CHAPTER 13 Radiation Therapy Effects and Considerations
For more than 100 years, the treatment of breast cancer was exclusively surgical. The paradigm of breast malignancy management was eventually challenged by several landmark breast preservation trials, which led to the wide acceptance of treatment of most stage I and II breast cancer patients with whole-breast irradiation following tumor excision. In 1973, in Milan, Italy, women with clinically localized breast carcinoma were randomly assigned treatment with total mastectomy or breast conservation therapy (BCT) with partial mastectomy (quadrantectomy) and whole-breast irradiation. By 1980, a total of 701 patients were randomized and treated. In the United States, a similar trial was initiated in 1976. The National Surgical Adjuvant Breast Project B-06 (NSABP B-06) randomized 2163 women with T1–T2 and N0–N1 breast cancer to one of three treatments: total mastectomy, segmental mastectomy, or segmental mastectomy and radiation therapy (RT). In 2002, the results of both studies, with 20 years of follow-up, were published in the New England Journal of Medicine. The results of both trials supported the use of BCT as equally effective compared to mastectomy, in that disease-free survival and overall survival were not adversely affected by preserving the breast. The importance of the status of margins was stressed in NSABP B-06. These two studies and many subsequent international and multi-institutional trials led to a consensus statement by the National Cancer Institute in June 1990 recommending BCT as preferable to mastectomy in appropriately selected patients.1–5
BREAST PRESERVING WHOLE-BREAST IRRADIATION (THREE-DIMENSIONAL NON-INTENSITY–MODULATED RADIATION THERAPY OR FORWARD PLANNING TECHNIQUE)
The patient is examined at consultation, paying special attention to the patient’s breast size, location of the tumor, and any wound issues. Pathology and imaging studies are reviewed. Patients requiring chemotherapy often receive a full course of systemic treatment before radiation. Before RT starts, a mammogram of the involved breast may be obtained. This will serve as a baseline study for follow-up mammograms. Case 1 in this chapter is an illustration of the potential utility of preradiation mammography. Questionable abnormalities need to be worked up before initiation of RT.
Treatment Planning
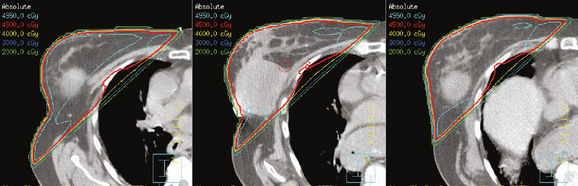
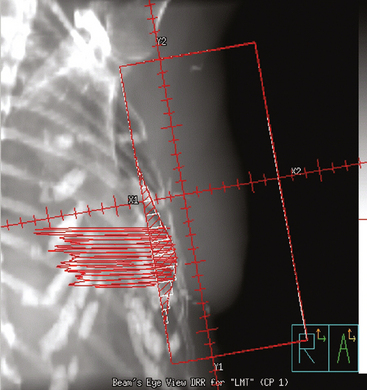
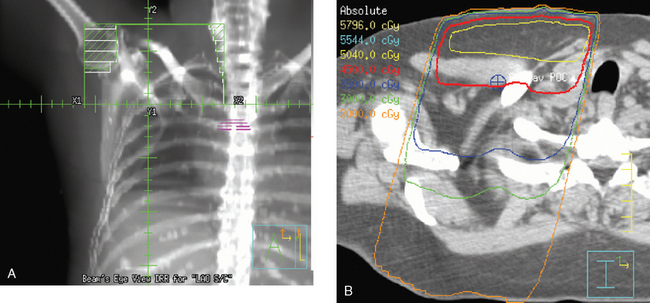
The computerized treatment planning process starts with the radiation oncologist outlining the volumes of interest (tumor bed, heart, lungs). The physicist or dosimetrist then creates an optimal arrangement of the tangential fields to cover the breast with a uniform isodose distribution (Figure 1). The dose-volume histograms are created, and the plan is reviewed and approved by the radiation oncologist. The patient is brought in for port films (Figure 2) and visual check of the fields on the skin to ensure a reproducible setup.
Tumor Bed Boost
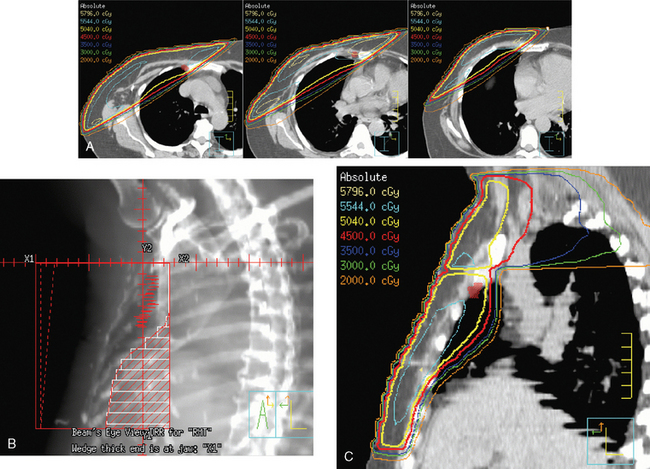
Studies have shown that a boost dose of about 1600 cGy to the tumor bed improves local control. Radiation oncologists use the findings on physical examination, as well as pretreatment imaging studies, and correlate these with the pathology and operative reports to design the volume of tissue to be included in the boost. The patient is placed on a simulation table in a position to even out the surface of the skin over the tumor bed. The volume is outlined and marked with a wire (Figure 3). Ultrasound or CT is obtained to verify the coverage of the tumor bed and to calculate the energy of the electron beam necessary to adequately treat the surgical bed. Once the coverage is approved by the radiation oncologist, a custom lead alloy cut-out is created. The optimal energy of the electron beam is selected to adequately cover the depth of the postsurgical bed, without extending into underlying structures.6–8
WHOLE-BREAST IRRADIATION USING INTENSITY-MODULATED RADIATION THERAPY (IMRT) OR FORWARD TREATMENT PLANNING TECHNIQUE
The continuing search for improved delivery of the conformal dose of radiation to the breast, while protecting the underlying structures and minimizing exposure of the contralateral breast, led to development of treatment planning algorithms using IMRT. Based purely on dosimetry, the superiority of treatment plans created using IMRT or forward planning technique (without wedges, using multiple-shaped fields) over threedimensional conformal opposed tangential fields (using wedges to compensate for uneven breast contours) is readily evident (Figure 4). The dose to the breast is more uniform, there is less of a “hot spot,” and the isodose curves are concave, conforming to the shape of the underlying chest wall. The dose to the ipsilateral lung can thus be reduced, and in cases of left breast treatment, the heart can be spared to a greater extent.
There are challenges associated with implementation of a breast cancer IMRT program on a routine basis. The planning and quality assurance processes are extensive, and the treatment time is longer (not an insignificant factor in a busy department). The patients tolerate treatment better, with less acute skin toxicity. Reduction in long-term toxicity is expected. Further efforts to improve the therapeutic index of RT (optimal dosing to the target volume and maximal protection of the surrounding tissues) involve alternative patient setup (prone), breath-hold and deep inspiration technique, and use of different fractionation. To further spare the surrounding tissues, techniques of partial-breast RT using IMRT have been developed. Partial-breast irradiation is being tested against whole-breast treatment in the ongoing NSABP B-39/Radiation Therapy Oncology Group (RTOG) 0413 study.9–11
TREATMENT OF LYMPH NODES
The radiation of axillary lymph nodes can be achieved with either inclusion of the level I and II lymph nodes in the tangential fields (if the CT simulation confirms good coverage and if the lung and heart can be spared) or use of a separate oblique field matched inferiorly to the upper border of the tangential breast fields. Depending on the patient’s anatomy, the use of an additional posterior axillary boost (PAB) field may be required. Coverage of the supraclavicular region depends on the available information delineating the exact location of the nodal basin and the brachial plexus (Figure 5).12–14
Internal Mammary Lymph Node Radiation Therapy
Primary lymphatic drainage into the internal mammary (IM) lymph nodes may occur in 17% to 25% of tumors located in medial, 29% in central, and 27% in lower outer quadrants of the breast. Information from lymphoscintigraphy or positron emission tomography (PET), CT, or MRI, if available, can help in localization of IM lymph nodes. Treatment of IM lymph nodes is individualized and dependent on many factors (Figure 6). Central, medial, or lower outer quadrant breast tumors with multiple positive axillary lymph nodes may require treatment of the IM lymph nodes. However, isolated IM failure is uncommon. The most commonly involved IM lymph nodes are in the second, third, and first interspaces, in that order. The potential for increased cardiopulmonary toxicity warrants caution. Published treatment guidelines give no definitive recommendation for routine treatment of IM lymph nodes and leave this decision to the discretion of the treating radiation oncologist.15,16
POST MASTECTOMY RADIATION THERAPY
Currently, postmastectomy RT is indicated in patients with large tumors (≥5 cm), involvement of four lymph nodes or more, close or positive margins, and sometimes multicentric tumors. A Danish study reported in the New England Journal of Medicine in 1997 on results of a trial in which 1708 women with stage II to III breast cancer were treated with mastectomy and then randomized to either eight cycles of Cytoxan, methotrexate, 5-fluorouracil (CMF) chemotherapy and chest wall and regional lymphatic RT, or nine cycles of CMF chemotherapy and no RT. After 114 months of median follow-up, the locoregional failure rate was 9% in the arm treated with RT, compared with 32% without RT. The 10-year overall survival rates were 54% and 45%, respectively. A Canadian study, also reported in the New England Journal of Medicine in 1997, reported results of a trial of 318 premenopausal node-positive patients after mastectomy who were randomized to undergo either CMF chemotherapy and RT or CMF chemotherapy alone. The arm treated with chemotherapy and RT received three cycles of CMF, followed by RT, followed by another three cycles of CMF. After 150 months of median follow-up, there was a 33% reduction in recurrences, a 17% improvement in disease-free survival, and a 29% reduction in breast cancer–related mortality.
As the use of postmastectomy RT has increased, so have concerns regarding the effect of RT on reconstructive tissue, implants, and the overall cosmetic treatment outcome. The Therapeutic Index of Loco/Regional Treatment must first account for optimal tumor control, while resulting in the best cosmetic outcome. It is not just cosmesis that is at stake. Development of implant encapsulation can be painful. Fat necrosis can be difficult to differentiate from tumor recurrence and often leads to biopsy, causing patients anxiety. Development of infection or seromas in the reconstructed breast may lead to the removal of transplanted or implanted tissue. Although all these adverse outcomes can occur without use of RT, the incidence does increase with exposure of transplanted tissue or implanted devices to radiation. It is thus extremely important that any patient with even a remote possibility of needing postmastectomy RT be thoroughly evaluated by the whole team to select the best surgical option.17–21
POSTMASTECTOMY RADIATION AND BREAST RECONSTRUCTION
Before mastectomy, patients should undergo an extensive consultation with review of the available reconstructive options. Clinically staged patients who are not expected to require postmastectomy RT are excellent candidates for immediate reconstruction. There are many factors that need to be taken into account while selecting the best surgical reconstruction option. Tumor-related considerations include the tumor size and location, multifocality or multicentricity, lymph node involvement, and chest wall fixation. Patient-related factors include body habitus, medical conditions affecting tissue healing (e.g., collagen vascular diseases), prior chest RT, the location of prior breast surgery scars, smoking history, and prior or planned chemotherapy. Patients with larger tumors (>5 cm), more than three lymph nodes involved, or close margins will require postmastectomy RT. Reconstructive surgery in this population of patients has to be planned carefully. Optimal approaches and timing of reconstruction are the subjects of ongoing discussion and continuing evolution. At many institutions, the anticipated need for postmastectomy RT is a relative contraindication to immediate breast reconstruction using implants. Delayed immediate reconstruction is a viable option in management of patients at high risk for needing postmastectomy RT. During mastectomy, tissue expanders are placed. While the patient is recovering from surgery and, if applicable, undergoing chemotherapy, tissue expansion is achieved by ongoing repetitive injections of saline. This process must be completed before the simulation and radiation treatment planning process can begin (Figure 7).21
Transverse rectus abdominis myocutaneous (TRAM) flap reconstruction surgery should be delayed if possible, until after completion of the full course of radiation. This allows for an optimal cosmetic outcome22,23 and improved technical coverage with the radiation fields.24,25
LOCOREGIONAL TREATMENT OF BREAST CANCER IN THE ERA OF NEOADJUVANT CHEMOTHERAPY
In an attempt to reduce false-negative sentinel lymph node findings after neoadjuvant chemotherapy, many surgeons favor routine sentinel lymph node sampling before initiation of chemotherapy. Positive sentinel lymph node biopsy would lead to completion axillary dissection, before or after chemotherapy. Pathology from prechemotherapy sentinel lymph node biopsy is used by the radiation oncologist to plan the extent of the radiation fields.26,27
NEOADJUVANT CHEMOTHERAPY TO ATTEMPT BREAST PRESERVATION
The increasing use of neoadjuvant chemotherapy for BCT necessitates even closer cooperation of the multidisciplinary team managing these patients. Unless there are obvious microcalcifications to mark the tumor, the lesion needs to be marked with a clip either before or within the first two cycles of chemotherapy. This is usually done with ultrasound or mammography guidance. In patients who reach clinical complete remission, these clips will guide the surgeon in planning the lumpectomy and the radiation oncologist in planning the extent of the tumor bed boost.28
RADIATION THERAPY IN THE MANAGEMENT OF DUCTAL CARCINOMA IN SITU
Three randomized studies comparing tumor resection (with clear margins) with or without RT reported a strong beneficial effect of RT on local recurrence rates, but no effect on overall survival. Chemoprevention with tamoxifen further protects the involved, as well as the contralateral, breast. The technique of RT is the same as that for invasive tumors. There is no need for treatment of lymph nodes, although sentinel lymph node biopsy may be indicated in high-grade or large tumors.29–31
EVOLUTION OF RADIATION THERAPY IN BREAST CONSERVATION THERAPY
Rationale and Options for Partial Breast Radiation
Numerous randomized trials32–35 comparing whole-breast radiation after tumor excision to tumor excision alone have shown that most breast tumors recur in the tumor cavity. Additionally, these trials have shown that the risk for recurrence outside the tumor cavity is similar whether or not whole-breast radiation was given. This suggests that additional radiation given outside the tumor cavity may not be of additional benefit to patients.
Patients are potential candidates for accelerated partial breast RT if they have stage 0, I, or II tumors, a single tumor less than 3 cm in maximal dimension, minimal nodal involvement, and clear surgical margins. Typically, partial-breast radiation is delivered twice a day, with treatments given at least 6 hours apart, for a total of 10 fractions.
Interstitial breast brachytherapy alone has been successfully used at some U.S. centers for more than 10 years following breast-conserving surgery. A trial was started by Vicini and colleagues in 1993 using brachytherapy as the only radiation treatment modality for patients following breastconserving surgery.36,37 By 2001, 120 patients were enrolled in this trial. Four patients developed local recurrence at a median follow-up of 82 months. During 1997 to 2000, 100 patients were enrolled in an RTOG prospective phase I/II study of breast brachytherapy. At a median follow-up of 2.7 years, most patients experienced only mild treatment-related toxicities.38
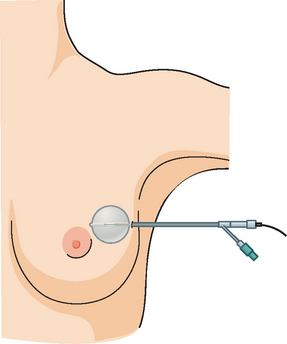
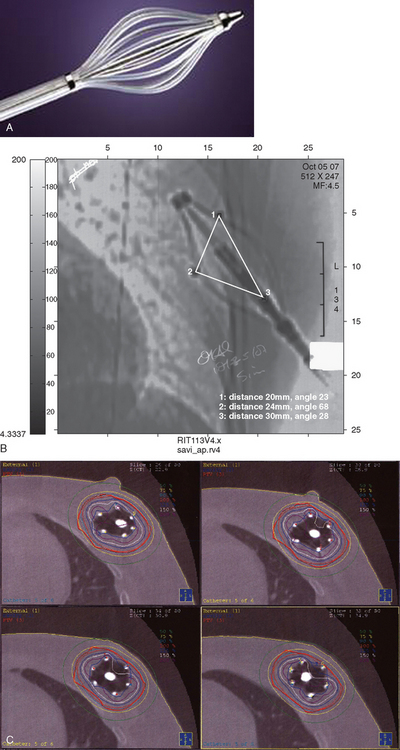
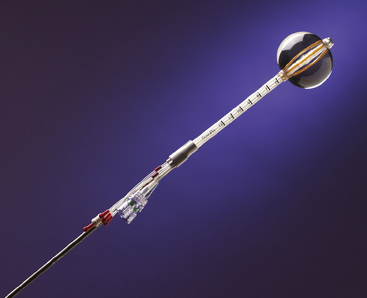
In 2002, the U.S. Food and Drug Administration approved the Proxima Therapeutics MammoSite balloon catheter for intracavitary high-dose-rate breast brachytherapy (Figure 8). Seventy patients were initially enrolled in a prospective multicenter trial evaluating the safety of the MammoSite balloon catheter. Subsequent evaluation of 43 patients eligible for the therapy revealed only mild to moderate self-limited side effects.39 Advantages of the balloon catheter are that it is easier to place in the cavity, placement is more reproducible, and patient comfort is improved. Only a single catheter needs to be temporarily placed in the lumpectomy cavity, as opposed to 10 to 20 catheters with traditional interstitial implants. However, the balloon needs to “conform” properly to the tumor cavity and optimal dosimetry could be problematic if a large air pocket develops along the periphery of the cavity. In addition, balloon catheters may not be appropriate for tumors near the skin surface. A more recent addition to the partial-breast RT armamentarium is the SAVI device, which allows a radiation oncologist to selectively direct radiation through up to 7 catheter channels, allowing more tailored manipulation of the isodose (Figure 9). The Contura multilumen balloon catheter combines features of these partial-breast RT devices, with multiple offset lumens around a balloon, also allowing dose-shaping opportunities to minimize skin and rib doses (Figure 10).
Three-dimensional conformal radiation technology has been developed and improved in recent years. This technique of accelerated partial breast radiation has the advantage of being noninvasive, eliminating an additional procedure and allowing many medical groups that do not perform brachytherapy to offer partial breast RT. No adverse side effects were seen in 28 patients treated with three-dimensional conformal radiation in a 1999 pilot study.40 A potential disadvantage is that the breast is not a stationary target, and there is the potential for a geographic miss with external RT to a small target.
In 2005, the NSABP, along with the RTOG, activated a phase III randomized trial comparing whole-breast RT and partial-breast RT in women with stage 0, I, or II breast cancer. The trial is currently open and is expected to accrue 3000 patients over a period of about 2 years and 5 months. This trial will be comparing overall survival, recurrence-free survival, distant recurrence-free survival, and quality-of-life issues in women receiving whole-breast or partial-breast RT.32–40
RADIATION AFTER AUGMENTATION
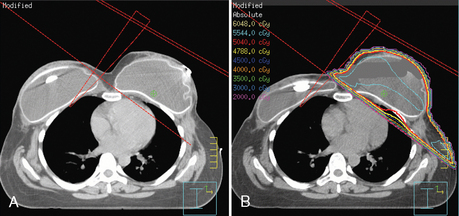
Patients who develop breast cancer after they have undergone prior breast augmentation present additional challenges in designing their treatment to achieve optimal locoregional control and the best possible cosmetic outcome. With careful dosimetry, using IMRT or three-dimensional treatment planning, breast tissue coverage with the desired isodose can be achieved, although there are technical challenges associated with the presence of the implanted device.25,41
These patients should receive an extensive review of the expected effects of radiation on the augmented breast. They need to be evaluated by the radiation oncologist, breast surgeon, and plastic surgeon (if not the same) before the definitive cancer procedure is scheduled. Often the decision whether to retain, remove, or modify the existing augmentation device is patient driven. However, the patient has to be given appropriate information so that she can make an informed decision. One of the concerns frequently voiced by patients is the effectiveness of follow-up imaging. Cases illustrating the range of postoperative appearances of a variety of reconstruction options, as well as manifestations of recurrences and treatment-related changes, are presented throughout this text, particularly in Chapter 6.41
IMAGING FOLLOW-UP OF RADIATION THERAPY–TREATED BREAST CANCER PATIENTS
BCT and RT induce readily visualized and potentially confusing changes in the treated breast on imaging, particularly on mammograms, CT scans, and breast MRI, with fewer effects generally noted on ultrasound and PET. Early postirradiation mammograms in particular can show variable degrees of skin thickening and a generalized increase in breast density and reticulation. These changes usually regress with time. In addition to routine follow-up imaging, clinically detected change should prompt imaging evaluation for possible recurrence. As with all breast imaging, mammography is the cornerstone modality for initial imaging, liberally supplemented with ultrasound. In cases in which there is persistent concern for possible recurrence, breast MRI can be very helpful, particularly more than 18 months after treatment, when little if any enhancement is seen in normal postoperative scars. Significantly enhancing scars older than 18 months should be further investigated, such as with biopsy, to exclude recurrent disease. A scar complicated by fat necrosis or fat necrosis developing in the treatment bed can at times perfectly mimic a local recurrence. Examples are presented in Chapters 6 and 7. A documented local recurrence should prompt systemic evaluation, to confirm that the recurrence is local only, before decision making on additional therapy. This is best accomplished with PET/CT and bone scan.
Radiologic follow-up after reconstruction depends on the technique of reconstruction used and the clinical question. Detection of recurrences in implant and TRAM flap reconstruction patients has traditionally relied on physical examination, supplemented with imaging when questions arise, rather than on routine imaging surveillance with mammography. This has been advocated by some authors42 as potentially enabling earlier diagnosis of recurrent disease than can be achieved by relying on physical examination. Familiarity with normal appearances of breast reconstructions is important to imagers interpreting these studies.43–45
RT COMPLICATIONS
Carcinogenesis: Radiation-Induced Sarcoma
A rare complication of radiation is a radiation-induced sarcoma. Primary sarcoma of the breast is very rare. Secondary sarcomas have been reported developing in breasts exposed to prior radiation or with chronic lymphedema from prior surgery. It is thought to be related to chronic changes in the breast resulting in prolonged edema (lymphedema). The most common histology is an angiosarcoma. This was initially described as Stewart-Treves syndrome and was first recognized in patients with long-standing extremity lymphedema. Clinically, it can present as a violaceous discoloration of the skin and a rapidly growing mass. Examples are Case 18 in Chapter 6 and Case 9 in this chapter.46,47
Carcinogenesis: Lung Cancer
A correlation has been suggested between RT to the breast and the eventual development of a lung malignancy. Patients who have been exposed to thoracic RT for treatment of breast cancer or Hodgkin’s disease should be on heightened lung surveillance. Newer techniques of breast RT better protect the underlying lung, but there is still a small amount of lung parenchyma exposed, and the risk for lung carcinogenesis, even though markedly reduced, still exists.48–50
Carcinogenesis: Risk for Contralateral Breast Malignancy
An increased risk of contralateral breast cancer due to RT after BCT has also been suggested. Doses delivered to the contralateral breast from primary radiation could be a cause. There are several treatment techniques that can be used to minimize contralateral breast dose, such as elimination of a medial wedge. Nevertheless, the increased risk appears to be minimal, and there are conflicting reports about the patient subpopulations at risk (e.g., <45 years old versus >50 years old). Obviously, the patient with a previous diagnosis of breast malignancy is already at increased risk for a second breast cancer, independent of radiation treatment, and requires ongoing follow-up with mammography supplemented with ultrasound or MRI.51–54
Cardiotoxicity
Long-term follow-up data on patients treated with RT in the 1960s and 1970s showed that even though the use of RT resulted in decreased breast cancer–specific deaths, overall survival suffered as a result of long-term cardiac toxicity associated with RT. This effect has been steadily diminishing with the implementation of modern techniques. More recent data suggest little increase in cardiac death rates due to RT, even with left breast tumors (without IM lymph node RT). Additionally, radiation doses delivered to the heart could be significantly reduced or virtually eliminated with newer treatment techniques such as forward planning IMRT or partial-breast irradiation. However, an increased risk for coronary artery disease in patients treated with radiation for left breast tumors has been suggested.55–57
1 Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233-1241.
2 Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997-1003.
3 Holli K, Saaristo R, Isalo J, et al. Lumpectomy with or without postoperative radiotherapy for breast cancer with favorable prognostic features: results of a randomized study. Br J Cancer. 2001;84:164-169.
4 Clark RM, Whalen T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. Ontario Clinical Oncology Group. J Natl Cancer Inst. 1996;88:1659-1664.
5 NIH Office of the Director: NIH Consensus Statement. Nov 1–3, 2000; 17, (4).
6 Bartelink H, Collette L, Fourquet A, et al. Impact of a boost dose of 16 Gy on the local control and cosmesis in patients with early breast cancer: The EORTC ‘boost versus no boost’ trial. Int J Radiat Oncol Biol Phys. 2000;48(3):111. abstract 1
7 Romestaing P, Lehingue Y, Delaunay D, et al. Role of a 10-Gy boost in the conservation treatment of early breast cancer: results of randomized clinical trial in Lyon, France. Int J Radiat Oncol Biol Phys. 51(3), 2001. abstract 1
8 Horst KC, Goffinet DR. 10 Gy vs 16 Gy electron beam boost dose in breast conservation therapy for early breast cancer: a decision analysis. Int J Radiat Oncol Biol Phys. 2002;54(2):307.
9 Vicini FA, Sharpe MB, Kestin LL, et al. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54(5):1336-1344.
10 Remouchamps VM, Vicini FA, Sharpe MB, et al. Significant reduction in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast radiation. Int J Radiat Oncol Biol Phys. 2003;55(2):392-406.
11 Hurkmans CW, Cho BCJ, Damen E, et al. Reduction of cardiac and lung complication probabilities after breast irradiation using conformal radiotherapy with or without intensity modulation. Radiother Oncol. 2002;62(2):163-171.
12 Veronesi U, Rilke F, Luini A, et al. Distribution of axillary node metastases by level of Invasion. Cancer. 1987;59:682.
13 Fisher B, Wolmark N, Bauer M, et al. The accuracy of clinical nodal staging and of limited axillary dissection as a determinant of histologic nodal status in carcinoma of the breast. Surg Gynecol Obstet. 1981;152:765.
14 Dewar JA, Sarrazin D, Benhamou E, et al. Management of the axilla in conservatively treated breast cancer: 592 patients treated at Institut Gustave-Roussey. Int J Radiat Oncol Biol Phys. 1987;13:375.
15 Byrd DR, Dunnwald LK, Mankoff DA, et al. Internal mammary lymph node drainage patterns in patients with breast cancer documented by breast lymphoscintigraphy. Ann Surg Oncol. 2001;8(3):234-240.
16 Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1539-1569.
17 Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949-954.
18 Ragaz J, Jackson SM, Ne N, et al. Adjuvant radiotherapy and chemotherapy in node positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956-962.
19 Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1539-1569.
20 Pierce L. The use of radiotherapy after mastectomy: a review of the literature. J Clin Oncol. 2003;23(8):1706-1717.
21 Taghian A, Jeong J-H, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project ran-domized clinical trials. J Clin Oncol. 2004;22:4247-4254.
22 Barreau-Pouhear I, Le MG, Rietjens M, et al. Risk factors for failure of immediate breast reconstruction with prosthesis after total mastectomy for breast cancer. Cancer. 1992;70:1145-1151.
23 Pomahac B, Recht A, May JW, et al. New trends in breast cancer management: is the era of immediate breast reconstruction changing? Ann Surg. 2006;244(2):282-288.
24 Javaid M, Song F, Leinster S, et al. Radiation effects on the cosmetic outcomes of immediate and delayed autologous breast reconstruction: an argument about timing. J Plast Reconstr Aesthet Surg. 2006;59(1):16-26.
25 Motwani SB, Strom EA, Schechter NR, et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(1):76-82.
26 Danforth DNJr, Lippman ME, McDonald H, et al. Effect of pre-operative chemotherapy on mastectomy for locally advanced breast cancer. Am Surg. 1990;56:6-11.
27 Xing Y, Ding M, Ross M, et al. Metaanalysis of sentinel lymph node biopsy following pre operative chemotherapy in patients with operable breast cancer. ASCO Ann Meeting 2004; abstract 561.
28 Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine year results from National Surgical Adjuvant Breast and Bowel Project B-18. Natl Cancer Inst Monogr. 2001:96-102.
29 Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer. Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441-452.
30 Julien JP, Bijker N, Fentiman IS, et al. Radiotherapy in breast conserving treatment for ductal carcinoma in situ: first results of the EORTC randomized phase III trial 10853. Lancet. 2000;355:528-533.
31 Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia and New Zealand: randomised controlled trial. Lancet. 2003;362:95-102.
32 Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-1241.
33 Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997-1003.
34 Holli K, Saaristo R, Isalo J, et al. Lumpectomy with or without postoperative radiotherapy for breast cancer with favorable prognostic features: results of a randomized study. Br J Cancer. 2001;84:164-169.
35 Clark RM, Whalen T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. Ontario Clinical Oncology Group. J Natl Cancer Inst. 1996;88:1659-1664.
36 Vicini F, Baglan K, Kestin L, et al. Accelerated treatment of breast cancer. J Clin Oncol. 2001;19:1993-2001.
37 Vicini F, Kini V, Chen P, et al. Irradiation of the tumor bed alone after lumpectomy in selected patients with early-stage breast cancer treated with breast conserving therapy. J Surg Oncol. 1999;70:33-40.
38 Kuske R, Winter K, Arthur D, et al. A phase I/II trial of brachytherapy alone following lumpectomy for select breast cancer: toxicity analysis of Radiation Therapy Oncology Group 95–17. Int J Radiat Oncol Biol Phys. 2002;54:87.
39 Keisch M, Vicini F, Kuske R, et al. Two-year cosmetic outcome with the MammoSite® breast brachytherapy applicator: technical factors associated with optimal results when performing partial breast irradiation. Proceedings of the 45th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, Salt Lake City, Utah, October 2003.
40 Vicini F, Remouchamps V, Wallace M, et al. Ongoing clinical experience utilizing 3D conformal external beam radiotherapy to deliver partial-breast irradiation in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003;57:1247-1253.
41 Schechter NR, Strom EA, Perkins GH, et al. Immediate breast reconstruction can impact postmastectomy irradiation. Am J Clin Oncol. 2005;28:485.
42 Helvie MA, Bailey JE, Roubidoux MA, et al. Mammographic screening of TRAM flap breast reconstructions for detection of nonpalpable recurrent cancer. Radiology. 2002;224:211-216.
43 Kim SM, Park JM. Mammographic and ultrasonographic features after autogenous myocutaneous flap reconstruction mammoplasty. J Ultrasound Med. 2004;23(2):275-282.
44 LePage MA, Kazerooni EA, Helvie MA, Wilkins EG. Breast reconstruction with TRAM flaps: normal and abnormal appearance at CT. RadioGraphics. 1999;19:1593-1603.
45 Devon RK, Rosen MA, Mies C, et al. Breast reconstruction with a transverse rectus abdominis myocutaneous flap: spectrum of normal and abnormal MR imaging findings. RadioGraphics. 2004;24(5):1287-1299.
46 Monroe AT, Feigenberg SJ, Mendenhall NP. Angiosarcoma after breast-conserving therapy. Cancer. 2003;97(8):1832-1840.
47 Stokkel MP, Peterse HL. Angiosarcoma of the breast after lumpectomy and radiation therapy for adenocarcinoma. Cancer. 1992;69(12):2965-2968.
48 Neugut AI, Robinson E, Lee WC, et al. Lung cancer after radiation therapy for breast cancer. Cancer. 1993;71(10):3054-3057.
49 Ford MB, Sigurdson AJ, Petrulis ES, et al. Effects of smoking and radiotherapy on lung carcinoma in breast carcinoma survivors. Cancer. 2003;98:1457-1464.
50 Scanlon EF, Suh O, Murthy SM, et al. Influence of smoking on the development of lung metastases from breast cancer. Cancer. 1995;75(11):2693-2699.
51 Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-2106.
52 Boice JD, Harvey EB, Blettneret M, et al. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992;326(12):781-785.
53 Obedian E, Fischer DB, Haffty BG. Second malignancies after treatment of early-stage breast cancer: lumpectomy and radiation therapy versus mastectomy. J Clin Oncol. 2000;18(12):2406-2412.
54 Kelly CA, Wang XY, Chu JC, et al. Dose to the contralateral breast: a comparison of four primary breast irradiation techniques. Int J Radiat Oncol Biol Phys. 1996;34(3):727-732.
55 Early Breast Cancer Trialists’ Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomized trials. Lancet. 2000;355:1757-1770.
56 Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15 years survival: an overview of randomized trials. Lancet. 2005;366:2087-2106.
57 Harris EER, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24(25):4100-4106.
CASE 1 Utility of preradiation mammography
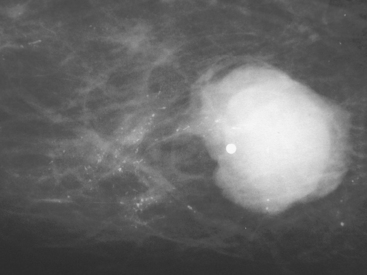
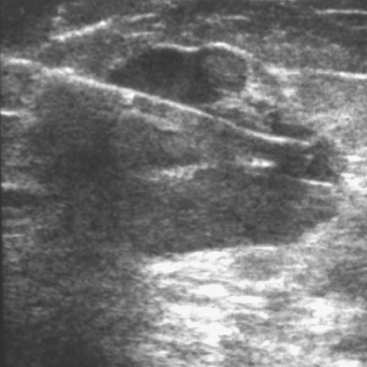
A 39-year-old woman with limited ability to communicate was brought in by her family after the patient pointed to a breast lump. The patient had a variety of severe congenital impairments, including blindness, deafness, spasticity, ataxia, and mental retardation. A palpable, 2-cm left upper inner quadrant mass was confirmed on physical examination by her primary care physician. Mammography confirmed a lobular, dense mass with ductal extension at the site of the palpable lump (Figure 1). Extending anteriorly toward the nipple from the dominant mass was a wide expanse of pleomorphic microcalcifications over an additional 4 cm. Ultrasound identified the lobular mass and served as guidance for performance of core biopsy, confirming mucinous carcinoma (Figure 2).
TEACHING POINTS
How did it happen that this patient’s suspicious DCIS calcifications were not excised at the time of her lumpectomy? Quality control happens at many different levels, and perhaps with additional checks in the system, these calcifications might not have “slipped through the cracks.” The initial interpretation clearly indicated that the microcalcifications were extensive and suspicious. The palpable lump corresponded to the dominant mass. One problem was the use of palpation guidance for localization of the abnormality in surgery. This proved effective for excision of the dominant mass, but made no provision for excision of the calcifications. In retrospect, they probably should have been needle-localized to guide more complete surgical excision.
CASE 2 Dramatic breast radiation therapy changes on mammography and MRI
Bilateral breast and peripheral lymphatic radiation therapy was given. The right regional lymphatics were treated because there was no axillary sampling. Radiation therapy was poorly tolerated with skin desquamation and fatigue.
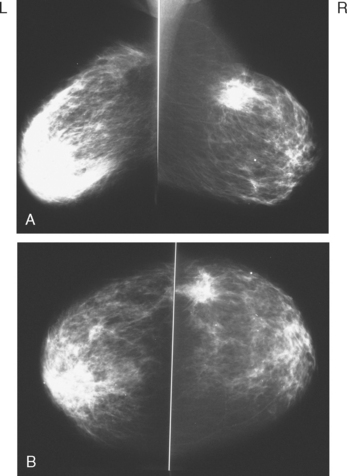
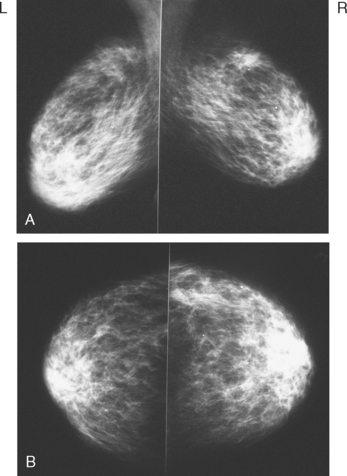
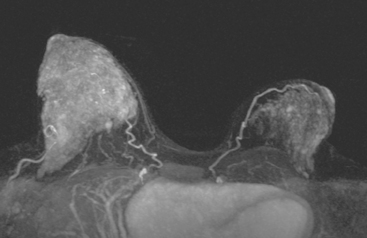
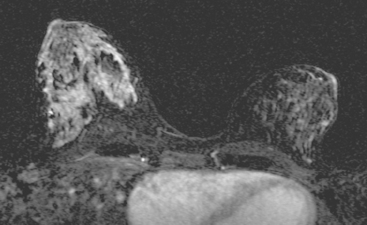
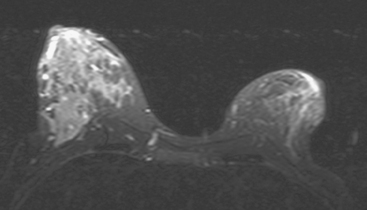
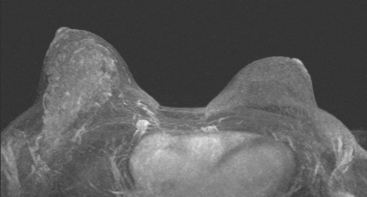
Both breasts were treated to doses of 50.40 Gy. The left lumpectomy cavity was boosted to 60.40 Gy. Planned right lumpectomy boost was not completed because of patient intolerance, and received a dose of 54.40. Mammograms were obtained before starting radiation therapy (Figure 1) and 4 months after completing radiation therapy (Figure 2).
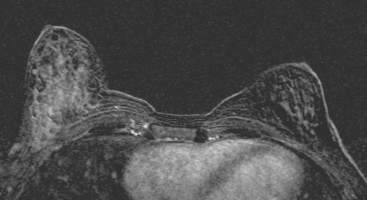
Breast MRI obtained for surveillance 4 months after completing radiation therapy shows marked bilateral changes of radiation therapy (Figure 3).
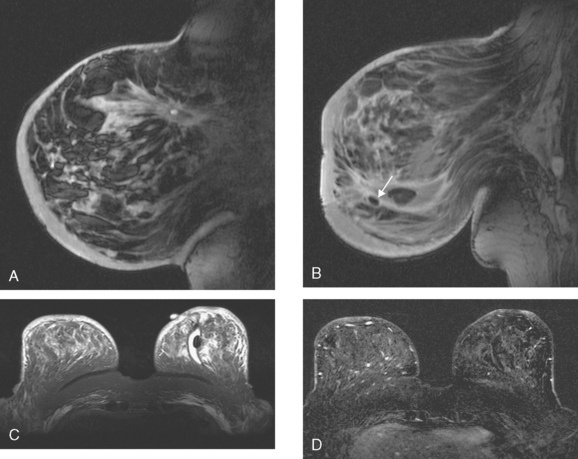
FIGURE 3 Breast MRI, concurrent with the mammogram in Figure 2, obtained 4 months after completing bilateral radiation therapy and 9 months after bilateral lumpectomies, shows dramatic radiation therapy changes. A, Unenhanced sagittal, T1-weighted, fat-saturated gradient echo image through the right lateral breast shows the UOQ scar. Architectural distortion surrounds a small fluid collection, which contains a small hyperintense focus of blood (methemoglobin). Note the marked skin thickening. B, Unenhanced sagittal, T1-weighted, fat-saturated gradient echo image through the left medial breast shows the LIQ scar as a fluid collection with surrounding distortion and scarring, containing a hypointense focus of clot (arrow). Note the marked skin thickening. C, Axial short tau inversion recovery (STIR) MRI of both breasts, through the left LIQ scar, shows hyperintense fluid outlining hypointense clot in the left scar. The skin is markedly thickened and edematous. Increased edema signal and increased trabeculation are seen throughout both breasts. Increased edema signal is also seen in the chest wall bilaterally. D, Despite the above changes, there is no notable enhancement on subtracted, enhanced, T1-weighted, gradient echo imaging, obtained at the same level as C. No enhancement outlines the left LIQ lumpectomy cavity. Only minimal enhancement of the lateral breast skin is seen bilaterally.
TEACHING POINTS
One curious finding in this case is the preradiation mammographic appearance of the left breast, seen in Figure 1, which strongly mimics radiation effects in appearance. This was 3 months after lumpectomy and axillary lymph node dissection, with 7 of 10 lymph nodes involved. This preradiation appearance was most likely due to lymphedema, given the extent of her axillary involvement and the recent surgery.
CASE 3 Two-month-old radiation therapy effects on lung (CT and PET)
A 50-year-old woman developed right breast swelling and skin changes. The prior year, she had been treated with breast conservation for a high-risk, 4.7-cm left breast cancer, estrogen receptor positive, progesterone receptor negative, unknown HER-2/neu, with angiolymphatic invasion and 10 of 11 lymph nodes involved and with focal extracapsular extension. She had been treated with chemotherapy with doxorubicin (Adriamycin), cyclophosphamide (Cytoxan), and paclitaxel (Taxol), completed 3 months before, and started tamoxifen. Radiation therapy was concluded less than 2 months before she presented with the new right breast symptoms. Physical examination showed a dominant, mobile, irregular, hard mass in the right upper breast with peau d’orange changes and periareolar erythema.
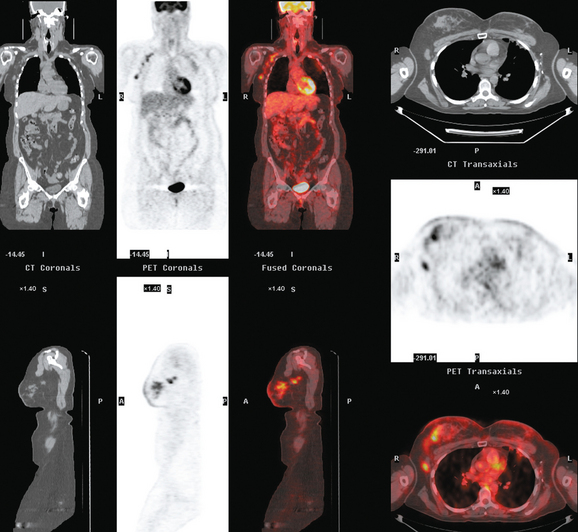
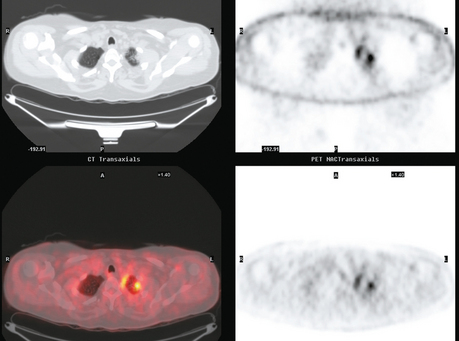
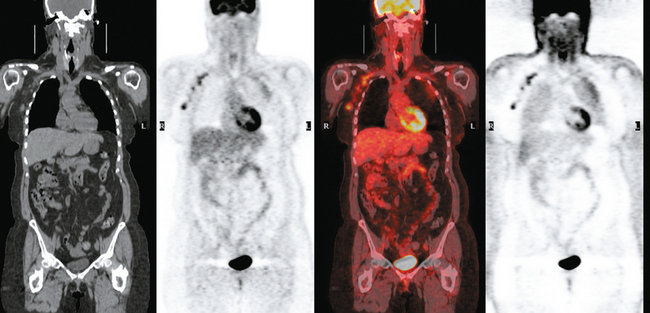
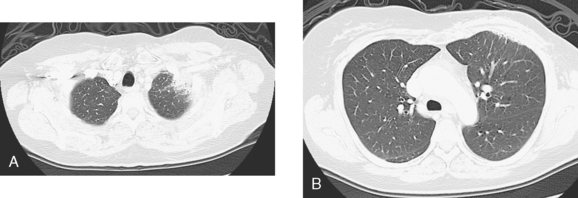
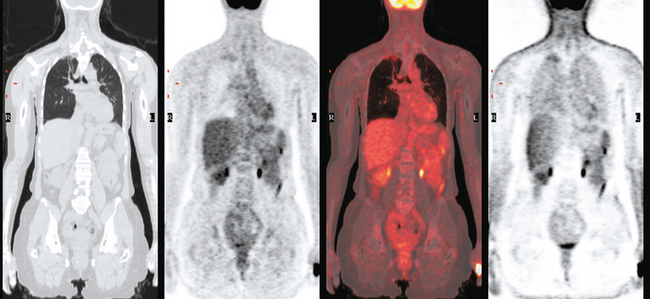
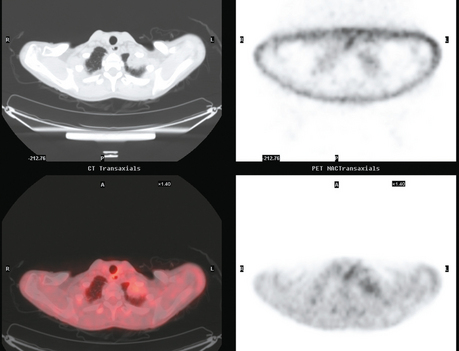
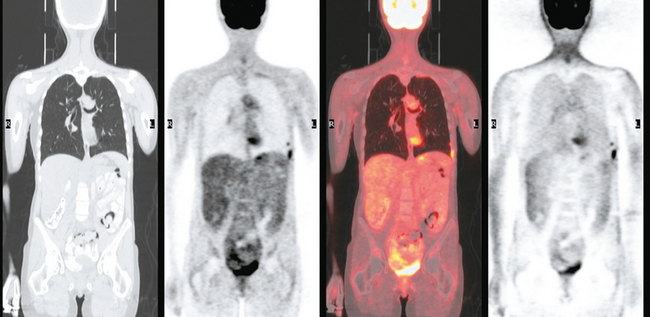
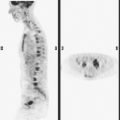
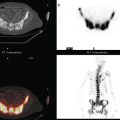
Staging for the clinically suspected inflammatory breast cancer was performed with positron emission tomography (PET)/CT. The dominant right breast cancer was intensely hypermetabolic, as were multiple right axillary lymph nodes (Figure 1). The right breast skin was thickened and increased in metabolic activity, supporting the suspected diagnosis of inflammatory cancer (subsequently confirmed by skin biopsy). In the left lung, moderately intense linear apical and subpleural parenchymal activity was seen, commensurate with recent radiation therapy (Figures 2, 3, 4, and 5). There was no evidence of distant metastases.
The patient’s chemotherapy regimen was changed to Taxol and bevacizumab (Avastin). After two cycles, her response was assessed with repeat PET/CT. These studies confirmed liver metastases, with growth of the previously seen lesions, and development of multiple, new, PET-positive liver masses. Development of right superior mediastinal and supraclavicular nodal foci of activity was also noted. The patient’s regimen was changed to gemcitabine.
TEACHING POINTS
This is an unusual case of an aggressive inflammatory breast cancer developing on the heels of a high-risk contralateral breast cancer. From an imaging point of view, it provides several take-off points for discussion. The significance and implications of breast skin activity and thickening are completely different between the two sides, emphasizing the importance of accurate clinical history and correlation. On the right, the skin changes reinforced the clinical suspicion of an inflammatory breast cancer, and dermallymphatic involvement was subsequently confirmed by skin biopsy. On the left, the skin activity and thickening are entirely commensurate with the fact that external-beam radiation to this breast concluded only 2 months before. The distribution of the left lung changes on CT are typical of radiation fibrosis, and the accompanying activity on PET is commensurate with the time course since treatment and would be expected to decline in intensity on subsequent follow-ups.
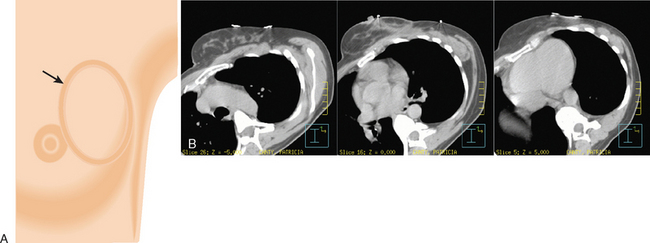
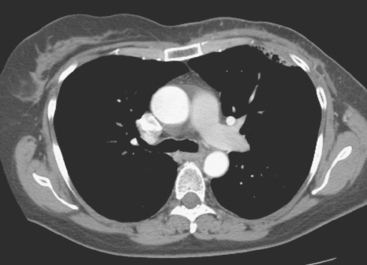
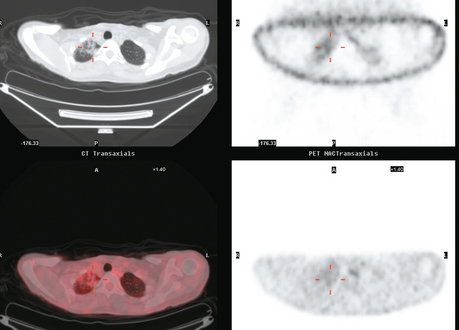
Axillary nodal involvement on the right was suspected both clinically and by imaging with ultrasound, and confirmed by FNA. However, the combination of PET and CT provides the best noninvasive estimate of the extent of involvement. In this case, we see evidence of involvement of axillary levels I, II, and III. On coronal PET/CT, we see a line of hypermetabolic right axillary lymph nodes ascending high into the axilla (Figure 5). Correlation with axial CT images allows us to localize these lymph nodes more precisely, with axillary lymph nodes lateral to the pectoralis minor designated level I, under the muscle level II, and those medial to it level III (Figure 6).
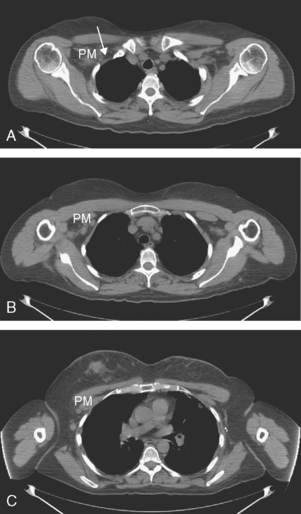
FIGURE 6 Axial unenhanced chest CT images for correlation with Figure 5 (A to C, from above to below). A, A cluster of five mildly enlarged right axillary level III lymph nodes is seen medial to the pectoralis minor muscle (PM) (arrow). B, Two enlarged right axillary level II lymph nodes are seen deep to the PM. C, Two enlarged right axillary level I lymph nodes are seen lateral to the PM. A portion of the patient’s right IDC can be seen on this section. Surgical clips can be seen on the left at axillary level I from prior lymph node dissection.
CASE 4 Breast cancer radiation–induced lung changes on CT and PET
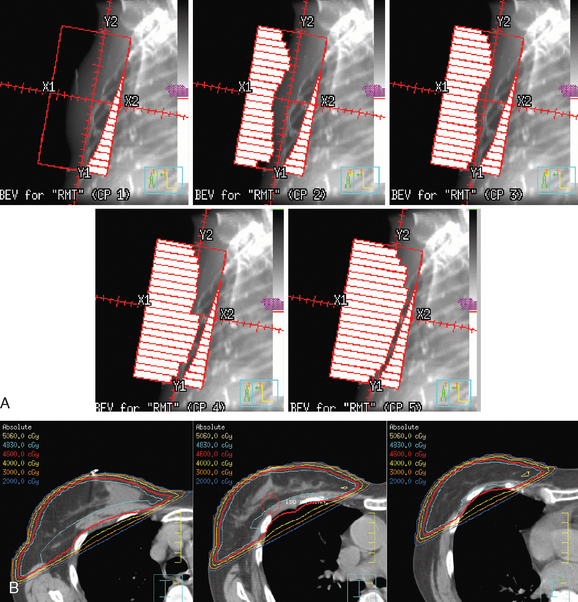
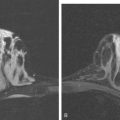
A 55-year-old woman with a large, locally advanced palpable left upper outer quadrant (UOQ) mass was treated for biopsy-proven infiltrating ductal carcinoma (IDC) with dermal lymphatic carcinoma. Clinically, the manifestations of inflammatory carcinoma were subtle at presentation, with faint periareolar erythema extending into the lower quadrants. She underwent four cycles of doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) (AC) neoadjuvant chemotherapy, followed by modified radical mastectomy, radiation to the chest wall, supraclavicular region, and posterior axilla (Figure 1).
After completion of radiation therapy, additional chemotherapy was given with four cycles of docetaxel (Taxotere). Margins of the mastectomy specimen were positive for both IDC and ductal carcinoma in situ (DCIS). The tumor was a 7- to 8-cm IDC with a 3.5-cm region of intermediate-grade DCIS, with extensive angiolymphatic invasion. Seven of 16 lymph nodes were involved. Final stage was stage IIIB, T4N2Mx, left breast inflammatory carcinoma, estrogen receptor and progesterone receptor positive, HER-2/neu negative.
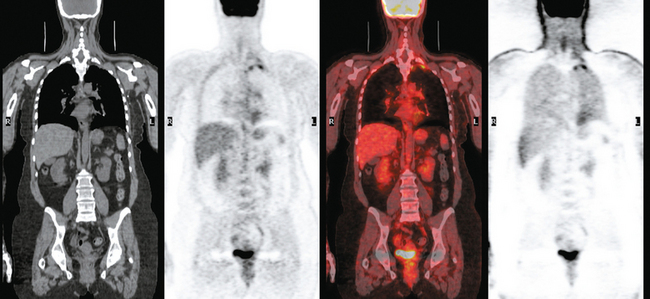
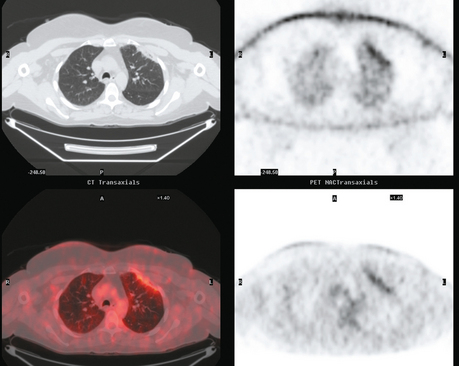
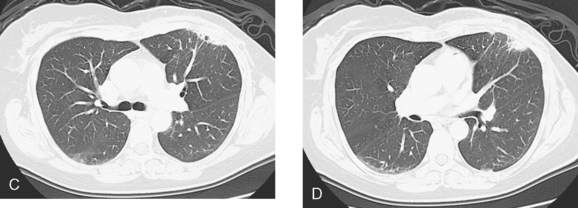
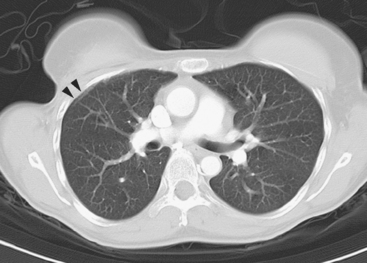
Imaging with PET/CT 4 months after the conclusion of radiation therapy showed characteristic changes of early radiation fibrosis of the underlying lung on both CT and PET (Figures 2, 3, and 4).
TEACHING POINTS
The radiation-induced changes of the lung parenchyma seen in this case delineate the most typical radiation portals utilized in breast cancer patients. The chest wall is radiated using tangential beams, which overlap with the underlying lung up to 3 cm. Administration of radiation to the supraclavicular fossa and posterior axillary boost doses include the lung apex. The most typical early lung changes visualized immediately after radiation therapy (in the first 3 months) are ground-glass opacity (see Case 8 in Chapter 6 for an example). The changes seen here show more dense lung consolidation. Lucencies within the band of consolidated lung are air bronchograms. These changes are typical of radiation-induced lung fibrosis, which is most commonly seen after 6 months or so. This may be progressive, but generally stabilizes by 2 years after treatment. Both radiation pneumonitis and fibrosis can be metabolically active on PET. The more recent the treatment, the higher the activity level can be, and for this reason, it is desirable when possible to delay PET imaging until 2 to 3 months after conclusion of radiation.
CASE 5 Typical apical changes from supraclavicular radiation therapy on CT, MRI, and PET
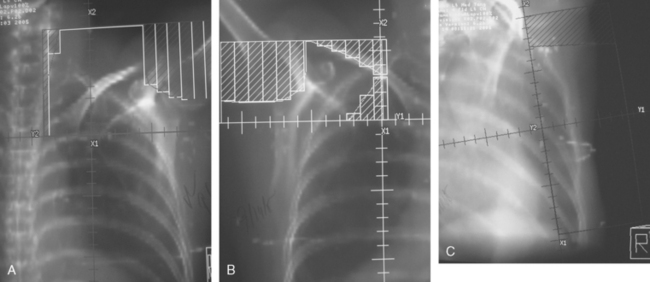
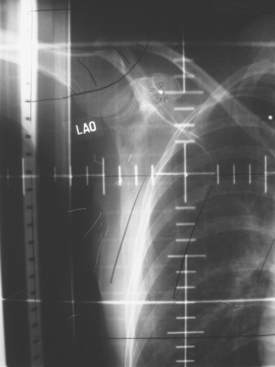
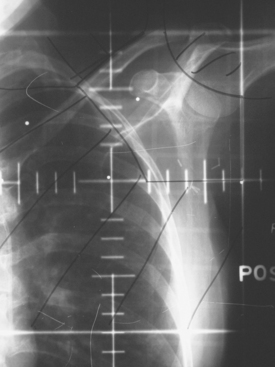
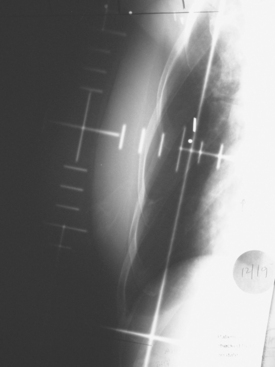
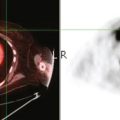
Details of her radiation therapy were as follows: Radiation fields covered the right supraclavicular fossa (SCF) and the upper axillary lymph nodes with a left anterior oblique field (Figure 1). Radiation dose of 180 cGy was given daily. After 28 fractions, the SCF received 5040 cGy. In order to bring the deeper tissue of the mid-axilla to a full dose, a daily posterior axillary boost (PAB) dose of 27 cGy was used (Figure 2). The breast was treated with non-coplanar tangential fields (Figure 3) delivering a daily dose of 180 cGy. The whole breast received 5040 cGy. The breast and regional lymphatics were treated with 9-MV photons. Technical details of the setup included use of half-beam block, wedges, table kick-out, and non-coplanar tangential orientation.
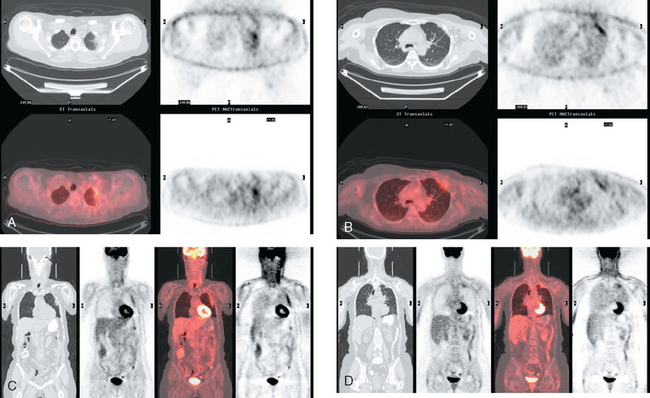
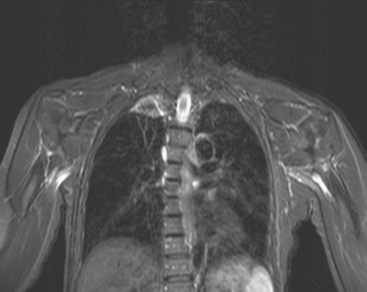
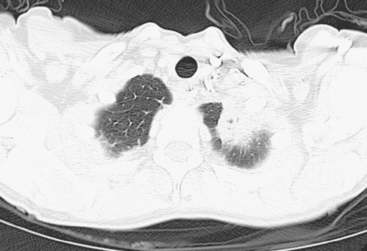
A coronal STIR sequence of the thorax (obtained in conjunction with the breast MRI) was interpreted as showing abnormal right apical signal intensity of uncertain significance (Figure 4), and a chest CT was recommended for further evaluation. As it happened, the patient had been previously evaluated with positron emission tomography (PET) and CT the year before for an unexplained rise in CA 27.29. No evidence of metastatic disease or recurrence was found at the time, and the tumor marker had subsequently normalized without treatment. Correlation with these studies showed stable findings of right apical radiation fibrosis (Figures 5, 6, 7, and 8).
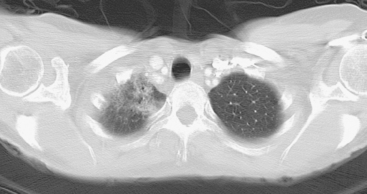
FIGURE 7 Lung window from a contrast-enhanced chest CT through the same level as Figure 6 better shows the apical consolidation.
TEACHING POINTS
Patients treated with breast-conserving surgery generally also undergo radiation therapy. Radiation effects will often be noted on subsequent imaging, and so it is important for imagers to be familiar with the typical resulting findings. Tangential fields used in breast or chest wall radiation include up to 3 cm of the underlying peripheral lung, which may develop changes that can be visualized on chest x-ray or, more commonly, on CT. Early changes, with hazy opacity, can be seen within a month after radiation therapy (range, 1 to 3 months). This may evolve to coalescent consolidation, which may subsequently dissipate or may progress to a relatively sharply demarcated zone of lung fibrosis over a period of 6 months to 2 years. The typical anterolateral peripheral lung location of tangential beam radiation changes is well illustrated by Figure 8.
The apical lung parenchymal changes depicted in Figures 4 to 7, with a sharp inferior linear edge, delineate the inferior extent of a supraclavicular port, which generally will be at the first or second intercostal space level. Mature effects such as these generally develop by 2 years after treatment and remain stable thereafter. The low-level apical cap of activity seen on PET is also typical and may persist for years or indefinitely.
Patients such as this, previously treated with lumpectomy for a high-risk tumor and with breast implants, are more difficult to image with mammography. Mammography is still useful, but postsurgical distortion and implants can limit its utility. The imaging surveillance of difficult-to-image patients such as this can be supplemented with ultrasound and MRI. MRI in particular overcomes limitations of breast density, postsurgical alterations, and the presence of implants. It is the most sensitive modality for the detection of occult breast malignancies.
CASE 6 Mass-like radiation fibrosis
CT scans obtained at the time of the current left chest wall recurrence (same patient as Case 11 in Chapter 10) showed a spiculated, mass-like finding in the left lung apex (Figure 1). This was associated with only minimal, linear, low-level positron emission tomography (PET) scan activity (Figures 2 and 3) and correlated with the history of supraclavicular radiation therapy 10 years before, as well as axillary radiation 7 years before. Review of the patient’s prior records identified a prior CT scan with similar findings 3 years before, at which time she had undergone a biopsy, with no malignancy obtained.
CASE 7 Early postradiation changes on breast MRI
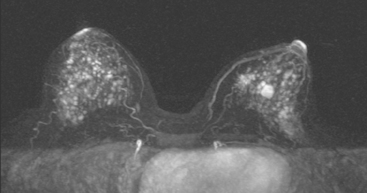
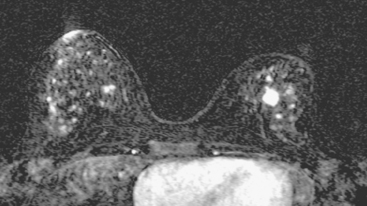
A 53-year-old perimenopausal woman underwent lumpectomy for a stage I, T1N0, 1.2-cm left infiltrating ductal carcinoma (IDC), which was estrogen receptor and progesterone receptor positive and HER-2/neu negative. One sentinel lymph node was negative. Breast MRI was obtained during her preoperative evaluation because of extreme breast density (Figures 1 and 2). Extensive stippled fibrocystic type foci of enhancement made the evaluation of the opposite breast difficult, for which repeat breast MRI was recommended in 6 months. In the meantime, the patient underwent surgery on the left for the known IDC and completed radiation therapy 3 months before returning for repeat breast MRI (Figure 3).
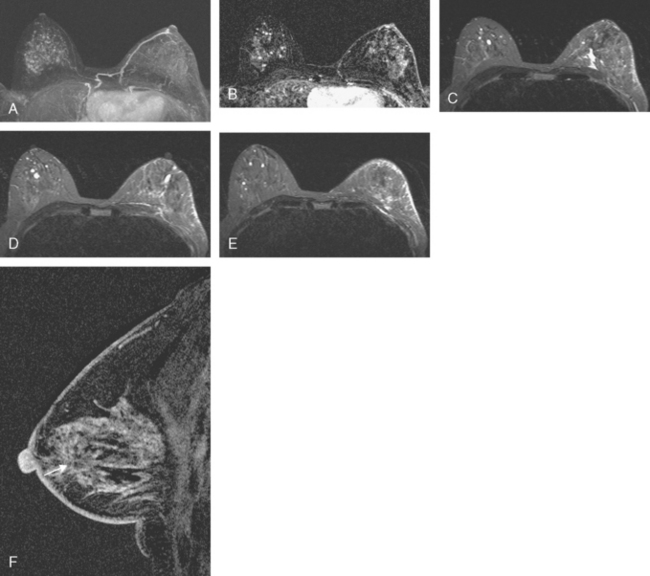
FIGURE 3 Repeat breast MRI, 6 months later, obtained to follow up the right breast, because of concern at the time of the first MRI that the extensive enhancement could be obscuring an abnormality. The patient had finished radiation therapy to the left breast 3 months before this study, and multiple post-treatment findings are seen as follows: A, Enhanced, subtracted, axial MIP view of both breasts shows a similar pattern of diffuse punctate foci of enhancement on the right. The pattern has changed considerably on the left. The dominant mass has been resected and is no longer seen. The enhancement is lower level, diffuse, and subdued (compare with Figure 1). B, A single slice from early in the enhanced, subtracted, dynamic axial series of this follow-up breast MRI shows new left breast skin thickening and mild enhancement, secondary to the radiation therapy. There also is new, linear enhancement of the left chest wall. C to E, Axial STIR images, from above to below show a postsurgical fluid collection on the left centrally, which tracks posteriorly to the pectoral muscle level. Radiation therapy effects are evident, with diffuse edema and reticulation of the left breast. Thickening and edema are noted of the left breast skin, particularly laterally. Increased signal from edema is also noted within the left pectoral and chest wall musculature. On the right side, scattered tiny breast cysts are seen. F, Enhanced, subtracted, sagittal view of the left breast, obtained about 5 minutes after contrast administration, shows the scar as architectural distortion in the retroareolar area (arrow), which enhances to the same degree as the breast parenchyma. Surgery was performed 6 months before this study.
TEACHING POINTS
In general, treatment effects from prior surgery and radiation are readily identifiable and generally do not significantly hamper breast MRI interpretation. That said, it is important to have accurate clinical information about the location of prior surgeries and the time course since interventions like surgery or radiation therapy. Breast MRI technologists should mark with vitamin E capsules or another suitable marker the locations of any palpable lumps that the patient can identify, as well as the locations of surgical scars or biopsy sites. The interpreter needs to bear in mind that if prior biopsies were image-guided, marker placements at the skin entry site may correspond in location only generally to the actual sites of underlying lesions.
One late effect of radiation therapy that we have observed on a number of occasions is demonstrated here but has not been well documented in the literature. Radiated breasts often have marked reduction of their baseline “stippled” pattern of enhancement after treatment. Presumably, this is due to the diffuse tissue and vascular damage induced by radiation to the treated field. The processes underlying radiation injury of tissues are still being delineated. Depletion of certain target cells, leading to fewer source cells for repopulation during post-treatment recovery, appears to be implicated. See also Case 8 for another example.
CASE 8 Late postradiation changes on breast MRI
Breast MRI showed striking asymmetry between the two sides in enhancement (Figure 1). The right side showed the expected pregnancy-induced hormonal effects, with intense, diffuse enhancement, making it difficult to exclude a breast cancer. The previously radiated left side was smaller and showed diffuse but subdued enhancement compared with the untreated side (Figures 2 and 3).
Repeat breast MRI, obtained a year later (8 months postpartum), showed persistent asymmetry between sides, but both sides showed marked interval regression of the pregnancy-related hormonal effects and enhancement (Figures 4 and 5).
TEACHING POINTS
Breast imaging during pregnancy is difficult. Hormonal changes of pregnancy result in growth of the breasts, which double in weight, and blood flow increases dramatically (180%). When a patient who has previously been treated for breast cancer becomes pregnant, her physicians may request imaging “clearance” of the breasts. Two-view film screen mammography with abdominal shielding results in a fetal dose estimated to be 500 μGy, which is considered acceptable. Of course, in a patient as young as this, mammography during pregnancy may well be limited because of breast density. There are data to suggest that mammographic density does not significantly change during pregnancy from baseline.
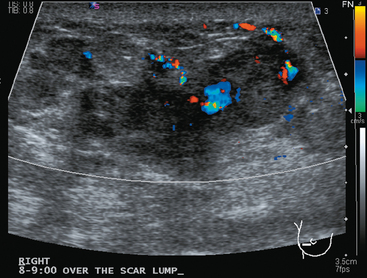
CASE 9 Radiation-induced angiosarcoma
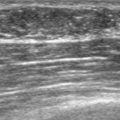
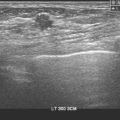
Imaging evaluations were highly suspicious for a multifocal recurrence. Ultrasound showed a highly irregular and very vascular dominant mass (Figure 1), with smaller additional sites of involvement suggested as well by ultrasound. Ultrasound-guided core needle biopsy confirmed a high-grade angiosarcoma.
TEACHING POINTS
This case represents a late, rare complication of breast radiation therapy, with induction of an angiosarcoma. Breast angiosarcoma is rare, accounting for 0.04% of all breast malignancies. Breast angiosarcomas may be primary or secondary; secondary angiosarcoma occurs in patients previously treated for breast cancer with radiation therapy or complicated by lymphedema. Primary angiosarcomas tend to occur in younger women (second to fourth decades of life) than secondary angiosarcomas, which generally occur in women after the age of 50 years, with a latency period after treatment of 4 to 7 years.
The prognosis is uniformly poor for patients such as this with high-grade disease.
Liberman L, Dershaw DD, Kaufman RJ, et al. Angiosarcoma of the breast. Radiology. 1992;183:649. (Retrospective review of 29 cases of primary angiosarcoma of the breast.)
Marchant LK, Orel SG, Perez-Jaffe LA, et al. Bilateral angiosarcoma of the breast on MR imaging. AJR Am J Roentgenol. 1997;169:1009-1010. (Case report of bilateral primary angiosarcoma appearance on breast MRI.)