reconstructed tongue the ability to elevate superiorly toward the palate, thus enhancing the swallowing mechanism.
The rectus abdominis free flap is another viable option for selected defects of the oropharynx. It, too, has been used extensively for total glossectomy defects,43–45 although it lacks the potential for convenient motor reinnervation. The flap is based on the deep inferior epigastric artery and vein, which provide several myocutaneous perforators that traverse the rectus muscle and terminate in the periumbilical skin. The skin paddle is designed with its epicenter above and lateral to the umbilicus because myocutaneous perforators are more abundant in this area. The superior portion of the skin paddle is incised, and the dissection is taken through the anterior rectus sheath to the underlying muscle. The fascia is then split vertically above the skin paddle to the costal margin. The lateral and inferior portions of the skin paddle are next incised, and the dissection is once again taken to the fascial level. A small cuff of anterior rectus fascia is preserved medially and laterally, taking care not to disrupt the cutaneous perforators. The fascia is then split vertically down to the pubic region. The rectus muscle is divided superiorly, taking care to ligate the superior pedicle. The muscle is then dissected free from the posterior rectus sheath, and the dissection is then taken below the arcuate line, noting that the posterior rectus sheath is absent below this level. The vascular pedicle is identified below the arcuate line along the lateral deep aspect of the muscle. The inferior portion of the muscle is divided after a tunnel is made between the pedicle and the overlying muscle. The pedicle is then dissected inferiorly to its origin off the external iliac system. The anterior rectus sheath is reconstructed with a patch of Gore-Tex or Mersilene mesh, whereas the cutaneous defect is closed primarily after wide undermining of the abdominal skin. The flap is highly reliable in the head and neck. A review of 73 flaps used in the head and neck revealed only one flap failure.46 The vascular pedicle is long and of excellent caliber. The flap can be easily harvested and can be performed simultaneously with the ablative team. The main disadvantage of this flap is the donor site morbidity, progressive flap muscle atrophy, and the chance of abdominal wall herniation.
Osteocutaneous
Free osteocutaneous flaps are not generally used in isolated defects of the oropharynx. However, as stated previously, these defects often accompany other sites in the head and neck. These flaps are commonly used for extensive mandibular defects that extend to the central segment of the mandible or that portion anterior to the mental foramen. Our flap of choice for anterior mandibular defects has been the fibular osteocutaneous flap. Other widely used flaps for this purpose are the iliac crest and scapular flap. These defects are not addressed in this chapter. Lateral mandibular defects, however, are commonly found in combination with oropharyngeal defects, particularly in the case of retromolar trigone primary tumors. These mandibular defects are effectively reconstructed with a soft tissue flap with or without a mandibular reconstruction plate. A recent study at the M. D. Anderson Cancer Center specifically addressed posterior mandibular defects reconstructed with vascularized bone compared with those reconstructed with a free soft-tissue flap.47 They report that vascularized bony reconstruction of posterior mandibular defects results in superior speech, diet, and midline symmetry. The rectus abdominis free flap was used in 75% of the soft tissue reconstructions, which may have provided too much bulk for adequate function. Also, the group failed to perform detailed analysis of the oropharyngeal portion of the defect and the radiation protocols used on the cohort of patients. The authors’ preference has been to reconstruct all defects of the pharynx with a single flap.48 Several others, however, recommend using a free bone flap for mandibular reconstruction in combination with a soft tissue flap for reconstructing the remainder of the defect.
Site-Specific Defects
Soft Palate
The soft palate is critical for velopharyngeal competence because it serves as a dynamic muscular structure to effectively separate the oral and nasal passageways. Ablative surgery to the area may result in hypernasal speech, with obvious air escape or nasal regurgitation of food with swallowing. These problems can be greatly improved with functional palatal reconstruction. The goals of palatal reconstruction are to recreate a functional myomucosal velum and to establish closure of the oronasal communication.49 Several options are available for palatal reconstruction including the use of local, regional, and free flaps. Also, more conservative methods such as the use of a palatal prosthesis may be considered. Primary closure and healing by secondary intention are rarely indicated but may be considered when a marginal resection of the soft palate is performed and the muscular raphe of the palate is not violated. Skin grafting of soft palatal defects is of little use because it does not adequately reestablish the bulk necessary for through-and-through palatal defects, therefore leading to contracture and palatal dysfunction. We suggest referring to the reconstructive ladder for all defects of the oropharynx.
Prosthetic obturation is the traditional means of reconstructing palatal defects. It is available at most academic centers and is usually performed by a maxillofacial prosthodontist. It remains the method of choice for defects of the hard palate.50 A prosthesis can also be used effectively to obturate a combined defect of the hard and soft palate or a total soft palate defect. Isolated defects of the soft palate, however, are among the most difficult to treat with a prosthesis.51 Prosthetic rehabilitation of soft palatal defects is often functionally inferior because the prosthesis lacks the dynamic capability of the native palate.52 Also, the prosthesis may irritate the remnant palate and result in patient discomfort and the need for multiple adjustments. Yoshida and colleagues53 compared surgical and prosthetic reconstruction for speech disorders after ablative surgery of the soft palate. All surgical reconstructions were performed with a free radial forearm flap. They concluded that defects extending into the posterior edge of the soft palate are best reconstructed surgically because they believe patients achieve better restoration of speech and function. In cases in which a posterior band of soft palate remained, half of the patients reconstructed with an obturator had excellent restoration as tested by a standard speech intelligibility score.
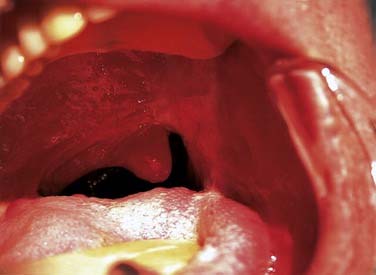
Local flaps are an excellent option for reconstructing soft palatal defects in cases in which less than 50% of the palate has been resected. The uvulopalatal flap has been used successfully for marginal defects of the posterior soft palate. The uvula and contralateral soft palate must be intact for this flap to be used (see Fig. 101-3). Zohar and colleagues49 first reported on the use of this flap in five patients to close small to moderate defects of the lateral soft palate. None of the patients had evidence of velopharyngeal insufficiency postoperatively. Zohar and colleagues concluded that the uvulopalatal flap is a technically simple technique without significant donor site morbidity and satisfies the two goals of soft palatal reconstruction. More recently, Gillespie and Eisele14 reported on 18 patients in whom the uvulopalatal flap was used for soft palatal reconstruction. A total of 11 patients had the flap used alone, whereas the flap was used in combination with a free radial forearm flap, pectoralis major flap, and a skin graft in four, two, and one patients, respectively. All flaps survived. One flap partially dehisced but healed uneventfully. Of the 11 patients who had reconstructions with the flap alone, nine had normal speech, whereas two had mild hypernasal speech. All patients with T1 tumors had normal speech (six of six). The remaining five patients had T2 lesions. By comparison, all six patients with T1 tumors had no evidence of dysphagia, whereas 40% (two of five) of the patients with T2 tumors had dysphagia requiring G-tube alimentation. The authors concluded that postoperative speech and swallowing function depends on initial tumor stage and the extent of the resection and that the uvulopalatal flap is an effective method of soft palate reconstruction either alone or in combination with other methods of reconstruction for selected oropharyngeal defects. We have used the uvulopalatal flap successfully in our practices. One of the authors (SMT) has used this flap in combination with the platysmal myocutaneous flap for tumors of the retromolar trigone. The uvulopalatal flap is used for the soft palatal defect, and the platysmal flap is used for the lateral pharyngeal and retromolar trigone reconstruction.
The buccinator musculomucosal flap is another option for limited defects of the soft palate. Originally used in cleft palate repair and for closure of septal perforations, the buccinator musculomucosal flap has been relatively overlooked in the otolaryngologic literature. The flap was originally described by Bozola and colleagues,54 and the vascularity of the flap was defined. The maximal flap size available is 7 cm in length by 4 cm in width. The flap is elevated in an anterior to posterior direction. Care is taken to stay 3 to 5 mm below the opening of Stensen’s duct and 1 cm posterior to the oral commissure. The buccal mucosa and the buccinator muscle are incised, and the dissection is taken to the level of the buccopharyngeal fascia. Posteriorly the buccal artery, which serves as the feeding vessel, and buccinator nerve are identified and preserved (Fig. 101-11). The flap is transposed into the defect, and the donor site is closed primarily. A recent article reported on eight patients who had this flap used for reconstructing defects of the soft palate, retromolar trigone, and oral cavity.15 The distal 3 mm of one flap underwent necrosis; however, this patient had had previous surgery at the site and was treated with radiotherapy after the initial surgery. The wound was débrided and healed uneventfully by secondary intention. Two of the eight patients had soft palatal reconstructions, and both were staged as T1 tumors. The two patients had no problems with swallowing. Speech results were not reported. All patients reported light single-point touch sensation over the flap 2 weeks after surgery. The authors concluded that the flap is dependable, sensate, and has a well-defined neurovascular pedicle, which can be readily used for intraoral reconstructions. Our experience with this flap has been limited, and further studies are necessary to determine the usefulness of the flap for palatal reconstruction. A sound anatomic basis for the flap has been demonstrated by Licameli and Dolan,15 which makes the flap somewhat promising. The main problem with the flap, however, is the limited amount of tissue available for transfer and the thinness of the flap. The main advantage of the flap is the defined sensory nerve supply to the flap, which may aid in oropharyngeal rehabilitation. Also, the flap is relatively easy and quick to harvest, with minimal donor site morbidity, and eliminates the need for extraoral reconstructions when a transoral resection has been performed.
The Superior Constrictor Advancement-Rotation Flap (SCARF) is another local flap that has been used for semisoft palate defects. The basic approach of flap design and use was initially described by Strong and colleagues55 and Healy and colleagues.56 More recently, Zeitels and Kim16 have reported on the use of this flap to reconstruct defects of 35% to 65% of the soft palate. They state that the use of this flap achieves circumferential closure of the velopharynx, which reestablishes its valvular sphincteric function. The flap was used to reconstruct 10 patients with transmural defects from the paramidline posterior pharyngeal wall to the soft palate. Two cases had temporalis flaps transposed into the lateral pharyngeal wall defect in combination with the SCARF. The SCARF reestablished velopharyngeal competence in all 10 patients. Two patients required secondary procedures because of partial dehiscence of the flap. Both subsequently healed uneventfully. All patients were able to resume a normal oral diet without significant nasopharyngeal regurgitation. Three patients had evidence of mild hypernasality, but these patients were not dissatisfied with their voice quality.
The SCARF is elevated by first transecting the anterior and posterior tonsillar pillars of the residual soft palate as low as possible without violating the tongue. The lateral oropharyngeal musculature is transected as the dissection is carried toward the mandibular ramus, therefore dividing the palatoglossus, the palatopharyngeus, and the superior constrictor. A stab incision is then made in the remnant of the soft palate just medial to the pterygoid hamulus. Dissection is performed bluntly with a Kelly clamp or a hemostat deep to the superior constrictor, and finger dissection is then used posteriorly until the cervical spine is palpable and the retropharynx is reached. These maneuvers effectively mobilize the soft palate, and the cut surface of the soft palate can then be rotated superolaterally and sutured to the exposed superior constrictor on the posterior pharyngeal wall (Fig. 101-12). The remainder of the wound including the stab incision on the soft palate is left open to heal by secondary intention. A pedicled muscle flap can be used to augment the lateral pharyngeal wall if necessary. Patients are started on an oral diet in approximately 7 days. The neovelopharyngeal aperture is narrow initially but widens as the lateral pharyngeal wall heals by secondary intention. Also, the neovelopharynx is sensate and has dynamic capability. This reconstructive approach may be feasible in cases in which the resection has been performed by way of a transoral technique and no communication with the neck exists. However, it necessitates surgical interruption of adjacent, normal palate and pharynx. The authors have had limited experience with this method of reconstruction and have favored the use of free fasciocutaneous flaps to reconstruct large defects of the soft palate, tonsil, and lateral pharyngeal wall.
Free flap reconstruction of the soft palate has become the most common method of reconstruction at our institutions (Halifax, Nova Scotia, Canada, and St. Louis). The authors most commonly encounter these defects after extensive resections of the oropharynx for tonsillar carcinomas. The radial forearm flap has become our flap of choice for this, although other flaps that may be considered are the anterolateral thigh and lateral arm free flap. The anatomy and technique of harvest for the radial forearm flap was described earlier in the chapter. The palatal defect is measured from the nasopharyngeal resection margin to the proposed neopalatal margin and is then doubled to take into account our folding technique. The flap is designed with the distal aspect of the forearm flap used for the palatal reconstruction. The width of the distal flap is determined by the preceding measurement, and the remainder of the flap is planned accordingly. The distal portion of the flap is inset first, with the distal midpoint of the flap sutured to the inferior remnant of the soft palate (Fig. 101-13). The nasopharyngeal surface is then reconstituted by folding the flap on itself. The oral surface of the palate is then reconstructed. The authors do not routinely perform a pharyngoplasty to create an adhesion between the flap and the posterior pharyngeal wall but consider this in cases where greater than 50% of the soft palate has been resected. This method nicely reestablishes the velopharynx, but it does lack dynamic capability.
Another option is to use the Gehanno method in combination with a free tissue transfer as suggested by Kimata and colleagues.57 In the Gehanno method the lateral and posterior pharyngeal wall is mobilized, and this advancement flap is turned inside out and sutured to the posterior surface of the remaining soft palate, thus reconstituting a mobile neovelopharynx.58 The free flap is then used to patch the anterior aspect of the soft palate and lateral pharyngeal wall. Kimata recommends the use of the Gehanno method in combination with a free flap (they used the rectus abdominis musculocutaneous flap in 26 of 40 reconstructions) for defects that extend to the contralateral soft palate because velopharyngeal function is superior due to a decreased incidence of wound dehiscence.57
Lacombe and Blackwell59 have reported their results with a folding technique similar to ours with a radial forearm flap. However, they routinely perform a midline adhesion in cases in which more than one third of the soft palate is reconstructed. They accomplish this by deepithelializing a portion of the forearm flap and suturing it to a denuded area on the posterior pharyngeal wall. Red rubber catheters (16 F) are used to establish lateral ports, essentially converting the result to look and function like a pharyngeal flap. Their study reviewed 15 patients who had soft palatal reconstruction with the free radial forearm flap. Thirteen of 15 patients achieved an oral diet, whereas two were dependent on enteral feeding. Similarly, 13 patients were reported as having good velopharyngeal function without revision surgery or use of a prosthesis. One patient required a palatal-lift prosthesis for nasal regurgitation, and another patient had a secondary pharyngeal flap to decrease the size of one lateral velopharyngeal port. As with pharyngeal flaps, judgment of the size of the velopharyngeal ports needs to be correct in order to avoid nasal obstruction and sleep apnea.
Several other groups have reported on their experience with soft palate reconstruction with a free radial forearm flap. Two used a forearm flap in conjunction with a superiorly based pharyngeal flap. Brown and colleagues60 and Penfold and collegues61 used this approach in 11 and 3 patients, respectively. They noted good functional results for swallowing, speech intelligibility, and velopharyngeal sufficiency. The larger of the two studies done by Brown used objective measures of function such as videofluoroscopy, voice intelligibility recordings, and functional outcome evaluation by means of a questionnaire. We would caution the use of the pharyngeal flap in cases in which a significant volume of the pharynx has already been resected, and further sacrifice with the pharyngeal flap may further compromise swallowing.
Speech function after palatal reconstruction was specifically addressed by Yoshida and colleagues,53 who reported on five patients who had soft palate reconstructions with a free radial forearm flap. Three of the five patients reconstructed with forearm flaps had a speech intelligibility score greater than 70%. The other two patients had scores of 40.8% and 57.8%. Two patients had a slight improvement of their intelligibility scores with placement of a special prosthesis. Eighty percent (four of five) of the patients were endoscopically and aerodynamically shown to have evidence of velopharyngeal incompetence. McCombe62 reported on velopharyngeal function in patients following reconstruction of defects between 50% and 100% of the soft palate. All patients were reconstructed with a free radial forearm flap. Results were disappointing and the authors recommend a reduction of the velopharyngeal aperture to compensate for the lack of mobility in the soft tissue reconstruction. This had been scientifically previously shown by Seikaly and colleagues,63 who found that patients with 50% or more of the soft palate resected and reconstructed with a radial forearm flap had significantly higher nasalance values and velopharyngeal orifice areas. Another recent report by Sinha and colleagues64 on 16 folded forearm flap reconstructions for soft palatal defects found that all patients had intelligible speech and 14 of the 16 patients were able to tolerate a normal or soft diet within 6 weeks. Figure 101-14 presents an algorithm for reconstruction of defects of the soft palate.
Tonsil and Pharyngeal Wall
Early tonsillar cancers can be easily resected transorally with the use of the carbon dioxide laser and the wound left to heal by secondary intention.65 The authors have used this technique for T1 lesions of the tonsil and lateral pharyngeal wall, thereby leaving defects of up to 4 cm. The resection is taken down through the superior constrictor to the parapharyngeal fat pad. It is important to leave the fat undisturbed if possible because this serves to protect the carotid artery from intraoral contents. Patients can be started on an oral diet the same day of surgery. The authors have found that this technique is well tolerated, and patients heal well with little if any distortion of native tissue. Close examination of the healed defect usually reveals slight inferior displacement of the soft palate on the side of the resection, but velopharyngeal insufficiency is rare. This has been exemplified in cases reconstructed with the SCARF because the entire basis of its use is to reconstruct the soft palate with local tissue while allowing the lateral pharyngeal wall to heal secondarily. Healing by secondary intention is also possible for posterior pharyngeal wall defects. This is usually accomplished by suturing the margins of the defect to the prevertebral fascia and allowing the defect to remucosalize over the exposed fascia.
Primary closure is another option for reconstructing defects of the tonsil or pharyngeal wall. No specific guidelines apply for defects that are amenable to primary closure. The general principles include the absence of tension on the pharyngeal wound closure and lack of distortion of the normal anatomy of the region. As stated previously, this technique must be used with caution in patients who have been previously radiated. McConnel and colleagues66 studied the functional results of primary closure versus flap reconstruction of the oropharynx. This was a prospective case-comparison study drawing from a database of 284 patients treated at four head and neck cancer institutions. Patients were matched for tumor location and the extent of resection. Speech and swallowing function was assessed preoperatively and 3 months after healing. They report that patients who had primary closure had less pharyngeal residue, were more efficient at swallowing liquids, and had higher conversational intelligibility than patients who had reconstruction with a distal flap. The primary closure patients had a longer oral transit time than the distal flap group. Compared with the free flap cohort, the primary closure patients had more efficient swallowing of liquids, less pharyngeal residual, and shorter pharyngeal delay times with paste. The authors did not find any difference in speech and swallowing function between the distal myocutaneous flap and the free flap groups. They conclude that primary closure results are equal or better in function than the use of flap reconstruction. This article is particularly controversial because it goes against most of our current trends in oropharyngeal reconstruction. Several questions have been raised about this study, and the authors of this chapter suggest reviewing the letter to the editor submitted by Reece and colleagues67 with regard to this. A more recent study by Zuydam and colleagues showed improved speech and swallowing in patients closed primarily as compared with those reconstructed with free flaps.68 Other published studies, however, support the use of primary closure for selected defects of the tonsil, pharyngeal wall, and tongue base.11,69 These studies do highlight the importance of considering this modality for selected cases and the need to objectively study speech and swallowing results achieved with the various means of reconstruction.
Skin grafting of tonsil/pharyngeal wall defects is another uncomplicated method of reconstruction available to the surgeon. Pauloski and colleagues69 described this technique in 14 patients with tonsillar defects in combination with varying defects of the tongue base and soft palate. The study specifically addressed variables affecting speech in patients with oral and oropharyngeal cancer including methods of reconstruction. Results were favorable for those reconstructed with a skin graft because they had more limited defects than the flap reconstruction group. Skin grafting may also be used to reconstruct defects of the posterior pharyngeal wall. The skin graft is sutured to the margins of the defect, and a bolster dressing is used to decrease the shearing forces on the graft. This technique can be used after a transoral or transcervical approach. A tracheotomy is recommended for airway protection in cases in which a skin graft is used.
Local flap reconstruction of tonsil and pharyngeal wall defects is uncommon. The longus coli muscle flap has been reported to be useful for defects of the lateral pharyngeal wall.28 The longus colli muscles arise from the anterior tubercles and transverse processes of the vertebral column from the arch of the atlas to the third thoracic vertebra and lie deep to the prevertebral fascia but anterior to the ligaments of the cervical spine. The muscle extends inferiorly to the level of the cervical esophagus. Innervation is from the cervical plexus (C2-C7), whereas the blood supply arises from muscular perforators of the vertebral artery. The muscle is thicker laterally, measuring approximately 0.5 to 1 cm in thickness. The flap is mobilized by dissecting free the lateral border of the muscle and then bluntly dissecting dorsal to the muscle. The muscle is rotated anteriorly into the defect without transecting it. Collins28 reports that up to 4 to 5 cm of flap width is available for use, and the procedure takes from 5 to 10 minutes. She reported on 16 patients who were reconstructed with this flap. A total of 13 had primary tumors of the pharyngeal wall. Eighty-eight percent of patients had stage 3 or 4 disease, and 50% had T3 or T4 primary tumors. She reports that there were no wound infections or fistulas, although one of two previously radiated patients had a transient wound-healing problem. Two thirds of patients achieved an oral diet, and 58% (7 of 12) of patients who had laryngeal preservation procedures were decannulated. The author concludes that the longus colli muscle flap is useful for defects of the lateral pharyngeal wall on the basis of the reliability of wound healing and the absence of a negative impact on oncologic and functional results.
The palatal island flap is another local flap option appropriate for limited defects of the tonsil and pharyngeal wall. The entire hard palate mucoperiosteum based on a single greater palatine neurovascular pedicle is available to be transferred with this technique (see Fig. 101-2). The flap is ideal for defects less than 5 cm involving the upper tonsil/pharyngeal wall and retromolar trigone. Gullane and Arena13 reported a 5% failure rate in a series of 53 palatal island flaps. They caution the use of this flap after irradiation, previous palatal surgery, or ligation of the external carotid or internal maxillary artery. Care must be taken to inset the flap without tension. The donor site is left open, and remucosalization occurs rapidly with little morbidity.
Regional flap reconstruction of the tonsil and pharyngeal wall remains a common reconstructive technique. The pectoralis major flap is without question the most commonly used regional flap for this purpose. This flap was first described by Ariyan1 in 1979 for head and neck reconstruction and revolutionized the field. It can be used as a musculocutaneous, myofascial, or myogenous transfer and may be tailored to fit almost any defect of the head and neck. The anatomy of the flap has been outlined previously with the pectoral branch of the thoracoacromial artery serving as the flap’s main blood supply. The flap is simple to harvest, and some surgeons prefer to do this through a deltopectoral-sparing incision starting in the axilla and coursing along the lateral border of the muscle inferiorly. If a cutaneous paddle is to be included, the lateral component is first incised, confirming correct planning of the skin paddle over the distal muscle. An avascular plane exists between the pectoralis major and the underlying pectoralis minor. The muscle is divided medially and laterally from its sternal and humeral attachments, respectively, after completing incision of the skin paddle. The skin paddle is then sutured to the underlying muscle to prevent shearing on the musculocutaneous perforators. The pedicle is identified medial and anterior to the pectoralis minor, and the flap can be narrowed superiorly or completely skeletonized on the pedicle alone, as the clavicle is approached. This assists tunneling into the neck.
Baek2 first reported on the use of the pectoralis major flap for oropharyngeal reconstruction. Since then, several groups have reported their experience with this flap. The largest study to date was a series of 371 patients who had pectoralis major myocutaneous flaps performed for head and neck defects. The overall complication rate was 36% with a 2.4% incidence of total flap necrosis.70 Koh has also recently reported on the use of this flap for oropharyngeal reconstruction and concluded that the pectoralis major flap is not only an alternative to a free flap but in fact may be a better choice for patients because the complication rates at their institution were lower in the pectoralis cohort compared with the free flap group.71 One of the limiting factors of the flap for pharyngeal wall reconstruction is its excessive bulk. For this reason, we have preferred to use this flap as a myofascial transfer because it can be easily molded to the pharyngeal defect and the accompanying fascia is useful for suture placement.72 Righi and colleagues73 reported a flap complication rate of 22% with their series of myofascial flaps. We have used this flap primarily in tonsillar/pharyngeal wall defects in combination with lateral oromandibular defects in patients believed to be poor candidates for free tissue transfer (Fig. 101-15).
The platysmal myocutaneous flap is another viable option for tonsillar/pharyngeal wall reconstruction. It is ideal for this purpose because of the thinness and pliability of the flap. It can be harvested in 20 to 30 minutes with little, if any, donor site morbidity. No special skills or equipment is necessary. The flap is designed low in the neck, with the skin paddle planned over the midportion of the platysma muscle. The upper skin paddle incision is made first, and a subcutaneous flap is raised to the level of the mandible. Next, the inferior portion of the skin paddle is incised and carried through the platysma. A subplatysmal flap is then elevated to the level of the mandible, which essentially frees the muscle and the overlying skin paddle (see Fig. 101-5). The flap has been used mostly for intraoral reconstruction; however, most series include patients in whom the flap has been used in the oropharynx. Ruark and colleagues21 reported on 13 of a total of 41 patients who had primary tumors of the oropharynx reconstructed with this technique. This is the largest series of platysmal flaps reported in the literature for oropharyngeal reconstruction. They did not specifically analyze the subsites reconstructed, but the flap was used for tonsil, lateral pharyngeal wall, and soft palate defects. Other than this report, a total of 29 myocutaneous oropharyngeal reconstructions are reported in the literature.19–22 Twelve were used for tonsil/pharyngeal wall reconstruction, whereas 17 were used in the tongue base. The complication rate for the tonsil/pharyngeal wall reconstructions was 67% (8 of 12). A more recent study by Jackel74 reported on six patients reconstructed with a platysmal myofascial flap. These patients had tonsillar primaries resected with transoral laser microsurgery and all were free of complications.
The sternocleidomastoid myocutaneous flap may also be used for tonsil/pharyngeal wall reconstruction. The flap can be used either as a superiorly or inferiorly based flap based, respectively, on the occipital and transverse cervical arteries. The superior thyroid artery supplies the midportion of the muscle. Ariyan75 reported on 31 consecutive reconstructions using this flap, of which 6 were used for the tonsil or pharyngeal wall. The remainder were used for defects of the oral cavity. Superiorly based flaps were used in two thirds of the cases. Fourteen patients had previous radiotherapy, and 17 had concomitant modified neck dissections. The complication rate in his series was 52%. Thirteen of 31 had partial loss of the skin paddle develop, whereas one was lost completely. Two patients had fistulas develop, despite no loss of the skin paddle. All healed without additional surgery. There was no difference in complication rates for patients who had neck dissections or previous radiation. The location of the blood supply (inferior vs. superior) was not found to be significant. Significant concerns exist with regard to the use of this flap. First, the reliability of the skin paddle is questionable on the basis of the preceding and other studies. The vascularity of the skin paddle may be enhanced by preserving the superior thyroid pedicle.76 One other major criticism of the use of this flap is its intimate relationship with potential nodal metastasis in the neck. The largest published study in the literature addresses these two issues. Sebastian and colleagues77 reported on 121 superiorly based flaps for oral reconstruction in patients with clinically N0 necks. Total flap loss occurred in 7% of cases, whereas superficial skin loss was seen in 23%. The fistula rate in the series was 12%. Radiation was found to increase the incidence of flap complications. Pathologically N0 necks had a recurrence rate of 6% compared with 17% in necks that were pathologically positive.
The temporal system of flaps may be useful for reconstructing tonsil/pharyngeal wall defects as well. The techniques of harvesting the temporalis and the temporoparietal fascia flap have been outlined previously in “Regional Flaps.” The flap’s ease of harvest and proximity to the defect make it a viable option. It may be particularly useful in cases in which the coronoid process of the mandible has been resected, leaving the muscle functionless. We have tended not to use this flap routinely for oropharyngeal defects given its bulk, difficulty of inset, and donor site morbidity. We previously discussed the use of this flap in conjunction with the SCARF, where the flap was used to augment the lateral pharyngeal wall defect in two cases.16 Huttenbrink78 reported on the use of this flap specifically for oropharyngeal reconstruction, and this represents the only dedicated article to this in the English literature. The transfer used by Huttenbrink consisted of muscle, fascia, and periosteum. He stresses the importance of subperiosteal dissection along the deep aspect of the flap to protect the deep temporal vascular pedicle. A total of 11 patients with oropharyngeal defects were reconstructed with the temporalis flap. One flap developed partial necrosis of the fascial layer, but the muscle healed uneventfully. There were no problems with the other 10 flaps. No fistulas or flap dehiscence occurred. The author was nonspecific with reporting of functional outcomes and simply reported that nearly all patients could swallow and speak without restraint. A subsequent article by Huttenbrink in the German literature expanded on his experience and reported on 26 patients in whom the flap was used.79 A more recent study by Smith and colleagues80 reported on 26 temporalis flaps used for oropharyngeal and nasopharyngeal reconstruction. They reported two cases of minor flap loss and two cases of transient frontal nerve weakness. More than 50% of patients were able to tolerate a normal diet, and 8% were G-tube dependent.
The temporoparietal fascial flap may be used in a similar fashion. The thinness, pliability, and vascularity make it ideal for this purpose. Flap harvest is relatively simple and was described in detail previously in this chapter. Donor site morbidity is rare, with frontal nerve injury and alopecia being the most significant. Cheney and colleagues23 have nicely outlined the application of this flap in head and neck reconstruction. They report on the variety of defects that can be reconstructed with this flap including the pharyngeal wall. The flap can be used either as a pedicled or free tissue transfer. Most defects of the pharyngeal wall should be amenable to pedicled flap reconstruction because of the proximity of the donor site and the thinness of the flap to be tunneled into the defect. Objective measures of functional outcomes that use this method of reconstruction are lacking.
Free flap reconstruction is now the gold standard reconstructive modality for extensive defects of the tonsil and pharyngeal wall. Usually, these defects occur in combination with defects of the soft palate and tongue base, and we focus on these two subsites in this section because published data on free flap reconstruction for isolated defects of the pharyngeal wall are scant. The radial forearm flap is the “workhorse” free flap in this area and has gained widespread popularity because of its thinness and pliability, ease of harvest, and minimal donor site morbidity. The forearm flap has become the most common free flap used in oral and pharyngeal reconstruction. Compared with the jejunum and gastro-omental free flaps for this purpose, there was no difference in speech, swallowing, or control of saliva between the different reconstructive modalities.81 The gastro-omental cohort, however, was found to have a higher complication rate. The authors make no reference as to whether the forearm flaps were neurotized at the time of surgery. We prefer the use of the forearm flap over the jejunum and gastro-omental flaps because the forearm flap may be reinnervated, which may improve pharyngeal sensation, thus preventing aspiration. Also, the forearm flap avoids the laparotomy required for harvest of the other two flaps.
A recent Finnish study addressed free flap reconstruction in the management of oral and pharyngeal cancer.82 Of the 50 patients included in the study, seven had defects of the tonsil and pharyngeal wall. All seven patients were reconstructed with a free radial forearm flap. Twenty percent of the flaps required reexploration, and the overall flap success rate was 96%. Patients who had primary tumors of the tonsil had the best 3- and 5-year survival rates at 85% and 57%, respectively. This point stresses the need for functional reconstruction because prognosis at this subsite tends to be good compared with the hypopharynx and oral cavity. Schwager83 reported the use of the forearm flap in reconstructing 62 patients with pharyngeal carcinoma. A regular diet was achieved an average of 14 days postoperatively. All patients had a tracheotomy performed at the time of surgery, and 90% were decannulated at 1 year. Another European article by Barzan84 reported on four patients with isolated posterior pharyngeal wall defects. All patients were reconstructed with a free radial forearm flap. No flap problems, fistulas, or donor site morbidity was noted. Speech and swallowing function was not noted.
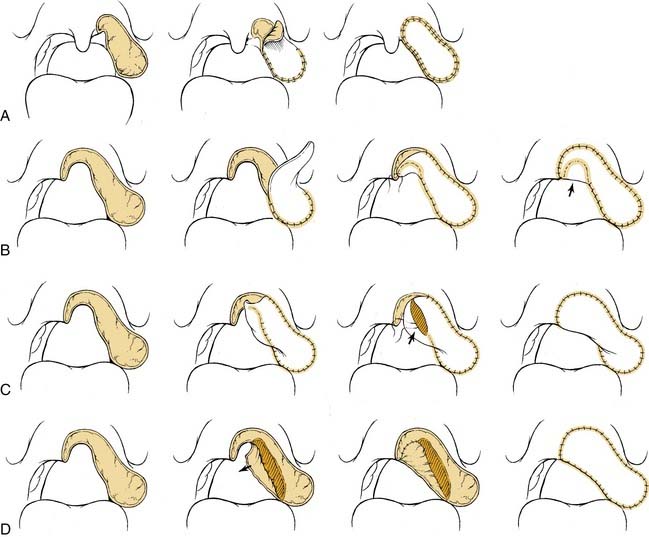
Combined defects of the lateral and superior (soft palate) pharyngeal wall have been addressed by Kimata and colleagues.57 All 40 patients had extensive defects of the lateral pharyngeal wall combined with varying defects of the soft palate. They classified the defects as follows: type 1, localized in the lateral pharyngeal wall and including palatopharyngeal, palatoglossal, and superior pharyngeal muscles; type 2, extending to the soft palate and including the tensor veli palatini and levator palatini muscles; and type 3, extending to the contralateral soft palate. Free flap reconstructions were performed on all patients. The rectus abdominis flap was used in 26 patients, an anterolateral thigh flap in 7 patients, and a forearm flap in 5 patients. Jejunum and superior iliac crest flaps were used in one patient each. Six flaps underwent partial necrosis, and 34 survived completely. The authors describe four different methods of reconstructing these defects, namely the patch, jump, denude, and Gehanno methods (Fig. 101-16). Wound dehiscence between the remnant soft palate and flap developed in 7 of the 40 patients. This occurred in 4 of 8 (50%) patients treated with the denude method and 3 of 10 (30%) patients treated with the Jump method. Of the seven patients with wound dehiscence, five had a wide fissure develop between the flap and the soft palate that resulted in postoperative hypernasality. As expected, patients with type 1 defects had the best overall velopharyngeal function. All 10 patients in this group had satisfactory postoperative function. Sixteen patients had type 2 defects. The authors report that most of these patients showed satisfactory function. Five of these patients had mild or severe nasal emission during soft blowing, and seven had mild nasal regurgitation with eating. Fourteen patients had type 3 pharyngeal defects. Of these, five patients had satisfactory function without nasal emission with soft blowing. The five patients who had flap dehiscence had severe velopharyngeal incompetence. The remaining four patients initially had intact velopharynges, but as the rectus free flap atrophied, they had progressive incompetence develop. Overall, patients with type 3 defects had low speech intelligibility scores, and 9 of 14 patients had evidence of hypernasality. The authors conclude that flap dehiscence was directly correlated to poor speech intelligibility, hypernasality, and nasal emission. The rates of wound dehiscence were lower with the patch and Gehanno methods. They suggest the use of the Gehanno method for extensive defects of the oropharynx and for all type 3 defects of the soft palate.
The lateral arm and thigh free flaps are other options for reconstructing defects of the tonsil and pharyngeal wall. Civantos41 reported on 28 patients who had the lateral arm flap used in head and neck reconstruction. Of these, 14 patients had large oropharyngeal defects, four of which involved the tonsil and pharyngeal wall. Eight of the 14 patients resumed early oral feeding, and all eventually were able to obtain nutrition orally. Swallowing was compared with a simultaneous control group that was reconstructed with pectoralis major flaps. The swallowing ability of the lateral arm cohort was found to be superior to the group reconstructed with the pectoralis flaps. The authors concluded that the lateral arm free flap is ideal for oropharyngeal reconstruction because the flap incorporates thin skin from the proximal forearm and thicker skin from the upper arm. They suggest that the thin forearm skin may be used in the lateral pharyngeal wall, whereas the thicker upper arm skin may be used to reconstruct the tongue base. Also, the flap is amenable to neurotization. Hayden and Deschler34 make similar recommendations with regard to the use of the free lateral thigh flap. They reported their experience with the lateral thigh flap in 58 cases. Most flaps were used for pharyngoesophageal reconstruction, but two involved the pharyngeal wall. The authors of this chapter have used the lateral thigh flap in the past as their second line flap but now prefer the anterolateral thigh flap in patients who are not candidates for radial forearm flap reconstruction or have very thin lower extremities. Figure 101-17 reviews an algorithmic approach to defects of the tonsil and pharyngeal wall.
Tongue Base
Reconstruction of the tongue base is challenging because of its proximity to the larynx and the resultant risk of aspiration. These defects may occur as an isolated entity but also may occur in combination with defects of the anterior tongue, with the extreme being the total glossectomy defect with or without laryngeal preservation. The goals of tongue base reconstruction in order of importance are (1) maintenance of the airway; (2) swallowing; and (3) articulation. The tongue base is critical for the first and second goals, with the anterior tongue being more significant with regard to articulation. The ideal tongue base reconstruction provides the necessary bulk to create a shelf above the laryngeal inlet. This serves to better direct the oral bolus down the posterior pharyngeal wall and away from the larynx. The form of reconstruction chosen should provide pliable tissue that has dynamic capability, which is required for adequate speech and swallowing. Last, reinnervation of the flap from both a sensory and motor standpoint would be ideal because this would improve pharyngeal sensation, thus decreasing the risk of aspiration, whereas motor function would enhance tongue and laryngeal elevation, resulting in a more physiologic swallow. These factors are critical in cases in which partial laryngectomy is performed because the risk of aspiration is increased.84
With the growing popularity of minimally invasive techniques, endoscopic resection of tongue base tumors with the use of the CO2 laser has emerged in selected centers. We have performed endoscopic resections of T1 to T3 carcinomas of the tongue base, leaving up to 6 cm to heal by secondary intention. With time, the surgical bed granulates in and forms a neotongue that closely resembles and functions like the native tongue base. This form of reconstruction is simple and reliable in the nonirradiated patient, although it may also be used as a salvage technique, with healing and local control being less predictable. Overall patient functioning is generally directly related to the extent of the resection performed.69 Primary closure is an excellent option for reconstructing selected defects of the tongue base. This approach has been championed by McConnel and colleagues.10,66 We have previously addressed this study in detail in “Tonsil and Pharyngeal Wall.” Tongue base defects of up to 60% were closed primarily, and these were matched against similar defects reconstructed with free and pedicled flaps. They report that there is no significant improvement in swallowing efficiency between patients having flaps and patients with primary closure. Conversely, patients with primary closure had better swallowing efficiency than both the free flap and pedicled flap groups. This report is controversial because it represents a paradigm shift in conventional reconstructive ideals. Currently, most would recommend flap reconstruction for defects involving far less than 60%, with one group suggesting flaps are necessary in cases where more than 20% of the tongue base is resected.67 Clearly, there are no definite guidelines to follow, and much is based on surgeon preference and experience. As a general rule, we have tended to use free tissue transfer in cases in which a mandibulotomy was performed for exposure, while reserving consideration of primary closure for transoral, transhyoid, or pharyngotomy approaches. Basic principles still apply for the use of primary closure because the wound must be free of tension, and there should be minimal or no distortion of the native anatomy. We have used primary closure for tongue base defects of 30% or less and favor the use of free flaps for more extensive defects.
Regional flap reconstruction of the base of tongue remains an option. The pectoralis major myocutaneous flap is the tried, tested, and true regional flap used for this purpose. Soon after the description of this flap, Conley and colleagues86 reported their experience with reinnervated pectoralis myocutaneous flaps for reconstructing total glossectomy defects. The pectoral nerves were anastomosed to the stump of the hypoglossal nerve in all cases. Electromyographic recordings confirmed that reinnervation had occurred. Although flap atrophy was prevented with the reinnervation of the muscle, coordinated contraction and movement of the neotongue was not observed. Even if muscular contraction had been observed, this would result in inferior movement of the flap, which is opposite to the desired upward movement seen in normal swallowing. Sultan and Coleman87 reported on a group of 11 patients who were reconstructed with pedicled pectoralis flaps. They proposed that delayed sagging of the flap leads to decreased laryngeal mobility. There is a scarcity of functional outcomes reported with this form of reconstruction. Weber and colleagues88 reported on swallowing results in 27 patients who had subtotal or total glossectomies with laryngeal preservation. All patients were reconstructed with a pectoralis major flap. Of the 27 patients, 18 (67%) were able to obtain an oral diet; however, with time, this number decreased to 12 (45%), thus confirming the subjective observations of Sultan and Coleman. Gehanno85 reported his experience with 80 patients with advanced carcinoma of the tongue who were treated with total glossectomy. Reconstruction was performed with a pedicled musculocutaneous flap in 75 patients (64 pectoralis major, 8 trapezius, and 3 latissimus) and a gastro-omental free flap in 5 patients. Twenty-six (32%) patients had a pharyngocutaneous fistula develop, and half of these patients required further surgery. Half of the patients were decannulated within 30 days of their initial surgery. At 3 months, 41 (53%) patients were able to eat soft food, and speech was intelligible in 49 (63%) patients. Five-year survival of the study patients was a dismal 12%.
Another regional musculocutaneous flap that may be used for total and subtotal glossectomy defects is the latissimus dorsi. This is our flap of choice for this defect. However, we have abandoned its use as a pedicled transfer and use it solely as a free flap for this purpose. Haughey42 has reported his initial experience with this flap as a pedicled transfer in tongue reconstruction. Of his first four latissimus flaps for tongue reconstruction, three were used as pedicled transaxillary transfers. Two of these three flaps failed, and both were salvaged with pectoralis major myocutaneous flaps. We have abandoned the pedicled transfer for tongue reconstruction because of the unreliability of the flap for this purpose and the potential limitations of neotongue mobility caused by pedicle tethering.
The platysmal myocutaneous flap has recently been reported to be appropriate for use in the tongue base. Koch20 reported on nine patients with base of tongue defects that were reconstructed with the platysma flap. Despite the high rate of complications reported with this flap by some, all nine flaps survived entirely. One patient had a pharyngocutaneous fistula develop that healed uneventfully with conservative measures. Eight of the nine patients were able to achieve an oral diet. The one patient unable to do so had a resection of a T3 tumor of the tongue base after failure of radiation and chemotherapy. None of the patients had evidence of aspiration. The flap was used for tongue base primary tumors in approximately 25% of the reported cases (9 of 34) in this series. A review of other published data on the platysma flap revealed only eight other cases in which this flap was used for tongue base reconstruction.12,22,89 Seven of the eight flaps survived entirely, and one flap developed partial necrosis of the skin paddle. Functional results for these patients are not apparent. The overall flap survival rate for the platysmal flap for tongue base reconstruction is 94% (17 of 18). The advantages of this flap for tongue base reconstruction are the flap’s simplicity and proximity to the defect, thus allowing a tension-free closure. Disadvantages of this technique are the lack of flap bulk and sensory potential.
Free tissue transfer has become the “go to” method of reconstruction for defects of the tongue base. Fasciocutaneous and musculocutaneous flaps are most commonly used for this purpose. Sensory recovery has been documented with fasciocutaneous flaps in tongue reconstruction.90 Seventeen patients who underwent reconstruction of glossectomy defects with reinnervated forearm flaps were studied 8 months after surgery. Sensory recovery occurred in all 17 patients. The free flap reconstructions had superior sensation compared with the contralateral forearm donor site and approached that of the native tongue. Despite this, no direct link between sensory recovery and functional recovery has been conclusively shown.90,91 We also published our experience with fasciocutaneous flaps for tongue and floor of mouth reconstruction.92 Forty-three reconstructions were performed, of which 13 were centered in the tongue base and did not extend into the oral tongue or floor of mouth for more than 2 cm. The defects were reconstructed with a planar inset of a forearm flap between the hyoid inferiorly and the oral tongue superiorly. No patients had a supraglottic laryngectomy or a delayed total laryngectomy for aspiration. In the presence of a contiguous pharyngeal wall defect, the flap was folded 90 degrees from the plane of the tongue base to provide coverage of the pharyngeal component. One lingual artery and hypoglossal nerve were preserved in all cases. Laryngeal suspensions were performed in all cases. All patients were decannulated at an average of 14.6 days. The Functional Outcomes Swallowing Score was used to assess swallowing outcomes.93 The tongue base cohort had a mean score of 1.6, which was superior to the oral tongue group at 2.4, but this was not statistically significant. Finally, speech intelligibility was greater than 90% in all patients, with a mean score of 98%. We believe this was partially due to the flap’s allowing for excellent anterior tongue remnant mobility.
Urken94 contended for the importance of reconstructing isolated base of tongue defects with tissue that provides both bulk and sensation. His flap of choice for this defect is also the radial forearm flap because it can be harvested with large amounts of additional vascularized subcutaneous fat to impart the necessary bulk while also providing sensation. He has described a tripartite subcutaneous extension from the body of the flap to reconstruct these defects. The bulk that is provided with these extensions does not diminish as is seen in vascularized muscle. All forearm flaps were sensate in his series. The earliest signs of sensation were noted after 4 months in most patients. The degree of discrimination did not seem to be age dependent. Improvements that suggested a role for sensory recovery were a reduction or disappearance in coughing and decreased use of adaptive techniques for aspiration during the time when sensation returned in the neotongue. However, no swallowing measures or quality of life instruments were used to support the contention that bulk or sensation was helpful, and the absence of a control group further confuses the reinnervation issue.11 Rieger and colleagues95 reported on their functional results on 32 patients following reconstruction of the base of tongue with a free radial forearm flap. Speech intelligibility was above 90% for most patients. Only five patients required a feeding tube at some point over the year of postoperative evaluation, and one patient used a tube as the primary source of nutrition. These results contrast with those of Borggreven,5 which found that patients with advanced tumors (T3-T4 vs. T2) and combined base of tongue and soft palate reconstructions were associated with profound swallowing problems. Despite giving details of sensory reinnervation, swallowing and rates of decannulation were not reported. Urken96 expanded the use of the sensate forearm flap and has described its use in combination with the iliac crest free flap for reconstruction of significant glossectomy-mandibulectomy defects.
The lateral arm free flap may also be used for base of tongue defects. Civantos41 reported on 17 patients who underwent major oropharyngeal resections, of whom 12 had more than half of the tongue base removed. Thirteen of these patients had reinnervation of their lateral arm flaps. Three patients had a total laryngectomy for oncologic reasons, and all were able to swallow. Of the remaining 14 patients, 12 could be evaluated between 6 months and 1 year postoperatively. Eight patients (67%) were able to swallow within 2 months, and 11 patients (92%) were able to feed orally by 8 months. All surviving patients were eventually able to achieve an oral diet. Salibian and colleagues97 reported on seven patients who had ulnar forearm flaps for reconstruction of subtotal glossectomy defects. All patients were able to feed orally. Four patients were able to resume a normal diet, whereas the remaining three were tolerating a soft diet.
Total or subtotal glossectomy defects are a particularly challenging problem for the reconstructive surgeon. The complexity of the reconstruction is due to the multitude of functions provided by the native tongue. These functions are possible because of the array of intrinsic and extrinsic musculature along with the strategic position of the tongue above the larynx. Our flap of choice when faced with these defects is the innervated free latissimus dorsi musculocutaneous flap. Haughey42 has reported on a design for latissimus dorsi flaps in tongue reconstruction. The flap is designed in such a fashion that the skin paddle is oriented perpendicular to the underlying muscle. The dimensions of the skin paddle are obtained by measuring the distance from the mandibular symphysis to the inferior extent of the resection in the base of tongue, vallecula, or supraglottic larynx. Allowance is made dorsally so that the flap will contact the palate once inset, and lateral wings are added to the skin paddle if a tonsillar defect is present. Also, a triangular wedge of anterior skin is deepithelialized, imbricated, and repaired to create a “cupula,” which rises from the anterior neotongue. An allowance is then required anteriorly in the skin paddle design to provide sufficient tissue for this maneuver. The key to this technique is in the inset of the flap. The flap is inset from an inferior to superior direction. If the epiglottis is preserved, the flap is sutured as close to its base as possible. The flap is then suspended from the skull base bilaterally to form a sling under the mandible. This is performed by suturing the latissimus muscle to the pharyngeal constrictors, medial pterygoids, or the masseter if the mandibular ramus was resected (Fig. 101-18). The thoracodorsal nerve was anastomosed to the hypoglossal nerve in all free flap cases. Postoperative superior movement of the flap was observed presumably from reinnervation of the latissimus muscle and was documented by video-oropharyngography in two patients. Fifteen cases were included in the study including three pedicled flaps and one free innervated radial forearm flap. Two of the three pedicled flaps and one of the 12 free flaps failed. Pedicled flap reconstruction with this technique is no longer recommended by the author. Four patients underwent a laryngectomy or laryngeal closure, and one died perioperatively. Of the 10 remaining patients, 8 were decannulated at a median interval of 3.2 weeks. An oral diet was obtained in 10 patients. Two patients in the series were G-tube dependent including one patient whose flap failed and was salvaged with a pectoralis major flap. Three patients who had died did not have swallowing results available. Speech intelligibility was not formally studied but was estimated by a speech language pathologist to be in the range of 85% to 90% on the basis of observations made in three patients. Videos of active, voluntary movement in the flap initiated by the patient are available from the author (BHH) upon request.
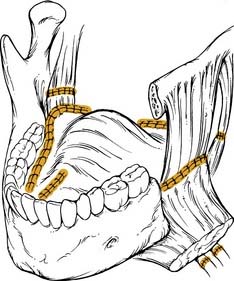
(Adapted from Haughey B. Tongue reconstruction: concepts and practice. Laryngoscope. 1993;103:1136.)
Kimata and colleagues43 reported on functional results and complications after total glossectomy with microvascular reconstruction. Thirty patients were included in the study, all of whom had a total glossectomy with laryngeal preservation. The rectus abdominis musculocutaneous flap was the authors’ flap of choice, and this was used in 23 patients. Other flaps used include combinations of the latissimus dorsi, serratus anterior, anterolateral thigh, and radial forearm flaps. Flaps were designed as wide and thick as possible while still allowing for primary closure of the donor site. The flaps were inset from an inferior to superior fashion, with slight anterior and superior traction in an attempt to elevate the larynx. A formal laryngeal suspension to the mandible was performed on four patients. There was one flap failure, and marginal necrosis occurred in five flaps. Orocutaneous fistulas developed in nine patients (27%), and two of these required additional surgery. Of the 30 patients in the study, 21 (70%) were decannulated with laryngeal preservation, and all of these patients were able to achieve an oral diet. In 9 of 30 patients, laryngeal function could not be preserved. Preoperative cerebral dysfunction, resection of the epiglottis, and postoperative orocutaneous fistulas were found to be statistically significant with regard to failure of laryngeal preservation. Speech was judged to be intelligible in 16 of 19 patients evaluated. At the conclusion of follow-up at a mean duration of 29 months, 10 patients were alive without disease. Similarly, Lyos and colleagues45 reported their experience with the rectus abdominis flap for tongue reconstruction. This was a retrospective outcomes analysis of 14 patients who had a minimum of 75% of the tongue resected with preservation of the mandible and larynx. Eight patients had a total glossectomy, whereas six had subtotal resections. Decannulation was possible in 12 patients (86%) at an average interval of 3.5 months postoperatively. One patient required a laryngectomy for intractable aspiration, and three patients required G-tubes for severe dysphagia. Eleven of 14 patients (81%) were able to tolerate an oral diet of some variety. A palatal augmentation device was found to be of benefit in eight patients because of insufficient vertical height of the free flap reconstruction. This is due to the atrophy of the rectus muscle and subsequent displacement of the skin paddle inferiorly. Speech was judged as being acceptable in nine patients (64%).
A money pouch–like reconstructive method after total glossectomy without laryngectomy has been described with a free rectus abdominis myocutaneous flap.44 With this technique, the authors established a money pouch–like neotongue by harvesting a skin paddle that is 20% larger than the defect in both width and length. When the skin paddle is sutured to the defect, the excess skin and fat fold into the shape of a money pouch, thus establishing the vertical height needed for successful tongue reconstruction (Fig. 101-19). They report on only three patients in this study; however, a normal swallow was achieved in all patients by 6 weeks with the assistance of oral ingestion training. Speech intelligibility was about 50% to 60% in the patients. Although this seems to be a novel technique, other descriptions of the rectus flap for tongue reconstruction have attempted to overcorrect to achieve this vertical height of the reconstruction.43 The rectus flap may also be inset as a sling under the mandible to support the overlying position of the skin paddle. Urken46 has preferred the use of the rectus flap over the latissimus for this purpose because of the tendinous inscriptions, which are able to hold sutures. He also advocates the use of drill holes in the mandible for suspension of the rectus flap. Atrophy of the rectus is inevitable, but this may be reduced by reinnervating the muscle with a hypoglossal nerve stump, although meaningful movement of the muscle has not been documented.94
Hayden34 reported on the use of the lateral thigh flap for reconstructing nine patients with total or near-total glossectomy defects. All flaps survived completely, and no patients had postoperative fistulas develop. Functional results in this cohort of patients are not reported. The author states that the flap is ideal for this purpose (in selected cases with appropriately fat thighs) because the distal portion of the flap can be deepithelialized and used as a platform under the mandible, while the proximal thicker portion of the flap is used for reconstruction of the neotongue.34 The free anterolateral thigh flap has been reported for total and near-total glossectomy defects. The flap is useful for this purpose because it provides significant bulk and also the possibility of sensory reinnervation. Yu98 reported on 13 patients reconstructed with this flap, 8 of which were reinnervated. Innervated flaps were found to have superior sensory recovery, which resulted in improved swallowing function and patient satisfaction. Figure 101-20 presents an algorithm for reconstructing defects of the tongue base.
Baek SM. Two new cutaneous free flaps: the medial and lateral thigh flaps. Plast Reconstr Surg. 1983;71:354-365.
Brown JS, Zuydam AC, Jones DC, et al. Functional outcome in soft palate reconstruction using a radial forearm free flap in conjunction with a superiorly based pharyngeal flap. Head Neck. 1997;19:524-534.
Colangelo LA, Logemann JA, Pauloski BR, et al. T stage and functional outcome in oral and oropharyngeal cancer patients. Head Neck. 1996;18:259-268.
Ethier JL, Taylor SM, Trites JRB. The pectoralis major myofascial flap in head and neck reconstruction: indications and outcomes. J Otolaryngol Head Neck Surg. In press, 2009.
Gillespie MB, Eisele DW. The uvulopalatal flap for reconstruction of the soft palate. Laryngoscope. 2000;110:612-615.
Gullane PJ, Arena S. Extended palatal island mucoperiosteal flap. Arch Otolaryngol. 1985;111:330-332.
Haughey BH. Tongue reconstruction: concepts and practice. Laryngoscope. 1993;103:1132-1141.
Haughey BH, Taylor SM, Fuller D. Fasciocutaneous flap reconstruction of the tongue and floor of mouth: outcomes and techniques. Arch Otolaryngol Head Neck Surg. 2002;128:1388-1395.
Kimata Y, Uchiyama K, Sakuraba M, et al. Velopharyngeal function after microsurgical reconstruction of lateral and superior oropharyngeal defects. Laryngoscope. 2002;112:1037-1042.
Koch WM. The platysma myocutaneous flap: underused alternative for head and neck reconstruction. Laryngoscope. 2002;112:1204-1208.
Lacombe V, Blackwell KE. Radial forearm free flap for soft palate reconstruction. Arch Facial Plast Surg. 1999;1:130-132.
Makitie AA, Beasley NJ, Neligan PC, et al. Head and neck reconstruction with anterolateral thigh flap. Otolaryngol Head Neck Surg. 2003;129:547-555.
Markkanen-Leppanen M, Suominen E, Lehtonen H, et al. Free flap reconstructions in the management of oral and pharyngeal cancer. Acta Otolaryngol. 2001;121:425-429.
McConnel FM, Pauloski BR, Logemann JA, et al. Functional results of primary closure vs flaps in oropharyngeal reconstruction: a prospective study of speech and swallowing. Arch Otolaryngol Head Neck Surg. 1998;124:625-630.
McConnel FM, Teichgraeber JF, Adler RK. A comparison of three methods of oral reconstruction. Arch Otolaryngol Head Neck Surg. 1987;113:496-500.
Netscher DT, Meade RA, Goodman CM, et al. Quality of life and disease-specific functional status following microvascular reconstruction for advanced (T3 and T4) oropharyngeal cancers. Plast Reconstr Surg. 2000;105:1628-1634.
Penfold CN, Brown AE, Lavery KM, et al. Combined radial forearm and pharyngeal flap for soft palate reconstruction. Br J Oral Maxillofac Surg. 1996;34:322-324.
Rieger JM, Zalmanowitz JG, Li SY, et al. Functional outcomes after surgical reconstruction of the base of tongue using the radial forearm free flap in patients with oropharyngeal carcinoma. Head Neck. 2007;29:1024-1032.
Seikaly H, Rieger J, Wolfaardt J, et al. Functional outcomes after primary oropharyngeal cancer resection and reconstruction with the radial forearm free flap. Laryngoscope. 2003;113:897-904.
Sinha UK, Young P, Hurvitz K, et al. Functional outcomes following palatal reconstruction with a folded radial forearm free flap. Ear Nose Throat J. 2004;83:45-48.
Taylor SM, Haughey BH. Combined pharyngoesophageal and cervical skin reconstruction using a single radial forearm flap. Laryngoscope. 2002;112:1315-1318.
Urken ML, Moscoso JF, Lawson W, et al. A systematic approach to functional reconstruction of the oral cavity following partial and total glossectomy. Arch Otolaryngol Head Neck Surg. 1994;120:589-601.
Urken ML, Weinberg H, Vickery C, et al. The neurofasciocutaneous radial forearm flap in head and neck reconstruction: a preliminary report. Laryngoscope. 1990;100:161-173.
Zeitels SM, Kim J. Soft-palate reconstruction with a “SCARF” superior-constrictor advancement-rotation flap. Laryngoscope. 1998;108:1136-1140.
Zuydam AC, Lowe D, Brown JS, et al. Predictors of speech and swallowing function following primary surgery for oral and oropharyngeal cancer. Clin Otolaryngol. 2005;30:428-437.
1. Ariyan S. The pectoralis major myocutaneous flap. A versatile flap for reconstruction in the head and neck. Plast Reconstruct Surg. 1979;63:73-81.
2. Baek SM, Biller HF, Krespi YP, et al. The pectoralis major myocutaneous island flap for reconstruction of the head and neck. Head Neck Surg. 1979;1:293-300.
3. Rich J, Milov S, Lewis J, Thorstadt W, Adkins D, Haughey BH. Transoral laser microsurgery ± adjuvant therapy for advanced oropharyngeal cancer. Laryngoscope. 2009. In press
4. Colangelo LA, Logemann JA, Pauloski BR, et al. T stage and functional outcome in oral and oropharyngeal cancer patients. Head Neck. 1996;18:259-268.
5. Borggreven PA, Verdonck-de Leeuw I, Rinkel RN, et al. Swallowing after major surgery of the oral cavity or oropharynx: a prospective and longitudinal assessment of patients treated by microvascular soft tissue reconstruction. Head Neck. 2007;29:638-647.
6. Kroll SS, Evans GR, Goldberg D, et al. A comparison of resource costs for head and neck reconstruction with free and pectoralis major flaps. Plastic Reconstruct Surg. 1997;99:1282-1286.
7. Tsue TT, Desyatnikova SS, Deleyiannis FW, et al. Comparison of cost and function in reconstruction of the posterior oral cavity and oropharynx. Free vs pedicled soft tissue transfer. Arch Otolaryngol Head Neck Surg. 1997;123:731-737.
8. Smeele LE, Goldstein D, Tsai V, et al. Morbidity and cost differences between free flap reconstruction and pedicled flap reconstruction in oral and oropharyngeal cancer: Matched control study. J Otolaryngology. 2006;35:102-107.
9. Netscher DT, Meade RA, Goodman CM, et al. Quality of life and disease-specific functional status following microvascular reconstruction for advanced (T3 and T4) oropharyngeal cancers. Plast Reconstruct Surg. 2000;105:1628-1634.
10. McConnel FM, Teichgraeber JF, Adler RK. A comparison of three methods of oral reconstruction. Arch Otolaryngol Head Neck Surg. 1987;113:496-500.
11. Logemann JA, Pauloski BR, Rademaker AW, et al. Speech and swallow function after tonsil/base of tongue resection with primary closure. J Speech Hear Res. 1993;36:918-926.
12. McGuirt WF, Matthews BL, Brody JA, et al. Platysma myocutaneous flap: caveats reexamined. Laryngoscope. 1991;101:1238-1244.
13. Gullane PJ, Arena S. Extended palatal island mucoperiosteal flap. Arch Otolaryngol. 1985;111:330-332.
14. Gillespie MB, Eisele DW. The uvulopalatal flap for reconstruction of the soft palate. Laryngoscope. 2000;110:612-615.
15. Licameli GR, Dolan R. Buccinator musculomucosal flap: applications in intraoral reconstruction. Arch Otolaryngol Head Neck Surg. 1998;124:69-72.
16. Zeitels SM, Kim J. Soft-palate reconstruction with a “SCARF” superior-constrictor advancement-rotation flap. Laryngoscope. 1998;108:1136-1140.
17. Freeman JL, Walker EP, Wilson JS, et al. The vascular anatomy of the pectoralis major myocutaneous flap. Br J Plast Surg. 1981;34:3-10.
18. Moloy PJ, Gonzales FE. Vascular anatomy of the pectoralis major myocutaneous flap. Arch Otolaryngol Head Neck Surg. 1986;112:66-69.
19. Futrell JW, Johns ME, Edgerton MT, et al. Platysma myocutaneous flap for intraoral reconstruction. Am J Surg. 1978;136:504-507.
20. Koch WM. The platysma myocutaneous flap: underused alternative for head and neck reconstruction. Laryngoscope. 2002;112:1204-1208.
21. Ruark DS, McClairen WCJr, Schlehaider UK, et al. Head and neck reconstruction using the platysma myocutaneous flap. Am J Surg. 1993;165:713-718.
22. Verschuur HP, Dassonville O, Santini J, et al. Complications of the myocutaneous platysma flap in intraoral reconstruction. Head Neck. 1998;20:623-629.
23. Cheney ML, Varvares MA, Nadol JBJr. The temporoparietal fascial flap in head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 1993;119:618-623.
24. McCarthy JG, Cutting CB, Shaw WW. Vascularized calvarial flaps. Clin Plast Surg. 1987;14:37-47.
25. Browne JD, Holland BW. Combined intraoral and lateral temporal approach for palatal malignancies with temporalis muscle reconstruction. Arch Otolaryngol Head Neck Surg. 2002;128(May (5)):531-537.
26. Maves MD, Panje WR, Shagets FW. Extended latissimus dorsi myocutaneous flap reconstruction of major head and neck defects. Otolaryngol Head Neck Surg. 1984;92:551-558.
27. Watson JS, Lendrum J. One stage pharyngeal reconstruction using a compound latissimus dorsi island flap. Br J Plast Surg. 1981;34:87-90.
28. Collins SL. The longus colli muscle flap for reconstruction of the lateral pharyngeal wall. Head Neck. 1997;19:297-308.
29. Urken ML, Weinberg H, Vickery C, et al. The neurofasciocutaneous radial forearm flap in head and neck reconstruction: a preliminary report. Laryngoscope. 1990;100:161-173.
30. Close LG, Truelson JM, Milledge RA, et al. Sensory recovery in noninnervated flaps used for oral cavity and oropharyngeal reconstruction. Arch Otolaryngol Head Neck Surg. 1995;121:967-972.
31. Lvoff G, O’Brien CJ, Cope C, et al. Sensory recovery in noninnervated radial forearm free flaps in oral and oropharyngeal reconstruction. Arch Otolaryngol Head Neck Surg. 1998;124:1206-1208.
32. Urken ML, Futran N, Moscoso JF, et al. A modified design of the buried radial forearm free flap for use in oral cavity and pharyngeal reconstruction. Arch Otolaryngol Head Neck Surg. 1994;120:1233-1239.
33. Baek SM. Two new cutaneous free flaps: the medial and lateral thigh flaps. Plast Reconstruct Surg. 1983;71:354-365.
34. Hayden RE, Deschler DG. Lateral thigh free flap for head and neck reconstruction. Laryngoscope. 1999;109:1490-1494.
35. Song YG, Chen GZ, Song YL. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg. 1984;37:149-159.
36. Koshima I, Fukuda H, Soeda S. The anterolateral thigh flap; variations in its vascular pedicle. Br J Plast Surg. 1989;42:260-262.
37. Makitie AA, Beasley NJ, Neligan PC, et al. Head and neck reconstruction with anterolateral thigh flap. Otolaryngol Head Neck Surg. 2003;129:547-555.
38. Wei FC, Jain V, Celik N, et al. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstruct Surg. 2002;109:2219-2226.
39. Song R, Song Y, Yu Y, et al. The upper arm free flap. Clin Plast Surg. 1982;9:27-35.
40. Sullivan MJ, Carroll WR, Kuriloff DB. Lateral arm free flap in head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 1992;118:1095-1101.
41. Civantos FJJr, Burkey B, Lu FL, et al. Lateral arm microvascular flap in head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 1997;123:830-836.
42. Haughey BH. Tongue reconstruction: concepts and practice. Laryngoscope. 1993;103:1132-1141.
43. Kimata Y, Uchiyama K, Ebihara S, et al. Postoperative complications and functional results after total glossectomy with microvascular reconstruction. Plast Reconstruct Surg. 2000;106:1028-1035.
44. Kiyokawa K, Tai Y, Inoue Y, et al. Functional reconstruction of swallowing and articulation after total glossectomy without laryngectomy: money pouch-like reconstruction method using rectus abdominis myocutaneous flap. Plast Reconstruct Surg. 1999;104:2015-2020.
45. Lyos AT, Evans GR, Perez D, et al. Tongue reconstruction: outcomes with the rectus abdominis flap. Plast Reconstruct Surg. 1999;103:442-447.
46. Urken ML, Turk JB, Weinberg H, et al. The rectus abdominis free flap in head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 1991;117:857-866.
47. King TW, Gallas MT, Robb GL, et al. Aesthetic and functional outcomes using osseous or soft-tissue free flaps. J Reconstruct Microsurg. 2002;18:365-371.
48. Taylor SM, Haughey BH. Combined pharyngoesophageal and cervical skin reconstruction using a single radial forearm flap. Laryngoscope. 2002;112:1315-1318.
49. Zohar Y, Buler N, Shvilli Y, et al. Reconstruction of the soft palate by uvulopalatal flap. Laryngoscope. 1998;108:47-50.
50. Johnson JT, Aramany MA, Myers EN. Palatal neoplasms: reconstruction considerations. Otolaryngol Clin North Am. 1983;16:441-456.
51. Aramany MA, Matalon V. Prosthetic management of postsurgical soft palate defects. J Prosthet Dent. 1970;24:304-311.
52. Shapiro BM, Komisar A, Silver C, et al. Primary reconstruction of palatal defects. Otolaryngol Head Neck Surg. 1986;95:581-585.
53. Yoshida H, Michi K, Yamashita Y, et al. A comparison of surgical and prosthetic treatment for speech disorders attributable to surgically acquired soft palate defects. J Oral Maxillofac Surg. 1993;51:361-365.
54. Bozola AR, Gasques JA, Carriquiry CE, et al. The buccinator musculomucosal flap: anatomic study and clinical application. Plast Reconstruct Surg. 1989;84:250-257.
55. Strong MS, DiTroia JF, Vaughan CW. Carcinoma of the palatine arch. A review of 73 patients. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:957-967.
56. Healy GB, Strong MS, Uchmakli A, et al. Carcinoma of the palatine arch. Am J Surg. 1976;132:498-503.
57. Kimata Y, Uchiyama K, Sakuraba M, et al. Velopharyngeal function after microsurgical reconstruction of lateral and superior oropharyngeal defects. Laryngoscope. 2002;112:1037-1042.
58. Gehanno P, Guédon C, Véber F, et al. [Velopharyngeal rehabilitation after transmaxillary buccopharyngectomy extending to the soft palate]. Ann Otolaryngol Chir Cervicofac. 1985;102:135-137.
59. Lacombe V, Blackwell KE. Radial forearm free flap for soft palate reconstruction. Arch Facial Plast Surg. 1999;1:130-132.
60. Brown JS, Zuydam AC, Jones DC, et al. Functional outcome in soft palate reconstruction using a radial forearm free flap in conjunction with a superiorly based pharyngeal flap. Head Neck. 1997;19:524-534.
61. Penfold CN, Brown AE, Lavery KM, et al. Combined radial forearm and pharyngeal flap for soft palate reconstruction. Br J Oral Maxillofac Surg. 1996;34:322-324.
62. McCombe D, Lyons B, Winkler R, et al. Speech and swallowing following radial forearm flap reconstruction of major soft palate defects. Br J Plast Surg. 2005;58:306-311.
63. Seikaly H, Rieger J, Wolfaardt J, et al. Functional outcomes after primary oropharyngeal cancer resection and reconstruction with the radial forearm free flap. Laryngoscope. 2003;113:897-904.
64. Sinha UK, Young P, Hurvitz K, et al. Functional outcomes following palatal reconstruction with a folded radial forearm free flap. Ear Nose Throat J. 2004;83:45-48.
65. Panje WR, Scher N, Karnell M. Transoral carbon dioxide laser ablation for cancer, tumors, and other diseases. Arch Otolaryngol Head Neck Surg. 1989;115:681-688.
66. McConnel FM, Pauloski BR, Logemann JA, et al. Functional results of primary closure vs flaps in oropharyngeal reconstruction: a prospective study of speech and swallowing. Arch Otolaryngol Head Neck Surg. 1998;124:625-630.
67. Reece GP, Kroll SS, Miller MJ, et al. Functional results after oropharyngeal reconstruction: a different perspective. Arch Otolaryngol Head Neck Surg. 1999;125:474-477.
68. Zuydam AC, Lowe D, Brown JS, et al. Predictors of speech and swallowing function following primary surgery for oral and oropharyngeal cancer. Clin Otolaryngol. 2005;30:428-437.
69. Pauloski BR, Logemann JA, Colangelo LA, et al. Surgical variables affecting speech in treated patients with oral and oropharyngeal cancer. Laryngoscope. 1998;108:908-916.
70. Vartanian JG, Carvalho AL, Carvalho SM, et al. Pectoralis major and other myofascial/myocutaneous flaps in head and neck cancer reconstruction: experience with 437 cases at a single institution. Head Neck. 2004;26:1018-1023.
71. Koh KS, Eom JS, Kirk I, et al. Pectoralis major musculocutaneous flap in oropharyngeal reconstruction: revisited. Plast Reconstruct Surg. 2006;118:1145-1149.
72. Ethier JL, Taylor SM, Trites JRB: The pectoralis major myofascial flap in head and neck reconstruction: indications and outcomes. J Otolaryngol Head Neck Surg. In Press, 2009.
73. Righi PD, Weisberger EC, Slakes SR, et al. The pectoralis major myofascial flap: clinical applications in head and neck reconstruction. Am J Otolaryngol. 1998;19:96-101.
74. Jackel MC. Platysma myofascial flap for reconstruction of oropharyngeal defects after transoral laser microsurgery of locally advanced carcinomas. J Laryngol Otol. 2006;120:1055-1058.
75. Ariyan S. Further experiences with the sternocleidomastoid myocutaneous flap: a clinical appraisal of 31 cases. Plast Reconstruct Surg. 1997;99:61-69.
76. Marx RE, McDonald DK. The sternocleidomastoid muscle as a muscular or myocutaneous flap for oral and facial reconstruction. J Oral Maxillofac Surg. 1985;43:155-162.
77. Sebastian P, Cherian T, Ahamed MI, et al. The sternomastoid island myocutaneous flap for oral cancer reconstruction. Arch Otolaryngol Head Neck Surg. 1994;120:629-632.
78. Huttenbrink KB. Temporalis muscle flap: an alternative in oropharyngeal reconstruction. Laryngoscope. 1986;96:1034-1038.
79. Huttenbrink KB. [The temporalis muscle fascia flap for covering defects in the oropharynx. Report of 4 years experiences with 26 cases]. Laryngorhinootologie. 1989;68:272-277.
80. Smith JE, Ducic Y, Adelson R. The utility of the temporalis muscle flap for oropharyngeal, base of tongue, and nasopharyngeal reconstruction. Otolaryngol Head Neck Surg. 2005;132:373-380.
81. Smith GI, Brennan PA, Scott PJ, et al. Outcome after radial forearm, gastro-omental, and jejunal free flaps in oral and oropharyngeal reconstruction. Br J Oral Maxillofac Surg. 2002;40:330-333.
82. Markkanen-Leppanen M, Suominen E, Lehtonen H, et al. Free flap reconstructions in the management of oral and pharyngeal cancer. Acta Otolaryngol. 2001;121:425-429.
83. Schwager K, Hoppe F, Hagen R, et al. [Outcome after resection of extensive oropharyngeal carcinomas and defect coverage by microvascular anastomosis of a radialis flap]. Laryngorhinootologie. 1999;78:259-262.
84. Barzan L, Comoretto R. [Free forearm skin flap with vascular microanastomosis in the reconstruction of the posterior pharyngeal wall]. Acta Otorhinolaryngol Ital. 1991;11:111-116.
85. Gehanno P, Guedon C, Barry B, et al. Advanced carcinoma of the tongue: total glossectomy without total laryngectomy. Review of 80 cases. Laryngoscope. 1992;102:1369-1371.
86. Conley J, Sachs ME, Parke RB. The new tongue. Otolaryngol Head Neck Surg. 1982;90:58-68.
87. Sultan MR, Coleman JJ3rd. Oncologic and functional considerations of total glossectomy. Am J Surg. 1989;158:297-302.
88. Weber RS, Ohlms L, Bowman J, et al. Functional results after total or near total glossectomy with laryngeal preservation. Arch Otolaryngology Head Neck Surg. 1991;117:512-515.
89. Conley JJ, Lanier DM, Tinsley PJr. Platysma myocutaneous flap revisited. Arch Otolaryngol Head Neck Surg. 1986;112:711-713.
90. Kuriakose MA, Lorce TR, Spies A, et al. Sensate radial forearm free flaps in tongue reconstruction. Arch Otolaryngol Head Neck Surg. 2001;127:1463-1466.
91. Boyd B, Mulholland S, Gullane P, et al. Reinnervated lateral antebrachial cutaneous neurosome flaps in oral reconstruction: are we making sense? Plast Reconstruct Surg. 1994;93:1350-1359.
92. Haughey BH, Taylor SM, Fuller D. Fasciocutaneous flap reconstruction of the tongue and floor of mouth: outcomes and techniques. Arch Otolaryngol Head Neck Surg. 2002;128:1388-1395.
93. Salassa JR. A functional outcome swallowing scale for staging oropharyngeal dysphagia. Digestive Diseases (Basel, Switzerland). 1999;17:230-234.
94. Urken ML, Moscoso JF, Lawson W, et al. A systematic approach to functional reconstruction of the oral cavity following partial and total glossectomy. Arch Otolaryngol Head Neck Surg. 1994;120:589-601.
95. Rieger JM, Zalmanowitz JG, Li SY, et al. Functional outcomes after surgical reconstruction of the base of tongue using the radial forearm free flap in patients with oropharyngeal carcinoma. Head Neck. 2007.
96. Urken ML, Weinberg H, Vickery C, et al. The combined sensate radical forearm and iliac crest free flaps for reconstruction of significant glossectomy-mandibulectomy defects. Laryngoscope. 1992;102:543-558.
97. Salibian AH, Allison GR, Krugman ME, et al. Reconstruction of the base of the tongue with the microvascular ulnar forearm flap: a functional assessment. Plast Reconstruct Surg. 1995;96:1081-1089.
98. Yu P. Reinnervated anterolateral thigh flap for tongue reconstruction. Head Neck. 2004;26:1038-1044.