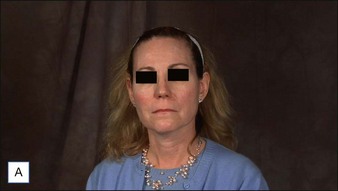
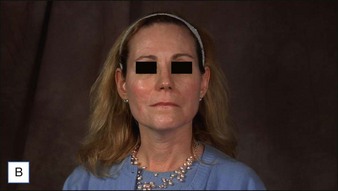
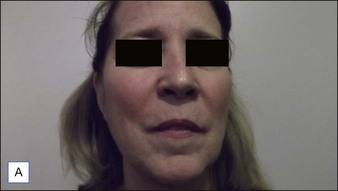
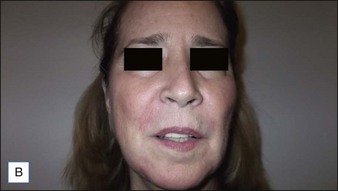
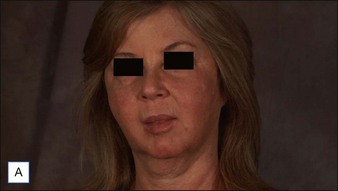
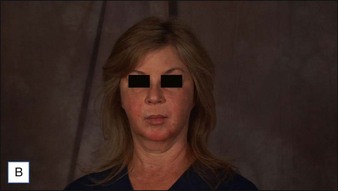
6 Radiesse® / Radiesse® with lidocaine
Summary and Key Features
• The ideal soft tissue filler for facial augmentation should be non-allergenic, and provide a natural look and feel
• The ideal soft tissue filler should require minimal downtime and exhibit few side effects
• Fillers can be permanent or temporary
• Calcium hydroxylapatite (CaHA) is well suited for facial augmentation and contouring
• CaHA has demonstrated a favorable safety profile
• CaHA longevity is approximately 15 months in active tissues of the face and may approach 24 months in more static locations
• CaHA is approved by the US Food and Drug Administration for correction of moderate to severe facial lines and folds and correction of soft tissue loss from HIV lipoatrophy
• Unlike collagen or hyaluronic acid filler materials, CaHA injection does not require overcorrection
• CaHA particles become fixed in position over time, do not migrate, and take on the natural characteristics of the soft tissue
• CaHA filler showed a high overall level of patient satisfaction, a remarkable safety profile, and clinical longevity
Injection techniques
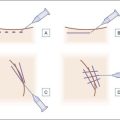
Cross-hatching / fanning
The fanning (Fig. 6.3) and cross-hatching techniques are variations of linear threading that allow filling of larger defects or facial contouring. In the fanning technique a single needle puncture allows ‘fan-like’ placement of successive linear threads by radially changing the needle direction. Cross-hatching delivers linear threads in a predetermined grid by multiple punctures. Both techniques are useful in the malar region and oral commissures to build a scaffold in deeper defects.
Alam M, Havey J, Pace N, et al. Large-particle calcium hydroxylapatite injection for correction of facial wrinkles and depressions. Journal of the American Academy of Dermatology. 2011;65:92–96.
Beer K, Yohn M, Cohen JL. Evaluation of injectable CaHA for the treatment of mid-face volume loss. Journal of Drugs in Dermatology. 2008;7:359–366.
Bray D, Hopkins C, Roberts DN. A review of dermal fillers in facial plastic surgery. Current Opinion in Otolaryngology & Head and Neck Surgery. 2010;18:295–302.
Busso M. Calcium hydroxylapatite (Radiesse): safety, techniques and pain reduction. Journal of Drugs in Dermatology. 2009;8:S21–S23.
Busso M, Applebaum D. Hand augmentation with Radiesse (calcium hydroxylapatite). Dermatologic Therapy. 2007;20:385–387.
Busso M, Karlsberg P. Cheek augmentation and rejuvenation using injectable calcium hydroxylapatite (Radiesse). Cosmetic Dermatologic Surgery. 2006;19:583–588.
Busso M, Voigts R. An investigation of changes in physical properties of injectable calcium hydroxylapatite in a carrier gel when mixed with lidocaine and with lidocaine/epinephrine. Dermatologic Surgery. 2008;34(suppl 1):S16–S23. discussion S4
Carruthers A, Liebeskind M, Carruthers J, et al. Radiographic and computed tomographic studies of calcium hydroxylapatite for treatment of HIV-associated facial lipoatrophy and correction of nasolabial folds. Dermatologic Surgery. 2008;34(suppl 1):S78–S84.
Fakhre GP, Perdikis G, Shaddix KK, et al. An evaluation of calcium hydroxylapatite (Radiesse) for cosmetic nasolabial fold correction: a meta-analysis and patient centric outcomes study. Annals of Plastic Surgery. 2009;63:486–489.
Glaich AS, Cohen JL, Goldberg LH. Injection necrosis of the glabella: protocol for prevention and treatment after use of dermal fillers. Dermatologic Surgery. 2006;32:276–281.
Hanke CW, Rohrich RJ, Busso M, et al. Facial Soft-Tissue Fillers conference: Assessing the State of the Science. Journal of the American Academy of Dermatology. 2011;64:S66–S85. e1-136
Jansen DA, Graivier MH. Evaluation of a calcium hydroxylapatite-based implant (Radiesse) for facial soft-tissue augmentation. Plastic and Reconstructive Surgery. 2006;118:S22–S30. discussion S1-S3
Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plastic Surgery. 2003;27:354–366. discussion 67
Marmur ES, Phelps R, Goldberg DJ. Clinical, histologic and electron microscopic findings after injection of a calcium hydroxylapatite filler. Journal of Cosmetic and Laser Therapy. 2004;6:223–226.
Marmur E, Green L, Busso M. A controlled, randomized study of pain levels in subjects treated with calcium hydroxylapatite (Radiesse) premixed with lidocaine for correction of nasolabial folds. Dermatologic Surgery. 2009;2010; 36(3):309–315.
Marmur ES, Taylor SC, Grimes PE, et al. Six-month safety results of calcium hydroxylapatite for treatment of nasolabial folds in Fitzpatrick skin types IV to VI. Dermatologic Surgery. 2009;35(suppl 2):1641–1645.
Moers-Carpi MM, Tufet JO. Calcium hydroxylapatite versus nonanimal stabilized hyaluronic acid for the correction of nasolabial folds: a 12-month, multicenter, prospective, randomized, controlled, split-face trial. Dermatologic Surgery. 2008;34:210–215.
Moers-Carpi M, Vogt S, Santos BM, et al. A multicenter, randomized trial comparing calcium hydroxylapatite to two hyaluronic acids for treatment of nasolabial folds. Dermatologic Surgery. 2007;33(suppl 2):S144–S151.
Radiesse 2009 [package insert]. Bioform Medical I.
Ridenour B, Kontis TC. Injectable calcium hydroxylapatite microspheres (Radiesse). Facial Plastic Surgery. 2009;25:100–105.
Schanz S, Schippert W, Ulmer A, et al. Arterial embolization caused by injection of hyaluronic acid (Restylane). British Journal of Dermatology. 2002;146:928–929.
Silvers SL, Eviatar JA, Echavez MI, et al. Prospective, open-label, 18-month trial of calcium hydroxylapatite (Radiesse) for facial soft-tissue augmentation in patients with human immunodeficiency virus-associated lipoatrophy: one-year durability. Plastic and Reconstructive Surgery. 2006;118:S34–S45.
Smith S, Busso M, McClaren M, et al. A randomized, bilateral, prospective comparison of calcium hydroxylapatite microspheres versus human-based collagen for the correction of nasolabial folds. Dermatologic Surgery. 2007;33(suppl 2):S12–S21. discussion S21
Tzikas TL. A 52-month summary of results using calcium hydroxylapatite for facial soft tissue augmentation. Dermatologic Surgery. 2008;34(suppl 1):S9–S15.