Reconstruction of the Auricle
Anatomy and Embryology
Topographic landmarks of the auricle are illustrated in Figure 22-1. The cartilaginous framework is composed of several distinct three-dimensional components. Medially, these components are the conchal, antihelical, antitragal, helical, and lobular complexes.1 These units are critical in planning the reconstruction of defects involving cartilage replacement.2
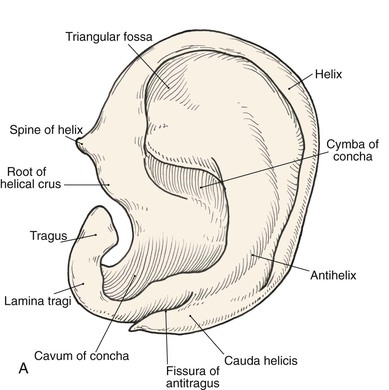
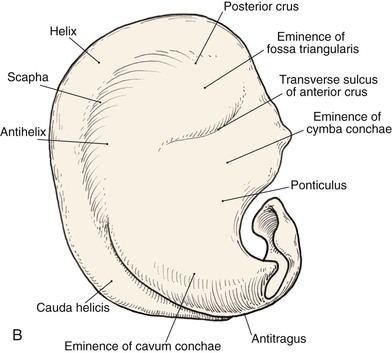
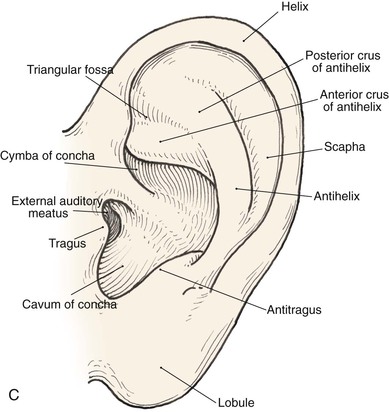
FIGURE 22-1 Auricular cartilage anatomy. A, Lateral aspect. B, Medial aspect. C, Topographic landmarks of the lateral surface of auricle.
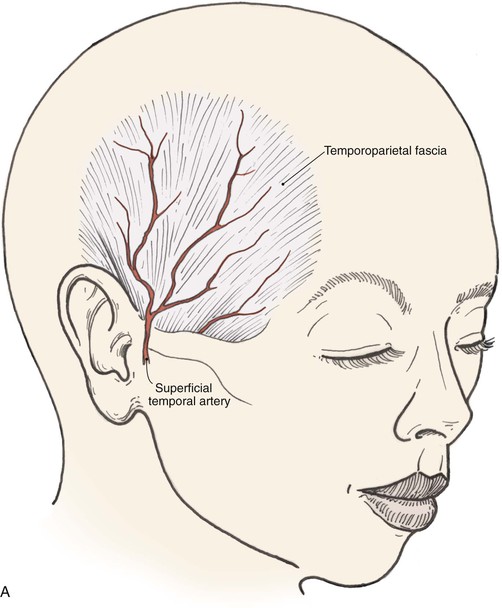
The arterial supply to the auricle is derived from branches of the superficial temporal artery and the postauricular artery. Both vessels arise from the external carotid artery.3 The medial surface of the ear is supplied by the postauricular artery. The lateral surface is supplied by both the postauricular and superficial temporal arteries, creating two arterial networks.4 The triangular fossa—scapha network is supplied by the helical branch of the superficial temporal artery. The conchal network is supplied by the postauricular artery, which provides septal perforators to the conchal floor (Fig. 22-2). Flap planning and design should give due consideration to these arterial networks. Venous drainage is through the postauricular vein, which drains into the external jugular system. There is supplemental venous drainage from the superficial temporal and retromandibular veins. Lymphatic drainage of the auricle is primarily to the preauricular, infra-auricular, and mastoid lymph nodes.
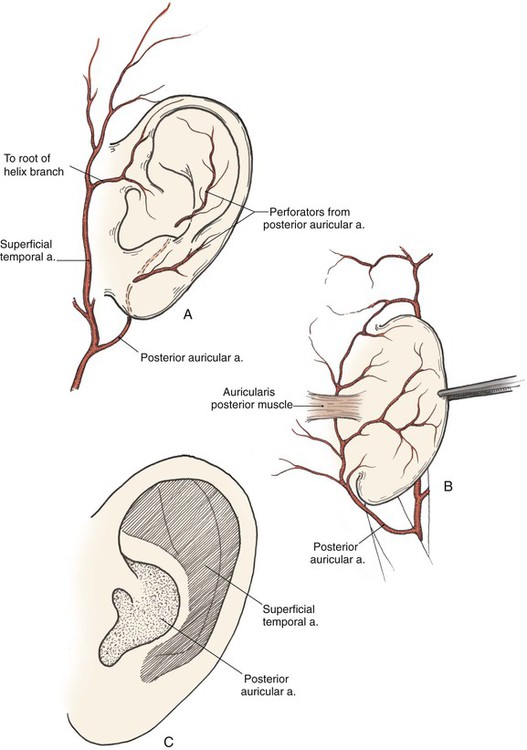
FIGURE 22-2 Arterial blood supply to auricle. A, Lateral aspect. B, Medial aspect. C, Lateral aspect of auricle, with indicated zones supplied by superficial temporal and postauricular vascular systems.
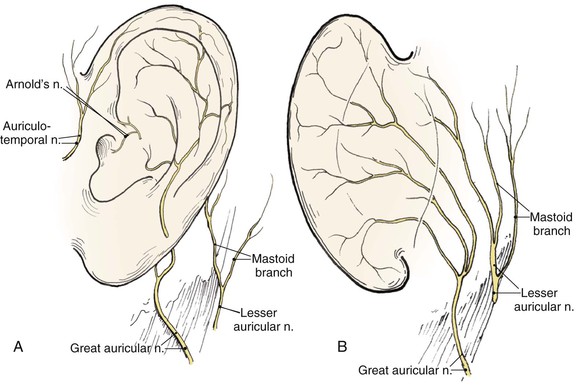
The auricle is innervated by contributions from both cranial and spinal nerves. They include the great auricular nerve (C2, C3), the auriculotemporal nerve (V3), the lesser occipital nerve (C2, C3), and a branch of the vagus nerve (X, Arnold’s nerve) (Fig. 22-3). The great auricular nerve divides into an anterior and posterior branch. The anterior branch supplies the lower half of the lateral aspect of the auricle. The posterior branch supplies a similar area on the medial surface of the auricle. The auriculotemporal nerve supplies the superolateral surface of the auricle. The lesser occipital nerve supplies the superior aspect of the ear on the medial side. The concha is supplied by Arnold’s nerve.
The auricle arises embryologically from the first and second branchial arches.5 Hillocks appear in these arches during the sixth week of gestation. The anterior hillock gives rise to the tragus, the root of the helix, and the superior helix; the posterior hillock becomes the antihelix, tragus, and lobule. The first branchial groove forms the external auditory meatus and concha.
Auricular Architecture
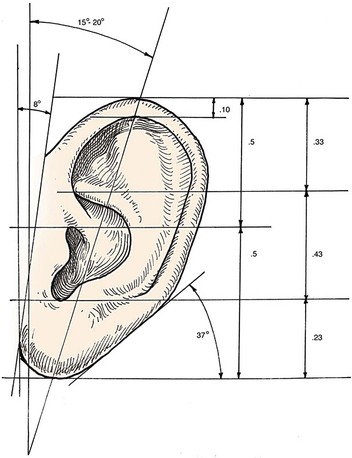
Analysis of the dimensions and proportions of the external ear is important for planning reconstructive procedures.6–9 The vertical height of the ear roughly matches the distance between the lateral orbital rim and the root of the helix, at the level of the eyebrow. Ear width is approximately 55% of its length (Fig. 22-4), and the helical rim protrudes 1- to 2.5-cm from the skull. The angle of protrusion from the skull typically ranges from 25° to 30°. The vertical axis of the ear is inclined posteriorly at an angle of approximately 15° to 20°.10,11 The original anatomic descriptions of auricular orientation suggested that the long axis of the ear was roughly parallel to the dorsum of the nose, but it is now widely recognized that the ear is rotated 10° anterior to that plane. The superior level of the ear matches the level of the lateral eyebrow. To account for individual variations in topographic features, positioning, and symmetry, the contralateral ear is used as a template in planning reconstruction, rather than strict adherence to textbook-defined norms. The most important principle of auricular reconstruction is that size, location, and orientation of the reconstructed ear be similar to the normal ear. The dominant features that make an ear recognizable are the helix, tragus, antitragus, and conchal bowl.8,9
General Principles
Auricular defects may be divided into defects of cutaneous coverage, with or without intact cartilage, and defects that are full thickness. Small cutaneous defects of the helical rim may be reconstructed with primary wound closure and occasionally require small excisions of cartilage to avoid distortion. Defects of the densely adherent lateral auricular skin can rarely be closed primarily, whereas those of the more pliable medial surface can often be repaired primarily. The pliability of medial auricular skin permits the harvest of relatively large postauricular skin grafts with primary closure of the donor site. Lateral cutaneous defects that have intact cartilage are best treated with skin grafts, provided there is intact perichondrium over the exposed cartilage. The contralateral postauricular skin is an excellent donor site for a full-thickness skin graft used to resurface the lateral ear. Preauricular skin may also be used with minimal donor site morbidity. In cases in which the perichondrium is absent, resulting in an avascular surface, cartilage may be removed if it is not critical to auricular shape (see examples in Chapter 15). Windows created through the cartilage provide surface contact between the graft and the vascularized subcutaneous tissue of the medial aspect of the auricle. The cartilage of the concha, fossa triangularis, and scapha are amenable to partial removal without loss of structural integrity of the ear. The cartilaginous windows provide access to the perichondrium of the medial aspect of the ear, which in turn provides sufficient vascularity for successful skin grafting.
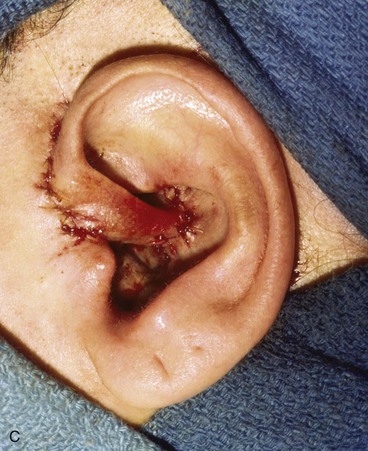
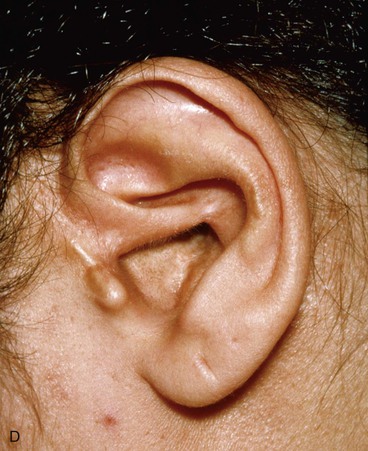
Surgical approaches for reconstruction of defects of skin and underlying cartilage include converting the defect into a full-thickness wedge-shaped excision, followed by primary wound closure, and employing a composite graft or flap that incorporates structural support. The size and location of the defect dictate which alternative is most appropriate. Defects less than 1.5 cm of the helix or antihelix are best repaired by conversion to a wedge-shaped excision. The wound is then repaired primarily and results in a minimally visible scar. Whereas this approach does reduce vertical height of the ear, the difference with the contralateral ear is usually not conspicuous. When a wedge-shaped excision of the auricle is performed, excessive wound closure tension is avoided by removing a portion of the concha to accommodate advancement of the two borders of the ear defect. If this is not performed, cupping and distortion of the ear will occur. Medium-sized helical defects, from 1.5- to 2-cm, can be managed by composite grafting from the contralateral ear.12 Typically, a composite graft half the size of the defect is transplanted from the opposite ear, thereby creating two ears of equal size (Fig. 22-5). The cartilage edges of the graft and the recipient site are securely approximated with a row of interrupted sutures. Graft immobilization is essential for proper healing and can be accomplished by use of bolster sutures to secure a splint over the ear. De-epithelialization of the medial aspect of the composite graft and its implantation into a subcutaneous recipient site have been described. The graft is released from its soft tissue attachment in a second surgical stage. This approach is designed to increase graft survival. Transfer of a composite graft from the contralateral ear exposes both ears to some risk of infection and deformity, so its use must be carefully considered in view of this risk.

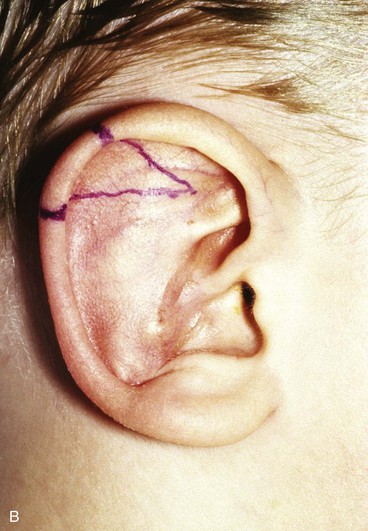
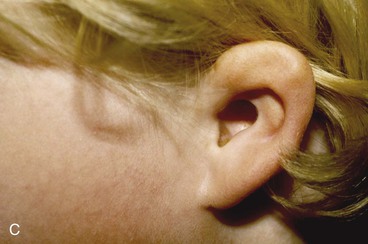
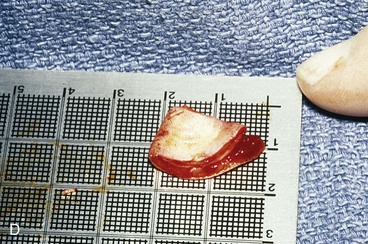
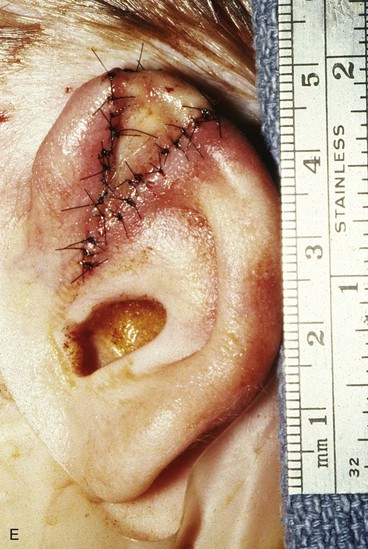
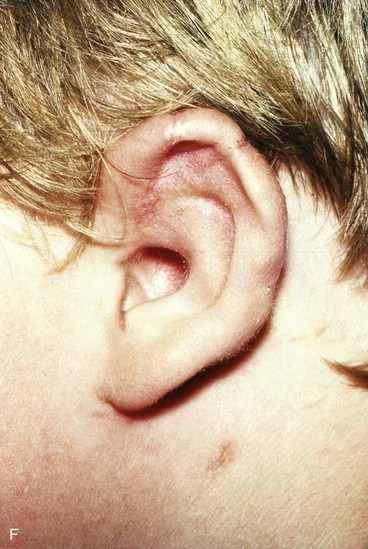
FIGURE 22-5 Left congenital auricular deformity with more than 20-mm difference in vertical height between auricles. A, Preoperative view. B, Donor ear composite graft outlined (half size of recipient deficit height). C, Preoperative view of affected ear. D, Harvested composite graft. E, Graft in place. Vertical height increased by 1 cm. Epithelium removed from medial aspect of graft, and medial aspect implanted in subcutaneous pocket. F, Postoperative result at 12 months.
Many local flaps have been described for repair of full-thickness auricular defects.13–22 Chondrocutaneous composite advancement flaps are best suited to repair of full-thickness helical rim defects. Helical chondrocutaneous composite flaps take advantage of the available tissue of the remaining helical rim and the redundant lax skin of the lobule. Anteriorly, a V-Y advancement of the helical root designed as an island flap is usually combined with a posterior chondrocutaneous composite advancement flap to achieve closure of helical rim defects (Fig. 22-6). Chondrocutaneous composite flaps may be advanced with the medial skin intact or incised. Although Antia originally described keeping the medial skin intact, there is greater ease of flap advancement with at least a limited incision of the medial skin. The arterial network of the helix-scapha region allows the creation of long chondrocutaneous composite flaps on a narrow pedicle. Depending on the size of the helical defect, Burow triangles are excised to facilitate advancement of the flaps (Figs. 22-7 and 22-8).23 Chondrocutaneous flaps in the form of transposition flaps may also be used for reconstruction of nonhelical defects.
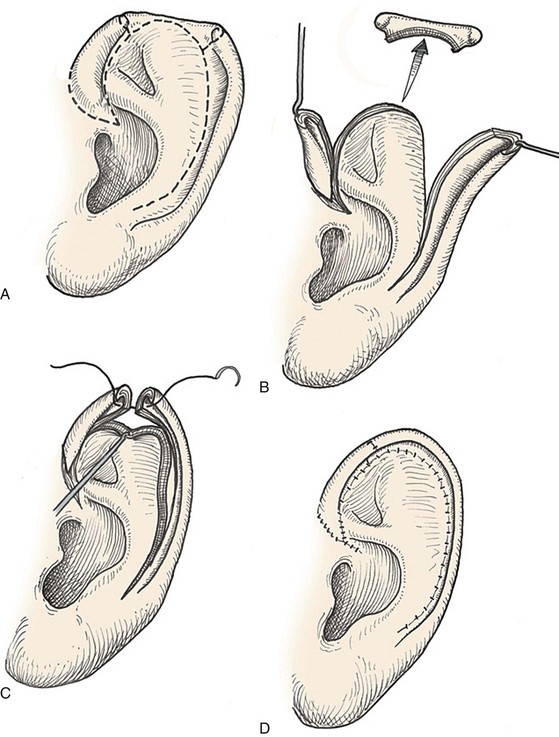
FIGURE 22-6 Auricular chondrocutaneous composite flaps. A, Helical defect. Broken lines indicate incisions through skin and cartilage. B, Island advancement flap consisting of helical root advanced on medial soft tissue pedicle. Remaining helix pedicled on earlobe. C, Flaps advanced. D, Wound closure.
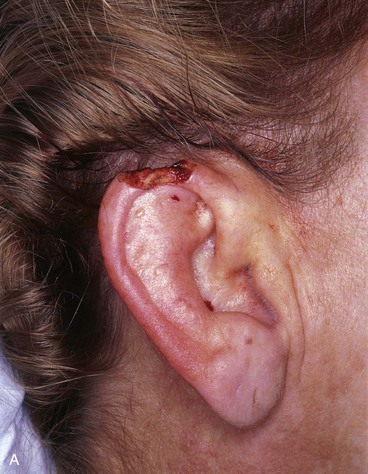
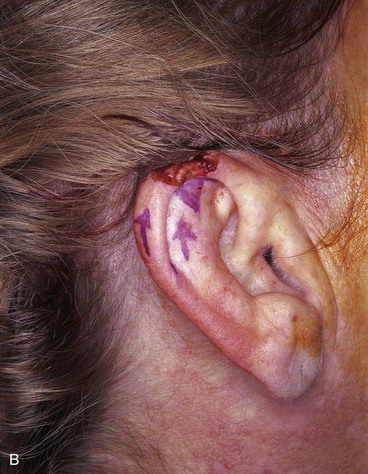
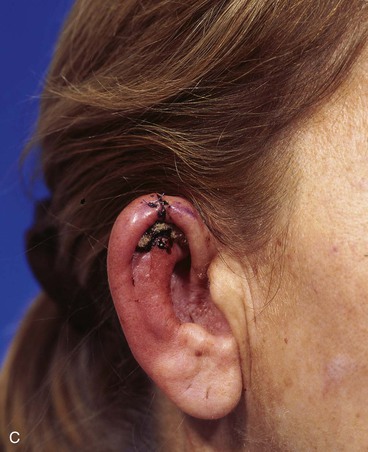
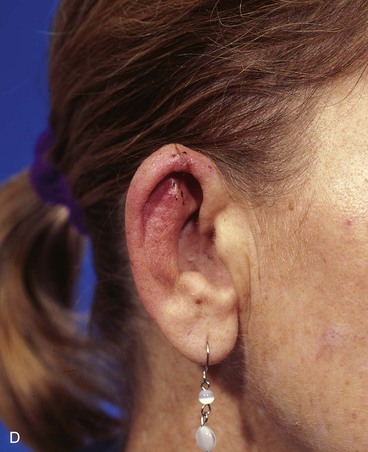
FIGURE 22-7 A, A 1.5-cm full-thickness defect of helix. B, Unilateral chondrocutaneous composite helical advancement flap planned for repair. Burow triangle marked for excision to facilitate advancement. C, Flap in place. Bolster dressing secured with sutures to maintain contour of scapha. D, Postoperative result at 7 days. (Courtesy of Shan R. Baker, MD.)
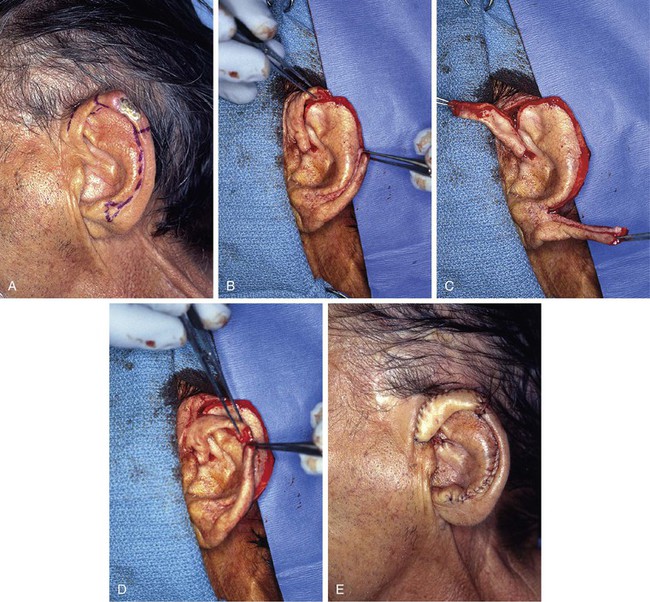
FIGURE 22-8 A, Squamous cell carcinoma of helix marked for excision. Broken lines outline planned incisions for bilateral chondrocutaneous composite helical advancement flaps after excision of tumor. B, Tumor excised. One-third of helix removed. C, Advancement flaps incised. D, Apposition of flaps demonstrates requirement for reduction of scapha and superior antihelix. E, Wound closed after reduction. (Courtesy of Shan R. Baker, MD.)
Both postauricular skin and preauricular skin serve as ideal donor sites for the creation of interpolated tube flaps. These flaps are used to reconstruct longer segments of the helical rim that are too large for reconstruction with a chondrocutaneous advancement flap. Initially, the flap is dissected as a bipedicled flap that is tubed on itself (Fig. 22-9A). After 3 weeks, one end of the cutaneous tube is detached from its origin and secured to one border of the helical rim defect. Three weeks later, the other end of the tube is incised and inset in the other border of the helical defect. Interpolated tube flaps are most useful for reconstruction of defects confined to the helical rim that are more than 2.5 cm in length (Fig. 22-10E, F).
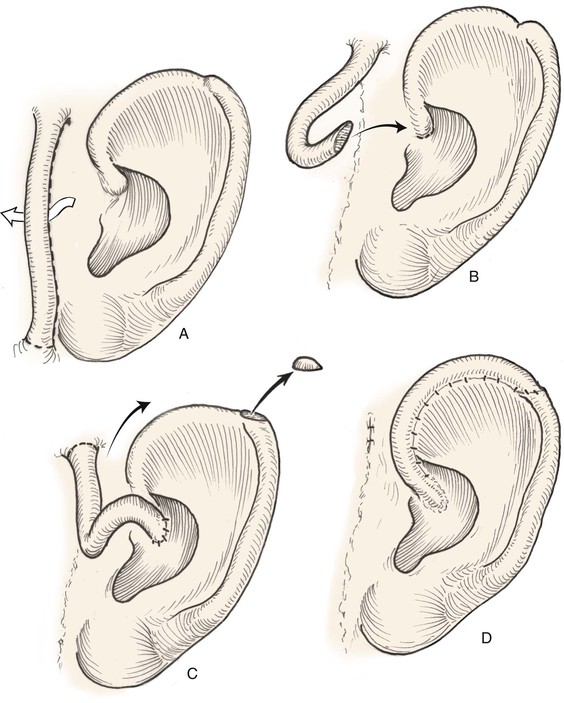
FIGURE 22-9 Preauricular interpolated cutaneous tube flap for repair of defects confined to helix. A, First stage: tube created from preauricular skin. B, Second stage: division of inferior pedicle and flap transfer to helical root. C, Third stage: division of superior pedicle and flap inset. D, Final appearance after inset.
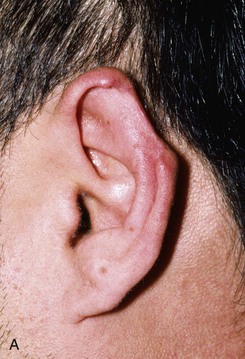
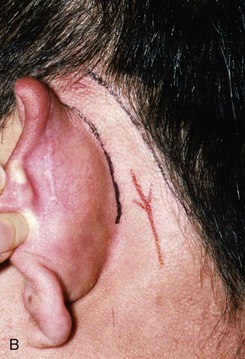
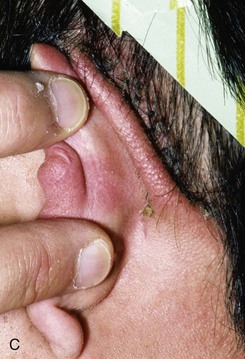
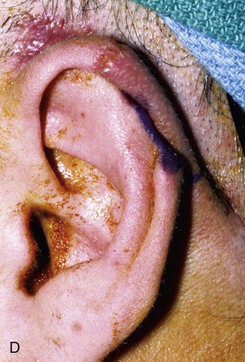
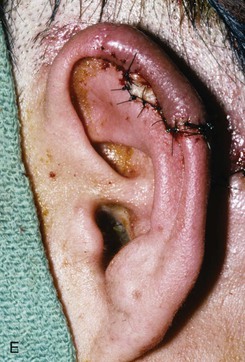
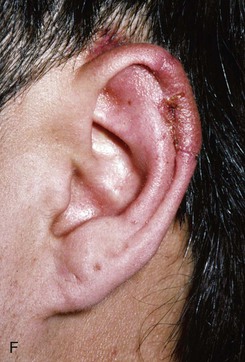
FIGURE 22-10 A, Helical defect. B, Postauricular interpolated cutaneous tube flap designed. C, Three weeks after first-stage tube creation. D, After second-stage transfer of flap to ear. E, Third-stage flap inset. F, One month after third surgical stage.
Postauricular island advancement flaps work well for conchal reconstruction and repair of full-thickness defects of the nonhelical portions of the ear.24,25 The postauricular flap is based on the auricular branch of the postauricular artery. The flap is designed as an island based on a subcutaneous tissue pedicle. A significant advantage is that it provides a single-stage repair with easy closure of the postauricular donor site. When it is designed as an island, the flap is outlined and incised deeply through the postauricular muscle. Posteriorly, the mastoid periosteum is incised, and the dissection proceeds in a superior and anterior direction. Care is taken to preserve the vascular supply to the flap as it enters the flap inferiorly. With the subcutaneous pedicle intact, the flap is advanced into its respective conchal or nonmarginal auricular defect (Fig. 22-11). Postauricular flaps may also be developed as interpolated flaps, requiring a subsequent stage to inset the flap. Interpolated postauricular flaps have been described as a single-stage procedure, which is accomplished by de-epithelialization of the skin of the flap’s pedicle.15,24 A modification has also been described that consists of harvesting of the flap from the medial surface of the auricle to repair a skin defect of the lateral surface. The technique requires de-epithelialization of a portion of the pedicle.16
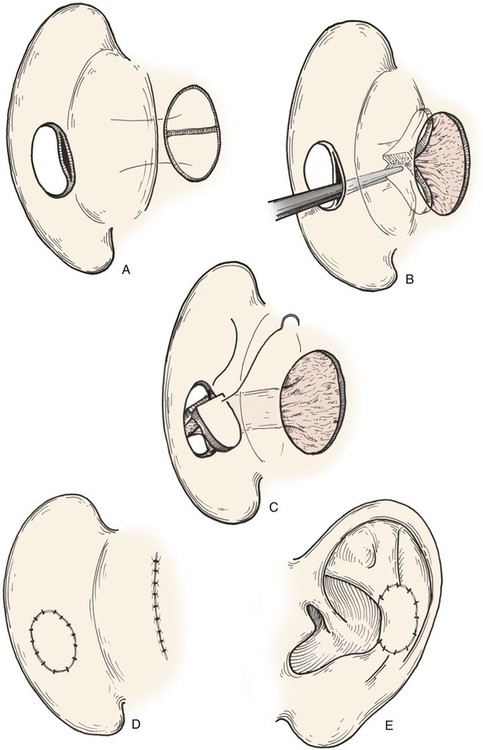
FIGURE 22-11 A, Subcutaneous tissue pedicled island advancement flap designed on postauricular skin. Flap is divided to provide medial and lateral surface cover for defect. B, Subcutaneous tissue pedicle tunneled beneath medial auricular skin. C, Flap folded on itself to provide skin cover of medial and lateral aspect of defect. D, E, Flap sutured in place. Donor defect closed.
The principles set forth by Tanzer and colleagues for microtia reconstruction are germane to reconstruction of large acquired auricular defects.26,27 A three-dimensional autologous rib cartilage framework and soft tissue coverage are the mainstays of microtia repair. Similar structural frameworks may be used for reconstruction of large full-thickness auricular defects. Success depends on the use of supple, thin, and highly vascular skin flaps to cover cartilage grafts and to ensure refined contour of the reconstructed ear. When local skin is scarred or absent, as is often the case with acquired defects, a temporoparietal fascia flap may provide the necessary thin, highly vascular covering for cartilage grafts and concomitantly serve as a recipient site for overlying split- or full-thickness skin grafting. When the vertical height of the ear is likely to diminish by more than 20 mm by use of a local flap, repair with a regional flap is more appropriate. The temporoparietal fascia flap, combined with autologous cartilage grafting to replace auricular framework deficiencies, has proved to be the most versatile regional flap for reconstruction of larger full-thickness ear defects.
Auricular Reconstruction Based on Anatomic Location
Defects of Conchal Bowl and Root of Helix
Conchal bowl defects may be allowed to heal by secondary intention, which usually results in minimal or no auricular distortion. In subtotal conchal bowl defects, full-thickness skin grafts provide adequate aesthetic results and permit easy postoperative monitoring of the area after extirpation of a cutaneous malignant neoplasm (Fig. 22-12). If exposed cartilage is devoid of perichondrium, it can be safely resected because conchal cartilage is not necessary for auricular form. The medial auricular skin becomes the nutrient bed for the skin graft. Graft immobilization is achieved with a bolster dressing.
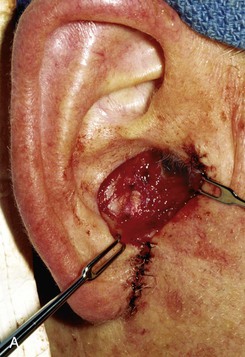
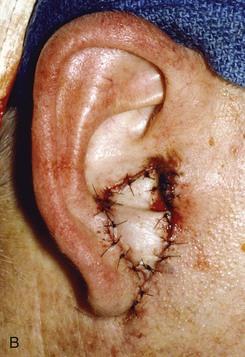
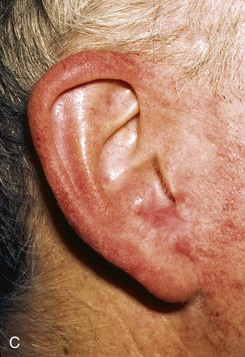
FIGURE 22-12 A, Full-thickness defect of concha cavum, with intact helical rim. B, Defect repaired with postauricular island advancement flap pedicled on postauricular muscle and subcutaneous tissue. Skin island folded to provide medial and lateral coverage. C, Postoperative result at 6 months.
A postauricular subcutaneous tissue pedicled island advancement flap is well suited for reconstruction of deep full-thickness conchal defects because of the flap’s proximity to the defect. This flap is an excellent choice for repair when lateral skin and cartilage of the concha are missing because it eliminates the requirement for a full-thickness skin graft. The flap is transferred from posterior to anterior toward the concha, followed by primary closure of the postauricular donor site (Fig. 22-11). The flap is dissected from the surface of the mastoid bone in a posterior to anterior direction to maintain a thick subcutaneous tissue pedicle that incorporates branches of the postauricular artery and, if present, the postauricular muscle. The flap may be bivalved, as shown in Figure 22-11, when there is a requirement for both medial and lateral skin coverage.
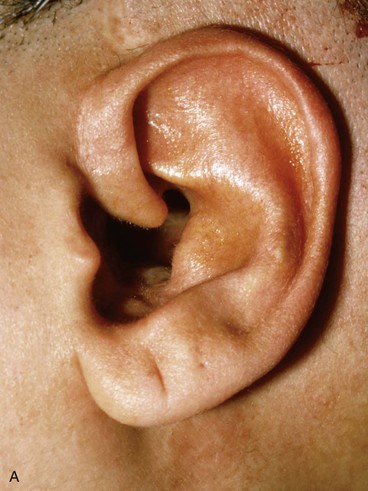
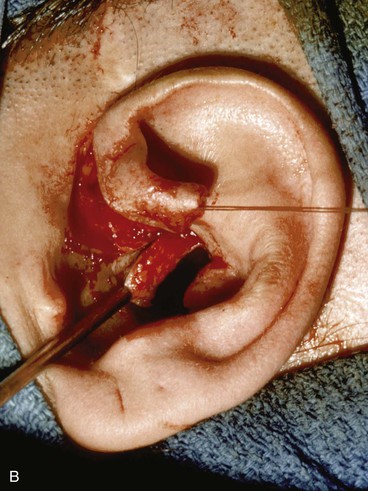
The helical root provides one of the four basic contour lines of the ear. Defects of the root of the helix are seen after resection of cutaneous lesions or after otologic surgery. Limited defects of the root may be reconstructed with a helical advancement flap, in which the remaining helix is advanced inferiorly toward the defect. An incision is made in the lateral skin and cartilage along the helical rim and the rim is advanced in a Y to V fashion to restore the helical root. The flap is composed of both lateral skin and cartilage. It derives its random blood supply from medial soft tissue attachments. The technique is effective for small defects (Fig. 22-13). Larger defects may require the excision of a small Burow triangle of skin and cartilage from the scapha to ensure adequate advancement.
Defects of Superior Third of Auricle
Helical chondrocutaneous advancement flaps are preferable to primary wound closure when the defect is limited to the helix and is between 1.5- and 2.5-cm in linear width (Fig. 22-14).22 This flap takes advantage of the movement of tissue that is possible when the helix is advanced once it is released from its attachment to the ear. An additional opposing advancement may be achieved with a V-Y island advancement flap consisting of the root of the helix. When necessary, incising both lateral and medial auricular skin to create the flap allows even greater helical mobility. The cartilage and skin of the flap are approximated independently of each other with sutures, avoiding wound closure tension. It may be necessary to excise portions of the scapha to prevent excess wound closure tension or cupping of the ear (Fig. 22-15).
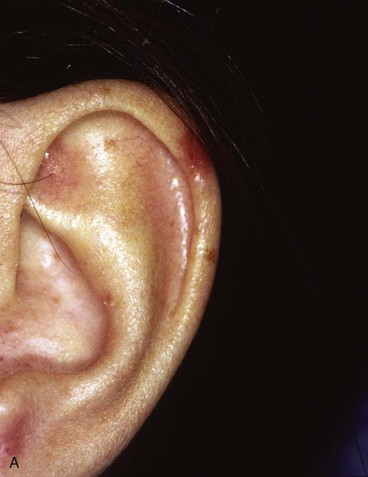
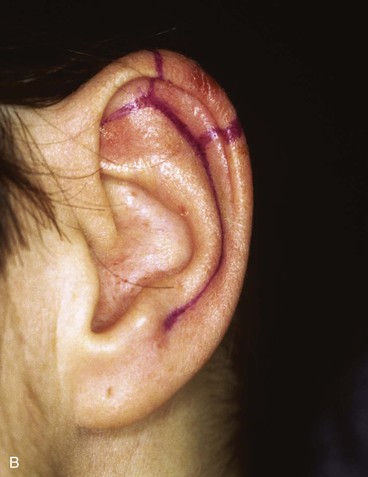
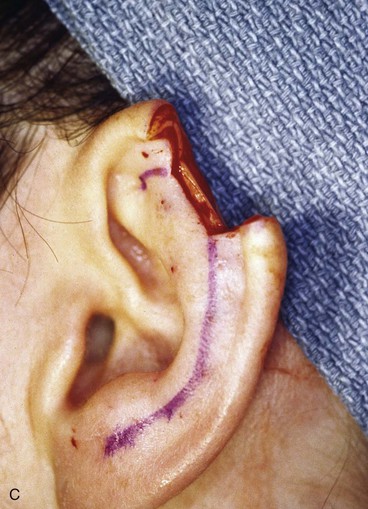
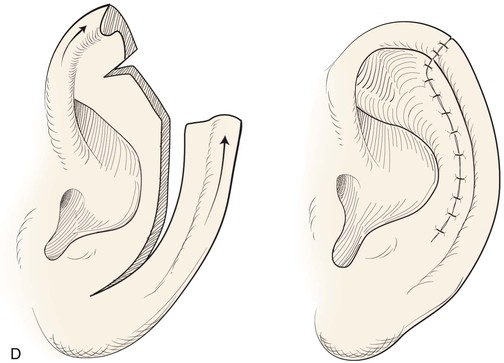
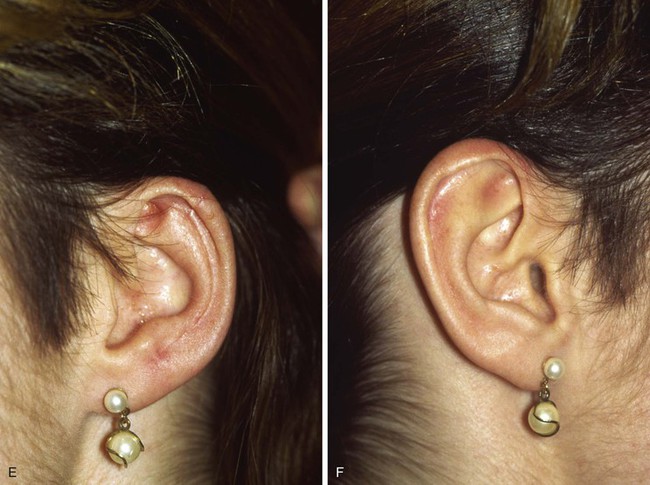
FIGURE 22-14 Reconstruction of helix with chondrocutaneous advancement flaps. A, Superficial melanoma of helix. B, Outline for resection and design of chondrocutaneous advancement flaps. C, Defect, measuring 2.5 cm. D, Drawing showing two advancement flaps. E, Postoperative result at 6 months with 1 cm of vertical shortening. F, Contralateral ear that was not operated on.
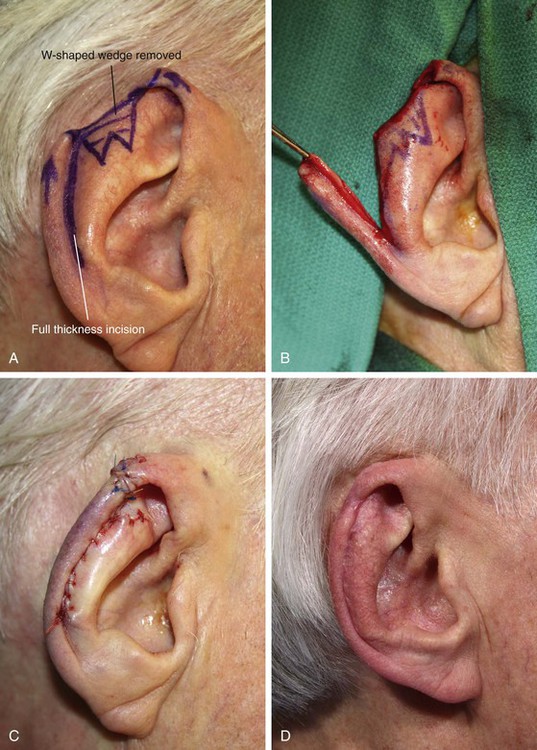
FIGURE 22-15 A, A 2-cm defect of helix. W-plasty designed for anticipated need to excise portions of scapha and antitragus. Incision for chondrocutaneous advancement flap marked. B, Inferior-based flap incised. C, Flap in place. D, Postoperative result at 6 months. (Courtesy of Shan R. Baker, MD.)
For defects larger than 2.5 cm in width and confined to the helical rim, a postauricular or preauricular interpolated tube flap is optimal for reconstruction (see Fig. 22-9).28,29 This flap is randomly based, and the operation is a multistaged procedure. The preauricular skin provides an excellent donor site to create a flap used to reconstruct the anterior helix. The first stage of this procedure requires the formation of a skin tube with both superior and inferior attachments. In the second stage, one end of the tube is transferred to the auricle. The other end of the flap is inset in the helical defect in a third surgical stage. Incorporation of subcutaneous fat into the substance of the tube helps provide contour to the reconstructed helix, but the tube does not contain rigid structural support and thus should be used only for isolated helical rim defects.
Full-thickness defects of the superior auricle, with an intact helical rim, present an unusual challenge (Fig. 22-16). Flaps used to repair such defects must be well vascularized. The postauricular subcutaneous tissue pedicled island advancement flap provides skin and soft tissue with a reliable blood supply to repair such defects.16 This flap provides excellent color match, and the scar from closure of the donor site of the flap is hidden in the postauricular sulcus. Based on the postauricular artery and vein, the flap incorporates postauricular skin and the postauricular muscle. It can be transferred, folded on itself, and used to reconstruct full-thickness defects of the superior and central portions of the auricle. If the defect involves structurally important elements of the auricle, a cartilage graft is incorporated within the substance of the flap.
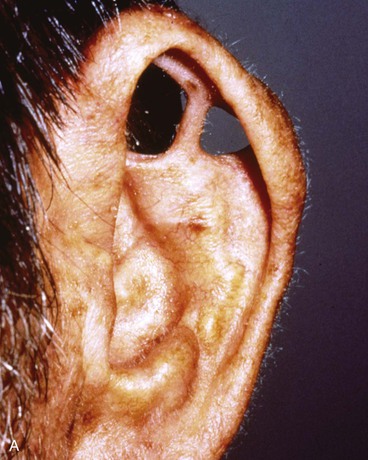
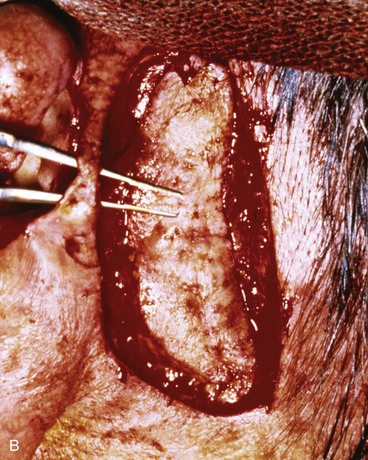
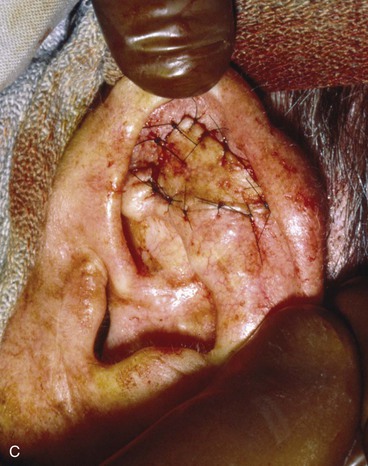
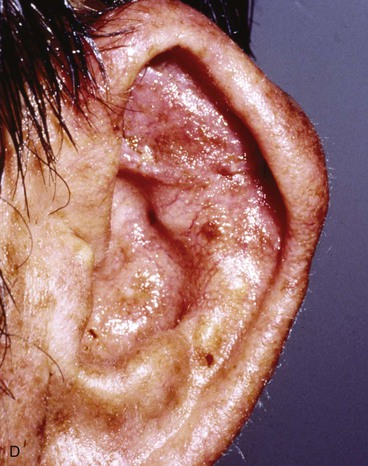
FIGURE 22-16 A, Full-thickness defects of the central portion of superior auricle. B, Postauricular subcutaneous tissue pedicled island advancement flap incised. C, Flap sutured in place. Skin island folded to provide lateral and medial surface coverage. D, Postoperative result at 6 months.
A composite chondrocutaneous advancement flap is another alternative for reconstruction of full-thickness defects of the superior auricle that do not involve the helix. The antihelix is advanced as a composite flap superiorly into the defect located in the area of the triangular fossa (Fig. 22-17). The flap is created by making a full-thickness incision in the scapha from the defect inferiorly to the earlobe. Depending on the size of the defect and the corresponding distance of advancement required, a Burow triangle may be excised from the substance of the earlobe.
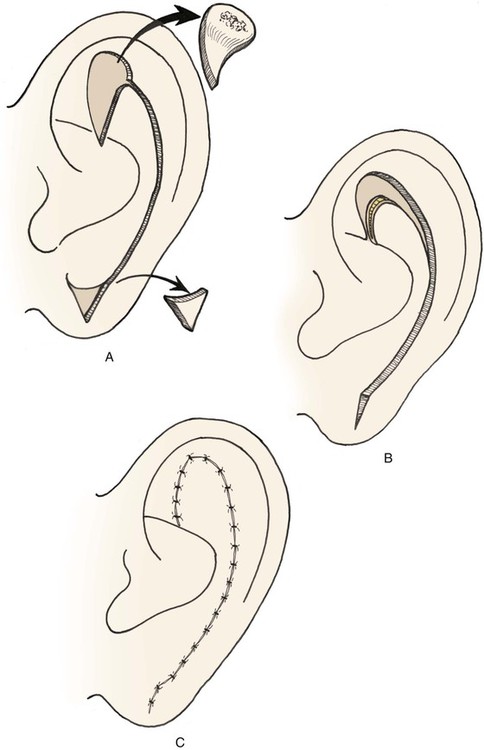
FIGURE 22-17 Auricular composite advancement flap consisting of medial and lateral auricular skin and intervening cartilage used for repair of full-thickness defect of antihelix and triangular fossa.
Small lateral skin defects on the central portion of the superior and middle third of the auricle may be closed with rotation advancement flaps (Fig. 22-18).30 An advantage of these flaps is that the incisions may be hidden in the scapha to make them inconspicuous. When the defect requires extensive soft tissue mobilization, with the risk of distortion of the helix, a full-thickness skin graft may be a better alternative (Fig. 22-19).
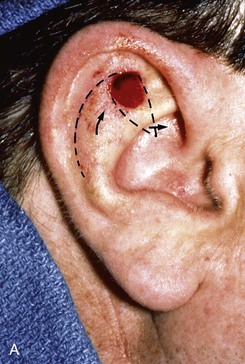
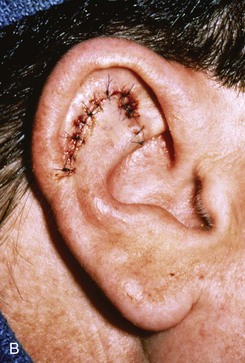
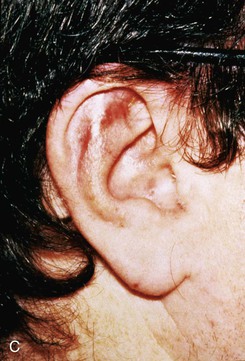
FIGURE 22-18 Chondrocutaneous advancement skin flap for skin defect of triangular fossa. A, Lateral auricular skin defect. Broken line indicates full-thickness incisions. B, Immediately after repair with rotation advancement flap. C, Postoperative result at 6 months.
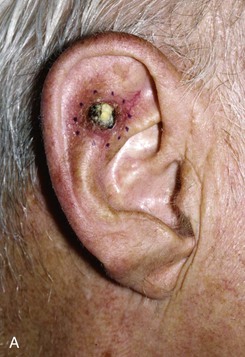
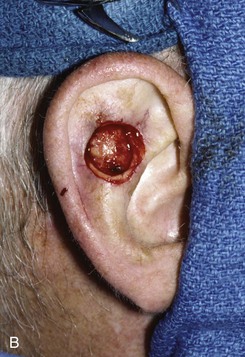
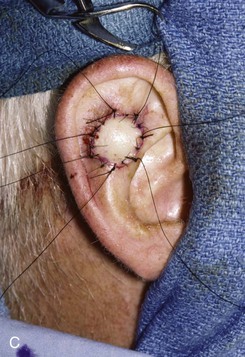
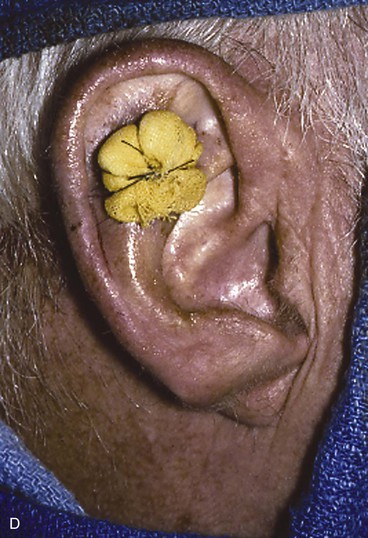
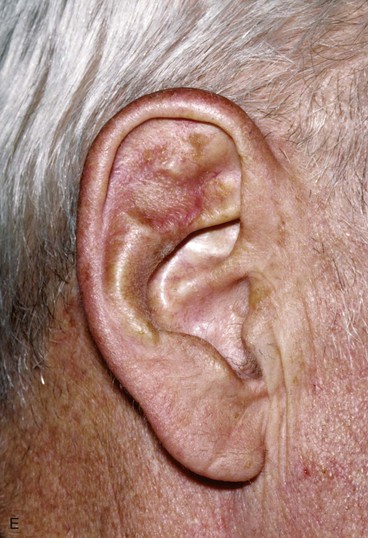
FIGURE 22-19 Full-thickness skin graft repair of defect involving lateral skin and underlying cartilage. A, Proposed surgical excision. B, Defect of lateral skin and cartilage after excision . C, Full-thickness skin graft, ready for bolster dressing. D, Bolster dressing in place. E, Postoperative result at 6 months.
Reconstruction of large defects of the superior third of the auricle, where cartilage from the helix, scapha, and triangular fossa are missing, mandates incorporation of a rigid framework fashioned from autogenous rib cartilage. The framework is covered by a temporoparietal fascia flap, which in turn is covered with a skin graft (Fig. 22-20).
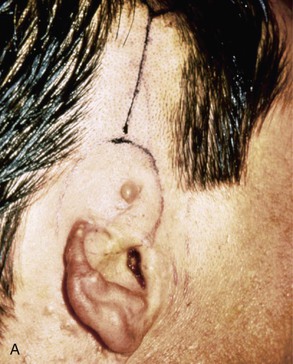
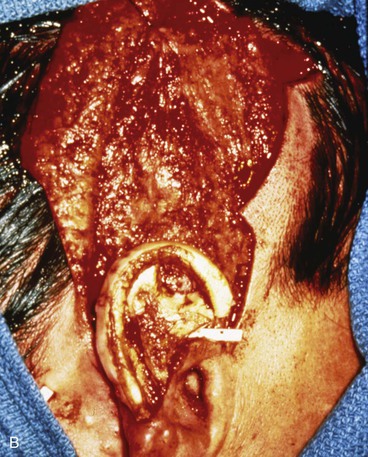
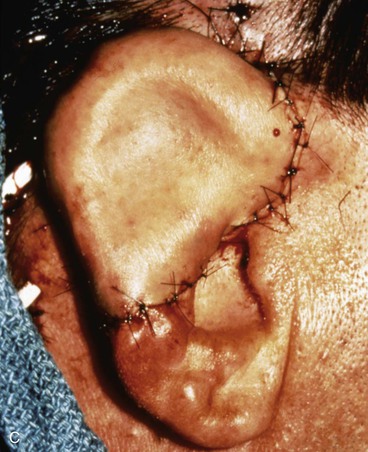
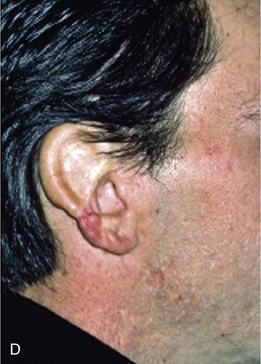
FIGURE 22-20 Autogenous cartilage framework with overlying temporoparietal fascia flap and skin graft to reconstruct superior auricle. A, Hemiauricular defect from human bite injury. Incision marked for dissection of temporoparietal fascia flap. B, Scalp elevated to expose temporoparietal fascia in preparation for coverage of costal cartilage framework. C, Flap turned down to cover cartilage. Flap covered with skin graft. D, Postoperative result at 8 months, after creation of superior auricular sulcus.
Defects of Middle Third of Auricle
Absence of the central third of the auricle is a conspicuous deformity. Small defects (<1.5 cm) may be closed by converting the defect into a wedge-shaped excision, although this approach will have a direct impact on the vertical height of the ear. Full-thickness helical defects less than 2.5 cm are amenable to helical chondrocutaneous advancement flaps. Defects that are larger than 2.5 cm in width and confined to the helical rim may be repaired with an interpolated cutaneous tube flap.31 Large defects of the central third of the auricle involving the helix and antihelix require cartilage grafting to achieve the necessary structural support.32,33 In some cases, repair is accomplished by use of a two-stage postauricular flap (Fig. 22-21). The first stage involves creating a cutaneous advancement flap based posteriorly on the scalp. The width of the flap is equal to the height of the defect, and the flap is elevated from the skin of the medial aspect of the remaining auricle and from the postauricular sulcus skin immediately adjacent to the defect. The flap is advanced over the lateral aspect of the defect. The medial aspect of the auricular cartilage is exposed as a result of dissecting the skin away from it in the process of creating the flap. The exposed cartilage is attached to and nourished by the denuded postauricular soft tissue. During the first stage, septal or conchal cartilage is implanted beneath the advancement flap to replace missing portions of the auricular cartilage. The cartilage graft is sutured directly to the borders of the auricular cartilage defect. The advancement flap provides soft tissue coverage for the cartilage graft and replaces the missing skin of the lateral ear. The second surgical stage, performed 3 weeks later, involves detachment of the advancement flap from the scalp. The flap is then folded on itself as a hinge flap to cover the medial aspect of the cartilage graft. For this to occur, the medial aspect of the cartilage graft and the auricular cartilage attached to the postauricular denuded soft tissue during the first surgical stage are released from their attachments to the postauricular soft tissue. Flap inset must provide adequate skin coverage of the cartilage graft and prevent exposure of any auricular cartilage medially. A full-thickness skin graft is used to close the postauricular donor area if it cannot be closed primarily. In cases of extensive cartilage loss in the central third of the ear, a more complex framework using the sixth to eighth ribs is carved and tailored to the missing segment of auricular cartilage. The framework is then covered by a postauricular skin advancement flap (Fig. 22-22). This procedure requires release of the framework cartilage graft from the head in a staged procedure with full-thickness skin grafting of the postauricular sulcus.
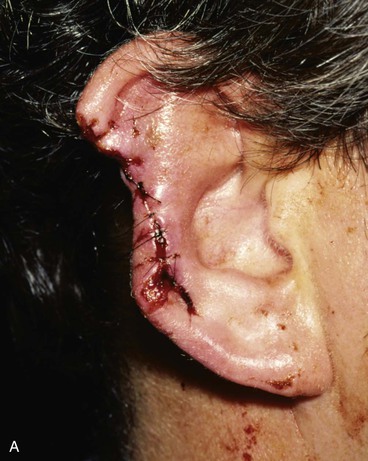
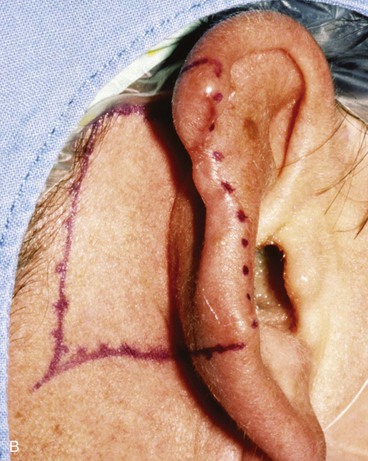
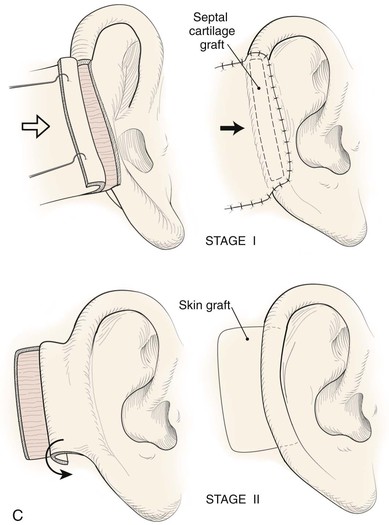
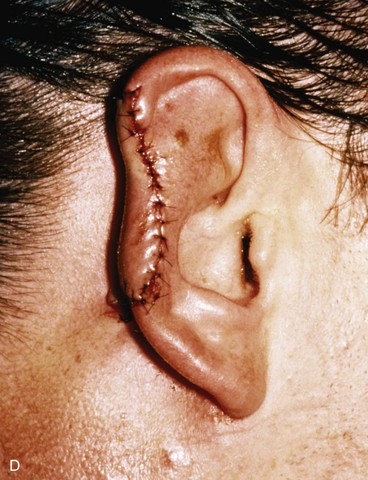
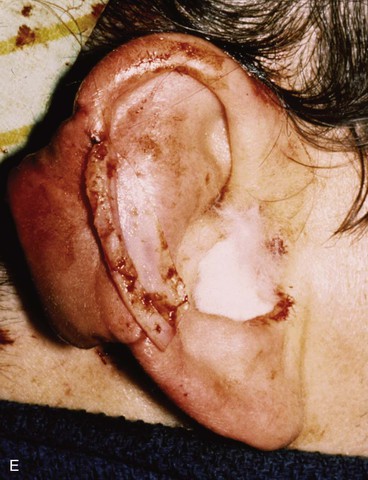
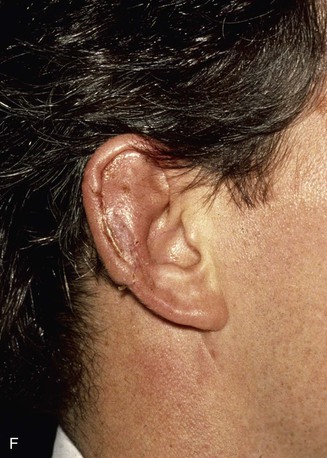
FIGURE 22-21 Two-stage postauricular advancement flap for repair of defect of central third of auricle. A, Traumatic auricular defect. B, Design of postauricular advancement flap. C, Drawings showing stages of reconstruction. D, First stage completed. Skin from medial aspect of ear and postauricular sulcus advanced to provide skin to cover lateral border of defect. E, Second stage. Release of flap from posterior attachment and incorporation of supporting septal cartilage graft. Graft, shown outside of flap, was inserted beneath flap to provide framework for constructed helix. F, Postoperative result at 6 months.
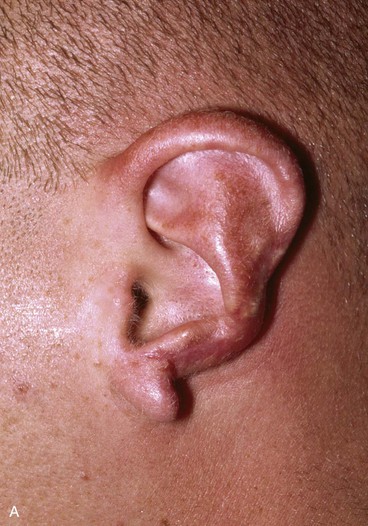
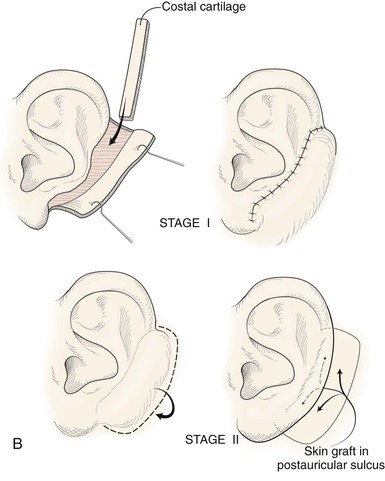
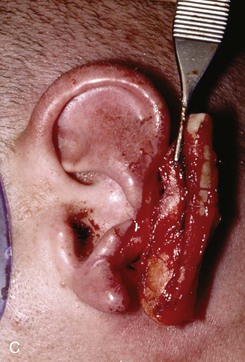
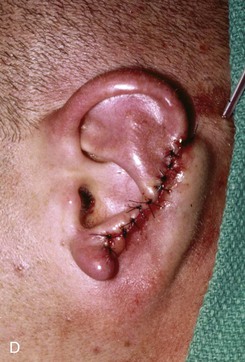
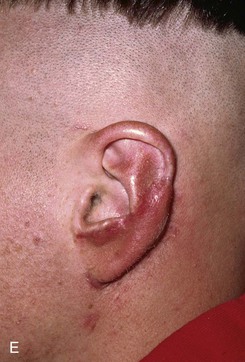
FIGURE 22-22 Reconstruction of central and inferior auricular defect with costal cartilage framework. A, Defect resulting from motor vehicle accident. B, Drawings showing stages of reconstruction. C, Costal cartilage graft tailored to replace missing cartilaginous segment. D, Framework implanted beneath postauricular skin. E, Postoperative result 4 months after framework elevation with overlying skin and full-thickness skin grafting of medial surface of constructed area.
Another approach to reconstruction of the middle third of the helix when the skin of the medial aspect of the concha and the postauricular sulcus are intact is to use a postauricular interpolated advancement flap in two surgical stages. Unlike most interpolated flaps that are transferred by pivotal movement, this flap is transferred by advancement over the intervening skin of the postauricular sulcus. In contrast to the previous discussion, in which the skin of the medial ear is incorporated into the advancement flap, in this case the skin of the medial aspect of the ear and of the postauricular sulcus is left undisturbed. The advancement flap is incised posterior to the postauricular sulcus. It is advanced over the skin of the postauricular sulcus and sutured to the margins of the lateral auricular skin defect. This creates a skin-lined channel representing the postauricular sulcus beneath the pedicle of the flap (Fig. 22-23).
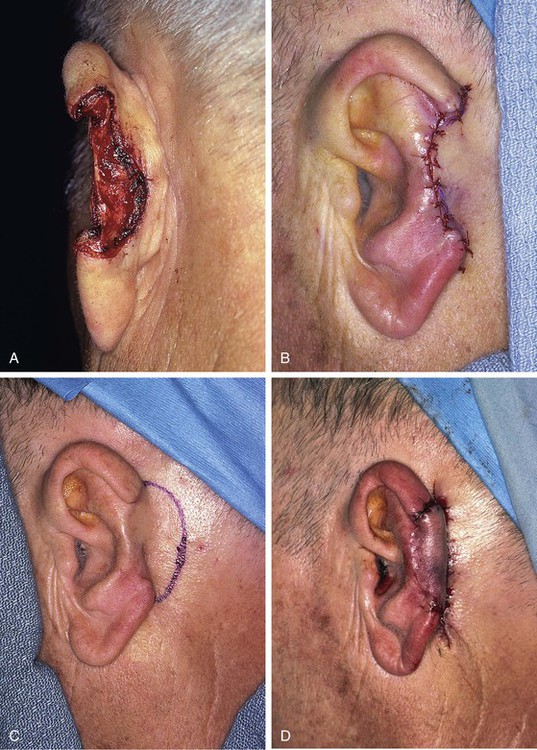
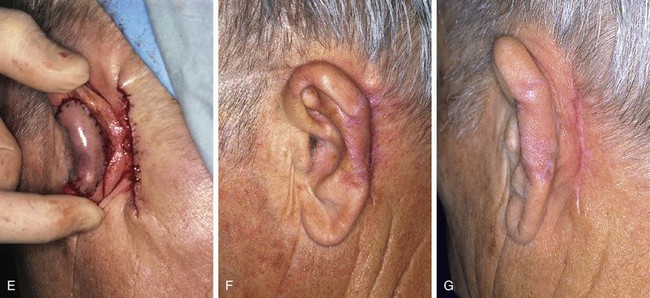
FIGURE 22-23 A, Loss of central third of helix after micrographic surgery for basal cell carcinoma. B, First stage. Interpolated flap from postauricular skin attached to lateral margin of auricular defect. Skin of postauricular sulcus left undisturbed. C, Hinge flap based on auricle is designed for reconstruction of helix. D, Second stage. Pedicle of interpolated flap divided and hinge flap folded medially and sutured to exposed skin margin of postauricular sulcus. E, Donor defect from inset of interpolated flap closed primarily. F, G, Four months after inset of flap. (Courtesy of Shan R. Baker, MD.)
The advantage of using the interpolated postauricular advancement flap compared with the technique of incorporating the postauricular skin into an advancement flap is that the medial aspect of the concha is not denuded and does not require subsequent release from attachments to the mastoid periosteum. A skin graft is not required to cover the medial aspect of the reconstructed ear and any cartilage graft that may have been used for the framework of the ear. In addition, skin grafts are not required to restore the postauricular sulcus because the skin of the postauricular sulcus is left in situ by the interpolated flap technique (Fig. 22-24).
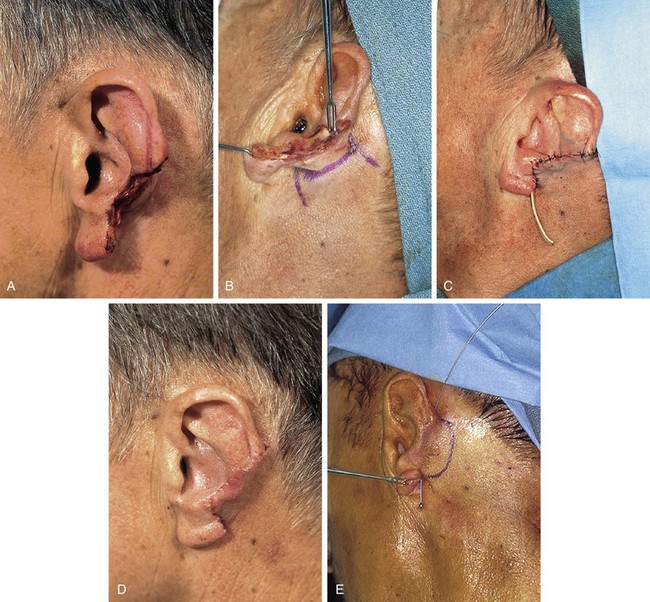
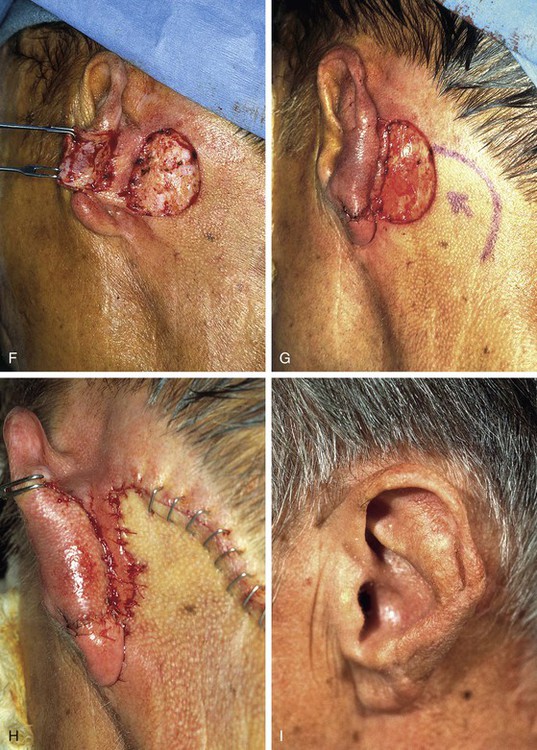
FIGURE 22-24 A, Defect of inferior third of helix and antihelix after micrographic excision of basal cell carcinoma. B, Interpolated flap from postauricular skin designed for reconstruction of ear. Skin of postauricular sulcus left undisturbed. C, First stage completed. Interpolated flap sutured to lateral margin of auricular defect. Rubber band drain placed in postauricular sulcus beneath pedicle of flap. D, One week after transfer of flap. E, Two months after transfer of interpolated flap. Probe has been inserted beneath pedicle of flap in postauricular sulcus. Hinge flap based on auricle is designed for reconstruction of ear. F, Pedicle of interpolated flap divided. G, Second stage completed. Hinge flap folded medially and sutured to exposed skin margin of postauricular sulcus. Rotation scalp flap designed for closure of donor defect of hinge flap. H, Donor defect closed. Skin of postauricular sulcus left undisturbed. I, Postoperative result at 2 months. (Courtesy of Shan R. Baker, MD.)
When the skin of the medial ear and postauricular sulcus is absent from traumatic loss or from excision, the remaining auricle is sutured to the postauricular skin as the first surgical stage. When the ear has completely healed, a second stage is performed in which a hinge flap based on the auricle is designed so that the flap can be constructed from the postauricular skin. Cartilage grafts are included during the second stage to provide a framework for the constructed ear. The donor site of the hinge flap is covered with a full-thickness skin graft harvested from the groin or supraclavicular fossa or closed with a scalp advancement flap (Fig. 22-25).
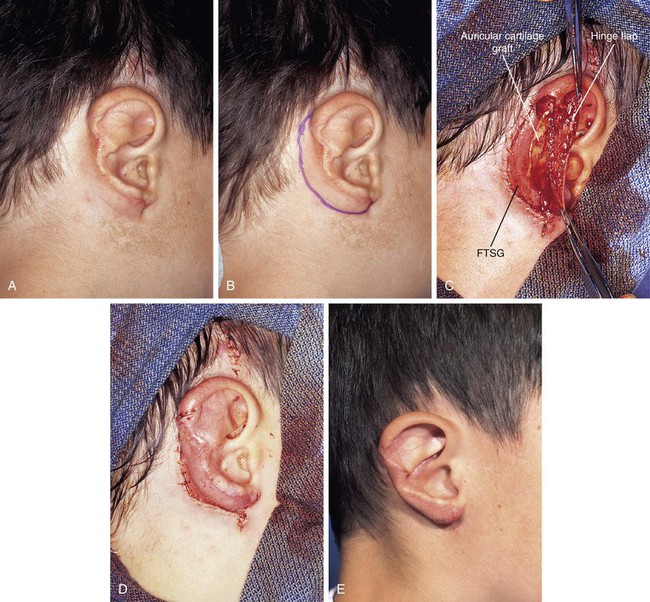
FIGURE 22-25 A, Traumatic avulsion of inferior two-thirds of auricle with loss of skin of postauricular sulcus and medial ear. Skin margin of antihelix was sutured to postauricular skin margin. B, Hinge flap based on auricle is designed for reconstruction of ear. C, Hinge flap dissected. Auricular cartilage graft harvested from contralateral ear inserted beneath flap for framework. Full-thickness skin graft (FTSG) in place to cover donor defect of hinge flap. D, Hinge flap folded medially over cartilage graft and sutured to skin graft used to reconstruct postauricular sulcus. E, Postoperative result at 1 year. No revision surgery performed. (Courtesy of Shan R. Baker, MD.)
Defects of Inferior Third of Auricle
Reconstruction of defects of the inferior third of the auricle is generally less difficult than repair of defects elsewhere on the ear because of the relative pliability and laxity of auricular and periauricular skin in this region. Up to 50% of the earlobe may be removed by a wedge-shaped excision followed by primary wound closure, yielding only minimal deformity. Tissue from the earlobe provides a resource for construction of small advancement flaps used to repair helical defects of the inferior third of the ear. When the entire inferior third of the ear is being reconstructed, cartilage grafting is necessary for an aesthetic result. Total earlobe reconstruction is performed by employing a composite flap containing conchal cartilage in a two-staged procedure (Fig. 22-26). First, a segment of cartilage, the shape of the earlobe obtained from the contralateral concha, is embedded in a subcutaneous pocket below the auricle in a location corresponding to the desired position of the earlobe. Six weeks later, the cartilage and overlying skin are elevated as a composite flap, based superiorly on the auricle. The medial surface of the composite flap is skin grafted and the donor site of the composite flap is closed primarily by advancing the adjacent skin. This technique provides an excellent skin color match between the flap and the ear, results in minimal local distortion of tissues, produces a naturally shaped and contoured earlobe, and allows precise earlobe positioning.
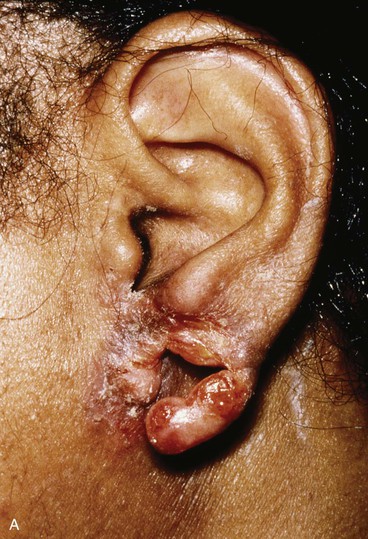
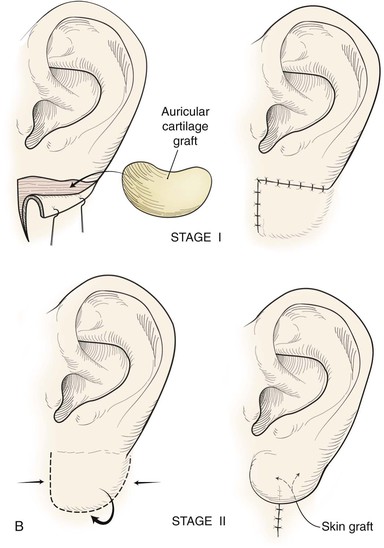
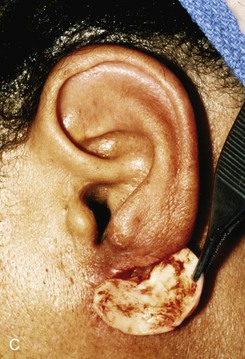
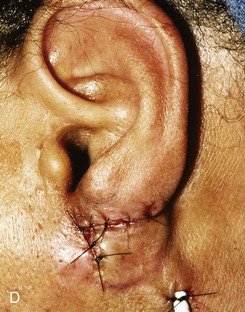
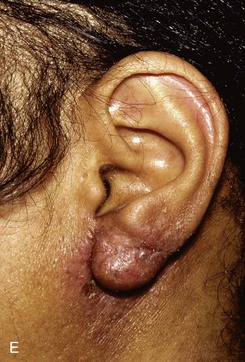
FIGURE 22-26 Earlobe reconstruction. A, Traumatic defect of earlobe. B, Drawings showing stages of reconstruction. C, Contralateral conchal cartilage sculpted into appropriate shape for framework of new earlobe. D, Conchal cartilage graft placed in subcutaneous tissue pocket for 6 weeks. E, Six months postoperatively, after second-stage lobular release and full-thickness skin grafting of medial surface of reconstructed earlobe.
Defects of Preauricular Area
Preauricular defects arise most commonly from excision of cutaneous malignant neoplasms. The function of the facial nerve should be carefully examined before reconstruction is undertaken. Surgical options for repair of preauricular cutaneous defects include primary wound closure and transposition, unipedicle advancement, and V-Y subcutaneous tissue pedicled advancement flaps34 (Fig. 22-27). With careful planning, scars may frequently be positioned within the preauricular crease, with excellent scar camouflage.
Large Auricular Defects
For ear defects encompassing one-third or more of the entire auricle, reconstruction often involves use of a temporoparietal fascia flap (TPFF). Large auricular defects may require only a portion of the standard fabricated cartilaginous framework required for microtia repair (Fig. 22-20). Skin adjacent to the defect may be adequate for coverage of the framework cartilage grafts, but often it is insufficient or scarred, causing it to be nonpliable with suboptimal vascularity. In these circumstances, the framework should be covered with a TPFF and an overlying split-thickness skin graft.35
Principles of Microtia Reconstruction with Temporoparietal Fascia Flaps
In reconstruction of large auricular defects, alloplastic implants may be avoided by employing a framework fashioned from autogenous cartilage. Synthetic frameworks are at greater risk of becoming infected and are fraught with problems of extrusion. 36,37 The cartilaginous portions of the sixth, seventh, and eighth ribs provide the raw material for creation of a framework for construction of the ear (Fig. 22-28). The synchondrosis between the sixth and seventh ribs serves as the body of the framework, forming the concha, antitragus, and curve of the antihelix. The helix is fashioned from the eighth rib, identical to the technique employed in microtia repair. The size and individual shape of the framework are determined by use of a template designed from the contralateral auricle. The helix is customized by removing the perichondrium from the medial aspect of the eighth rib and progressively thinning the cartilage until it becomes sufficiently flexible to simulate the curvature of the helix. The cartilaginous segment is then secured to the body framework by sutures, with use of a combination of 3-0 stainless steel wire and 4-0 clear nylon sutures. Care is taken in attaching the root of the sculpted helix to the body framework. Final contour is achieved with 5- and 7-mm gouges to create the scaphoid and triangular fossa. Once it is crafted, the cartilaginous framework graft is stabilized at the recipient site and properly positioned on the basis of preoperative measurements.38,39
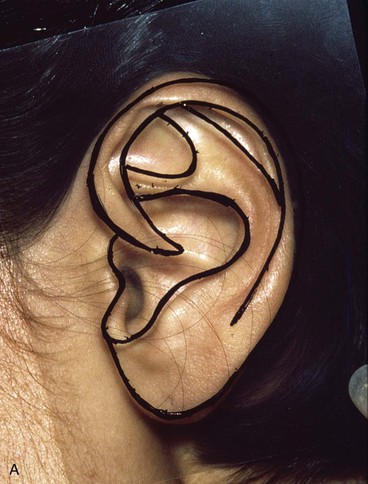
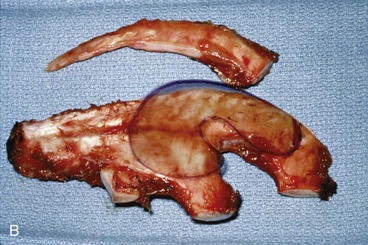
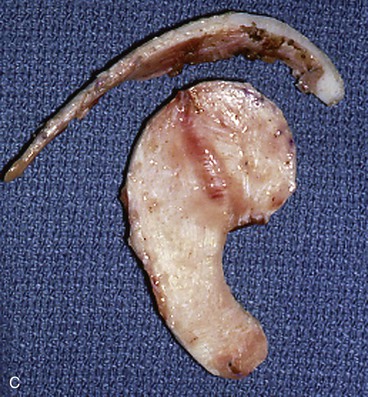
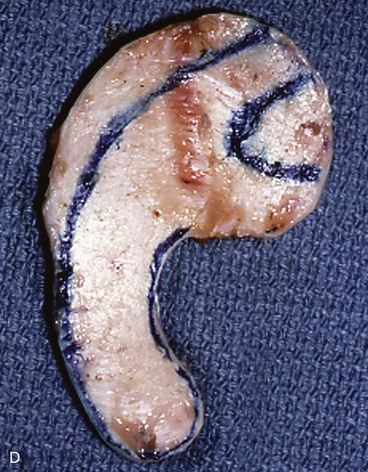
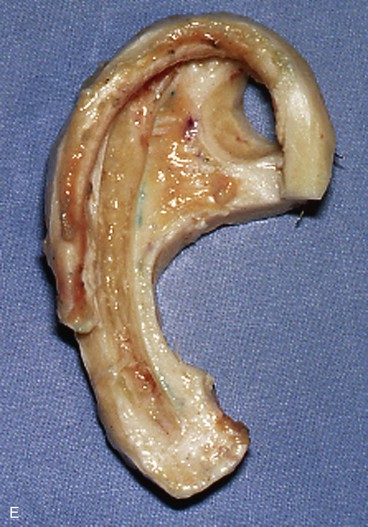

FIGURE 22-28 Carving of costal cartilage framework for auricular reconstruction. A, Template created from contralateral, normal ear. B, Costal cartilage harvested for auricular framework. C, D, Template used to sculpt segments for construction of helix and body of framework. E, Body of framework consisting of scapha and triangular fossa and helix sculpted from costal cartilage. Segments of cartilage integrated with 3-0 stainless steel wire and clear nylon sutures. F, Vertical and horizontal dimensions of framework should match template.
The cartilaginous framework must be covered with a thin, vascular, hairless, soft tissue flap capable of nourishing a skin graft. A pedicled TPFF satisfies these requirements and is frequently used to cover the cartilage framework.40–43 The TPFF also has the following advantages: the anatomy of the flap is well known to head and neck surgeons; a large surface area of tissue (4 × 12 cm) may be harvested; the flap may be pivoted inferiorly to cover a wide range of auricular defects; and the fascia may be transferred to the contralateral auricular region, if necessary, by microsurgical techniques (Fig. 22-29).44–46
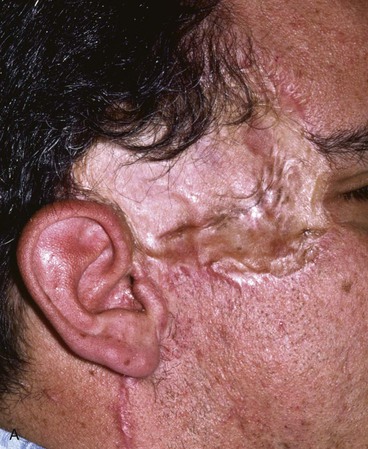
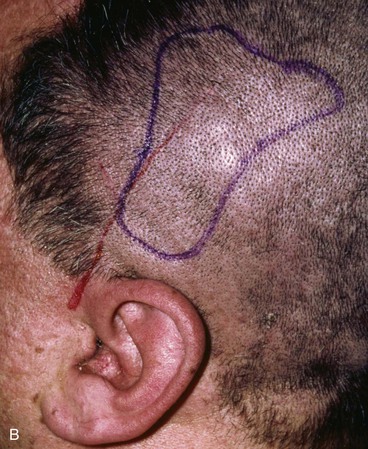
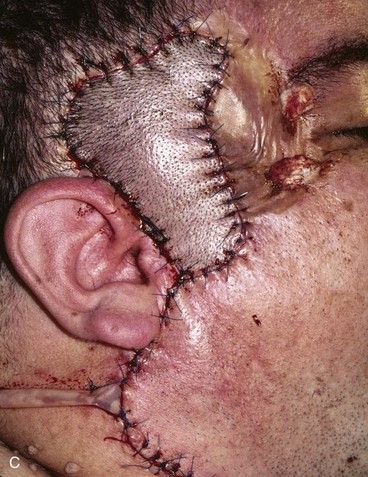
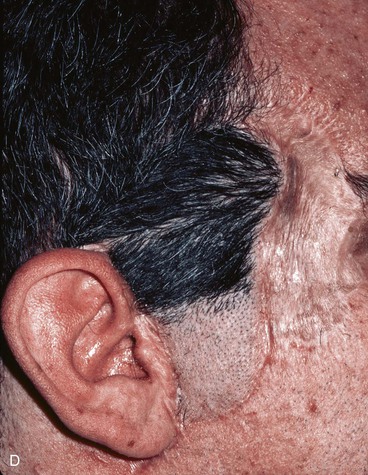
FIGURE 22-29 Microsurgical hair-bearing temporoparietal scalp flap used to reconstruct area of alopecia and scarring. A, Preoperative photograph showing site of prior excision of basal cell carcinoma with skin graft coverage. B, Microsurgical scalp flap outlined on contralateral temporal area. C, Flap transferred to recipient site. D, Postoperative result at 6 months.
Anatomy
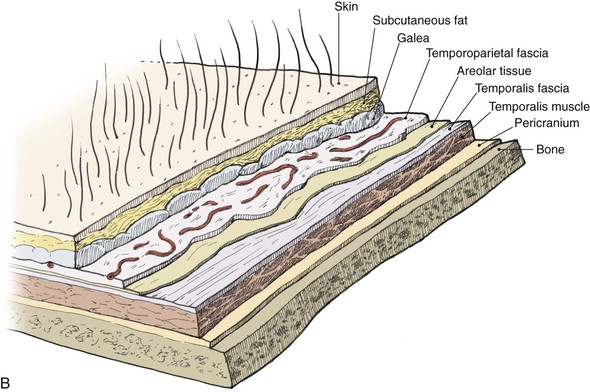
The temporoparietal fascia, also known as the superficial temporal fascia, is continuous with the superficial musculoaponeurotic system as well as the galea (Fig. 22-30). It lies deep to the skin and subcutaneous tissue of the scalp, to which it is firmly bound, particularly in the area of the temporal line. This fascia should not be confused with the fascial layer surrounding the temporalis muscle. The temporoparietal fascia attaches to the zygomatic arch, whereas the temporalis muscle fascia is deeper, is thicker, and divides to envelop the zygomatic arch. Measuring on average 2 to 3 mm in thickness over the parietal area, the temporoparietal fascia is highly vascular, with an abundant and consistent blood supply provided by the superficial temporal artery and vein.3,47
Technique
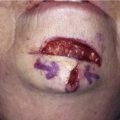
To elevate the TPFF, a 6-cm-long vertical incision is made in the scalp immediately above the auricular defect, exposing the superficially located temporoparietal fascia. In harvesting of the flap, the hair follicles and subcutaneous fat must remain a part of the overlying scalp. Careful avoidance of injury to the hair follicles prevents iatrogenic alopecia. The deep plane of flap elevation is just superficial to the temporalis muscle fascia in the loose connective tissue that separates the temporoparietal fascia from the temporalis fascia. The vascular pedicle is identified and protected. The anterior limit of the flap remains posterior to the location of the temporal branch of the facial nerve. The posterior margin of the flap should capture the posterior branches of the superficial temporal artery and vein and can be back cut to the level of these vessels to facilitate flap transfer. The flap is turned 180° downward from superior to inferior in a hinge fashion such that the lateral surface of the flap becomes the medial surface adherent to the auricular cartilage framework. The edges of the flap are tucked under the existing skin edges of the defect. Split-thickness skin grafts provide external coverage over the fascia flap. For the first 72 hours after flap transfer, a wound drain is attached to a closed system suction device that is fixed to the patient’s dressing. The TPFF may also be used to cover exposed native auricular cartilage when large areas of the cartilage are exposed (Fig. 22-31).48 Split-thickness skin grafts applied directly to auricular cartilage without a surface covering of perichondrium have limited success. Incorporation of a TPFF to serve as the recipient bed for the split-thickness skin graft markedly enhances healing.
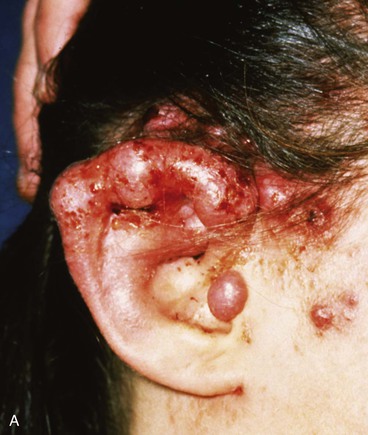
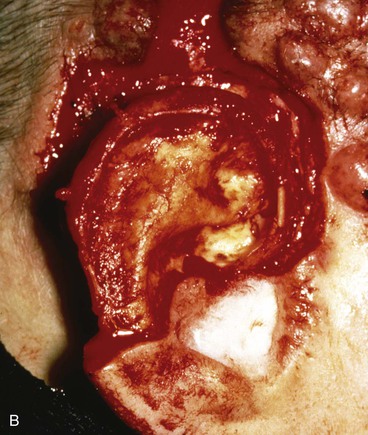
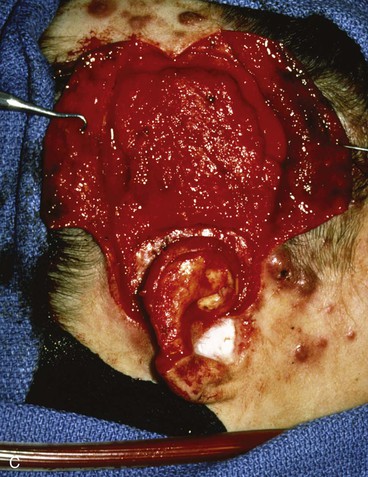
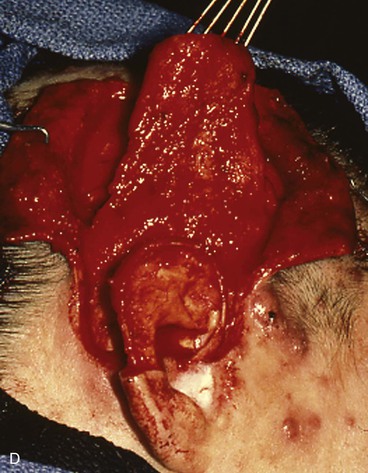
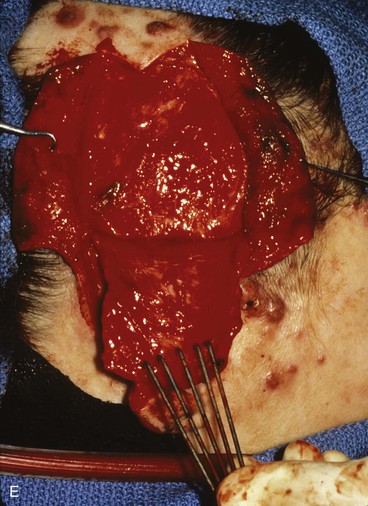
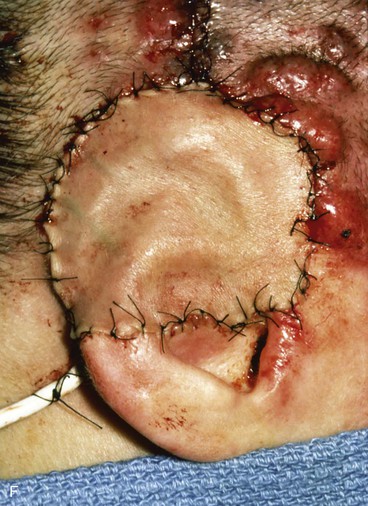
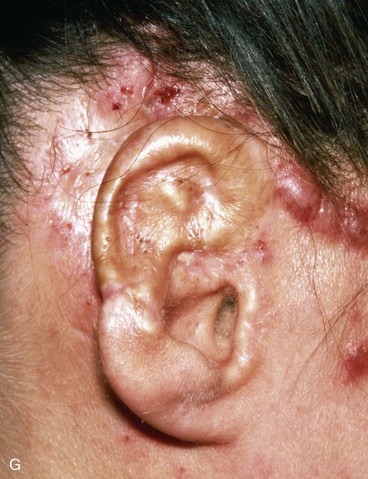
FIGURE 22-31 Angiolymphoid hyperplasia of auricle affecting medial and lateral skin but not auricular cartilage. Total resurfacing of cartilage required. A, Preoperative view. B, Defect after complete removal of diseased soft tissue. C, D, Temporoparietal fascia flap dissected and ready for transfer over framework. E, Temporoparietal fascia flap transferred as an inferiorly based hinge flap to cover native auricular cartilage. F, Skin graft placed over fascia flap. G, Postoperative result at 12 months, after superior auricular sulcus creation with skin graft.
Resurfacing of Temporal Cavity After Auriculectomy
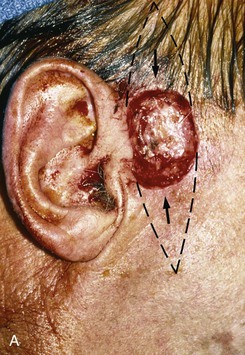
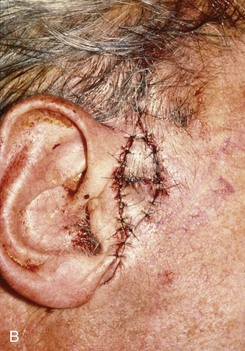
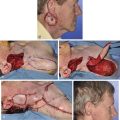
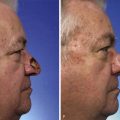
On occasion, large cancers of the auricle that do not involve the temporal bone necessitate complete removal of the ear. Several options are available for resurfacing of the exposed bone after total auriculectomy, including bilobed cutaneous flaps transposed over the temporal bone. These can be designed to preserve the external auditory canal (Fig. 22-32). Cervical rotation flaps, regional chest and shoulder flaps, and microsurgical tissue transfer may also be used. A disadvantage of a musculocutaneous flap is that the bulk of the flap may constrict the external auditory canal, compromising hearing. In certain cases, temporal bone defects may be covered with a TPFF and skin grafted. In these situations, the patient may subsequently be fitted for an auricular prosthesis (Fig. 22-33).
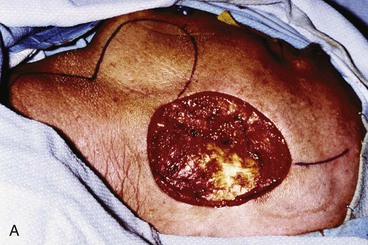
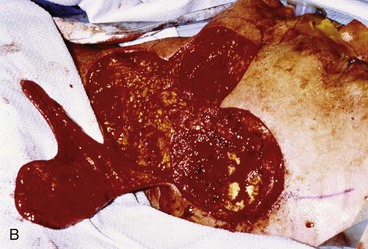
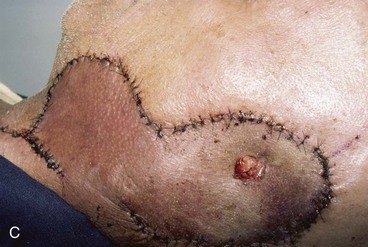
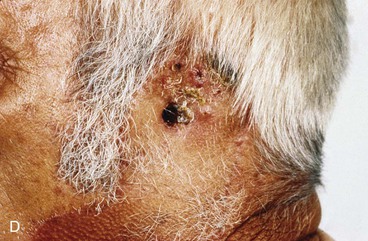
FIGURE 22-32 Total auriculectomy defect repaired with bilobed cervical flap. A, Total auriculectomy defect, with posteriorly based cervical bilobed cutaneous flap designed for repair. Incision marked at superior border of defect not used. B, Flap elevated. C, Flap in place. D, Postoperative result at 6 months.
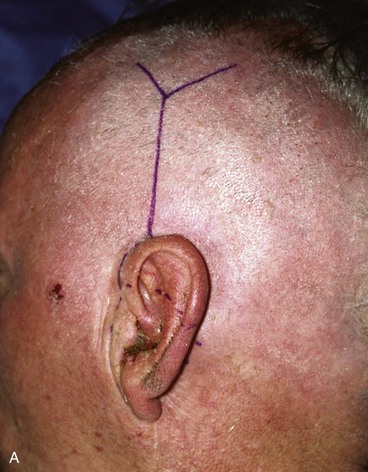
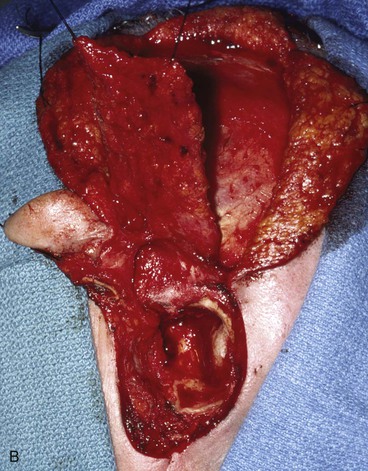
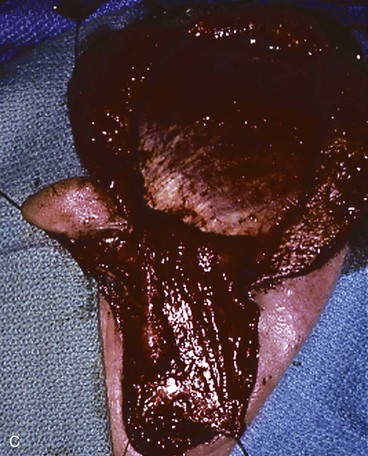
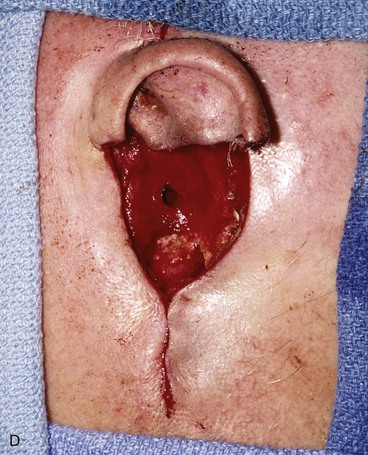
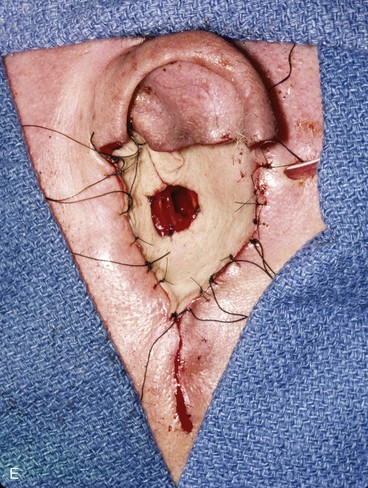
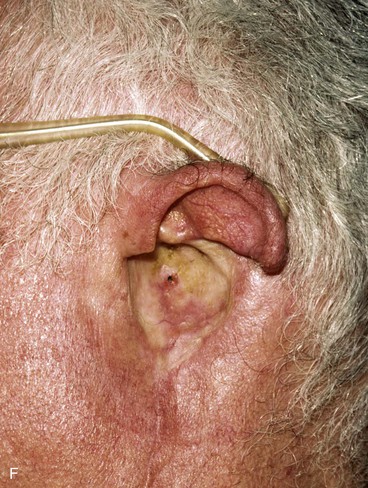
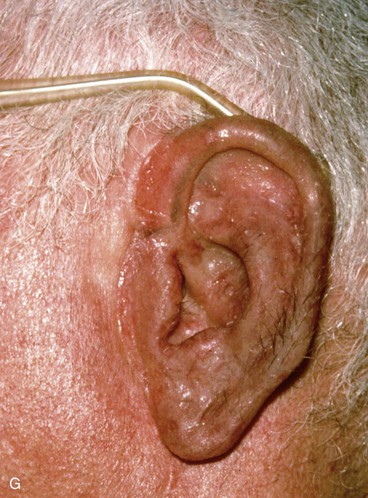
FIGURE 22-33 Repair of subtotal auriculectomy defect and external auditory canal preservation. A, Extensive squamous cell carcinoma of conchal bowl. Incision marked for elevation of temporoparietal fascial flap. B, Resection of cancer, with preservation of superior remnant of auricle, and elevation of fascial flap. C, Flap hinged downward to cover wound. D, Flap in place over mastoid cavity. E, Flap covered with skin graft with hole for external auditory canal. F, Postoperative result at 6 months. G, Auricular prosthesis in place.
References
1. Allison, G. Anatomy of the external ear. Clin Plast Surg. 1978; 5:419.
2. Hayashi, R, Matsuo, K, Hirose, T. Tension lines of the auricular cartilage. Plast Reconstr Surg. 1991; 87:869.
3. Abul-Hassan, HS, von Drasek Ascher, G, Acland, RD. Surgical anatomy and blood supply of the fascial layers of the temporal region. Plast Reconstr Surg. 1986; 77:17.
4. Park, C, Lineaweaver, WC, Rumly, TO, Buncke, HJ. Arterial supply of the anterior ear. Plast Reconstr Surg. 1992; 90:38.
5. Adamson, JE, Horton, CE, Crawford, HH. The growth pattern of the external ear. Plast Reconstr Surg. 1965; 36:466.
6. Farkas, LG. Growth of normal and reconstructed auricles. In: Tanzer RC, Edgerton MT, eds. Symposium on reconstruction of the auricle. St. Louis: Mosby, 1974.
7. Thomson, HG, Correa, A. Unilateral microtia reconstruction: is the position symmetrical? Plast Reconstr Surg. 1993; 92:852.
8. Tolleth, H. Artistic anatomy, dimensions and proportions of the external ear. Clin Plast Surg. 1978; 36:466.
9. Tolleth, H. A hierarchy of values in the design and construction of the ear. Clin Plast Surg. 1978; 36:466.
10. Postnick, JC, Al-Qatta, MM, Whitaker, LA. Assessment of the preferred vertical position of the ear. Plast Reconstr Surg. 1993; 91:1198.
11. Skiles, MS, Randall, P. The aesthetics of ear placement: an experimental study. Plast Reconstr Surg. 1983; 72:133.
12. Brent, B. Reconstruction of traumatic ear deformities. Clin Plast Surg. 1978; 5:437.
13. Bumsted, RM, Ceilley, RI. Auricular malignant neoplasms. Arch Otolaryngol. 1982; 108:225.
14. Cotlar, SW. Reconstruction of the burned ear using a temporalis fascial flap. Plast Reconstr Surg. 1983; 71:45.
15. Donelan, MB. Conchal transposition flap for postburn ear deformities. Plast Reconstr Surg. 1989; 83:641.
16. Elsahy, NI. Ear reconstruction with a flap from the medial surface of the auricle. Ann Plast Surg. 1985; 14:169.
17. Eriksson, E, Vogt, PM. Ear reconstruction. Clin Plast Surg. 1992; 19:637.
18. Herberhold, C. Reconstruction of the auriculum. Facial Plast Surg Monogr. 1988; 5:385.
19. Rosenthal, JS. The thermally injured ear: a systematic approach to reconstruction. Clin Plast Surg. 1992; 19:645.
20. Song, R, Chen, Z, Yang, P, Yue, J. Reconstruction of the external ear. Clin Plast Surg. 1982; 9:49.
21. Songharoen, S, Smith, RA, Jabaley, ME. Tumors of the external ear and reconstruction of defects. Clin Plast Surg. 1978; 5:447.
22. Antia, NH, Buch, VI. Chondrocutaneous advancement flap for the marginal defect of the ear. Plast Reconstr Surg. 1967; 39:472.
23. Ramirez, OM, Heckler, FR. Reconstruction of nonmarginal defects of the ear with chondrocutaneous advancement flaps. Plast Reconstr Surg. 1989; 84:32.
24. Gingrass, RP, Pickrell, KL. Techniques for closure of conchal and external auditory canal defects. Plast Reconstr Surg. 1968; 14:568.
25. Krespi, YP, Ries, WR, Shugar, JMA, Sisson, GA. Auricular reconstruction with postauricular myocutaneous flap. Otolaryngol Head Neck Surg. 1983; 91:191.
26. Tanzer, RC, Bellucci, RJ, Convers, JM, Brent, B. Deformities of the auricle. In: Converse JM, ed. Reconstructive plastic surgery. Philadelphia: WB Saunders, 1977.
27. Tanzer, RC. Total reconstruction of the external ear. Plast Reconstr Surg. 1959; 23:1.
28. Renard, A. Postauricular flaps based on a dermal pedicle for ear reconstruction. Plast Reconstr Surg. 1981; 68:159.
29. Scott, MJL, Klaassen, MF. Immediate reconstruction of the helical rim after bite injury using the posterior auricular flap. Injury. 1992; 23:333.
30. Elsahy, NI. Ear reconstruction with rotation-advancement composite flap. Plast Reconstr Surg. 1985; 77:567.
31. Lawson, VG. Reconstruction of the pinna using preauricular flaps. J Otolaryngol. 1984; 13:191.
32. Millard, DR. The chondrocutaneous flaps in partial auricular repair. Plast Reconstr Surg. 1966; 37:523.
33. Millard, DR. Reconstruction of one-third plus of the auricular circumference. Plast Reconstr Surg. 1992; 90:475.
34. Sutton, AE, Quatela, VC. Bilobed flap reconstruction of the temporal forehead. Arch Otolaryngol Head Neck Surg. 1992; 118:978.
35. Bardsley, AF. Primary reconstruction of a severed ear fragment using a flap of temporoparietal fascia. Br J Plast Surg. 1986; 39:524.
36. Brent, B. Auricular repair with autogenous rib cartilage grafts: two decades of experience with 600 cases. Plast Reconstr Surg. 1992; 90:355.
37. Nagata, S. A new method of total reconstruction of the auricle for microtia. Plast Reconstr Surg. 1993; 92:187.
38. Brent, B. The acquired auricular deformity: a systematic approach to its analysis and reconstruction. Plast Reconstr Surg. 1977; 59:475.
39. Brent, B. The correction of microtia with autogenous cartilage grafts. I: The classic deformity. II: Atypical and complex deformities. Plast Reconstr Surg. 1980; 66:1.
40. Brent, B, Byrd, HS. Secondary ear reconstruction with cartilage grafts covered by axial, random, and free flaps of temporoparietal fascia. Plast Reconstr Surg. 1983; 72:141.
41. Brent, B, Upton, J, Acland, RD, et al. Experience with the temporoparietal fascial free flap. Plast Reconstr Surg. 1985; 76:177.
42. Jenkins, AM, Finucan, T. Primary nonmicrosurgical reconstruction following ear avulsion using the temporoparietal fascial island flap. Plast Reconstr Surg. 1989; 83:148.
43. Rose, EH, Norris, MS. The versatile temporoparietal fascial flap: adaptability to a variety of composite defects. Plast Reconstr Surg. 1990; 85:224.
44. Tegtmeier, RE, Gooding, RA. The use of a fascial flap in ear reconstruction. Plast Reconstr Surg. 1977; 60:406.
45. Smith, RA. The free fascial scalp flap. Plast Reconstr Surg. 1980; 66:204.
46. Cheney, ML, Varvares, M, Nadol, JB. The temporoparietal fascia flap in head and neck reconstruction. Arch Otolaryngol Head Neck Surg. 1993; 19:618.
47. Marty, F, Montandon, D, Gumener, R, Zbrodowski, A. Subcutaneous tissue in the scalp: anatomical physiological and clinical study. Ann Plast Surg. 1977; 60:406.
48. Cheney, ML, Bhatt, S, Googe, P, Hibbard, D. Angiolymphoid hyperplasia with eosinophilia. Ann Otol Rhinol Laryngol. 1993; 102:303.