CHAPTER 55 Rationale of Minimally Invasive Spine Surgery
Anatomy of the Posterior Paraspinal Muscles
The posterior lumbar paraspinal muscles are part of a larger biomechanical system that includes the abdominal muscles and their fibrous attachment to the spine through the dorsolumbar fascia. This network of muscles is responsible for generating movements of the spine while maintaining its stability.1,2 In addition to maintaining spinal posture in its neutral position, the paraspinal muscles guard the spine from excessive bending that would otherwise endanger the integrity of the intervertebral discs and ligaments.3 Panjabi and colleagues4,5 have proposed that the paraspinal muscles apply minimal resistance inside the neutral zone (NZ) but increase their stiffness exponentially once the range of motion falls outside this NZ. This dynamic stabilizing system is controlled by an interconnected chain of mechanoreceptors embedded in the muscle fascicles, the disc annulus, and the spinal ligaments.6 Functional electromyographic (EMG) studies reveal that spinal stability is achieved by the simultaneous contraction of several agonist-antagonist muscles.3,7,8 Architectural studies suggest that the individual paraspinal muscles may have different primary roles as either movers or stabilizers of the spinal column.9
Multifidus Muscle
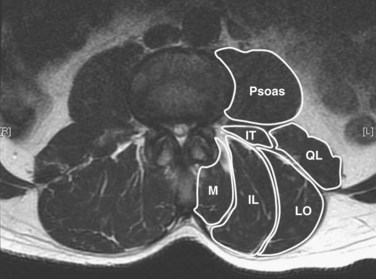
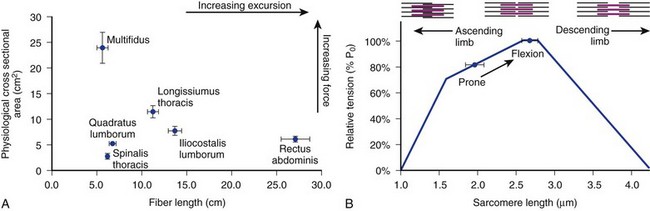
The posterior paraspinal muscles are composed of two muscle groups: (1) the deep paramedian transversospinalis muscle group, which includes the multifidus, interspinales, intertransversarii, and short rotators, and (2) the more superficial and lateral erector spinae muscles that include the longissimus and iliocostalis (Fig. 55–1). These muscles run along the thoracolumbar spine and attach caudally to the sacrum, sacroiliac joint, and iliac wing. The multifidus is the most medial of the major posterior paraspinal muscles and is the largest muscle that spans the lumbosacral junction. It is believed to be the major posterior stabilizing muscle of the spine.3,9,10 Compared with other paraspinal muscles, the multifidus muscle is short and stout. It has a large physiologic cross-sectional area (PCSA) but short fiber lengths. This unique architectural anatomy is designed to create large forces over relatively short distances (Fig. 55–2A).9 Furthermore, the multifidus sarcomere length is positioned on the ascending portion of the length-tension curve (Fig. 55–2B). When our posture changes from standing erect to bending forward, the multifidus can produce more force as the spine flexes forward. This serves to protect the spine at its most vulnerable position.
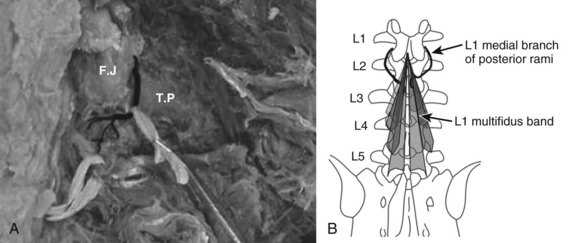
The multifidus is the only muscle that is attached to both the posterior parts of the L5 and S1 vertebrae and is therefore the sole posterior stabilizer that both originates and inserts to this segment. The morphology of the lumbar multifidus is complex.11 Unlike the other paraspinal muscles that have specific origins and insertions, the multifidus muscle is formed by five separate bands, each having its own origin and several different insertion sites. Each band consists of several fascicles arising from the tip of the spinous process and the lateral surface of the vertebral lamina. Caudally, the different fascicles diverge to separate attachments into the mammillary processes of the caudal vertebrae two to five levels below their origin and downward through each vertebra to the sacrum. For example, fibers from the L1 band insert into the mammillary processes of the L3, L4, and L5 vertebrae to the dorsal part of S1 and then to the posterior superior iliac spine. Biomechanical analyses, based on the multifidus muscle anatomy, show that it produces posterior sagittal rotation of the vertebra, which opposes a counter rotation generated by the abdominal muscles. The multifidus can further increase lumbar spine stability through a ‘bowstring’ mechanism in which the muscle, positioned posterior to the lumbar lordosis, produces compressive forces on the vertebrae interposed between its attachments.12
Erector Spinae Muscles
The erector spinae muscles are composed of the longissimus, iliocostalis, and spinalis (in the thoracic area).11,13 In the lumbar spine, the longissimus is positioned medially and arises from the transverse and accessory processes and inserts caudally into the ventral surface of the posterior superior iliac spine. The laterally positioned iliocostalis arises from the tip of the transverse processes and the adjacent middle layer of thoracolumbar fascia and inserts into the ventral edge of the iliac crest caudally.14 Unilateral contraction of the lumbar erector spinae laterally flexes the vertebral column; bilateral contraction produces extension and posterior rotation of the vertebrae in the sagittal plane. In addition to their role as the major extensor muscles of the trunk, the iliocostalis and the longissimus also exert large compressive loads and lateral and posterior shear forces at the L4 and L5 segments. Although these forces increase the stiffness and stability of the normal vertebral column, the shearing forces may also exacerbate instability and deformity in a malaligned spine.15 In contrast to the multifidus muscle, microarchitectural studies reveal that these muscles are designed as long muscle fascicles with relatively small PCSA. This anatomic morphology suggests that they serve to move the trunk to extension, lateral bending, and rotation. With this type of design, they are less likely to act as primary stabilizers of the vertebral column.16
Interspinales, Intertransversarii, and Short Rotator Muscles
The interspinales, intertransversarii, and short rotator muscles are short flat muscles that lie dorsal to the intertransverse ligament (see Fig. 55–1). The intertransversarii and interspinales run along the intertransverse and interspinous ligaments of each segment. The short rotators originate from the posterior-superior edge of the lower vertebra and attach to the lateral side of the upper vertebral lamina. Because of their small PCSA, they are unable to generate the forces necessary for movement or stability of the spinal column. More likely, they act as proprioceptive sensors rather than force-generating structures.17
Innervation of the Posterior Paraspinal Muscles
The innervation of all of the posterior paraspinal muscles is derived from the dorsal rami. The iliocostalis is innervated by the lateral branch, while the lumbar fibers of the longissimus receive innervation from the intermediate branch.18 The multifidus is innervated by the medial branch of the dorsal rami (Fig. 55–3). The medial branch curves around the root of the superior articular process and passes between the mammillary and accessory processes to the vertebral lamina, where it branches to supply the multifidus muscle, intertransversarii and interspinales muscles, and zygapophyseal joints.19
During its extramuscular course, the medial branch is strongly attached to the vertebral body in two locations. The first attachment is to the periosteum lateral to the zygapophyseal joints by fibers of the intertransverse ligament. The mamillo-accessory ligament provides the second attachment in the lumbar spine. This strong ligament covers the medial branch and is often calcified.20 These attachments to the vertebra are of clinical importance because they expose the medial branch to possible damage during a midline posterior surgical approach.21
Direct damage to the nerve is also possible during insertion of pedicle screws.22 Insertion of a pedicle screw in the area of the mammillary process can injure the medial branch arising from the cephalad level nerve root, causing denervation injury and consequent atrophy to the multifidus fascicles that arise from the adjacent cephalad level.19,23 For instance, pedicle screws placed at L2 may damage the L1 nerve, which denervates the multifidus bands that originate at L1 and insert into the vertebrae caudally. Moreover, the mono-segmental innervation of the multifidus makes it particularly susceptible to atrophy because it lacks a collateral nerve supply from adjacent muscle segments.24 It is intriguing to consider that dysfunction of this muscle could contribute to adjacent level disc degeneration.
Fiber Type Characteristics of the Paraspinal Muscles
Fiber type analysis can provide important information about the use pattern of a muscle.25,26 There are two major fiber types in skeletal muscles: type I, also known as “slow twitch” and type II or “fast twitch.” The type I fibers possess low ATPase activity, prolonged twitch duration (hence slow twitch), and a low maximal velocity. In addition, type I fibers contain higher mitochondrial content and greater oxidative enzyme complements than type II fibers. Type II fibers are characterized by higher ATPase activities and correspondingly shorter isometric twitch durations. With this design, they are better suited to support the regeneration of ATP through anaerobic mechanisms. Type II fibers can be further subdivided into type IIa and IIx fibers. Type IIx fibers are generally more extreme in each of these respects than the type IIa.26–28
One of the most striking features of the lumbar paraspinal muscles is the predominance of type I muscle fibers compared with other skeletal muscles. Polgar and Johnson studied the distribution of fiber types in 36 human muscles.29,30 A significantly larger type I–to–type II fiber ratio was observed in the multifidus, longissimus, and iliocostalis muscles compared with muscles of the extremities. The predominance of type I fibers and selective type II atrophy have been found in other studies that analyzed fiber type distribution and size in normal paraspinal muscles. It is presumed that along with the adaptation to their stabilizing tonic work characteristics, the phenomenon of type II atrophy can be explained by the sedentary modern life style that deprives these muscles of stimulation from exercise.31 The relatively larger size of type II fibers in professional athletes further supports this assumption.32,33
The morphology of fiber type distribution between the different paraspinal muscles and in different areas inside the muscle is well known. Jorgensen and colleagues34 reported a higher proportion of type I fibers in the longissimus than in the multifidus or iliocostalis muscles. Furthermore, the multifidus muscle is composed of a relatively high percentage of type I fibers, consistent with a postural function. The psoas muscle, on the other hand, is composed of a higher percentage of type II fibers such as in the appendicular muscles.35 Mannion and colleagues36 showed that in women the mean size of the type I fiber is significantly greater than that of either the type IIA or the type IIX, while men have relatively larger-sized type II fibers. In the older population, a loss of muscle mass leads to a decrease in both fiber type sizes with slightly more effect in type II fibers.37–39 The most profound changes in fiber size and fiber type distribution occur in patients with degenerative conditions of the spine.27 Compared with the control, the muscle of low back pain (LBP) patients had a significantly higher proportion of type IIx fiber than type I fibers. They proposed that the relatively low proportion of type I fibers in patients with LBP render them less resistant to fatigue and more susceptible to injury.
Paraspinal Muscle Injury
Characteristics of Paraspinal Muscles in the Postsurgical Spine
Spine surgery inherently causes damage to surrounding muscles.40 This injury can be followed by atrophy of the muscles and subsequent loss of function. Among the different surgical approaches to the spine, it appears that injury to the muscle is greatest when using the midline posterior approach.41 The multifidus muscle is most severely injured when using this approach. Muscle atrophy coincides with decreased muscle cross-sectional area (CSA), which in turn correlates with decreased force production capacity of the muscle.40,42–50
Muscle biopsies obtained from patients undergoing revision spinal surgery exhibit an array of pathologic features that include selective type II fiber atrophy, widespread fiber type grouping (a sign of reinnervation), and “moth-eaten” appearance of muscle fibers.39 Although these pathologic changes can occasionally be found in biopsies from normal individuals, the pathologic changes are more prevalent after surgery.51 Atrophy of the paraspinal muscles can readily be seen in postsurgical back patients.47 Reductions in the CSA of the paraspinal muscles is greatest following a midline approach for a posterolateral fusion.41,48,52
Mechanism of Paraspinal Muscle Injury During Surgery
The factors responsible for muscle injury during surgery have been well studied in both animals and humans. Muscle damage can be caused by several different mechanisms. Direct injury to the muscle is caused by dissection and stripping of tendinous attachments from the posterior elements of the spine. Additionally, extensive use of the electrocautery causes localized thermal injury and necrosis to the tissues. The most significant factor responsible for muscle injury is likely the use of forceful self-retaining retractors. Kawaguchi and colleagues53–56 quantified the factors responsible for muscle necrosis following a standard open midline posterior approach. They proposed that injury is induced by a crush mechanism similar to that caused by a pneumatic tourniquet during surgery of the limbs. During the application of self-retaining retractors, elevated pressures lead to decreased intramuscular perfusion.57–60 The severity of the muscle injury is closely correlated to the degree of the intramuscular pressure and the length of retraction time. A pressure-time parameter can be calculated by multiplying the intramuscular pressure and the length of time of the surgery. A high pressure-time product was shown to be tightly correlated to muscle necrosis. They concluded that muscle damage can be reduced by intermittent release of the retractors during prolonged surgery combined with a relatively longer incision that allows reduced retraction pressures.
Denervation is yet another mechanism that leads to muscle degeneration and atrophy following surgery. Muscle denervation can occur in a discrete location along the supplying nerve or be located in several points along the nerve and the neuromuscular junction. As previously described, nerve supply to the multifidus is especially vulnerable to injury because of its mono-segmental innervation pattern.24 Muscle denervation is also possible through damage to the neuromuscular junction following long muscle retraction and necrosis. Shorter retraction time or an intermittent release of muscle retraction has been shown to significantly decrease degeneration and denervation of the muscles.59 Gejo and colleagues examined the relationship between retraction time and postoperative damage to the paraspinal muscle, by measuring postoperation signal intensity of the multifidus muscle, using T2-weighted magnetic resonance imaging (MRI).61 Long retraction time during surgery correlated with high-signal intensity in the multifidus muscle even at 6 months postsurgery. They proposed that these findings reflect chronic denervation of the muscle caused by damage to the neuromuscular synapses.
Correlation of Muscle Injury with Clinical Outcomes
There appears to be a correlation between muscle damage and long-term postoperative pain. Shivonen and colleagues62 found signs of severe denervation of the multifidus muscle in patients with failed back syndrome. Muscle biopsies showed signs of advanced chronic denervation consisting of group atrophy, marked fibrosis, and fatty infiltration. Moreover, fiber type grouping, a histologic sign of reinnervation, was rare. They hypothesized that the denervation injury resulted from direct damage to the medial branch of the posterior rami during muscle retraction associated with the posterior midline approach. The lack of reinnervation was thought to result from the absence of intersegmental nerve supply to the multifidus. Signs of severe denervation of the paraspinal muscles correlate with poor outcome of postsurgical patients. They also showed that poor clinical outcomes are associated with abnormal EMG patterns 2 to 5 years postsurgery. Although a correlation between the degree of muscle atrophy following surgery and the incidence of failed back syndrome was found, it is not clear what specific pathogenic factors are responsible.
Preservation of Muscle Function and Integrity
Minimally invasive spine surgery techniques strive to minimize muscle injury during surgery. By decreasing/minimizing the use of self-retaining retractors, intramuscular retraction pressure is reduced and thereby leads to less crush injury. Furthermore, focusing the surgical corridor directly over the surgical target site allows for less muscle stripping that may otherwise disrupt its tendinous attachments or damage their neurovascular supply. Kim and colleagues63 compared trunk muscle strength between patients treated with open posterior instrumentation versus percutaneous instrumentation. Tests were performed isometrically at multiple flexion positions. Patients undergoing percutaneous instrumentation displayed more than 50% improvement in extension strength, while patients undergoing traditional midline open surgery had no significant improvement in lumbar extension strength. Extension strength correlated with preservation of multifidus CSA as measured on MRI. In a similar study, Stevens and colleagues64 assessed the postsurgical appearance of the multifidus muscle using a high-definition MRI sequence. In patients treated via an open posterior transforaminal lumbar interbody fusion (TLIF) technique, marked intermuscular and intramuscular edema was observed on postsurgical MRI at 6 months. In contrast, patients in the MIS TLIF group had a normal appearance on MRI postsurgery.
Hyun and colleagues46 retrospectively assessed a group of patients that underwent unilateral TLIF with ipsilateral instrumented posterior spinal fusion via an open technique. Contralateral instrumented posterior spinal fusion was performed at the same level employing a paramedian, intermuscular (Wiltse), minimally invasive approach. Postoperatively, there was a significant decrease in the CSA of the multifidus on the side of the open approach while no reduction in the multifidus CSA on the contralateral side was observed.
Decreases in tissue trauma have local effects but also alter overall systemic physiology. Kim and colleagues65 studied circulating markers of tissue injury in patients undergoing open versus MIS fusions. Markers of skeletal muscle injury (creatinine kinase, aldolase); proinflammatory cytokines (IL-6, IL-8); and anti-inflammatory cytokines (IL-10, IL-1 receptor antagonist) were analyzed with ELISA techniques. Two to sevenfold increases in all markers were observed in the open surgery group. The greatest difference between the groups occurred on the first postoperative day. Most markers returned to baseline in 3 days for the MIS group, whereas the open-surgery group required 7 days. IL-6 and IL-8 are known cytokines that participate in various systemic inflammatory reactions.66,67 It is possible that such elevations in inflammatory cytokines have direct effects beyond the surgical site. As such, persistently elevated levels of proinflammatory cytokines have been associated with organ failure in postsurgical patients.68
Preservation of the Bone-Ligament Complex
It is well accepted that excessive facet resection leads to altered motion and spinal instability.69–72 Furthermore, a laminectomy leads to loss of the midline supraspinous/interspinous ligament complex, which can contribute to flexion instability.73,74 In cases where significant bony resection is required, or when there is an underlying relative instability (such as in spondylolisthesis), concomitant fusion is often recommended following a decompressive laminectomy.73–76 Efforts to limit such potentially destabilizing surgery have been pursued via unilateral laminotomies in which the spinous processes and corresponding tendinous attachments of the multifidus muscle and the supraspinous/interspinous ligaments are preserved (Fig. 55–4). When this technique is combined with minimally invasive tubular retractors, bilateral decompression for stenosis can be achieved with good clinical results.77,78 The long-term outcome of such MIS procedures and their effect on spinal stability have yet to be shown clinically. However, biomechanical studies suggest that such MIS techniques have significant effects on spinal stability.
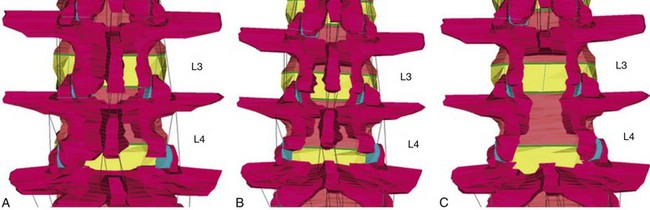
Finite element analyses have been used to assess the consequences of various lumbar decompressive procedures on spinal motion. Fessler and colleagues79 compared three decompressive techniques to treat two-level spinal stenosis: open laminectomies versus interlaminar midline decompression (which retains the spinous process but sacrifices the interspinous/supraspinous ligaments) versus MIS unilateral laminotomies (Fig. 55–5). These studies show that open laminectomy produces marked increases in flexion, extension, and axial rotation. For flexion-extension, there is a greater than twofold increase in motion, which leads to increased stress on the annulus. No changes in flexion were noted when the interlaminar or MIS models were studied. Axial rotation increased by 2.5-fold in the open and interlaminar groups but only 1.3-fold in the MIS group. These findings lend further support to the concept that MIS techniques have relevant effects on spinal motion and stability.
1 Gejo R, Matsui H, Kawaguchi Y, et al. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine (Phila Pa 1976). 1999;24:1023-1028.
2 Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976). 2005;30:123-129.
3 Kim KT, Lee SH, Suk KS, Bae SC. The quantitative analysis of tissue injury markers after mini-open lumbar fusion. Spine (Phila Pa 1976). 2006;31:712-716.
4 MacIntosh JE, Bogduk N. The morphology of the lumbar erector spinae. Spine. 1987;12:658-668.
5 Ward SR, Kim CW, Eng CM, et al. Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. J Bone Joint Surg Am. 2009;91:176-185.
1 Brown SH, McGill SM. Muscle force-stiffness characteristics influence joint stability: a spine example. Clin Biomech (Bristol, Avon). 2005;20:917-922.
2 Brown SH, Potvin JR. Constraining spine stability levels in an optimization model leads to the prediction of trunk muscle co-contraction and improved spine compression force estimates. J Biomech. 2005;38:745-754.
3 Cholewicki J, Panjabi MM, Khachatryan A. Stabilizing function of trunk flexor-extensor muscles around a neutral spine posture. Spine (Phila Pa 1976). 1997;22:2207-2212.
4 Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5:390-396. discussion 7
5 Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5:383-389. discussion 97
6 Panjabi MM, White AA3rd. Basic biomechanics of the spine. Neurosurgery. 1980;7:76-93.
7 McGill SM. Electromyographic activity of the abdominal and low back musculature during the generation of isometric and dynamic axial trunk torque: implications for lumbar mechanics. J Orthop Res. 1991;9:91-103.
8 Cholewicki J, McGill SM, Norman RW. Lumbar spine loads during the lifting of extremely heavy weights. Med Sci Sports Exerc. 1991;23:1179-1186.
9 Ward SR, Kim CW, Eng CM, et al. Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. J Bone Joint Surg Am. 2009;91:176-185.
10 Donisch EW, Basmajian JV. Electromyography of deep back muscles in man. Am J Anat. 1972;133:25-36.
11 MacIntosh JE, Bogduk N. The morphology of the lumbar erector spinae. Spine. 1987;12:658-668.
12 Bogduk N, Macintosh JE, Pearcy MJ. A universal model of the lumbar back muscles in the upright position. Spine (Phila Pa 1976). 1992;17:897-913.
13 Macintosh JE, Bogduk N. The attachments of the lumbar erector spinae. Spine (Phila Pa 1976). 1991;16:783-792.
14 Bustami FM. A new description of the lumbar erector spinae muscle in man. J Anat. 1986;144:81-91.
15 Bogduk N. A reappraisal of the anatomy of the human lumbar erector spinae. J Anat. 1980;131:525-540.
16 Delp SL, Suryanarayanan S, Murray WM, et al. Architecture of the rectus abdominis, quadratus lumborum, and erector spinae. J Biomech. 2001;34:371-375.
17 Bogduk N. Proceedings: The posterior lumbar muscles and nerves of the cat. J Anat. 1973;116:476-477.
18 Bogduk N. The innervation of the lumbar spine. Spine (Phila Pa 1976). 1983;8:286-293.
19 Bogduk N, Long DM. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg. 1979;51:172-177.
20 Bogduk N. The lumbar mamillo—accessory ligament. Its anatomical and neurosurgical significance. Spine (Phila Pa 1976). 1981;6:162-167.
21 Boelderl A, Daniaux H, Kathrein A, Maurer H. Danger of damaging the medial branches of the posterior rami of spinal nerves during a dorsomedian approach to the spine. Clin Anat. 2002;15:77-81.
22 Regev GJ, Lee YP, Taylor WR, et al. Nerve injury to the posterior rami medial branch during the insertion of pedicle screws: comparison of mini-open versus percutaneous pedicle screw insertion techniques. Spine (Phila Pa 1976). 2009;34:1239-1242.
23 Dreyfuss P, Stout A, Aprill C, et al. The significance of multifidus atrophy after successful radiofrequency neurotomy for low back pain. PM R. 2009;1:719-722.
24 Macintosh JE, Bogduk N. 1987 Volvo award in basic science. The morphology of the lumbar erector spinae. Spine (Phila Pa 1976). 1987;12:658-668.
25 Pette D. The Dynamic State of Muscle Fiberse. Berlin: Walter de Gruyter & Company; 1990.
26 Edgerton VR, Roy RR. Regulation of skeletal muscle fiber size, shape and function. Journal of Biomechanics. 1991;21:123-133.
27 Mannion AF, Kaser L, Weber E, et al. Influence of age and duration of symptoms on fiber type distribution and size of the back muscles in chronic low back pain patients. Eur Spine J. 2000;9:273-281.
28 Mannion AF. Fiber type characteristics and function of the human paraspinal muscles: normal values and changes in association with low back pain. J Electromyogr Kinesiol. 1999;9:363-377.
29 Polgar J, Johnson MA, Weightman D, Appleton D. Data on fiber size in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;19:307-318.
30 Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fiber types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18:111-129.
31 Crossman K, Mahon M, Watson PJ, et al. Chronic low back pain-associated paraspinal muscle dysfunction is not the result of a constitutionally determined “adverse” fiber-type composition. Spine (Phila Pa 1976). 2004;29:628-634.
32 Puustjarvi K, Tammi M, Reinikainen M, et al. Running training alters fiber type composition in spinal muscles. Eur Spine J. 1994;3:17-21.
33 Short KR, Vittone JL, Bigelow ML, et al. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol. 2005;99:95-102.
34 Jorgensen K, Nicholaisen T, Kato M. Muscle fiber distribution, capillary density, and enzymatic activities in the lumbar paravertebral muscles of young men. Significance for isometric endurance. Spine (Phila Pa 1976). 1993;18:1439-1450.
35 Regev GJ, Kim CW, Thacker BE, et al: Regional myosin heavy chains distribution in selected paraspinal muscles. Spine (Phila Pa 1976) in press.
36 Mannion AF, Weber BR, Dvorak J, et al. Fiber type characteristics of the lumbar paraspinal muscles in normal healthy subjects and in patients with low back pain. J Orthop Res. 1997;15:881-887.
37 Rantanten J, Rissanen A, Kalimo H. Lumbar muscle fiber size and type distribution in normal subjects. European Spine. Journal. 1994;3:331-335.
38 Zhu XZ, Parnianpour M, Nordin M, Kahanovitz N. Histochemistry and morphology of erector spinae muscle in lumbar disc herniation. Spine (Phila Pa 1976). 1989;14:391-397.
39 Mattila M, Hurme M, Alaranta H, et al. The multifidus muscle in patients with lumbar disc herniation. A histochemical and morphometric analysis of intraoperative biopsies. Spine (Phila Pa 1976). 1986;11:732-738.
40 Gejo R, Matsui H, Kawaguchi Y, et al. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine (Phila Pa 1976). 1999;24:1023-1028.
41 Gille O, Jolivet E, Dousset V, et al. Erector spinae muscle changes on magnetic resonance imaging following lumbar surgery through a posterior approach. Spine (Phila Pa 1976). 2007;32:1236-1241.
42 Datta G, Gnanalingham KK, Peterson D, et al. Back pain and disability after lumbar laminectomy: is there a relationship to muscle retraction? Neurosurgery. 2004;54:1413-1420. discussion 20
43 Franzini A, Ferroli P, Marras C, Broggi G. Huge epidural hematoma after surgery for spinal cord stimulation. Acta Neurochir (Wien). 2005;147:565-567. discussion 7
44 Granata C, Cervellati S, Ballestrazzi A, et al. Spine surgery in spinal muscular atrophy: long-term results. Neuromuscul Disord. 1993;3:207-215.
45 Hutchinson D, Kozin SH, Mayer N, et al. Dynamic electromyographic evaluation of adolescents with traumatic cervical injury after biceps to triceps transfer: the role of phasic contraction. J Hand Surg Am. 2008;33:1331-1336.
46 Hyun SJ, Kim YB, Kim YS, et al. Postoperative changes in paraspinal muscle volume: comparison between paramedian interfascial and midline approaches for lumbar fusion. J Korean Med Sci. 2007;22:646-651.
47 Mayer TG, Vanharanta H, Gatchel RJ, et al. Comparison of CT scan muscle measurements and isokinetic trunk strength in postoperative patients. Spine (Phila Pa 1976). 1989;14:33-36.
48 Motosuneya T, Asazuma T, Tsuji T, et al. Postoperative change of the cross-sectional area of back musculature after 5 surgical procedures as assessed by magnetic resonance imaging. J Spinal Disord Tech. 2006;19:318-322.
49 Rantanen J, Hurme M, Falck B, et al. The lumbar multifidus muscle five years after surgery for a lumbar intervertebral disc herniation. Spine (Phila Pa 1976). 1993;18:568-574.
50 Kawaguchi Y, Matsui H, Gejo R, Tsuji H. Preventive measures of back muscle injury after posterior lumbar spine surgery in rats. Spine (Phila Pa 1976). 1998;23:2282-2287. discussion 8
51 Weber BR, Grob D, Dvorak J, Muntener M. Posterior surgical approach to the lumbar spine and its effect on the multifidus muscle. Spine (Phila Pa 1976). 1997;22:1765-1772.
52 Suwa H, Hanakita J, Ohshita N, et al. Postoperative changes in paraspinal muscle thickness after various lumbar back surgery procedures. Neurol Med Chir (Tokyo). 2000;40:151-154. discussion 4-5
53 Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 2: Histologic and histochemical analyses in humans. Spine (Phila Pa 1976). 1994;19:2598-2602.
54 Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 1: Histologic and histochemical analyses in rats. Spine (Phila Pa 1976). 1994;19:2590-2597.
55 Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine (Phila Pa 1976). 1996;21:941-944.
56 Kawaguchi Y, Yabuki S, Styf J, et al. Back muscle injury after posterior lumbar spine surgery. Topographic evaluation of intramuscular pressure and blood flow in the porcine back muscle during surgery. Spine (Phila Pa 1976). 1996;21:2683-2688.
57 Styf J. Pressure in the erector spinae muscle during exercise. Spine (Phila Pa 1976). 1987;12:675-679.
58 Styf J, Lysell E. Chronic compartment syndrome in the erector spinae muscle. Spine (Phila Pa 1976). 1987;12:680-682.
59 Styf JR, Willen J. The effects of external compression by three different retractors on pressure in the erector spine muscles during and after posterior lumbar spine surgery in humans. Spine (Phila Pa 1976). 1998;23:354-358.
60 Taylor H, McGregor AH, Medhi-Zadeh S, et al. The impact of self-retaining retractors on the paraspinal muscles during posterior spinal surgery. Spine (Phila Pa 1976). 2002;27:2758-2762.
61 Gejo R, Kawaguchi Y, Kondoh T, et al. Magnetic resonance imaging and histologic evidence of postoperative back muscle injury in rats. Spine (Phila Pa 1976). 2000;25:941-946.
62 Sihvonen T, Herno A, Paljarvi L, et al. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine (Phila Pa 1976). 1993;18:575-581.
63 Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976). 2005;30:123-129.
64 Stevens KJ, Spenciner DB, Griffiths KL, et al. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech. 2006;19:77-86.
65 Kim KT, Lee SH, Suk KS, Bae SC. The quantitative analysis of tissue injury markers after mini-open lumbar fusion. Spine (Phila Pa 1976). 2006;31:712-716.
66 Igonin AA, Armstrong VW, Shipkova M, et al. Circulating cytokines as markers of systemic inflammatory response in severe community-acquired pneumonia. Clin Biochem. 2004;37:204-209.
67 Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunol Today. 1994;15:127-133.
68 Ogawa M. Acute pancreatitis and cytokines: “second attack” by septic complication leads to organ failure. Pancreas. 1998;16:312-315.
69 Zander T, Rohlmann A, Klockner C, Bergmann G. Influence of graded facetectomy and laminectomy on spinal biomechanics. Eur Spine J. 2003;12:427-434.
70 Natarajan RN, Andersson GB, Patwardhan AG, Andriacchi TP. Study on effect of graded facetectomy on change in lumbar motion segment torsional flexibility using three-dimensional continuum contact representation for facet joints. J Biomech Eng. 1999;121:215-221.
71 Lee KK, Teo EC, Qiu TX, Yang K. Effect of facetectomy on lumbar spinal stability under sagittal plane loadings. Spine (Phila Pa 1976). 2004;29:1624-1631.
72 Abumi K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976). 1990;15:1142-1147.
73 Tuite GF, Doran SE, Stern JD, et al. Outcome after laminectomy for lumbar spinal stenosis. Part II: Radiographic changes and clinical correlations. J Neurosurg. 1994;81:707-715.
74 Tuite GF, Stern JD, Doran SE, et al. Outcome after laminectomy for lumbar spinal stenosis. Part I: Clinical correlations. J Neurosurg. 1994;81:699-706.
75 Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997;22:2807-2812.
76 Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802-808.
77 Palmer S, Turner R, Palmer R. Bilateral decompression of lumbar spinal stenosis involving a unilateral approach with microscope and tubular retractor system. J Neurosurg. 2002;97:213-217.
78 Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila Pa 1976). 2002;27:432-438.
79 Bresnahan L, Ogden AT, Natarajan RN, Fessler RG. A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine (Phila Pa 1976). 2009;34:17-23.