Reconstruction of the Cheek
Introduction
Achieving aesthetic surgical outcomes after reconstruction of cutaneous cheek defects requires knowledge of cheek anatomy and facial aesthetic regions. The size and location of the defect and the structures adjacent to the defect are assessed to determine where tissue can be recruited for construction of skin flaps without adversely affecting the nose, lips, and eyelids by secondary tissue movement. Analysis of each cheek defect is essential, and fundamental concepts of cutaneous flap biomechanics should be addressed (see Chapter 3) to facilitate tension-free wound closures with optimally positioned scars.
Fundamental Concepts
Anatomy and Aesthetic Units
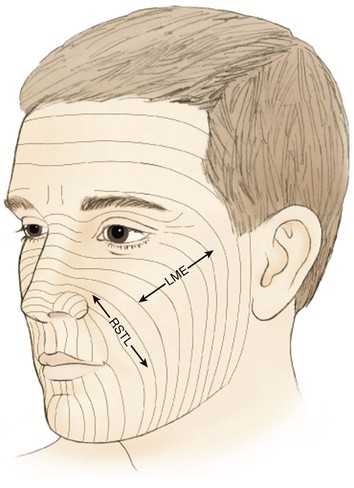
The cheek is the largest facial aesthetic region and is juxtaposed to the orbital, nasal, and lip aesthetic regions. The aesthetic region of the cheek has the following boundaries: inferior bony orbital rim, nasofacial (cheek-nose junction) sulcus, line from the lateral canthus to the root of the helix, preauricular crease, melolabial crease, labiomandibular crease, and inferior border of the mandible (Fig. 20-1). The cheek can be conceptually divided into areas based on the difference of underlying bone, soft tissue, skin quality, and skin laxity. On the basis of these anatomic differences, there are four aesthetic units: medial, lateral, zygomatic, and buccal. The skin of the medial and buccal units is thicker and more mobile than the skin of the other units of the cheek. The skin of the lateral unit is moderately fixed to the underlying fascia, and the skin of the zygomatic area is quite fixed, in part by the cutaneous retaining ligaments in the region. This unit also has the least skin laxity.1
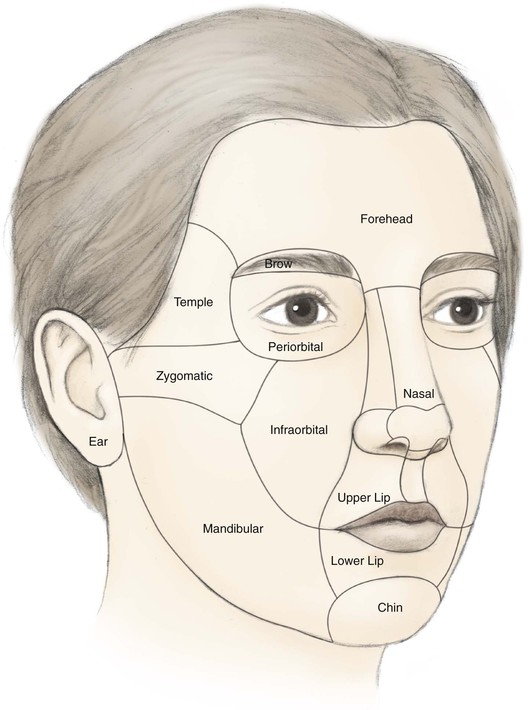
FIGURE 20-1 Aesthetic boundaries of cheek aesthetic region: inferior bony orbital rim, nasofacial sulcus, melolabial crease, labiomandibular crease, inferior border of mandible, preauricular crease, and line from lateral canthus to root of helix. Aesthetic region of cheek based on inherent mobility and thickness of skin may be divided into units: infraorbital (medial, buccal), zygomatic, and mandibular (lateral).
Several important ligaments collectively referred to as cheek retaining ligaments support the soft tissues of the cheek. Retaining ligaments in the area of the zygoma (known as McGregor’s patch)1 serve as strong bone anchors for supporting the skin overlying the zygoma. More inferiorly and anteriorly, a dense attachment to the skin known as the mandibular retaining ligament is present at the cheek where it joins the lower lip. This ligament accounts for the formation of the labiomandibular crease. Fibrous attachments of the superficial musculoaponeurotic system (SMAS) to the cheek skin at the junction of cheek and upper lip create the melolabial crease. The labiomandibular and melolabial creases provide important landmarks for concealing incisions and represent aesthetic boundary lines between the cheek and the upper and lower lips. The contour of the cheek is due to the convexity of the maxilla and the malar fat pad. The fat pad is well known to plastic surgeons for its relationship to aging of the midface. In addition to retaining ligaments, the cheek is supported by the muscles of facial expression. Most notable is the zygomaticus major positioned deep to the malar fat pad and invested by the SMAS.1
Lymphatic drainage of the cheek is primarily to the submental, submandibular, and preauricular lymph nodes. Additional drainage occurs to the second echelon of lymph nodes in the superior jugular chain.2 The vascular supply to the cheek skin is derived from three major sources, which perfuse the dermal and subdermal plexuses.1 The superficial temporal artery provides a minor supply through the transverse facial branch. The infraorbital artery provides collateral supply to the midcheek with anastomotic connections to the facial artery. The facial artery and its continuation as the angular artery provide the majority of blood to the skin of the cheek. The venous drainage is based on vessels that follow the named arterial supply.
The facial nerve provides motor innervation to the muscles of facial expression that form a base on which the subcutaneous tissue and skin of the cheek rest. The majority of sensory innervation of the cheek is provided by branches of the second division of the trigeminal nerve through the infraorbital nerve. The third division of the trigeminal nerve contributes to the sensory supply of the lateral cheek through the auriculotemporal nerve and of the inferior cheek through the mental nerve.
Flap Planning
When possible, scars should be positioned at the border of aesthetic units to provide the best camouflage. When scars cannot be placed in borders of aesthetic units, they are aligned parallel to relaxed skin tension lines (RSTLs), which are curvilinear in the cheek (Fig. 20-2). RSTLs are a result of the intrinsic elasticity of the skin and are perpendicular to lines of maximal extensibility.3 Therefore, wound closure oriented in an axis parallel to RSTLs results in less wound closure tension and more favorable scar camouflage. Wrinkles located in the lateral canthal region are largely due to the action of the orbicularis oculi muscle and provide a useful site in which to place incisions. In general, the boundaries of cheek aesthetic units and facial creases are more favorable locations than the RSTLs for positioning of scars.3
Medial Cheek Defects
Healing by secondary intention of small defects of the medial cheek (1 cm or less) can result in an acceptable appearance, particularly on concave surfaces such as the perialar and melolabial regions. However, most skin defects of the cheek do not heal with an acceptable aesthetic result when they are allowed to heal by secondary intention. This may be related to facial motion and the slightly convex surface topography of the cheek.
Primary wound closure is an excellent option for repair of smaller cutaneous defects of the cheek. Typically, it results in a linear suture line with minimal tension on the closure because wide skin undermining is possible. In general, undermining up to 4 cm beyond the borders of the defect improves tissue recruitment, whereas undermining more widely adds little to decreasing wound closure tension (see discussion of this in Chapters 3 and 6).4 Primary wound closure is a particularly good option when the resulting scar can be placed in the borders of the cheek aesthetic region or parallel to RSTLs. An example is a defect adjacent to the junction of the cheek and nasal sidewall (nasofacial sulcus) repaired with primary wound closure, resulting in a scar positioned in the sulcus, thus providing excellent camouflage (Fig. 20-3). Because most cutaneous defects created by micrographic surgery are circular, an ellipse can be designed such that the resulting scar is parallel to RSTLs. When the ends of the ellipse encroach on a facial structure or extend beyond the borders of an aesthetic unit, an M-plasty may be used to shorten the length of the closure. This technique eliminates or reduces the necessity of the scar’s crossing boundaries of aesthetic units (Fig. 20-4).
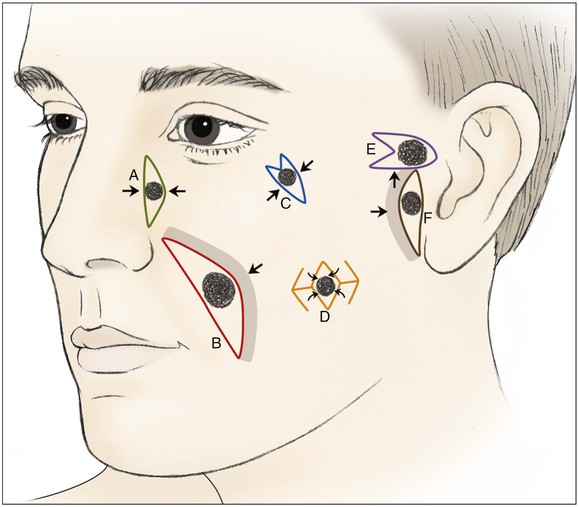
FIGURE 20-3 Various reconstructive techniques for small and medium-sized defects of cheek: primary closure (A); primary closure with asymmetric skin undermining (B; shaded area represents greater undermining); elliptical closure with M-plasty (C); rhombic flaps (D); advancement with M-plasty (E); and primary closure with asymmetric skin undermining (F).
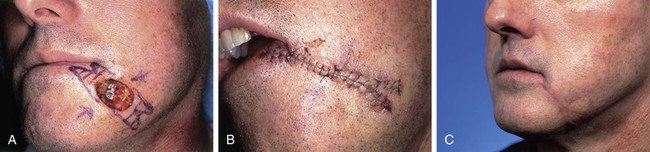
FIGURE 20-4 A, A 3 × 2-cm skin defect of inferior medial cheek. Primary wound closure planned with bilateral M-plasties designed. B, Wound repaired. C, Postoperative result at 3 weeks. (Courtesy of Shan R. Baker, MD.)
The V-Y subcutaneous tissue pedicle island advancement flap is an effective way of reconstructing small defects of the medial cheek adjacent to the alae (Fig. 20-5). The flap is designed as an island of skin with a triangle shape, preserving the subcutaneous tissue beneath the skin island. Extending the incision used to create the flap to the proper depth is important. If incisions are too shallow, the ability to advance the flap will be restricted; and if incisions are too deep, the nerve supply to the facial musculature may be compromised. The flap is advanced and the wound closed so that the suture line assumes a Y-shaped configuration. The blood supply to the flap is from perforators that enter the subcutaneous tissue pedicle. The margins of the flap can be conservatively undermined to allow better mobilization and skin edge eversion during wound closure. The island flap is particularly useful for repair of defects adjacent to the nasal alae (see discussion in Chapters 6, 9, and 12). This area has a natural triangle shape, and part of the resulting scar will typically be in the melolabial crease.5
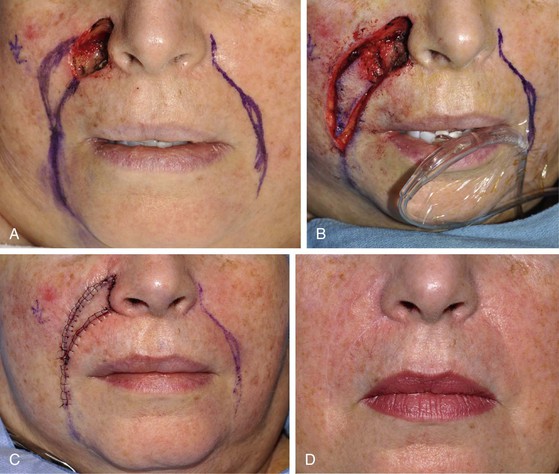
FIGURE 20-5 A, A 2.5 × 1.5-cm skin defect of medial cheek. V-Y subcutaneous tissue pedicle island advancement flap designed for repair. B, Flap dissected before transfer. C, Flap in place. D, Postoperative result at 5 months.
Medium-sized (2 to 3 cm) medial cheek cutaneous defects can be reconstructed by a number of different methods. Primary wound closure with extended incisions to remove standing cutaneous deformities may be used when defects are located near the border of the cheek aesthetic region. The surgeon may use asymmetric or differential undermining of the wound margins to position the wound closure suture line at or parallel to the border of the aesthetic region. This approach may provide excellent scar camouflage as the borders of the region are where natural shadows or lines of topographic transition occur. Primary wound closure is a particularly useful technique when defects are on or adjacent to the superior aspect of the melolabial fold (Fig. 20-6). When defects are not directly in the junction of the cheek with the lips or nose, the intervening skin may sometimes be excised to position the suture line in the aesthetic border of the medial cheek. Defects that are adjacent to the nasofacial sulcus are particularly amenable to primary wound closure (Fig. 20-7). In such cases, the skin lateral to the wound is advanced medially and anchored to the periosteum at the nasofacial sulcus by a 4-0 long-lasting absorbable suture. This may prevent effacement of the nasofacial sulcus and reduce wound closure tension.
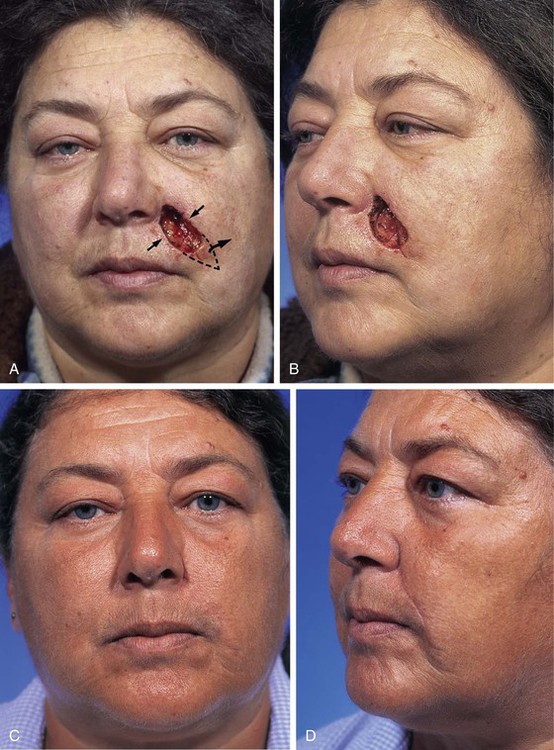
FIGURE 20-6 A, B, A 3 × 2-cm skin defect of medial cheek involving melolabial crease. Wound closed primarily. Standing cutaneous deformity (broken line) excised in melolabial crease. C, D, Postoperative views at 7 months. Scar is in aesthetic boundary of melolabial crease. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
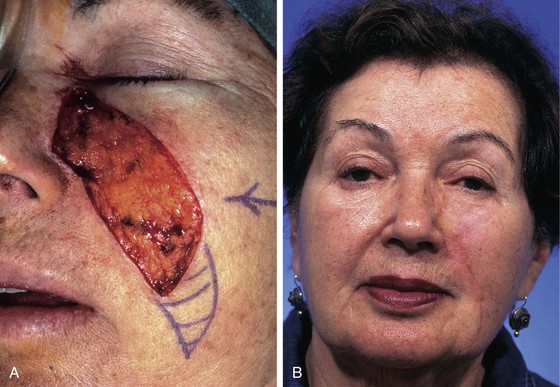
FIGURE 20-7 A, A 4 × 2-cm skin defect of medial cheek. Wound closed primarily. Anticipated standing cutaneous deformity marked with horizontal lines. B, Postoperative result at 2 months. (Courtesy of Shan R. Baker, MD.)
Transposition flaps are useful for repair of medium-sized defects of the medial cheek (Fig. 20-8). However, advancement flaps or rotation advancement flaps are easier to design and to dissect because of the considerable mobility of the skin of the medial cheek (Fig. 20-9). This mobility is related to the ample subcutaneous fat beneath the skin of the medial cheek. Although the type of flap selected depends on the position and size of the defect, flaps used to repair medial cutaneous cheek defects generally involve some component of pivotal and advancement motion (Fig. 20-10). As the diameter of the medial cheek defect increases, the length of the laterally based flap increases, resulting in a flap with more medial advancement and less pivotal movement (see Fig. 20-10). Regardless of the amount of rotation or advancement, it is essential to minimize an inferiorly oriented vector of tension that increases the risk of postoperative lower lid retraction (Figs. 20-11 and 20-12). The flap is typically undermined in the subcutaneous tissue plane, but SMAS flaps may also be elevated to correct contour defects or to reduce wound closure tension. When using any cheek flap to repair defects adjacent to the nose or lower eyelid, the surgeon must make several decisions about flap design. First, the surgeon must decide whether the tissue medial to the defect should be sacrificed to position incisions at the nasofacial junction (see Fig. 20-12). Widening the initial defect to the position of the nasofacial sulcus allows the standing cutaneous deformity that forms from advancement inferior to the defect to be excised in the melolabial crease. Another consideration is whether to excise eyelid skin to position the scar in a subciliary position (see Fig. 20-12). Placement of the incision in the subciliary line results in a less conspicuous scar and avoids persistent lower eyelid edema but requires the removal of normal eyelid skin. A disadvantage of advancing cheek skin to the level of the subciliary line is a greater risk of ectropion or inferior eyelid retraction, particularly in older individuals with poor muscle tone. The ability of the eyelid to tolerate scar contraction can be estimated by the snap test in a fashion similar to evaluation of the muscle tone of the eyelid before blepharoplasty surgery. The subciliary line is the preferred location for positioning of scars in younger patients because of the excellent scar camouflage it provides. Properly performed advancement flaps using suture suspension of the flap to the periosteum of the bony orbital rim rarely lead to problems with eyelid retraction. However, in older patients and patients with poor orbicularis muscle tone, the eyelid skin should not be excised. If in doubt, it is far better to preserve all of the skin of the eyelid and to attempt to position scars along the inferior bony orbital rim in the aesthetic border between the cheek and the eyelid. Some authors advocate routine lateral canthopexy to tighten the lower eyelid to prevent retraction when cheek advancement flaps are used to repair cheek defects in the vicinity of the lower eyelid.6 Regardless of incision placement, it is important to anchor the flap to the periosteum of the inferior bony orbital rim to prevent excessive downward traction on the eyelid. The superior medial tip of an advancement flap may be de-epithelialized and the peninsula of dermal tissue used to anchor the flap to the periosteum of the medial canthal region to provide additional medial support to the flap.
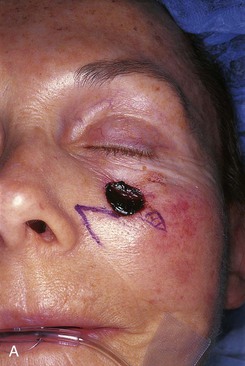
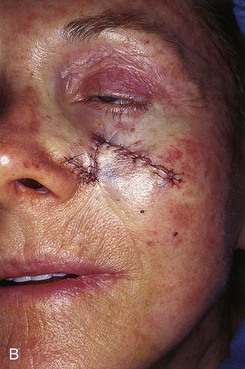
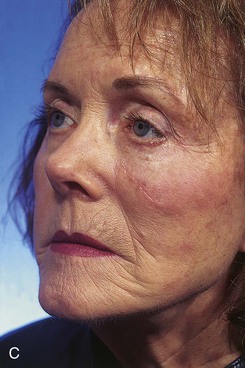
FIGURE 20-8 A, A 1.5 × 1.5-cm skin defect of medial cheek. Transposition flap designed for repair. Anticipated standing cutaneous deformity marked inferior and lateral to defect. B, Flap in place. C, Postoperative result at 2 months. (Courtesy of Shan R. Baker, MD.)
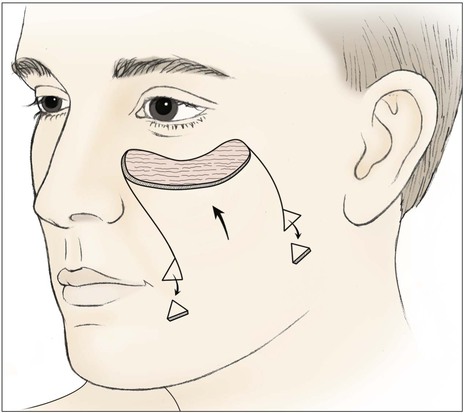
FIGURE 20-9 Unipedicle advancement flap designed to repair medial cheek defect. Standing cutaneous deformities excised at base of flap.
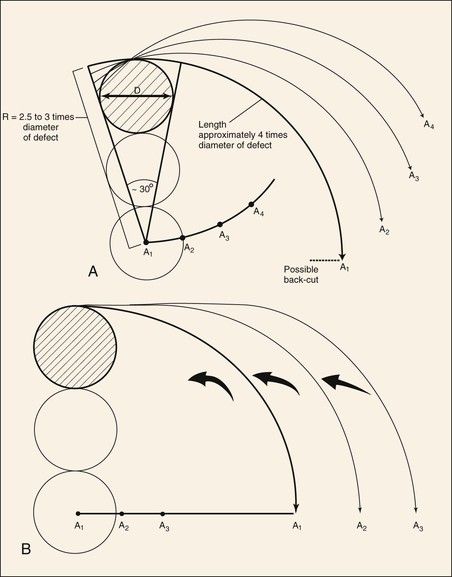
FIGURE 20-10 Dynamics of rotation advancement flaps. A, Rotation (pivotal) flaps are drawn using radius (R) that is 2.5 to 3 times diameter (D) of defect. Location of pivotal points (A1-A4) may be modified to cause flap design to change from pure pivotal (A1) to primarily advancement (A4). Note that as pivotal points progress from A1 to A4, base and tip of modified flaps become wider and flap is designed for greater advancement and less pivotal movement. B, If pivotal point (A1-A3) is moved on a line perpendicular to vertical axis of defect, flap will manifest greater advancement and less pivotal movement. (From Murakami CS, Nishioka GJ: Essential concepts in the design of local skin flaps. Facial Plast Surg Clin North Am 4:455, 1996.)
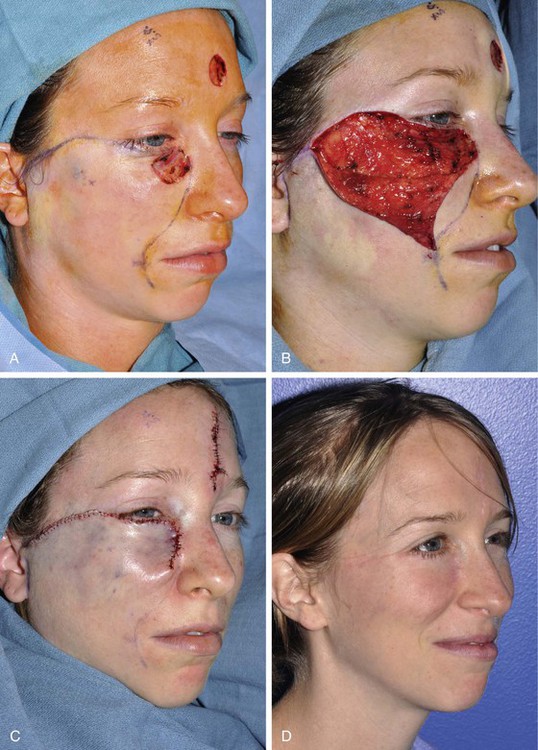
FIGURE 20-11 A, A 2 × 2.5-cm defect of medial cheek and eyelid. Rotation advancement flap designed for repair. B, Rotation advancement flap elevated in subcutaneous plane, with greater degree of advancement as flap is extended laterally toward preauricular crease. Flap reflected inferiorly. C, Flap in place. Standing cutaneous deformity at base maintained to optimize vascularity to distal aspect of flap. Deformity removed 2 to 3 weeks later. D, Postoperative result at 6 months.
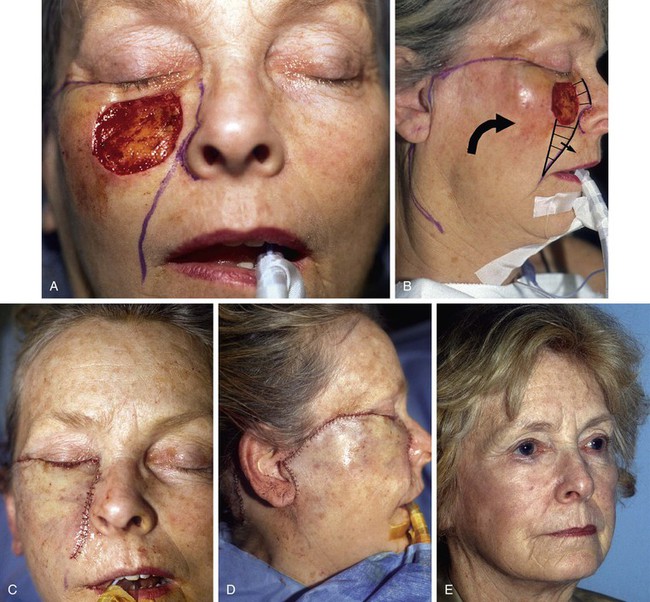
FIGURE 20-12 A, A 4 × 3-cm medial cheek defect. Rotation flap designed for repair. Incision for flap placed in subciliary line. Nasofacial sulcus and melolabial crease marked. Skin between defect and nasofacial sulcus and melolabial crease removed to position advancing border of flap in aesthetic boundary. B, Incision for flap extended to preauricular crease and posterior auricular sulcus. Anticipated standing cutaneous deformity marked with horizontal lines on melolabial fold. C, D, Flap in position. Note incision lines at level of lateral canthus. Medial border of flap positioned in nasofacial sulcus and melolabial crease. E, Postoperative result with normal eyelid position and well-camouflaged scars.
Inferiorly based pivotal advancement flaps are commonly used for reconstruction of the medial cheek, especially for defects located in the superior aspect of the medial cheek unit. The flap is dissected by making a single incision in the nasofacial sulcus and melolabial crease, maintaining the nasolabial isthmus (Fig. 20-13). The flap is undermined in the subcutaneous tissue plane and advanced superiorly and pivoted laterally. A standing cutaneous deformity develops superior and lateral to the defect. Depending on the location of the defect, the deformity may be excised, placing the resulting scar at the eyelid-cheek junction line or in the subciliary line (Figs. 20-14 and 20-15). This is in contrast to laterally based cheek rotation advancement flaps used to repair the same defect that develops standing cutaneous deformities inferiorly and medially at the base of the flap (see Fig. 20-11). An equalizing Burow triangle excision, Z-plasty, or back cut may be made at the inferior aspect of the incision used to create the inferiorly based pivotal advancement flaps to improve tissue movement and to decrease wound closure tension. Similar to other cheek flaps, inferiorly based pivotal advancement flaps are at risk for causing lower eyelid malposition. As a result, techniques such as periosteal anchoring sutures, flap tip de-epithelialization with medial canthal anchoring, and lateral canthoplasty may be used in conjunction with the flap for optimal results.7
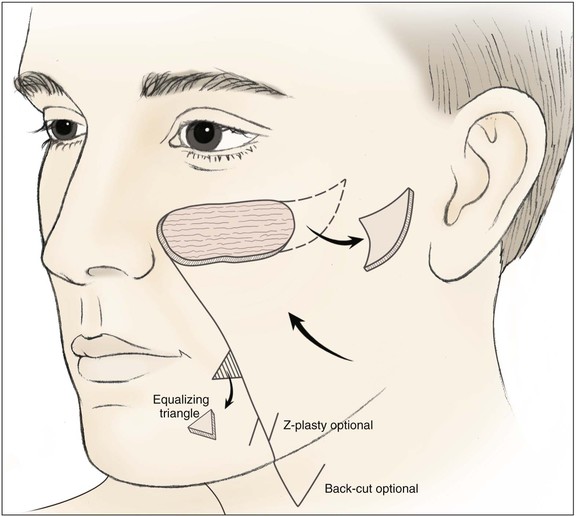
FIGURE 20-13 Pivotal advancement cheek flap. Incision for flap is in or parallel to nasofacial sulcus and melolabial crease for variable distances, depending on size of flap. Back cut or Z-plasty may be used to reduce tension on closure. An equalizing Burow triangle is excised to facilitate wound closure. Standing cutaneous deformity formed by advancement and pivotal movement of flap excised lateral to defect.
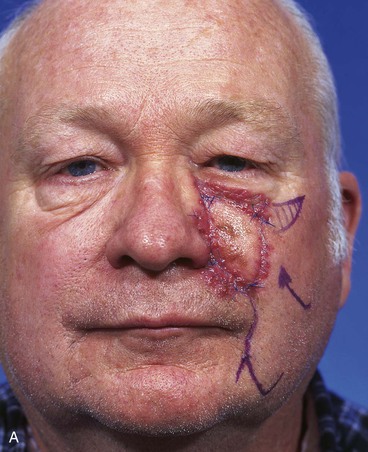
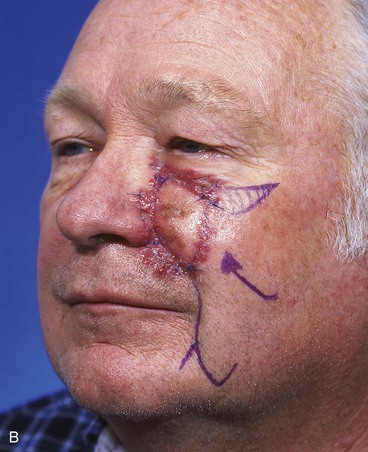
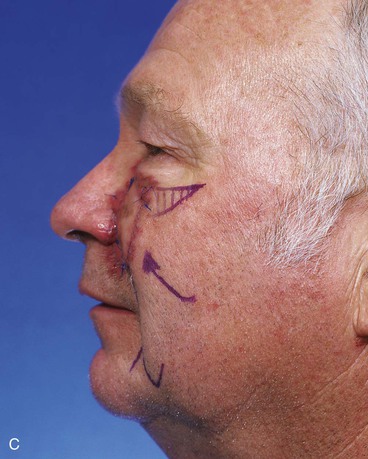
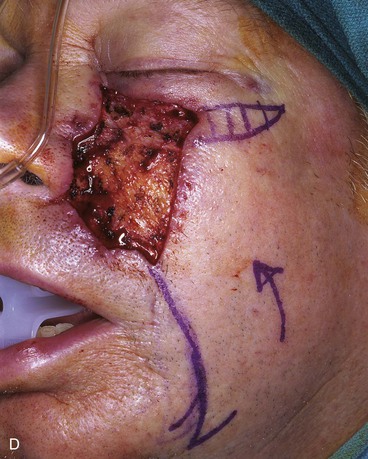
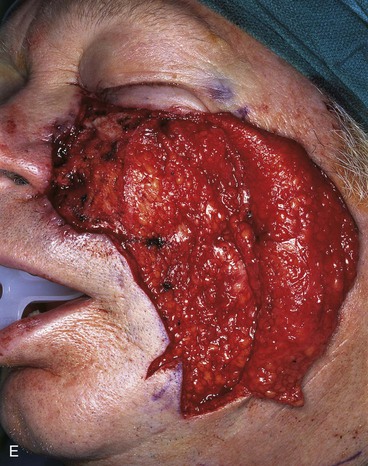
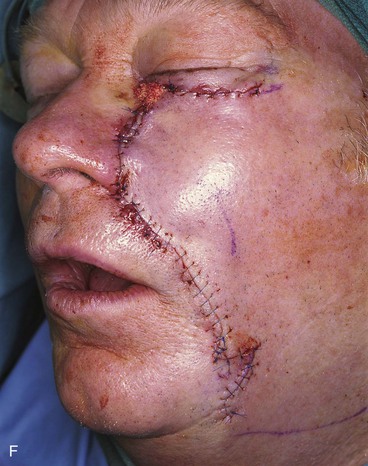


FIGURE 20-14 A-C, Melanoma in situ of medial cheek. A 5 × 3-cm area is marked for excision by the square technique to ensure tumor-free margins. Pivotal advancement flap designed for repair of defect after resection of lesion. Anticipated standing cutaneous deformity marked with vertical lines. Z-plasty designed at base of flap to eliminate need for an equalizing Burow triangle. D, E, Melanoma excised, flap dissected in subcutaneous plane. F, Flap transferred to recipient site. G, H, Postoperative views at 8 months. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
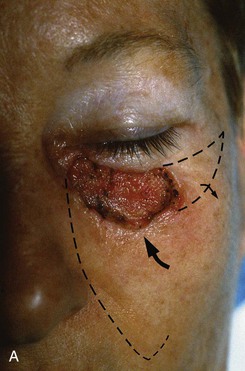

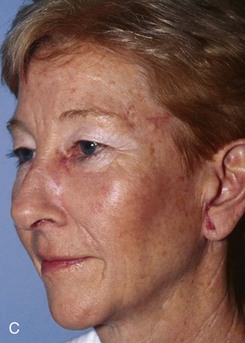
FIGURE 20-15 A, A 3 × 1.5-cm skin defect of medial cheek and lower eyelid. Pivotal advancement cheek flap designed for repair. Incision with back cut placed in nasofacial sulcus and superior melolabial crease (broken line). Standing cutaneous deformity excised laterally parallel to inferior bony orbital rim. B, C, Postoperative result with normal eyelid position.
Medium-sized cutaneous defects of the medial cheek can often be easily repaired with a V-Y subcutaneous tissue pedicle island advancement flap (Fig. 20-16). In the medial cheek, the flap depends on the sliding motion of its subcutaneous tissue pedicle for advancement. The region of the face with the most abundant fat is the medial cheek. Thus, island advancement flaps are effective methods of repairing skin and soft tissue defects of the medial cheek and lateral upper lip. The flap is particularly well suited for cutaneous defects immediately adjacent to the alae (Fig. 20-17; see also Fig. 20-5). This flap is discussed in detail in Chapter 12.
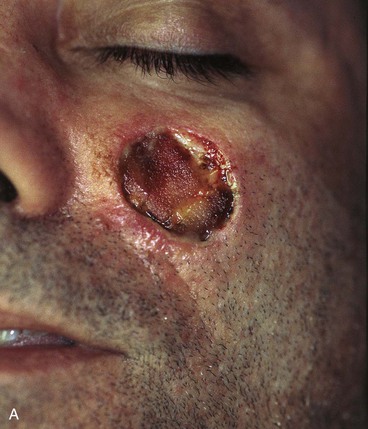
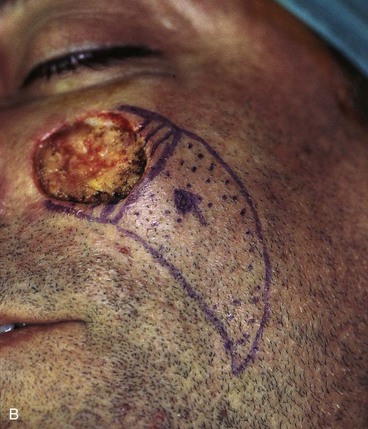
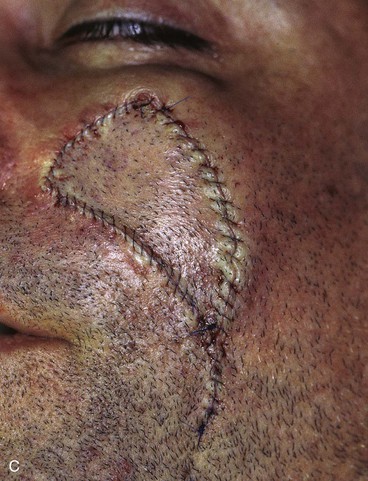
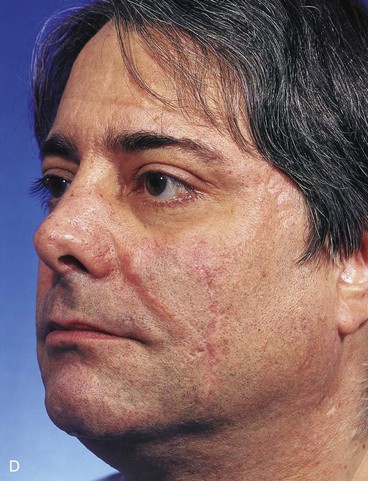
FIGURE 20-16 A, A 3 × 2-cm skin defect of medial cheek. B, Subcutaneous tissue pedicle island advancement flap designed for repair. Area marked with vertical lines excised to create square defect. Stippled area on flap indicates degree of subcutaneous undermining of flap. Nonstippled area marks location of flap’s subcutaneous tissue pedicle. C, Flap advanced and wound closed. D, Postoperative result at 1 year, 3 months. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
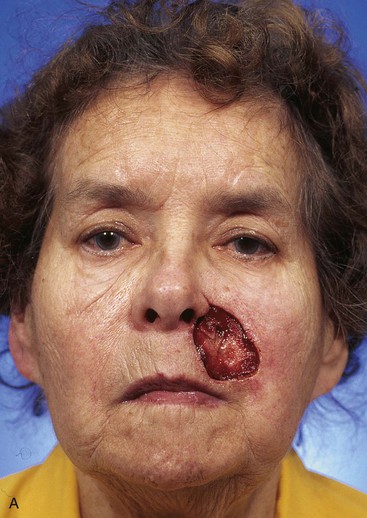
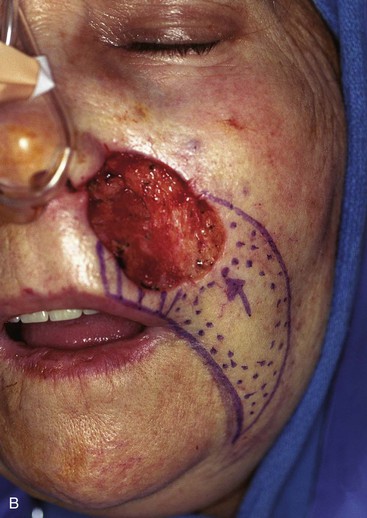
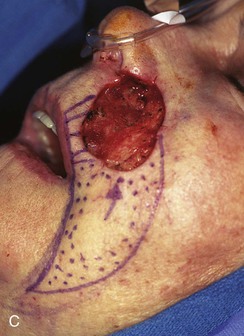
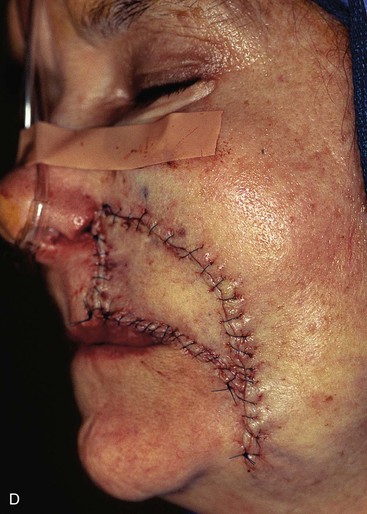
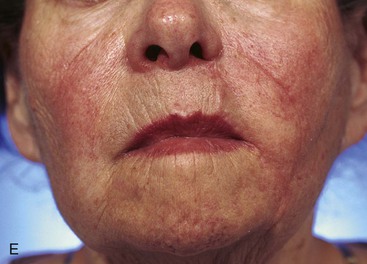
FIGURE 20-17 A, A 3 × 3.5-cm skin defect of medial cheek and upper lip. B, C, Subcutaneous tissue pedicle island advancement flap designed for repair. Vertical lines mark skin for excision so flap border will lie along vermilion border. Stippled area on flap indicates degree of subcutaneous undermining of flap. Nonstippled area marks location of flap’s subcutaneous tissue pedicle. D, Flap advanced and wound closed. E, Postoperative result at 3 months. (Courtesy of Shan R. Baker, MD.)
Reconstruction of large (more than 3 cm) medial cheek defects presents a greater challenge to the surgeon than repair of smaller cutaneous defects. Reconstruction can frequently be accomplished by transferring the lateral cheek skin medially in the form of a rotation flap (Fig. 20-18). Rotation flaps are pivotal flaps with a curvilinear border. The incision for creating such flaps usually extends to the preauricular crease and then inferiorly into the neck. Larger medial cheek cutaneous defects repaired with lateral cheek skin are usually reconstructed with local flaps that exhibit both pivotal and advancement movement to maximize the transfer of skin from the regions of the posterior cheek and superior neck (Fig. 20-19). A standing cutaneous deformity in or parallel to the melolabial crease usually develops. For larger flaps, this deformity is often maintained at the time of initial repair to optimize vascularity to the distal aspects of the flap. It is then excised a few weeks postoperatively under local anesthesia.
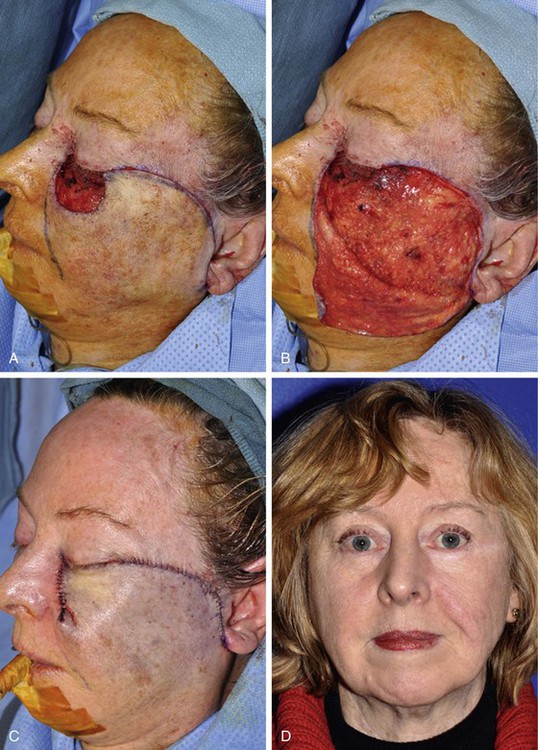
FIGURE 20-18 A, A 4 × 3-cm defect of medial cheek and lower eyelid. Rotation advancement flap designed for repair. B, Flap elevated in a subcutaneous plane. Preauricular incision extended to lobule of ear. Flap reflected inferiorly. C, Flap in place with standing cutaneous deformity parallel to melolabial crease. D, Postoperative result at 12 months. Persistent fullness of medial cheek due to incomplete removal of standing cutaneous deformity.
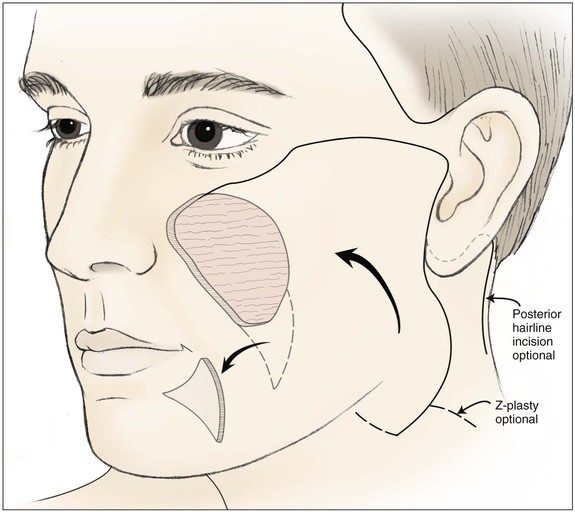
FIGURE 20-19 Cervicofacial rotation advancement flap designed for large medial cheek defect. Standing cutaneous deformity excised in melolabial crease. Incision for flap may extend into neck skin crease or posterior to ear along postauricular hairline.
The cervicofacial rotation advancement flap is a workhorse flap for reconstruction of large defects of the medial cheek. Depending on the location of the defect and its size, the flap is designed with incisions positioned either at the inferior bony orbital rim or in the subciliary line as discussed with cheek advancement flaps. If a subciliary incision is used, it is carried out beyond the lateral canthus in a curvilinear fashion. A tendency to drift inferiorly with the lateral incision should be avoided to help prevent lateral lower lid retraction on wound healing. Extending the lateral incision slightly higher than the lateral palpebral fissure allows the incision to be hidden in the crow’s-feet wrinkles and provides added support to the lower eyelid when the flap is transferred. From this lateral extension, the incision curves inferiorly to the preauricular crease. Once the incision reaches the lobule of the ear, it may be carried inferiorly and medially into the neck or extend behind the ear in the postauricular sulcus and along the hairline (see Fig. 20-19).7 The posterior extension of the incision helps camouflage part of the donor site scar behind the ear and along the postauricular hairline. When the incision is positioned behind the ear, the flap can be designed as a large modified bilobe flap. This style of flap is useful in the repair of large defects; however, the aesthetic advantage of using a postauricular incision may be lost when the two flaps are transferred to the cheek, giving rise to suture lines outlining both flaps rather than a single suture line necessary for a standard cervicofacial rotation advancement flap (Fig. 20-20).8 With larger defects, significant pivotal and advancement tissue movement of the flap is required, resulting in a standing cutaneous deformity inferior to the leading border of the flap. The deformity can be excised primarily, with the incision optimally placed in or parallel to the melolabial crease. In patients who have tenuous flaps or are at risk for ischemia (prior irradiation, smokers), the standing cutaneous deformity can be left in place and excised at a later date once the flap has healed sufficiently and survival is ensured (see Fig. 20-20). The cervicofacial rotation advancement flap is most frequently elevated in the subcutaneous tissue plane, preserving the subdermal plexus. Alternatively, the flap may be elevated in the sub-SMAS plane when a deep defect requires a thicker flap to avoid a contour irregularity (see discussion of contour defects). Anchoring the flap to the periosteum located at the inferior bony orbital rim and the nasofacial sulcus with long-lasting absorbable sutures minimizes distortion of adjacent facial structures and retraction of the lower eyelid. A back cut or an equalizing Burow triangle excision at the base of the flap is also useful to decrease wound closure tension.
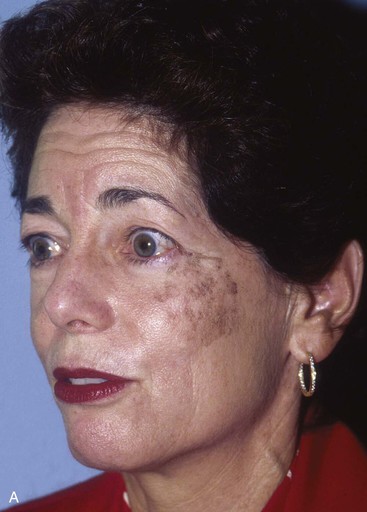
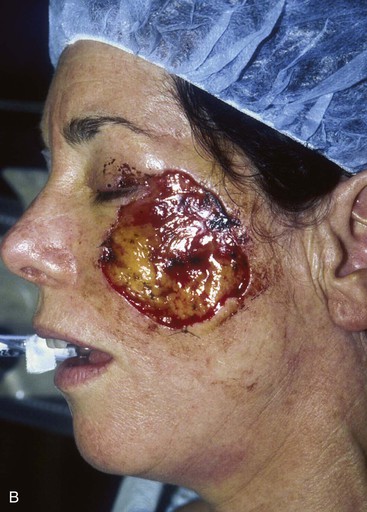
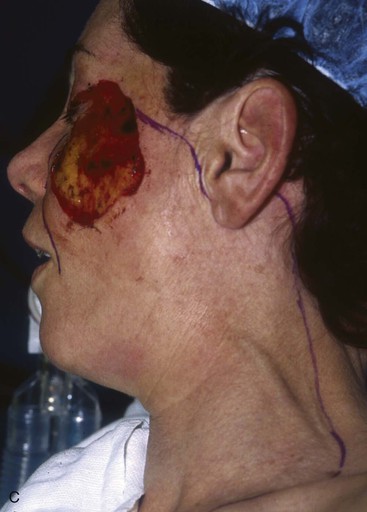
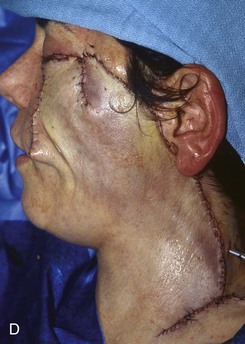
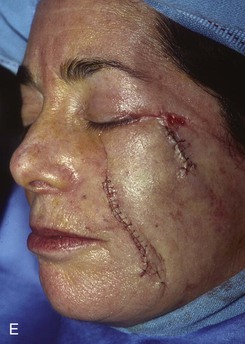

FIGURE 20-20 A, Melanoma in situ of cheek. B, A 4 × 5-cm skin defect after excision. Defect involves zygomatic and buccal aesthetic units of cheek. C, Cervicofacial rotation advancement flap designed for reconstruction. Incision line extends to lower neck to create large flap. Flap has slight bilobed configuration to place incision line more posteriorly. D, Flap in place. Back cut made at base of flap to reduce wound closure tension. E, Standing cutaneous deformities excised in second stage to ensure vascularity of flap at time of initial flap transfer. F, Postoperative result.
Sizable cutaneous defects of the medial cheek may be reconstructed with very large V-Y subcutaneous tissue pedicle island advancement flaps. This is because of the ample subcutaneous fat in the medial cheek. The abundant fat affords considerable advancement of an island of skin based on a subcutaneous pedicle (Fig. 20-21). The limiting factor for use of such flaps is the superior extent of the defect. It is usually not possible to advance island cheek flaps superior to the level of the inferior bony orbital rim.
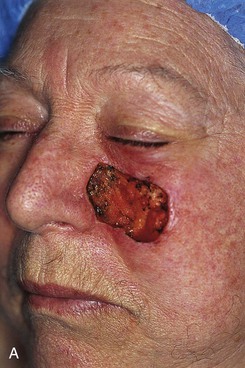
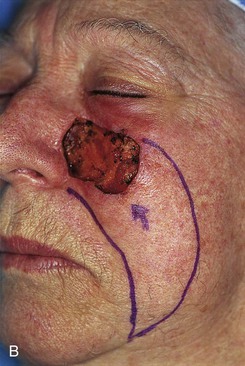
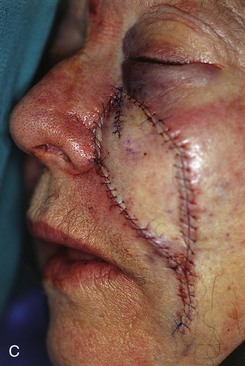


FIGURE 20-21 A, A 4 × 3-cm skin defect of medial cheek. B, Subcutaneous tissue pedicle island advancement flap designed for repair. C, Flap advanced and wound closed. D, E, Postoperative views at 1 year, 3 months. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
A skin graft may be required for reconstruction when cutaneous defects of the medial cheek are too large to be repaired with island advancement flaps or with flaps consisting of skin from the posterior cheek and superior neck. Skin grafting is infrequently used for cheek reconstruction because it generally provides inferior aesthetic results compared with the use of local flaps. However, skin grafting is particularly useful for repair of very large cutaneous defects and defects secondary to malignant disease that require careful surveillance of the area involved with the malignant neoplasm. The most frequent example of this situation is a patient with melanoma who may require wide surgical margins and prolonged surveillance. Skin grafts used for this purpose usually consist of a full-thickness graft, which may be harvested from a number of sites. The preauricular region of the opposite cheek provides skin with excellent color and textural match to native skin and allows the resultant donor scar to be well camouflaged in a pretragal crease. Postauricular skin provides a graft with a reasonable color match to the skin of the cheek and offers an inconspicuous donor site scar. The supraclavicular fossa provides an abundant source of skin with good color match to cheek skin and ensures a donor site scar easily hidden under clothing. The contralateral melolabial fold is also a donor source of skin grafts and provides the best possible color and textural match to the skin of the medial cheek. Split-thickness skin grafts contract more than full-thickness grafts and provide poorer aesthetic results. Split-thickness grafts often result in a thickness mismatch between native cheek skin and the graft. However, when a large skin graft is necessary, split-thickness grafts can more easily be harvested than full-thickness grafts.
Buccal Cheek Defects
Primary wound closure is a useful technique for repairing small (1 cm or less) buccal skin defects. The principles of primary wound closure of small buccal cheek defects are similar to those described for the medial cheek aesthetic unit. The central location of the buccal aesthetic unit of the cheek causes difficulty with positioning of scars along aesthetic boundary lines when cutaneous flaps are designed. Because many small defects resulting from micrographic surgery are circular or oval, primary wound closure in the form of an ellipse that parallels RSTLs is the preferred technique. Some defects that are close to the melolabial crease can be repaired with the scar placed in the crease. Asymmetric undermining is useful to reduce the tendency to displacement of the scar laterally toward the center of the buccal aesthetic unit. Defects that are not immediately adjacent to the melolabial crease but are in reasonable proximity can be repaired with flaps that enable placement of the resulting scar in the melolabial crease. This is accomplished by excising intervening skin between the defect and the crease and using wide asymmetric skin undermining (see Fig. 20-3).
Medium-sized (2 to 3 cm) defects that have a linear axis parallel to RSTLs can often be repaired primarily (Fig. 20-22). There are a multitude of cutaneous flaps that can be used to reconstruct medium-sized defects of the buccal area when primary wound closure is not ideal. Many of these flaps are of the transposition or advancement type. The disadvantage of such flaps is that the resulting scars often lie in the center of the aesthetic unit and are oriented in several different directions so that portions of the scars are not aligned with RSTLs. The main transposition flaps used in cheek reconstruction are reviewed here, and only key points are highlighted because details of these flaps are covered elsewhere in this volume. In general, the authors prefer pivotal advancement flaps or primary closure rather than transposition flaps for medium-sized defects.
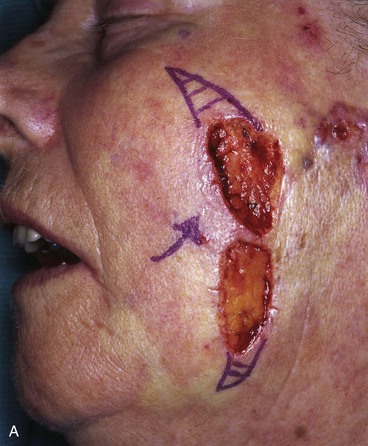
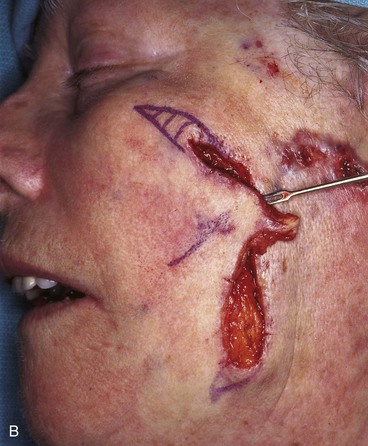
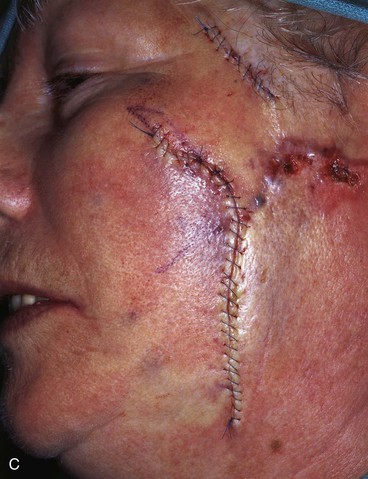
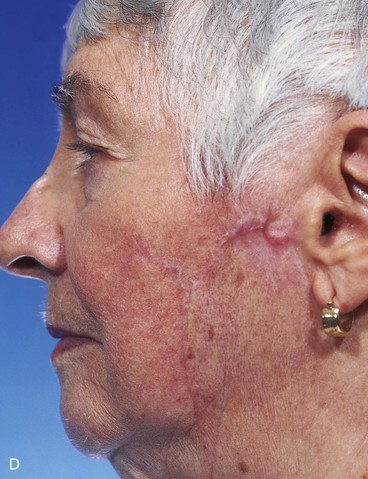
FIGURE 20-22 A, B, Two skin defects of buccal aesthetic unit oriented parallel to RSTLs. Primary wound closure planned. Anticipated standing cutaneous deformities marked by horizontal lines. C, Wound repaired. D, Postoperative result at 6 months. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
The classic Limberg flap was constructed as a rhombus with two 60° and two 120° internal angles. The geometry of the flap is precise and requires a 60° to 120° rhombus-shaped defect for the flap to properly close the cutaneous defect.9 The Dufourmentel variant of the Limberg flap enables repair of defects with less excision of normal skin to create a rhombus-shaped defect. Like the Limberg flap, the Dufourmentel flap requires a defect with a rhombus configuration; however, the internal angles of the defect may vary from the 60° and 120° angles required for the Limberg flap.10
The Webster modification of the Limberg flap uses an M-plasty at the base of the flap to limit the length of excision of the standing cutaneous deformity formed when the flap is transferred. For wound closure, the flap relies on both primary and secondary tissue movement.9,11
Bilobe flaps are double transposition flaps and consist of the movement of two flaps over an incomplete bridge of tissue.12–14 The flap may be designed in a number of different ways; the details of these designs are discussed in Chapter 10. Bilobe flaps designed with a limited pivotal arc of approximately 90° are most commonly used. The flap may be designed by creating multiple adjacent rows of circles around the defect, with the first row of circles having a surface area 80% of the defect size. A second row of circles peripheral to the first row is constructed with a surface area that is 70% of the defect size. The circles provide a template on which to design a bilobe flap. The first row of circles represents alternative positions for designing the first lobe of the flap. The second row of circles represents the size and possible locations of the second lobe of the flap. Selection of a circle from the first row for the first lobe of the bilobe flap dictates that the immediately adjacent circle from the second row will serve as the template for the second lobe of the flap. Circle selection and thus location for the flap design depend on the facial structures in the vicinity of the defect and the skin laxity in the area of the circles. The surface area of the circles is less than that of the defect because closure of the entire wound (defect plus donor sites of the lobes) depends in part on secondary tissue movement.
Cheek pivotal advancement (noncurvilinear) and cervicofacial rotation advancement (curvilinear) flaps have been discussed, and the principles for their use to repair buccal defects parallel those guiding their use for medial cheek defects. The leading border of the flap will lie in the center of the aesthetic region of the cheek (Fig. 20-23). This is in contrast to advancement flaps used to reconstruct medial defects, where the flap may often be advanced to the nasofacial sulcus and melolabial crease with minimal sacrifice of normal tissue. This is not practical in repairing most defects of the buccal unit of the cheek because of its central location.
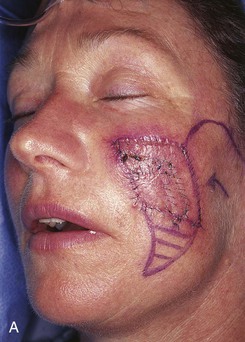
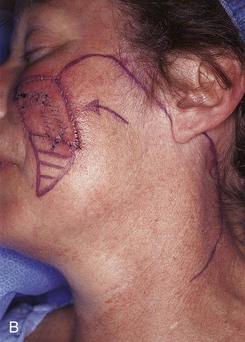
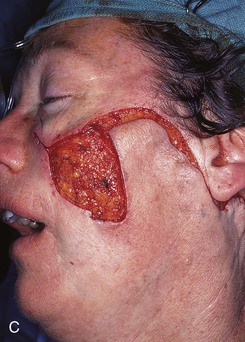
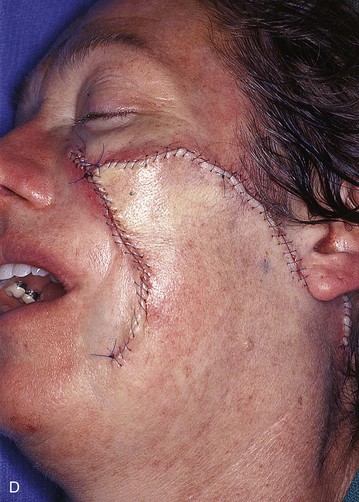
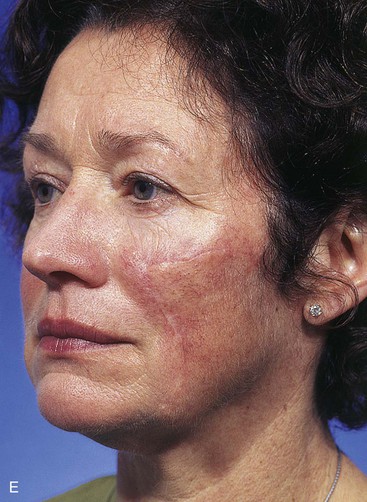
FIGURE 20-23 A, B, A 4 × 5-cm melanoma in situ marked for excision. Large cervicofacial rotation advancement flap designed for repair after excision. Anticipated standing cutaneous deformity marked with horizontal lines. C, Melanoma excised, flap dissected. D, Flap in place. Standing cutaneous deformity excised parallel to melolabial crease. E, Postoperative result at 6 months. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
Repair of large (more than 3 cm) cheek defects of the buccal area of the cheek that cannot be repaired with cervicofacial rotation advancement flaps may require the use of a full-thickness skin graft. Skin grafting techniques are similar to those performed at other locations on the face, and no special considerations exist. Meticulous hemostasis is always required to prevent hematoma formation as the initial survival of the graft relies on imbibition. Sewn-on bolster dressings and tacking sutures to anchor the graft to the wound bed are helpful techniques. Bolster dressings may consist of Vaseline or Xeroform gauze configured in a dome over the graft. The bolster is secured over the skin graft by interrupted sutures that are tied over the bolster in a radial fashion. Skin grafting and the use of skin grafts in combination with local flaps are discussed in Chapters 15 and 16.
Lateral Defects
Medium-sized (2 to 3 cm) cutaneous defects of the lateral unit of the cheek may be reconstructed with local flaps having a number of flap designs. Cheek advancement flaps are useful for closure of preauricular defects. The cheek skin in the vicinity of the defect is undermined in the subcutaneous tissue plane, with care taken to preserve the subdermal plexus. If the defect is circular, standing cutaneous deformities will develop and need to be removed; they often can be positioned in a preauricular crease. Inferior lateral defects can often be closed by advancing cervical skin upward toward the ear, with the resultant scar positioned in a preauricular crease (Figs. 20-24 and 20-25). If possible, skin defects in proximity to the preauricular crease but not immediately adjacent to the crease are enlarged, discarding the preauricular skin such that the scar can be placed preauricularly rather than in the middle of the aesthetic unit (see Fig. 20-3).
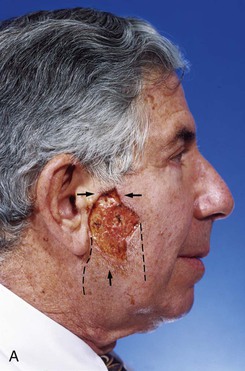
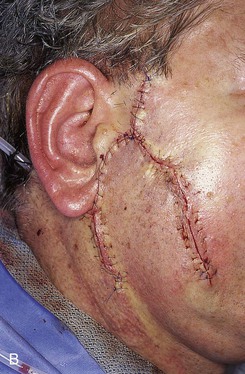
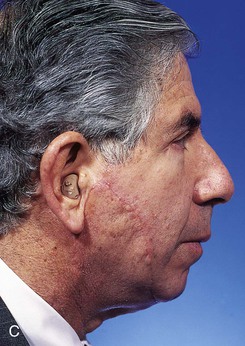
FIGURE 20-24 A, A 3.5 × 2.5-cm skin defect of lateral cheek. Broken lines indicate incisions for advancement flap used for repair of wound. Superior portion of wound closed primarily. B, Flap in place. Standing cutaneous deformities removed from either side of flap base. C, Postoperative result at 1 month. (Courtesy of Shan R. Baker, MD.)
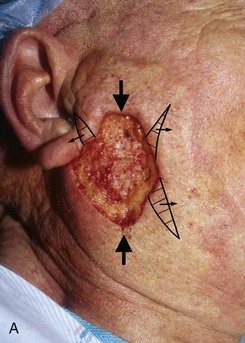
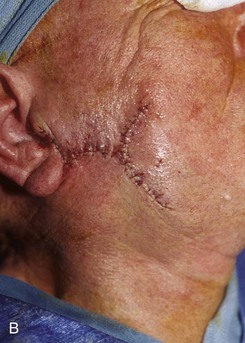
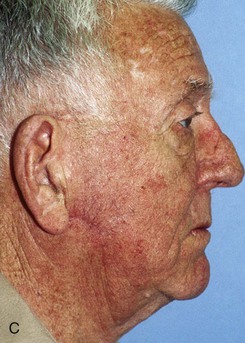
FIGURE 20-25 A, A 3 × 4-cm skin defect of lateral cheek. Large arrows indicate direction of advancement. Triangles represent anticipated excision of standing cutaneous deformities resulting from advancement. B, Skin advanced and deformities excised. C, Postoperative result.
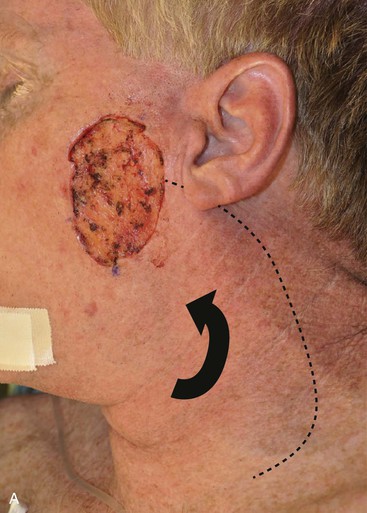
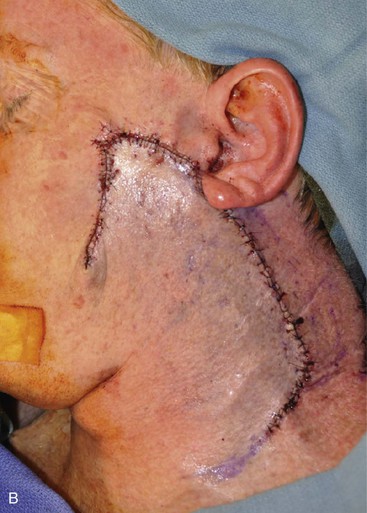
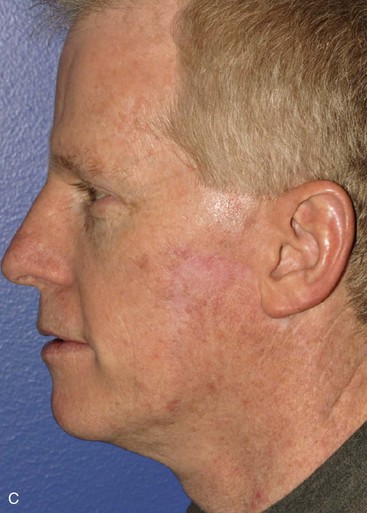
Cervicofacial rotation advancement flaps are probably the most useful flaps for reconstruction of large (more than 3 cm) lateral cheek defects. An incision is made from the lateral border of the defect toward the preauricular crease and is then curved inferiorly along the ear lobule into the neck along a skin crease. Making the incision in a skin crease of the lateral neck provides the flap with greater mobility than when the incision is placed along the postauricular hairline and border of the trapezius muscle. A more anterior positioning of the incision also creates a donor site that is easier to close, but extending the incision behind the ear and along the postauricular hairline often provides better scar camouflage (Fig. 20-26). The flap is elevated in a subcutaneous plane and for significantly large defects can be extended toward the clavicle to allow greater mobilization. A drain is often placed to minimize the risk of seroma and hematoma formation. A standing cutaneous deformity is created medial and inferior to the defect as the flap is moved toward the defect and can pose a challenge because of a tendency for the deformity to extend into the central portion of the cheek. The standing cutaneous deformity may be excised but is often intentionally maintained, primarily to optimize vascularity to distal aspects of the flap, and excised at a later date under local anesthesia (Fig. 20-27). An M-plasty is useful in reducing the length of the scar resulting from excision of the deformity.
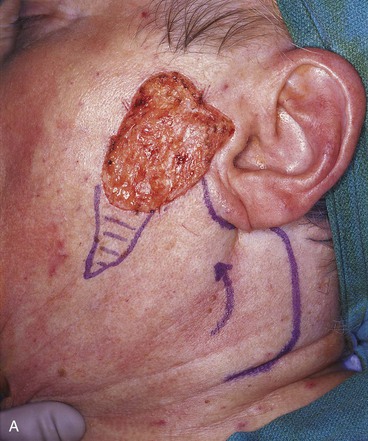
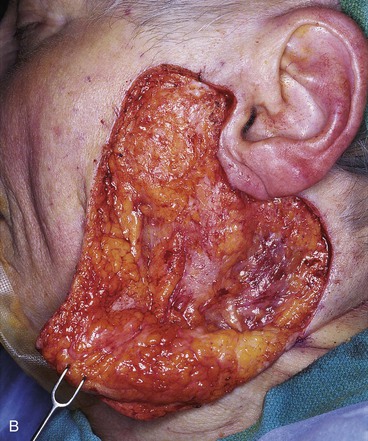
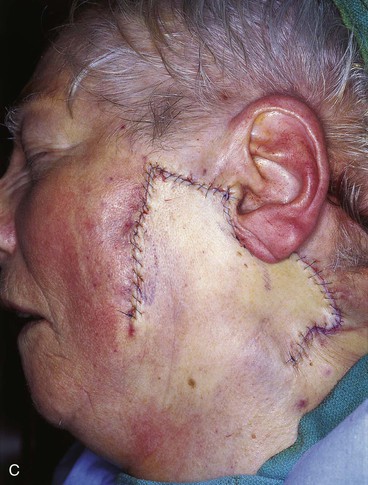
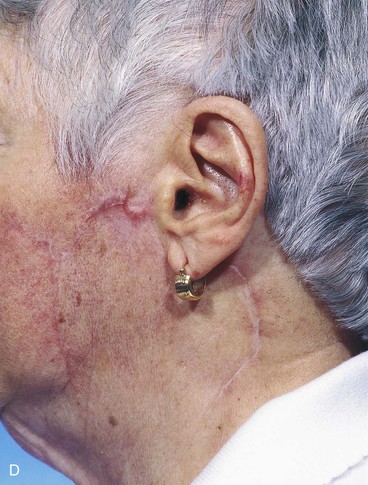
FIGURE 20-26 A, A 3.5 × 3-cm skin defect of lateral cheek. Rotation advancement flap designed for repair. Anticipated standing cutaneous deformity marked with horizontal lines. B, Flap dissected in subcutaneous plane. C, Flap in place. D, Postoperative result at 7 months. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
Zygomatic Cheek Defects
The zygomatic aesthetic unit of the cheek is the region of the cheek between the buccal unit and the temple. It represents the skin covering the zygomatic arch and malar eminence. Areas that usually provide the most mobile skin for construction of flaps used to repair cutaneous defects of the zygomatic aesthetic unit are located in the temple and buccal areas. Care is taken not to distort the lateral canthus or lower eyelid when cutaneous defects of this unit are reconstructed.
Transposition flaps are very useful for reconstruction of medium-sized (2 to 3 cm) cutaneous defects of the zygomatic unit (Fig. 20-28). Transposition flaps have been discussed before, and their design and implementation are the same for the zygomatic region of the cheek. However, the use of transposition flaps requires more forethought in this region to provide low-tension wound closures that do not distort the eyelid or lateral canthus. Particular attention is paid to the vectors of wound closure tension and lines of maximal extensibility. As an example, there are fewer alternative rhombic flaps that may be used to repair a defect in this area compared with a similar-sized defect in the buccal aesthetic unit. This is related to the inability to recruit skin from the periorbital area.
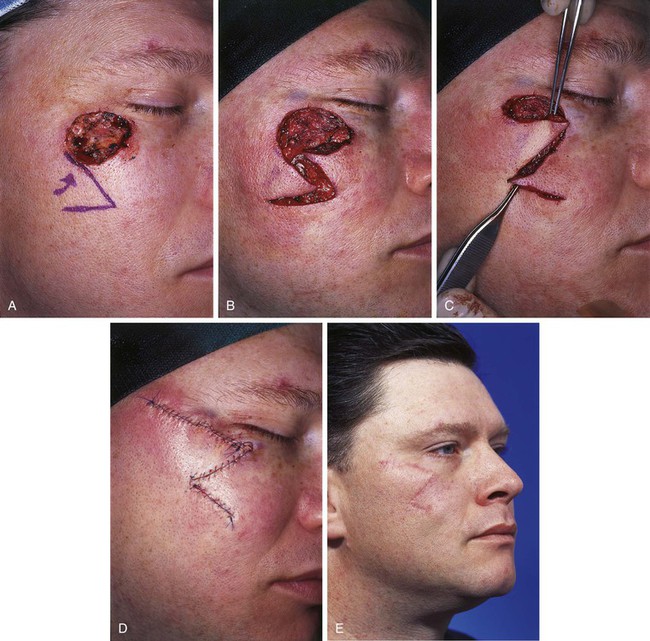
FIGURE 20-28 A, A 2.5 × 3-cm skin defect of zygomatic aesthetic cheek unit. Transposition flap designed for repair. B, C, Flap incised and transposed. D, Wound closed. Standing cutaneous deformity excised superior and lateral to defect. E, Postoperative result at 5 months. (Courtesy of Shan R. Baker, MD.)
The temporal branch of the facial nerve is relatively superficial in the zygomatic region (see discussion in Chapter 21), and the plane of dissection must be more precise to avoid injury to the nerve. Consisting of three to five branches, the temporal branch of the facial nerve traverses the midportion of the zygomatic arch. These branches are superficial relative to other branches of the facial nerve. Several methods have been used to determine their location. Correia and Zani describe temporal branches crossing the arch in the zone formed by a triangle with the apex at the ear lobe and the base formed by a line extending between the lateral brow and the temporal hairline. The temporal branches cross the zygomatic arch 2 cm posterior to the anterior end of the zygomatic arch, approximately one fingerbreadth posterior to the lateral bony orbital rim. The most posterior branch lies approximately 1.8 cm anterior to the anterior aspect of the helix. Owing to the proximity of the temporal branches to the surface of the skin of the zygomatic aesthetic unit, skin flaps are elevated above the temporoparietal fascia in the superficial subcutaneous fat immediately beneath the skin without injury to the subdermal vascular plexus. When transposition flaps are used to repair skin defects of the zygomatic area, a standing cutaneous deformity is often formed inferiorly. Depending on the precise location of the defect, the deformity may extend into the midportion of the cheek, and the scar from excision may be more conspicuous. An M-plasty may be used in this situation to reduce the inferior extent of the scar.
Multiregional Cheek Defects
Periorbital and Cheek Defects
Cutaneous defects that extend from the cheek into the lower eyelid obviate the decision regarding placement of incisions in the subciliary line versus along the inferior bony orbital rim (see Figs. 20-11 and 20-18). Properly designed cheek flaps can resurface both defects effectively. The extent of the eyelid defect in terms of thickness must be assessed. Tacking the cheek flap to the periosteum of the inferior or lateral bony orbital rim avoids excessive inferior pull by the flap on the lower eyelid margin. In patients with poor lower eyelid muscle tone, lid tightening procedures or auricular cartilage grafts may be used to support the eyelid. Auricular cartilage is used in patients at risk for eyelid retraction or secondarily in patients who have suffered unexpected eyelid retraction after repair of a cutaneous defect of the eyelid. Lower eyelid retractions are difficult to treat, and addressing them early while they are less severe and before permanent scar contracture occurs is advantageous. The use of a lateral canthopexy or superior retinacular suspension to tighten the lower eyelid is advisable in older patients during the process of reconstruction of lower eyelid defects that extend into the cheek.
Nasal and Cheek Defects
Cutaneous defects of the medial cheek that extend into the nose are best reconstructed by repairing each aesthetic region independently. The most common scenario involves repair of skin defects of the medial cheek and lateral nasal sidewall or ala. Reconstruction of these complex cheek and nasal defects usually calls for the use of an advancement cheek flap to repair the cheek component of the defect and a separate flap or skin graft to repair the nasal component of the defect (Figs. 20-29 and 20-30). When a cheek advancement flap is used, the flap is advanced medially to the nasofacial sulcus and the underside of the flap is tacked to the periosteum of the nasal process of the maxilla to minimize effacement of the nasofacial sulcus. If the nasal portion of the defect is small, undermining of the nasal skin may allow primary closure with the resulting scar in the nasofacial sulcus. Larger defects that cannot be repaired primarily may be repaired with a full-thickness skin graft or an interpolated paramedian forehead flap, depending on the size and depth of the nasal defect. For medial cheek defects that also require full-thickness repair of the ala, staging the reconstruction by repairing only the cheek defect at the first operation is considered. Surgical staging during a period of a few weeks allows any lateralization of the cheek flap to occur, resulting in a stable cheek platform on which to construct the ala. This minimizes the risk of any lateralization of the alar base that can occur when both defects are repaired at the same setting (see Fig. 20-30). Staging must be balanced with the patient’s desires and ability to tolerate multiple surgical procedures.
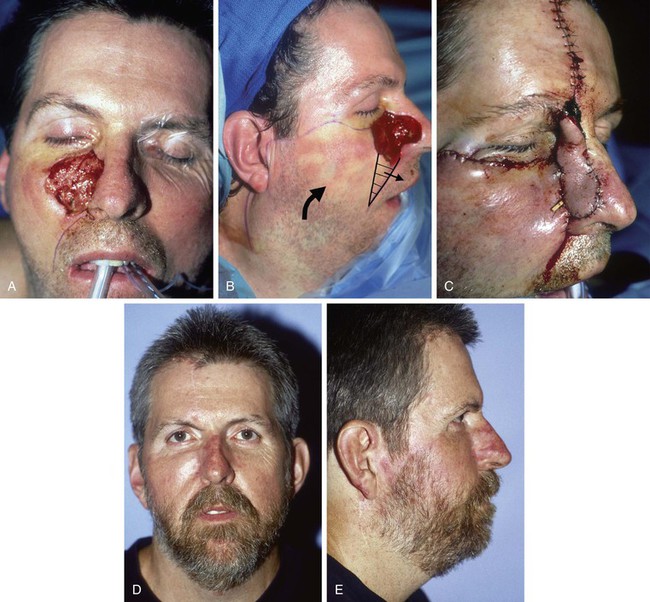
FIGURE 20-29 A, Skin defect of medial cheek and nasal sidewall after excision of basal cell carcinoma. B, Cervicofacial rotation advancement flap designed for repair. Superior border of flap designed at level of inferior bony orbital rim. Anticipated standing cutaneous deformity marked with horizontal lines. C, Flap in place. Interpolated paramedian forehead flap used to repair nasal sidewall. D, E, Postoperative result. (From Tollefson TT, Murakami CS, Kriet JD: Cheek repair. Otolaryngol Clin North Am 34:640, 2001.)
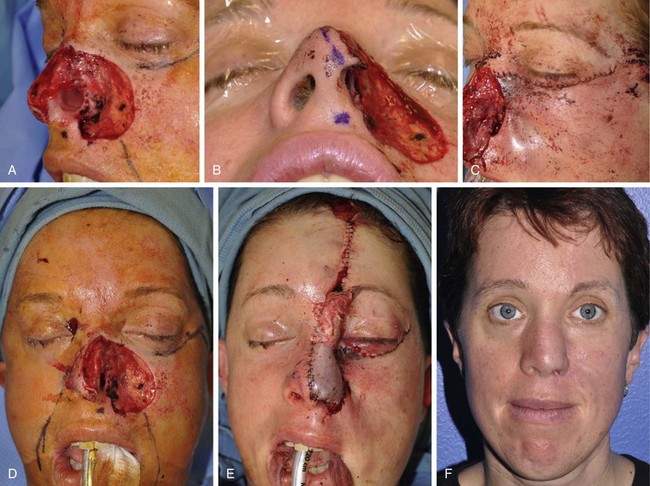
FIGURE 20-30 A-C, Full-thickness nasal defect of ala, tip, and sidewall with extension into medial cheek. D, Laterally based cheek advancement flap incised along subciliary crease and advanced to nasofacial sulcus. Lateral aspect of flap secured to lateral orbital rim periosteum to prevent lower lid retraction. Ipsilateral septal mucoperichondrial hinge flap reflected outward for lining of ala. E, Defect repaired with cartilage grafts and paramedian forehead flap. Low hairline resulted in hair follicles transposed to distal portion of flap. F, Postoperative result at 2 years. Postoperative laser therapy performed to eradicate hair follicles on nose. (From Bhrany AD: Complex nasal reconstruction: a case study: reconstruction of a full-thickness nasal defect. Facial Plast Surg Clin North Am 19:183-195, 2011.)
Contour Defects: Subcutaneous Adipose Flap and SMAS Flap
The SMAS is a fibrofatty tissue layer that supports the overlying skin. The SMAS is infrequently involved with cutaneous malignant neoplasms and is therefore usually available for use in reconstruction of defects after micrographic surgery. SMAS defects occur only in more advanced tumors that require deeper resection. In these more advanced cases, two options exist for addressing contour problems that may occur after reconstruction. The surgeon may dissect the flap used for the repair in a deeper tissue plane, including a greater amount of subcutaneous fat within the flap. When sufficient subcutaneous fat is not available, the other option is to dissect the SMAS flap independent of a cutaneous flap. The SMAS flap is used to fill the depths of the wound; the skin flap is used to cover the defect. The SMAS extends over the cheek, inserting at the melolabial crease, and is continuous with the platysma inferiorly. Superiorly, the SMAS is discontinuous at the zygomatic arch but is present in the temporal region in the form of the temporoparietal fascia and then as the galea of the scalp. A flap of SMAS tissue is elevated superficial to the zygomaticus major muscle to prevent injury to the buccal branches of the facial nerve. When the SMAS is undermined laterally to medially, the zygomaticus major is most easily and safely identified at its origin from the zygoma. The muscle is then followed inferiorly, remaining superficial to the muscle to protect the facial nerve branches that innervate the muscle on its deep surface. Because the SMAS invests the zygomaticus major, the natural plane is to continue beneath the zygomaticus major. This path of least resistance must be avoided, and a transition to a more superficial plane should be made as the muscle is encountered. Finding the muscle superiorly at its origin allows the surgeon to define tissue planes with greater ease and confidence. This maneuver is familiar to surgeons who perform deep plane rhytidectomies.15,16
Neck and Cheek Defects
Advanced cutaneous malignant neoplasms of the cheek that involve the parotid gland and neck often result in large defects that require significant bulk for adequate reconstruction. The supraclavicular artery island flap is a pedicled fasciocutaneous flap useful to repair these defects.17,18 The flap is based on the supraclavicular artery as it arises from the transverse cervical artery (Fig. 20-31). Intraoperatively, a Doppler signal confirms the location of the artery in the triangular fossa bordered by the clavicle and the sternocleidomastoid and trapezius muscles. The flap is then designed, reliably having dimensions of up to 24 × 8 cm, with the ability to reach lateral, buccal, and zygomatic cheek defects.18,19 Proximal portions of the flap are de-epithelialized and tunneled subcutaneously under the neck skin to reach facial defects. Advantages of the flap include low donor site morbidity and the option to reconstruct large, deep defects with cervical skin and soft tissue without the need for free tissue transfer.
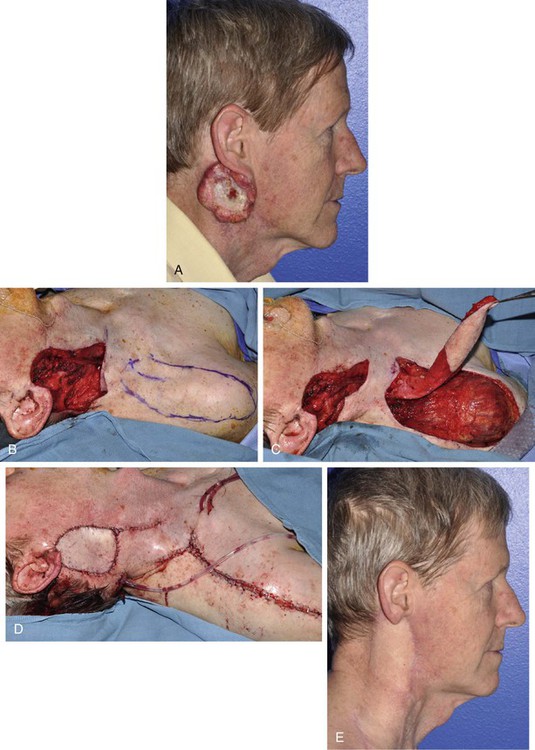
FIGURE 20-31 A, Squamous cell carcinoma involving ear, cheek, neck, and parotid gland. B, A 9 × 7-cm defect after resection, with X identifying supraclavicular artery Doppler site and flap designed extending to shoulder. C, Flap elevated, proximal portion de-epithelialized and tunneled under neck skin. D, Flap in place. E, Postoperative result at 4 months.
Summary
The cheek, which has a broad, slightly convex surface, is the largest facial aesthetic region, and the surgeon is frequently required to reconstruct cutaneous defects in this area. The cheek has a distinct anatomic configuration that assists with reconstruction by local skin flaps. The relative abundance of skin and subcutaneous fat of the cheek provides multiple surgical options for repair of cutaneous defects. A framework for selection of the proper flap based on defect location and size is presented to assist the reader in selecting the ideal method of repair. Local flap design is based on the orientation of RSTLs in relationship to the cheek aesthetic units.
References
1. La Trenta, GS. Atlas of aesthetic face and neck surgery. Philadelphia: WB Saunders; 2004.
2. Janfaza, P, Nadol, JB, Galla, RJ, et al. Surgical anatomy of the head and neck. Philadelphia: Lippincott Williams & Wilkins; 2000.
3. Pierard, GE, Lapiere, CM. Microanatomy of the dermis in relation to relaxed skin tension lines and Langer’s lines. Am J Dermatopathol. 1987; 9:219.
4. Larrabee, WF, Jr., Sutton, D. The biomechanics of advancement and rotation flaps. Laryngoscope. 1981; 91:726.
5. Rustad, TJ, Hartshorn, DO, Clevens, RA, et al. The subcutaneous pedicle flap in melolabial reconstruction. Arch Otolaryngol Head Neck Surg. 1998; 124:1163.
6. Jelks, GW, Jelks, EB. Prevention of ectropion in reconstruction of facial defects. Clin Plast Surg. 2001; 28:297.
7. Menick, FJ. Reconstruction of the cheek. Plast Reconstr Surg. 2001; 108:496.
8. Cook, TA, Israel, JM, Wang, TD, et al. Cervical rotation flaps for midface resurfacing. Arch Otolaryngol Head Neck Surg. 1991; 117:77.
9. Larrabee, WF, Jr., Trachy, R, Sutton, D, Cox, K. Rhomboid flap dynamics. Arch Otolaryngol. 1981; 107:755.
10. Lister, GD, Gibson, T. Closure of rhomboid skin defects: the flaps of Limberg and Dufourmentel. Br J Plast Surg. 1972; 25:300.
11. Murakami, CS, Nishioka, GJ. Essential concepts in the design of local skin flaps. Facial Plast Surg Clin North Am. 1996; 4:455.
12. Murakami, CS, Odland, PB. Bilobe flap variations. Oper Tech Otolaryngol Head Neck Surg. 1993; 4:76.
13. McGregor, JC, Soutar, DS. A critical assessment of the bilobed flap. Br J Plast Surg. 1981; 34:197.
14. Zitelli, JA. The bilobed flap for nasal reconstruction. Arch Dermatol. 1989; 125:957.
15. Mitz, V, Peyronie, M. The superficial musculo-aponeurotic system (SMAS) in the parotid and cheek area. Plast Reconstr Surg. 1976; 58:80.
16. Coppini, L, Zigiotti, GL, Messerotti, G, et al. The superficial musculo-aponeurotic system (SMAS) of the face and neck. Anatomy and surgical dissection. Acta Otorhinolaryngol Ital. 1983; 3:427.
17. Vinh, VQ, Anh, TV, Ogawa, R, Kyakusoku, H. Anatomical and clinical studies of the supraclavicular flap: analysis of 103 flaps used to reconstruct neck scar contractures. Plast Reconstr Surg. 2009; 123:1471.
18. Chiu, ES, Li, PH, Friedlander, PL. Supraclavicular artery island flap for head and neck oncologic reconstruction: indications, complications and outcomes. Plast Reconstr Surg. 2009; 124:115.
19. Chan, JWH, Wong, C, Ward, K, et al. Three- and four-dimensional computed tomographic angiography studies of the supraclavicular artery island flap. Plast Reconstr Surg. 2010; 125:525.