Chapter 102 Radiation Therapy and Radiosurgery in the Management of Craniopharyngiomas
Craniopharyngiomas are benign extra-axial epithelial tumors that arise from squamous epithelial remnants of Rathke’s pouch, near the pituitary gland.1 These cells may extend from the nasopharynx to the tuber cinereum and may arise within the sphenoid bone, the sella, or the suprasellar region. Although craniopharyngiomas are rare, they are the most common suprasellar tumor in the pediatric age group, accounting for as many as 5% of all intracranial tumors or up to 10% of pediatric brain tumors.2 Its incidence has been estimated to be about 1.5 per million persons per year,3,4 but may be considerably higher in specific ethnic groups, such as Japanese children (5.25 per million).5 Craniopharyngiomas have a bimodal age distribution, generally appearing in young patients between the ages of 5 and 14 years and in adults between 50 and 74 years.
Despite being histologically benign, craniopharyngiomas can cause severe and often permanent damage to nearby hypothalamic, visual, and endocrine apparatus. The presentation of these tumors may include symptoms related to endocrine derangement of the hypothalamic–pituitary axis, with severity dependent upon location, size, and rate of growth. Mass effect from the tumor may result in increased intracranial pressure presenting as headache, nausea, and vomiting. Cases with larger mass lesions may also present with hydrocephalus (seen more commonly in children than in adults), as a result of the obstruction of the cerebral aqueduct or the interventricular foramina.6,7 Compression of the nearby optic apparatus typically results in visual field defects, such as chiasmal syndrome and papilledema. Endocrine disruption often manifest as amenorrhea, hypothyroidism, and diabetes insipidus.8,9
Current treatment strategies include cystic drainage, intracavity chemotherapy, limited or gross total resection, and radiation therapy. These strategies are often combined in a patient-specific treatment plan based on age at presentation, tumor size, relation to optic chiasm and third ventricle, presence of hydrocephalus, and degree of pituitary endocrinopathy. If total excision can be safely performed with minimal risk to these structures, then surgery remains the treatment of choice as this allows rapid decompression, minimizes recurrence, and provides a histologic diagnosis. However, judgment of minimal risk is often unclear as some favor subtotal resection coupled with adjunctive therapy to achieve similar outcomes.10–18 Although surgical approaches are often curative, they harbor high treatment-related morbidity and mortality due to the close proximity of crucial neurovascular structures. Recurrent craniopharyngiomas must be considered separately, as secondary surgery is associated with higher risk of complications and a lower cure rate.16,19–23 More recently, stereotactic radiosurgery techniques have become increasingly utilized as either a primary or secondary treatment for craniopharyngioma patients.
Surgical Outcomes
Complete surgical resection is a primary objective and has curative potential. In a recent series by Shi et al., 284 patients (58 children) were treated surgically, without adjunctive therapy, between 1996 and 2006. Total, subtotal, and partial removal of the tumors were achieved in 237 (83.5%), 34 (12.0%), and 13 (4.5%) patients, respectively.24,25 Upon follow-up, 23 (14.1%) patients experienced recurrence 1 to 3.5 years after total resection, and 24 (64.9%) recurred after 0.25 to 1.5 years after subtotal or partial resection. In this series, the early mortality rate was 4.2%. In another 25-year retrospective study by Van Effenterre and Boch, 122 patients underwent gross total (59%), subtotal (29%), or partial (12%) surgical resection. During the follow-up period, 29 patients (24%) experienced one or more recurrences. The delay to recurrence was 1 to 180 months (mean 42 months, median 12 months). Patients that underwent total, subtotal, or partial removal experienced 13%, 33%, and 69% recurrence, respectively. Radiotherapy was reserved only for cases of recurrence. The surgical mortality rate was 2.5% and overall survival was 95% at two years, 91% at 5 years, and 83% at 10 years.26
The comparison of surgical complications across various series produces a variable picture. Most of the recent large series report a total resection rate of 59% to 90%.16,26–28 The 10-year recurrence-free survival rates have been reported as 74% to 81% for gross total resection,23,29,30 41% to 42% after partial removal,31,32 and 83% to 90% after combined surgery and radiotherapy.18,42 Surgical mortality rates vary between 1.1% and 4.2%.16,26,28,33 It is well documented that recurrent tumors are associated with significantly higher risk and poorer outcome, with overall mortality rates reported between 10.5% to 40.6%.16, 28 Pituitary dysfunction may occur in 50% to 100% of patients, the most common being diabetes insipidus. Visual deterioration may occur in up to 50% of patients undergoing gross total resection.34
Radiation Therapy for Craniopharyngiomas
Endocavitary Radiation Therapy
Endocavitary/intracavitary irradiation with a beta-emitter (186Re, 32P, 198Au, 90Y) or an antitumoral antibiotic (bleomycin) can be used to treat purely cystic or cystic components of craniopharyngiomas.35 This treatment modality requires the use of stereotactic technique to achieve intracystic instillation of radioactive agents. In a recent retrospective study of endocavitary irradiation (186Re) treatment by Derrey et al., of 48 patients treated, complete cystic resolution was achieved in 17 patients (44%) and partial resolution in another 17 patients (44%). Visual function improved in 12 patients, while baseline endocrine function was preserved.35 Similarly, Julow and colleagues observed an 80% reduction in 47 patients and complete disappearance of cyst in 27 patients within 1 year after treatment with intracystic colloidal yttrium-90.36 Across several studies, the response rate of tumors to endocavitary/intracavitary irradiation is 71% to 88%.37,38 However, because intracavitary irradiation is limited to cystic tumors, recurrence, and survival rates with this type of therapy alone are considered inferior to surgery or external radiotherapy.24,38 Additionally, the risk of visual deterioration is considerable, possibly due to unpredictable radiation dose to the optic pathway and radiation damage from leakage. In a review by Caceres, no change or improvement in visual acuity after intra-cavitary irradiation ranged from 42% to 99% while 31% to 58% experienced deterioration in visual function.39
External Beam Radiation Therapy
Fractionated radiation therapy improves craniopharyngioma control and survival32,40–44 and is the standard treatment for residual or recurrent tumor. Most series demonstrate that when combined with subtotal resection, adjuvant radiotherapy allows for greater tumor control and survival than surgery alone.31, 45–49 In a study by Varlotto et al.,49 an 89% tumor control rate was seen in patients who received both subtotal resection and external beam irradiation.39 Stripp and colleagues48 compared 57 patients treated only with surgery to 18 treated with subtotal resection combined with radiation therapy, demonstrating a 10-year tumor control rate of 42% and 84%, respectively. The case for primary radiation therapy for recurrent craniopharyngioma is even stronger for lower risk and better outcome (30% vs. 90% 10-year progression-free survival).12,50–52 Finally, Karavitaki and colleagues examined the records of 121 patients and subdivided the patients into four treatment categories: gross total removal, gross total removal with radiotherapy, partial removal, and partial removal with radiotherapy. The recurrence-free survival rate was 100% at 10 years in the gross total removal and gross total removal with radiotherapy groups, 38% in the partial removal group, and 77% in the partial removal with radiotherapy group.34
With radiotherapy, the risk of neurotoxicity from radiation injury should be considered alongside gains in potential tumor control. Conventionally fractionated focal radiation therapy around the sellar–suprasellar region is also associated with risks similar to surgery. Disruption of the hypothalamic-pituitary axis (30% to 70%) may result in diabetes insipidus, panhypopituitarism, hypogonadism, hypothalamic obesity, or sleep disturbance.53–55 The normal optic apparatus is particularly sensitive to radiation, and optimized dose and fractionation regimes carry a 3% risk of optic neuropathy.56–58 There is also considerable discussion about the effect of radiation on cognitive function, an issue particularly pertinent in the pediatric population. Additionally, radiation itself carries the risk of secondary malignancies,59–61 radiation necrosis,61,62 and vasculopathy, which also have end-neurodegenerative effects.
Typically, craniopharyngiomas are treated with doses between 45 and 60 Gy in 1.8- to 2-Gy fractions to prevent growth of tumor and minimize injury to the visual pathways. Long-term (10 years) local control ranges from 31% to 42% with surgery alone compared with 57% to 89% with surgery and radiotherapy.31,32,45,47–49 However, there are limitations as the wide treatment field includes irradiating many structures, such as the optic apparatus, pituitary gland, hypothalamus, and medial temporal lobe. The risk may only manifest itself after a long delay, but this is particularly important since benign conditions such as craniopharyngiomas confer favorable long-term survival and its predilection for the pediatric population. Another limitation is when conventional radiotherapy fails, it almost inevitably precludes further radiotherapy treatment to the recurrent tumor. Finally, although of minor importance, conventional fractionated radiotherapy usually takes place over a 6-week course, which is less attractive to patients when compared to other shorter treatment courses. For these reasons, radiosurgery (particularly multisession radiosurgery) may present a more amenable option, especially to tumors surrounding the optic apparatus.
Stereotactic Radiosurgery
Stereotactic radiosurgery (SRS) is a relatively recent therapeutic option that has significantly improved the effectiveness of and morbidity associated with radiation therapy. With SRS, one to five sessions of radiation are utilized to treat residual or recurrent lesions. The application of stereotaxis for target localization, treatment planning, and daily treatment immobilization allows for a more precise delivery of radiation dose with a steeper dose gradient between tumor and parenchymal tissue to prevent further neurologic deficit. The irradiation dose can be delivered using either a multiple cobalt-60, gamma radiation-emitting source such as a gamma knife (GK) or a modified linear accelerator (LINAC, CyberKnife). Most stereotactic systems can deliver a radiation beam with no more than approximately 1 mm of error. Historically, SRS for craniopharyngiomas was limited to tumors 3 cm or less that are 3 to 5 mm away from the optic chiasm and nerves. In the case of single-session radiosurgery, the optic chiasm becomes a limiting anatomic structure capable of only receiving 8 to 10 Gy per session before the incidence of optic neuropathy increases.63,64 More recent multisession radiosurgery using image-guided radiosurgical techniques has allowed for treatment of craniopharyngiomas immediately adjacent to the anterior visual pathways.65
In the current literature, several studies have reported safe and effective long-term results with the application of SRS using a GK for the treatment of craniopharyngiomas.66–69 Kobayashi et al. published the largest treatment and outcomes series with 98 cases. At a mean marginal dose of 11.5 Gy and a mean tumor size of 3.5 cm3, Kobayashi and colleagues observed a tumor control rate of 79.6%, with a complete response in 19.4% and partial response in 67.4% of the cases.70 The actuarial 5- and 10-year survival rates were 94.1% and 91% with respective progression-free survival rates of 60.8% and 53.8%. Yomo and colleagues demonstrated the outcomes of 18 patients with residual or recurrent craniopharyngioma who were treated by the Leksell Gamma Knife Model C. Tumor growth (mean tumor volume of 1.8 cm3 and a mean marginal irradiation dose of 11.6 Gy) was controlled in 17 cases (94%), and volume reduction was attained in 13 cases (72%).71 No new endocrinopathy was observed and 3 patients experienced substantial improvement of visual functions following shrinkage of the neoplasm. In another study by Chung et al., tumor control was achieved in 87% of the 31 patients and 84% had fair to excellent clinical outcome.72 Finally, Minniti et al. completed a meta-analysis of eight published studies that includes 252 patients who underwent either unfractionated radiosurgery or GK therapy, demonstrating a tumor control rate of 69%. Taken together (Table 102-1), the published studies on GK therapy for craniopharyngiomas demonstrate an average control rate of 90% for solid tumors, 88% for cystic tumors, and 60% for mixed tumors.73 Tumor control was achieved with a mean marginal dose of 12 Gy and recurrence of tumor was observed in 85% of cases that received a marginal dose of less than 6 Gy.
With the current advances in image-guided radiosurgical technology, the principle of multi-session delivery of radiosurgery can be incorporated with the anatomic precision and conformality of radiosurgery. This allows for the precise delivery of potentially safer radiation doses than encountered in single session radiosurgery, while exploiting the volume effect by applying higher and more effective doses than was possible with conventional radiation therapy. The multisession delivery approach is particularly pertinent in treating craniopharyngiomas, which are often located near delicate neurovascular structures. The tolerance of these critical structures to radiation depends on the amount of radiation being received, volume of tissue irradiated, previous insult, and prior radiotherapy. Due to the proximity of the tumors to the optic apparatus, single doses of 8 to 10 Gy appear tolerable to avoid damage to nearby structures.24 Higher doses to optic nerve are associated with increasing rates of deficit. Leber et al. reported that optic neuropathy occurred in 22 (26.7%) patients who received 10 to 15 Gy and 13 (78%) of patients who received more than 15 Gy, while 31 patients who received less than 10 Gy were without optic insult.64 Likewise, Stafford and colleagues observed optic neuropathy in <2% of patients who received 8 to 10 Gy and in 6.9% who received more than 12 Gy after treatment with RS for benign tumors of the sellar or parasellar region.73,74
Cyberknife SRS for Craniopharyngiomas: Our Experience
In a study by Lee et al.,75 11 patients with residual craniopharyngiomas within 2 mm of the optic apparatus or pituitary gland were treated with the CyberKnife SRS System (Accuray, Sunnyvale, CA). The clinical presentation, surgical history, radiation received, and outcome of these 5 male and 6 female patients with an average age of 34.5 years are documented in Tables 102-2 and 102-3. A mean marginal dose of 21.6 Gy prescribed to a mean isodose line of 75% was applied over multiple sessions (Figure 102.1). The mean maximum dose was 29.9 Gy and the mean target volume was 6 cm3. Patient outcomes were quantified using magnetic resonance imaging (MRI) and formal Goldman visual field assessments at 6 months intervals for two years, then once every year. Prior to CyberKnife therapy, 10 patients suffered from a degree of visual loss while 5 had endocrine abnormalities requiring hormonal replacement. Ten patients had operative reports documenting a subtotal resection with radiologic confirmation and one had a complete resection with follow-up MRI demonstrating recurrence 1 year after surgery. Residual tumor was most often located in the suprasellar region and in 10 cases was found to be against or displacing the optic nerve or chiasm. The pituitary stalk alone was compressed in 1 patient.
Table 102-2 Patient Characteristics Undergoing CyberKnife Radiosurgery for Residual Craniopharyngioma between 2000 and 2007
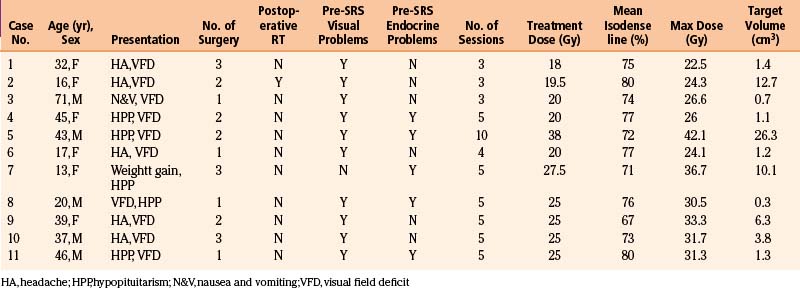
Table 102-3 Summary of 11 Patients and Treatment Planning Characteristics Included in Analysis
| Gender (No.) | |
| Male | 5 |
| Female | 6 |
| Age (years) | |
| Mean | 34.5 (range, 13–71) |
| Previous Surgery (No.) | |
| 1 | 4 |
| 2 | 4 |
| 3 | 3 |
| Previous Radiotherapy (No.) | 1 |
| Extent of Last Resection (No.) | |
| Complete | 1 |
| Subtotal | 10 |
| Site of residual or recurrent tumor | |
| Intrasellar | 1 |
| Suprasellar | 9 |
| Both | 1 |
| Against optic apparatus | 8 |
| Against pituitary stalk or gland | 1 |
| Both | 2 |
| CyberKnife Sessions (No.) | |
| 3 | 3 |
| 4 | 1 |
| 5 | 6 |
| 10 | 1 |
| Mean Target Volume (cm3) | 6 (range, 0.3–26.3) |
| Mean Marginal Dose (Gy) | 21.6 (range, 18–38) |
| Mean Maximal Dose (Gy) | 29.9 (range, 24.1–42.1) |
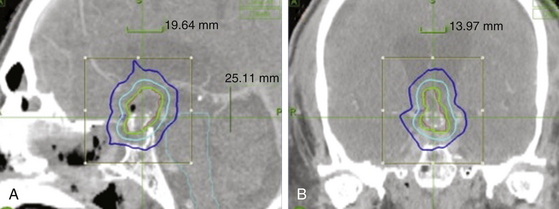
FIGURE 102-1 A typical CyberKnife treatment plan for a craniopharyngioma tumor shown in sagittal (A) and coronal (B) views.
The mean follow-up time was 15.4 months (range, 4 to 64 months). All 10 patients with visual field or acuity problems either improved or remained stable after CyberKnife radiosurgery. In this series, treatment plans were designed to keep the dose experienced by the optic apparatus less than 5 Gy during any single session. The volume of the optic apparatus that received 80% of the prescribed dose was less than 0.05 cm3, whereas the volume that received 50% of the dose was less than 0.5 cm3. Therefore, the actual volume of the optic segment that received 5 Gy would be small relative to the total volume of the optic apparatus. Preservation of baseline visual function is supported by our previous work which showed that the risk of visual loss with multi-session radiosurgery appears to be low for perioptic tumors.65,76 Radiation-induced optic neuropathy is a known entity that tends to present over the course of several years; however, our study’s short follow-up prevents definitive conclusions regarding the effect of multisession therapy.
There were no new neuroendocrine problems and the five patients with endocrine derangement remained stable with no new deterioration after CyberKnife treatment. Tumor shrinkage was seen in seven patients, with three staying the same at 2 years post-treatment, resulting in a 91% tumor control rate. One patient developed a cystic enlargement of the residual tumor without any worsening symptoms or signs. Irradiation of cystic craniopharyngiomas may result in cystic enlargement, which does not represent tumor recurrence and may later regress.77 In our series, the patient’s symptoms remained stable; however, rigorous clinical and radiologic including visual and neuroendocrine assessment is critical. We believe that multisession treatment regimens minimize the risk to the optic apparatus and pituitary gland while delivering an appropriate amount of radiation for disease control.
Disclosure and Acknowledgment
Drs. Adler and Chang are shareholders of Accuray, Inc. Steven D. Chang, MD, is supported in part by a research gift from Robert C. and Jeannette Powell.
Adler J.R.Jr., Gibbs I.C., Puataweepong P., et al. Visual field preservation after multisession CyberKnife radiosurgery for perioptic lesions. Neurosurgery. 2006;59:244-254. discussion 244–254
Derrey S., Blond S., Reyns N., et al. Management of cystic craniopharyngiomas with stereotactic endocavitary irradiation using colloidal 186. Re: a retrospective study of 48 consecutive patients. Neurosurgery. 2008;63:1045-1052. discussion 1052-1053
Fisher B.J., Gaspar L.E., Noone B. Radiation therapy of pituitary adenoma: delayed sequelae. Radiology. 1993;187:843-846.
Flickinger J.C., Lunsford L.D., Singer J., et al. Megavoltage external beam irradiation of craniopharyngiomas: analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys. 1990;19:117-122.
Garre M.L., Cama A. Craniopharyngioma: modern concepts in pathogenesis and treatment. Curr Opin Pediatr. 2007;19:471-479.
Girkin C.A., Comey C.H., Lunsford L.D., et al. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997;104:1634-1643.
Gopalan R., Dassoulas K., Rainey J., et al. Evaluation of the role of gamma knife surgery in the treatment of craniopharyngiomas. Neurosurg Focus. 2008;24:E5.
Hetelekidis S., Barnes P.D., Tao M.L., et al. 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys. 1993;27:189-195.
Honegger J., Buchfelder M., Fahlbusch R. Surgical treatment of craniopharyngiomas: endocrinological results. J Neurosurg. 1999;90:251-257.
Jane J.A.Jr., Laws E.R. Craniopharyngioma. Pituitary. 2006;9:323-326.
Karavitaki N., Cudlip S., Adams C.B., et al. Craniopharyngiomas. Endocr Rev.. 2006;27:371-397.
Kobayashi T. Long-term results of gamma knife radiosurgery for 100 consecutive cases of craniopharyngioma and a treatment strategy. Prog Neurol Surg. 2009;22:63-76.
Leber K.A., Bergloff J., Pendl G. Dose–response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88:43-50.
Minniti G., Esposito V., Amichetti M., et al. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg Rev. 2009;32:125-132. discussion 132
Moon S.H., Kim I.H., Park S.W., et al. Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas–a study in single institute. Childs Nerv Syst. 2005;21:799-807.
Pollock B.E., Lunsford L.D., Kondziolka D., et al. Phosphorus-32 intracavitary irradiation of cystic craniopharyngiomas: current technique and long-term results. Int J Radiat Oncol Biol Phys. 1995;33:437-446.
Rajan B., Ashley S., Gorman C., et al. Craniopharyngioma–long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993;26:1-10.
Shi X.E., Wu B., Zhou Z.Q., et al. Microsurgical treatment of craniopharyngiomas: report of 284 patients. Chin Med J (Engl). 2006;119:1653-1663.
Stafford S.L., Pollock B.E., Leavitt J.A., et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177-1181.
Stripp D.C., Maity A., Janss A.J., et al. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys. 2004;58:714-720.
Ulfarsson E., Lindquist C., Roberts M., et al. Gamma knife radiosurgery for craniopharyngiomas: long-term results in the first Swedish patients. J Neurosurg. 2002;97:613-622.
Van Effenterre R., Boch A.L. Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg. 2002;97:3-11.
Varlotto J.M., Flickinger J.C., Kondziolka D., et al. External beam irradiation of craniopharyngiomas: long-term analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys. 2002;54:492-499.
Yasargil M.G., Curcic M., Kis M., et al. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg. 1990;73:3-11.
Yomo S., Hayashi M., Chernov M., et al. Stereotactic radiosurgery of residual or recurrent craniopharyngioma: new treatment concept using Leksell Gamma Knife Model C with automatic positioning system. Stereotact Funct Neurosurg. 2009;87:360-367.
1. Karavitaki N., Cudlip S., Adams C.B., et al. Craniopharyngiomas. Endocr Rev. 2006;27:371-397.
2. Rickert C.H., Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17:503-511.
3. Bunin G.R., Surawicz T.S., Witman P.A., et al. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89:547-551.
4. Haupt R., Magnani C., Pavanello M., et al. Epidemiological aspects of craniopharyngioma. J Pediatr Endocrinol Metab. 2006;19(Suppl 1):289-293.
5. Kuratsu J., Ushio Y. Epidemiological study of primary intracranial tumors in childhood. A population-based survey in Kumamoto Prefecture, Japan. Pediatr Neurosurg. 1996;25:240-246. discussion 247
6. Jane J.A.Jr., Laws E.R. Craniopharyngioma. Pituitary. 2006;9:323-326.
7. Garre M.L., Cama A. Craniopharyngioma: modern concepts in pathogenesis and treatment. Curr Opin Pediatr. 2007;19:471-479.
8. Garnett M.R., Puget S., Grill J., et al. Craniopharyngioma. Orphanet J Rare Dis. 2007;2:18.
9. Gopalan R., Dassoulas K., Rainey J., et al. Evaluation of the role of Gamma Knife surgery in the treatment of craniopharyngiomas. Neurosurg Focus. 2008;24:E5.
10. Caldarelli M., Massimi L., Tamburrini G., et al. Long-term results of the surgical treatment of craniopharyngioma: the experience at the Policlinico Gemelli, Catholic University, Rome. Childs Nerv Syst. 2005;21:747-757.
11. Isaac M.A., Hahn S.S., Kim J.A., et al. Management of craniopharyngioma. Cancer J. 2001;7:516-520.
12. Kalapurakal J.A., Goldman S., Hsieh Y.C., et al. Clinical outcome in children with craniopharyngioma treated with primary surgery and radiotherapy deferred until relapse. Med Pediatr Oncol. 2003;40:214-218.
13. Thompson D., Phipps K., Hayward R. Craniopharyngioma in childhood: our evidence-based approach to management. Childs Nerv Syst. 2005;21:660-668.
14. Tomita T., Bowman R.M. Craniopharyngiomas in children: surgical experience at Children’s Memorial Hospital. Childs Nerv Syst. 2005;21:729-746.
15. Zuccaro G. Radical resection of craniopharyngioma. Childs Nerv Syst. 2005;21:679-690.
16. Fahlbusch R., Honegger J., Paulus W., et al. Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg. 1999;90:237-250.
17. Honegger J., Barocka A., Sadri B., et al. Neuropsychological results of craniopharyngioma surgery in adults: a prospective study. Surg Neurol. 1998;50:19-28. discussion 28-29
18. Honegger J., Buchfelder M., Fahlbusch R. Surgical treatment of craniopharyngiomas: endocrinological results. J Neurosurg. 1999;90:251-257.
19. Caldarelli M., di Rocco C., Papacci F., et al. Management of recurrent craniopharyngioma. Acta Neurochir (Wien). 1998;140:447-454.
20. Fisher P.G., Jenab J., Gopldthwaite P.T., et al. Outcomes and failure patterns in childhood craniopharyngiomas. Childs Nerv Syst. 1998;14:558-563.
21. Gupta D.K., Ojha B.K., Sarkar C., et al. Recurrence in pediatric craniopharyngiomas: analysis of clinical and histological features. Childs Nerv Syst. 2006;22:50-55.
22. Minamida Y., Mikami T., Hashi K., et al. Surgical management of the recurrence and regrowth of craniopharyngiomas. J Neurosurg. 2005;103:224-232.
23. Vinchon M., Dhellemmes P. Craniopharyngiomas in children: recurrence, reoperation and outcome. Childs Nerv Syst. 2008;24:211-217.
24. Honegger J., Tatagiba M. Craniopharyngioma surgery. Pituitary. 2008;11:361-373.
25. Shi X.E., Wu B., Zhou Z.Q., et al. Microsurgical treatment of craniopharyngiomas: report of 284 patients. Chin Med J (Engl). 2006;119:1653-1663.
26. Van Effenterre R., Boch A.L. Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg. 2002;97:3-11.
27. Hoffman H.J., De Silva M., Humphreys R.P., et al. Aggressive surgical management of craniopharyngiomas in children. J Neurosurg. 1992;76:47-52.
28. Yasargil M.G., Curcic M., Kis M., et al. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg. 1990;73:3-11.
29. Department of Neurosurgery, Mayo Clinic. Long-term outcomes for surgically resected craniopharyngiomas. Neurosurgery. 2000;46:291-302. discussion 302-305
30. Fischer E.G., Welch K., Shillito J.Jr., et al. Craniopharyngiomas in children. Long-term effects of conservative surgical procedures combined with radiation therapy. J Neurosurg. 1990;73:534-540.
31. Hetelekidis S., Barnes P.D., Tao M.L., et al. 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys.. 1993;27:189-195.
32. Rajan B., Ashley S., Gorman C., et al. Craniopharyngioma–a long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993;26:1-10.
33. Shi X.E., Wu B., Fan T., et al. Craniopharyngioma: surgical experience of 309 cases in China. Clin Neurol Neurosurg. 2007;10(2):151-159.
34. Karavitaki N., Brufani C., Warner J.T., et al. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf). 2005;62:397-409.
35. Derrey S., Blond S., Reyns N., et al. Management of cystic craniopharyngiomas with stereotactic endocavitary irradiation using colloidal 186. Re: a retrospective study of 48 consecutive patients. Neurosurgery. 2008;63:1045-1052. discussion 1052-1053
36. Julow J., Backlund E.O., Lanyi F., et al. Long-term results and late complications after intracavitary yttrium-90 colloid irradiation of recurrent cystic craniopharyngiomas. Neurosurgery. 2007;61:288-295. discussion 295-296
37. Pollock B.E., Lunsford L.D., Kondziolka D., et al. Phosphorus-32 intracavitary irradiation of cystic craniopharyngiomas: current technique and long-term results. Int J Radiat Oncol Biol Phys. 1995;33:437-446.
38. Voges J., Sturm V., Lehrke R., et al. Cystic craniopharyngioma: long-term results after intracavitary irradiation with stereotactically applied colloidal beta-emitting radioactive sources. Neurosurgery. 1997;40:263-269. discussion 269-270
39. Caceres A. Intracavitary therapeutic options in the management of cystic craniopharyngioma. Childs Nerv Syst. 2005;21:705-718.
40. Flickinger J.C., Lunsford L.D., Singer J., et al. Megavoltage external beam irradiation of craniopharyngiomas: analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys.. 1990;19:117-122.
41. Merchant T.E., Kiehna E.N., Sanford R.A., et al. Craniopharyngioma: the St. Jude Children’s Research Hospital experience 1984-2001. Int J Radiat Oncol Biol Phys. 2002;53:533-542.
42. Pemberton L.S., Dougal M., Magee B., et al. Experience of external beam radiotherapy given adjuvantly or at relapse following surgery for craniopharyngioma. Radiother Oncol. 2005;77:99-104.
43. Schubert T., Trippel M., Tacke U., et al. Neurosurgical treatment strategies in childhood craniopharyngiomas: is less more? Childs Nerv Syst. 2009;25:1419-1427.
44. Kramer S., Southard M., Mansfield C.M. Radiotherapy in the management of craniopharyngiomas: further experiences and late results. Am J Roentgenol Radium Ther Nucl Med. 1968;103:44-52.
45. Habrand J.L., Ganry O., Couanet D., et al. The role of radiation therapy in the management of craniopharyngioma: a 25-year experience and review of the literature. Int J Radiat Oncol Biol Phys.. 1999;44:255-263.
46. Rajan B., Ashley S., Thomas D.G., et al. Craniopharyngioma: improving outcome by early recognition and treatment of acute complications. Int J Radiat Oncol Biol Phys. 1997;37:517-521.
47. Regine W.F., Mohiuddin M., Kramer S. Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol. 1993;27:13-21.
48. Stripp D.C., Maity A., Janss A.J., et al. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys. 2004;58:714-720.
49. Varlotto J.M., Flickinger J.C., Kondziolka D., et al. External beam irradiation of craniopharyngiomas: long-term analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys. 2002;54:492-499.
50. Jose C.C., Rajan B., Ashley S., et al. Radiotherapy for the treatment of recurrent craniopharyngioma. Clin Oncol (R Coll Radiol). 1992;4:287-289.
51. Kalapurakal J.A., Goldman S., Hsieh Y.C., et al. Clinical outcome in children with recurrent craniopharyngioma after primary surgery. Cancer J. 2000;6:388-393.
52. Moon S.H., Kim I.H., Park S.W., et al. Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas–a study in single institute. Childs Nerv Syst. 2005;21:799-807.
53. Agha A., Sherlock M., Brennan S., et al. Hypothalamic-pituitary dysfunction after irradiation of nonpituitary brain tumors in adults. J Clin Endocrinol Metab. 2005;90:6355-6360.
54. Darzy K.H., Shalet S.M. Hypopituitarism after cranial irradiation. J Endocrinol Invest. 2005;28:78-87.
55. McCollough W.M., Marcus R.B.Jr., Rhoton A.L.Jr., et al. Long-term follow-up of radiotherapy for pituitary adenoma: the absence of late recurrence after greater than or equal to 4500 cGy. Int J Radiat Oncol Biol Phys. 1991;21:607-614.
56. Fisher B.J., Gaspar L.E., Noone B. Radiation therapy of pituitary adenoma: delayed sequelae. Radiology. 1993;187:843-846.
57. McCord M.W., Buatti J.M., Fennell E.M., et al. Radiotherapy for pituitary adenoma: long-term outcome and sequelae. Int J Radiat Oncol Biol Phys. 1997;39:437-444.
58. Movsas B., Movsas T.Z., Steinberg S.M., et al. Long-term visual changes following pituitary irradiation. Int J Radiat Oncol Biol Phys. 1995;33:599-605.
59. Brada M., Ford D., Ashley S., et al. Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. Bmj. 1992;304:1343-1346.
60. Sachs R.K., Brenner D.J. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci U S A. 2005;102:13040-13045.
61. Tsang R.W., Brierley J.D., Panzarella T., et al. Radiation therapy for pituitary adenoma: treatment outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 1994;30:557-565.
62. Hoshi M., Hayashi T., Kagami H., et al. Late bilateral temporal lobe necrosis after conventional radiotherapy. Neurol Med Chir (Tokyo). 2003;43:213-216.
63. Girkin C.A., Comey C.H., Lunsford L.D., et al. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997;104:1634-1643.
64. Leber K.A., Bergloff J., Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88:43-50.
65. Adler J.R.Jr., Gibbs I.C., Puataweepong P., et al. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery. 2006;59:244-254. discussion 244-254
66. Chiou S.M., Lunsford L.D., Niranjan A., et al. Stereotactic radiosurgery of residual or recurrent craniopharyngioma, after surgery, with or without radiation therapy. Neuro Oncol. 2001;3:159-166.
67. Kobayashi T., Kida Y., Mori Y., et al. Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg. 2005;103:482-488.
68. Selch M.T., DeSalles A.A., Wade M., et al. Initial clinical results of stereotactic radiotherapy for the treatment of craniopharyngiomas. Technol Cancer Res Treat. 2002;1:51-59.
69. Ulfarsson E., Lindquist C., Roberts M., et al. Gamma knife radiosurgery for craniopharyngiomas: long-term results in the first Swedish patients. J Neurosurg. 2002;97:613-622.
70. Kobayashi T. Long-term results of gamma knife radiosurgery for 100 consecutive cases of craniopharyngioma and a treatment strategy. Prog Neurol Surg. 2009;22:63-76.
71. Yomo S., Hayashi M., Chernov M., et al. Stereotactic radiosurgery of residual or recurrent craniopharyngioma: new treatment concept using Leksell gamma knife model C with automatic positioning system. Stereotact Funct Neurosurg. 2009;87:360-367.
72. Chung W.Y., Pan D.H., Shiau C.Y., et al. Gamma knife radiosurgery for craniopharyngiomas. J Neurosurg. 2000;93(Suppl 3):47-56.
73. Minniti G., Esposito V., Amichetti M., et al. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg Rev. 2009;32:125-132. discussion 132
74. Stafford S.L., Pollock B.E., Leavitt J.A., et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177-1181.
75. Lee M., Kalani M.Y., Cheshier S., et al. Radiation therapy and CyberKnife radiosurgery in the management of craniopharyngiomas. Neurosurg Focus. 2008;24(5):E4.
76. Pham C.J., Chang S.D., Gibbs I.C., et al. Preliminary visual field preservation after staged CyberKnife radiosurgery for perioptic lesions. Neurosurgery. 2004;54:799-810. discussion 810-812
77. Constine L.S., Randall S.H., Rubin P., et al. Craniopharyngiomas: fluctuation in cyst size following surgery and radiation therapy. Neurosurgery. 1989;24:53-59.
78. Minniti G., Saran F., Traish D., et al. Fractionated stereotactic conformal radiotherapy following conservative surgery in the control of craniopharyngiomas. Radiother Oncol. 2007;82(1):90-95.
79. Miyazaki ST, Takusagawa Y, Fukushima T. Multisession CyberKnife® radiosurgery in the management of craniopharyngioma CyberKnife® Users’ Meeting Saturday, February 7, 2009.
80. Combs S.E., Thilmann C., Huber P.E., et al. Achievement of long-term local control in patients with craniopharyngiomas using high precision stereotactic radiotherapy. Cancer. 2007;109(11):2308-2314.
81. Giller C.A., Berger B.D., Pistenmaa D.A., et al. Robotically guided radiosurgery for children. Pediatr Blood Cancer. 2005;45(3):304-310.
82. Albright A.L., Hadjipanayis C.G., Lunsford L.D., et al. Individualized treatment of pediatric craniopharyngiomas. Childs Nerv Syst. 2005;21(8-9):649-654.
83. Amendola B.E., Wolf A., Coy S.R., Amendola M.A. Role of radiosurgery in craniopharyngiomas: a preliminary report. Med Pediatr Oncol. 2003;41(2):123-127.
84. Yu X., Liu Z., Li S. Combined treatment with stereotactic intracavitary irradiation and gamma knife surgery for craniopharyngiomas. Stereotact Funct Neurosurg. 2000;75(2-3):117-122.
85. Mokry M. Craniopharyngiomas: A six year experience with gamma knife radiosurgery. Stereotact Funct Neurosurg. 1999;72(suppl 1):140-149.
86. Prasad D., Steiner M., Steiner L., et al. Gamma knife surgery for craniopharyngioma. Acta Neurochir (Wien). 1995;134(3-4):167-176.