CHAPTER 11 Reconstruction of the Medial Patellofemoral Ligament
Recurrent lateral instability of the patella after traumatic patellar dislocation or subluxation usually requires attention to the medial soft tissue restraints for prevention of future pathologic lateral translation. Historically, procedures for addressing medial restraint insufficiency include reefing, vastus medialis obliquus (VMO) advancements, and nonanatomic tendon transfer procedures.1,2 In addition to shortening or imbrication of the medial soft tissue restraints, several authors have described reconstructing or reinforcing the medial patellar structures with fascia lata, nylon, and even preserved skin.3–6 Earlier proximal procedures for patellar instability can be summarized into two categories—advancement from structures above the patella or nonanatomic attachment from tendons anchored below the patella. Numerous earlier techniques of proximal VMO advancement and extensor mechanism equilibration have been summarized best by Ficat,7 who described these proximal muscle transfers as a dynamic means of patellar control. Baker and colleagues8 have summarized some of the early work of Galliazzi, with emphasis on a nonanatomic static semitendinosus tenodesis for the prevention of lateral subluxation.
ANATOMY
The MPFL is a thickened band of retinacular tissue originating in the saddle area between the medial epicondyle and adductor tubercle and inserting on the proximal third of the medial border of the patella. Typically, the MPFL is approximately 55 mm long and is overlaid by the distal part of the VMO, with fibers merging into the deep aspect of the muscle.9 Current techniques for addressing post-traumatic patellar instability have shifted focus to a restoration of the normal anatomy and the importance of the MPFL. The primary restraints against lateral patellar mobility are passive.10 The trochlea is the most important patellar stabilizer in normal knees beyond 20 degrees of flexion. If the patella is dislocating laterally, the medial restraints must be abnormally lax or deficient.
The MPFL has been shown to be the primary soft tissue restraint to lateral translation of the patella; it functions primarily in the first 30 degrees (before patellar engagement in the trochlea) and becomes lax in flexion.6,10–13 Additionally, if the trochlear groove is deficient (dysplastic), the MPFL takes on an even greater role. The MPFL is believed always to sustain some form of injury during traumatic lateral dislocation of the patella. Logically, a repair or reconstruction of this structure should provide the most anatomic reproduction of normal patellar biomechanics. Even though other medial tissues, such as the medial patellotibial ligament, may contribute to patellar stability, addressing the primary static restraint to lateral patellar subluxation provides the most reproducible and reliable outcomes at present.14 Reconstruction of the MPFL restores tracking to near-normal when the medial restraints are deficient. Current techniques for MPFL reconstruction differ from historical models and VMO advancement because of the emphasis on avoiding the creation of increased patellofemoral contact forces. VMO advancements and proximal reefings tend to tighten, therefore increasing the patellar joint contact forces in flexion. By anatomically reconstructing damaged normal anatomy, we attempt to correct patellar pathology without introducing a mechanism that will lead to medial facet overload and subsequent degenerative patellar disease.
PATIENT EVALUATION
History and Physical Examination
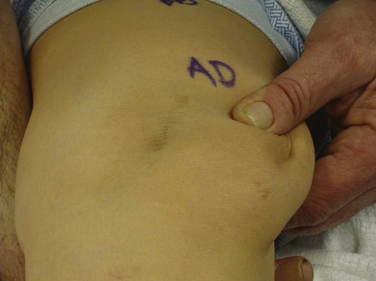
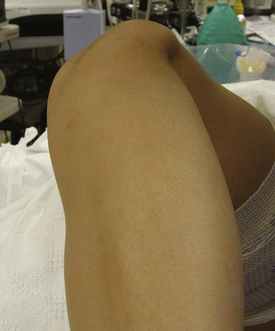
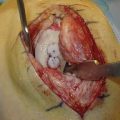
Success, as with any operation, relies mainly on proper patient selection. After an extensive history, the physical examination begins with examining the patient’s standing leg alignment and gait. A standing squat is helpful for demonstrating painful arc of motion and crepitus. We then have the patient flex and extend the knee while seated on the edge of the examination table to assess patellar tracking, specifically checking to see if the patella slides laterally with full extension, a J sign. Palpation of the patella with slight posterior pressure allows us to feel for crepitus and isolate the exact arc of pain better during active range of motion. With the patient supine, we assess lateral patellar translation, looking for abnormal increased glide. Specifically, we examine both knees at full extension and at 30 degrees of flexion (Fig. 11-1). We subdivide the patella into four quadrants and determine the extent (the number of quadrants) that the patella can be shifted laterally over the edge of the trochlea; MPFL patholaxity typically corresponds to 3+ to 4+ lateral glide at 30 degrees of flexion (Fig. 11-2). We also try to determine whether there is a hard or soft end point to translation, which can be helpful in determining whether there is any residual ligament function. Passive patellar tilt is examined to determine tightness of the lateral retinaculum. We have found the Sage sign, passive medial translation of the patella performed at 15 to 20 degrees of flexion, to be helpful in determining the need for lateral release. If the patella can be translated two quadrants or more, lateral release is not necessary, and may lead to debilitating medial instability if performed in this situation. In our practice, we often see patients with previously failed reefing procedures, previous lateral releases, and trochlear dysplasia, which may contribute to a more progressive laxity of medial restraints. In these cases, it is especially important to use the Fulkerson jump test to rule out iatrogenic medial patellar dislocations (postexcessive lateral release), which can present with the patient mistakenly reporting lateral dislocations.
Diagnostic Imaging
Standard imaging includes anteroposterior (AP) weight bearing, posteroanterior (PA) flexed weight bearing, a true lateral at 30 degrees of flexion to assess patellar height and trochlear dysplasia, and axial views (at 30, 60, and 90 degrees of flexion) to look at congruence and patellar rotation or tilt. Assessment of patellar height on the 30-degree lateral is performed using the Blackburn-Peel method, measured off the tibial plateau and articular surface of the patella, rather than the Insall-Salvati method. This eliminates the chance of misreading patella alta or infera, occasionally caused by a long nonarticular “nosed” portion of a patella, such as with a Cyrano-type morphology.15 Trochlear dysplasia is also best assessed on the true lateral view, looking for the crossing sign and presence of a trochlear bump.
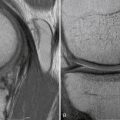
We routinely perform a calibrated computed tomography (CT) patellar tracking study with midaxial cuts from 0 to 60 degrees of knee flexion in 10-degree increments to assess patellar tracking from 0 to 60 degrees. This is the range in which stability occurs. The CT study also includes fixed-frame images from the trochlear groove to the tibial tubercle to measure tibial tuberosity-trochlear groove (TTTG) distance. From the CT cuts and Merchant view at 30 degrees, we can determine whether the patella is tilted. Normally, the patella will be centered in the trochlea; however, if the lateral facet is parallel to the anterior trochlea or if the angle formed by the lateral facet and the anterior trochlea is open medially, the patella is tilted. The congruence angle on a standard Merchant view should normally demonstrate that the patellar apex is medial to the bisected trochlea. The sulcus angle can be measured and should be 137 ± 6 degrees.16 A higher measured angle helps us identify a flat, shallow, dysplastic trochlea. Finally, magnetic resonance imaging (MRI) can be helpful in determining the extent of chondral damage in the patellofemoral joint and the status of the soft tissue restraints, as well as ruling out other pathology. We prefer MR arthrography if advanced cartilage sequencing is not available.
This compilation of information is predicated on the understanding that patellofemoral problems are often multifactorial. Factors such as the presence of trochlear dysplasia, poor tissue quality, patellar cartilage damage, hip rotation, alignment, and status of the tibiofemoral joint may influence operative decisions. As a stand-alone procedure, MPFL reconstruction is solely used for instability, whereas distal realignment surgery is better used to address tubercle malalignment with patellofemoral pain and/or instability. It is our experience that a Q angle more than 20 degrees at 30 degrees of flexion or a corresponding TTTG value more than 15 to 20 mm will bring the necessity of a distal osteotomy into serious consideration. In many cases, the presence of both scenarios may require a combined proximal and distal approach. Normal TTTG values, an increased lateral patellar glide, and a history of recurrent patellar dislocations following trauma suggest MPFL incompetence and the need for reconstruction.
TREATMENT
Arthroscopic Technique
Graft Selection
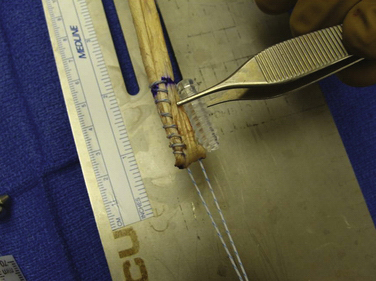
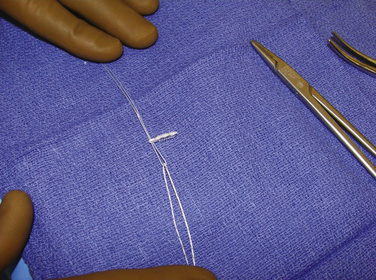
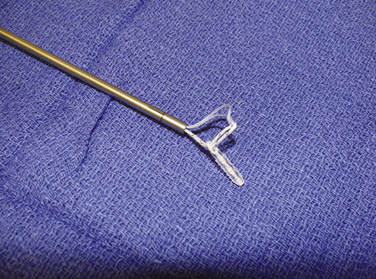
We have used semitendinosus autograft in the past, but it is our current preference to use a semitendinosus allograft. Of note, in petite patients a gracilis tendon is often sufficient. We have found that the allograft performs just as well in this richly vascular, extra-articular environment. If an autograft is used, this is harvested though a separate incision over the pes anserine insertion. This tendon far exceeds the 200 N associated with native MPFL rupture in cadaver models; the doubled graft requires approximately 2000 N for rupture. It is also an order of magnitude stronger (150 N/ mm) than the average stiffness of the MPFL (12 N/mm).10,17 The advantages of such a stiff graft include the resistance to stretching out over time and the ability to perform long term in high-demand situations, such as with trochlear dysplasia. The main disadvantage, however, is that it can place high compressive force on the patella if placed improperly or overtensioned. The graft is first doubled on itself on a standard graft preparation board. Next, a running baseball stitch (using no. 2 FiberWire [Arthrex, Naples, Fla] or comparable suture) for a distance of 25 mm is performed at each free end, creating a Y-shaped graft (Fig. 11-3). The doubled end is sutured for a distance of 25 mm and then sized (Fig. 11-4).
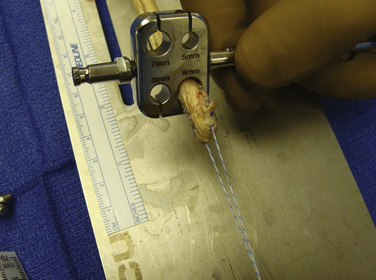
FIGURE 11-4 Only the first 3 cm of the doubled end of the tendon site needs to be sized after it has been
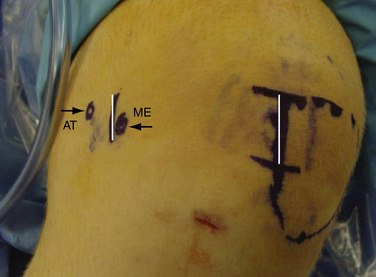
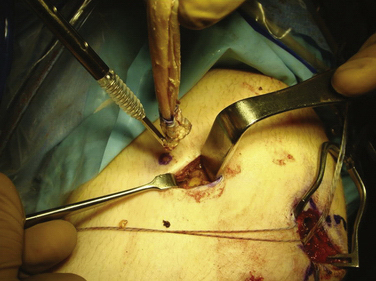
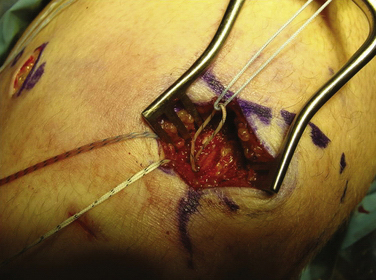
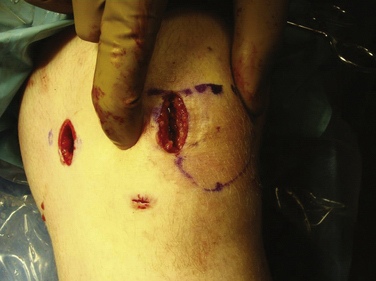
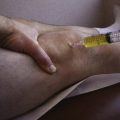
Arthroscopy is routinely carried out, assessing and addressing any chondral lesions and concomitant pathology. Lateral release is performed on an individual basis and can be done arthroscopically. A two-incision approach is used; however, one long medial parapatellar incision could be used if a concomitant procedure such as cartilage restoration of the patella or trochlea is simultaneously performed. The first 3-cm longitudinal incision is made along the proximal medial border of the patella. Dissection is continued down subperiosteally along the proximal half of the medial patella border to the interval between layers 2 and 3 (between the MPFL and capsular layer; Fig. 11-5). This interval is bluntly developed medially toward the medial epicondyle using a curved Kelly clamp. The graft should always be placed extra-articularly (superficial) adjacent to the capsule. The second incision is made over the tip of the clamp as it overlies the saddle between the epicondyle and adductor tubercle (Figs. 11-6 and 11-7). The femoral attachment of the MPFL is identified using the landmarks of the medial epicondyle, medial collateral ligament (MCL), and adductor tubercle. The femoral attachment of the MPFL resides in the saddle between the adductor tubercle and medial epicondyle. The fascia is incised and a 2.4-mm guide pin (Bio-Tenodesis fixation set; Arthrex) is placed just proximal and posterior to the epicondyle and distal and anterior to the adductor tubercle. A C arm can be helpful for identifying the correct pin placement properly, also known as Schöttle’s point (Fig. 11-8).18 A no. 2 suture is wrapped around this guide pin; the free ends are passed to the patellar incision with the clamp through the same MPFL–capsular interval layer and sutured into the patellar attachment site of the MPFL, which is along the proximal half of the medial patella. Note that the arms of the suture are at the proximal and distal extent of the patellar MPFL attachment footprint (Fig. 11-9). The suture is tied with all slackness removed, with the knee at 30 degrees of flexion. The knee is then placed through range of motion. The suture should become lax with increasing flexion and minimally change or slightly tighten in terminal extension (Fig. 11-10). The planned attachments sites and MPFL length should not overconstrain, overtension, or tilt the patella medially at any point during full range of motion.
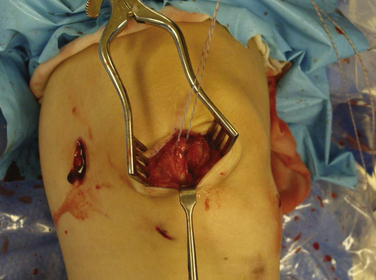
FIGURE 11-5 Dissection is carried down between layers 2 and 3 as a tag suture is placed in layers 1 and 2. sutured.
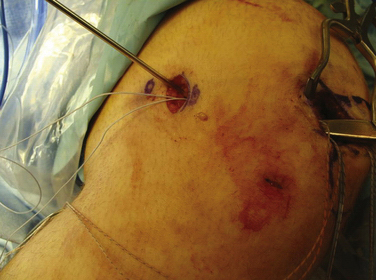
FIGURE 11-8 If the first guide pin placement is incorrect, it can be left as a reference while another pin is drilled.
The key to this operation is carefully determining the proper femoral attachment point.18 The two factors most critical for preventing increased patellofemoral contact forces are determining the proper fixation point and avoiding graft overtensioning. The location of the femoral attachment site alters the graft distances (and therefore the graft tension) through knee range of motion much more than the patellar site as a result of the cam shape of the medial femoral condyle. This means that there is no true isometric point but rather an anisometric point, where the graft can have proper changes of length through a range of motion.14 Even slight variations in position of the femoral attachment site can have major implications on the patellar tracking and contact forces.19 If the suture does not exhibit the desired length changes during range of motion, the pin in the epicondyle must be repositioned. Usually, if there is excessive tightening in extension, the graft is too distal or posterior, and, if there is increased tension in flexion, the femoral point is too proximal or anterior. Distal-proximal variation has the greatest effect. Overconstraining not only causes abnormal contact forces on the medial facet of the patella, but also makes it more difficult to regain flexion in the postoperative rehabilitation. Our ultimate goal is to re-create a passive restraint to excessive lateral patellar displacement, specifically in extension and the early phases of flexion. It should re-create normal anatomy and therefore allow a normal degree of lateral patellar mobility, as compared with the contralateral knee. The graft should not be used forcefully to pull the patella medially, nor should it tighten in flexion when the patella engages the trochlea; This could result in loss of motion and markedly increased pressures on the medial facet.
Femoral Socket Preparation
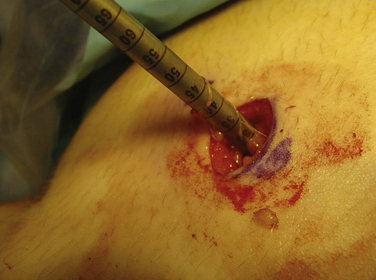
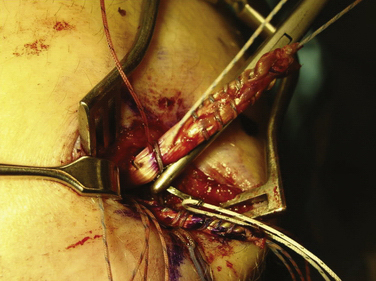
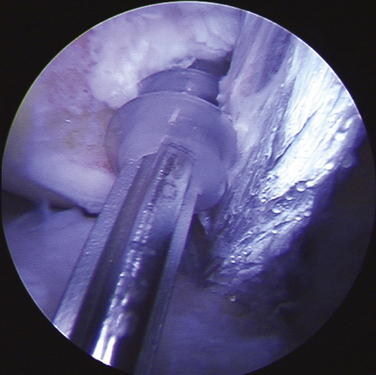
When the appropriate femoral attachment site has been determined, a cannulated drill bit from the Bio-Tenodesis system is chosen that is 0.5 to 1 mm larger than the size of the doubled sutured end of the graft. The femoral socket is then drilled to a depth 2 mm longer than the designated screw size length, usually 25 mm (Fig. 11-11). In most cases, a 7- × 23-mm or 8- × 23-mm Bio-Tenodesis screw is used. The appropriately sized screw is loaded onto the Bio-Tenodesis driver. A no. 2 FiberWire suture loop or no. 2 FiberWire suture snare is passed through the center cannulation of the driver tip. The sutures from the graft are placed through the suture loop. The sutures extending from the graft are then pulled up into the center cannulation of the driver and secured as they exit the Bio-Tenodesis driver handle. The Bio-Tenodesis screwdriver tip (Bio-Tenodesis fixation set) is then advanced into the socket, pulling the graft into the socket with the screw in the posterior aspect of the socket and the tendon placed anteriorly (Fig. 11-12). The screw is then advanced into the socket until it is flush to the cortical bone rim. This technique allows for fixation into a closed socket, without requiring drilling through the opposite cortex.
Graft Length Selection and Patellar Fixation
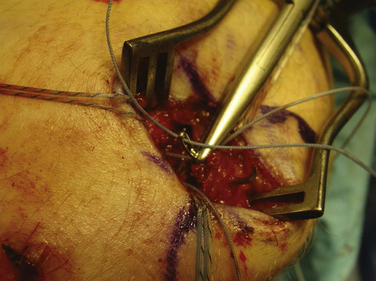
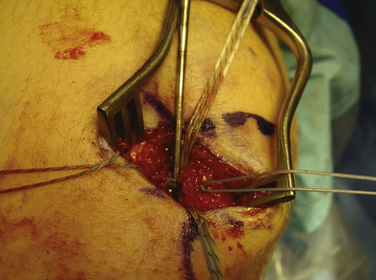
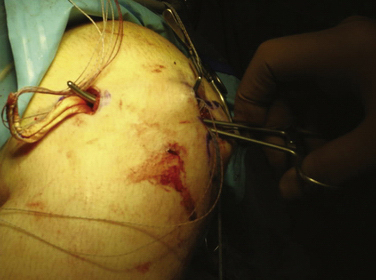
We currently use a Bio-Suture Tak (Arthrex) reverse loop technique. Two doubly loaded 2.9- or 3.7-mm Bio-Suture Tak anchors are reloaded in a loop-to-loop fashion with the FiberWire loop end rerouted through the suture anchor loop. This technique allows an easier method to fine tune graft tension and increase the surface area of tendon to bone contact. It involves removing the anchor from the insertion handle and removing both sutures from the eyelet. One of the sutures from the anchor is doubled on itself and the looped end is rethreaded through the eyelet. The second suture from the anchor is then passed through the loop of the first suture. It does not need to be rethreaded through the eyelet; it is captured in the loop of the first suture (Fig. 11-13). The four tails of the sutures are then passed back down the insertion handle using a passing wire so that the anchor can be reloaded onto the handle (Fig. 11-14). The suture anchors are placed in a trough, created with a small rongeur, in the medial edge of the patella (from the midwaist, superiorly) anterior to the articular cartilage, approximately 2 cm apart (Fig. 11-15). The two free arms of the allograft tendon are brought through the developed soft tissue tunnel interval from the femoral attachment to the patella (Fig. 11-16). After pulling the sutures and graft tails through the soft tissue interval, the two free ends of the graft are pulled through the anchor suture loops (Fig. 11-17). One tail of the Y graft is through the midwaist loop and the other tail is through the proximal loop. The secondary suture loops from each anchor are then removed. The suture loops are temporarily cinched tight around the graft and held with hemostats (Fig. 11-18). The initial graft length is set at approximately 30 degrees of flexion, with both grafts pulled to length with slight tension and no laxity. The knee should be brought through a full range of motion, with the graft only slightly tightening between 30 and 0 degrees and becoming progressively lax in flexion. The graft tension should allow for identical lateral patellar translation as compared with the contralateral knee, or 1+ to 2+ lateral translation of the patella at 0 and 30 degrees of knee flexion if the other side is affected (Fig. 11-19).
FIGURE 11-15 Trough being created with the rongeur. Another option is to use a light burring technique.
PEARLS& PITFALLS
PEARLS
PITFALLS
CONCLUSIONS
Although reconstruction of the medial patellofemoral ligament prevents recurrent dislocation of the patella, the biomechanics of the medial patellofemoral restraints are still not fully understood. Specifically, the role of the medial patellar meniscal ligament (MPML) and medial patellar tibial ligament (MPTL) are not completely understood. It has been shown that they are less important than the MPFL, but they may still have a role in patellar stability. The interaction of patella alta and trochlear dysplasia with MPFL reconstruction are other areas that warrant further investigation. Also, other authors have suggested alternative techniques for reconstruction. Instead of the reverse-loaded anchors, drill holes in the patella can be used for fixation,20 the graft can be fixed into a closed patellar socket,14 MPFL and MTFL ligaments can be reconstructed concurrently,21 and other grafts such as the adductor longus can be used.22
1. Hauser EW. Total tendon transplant for slipping patella. Surg Gynecol Obstet. 1938;66:199.
2. Insall J, Falvo KA, Wise DW. Chondromalacia patella. J Bone Joint Surg Am. 1976;58:1-8.
3. Bonvallent JM. Resultats de la reflection a la peau de l’aileron rotulien interne dans les luxatins recidivantes de la rotule. Chirurgie. 1973;99:124-128.
4. Davis DK, Fithian DC. Techniques of medial retinacular repair and reconstruction. Clin Orthop. 2002;402:38-52.
5. Fulkerson JP. Disorders of the Patellofemoral Joint, 3rd ed. Baltimore: Williams and Wilkins; 1997.
6. Nomura E, Fujikawa T, et al. Anatomical study of the medial patellofemoral ligament. Orthop Surg Suppl. 1992;22:2-5.
7. Ficat P. Pathologie Femoror-Patellaire. Paris: Masson; 1970.
8. Baker RH, Carroll N, Dewar P, Hall J. Semitendinosus tenodesis for recurrent dislocation of the patella. J Bone Joint Surg Am Br. 1972;(54):103-109.
9. Amis, AA, Firer P, Mountney J, et al. Anatomy and biomechanics of the medial patellofemoral ligament. Knee. 2003;10:215-220.
10. Conlan T, Garth WPJr, Lemons JE. Evaluation of the medial soft tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75:682-693.
11. Burks RT, Desio SM, Bachus KN, et al. Biomechanical evaluation of lateral patellar dislocations. Am J Knee Surg. 1998;11:24-31.
12. Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26:59-65.
13. Hautamaa PV, Fithian DC, Kaufman KR, et al. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
14. Farr J, Schepsis AA. Reconstruction of the medial patellofemoral ligament for recurrent patellar instability. J Knee Surg. 2006;19:307-316.
15. Grelsamer RP, Proctor CS, Bazos AN. Evaluation of patellar shape in the sagittal plane. Am J Sports Med. 1994;22:61-66. A clinical analysis
16. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2:19-26.
17. Hamner DL, Brown CHJr, Steiner ME, et al. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am. 1999;81:549-557.
18. Steensen RN, Dopirak RM, McDonald WG 3rd. The anatomy and isometry of the medial patellofemoral ligament: implications for reconstruction. Am J Sports Med. 2004;32:1509-1513.
19. Elias JJ, Cosgarea AJ. Tension in a reconstructed MPFL could overload medial patellofemoral cartilage: a computational analysis. Am J Sports Med. 2006;34:1478-1485.
20. Fithian DC, Paxton EW. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32:1114-1121.
21. Drez DJr, Edwards TB. Results of medial patellofemoral ligament reconstruction and treatment of patellar dislocation. Arthroscopy. 2001;17:298-306.
22. Teitge RA, Spak RT. Lateral patellofemoral ligament reconstruction. Arthroscopy. 2004;20:998-1002.