CHAPTER 39 Radiation Injury
Early and late gastrointestinal injury may occur following irradiation of thoracic, abdominal, and pelvic malignancies of gastrointestinal and non-gastrointestinal origin. Toxicity to the gastrointestinal tract frequently limits radiation doses that can be delivered for many tumor types. As with most other toxicities associated with radiotherapy, gastrointestinal side effects are categorized into two broad types: early or acute reactions, such as diarrhea and nausea, experienced during and soon following the completion of a course of therapy; and late or chronic reactions, such as ulceration, stricture formation, and bowel obstruction, that can arise months to years after the course of radiation therapy. The incidence and severity of radiation-induced morbidity depend on total radiation dose, radiation fraction size, treatment volume, treatment techniques, and the presence or absence of other treatment modalities including systemic chemotherapy and surgery. A grading system for adverse events (severity, one to five) has been developed.1 This chapter discusses the early and late responses of the esophagus, stomach, small and large intestine, rectum, anus, and liver to radiation and combined radiation-chemotherapy treatment regimens.
MOLECULAR MECHANISMS OF RADIATION-INDUCED GASTROINTESTINAL DAMAGE
APOPTOSIS
In animal studies, a rapid increase in the rate of programmed cell death (apoptosis) of intestinal crypts cells can be observed after exposure to low-dose radiation (1 to 5 cGy). The rate of apoptosis is radiation dose dependent and reaches a plateau at 1 Gy. Radiation exposure also increases expression of the tumor suppressor gene P53 in the stem cell region. The apoptosis induced by radiation is dependent on the presence of P53. Additionally, the rate of spontaneous and radiation-induced apoptosis is significantly increased in animals lacking bcl-2, suggesting a protective effect of bcl-2 against radiation-induced apoptosis.2 It is therefore postulated that P53 promotes apoptosis after irradiation and that bcl-2 protects the mucosa. The expression of higher levels of bcl-2 may explain the increased tolerance of the colorectal mucosa to radiation as compared with the small intestine (see following).
ROLE OF CYTOKINES
Ionizing radiation activates the translation of the gene coding for transforming growth factor-β (TGF-β) in the intestines. TGF-β is a potent fibrogenic and proinflammatory cytokine, leading to hyperplasia of connective tissue mast cells and leukocyte migration into the intestinal wall. TGF-β promotes intestinal fibrosis by stimulating the expression of collagen and fibronectin genes and the chemotaxis of fibroblasts. The extracellular matrix of the intestine is also increased as TGF-β inhibits its degradation. The increased expression of TGF-β is especially enhanced in areas with histopathologic changes consistent with radiation damage: areas with mucosal ulceration, mucosal and serosal thickening, inflammatory cell infiltrates, and vascular sclerosis.3,4 TGF-β exists in three isoforms: TGF-β1, TGF- β2, and TGF- β3. All three isoforms are overexpressed in the early postradiation phase. However, only isoform β1 remains elevated six months after radiation exposure. In the first 2 weeks after radiation, TGF-β1 messenger RNA is increased in epithelial cells, fibroblasts of the submucosa and subserosa, vascular endothelial cells, and smooth muscle cells of the intestinal wall. However, at 26 weeks, the expression of TGF-β1 of epithelial cells returns to baseline level but TGF-β1 expression remains elevated in vascular endothelial cells, fibroblasts, and smooth muscle cells.5 Compared with control mouse intestine, the TGF-β1 immunoreactivity or overexpression is substantially increased in areas of radiation-induced acute and late bowel injury.6,7 In addition, pathologic examination of bowel specimens from patients undergoing surgery for radiation enteropathy showed an increased TGF-β in areas with vascular sclerosis and fibrotic areas of the serosa and muscularis propria as compared with patients who have surgery for other causes.8 Neutralizing antibodies to TGF-β and gene therapy using decorin (a natural TGF-β inhibitor) have been shown to suppress or reverse fibrosis in preclinical models.9
Epidermal growth factors, interleukins, and tumor necrosis factors are also being investigated for their effects in chronic radiation injury.10 Another cytokine implicated in the development of radiation injury is connective tissue growth factor (CTGF). The CTGF expression is increased in intestinal radiation fibrosis associated with chronic radiation injury.11 CTGF is found commonly in the extracellular region surrounding the area of active fibrosis or neovascularization. TGF-β1 may induce CTGF, which in turn functions as a mediator of intestinal radiation fibrosis by sustaining the activation of fibrogenesis in the irradiated gastrointestinal tract. Mechanisms underlying the pathogenesis of radiation-induced gastrointestinal damage remain an active area of investigation.
ESOPHAGUS
INCIDENCE AND CLINICAL FEATURES
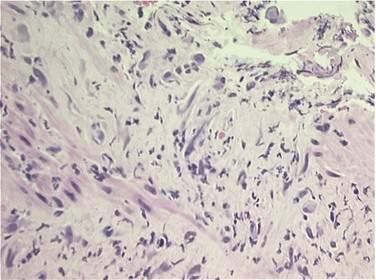
Early and late injury of the esophagus often occurs following irradiation of malignances of the thorax and upper abdomen (e.g., esophageal/gastroesophageal junctional carcinomas and lung carcinomas). The normal esophageal mucosa undergoes continuous renewal or turnover. Acute esophageal side effects are believed to be primarily related to radiation damage to the basal epithelial layer, manifested histologically by vacuolization, resulting in epithelial thinning followed by denudation (Fig. 39-1). These changes manifest clinically as dysphagia, odynophagia, and substernal discomfort, usually occurring within two to three weeks following initiation of radiation therapy. Patients may describe a sudden, sharp, severe chest pain radiating to the back. As treatment progresses, pain may become constant and may not necessarily be related to swallowing. The symptoms may be confused with Candida esophagitis, which may occur in conjunction with radiation esophagitis. Concurrent chemotherapy exacerbates these toxic effects. Endoscopically, mucositis and ulceration may be observed. Perforation and bleeding are rare in the acute phase.12 After treatment completion, basal proliferation returns and regeneration occurs, usually within three weeks.13
Following recovery from acute injury, late effects such as benign stricture, persistent ulceration and fistula formation may occur months to years following treatment. These effects are believed primarily due to inflammation and scar formation within the esophageal muscle. The connective tissues surrounding the esophagus may also exhibit severe fibrosis over time.14 Small vessel telangiectasias may be seen endoscopically. Histologic studies of the esophagus in previously irradiated patients have demonstrated epithelial thickening, chronic inflammation, fibrosis of the submucosa and muscularis propria, and (rarely) chronic ulceration. Complete epithelial recovery from radiation effects may take 3 to 24 months.15 Late effects often manifest as dysphagia due to stricture as well as altered motility due to fibrosis or muscular damage, possibly with accompanying nerve injury. Fistula formation is unusual and radiation dose dependent. Barium swallow examination may show strictures as well as disruption of peristalsis at the level of the irradiated esophagus with repetitive and nonperistaltic waves above and below the irradiated region. Abnormal peristalsis has been reported at 1 to 3 months following treatment completion, whereas most strictures occur 4 to 8 months following treatment completion. Late effects are usually not seen until 3 months following completion of radiation therapy, with a median time to onset of 6 months in some series.16,17
Development of radiation-related late complications is dose related. The TD5/5 (i.e., dose at which 5% of patients will develop complications at five years) has been estimated to be 60 Gy when one third of the length of the esophagus is irradiated.18 Other series have reported complication rates of less than 1% to 30% with doses of approximately 60 Gy, with benign stricture as the primary complication. Seaman and Ackerman treated patients to doses of 60 to 75 Gy, resulting in severe esophagitis and stricture formation in some patients. They concluded that the upper limit of dose tolerance of the esophagus was 60 Gy given at 10 Gy per week.19 Other studies reported that patients receiving 60 Gy had late complication rates of 1.2% to 18%. With contemporary radiation doses of 50 Gy, late complication rates have been observed in 2% of patients or less.20,21
Brachytherapy (the temporary insertion of a radioactive source into or adjacent to a tumor) has also been used as a technique for radiation dose escalation in esophageal cancer. Gaspar and colleagues reported the results of a phase I/II study examining the role of brachytherapy in addition to external beam radiation therapy in the treatment of esophageal cancer. The one-year actuarial fistula formation rate was 18%, and the authors recommended caution in the use of this approach, particularly in conjunction with concurrent chemotherapy.22 In contrast, other authors have reported much lower rates of fistula formation with brachytherapy.23 The length of esophagus being irradiated may not closely correlate with the incidence of esophagitis after radiotherapy.24,25 However, the intensity of cancer treatment such as use of concurrent chemotherapy with radiation therapy increases the rate of acute esophagitis.26 Maguire and colleagues evaluated 91 patients treated with radiation therapy for non–small cell lung cancer and found that the percent esophageal volume and surface area treated to greater than 50 Gy predicted late esophageal toxicity. Patients who had preexisting gastroesophageal reflux disease and esophageal erosions secondary to tumor were at increased risk for late toxicity. Hyperfractionation (multiple daily radiation treatments) was also associated with increased acute toxicity.27 Singh and associates studied patients with non–small cell lung cancer who received conformal daily radiation therapy with or without concurrent chemotherapy; they found that a maximal esophageal “point” dose of 69 Gy (radiation therapy alone) and 58 Gy (with concurrent chemotherapy) predicted significant toxicity. Twenty-six percent of patients receiving concurrent chemoradiotherapy developed grade 3 or higher esophageal toxicity, whereas only 1.3% of patients who received radiation therapy alone experienced this degree of toxicity.28 Ahn and colleagues found that the most powerful predictor of late esophageal toxicity in 254 patients treated for non–small cell lung cancer was the severity of acute esophageal toxicity. Severe acute toxicity was predicted by the use of twice daily radiation, older age, increasing nodal stage and a variety of dosimetric parameters. The overall incidence of late toxicity was 7%, with a median and maximal time to onset of 5 and 40 months, respectively.29 The Radiation Therapy Oncology Group, using standard radiation therapy techniques, reported grade 3 or higher acute esophageal toxicity in 34% of patients treated with concurrent hyperfractionated radiation therapy and chemotherapy versus 1.3% of patients treated with standard thoracic radiotherapy alone.30 Based on these and other data, it is clear that the addition of concurrent chemotherapy to radiation therapy increases the incidence of esophageal toxicity.
TREATMENT AND PREVENTION
The treatment and prevention of radiation-induced esophagitis have come under increased attention with the use of aggressive combination chemotherapy and radiation therapy regimens. The treatment of acute esophagitis is based on the grade of symptoms experienced by the patient. Treatment interruptions may ease the symptoms of acute esophagitis, but may also compromise the patient’s cancer treatment. Treatment interruption is reserved for severe cases. The management of acute esophagitis usually includes symptomatic management such as topical anesthetics (including viscous lidocaine-based regimens), oral analgesics (including anti-inflammatory agents and narcotics), gastric antisecretory drugs (histamine [H2] blockers, proton pump inhibitors), promotility agents (such as metoclopramide), and treatment of superimposed infection (candidiasis). Dietary modification, including bland foods as well as pureed or soft foods and soups, can help patients maintain food and liquid intake. Other modifications include avoidance of smoking, alcohol, coffee, spicy or acidic foods or liquids, chips, crackers, fatty foods, and indigestible foods. A study of dietary modifications and pharmacologic prophylaxis for radiation-induced esophagitis reported decreased toxicity and fewer treatment interruptions. It was recommended to drink between meals and to eat six smaller meals per day, consisting of semisolid food, soup, high-calorie supplements, purees, puddings, milk, and soft breads.31 Additionally, ingestion of hot or cold foods should be avoided if possible; instead foods and liquids should be at room temperature. In severe cases, feeding tube placement may be required.
Radioprotective chemical agents have been investigated as a means of mitigating radiation-induced normal tissue toxicity. The best-studied of these is amifostine, an organic thiophosphate. This agent is a scavenger of free radicals and serves as an alternative target to nucleic acids for alkylating or platinum agents.32 In one trial, patients treated with chemotherapy and radiation therapy for non–small cell lung cancer were randomized to receive amifostine or no drug. Amifostine did not significantly reduce grade 3 or higher esophagitis in these patients. However, patient self-assessments suggested a significantly lower incidence of acute esophagitis in those who received amifostine.33 Other trials have demonstrated a protective effect,34,35 whereas others have not confirmed this.36 Larger, randomized and placebo-controlled trials are needed to determine the ultimate efficacy of amifostine in preventing radiation injury of the esophagus.
STOMACH
INCIDENCE AND CLINICAL FEATURES
The stomach may be damaged following irradiation of the upper abdomen for cancer, including esophageal-GE junctional, gastric, and pancreatic carcinomas. Radiation to the stomach in animals using a very high single dose of irradiation results in erosive and ulcerative gastritis. A slightly lower single dose (23 Gy) results in gastric dilatation and gastroparesis, with replacement of the normal gastric mucosa by hyperkeratinized squamous epithelium. With even lower doses, gastric obstruction occurring months after irradiation was observed, with an atrophic gastric mucosa and intestinal metaplasia seen in surviving animals.37
Studies in which serial gastric biopsies were obtained following irradiation of patients for peptic ulcer disease noted coagulation necrosis of chief and parietal cells with mucosal thinning, edema, and chronic inflammatory infiltration.17,38 In addition, gastric acid production decreased after relatively low doses of gastric irradiation. In the past, radiotherapy had been used to decrease acid production in patients with peptic ulcer disease. Even with a relatively low dose of 18 Gy delivered in 10 fractions, approximately 40% of ulcer patients had a 50% reduction in gastric acid secretion that lasted for a year or more.39
Clinically, radiation-induced gastritis may occur within a week of starting radiotherapy, with microscopic changes including edema, hemorrhage, and exudation. Histologic changes may also include disappearance of cytoplasmic details and granules in parietal and chief cells as early as one week into therapy. Cell damage and subsequent cell death are often seen first in the depths of glands, followed by thinning of the gastric mucosa.40 Additional mucosal changes include deepening of the glandular pits and proliferation of cells in the glandular neck. Loss of glandular architecture and thickening of the mucosa can be seen by the third week of radiotherapy. Approximately three weeks after completing radiotherapy, histologic recovery may be seen. Signs of recovery of early radiation injury to the stomach include re-epithelialization and fibrosis.
Symptoms of acute radiation injury of the stomach consist primarily of nausea and vomiting, dyspepsia, anorexia, abdominal pain, and malaise. These are more common with the concurrent administration of chemotherapy. Radiation-induced nausea and vomiting may occur within the first 24 hours following treatment. It is estimated that approximately half of patients receiving upper abdominal radiation will experience emesis within two to three weeks following initiation of irradiation.41
Late effects of gastric irradiation have been classified into four categories: (1) acute ulceration (occurring shortly after completion of radiation therapy); (2) gastritis with smoothened mucosal folds and mucosal atrophy on endoscopy accompanied by radiographic evidence of antral stenosis (1 to 12 months following irradiation) (see Chapter 51); (3) dyspepsia, consisting of vague gastric symptoms without obvious clinical correlate (6 months to four years following irradiation); and (4) late ulceration (averaging 5 months after irradiation).17,42 The TD5/5 for treatment of the entire stomach has been estimated to be 50 Gy. Large studies of upper abdominal irradiation have suggested that prior abdominal surgery as well as using a higher radiation dose per fraction of radiation may increase the risk of late effects.43 Studies from Walter Reed Army Medical Center delivering abdominal radiation using now antiquated techniques in testicular cancer patients have suggested that higher radiation doses lead to an increasing risk of late ulceration and perforation, with ulceration occurring in approximately 6% of patients treated to 45 to 50 Gy, 10% of patients treated to 50 to 60 Gy, and 38% of patients treated to greater than 60 Gy. No ulceration was seen in patients receiving less than 45 Gy. In this series, symptomatic gastritis occurred approximately 2 months following radiation completion, with ulcer formation occurring at a median of 5 months. Six of 233 patients (3%) required surgery for ulcer hemorrhage or pain related to ulcer disease, almost all of whom had received doses of greater than 50 Gy.17,45 Other studies of patients treated with radiation therapy for Hodgkin’s lymphoma or for testicular, gastric, or cervical cancer have established tolerance limits for gastric irradiation.43–46 These studies delivered doses of 40 to 60 Gy. Patients who received doses greater than 50 Gy experienced gastric ulceration and gastric ulcer–associated perforation at rates of 15% and 10%, respectively. If indicated, the dose to the entire stomach with conventionally administered radiation therapy is limited to 45 to 50 Gy, with an estimated 5% to 10% risk of severe radiation toxicity. Where appropriate, reduced field boosts can be given to treat to doses up to 55 Gy with acceptable toxicity.
TREATMENT AND PREVENTION
Acute symptoms of gastric radiation in toxicity are treated with antiemetics (5-hydroxytryptamine-3 [5-HT3] antagonists, phenothiazines, metoclopramide, glucocorticoids, benzodiazepines, antihistamines, or anticholinergics), as well as consumption of a light meal prior to delivery of radiation therapy. Randomized trials of prophylactic 5-HT3 inhibitors have shown efficacy compared with placebo in preventing radiation-induced nausea and vomiting.47 A randomized trial of 211 patients receiving upper abdominal radiation compared the 5-HT3 inhibitor ondansetron given twice daily, with or without dexamethasone delivered daily for the first five fractions of treatment. Patients receiving dexamethasone showed a trend toward improved complete control of nausea (50% versus 38%) and significant improvement in complete control over emesis. The authors concluded that the addition of dexamethasone resulted in modest improvement in protection against radiation-induced emesis.48 Narcotic and non-narcotic agents are often used for pain. Additionally, it is generally recommended that patients be placed on antacid medications, including proton pump inhibitors. Careful nutritional support along with antiemetic therapy is essential for patients undergoing radiotherapy to the abdomen. Acute symptoms generally resolve within one to two weeks following completion of radiation therapy.
SMALL INTESTINE
Small bowel injury, or radiation enteritis, is the primary treatment-limiting toxicity in the radiotherapeutic management of abdominal and pelvic malignancies. The small bowel can be damaged during radiation treatment of malignances of the stomach, pancreas, rectum, and anus, as well as during treatment of gynecologic malignancies. The first case of radiation enteropathy was described in 1897.49
INCIDENCE AND CLINICAL FEATURES
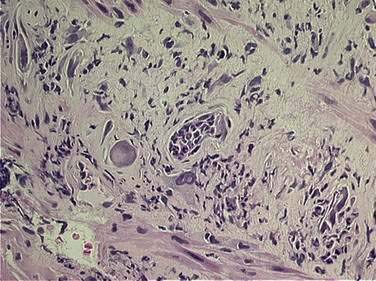
The epithelium of the gastrointestinal tract has a high proliferative rate, making it susceptible to radiation- and chemotherapy-induced mucositis. The intestinal lining is normally replaced every three to five days, reflecting this high cellular turnover rate. Irradiation of intestinal mucosa primarily affects the clonogenic intestinal stem cells within the crypts of Lieberkühn (cells that provide, via self-replication and eventual maturation, replacement cells in the intestinal villi). Stem cell damage, either as a primary result of radiation damage or as a result of radiation-induced microvascular damage, leads to a decrease in cellular reserves for the intestinal villi, resulting in mucosal denudement, shortened villi, and decreased absorptive area, with associated intestinal inflammation and edema. Histologic changes are seen within hours of irradiation. Within two to four weeks, an infiltration of leukocytes with crypt abscess (microabscess) formation can be seen (Fig. 39-2). Ulceration may also occur. This acute injury can result in impaired absorption of fats, carbohydrates, proteins, bile salts, and vitamin B12, with associated loss of water, electrolytes, and protein. Additionally, impaired ileal bile salt absorption increases loads of conjugated bile salts entering the colon, which are in turn deconjugated by colonic bacteria, resulting in intraluminal water retention with resultant diarrhea. Furthermore, impaired digestion of lactose may occur following radiation, leading to increased bacterial fermentation with associated flatulence, distention, and diarrhea, possibly accompanied by bacterial overgrowth. There is also evidence of acutely altered gut motility following radiation therapy.50
Patients with acute radiation enteritis experience diarrhea, abdominal cramping or pain, nausea and vomiting, anorexia, and malaise. Radiation-induced diarrhea often appears during the third week of a fractionated radiation course, with reported rates of 20% to 70%.51 Acute radiation enteropathy with diarrhea may be seen in some patients after delivery of doses of 18 to 22 Gy using conventional fractionation, and in most patients receiving doses of 40 Gy. The symptoms and pathologic findings typically subside and spontaneously disappear two to six weeks following completion of radiation therapy.52 However, growing evidence suggests that patients who develop acute small intestine toxicity may be at higher risk for chronic effects.53
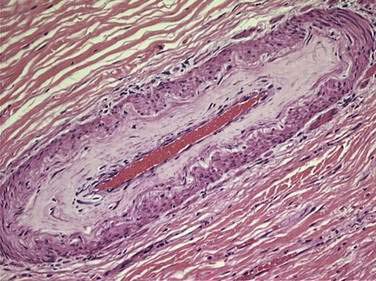
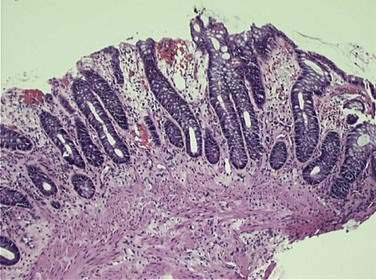
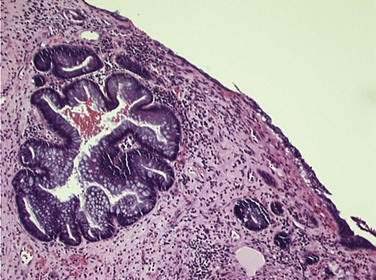
Histologic changes of chronic toxicity to the small intestine include progressive occlusive vasculopathy with foam cell invasion of the intima and hyaline thickening of the arteriolar walls, as well as collagen deposition and fibrosis, often in the submucosal layer of the bowel wall. The small bowel becomes thickened with development of telangiectasias, whereas the vessel walls of small arterioles are obliterated, causing ischemia (Fig. 39-3).54 As the vasculopathy progresses, mucosal ulceration, necrosis, and occasionally perforation of the intestinal wall can be seen, resulting in fistula and abscess formation. Lymphatic damage results in constriction of the lymphatic channels, which contributes to mucosal edema and inflammation.55 Histologically, the mucosa atrophies, with atypical hyperplastic glands and intestinal wall fibrosis (Fig. 39-4).15 As the ulcers heal, there can be fibrosis with narrowing of the intestinal lumen with subsequent stricture formation and even obstruction with dilatation of the proximal bowel. Bacterial overgrowth may be an indirect complication arising from stasis in a dilated loop of bowel proximal to the stricture. Although the affected segments of intestine and serosa appear thickened with areas of telangiectasias,56 it should be noted that even if the gut appears normal, patients can still be at risk of spontaneous perforation.57
Chronic radiation enteritis can cause significant morbidity. This complication tends to be progressive, with an onset at least 6 months after radiotherapy. Late radiation injury to the small intestine occurs at a median of 8 to 12 months following radiation therapy, though it can appear years later.17 There are numerous clinical manifestations of the chronic phase of radiation enteritis (Table 39-1). These include malabsorption and diarrhea, with more rapid transit times occurring in the affected bowel. Rarely, chronic malnutrition may develop, resulting in anemia and hypoalbuminemia. There can be bleeding from ulceration and pain and bloating from strictures, as well as fevers from abscess. Fibrosis and vasculitis of the bowel may lead to dysmotility, stricture, and malabsorption.58,59 Malabsorption and other complications may require surgical intervention and parenteral alimentation (discussed in Chapters 4 and 5 and following). Patients with severe chronic radiation enteritis have a poor long-term prognosis with a mortality rate of approximately 10%.60–66 The overall incidence of chronic radiation enteritis has not been precisely defined. Retrospective series suggest an incidence of 5% to 15%; however, these studies often include a large number of patients who were lost to follow-up or died between the end of radiation therapy and the completion of the study. A review of randomized trials of adjuvant radiation therapy for rectal cancer shows severe long-term complications as low as 1.2% and as high as 15%.67 In older series of radiotherapy for abdominal cancers, symptoms of chronic bowel dysfunction were present in many patients, although such symptoms are often multifactorial and influenced by other treatment modalities including surgery and chemotherapy.68,69 However, it appears that advances in radiation therapy treatment techniques have reduced chronic small intestinal toxicity rates.
Table 39-1 Clinical Complications of Chronic Radiation Enteritis or Proctitis*
| COMPLICATION | LESION(S) | SYMPTOMS |
|---|---|---|
| Obstruction | Stricture | Constipation, nausea, vomiting, postprandial abdominal pain |
| Infection | Abscess | Abdominal pain, fever, chills, sepsis, peritonitis |
| Fistulization | Fistula | Fecal, vaginal, or bladder discharge; pneumaturia |
| Bleeding | Ulceration | Rectal pain, tenesmus, rectal bleeding, anemia |
| Malabsorption | Small bowel damage | Diarrhea, steatorrhea, weight loss, malnutrition, cachexia |
* From Girvent M, Carlson GL, Anderson I, et al: Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl 2000; 82:198.
Certain factors have been found to predispose patients to radiation toxicity to the small intestine. Women, older patients, and thin patients may have a larger amount of small bowel in the pelvic cul-de-sac, which can increase the probability of radiation injury in the treatment of pelvic malignancies.70 Patients with a history of pelvic inflammatory disease or endometriosis also appear to be at higher risk of radiation complications.71,72 Patients who have had previous abdominal surgery can develop adhesions that decrease the mobility of the small bowel, thereby allowing it to be consistently exposed to fractionated radiation therapy.73,74 In addition, patients with prior pelvic surgery may have an increase in the amount of small bowel within the pelvis, allowing increased exposure during pelvic irradiation. In a series published by Eifel and associates, the risk of small bowel complications was significantly higher in women who had undergone a previous laparotomy.75 With modern treatment approaches (allowing direct visualization of the volume of bowel in treatment field) and use of improved treatment techniques (discussed later), as well as a shift toward neoadjuvant approaches, this risk factor may not be as relevant as in prior years. Patients with diabetes, hypertension, and cardiovascular disease also have an increased risk of pre-existing vascular damage or occlusion.76 These comorbid conditions are compounded by the pathologic changes of chronic radiation injury, which include vasculopathy and ischemia, predisposing these patients to radiation-related small bowel toxicity. Patients with collagen vascular disease and inflammatory bowel disease also have a higher risk of acute as well as chronic radiation-induced injury. Patients with these diseases may have pathologic changes which include transmural fibrosis, collagen deposition, and inflammatory infiltration of the mucosa. The late effects induced by radiation therapy to the small bowel are likely additive to these preexisting changes, and studies have shown that these patients have a lower gastrointestinal tolerance to radiation therapy.77–79 Patients whose inflammatory bowel disease or nonmalignant systemic disease is quiescent or well controlled appear to fare better than patients with active disease.
Studies have also addressed the effect of radiation dose on occurrence of small bowel toxicity. Volume of the treatment field, volume of irradiated small bowel, total radiation dose, fraction size, treatment time, and treatment technique all influence small bowel tolerance. The TD5/5 for small volumes of small bowel has been estimated to be 50 Gy. Patients can generally receive 45 to 50 Gy in 1.8 to 2 Gy daily fractions to a pelvic field without a significant rate of toxicity.80 For postoperative patients, radiation to 45 to 50 Gy in five weeks is associated with an approximate 5% incidence of small bowel obstruction requiring surgery, whereas at doses greater than 50 Gy, the incidence rises to as high as 25% to 50%.70 Doses greater than 2 Gy per fraction in the postoperative setting also increase the risk of toxicity.81 At radiation doses of 70 Gy or greater, the incidence of toxicity rises precipitously.82 A study of different treatment techniques to minimize the effect of pelvic radiation on the small bowel showed that irradiating smaller volumes of bowel yielded less toxicity.83 In addition, treating patients in the prone position with external compression and bladder distention decreased side effects, likely from exclusion of portions of the small bowel from the radiation field. Another study treating postoperative patients with pelvic radiation therapy noted less small bowel toxicity by placing patients in the decubitus position.84 Studies have also analyzed dose-volume parameters associated with acute small bowel toxicity in patients undergoing treatment with 5-FU–based chemoradiation therapy for rectal cancer.85,86 These found strong correlations between acute toxicity and the amount of small bowel irradiated at each dose level analyzed. Another analysis evaluating rectal cancer patients treated preoperatively with chemoradiotherapy also showed a strong correlation between the occurrence of severe diarrhea and irradiated small bowel volume, surmising that limiting the volume of small bowel receiving greater than 15 Gy may significantly improve treatment tolerance.87 These and other studies imply that attention to detail in radiation planning with the use of modern treatment techniques are important considerations in patient treatment.
The combination of radiation and chemotherapy (e.g., 5-FU) increases the risk of small bowel toxicity. In a randomized trial delivering 40 to 48 Gy using parallel, opposed fields to patients with rectal cancer, the incidence of severe small bowel complications was significantly higher in patients who received chemotherapy and radiation therapy than in patients who received radiation therapy alone. There were two treatment-related deaths (4%) in the combination therapy arm.88,89 In other trials in which multiple field radiation techniques were used, bolus 5-FU and radiation therapy showed no increase in chronic toxicity when chemotherapy and radiation therapy were combined. There was, however, a mild increase in acute diarrhea symptoms.83,90 The use of continuous infusional 5-FU as opposed to bolus 5-FU combined with radiation therapy also has been studied. Continuous 5-FU with radiation to 50.4 Gy in 1.8-Gy fractions was associated with more acute diarrhea, but no significant increase in chronic or severe small bowel toxicity as compared with bolus 5-FU therapy.91 Capecitabine, a prodrug that is converted to 5-FU in the tumor, also appears to enhance acute diarrhea rates when combined with radiation therapy.92 Although the addition of concurrent chemotherapy increases the acute toxicity of external radiotherapy (e.g., diarrhea, bowel frequency, cramping), its contribution to late bowel toxicity is poorly defined.17
There is investigation in integration of novel chemotherapeutic and “targeted” agents with radiation therapy in the neoadjuvant therapy of gastrointestinal cancers. Data from phase I and phase II trials using novel agents such as oxaliplatin, irinotecan, and epidermal growth factor receptor inhibitors suggest that the addition of these agents may significantly increase grades 3 and 4 gastrointestinal toxicity rates relative to conventional neoadjuvant chemoradiotherapy regimens, further emphasizing the importance of careful radiation planning to maximize normal tissue sparing in these patients.93–95
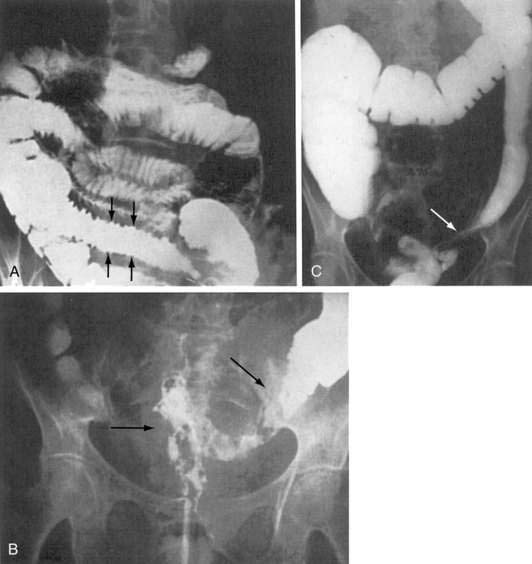
Diagnosis of chronic enteropathy is often a clinical one. The cause of symptoms can be variable from patient to patient. Therefore, individualization of diagnostic and therapeutic approaches is indicated. General diagnostic evaluation procedures and therapeutic options are displayed in Tables 39-2 and 39-3. Consultation with the treating radiation oncologist should be requested if the clinical presentation is consistent with radiation enteritis. Review of the patient’s previous radiation treatment record will reveal the total dose, fractionation, volume of treatment, and other radiation parameters. Analysis of the treatment plan may show areas of high dose, especially if the patient had an intracavitary implant or brachytherapy. Lesions encountered at endoscopy or x-ray studies are usually localized in the area of high dose. Ulceration of the mucosa, thickening of jejunal folds, and thickening of the intestinal loops are radiologic signs that suggest radiation damage to the small bowel (Fig. 39-5). Faster intestinal transit and reduced bile acid and lactose absorption are observed in patients with chronic radiation enteritis.96 These effects may be improved after the administration of loperamide. Antibiotics are indicated if there is small bowel bacterial overgrowth syndrome (see Chapter 102).97,98
Table 39-2 Pathophysiologic Features of Patients with Late Radiation Enteropathy
| PATHOPHYSIOLOGIC FEATURE | CLINICAL SYMPTOM OR SIGN |
|---|---|
| Mucosal dysfunction | Lactose intolerance |
| Vitamin B12 deficiency | |
| Steatorrhea | |
| Stricture or blind loop syndrome with bacterial overgrowth | Diarrhea |
| Intestinal dysmotility | Bloating |
| Constipation | |
| Diarrhea | |
| Abnormal bile acid recirculation | Cholerrheic diarrhea |
From Hauer-Jensen M, Wang J, Denham J. Bowel injury: Current and evolving management strategies. Semin Radiat Oncol 2003; 13:357-371.
Table 39-3 Therapeutic Options for Patients with Late Radiation Enteropathy
| UNDERLYING PATHOPHYSIOLOGIC FEATURE | THERAPEUTIC OPTIONS |
|---|---|
| Nutritional deficits | Correction of specific deficits |
| Low-fat diet | |
| Lactose-free diet | |
| Elemental diet | |
| Total parenteral nutrition | |
| Intestinal dysmotility (increased or decreased) | LoperamideOctreotide |
| Prokinetic agents | |
| Bile acid malabsorption | Bile-salt binding agent |
| Bacterial overgrowth | Antibiotics |
From Hauer-Jensen M, Wang J, Denham J. Bowel injury: Current and evolving management strategies. Semin Radiat Oncol 2003; 13:357-371.
TREATMENT AND PREVENTION
The management of acute radiation small bowel toxicity should be based on the severity of symptoms. Most cases of acute radiation enteritis are self-limited, requiring only supportive treatment. Diarrhea, nausea, vomiting, and abdominal cramping are treated symptomatically. Antidiarrhea medications such as loperamide, diphenoxylate with atropine, anticholinergic agents, and opiates can be used. Antiemetic agents may also be effective. A low-fat, lactose-free diet also may improve symptoms. A study of oral sulcralfate in patients receiving pelvic irradiation showed a decrease in frequency and improvement in consistency of bowel movements. In this study, not only were acute symptoms improved, but also chronic symptoms a year after completion of radiation seemed to be improved.99 Cholestyramine to treat diarrhea from bile acid malabsorption has shown some benefit,100 and treatment with anti-inflammatory agents has decreased some symptoms.101 Intractable diarrhea during the combined-modality treatment may require hospital admission for administration of parenteral fluids and electrolytes. Patients who are refractory to conventional antidiarrhea medications may benefit from administration of a synthetic somatostatin analog such as octreotide.
Chronic effects of diarrhea are managed symptomatically with low-residue diet. Fiber supplementation (e.g., Metamucil, Citrucel) has shown benefit in some cases. In the rare setting of malnutrition related to chronic radiation injury, total parenteral nutrition (TPN) can improve clinical outcome, and methylprednisolone may add to the effects of TPN.72 However, the five-year survival rate for patients undergoing TPN ranges from 36% to 54%.62,102 It has been estimated that overall mortality rates associated with chronic radiation enteropathy are approximately 10%.103
Small bowel obstruction is generally managed conservatively with bowel rest and tube decompression. If the obstruction is severe or chronic, bowel resection or lysis of adhesions may be required, although the need for surgery is relatively uncommon. It is difficult to perform surgery for chronic radiation enteritis because of the diffuse fibrosis and alterations in the intestine and mesentery. The risk of anastomotic leak is high if the anastomosis is performed using irradiated bowel.103 The risk of leak can be lowered if at least one limb of the anastomosis is previously unirradiated.104 However, it may be difficult to distinguish between the area of normal tissue and the irradiated portion of the intestines by gross examination during surgery, even when the fresh tissue is sent for frozen section. Another method the surgeon can use to circumvent this technical difficulty is to create the anastomosis with unirradiated colon. The accuracy in localizing injured bowel may be improved by intraoperative endoscopic examination, which can detect radiation-induced mucosal injury.105
Limited resection of the diseased intestine is the goal, but if the lesion is too diffuse, a bypass procedure may be attempted. If feasible, resection of the affected bowel results in a superior outcome than an enteric bypass procedure. However, extensive surgical resection of the diseased intestines may lead to short bowel syndrome (see Chapter 103) and increase the need for total parenteral nutrition. Because of the progressive evolution of the fibrosis, the patient may require additional surgery. Surgical bypass of the injured bowel may be associated with a blind loop syndrome, and the patient still may be at risk for perforation, bleeding, abscess, and fistulas due to the persistence of the affected bowel. Bypass procedures should be performed when resection is not possible or as a temporary management before resection at a later date. Surgery should be performed by an experienced team familiar with the management of radiation enteritis. Perforations and fistulae are best managed surgically. It should be noted that many patients with chronic small bowel radiation toxicity are nutritionally depleted and are more susceptible to anastomotic leakage and dehiscence after surgery. The postoperative mortality of these patients may be significant and must be taken into consideration before a decision to proceed with surgery is made.
A recent approach to treatment of chronic radiation enteritis is the application of hyperbaric oxygen.106,107 The rationale for hyperbaric oxygen is the creation of an oxygen gradient in hypoxic tissue that stimulates the formation of new blood vessels. Neoangiogenesis improves the blood supply and decreases the ischemia and necrosis responsible for severe complications. In a retrospective study of 36 patients with severe radiation enteritis refractory to medical management, improvement of clinical symptoms was reported in two thirds of the patients treated with hyperbaric oxygen.108 Hyperbaric oxygen may be helpful in management of bleeding due to chronic radiation enteritis in patients who are not controlled with conservative measures such as formalin and laser therapy.109,110 A large clinical series of 65 consecutive patients with chronic radiation enteritis (rectal and small bowel), primarily manifested as chronic bleeding, were treated with hyperbaric oxygen. Response rates for rectal and more proximal sites were 65% and 73%. The response rate for bleeding was 70% and for other symptoms (pain, diarrhea, weight loss, fistula, and obstruction) was 58%. The authors concluded that hyperbaric oxygen therapy resulted in healing or clinically significant improvement in two thirds of patients with chronic radiation enteritis.111 The optimal application of hyperbaric oxygen treatments in chronic enteritis remains a topic of ongoing investigation.
Because chronic radiation enteritis is complex and rarely curable, prevention is key and measures to decrease its incidence are imperative. Pancreatic enzymes can exacerbate acute intestinal radiation toxicity,113 and reducing pancreatic secretion with a synthetic somatostatin receptor analog such as octreotide may reduce early and delayed radiation enteritis in animal studies.114 One of the major risk factors for injury is previous abdominopelvic surgery, which leads to the prolapse of the small intestines into the pelvis, exposing them to more radiation. Anticipation for the need of radiation and chemotherapy before or after surgery requires close collaboration among surgical, radiation, and medical oncologists. If gross residual tumor is found unexpectedly at surgery, outlining the tumor bed with surgical clips to facilitate postoperative treatment planning and surgical techniques to keep the small intestine outside the pelvis (e.g., omentoplasty or polyglycolic mesh) may significantly decrease the rate of complications (see following).
Postoperative bowel adhesions may also increase the volume of bowel irradiated compared with normal small intestine, which is usually mobile and can move out of the radiation field. If radiation therapy is anticipated after surgery, attempts should be made at the time of surgery to displace the bowel outside the radiation field.115 One simple technique is the surgical placement of a polyglycolic, biodegradable mesh that moves the intestines out of the pelvis.116,117 This procedure has minimal morbidity and it does not significantly increase operating time. It also does not require a second operation to remove the mesh because it is absorbed three to four months after surgery. Magnetic resonance imaging (MRI) can be used after surgery to verify the position of the mesh, the small bowel, and eventual disappearance of the mesh. A reduction of 50% of the volume of the small bowel exposed to the radiation has been demonstrated with placement of a mesh during surgery, allowing a higher dose of radiation to be given postoperatively where indicated.118,119 Other techniques such as pelvic reconstruction, omentoplasty, and transposition of the colon may also significantly decrease the volume of bowel exposed to radiation therapy.119–122
Radiation therapy technique is critical in reducing the rate of complications. The use of only anterior and posterior fields for pelvic radiation should be avoided if possible because of the high dose and large volume of bowel irradiated. A higher rate of operative mortality was reported in trials using this technique preoperatively for rectal cancers.123,124 As discussed, the toxicity of radiation therapy correlates with the volume of small bowel irradiated.125 In many patients, treatment in the prone position with a special “belly board” allows the displacement of the small intestines out of the radiation field.126,127 Patients should be instructed to maintain a full bladder during the radiation session, which further displaces the intestines out of the pelvis.74 Three-dimensional treatment planning optimizes the treatment technique by facilitating more accurate dose distributions. A three-dimensional treatment algorithm ensures the sparing of excessive radiation dose to normal tissues by the judicious use of multiple fields to the target volume from multiple geometries.128 In gynecologic brachytherapy, appropriate packing to displace the rectum and bladder away from the radioactive sources will decrease the risk of complications. In addition, newer treatment techniques such as intensity-modulated radiotherapy (IMRT) use sophisticated planning techniques to avoid critical structures (discussed following). Preventive therapeutic strategies also include investigation of antioxidants, free-radical scavengers and other cytoprotectant agents, cytokine modification, enterotrophic (growth-promoting) strategies, novel anti-inflammatory agents, modulators of intraluminal contents, modulators of endothelial dysfunction, as well neuroimmunomodulators to prevent this complication.103
LARGE INTESTINE
INCIDENCE AND CLINICAL FEATURES
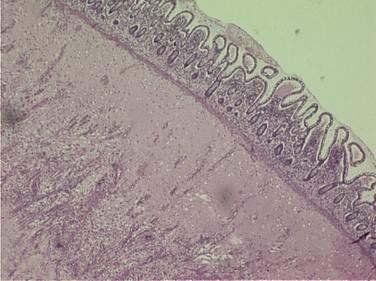
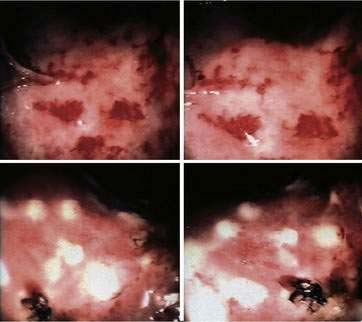
Acute and chronic injury of the large intestine is similar to injury of the small intestine just discussed. Acutely, there is a decrease in the stem cell mitotic rate, resulting in a depletion of precursor cells required to replenish the epithelium as it normally sheds. Acute injury can be accompanied by superficial mucosal erosions and lamina proprial hemorrhage. There is also a thickening of the mucosa with proliferation of fibroblasts (Fig. 39-6).129 Late changes include vascular fibrosis with associated ischemia and formation of telangiectasias which can be a source of bleeding (Fig. 39-7). Late radiation bowel changes can lead to fluid and electrolyte malabsorption, obstruction, chronic proctitis, and fistula formation. Ischemic changes also include ulceration (Fig. 39-8), perforation, and fistulae.56 Bowel wall fibrosis may also occur, causing decreased motility, stricture and compliance.130 A decrease in rectal compliance may reduce the ability of the rectum to act as a reservoir, leading to fecal frequency, urgency, and incontinence.
Acute colitis from radiation therapy manifests clinically as diarrhea, cramping, tenesmus, urgency, incontinence, and less commonly as mucoid or bloody rectal discharge. These symptoms can result from rectal inflammation, edema, and spasm. Symptoms often begin two to three weeks into treatment, and usually resolve within several weeks to three months following radiation completion. A relationship between the incidence of acute and chronic radiation injury is uncertain.131,132 Chronic changes appear within six months to two years and beyond following completion of radiation therapy, with symptoms similar to acute injury. Patients may present with tenesmus, bleeding, low-volume diarrhea, rectal pain, and occasionally low-grade obstruction or fistulae.133 Patients can develop a pancolitis that mimics inflammatory bowel disease. It should be remembered that pelvic irradiation is a risk factor for development of rectal cancer.134
The large intestine is less radiosensitive than the small intestine. This may be partially explained by the fact that higher doses of radiation are often delivered to smaller volumes of the rectum compared with small intestine (i.e., focal “collateral” rectal irradiation in prostate cancer therapy, focal collateral rectal irradiation in high-dose gynecologic malignancy implants, and so on). The rectum is also a readily accessible organ by endoscopy, allowing early diagnosis as well as intervention, possibly preventing symptomatic progression. Series have reported the risk of serious late rectal complications is 5% or less when less than 80 Gy is delivered.17 Radiation injury of the large intestine occurs most frequently in the rectum due to its location adjacent to the prostate, bladder, cervix, uterus, and ovaries, exposing it to collateral radiation dose with treatment of these organs.15 Acute rectal injury is often self-limited, but the incidence of chronic radiation proctitis is increasing with increased use of pelvic radiation therapy and radiation dose escalation.135,136
As is true with the other sites, the incidence of large bowel toxicity is associated with radiation dose, volumes treated, and the use of concurrent chemotherapy. In an early study of radiation therapy for testicular cancer, radiation injury to the colon occurred in 18% of patients after 45 Gy and in 37% of patients after 60 to 64 Gy.137 The treatment of rectal cancer commonly uses doses of 45 to 54 Gy, whereas treatment of prostate and cervical cancer uses higher doses, ranging from 60 to 80 Gy. The incidence of severe rectosigmoid toxicity in cervical cancer patients was 4% or less for patients receiving doses below 80 Gy, and 13% for doses greater than 95 Gy.138 The treatment of prostate cancer with doses of 60 to 70 Gy has been associated with an incidence of severe proctitis below 8%.139 Radiation doses of 60 to 70 Gy for anal cancer yield an incidence of severe rectal toxicity of 5% or less.140–142 Treatment using conformal radiation, three-field, and four-field techniques further decreases the risk of rectal toxicity.138,143 A trial of conformal versus conventional radiation for prostate cancer reported less radiation proctitis (5% versus 15%, respectively).144 The use of IMRT (discussed later) may further improve this rate, as demonstrated by a recent series treating of prostate cancer patients to doses greater than 80 Gy. Of 772 patients, 4.5% developed acute grade 2 rectal symptoms and no patient developed acute grade 3 or above rectal symptoms. Eleven patients (1.5%) developed late grade 2 rectal bleeding and four patients (0.1%) experienced late grade 3 rectal toxicity requiring either transfusion or laser cauterization. No late grade 4 rectal complications were observed.145
Combining chemotherapy with radiation therapy increases toxicity rates. A study of 5-FU plus cisplatin and radiation therapy for cervical cancer showed a combined incidence of severe small and large bowel toxicity of 18%.146 In contrast, a combination of 5-FU and mitomycin C with radiation doses of 40 to 55 Gy in the treatment of anal cancer was associated with a less than 5% risk of severe rectal complications.147 Multiple trials have combined 5-FU–based chemotherapy and radiation therapy as neoadjuvant and adjuvant treatment for rectal cancer.88,91,148,149 The toxic effect of combined chemotherapy and radiation has varied, from no significant increase in toxicity to a 24% incidence of severe diarrhea and a 25% incidence of chronic bowel injury.150 Because of the increase in toxicity seen with single or opposed-only radiation fields, the use of conformal and multifield techniques is necessary when using combination therapy. The increasing use of neoadjuvant chemoradiotherapy has also raised the concern of increased postoperative complications in these patients. However, a large randomized trial showed a significant reduction in the rates of acute and chronic gastrointestinal toxicity in patients treated neoadjuvantly versus patients treated adjuvantly.149
In contrast to small bowel injury, previous abdominopelvic surgery does not appear to predispose the rectum to radiation injury, likely due to the fact it is not otherwise mobile. However, because of the similarity of vascular changes seen with small bowel radiation injury, a history of diabetes, hypertension, cardiovascular disease, or peripheral vascular disease may predispose large bowel to radiation toxicity.76–79 Patients with collagen vascular disease and inflammatory bowel disease also have an increased propensity for large bowel radiation toxicity (as discussed in the section on small bowel toxicity).
TREATMENT AND PREVENTION
Management of large bowel radiation toxicity is based on symptom control. Acute toxicity is treated with antimotility agents, such as loperamide or diphenoxylate with atropine, and a low-residue diet. Opiates and anticholinergics may also be of benefit. Glucocorticoid-containing suppositories may be helpful in the management of patients with anorectal inflammation. Colonoscopy should be avoided if possible because of the potential risk of perforation associated with the friability of the rectal mucosa during radiation.151
For chronic diarrhea due to decreased rectal compliance, stool softeners or fiber supplements can help. As in acute proctitis, steroid suppositories may be beneficial. The benefit of steroid retention enemas is unclear.152 Sucralfate enemas, also controversial, may be used for ulcerative lesions. Short-chain fatty acids, which nourish the colonic mucosa, have been studied in acute and chronic radiation proctitis. Initial relief of symptoms can be seen, but symptoms recur shortly after stopping treatment.153 Hyperbaric oxygen has been used to stabilize bleeding related to telangiectasias, but this treatment is not widely available and requires many treatments before any effect is seen.154,155 Nonetheless, a randomized trial in patients with refractory chronic radiation proctitis reported that hyperbaric oxygen therapy significantly improved healing.156
Treatment of colorectal ulcerations associated with bleeding is initially endoscopic, with the use of coagulation techniques, such as argon plasma coagulation. Bleeding due to radiation proctopathy is usually minor and often controlled endoscopically with conservative measures such as cauterization of the telangiectasias with laser treatment (see Fig. 39-7),157–160 or by application of formalin.161–164 Sucralfate enemas may alleviate radiation proctopathy by forming a protective complex with the rectal mucosa. It also increases the local levels of fibroblast growth factors and prostaglandins. Sucralfate enemas appear to be helpful in chronic proctopathy but their benefit is unclear during the acute period.165–167 Short-chain fatty acid enemas may be also helpful for management of chronic hemorrhagic radiation proctopathy by inhibiting the inflammatory response including the NF-κB pathway168,169 (see Chapter 2).
Strictures can also be endoscopically dilated. For patients who have refractory bleeding, stricture, perforation, or fistulae, surgical management may (rarely) be necessary. Management of a pelvic fistula (e.g., vaginal or bladder fistula) is complex and requires fecal diversion before the corrective surgery. A thorough radiographic investigation such as barium enema, small bowel follow-through, or enteroclysis to delineate the extent of the fistula should be performed before surgery. Patients with fistulas may present with additional challenges such as electrolyte imbalance, malnutrition, and infections. Many surgical techniques have been described to repair fistulas, but the corrective surgery is best done when the patient is medically stable and enough time has elapsed after the surgical diversion. This allows healing and decreased inflammation of the affected tissues.170,171
Prevention of large bowel toxicity from radiation has been studied. Prostaglandins have been investigated as a potential radioprotector. Prostaglandin E2 and prostaglandin analogs display radiation protection in animal studies.172–175 Misoprostol suppositories also have been shown to reduce symptoms of acute radiation enteritis in patients undergoing radiation therapy for prostate cancer.176 However, a randomized placebo-controlled trial from Germany in patients with prostate cancer undergoing irradiation found that significantly more patients experienced rectal bleeding in the misoprostol group.177
Amifostine is a sulfhydryl compound that is converted intracellularly to an active metabolite, which in turn binds to free radicals and protects the cell from radiation injury.178 Amifostine has been investigated for the prevention of chronic radiation enteritis. Amifostine has demonstrated protection of the small as well as the large intestines in preclinical studies.179 Amifostine has also been shown to reduce the incidence of early and delayed radiotherapeutic injuries at several anatomic sites. In one randomized study the late effects of radiation were significantly reduced in the group receiving parenterally administered amifostine.180 However, the median follow-up was quite short (24 months), and longer follow-up is necessary to confirm the benefits of the medication given the incidence of late complications increases with time. Another randomized trial evaluated 205 patients with pelvic malignancies who received radiation therapy, alone or with intravenous amifostine. Patients receiving amifostine experienced a significantly lower incidence of grades 2 and 3 bladder and lower gastrointestinal tract toxicity, with no significant difference between the two groups in tumor response to treatment.181 There is also evidence to suggest that intrarectal application of amifostine may reduce the risk of proctitis in patients undergoing radiotherapy for prostate cancer.182
A preclinical study showed a possible role for anti–TGF-β1 interventions to reduce delayed radiation fibrosis and enteropathy.183 Many special diets and nutrients such as fiber, elemental diets, short-chain fatty acids, and amino acids such as glutamine may reduce radiation toxicity to the intestine. However, consistent clinical results were not observed.184–189
ANUS
INCIDENCE AND CLINICAL FEATURES
The anal canal is typically spared from significant radiation exposure except in irradiation for anal, low rectal, and gynecologic cancers. The primary acute toxicity from anal cancer irradiation is diarrhea from the exposure of the large bowel to irradiation. However, damage to the anus itself can occur in the form of acute desquamation or ulceration, with later development of ulcers, strictures, anorectal fistulae, and incontinence.190 The primary data on anal toxicity from radiation therapy come from studies using radiation or chemoradiotherapy for the treatment of anal cancer itself. Anal toxicity manifests as mucosal edema and friability.191 These changes are often exacerbated by diarrhea that occurs from rectal toxicity. Chronically, anal fibrotic changes may evolve.
Clinically, anal toxicity presents initially as a perianal skin reaction that ranges from minimal skin changes to moist desquamation and erythema, as well as diarrhea. These changes are self-limited and usually resolve within a few weeks of completion of therapy. Acute toxicity can lead to an interruption of therapy, although this may be less common with modern radiation treatment techniques.147,191,192 The incidence of acute toxicity is high, and is increased with concurrent chemotherapy delivery or use of a large dose per fraction.142,193–195 Phase III studies and series of patients treated with combined chemotherapy and radiation therapy have noted an incidence of skin toxicity of grade 3 or above in 26% to 78% of patients using doses of 45 to 60 Gy in 1.8- to 2.25-Gy fractions.147,190,195–199 A study using conformal radiation techniques (50.4 Gy in 1.8-Gy fractions) combined with chemotherapy reported grade 3 (or higher) acute skin toxicity in only 20% of patients.200 In a recent multi-institutional experience of anal cancer patients treated with IMRT-based chemoradiotherapy, grade 3 skin toxicity was seen in 38% of patients with no grade 4 toxicity observed, comparing favorably to the results of previous randomized trials.192 Further studies of advanced radiation therapy techniques are needed to confirm this improvement.
Late anal toxicity occurs within months to years following completion of therapy. The most common late complication is anorectal ulceration. Patients also may develop anal stricture or stenosis, incontinence, anal pain, or anorectal fistulae.190,194,195,201 There does not appear to be an increase in the occurrence of chronic anal toxicity with the addition of chemotherapy to radiation therapy.147,199,201 Doses of 45 to 60 Gy in fractions of 1.8 to 2 Gy are considered safe, resulting in chronic grade 3 (or higher) toxicity rates of zero to 22%.140,141,193,195,202,203 Doses greater than 65 Gy or fraction size greater than 2 Gy results in a high incidence of anal toxicity.194 Patients with HIV and anal cancer who are treated with combined chemotherapy and radiation therapy have an increased risk of acute and late anal toxicity.204
TREATMENT
Treatment of acute toxicity is primarily supportive, including proper skin care, dietary modifications, pain medications and topical steroid medications, with breaks in radiation treatment if severe. The effects are self-limited, and usually resolve within weeks of conclusion of therapy. Treatment for chronic toxicity with anal stricture and stenosis includes sphincter dilatation. Rare patients can require colostomy for severe symptoms. Small studies of hyperbaric oxygen therapy have shown efficacy in treating chronic anorectal ulcers.205 There is also a report of oral vitamin A therapy as a treatment for anorectal ulceration, but confirmatory studies are lacking.191
LIVER
INCIDENCE AND CLINICAL FEATURES
Radiation-induced liver disease (RILD) is seen in approximately 5% of patients when the whole liver radiation dose reaches 30 to 35 Gy at 2 Gy per fraction.206,207 The pathologic lesion in RILD is central vein thrombosis at the lobular level (veno-occlusive disease), which results in marked sinusoidal congestion leading to lobular hemorrhage and secondary injury to surrounding hepatocytes.208 Fibrin deposition in the central veins is thought to be the cause of the veno-occlusive injury. It is unknown what stimulates the fibrin deposition, but there are hypotheses that suggest that TGF-β is increased in the setting of exposure to radiation, and this in turn stimulates fibroblast migration to the site of injury, causing fibrin and collagen deposition. Foci of necrosis are found in the affected portion of the lobules.209 Severe acute hepatic toxicity may progress to fibrosis, cirrhosis, and liver failure.
Recent studies have emphasized the effect of the liver volume irradiated in addition to dose.208 Although radiation hepatopathy can occur after doses of 35 to 40 Gy to the entire liver, significantly higher doses can be given with few clinical complications if sufficient normal liver is spared. Studies by Lawrence and colleagues report that if less than 25% of the normal liver is treated with radiation therapy there may be no upper limit on dose associated with radiation hepatopathy.208 Estimates of the hepatic irradiation doses associated with a 5% risk of RILD for uniform irradiation of one third, two thirds, and the whole liver are 90 Gy, 47 Gy, and 31 Gy, respectively. Combining chemotherapy and radiation can increase liver damage, particularly if the chemotherapeutic agents are hepatotoxic. Chlorambucil, busulfan, and platinum drugs are used with radiation in bone marrow transplantation and are hepatotoxic agents. In contrast, fluoropyrimidines do not seem to increase radiation-related hepatotoxicity.207,210
THERAPEUTIC TECHNIQUES TO REDUCE TOXICITY
In the past, radiation therapy plans were based on two-dimensional (2D) planning, in which treatment fields were defined using x-ray images and known anatomic landmarks. With improvements in imaging and computer capabilities, three-dimensional (3D) treatment planning became available in the 1980s. An advanced form of 3D planning, intensity-modulated radiation therapy (IMRT) has now been implemented in clinical practice.211,212 IMRT is a potentially significant advance in achieving these goals. As opposed to conventional “static” fields, IMRT uses the principle of multiple “fields-within-fields” that more accurately conform radiation dose to target tissues while sparing normal structures. IMRT requires target tissues and normal organs are accurately defined. Dose constraints are assigned to these organs along with a desired (prescription) dose to the target volume(s). “Inverse planning,” whereby computer search algorithms establish multiple (and sometimes unconventional) beam or field designs is then performed, attempting to meet the prescribed target dose and normal tissue dose constraints. Individual fields are treated with multiple, small “beamlets” rather than one uniform beam, and each beam delivers a different dose (intensity) to the different parts of the target. This allows close conformation of radiation dose to the shape of the target and preferential sparing of normal surrounding organs from the high-dose areas.
Collectively, early clinical results in varying cancers using IMRT-based chemoradiotherapy have shown significant decreases in treatment-related toxicities, with cancer-related outcomes similar to conventional radiotherapy approaches. For example, Mundt and associates showed a marked improvement in small bowel dosimetry for patients with gynecologic malignancies treated with IMRT compared with conventional 3D planning. An experience of 36 patients with gynecologic malignancies treated with intensity-modulated whole pelvic radiotherapy were compared with outcomes of 30 patients treated at the same institution with 3D conformal radiotherapy. Patients were well matched with respect to demographic and treatment factors. Significantly lower rates of chronic gastrointestinal toxicity were seen in the IMRT group, with only 11% of women treated with IMRT experiencing grades 1 to 3 toxicity (0% grade 3) versus 50% in the non-IMRT group.213 In a different series, Salama and colleagues reported on 53 patients with anal carcinoma treated with IMRT-based chemoradiotherapy. The median radiation doses to the pelvis and the primary disease were 45 and 52 Gy, respectively. Fifteen percent of patients experienced acute grade 3 gastrointestinal toxicity with no grade 4 toxicity observed, comparing favorably to observed rates of severe gastrointestinal toxicity in contemporary trials using conventional radiation planning.192 This is especially notable given the significantly higher pelvic doses delivered in patients receiving IMRT in this series. These techniques will require further demonstration of meaningful clinical benefit in patient outcomes to further solidify their routine use in clinical practice, with cooperative group trials underway.
Ahn SJ, Kahn D, Zhou S, et al. Dosimetric and clinical predictors for radiation-induced esophageal injury. Int J Radiat Oncol Biol Phys. 2005;61:335-47. (Ref 29.)
Clark R, Tenorio L, Husse J, et al. Hyperbaric oxygen treatment for chronic refractory radiation proctitis: A randomized and controlled double blind cross-over trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2008. March 12 [Epub ahead of print]. PMID 18342453. (Ref 156.)
Coia LR, Myerson RJ, Tepper JE. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys. 1995;31:1213-36. (Ref 17.)
Dawson LA, Ten Haken RK, Lawrence T. Partial irradiation of the liver. Semin Radiat Oncol. 2001;15:240-6. (Ref 208.)
Gunnlaugsson A, Kjellen E, Nilsson P, et al. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol. 2007;46:937-44. (Ref 87.)
Hauer-Jensen M, Wang J, Denham JW. Bowel injury: Current and evolving management strategies. Semin Radiat Oncol. 2003;13:357-71. (Ref 103.)
Hille A, Schmidberger H, Hermann RM, et al. A phase III randomized, placebo-controlled, double-blind study of misoprostol rectal suppositories to prevent acute radiation proctitis in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1488-93. (Ref 177.)
Horiot JC, Aapro M. Treatment implications for radiation-induced nausea and vomiting in specific patient groups. Eur J Cancer. 2004;40:979-87. (Ref 47.)
Johnston M, Robertson G, Frizelle F, et al. Management of late complications of pelvic radiation in the rectum and anus. Dis Colon Rectum. 2003;46:247-59. (Ref 136.)
Marshall GT, Thirlby RC, Bredfeldt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42. (Ref 112.)
Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: Radiation therapy oncology group trial 98-01. J Clin Oncol. 2005;23:2145-54. (Ref 33.)
O’Rourke IC, Tiver K, Bull C, et al. Swallowing performance after radiation therapy for carcinoma of the esophagus. Cancer. 1988;61:2022-6. (Ref 16.)
Willett CG, Ooi CJ, Zietman AL, et al. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. Int J Radiat Oncol Biol Phys. 2000;46:995-8. (Ref 79.)
Wong RK, Paul N, Ding K, et al. 5-Hhydroxytryptamine-3 receptor antagonist with or without short-course dexamethasone in the prophylaxis of radiation induced emesis: A placebo-controlled randomized trial of the National Cancer Institute of Canada Clinical Trials Group (SC19). J Clin Oncol. 2006;24:3458-64. (Ref 48.)
1. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTC, NCI, NIH, DHHS March 31, 2003 (http://ctep.cancer.gov), Publish date: June 10, 2003.
2. Potten C, Booth C. The role of radiation induced and spontaneous apoptosis in the homeostasis of the gastrointestinal epithelium. Comp Biochem Physiol. 1997;3:473.
3. Landberg C, Hauer-Jensen M, Sung C, et al. Expression of fibrogenic cytokines in rat small intestine after fractionated irradiation. Radiother Oncol. 1994;32:29.
4. Richter K, Langberg C, Sung C, et al. Increased transforming growth factor β (TGF-β) immunoreactivity is independently associated with chronic injury in both consequential and primary radiation enteropathy. Int J Radiat Oncol Biol Phys. 1997;19:187.
5. Wang J, Zheng H, Sung C, et al. Cellular sources of transforming grow factor-β isoforms in early and chronic radiation enteropathy. Am J Pathol. 1998;5:1531.
6. Wang J, Richter K, Sung C, et al. Upregulation and spatial shift in the localization of the mannose 6-phosphate/insulin-like growth factor II receptor during radiation enteropathy development in the rat. Radiother Oncol. 1999;50:205.
7. Skwarchuk M, Travis L. Changes in histology and fibrogenic cytokines in irradiated colorectum of two murine strains. Int J Radiat Oncol Biol Phys. 1998;42:169.
8. Richter K, Fink L, Hughes B, et al. Is the loss of endothelial thrombomodulin involved in the mechanism of chronicity in late radiation enteropathy? Radiother Oncol. 1997;44:65.
9. Isaka Y, Brees D, Ikegaya K, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2:418.
10. Herskind C, Bamberg M, Roderman H. The role of cytokines in the development of normal tissue reactions after radiotherapy. Strahlenther Onkol. 1998;174:12.
11. Vozenin-Brotons M-C, Fabien M, Sabourin J-C, et al. Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int J Radiat Oncol Biol Phys. 2003;56:561.
12. Chowan N. Injurious effects of radiation on the esophagus. Am J Gastroenterol. 1990;85:115-20.
13. Phillips TL, Ross G. Time-dose relationships in the mouse esophagus. Radiology. 1974;113:435-40.
14. Coia L. The esophagus. Moss’ Radiation Oncology: Rationale, Techniques, Results, 7th ed. 1994. Mosby–Year Book, St. Louis, Mo.
15. Berthrong M, Fajardo LF. Radiation injury in surgical pathology. Part II. Alimentary tract. Am J Surg Pathol. 1981;5:153-78.
16. O’Rourke IC, Tiver K, Bull C, et al. Swallowing performance after radiation therapy for carcinoma of the esophagus. Cancer. 1988;61:2022-6.
17. Coia LR, Myerson RJ, Tepper JE. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys. 1995;31:1213-36.
18. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-22.
19. Seaman WB, Ackerman LV. The effect of radiation on the esophagus; a clinical and histologic study of the effects produced by the betatron. Radiology. 1957;68:534-41.
20. Morichau-Beauchant M, Touchard G, Battandier D, et al. Chronic radiation-induced esophagitis after treatment of oropharyngolaryngeal cancer: A little-known anatomo-clinical entity. Gastroenterol Clin Biol. 1983;7:843-50.
21. Kramer S, Gelberr R, Snow J. Preoperative versus postoperative radiation therapy in advanced squamous cell carcinoma of the head and neck: Final report on RTOG 73-03. Am J Clin Oncol (CCT). 1983;6:150.
22. Gaspar LE, Winter K, Kocha WI, et al. A phase I/II study of external beam radiation, brachytherapy, and concurrent chemotherapy for patients with localized carcinoma of the esophagus (Radiation Therapy Oncology Group Study 9207): Final report. Cancer. 2000;88:988-95.
23. Vuong T, Szego P, David M, et al. The safety and usefulness of high-dose-rate endoluminal brachytherapy as a boost in the treatment of patients with esophageal cancer with external beam radiation with or without chemotherapy. Int J Radiat Oncol Biol Phys. 2005;63:758-64.
24. Ball D, Bishop J, Smith J, et al. A phase III study of accelerated radiotherapy with and without carboplatin in non-small cell lung cancer: An interim toxicity analysis of the first 100 patients. Int J Radiat Oncol Biol Phys. 1995;31:267.
25. Choy H, LaPorte K, Knill-Selby E, et al. Esophagitis in combined modality therapy for locally advanced non-small cell lung cancer. Sem Radiat Oncol. 1999;9:90.
26. Werner-Wasik M, Pequignot E, Leeper D, et al. Predictors of severe esophagitis include use of concurrent chemotherapy, but not the length of irradiated esophagus: A multivariate analysis of patients with lung cancer treated with non-operative therapy. Int J Radiat Oncol Biol Phys. 2000;48:689.
27. Maguire PD, Sibley GS, Zhou SM, et al. Clinical and dosimetric predictors of radiation-induced esophageal toxicity. Int J Radiat Oncol Biol Phys. 1999;45:97-103.
28. Singh AK, Lockett MA, Bradley JD. Predictors of radiation-induced esophageal toxicity in patients with non-small-cell lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:337-41.
29. Ahn SJ, Kahn D, Zhou S, et al. Dosimetric and clinical predictors for radiation-induced esophageal injury. Int J Radiat Oncol Biol Phys. 2005;61:335-47.
30. Byhardt RW, Scott C, Sause WT, et al. Response, toxicity, failure patterns, and survival in five Radiation Therapy Oncology Group (RTOG) trials of sequential and/or concurrent chemotherapy and radiotherapy for locally advanced non-small-cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1998;42:469-78.
31. Sasso FS, Sasso G, Marsiglia HR, et al. Pharmacological and dietary prophylaxis and treatment of acute actinic esophagitis during mediastinal radiotherapy. Dig Dis Sci. 2001;46:746-9.
32. Capizzi RL, Oster W. Chemoprotective and radioprotective effects of amifostine: An update of clinical trials. Int J Hematol. 2000;72:425-35.
33. Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: Radiation therapy oncology group trial 98-01. J Clin Oncol. 2005;23:2145-54.
34. Antonadou D, Throuvalas N, Petridis A, et al. Effect of amifostine on toxicities associated with radiochemotherapy in patients with locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;57:402-8.
35. Werner-Wasik M, Axelrod RS, Friedland DP, et al. Phase II: trial of twice weekly amifostine in patients with non-small cell lung cancer treated with chemoradiotherapy. Semin Radiat Oncol. 2002;12:34-9.
36. Arquette M, Wasserman T, Govindan R, et al. Phase II evaluation of amifostine as an esophageal mucosal protectant in the treatment of limited-stage small cell lung cancer with chemotherapy and twice-daily radiation. Semin Radiat Oncol. 2002;12:59-61.
37. Breiter N, Trott KR, Sassy T. Effect of X-irradiation on the stomach of the rat. Int J Radiat Oncol Biol Phys. 1989;17:779-84.
38. Goldgraber MB, Rubin CE, Palmer WL, et al. The early gastric response to irradiation; a serial biopsy study. Gastroenterology. 1954;27:1-20.
39. Palmer W. Gastric irradiation in peptic ulcer. Chicago: University of Chicago Press; 1974.
40. Stevens K. The stomach and small intestine. Moss’ Radiation Oncology: Rationale, Techniques, Results, 7th ed. 1994. Mosby–Year Book, St. Louis, Mo.
41. Henriksson R, Bergstrom P, Franzen L, et al. Aspects on reducing gastrointestinal adverse effects associated with radiotherapy. Acta Oncol. 1999;38:159-4.
42. Sell A, Jensen TS. Acute gastric ulcers induced by radiation. Acta Radiol Ther Phys Biol. 1966;4:289-97.
43. Cosset JM, Henry-Amar M, Burgers JM, et al. Late radiation injuries of the gastrointestinal tract in the H2 and H5 EORTC Hodgkin’s disease trials: Emphasis on the role of exploratory laparotomy and fractionation. Radiother Oncol. 1988;13:61-8.
44. Pearson JG. The present status and future potential of radiotherapy in the management of esophageal cancer. Cancer. 1977;39:882-90.
45. Hamilton CR, Horwich A, Bliss JM, et al. Gastrointestinal morbidity of adjuvant radiotherapy in stage I malignant teratoma of the testis. Radiother Oncol. 1987;10:85-90.
46. Gunderson LL, Hoskins RB, Cohen AC, et al. Combined modality treatment of gastric cancer. Int J Radiat Oncol Biol Phys. 1983;9:965-75.
47. Horiot JC, Aapro M. Treatment implications for radiation-induced nausea and vomiting in specific patient groups. Eur J Cancer. 2004;40:979-87.
48. Wong RK, Paul N, Ding K, et al. 5-Hydroxytryptamine-3 receptor antagonist with or without short-course dexamethasone in the prophylaxis of radiation induced emesis: A placebo-controlled randomized trial of the National Cancer Institute of Canada Clinical Trials Group (SC19). J Clin Oncol. 2006;24:3458-64.
49. Walsh D. Deep tissue traumatism from roentgen ray exposure. BMJ. 1897;2:272-3.
50. Erickson BA, Otterson MF, Moulder JE, et al. Altered motility causes the early gastrointestinal toxicity of irradiation. Int J Radiat Oncol Biol Phys. 1994;28:905-2.
51. Claben J, Belka C, Paulson F, et al. Radiation-induced gastrointestinal toxicity: pathophysiology approaches to treatment and prophylaxis. Strahlenther Onkol. 1998;147:82-4.
52. Hauer-Jensen M. Late radiation injury of the small intestine. Clinical, pathophysiologic and radiobiologic aspects. A review. Acta Oncol. 1990;29:401-15.
53. Buchler DA, Kline JC, Peckham BM, et al. Radiation reactions in cervical cancer therapy. Am J Obstet Gynecol. 1971;111:745-50.
54. Shofield P, Carr N, Holden D. Pathogenesis and treatment of radiation bowel disease. J R Soc Med. 1986;79:30-2.
55. Greenberger N, Isselbacher K. Malabsorption following radiation injury to the gastrointestinal tract. Am J Med. 1964;36:450-6.
56. Haselton P, Carr N, Schofield P. Vascular changes in radiation bowel disease. Histopathology. 1985:517-34.
57. Galland RB, Spencer J. Spontaneous postoperative perforation of previously asymptomatic irradiated bowel. Br J Surg. 1985;72:285.
58. Husebye E, Hauer-Jensen M, Kjorstad K, et al. Severe late radiation enteropathy is characterized by impaired motility of proximal small intestine. Dig Dis Sci. 1994;39:2341.
59. Husebye E, Skar V, Hoverstad T, et al. Abnormal intestinal motor patterns explain enteric colonization with gram negative bacilli in late radiation enteropathy. Gastroenterology. 1995;109:1078.
60. Galland R, Spender J. The natural history of clinically established radiation enteritis. Lancet. 1985;1:1257.
61. Harling H, Balslev I. Long-term prognosis of patients with severe radiation enteritis. Am J Surg. 1988;155:517.
62. Silvain C, Besson I, Ingrand P, et al. Long term outcome of severe radiation enteritis treated by total parenteral nutrition. Dig Dis Sci. 1992;37:1065.
63. Fischer L, Kimose H, Spjeldnaes N, et al. Late radiation injuries of the small intestine: Management and outcome. Acta Chir Scand. 1989;155:47.
64. Kimose H, Fischer L, Spjeldnaes N, et al. Late radiation injury of the colon and rectum: Surgical management and outcome. Dis Colon Rectum. 1989;32:684.
65. Regimbeau J-M, Panis Y, Gouzi J-L, et al. Operative and long term results after surgery for chronic radiation enteritis. Am J Surg. 2001;182:237.
66. Rodier J. Radiation enteropathy-incidence, aetiology, risk factors, pathology and symptoms. Tumori. 1995;81:122.
67. Ooi B, Tjandra J, Green M. Morbidities of adjuvant chemotherapy and radiotherapy for resectable rectal cancer. An overview. Dis Col Rect. 1999;42:403.
68. Yeoh E, Sun W, Russo A, et al. A retrospective study of the effects of pelvic irradiation for gynecological cancer on anorectal function. Int J Radiat Oncol Biol Phys. 1996;35:1003.
69. Fransson P, Widmark A. Late side effects unchanged 4-8 years after radiotherapy for prostate carcinoma. Cancer. 1999;85:678.
70. Letschert G, Lebesque J, Aleman B, et al. The volume effect in radiation-related late small bowel complications: results of a clinical study of the EORTC Radiotherapy Cooperative Group in patients treated for rectal carcinoma. Radiother Oncol. 1994;32:116-23.
71. Stockbrine M, Hancock J, Fletcher G. Complications in 831 patients with squamous cell carcinoma of the intact uterine cervix treated with 3000 rads or more whole pelvis radiation. Am J Roentgenol. 1970;108:283-304.
72. Louidice T, Lang J. Treatment of radiation enteritis: A comparison study. Am J Gastroenterol. 1983;78:481-7.
73. Louidice T, Baxter D, Bailint J. Effects of abdominal surgery on the development of radiation enteropathy. Gastroenterol. 1977;73:1093-7.
74. Green N. The avoidance of small intestine injury in gynecologic cancer. Int J Radiat Oncol Biol Phys. 1983;9:1385-90.
75. Eifel PJ, Levenback C, Wharton JT, et al. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1995;32:1289-300.
76. DeCosse JJ, Rhodes RS, Wentz WB, et al. The natural history and management of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369-84.
77. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136-43.
78. Song DY, Lawrie WT, Abrams RA, et al. Acute and late radiotherapy toxicity in patients with inflammatory bowel disease. Int J Radiat Oncol Biol Phys. 2001;51:455-9.
79. Willett CG, Ooi CJ, Zietman AL, et al. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. Int J Radiat Oncol Biol Phys. 2000;46:995-8.
80. Kao M. Intestinal complications of radiotherapy in gynecologic malignancy—Clinical presentation and management. Int J Obstet Gynecol. 1995;49:S69-75.
81. Hanks GE, Herring DF, Kramer S. Patterns of care outcome studies. Results of the national practice in cancer of the cervix. Cancer. 1983;51:959-67.
82. Perez CA, Fox S, Lockett MA, et al. Impact of dose in outcome of irradiation alone in carcinoma of the uterine cervix: Analysis of two different methods. Int J Radiat Oncol Biol Phys. 1991;21:885-98.
83. Gallagher MJ, Brereton HD, Rostock RA, et al. A prospective study of treatment techniques to minimize the volume of pelvic small bowel with reduction of acute and late effects associated with pelvic irradiation. Int J Radiat Oncol Biol Phys. 1986;12:1565-73.
84. Duttenhaver JR, Hoskins RB, Gunderson LL, et al. Adjuvant postoperative radiation therapy in the management of adenocarcinoma of the colon. Cancer. 1986;57:955-63.
85. Baglan KL, Frazier RC, Yan D, et al. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:176-83.
86. Tho LM, Glegg M, Paterson J, et al. Acute small bowel toxicity and preoperative chemoradiotherapy for rectal cancer: investigating dose-volume relationships and role for inverse planning. Int J Radiat Oncol Biol Phys. 2006;66:505-13.
87. Gunnlaugsson A, Kjellen E, Nilsson P, et al. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol. 2007;46:937-44.
88. Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465-72.
89. Douglass HOJr, Moertel CG, Mayer RJ, et al. Survival after postoperative combination treatment of rectal cancer. N Engl J Med. 1986;315:1294-5.
90. Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709-15.
91. O’Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502-7.
92. Dunst J, Reese T, Sutter T, et al. Phase I trial evaluating the concurrent combination of radiotherapy and capecitabine in rectal cancer. J Clin Oncol. 2002;20:3983-91.
93. Czito BG, Willett CG, Bendell JC, et al. Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer: phase I trial results. J Clin Oncol. 2006;24:656-62.
94. Ryan DP, Niedzwiecki D, Hollis D, et al. Phase I/II study of preoperative oxaliplatin, fluorouracil, and external-beam radiation therapy in patients with locally advanced rectal cancer: Cancer and Leukemia Group B 89901. J Clin Oncol. 2006;24:2557-62.
95. Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol. 2006;24:650-5.
96. Yeoh E, Horowitz M, Russo A, et al. Gastrointestinal function in chronic radiation enteritis—Effects of loperamide-N-oxide. Gut. 1993;34:476.
97. Meyers J, Ehrenpreis E, Craig R. Small intestinal bacterial overgrowth syndrome. Curr Treat Options Gastroenterol. 2001;4:7.
98. Attar A, Flourie B, Rambaud J, et al. Antibiotic efficacy in small intestinal bacterial overgrowth-related chronic diarrhea: A crossover, randomized trial. Gastroenterology. 1999;117:794.
99. Henriksson R, Franzen L, Littbrand B. Effects of sucralfate on acute and late bowel discomfort following radiotherapy of pelvic cancer. J Clin Oncol. 1992;10:969-75.
100. Berk RN, Seay DG. Cholerheic enteropathy as a cause of diarrhea and death in radiation enteritis and its prevention with cholestyramine. Radiology. 1972;104:153-6.
101. Mennie AT, Dalley VM, Dinneen LC, et al. Treatment of radiation-induced gastrointestinal distress with acetylsalicylate. Lancet. 1975;2:942-3.
102. Van Gossum A, Bakker H, Bozetti F, et al. Home parenteral nutrition in adults: A European multicentre survey in 1997. Clin Nutr. 1999;18:135.
103. Hauer-Jensen M, Wang J, Denham JW. Bowel injury: current and evolving management strategies. Semin Radiat Oncol. 2003;13:357-71.
104. Girvent M, Carlson G, Anderson I, et al. Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl. 2000;82:198.
105. Galland R, Spender J. Surgical management of radiation enteritis. Surgery. 1986;99:133.
106. Kuroki F, Iida M, Matsui T, et al. Intraoperative endoscopy for small intestinal damage in radiation enteritis. Gastroenterol Endosc. 1992;38:196.
107. Neurath M, Branbrink A, Meyer K, et al. A new treatment for severe malabsorption due to radiation enteritis. Lancet. 1996;347:1302.
108. Hamour A, Denning D. Hyperbaric oxygen therapy in a woman who declined colostomy. Lancet. 1996;348:197.
109. Gouello J, Bouachour G, Person B, et al. Interet de l’oxygenotherapie hyperbare dans la pathologie digestive post radique. La Presse Med. 1993;28:1053.
110. Zimmermann F, Feldmann H. Radiation proctitis: Clinical and pathological manifestations, therapy and prophylaxis of acute and late injurious effects of radiation on the rectal mucosa. Strahlenther Onkol. 1998;174:85.
111. Feldmeier J, Heimbach R, Davolt D, et al. Hyperbaric oxygen an adjunctive treatment for delayed radiation injuries of the abdomen and pelvis. Undersea Hyperb Med. 1996;24:215.
112. Marshall GT, Thirlby RC, Bredfeldt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
113. Sokol A, Lipson L, Morgenstern L, et al. Protection against lethal irradiation injury by pancreatic enzyme exclusion. Surg Forum. 1967;18:387.
114. Wang J, Zheng H, Hauer-Jensen M. Influence of short-term octreotide administration on chronic tissue injury, transforming growth factor β (TGF-β) overexpression, and collagen accumulation in irradiated rat intestine. J Pharmacol Exp Ther. 2001;297:35.
115. Waddell B, Rodriguez M, Lee R, et al. Prevention of chronic radiation enteritis. J Am Coll Surg. 1999;189:611.
116. Meric F, Hirschl R, Womer R, et al. Prevention of radiation enteritis in children, using a pelvic mesh sling. J Pediatr Surg. 1994;29:917.
117. Rodier J, Janser J, Rodier J, et al. Prevention of radiation enteritis by an absorbable polyglycolic acid mesh sling. Cancer. 1991;68:2545.
118. Dasmahapatra K, Swaminathan A. The use of a biodegradable mesh to prevent radiation associated small bowel injury. Arch Surg. 1991;126:366.
119. Logmans A, van Lent M, van Geel A, et al. The pedicled omentoplasty, a simple and effective surgical technique to acquire a safe pelvic radiation field: Theoretical and practical aspects. Radiat Oncol. 1994;33:269.
120. Logmans A, Trimbos J, van Lent M. The omentoplasty: A neglected ally in gynecologic surgery. Eur J Obstet Gynecol. 1995;58:167.
121. Smedh K, Moran B, Heald R. Fixed rectal cancer at laparotomy: A simple operation to protect the small bowel from radiation enteritis. Eur J Surg. 1997;163:547.
122. Chen J, Changchien C, Wang J, et al. Pelvic peritoneal reconstruction to prevent radiation enteritis in rectal carcinoma. Dis Colon Rectum. 1992;35:897.
123. Group: SRCS. Preoperative short term radiation therapy in operable rectal cancer: A prospective randomized trial. Cancer. 1990;66:49.
124. Goldberg P, Nicholls R, Porter NH, et al. Long term results of a randomized trial of short course low dose adjuvant preoperative radiotherapy for rectal cancer: Reduction in local treatment failure. Eur J Cancer. 1994;30A:1602.
125. Letschert J, Lebesque J, de Boer R, et al. Dose-volume correlation in radiation induced late small bowel complications: A clinical study. Radiother Oncol. 1990;18:307.
126. Caspars R, Hop W. Irradiation of true pelvis for bladder and prostatic carcinoma in supine, prone or Trendelenburg position. Int J Radiat Oncol Biol Phys. 1983;9:589.
127. Shanahan T, Mehta M, Berterud K, et al. Minimization of small bowel volume within treatment fields utilizing customized belly boards. Int J Radiat Oncol Biol Phys. 1990;19:469.
128. Kolbl O, Richter S, Flentje M. Influence of treatment technique on dose-volume histogram and normal tissue complication probability for small bowel and bladder: A prospective study using a 3-D planning system and a radiobiological model in patients receiving postoperative pelvic irradiation. Strahlenther Onkol. 2000;176:105.
129. Haboubi N, Schofield P, Rowland P. The light and electron microscopic features of early and late phase radiation-induced proctitis. Am J Gastroenterol. 1988;83:1140-4.
130. Anseline P, Lavery I, Fazio V, et al. Radiation injury of the rectum. Ann Surg. 1981;194:716-24.
131. Ajlouni M. Radiation-induced proctitis. Curr Treat Options Gastroenterol. 1999;2:20-6.
132. Wang CJ, Leung SW, Chen HC, et al. The correlation of acute toxicity and late rectal injury in radiotherapy for cervical carcinoma: Evidence suggestive of consequential late effect (CQLE). Int J Radiat Oncol Biol Phys. 1998;40:85-91.
133. Jao SW, Beart RWJr, Gunderson LL. Surgical treatment of radiation injuries of the colon and rectum. Am J Surg. 1986;151:272-7.
134. Kimura T, Iwagaki H, Hizuta A, et al. Colorectal cancer after irradiation for cervical cancer—Case reports. Anticancer Res. 1995;15:557-8.
135. Chapuis P. Challenge of radiation-induced rectal bleeding. Aust N Z J Surg. 2001;71:200-1.
136. Johnston M, Robertson G, Frizelle F, et al. Management of late complications of pelvic radiation in the rectum and anus. Dis Colon Rectum. 2003;46:247-59.
137. Friedman M. Calculated risks of radiation therapy of normal tissue in the treatment of cancer of the testis. New York: The American Cancer Society. National Cancer Institute, USPHS Federal Science Agency; 1952.
138. Perez CA, Breaux S, Bedwinek JM, et al. Radiation therapy alone in the treatment of carcinoma of the uterine cervix. II. Analysis of complications. Cancer. 1984;54:235-46.
139. Pilepich MV, Krall JM, Sause WT, et al. Correlation of radiotherapeutic parameters and treatment related morbidity in carcinoma of the prostate—Analysis of RTOG study 75-06. Int J Radiat Oncol Biol Phys. 1987;13:351-7.
140. Cantril ST, Green JP, Schall GL, et al. Primary radiation therapy in the treatment of anal carcinoma. Int J Radiat Oncol Biol Phys. 1983;9:1271-8.
141. Eschwedge F, Lasser P, Chavy A, et al. Squamous cell carcinoma of the anal canal. Treatment by external beam irradiation. Radiother Oncol. 1985;3:145.
142. Cummings B, Keane T, Thomas G, et al. Results and toxicity of the treatment of anal canal carcinoma by radiation therapy or radiation therapy and chemotherapy. Cancer. 1984;54:2062-8.
143. Weil MD, Crawford ED, Cornish P, et al. Minimal toxicity with 3-FAT radiotherapy of prostate cancer. Semin Urol Oncol. 2000;18:127-32.
144. Dearnaley DP, Khoo VS, Norman AR, et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: A randomised trial. Lancet. 1999;353:267-72.
145. Zelefsky M, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111-16.
146. Grigsby PW, Perez CA. Efficacy of 5-fluorouracil by continuous infusion and other agents as radiopotentiators for gynecological malignancies. Berlin and Heidelberg, Germany: Springer, Verlag; 1991.
147. Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527-39.
148. Miller R, Sargent D, Martenson JA, et al. Acute diarrhea during and after adjuvant bolus and continuous infusion 5-fluorouracil chemotherapy and pelvic radiation therapy. A detailed analysis of toxicity from a randomized intergroup trial. Proc Am Soc Clin Oncol. 1999;18:240a.
149. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-40.
150. Enker WE, Merchant N, Cohen AM, et al. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg. 1999;230:544-52.
151. Nussbaum M, Campana T, Wees J. Radiation induced intestinal injury. Clin Plast Surg. 1993;20:573.
152. Babb RR. Radiation proctitis: A review. Am J Gastroenterol. 1996;91:1309-11.
153. Talley NA, Chen F, King D, et al. Short-chain fatty acids in the treatment of radiation proctitis: A randomized, double-blind, placebo-controlled, cross-over pilot trial. Dis Colon Rectum. 1997;40:1046-50.
154. Charmeau J, Bouachour G, Person B, et al. Severe hemorrhagic radiation proctitis advancing to gradual cessation with hyperbaric oxygen. Dig Dis Sci. 1991;36:373-5.
155. Carl UM, Peusch-Dreyer D, Frieling T, et al. Treatment of radiation proctitis with hyperbaric oxygen: What is the optimal number of HBO treatments? Strahlenther Onkol. 1998;174:482-3.
156. Clark R, Tenorio L, Husse J, et al. Hyperbaric oxygen treatment for chronic refractory radiation proctitis: A randomized and controlled double blind cross-over trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2008. March 12 [Epub ahead of print]. PMID 18342453
157. Fantin A, Binek J, Suter W, et al. Argon beam coagulation for treatment of symptomatic radiation-induced proctitis. Gastrointest Endosc. 1999;49:515.
158. Tam W, Moore J, Schoeman M. Treatment of radiation proctitis with argon plasma coagulation. Endoscopy. 2000;32:667.
159. Smith S, Wallner K, Dominitz J, et al. Argon beam coagulation for rectal bleeding after prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2001;51:636.
160. Kaassis M, Oberti E, Burtin P, et al. Argon plasma coagulation for the treatment of hemorrhagic radiation proctitis. Endoscopy. 2002;32:673.
161. Seow-Choen F, Goh H, Eu K, et al. A simple and effective treatment for hemorrhagic radiation proctitis using formalin. Dis Colon Rectum. 1993;36:135.
162. Rubinstein E, Ibsen T, Rasmussen R, et al. Formalin treatment of radiation-induced hemorrhagic proctitis. Am J Gastroenterol. 1986;81:44.
163. Saclarides T, King D, Franklin J, et al. Formalin instillation for refractory radiation-induced hemorrhagic proctitis. Dis Colon Rectum. 1996;39:196.
164. Counter S, Froese D, Hart M. Prospective evaluation of formalin therapy for radiation proctitis. Am J Surg. 1999;177:396.
165. O’Brien P, Franklin C, Dear K, et al. A phase III double-blind randomized study of rectal sucralfate suspension in the prevention of acute radiation proctitis. Radiother Oncol. 1997;45:117.
166. Martenson J, Bollinger J, Sloan J, et al. Sucralfate in the prevention of treatment-induced diarrhea in patients receiving pelvic radiation therapy: A North Central Cancer Treatment Group phase III double-blind placebo-controlled trial. J Clin Oncol. 2000;18:1239.
167. Kneebone A, Mameghan H, Bolin T, et al. The effect of oral sucralfate on the acute proctitis associated with prostate radiotherapy: A double-blind, randomized trial. Int J Radiat Oncol Biol Phys. 2001;51:628.
168. Pinto A, Fidalgo P, Cravo M, et al. Short chain fatty acids are effective in short-term treatment of chronic radiation proctitis. Dis Colon Rectum. 1999;42:788.
169. Vernia P, Fracasso P, Casale V, et al. Topical butyrate for acute radiation proctitis: Randomized, crossover trial. Lancet. 2000;356:1232.
170. Frileux P, Berger A, Zinzindohoue F, et al. Fistules recto-vaginales de l’adulte. Ann Chir. 1994;48:412.
171. Mann W. Surgical management of radiation enteropathy. Surg Clin North Am. 1991;71:977.
172. Hanson W, Thomas C. 16,16-Dimethyl Prostaglandin E2 increases survival of murine intestinal stem cells when given before photon radiation. Radiat Res. 1983;96:393.
173. Tomas-de la Vega J, Banner B, Hubbard M, et al. Cytoprotective effect of prostaglandin E2 in irradiated rat ileum. Surg Gynecol Obstet. 1984;158:39.
174. Keelan M, Walker K, Cheeseman C, et al. Two weeks of oral synthetic E2 prostaglandin (enprostil) improves the intestinal morphological but not the absorptive response of the rat to abdominal irradiation. Digestion. 1992;53:101.
175. Delaney J, Bonsack M, Felemovicius I. Misoprostol in the intestinal lumen protects against radiation injury of the mucosa of the small bowel. Radiat Res. 1994;137:405.
176. Khan A, Birk J, Anderson J, et al. A prospective randomized placebo-controlled double-blinded pilot study of misoprostol rectal suppositories in the prevention of acute and chronic radiation proctitis syndrome in prostate cancer patients. Am J Gastroenterol. 95, 2000.
177. Hille A, Schmidberger H, Hermann RM, et al. A phase III randomized, placebo-controlled, double-blind study of misoprostol rectal suppositories to prevent acute radiation proctitis in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1488-93.
178. Door R. Radioprotectants: Pharmacology and clinical applications of Amifostine. Semin Radiat Oncol. 1998;8:10.
179. Ito H, Meistrich M, Barkley T, et al. Protection of acute and late radiation damage of the gastrointestinal tract by WR-2721. Int J Radiat Oncol Biol Phys. 1986;12:211.
180. Liu T, Liu Y, He S, et al. Use of radiation with or without WR-2721 in advanced rectal cancer. Cancer. 1992;69:2820.
181. Athanassiou H, Antonadou D, Coliarakis N, et al. Protective effect of amifostine during fractionated radiotherapy in patients with pelvic carcinomas: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2003;56:1154-60.
182. Ben-Joseph E, Han S, Tobi M, et al. Intrarectal application of amifostine for the prevention of radiation-induced rectal injury. Semin Radiat Oncol. 2002;12:81.
183. Zheng H, Wang J, Koteliansky V, et al. Recombinant soluble transforming growth factor-b type II receptor ameliorates radiation enteropathy in the mouse. Gastroenterology. 2000;119:1286.
184. Klimberg V, Souba W, Olson D, et al. Prophylactic glutamine protects intestinal mucosa from radiation injury. Cancer. 1990;66:62.
185. Campos F, Waitzberg D, Mucerino D, et al. Protective effects of glutamine enriched diets on acute actinic enteritis. Nutr Hosp. 1996;11:167.
186. McArdle A. Elemental diets in treatment of gastrointestinal injury. Adv Biosci. 1994;94:201.
187. Huang E, Leung S, Wang C, et al. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial. Int J Radiat Oncol Biol Phys. 2000;46:535.
188. Foster K, Brown M, Alberti K, et al. The metabolic effects of abdominal irradiation in man with and without dietary therapy with and elemental diet. Clin Radiol. 1980;31:13.
189. McArdle A, Reid E, Laplante M, et al. Prophylaxis against radiation injury. The use of elemental diet prior to and during radiotherapy for invasive bladder cancer and in early postoperative feeding following radical cystectomy and ileal conduit. Arch Surg. 1986;121:879.
190. Gabriele AM, Rovea P, Sola B, et al. Radiation therapy and chemotherapy in the conservative treatment of carcinoma of the anal canal: Survival and late morbidity in a series of 25 patients. Anticancer Res. 1997;17:653-6.
191. Levitsky J, Hong JJ, Jani AB, et al. Oral vitamin a therapy for a patient with a severely symptomatic postradiation anal ulceration: Report of a case. Dis Colon Rectum. 2003;46:679-82.
192. Salama JK, Mell LK, Schomas DA, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: A multicenter experience. J Clin Oncol. 2007;25:4581-6.
193. Myerson RJ, Kong F, Birnbaum EH, et al. Radiation therapy for epidermoid carcinoma of the anal canal, clinical and treatment factors associated with outcome. Radiother Oncol. 2001;61:15-22.
194. Mitchell SE, Mendenhall WM, Zlotecki RA, et al. Squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2001;49:1007-13.
195. Flam MS, John MJ, Mowry PA, et al. Definitive combined modality therapy of carcinoma of the anus. A report of 30 cases including results of salvage therapy in patients with residual disease. Dis Colon Rectum. 1987;30:495-502.
196. Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040-9.
197. Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049-54.
198. Rich TA, Ajani JA, Morrison WH, et al. Chemoradiation therapy for anal cancer: Radiation plus continuous infusion of 5-fluorouracil with or without cisplatin. Radiother Oncol. 1993;27:209-15.
199. John M, Flam M, Palma N. Ten-year results of chemoradiation for anal cancer: Focus on late morbidity. Int J Radiat Oncol Biol Phys. 1996;34:65-9.
200. Vuong T, Devic S, Belliveau P, et al. Contribution of conformal therapy in the treatment of anal canal carcinoma with combined chemotherapy and radiotherapy: Results of a phase II study. Int J Radiat Oncol Biol Phys. 2003;56:823-31.
201. Peiffert D, Bey P, Pernot M, et al. Conservative treatment by irradiation of epidermoid cancers of the anal canal: Prognostic factors of tumoral control and complications. Int J Radiat Oncol Biol Phys. 1997;37:313-24.
202. Tanum G, Tveit K, Karlsen KO, et al. Chemotherapy and radiation therapy for anal carcinoma. Survival and late morbidity. Cancer. 1991;67:2462-6.
203. John M, Pajak T, Flam M, et al. Dose escalation in chemoradiation for anal cancer: Preliminary results of RTOG 92-08. Cancer J Sci Am. 1996;2:205-11.
204. Kim JH, Sarani B, Orkin BA, et al. HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients. Dis Colon Rectum. 2001;44:1496-502.
205. Bem J, Bem S, Singh A. Use of hyperbaric oxygen chamber in the management of radiation-related complications of the anorectal region: Report of two cases and review of the literature. Dis Colon Rectum. 2000;43:1435-8.
206. Constine LS, Williams JP, Morris M, et al. Late effects of cancer treatment on normal tissues: Principles and practice of radiation oncology. Philadelphia: Lippincott Williams & Wilkins; 2004. p 357
207. Lawrence TS, Robertson JM, Anscher MS, et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237-48.
208. Dawson LA, Ten Haken RK, Lawrence T. Partial irradiation of the liver. Semin Radiat Oncol. 2001;15:240-46.
209. Ogata K, Hizawa K, Yoshida M, et al. Hepatic injury following irradiation—A morphologic study. Tokushima J Exp Med. 1963;43:240-51.
210. Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781-8.
211. Bortfeld T, Boyer AL, Schlegel W, et al. Realization and verification of three-dimensional conformal radiotherapy with modulated fields. Int J Radiat Oncol Biol Phys. 1994;30:899-908.
212. Burman C, Chui CS, Kutcher G, et al. Planning, delivery, and quality assurance of intensity-modulated radiotherapy using dynamic multileaf collimator: A strategy for large-scale implementation for the treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1997;39:863-73.
213. Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56:1354-60.