9
Radiotherapy and chemotherapy in treatment of oesophageal and gastric cancer
Introduction
The identification of improved activity when chemotherapy and radiotherapy are given synchronously has already led to chemoradiotherapy (CRT) becoming the primary organ-preserving approach in anal, cervix and certain head and neck cancers, with surgery being reserved for salvage.1,2 There is now good evidence that primary CRT has a role in oesophageal cancer treatment.
Oesophageal cancer
Potentially curative treatment
Theoretical and generic issues of preoperative versus postoperative therapy treatment include:
• a more easily defined target volume;
• improved tumour oxygenation at the time of treatment;
• the potential to improve resectability and reduce the impact of tumour cell spillage at surgery;
• improved chance of an R0 resection and thereby the reduction of local recurrence;
• improved chance of treating micrometastatic disease;
• patient likely to be better able to tolerate adjuvant therapy prior to major surgery than following it;
• may improve swallowing and therefore nutrition prior to surgery;
• spare those patients that progress early with metastatic disease major surgery.
• the overtreatment of some patients that will not benefit from non-targeted therapy as opposed to targeting patients with factors that may determine the likely risk and site of residual disease;
• may make patient less well physiologically prior to major surgery, increasing risk of perioperative morbidity and mortality;
• may allow disease progression prior to definitive treatment.
Preoperative radiotherapy alone
This approach has been shown to be of value in rectal cancer.3 There have been six randomised trials of preoperative radiotherapy. Three trials were restricted to squamous carcinoma. One of these, by Gignoux et al., reported an improvement in local/regional recurrence (46% vs. 67%).4 Nygaard et al. report improved survival, but this series is complicated by the inclusion of some patients also receiving chemotherapy.5 One trial included both squamous and adenocarcinoma,6 and two do not specify the histology. Overall it is difficult to draw firm conclusions from these trials.
Postoperative radiotherapy
There are four randomised trials in the literature. The numbers are small (totalling 843 adjuvant patients), and three out of the four include only squamous carcinoma. Teniere et al.8 showed no survival advantage in 221 patients. There was a small improvement in the failure rate but at the cost of significant side-effects. The benefit appears to be limited to node-negative patients. Fok et al.9 included both adenocarcinoma and squamous carcinoma. Whilst both curative and palliative resections were included, the patients were separately analysed and received different radiotherapy doses. The results show a significant morbidity (37%) and mortality related to bleeding from the transposed intrathoracic stomach. It should be noted that the dose per fraction of the radiotherapy was high (3.5 Gy), which may be significant. There was a lower intrathoracic recurrence rate, particularly relating to tracheobronchial disease.
A larger randomised study from China included 495 well-staged patients with squamous carcinoma randomised to receive either surgery alone (S) or surgery and postoperative radiotherapy (S + R).10 Whilst there are significant concerns about the ethics (the patients were not aware they were in a trial and so did not give appropriate consent), the study was still published because of its significant results. The surgery appears to be of a high standard and included a radical lymph node dissection. The radiotherapy was wide field and included the bilateral supraclavicular fossae (SCF), mediastinum and anastomosis to an initial dose of 40 Gy. A further 10 Gy was given to the SCF and 20 Gy to the mediastinum by a different technique, allowing a maximum dose to the transposed stomach of 50 Gy. There was a relatively high proportion of earlier stage IIA disease in the study compared with a UK population. The analysis showed a highly significant difference in 1-, 3- and 5-year survival in stage III disease between the S and S + R arms (67.5%, 23.3%, 13.1% vs. 75.5%, 43.2%, 35.1%, respectively). The pattern of relapse was different between the two arms, with significantly fewer recurrences in the neck, SCF and mediastinum. Unlike other studies, toxicity to the transposed stomach was minimal.
The role of postoperative radiotherapy-based treatment in the case of a histological R1 resection is even less clear. There have been no randomised trials addressing this group of patients; indeed, the quality of reporting of circumferential resection margin (CRM) involvement by microscopic disease, which is influenced by postoperative surgical dissection of the operative specimen, is variable. In the absence of randomised evidence, the knowledge that radiotherapy has a proven role in oesophageal cancer probably justifies considering patients with longitudinal resection margin involvement for postoperative radiotherapy on an individual patient basis. When undertaken, there is some evidence that one should attempt to encompass both the anastamosis and the tumour bed but in the case of a high anastomosis for a lower oesophageal cancer, which is difficult to see radiologically, this can be challenging and requires specialised multidisciplinary input. The role for radiotherapy treatment in the case of CRM involvement is unclear, but it would seem sensible to target those patients where the risk of systemic disease relapse is lower, i.e. those with a lower ratio of involved lymph nodes.11
Preoperative chemotherapy
Preoperative chemotherapy in both squamous and adenocarcinoma appears to achieve consistently good clinical response rates, ranging from 47% to 61%.12,13 Early studies, predominantly in squamous carcinoma, used combinations of cisplatin, vindesine and bleomycin. More recently, cisplatin and 5-fluorouracil (5-FU) combinations have been used in important randomised trials. New 5-hydroxytryptamine-3 (5-HT3) antagonist antiemetic drugs have allowed cisplatin to be used with dramatically reduced toxicity. Protracted venous infusion (PVI) of 5-FU, and more recently capecitabine, an oral 5-FU prodrug, in combination with cisplatin and epirubicin (the ECF regimen) has produced increased response rates in non-randomised studies. These more modern cisplatin–5-FU combinations seem to be active in both squamous14 and adenocarcinoma,13 although the benefit of anthracycline therapy, i.e. epirubicin, in squamous cell carcinoma is less certain and is therefore often omitted.
Randomised trials of preoperative chemotherapy
The American Intergroup Trial (INT 0113) produced data on 440 randomised patients with a median follow-up of 46.5 months.15 Adenocarcinoma (54%) was the predominant histology. The chemotherapy given was three preoperative courses (cisplatin and 5 days of infusional 5-FU) and in stable or responding patients two postoperative courses. Overall, 83% of patients received the intended two preoperative cycles of chemotherapy. However, only 32% of patients received both postoperative chemotherapy cycles. There was no difference in treatment-related mortality between the two arms (6% surgery (S) vs. 7% chemotherapy (C) + surgery (S); P = 0.33). On an intent-to-treat basis there was no difference in median survival (16.1 months C + S vs. 14.9 months S), and 1-, 2- and 3-year (23% C + S vs. 26% S) survivals. Disappointingly, there was no difference in the pattern of metastatic disease between the two arms. However, there was a significantly higher rate of R1 resections in the surgery-alone arm.
The Medical Research Council (MRC) OEO2 study is the largest and arguably the most influential trial in this area.16 A total of 802 patients were randomised to receive two courses of cisplatin and a 4-day infusion of 5-FU followed by surgery (CS) after 3–5 weeks or immediate surgery alone (S) and showed a significant survival advantage for patients receiving preoperative chemotherapy.
The overall survival rate was significantly improved with preoperative chemotherapy (P = 0.004; hazard ratio 0.79, CI 0.67–0.93), with an estimated reduction in risk of death of 21% and 2-year survival figures of 43% CS vs. 34% S. There was no evidence that the effect of chemotherapy varied with histology. Long-term follow-up with a median follow-up of 6 years has confirmed these results, with 5-year survivals of 23% CS vs. 17% S.17
The recently completed MRC/NCRI trial in the UK (OEO5) compared OEO2 chemotherapy with four cycles of ECX (epirubicin–cisplatin–capecitabine) in adenocarcinoma alone. The high completion rate and positive results of preoperative chemotherapy in the MRC MAGIC (ST02) study19 for gastric and gastro-oesophageal cancer pointed to the strategy of using a modified ECF regimen, which is accepted in the UK as the best standard of care for advanced gastro-oesophageal cancer, and using it in a neoadjuvant setting to try and improve on the results of OEO2. The results of the REAL2 study,20 a phase III trial of palliative chemotherapy, showed that the oral fluoropyrimidine (capecitabine) could be substituted for infusional 5-FU with safety and at least equivalent efficacy. The advantage of easier chemotherapy delivery without the use of Hickman lines and their associated morbidity is a step forward. This study is also important in that it places an emphasis on high-quality assurance of staging, surgery, chemotherapy and pathology. There is little doubt that at least one of the reasons for differing results in trials in the whole area of gastro-oesophageal cancer has been a wide variation in the quality of staging modalities and surgery, as well as the heterogeneity in the regimens tested and trial design. The MRC OEO5 trial attempted to set high standards that should translate into improved patient selection and outcomes, even within the control arm.
Postoperative chemotherapy
There are few useful trials that address the question of adjuvant postoperative chemotherapy. The trials reported by Roth et al.21 and Kelsen et al.15 both have an adjuvant component, coupled with preoperative treatment. The fact that only 32% completed the postoperative phase in the Intergroup study underlines a problem with this approach.15 Patients undergoing major resections for oesophageal carcinoma often have a prolonged postoperative phase. The start of chemotherapy may be delayed due to performance status. Patients may also choose not to continue. A strategy that relies solely on postoperative treatment may have significant problems. Improved patient selection and postoperative supportive care may allow this approach to be practical. The MAGIC gastric cancer trial latterly included tumours of the gastro-oesophageal junction and lower oesophagus and intended three postoperative courses of ECF as well as three given preoperatively in the protocol. Again, only 40% completed the postoperative chemotherapy. The trial has shown an improvement in overall survival, as described in the section on gastric cancer,19 which lends further support for the concept of neoadjuvant chemotherapy for cancers of the oesophagus or gastro-oesophageal junction.
Preoperative chemoradiotherapy
Non-randomised studies of CRT have appeared in the literature since the late 1980s. The review article by Geh et al.22 summarises 46 trials containing 20 patients or more. Overall, pooled data from these studies show that, of 2704 patients (squamous 68% and adenocarcinoma 32%), 79% were operated on with a pCR rate of 24% of those treated and 32% of those resected. As experience with this modality of treatment has grown, lessons have been learned. Attempts to escalate the dose of radiotherapy can lead to unacceptable rates of morbidity, especially if higher doses per fraction are used.23,24 Reported CRT-related deaths in the non-randomised series ranged from 0% to 15% (mean 3%). Postoperative deaths ranged from 0% to 29% (mean 9%). Adult respiratory distress syndrome, anastomotic leak and breakdown, pneumonia and sepsis were the commonest causes of death following oesophageal resection. Treatment-related deaths ranged from 3% to 25% (mean 9%) of all patients treated. It seems clear that the risk of chemotherapy-related toxicity, particularly myelosuppression, rises with the number of drugs used and the intensity of the CRT regimen.25,26 An increased risk of tracheobronchial fistula has been reported.27 However, most of the reported series did not have the latest sophisticated radiotherapy techniques that allow greater precision and sparing of organs and tissues to within normal tissue tolerance.
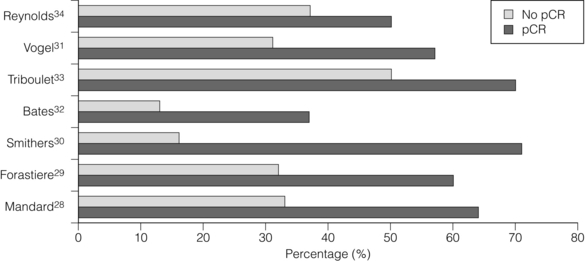
Consistent reporting of pathology is important, and a grading of CRT response has been described by Mandard et al.28 Five grades of response ranging from no identifiable tumour to complete absence of regression allow a more objective approach to be adopted. In this paper the significant predictor of disease-free survival after multivariate analysis was the tumour regression grade. There is evidence that pCR confers a survival advantage over those patients not achieving pCR.29–34 In Fig. 9.1, different comparative outcomes, such as median survival in months, overall or disease-free survival in years, are plotted together in the series, quoting outcomes separately. The importance is in the consistent nature of the difference in outcomes in each series. It becomes clear that prediction of this response prior to treatment either through molecular markers or PET activity after induction chemotherapy alone might allow very different algorithms of treatment modalities (also see Chapter 3).
Table 9.1 summarises nine reported randomised trials of preoperative CRT compared with surgery alone. In four of these the chemotherapy was given sequentially to the radiotherapy and in four synchronously. Two trials using sequential treatment in squamous carcinoma received relatively low doses of radiotherapy and showed no convincing evidence of improved survival with the combined treatment.6,35 In a larger European Organisation for Research and Treatment of Cancer (EORTC) trial involving 282 patients, the cisplatin chemotherapy was given in close sequence with the radiotherapy.24 The radiotherapy was given in a split course and at a relatively high dose per fraction (two courses of 18.5 Gy in five daily fractions split 2 weeks apart). The CRT patients were more likely to have a curative resection. The disease-free survival was significantly longer (3-year CRT + S 40% vs. S 28%). There was no difference in the overall survival, largely due to a significantly higher postoperative mortality in the CRT arm (12% vs. 4%). Apinop et al.36 reported a synchronous CRT series of 69 squamous histology patients with no improvement in survival.
Table 9.1
Randomised trials of preoperative chemoradiation
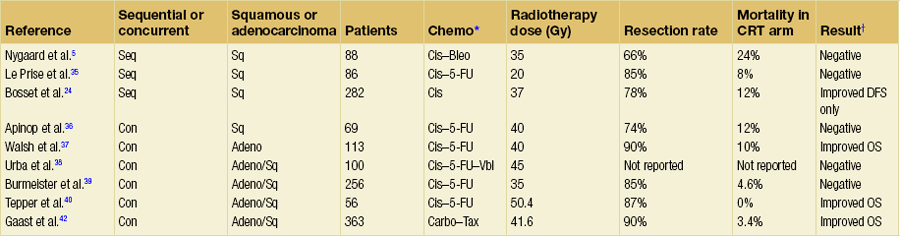
*Bleo, bleomycin; Carbo, carboplatin; Cis, cisplatin; 5-FU, 5-fluorouracil; Tax, paclitaxel; Vbl, vinblastine.
There are four larger trials of preoperative synchronous CRT.
The University of Michigan trial38 randomised 100 patients including both squamous and adenocarcinoma. The surgery was a transhiatal resection. Patients in the CRT arm received 45 Gy in 30 fractions with cisplatin, 5-FU and vinblastine. At first analysis there was no significant difference between the arms but at 3 years a statistically significant benefit to the combined treatment emerged, with overall survival of 32% vs. 15%. A final analysis has shown no survival advantage and demonstrates the danger of early publication of a trial that was essentially underpowered.
The results of the Australasian Gastro-Intestinal Trials Group (AGITG)39 have been criticised for having a low radiotherapy dose and only one cycle of cisplatin and 5-FU chemotherapy. Although the trial was negative overall there are some clues for the direction of future approaches. There was a significant survival difference in patients with squamous histology (36% of the total) with the addition of CRT and a much higher pathological complete response rate.
The US trial NCCTG-C9781 (CALGB 9781) closed prematurely as a result of poor recruitment due to a reluctance to recruit patients to a trial with a no treatment arm. However, mature results from CALGB 9781 are available and despite small numbers show a significant improvement in overall survival in preoperative CRT compared to surgery alone (5-year survival of 39% vs. 16%).40 Resection rates were high in the preoperative CRT arm (87%) and there was no increase in operative mortality. The trial included higher quality staging and surgery.
Interpretation of such heterogeneous trials, in the regimen being tested, design and outcomes, is difficult. Nevertheless, a meta-analysis of randomised trials has shown that this approach increases R0 resection rates, reduces locoregional recurrence and improves survival compared with surgery alone.41 More recently, and not included in the meta-analysis above, a randomised phase III study comparing surgery alone to preoperative CRT has shown a near doubling of overall survival (OS) in favour of the preoperative arm (OS 49 vs. 26 months, hazard ratio (HR) 0.67), a pCR rate of 32% and no increase in surgical mortality (3.8% (S) vs. 3.4% (CRT-S)).42 In the ‘CROSS’ trial, 363 patients with operable oesophageal or gastro-oesophageal junction tumours were randomised to surgery alone or to a preoperative CRT regimen of weekly carboplatin (AUC2) and paclitaxel (50 mg/m2) concurrent with radiotherapy (41.4 Gy in 23 fractions). Of the 175 patients assigned to the CRT arm, 163 completed protocol treatment and the study reported a low incidence of grade 3/4 CRT toxicity (haematological, 6.8%; non-haematological, 16%). The R0 resection rates in the surgery and CRT + surgery arms were 67% and 92.3%, respectively (P = 0.002). The results of this study, performed in patients with a similar stage and morphological distribution to those in the UK, would suggest that where preoperative CRT is delivered safely, this may lead to a significant improvement in outcome.
Neoadjuvant chemoradiotherapy or chemotherapy?
There is rightly a clear separation in future trials for adenocarcinoma and squamous carcinoma. As the trend moves towards squamous cancers being treated with primary CRT, the role of preoperative CRT may be revisited as a means of improving the outcome for patients with adenocarcinoma. The majority of such patients will present with stage III disease (at least T3 with lymph node metastases). Such tumours frequently threaten the circumferential margin of surgical resection (CRM), although a clear plane for surgical excision does not exist as it does for other anatomical sites such as the rectum. Disease present at or within 1 mm of the circumferential margin (R1) occurs in more than 50% of stage III cases11,44 and is a poor prognostic factor. In the OEO2 study, the 3-year and median survival for patients with R0 and R1 resection were reported as 42.4% vs. 18% and 2.1 years vs. 1.1 years, respectively.16 Preoperative chemoradiotherapy (CRT) has become a standard management strategy in rectal cancer for patients who have a threatened CRM on preoperative staging.
There has been only one randomised phase III trial comparing preoperative chemotherapy with preoperative CRT. This study by Stahl et al. aimed to recruit 354 patients to detect a 10% improvement in OS in favour of CRT (from 25% to 35%) but closed early as only 126 patients could be recruited in 5 years. Nonetheless, it showed a non-significant trend towards improved 3-year survival in favour of CRT (47.4% vs. 27.7%, P = 0.07).45
The undoubted extra toxicity may be justified for this selected group and is infinitely preferable to postoperative treatment. New radiotherapy technology allows more accurate treatment delivery and lower morbidity, and when coupled with higher quality surgery and perioperative care should allow the sort of overall results from the Dutch trial42 to be reproduced. Whatever improvements in locoregional treatments are proposed, the highest risk to be faced and addressed with new trials for stage III adenocarcinoma is ultimate systemic relapse. Trials with new biological agents added to standard chemotherapy or selective CRT are likely to be the next step, with advance knowledge from their use in the advanced and metastatic disease setting.
Definitive radiotherapy and chemoradiotherapy
Definitive radiotherapy
Classical figures quoted for survival from radical radiotherapy come from the paper from Earlam and Cunha-Melo.46 Mean survival figures of 8489 patients at 1, 2 and 5 years were 18%, 8% and 6%, respectively. Approximately 50% of patients were treated with curative intent. Older series tend to be of squamous carcinoma treated with radiotherapy alone. Modern radiotherapy in more selected patients can produce impressive survival results. In a series of 101 patients treated at the Christie Hospital in Manchester between 1985 and 1994, 3- and 5-year survival figures of 27% and 21%, respectively, were recorded.47 There was a slightly better survival for adenocarcinoma, but not reaching statistical significance. The majority of tumours (96/101) were of 5 cm or less in length. Importantly, the only significant prognostic factor was the use of diagnostic CT, introduced during the latter part of the study. This was used to plan the radiotherapy and led to an increase in field sizes. The conclusion of the paper was that radiotherapy provided an effective alternative to surgery and that modern radiotherapy planning techniques may improve results.
There is no reason to compromise on staging or treatment planning standards and with modern technology high doses can be given with low morbidity. A selected series of 51 patients 80 years and over with squamous carcinoma treated with 66 Gy of radiotherapy in Japan produced median survival of 30 months and a 3-year survival rate of 39%.48
Definitive chemoradiotherapy
The adoption of CRT stems from high response rates and in particular high pCR rates seen in patients going on to resection. There are four randomised trials comparing radiotherapy alone with CRT. Three of these use low doses or low intensity of chemotherapy. A small series of 59 patients from Brazil did not demonstrate a significant survival advantage.49 The response rates and 5-year survival rates (6% vs. 16%) were better in the CRT arm but at a cost of increased acute toxicity. An important non-randomised series is reported by Coia et al.50 Treatment was with infusional 5-FU and mitomycin C with 60 Gy of radiotherapy. Patients with early-stage disease are reported separately. The respective 5-year survival and local failure rates, in clinical stages I and II combined, were 30% and 25%. There was no treatment-related mortality, although there was increased acute toxicity (22% grade III and 6% grade IV).
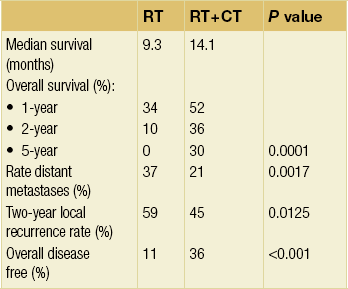
The high local failure rate of 45% in the Herskovic trial led to the Intergroup study 00123 (Minsky) that compared a regimen similar to the Herskovic regimen (modified with narrower radiotherapy fields, radiotherapy using 1.8 Gy/fraction and an alteration in the chemotherapy schedule to reduce anticipated toxicity), to the same schedule but with a higher dose of radiotherapy (64.8 Gy in 36 fractions).52 In total, 236 patients, once again predominantly with squamous cell cancer, were randomised within this study. The trial had to be closed prematurely due to an excess of treatment-related deaths in the experimental arm (11 vs. 2), although the majority of these occurred before the higher dose section of the treatment protocol had been received. Although this trial did not show better disease control with higher doses of radiotherapy (56% failure at 2 years compared to 52% in the standard arm) it did demonstrate remarkably consistent outcomes of, approximately 30% survival at 3 years with definitive chemoradiotherapy.
Another approach to improve local control was to use brachytherapy to intensify the radiotherapy dose to the tumour. Study RTOG 92-07 used the 50-Gy external beam and chemotherapy protocol from the Herskovic protocol and added an intraluminal brachytherapy boost with one of two methods of delivery, high dose rate or low dose rate.53 Six of the 35 patients developed an oesophageal fistula and this toxicity was deemed unacceptable.
Following successful CRT or radiotherapy alone there is a significant rate of benign stricture formation. This ranges from 12%54 to 25%50 in more modern studies. However, good swallowing function can be maintained in the majority of patients. Even in those with a benign stricture, a full or soft diet can be maintained by dilations in 71% of cases.55 The treatment of post-CRT benign stricture with stents has not been successful in the authors’ experience and gives rise to mediastinal pain.
Future directions in definitive chemoradiation
The ability to predict which patients will respond to chemotherapy or CRT would allow greater certainty in a primary non-surgical approach. Molecular markers predicting response to chemotherapy hold some promise.56–58 Conventional reassessment following treatment, with a negative endoscopic biopsy35 and CT,59 appears unreliable. However, the use of a positive surveillance endoscopic biopsy to direct salvage surgery in squamous carcinoma treated with definitive CRT has been reported.60 Reports of the value of endoscopic ultrasound (EUS) are more variable, with some showing a good correlation with final pathological stage61 and others suggesting it is not reliable.62 There are reports advocating that this failure to reliably predict pCR necessitates resection.34
Improvements in CRT outcomes are likely to come from refinements in chemotherapy and radiotherapy technique. Results from preoperative phase II studies suggest a steady improvement in pCR rates, with more acceptable toxicity. The rates of pCR range from 24%31 in 1993 to reports of 56%64 in 1998. Care must be taken in interpreting the literature as pCR rates can vary depending upon whether rates are quoted as intent to treat or of completed resections, and in the protocols and quality assurance procedures associated with the histological examination. Careful staging can ensure that patients with established metastatic disease are appropriately managed. There has been a trend to accept lower standards of staging in non-surgical series. It is important that all patients who are deemed to have a potentially curative therapy have access to comparable staging, including EUS and PET. In the preoperative setting new protocols can be assessed for toxicity and response rates before use in a phase III randomised setting.65
Central to improving treatment strategies is an understanding of patterns of treatment failure. An important series of a detailed analysis of CRT has been published from Australia using combined data from Trans-Tasman Radiation Oncology Group studies.66 This looks at results from 274 patients treated with definitive CRT and 92 patients treated with preoperative CRT. A summary of survival and recurrence patterns is given in Table 9.3. The overall local control rate for definitive CRT is almost 55%, rising to 70% in upper squamous cancers. The striking difference in outcome for these upper cancers includes an apparently lower distant failure rate and improved overall survival. It may be that these tumours are inherently different and respond more like squamous carcinomas of the head and neck. The persisting high distant failure rate in adenocarcinoma treated with CRT and surgery underlines a need for either earlier diagnosis and treatment or improved systemic therapy. There is no doubt that the success of CRT as definitive treatment is determined by similar factors to the outcomes of surgery, namely stage, performance status and the length of the tumour.
Table 9.3
Five-year survival and cumulative incidence of relapse in the study of Denham et al. (2003)66
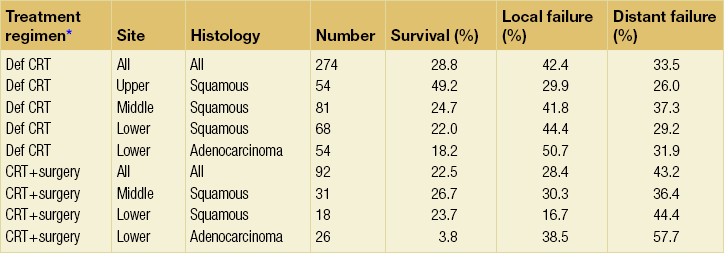
*Def CRT, definitive chemoradiation; CRT + surgery, preoperative chemoradiation + surgery.
There are huge changes in the technology available for radiotherapy treatment. The development of three-dimensional and conformal radiotherapy treatment planning systems directly linked to spiral CT data allows the shape of radiotherapy fields to be individually tailored to an irregular-shaped target volume. In order for this to be successful, however, reliable imaging techniques are essential, including using EUS67 and PET68 to help delineate the gross tumour volume, and also localise the tumour on the axial planning CT scans. A reduction in normal tissue damage and so potentially the toxicity of combining therapy will be possible. The ability to define varying dose intensity within a radiotherapy field (intensity-modulated radiotherapy treatment, IMRT) may be helpful in being able to safely increase the dose, especially to tumours in the upper third of the oesophagus. One of the major concerns for tumours in the lower third is the movement of these tumours during treatment as a result of peristalsis, cardiac ejection cycles and especially respiratory motion. This can be up to 2 cm in the superior–inferior direction. There is no doubt that even better distributions of dose can be achieved with proton therapy but availability and cost may preclude this being feasible for some years. Improvements in CRT will also come from a better understanding of the effects on normal tissue near the clinical target volume, such as heart and lung.
The current NCRI study of definitive CRT (SCOPE 1) aims to compare cisplatin and capecitabine with 50 Gy of radiotherapy in the control arm and add the epidermal growth factor receptor (EGFR) monoclonal antibody cetuximab to the investigational arm. There is evidence that one mechanism of radiotherapy resistance is through activation of the EGFR pathways and clinical evidence from a randomised trial in squamous cancer of the head and neck of improved local control and overall survival.69 This study is important in also defining very high radiotherapy technical standards for the UK, ensuring the accuracy of target volume definition and minimising normal tissue morbidity. It is open to both adenocarcinoma and squamous carcinoma selected by multidisciplinary teams, the entry criteria broadly being patients with disease treatable by chemoradiotherapy who have been deemed unsuitable for surgery due to the extent of local disease, patient comorbidities or patient choice.
Definitive CRT versus surgery
Definitive CRT treatments now report good survival figures51,59 rivalling those of surgery, stage for stage.70,71
There have been few trials that allow a direct comparison between a primary CRT policy and surgery, and indeed CRT studies may have had a selection bias against them. The results of CRT alone tend to have 5-year survival figures generally comparable to those seen in Tables 9.2 and 9.3, of the order of 30% overall, which are similar to surgical series. Squamous cancers, in particular of the mid and upper thirds, have better outcomes. It does allow CRT to be considered as a viable option to chemotherapy and surgery for adenocarcinoma and as primary treatment for squamous carcinoma.
In a French study, patients were assessed after induction CRT using 5-FU and cisplatin.73 If they had achieved an objective response they were randomised (295 of 455 patients) to carry on with CRT or go to surgery. There was no significant difference between the 2-year survival rates for patients who had surgery (33.6%) and those who had CRT alone (39.8%). There were more early deaths in the surgery arm but CRT required more dilatations and stents.
In a German trial, 177 patients with T3 or T4 squamous carcinoma were randomised to receive CRT + surgery or CRT alone.74 The rate of response to initial CRT was the same for both arms. There was a strong trend towards improved local tumour control in the arm with surgery. In responding patients the 3-year survival (45% and 44%, respectively) was equivalent in both arms, whereas in non-responding patients the rates were 18% and 11%, respectively. The 3-year survival rate improved to 35% in non-responding patients undergoing complete tumour resection, implying that a subgroup of non-responding patients may benefit from surgery as an elective salvage procedure. Longer-term results confirm no clear survival difference between a surgical versus CRT approach. This trial did show that a clinical response to induction chemotherapy may be a valuable surrogate for predicting prognosis.
There is evidence collected from the literature that selected salvage surgery is possible after CRT failure, with acceptable operative mortality of 11.4% and 5-year survival rates of 25–35%.75 Clearly such a high-risk policy should be after CT-PET restaging and only within the context of a tertiary MDT with audited results for the safe delivery of both chemoradiation and surgery.
Small-cell oesophageal cancer
The treatment of SCOC is dependent on a separation between limited disease and extensive disease. Table 9.4 shows the outcomes in two of the larger and most recent series in the literature.76,77 Both have good references and discussion.
Gastric cancer
Potentially curative treatment
Perioperative adjuvant chemotherapy
The MRC STO2 (MAGIC) trial was opened in 1994 and aimed to recruit 500 patients testing the role of three courses of ECF before and after resection in operable gastric cancer. The results suggest a significant downstaging effect of the chemotherapy.19 As the MRC OEO2 neoadjuvant oesophageal trial was completed, the eligibility criteria were widened in 1999 to include adenocarcinoma of the lower oesophagus. The type of resection was left to the discretion of the participating surgeon and the staging was relatively permissive by modern standards. The arms of the study were well balanced and included 74% stomach, 14% oesophageal and 12% junctional cancers. Toxicity of the chemotherapy was acceptable but only 40% of patients received both cycles of postoperative treatment. In fact, the majority of resections were at least D1, with 40% having a D2. The proportion deemed to have had a potentially curative resection was 10% higher with chemotherapy (79% vs. 69%). There was a significant effect on tumour size, T stage and nodal status. Recent results with a median follow-up of > 3 years have demonstrated an improvement in overall survival (hazard ratio of 0.75, P = 0.009), with 5-year survival rates of 36% for chemotherapy and surgery vs. 23% for surgery alone. Progression-free survival was also significantly prolonged.
In a similar French multicentre trial, of perioperative fluorouracil plus cisplatin in resectable gastro-oesophageal adenocarcinoma, 224 patients with resectable adenocarcinoma of the lower oesophagus, gastro-oesophageal junction (GEJ) or stomach were randomly assigned to either perioperative chemotherapy and surgery (CS group; n = 113) or surgery alone (S group; n = 111).81 Chemotherapy consisted of two or three preoperative cycles of intravenous cisplatin (100 mg/m2) on day 1, and a continuous intravenous infusion of fluorouracil (800 mg/m2/day) for 5 consecutive days (days 1–5) every 28 days and three or four postoperative cycles of the same regimen. The CS group had a better overall survival (5-year rate 38% vs. 24%; HR for death: 0.69; 95% CI 0.50–0.95; P = 0.02). The postoperative morbidity was similar in the two groups.
Intraperitoneal chemotherapy
The pattern of peritoneal and hepatic recurrence in gastric cancer makes the early use of intraperitoneal chemotherapy attractive. The most positive trial is from Japan,82 using mitomycin C adsorbed on to activated charcoal, acting as a delayed-release preparation. Fifty patients with serosal involvement were randomised to immediate treatment or observation. A highly significant difference in survival at 2 years was seen (68.6% vs. 26.9%), with the treatment group maintaining its advantage at 3 years. The treatment was reported to be well tolerated. However, when an attempt was made to repeat these results, in an Austrian multicentre study,83 serious toxicity caused the trial to be suspended. A significantly higher postoperative complication rate (35% vs. 16%) and 60-day mortality rate (11% vs. 2%) were seen in the treatment arm of the study. No benefits were found in overall or recurrence-free survival.
Postoperative chemoradiotherapy
In the British Stomach Cancer Group trial,84 postoperative radiotherapy was one of the arms of the study. The other arms were FAM chemotherapy and a control surgery-only group. There was no difference in survival but the local recurrence rate was significantly better (54% surgery vs. 32% with radiotherapy; P < 0.01).
The regimen consisted of 5-FU–leucovorin (folinic acid) given in the first and last weeks of radiotherapy (45 Gy) and two 5-day courses of 5-FU–leucovorin given monthly. With a median follow-up of 3.3 years both the disease-free survival (49% vs. 32%) and overall survival (52% vs. 41%) were improved in the CRT arm. There was some significant haematological and gastrointestinal morbidity. However, the treatment-related mortality was only 1%. The need for great care in the technical quality and placement of the radiotherapy was apparent. However, a significant proportion of the patients (54%) had only a D0 resection and the survival in the surgery-alone arm was relatively poor (41% 3-year survival). It is possible that the CRT is making up for less than adequate surgery, and may not translate into routine practice where more extensive surgery is undertaken. It is the most obvious source of criticism of the trial. However, in a subsequent paper,86 using a different surgical quality assurance measure for the likelihood of undissected disease (the Maruyama Index), the group concluded that surgical under-treatment clearly undermines survival. Major concerns about the toxicity and chemotherapy used and the poor radiotherapy technique are being addressed, which should significantly reduce the potential for long-term morbidity and make the most of sophisticated IMRT and radiotherapy planning techniques. Despite criticisms, postoperative CRT has been patchily adopted throughout the world.
Palliative chemotherapy
Squamous carcinoma of the oesophagus
Cisplatin-containing combination chemotherapy is the standard for the treatment of advanced and recurrent squamous carcinoma. The indications for use are limited by the relative infrequency of the disease, and in particular the age and performance status of patients requiring palliation. Very often the indication is to improve symptoms and quality of life caused by the primary leasion and local therapy with a stent or radiotherapy will be adequate. However, good response rates of the order of 35% can be achieved with cisplatin and 4- or 5-day 5-FU infusion.87 Response duration is variable and can range from 3 to 6 months. Consideration should be given to consolidation palliative radiotherapy after successful chemotherapy to improve local control where recurrent growth may produce symptoms for patients with a better performance status and expectation of life. There is some evidence that the improved response rates seen with PVI of 5-FU in adenocarcinoma can be achieved in squamous carcinoma.14 New agents such as paclitaxel are clearly active as single agents but have yet to demonstrate their clear superiority in combination regimens. Some results are promising, with response rates nearer 50%.88
Adenocarcinoma of the oesophagus and stomach
The FAM regimen (5-FU, doxorubicin and mitomycin) initially seemed to have a high response rate of 40%.92 However, in the setting of a randomised trial by the North Central Cancer Treatment Group, it seemed to be no better than 5-FU alone.93 In an attempt to modulate the activity of 5-FU within the FAM regimen, high-dose methotrexate was given 1 hour before the 5-FU in the FAMTX regimen (fluorouracil, doxorubicin and methotrexate). Klein produced impressive results in a study of 100 patients.94 The response rate was 58%, with a complete remission rate of 12%. There were only 3% treatment-related deaths and a long-term survival rate of 6%. The response rate seen in subsequent studies was slightly lower but still confirmed acceptable toxicity. This regimen has now been tested against other combinations. A randomised EORTC trial with 208 evaluable patients demonstrated its superiority against FAM.95 Median survival was better (42 weeks vs. 29 weeks; P = 0.004), with 41% and 9% of the FAMTX patients alive at 1 and 2 years, respectively, compared with 22% and 0% for FAM patients. The EAP regimen (etoposide, doxorubicin and cisplatin) was found to have similar survivals, similar overall response rates but lower complete remission rates and was significantly more toxic.96 The recent EORTC trial has compared three regimens, FAMTX, ELF (etoposide, leucovorin and bolus 5-FU) and FUP (infusional 5-FU and cisplatin), in 399 randomised patients.97 There was no significant difference in median survivals between the regimens. The response rates were lower than in some previous trials (ELF 9%, FUP 20%, FAMTX 12%) but this trial had tight objective response criteria and required measurable disease. The conclusion is that they all produce modest response with comparable survival and toxicity.
Patients were predominantly of good performance status with a median age of 60 years. The overall objective response rate was 45% in the ECF arm and 21% in the FAMTX arm (P = 0.0002). The response of locally advanced disease to ECF has previously been shown to be higher than in metastatic disease.13 This was confirmed in both arms of the trial (56% ECF vs. 23% FAMTX). Of the 121 patients receiving ECF, 10 were able to undergo a resection due to improved status, six of whom remain disease free. There were three cases of histological pCR. Only 5% of patients had progression whilst on either chemotherapy regimen.
In a study from Leeds of advanced upper gastrointestinal cancer patients, oral UFT and leucovorin were substituted for PVI of 5-FU in ECF in an attempt to create a more practical, acceptable and cheaper alternative (the ECU regimen) without the need for central lines and pumps.99 In this dose-escalation pilot study 30 patients were treated. Toxicity was acceptable and of 20 assessable patients, nine of the 15 with gastro-oesophageal cancer had an objective response and two of these were complete radiological responses.
The NCRI REAL2 trial was designed to address some practical problems that surrounded delivery of the gold standard ECF regime.20 Infusional 5-FU has problems associated with Hickman lines, particularly thrombosis and infection. Cisplatin causes renal toxicity and requires prehydration and inpatient admission for higher doses. It tested the toxicity and response rates of oxaliplatin as a substitute for cisplatin, and of capecitabine (an oral fluoropyrimidine) as a substitute for infusional 5-FU in a randomised 2 × 2 study based on statistics of non-inferiority against ECF.
The EOX regimen has been taken forward as the control arm in the next REAL3 study, with the addition of panitumumab (an EGFR antibody) in the investigational arm. Other attempts to improve treatment outcomes using the addition of docetaxel to cisplatin and 5-FU have uncovered high potential toxicities with neutropenia, treatment withdrawal rates of nearly 50% due to grade 3 and 4 toxicity, and no improvement in response rates or survival, raising a question as to whether a plateau has been nearly reached with conventional approaches to chemotherapy.100 New biological agents possibly bring new distinctions between different agents even within antibodies to the same receptor and very different response rates of gastric and gastro-oesophageal/oesophageal cancers.101 This emphasises the need for good tissue collection and analysis in parallel with clinical studies. The recent closure of REAL3 as a result of insufficient benefits seen in the trial arm of EOX plus panitumumab suggests that we have not yet found the right targets or the optimum agents with which to counter these targets.
Trastuzumab (herceptin), a monoclonal antibody against human epidermal growth factor receptor 2 (HER-2; also known as ERBB-2), was investigated in combination with chemotherapy for first-line treatment of HER-2-positive advanced gastric or gastro-oesophageal junction cancer. The ToGA trial was an international phase 3 study undertaken in 594 patients, randomised to capecitabine or fluorouracil plus cisplatin plus or minus trastuzumab.102 Median overall survival was 13.8 months (95% CI 12–16; 16.0 months in those who would be considered HER-2 positive by today’s definition (HER-2 3 + or HER-2 2 + + FISH +)) in those assigned to trastuzumab plus chemotherapy compared with 11.1 months (95% CI 10–13) in those assigned to chemotherapy alone (HR 0.74; P = 0 · 0046). There were no significant differences in toxicities, including cardiac, between the two groups. The proportion of HER-2-positive tumours ranges from approximately 10% to 30% of all gastric cancers, being higher in gastro-oesophageal junctional cancers, Caucasians and intestinal type pathology.
The selection of patients who are likely to benefit from palliative chemotherapy may be helped by the development of prognostic scoring methods. One study has demonstrated that performance status, liver metastases, peritoneal metastases and alkaline phosphatase can be used to separate different risk groups.103 Problems in the literature with myelosuppression, and in particular toxic deaths, may be avoided by the use of growth factors to reduce the incidence of neutropenic sepsis. Many of the problems of severe emesis have already been improved by the use of 5-HT3 antiemetic drugs.
Palliative radiotherapy
There is a major difference between the fractionation regimens used in the USA and in the UK. ‘Palliative’ doses of 40–60 Gy in 4–6 weeks are quoted in the US literature. These are in the radical dose range and are felt to be inappropriate for UK practice, where doses of 20–30 Gy in 1 or 2 weeks are more likely to be used. These can be combined with brachytherapy. Good resolution of tumour and symptom relief in a majority of patients have been reported.104 Often, however, whichever palliative technique is used first, other modalities have a role for patients with longer survival, to maintain swallowing.
Brachytherapy
Brachytherapy involves the placement of a high-dose-rate radioactive source, usually iridium-192, down the oesophagus in proximity to the tumour. The aim is to get direct tumour cell kill, thereby relieving dysphagia, or in the case of its use as a boost to external beam radiotherapy, to achieve an increased dose to the tumour with minimal dose to surrounding normal tissues. It does not require a general anaesthetic and can be done as a day-case procedure. Occasionally, placement of a nasogastric guide tube is required under endoscopic vision. Pagliero and Rowlands105 describe a single dose of 15 Gy with a response rate of about 60% measured at 6 weeks from treatment. It can be repeated in cases of symptomatic relapse.
The optimum dose of brachytherapy has been addressed in a randomised trial using three schedules.106 Three doses and schedules were tested in 172 patients with advanced oesophageal cancer These were 12 Gy/two fractions (A), 16 Gy/two fractions (B) and 18 Gy/three fractions (C).
Future strategies
In order to achieve the best outcomes for patients, assessment, staging and treatment need to be closely coordinated and integrated in a multidisciplinary setting. Poor outcomes from single-modality therapy and increasing evidence of the value of multiple modalities will be powerful drivers towards higher quality and more centralised services. Site specialist clinicians and support services can only meet demands for quality assurance in all possible modalities of treatment with appropriate resources and infrastructure. The essential role of high-quality radiology, including EUS, and expert pathology cannot be underestimated. The routine use of PET, both as a diagnostic tool to pick up early metastatic disease, and also to help target volume localisation in radiotherapy planning and predict response to non-surgical treatment, seems likely to become a key decision-making tool (see Chapter 3). Support services such as specialist nursing and dietetic services are particularly important in this area of disease management.
As with radiotherapy treatments, systemic therapy is moving towards an era of personalised oncology. This is where treatments are selected based on the characteristics of the individual tumour rather than on prognostic risk factors. Many patients will never benefit from current chemotherapy and biological therapies, including monoclonal antibodies, and biomarkers are needed to select those that will benefit the most from what may be relatively toxic and/or expensive treatment. We have seen the first of these in the form of HER-2-positive oesophagogastric cancer, which predicts the benefit of the addition of herceptin to combination chemotherapy.102 ERCC1 may predict for cisplatin resistance and future studies must define the role of these biomarkers in routine treatment. If the lessons from past trials are to be learned, namely the poor and variable results in control arm treatments, attention will have to be paid to rigorous quality assurance within each area of defined treatment. This will aid the process of new high-quality research trials aiming to develop new treatment strategies.
References
1. Nigro, N.D., Seydel, H.G., Considine, B., et al, Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer 1983; 51:1826–1829. 6831348
2. Northover, J.M., Epidermoid cancer of the anus – the surgeon retreats. J R Soc Med 1991; 84:389–390. 1865441
3. Swedish Rectal Cancer Trial, Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997; 336:980–987. 9091798
4. Gignoux, M., Roussel, A., Paillot, B., et al, The value of preoperative radiotherapy in esophageal cancer: results of the EORTC. World J Surg 1987; 11:426–432. 3630187
5. Nygaard, K., Hagen, S., Hansen, H.S., et al, Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicentre study of pre-operative radiotherapy and chemotherapy. The Second Scandinavian Trial in esophageal cancer. World J Surg 1992; 16:1104–1110. 1455880
6. Arnott, S.J., Duncan, W., Kerr, G.R., et al, Low-dose preoperative radiotherapy for carcinoma of the oesophagus: results of a randomized clinical trial. Radiother Oncol 1992; 24:108–113. 1496141
7. Arnott, S.J., Duncan, W., Gignoux, M., et al, Preoperative radiotherapy in esophageal carcinoma: a meta-analysis using individual patient data (Oesophageal Cancer Collaborative Group). Int J Radiat Oncol Biol Phys 1998; 41:579–583. 9635705
8. Teniere, P., Hay, J., Fingethut, A., et al, Postoperative radiation therapy does not increase survival after curative resection for squamous carcinoma of the middle and lower oesophagus as shown by a multi-center controlled trial. Surg Gynaecol Obstet 1991; 173:123–130. 1925862
9. Fok, M., Sham, J.S.T., Choy, D., et al, Postoperative radiotherapy for carcinoma of the esophagus: a prospective randomized controlled trial. Surgery 1993; 113:138–147. 8430362
10. Xiao, Z.F., Yang, Z.Y., Liang, J., et al, Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg 2003; 75:331–336. 12607634
11. Dexter, S.P., Sue-Ling, H., McMahon, M.J., et al, Circumferential resection margin involvement: an independent predictor of survival following surgery for oesophageal cancer. Gut. 2001;48(5):667–670. 11302966
12. Schlag, P.M., Randomized trial of preoperative chemotherapy of squamous cell cancer of the esophagus. Arch Surg 1992; 127:1446–1450. 1365692
13. Bamias, A., Hill, M.E., Cunningham, D., et al, Epirubicin, cisplatin and protracted venous infusion of 5-fluorouracil for esophagogastric adenocarcinoma. Cancer 1996; 77:1978–1985. 8640659
14. Andreyev, H.J.N., Norman, A.R., Cunningham, D., et al, Squamous oesophageal cancer can be downstaged using protracted venous infusion of 5-fluorouracil with epirubicin and cisplatin (ECF). Eur J Cancer 1995; 31A:2209–2214. 8652244
15. Kelsen, D.P., Ginsberg, R., Pajak, T.F., et al, Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998; 339:1979–1984. 9869669
16. Medical Research Council Oesophageal Cancer Working Party, Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002; 359:1727–1733. 12049861
17. Allum, W.H., Fogaty, P.J., Stenning, S.P., et al. Long term results of the MRC OEO2 randomized trial of surgery with or without preoperative chemotherapy in resectable esophageal cancer. Proc ASCO GI Cancer Symp. 2008. [abstr. 9].
18. Malthaner, R., Fenlon, D., Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev. 2003;(4):CD001556. 14583936
19. Cunningham, D., Allum, W.H., Stenning, S.P., et al, Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355:11–20. 16822992
20. Cunningham, D., Starling, N., Rao, S., et al, Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358:36–46. 18172173
21. Roth, J.A., Pass, H.I., Flanagan, M.M., et al, Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine and bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Surg 1988; 96:242–248. 2456424
22. Geh, I.J., Crellin, A.M., Glynne-Jones, R., A review of the role of preoperative (neoadjuvant) chemoradiotherapy in oesophageal carcinoma. Br J Surg 2001; 88:338–356. 11260097
23. Urba, S.G., Orringer, M.B., Perez-Tamayo, C., et al, Concurrent preoperative chemotherapy and radiation therapy in localized esophageal adenocarcinoma. Cancer 1992; 69:285–291. 1728358
24. Bosset, J.F., Gignoux, M., Triboulet, J.P., et al, Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997; 337:161–167. 9219702
25. MacKean, J., Burmeister, B.H., Lamb, D.S., et al, Concurrent chemoradiation for oesophageal cancer: factors influencing myelotoxicity. Aust Radio 1996; 40:424–429. 8996905
26. Minsky, B.D., Neuberg, D., Kelsen, D.P., et al, Final report of Intergroup trial 0122 (ECOG PE-289, RTOG 90-12): phase II trial of neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 1999; 43:517–523. 10078631
27. Bartels, H.E., Stein, H.J., Siewert, J.R., Tracheobronchial lesions following oesophagectomy: prevalence, predisposing factors and outcome. Br J Surg 1998; 85:403–406. 9529504
28. Mandard, A.M., Dalibard, F., Mandard, J.C., et al, Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Cancer 1994; 73:2680–2686. 8194005
29. Forastiere, A.A., Orringer, M.B., Perez-Tamayo, C., et al, Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol 1993; 11:1118–1123. 8501498
30. Smithers, B.M., Devitt, P., Jamieson, G.G., et al, A combined modality approach to the management of oesophageal cancer. Eur J Surg Oncol 1997; 23:219–223. 9236895
31. Vogel, S.B., Mendenhall, W.M., Sombeck, M.D., et al, Downstaging of esophageal cancer after preoperative radiation and chemotherapy. Ann Surg 1995; 221:685–695. 7794073
32. Bates, B.A., Detterbeck, F.C., Bernard, S.A., et al, Concurrent radiation therapy and chemotherapy followed by esophagectomy for localized esophageal carcinoma. J Clin Oncol 1996; 14:156–163. 8558191
33. Triboulet, J.P., Amrouni, H., Guillem, P., et al, Long-term results of resected esophageal cancer with complete remission to pre-operative chemoradiation. Ann Chir 1998; 52:503–508. 9752498
34. Reynolds, J.V., Muldoon, C., Hollywood, D., et al, Long-term outcomes following neoadjuvant chemoradiotherapy for oesophageal cancer. Ann Surg 2007; 245:707–716. 17457163
35. Le Prise, E., Etienne, P.L., Meunier, B., et al, A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 1994; 73:1779–1784. 8137201
36. Apinop, C., Puttisak, P., Preecha, N., A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology 1994; 41:391–393. 7959579
37. Walsh, T.N., Noonan, N., Hollywood, D., et al, A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996; 335:462–467. 8672151
38. Urba, S., Orringer, M., Turrisi, A., et al. A randomized trial comparing surgery (S) to preoperative concomitant chemoradiation plus surgery in patients (pts) with resectable esophageal cancer (CA): updated analysis. Proc Am Soc Clin Oncol. 1997; 16:277.
39. Burmeister, B.H., Smithers, B.M., Fitzgerald, L., et al. A randomised phase III trial of preoperative chemoradiation followed by surgery (CR-S) versus surgery alone (S) for localized resectable cancer of the esophagus. Proc Am Soc Clin Oncol. 2002; 21:518.
40. Tepper, J., Krasna, M.J., Niedzwiecki, D., et al, Phase III trial of trimodality therapy with cisplatin; fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008; 26:1086–1092. 18309943
41. Urschel, J.D., Vasan, H., A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185(6):538–543. 12781882
42. van Hagen, P., Hulshof, M.C.C.M., Lanschot, J.J.B., et al, Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366:2074–2084. 22646630
43. Gebski, V., Burmeister, B., Foo, K., et al, Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007; 8:226–234. 17329193
44. Khan, O.A., Cruttenden-Wood, D., Toh, S.K., Is an involved circumferential resection margin following oesphagectomy for cancer an important prognostic indicator? Interact Cardiovasc Thorac Surg 2010; 11:645–648. 20736225
45. Stahl, M., Walz, M.K., Stuschke, M., et al, Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009; 27:851–856. 19139439
46. Earlam, R., Cunha-Melo, J.R., Oesophageal squamous cell carcinoma I. A critical review of radiotherapy. Br J Surg 1980; 67:457–461. 6158354
47. Sykes, A.J., Burt, P.A., Slevin, N.J., et al, Radical radiotherapy for carcinoma of the oesophagus: an effective alternative to surgery. Radiother Oncol 1998; 48:15–21. 9756167
48. Kawashima, M., Kagami, Y., Toita, T., et al, Prospective trial of radiotherapy for patients 80 years of age or older with squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 2006; 64:1112–1121. 16376491
49. Araujo, C.M., Souhami, L., Gil, R.A., et al, A randomized trial comparing radiation therapy versus concomitant radiation therapy and chemotherapy in carcinoma of the thoracic esophagus. Cancer. 1991;67(9):2258–2261. 1707338
50. Coia, L.R., Engstrom, P.F., Paul, A.R., et al, Long-term results of infusional 5-FU, mitomycin-C, and radiation as primary management of esophageal cancer. Int J Radiat Oncol Biol Phys 1991; 20:29–36. 1704362
51. Al-Sarraf, M., Martz, K., Herskovic, A., et al, Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an Intergroup study. J Clin Oncol 1997; 15:277–284. 8996153
52. Minsky, B.D., Neuberg, D., Kelsen, D.P., et al, Neoadjuvant chemotherapy plus high-dose radiation for squamous cell carcinoma of the esophagus: a preliminary analysis of the phase II Intergroup Trial 0122. J Clin Oncol. 1996;14(1):149–155. 8558190
53. Gaspar, L.E., Qian, C., Kocha, W.I., et al, A phase I/II study of external beam radiation, brachytherapy and concurrent chemotherapy in localized cancer of the esophagus (RTOG 92-07): preliminary toxicity report. Int J Radiat Oncol Biol Phys. 1997;37(3):593–599. 9112458
54. Coia, L.R., Soffen, E.M., Schultheiss, T.E., et al, Swallowing function in patients with esophageal cancer treated with concurrent radiation and chemotherapy. Cancer 1993; 71:281–286. 8422619
55. O’Rourke, I.C., Tiver, K., Bull, C., et al, Swallowing performance after radiation therapy for carcinoma of the esophagus. Cancer 1988; 61:2022–2026. 2452006
56. Ribiero, U., Finklestein, S.D., Safatle-Ribiero, A., et al, P53 sequence predicts treatment response and outcome of patients with esophageal carcinoma. Cancer 1998; 83:7–18. 9655287
57. Yamamoto, M., Tsujinaka, T., Shiozaki, H., et al, Metallothionein expression correlates with the pathological response of patients with esophageal cancer undergoing preoperative chemoradiation therapy. Oncology 1999; 56:332–337. 10343199
58. Beardsmore, D.M., Verbeke, C.S., Davies, C.L., et al, Apoptotic and proliferative indexes in esophageal cancer: predictors of response to neoadjuvant therapy apoptosis and proliferation in esophageal cancer. J Gastrointest Surg 2003; 7:77–87. 12559188
59. Jones, D.R., Parker, L.A., Detterbeck, F.C., et al, Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer 1999; 85:1026–1032. 10091784
60. Lim, J.T.W., Truong, P.T., Berthelet, E., et al, Endoscopic response predicts for survival and organ preservation after primary chemoradiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2003; 57:1328–1335. 14630270
61. Giovannini, M., Seitz, J.F., Thomas, P., et al, Endoscopic ultrasonography for assessment of the response to combined radiation therapy and chemotherapy in patients with esophageal cancer. Endoscopy 1997; 29:4–9. 9083729
62. Mallery, S., DeCamp, M., Bueno, R., et al, Pretreatment staging by endoscopic ultrasonography does not predict complete response to neoadjuvant chemoradiation in patients with esophageal carcinoma. Cancer 1999; 86:764–769. 10463973
63. Wieder, H.A., Brucher, B., Zimmermann, F., et al, Time course of tumour metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol 2004; 22:900–908. 14990646
64. Raoul, J.L., Le Prise, E., Meunier, B., et al, Neoadjuvant chemotherapy and hyperfractionated radiotherapy with concurrent low-dose chemotherapy for squamous cell esophageal carcinoma. Int J Radiat Biol Phys 1998; 42:29–34. 9747816
65. Crellin, A.M., Sebag-Montefiore, D., Martin, I., et al. Preoperative chemotherapy and radiotherapy, plus excision (CARE): a phase II study in esophageal cancer. Proc Am Soc Clin Oncol. 2000; 19:A1128.
66. Denham, J.W., Steigler, A., Kilmurray, J., et al, Relapse patterns after chemo-radiation for carcinoma of the oesophagus. Clin Oncol 2003; 15:98–108. 20035424
67. Thomas, E., Crellin, A., Harris, K., et al, The role of endoscopic ultrasound (EUS) in planning radiotherapy target volumes for oesophageal cancer. Radiother Oncol 2004; 73:149–151. 15542161
68. Leong, T., Everitt, C., Yuen, K., et al, A prospective study to evaluate the impact of FDG-PET on CT-based radiotherapy treatment planning for oesophageal cancer. Radiother Oncol 2006; 78:254–261. 16545881
69. Bonner, J.A., Harari, P.M., Giralt, J.L., et al, Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354:567–578. 16467544
70. Chan, A., Wong, A., Is combined chemotherapy and radiation therapy equally effective as surgical resection in localized esophageal carcinoma? Int J Radiat Oncol Biol Phys. 1999;45(2):265–270. 10487544
71. Murakami, M., Kuroda, Y., Nakajima, T., et al, Comparison between chemoradiation protocol intended for organ preservation and conventional surgery for clinical T1–T2 esophageal carcinoma. Int J Radiat Oncol Biol Phys. 1999;45(2):277–284. 10487546
72. Wilson, K.S., Lim, J.T., Primary chemotherapy–radiotherapy and selective oesophagectomy for oesophageal cancer: goal of cure with organ preservation. Radiother Oncol 2000; 54:129–134. 10699475
73. Bedenne, L., Michel, P., Bouche, O., et al, Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007; 25:1160–1168. 17401004
74. Stahl, M., Wilke, H., Lehmann, N., et al. Long-term results of a phase III study investigating chemoradiation with and without surgery in locally advanced squamous cell carcinoma (LA-SCC) of the esophagus. J Clin Oncol. 26(Suppl.), 2008. [Abstr. 4530].
75. Gardner-Thorpe, J., Hardwick, R., Dwerryhouse, S.J., Salvage oesophagectomy after local failure of definitive chemoradiotherapy. Br J Surg 2007; 94:1059–1066. 17657720
76. Hudson, E., Powell, J., Mukherjee, S., et al, Small cell oesophageal carcinoma: an institutional experience and review of the literature. Br J Cancer 2007; 96:708–711. 17299393
77. Ku, G.Y., Minsky, B.D., Rusch, V.W., et al, Small-cell carcinoma of the esophagus and gastroesophageal junction: review of the Memorial Sloan-Kettering experience. Ann Oncol 2008; 19:533–537. 17947223
78. Hermans, J., Bonenkamp, J.J., Ban, M.C., et al, Adjuvant therapy after curative resection for gastric cancer: a meta-analysis of randomized trials. J Clin Oncol 1993; 11:1441–1447. 8336183
79. Earle, C.C., Maroun, J.A., Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35(7):1059–1064. 10533448
80. Mari, E., Floriani, I., Tinazzi, A., et al, Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi della Apparato Digerente). Ann Oncol. 2000;11(7):837–843. 10997811
81. Ychou, M., Boige, V., Pignon, J.-P., et al, Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. Clin Oncol. 2011;29(13):1715–1721. 21444866
82. Hagiwara, A., Takahashi, T., Kojima, O., et al, Prophylaxis with carbon-adsorbed mitomycin against peritoneal recurrence of gastric cancer. Lancet. 1992;339(8794):629–631. 1347336
83. Rosen, H.R., Jatzko, G., Repse, S., et al, Adjuvant intraperitoneal chemotherapy with carbon-adsorbed mitomycin in patients with gastric cancer: results of a randomized multicenter trial of the Austrian Working Group for Surgical Oncology. J Clin Oncol. 1998;16(8):2733–2738. 9704725
84. Hallissey, M.T., Dunn, J.A., Ward, L.C., et al, The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet. 1994;343(8909):1309–1312. 7910321
85. Macdonald, J.S., Smalley, S.R., Benedetti, J., et al, Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345:725–730. 11547741
86. Hundahl, S.A., Macdonald, J.S., Benedetti, J., Southwest Oncology Group and the Gastric Intergroup, Surgical treatment variation in a prospective randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment for the. Ann Surg Oncol. 2002;9(3):278–286. 11923135
87. Bleiberg, H., Jacob, J.H., Bedenne, L., et al. A randomized phase II trial of 5-fluorouracil (5FU) and cisplatin (DDP) versus DDP alone in advanced esophageal cancer. Proc Soc Clin Oncol. 1991; 10:A447.
88. Zhang, X., Shen, L., Li, J., et al, A phase II trial of paclitaxel and cisplatin in patients with advanced squamous-cell carcinoma of the esophagus. Am J Clin Oncol 2008; 31:29–33. 18376224
89. Murad, A., Santiago, F., Petroianu, A., et al, Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993; 72:37–41. 8508427
90. Pyrhonen, S., Kuitunen, T., Nvandoto, P., et al, Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995; 71:587–591. 7533517
91. Glimelius, B., Ekstrom, K., Hoffman, K., et al, Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997; 8:163–168. 9093725
92. Macdonald, J., Schein, P., Woolley, P., et al, 5-Fluorouracil, doxorubicin and mitomycin (FAM) combination chemotherapy for advanced gastric cancer. Ann Intern Med 1980; 93:533–536. 7436184
93. Cullinan, S., Moertel, C., Fleming, T., et al, A comparison of three chemotherapeutic regimens in the treatment of advanced pancreatic and gastric cancer. JAMA 1985; 253:2061–2067. 2579257
94. Klein, H.O., Long term results with FAMTX (5-fluorouracil, adriamycin, methotrexate) in advanced gastric cancer. Cancer Res. 1989;9(4):1025–1026. 2817784
95. Wils, J.A., Klein, H.O., Wegener, D.J.T., et al, Sequential high-dose methotrexate and fluorouracil combined with doxorubicin: a step ahead in the treatment of advanced gastric cancer. A trial of the European Organisation for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol 1991; 9:827. 2016625
96. Kelsen, D., Atiq, O., Saltz, L., et al, FAMTX versus etoposide, doxorubicin and cisplatin: a random assignment in gastric cancer. J Clin Oncol 1992; 10:541–548. 1548519
97. Vanhoefer, U., Rougier, P., Wilke, H., et al, Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol 2000; 18:2648–2657. 10894863
98. Webb, A., Cunningham, D., Scarffe, J.H., et al, Randomized trial comparing epirubicin, cisplatin and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997; 15:261–267. 8996151
99. Seymour, M.T., Dent, J.T., Papamichael, D., et al, Epirubicin, cisplatin and oral UFT with leucovorin (ECU): a phase I–II study in patients with advanced upper gastrointestinal tract cancer. Ann Oncol. 1999;10(11):1329–1333. 10631461
100. Van Cutsem, E., Moiseyenko, V., Tjulandin, S., et al, Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol 2006; 24:4991–4997. 17075117
101. Dragovich, T., McCoy, S., Fenoglio-Preiser, C., et al, Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol 2006; 24:4922–4927. 17050876
102. Bang, Y.-J., Van Cutsem, E., Feyereislova, A., et al, Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376:687–697. 20728210
103. Chau, I., Norman, A., Cunningham, D., et al, Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer – pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol 2004; 22:2395–2403. 15197201
104. Dawes, P.J.D.K., Clague, M.B., Dean, E.M., Combined external beam and intracavitary radiotherapy for carcinoma of the oesophagus. Brachytherapy 2. Proceedings of the 5th International Selectron User’s Meeting 1988. Nucleotron International, 1989:442–444.
105. Pagliero, K.M., Rowlands, C.G., The place of brachytherapy in the treatment of carcinoma of the oesophagus. Brachytherapy HDR and LDR. Proceedings of a brachytherapy meeting: remote afterloading; state of the art. Nucleotron Corporation, 1990:44–51.
106. Sur, R.K., Donde, B., Levin, V.C., et al, Fractionated high dose rate brachytherapy in palliation of advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;40(2):447–453. 9457834
107. Gaspar, L.E., Nag, S., Hersokic, A., et al, American Brachytherapy Society (ABS) consensus guidelines for brachytherapy of esophageal cancer. Int J Radiat Oncol Biol Phys. 1997;38(1):127–132. 9212013
108. Homs, M.Y., Steyerberg, E.W., Eijkenboom, W.M., et al, Single-dose brachytherapy verus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomized trial. Lancet 2004; 364:1497–1504. 15500894