Chapter 11 Radiation Safety and Complications of Fluoroscopy, Ultrasonography, and Computed Tomography
 Patient doses can be minimized by limiting the time of fluoroscopy; proper positioning of the C-arm; use of collimation; and if necessary, shielding the patient.
Patient doses can be minimized by limiting the time of fluoroscopy; proper positioning of the C-arm; use of collimation; and if necessary, shielding the patient. Scattered radiation exposure, the major radiation risk to procedure room staff, can be reduced by use of personal protective equipment such as lead aprons, lead shielding, and maximizing the distance from the x-ray source.
Scattered radiation exposure, the major radiation risk to procedure room staff, can be reduced by use of personal protective equipment such as lead aprons, lead shielding, and maximizing the distance from the x-ray source.Terminology
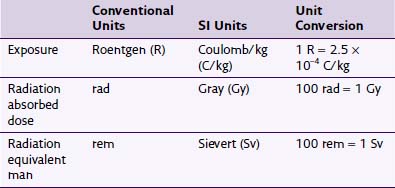
Any discussion of radiation safety requires a basic understanding of the nomenclature specific to this field. The literature is inconsistent in its use of units, with no standardization on using conventional or SI units. Fortunately, for the purposes of the clinician using fluoroscopy, many of these units can often be considered equivalent. Exposure is a quantity of radiation intensity. It is expressed in the conventional units of Roentgen (R) and the SI units of Coulomb/kg (C/kg). The energy absorbed from the exposure is described in conventional units as radiation absorbed dose (rad) and in SI units as Gray (Gy). Different types of radiation cause different biologic effects despite having similar absorbed doses. To predict the biologic effect from different types of radiation, rad is converted to radiation equivalent man (rem) or Sievert (Sv) in SI units. This conversion is accomplished by multiplying either rad or Gy by a quality factor unique to the type of radiation. As an example, whereas the quality factor for x-ray radiation is 1, it is 20 for α particle or fast neutron radiation.1 This quality factor of 1 allows exposure, dose, and dose equivalent to be considered equal for practical purposes despite their different meanings and uses, so 1 R ≈ 1 rad ≈ 1 rem. Conversions between units are summarized in Table 11-1.
Radiation Physics
The image produced by the X-radiation can be altered by either modulating the current or voltage of the x-ray tube. A higher tube current (mA) will cause an increase in the number of electrons striking the anode and thus increase the number of x-rays produced. By increasing the voltage (kVp), the released electrons will have higher energy, and thus the X-radiation will have higher energy and penetrance. The amount of radiation reaching the image intensifier over a period of time is called the radiographic density; the higher the amount of radiation, the brighter the image appears on the fluoroscope monitor. In general, a 15% increase in kVp will have the same effect on radiographic density as doubling the mA.2 Modern C-arms incorporate automatic brightness control (ABC) to optimize the brightness and contrast of the image. This is accomplished by automatic adjustment of mA and kVp.1
Background radiation is unavoidable radiation from both natural sources as well as medical procedures. The average individual receives approximately 3.6 mSv/yr with 15% of this coming from medically necessary radiation when averaged across the population.2 The amount of background radiation from natural sources varies according to region.3
Basics of C-Arm Design
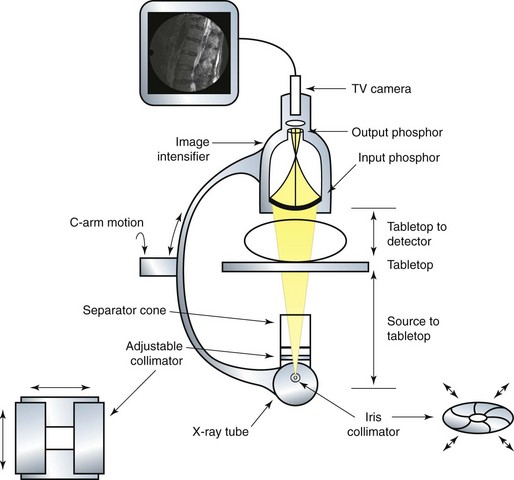
X-radiation is produced in the x-ray tube and directed toward the image intensifier. The image intensifier is composed of two phosphor screens. The input phosphor is spherical, and in combination with the output phosphor, the x-ray image is amplified and converted to visible light, allowing a TV camera to then transmit the image to a screen distant from the image intensifier. Collimation is the process of restricting the x-ray beam to only the clinically important anatomic area. The two types of collimators are iris and adjustable. An adjustable collimator is composed of lead shutters that can be closed to create a rectangular field. An iris variable aperture collimator creates a smaller circular field of radiation. Both collimators reduce the radiation field that reaches the image intensifier and the overall dose to the patient. A basic anatomy of a typical C-arm can be seen in Fig. 11-1.
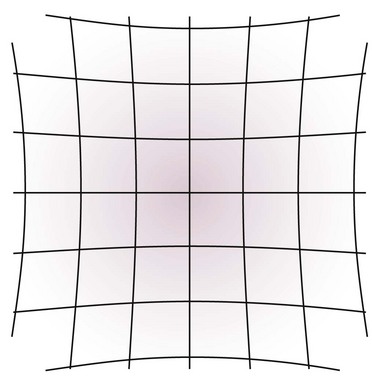
Two types of distortion can occur because of the construction of the image intensifier. Vignetting is the decreased spatial resolution and brightness at the periphery of the fluoroscopic image. The second is pincushion distortion, which is caused by the spherical construction of the input phosphor.2 This creates a fisheye effect as signal from the spherical input phosphor is transmitted to the flat output phosphor. A visual description of this effect can be seen in Fig. 11-2. Both of these distortions can be avoided by placing the anatomic structure of interest in the center of the fluoroscopic image.
Radiation Biology
Damage to the body from radiation occurs from direct cellular damage and indirect damage from creation of reactive oxygen species. Direct cellular damage is most likely to occur in cells that are in the G1 or M phases of the cell cycle. In the G1 stage, proteins are synthesized that prepare the cell for replication. During the M stage, DNA is packaged tightly into chromosomes, and there is an increased risk of a lethal double-strand DNA break.4 Not all radiation damage is lethal to the cell because complex mechanisms repair both single- and double-strand DNA breaks.5 The repair process is usually completed in 1 to 2 hours, so an increase in time between radiation doses causes an increase in cell survival.4
Indirect cellular damage is the result of hydrolysis of water, resulting in production of reactive oxidative species. Two-thirds of radiation-induced DNA damage is attributable to hydroxyl radicals.4 For example, a reactive oxygen species may combine with protein, resulting in the loss of important enzymatic activity in the cell.6 Antioxidants that can scavenge free radicals are therefore important in minimizing this type of damage. Molecules that have sulfhydryl groups and amine groups can scavenge these destructive entities.4
Damage Caused by Radiation
The effects of radiation on the body are divided into deterministic (nonstochastic) and probabilistic (stochastic). Deterministic effects are directly related to the dose received and exhibit a threshold below which the effect does not normally occur and above which the effect is dose dependent. Typically, these effects are related to cell death. An example is found in skin injury from exposure to radiation. An acute dose of 2 Gy causes early transient erythema, which occurs several hours after the dose and resolves within days.7 At an acute dose of 7 Gy, permanent epilation (loss of hair) occurs in about 3 weeks. Table 11-2 gives a more complete enumeration of deterministic effects.8 As described later in this chapter, many of the deterministic effects are not seen because of relatively low doses of radiation administered in the typical interventional pain fluoroscopy suite.
Table 11-2 Skin Entrance Dose Thresholds for Radiation-Induced Skin Injury
| Effect | Dose (Gy) | Onset |
|---|---|---|
| Early transient erythema | 2 | Hours |
| Main erythema | 6 | 10 days |
| Permanent epilation | 7 | 3 weeks |
| Dry desquamation | 14 | 4 weeks |
| Moist desquamation | 18 | 4 weeks |
| Secondary ulceration | 24 | 6 weeks |
| Late erythema | 15 | 8–10 weeks |
| Ischemic dermal necrosis | 18 | >10 weeks |
| Dermal atrophy | 10 | 12 weeks–1 year |
| Induration (invasive fibrosis) | 10 | >1 year |
| Telangiectasia | 10 | >1 year |
| Late dermal necrosis | >12 | >1 year |
From Brown KR, Rzucidlo ER: Acute and chronic radiation injury, J Vasc Surg 53(suppl):15S-21S, 2011.
As the probabilistic risks of radiation exposure have no threshold value, the guiding concept in radiation safety has been to keep doses “as low as reasonably achievable.” This is known as the ALARA concept and is accepted by all regulatory agencies.1 The maximum permissible dose (MPD) is the upper limit of rem one could receive without substantial risk of a clinically significant reaction. Table 11-3 lists the annual MPDs for different anatomic structures. A dose of 25 rem can lead to measurable hematologic depression, and a whole-body total radiation dose of 100 rem can lead to radiation sickness, nausea, fatigue, hemapoietic disturbances, alopecia, and radiation dermatitis.9 It is thought that x-rays may account for 1% of all cancers in the United States.10
Table 11-3 Annual Maximum Permissible Dose Per Target Organ or Area
| Organ or Area | Annual Maximum Permissible Dose (rem) |
|---|---|
| Thyroid | 50 |
| Extremities | 50 |
| Lens of the eye | 15 |
| Gonads | 50 |
| Whole body | 5 |
| Pregnant women | 0.5 |
From National Council on Radiation Protection and Measurements (NCRP): Report No. 116. Limitation of exposure to ionizing radiation, Bethesda, MD, 1993, NCRP Publications.
Patient Safety
The goal of patient safety is accomplished by reducing the radiation dose to the minimum amount needed to perform the diagnostic or interventional procedure. Certain patients are at higher risk from a given absorbed dose. Patients with connective tissue disorders such as lupus or DNA repair abnormalities such as xeroderma pigmentosum appear to have radiation hypersensitivity.8 Obesity is also a risk factor because higher doses of x-radiation are necessary to obtain the same images as in a thin person. Lastly, different medications are known radiosensitizers. Use of chemotherapeutic agents such as doxorubicin, bleomycin, and methotrexate can increase the risk of radiation-induced injury.8
Several methods are used for increasing patient safety. Fractionating the dose of radiation and thus allowing time for healing between exposures is known to increase tolerance to the damaging effects of radiation. Molecular repair of the cell begins within hours of the radiation dose, and cellular repopulation of the tissue begins within days of the radiation dose.8 Positioning of the C-arm also changes the dose of radiation. Varying the position of the beam helps protect the skin by decreasing the dose in any given area of skin. Increasing the angle of entry of the beam also puts the skin closer to the x-ray source.11 By reducing the overall amount of tissue the beam must traverse, the total dose can be minimized while adequate image quality is maintained. This is accomplished by keeping extraneous tissue, such as arms or breast tissue, out of the path of the beam. By removing the extraneous tissue, the ABC algorithm will thus calculate a lower dose of radiation to obtain an equivalent image.
Minimizing the amount of fluoroscopy time is also crucial to reduce the dose. Continuous fluoroscopy delivers high doses of radiation. One minute of continuous or cinefluoroscopy typically delivers an exposure of 1 to 10 R/min. As a comparison, a typical single posteroanterior chest radiograph has uses an exposure of 15 mR. At a typical exposure of 2 R/min, 1 minute of continuous fluoroscopy can deliver an equivalent exposure of approximately 130 chest radiographs.4 Using short bursts of fluoroscopy instead of continuous fluoroscopy can markedly decrease the delivered dose. Modern fluoroscopy units include a pulsed fluoroscopy mode. This mode uses frequent periodic spot images with periods between without any exposure. Using pulsed fluoroscopy instead of continuous fluoroscopy can allow up to a 40% decrease in absorbed dose.12 This mode is usually acceptable in procedures in which continuous fluoroscopy has been typically used, such as placement of spinal cord stimulator leads. Features of the C-arm imaging software, such as last image hold, reduce the need for repeat images and thereby reduce the radiation dose.13
Patient exposure can also be reduced by optimizing equipment factors. Appropriate filtration of 2.5 mm total aluminum equivalent should be in place at the x-ray source. This eliminates the low-power x-ray waves that do not contribute to creation of the image but do contribute to the total patient dose. Collimation should be used when possible to reduce radiation exposure.14 This not only reduces the amount of x-radiation received by the patient but can also improve image quality by excluding areas of significantly different densities. Keeping the image intensifier as close to the patient as possible also helps to reduce patient radiation dose.2
Practitioner Safety
By putting patient safety first through adherence to the ALARA principle, practitioners also maximize the safety of the staff present in the procedure center. The main sources of radiation exposure to practitioners are from leakage from the x-ray tube and scatter from the patient and surroundings.13 Leakage from the x-ray tube is decreased through proper shielding and maintenance of the C-arm. Scatter occurs through two different mechanisms. The Compton effect occurs when an x-ray impacts an outer shell electron and is deflected. The photoelectric effect occurs when an x-ray impacts an inner shell electron and ejects it. An outer shell electron will then occupy the vacant shell and thus emit an x-ray as secondary radiation.1 Both of these effects cause the x-radiation to deviate or scatter from the intended path of the beam.
The patient is the major source of radiation exposure to the practitioner because of scatter. The scatter exposure level from the patient is often 0.1% of the entrance skin exposure. At a typical exposure of 2 R/min, the scatter exposure 1 m from the patient would be 2 mR/min. However, the amount of scatter is increased two to three times if the radiation source is on the same side of the table as the practitioner, as in a cross-table lateral view.1
Maximizing the distance from the radiation source is an effective means of decreasing the radiation exposure for practitioners. Radiation exposure falls as the square of the distance.1 Standing away from the patient while performing spot images is useful. The use of forceps or other remote handling devices when manipulating an object in the field and the use of extension tubing while injecting under continuous fluoroscopy are ways to minimize exposure.15,16
Proper shielding plays in important role in reducing the radiation dose. Lead aprons absorb approximately 90% to 95% of the scattered radiation that reaches them.17 Wrap-around aprons should be used by anyone present in the procedure room who spends a significant amount of time with their backs to the radiation source. Thyroid shields also are an important adjunct to the lead apron, and similar to lead aprons, they should be 5 mm lead equivalent at a minimum.1 X-ray attenuating sterile surgical gloves provide extra protection but should not be considered a substitute for the practitioner keeping his or her hands out of the field. Additionally, ABC increases the output of radiation if a protective glove is in the field, overcoming any protective effects of the glove.
Radiation-induced cataracts have been described in interventional radiologists with a lens dose that approached 150 mSv/yr.18 Protective eyeglasses can significantly attenuate scatter to the lens and should have a minimal lead equivalent of 0.35 mm.19 These are recommended for personnel with collar badge readings of greater than 400 mrem per month.2 Use of these glasses reduce exposure to 2% to 3% of baseline dose, resulting in a total annual dose of only a few µSv.20
Another major concern is overall cancer risk. In a longitudinal study of 88,766 U.S. radiation technologists by the National Cancer Institute and University of Minnesota, there was no increase in all-cause mortality, cancer, or cardiovascular disease in technologists who work with fluoroscopy compared with those who did not. The exception to this was in workers who started before 1950, when doses were higher. In this subpopulation there was some increased risk of leukemia, thyroid disease, and female breast cancer.2 Jartti et al21 studied physicians who worked with x-rays using exposure data from 1970 to 2001 and found a slight increase in female breast cancer but no statistically significant change in overall mortality or cancer risk from the baseline population.
Happily for patients and practitioners alike, interventional pain procedures require very little radiation exposure compared with other diagnostic studies and interventions. Botwin et al14 performed 100 transforaminal epidural steroid injections (TFESIs) under fluoroscopic guidance using spot images. The average fluoroscopy time to perform the injections was 15.16 seconds. Cumulative radiation doses to the practitioner were measured both inside and outside the lead apron and at the hand and eye. There was a cumulative dose of 30 mrem outside the apron and 0 mrem inside. The total dose was 40 mrem at the eye and 70 mrem at the hand of the practitioner. The average dose per procedure was shown to be 0.3 mrem outside the apron, 0 mrem inside the apron, 0.4 mrem at the eye, and 0.7 mrem at the hand.14 These doses indicate that one could perform thousands of TFESIs per year and still be within the MPD. Manchikanti et al13 reported on the dose to the practitioner from 509 patients undergoing 800 procedures, including interlaminar epidural, transforaminal epidural, and facet joint nerve injections. They reported a mean dose of 0.629 mrem outside the apron at the chest per patient to the practitioner.13
Advanced interventions demand more frequent visualization of the anatomy and often require some use of continuous fluoroscopy during critical portions of the procedure. This results in a higher dose of radiation for both the patient and practitioner. Botwin et al22 also studied the dose of radiation delivered to the practitioner during lumbar discography. A total of 37 patients underwent 106 discograms (levels). The average dose per level was determined to be 2.35 mrem outside the apron, 0.18 mrem inside the apron, 1.49 mrem at the eye, and 3.66 mrem at the hand. Fluoroscopy time per level averaged 57.77 seconds.22 Boszczyk et al23 reported on 15 sessions of kyphoplasty with 27 levels performed. Patients received an average total entrance skin dose of 1 Gy.
Contrast Media
One of the benefits of fluoroscopy is the ability to confirm needle placement in real time. This ability is augmented by the use of contrast media because it allows confirmation that the needle is not in the subdural or intravascular space. All of the currently used contrast media are based on the 2,4,6-triiodinated benzene ring. They have a higher viscosity and greater osmolality than blood, plasma, or cerebrospinal fluid.24 Those most commonly used in interventional pain procedures, such as Omnipaque, Isovue, and Visipaque, are considered low-osmolality contrast media (LOCM) because their osmolality is only two to three times that of serum.24 LOCM have a much lower incidence of mild and moderate contrast reactions (0.2% vs. 6% to 8% for high-osmolality contrast media), but the incidence of severe reactions is similar. Anaphylactoid reactions are less common with LOCM.25
Contrast reactions fall into two groups: anaphylactoid or idiosyncratic and nonanaphylactoid. Anaphylactoid reactions are the most serious type of reaction, are independent of dose, and occasionally lead to fatal outcomes. These reactions are more common in patients with asthma, patients with previous reactions, patients with cardiovascular disease and renal disease, and patients taking β-blockers.24 Symptoms associated with anaphylactoid reactions range from skin rash, nausea, and itching to severe reactions such as hypotension, overt bronchospasm, laryngeal edema, seizures, and life-threatening arrhythmias. The overall risk for severe reactions from LOCM is 0.03%.26
Nonanaphylactoid reactions depend on the ionicity, osmolality, iodine concentration of the media, volume, and route of administration. Higher volumes and intraarterial injection are more likely to cause a reaction.27 Reactions are believed to be caused by pertubation of homeostasis of the body, specifically blood circulation. The respiratory, gastrointestinal, and nervous systems are also commonly affected. Symptoms are typically warmth, a metallic taste, nausea, vomiting, bradycardia, hypotension, vasovagal reactions, neuropathy, and delayed reactions.24 Pretreatment with a corticosteroid, antihistamine, or both may be considered in a patient with previous reactions or with significant risk factors for a reaction.
Computed Tomography–Guided Interventional Procedures
Fluoroscopy may be the most familiar imaging modality among interventional pain practitioners, but computed tomography (CT)–guided injections are becoming more common. These have typically been performed by interventional radiologists and deliver large doses of x-radiation.28 CT fluoroscopy is a recently developed mode of image acquisition that allows for faster image reconstruction, near-continuous image update, and in-room table control and image viewing.28 There is reduced spatial resolution compared with typical CT images. The rate of image acquisition is typically four to eight images per second, similar to pulsed fluoroscopy. Carlson et al28 performed 203 CT fluoroscopy–guided procedures such as biopsies, aspirations, and catheter drainages. CT fluoroscopy times ranged anywhere from 7.5 seconds for aspirations to 13.8 seconds for catheter drainages. Patient doses ranged from a mean of 34 mGy (3400 mrad) for aspiration to 53 mGy (5200 mrad) for catheter drainages. In comparison, conventional CT guidance doses were 738 mGy for aspirations and 936 mGy for catheter drainages.28 Silverman et al16 performed a similar study using CT fluoroscopic guidance but reported much higher doses. The mean reported patient dose was 300 mGy (30,000 mrad) with a mean CT fluoroscopy time of 79 seconds from 107 abdominal biopsy and catheter drainage procedures.
Wagner29 reported on the use of CT fluoroscopy for selective nerve root blocks. In a subset of 54 patients, he reported a mean CT fluoroscopy time of 2 seconds with a mean dose to the practitioner of 0.73 mrem per procedure. Compared with the study by Botwin et al,14 this is approximately twice the dose received by the practitioner but is similar to the dose reported by Manchikanti et al.13 Many studies referenced in this study did not use contrast media to verify placement of the needle. This is because of the improved spatial resolution of CT versus conventional fluoroscopy and thus presumed superiority in determining needle placement. One purported advantage of CT guidance is the ability to avoid contrast. However, contrast is still useful for determination of intravascular injection because negative aspiration is not a reliable indicator of proper needle placement.
Ultrasound-Guided Interventional Procedures
Ultrasound guidance for interventional pain procedures has recently been advanced as an alternative to fluoroscopic guidance in chronic pain interventions. The advantages of using ultrasound guidance are real-time visualization of the soft tissues, nerves, vessels, and injectate around the nerve.30 Additionally, exposure to ionizing radiation is avoided. The disadvantages are the bony artifacts and limited resolution of deep tissues.30 Although some risks, such as cavitation and an increase in temperature, are associated with ultrasonography, its widespread use in obstetrics underlies the inherent safety of this modality.31 Successful ultrasound-guided blocks for chronic pain have been described for lumbar medial branch blocks, lumbar facet injections, lumbar selective nerve root blocks, cervical selective nerve root blocks, occipital nerve blocks, cervical medial branch blocks, cervical facet injections, stellate ganglion blocks, and transabdominal celiac plexus neurolysis.30,32–38 Despite the significant interest in this imaging modality, most of the publications are feasibility studies, and few randomized controlled trials have been published.
1 Bushberg TB, Seibert JA, et al. The essential physics of medical imaging, ed 2. Philadelphia: Lippincott Williams & Wilkins; 2002.
2 Fishman SM, Smith H, Meleger A, Seibert JA. Radiation safety in pain medicine. Reg Anesth Pain Med. 2002;27(3):296-305.
3 Zeng W. Communicating radiation exposure; a simple approach. J Nucl Med Technol. 2001;29(3):156-158.
4 Brown KR, Rzucidlo ER. Acute and chronic radiation injury. J Vasc Surg. 2011;53(suppl):15S-21S.
5 Peterson CL, Cote J. Cellular machineries for chromosomal DNA repair. Genes Dev. 2004;18:602-616.
6 Dowd S, Tilson E. Practical radiation protection and applied radiobiology. Philadelphia: Saunders; 1999.
7 Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment. J Am Acad Dermatol. 2006;54:23-46.
8 Koenig TR, Wolff D, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11.
9 National Council on Radiation Protection and Measurements (NCRP). Report No. 116. Limitation of exposure to ionizing radiation. Bethesda, MD: NCRP Publications; 1993.
10 Berrington de Gozalez A, Darby S. Risk of cancer from diagnostic x-rays: estimates for the UK and 14 other countries. Lancet. 2004;363:345-351.
11 Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: Part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20.
12 Wininger KL, Deshpande KK, Deshpande KK. Radiation exposure in percutaneous spinal cord stimulation mapping: a preliminary report. Pain Physician. 2010;13:7-18.
13 Manchikanti L, Cash KA, Moss TL, Pampati V. Effectiveness of protective measures in reducing risk of radiation exposure in interventional pain management: a prospective evaluation. Pain Physician. 2003;6:301-305.
14 Botwin KP, Thomas S, Gruber RD, et al. Radiation exposure of the spinal interventionalist performing fluoroscopically guided lumbar transforaminal epidural steroid injections. Arch Phys Med Rehabil. 2002;83(5):697-701.
15 Nawfel RD, Judy PF, Silverman SG, et al. Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology. 2000;216:180-184.
16 Silverman SG, Tuncali K, Adams DF, et al. CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology. 1999;212:673-681.
17 Statkiewicz-Sherer MA, Viscanti PJ, et al. Radiation protection in medical radiography, ed 3. St. Louis: Mosby; 1998.
18 Vañó E, González L, Beneytez F, Moreno F. Lens injuries induced by occupational exposure to non-optimized interventional radiology laboratories. Br J Radiol. 1998;71:728-733.
19 National Council on Radiation Protection and Measurements (NCRP). Report No. 93 Ionizing radiation exposure of the population of the United States. Bethesda, MD: NCRP Publications; 1987.
20 Vano E, Gonzalez L, Fernández JM, Haskal ZJ. Eye lens exposure to radiation in interventional suites: caution is warranted. Radiology. 2008;248:945-953.
21 Jartti P, Pukkala E, Uitti J, Auvinen A. Cancer incidence among physicians occupationally exposed to ionizing radiation in Finland. J Work Environ Health. 2006;32:368-373.
22 Botwin KP, Fuoco GS, Torres FM, et al. Radiation exposure to the spinal interventionalist performing lumbar discography. Pain Physician. 2003;6:295-300.
23 Boszczyk BM, Bierschneider M, Panzer S, et al. Fluoroscopic radiation exposure of the kyphoplasty patient. Eur Spine J. 2006;15:347-355.
24 Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol. 2008;36:69-74.
25 Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol. 2001;176:1385-1388.
26 Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5:28-31.
27 Limbruno U, De Caterina R. Vasomotor effects of iodinated contrast media: just side effects? Curr Vasc Pharmacol. 2003;1:321-328.
28 Carlson SK, Bender CE, Classic KL, et al. Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology. 2001;219:515-520.
29 Wagner AL. Selective lumbar nerve root blocks with CT fluoroscopic guidance: technique, results, procedure time, and radiation dose. Am J Neuroradiol. 2004;25:1592-1594.
30 Narouze SN, Vydyanathan A, Kapural L, et al. Ultrasound-guided cervical selective nerve root block: a fluoroscopically-controlled feasibility study. Reg Anesth Pain Med. 2009;34:343-348.
31 American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 101: ultrasonography in pregnancy. Obstet Gynecol. 2009;113(2 Pt 1):451-461.
32 Shim JK, Moon JC, Yoon KB, et al. Ultrasound-guided Lumbar medial-branch block: a clinical study with fluoroscopy control. Reg Anesth Pain Med. 2006;31:451-454.
33 Galiano K, Obwegeser AA, Bodner G, et al. Ultrasound guidance for facet joint injections in the lumbar spine: a computed tomography-controlled feasibility study. Anesth Analg. 2005;101:579-583.
34 Galiano K, Obwegeser AA, Bodner G, et al. Real-time sonographic imaging for periradicular injections in the lumbar spine: a sonographic anatomic study of a new technique. J Ultrasound Med. 2005;24:33-38.
35 Eichenberger U, Greher M, Kapral S, et al. Sonographic visualization and ultrasound-guided block of the third occipital nerve: prospective for a new method to diagnose C2-C3 zygapophysial joint pain. Anesthesiology. 2006;104:303-308.
36 Galiano K, Obwegeser AA, Bodner G, et al. Ultrasound-guided facet joint injections in the middle to lower cervical spine: a CT-controlled sonoanatomic study. Clin J Pain. 2006;22:538-543.
37 Kapral S, Krafft P, Gosch M, et al. Ultrasound imaging for stellate ganglion block: direct visualization of puncture site and local anesthetic spread. Reg Anesth. 1995;20:323-328.
38 Bhatnagar S, Gupta D, Mishra S, et al. Bedside ultrasound-guided celiac plexus neurolysis with bilateral paramedian needle entry technique can be an effective pain control technique in advanced upper abdominal cancer pain. J Palliat Med. 2008;11(9):1195-1199.