Reconstruction of Congenital Auricular Malformations
Anatomy and Embryology
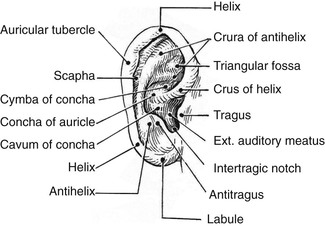
Topographic landmarks of a normal ear are shown in Figure 23-1. Development of the auricle is first observed in the 5-week-old embryo. The auricle begins as six mesenchymal proliferations at the dorsal ends of the first and second pharyngeal arches surrounding the first pharyngeal cleft. Initially, the external ear is located in the lower neck of the embryo. As the mandible develops, the ear ascends to the side of the head at the level of the eyes. The commonly accepted embryologic theory (Fig. 23-2) is that the six hillocks correlate directly with the tragus, helix, cymba, scapha, antihelix, and antitragus.1
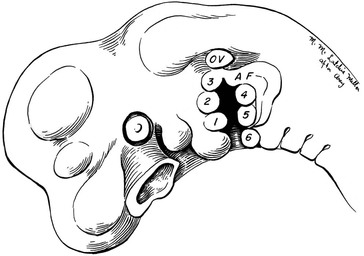
FIGURE 23-2 Auricle in 5-week human embryo develops from six hillocks. OV, otic vesicle; AF, auricular fold.
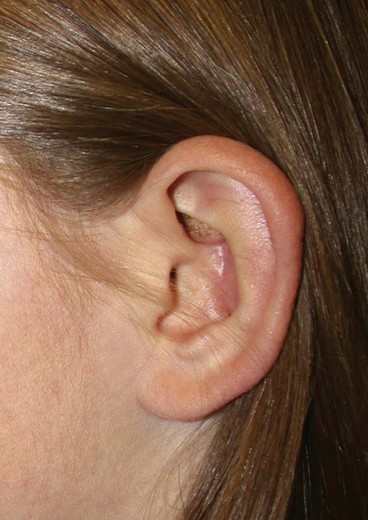
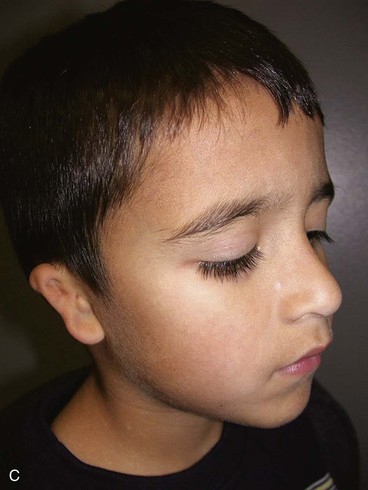
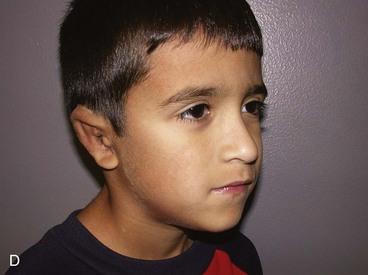
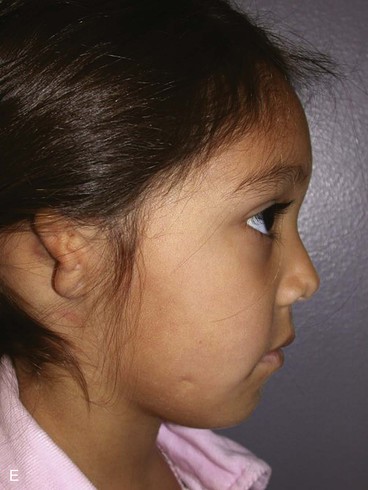
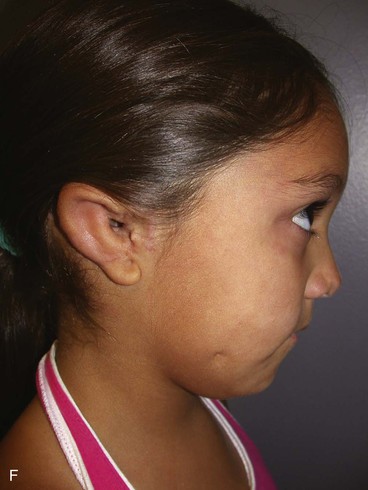
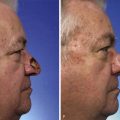
A normal auricle is shown in Figure 23-3. The vertical axis of the normal auricle is tilted posteriorly approximately 20°. The vertical height of the normal ear is approximately equal to the distance between the lateral bony orbital rim and the root of the helix at the level of the eyebrow (about 6-cm). The width of the ear is approximately 55% of its height. Typically, the helical rim protrudes 1- to 2-cm from the skull, and the angle of protrusion should be between 25° and 30°. The superior aspect of the ear is usually level with the eyebrow.2
Preoperative Considerations
A number of congenital auricular deformities exist that may require surgical intervention. To achieve the most natural appearing ear possible through surgery, the surgeon must assess the deformed ear for the presence of several important auricular landmarks. Recognizing and grading the deformity will allow the surgeon to craft the proper surgical options. Involving family members of the patient with an ear deformity is important. Eavey3 observed that children with microtia and significant auricular malformations require comprehensive attention directed to early family counseling and evaluation of the patient for expected and unexpected hearing loss, impaired language development, and associated medical conditions. Both auricular and otologic reconstructive procedures may be necessary and must be coordinated. In 1999, Wang4 identified that the early use of an auricular prosthesis is psychologically beneficial to children who have an ear defect resulting from congenital malformation. Wang also described techniques to establish the proper location and orientation of auricular implants used to secure auricular prostheses by using information from computed tomography (CT) scans. The techniques were applied to children and adults to ensure accurate implant placement, leading to improved positioning and orientation of auricular prostheses.
Congenital Malformations
External ear deformities exist in 1% of births. Correcting major congenital malformations of the auricle tests the plastic surgeon’s surgical skills. Microtia has been the subject of numerous publications as clinicians have attempted to establish a method of classification of the severity of a given deformity. Tanzer5,6 in 1959 published the first scientific article on auricular reconstruction with autogenous rib cartilage. In 1966, Cronin7 popularized the use of silicone as an implant material to reconstruct auricles. Brent,8 who first reported his work in 1974, is considered the world’s foremost authority on auricular reconstruction.
Classification
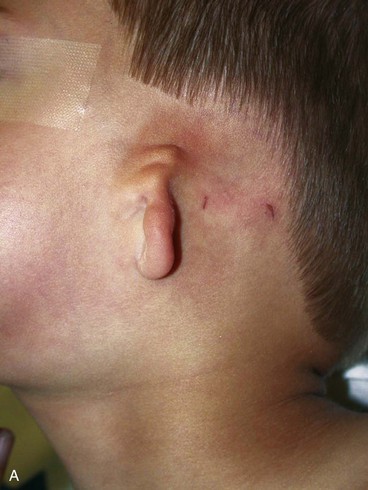
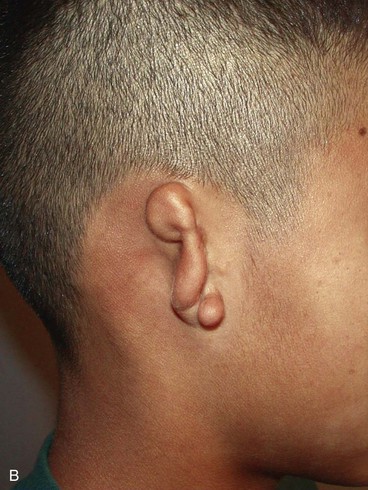
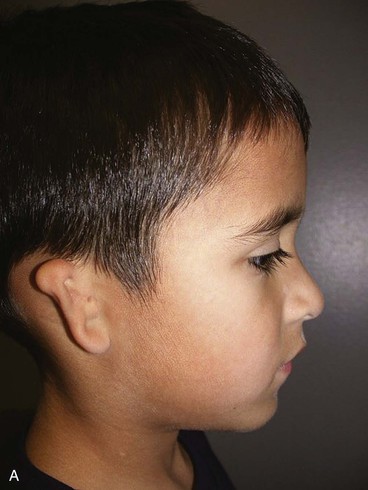
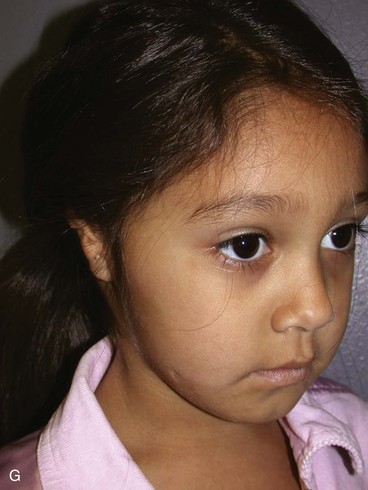
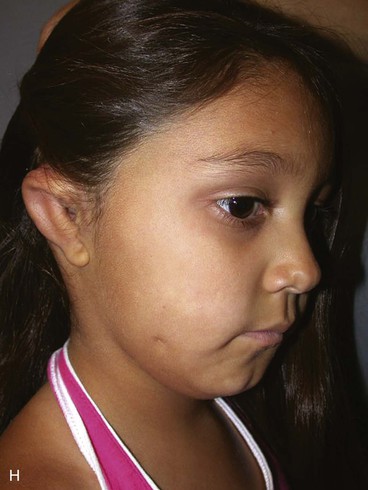
In 1988, Aguilar and Jahrsdoerfer9 amended the 1926 grading system used to classify the severity of microtia described by Marx. In 1996, Aguilar10 reaffirmed this concise classification of congenital ear malformations: grade I is a normal ear; grade II has some of the auricular framework present, but there are obvious deformities; and grade III is the standard “peanut ear” deformity (Fig. 23-4), which encompasses anotia (Marx’s grade IV).
In 1977, Tanzer11 proposed a clinical classification of auricular deformities. This has been used by others and consists of the following:
Complete hypoplasia (microtia)
Hypoplasia of the middle third of the auricle
In 1974, Rogers12 published a similar classification that divided deformities into four groups: macrotia, lop ear, cup ear, and prominent ear. Weerda13 combined all of the classifications proposed by Marx and Tanzer and modified by Rogers.14 This classification is included for completeness, and demonstrates the awkwardness of classification schemes that are too comprehensive or detailed. Weerda’s system included surgical guidance for each classification:
First-degree dysplasia. Average definition: most structures of a normal auricle are recognizable (minor deformities). Surgical definition: reconstruction normally does not require the use of additional skin or cartilage.
Protruding ears; synonyms: prominent ears, bat ears
Cryptotia; synonyms: pocket ear, group IV B (Tanzer)
Small deformities: absence of the tragus, satyr ear, darwinian tubercle, additional folds (Stahl ear)
Colobomas; synonyms: clefts, transverse coloboma
Lobule deformities; synonyms: pixed lobule, macrolobule, absence of lobule, lobule colobomas (bifid lobule)
Type I: Cupped upper portion of the helix, hypertrophic concha, reduced height; synonyms: lidding helix, constricted helix, group IV A (Tanzer), lop ear, minor (mild or moderate) cupping
Type II: More severe lopping of the upper pole of the ear; rib cartilage is used as support when a short ear must be expanded or the auricular cartilage is limp
Second-degree dysplasia. Average definition: some structures of a normal auricle are recognizable. Surgical definition: partial reconstruction requires the use of some additional skin and cartilage. Synonym: second-degree microtia (Marx).
Cup ear deformity, type III: the severe cup ear deformity is malformed in all dimensions; synonyms: cockleshell ear, constricted helix, group IV (Tanzer), snail-shell ear
Third-degree dysplasia. Average definition: none of the structures of a normal auricle are recognizable. Surgical definition: total reconstruction requires the use of skin and large amounts of cartilage. Synonyms: complete hypoplasia group II, peanut ear, third-degree microtia (Marx); normally there is a concomitant congenital atresia.
Unilateral: one ear is normal; no middle ear reconstruction is performed on any child; auricular reconstruction is begun at age 5 or 6 years
Bilateral: bone conduction hearing aid before the first birthday; middle ear surgery at age 4 years without transposition of the vestige; bilateral reconstruction of the auricle at age 5 or 6 years
The treatment recommendations by Rogers are debatable because of certain alternative options. For bilateral microtia, a bone conduction hearing aid is usually implemented at birth. In addition, even in bilateral cases, middle ear surgery can follow the first two stages of auricular reconstruction rather than being the first procedure.15
Surgical Reconstruction of Auricular Deformities
In cases of congenital microtia and concomitant atresia, there should be complete coordination between the otologist and the plastic surgeon. Aguilar10 in 1996 presented the concept of the integrated auricular reconstruction protocol. As shown in Table 23-1, he recommended that microtia repair be accomplished in five stages. The work of the plastic surgeon should be performed first, and the operations should be staged to facilitate total reconstruction of the microtia-atresia complex.
TABLE 23-1
Treatment of Congenital Malformation of the Auricle
| Stage | Treatment |
| I | Creation of a cartilaginous framework with autogenous rib cartilage |
| II | Lobule transposition |
| III | Atresia repair (by otologist) |
| IV | Tragal construction |
| V | Elevation |
In 1997, Williams et al16 reported on the use of polyethylene (Medpor) implants in auricular reconstruction. The implants can be used with skin grafts to reconstruct auricles with deficient auricular cartilage. Animal studies demonstrated that the implant material is well tolerated and could serve as replacement for native cartilage in auricular reconstruction. Williams et al16 reported that wounds in which polyethylene (Medpor) implants were exposed to the exterior as early as 4 days after implantation could heal by secondary intention and subsequently support a skin graft placed over the implant. The authors concluded that this was possible because of the extent of the fibrous tissue ingrowth from surrounding tissue into the pores of the implant. The porous nature of the implant material served as a scaffolding for ingrowth of native tissue. However, in the clinical situation, the use of this type of implant has been a problem. Exposure of the Medpor implant usually requires débridement and frequently removal of the implant. Historically, the use of other materials for auricular frameworks has generally been unsuccessful. Neither irradiated cartilage nor Silastic implants have stood the test of time. Irradiated cartilage tends to be absorbed over time, and Silastic implants are notorious for their inability to withstand trauma, resulting in a tendency to extrude with time.
Surgical Planning and Treatment
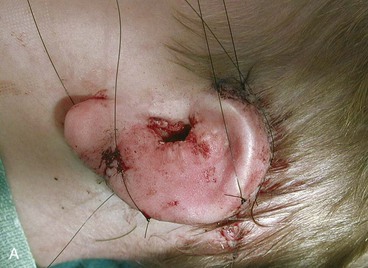
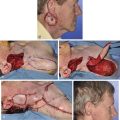
The first surgical stage of auricular reconstruction begins with framework construction from rib grafts and implantation of the fabricated framework beneath the skin at the recipient site. The dissection of rib cartilage is extraperichondrial, and no stripping of the perichondrium of the graft is performed (Fig. 23-5). The technique to sculpt the costal cartilage framework has been described by Brent.17 The framework is constructed of segments of the sixth, seventh, and eighth ribs, which are joined together with 5-0 stainless steel wire (Fig. 23-6). The framework is placed beneath the skin of the lateral face with proper positioning and orientation as determined by the preoperative patient assessment (Fig. 23-7). When it is performed by trained surgeons, the technique produces reliable results. The most common complication that occurs from the first-stage procedure is atelectasis. Other complications include pleural tear (not a complication unless it is not recognized intraoperatively), pneumothorax, pneumomediastinum, chest wall deformity, hypertrophic scarring, and pneumonia.
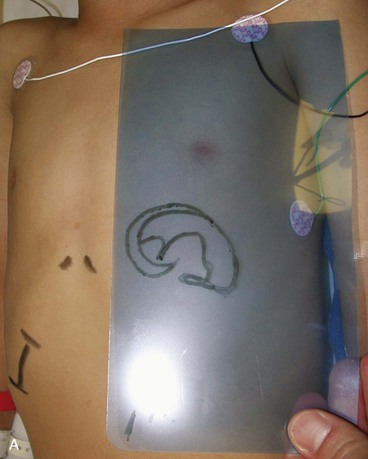
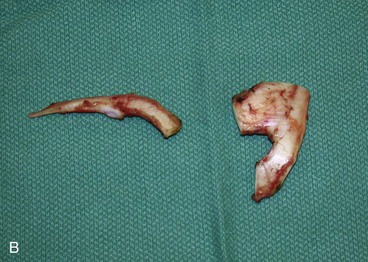
FIGURE 23-5 A, B, Costal cartilage harvested from sixth, seventh, and eighth ribs and dissected extraperichondrially.
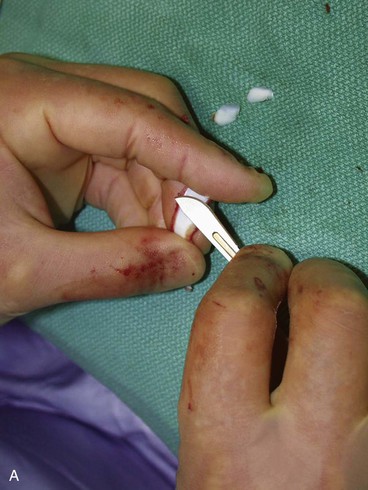
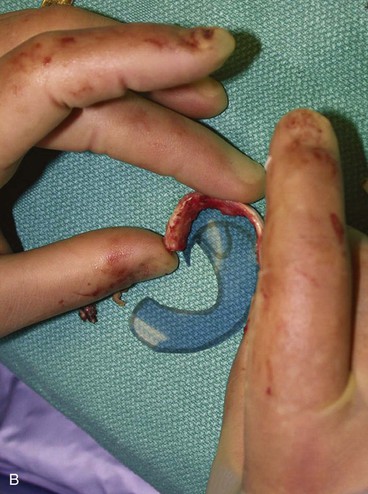
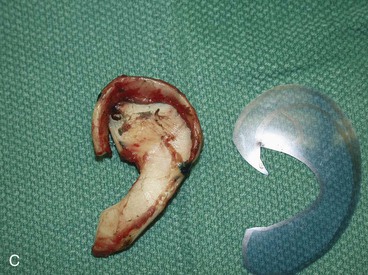
FIGURE 23-6 By use of template as guide, cartilage framework is sculpted from costal cartilage and assembled with 5-0 stainless steel wire. A-C, Sculpting of left auricular framework.
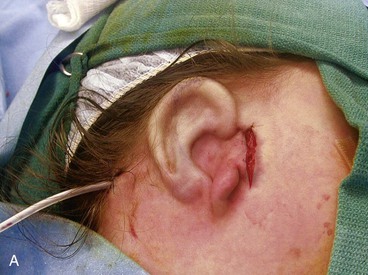
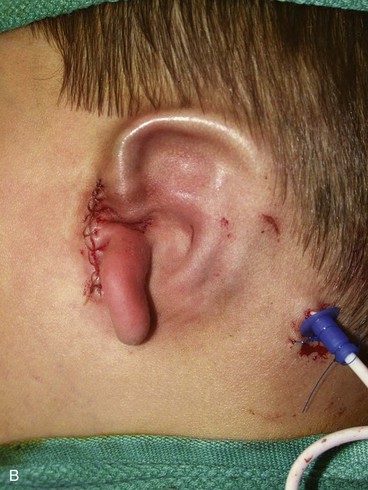
FIGURE 23-7 A, B, Cartilaginous framework beneath skin for reconstruction of right and left auricles.
The second surgical stage consists of transposing the lobule. To avoid protrusion of the lobule, the incision on the medial aspect of the lobe should be fairly high. The inferiorly based pedicle of the earlobe flap is quite thin; thus, great care is taken in its handling (Fig. 23-8).
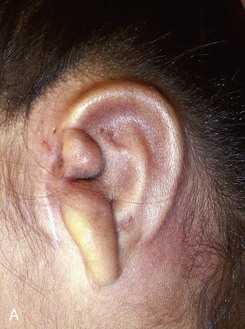
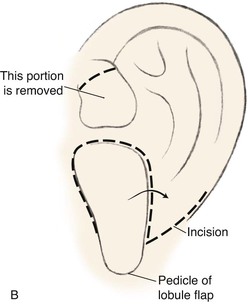
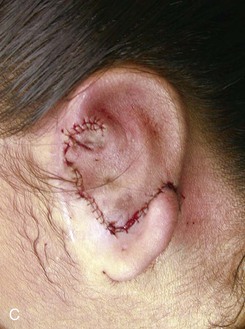
FIGURE 23-8 A, Cartilaginous framework beneath skin and malpositioned lobule. B, Drawing showing incisions for transposition of lobule. C, Lobule transposed. Superior aspect of malpositioned lobule discarded.
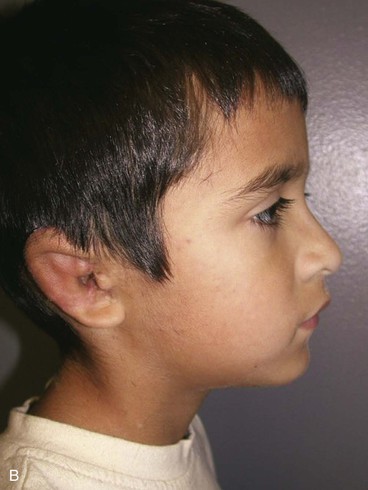
On completion of the first two surgical stages for reconstruction of the auricle, attention is then turned to the surgery required to restore hearing in cases of associated atresia of the middle ear. This is considered the third stage of treatment and is performed by an otologist. The location of the temporal bone remnant dictates the location for the opening of the external canal in the overlying skin. It is easy to manipulate the fabricated framework and position it so that an otologist may properly reconstruct the external auditory canal. If the external canal is created before the first two stages of auricular reconstruction, the complication rate of microtia repair is much higher. This is because it is difficult to properly position and orient the auricular framework around an external auditory canal as a result of the adjacent scarring created by the canal surgery. The scarring also impairs the vascular supply to the region of reconstruction, and this may contribute to an increased risk of complications.
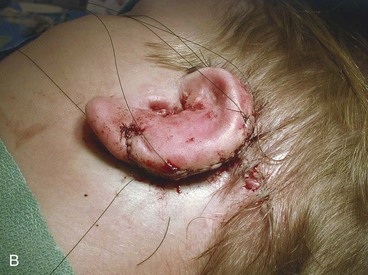
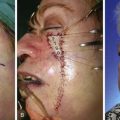
The third stage of auricular reconstruction (fourth stage of treatment) is performed after the creation of the external auditory canal. It is directed toward reconstruction of the tragus (Fig. 23-9). This is accomplished by obtaining a composite auricular graft from the opposite ear. The composite graft is fashioned into a triangle-shaped graft, which is transferred to the appropriate location at the reconstructive site. Auricular elevation with creation of a supra-auricular and postauricular sulcus is performed during stage V of treatment (Fig. 23-10). This is accomplished by dissecting the cartilaginous framework with the overlying skin as a large composite flap. The flap is elevated away from the head superiorly and posteriorly, creating a tissue void where the anticipated superior and posterior sulci will be. The area of the tissue void as well as the medial aspect of the dissected flap is covered by a full-thickness skin graft.
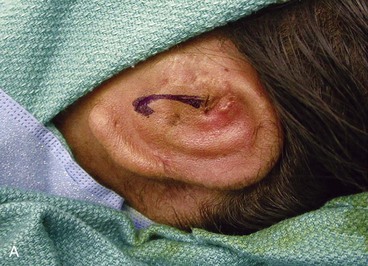
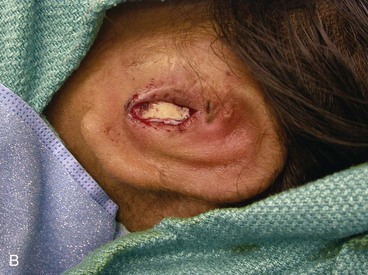
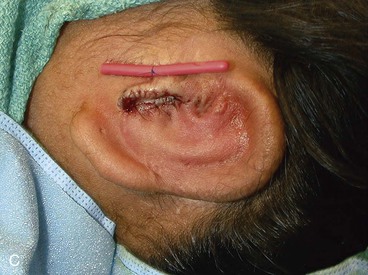
FIGURE 23-9 Tragus reconstructed with composite auricular graft from contralateral ear. A, Recipient site incision. B, Conchal bowl excavated. Skin graft beneath bolster resurfaces conchal bowl. C, Composite graft sutured in place. Rubber catheter stent used to maintain position and orientation of graft.
Summary
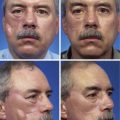
Auricular reconstruction is difficult, and with each of the steps described, complications may occur, even in the hands of experienced surgeons. Reconstruction always requires postoperative evaluations, and often the surgeon will not be totally satisfied. Surgical correction of congenital microtia requires commitment by the plastic surgeon to be adept at the various reconstructive surgical techniques. The surgeon should be performing more than five such operations per year to maintain proficiency (Fig. 23-11). The team approach described here is invaluable to the patient and the patient’s family. Failure to offer such an approach to treatment is a significant disservice to the patient. The field of tissue engineering promises to change auricular reconstruction. The nexus of cell growth biology and cell scaffolding technology is rapidly changing and may at some point in the future provide a biocompatible synthesized implant that serves as an auricular framework. The technology must overcome issues such as tissue rejection, integrity of form, and structural strength for synthesized implants to withstand overlying skin tension after implantation.
References
1. Gulya, AJ. Developmental anatomy of the ear. In Glascock ME, III., Shambaugh GE, Jr., eds.: Surgery of the ear, 4th ed, Philadelphia: WB Saunders, 1990.
2. Farkas, LG. Anthropometry of normal and anomalous ears. Clin Plast Surg. 1978; 5:401.
3. Eavey, RD. Microtia and significant auricular malformation. Ninety-two pediatric patients. Arch Otolaryngol Head Neck Surg. 1995; 121:57.
4. Wang, R. Presurgical confirmation of craniofacial implant locations in children requiring implant-retained auricular prosthesis. J Prosthet Dent. 1999; 81:492.
5. Tanzer, RC. Total reconstruction of the external ear. Plast Reconstr Surg. 1959; 23:1.
6. Tanzer, RC, Correction of microtia with autogenous costal cartilage. Symposium on reconstruction of the auricle. Tanzer, R, Edgerton, M, eds. Symposium on reconstruction of the auricle; vol. 10. CV Mosby, St. Louis, 1974.
7. Cronin, TD. Use of a Silastic frame for total and subtotal reconstruction of the external ear: preliminary report. Plast Reconstr Surg. 1966; 37:399.
8. Brent, B. Ear reconstruction with an expansible framework of autogenous rib cartilage. Plast Reconstr Surg. 1974; 53:619.
9. Aguilar, EA, III., Jahrsdoerfer, RA. The surgical repair of congenital microtia and atresia. Arch Otolaryngol Head Neck Surg. 1988; 98:600.
10. Aguilar, EA, III. Auricular reconstruction of congenital microtia (grade III). Laryngoscope. 1996; 106(Suppl. 82):1.
11. Tanzer, RC, Congenital deformities. Reconstructive plastic surgery. 2nd ed. Converse, JM, eds. Reconstructive plastic surgery; vol. 3. WB Saunders, Philadelphia, 1977.
12. Rogers, B, Anatomy, embryology and classification of auricular deformities. Symposium on reconstruction of the auricle. Tanzer, R, Edgerton, M, eds. Symposium on reconstruction of the auricle; vol. 10. CV Mosby, St. Louis, 1974.
13. Weerda, H. Classification of congenital deformities of the auricle. Facial Plast Surg. 1988; 5:385.
14. Rogers, B. Microtic, lop, cup and protruding ears. Plast Reconstr Surg. 1968; 41:208.
15. Aguilar, EA, III. Classification of auricular congenital deformities. In: Papel ID, Nachlas NE, eds. Facial plastic and reconstructive surgery. St. Louis: Mosby−Year Book, 1992.
16. Williams, JD, Romo, T, III., Scalfani, AP, et al. Polyethylene implants in auricular reconstruction. Arch Otolaryngol Head Neck Surg. 1997; 123:578.
17. Brent, B. Auricular repair with autogenous rib cartilage grafts: two decades of experience with 600 cases. Plast Reconstr Surg. 1992; 90:355.