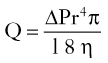Reconstruction of the Eyelids
Physiology of the Ocular Anterior Segment
Interruption of tear drainage from damage to the lacrimal outflow system is common after eyelid reconstruction. The lacrimal puncta are normally in contact with the ocular surface and eversion or ectropion may occur, especially with lower eyelid reconstruction. The lacrimal canaliculi connect the puncta with the lacrimal sac, located posterior to the palpable anterior limb of the medial canthal tendon. The upper and lower canaliculi are encased in fibers of the orbicularis oculi muscle, which insert onto the lateral wall of the lacrimal sac. Contraction of the orbicularis oculi during blinking pumps the tears into the lacrimal sac. From there, the tears drain to the inferior nasal meatus via the nasolacrimal duct through the maxilla. Surgery in the region of the medial canthus frequently results in epiphora caused by injury to the canaliculi, the lacrimal sac, or the orbicularis oculi fibers that form the lacrimal pump. Primary repair of the canaliculi is obligatory when trauma or tumor excision results in their interruption.
Surgical Anatomy of the Eyelids
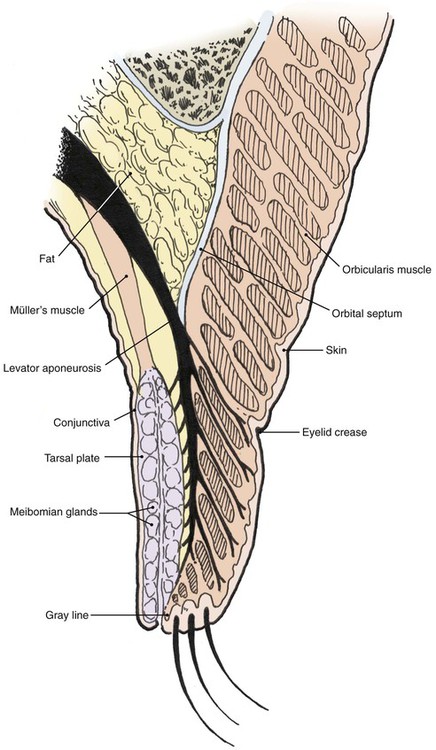
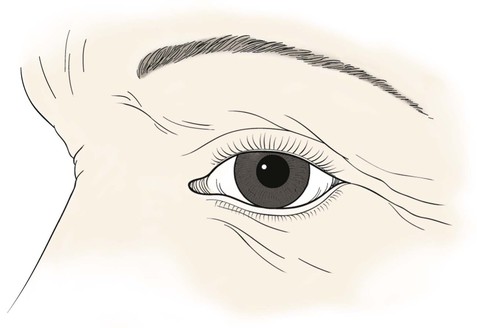
The multilaminar structure of the eyelids varies according to distance from the palpebral fissure. For the upper eyelid below the lid crease, the layers include the epidermal skin with minimal dermis, orbicularis oculi muscle, levator aponeurosis, tarsus, and conjunctiva. Above the lid crease, the layers include skin, orbicularis oculi, orbital septum, orbital fat, levator aponeurosis, Müller’s muscle, and conjunctiva (Fig. 17-1).
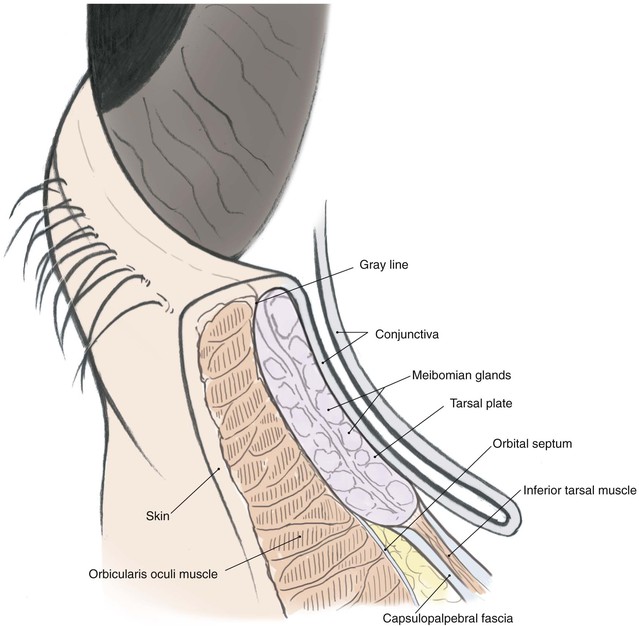
The lower eyelid has similar structures, except that the retractor (analogous to the levator muscle) is the capsulopalpebral fascia. This fascia is an extension of the inferior rectus muscle sheath that inserts at the inferior border of the lower eyelid tarsus and causes passive downward movement of the lower eyelid in downward gaze. A sympathetically innervated inferior tarsal muscle analogous to Müller’s muscle is also present in most individuals (Fig. 17-2).
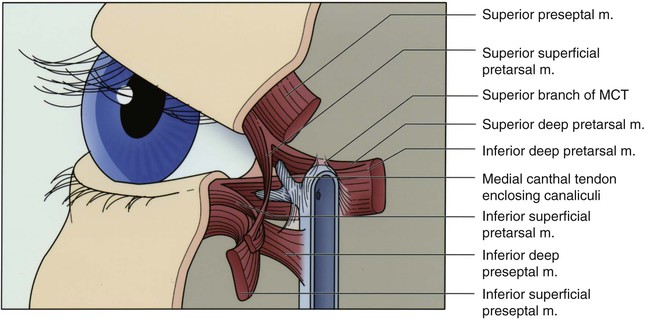
The lateral canthal tendon anchors the tarsal plates posteriorly and superolaterally to Whitnall’s tubercle inside the orbital rim. The lateral eyelids are thus kept snugly against the globe in all positions of gaze. Medially, the medial canthal tendon is the centerpiece of medial canthal anatomy. The medial canthal tendon has an elastic lateral portion that supports the lacrimal canaliculi and then splits into anterior, superior, and posterior limbs, all of which blend with the lacrimal sac fascia.1 The thick anterior limb inserts on the maxillary bone, and the superficial preseptal orbicularis muscle fibers insert on the anterior limb (Fig. 17-3). The superior branch extends to the lacrimal sac apex and covers the anterosuperior portion of the lacrimal sac.2 The thin posterior limb forms the anterior fascia of the pretarsal orbicularis muscle and inserts on the posterior lacrimal crest formed by the lacrimal bone. It acts as a horizontal supporting band that posteriorly directs forces generated by the pretarsal muscle fibers. The lateral portion of the medial canthal tendon invests the fragile lacrimal canaliculi. Reconstruction at either canthus must re-create the deep attachments of the canthal tendons inside the orbit to avoid symptomatic eyelid malpositions.
The tarsal plates are composed of dense connective tissue and house the meibomian glands that produce oil for the tears. The upper eyelid tarsus is 10- to 12-mm in height and tapers medially and laterally. It is 16- to 20-mm in length and approximately 1-mm thick. The lower eyelid tarsus is 4- to 5-mm in height but similar in length and thickness to the upper tarsus. Both are anchored medially and laterally to the orbital rim by the canthal tendons, as described before. The posterior surface in each case is lined with densely adherent conjunctiva.
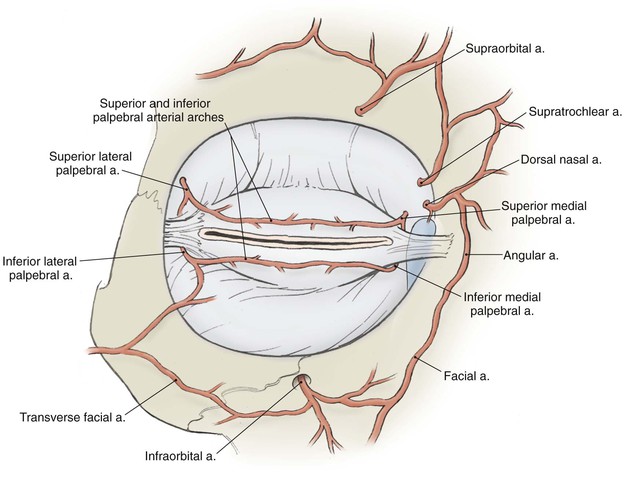
The vascular supply to the region comes from the internal and external carotid systems. Branches from the ophthalmic artery in the posterior orbit pass forward as the anterior ciliary arteries and contribute to the superior and inferior tarsal arcades in the eyelids (Fig. 17-4). Branches from the facial artery supply the medial and lateral canthus and also contribute to the eyelid vascular arcades. Risk of eyelid vascular compromise exists if the arteries are interrupted both medially and laterally.
Anesthesia
Topical tetracaine 1% or proparacaine 0.5% eye drops are used to anesthetize the ocular surface and are repeated as needed for patient comfort. Frequent lubrication of the cornea with ophthalmic petrolatum ointment or hydroxypropyl cellulose eye drops will also improve patient comfort during and after surgery.
Reconstructive Objectives
1. Nonkeratinizing mucosal epithelium to line the inside of the reconstructed eyelid to protect and to wet the cornea
2. Rotationally stable eyelid margin with a mucocutaneous junction to protect the eye from skin, lanugo hairs, and lashes
3. Posterior apposition to the globe in all areas of the reconstructed eyelid
4. Moderately flexible, firm connective tissue frame to provide support and shape for the eyelid
5. Adequate protractor muscle to close the eyelids, to provide a normal blink, and to assist in posterior apposition of the eyelid to the eye
6. Lack of orbital septal contracture or attachments that restrict eyelid excursion
7. Supple, thin skin that permits normal eyelid excursion
8. Adequate levator muscle function to provide upper lid clearance of the pupil
9. Appropriate height and shape of the reconstructed eyelid and the medial and lateral canthi to maintain symmetry with the contralateral side
The single most useful reconstructive strategy to achieve most of these objectives is conversion of vertical surgical tension to horizontal tension in the eyelids whenever possible.
Surgical planning is facilitated by conceptually dividing the eyelids into anterior and posterior lamellae. The anterior lamella is composed of the skin and the orbicularis muscle; the posterior lamella is composed of the conjunctiva, tarsus, and eyelid retractors. For full-thickness defects, both lamellae usually require reconstruction. In these cases, at least one of the reconstructed lamellae typically must include a blood supply. Commonly used, reliable surgical techniques are listed in Tables 17-1 and 17-2 and involve various flaps and grafts. Selection will depend on the size, location, depth, and configuration of the defect. Some superficial defects may require only reconstruction of the anterior lamella, and choice of flap or graft is primarily dependent on the need to restore a mobile, cosmetically satisfactory eyelid or to restore normal canthal position. When necessary, reconstruction of the lacrimal tear drainage system is accomplished concomitantly.
TABLE 17-1
Algorithm for Full-Thickness Lower Eyelid Reconstruction
| Size of Eyelid Margin Defect (eyelid width) | Repair |
| <25% | Direct closure |
| 25%-50% | Direct closure with lateral cantholysis |
| 25%-80% | Tarsoconjunctival graft and skin-muscle flap |
| 33%-66% | Semicircular flap |
| 50%-75% | Semicircular flap with periosteal flap |
| 50%-100% | Tarsoconjunctival flap and skin graft |
Algorithm for Full-Thickness Upper Eyelid Reconstruction
| Size of Eyelid Margin Defect (eyelid width) | Repair |
| <25% | Direct closure |
| 25%-50% | Direct closure with lateral cantholysis |
| 25%-50% | Tarsal rotation flap and skin-muscle flap or skin graft |
| 25%-75% | Tarsoconjunctival graft and skin-muscle flap |
| 33%-66% | Semicircular flap with periosteal flap |
| 50%-100% | Cutler-Beard flap |
Relaxed Skin Tension Lines
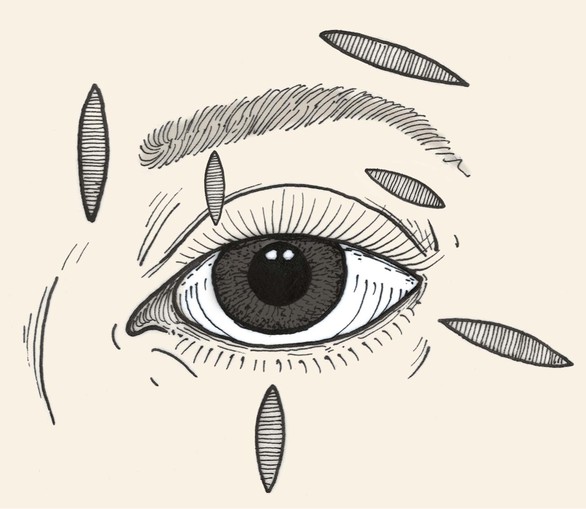
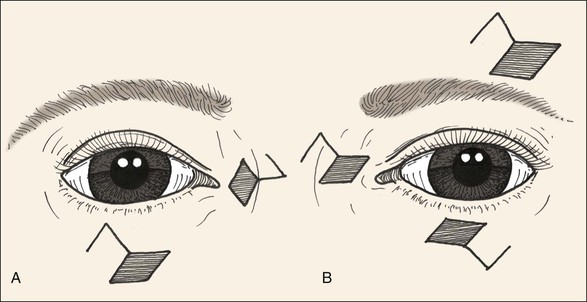
Relaxed skin tension lines (RSTLs) are a generally useful guide for the reconstructive surgeon in the attempt to minimize cutaneous scars.3 Around the eyes, these lines follow the lines of facial expression (Fig. 17-5) and are oriented horizontally in the upper and lower eyelid skin. Unfortunately, vertical tension created by closure of elliptical defects oriented along RSTLs in the eyelids has a substantial risk of creating iatrogenic cicatricial eyelid malpositions, including retraction and ectropion. Asymptomatic laxity in the medial and lateral canthal tendons, commonly present in the elderly, will permit downward migration of the lower lids. Similarly, ineffective senescent or neuropathic orbicularis muscle tone will predispose patients to lower eyelid ectropion or upper eyelid retraction and lagophthalmos. For these reasons, horizontal tension is usually preferred for closure of eyelid wounds, and the long axis of any ellipse will be perpendicular to the eyelid margin (Fig. 17-6). Excessive dermatochalasis in the upper eyelid that would otherwise require blepharoplasty may permit the use of horizontally oriented ellipses in the lid crease for some individuals. Conversely, for the glabella, medial canthus, eyebrows, and lateral canthus, use of RSTLs for wound orientation will produce satisfactory outcomes (see Fig. 17-6).
Local Skin Flaps for Superficial Defects
The simple ellipse is often used for excision of small facial lesions, including those in the periocular area. Whereas the long axis of such an ellipse is usually oriented parallel to RSTLs, ellipses are oriented perpendicular to RSTLs in the eyelids to minimize vertical tension and the risk of cicatricial ectropion. Because the ellipse sacrifices up to 160% of the surface area of the excised lesion of interest, modified ellipses such as the double S ellipse and O-Z plasty can be used to conserve normal tissue (Fig. 17-7).
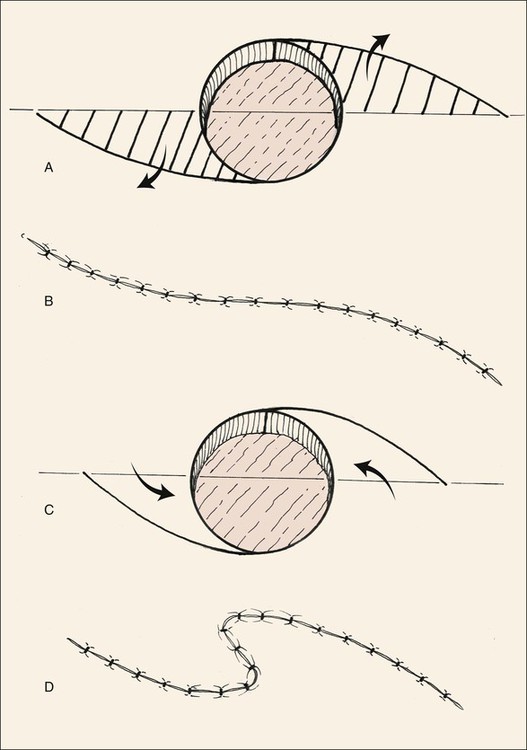
FIGURE 17-7 A, S-shaped ellipse in which alternate halves of ellipse are excised (lined area), sacrificing less normal tissue. B, Wound is closed in O-to-S plasty. C, O-to-Z plasty. Curved incisions are made from opposite tangents of circular defect. D, Wound closure assumes Z configuration.
Local skin flaps are preferred to free skin grafts for reconstruction of anterior lamellar defects in the periocular region for many reasons.4 Adjacent skin usually provides a better texture and color match than with grafts from distant sites, and flaps undergo substantially less contraction during healing. In addition, the rich vascular supply in the region permits the use of flaps that would be more tenuous in other parts of the body. Furthermore, the ample vascular supply of local flaps can be used to support free grafts for posterior lamellar reconstruction. Prior irradiation or multiple prior surgeries may reduce local circulation.
Rhomboid transposition flaps are the most useful adjacent skin flap in the periocular area, especially for medial5 and lateral canthal defects. The principles of design can be found in Chapter 11 of this text. Specific to this region is the need to avoid distortion of the eyebrow, eyelid crease, eyelid margin, and canthal angles. Common flap designs are shown in Figure 17-8. The vector of maximal wound closure tension must be parallel to the lid margin in the lower lid and most commonly in the upper lid as well. Maximal tension may be vertical in the lateral and medial canthal regions, provided the canthal position is not changed.
Other advancement and transposition flaps can be used to repair certain anterior lamellar defects (Figs. 17-9 to 17-12). Among those commonly used are the standard unipedicle and bipedicle rectangle-shaped advancement flaps and V-Y and Y-V advancement flaps. The unipedicle rectangular advancement flap is well suited to the region and can be used to close defects up to 25 cm2. Cutaneous defects of the eyebrow or the anterior lamella of the medial upper and lower eyelids can be repaired with this flap. The resulting scars fall into or parallel to the lid creases and avoid injury to the lid margin. V-Y advancement flaps can be used to lengthen the palpebral fissure or to close donor sites.6 Y-V advancement flaps are useful in the management of epicanthal folds7 and scar contractures.
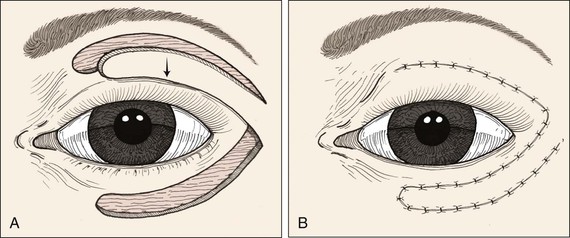
FIGURE 17-9 Transposition flap used to repair anterior lamellar defect of lower eyelid. Flap can be transferred as cutaneous or musculocutaneous flap.
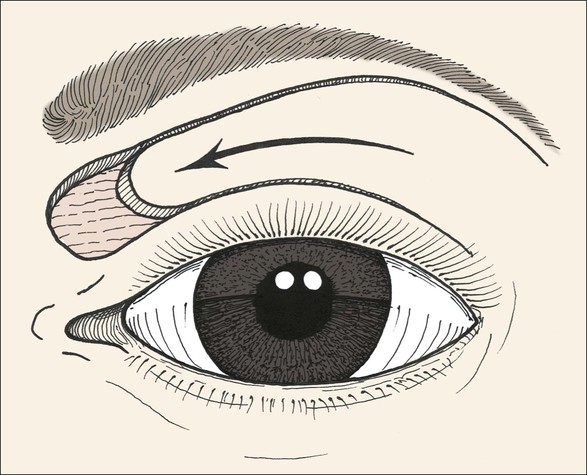
FIGURE 17-11 Musculocutaneous advancement flap used to repair medial upper eyelid defect. When it is combined with lower eyelid and cheek advancement flaps, large medial canthal skin defects can be successfully repaired.
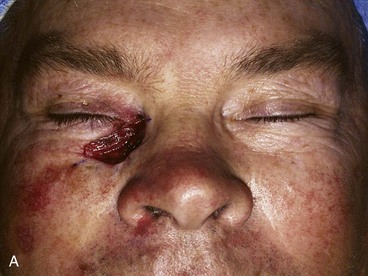
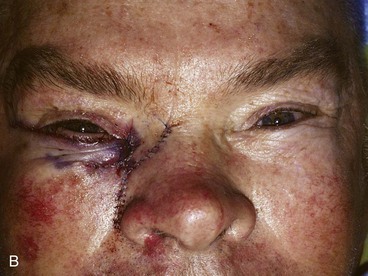
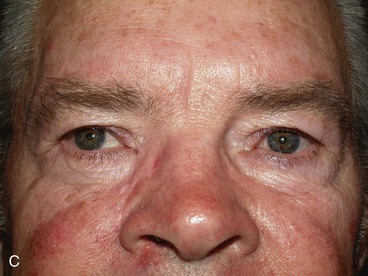
FIGURE 17-12 Eyelid defect repaired with anterior lamella flaps. A, Lower eyelid and medial canthal defect. B, Defect closed with medial canthopexy to reinforce medial canthal tendon, rhombic flap from nose, and advancement flap from melolabial fold. C, Postoperative view at 1 year. Note maintenance of lower eyelid height.
Healing by second intention is generally not an accepted strategy for eyelid wounds because the incidence of cicatricial ectropion is substantial. However, in the medial canthus, healing by second intention (or laissez-faire) is more often successful,8,9 especially if the defect is 1 cm or less in diameter and centered between the upper and lower eyelids.
During surgery to reconstruct superficial lower eyelid defects, if significant lower eyelid retraction or ectropion occurs, it is advisable to perform concomitant lateral canthoplasty to avoid postoperative eyelid malposition. Many canthoplasty methods have been described; however, simple horizontal tightening of the lateral canthal tendon with the lateral tarsal strip procedure10 is reliable and should be mastered by reconstructive surgeons working in this area.
Primary Closure of Full-Thickness Defects
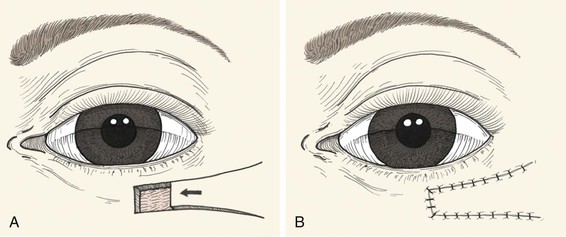
Excision of eyelid scars and neoplasia as well as repair of colobomas frequently requires reconstruction of a full-thickness defect. In the simplest case, horizontal laxity of the eyelid and canthal tendons will permit direct closure under reasonable tension. This is usually possible with defects that involve 33% or less of the horizontal length of the eyelid, although some authors find it useful in up to 50% of the horizontal length.11 A few additional millimeters can be gained with concomitant lateral canthotomy, although this may result in inferior displacement of the reconstructed lower eyelid. The advantage of primary wound closure is preservation of the eyelid margin and lashes in a single-stage procedure. As with repair of other multilaminar structures, the primary strategy is to restore tissue integrity by matching corresponding layers. Creation of (or conversion to) a pentagonal defect greatly eases this repair by eliminating standing cutaneous deformities. Establishing clean tarsal edges perpendicular to the lid margin ensures re-creation of reasonably normal eyelid contour without notching or misalignment.
The first suture must establish perfect vertical alignment of the eyelid margin and should be replaced unless it is ideal. It is placed to approximate the tarsal edges just peripheral to the eyelid margin, at 90% depth, using a trapezoidal passage that places the knot farther from the margin than the deep suture pass (Fig. 17-13). A 5-0 or 6-0 polyglactin suture on an S-14 spatula needle is preferred, although Monocryl and polydioxanone sutures may also be used. Chromic and plain gut sutures dissolve too quickly to be useful for this deep closure. After the initial suture placement, additional interrupted sutures are placed to completely approximate the tarsus. The eyelid retractors (levator aponeurosis or capsulopalpebral fascia) are repaired similarly, avoiding full-thickness suture passage that will later abrade the cornea.
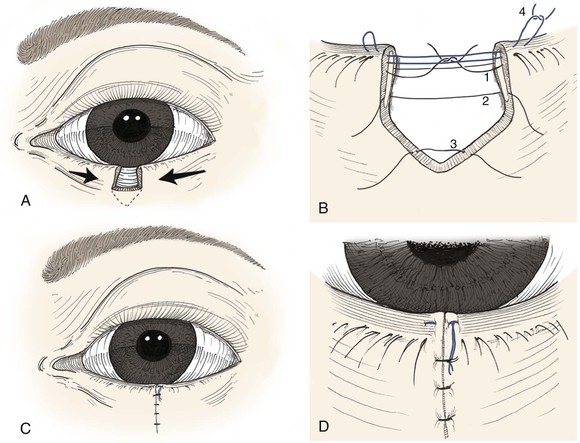
FIGURE 17-13 A, Full-thickness defect of eyelid. Lateral cantholysis may be performed to facilitate primary wound closure. B, To avoid corneal irritation, sutures are passed through anterior two-thirds of tarsal plate (1, 2) and capsulopalpebral fascia/levator (3). Precise approximation of eyelid margin is achieved with a vertical mattress suture (4). C, The mattress suture ends are incorporated into the skin closure to avoid corneal irritation. D, Detail view.
Once perfect vertical alignment has been established, anteroposterior alignment of lid margin structures must also be restored. Eversion of the eyelid margin wound is necessary to avoid notching and is accomplished during this phase of tarsal repair. The most consistently successful technique is to place a vertical mattress suture at the gray line, tied to create significant eversion. A 4-0 silk suture on a G-1 or G-3 needle is well suited to this application, and once tied, the ends are left long and incorporated into the skin closure to avoid corneal irritation (Figs. 17-13 and 17-14). Alternatively, 6-0 silk interrupted sutures can be placed at the gray line and the lash line to create wound eversion.
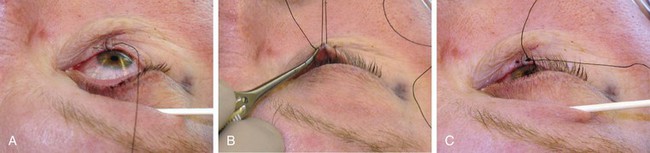
FIGURE 17-14 Intraoperative photographs of full-thickness defect repair. A, Tarsus repaired with 6-0 polyglactin, and eyelid margin alignment established with first pass of 4-0 silk. B, Second pass of silk vertical mattress suture facilitated by upward tension on free ends of first pass. C, Mattress suture tied with tension to cause mild eversion.
Final closure of the skin will typically result in a vertical scar. Suture approximation can be accomplished with any 6-0 material; pleasing results have been obtained with nylon, polypropylene, silk, and even polyglactin. Arrowhead flap closure of the skin defect may result in a shorter scar12 and is used when possible. Postoperative dressing consists only of erythromycin ophthalmic ointment, preferred for its low toxicity and allergenicity. Ointments or eye drops that contain steroids should be avoided. Removal of the vertical mattress silk suture at the lid margin is delayed for 10 to 14 postoperative days. Skin sutures may be removed earlier if desired. Examples of primary eyelid repair with the suture techniques discussed are seen in Figure 17-15.
Semicircular Flap Reconstruction of Larger Full-Thickness Defects
The Tenzel semicircular advancement flap is a useful technique in eyelid reconstruction and is well suited to close full-thickness defects of the eyelid that encompass 33% to 75% of the horizontal length of the lower eyelid and 30% to 66% of the upper eyelid. Initially described by Richard Tenzel in 1978,13 this flap is conceptually similar to the classic Mustarde cheek rotation flap14 for eyelid reconstruction but provides better dynamic reconstruction of the eyelid lamellae with considerably less tissue rearrangement. There are several variations in the technique that can be chosen on the basis of individual patient characteristics. In each case, the anterior lamella (skin and orbicularis muscle) and posterior lamella (tarsus and conjunctiva) of the eyelid are addressed in a single-stage operation (Fig. 17-16).
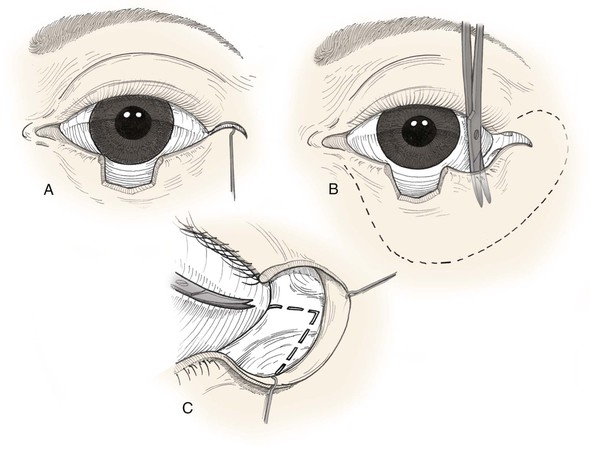
FIGURE 17-16 A, Tenzel flap incised. B, Flap consisting of skin and orbicularis muscle is elevated and widely undermined. C, Lateral canthotomy and cantholysis (broken line) are performed. Eyelid defect is then repaired as in primary wound closure.
Indications for use of the Tenzel flap include full-thickness central or lateral eyelid defects of 30% to 75% of the length of the original lower eyelid margin and 30% to 66% of the upper eyelid margin. Such defects are commonly a result of trauma, micrographic surgery for skin cancer, or congenital eyelid coloboma. The Tenzel flap is most useful when there is adequate lateral canthal skin laxity and availability of at least a small segment of full-thickness eyelid on both sides of the defect. This flap can be used when there is no tarsus on the lateral side of the defect, but optimal results will require a periosteal flap or a posterior lamellar graft such as hard palate mucosa or auricular cartilage (see later). In cases with a history of previous irradiation or burn, the Tenzel advancement flap is preferred to grafting as the flap provides its own blood supply. In general, the final postoperative appearance of the flap is superior to reconstruction with a skin graft or the larger Mustarde flap.
Reconstruction of the lower eyelid by the Tenzel flap is as follows. Beginning exactly at the lateral canthus, a semicircular superior arching line is drawn with a diameter of approximately 20-mm extending toward the level of the lateral eyebrow line (Figs. 17-16 and 17-17). For upper eyelid reconstruction, the mirror image is used. Next, the tarsal edges are freshened as necessary so that they are perfectly perpendicular to the lid margin, sacrificing as little tissue as possible. A Bard-Parker No. 15 blade is used for the initial incision and dissection of the musculocutaneous flap. Limited electrodissection with a sharp tungsten needle may also be used, with the generator set on minimum blend. A lateral canthotomy incision is made beneath the musculocutaneous flap, and the inferior ramus of the lateral canthal tendon is cut to completely mobilize the lateral lower eyelid segment. The musculocutaneous flap is thoroughly undermined and advanced medially so that the lateral lid tissue is advanced to the medial edge of the defect. In most cases, there is adequate skin laxity in the lateral canthal/temporal region for development of a Tenzel flap, but increased tissue advancement can occasionally be gained with rapid intraoperative tissue expansion with a Foley catheter placed beneath the skin of the temple. Two 5-minute cycles of skin expansion are used before flap advancement.15
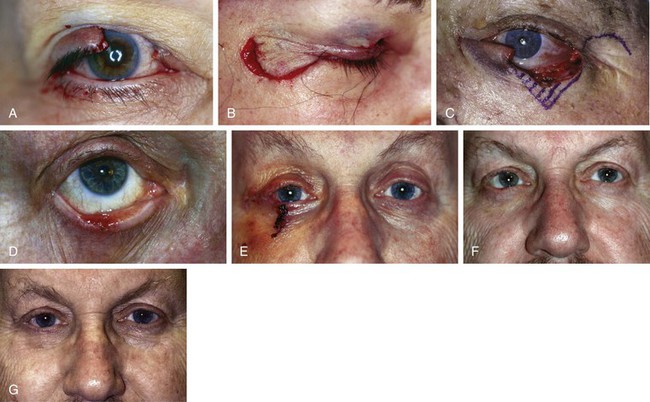
FIGURE 17-17 Tenzel semicircular flaps. A, Upper eyelid defect. B, Defect shown in A closed with Tenzel flap. C, Full-thickness lower eyelid defect. Tenzel flap designed for repair. D, Right lower eyelid squamous cell carcinoma. E, Same patient as shown in D 1 week after repair with Tenzel flap. F, G, Same patient as shown in D. Preoperative and 1-year postoperative views.
Lid margin sutures are placed in the gray line to perfectly align the lamellae of the eyelid at the wound edges. When enough full-thickness eyelid tissue is available on either side of the wound, the preferred technique is to place a vertical mattress 4-0 silk suture at the gray line for improved eversion of the superior eyelid margin. If instead one edge of the wound consists of a very small portion of full-thickness eyelid, simple interrupted 6-0 silk sutures are placed at the gray line and the lash line. In either case, to avoid corneal irritation, the ends of the sutures are left long after tying so that later they can be incorporated into a knot inferiorly on the skin.
To prevent lateral sagging of the eyelid, lateral canthal fixation of the lateral flap tissue must be performed. A 4-0 polyglactin 910 suture on a semicircular needle (e.g., P-2, ME-2, OPS-5) may be used to secure the deep surface of the flap to the inner aspect of superolateral orbital rim periosteum. However, improved eyelid contour can be obtained by developing a periosteal flap16 from the lateral orbital rim that is left hinged at the arcus marginalis and sutured to the inner aspect of the musculocutaneous flap as far medially as possible. The periosteal flap must be based high enough (at least midpupil) to provide upward pull on the lid and should be angled superiorly 45° to follow the lower eyelid contour. It should be 8- to 10-mm wide, and the length is determined by measuring the distance from the lateral orbital bony rim to the lateral end of the tarsus. If necessary, adjacent deep temporal fascia may be incorporated by extending the periosteal incisions laterally. In all cases, the goal is to re-create a tight eyelid with proper posterior contour at the lateral canthus (Fig. 17-18).
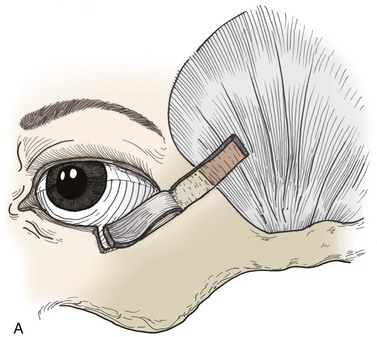
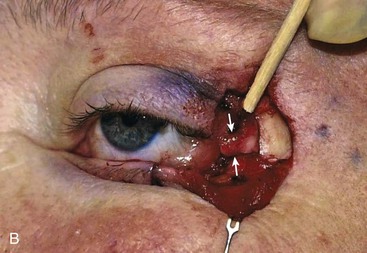
FIGURE 17-18 A, Periosteal and temporalis fascial flap used to reconstruct posterior lamella defects of temporal eyelid. Flap is angled 45° so lower eyelid contour is maintained after strip is reflected inferomedially. B, Periosteal inversion flap used to support lateral lower eyelid reconstruction. Wood stick and white arrows indicate flap elevated for repair of lower eyelid. Note that orientation of flap provides vertical support.
Wound dressing consists of erythromycin ophthalmic ointment. Cotton gauze dressing is generally not necessary but may help prevent hematoma. If it is used, it should be applied carefully as a compression patch over the closed eyelids to avoid abrading the cornea. The patch can be removed in 1 or 2 days, and antibiotic ointment is then applied to the wounds and the eye twice daily. Erythromycin ophthalmic ointment is preferred as it is the least likely to cause corneal irritation or allergic reaction.
Complications of the Tenzel flap include eyelid notching, ectropion, lateral canthal webbing, trap-door deformity, and symblepharon formation (Fig. 17-19).
Free Tarsoconjunctival Grafts for Lower Eyelid Reconstruction
A free tarsoconjunctival graft can be used to extend the posterior lamellar reconstruction accompanying an upper or lower eyelid Tenzel semicircular flap. It also is an important alternative to the semicircular flap for repair of lower eyelid defects involving 33% to 80% of the lower lid in some situations. It was initially described in 1918 and has been popularized more recently.17,18 The free tarsoconjunctival graft avoids the lateral canthal dissection and rearrangement that can rarely result in deformity of the lateral aspect of the reconstructed lower lid when the Tenzel approach is used. It has an advantage over the Hughes tarsoconjunctival flap in that occlusion of the eye is avoided. It requires the harvest of a free tarsoconjunctival graft from the upper lid to repair the posterior lamella of a lower lid defect, with concomitant repair of the anterior lamellar defect with a musculocutaneous flap. A full-thickness skin graft can be used to repair the anterior lamella, provided an orbicularis muscle flap is advanced over the free tarsoconjunctival graft.19 A musculocutaneous pedicle flap will typically result in a better color and thickness match.
The free tarsoconjunctival graft is usually harvested from the ipsilateral upper lid, although it is possible to take it from the opposite upper lid, another advantage over the Hughes and Tenzel flaps. Local anesthetic is infiltrated into the area of the recipient lower lid defect and at the donor upper lid subcutaneously and above the superior tarsal border subconjunctivally. A 4-0 silk traction suture is placed in the donor upper lid margin, and the lid is everted over a Desmarres retractor or a cotton-tipped applicator. The desired graft is always a little smaller than the size of the original defect, which can be assessed by grasping both ends of residual eyelid margin tissue and bringing them together with skin hooks or toothed forceps. The width of the tarsoconjunctival flap will be slightly less than the measured size of the defect, so that there will be adequate horizontal tension in the reconstructed eyelid after postoperative canthal tendon relaxation to prevent late ectropion or retraction. The average vertical tarsal height varies on the basis of race; for whites, it is typically 10-mm in the upper lid and 4-mm in the lower lid, whereas in Asian patients these measurements are 8-mm for the upper lid and 5-mm for the lower lid.20 The lower 4-mm of the upper tarsus (closest to the lid margin) must be preserved to prevent postoperative upper lid entropion or deformity. This works out well in most cases as only 4- to 5-mm of vertical height of the free tarsoconjunctival graft is required to replace the missing posterior lamella of the lower lid defect. Calipers and a marking pen are used to carefully outline the tarsoconjunctival graft before harvest. It is best to take the graft from the central portion of the upper lid, where the tarsus is highest, rather than to take it from the medial or lateral areas, where the tarsal height tapers. A No. 15 scalpel or No. 69 Beaver blade is used to cut through conjunctiva and tarsus inferiorly and on each end of the graft. Blunt-tipped Westcott scissors can be used to dissect the tarsoconjunctival graft away from the levator aponeurosis, which is left intact and disturbed as little as possible. Superiorly it is helpful to harvest an extra 2-mm of conjunctiva as this will help form the new lower lid margin. Müller’s muscle is transected at the superior tarsal border and dissected as little as possible. Hemostasis is achieved, and the harvest site is left to heal spontaneously (Fig. 17-20; see video).
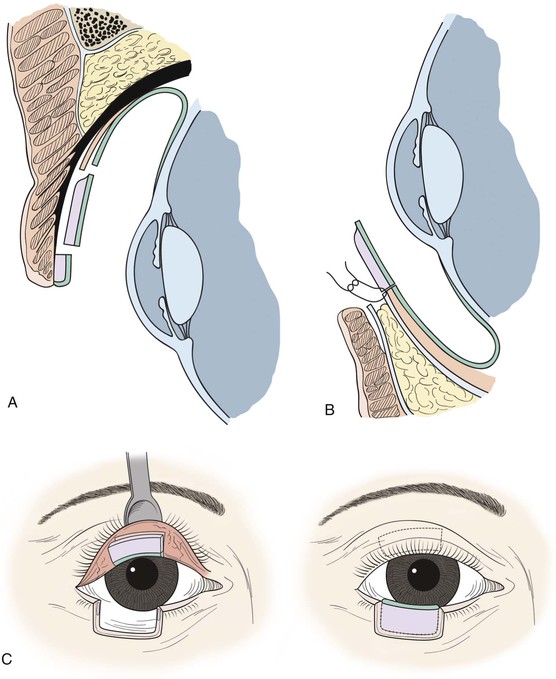
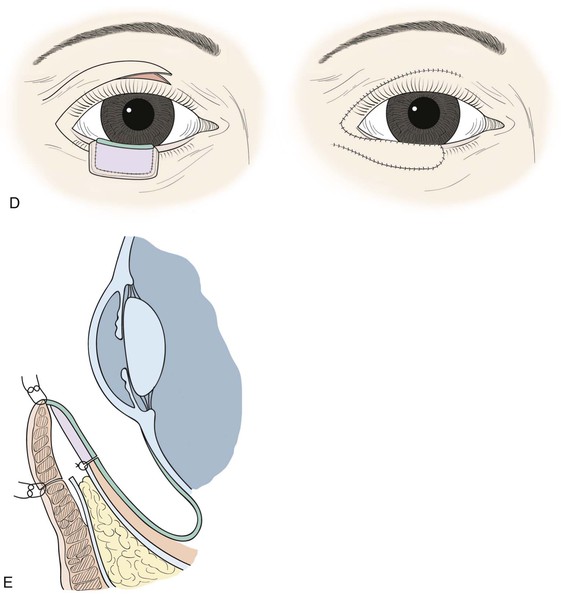
FIGURE 17-20 Lower eyelid reconstruction with autogenous tarsoconjunctival graft and local musculocutaneous transposition flap. A, Harvest of tarsoconjunctival graft from upper eyelid, sparing the inferior 4-mm of tarsus and minimally disturbing the levator aponeurosis. B, Placement of tarsoconjunctival graft at lower eyelid, where it is sutured to the capsulopalpebral fascia and conjunctiva but not the orbital septum. C, Frontal view of tarsoconjunctival graft harvest and placement to repair posterior lamellar defect; note that superior border of graft is oriented superiorly at lower eyelid. D, Local transposition flap harvested from upper eyelid and transposed to repair the lower eyelid anterior lamellar defect. E, At newly reconstructed eyelid margin, union of the transposition flap and tarsoconjunctival graft with absorbable suture.
The anterior lamella must next be reconstructed, preferably with a musculocutaneous flap. This can be harvested from the ipsilateral upper lid or sometimes advanced upward from below the defect. Musculocutaneous flaps have advantages including improved color and texture match for the reconstructed eyelid.21
Unfortunately, there are times when the defect is large and insufficient tissue remains to harvest a local flap. Full-thickness skin grafts taken from the preauricular, postauricular, supraclavicular, or upper inner arm areas are alternatives. However, there is hazard to laying a full-thickness skin graft on top of a free tarsoconjunctival graft. Because each of the grafts needs to acquire a blood supply to survive, some vascularized tissue must be brought between them. Orbicularis muscle is suitable for this task and can often be mobilized to cover the free tarsoconjunctival graft and also lay beneath the full-thickness skin graft. A 6-0 plain suture is used to join the orbicularis flap, the free tarsoconjunctival graft, and the skin graft at the lid margin (see Fig. 17-20 and video).
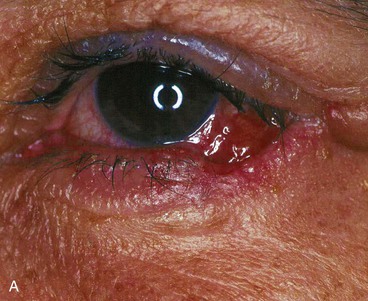
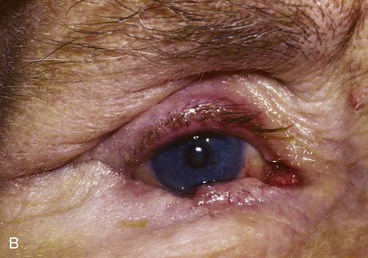
Potential complications from free tarsoconjunctival grafts include dehiscence of the graft; failure of the tarsoconjunctival graft, musculocutaneous flap, or skin graft; persistent redness of the reconstructed lid margin22; infections; deformity of the upper lid donor site with buckling of the lid margin; corneal irritation from lanugo hairs; entropion or ectropion; and ptosis or retraction of the upper lid.
Hughes Tarsoconjunctival Flap for Lower Eyelid Reconstruction
Full-thickness defects will sometimes involve all or most of the length of the lower eyelid. The Tenzel semicircular flap is well suited for repair of smaller defects that are not amenable to primary closure. However, larger defects encompassing 66% to 100% of the lower eyelid are typically not satisfactorily repaired with a semicircular flap. William Hughes first described the tarsoconjunctival pedicle flap named for him in 1937.23 The flap provides a relatively ideal posterior lamella replacement: vascularized autogenous tarsus lined with conjunctiva (Fig. 17-21). The anterior lamella is reconstructed with a full-thickness skin graft or a local advancement flap. Unfortunately, the Hughes flap causes temporary obstruction of vision until it is divided at a second-stage procedure, 7 to 45 days after the initial operation. Nevertheless, it continues to be a highly effective and popular technique for reconstruction of large lower eyelid defects.
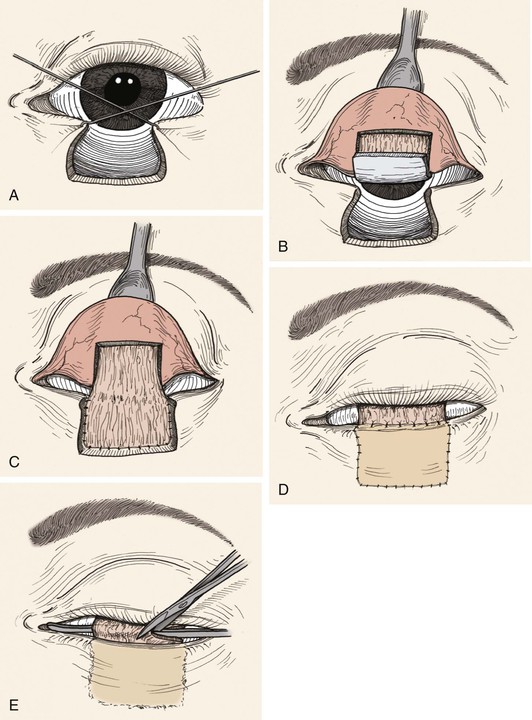
FIGURE 17-21 Hughes technique for reconstruction of lower eyelid. A, Hooks used to approximate wound margins and width of persistent defect measured. B, Three-sided flap consisting of conjunctiva and tarsus incised. Horizontal border of flap is at least 4-mm superior to lid margin. C, Composite flap is advanced into lower eyelid defect so that the upper border of tarsus contained within the flap is in alignment with remaining lower eyelid margin. D, Anterior lamella reconstructed with full-thickness skin graft. Superior border of skin graft is sutured to conjunctiva 1- to 2-mm above upper border of tarsus contained within tarsoconjunctival flap. E, Pedicle of tarsoconjunctival flap divided 2 to 4 weeks after initial reconstruction.
Local anesthetic infiltration in preparation for the Hughes tarsoconjunctival flap reconstruction must include both the recipient bed in the lower eyelid and the ipsilateral upper eyelid. The upper eyelid is everted, and 1 mL of local anesthetic is injected subconjunctivally above the superior tarsal border with a 30-gauge needle. Another 1 mL is injected subcutaneously at the eyelid crease, and proparacaine 0.5% eye drops are instilled for corneal anesthesia.
Satisfactory postoperative upper eyelid stability and contour requires that the most inferior 4-mm of tarsus be spared. To begin harvest of the tarsoconjunctival flap, the upper eyelid is everted over a Desmarres or small vein retractor (see Fig. 17-21). Calipers and a surgical marker are used to create a line parallel to the eyelid margin 4-mm superiorly on the posterior tarsal surface. Markings are also made for the medial and lateral borders of the planned flap. A No. 15 scalpel or No. 69 Beaver blade is used to make a horizontal incision along the marked line full thickness through the tarsus only. If the incision is partial thickness, blunt Westcott scissors can complete the incision. In this location, the next tissue plane encountered is the levator aponeurosis, which should be left intact.
Dissection with blunt-tipped scissors proceeds superiorly in an easily maintained tissue plane at the anterior surface of the tarsus, posterior to the levator aponeurosis. At the superior border of the tarsus, continued dissection in this plane will leave both Müller’s muscle and conjunctiva in the flap. Although the enhanced blood supply by including the muscle may occasionally be desirable, it is generally a mistake to include Müller’s muscle in the flap as upper eyelid retraction will often result after flap inset. Instead, the plane of dissection transitions to subconjunctival, posterior to Müller’s muscle. Care must be exercised at the superior border of the tarsus to avoid injury to the vascular arcade. Transitioning from the anterior surface of the tarsus to the new plane of dissection can be facilitated by infiltration of 0.5 mL of local anesthetic to hydrodynamically separate the tissues. Dissection is then continued superiorly until the flap can be advanced into the lower eyelid defect without excessive wound closure tension. Cautery of conjunctival vessels is kept to a minimum, and bipolar forceps are safest for the underlying cornea.
The flap will usually have 4- to 6-mm of tarsal height centrally, with tapering of the tarsal tissue medially and laterally. With use of an S-14 spatula needle, the flap is sutured to the medial and lateral tarsal stumps of the lower eyelid defect (or periosteal flaps elevated for the purpose in total eyelid reconstruction) with multiple interrupted 90% thickness 6-0 polyglactin sutures. The repair should be such that the superior tarsal border of the flap approximates the presurgical lower eyelid margin. Conjunctiva and retractors are advanced from the inferior fornix and secured to the lower border of the tarsoconjunctival flap with multiple interrupted 6-0 polyglactin sutures. At this point, the cornea will be covered and protected by the tarsoconjunctival flap and the upper eyelid will rest at approximately midpupil height.
Next, the anterior lamella must be reconstructed with a full-thickness skin graft or musculocutaneous advancement flap. Use of bipedicle orbicularis flaps or a musculocutaneous advancement flap for the anterior lamella has advantages including improved color and texture uniformity and postoperative neurologically active muscle in the lower eyelid24 that may prevent retraction. Often, however, there is inadequate local tissue for such flaps, and a full-thickness skin graft is necessary. Donor sites for skin grafts most commonly used include the ipsilateral or contralateral upper eyelid, preauricular25 or postauricular skin, and supraclavicular skin. A template is developed to estimate the size of the required skin graft to include coverage of the tarsoconjunctival flap up to the desired height of the reconstructed eyelid. The full-thickness graft is harvested in the usual way, thinned of connective tissue and fat, and sutured to the recipient site. Running 6-0 or 7-0 chromic gut is used for the superior border of the graft, with fixation to the conjunctiva just above the superior tarsal border. Fixation at the medial, lateral, and inferior borders can be accomplished with the same suture, but 6-0 silk is preferred because it can be used to easily create a tie-over bolster (e.g., cotton dental roll, Telfa, and cotton wool). Alternatively, an ocular compression patch left in place for 36 to 48 hours has also been effective in ensuring success of the skin graft.
Inset of the tarsoconjunctival flap is commonly performed at 2 to 4 weeks26 after the initial reconstruction, although reports indicate successful results as early as 1 week.27 This second stage is performed with topical proparacaine 0.5% eye drops and 1% lidocaine with 1 : 200,000 epinephrine concentration for local anesthesia. A narrow malleable retractor or a grooved director is inserted under the flap to protect the cornea. The flap is separated at the intended position of the new lower eyelid margin with scissors or a No. 15 scalpel, angled to leave a small conjunctival frill on the lower eyelid. The new lower eyelid margin is trimmed of excess tissue as needed, and gentle hemostasis is achieved. The ideal position for the mucocutaneous junction that will form with healing is just anterior to the apex of the reconstructed eyelid so that the keratinized skin and lanugo hairs of the graft do not contact the cornea. It may be helpful to suture the conjunctival edge anteriorly with 7-0 chromic gut. The remaining flap tissue still attached to the upper eyelid is transected flush with the conjunctival surface while the eyelid is everted, and hemostasis is achieved. If Müller’s muscle has been included in the flap, adjustment in upper eyelid height and contour may be necessary. This is accomplished with blunt dissection superiorly on the anterior surface of Müller’s muscle and then fixation to the levator aponeurosis with absorbable suture. The eye is dressed with ophthalmic antibiotic ointment (e.g., erythromycin) or antibiotic eye drops, and the patient is instructed to continue use twice daily for 1 week. Examples of eyelid reconstruction by the Hughes tarsoconjunctival flap are shown in Figure 17-22.
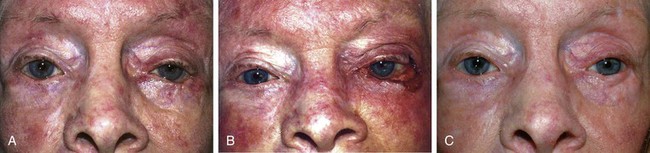
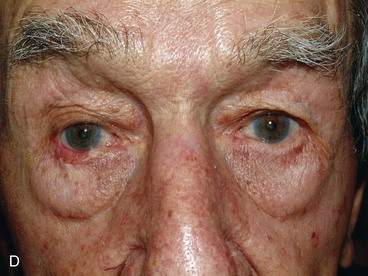
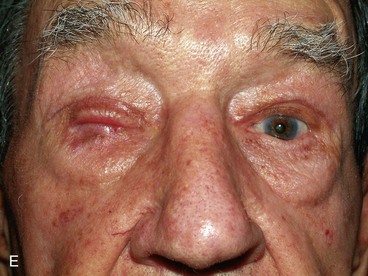
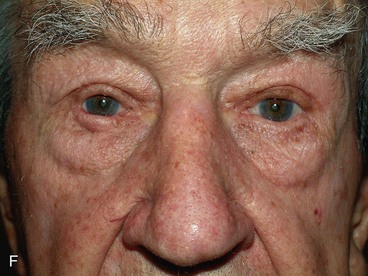
FIGURE 17-22 Hughes tarsoconjunctival flap reconstruction. A, Squamous cell carcinoma of left lower eyelid. B, Same patient as shown in A with 95% loss of lower eyelid after resection of cancer. C, Postoperative view 1 year after reconstruction with Hughes flap and skin graft. D, Morpheaform basal cell carcinoma of right lower eyelid. E, Same patient as shown in D before separation of Hughes flap. F, Postoperative view 6 months after reconstruction with Hughes flap and skin advancement flap.
Potential complications from Hughes tarsoconjunctival flap repair with full-thickness skin graft include failure of the skin graft, dehiscence of the tarsoconjunctival flap,28 infections, persistent erythema of the reconstructed lower eyelid margin, corneal irritation from lanugo hair trichiasis, pyogenic granuloma, lower and upper eyelid retraction, notching of the reconstructed eyelid, symblepharon, entropion, ectropion, and upper eyelid ptosis (Fig. 17-23).
Tarsal Transposition Flap for Lateral Upper Eyelid Reconstruction
Defects involving up to 50% of the upper eyelid can be closed with a tarsoconjunctival transposition flap, as initially described by Kersten et al29 in 1986. It is possible to combine it with a flap of periosteum from the lateral orbital rim to repair larger defects. It is a viable alternative to composite grafts, Tenzel semicircular flaps, and even the Cutler-Beard repair in lateral upper lid defects. It has the advantage of being a one-stage repair, using local vascularized eyelid tissue rather than grafts from the nose, ears, or other eyelids. It involves use of the ipsilateral remaining upper lid tarsus as a transposition flap to repair the posterior lamellar defect, followed by a musculocutaneous flap or skin graft for the anterior lamella.
The technique begins with local anesthetic administration to the involved upper lid subcutaneously, at the superior conjunctival fornix, and at the lateral canthus. A 4-0 silk traction suture is placed in the lid margin and used to evert the lid over a cotton-tipped applicator. An incision is planned in the remaining tarsus of the upper lid. A vertically oriented flap 3- to 4-mm wide and hinged at the eyelid margin is designed with a caliper and outlined with a marking pen. A No. 15 blade is used to incise conjunctiva and tarsus from the top of the tarsus to within 1.5- to 2-mm of the lid margin, after which the flap is sharply dissected free from its anterior attachments to the orbicularis oculi. The flap is then pivoted 90°. After transposition, there will be some tissue bunching at the lid margin, which can be carefully trimmed with fine scissors. The flap is sutured to the remaining soft tissue at the lateral canthus, or to a periosteal flap, with a 5-0 polyglactin suture on an S-14 spatula needle. The suture technique is important as dehiscence of this flap could lead to failure of the repair. A “crossed swords” method in which the two spatula needles of a double-armed suture are both passed through the tarsoconjunctival flap works well. Good purchase at the anchor point and avoidance of excessive tension on the flap are both important. The conjunctiva can usually be advanced to the superior border of the tarsoconjunctival transposition flap from the superior fornix and loosely fixed there with 7-0 chromic suture on a spatula needle. Existing attachments of the remaining levator aponeurosis are usually adequate for eyelid elevation so that manipulation of the levator is not necessary. The residual anterior lamellar defect can sometimes be repaired with a musculocutaneous advancement flap from the lateral aspect of the upper lid. If this is not possible, a full-thickness skin graft can also be used (Figs. 17-24 and 17-25; see video).
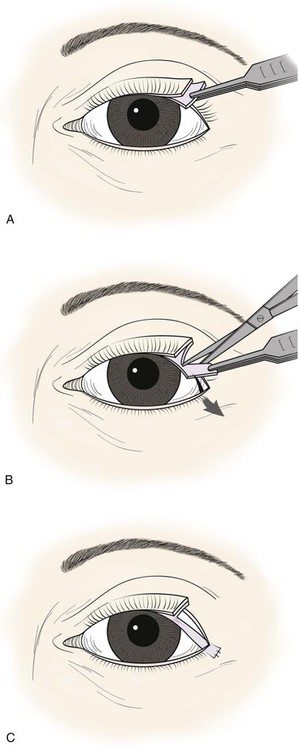
FIGURE 17-24 Upper eyelid reconstruction with tarsal transposition flap. A, Superior border of lateral tarsus incised horizontally. B, Vertical tarsal incision creates an inferiorly based transposition flap. C, Flap fixed to lateral orbital rim or lateral canthal tendon.
Eyelid and Simple Tarsal Composite Grafts
Composite grafts can be harvested from the contralateral upper or lower eyelid to provide similar autologous tissue for reconstruction. The primary disadvantage of this approach is violation of the integrity of the normal eyelid. Composite grafts of tarsus and conjunctiva can be used for posterior lamellar reconstruction alone,18 or alternatively, composite grafts of conjunctiva, tarsus, and skin including the lid margin30,31 can be used with an interposed blood supply to reconstruct short segments of the upper or lower eyelid. Microsurgical transfer of full-thickness eyelid free flaps has also been described in a rabbit model.32
Free tarsoconjunctival grafts can be harvested from the contralateral upper eyelid and used to reconstruct defects up to two-thirds the width of the posterior lamella of the ipsilateral upper eyelid or either lower eyelid. The graft is obtained by use of techniques previously described. The graft is transferred to the defect and secured to the remaining eyelid tarsus with interrupted partial-thickness 5-0 or 6-0 polyglactin sutures so that it is perfectly aligned and a 2-mm-wide conjunctival free edge is positioned at the lid margin. If necessary, 4-0 polyglactin is used to attach the lateral edge of the graft to the lateral canthal tendon or to the periosteum inside the lateral orbital rim. The anterior lamella is then reconstructed with a musculocutaneous advancement flap (e.g., semicircular flap or transposition flap as described before).
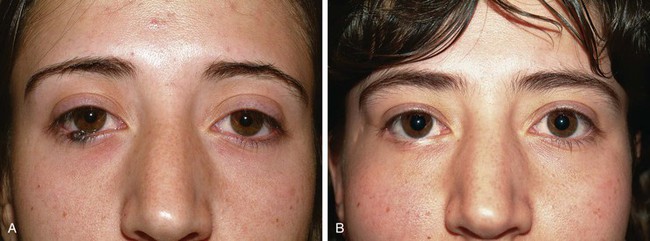
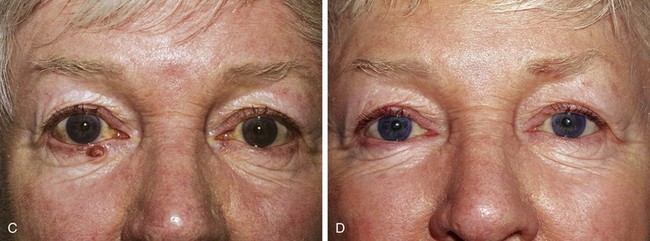
In cases in which the full-thickness lower eyelid defect is shallow (e.g., 5- to 10-mm in height) and there is substantial lack of skin laxity for the creation of a flap, a modified composite eyelid graft can be useful. This graft consists of a pentagon of full-thickness eyelid harvested from the contralateral upper or lower eyelid from which the orbicularis muscle has been excised, leaving the skin, lashes, tarsus, and conjunctiva (Figs. 17-26 and 17-27). Its width is limited by the laxity in the donor eyelid, but typically at least 10-mm can be harvested in elderly patients. The graft is supported by the rich blood supply of adjacent orbicularis muscle that is mobilized into the space left by the excised muscle. The donor defect is closed according to the method described in the section discussing primary closure of full-thickness defects. The eyelid composite graft is transferred to the defect, and the tarsal edges are sutured in perfectly aligned position with partial-thickness 5-0 or 6-0 polyglactin interrupted sutures. Adjacent orbicularis muscle, typically from the inferior wound edge, is undermined and sutured into the space between the skin and tarsus with 6-0 polyglactin. The skin edges of the graft are then sutured to the borders of the recipient site with 6-0 silk, and a bolster or compression patch is applied over the closed lids for 24 to 36 hours.
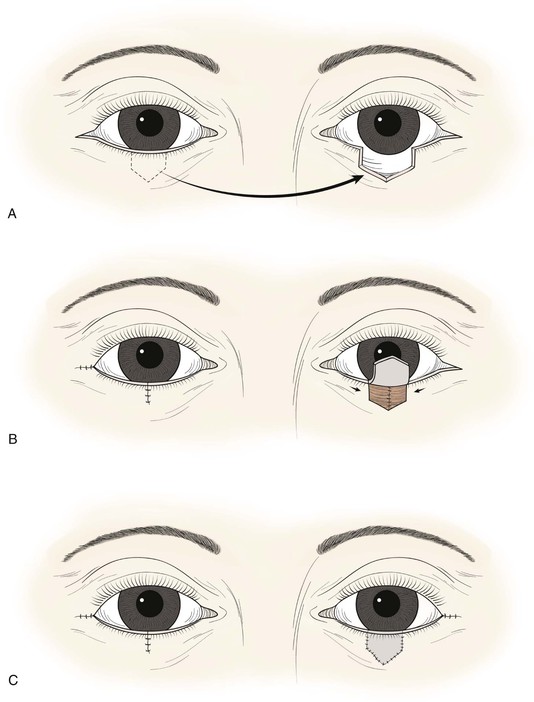
FIGURE 17-26 A, Modified composite graft consisting of skin, lashes, tarsus, and conjunctiva. Muscle removed to accommodate muscle advancement flaps at recipient site to nourish graft. B, Bilateral muscle flaps advanced into space left by excision of muscle of graft. Skin and lashes of graft are hinged to graft superiorly. C, Graft sutured in place. Donor site closed primarily.
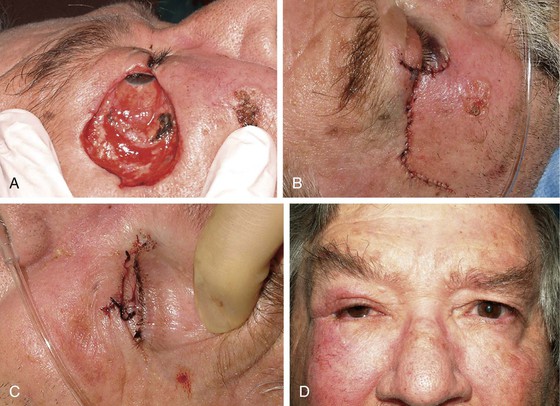
FIGURE 17-27 Reconstruction of eyelid with composite graft. A, Defect. B, Repair with composite graft to right upper eyelid. Lower eyelid repaired with rotation advancement skin flap and periosteal flap. C, Composite graft harvest site from left lower eyelid. D, Postoperative view at 2 months. Note preservation of lashes and maintenance of margin of upper lateral eyelid.
Cutler-Beard Flap for Complete Upper Eyelid Reconstruction
The Cutler-Beard lid-sharing flap33 is probably the best available technique for total upper eyelid reconstruction. It is a skin-muscle-conjunctiva flap from the lower eyelid advanced into the upper eyelid defect. It involves temporary closure of the involved eye for 4 to 6 weeks (Fig. 17-28). The other major disadvantage of this flap is that the rigid posterior lamella is not usually replaced, which may result in instability of the reconstructed eyelid margin over time.34 Free tarsoconjunctival grafts (as described earlier) or free autogenous hard palate mucosal grafts35,36 can be used to provide posterior lamellar support to the flap37 but are best added after flap inset so that corneal integrity can be monitored while risk of suture injury exists.
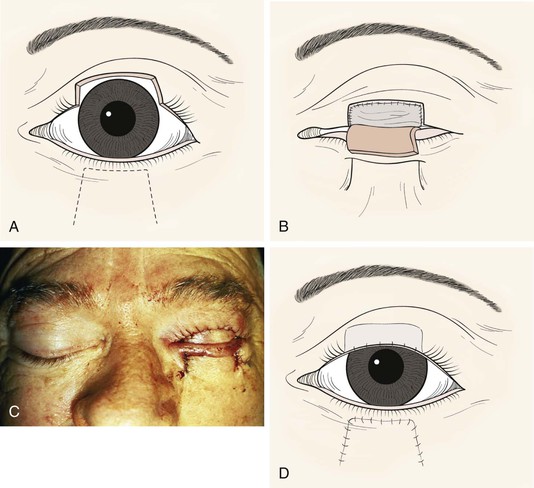
FIGURE 17-28 Cutler-Beard flap reconstruction of upper eyelid. A, Flap designed on lower eyelid. B, Posterior lamellar graft in place. C, Clinical appearance at surgery. D, After inset of flap.
The composite flap is then brought under the bridge of lower eyelid margin and sutured to the edges of the upper eyelid defect. The conjunctiva and lower eyelid retractors are sutured with 6-0 polyglactin to the remnants of tarsus or to the canthal tendons or periorbita medially and laterally. The borders of the orbicularis and skin are also approximated in layers with 6-0 polyglactin suture.
Flap inset is typically accomplished as a second stage 4 to 8 weeks after reconstruction, although successful inset at 2 weeks has been described.38 A longer time may be necessary for tissue vascularization and stretching in patients who have had local irradiation or who have particularly tight wound closures. A horizontal mark is made on the skin 2-mm below the anticipated new upper eyelid margin. With the cornea protected by a narrow malleable retractor, the flap is cut with straight scissors angled so that the cut is more inferior on the conjunctival side. After initial hemostasis, the conjunctival edge is then sutured to the skin edge with 7-0 chromic suture, ensuring that the new upper eyelid margin is not keratinized. It may be necessary to excise a small wedge of orbicularis muscle to permit advancement of the conjunctival edge anteriorly. The inferior edge of the lower eyelid bridge is then freshened, and the base of the lower eyelid flap is sutured to the bridge in a layered fashion with buried interrupted 6-0 polyglactin for the conjunctiva and retractors and interrupted or running 6-0 polypropylene, nylon, or plain gut for the skin. The skin and orbicularis may need to be undermined to prevent lower eyelid ectropion, and any horizontal laxity should be repaired with a lateral tarsal strip procedure.
Canalicular Reconstruction
Defects in the medial upper and lower eyelids or medial canthus will frequently involve the lacrimal drainage system, especially the puncta and canaliculi. Primary reconstruction of these tissues will often prevent epiphora and the need for more extensive procedures, such as conjunctivodacryocystorhinostomy with Jones tube. In cases involving loss of the punctum and partial proximal canaliculus, simple monocanalicular or bicanalicular silicone lacrimal intubation may be adequate to maintain lacrimal drainage. However, complete loss of the canaliculi is difficult to manage primarily, and postoperative epiphora may be best prevented in these cases by primary endoscopic conjunctivodacryocystorhinostomy with Jones tube,39 performed in conjunction with an ophthalmologist familiar with that procedure.
In cases in which there is only partial loss of the canaliculi or puncta, silicone intubation of the remaining lacrimal system should be performed.40,41 The Quickert-Dryden malleable silver probes and the Crawford olive-tipped probes attached to silicone tubing are commonly available and relatively easy to use for this purpose (Figs. 17-29 and 17-30). The probes should first be bent so that each forms a semicircle; this greatly facilitates retrieval from the inferior nasal meatus. One probe is then inserted into the proximal end of each remaining canaliculus or punctum. One at a time, each probe is then passed into the lacrimal sac until a firm “stop” is felt on the lacrimal bone. It is then redirected inferolaterally while gentle medial pressure is maintained. The probe will pass into the nasolacrimal duct and ultimately into the inferior nasal meatus, from which it is retrieved under headlight observation with a Crawford hook, Takahashi forceps, or other clamp. If the semicircular curve created before passage is ideal, the probe will often spontaneously exit from the naris. Once both ends have been passed in this fashion, the probes are cut from the silicone, and the two silicone ends are tied together with a square knot so that there is neither tension at the medial canthus nor a prolapse of the knot from the nostril. It can be fixed to the lateral nasal wall with 6-0 polypropylene suture or tied over a small silicone bolster to avoid excessive movement, but the surgeon must be careful to avoid tension at the medial canthus that will invariably cause undesired erosion of the remaining canaliculi. The silicone tubing is typically left in place at least 6 months. It can be removed by cutting the tube in the medial ocular commissure and then pulling sharply on one end or, if it is fixated in the nose, by pulling the knot out from the inferior nasal meatus.
References
1. Elner, VM, The histologic anatomy of the medial canthal ligament and surrounding structures Annual meeting of the American Society of Ophthalmic Plastic and Reconstructive Surgery. New Orleans. October 28, 1989.
2. Anderson, RL. Medial canthal tendon branches out. Arch Ophthalmol. 1977; 95:2051.
3. Grabb, WC, Smith, JW. Plastic surgery, 3rd ed. Boston: Little, Brown; 1979.
4. Patrinely, JR, Marines, HM, Anderson, RL. Skin flaps in periorbital reconstruction. Surv Ophthalmol. 1987; 31:249.
5. Shotton, F. Rhombic flap for medial canthal reconstruction. Ophthalmic Surg. 1973; 14:46.
6. Doxanas, MT, Anderson, RL. Clinical orbital anatomy. Baltimore: Williams & Wilkins; 1984.
7. Callahan, MA, Callahan, A. Ophthalmic plastic and orbital surgery. Birmingham: Aesculapius; 1979.
8. Lowry, JC, Bartley, GB, Garrity, JA. The role of second-intention healing in periocular reconstruction. Ophthal Plast Reconstr Surg. 1997; 13:177.
9. Sharkar, J, Nair, RG, Sullivan, SC. Management of peri-ocular skin tumours by laissez-faire technique: analysis of functional and cosmetic results. Eye (Lond). 2002; 16:50.
10. Anderson, RL, Gordy, DD. The tarsal strip procedure. Arch Ophthalmol. 1979; 97:2192.
11. Anderson, RL, Ceilley, RI. A multispecialty approach to the excision and reconstruction of eyelid tumors. Ophthalmology. 1978; 85:1150.
12. Stabel, JE, Hawes, MJ. The arrowhead skin-muscle flap in the closure of lower eyelid defects. Ophthal Plast Reconstr Surg. 1998; 14:222.
13. Tenzel, RR, Stewart, WB. Eyelid reconstruction by the semicircle flap technique. Ophthalmology. 1978; 85:1164.
14. Mustarde, JC. Repair and reconstruction in the orbital region. New York: Churchill Livingstone; 1971.
15. Foster, JA, Scheiner, AJ, Wulc, AE, et al. Intraoperative tissue expansion in eyelid reconstruction. Ophthalmology. 1998; 105:170.
16. Weinstein, GS, Anderson, RL, Tse, DT, et al. The use of a periosteal strip for eyelid reconstruction. Arch Ophthalmol. 1985; 103:357.
17. Leone, CR, Hand, SI. Reconstruction of the medial eyelid. Am J Ophthalmol. 1979; 87:797.
18. Hawes, MJ. Free autogenous grafts in eyelid tarsoconjunctival reconstruction. Ophthalmic Surg. 1987; 18:37.
19. Paridaens, D, van den Bosch, WA. Orbicularis muscle advancement flap combined with free posterior and anterior lamellar grafts. Ophthalmology. 2008; 115:189.
20. Wesley, RE, McCord, CD, Jones, NA. Height of the tarsus of the lower eyelid. Am J Ophthalmol. 1980; 90:102.
21. Leone, CR, Van Gemert, JV. Lower lid reconstruction using tarsoconjunctival grafts and bipedicle skin-muscle flap. Arch Ophthalmol. 1989; 107:758.
22. Hawes, MJ, Grove, AS, Hink, EM. Comparison of free tarsoconjunctival grafts and Hughes tarsoconjunctival flaps for lower eyelid reconstruction. Ophthal Plast Reconstr Surg. 2011; 27:219.
23. Hughes, WL. A new method for rebuilding a lower lid. Arch Ophthalmol. 1937; 17:1008.
24. Lowry, JC, Bartley, GB, Litchy, WJ. Electromyographic studies of the reconstructed lower eyelid after a modified Hughes procedure. Am J Ophthalmol. 1995; 119:225.
25. Dryden, RM, Wulc, AE. The preauricular skin graft in eyelid reconstruction. Arch Ophthalmol. 1985; 103:1579.
26. McNab, AA. Early division of the conjunctival pedicle in modified Hughes repair of the lower eyelid. Ophthalmic Surg Lasers. 1996; 27:422.
27. Leibovitch, I, Selva, D. Modified Hughes flap: division at 7 days. Ophthalmology. 2004; 111:2164.
28. Bartley, GB, Messenger, MM. Outcome of tarsoconjunctival flap dehiscence after eyelid reconstruction. Am J Ophthalmol. 2002; 134:627.
29. Kersten, RC, Anderson, RL, Tse, DT, Weinstein, GL. Tarsal rotational flap for upper eyelid reconstruction. Arch Ophthalmol. 1986; 104:918.
30. Putterman, AM. Viable composite grafting in eyelid reconstruction. Am J Ophthalmol. 1978; 85:237.
31. Werner, MS, Olson, JJ, Putterman, AM. Composite grafting for eyelid reconstruction. Am J Ophthalmol. 1993; 116:11.
32. Molnar, JA, Yan, JG, Matloub, HS. A prefabricated free flap for eyelid reconstruction. J Reconstr Microsurg. 1998; 14:479.
33. Cutler, NL, Beard, C. A method for partial and total upper eyelid reconstruction. Am J Ophthalmol. 1955; 39:1.
34. Carrol, RP. Entropion following the Cutler-Beard procedure. Ophthalmology. 1983; 90:1052.
35. Siegel, RJ. Palatal grafts for eyelid reconstruction. Plast Reconstr Surg. 1985; 76:411.
36. Bartley, GB, Kay, PP. Posterior lamellar eyelid reconstruction with a hard palate mucosal graft. Am J Ophthalmol. 1989; 107:609.
37. Fischer, T, Noever, G, Langer, M, Kammer, E. Experience in upper eyelid reconstruction with the Cutler-Beard technique. Ann Plast Surg. 2001; 47:338.
38. Hsuan, J, Selva, D. Early division of a modified Cutler-Beard flap with a free tarsal graft. Eye (Lond). 2004; 18:714.
39. Trotter, WL, Meyers, DR. Endoscopic conjunctivodacryocystorhinostomy with Jones tube placement. Ophthalmology. 2000; 107:1206.
40. Quickert, M, Dryden, RM. Probes for intubation in lacrimal drainage. Trans Am Acad Ophthalmol Otolaryngol. 1970; 74:431.
41. McCord, CD. Canalicular resection and reconstruction by canaliculostomy. Ophthalmic Surg. 1980; 11:440.