Chapter 30 Radionuclide Imaging in Heart Failure
INTRODUCTION
The epidemic of heart failure affects 5 million people in the United States, with 500,000 new diagnoses made every year.1 Physicians treating heart failure seek answers to several important clinical questions that impact on management:
ESTABLISHED USES OF RADIONUCLIDE IMAGING IN HEART FAILURE
Determining Heart Failure Etiology
Data from epidemiologic studies and clinical trials suggest that 60% to 70% of patients with heart failure have coronary artery disease (CAD). These data underscore the fact that CAD has now overtaken hypertension as the most common cause of heart failure. In a review of 13 randomized, multicenter clinical trials of patients with established heart failure reported in the New England Journal of Medicine, Gheorghiade and Bonow reported that 68% of patients had CAD.2 It is likely that these data underestimate the true prevalence of CAD in heart failure, since patients presenting with LV dysfunction due to acute MI were generally excluded from these trials. It is also important to note that the majority of these reports pertained to patients with chronic heart failure. However, adjudicating etiology is most relevant in patients with new-onset heart failure in whom the detection of significant CAD has important therapeutic and prognostic implications.3
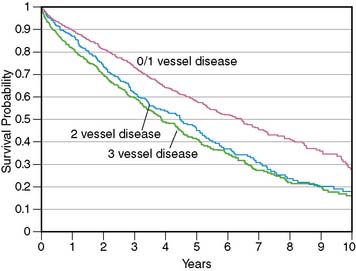
More important, in these clinical trials, an “ischemic” etiology of heart failure was generally attributed to patients with any significant epicardial CAD (usually defined as = 50% diameter stenosis). However, in patients with heart failure and limited-extent CAD (e.g., single-vessel disease) and severe LV dysfunction, the underlying pathophysiology is unlikely to be ischemic LV dysfunction but rather cardiomyopathy of other etiologies coexisting with minor CAD. This distinction between heart failure patients with LV dysfunction resulting from extensive CAD and those with nonischemic LV dysfunction coexisting with limited-extent CAD is prognostically important, as was demonstrated by Felker and colleagues, who followed up 1921 symptomatic heart failure patients enrolled in the Duke database.4 Patients were categorized as having ischemic and nonischemic cardiomyopathy based on two separate definitions. In the traditional definition, heart failure patients with any epicardial CAD equal to 75% were classified as having ischemic cardiomyopathy. In the modified definition, an ischemic etiology was attributed only to patients with extensive CAD, defined as significant left main or proximal left anterior descending coronary artery stenosis, three-vessel disease, or single-vessel disease with prior MI or coronary revascularization, that is, patients with extensive CAD more likely to be etiologically related to heart failure. Therefore, in the modified definition, heart failure patients with single-vessel disease without prior MI or coronary revascularization were categorized as having nonischemic cardiomyopathy. At the end of a median follow-up period of 3.1 years (interquartile range 1.0 to 6.2 years), the authors reported that the modified definition of ischemic cardiomyopathy was a more powerful discriminator of prognosis, and the prognosis of heart failure patients with single-vessel CAD without prior MI or coronary revascularization was identical to that of heart failure patients without CAD (Fig. 30-1). This important observation indicates that the standard definition of ischemic cardiomyopathy applied to most heart failure studies (≥50% diameter stenosis in any coronary artery) may be inadequate for the characterization of heart failure etiology and risk, since it does not enable a distinction between extensive CAD that is likely to be the etiology of heart failure and limited-extent CAD coexisting with heart failure of other etiologies. Making this distinction is a critical step in the evaluation of heart failure patients, since the presence of extensive CAD implies potential benefit from coronary revascularization.5
Current practice guidelines mandate coronary angiography in heart failure patients with angina.6 However, up to 60% of patients with ischemic LV dysfunction do not have angina,7 and the guidelines do not offer firm recommendations for the optimal initial evaluation strategy of these patients.6,8 Given this lack of a unified approach and the potential adverse consequences of not identifying extensive CAD, most heart failure patients are likely to undergo at least one coronary angiogram to exclude CAD, especially after the initial onset of symptoms. Many patients end up having repeat coronary angiograms when clinical symptoms worsen or left ventricular ejection fraction (LVEF) deteriorates.
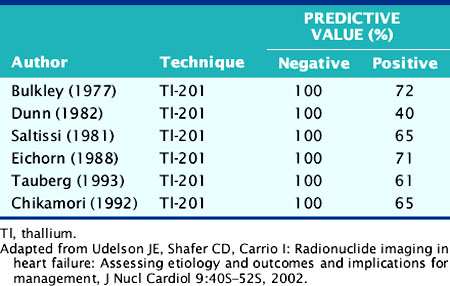
The role of myocardial perfusion imaging (MPI) to diagnose CAD noninvasively in patients with heart failure has been investigated.9 Many of these studies predated contemporary MPI methodology, for example, using nongated, planar thallium-(201Tl) scintigraphy, and yet showed a near-perfect sensitivity and negative predictive value (NPV) (Table 30-1). A more recent study using gated technetium-99m (99mTc) sestamibi single-photon emission computed tomographic (SPECT) MPI incorporating information on both perfusion and regional LV function demonstrated a high sensitivity of 94% but specificity of only 32%.10 Thus, these studies performed in patients with chronic heart failure suggest that MPI may be used to exclude significant CAD in heart failure patients with a high degree of accuracy, but because of the poor specificity of the technique, many heart failure patients without CAD will undergo coronary angiography based on falsely positive MPI results.
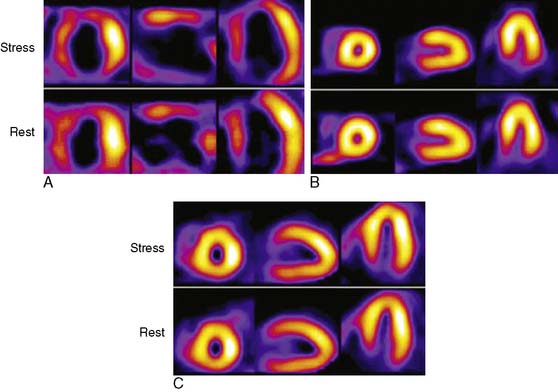
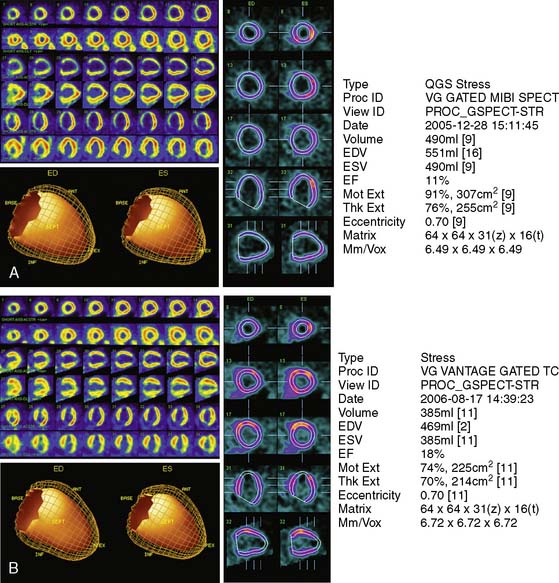
The Investigation of Myocardial Gated SPECT Imaging as Initial Strategy (IMAGING) in Heart Failure study prospectively explored the role of MPI in 201 patients presenting with new-onset heart failure, recruited from 14 hospitals in the United States and United Kingdom.11 Patients underwent exercise or pharmacologic stress MPI with 99mTc sestamibi gated SPECT imaging during or within 2 weeks of the index hospitalization for heart failure. The diagnosis of heart failure was based on the Framingham criteria.12 A subset of patients (37%) underwent coronary angiography based on clinical indications, and the performance characteristics of MPI for CAD diagnosis were derived from this angiographic cohort. A positive MPI was defined by a summed stress score greater than 3 (the sum of the individual segmental perfusion scores on stress MPI, using a 17-segment LV model and a 5-point semiquantitative perfusion score ranging from 0 = normal to 4 = absent perfusion), as validated previously.13 For the diagnosis of any angiographic CAD (= 70% diameter stenosis, prevalence 51%), the sensitivity, specificity, positive predictive value (PPV), and NPV of MPI were 82%, 57%, 67%, and 75%, respectively. For the diagnosis of more extensive CAD, indicating the presence of ischemic LV dysfunction (= 70% stenosis in the left main or proximal left anterior descending coronary artery, = 70% stenosis in two or more major epicardial coronary arteries, or any stenosis = 70% with prior MI or coronary revascularization, prevalence 36%), the corresponding values were 96%, 56%, 55%, and 95%, respectively (Table 30-2). Thus, the principal finding of this study in patients with new-onset heart failure was concordant with prior studies in patients with established heart failure in that MPI has a high NPV for excluding an ischemic etiology for LV dysfunction. Several limitations of this study should be noted. Although prospective and the first study to explore MPI in patients with new-onset heart failure, this was an observational cohort of nonconsecutive patients. Furthermore, coronary angiography was performed in only 37% of patients and was driven by clinical indications, including in some cases, the results of the MPI. Therefore the results were subject to verification bias, which is known to falsely lower specificity and overestimate sensitivity. Selection bias may have also been operative in patients with new-onset heart failure and known CAD, who are sometimes referred directly for coronary angiography. Thus, the applicability of these results to all patients with new-onset heart failure needs further confirmation. Nevertheless, this study in patients with new-onset heart failure, and prior studies in patients with chronic disease, indicate that heart failure patients with a normal MPI are highly unlikely to have significant, etiologically related CAD. Using MPI as an early investigative strategy might avoid the routine use of coronary angiography in some patients. Figure 30-2 shows characteristic patterns of perfusion and function in heart failure patients. Patients with cardiomyopathy of nonischemic etiologies generally have normal or near-normal perfusion. In these patients, coronary angiography can be safely avoided.
Table 30-2 Performance Characteristics of Gated SPECT 99mTc Sestamibi for CAD Diagnosis in Patients with New-Onset Heart Failure from the IMAGING in Heart Failure Study103
| CAD definition | Any CAD: ≥70% stenosis in any coronary artery | Extensive CAD: stenosis ≥ 70% in the LM or proximal LAD, ≥ 70% in ≥ two major epicardial coronary arteries, or any stenosis ≥ 70% with a prior MI or coronary revascularization |
| CAD prevalence by angiography | 51% (n = 38) | 36% (n = 27) |
| Sensitivity % (95% CI) | 82 (66-92) | 96 (81-99) |
| Specificity % (95% CI) | 57 (40-72) | 56 (41-71) |
| PPV % | 67 | 55 |
| NPV % | 75 | 96 |
Criteria for positive SPECT was summed stress score > 3.
CAD, coronary artery disease; LAD, left anterior descending coronary artery; LM, left main coronary artery; MPI, myocardial perfusion imaging; NPV, negative predictive value; PPV, positive predictive value; SPECT, single-photon emission computed tomography; Tc, technetium.
From Soman P, Lahiri A, Mieres JH, et al: Etiology and pathophysiology of new-onset heart failure: Evaluation by myocardial perfusion imaging, J Nucl Cardiol 16:82?91, 2009.
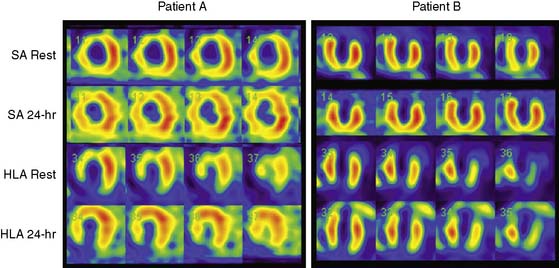
The predominant cause of false-positive MI is soft-tissue attenuation, although pathophysiologic phenomena such as myocardial fibrosis and endothelial dysfunction produce abnormal MPI in some patients with unobstructed epicardial coronary arteries.14,15 Although quantitative attenuation-correction techniques have been shown in clinical trials to improve diagnostic specificity of SPECT MPI, this approach has not been tested specifically in patients with heart failure.16 Given the propensity of attenuation artifacts in patients with a dilated LV, attenuation correction is likely to favorably impact specificity in this population. Figure 30-3 shows the gated SPECT images from a patient in whom the attenuation correction formed using a gadolinium-153 transmission scan was useful in correctly identified nonischemic LV dysfunction.
With the advent of cardiac CT, small, single-center studies have explored the utility of coronary calcium scoring and CT coronary angiography for the diagnosis of CAD in heart failure patients.17,18 Like MPI, coronary calcium scoring and CT coronary angiography have good NPV for this application. In the future, combined SPECT/CT imaging may improve diagnostic accuracy.
Measuring Left Ventricular (Dys)Function (See Chapters 12 and 13)
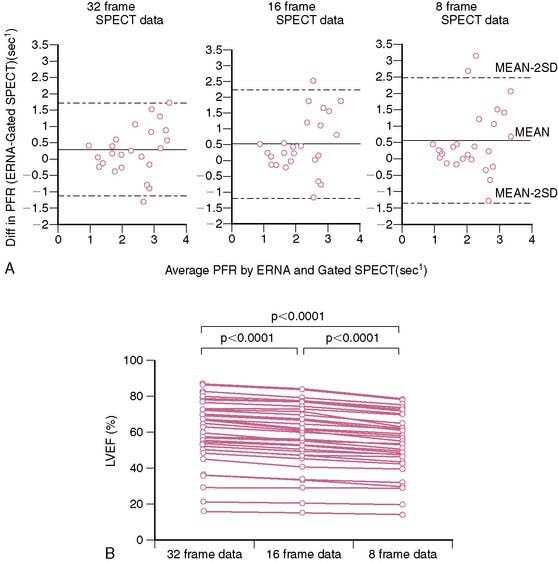
Radionuclide techniques are well validated and widely applied for the assessment of LV function. While radionuclide ventriculography (RVG) was the traditional radionuclide technique used to determine LV systolic and diastolic function, this method has been largely supplanted by gated SPECT imaging, which is extensively used for myocardial perfusion assessment and provides simultaneous information on LV systolic function. Sixteen-frame ECG gating provides an accurate assessment of LV systolic function, but it may not provide a sufficiently high-fidelity time-volume curve for diastolic function assessment (Fig. 30-4).19 Assessment of diastolic function can be performed with 32- or 64-frame gating, which can be impractical for routine MPI because of the long scanning time required for collection of adequate counts.19,20
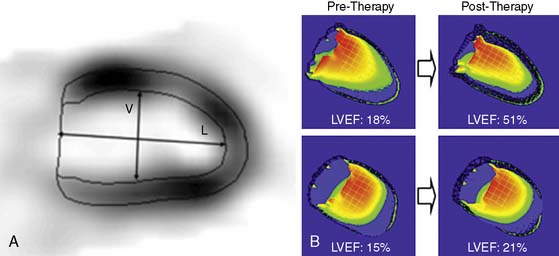
Assessment of ejection fraction by gated SPECT imaging has been validated against other established imaging modalities, including echocardiography, radionuclide ventriculography, and newer techniques with high spatial resolution, such as magnetic resonance imaging, with excellent correlation.20 However, it must be noted that the normal limits of ejection fraction vary depending on the modality used.21,22 This intermodality variability is exaggerated in patients with LV systolic dysfunction,22 an observation that has been overlooked even in the design of major clinical trials requiring precise measurement of LV function, such as the implantable cardioverter-defibrillator trials in heart failure patients. While these trials recruited patients based on specific ejection fraction criteria, a modality of ejection fraction measurement was usually not specified.23,24 Normal limits also vary based on gated SPECT methodology, with 8-frame gating resulting in lower ejection fraction values that correlate less well with RVG compared to 16-frame gating19,25 and with the software used for quantification of ejection fraction and volumes (see Fig. 30-4).26 In some laboratories including the author’s, the lower limit of normality for ejection fraction measurement by gated SPECT is set variably depending on the frame rate used for gating (45% and 50% for 8-frame and 16-frame gating, respectively). Compared to two-dimensional echocardiography, which is also widely used for assessment of LV systolic function, a significant advantage of gated SPECT estimation of LV systolic function is its fully automated application, resulting in high reproducibility. Studies indicate that the variability in serial estimations of LV ejection fraction from gated rest 99mTc SPECT scans is approximately ±5% (Fig. 30-5).27,28 Serial gated SPECT imaging can be effectively used to assess changes in perfusion and function following therapeutic interventions (Fig. 30-6A and B).
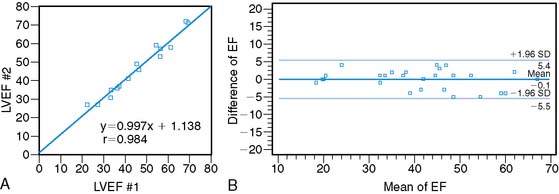
Figure 30-5 Reproducibility of left ventricular ejection fraction (LVEF) on serial resting gated SPECT imaging. A, Data from Johnson et al.27 on 15 patients who underwent serial rest thallium-201 imaging. LVEF obtained from the first and second studies are plotted on the x– and y-axes, respectively. The regression line is not significantly different from the line of identity, indicating close correlation between the two measurements. B, Data from Hyun et al.28 on 26 patients, showing that the 2-SD limits of difference in two serial measurements of LVEF by technetium-99m gated SPECT imaging assessed by Bland-Altman plotting was ± 5.5%. In this study, the variability between serial gated SPECT thallium-201 imaging was higher.
Predicting Benefit from Coronary Revascularization: Assessment of Myocardial Viability (See Chapters 37 and 38)
In patients with ischemic LV dysfunction, the assessment of myocardial viability is a critical step in management planning. On one hand, patients with severe LV systolic dysfunction undergoing coronary bypass grafting are at high risk for perioperative mortality.29 On the other, the presence of significant amounts of residual myocardial viability portends the potential for improvement in symptoms,30–32 regional LV function,33,34 global LV function,35,36 and prognosis5,37 following coronary revascularization. It is important to note that while the use of functional recovery is a convenient standard for viability studies, it may not capture the full benefit of revascularization, which may confer other important benefits such as attenuation of progressive remodeling and prevention of arrhythmia independently of functional recovery.38–40 LV function may also continue to improve for several months following revascularization, so a single assessment, if not timed appropriately, may underestimate the full extent of functional recovery.39 Finally, resting regional function is primarily determined by endocardial thickening, which is unlikely to improve in patients who have suffered a subendocardial MI despite preserved myocardial viability.41–43 A more important criterion for assessing the usefulness of noninvasive viability testing should be whether it can drive therapeutic decisions that ultimately improve patient outcome. While the field of viability testing is somewhat limited by the lack of randomized control studies, a meta-analysis by Allman and colleagues5 of 24 studies of viability testing with MPI or dobutamine echocardiography that reported on survival after coronary revascularization provides important insights. In patients with predominantly viable myocardium, follow-up on medical therapy was associated with a 16% annual mortality. Similar patients who were revascularized experienced an annual mortality of only 3.2%—that is, an 80% reduction in mortality. In contrast, the choice of medical therapy or revascularization had no significant impact on annual mortality (7.7% versus 6.2%, respectively) in patients with predominantly nonviable myocardium. Despite the known limitations of pooling data from observational cohort studies, which can bring into play selection biases that cannot be evaluated when meta-analyzing published literature, these data provide a strong signal that the presence of residual myocardial viability in patients with LV systolic dysfunction is a marker of very high risk without revascularization.
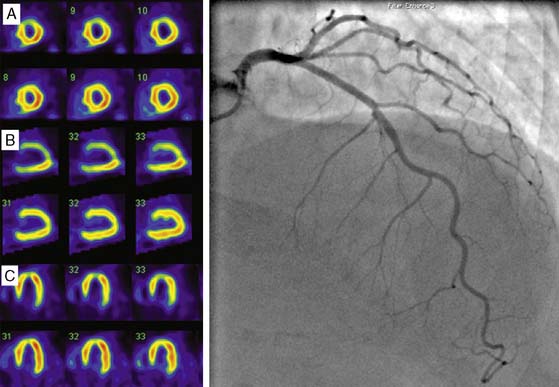
Clinical studies suggest that residual myocardial viability is prevalent among patients with ischemic LV dysfunction.44,45 For example, in the CHRISTMAS trial of 489 patients with chronic heart failure due to ischemic LV dysfunction (LVEF 29 ± 11%), 79% had viable myocardium demonstrated by 99mTc sestamibi SPECT imaging.45 It must be noted that viability exists as a continuum, ranging from patients with extensive scar tissue and minimal residual viability, to those with minimal scar tissue and predominantly viable but dysfunctional myocardium due to varying combinations of hibernating and repetitive stunning.46,47 Patient outcome is dependent on not only the presence but also the extent of viability, and a critical threshold mass of viable myocardium may be necessary for functional recovery and prognostic benefit to occur from revascularization.48 Therefore, while several clinical and laboratory parameters, including anginal symptoms, absence of Q waves on the electrocardiogram, and absence of thinning and akinesis on echocardiography, indicate the presence of some viability, a systematic assessment of the degree and extent of viability is often indicated for management planning and prognostication. Techniques used for viability assessment are based on the demonstration of preserved myocyte metabolism (F-18 fluorodeoxyglucose positron emission tomography [PET]), cellular integrity (SPECT with 201Tl or 99mTc ligands), contractile reserve (dobutamine echocardiography), microvascular integrity (myocardial contrast echocardiography), or the absence of scar tissue (gadolinium-enhanced cardiac magnetic resonance [CMR] imaging). The use of PET and SPECT for viability assessment is backed by a substantial literature, with SPECT having the advantages of wider availability and relative ease of use, and PET having a slightly higher overall accuracy.33 The property of redistribution confers an advantage to 201Tl compared with 99mTc agents for viability assessment in dysfunctional myocardial segments supplied by a critically stenosed coronary artery. Here the continued uptake of 201Tl over time results in higher tracer uptake on the delayed compared to early scan, thus signaling the presence of viable myocardium (Fig. 30-7).49,50 However, when used with nitrate enhancement and quantitative interpretation, 99mTc-sestamibi SPECT has comparable accuracy for viability assessment.51,52 When compared with other imaging modalities for viability assessment, radionuclide techniques have comparable predictive accuracy for functional recovery.33 Comparative studies also indicate that slight differences in sensitivity and specificity among these modalities may not impact on management decision making in a clinically meaningful way.53 Furthermore, other information that can be derived from PET and SPECT imaging, such as the degree of remodeling, may interact with the amount of residual viability to determine outcome after revascularization.54
EVOLVING APPLICATIONS OF RADIONUCLIDE TECHNIQUES IN HEART FAILURE
Targeted Molecular Imaging and Imaging Myocardial Metabolism (See Chapters 40 and 41)
The most recent American College of Cardiology/American Heart Association guidelines for the diagnosis and management of heart failure includes a preclinical class (stage A) consisting of patients with conditions that are associated with a high likelihood of the development of heart failure.6 It is established that molecular mechanisms mediating heart failure are already operative at this stage,55 and imaging these mechanisms may facilitate the important goals of enhancing our understanding of heart failure pathophysiology and testing early therapy to halt disease progression. Molecular and metabolic imaging at later stages in the evolution of heart failure helps identify specific processes that may predominate in individual patients or patient groups and explain the heterogeneity in response to therapy (e.g., β-blockers). Such an approach may in future facilitate personalized medicine for heart failure. Both SPECT- and PET-based imaging can be potentially applied to a wide array of molecular targets. PET has the advantage of better spatial resolution and quantification.56 The areas of heart failure where there are substantial ongoing research efforts directed to the application of molecular and metabolic imaging are (1) the imaging of cellular mechanisms underlying heart failure and (2) imaging of regenerative cell therapy.
Imaging Cellular Mechanisms in Heart Failure
Apoptosis: The imaging of programmed cell death (apoptosis) is one area where a substantial amount of animal work has already led to encouraging preliminary clinical data. The current approach uses radiolabeled annexin-V, a phosphatidyl-binding protein, to target phosphatidylserine, which is an intracellular cell-membrane phospholipid that is translocated to the cell surface during apoptosis. Small clinical studies using 99mTc annexin-V SPECT imaging have successfully imaged apoptosis in humans.57 Potential applications in heart failure include imaging myocarditis, chemotherapy-induced cardiomyopathy, and cardiac transplant rejection.58
Renin-Angiotensin System (RAS): 18F-Captopril and 18F-lisinopril have been used to image the activity of the angiotensin-converting enzyme (ACE), which is increased in heart failure and modulates fibrosis, inflammation, and apoptosis through angiotensin II. Many of the effects of the RAS are paracrine, by components produced locally in the heart, and 18F-lisinopril may have an advantage in that it has a higher affinity for tissue-bound ACE than 18F-captopril.55 An 99mTc-labeled angiotensin II type I receptor ligand is also being studied.55
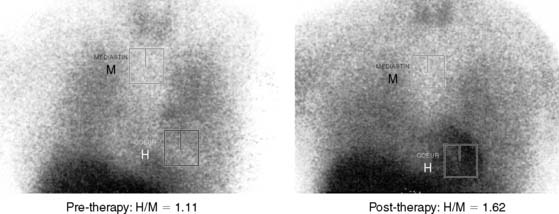
Myocardial Sympathetic Neuronal Activity: The heart is richly supplied with sympathetic nerves that are intricately involved in the control of heart rate and contractility. Sympathetic dysregulation is a prominent component of the pathophysiology of heart failure and has established prognostic significance.59,60 The use of 123I-metaiodobenzylguanidine (MIBG) for scintigraphic imaging of the myocardium was first reported by Wieland and colleagues in 1981.61 MIBG is an analogue of norepinephrine with identical mechanisms of uptake, storage, and release. However, unlike norepinephrine, it is retained in the sympathetic nerve endings unbound and largely unmetabolized, and it is therefore useful for scintigraphic imaging.62 Current protocols used for MIBG imaging are designed to obtain comprehensive information about the functional activation state and anatomic distribution of the myocardial sympathetic innervation. Early (15-minute) and late (4-hour) anterior planar and SPECT imaging are performed. The MIBG uptake of the myocardium is represented as the heart-to-mediastinum (H/M) ratio on planar imaging. The H/M ratio on the early planar image is a reflection of the degree of uptake of MIBG by the myocardial sympathetic neurons. The H/M ratio on the delayed images is a reflection of the washout rate of MIBG, calculated as the difference in the background-subtracted (heart counts − mediastinum counts) myocardial counts between the early and late planar images, expressed as a percentage of the counts in the early image (Fig. 30-8). The washout rate is an index of the state of activation of the myocardial sympathetic neurons. A high washout rate (and thus, a lower H/M ratio on the delayed image) is indicative of down-regulation of the myocardial sympathetic neurons, commonly seen in patients with heart failure. The degree of reduction in the H/M ratio is proportional to the degree of heart failure, varies directly with changes in LV systolic function (Fig. 30-9),62 and therefore can be used to follow response to therapy. Attempts to predict response to therapy, however, have produced mixed results.62 The H/M ratio and washout rate have been established as strong predictors of prognosis in heart failure, and in one study by Merlet et al., surpassed LV ejection fraction in this regard.63 An improvement in MIBG uptake with therapy has also been associated with a favorable prognosis in heart failure patients.64
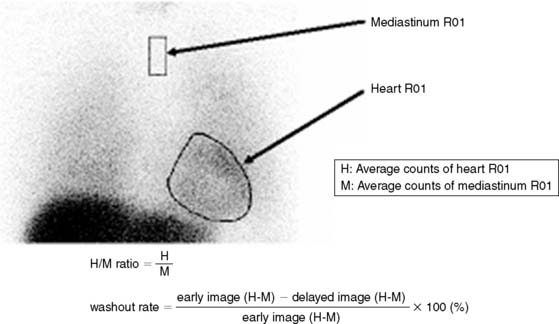
Figure 30-8 Method of calculating the heart-to-mediastinum (H/M) ratio and washout rate on MIBG planar images.
(From Yamashina S, Yamazaki Ji: Neuronal imaging using SPECT, Eur J Nucl Med Mol Imaging 34:939–950, 2007.)
Imaging Myocardial Metabolism: Ischemia shifts myocardial metabolism from long-chain fatty acids, which is the predominant substrate under aerobic conditions, to glucose.65 This finding prompted the investigation of several radionuclide-labeled fatty acid compounds for use in myocardial imaging. The uptake and washout kinetics of these agents are reflective of distinct pathways of myocardial fatty acid metabolism that are altered in acute ischemia, chronic ischemia, and the different states of dysfunctional but viable myocardium. Initial studies used C-11 palmitate and PET imaging, with encouraging results.66,67 Subsequent efforts were directed at adapting these tracers and protocols for the more widely available SPECT cameras and obviating the need for cyclotron production, resulting in the introduction of I-123-labeled agents.
123I-Iodophenylpentadecanoic acid (IPPA) is a straight-chain fatty acid that is taken up by the myocardium in proportion to regional perfusion. Under nonischemic conditions, this tracer is rapidly metabolized and released, resulting in rapid washout kinetics.68 Suppressed metabolism in the presence of ischemia results in longer myocardial retention and a “redistribution” pattern on serial imaging.69 Qualitative and semiquantitative analysis of IPPA uptake and washout have been used in various clinical trials to successfully diagnose coronary stenosis,68 detect myocardial ischemia,70 determine myocardial viability,71–73 and predict recovery of regional LV dysfunction following revascularization.74 One study, for example, demonstrated superiority of this agent over rest-redistribution 201Tl imaging for viability detection.73 However, the rapid dynamics of straight-chain fatty acid uptake and metabolism is a critical problem for SPECT imaging.
Methyl-branching of the fatty acid chain protects against beta oxidation and considerably slows down washout from the myocardium (metabolic trapping).75 Of the methyl-branched fatty acid tracers, 123I-(p-iodophenyl)-3-(R,S)-methylpentadecanoic acid (BMIPP)76 has been extensively studied in Japan and Europe and more recently in the United States. Excellent-quality images with high heart-to-background ratios can be obtained 15 to 30 minutes after tracer administration.77 Because of the metabolic modulation, however, separate imaging with 201Tl or one of the 99mTc ligands is required for perfusion assessment.
The clinical utility of BMIPP imaging stems from the fact that abnormalities of fatty acid metabolism resulting from transient ischemia persist for prolonged periods.77,78 Therefore, a defect on BMIPP imaging is indicative of recent ischemia, even when perfusion has returned to normal (ischemic memory).78 Detection of ischemic memory has potential utility in many clinical situations, including assessment of chest pain in the emergency department, diagnosis of CAD without stress testing, and diagnosis of vasospastic angina. There is also potential utility for risk stratification of patients with high likelihood of CAD.79 In dysfunctional myocardium, a disproportionately greater decrease in BMIPP compared to a perfusion trace uptake likely represents recurrent ischemia with stunning and has been shown to correlate with preserved inotropic reserve,80 histologic evidence of viability,81 and postrevascularization recovery of function.80,82,83 On the other hand, a concordant severe reduction in both BMIPP uptake and perfusion indicates scar tissue.
Monitoring Regenerative Cell Therapy: Regenerative cell therapy using embryonic stem cells, bone marrow–derived stem cells, and endothelial progenitor cells is being investigated for heart failure. Such therapy, delivered by intracoronary, intramyocardial, or systemic venous injection, requires monitoring of the homing, engraftment, and survival of the injected cells. In addition to providing information on these aspects, radionuclide imaging has the advantage of being able to assess myocardial remodeling, regional myocardial perfusion and viability, and regional and global function, which can be used as measures of outcome in regenerative therapy. Two approaches have been used. Direct radiolabeling of cells enables monitoring of homing and engraftment. 111Indium oxine and 99mTc exametazime labeling have been used for SPECT imaging84 and F-18 fluorodeoxyglucose for PET imaging.85 X-ray CT can be used conjointly to enable localization.86 Disadvantages of this approach include persistence of radioactivity despite cell death, which may provide unreliable information regarding cell survival, and the fact that the duration of monitoring is limited by radioactive decay of the label.87 Reporter gene imaging overcomes some of these limitations of direct radiolabeling. In this process, a reporter gene that encodes for a protein product is transferred to regenerative cells in vitro before they are applied therapeutically. After engraftment, the cells produce the protein product, which is then detected by the intravenous injection of highly specific radiolabeled reporter probes, followed by imaging. This method ensures that the imaging signals detected are from viable cells capable of expressing the reporter gene. Furthermore, repeated imaging can be performed to monitor cell survival and activity.87
Assessing LV Remodeling: Shape Indices
The cellular and interstitial changes that underlie the phenomenon of LV remodeling are accompanied by a transformation of the normally ellipsoid LV into a more spherical structure. The degree of remodeling can be assessed by calculating a sphericity index, defined as the ratio of the largest long-axis and short-axis diameters of the LV,88 which has established utility in predicting response to therapy and prognosis in heart failure.38,88–90 The sphericity index has generally been calculated based on echocardiographic measurements, but Fukuchi and colleagues manually determined the sphericity index on gated SPECT imaging as the ratio of the LV long-axis diameter to the LV vertical diameter on the end-diastolic vertical long-axis frame (Fig. 30-10).91 In 38 patients with idiopathic dilated cardiomyopathy treated with β-adrenergic blockers, the sphericity index at baseline identified patients who responded to therapy (defined as = 10% increase in EF at a mean follow-up of 4 months). The mean sphericity index was 1.77 ± 0.26 versus 1.52 ± 0.15 in responders and nonresponders, respectively; P < 0.05. The only other parameter predictive of response was the presence of myocardial perfusion defects in these patients with idiopathic dilated cardiomyopathy, presumably indicative of myocardial fibrosis.91 Abidov and colleagues92 recently reported an automated algorithm on gated SPECT MPI to define LV geometry. Taking advantage of the true three-dimensional nature of SPECT data and the completely operator-independent execution of the QGS program (Cedars-Sinai Medical Center, Los Angeles, CA), they developed an algorithm for the calculation of an LV shape index (LVSI), defined as the ratio of the maximum three-dimensional short- and long-axis dimensions of the left ventricle in systole, LVSIs, and diastole, LVSId. Normal limits were determined from 186 consecutive patients with = 5 likelihood of CAD and normal MPI, and then applied prospectively in 93 hospitalized patients, of whom 25 had a discharge diagnosis of worsening heart failure. Repeatability was tested in a group of 52 patients who had sequential MPI within 60 days of each other, without clinical events in between. The LVSI was significantly different in the CHF group compared to normal subjects and also the non-CHF group; it was superior to all conventional variables, including LVEF, for predicting CHF hospitalizations. A cutoff LVSIs of 0.54 had a sensitivity of 68% and specificity of 95% for predicting CHF hospitalizations, and a normalcy rate of 99% in the control population. LVSIs and LVSId had good reproducibility on serial measurement (R2 = 0.8497 and 0.8233, respectively). Gated SPECT imaging offers a widely available and reproducible method to determine LV geometry, which is an indicator of the degree of remodeling and has potential utility for the prediction of functional recovery and prognosis following therapeutic interventions in patients with LV systolic dysfunction.
Assessment of LV Mechanical (Dys)Synchrony
Cardiac resynchronization therapy (CRT) has been shown to improve symptoms and prognosis in selected patients with LV systolic dysfunction (EF = 35%), severe symptoms (NYHA class III or IV), and evidence of mechanical dyssynchrony (QRS duration = 120 ms on the surface electrocardiogram).93,94 Much effort has been directed toward optimizing patient selection for this invasive and costly therapy. Using the conventional criteria listed above, less than two-thirds of patients who undergo CRT derive symptomatic benefit from it. This disparity is most likely due to a dissociation between mechanical synchrony and the QRS duration in some patients.95 Echocardiography-derived indices of dyssynchrony using tissue Doppler imaging and speckle tracking have been used to improve detection of mechanical dyssynchrony and response to CRT, and encouraging results were obtained in individual studies, including studies in patients with LV systolic dysfunction and narrow QRS complexes.95 However, the poor reproducibility of echo-based assessments is a significant limitation to the widespread application of this approach, as was demonstrated in the recent multicenter Predictors of Response to Cardiac Resynchronization Therapy (PROSPECT) trial.96
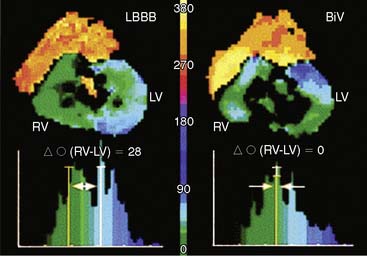
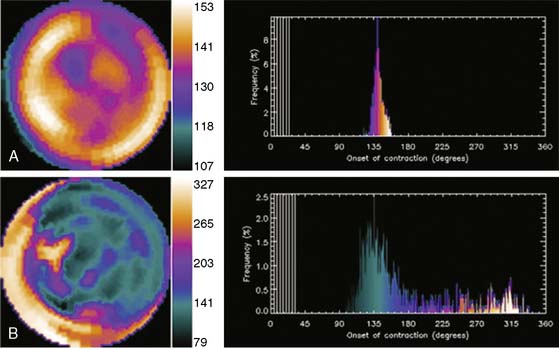
Phase analysis of radionuclide images of the heart is well suited for the assessment of ventricular synchrony (Fig. 30-11). This has hitherto been performed on radionuclide ventriculography, from which an indirect assessment of regional LV function is obtained by measuring changes in regional (counts) within the LV blood pool (indicative of regional shifts in blood volume related to regional wall motion).97 Phase analysis on gated-SPECT imaging has recently been investigated as a modality to assess the synchrony of LV mechanical contraction.98 Preliminary studies indicate ability to distinguish between patients with normal conduction and LV systolic function from those with LV systolic dysfunction, left bundle branch block, right bundle branch block, or right ventricular pacing.99 Small studies also suggest that baseline phase analysis variables can predict response to CRT.100 Advantages of gated SPECT imaging for this application are its existing widespread use in heart failure patients for perfusion imaging, completely automated function involving minimal operator input (resulting in excellent reproducibility), and the ability to simultaneously generate information on scar burden, viability, and remodeling, all of which may have important bearing on response to CRT. Phase analysis is based on the linear relationship between LV regional myocardial thickening and change in regional counts (brightening) between diastole and systole due to the partial volume effect.101 This change in regional counts can be used to determine the onset of thickening (mechanical contraction) in each region of the LV myocardium. A phase analysis is performed by applying Fourier transformation to the count variation over time in each myocardial voxel, thus generating a three-dimensional phase distribution. This phase distribution is displayed as a function of the R-R interval, so that the percentage of LV myocardium initiating mechanical contraction (thickening) at any point in the cardiac cycle can be determined.98 This information is used to determine how homogeneous or heterogeneous the onset of mechanical contraction (synchrony or dyssynchrony) is across the LV myocardium. The phase analysis tool is implemented in the Emory Cardiac Toolbox and described in the recent publication by Chen and colleagues.98 As shown in Figure 30-12, the phase analysis is represented as polar maps and phase histograms. The coordinates for the histogram are the cardiac cycle on the x-axis (the R-R interval divided into 360 degrees, which can be converted to milliseconds if the heart rate is known) and percentage of the myocardium initiating mechanical contraction during any gating frame on the y-axis. With coordinated ventricular contraction, most myocardial segments have nearly the same phase, resulting in a uniform phase image and a narrow and highly peaked phase histogram. The following previously validated indices with known normal limits are measured to quantify LV dyssynchrony:
1. Rosamond W., Flegal K., Friday G., Furie K., Go A., Greenlund K., Haase N., Ho M., Howard V., Kissela B., Kittner S., Lloyd-Jones D., McDermott M., Meigs J., Moy C., Nichol G., O’Donnell C.J., Roger V., Rumsfeld J., Sorlie P., Steinberger J., Thom T., Wasserthiel-Smoller S., Hong Y. for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics–2007 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171.
2. Gheorghiade M., Bonow R.O. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282-289.
3. Velagaleti R.S., Vasan R.S. Heart failure in the twenty-first century: is it a coronary artery disease or hypertension problem? Cardiol Clin. 2007;25:487-495.
4. Felker G.M., Shaw L.K., O’Connor C.M. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210-218.
5. Allman K.C., Shaw L.J., Hachamovitch R., Udelson J.E. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151-1158.
6. Hunt S.A., Abraham W.T., Chin M.H., Feldman A.M., Francis G.S., Ganiats T.G., Jessup M., Konstam M.A., Mancini D.M., Michl K., Oates J.A., Rahko P.S., Silver M.A., Stevenson L.W., Yancy C.W., Antman E.M., Smith S.C.Jr, Adams C.D., Anderson J.L., Faxon D.P., Fuster V., Halperin J.L., Hiratzka L.F., Hunt S.A., Jacobs A.K., Nishimura R., Ornato J.P., Page R.L., Riegel B. ACC/AHA 2005 Guideline update for the diagnosis and management of chronic heart failure in the adult—Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation. 2005;112:1825-1852.
7. Poole-Wilson P.A., Swedberg K., Cleland J.G., Di L., Hanrath P., Komajda M., Lubsen J., Lutiger B., Metra M., Remme W.J., Torp-Pedersen C., Scherhag A., Skene A., Carvedilol O. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13.
8. Klocke F.J., Baird M.G., Lorell B.H., Bateman T.M., Messer J.V., Berman D.S., O’Gara P.T., Carabello B.A., Russell R.O.J., Cerqueira M.D., St J., DeMaria A.N., Udelson J.E., Kennedy J.W., Verani M.S., Williams K.A., Antman E.M., Smith S.C.J., Alpert J.S., Gregoratos G., Anderson J.L., Hiratzka L.F., Faxon D.P., Hunt S.A., Fuster V., Jacobs A.K., Gibbons R.J., Russell R.O., American C., American H., American S. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation. 2003;108:1404-1418.
9. Udelson J.E., Shafer C.D., Carrio I. Radionuclide imaging in heart failure: assessing etiology and outcomes and implications for management. J Nucl Cardiol. 2002;9:40S-52S.
10. Danias P.G., Papaioannou G.I., Ahlberg A.W., O’Sullivan D.M., Mann A., Boden W.E., Heller G.V. Usefulness of electrocardiographic-gated stress technetium-99m sestamibi single-photon emission computed tomography to differentiate ischemic from nonischemic cardiomyopathy. Am J Cardiol. 2004;94:14-19.
11. Soman P., Lahiri A., Mieres J.H., Calnon D.A., Wolinsky D., Beller G.A., Sias T., Burnham K., Conway L., McCullough P.A., Daher E., Walsh M.N., Wight J., Heller G.V., Udelson J.E. Etiology and pathophysiology of new-onset heart failure: Evaluation by myocardial perfusion imaging. J Nucl Cardiol. 2009;16:82-91.
12. Di B., Pozzi C., Cavallini M.C., Innocenti F., Baldereschi G., De A., Antonini E., Pini R., Masotti G., Marchionni N. The diagnosis of heart failure in the community. Comparative validation of four sets of criteria in unselected older adults: the ICARe Dicomano Study. J Am Coll Cardiol. 2004;44:1601-1608.
13. Hachamovitch R., Hayes S.W., Friedman J.D., Cohen I., Berman D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single-photon emission computed tomography. Circulation. 2003;107:2900-2907.
14. Doi Y.L., Chikamori T., Tukata J., Yonezawa Y., Poloniecki J.D., Ozawa T., McKenna W.J. Prognostic value of thallium-201 perfusion defects in idiopathic dilated cardiomyopathy. Am J Cardiol. 1991;67:188-193.
15. Soman P., Dave D.M., Udelson J.E., Han H., Ouda H.Z., Patel A.R., Karas R.H., Kuvin J.T. Vascular endothelial dysfunction is associated with reversible myocardial perfusion defects in the absence of obstructive coronary artery disease. J Nucl Cardiol. 2006;13:756-760.
16. Heller G.V., Links J., Bateman T.M., Ziffer J.A., Ficaro E., Cohen M.C., Hendel R.C. American Society of Nuclear Cardiology and Society of Nuclear Medicine joint position statement: Attenuation correction of myocardial perfusion SPECT scintigraphy. J Nucl Cardiol. 2004;11:229-230.
17. Budoff M.J., Jacob B., Rasouli M.L., Yu D., Chang R.S., Shavelle D.M. Comparison of electron beam computed tomography and technetium stress testing in differentiating cause of dilated versus ischemic cardiomyopathy. J Comput Assist Tomogr. 2005;29:699-703.
18. Andreini D., Pontone G., Pepi M., Ballerini G., Bartorelli A.L., Magini A., Quaglia C., Nobili E., Agostoni P. Diagnostic accuracy of multidetector computed tomography coronary angiography in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2007;49:2044-2050.
19. Kumita S., Cho K., Nakajo H., Toba M., Uwamori M., Mizumura S., Kumazaki T., Sano J., Sakai S., Munakata K. Assessment of left ventricular diastolic function with electrocardiography-gated myocardial perfusion SPECT: Comparison with multigated equilibrium radionuclide angiography. J Nucl Cardiol. 2001;8:568-574.
20. Germano G., Kavanagh P.B., Slomka P.J., Van Kriekinge S.D., Pollard G., Berman D.S. Quantitation in gated perfusion SPECT imaging: The Cedars-Sinai approach. J Nucl Cardiol. 2007;14:433-454.
21. Rozanski A., Nichols K., Yao S.S., Malholtra S., Cohen R., DePuey E.G. Development and application of normal limits for left ventricular ejection fraction and volume measurements from 99mTc-sestamibi myocardial perfusion gates SPECT. J Nucl Med. 2000;41:1445-1450.
22. Bellenger N.G., Burgess M.I., Ray S.G., Lahiri A., Coats A.J.S., Cleland J.G.F., Pennell D.J. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? Eur Heart J. 2000;21:1387-1396.
23. Moss A.J., Zareba W., Hall W.J., Klein H., Wilber D.J., Cannom D.S., Daubert J.P., Higgins S.L., Brown M.W., Andrews M.L., the Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-883.
24. Bigger J.T., The Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. N Engl J Med. 1997;337:1569-1575.
25. Manrique A., Koning R., Cribier A., et al. Effect of temporal sampling on evaluation of left ventricular ejection fraction by means of thallium-201 gated SPET: comparison of 16- and 8-interval gating, with reference to equilibrium radionuclide angiography. Eur J Nucl Med Mol Imaging. 2000;27:694-699.
26. Nakajima K., Higuchi T., Taki J., Kawano M., Tonami N. Accuracy of ventricular volume and ejection fraction measured by gated myocardial SPECT: Comparison of 4 software programs. J Nucl Med. 2001;42:1571-1578.
27. Johnson L.L., Verdesca S.A., Aude W.Y., Xavier R.C., Nott L.T., Campanella M.W., Germano G. Postischemic stunning can affect left ventricular ejection fraction and regional wall motion on post-stress gated sestamibi tomograms. J Am Coll Cardiol. 1997;30:1641-1648.
28. Hyun I.Y., Kwan J., Park K.S., Lee W.H. Reproducibility of Tl-201 and Tc-99m sestamibi gated myocardial perfusion SPECT measurement of myocardial function. J Nucl Cardiol. 2001;8:182-187.
29. Muhlbaier L.H., Pryor D.B., Rankin J.S., Smith L.R., Mark D.B., Jones R.H., Glower D.D., Harrell F.E.J., Lee K.L., Califf R.M., et al. Observational comparison of event-free survival with medical and surgical therapy in patients with coronary artery disease. 20 years of follow-up. Circulation. 1992;86:II198-II204.
30. Di Carli M.F., Asgarzadie F., Schelbert H.R., Brunken R.C., Laks H., Phelps M.E., Maddahi J. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation. 1995;92:3436-3444.
31. Marwick T.H., Zuchowski C., Lauer M.S., Secknus M.A., Williams Lytle B.W. Functional status and quality of life in patients with heart failure undergoing coronary bypass surgery after assessment of myocardial viability. J Am Coll Cardiol. 1999;33:750-758.
32. Bax J.J., Poldermans D., Elhendy A., Cornel J.H., Boersma E., Rambaldi R., Roelandt J.R., Fioretti P.M. Improvement of left ventricular ejection fraction, heart failure symptoms and prognosis after revascularization in patients with chronic coronary artery disease and viable myocardium detected by dobutamine stress echocardiography. J Am Coll Cardiol. 1999;34:163-169.
33. Bax J.J., Wijns W., Cornel J.H., Visser F.C., Boersma E., Fioretti P.M. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: comparison of pooled data. J Am Coll Cardiol. 1997;30:1451-1460.
34. Kim R.J., Wu E., Rafael A., Chen E.L., Parker M.A., Simonetti O., Klocke F.J., Bonow R.O., Judd R.M. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453.
35. Meluzin J., Cigarroa C.G., Brickner M.E., Cerny J., Spinarova L., Frelich M., Stetka F., Groch L., Grayburn P.A. Dobutamine echocardiography in predicting improvement in global left ventricular systolic function after coronary bypass or angioplasty in patients with healed myocardial infarcts. Am J Cardiol. 1995;76:877-880.
36. Senior R., Kaul S., Raval U., Lahiri A. Impact of revascularization and myocardial viability determined by nitrate-enhanced Tc-99m sestamibi and Tl-201 imaging on mortality and functional outcome in ischemic cardiomyopathy. J Nucl Cardiol. 2002;9:454-462.
37. Pagley P.R., Beller G.A., Watson D.D., Gimple L.W., Ragosta M. Improved outcome after coronary bypass surgery in patients with ischemic cardiomyopathy and residual myocardial viability. Circulation. 1997;96:793-800.
38. Senior R., Lahiri A., Kaul S. Effect of revascularization on left ventricular remodeling in patients with heart failure from severe chronic ischemic left ventricular dysfunction. Am J Cardiol. 2001;88:624-629.
39. Bonow R.O. Identification of viable myocardium. Circulation. 1996;94:2674-2680.
40. Samady H., Elefteriades J.A., Abbott B.G., Mattera J.A., McPherson C.A., Wackers F.J. Failure to improve left ventricular function after coronary revascularization for ischemic cardiomyopathy is not associated with worse outcome. Circulation. 1999;100:1298-1304.
41. Myers J.H., Stirling M.C., Choy M., Buda A.J., Gallagher K.P. Direct measurement of inner and outer wall thickening dynamics with epicardial echocardiography. Circulation. 1986;74:164-172.
42. Lieberman A.N., Weiss J.L., Jugdutt B.I., Becker L.C., Bulkley B.H., Garrison J.G., Hutchins G.M., Kallman C.A., Weisfeldt M.L. Two-dimensional echocardiography and infarct size: relationship of regional wall motion and thickening to the extent of myocardial infarction in the dog. Circulation. 1981;63:739-746.
43. Kaul S. There may be more to myocardial viability than meets the eye. Circulation. 1995;92:2790-2793.
44. Auerbach M.A., Schoder H., Hoh C., Gambhir S.S., Yaghoubi S., Sayre J.W., Silverman D., Phelps M.E., Schelbert H.R., Czernin J. Prevalence of myocardial viability as detected by positron emission tomography in patients with ischemic cardiomyopathy. Circulation. 1999;99:2921-2926.
45. Cleland J.G., Pennell D.J., Ray S.G., Coats A.J., Macfarlane P.W., Murray G.D., Mule J.D., Vered Z., Lahiri A. Carvedilol hm. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet. 2003;362:14-21.
46. Camici P.G., Wijns W., Borgers M., De S., Ferrari R., Knuuti J., Lammertsma A.A., Liedtke A.J., Paternostro G., Vatner S.F. Pathophysiological mechanisms of chronic reversible left ventricular dysfunction due to coronary artery disease (hibernating myocardium). Circulation. 1997;96:3205-3214.
47. Wijns W., Vatner S.F., Camici P.G. Hibernating myocardium. N Engl J Med. 1998;339:173-181.
48. Ragosta M., Beller G.A., Watson D.D., Kaul S., Gimple L.W. Quantitative planar rest-redistribution 201Tl imaging in detection of myocardial viability and prediction of improvement in left ventricular function after coronary bypass surgery in patients with severely depressed left ventricular function. Circulation. 1993;87:1630-1641.
49. Pohost G.M., Zir L.M., Moore R.H., McKusick K.A., Guiney T.E., Beller G.A. Differentiation of transiently ischemic from infarcted myocardium by serial imaging after a single dose of thallium-201. Circulation. 1977;55:294-302.
50. Bonow R.O., Dilsizian V. Thallium-201 and technetium-99m-sestamibi for assessing viable myocardium. J Nucl Med. 1992;33:815-818.
51. Udelson J.E., Coleman P.S., Metherall J., Pandian N.G., Gomez A.R., Griffith J.L., Shea N.L., Oates E., Konstam M.A. Predicting recovery of severe regional ventricular dysfunction. Comparison of resting scintigraphy with 201Tl and 99mTc-sestamibi. Circulation. 1994;89:2552-2561.
52. Sciagra R., Bisi G., Santoro G.M., Zerauschek F., Sestini S., Pedenovi P., Pappagallo R., Fazzini P.F. Comparison of baseline-nitrate technetium-99m sestamibi with rest-redistribution thallium-201 tomography in detecting viable hibernating myocardium and predicting postrevascularization recovery. J Am Coll Cardiol. 1997;30:384-391.
53. Siebelink H.M., Blanksma P.K., Crijns H.J., Bax J.J., van B., Kingma T., Piers D.A., Pruim J., Jager P.L., Vaalburg W. Van d. No difference in cardiac event-free survival between positron emission tomography-guided and single-photon emission computed tomography-guided patient management: a prospective, randomized comparison of patients with suspicion of jeopardized myocardium. J Am Coll Cardiol. 2001;37:81-88.
54. Schinkel A.F., Poldermans D., Rizzello V., Vanoverschelde J.L., Elhendy A., Boersma E., Roelandt J.R., Bax J.J. Why do patients with ischemic cardiomyopathy and a substantial amount of viable myocardium not always recover in function after revascularization? J Thorac Cardiovasc Surg. 2004;127:385-390.
55. Shirani J., Narula J., Eckelman W.C., Narula N., Dilsizian V. Early imaging in heart failure: Exploring novel molecular targets. J Nucl Cardiol. 2007;14:100-110.
56. Shirani J., Dilsizian V. Molecular imaging in heart failure. Curr Opin Biotechnol. 2007;18:65-72.
57. Thimister P.W., Hofstra L., Liem I.H., Boersma H.H., Kemerink G., Reutelingsperger C.P., Heidendal G.A. In vivo detection of cell death in the area at risk in acute myocardial infarction. J Nucl Med. 2003;44:391-396.
58. Korngold E.C., Jaffer F.A., Weissleder R., Sosnovik D.E. Noninvasive imaging of apoptosis in cardiovascular disease. Heart Fail Rev. 2008;13:163-173.
59. Ferrari R., Ceconi C., Curello S., Visioli O. The neuroendocrine and sympathetic nervous system in congestive heart failure. Eur Heart J. 1998;19:F45-F51.
60. Schrier R.W., Abraham W.T. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577-585.
61. Wieland D.M., Brown L.E., Rogers W.L., Worthington K.C., Wu J.L., Clinthorne N.H., Otto C.A., Swanson D.P., Beierwaltes W.H. Myocardial imaging with a radioiodinated norepinephrine storage analog. J Nucl Med. 1981;22:22-31.
62. Yamashina S., Yamazaki J. Neuronal imaging using SPECT. Eur J Nucl Med Mol Imaging. 2007;34:939-950.
63. Valette H., Bourguignon M.H., Le Guludec D., Merlet P., Dove P.J., Kiger J.P., Slama M., Motte G., Raynaud C., Syrota A. ECG gated thallium 201 myocardial images: value in detecting multivessel disease in patients on anti anginal therapy 1–3 months after myocardial infarction. Eur J Nucl Med. 1988;13:551-556.
64. Fujimoto S., Inoue A., Hisatake S., Yamashina S., Yamashina H., Nakano H., Yamazaki J. Usefulness of meta-iodobenzylguanidine myocardial scintigraphy for predicting cardiac events in patients with dilated cardiomyopathy who receive long-term beta blocker treatment. Nucl Med Commun. 2005;26:97-102.
65. Neely J.R., Rovetto M.J., Oram J.F. Myocardial utilization of carbohydrate and lipids. Prog Cardiovasc Dis. 1972;15:289-329.
66. Goldstein R.A., Klein M.S., Welch M.J., Sobel B.E. External assessment of myocardial metabolism with C-11 palmitate in vivo. J Nucl Med. 1980;21:342-348.
67. Schon H.R., Schelbert H.R., Robinson G., Najafi A., Huang S.C., Hansen H., Barrio J., Kuhl D.E., Phelps M.E. C-11 labeled palmitic acid for the noninvasive evaluation of regional myocardial fatty acid metabolism with positron-computed tomography. I. Kinetics of C- 11 palmitic acid in normal myocardium. Am Heart J. 1982;103:532-547.
68. Reske S.N., Biersack H.J., Lackner K., Machulla H.J., Knopp R., Hahn N., Winkler C. Assessment of regional myocardial uptake and metabolism of omega-(p-123I-phenyl) pentadecanoic acid with serial single-photon emission tomography. Nuklearmedizin. 1982;21:249-253.
69. Yang J.Y., Ruiz M., Calnon D.A., Watson D.D., Beller G.A., Glover D.K. Assessment of myocardial viability using 123I-labeled iodophenylpentadecanoic acid at sustained low flow or after acute infarction and reperfusion. J Nucl Med. 1999;40:821-828.
70. Caldwell J.H., Martin G.V., Link J.M., Krohn K.A., Bassingthwaighte J.B. Iodophenylpentadecanoic acid-myocardial blood flow relationship during maximal exercise with coronary occlusion. J Nucl Med. 1990;31:99-105.
71. Murray G., Schad N., Ladd W., Allie D., vander Z., Avet P., Rockett J. Metabolic cardiac imaging in severe coronary disease: assessment of viability with iodine-123-iodophenylpentadecanoic acid and multicrystal gamma camera, and correlation with biopsy. J Nucl Med. 1992;33:1269-1277.
72. Hansen C.L., Heo J., Oliner C., Van D., Iskandrian A.S. Prediction of improvement in left ventricular function with iodine-123-IPPA after coronary revascularization. J Nucl Med. 1995;36:1987-1993.
73. Iskandrian A.S., Powers J., Cave V., Wasserleben V., Cassell D., Heo J. Assessment of myocardial viability by dynamic tomographic iodine 123 iodophenylpentadecanoic acid imaging: comparison with rest-redistribution thallium 201 imaging. J Nucl Cardiol. 1995;2:101-109.
74. Verani M.S., Taillefer R., Iskandrian A.E., Mahmarian J.J., He Z.X., Orlandi C. 123I-IPPA SPECT for the prediction of enhanced left ventricular function after coronary bypass graft surgery. Multicenter IPPA Viability Trial Investigators. 123I-iodophenylpentadecanoic acid. J Nucl Med. 2000;41:1299-1307.
75. Tamaki N., Tadamura E., Kawamoto M., Magata Y., Yonekura Y., Fujibayashi Y., Nohara R., Sasayama S., Konishi J. Decreased uptake of iodinated branched fatty acid analog indicates metabolic alterations in ischemic myocardium. J Nucl Med. 1995;36:1974-1980.
76. Knapp F.F.J., Goodman M.M., Callahan A.P., Kirsch G. Radioiodinated 15-(p-iodophenyl)-3,3-dimethylpentadecanoic acid: a useful new agent to evaluate myocardial fatty acid uptake. J Nucl Med. 1986;27:521-531.
77. Tamaki N., Kawamoto M., Yonekura Y., Fujibayashi Y., Takahashi N., Konishi J., Nohara R., Kambara H., Kawai C., Ikekubo K., et al. Regional metabolic abnormality in relation to perfusion and wall motion in patients with myocardial infarction: assessment with emission tomography using an iodinated branched fatty acid analog. J Nucl Med. 1992;33:659-667.
78. Dilsizian V., Bateman T.M., Bergmann S.R., Des P., Magram M.Y., Goodbody A.E., Babich J.W., Udelson J.E. Metabolic imaging with β-methyl-p-iodophenyl-pentadecanoic acid identifies ischemic memory after demand ischemia. Circulation. 2005;112:2169-2174.
79. Nishimura M., Tsukamoto K., Hasebe N., Tamaki N., Kikuchi K., Ono T. Prediction of cardiac death in hemodialysis patients by myocardial fatty acid imaging. J Am Coll Cardiol. 2008;51:139-145.
80. Hambye A.S., Vaerenberg M.M., Dobbeleir A.A., Van d., Franken P.R. Abnormal BMIPP uptake in chronically dysfunctional myocardial segments: correlation with contractile response to low-dose dobutamine. J Nucl Med. 1998;39:1845-1850.
81. Kudoh T., Tadamura E., Tamaki N., Hattori N., Inubushi M., Kubo S., Magata Y., Nishimura K., Matsuda K., Konishi J. Iodinated free fatty acid and 201T1 uptake in chronically hypoperfused myocardium: histologic correlation study. J Nucl Med. 2000;41:293-296.
82. Ito T., Tanouchi J., Kato J., Morioka T., Nishino M., Iwai K., Tanahashi H., Yamada Y., Hori M., Kamada T. Recovery of impaired left ventricular function in patients with acute myocardial infarction is predicted by the discordance in defect size on 123I-BMIPP and 201Tl SPECT images. Eur J Nucl Med. 1996;23:917-923.
83. Naruse H., Arii T., Kondo T., Morita M., Ohyanagi M., Iwasaki T., Fukuchi M. Clinical usefulness of iodine 123-labeled fatty acid imaging in patients with acute myocardial infarction. J Nucl Cardiol. 1998;5:275-284.
84. Barbash I.M., Chouraqui P., Baron J., Feinberg M.S., Etzion S., Tessone A., Miller L., Guetta E., Zipori D., Kedes L.H., Kloner R.A., Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation. 2003;108:863-868.
85. Hofmann M., Wollert K.C., Meyer G.P., Menke A., Arseniev L., Hertenstein B., Ganser A., Knapp W.H., Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198-2202.
86. Kraitchman D.L., Tatsumi M., Gilson W.D., Ishimori T., Kedziorek D., Walczak P., Segars W.P., Chen H., Fritzges D., Izbudak I., Young R.G., Marcelino M., Pittenger M.F., Solaiyappan M., Boston R.C., Tsui B.M.W., Wahl R.L., Bulte J.W.M. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial Infarction. Circulation. 2005;112:1451-1461.
87. Bengel F. Nuclear imaging in cardiac cell therapy. Heart Fail Rev. 2006;11:325-332.
88. Douglas P.S., Morrow R., Ioli A., Reichek N. Left ventricular shape, afterload and survival in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1989;13:311-315.
89. Lowes B.D., Gill E.A., Abraham W.T., Larrain J.R., Robertson A.D., Bristow M.R., Gilbert E.M. Effects of carvedilol on left ventricular mass, chamber geometry, and mitral regurgitation in chronic heart failure. Am J Cardiol. 1999;83:1201-1205.
90. Hall S.A., Cigarroa C.G., Marcoux L., Risser R.C., Grayburn P.A., Eichhorn E.J. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with β-adrenergic blockade. J Am Coll Cardiol. 1995;25:1154-1161.
91. Fukuchi K., Yasumura Y., Kiso K., Hayashida K., Miyatake K., Ishida Y. Gated myocardial SPECT to predict response to β-blocker therapy in patients with idiopathic dilated cardiomyopathy. J Nucl Med. 2004;45:527-531.
92. Abidov A., Slomka P.J., Nishina H., et al. Left ventricular shape index assessed by gated stress myocardial perfusion SPECT: Initial description of a new variable. J Nucl Cardiol. 2006;13:652-659.
93. Bristow M.R., Saxon L.A., Boehmer J., Krueger S., Kass D.A., De Marco T., Carson P., DiCarlo L., DeMets D., White B.G., DeVries D.W., Feldman A.M. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
94. Cleland J.G.F., Daubert J.C., Erdmann E., Freemantle N., Gras D., Kappenberger L., Tavazzi L. For the Cardiac Resynchronization-Heart Failure (Care-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
95. Gorcsan J. Role of echocardiography to determine candidacy for cardiac resynchronization therapy. Curr Opin Cardiol. 2008;23:16-22.
96. Chung E.S., Leon A.R., Tavazzi L., Sun J.P., Nihoyannopoulos P., Merlino J., Abraham W.T., Ghio S., Leclercq C., Bax J.J., Yu C.M., Gorcsan J., St J., De S., Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608-2616.
97. Kerwin W.F., Botvinick E.H., O’Connell J.W., Merrick S.H., DeMarco T., Chatterjee K., Scheibly K., Saxon L.A. Ventricular contraction abnormalities in dilated cardiomyopathy: effect of biventricular pacing to correct interventricular dyssynchrony. J Am Coll Cardiol. 2000;35:1221-1227.
98. Chen J., Henneman M.M., Trimble M.A., Bax J.J., Borges-Neto S., Iskandrian A.E., Nichols K.J., Garcia E.V. Assessment of left ventricular mechanical dyssynchrony by phase analysis of ECG-gated SPECT myocardial perfusion imaging. J Nucl Cardiol. 2008;15:127-136.
99. Trimble M.A., Borges-Neto S., Smallheiser S., Chen J., Honeycutt E.F., Shaw L.K., Heo J., Pagnanelli R.A., Tauxe E.L., Garcia E.V., Esteves F., Seghatol-Eslami F., Kay G.N., Iskandrian A.E. Evaluation of left ventricular mechanical dyssynchrony as determined by phase analysis of ECG-gated SPECT myocardial perfusion imaging in patients with left ventricular dysfunction and conduction disturbances. J Nucl Cardiol. 2007;14:298-307.
100. Henneman M.M., Chen J., Dibbets-Schneider P., Stokkel M.P., Bleeker G.B., Ypenburg C., van der Wall E.E., Schalij M.J., Garcia E.V., Bax J.J. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med. 2007;48:1104-1111.
101. Marcassa C., Marzullo P., Parodi O., Sambuceti G., L’Abbate A. A new method for noninvasive quantitation of segmental myocardial wall thickening using technetium-99m 2-methoxy-isobutyl-isonitrile scintigraphy—results in normal subjects. J Nucl Med. 1990;31:173-177.