Chapter 32 Reanimation of the Paralysed Elbow
Conditions resulting in paralysis at the elbow
Absent muscle may result either from trauma destroying or damaging the muscle beyond functional restitution, or from congenital abnormalities including arthrogryposis multiplex congenita.1,2 As we examine the treatment of each of these causes of loss of elbow flexion, a number of treatment techniques will emerge and it will become apparent that all techniques have some application in most conditions. Choosing between these techniques will depend partly upon the repertoire of the surgical team and their experience, and upon patient preference influenced by their age, other conditions, and important factors such as appearance. For such a simple motion, elbow flexion can be remarkably difficult to achieve and it is not uncommon in the senior author’s practice for more than one technique to be employed in a single patient.
Prior to reanimating any elbow, consideration must be given to the passive range of motion. This need not be full or normal; indeed following the restoration of elbow flexion, some degree of contracture is desirable to facilitate the initiation of motion (by virtue of the increased moment arm of muscle action at rest). Similarly full active flexion is not always essential even for feeding. Many children with obstetric brachial palsy develop the ‘trumpet’ sign3 by which in order to get the hand to the mouth the humerus is abducted to 90° or more prior to completion of elbow flexion. This activity compensates for the absence of active external rotation at the shoulder but also excludes gravity from the elbow articulation making flexion easier. It also brings the prehensile radial border of the hand to the mouth in full supination, which is important for those children who have lost pronation.
In dealing with restoration of elbow movement it is important not to consider the elbow in isolation. Too often in adult brachial plexus injury the senior author sees patients in whom elbow flexion has been restored without addressing the instability, or lack of active external rotation, at the shoulder. This results in an elbow that flexes up the anterior chest wall to the anterior neck but does not support a hand that can be positioned in space. Whilst this simple function in a badly paralysed limb can be useful for augmenting brachiothoracic grasp, it is important when planning any brachial plexus reconstruction to consider the implications at the shoulder which may itself be managed by either reanimation or arthrodesis;4,5 a topic that is beyond the scope of this chapter. Furthermore, elbow flexion is valuable in the context of positioning a competent hand in space and so its place in the restoration of function of an upper limb whether from brachial plexus palsy, congenital defects or widespread trauma, must be considered at the outset. A coherent programme with6,7 an appropriate timescale should, therefore, be agreed with the patient at the start of reconstruction and rehabilitation.
Specific circumstances of lost elbow flexion and methods of treatment
Acute denervation
Because two nerves supply the three muscles responsible for elbow flexion, complete neurogenic loss of flexion is only usually associated with injuries to the upper trunk of the brachial plexus especially proximal to Erb’s point at the C5–C6 level. Isolated paralysis of the anterior compartment of the arm may result from an injury to the musculocutaneous nerve distally either through traction or more commonly as a result of a penetrating injury including iatrogenic causes. As with any acute denervation of the muscle the underlying principle is early restoration of nerve continuity. Where there has been a penetrating wound the diagnosis is obvious and exploration is mandatory wherever possible. Problems most commonly arise in the closed injury where the temptation is to ‘wait and see’. A full discussion of the indications for brachial plexus surgery is not appropriate here but we make some observations. The principle of ‘wait and see’ rests on the concept that a neurapraxia may be responsible for loss of nerve function and that this may be fully recoverable. Furthermore, some will argue, that even if the injury is a Sunderland Grade II or III8,9 surgical exploration would reveal a nerve in continuity and that given that the prognosis is uncertain, resection and grafting would not be appropriate. This is all well and good, but against this must be considered the fact that loss of nerve continuity even at the axonal level results in distal end-organ decay and proximal central cell death, and that these consequences are inversely related to the period since injury. For these reasons some surgeons set an arbitrary time of 3 months to assess if recovery is occurring. This would be appropriate particularly if one is able to monitor the progression of a Hoffman–Tinel phenomenon or there is strong circumstantial evidence (from history of low kinetic energy transfer or evidence from scans of thecal integrity) that a neurapraxia is likely. Recovery from a true neurapraxia is not sequential from proximal to distal but is sudden and anatomically more random as the functional conduction blocks recover.
In addition to the necessary investigations of electromyography (which can be useful in identifying neurapraxia) and nerve conduction studies10 (which are less useful because of the difficulty in achieving compound nerve axon potentials or sensory nerve axon potentials in the deeply placed musculocutaneous nerve), in the absence of scan evidence of avulsion, exploration of the upper trunk in the acute phase after injury in experienced hands can be invaluable. If the brachial plexus is explored within a week of injury the nature, extent and severity of the damage are immediately apparent. In the rare case that a lesion in continuity is so innocuous as to leave doubt about resection and repair, nothing is lost after a simple exploration and one can revert to a ‘wait and see’ policy. However in the vast majority of cases we have explored in such circumstances, the indication for treatment has been unequivocally apparent.11 This principle of open exploration at an early stage (especially in the first week before fibrosis clouds the picture) has guided us over 20 years in the management of upper trunk injuries and infraclavicular injuries to the brachial plexus. The only exception to this in adults has been the prolonged crush injury resulting from dislocation of the shoulder and delayed relocation where early exploration is unlikely to yield useful information since traction and disruption is not the cause of injury and the consequences of localized compression can be difficult to conclude from direct inspection.
The circumstances of Narakas12–14 type I and type II obstetric brachial palsies are similarly specific and the restoration of elbow flexion in these children by primary nerve grafting is the subject of considerable debate and at present there is no universally accepted protocol. The senior author’s own practice is to follow the multiple movement scale as developed in Toronto.15 At 12 weeks, if there is a low score then exploration should be undertaken. At this time the parents are told that the first part of the procedure will be simple exploration of the upper trunk or even the whole brachial plexus and that we will only proceed to surgical repair where the appearance indicates a very low probability of spontaneous or substantial recovery. This judgement requires experience.
In some cases of adult brachial plexus injury (and some cases of congenital brachial plexus injury) C5 and C6 roots are avulsed16–19 and no orthotopic restoration is possible. Here in the acute circumstance nerve transfers must be employed. The principle of a nerve transfer is that an undamaged distal nerve stump which is connected to the muscle in the normal way may be reanimated by neurosynthesis with a competent centrally connected proximal stump of a nerve that was serving another purpose. Clearly, as with tendon transfers, the donor nerve (that is the proximally connected one) must have a function that can be spared. Much has been written on this subject since Sir Herbert Seddon20–22 first described this procedure using intercostal nerves, although the indications in elbow flexion are now quite clear. As stated previously in the case of paralysis of the shoulder and elbow a clear decision must be made about the management of the shoulder whether by nerve transfers (such as the accessory nerve to the suprascapular nerve or the radial nerve to the axillary nerve) or by arthrodesis, at the same time that a management plan is made for the elbow. A number of nerve transfers have been advocated for the elbow and the author’s preferences are given below.
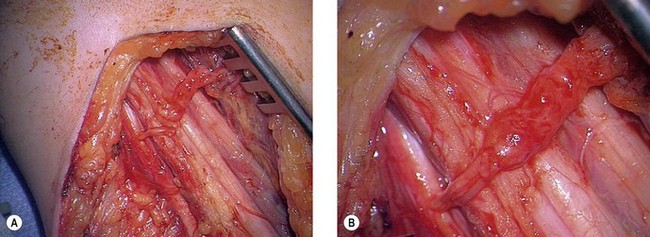
Oberlin transfer
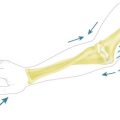
In 1994, Dr Christophe Oberlin23 described the transfer of fascicles of the ulnar nerve to the nerve supplying biceps brachialis. The theory was that fascicles of the ulnar nerve in the upper arm could be isolated using a neuro-stimulator in the case of the C5–C6 injury (where the hand remained competent) and that these fibres which originate from C8 or T1 could be transferred to the nerve to biceps brachialis and so reinnervate that muscle (Fig. 32.1). This is indeed the case and this is a most effective transfer. We would, however, make a number of observations. First, we are sceptical that fascicles to individual hypothenar muscles can be isolated in the mid-proximal arm. This does not detract from the value of the transfer but can be confusing for surgeons newly undertaking this procedure. Second, the median or the ulnar nerve can be used for this transfer and it is likely that the radial nerve can also be used under certain circumstances. The appeal of this transfer lies in the fact that the neurosynthesis is conducted close to the hilum of the muscle and reinnervation is prompt, mitigating the effects of distal target organ decay. This is our preferred nerve transfer in the arms of adults where orthotopic nerve repair is not possible and a good hand exists. We have never found a significant donor defect in the hand.
Intercostal nerve transfer
Sir Herbert Seddon made the discovery that intercostal nerves which are both sensory and motor could be transferred onto nerves in the arm with resultant function. This technique subsequently fell into some disrepute, although it has been resurrected in the past 20 years and we have found it extremely valuable in certain cases. The protocol for such transfers was well described by Chuang24,25 and our practice described here is based upon his work.
Once the musculocutaneous nerve in which orthotoptic repair is not possible has been isolated, its distal stump is prepared as far as possible by dissecting the nerve into the apex of the axilla to the retroclavicular portion where it forms the terminal branch of the upper trunk as the lateral head of the median nerve separates from it. Transected at that point, the distal stump of the musculocutaneous nerve can then be delivered into the axilla. Some surgeons have advocated skeletonizing that nerve into its sensory and motor component to avoid pushing motor fibres distally into the sensory territory after neurosynthesis. Dr Julia Terzis has proposed an alternative and ingenious solution which is to connect the lateral cutaneous nerve of the forearm (the terminal branch of the musculocutaneous nerve) to the nerve to brachioradialis making use of such misdirected fibres to reanimate another elbow flexor.7,26 This technique has the advantage that it does not damage the musculocutaneous nerve further.
Other nerve transfers
Other nerves18,27–35 have also been used to reanimate the musculocutaneous nerve or nerve to biceps brachialis. These included the phrenic nerve which (on the right side easily, and on the left side with some more difficulty) may be harvested endoscopically. This nerve should not be used in small children and certainly never below the age of 3 years, as significant and on occasion fatal respiratory difficulties may ensue. We have been reluctant to use the phrenic nerve generally because of the effect on respiration but where other nerves are not available it has been effective and the adult patient has rarely suffered an identifiable deficit. The phrenic nerve may be used at the same time as three intercostal nerves although a respiratory deficit is more likely.
The spinal accessory nerve can also be used. If the shoulder has been arthrodesed the distal spinal accessory nerve may be spared to reanimate the elbow, although this usually requires an interpositional nerve graft with consequent loss of axonal material at each neurosynthesis. Lastly the cross neck C7 nerve transfer has achieved remarkable popularity since first described by Gu36–38 in China in the early 1990s. The senior author was hesitant to undertake nerve transfers from the undamaged opposite C7 root but after examining the cases of other surgeons, began to undertake this transfer a number of years ago.
In our experience, there has been little if any donor defect. We have, however, subsequently abandoned this nerve transfer as in our hands it has resulted in little functional benefit. Almost all reports of function following this nerve transfer are really reports of muscle activity. This has been demonstrated by several authors.29,39,40 The concern here, however, is that none of our patients (who range from the age of 1 year at the time of transfer to mid-forties) have managed to separate the recovered muscle function from activity of the contralateral limb. It has therefore been a transfer that has clearly resulted in muscle activity, although this activity has in our patients never been translated into day-to-day function or integrated into the activity of the patient. This is in stark contrast to the utility of ipsilateral transfers.
Chronic denervation
There is some debate about what constitutes chronic denervation and from what time after nerve injury orthotopic repair is unlikely to succeed.11,41–45 We have no doubt that 1 year after injury in an adult, motor reinnervation is so unlikely to succeed as to be rarely worth attempting. The difficulty exists between 6 months and a year where some authors will enthusiastically undertake orthotopic or heterotopic repair while others will look to muscle transfers. The commonest circumstance in which this arises is either a patient whose care has been delayed for other reasons (usually other trauma) or the patient in whom repair of the brachial plexus has failed to result in recovery of function. In all cases of chronic denervation where orthotopic or heterotopic repair is inappropriate the afflicted muscle must be replaced or substituted. This can be done conventionally by tendon transfers or less conventionally by free functioning microvascular muscle transplantation.
Pectoralis major
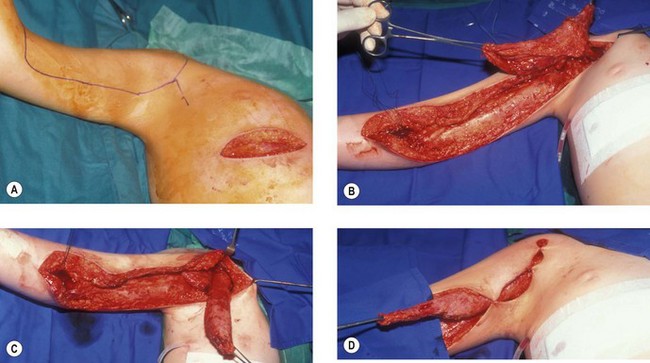
There are two main ways of transferring the pectoralis major. Its beautifully vorticeal tendon may be detached from the humerus and elongated distally to be inserted directly into the biceps brachii tendon. This unipolar transfer was first described in 1917.46 It does, however, produce a web across the anterior axillary fold and results in adduction of the humerus at the same time as flexion of the elbow.
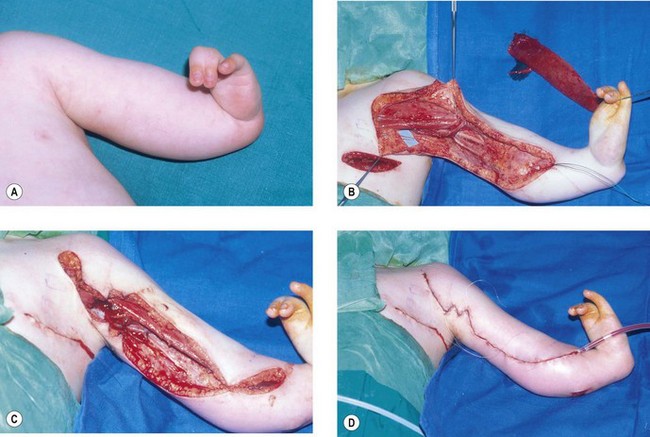
The alternative is the Clark47–49 transfer in which the origin of pectoralis major is detached from the lower ribs together with part of the anterior rectus sheath. The muscle can then be isolated by carefully dissecting proximally from the origin preserving the neurovascular pedicle on the under surface and isolating the muscle on that pedicle which is largely innervated by the lateral pectoral nerve. The fibres of the anterior rectus sheath are then used for the repair at the level of the biceps brachialis. A more contemporary modification sees the tendon of pectoralis major also detached and relocated either to the anterior clavicle laterally or more commonly to the coracoid process50,51 (Fig. 32.2). This transfer is very effective in children and can also be very useful in adults. In children the muscle can be harvested through both a short transverse upper abdominal and axillary incisions. So distensible is the skin of the child and so short the distances that the dissection can proceed satisfactorily through these two incisions. In the adult more extensive incisions are needed over the free border of the pectoralis major. These can be just as disfiguring as the loss of the muscle.
Steindler flexoplasty57
In some patients with denervation of the three prime flexors of the elbow, it can be observed that flexion is nonetheless achieved by clenching the fist, extending the wrist and allowing those fibres of the common flexor origin that pass anterior to the axis of the elbow joint to contract and flex the elbow. This is the so called Steindler effect, and Steindler’s contribution was to observe that this could be enhanced by moving the common flexor origin proximally so increasing the lever arm of these muscles at the elbow joint. In practice this can be a useful transfer.58–62
One of the pleasing things about this transfer is that no re-education is necessary. Furthermore the transfer invariably produces a flexion contracture at the elbow which facilitates the initiation of elbow flexion. The disappointing thing about this transfer in our hands has been that while it does result in good and quite powerful elbow flexion to well beyond 90°, this flexion requires clenching of the fist in most patients and thus the moment the object held in the hand is released the elbow flexion is lost. This can be circumvented by transferring only the wrist flexors,60–62 although this is a much more difficult and less certain undertaking. In practice we have used the Steindler flexorplasty to augment the initiation of elbow flexion or to strengthen other flexion transfers in the manner alluded to at the beginning of this chapter where more than one solution may be required to restore competent flexion.
Free functioning muscle transfer
A number of muscles have been recommended for this purpose including the rectus abdominus, the latissimus dorsi, the rectus femoris, the gastrocnemius and the gracilis muscle.63–67 We have experience with the gracilis muscle in over 60 cases and feel we can speak about this with most authority. The other muscle transfers can be examined on their merits in the published literature, although we will make brief comments about them here. The gastrocnemius muscle is appealing because of its bulk and the fact that its activity is to a considerable degree supplanted by soleus. However, it is a disfiguring donor site, and even if only one belly of the transfer is taken the damage to the calf is clear and permanent. It has the attraction of a clearly defined neurovascular pedicle and it certainly deserves consideration as a transfer, although the excursion of this muscle is not great.
The rectus femoris muscle can be harvested with relatively little donor morbidity and the anterior thigh scar is surprisingly good. We have every reason to believe that this is a valuable muscle for transfer but have experience with only one case in which it was difficult to match the length of the transfer to the shorter length of the upper arm. Like the gracilis muscle it has an excellent excursion, but has greater cross-sectional area (and so can develop greater force) and greater volume (so greater power and work capacity) than gracilis. The mismatch in length remains a problem, as does the donor site.68–70
Gracilis muscle
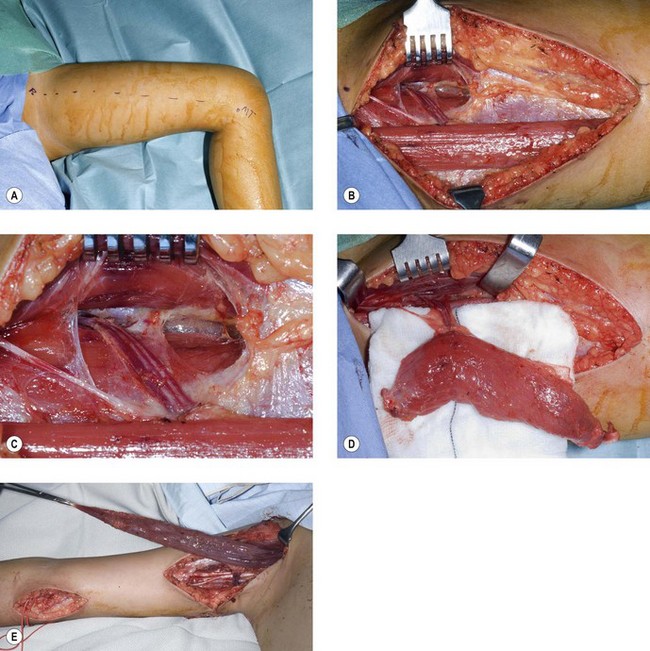
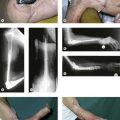
Our preferred elbow flexor is the gracilis muscle. This muscle of the medial thigh is ideal in so many ways. It is redundant, of reasonable cross-sectional area, of good length and excursion and has a fibrous tendon at either end. Furthermore it has a proximally located reliable neurovascular pedicle with a long nerve (the obturator nerve) to facilitate positioning the neurosynthesis (Figs 32.3, 32.4).
We have found the gracilis muscle to be an extremely useful replacement for an absent or chronically denervated elbow flexor mass.71 Everything, however, depends upon successful revascularization, which is easily achieved on the brachial vessels, and successful reinnervation, which is facilitated by the long motor nerve, all of whose fibres end within the muscle. We have most commonly reanimated this on an Oberlin transfer or intercostal nerve transfers (the Oberlin being preferred in adults, the intercostal nerves in children). We have attached the proximal tendinous origin of the gracilis to the coracoid process and woven the distal tendon skeletonized of its adventitial tissues into the biceps muscle and its tendon. In cases where the elbow flexors are absent this tendon of gracilis may be passed through a drill hole in the ulnar and secured on the dorsal surface. In this respect it is worth noting that for any elbow transfer orthotopic repair to the biceps tendon may not be possible. A more distal placement may be the only option. This does have the benefit of producing a flexion contracture of the elbow and facilitating the lever arm of the transfer.
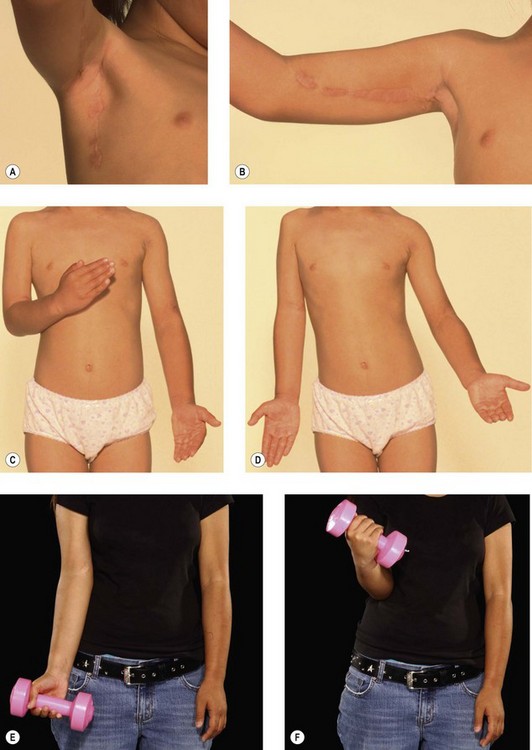
We have reviewed our results of free functioning muscle transfer to the elbow and found that transfers of the gracilis muscle in children, whether on intercostal or Oberlin transfers, are almost universally successful in providing powerful activity and adequate reanimation (Fig. 32.5). As with all transfers, however, they have been less successful in adults, although our improvement rate has been at least as good as and usually better than for pedicled transfers. This transfer has the disadvantage of requiring microsurgical familiarity and should only be conducted in a unit undertaking regular microvascular transfers with a high success rate. However, it is worth noting that should this transfer fail it can simply be repeated using the contralateral gracilis with no significant functional cost to the lower limbs. It is thus rare among free muscle transfers as being totally expendable.
Absent muscle of congenital origin
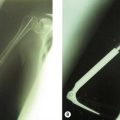
In radial dysplasia (Fig. 32.6) it is most commonly the wrist, the hand and the elbow that are affected and in this condition it is imperative that before consideration is given to correcting the wrist deformity an evaluation of the elbow is undertaken. In the absence of elbow flexion, correction of the wrist may disadvantage the child significantly.
1 Kozin SH. Congenital differences about the elbow. Hand Clin. 2009;25(2):277-291.
2 Chomiak J, Dungl P. Reconstruction of elbow flexion in arthrogryposis multiplex congenita type I. Part I: surgical anatomy and vascular and nerve supply of the pectoralis major muscle as a basis for muscle transfer. J Child Orthop. 2008;2(5):357-364.
3 Chuang DC, Ma HS, Wei FC. A new evaluation system to predict the sequelae of late obstetric brachial plexus palsy. Plast Reconstr Surg. 1998;101(3):673-685.
4 Chuang DC, Hattori Y, Ma HS, et al. The reconstructive strategy for improving elbow function in late obstetric brachial plexus palsy. Plast Reconstr Surg. 2002;109(1):116-126. discussion 27–9
5 Liao HT, Chuang DC, Ulusal AE, et al. Surgical strategies for brachial plexus polio-like paralysis. Plast Reconstr Surg. 2007;120(2):482-493.
6 Birch R. Brachial plexus injury: the London experience with supraclavicular traction lesions. Neurosurg Clin N Am. 2009;20(1):15-23. v
7 Terzis JK, Kostopoulos VK. The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg. 2007;119(4):73e-92e.
8 Flores AJ, Lavernia CJ, Owens PW. Anatomy and physiology of peripheral nerve injury and repair. Am J Orthop. 2000;29(3):167-173.
9 Sunderland S. Brachial plexus injuries. Clin Neurol Neurosurg. 1993;95(Suppl.):S1-S2.
10 Tsai YA, Chuang TY, Yen YS, et al. Electrophysiologic findings and muscle strength grading in brachioplexopathies. Microsurgery. 2002;22(1):11-15.
11 Jivan S, Kumar N, Wiberg M, et al. The influence of pre-surgical delay on functional outcome after reconstruction of brachial plexus injuries. J Plast Reconstr Aesthet Surg. 2009;62(4):472-479.
12 Narakas AO. Lesions found when operating traction injuries of the brachial plexus. Clin Neurol Neurosurg. 1993;95(Suppl.):S56-S64.
13 Narakas AO. The treatment of brachial plexus injuries. Int Orthop. 1985;9(1):29-36.
14 Kay SP. Obstetrical brachial palsy. Br J Plast Surg. 1998;51(1):43-50.
15 Clarke HM, Curtis CG. An approach to obstetrical brachial plexus injuries. Hand Clin. 1995;11(4):563-580. discussion 80–1
16 Uerpairojkit C, Leechavengvongs S, Witoonchart K, et al. Nerve transfer to serratus anterior muscle using the thoracodorsal nerve for winged scapula in C5 and C6 brachial plexus root avulsions. J Hand Surg (Am). 2009;34(1):74-78.
17 Gousheh J. Surgical technique for the treatment of C5 and C6 root avulsion. Acta Neurochir Suppl. 2007;100:13-14.
18 Leechavengvongs S, Witoonchart K, Uerpairojkit C, et al. Combined nerve transfers for C5 and C6 brachial plexus avulsion injury. J Hand Surg (Am). 2006;31(2):183-189.
19 Bertelli JA, Ghizoni MF. Reconstruction of C5 and C6 brachial plexus avulsion injury by multiple nerve transfers: spinal accessory to suprascapular, ulnar fascicles to biceps branch, and triceps long or lateral head branch to axillary nerve. J Hand Surg (Am). 2004;29(1):131-139.
20 Seddon HJ. Nerve grafting and other unusual forms of nerve repair. Spec Rep Ser Med Res Counc (GB). 1954;282:389-417.
21 Seddon HJ. The surgery of nerve injuries. Practitioner. 1960;184:181-187.
22 Seddon H. Advances in nerve repair. Triangle. 1968;8(7):252-259.
23 Oberlin C, Beal D, Leechavengvongs S, Salon A, Dauge MC, Sarcy JJ. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg (Am). 1994;19(2):232-237.
24 Chuang DC, Yeh MC, Wei FC. Intercostal nerve transfer of the musculocutaneous nerve in avulsed brachial plexus injuries: evaluation of 66 patients. J Hand Surg (Am). 1992;17(5):822-828.
25 Chuang DC, Epstein MD, Yeh MC, et al. Functional restoration of elbow flexion in brachial plexus injuries: results in 167 patients (excluding obstetric brachial plexus injury). J Hand Surg (Am). 1993;18(2):285-291.
26 Terzis JK, Kokkalis ZT. Elbow flexion after primary reconstruction in obstetric brachial plexus palsy. J Hand Surg Eur. 2009;34:449-458.
27 Wellons JC, Tubbs RS, Pugh JA, et al. Medial pectoral nerve to musculocutaneous nerve neurotization for the treatment of persistent birth-related brachial plexus palsy: an 11-year institutional experience. J Neurosurg Pediatr. 2009;3(5):348-353.
28 Park TS. Medial pectoral nerve transfer. J Neurosurg Pediatr. 2009;3(5):345-346. discussion 6–7
29 Songcharoen P. Management of brachial plexus injury in adults. Scand J Surg. 2008;97(4):317-323.
30 Monreal R. Restoration of elbow flexion by transfer of the phrenic nerve to musculocutaneous nerve after brachial plexus injuries. Hand (NY). 2007;2(4):206-211.
31 Liverneaux PA, Diaz LC, Beaulieu JY, et al. Preliminary results of double nerve transfer to restore elbow flexion in upper type brachial plexus palsies. Plast Reconstr Surg. 2006;117(3):915-919.
32 Moissonnier P, Cuvilliez V, Klein A, et al. Restoration of elbow flexion by performing contralateral lateral thoracic and thoracodorsal nerve transfers after experimental musculocutaneous nerve transection. J Neurosurg. 2005;103(1):70-78.
33 Novak CB, Mackinnon SE, Tung TH. Patient outcome following a thoracodorsal to musculocutaneous nerve transfer for reconstruction of elbow flexion. Br J Plast Surg. 2002;55(5):416-419.
34 Xu WD, Gu YD, Xu JG, et al. Full-length phrenic nerve transfer by means of video-assisted thoracic surgery in treating brachial plexus avulsion injury. Plast Reconstr Surg. 2002;110(1):104-109. discussion 10–11
35 Merrell GA, Barrie KA, Katz DL, et al. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the English literature. J Hand Surg (Am). 2001;26(2):303-314.
36 Gu Y, Xu J, Chen L, et al. Long term outcome of contralateral C7 transfer: a report of 32 cases. Chin Med J (Engl). 2002;115(6):866-868.
37 Gu YD. Contralateral C7 root transfer over the last 20 years in China. Chin Med J (Engl). 2007;120(13):1123-1126.
38 Gu YD, Shen LY. Electrophysiological changes after severance of the C7 nerve root. J Hand Surg Br. 1994;19(1):69-71.
39 Songcharoen P, Wongtrakul S, Mahaisavariya B, et al. Hemi-contralateral C7 transfer to median nerve in the treatment of root avulsion brachial plexus injury. J Hand Surg (Am). 2001;26(6):1058-1064.
40 Songcharoen P, Wongtrakul S, Spinner RJ. Brachial plexus injuries in the adult. nerve transfers: the Siriraj Hospital experience. Hand Clin. 2005;21(1):83-89.
41 Jivan S, Novikova LN, Wiberg M, et al. The effects of delayed nerve repair on neuronal survival and axonal regeneration after seventh cervical spinal nerve axotomy in adult rats. Exp Brain Res. 2006;170(2):245-254.
42 Belzberg AJ, Dorsi MJ, Storm PB, et al. Surgical repair of brachial plexus injury: a multinational survey of experienced peripheral nerve surgeons. J Neurosurg. 2004;101(3):365-376.
43 Kline DG. Timing for brachial plexus injury: a personal experience. Neurosurg Clin N Am. 2009;20(1):24-26. v
44 Kline DG. Timing for exploration of nerve lesions and evaluation of the neuroma-in-continuity. Clin Orthop Relat Res. 1982;163:42-49.
45 Kline DG, Hackett ER. Reappraisal of timing for exploration of civilian peripheral nerve injuries. Surgery. 1975;78(1):54-65.
46 Schulze Berge. Ersatz der Beuger des Vorderarmes (Bizeps und Brachialis) durch den Pectoralis major. Deutsche med Wochenschr. 1917;43:433.
47 Clark JPM. Reconstruction of biceps brachii by pectoral muscle transplantation. Br J Surg. 1946;34:180.
48 Brooks DM, Seddon HJ. Pectoral transplantation for paralysis of the flexors of the elbow; a new technique. J Bone Joint Surg (Br). 1959;41(1):36-43.
49 Matory WEJr, Morgan WJ, Breen T. Technical considerations in pectoralis major transfer for treatment of the paralytic elbow. J Hand Surg (Am). 1991;16(1):12-18.
50 Narakas AO. Muscle transpositions in the shoulder and upper arm for sequelae of brachial plexus palsy. Clin Neurol Neurosurg. 1993;95(Suppl.):S89-S91.
51 Wahegaonkar AL, Doi K, Hattori Y, et al. Surgical technique of pedicled bipolar pectoralis major transfer for reconstruction of elbow flexion in brachial plexus palsy. Tech Hand Up Extrem Surg. 2008;12(1):12-19.
52 Vekris MD, Beris AE, Lykissas MG, et al. Restoration of elbow function in severe brachial plexus paralysis via muscle transfers. Injury. 2008;39(Suppl. 3):S15-S22.
53 Kawamura K, Yajima H, Tomita Y, et al. Restoration of elbow function with pedicled latissimus dorsi myocutaneous flap transfer. J Shoulder Elbow Surg. 2007;16(1):84-90.
54 Arroyo JS, McGuire MH, Crosby LA. Latissimus dorsi transfer for elbow flexion in the paralytic limb. Nebr Med J. 1995;80(9):290-292.
55 Hirayama T, Tada H, Katsuki M, et al. The pedicle latissimus dorsi transfer for reconstruction of the plexus brachialis and brachium. Clin Orthop Relat Res. 1994;309:201-207.
56 Eggers IM, Mennen U, Matime AM. Elbow flexorplasty: a comparison between latissimus dorsi transfer and Steindler flexorplasty. J Hand Surg (Br). 1992;17(5):522-525.
57 Steindler A. Operative treatment of paralytic conditions of the upper extremity. J Orthop Surg. 1919;1:608.
58 Andrisano A, Porcellini G, Stilli S, et al. The Steindler method in the treatment of paralytic elbow flexion. Ital J Orthop Traumatol. 1990;16(2):235-239.
59 Beaton DE, Dumont A, Mackay MB, et al. Steindler and pectoralis major flexorplasty: a comparative analysis. J Hand Surg (Am). 1995;20(5):747-756.
60 Brunelli GA, Vigasio A, Brunelli GR. Modified Steindler procedure for elbow flexion restoration. J Hand Surg (Am). 1995;20(5):743-746.
61 Chen WS. Restoration of elbow flexion by modified Steindler flexorplasty. Int Orthop. 2000;24(1):43-46.
62 Ishida O, Sunagawa T, Suzuki O, et al. Modified Steindler procedure for the treatment of brachial plexus injuries. Arch Orthop Trauma Surg. 2006;126(1):63-65.
63 Adams JE, Kircher MF, Spinner RJ, et al. Complications and outcomes of functional free gracilis transfer in brachial plexus palsy. Acta Orthop Belg. 2009;75(1):8-13.
64 Wechselberger G, Hussl H, Strickner N, et al. Restoration of elbow flexion after brachial plexus injury by free functional rectus femoris muscle transfer. J Plast Reconstr Aesthet Surg. 2009;62(2):e1-e5.
65 Doi K. Management of total paralysis of the brachial plexus by the double free-muscle transfer technique. J Hand Surg Eur. 2008;33(3):240-251.
66 Chuang DC. Neurotization and free muscle transfer for brachial plexus avulsion injury. Hand Clin. 2007;23(1):91-104.
67 Bishop AT. Functioning free-muscle transfer for brachial plexus injury. Hand Clin. 2005;21(1):91-102.
68 Wechselberger G, Ninkovic M, Pulzl P, et al. Free functional rectus femoris muscle transfer for restoration of knee extension and defect coverage after trauma. J Plast Reconstr Aesthet Surg. 2006;59(9):994-998.
69 Chung DC, Carver N, Wei FC. Results of functioning free muscle transplantation for elbow flexion. J Hand Surg (Am). 1996;21(6):1071-1077.
70 Wei CY, Chuang DC, Chen HC, et al. The versatility of free rectus femoris muscle flap: an alternative flap. Microsurgery. 1995;16(10):698-703.
71 Kay S, Pinder R, Wiper J, et al. Microvascular free functioning gracilis transfer with nerve transfer to establish elbow flexion. J Plast Reconstr Aesthet Surg. 2010;63(7):1142-1149.