11 Reconstitution / Dilution
Summary and Key Features
• Prescribing information on all available products recommends reconstitution in unpreserved saline
• Studies in the literature suggest that other solutions can be used such as preserved saline, anesthetics (lidocaine or bupivacaine), with or without epinephrine and albumin
• In vivo studies demonstrate that onabotulinumtoxinA is not as fragile as originally thought and that foam during reconstitution does not inactivate the toxin
• Although manufacturers recommend storage at 2–8°C in the refrigerator, toxin administration within 4–24 hours, and avoiding freezing after reconstitution, it appears that some products can be stored for longer periods of time after reconstitution, in the refrigerator or the freezer, without loss of efficacy or contamination
• In regard to the best dilution to be used, when treating limb muscles, perhaps as a result of greater dispersion of the toxin, higher dilutions might be beneficial
• For facial muscles, more concentrated volumes are preferred, since they produce less discomfort for patients
• The literature suggests that toxins may be more sturdy and resistant to degradation than previously understood
Reported substances used in the reconstitution process
OnabotulinumtoxinA is provided as a vacuum-dried powder, whereas incobotulinumtoxinA, abobotulinumtoxinA, and BoNT-A from Lanzhou (China) are presented as lyophilized powders (Fig. 11.1). RimabotulinumtoxinB is provided as a ready-to-use sterile liquid and no reconstitution is required.
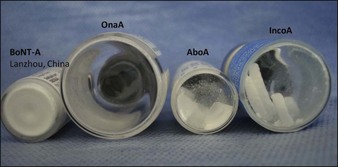
Figure 11.1 Appearance of various vacuum-dried and lyophilized BoNT-A powder products, before reconstitution.
Dilution issues
There is considerable discussion about the effect of dilution of BoNTs on clinical effect, duration and diffusion. Table 11.1 shows the BoNT-A manufacturers’ recommended volumes for reconstitution.
| Toxin | Dilution range | Concentration |
|---|---|---|
| OnabotulinumtoxinA | 1–8 mL of saline | 12.5–100 U/mL |
| AbobotulinumtoxinA | 300 U in 0.6–2.5 mL | 120–500 U/mL |
| IncobotulinumtoxinA | 1–8 mL | 12.5–100 U/mL |
Non-facial muscles
Hyperhidrosis
There is no standardized dilution for botulinum toxin treatment of focal hyperhidrosis. Dilutions reported for hyperhidrosis are detailed in Table 11.2.
| Toxin | Dilution range | Most used dilution |
|---|---|---|
| OnabotulinumtoxinA | 1–10 mL of saline | 2–5 mL |
| AbobotulinumtoxinA | 1.25–10 mL | 2.5–5 mL |
| IncobotulinumtoxinA | 1–10 mL of saline | 10 mL (one paper) |
Asher B, Talarico S, Casuto D, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit) – part I: upper facial wrinkles. Journal of the European Academy of Dermatology and Venereology. 2010;24(11):1285–1295.
Boyle MH, McGwin, Flanagan CE, et al. High versus low concentration botulinum toxin A for benign essential blepharospasm: does dilution make a difference? Ophthalmic Plastic and Reconstructive Surgery. 2009;25:81–84.
Carruthers J, Carruthers A. Botulinumtoxin in facial rejuvenation: an update. Dermatologic Clinics. 2009;27:417–425.
Carruthers A, Carruthers J, Cohen J. Dilution volume of botulinum toxin type a for the treatment of glabellar rhytides: does it matter? Dermatologic Surgery. 2007;33:S97–S104.
Carruthers J, Fagien S, Matarasso SL. Consensus recommendations on the use of botulinum toxin Type A in facial aesthetics. Plastic and Reconstructive Surgery. 2004;114(6 suppl):1S–22S.
Gracies JM, Lugassy M, Weisz DJ, et al. Botulinum toxin dilution and endplate targeting in spasticity: a double-blind controlled study. Archives of Physical Medicine and Rehabilitation. 2009;90:9–16.
Hexsel DM, Trindade de Almeida AR, Rutowithsch M, et al. Multicenter, double blind study of the efficacy of injections with botulinum toxin type A reconstituted up to six consecutive weeks before application. Dermatologic Surgery. 2003;29:523.
Hu GC, Chuang YC, Liu JP, et al. Botulinum toxin (Dysport) treatment of the spastic gastrocnemius muscle in children with cerebral palsy: a randomized trial comparing two injection volumes. Clinical Rehabilitation. 2009;23(1):64–71.
Kane M, Donofrio L, Ascher B, et al. Expanding the use of neurotoxins in facial aesthetics: A consensus panel’s assessment and recommendations. Journal of Drugs in Dermatology. 2010;9(1):S3–S22.
Kazim NA, Black EH. Botox: shaken, not stirred. Ophthalmic Plastic and Reconstructive Surgery. 2008;24(1):10–12.
Lee LR, Chuang YC, Yang BJ, et al. Botulinum toxin for lower limb spasticity in children with cerebral palsy: a single-blinded trial comparing dilution techniques. American Journal of Physical Medicine and Rehabilitation. 2004;83:766–773.
Mohammadi B, Kollewe K, Wegener M, et al. Experience with long-term treatment with albumin-supplemented botulinum toxin type A. Journal of Neural Transmission. 2009;116:437–441.
Parsa AA, et al. Reconstituted botulinum type A neurotoxin: clinical efficacy after long-term freezing before use. Aesthetic Plastic Surgery. 2007;31:188–191.
Shome D, Nair AG, Kapoor R, et al. Botulinum toxin A: is it really a fragile molecule? Dermatologic Surgery. 2010;36:2106–2110.
Toth SI, Smith LA, Ahmed SA. Extreme sensitivity of botulinum neurotoxin domains towards mild agitation. Journal of Pharmaceutical Sciences. 2009;98(9):3302–3311.
Trindade de Almeida AR, Kadunc BV, Di Chiacchio N, et al. Foam during reconstitution does not affect the potency of botulinum toxin A. Dermatologic Surgery. 2003;29:530.
Trindade de Almeida AR, Secco LC, Carruthers A. Handling botulinum toxins: an update literature review. Dermatologic Surgery. 2011;37(11):1553–1565.