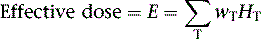Chapter 10 Radiation Considerations for Cardiac Nuclear and Computed Tomography Imaging
INTRODUCTION
The contribution of nuclear cardiology and computed tomography (CT) to the diagnosis and management of cardiovascular disease is undeniable. In contrast to other imaging modalities, they rely on the use of ionizing radiation, which has been associated with the risk of harmful effects. Of greatest concern is increasing the risk of cancer occurrence and mortality.1–4 In recent years, the number of diagnostic imaging studies using “low-level” doses of ionizing radiation increased dramatically. Concern over the risk to the population grew accordingly.2,3 Industry data show that cardiac imaging applications using single-photon emission computed tomography (SPECT), positron emission tomography (PET), and CT experienced greater relative increases compared with other imaging areas. Within these groups, there was an increase in the proportion of studies performed in women and pediatric populations, raising additional concern over exposure in these populations.3,4 These developments have fueled the debate over the accuracy and appropriateness of the estimated rates of cancer predicted by current models and assumptions that have been at times highly disputed. While it is generally accepted that excessive exposure to ionizing radiation can cause certain types of malignancies and other diseases, this relationship is far less accepted for the amounts of radiation used in most diagnostic imaging tests. In clinical practice, the debate is largely centered on the uncertainty in risk estimates relative to the counterprevailing risk of misdiagnosed disease or underutilization of appropriate testing.5 In parallel, advances in imaging technology have reduced radiation dose while preserving image quality, particularly for CT—and cardiac CT specifically—and are rapidly being adopted into clinical application.6 New SPECT reconstruction algorithms and detector configurations for myocardial perfusion imaging allow studies to be acquired in half the time or less7–9 of conventional protocols and theoretically may allow tradeoff of acquisition time advantage for imaging with less injection activity.7 Cardiac PET studies overall deliver favorable radiation dose compared with SPECT and are therefore an option for radiation dose reduction.
RADIATION DOSIMETRY
Fundamentals, Definitions, and Quantities
The amount of energy absorbed from incident radiation per unit mass of tissue is defined as the absorbed dose.10 It is expressed in units of rads, where 1 rad is defined as the absorption of 100 ergs per gram of tissue (or 1 joule per kilogram [J/kg]). The absorbed dose definition applies to most forms of radiation relevant to medical imaging, including photons, (x-rays, gamma) and particles with mass (e.g., electrons, positrons). The International System (SI) unit for absorbed dose is the gray (Gy); 1 gray is equal to 100 rads, or 1 rad = 10 mGy. Absorbed dose is also expressed in terms of the roentgen (R), a unit of radiation exposure defined as the amount of radiation producing 2.58 × 10−4 coulombs (C) of ionization charge per kilogram of air under standard conditions.11 Because this is such a complex definition to relate to various materials, the rad or gray are most frequently utilized.
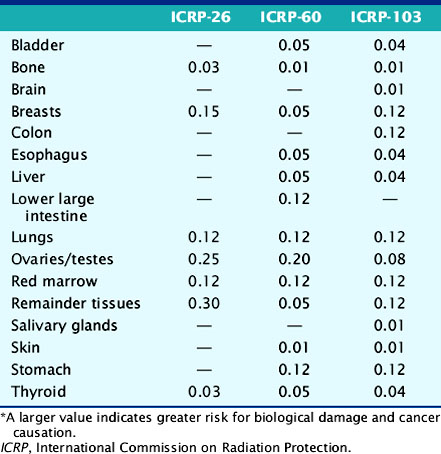
The deposition of energy in tissue occurs through a number of mechanisms that are dependent on the magnitude of energy and atomic properties of the tissue.10,11 Since the same amount of energy deposited by different forms of radiation can produce different amounts of biological damage, a quantity called the quality factor is used to describe the difference.12 The International Commission on Radiation Protection (ICRP), an important policy-setting body for radiation dosimetry, defines the quality factors as: 20 for alpha particles (helium nucleus), 10 for protons, and 1 for beta particles, gamma rays, and x-rays.12 Quality factors are dimensionless quantities defined such that the product of the absorbed dose and quality factor give an estimate of the biological damage for the type and amount of radiation. The resulting quantity is called the dose equivalent and is expressed in units of rem (roentgen equivalent man) or the SI units of sieverts (Sv).10 In principle, dose equivalent and absorbed dose have the same units, since the quality factors are dimensionless. However, to differentiate them, the units of dose equivalent are given the names rems or sieverts. The risk of cancer or harmful effects developing from the biological damage from ionizing radiation are derived from organ-specific dose equivalent values by use of a tissue weighting factor, wT, reflecting the sensitivity to developing fatal cancer, or, in the latest 2007 revision, including lethality and loss of life quality (ICRP-103). Two quantities commonly used to quantify risk are the effective dose (ED)12 and the effective dose equivalent (EDE).13 The ED has generally replaced the older concept of EDE. Recent changes in the wT values in 2007 are an increase for breast tissue (from 0.05 to 0.12), a decrease for gonads (from 0.2 to 0.08), and inclusion of more organs and tissues in the “remainder” component (from 0.05 to 0.12). Table 10-1 lists tissue weighting factors for representative organs from the ICRP-30, ICRP-60, and most recent ICRP-103 publications.13,14 The basic equation relating ED to these quantities is given as:
Table 10-1 Tissue Radiation Sensitivity Factors (wT) from the ICRP-36, ICRP-60, and the Recent ICRP-103 Reports*
where HT is the tissue-specific equivalent doses and wT is the tissue-specific weighting factors. Complex mathematic simulation programs are used with standardized anatomic models of the human body,15,16 kinetic parameters, and voiding assumptions to estimate HT. Initially in early ICRP models, organs were simulated as spheres containing uniform amounts of radioactivity, and only self-irradiation was included. Two systems exist for calculation of ED and EDE values: the Medical Internal Radiation Dose (MIRD) system,16 developed by the Society of Nuclear Medicine specifically for calculating patient radiation doses, and the ICRP system, developed initially for studying and regulating the protection of nuclear industry workers.13 The MIRD approach includes so-called S-factor’s17 which relate the cumulative activity16 (integral number of decays in an organ) to the exposure of other organs in order to calculate the dose equivalent. A limiting assumption of the MIRD approach is that the radioactivity in each organ is uniformly distributed. Organs containing no activity may have a non-zero absorbed dose value resulting from the surrounding sources. A total body radiation dose is then calculated from the contributions of all organs and compartments (blood, other structures) of the body by a weighted summation of the values that include the “risk” from each organ’s exposure resulting in an equivalent risk from a whole body uniform exposure. Note that the ED is not the same as a “total body dose” often reported on the package insert for many radiopharmaceuticals. The total body dose is the total energy deposited anywhere in the body divided by the total mass of the body. The intent is to provide ED and EDE values that quantify the risk in a single number so that exposures under different circumstances (internal, external, amount of radiation, type of radiation, rate and route of delivery, and other factors) can be compared as a common quantity. The values are intended to be independent of the number of exposures or whether a single or multiple organs were exposed.
Values reported for ED or EDE for a specific application and protocol often differ, leading to uncertainty in evaluating risk.18 This is attributable to differences in assumptions and methodologies in the models and differences in the input data origin. It is important to note that ED and EDE represent expected or average values for a given population. Application to an individual is inappropriate. While radiation dose estimates are provided for an “individual” study by modern CT scanners, it has yet to be implemented for nuclear imaging procedures, as is done for radiation therapy protocols using internal sources.
The package insert (PI) for a radiopharmaceutical is the primary resource for radiation dosimetry values. The dose values are indication-specific and expressed in either rads (rem) or grays (Sv) for a total amount of injected radiopharmaceutical, or “per unit” of injected radiopharmaceutical. The values are typically obtained from studies on normal volunteers under resting conditions, but stress values are included for stress/rest imaging protocols such as myocardial perfusion. The standard stress modality for the PI is exercise stress when given. However, values for pharmacologic stress are often published but are not routine, probably given the many types of pharmacologic stress agents and protocols. The PI also contains relevant dosimetry and safety information for both toxicity and radiation dose contributions from radionuclide contaminants resulting from the radioisotope or radiopharmaceutical manufacturing process.19
The use of ED and EDE as standards for risk is controversial but is the most accepted standard. Individual investigators and position statements reflecting the opinion of health-related professional societies20–22 have questioned the use of this approach. Issues cited in the debate include the omission of molecular repair mechanisms for biological damage in the risk calculations, differences in the tissue radiosensitivity values across species, or differences in the model and specific assumptions in the methodology. Many of the complex factors affecting the development and response by the body to the many forms of cancer are not well understood nor included in the models. The risk values are thus theoretical calculations with limited outcome of data. Adaptation of the EDE and ED is not universal, and some countries have not accepted them as a standard.
Environmental Sources of Radiation Exposure
When comparing risk values for ionizing radiation exposure, it is useful to contrast them with risk values resulting from environmental sources alone. The risk to the general population from background radiation of all types (naturally occurring and man-made) in the United States is estimated at 3.0 to 3.6 mSv annually.23,24 The worldwide annual mean value has been estimated at 2.4 mSv, considerably lower than the U.S. value, although some regions are considerably higher, with a range of 1.5 to 10 mSv.24 One-half to two-thirds of the background radiation dose in the United States is estimated to originate from the alpha particle emissions of radon absorbed by the lungs.23 Individuals living at higher elevations, where shielding by the atmosphere from high-energy cosmic rays is reduced may receive greater exposure. The background values can be helpful when responding to radiation dose questions.
Radiation Dosimetry of Single-Photon Emission Computed Tomography Myocardial Perfusion Tracers
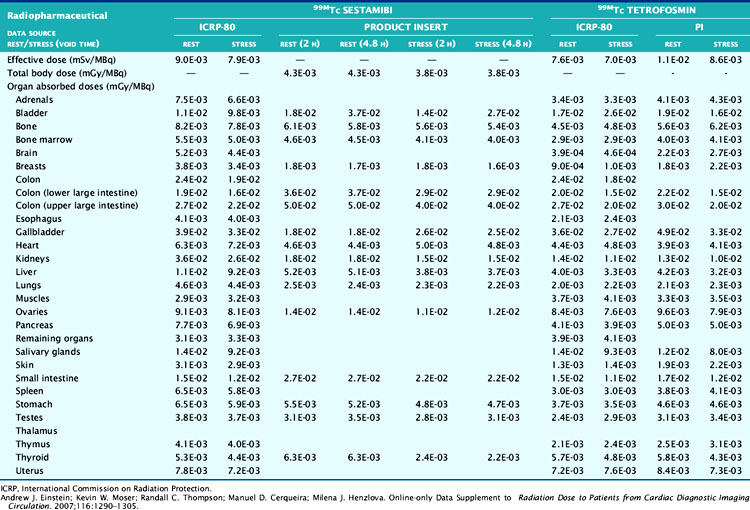
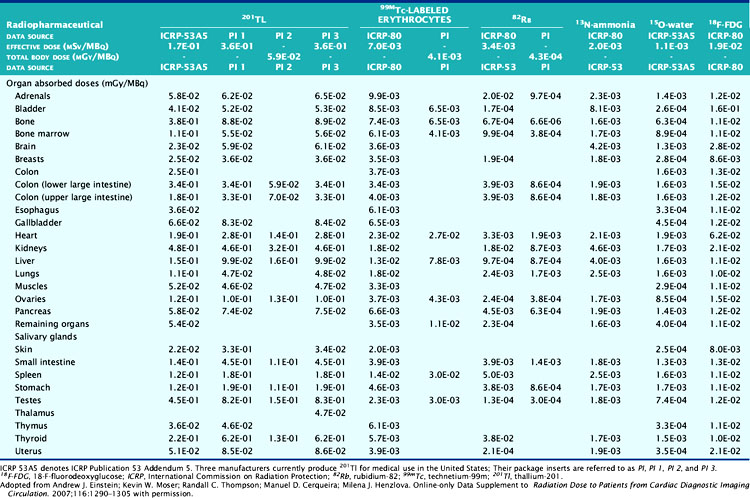
The technetium (Tc)-99m-labeled tracers (T1/2 = 6.02 hours) for myocardial perfusion imaging are Tc-99m sestamibi,25 Tc-99m tetrofosmin26 and Tc-99m-teboroxime,27 although the latter is not currently marketed. Thallium (Tl)-201 chloride (T1/2 = 72 hours) is used for myocardial perfusion and viability assessment in single- and dual-isotope protocols.28 Table 10-2 lists dosimetry values for these tracers from PIs and other sources. Tl-201 dosimetry has attracted particular interest recently because of its high ED and EDE values compared to the Tc-99m perfusion agents and the PET myocardial perfusion agents.3,18 Shown in Table 10-3 are values for Tl-201 SPECT protocols that can be more than double the values for the Tc-99m agents. Using the PI values, Tc-99m tetrofosmin shows an EDE value of 8.61E-03 mSv/MBq for exercise stress and 1.12E-02 mSv/MBq for the resting dose.18 Therefore, a conventional rest/stress SPECT imaging protocol using a 30 mCi (1110 MBq) stress injection and 10 mCi (370 MBq) rest injection yields a total EDE of approximately 14.0 mSv. A rest/stress protocol using Tc-99m sestamibi from the PI values for the same injected activity and protocol yields an absorbed radiation dose of 570 mrad.25 Based on the PI for Tl-201, a stress-redistribution protocol utilizing 3.0 mCi of activity results has an EDE of 37 mSv.18 A dual-isotope SPECT protocol using a 3.0 mCi dose of Tl-201 at rest and a 30 mCi dose of a Tc-99m agent at stress results in an EDE of approximately 41 mSv.3,18 Thus, the inclusion of a Tl-201 injection results in a significant increase in radiation dose compared with the other protocols. Thomas et al. recently examined the dose from Tl-201 chloride to the testes in adults and children, important for fertility considerations. The study recommended a downward revision from the current ICRP values for testicular dose by a factor of almost 2.29 The impact, however, on total ED or EDE values was not significant. For breastfeeding women who must have a nuclear medicine study, a temporary suspension of breastfeeding following the scans currently recommended. The delay (2 weeks) is somewhat unique for Tl-201 studies, given the longer physical half-life. The Nuclear Regulatory Commission (NRC)30 provides specific guidance for the recommended suspension of breastfeeding following a nuclear study, based on the radiopharmaceutical and protocol. Data suggesting that some studies may not warrant suspension of breastfeeding are provided in Refs. 31 and 32. Lastly, radiation dose considerations specific to males and females may be important if the same injected activity is given to both genders, due to organ mass differences, biokinetics, and other factors.32
Radiation Dosimetry for Cardiac Positron Emission Tomography Tracers
Table 10-3 lists radiation dose values for the approved cardiac PET tracers and other cardiac imaging tracers. Rubidium-82 chloride (Rb-82) (T1/2 = 75 sec) is a chemical analog of Tl-201 chloride used for the assessment of coronary artery disease with PET.19,33 Rb-82 is produced as the daughter radionuclide of strontium-82 (Sr-82) eluted from a portable on-site generator renewed approximately monthly due to the physical half-life of Sr-82 (T1/2 = 25 days).19,34 Assay of the eluent for trace contaminants of Strontium (Sr-85) (T1/2 = 64.8 days) and Sr-82 is required as part of daily quality control. SR-82 activity levels toward the end of the month result in a lower maximum available Rb-82 dose for injection, with a concordant reduced exposure.
The Rb-82 PI reports dosimetry values based on data from Kearfott et al.35 in animals and Ryan et al.36 in normal human volunteers. For an adult, an absorbed radiation dose of 0.95 mGy/2220 MBq or 0.096 rads for each 60 mCi injected is reported. Lodge et al.37 computed effective dose equivalent values of 5.5 mSv for combined rest/stress doses of 60 mCi each. Effective dose equivalent values were reported in ICRP-80 of 15.8 mSv for combined rest/stress (60 mCi total) injections.38 Recently, deKemp et al.39 reexamined ED values for Rb-82 myocardial perfusion PET, specifically the role of conservative assumptions used in early dose studies. The authors suggest that the ICRP-80 values may overestimate ED by as much as fivefold, lending weight to the PI values.
Nitrogen-13 ammonia chloride (N-13; T1/2 = 10 min) is used for PET imaging in the diagnosis of coronary artery disease40 and for quantitative assessment of myocardial bloodflow.41 N-13 production requires on-site cyclotron and radiochemistry facilities for rapid preparation and injection into the patient. By comparison, the positrons emitted from N-13 are significantly lower in energy (492 keV) compared to those from Rb-82 and contribute to less radiation dose from N-13 (Valentin et al.) in ICRP-80, reported values for N-13 ammonia chloride for rest/stress injections of 550 MBq (15 mCi) each, equal to 2.2 mSv.41
F-18-labeled fluorodeoxyglucose (F-18DG; T1/2 = 110 min) is utilized with PET cardiac imaging for the assessment of myocardial viability, evaluating the extent of scar, hibernating myocardium, and stunned myocardium.42 FDG provides complimentary information to poorly perfused or equivocal regions on rest/stress images. FDG cardiac imaging is performed conventionally at rest after metabolically preparing the myocardium for optimal FDG uptake. The FDG PI reports dose values for a single 10 mCi injection of FDG.43 ICRP-80 reports that for this amount of activity, the effective dose is 7 mSv.44 The Oak Ridge Associated Laboratories reported an effective dose equivalent 3.0E-02 mSv/MBq, which for a 10 mCi (370 MBq) injection yields 11.2 mSv.44 FDG is provided in unit dose syringes, and thus as with N-13 ammonia, there are important radiation safety considerations for the technologist and clinical personnel involved with the study.45,46 Shown for reference in Table 10-4 are representative values of doses from other common medical imaging procedures using ionizing radiation.
Table 10-4 Radiation Exposure Estimates from Common Medical Imaging Procedures
| Study Type | Relevant Organ | Relevant Organ Dose (mGy or mSv) |
|---|---|---|
| Dental radiography | Brain | 0.005 |
| Posterior-anterior chest radiography | Lung | 0.01 |
| Lateral chest radiography | Lung | 0.15 |
| Screening mammography | Breast | 3 |
| Adult abdominal CT | Stomach | 10 |
| Barium enema | Colon | 15 |
| Neonatal abdominal CT | Stomach | 20 |
CT, Computed tomography.
Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med 2007;357:2277-2284.
MODELS FOR RADIATION RISK
The Linear No-Threshold Model for Radiation Risk
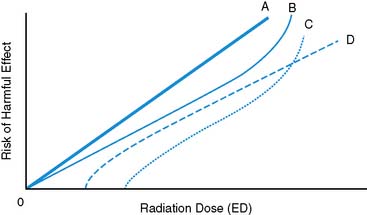
The linear no-threshold (LNT) model47,48 describes a relationship between risk of harmful effects and the amount of exposure to ionizing radiation (Fig. 10-1). The LNT model implies that there is no level of exposure to ionizing radiation below which there is zero risk of causing cancer. It also implies that risk will increase in direct linear proportion to the amount of radiation exposure, and typically refers to an “instantaneous” exposure and a lifetime risk of fatal cancer. The model stands in contrast to alternative proposed models suggesting that an exposure threshold exists below which the risk of cancer induction is negligible, nonexistent, or potentially beneficial. A key criticism of the LNT model centers on the extrapolation of data based primarily on observed health effects following the high-intensity exposures from the Hiroshima and Nagasaki events of World War II to the “low-level” ionizing radiation exposure values of diagnostic imaging. The appropriateness of the LNT model was reemphasized in the 2005 report by the Biological Effects of Ionizing Radiation (BEIR) Committee report number 7, following examination of the weight of current evidence.48 Of particular interest in the report was the implication for so-called low-level (<100 mSv) exposures typical of diagnostic imaging procedures. The cancer risk coefficient predicted from the LNT model is 4.8 × 10−4 per rem. Or, an additional five cancers would be expected above a baseline rate of 3000 for an individual receiving a 1-rem exposure in their lifetime. This would be approximately the same exposure from a conventional CT angiography study or SPECT rest/stress myocardial perfusion imaging (MPI) study with Tc-99m agents.
Attributable Lifetime Risk from Radiation Exposure
The theoretical risk of harmful effects from ionizing radiation exposure over a lifetime is dependent on the age of the individual at the time of exposure. Individual exposures at various times during a lifetime are combined through a weighting that takes into account the probabilistic nature of delay between exposure and development of cancer, which changes with age.49 The cumulative effect has been described by the attributable lifetime risk (ALR).49,50 The ALR differs from the simple additive approach of exposures occurring at times that are negligibly separated compared to the expected human lifetime. There are also differences in ALR for males and females.49 The issue of repeated cardiac CT studies on ALR has been reported in several studies recently, based on the models and data described in the BEIR VII report. Einstein et al.50 examined the ALR of fatal cancer from cardiac CT exposures using models and data from the BEIR VII report. Among their findings, the authors reported that ALR estimates for standard cardiac scans varied from 1 in 143 for a 20-year-old woman to 1 in approximately 3300 for an 80-year-old male. Sodicksin et al.51 examined data retrospectively from more than 190,000 CT studies focusing on ALR of individuals having repeat CT examinations over the previous 22 years. 33% of patients had 5 or more lifetime CT examinations, 5% had more than 22 over their lifetime, and 15% received more than 100 mSv collectively over their lifetime. The resulting ALR values had mean and maximum values of 0.3% and 12% for cancer incidence and 0.2% and 6.8% for cancer mortality, respectively, showing that cumulative exposure from CT examinations had an incremental lifetime of cancer above baseline. Importantly, the subjects of this study did not have the advantages of modern CT dose-reduction techniques, which hypothetically would have reduced the increased incidence for this population.
In a similar study on whole-body PET/CT using 18-FDG and comparing U.S. and Hong Kong populations, Huang et al. also found an increase in the ALR for cancer, which interestingly was significantly greater for the Hong Kong population.52 Quantitation of risk from “low-dose” ionizing radiation is controversial because of the lack of definitive outcome-based studies linking low levels of radiation exposure with health effects as acknowledged in the BEIR VII report. Approximately 2000 to 4000 of every 10,000 individuals will die of cancers of all origins.53 It has been estimated that 1 rem (10 mSv) of exposure received annually, or approximately three times the expected annual value, from background radiation sources for the U.S. population received in “small doses” over an individual’s lifetime will result in 5 to 6 additional fatal cancers per 10,000 individuals (1 in 2000 to 1 in 2500)54,55. It could therefore be concluded that up to 0.06% (6/10,000) additional deaths from cancer will result, or the 2000 possible deaths is increased to 2006 out of 10,000. If the average lifetime of an individual is 76 years, extrapolating this relationship to a 10-mSv exposure would increase the risk approximately threefold to 2018 out of 10,000 is implied.
Another important risk consideration is the so-called genetic effects that extend beyond an individual’s risk to the progeny through passing of genetic mutation. The genetically significant dose (GSD)56 represents the estimated dose to the population gene pool that can result in genetic effects in its offspring. GSD is defined as the dose that, if received by every member of the population, would produce the total genetic effect on the population as the sum of the individual doses actually received. It is a complex quantity related to age, number of expected children, gender, type of exposure and other factors. The GSD is currently estimated to be 20 mrad above the annual background radiation exposure levels. This dose is well below a detectable level and is well within radiation exposure tolerance for the population.
Occupational Radiation Exposure and Limitations
The NRC provides well-enforced legal limits for professional staff exposure to radiation in the provision of medical services.57 The key sources of exposure for a nuclear cardiac imaging facility likely include the management of waste and preparation of patient doses in the hot laboratory, exposure to radiopharmaceuticals at the time of injection, and secondary radiation exposure from the patient post injection, during monitoring or image acquisition. The cumulative exposure values are dependent upon the number of studies performed annually, the role of the individual in relation to interaction with the radiopharmaceutical and patient, and the properties of the radionuclides and protocols used at the facility. For CT facilities, the exposure can be considerably less than nuclear laboratories because of the separation of patients and staff during x-ray exposure and the cessation of exposure at the end of the study, compared with the finite half-lives of radiopharmaceuticals. Exposure to staff from combined SPECT/CT or PET/CT includes the additive exposure from both modalities. Schleipman et al.58 described the radiation exposure to staff in a nuclear medicine department performing Rb-82 rest/stress PET and Tc-99m sestamibi rest/stress myocardial perfusion studies. In particular, they examined the role of physical position of the technologist with respect to the patient. For this laboratory performing pharmacologic stress protocols, the dose to personnel was less than that for the Tc-99m studies. Additionally, the exposure to the personnel for routine stress testing at variable distances from the patient was equivalent to background.
“As Low As Reasonably Achievable” Principle
The “as low as reasonably achievable” (ALARA) principle59 applies to all aspects of radiation exposure, including diagnostic imaging. The principle is intended to promote a culture and mindset resulting in minimizing exposure to ionizing radiation at all reasonable costs. For patients, if no benefit is obtained, there is no justification for the medical radiation exposure. For laboratory personnel, there is no benefit receiving unnecessary radiation exposure, particularly if it is not essential to performing their duties. In general, perhaps with the exception of mammography, there are no federal regulations limiting the radiation dose to patients.60 Beyond these considerations, adherence to the structure imposed by the ALARA concept supports an overall efficiency of laboratory operations for all imaging modalities, and limitation of patient radiation dose is largely entrusted to the physician and technologist. In 1994, the ALARA principle was adopted as part of Title 10 of the Code of Federal Regulations (10 CFR 35.20), which is binding on all institutions as an NRC regulation.
COMPUTED TOMOGRAPHY (See Chapter 21)
CT scanner technology has advanced at an unprecedented pace, including increases in the number of slices, temporal resolution, spiral resolution and electrocardiogram (ECG)-gating strategies, and dual-source dual-energy capabilities.61 The temporal resolution and multislice capabilities are particularly important for improving imaging of the heart during the cardiac cycle by reducting image blurring. CT is also used routinely to obtain transmission scans for attenuation correction of PET images and calcium scoring studies. The growth in the number of CT studies has raised concern about the cumulative effect of radiation exposure and ALR for cancer. Brenner and Hall2 examined CT utilization, reporting the number of scans performed in the United States increased from 3 million in 1980 to 62 million in 2006. The authors highlight that the greatest proportional increase in CT studies has occurred in the pediatric population, citing advances in image acquisition speed (<1 second) and in the increased application to screening of asymptomatic adults. Children are more susceptible to radiation damage, having greater numbers of actively dividing cells and a greater number of years in which a “lifetime cancer” can be realized. In response to these factors, medical imaging societies have produced position statements, guidelines, and other publications to advance education on the issues of radiation exposure in diagnostic imaging.60,62,63
CT Radiation Dosimetry: Fundamentals, Definitions, and Quantities
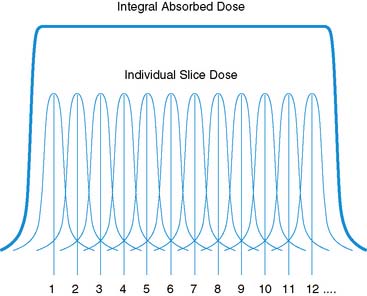
The approach to dosimetry for CT is similar in many ways to the internal dosimetry approach. Radiation risk calculations from x-ray CT exposure are based on the use of the CT dose index (CTDI).64–66 The CTDI is calculated as the integral over the range of exposure along the z-axis (system or patient axis) of the radiation dose profile. It represents the average absorbed dose for the series of contiguous irradiated sections of tissue. It is calculated assuming that the “tails” of the exposure profiles are included, which is a theoretical approximation (Fig. 10-2). Common variants of the CTDI include CTDIFDA, CTDI100, CTDIW, and CTDIvol; the definitions and differences are discussed in detail elsewhere.64–67 The differences are related to the multislice capabilities of the system, as expressed by pitch, calibration of the measurements against known geometries, and other factors. The CTDIvol is commonly implemented on modern CT scanners to provide individual patient dose estimates. The SI units of CTDI are the milligray (mGy). The American College of Radiology requires sites applying for accreditation to measure CTDI and ED values for a range of pediatric and adult imaging applications.
From the CTDI, the dose length product, which represents the overall energy absorbed for a CT scan, is obtained by integration of the CTDI over the z-axis length of the scan.67 DLP represents the potential biological effect delivered by the absorbed energy of the scan but is not yet a measure of risk, which requires incorporation of the specific sensitivity of each tissue to the type and amount of radiation. The DLP is most commonly calculated using Monte Carlo calculations, which track the path of individual x-rays and their ionization products and the location of energy deposition in models of standard anatomic geometries much like the MIRD approach described earlier. ED values for various studies and specific regions of the body (e.g., head, neck, abdomen, pelvis, thorax) can then be calculated as the product of the DLP and a factor, k, representing the proportion and sensitivity of tissues in the scan region.67 The units of k are mSv-mGy−1-cm−1, so that the product is expressed in units of mSv. The values of k are dependent upon the parameters of the acquisition (mAs, kVp, pitch), but the calibration is typically performed automatically by the system. The appropriateness of these values in larger patients or specific populations is a concern, and deviations in the values may be significant. It is important to note that in all cases, the ED represents an “expected” dose and is not an exact dose for each patient, as with the internal dosimetry values. However, while certain values have become standard, these models have intrinsic variability,67 as with other quantitative dose methods. It should be emphasized that at the time of this writing, CT system estimates for DLP are highly dependent upon specific ‘default’ study acquisition parameters defined by the manufacturer. Deviation from these default settings may result in erroneous DLP values, and therefore exposures that differ significantly from expected values. This would have the unintended consequence of potentially delivering a higher dose than suggested by the calculations. It is highly recommended that the DLP values be calibrated or confirmed to be accurate when acquisition parameters are manually adjusted. This is most effectively performed by a medical or health physicist with expertise in cardiac CT technologies and imaging protocols.
Dose Reduction Techniques for Cardiac Computed Tomography
Scanners with fewer slices have been reported to provide lower radiation exposure than those with a higher number of slices.68 However, this should now be considered on balance with radiation dose reduction methods; the generalization may not hold true. The application of highly standardized protocols emphasizing all aspects of dose limitation are important and needed. Hausleiter et al. recently69 reported the wide variability in radiation dose associated with cardiac CT angiography. In their study, 94% of systems were 64 slice and the remainder 16 slice, and primarily single source. They reported that radiation dose estimates differed significantly, ranging from 5.7 mSv to 36.5 mSv, with a median of 15.4 mSv. Furthermore, they reported the widely variable application of standardized dose reduction strategies when available. Extended discussion of other important considerations and in-depth detail for a range of imaging circumstances are described elsewhere.61
Tube Current Modulation
Modulation of the tube current (mA) or effective tube current (mAs) based on the patient’s anatomy, specific region of interest, or susceptibility to radiation exposure of certain organs is a highly effective method for optimizing radiation dose.70,71 Modulation of the dose may be applied along the z-axis of the patient (longitudinal variation), angularly to respond to thicker regions of the body, or both (angular-longitudinal).61 The current may be modulated to rise and fall with variable frequency and by various amounts, depending on the parameters of the acquisition. The principle is based on recognition that attenuations through different regions or at different angles through the body are quite different and can be exploited to reduce dose. In general, most methods utilize a “scout” or “topogram” image just prior to the study scan combined with a second lateral scan or an anteroposterior scan, with predictive algorithms to (1) estimate attenuation along projections through the body and (2) predict the expected image noise levels to avoid compromising image reconstruction accuracy at the expense of reduced dose. The small additional exposure from the scout scans is easily offset by the reduction in exposure of the modulated study scan. Body mass index (BMI) or other body habitus descriptors may be used in some systems. Based on the predicted optimal intensity required for each projection, the tube current is varied as the scanner translates through the acquisition. Additionally, tube current modulation may be used with automatic exposure-control algorithms that will assist in predicting overall noise or contrast-to-noise values requested by the operator.
ECG-Pulse Modulation
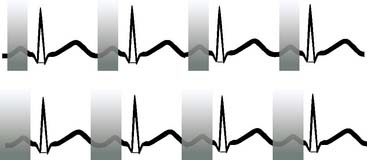
For cardiac imaging, minimizing the effects of heart motion during the cardiac cycle is critical to maximizing image quality. While tube current modulation can be applied to general radiologic CT studies, additional reduction in radiation dose for cardiac CT scans can be obtained from the use of ECG-pulse modulation techniques during image acquisition (Fig. 10-3).72,73 With these methods, the tube current rapidly rises to a plateau, followed by a rapid descent to a baseline, resulting in a proportional change in x-ray beam exposure triggered at points in the R-R cycle by the ECG.74 The technique may be applied in a retrospective or prospective mode, and the degree of dose reduction for each is very different. Retrospective ECG-gated pulse-modulation refers to the reconstruction of image data acquired as with a conventional acquisition, where projection data are obtained over the full R-R cycle. The image data are stored such that the user may select a specific subset of data from the cardiac cycle. Image data weighted toward the ED phase is typically used, since the heart is most static at this phase. Prospective ECG-pulse modulation utilizes a prediction algorithm to estimate the time of subsequent phases of the cardiac cycle to predict a preset window for imaging, such that image data are acquired only for this region of the cardiac cycle.73 By modulating the acquisition in this way, exposure during the other phases is avoided, with a proportional reduction in radiation dose.
Organ and Breast Shielding
A specific concern for CT radiation exposure has been related to the female population, where a higher effective dose to the breast results from elevated radiosensitivity of breast tissue75,76 and direct positioning in the beam. Several groups have recently reported the utilization of breast shielding methods to minimize exposure to certain organs, applied in pediatric studies and to women. A primary concern with these approaches has been the effect on image noise, which may compromise image quality, and the introduction of image artifacts related to projection sampling. At this time, these methods have not been widely adopted, owing to concerns about image quality, but they continue to be investigated and have shown effectiveness for dose reduction.
Dosimetry Values for Cardiac CT Procedures
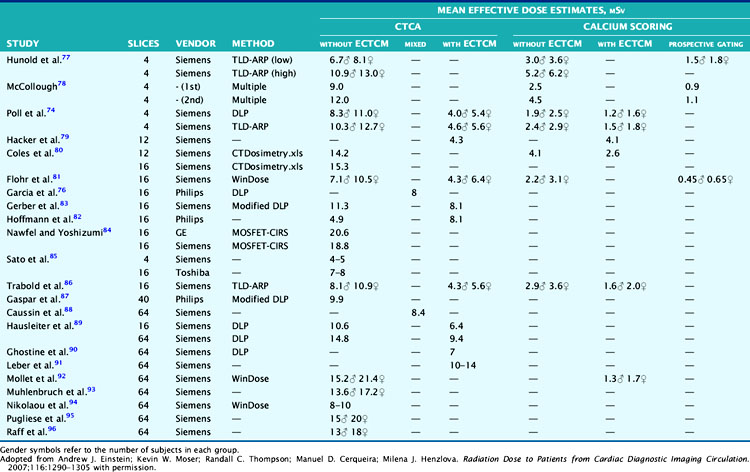
Table 10-5 lists radiation dose results from a large number of recent studies in the literature, conducted over various populations, methods, and CT systems.77–96 As can be seen, the results are variable. Additionally, studies using a form of modulation, whether ECG or contrast (exposure control) based, tend to yield ED values approximately one-half or less the values without. Additional information on the differences is captured in the recent publication by Hausleiter et al.69 concluding that dose reduction techniques, although available, are not routinely used and may reflect practical limitations that remain in their implementation.
X-RAY AND RADIONUCLIDE SOURCE–BASED RADIATION EXPOSURE FOR PET AND SPECT ATTENUATION CORRECTION
PET imaging requires attenuation correction as a standard for effective image accuracy. Some dedicated PET systems utilize radionuclide sources (germanium-68 or cesium-137) for transmission attenuation correction.97 Transmission imaging using radionuclide sources for SPECT remains an option,98 as well as CT99 using low-dose protocols. Absorbed dose values from transmission exposure for attenuation correction have been reported and show minimal additional dose is added compared with the internal radiation dose of most procedures: typically less than 1.0 mSv and often lower than 0.6 mSv. PET/CT and SPECT/CT hybrid systems, combined, therefore deliver greater radiation dose for attenuation correction compared with the radionuclide sources alone but the amount is minimal.
1. Mettler F.A.Jr, Thomadsen B.R., Bhargavan M., et al. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys. 2008;95:502-507.
2. Brenner D.J., Hall E.J. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284.
3. Einstein A.J., Moser K.W., Thompson R.C., Cerqueira M.D., Henzlova M.J. Radiation Dose to Patients from Cardiac Diagnostic Imaging. Circulation. 2007;116:1290-1305.
4. National Cancer Institute: Radiation Risks and Pediatric Computed Tomography (CT): A Guide for Health Care Providers. http://www.cancer.gov/cancertopics/causes/radiation-risks-pediatric-CT.
5. Zanzonico P., Rothenberg L.N., Strauss H.W. Radiation exposure of computed tomography and direct intracoronary angiography: risk has its reward. J Am Coll Cardiol. 2006;47:1846-1849.
6. McCollough C.H., Primak A.N., Braun N., Kofler J., Yu L., Christner J. Strategies for reducing radiation dose in CT. Radiol Clin North Am. 2009;47(1):27-40.
7. Patton J.A., Slomka P.J., Germano G., Berman D.S. Recent technologic advances in nuclear cardiology. J Nucl Cardiol. 2007;14(4):501-513.
8. Heller G.V., et al. A Multicenter Evaluation of a New Post-Processing Method with Depth-Dependent Collimator Resolution Applied to Full and Half-Time Acquisitions Without and With Simultaneously Acquired Attenuation. J Nucl Cardiol. 2009. (in press)
9. DePuey E.G., Gadiraju R., Clark J., Thompson L., Anstett F., Shwartz S.C. Ordered subset expectation maximization and wide beam reconstruction “half-time” gated myocardial perfusion SPECT functional imaging: a comparison to “full-time” filtered backprojection. J Nucl Cardiol. 2009. (in press)
10. Zanzonico P.B. Internal radionuclide radiation dosimetry: a review of basic concepts and recent developments. J Nucl Med. 2000;41(2):297-308.
11. Bushberg J.T., Siebert J.A., Leidholdt E.M., Boone J.M. The essential Physics of Medical Imaging, ed 1. Philadelphia: Williams & Wilkins, 1994.
12. International Commission on Radiological Protection. 1990 Recommendations of the International Commission on Radiological Protection. New York: Pergamon Press, 1991. ICRP Publication 60
13. International Commission on Radiological Protection. Limits for Intakes of Radionuclides by Workers. New York: Pergamon Press, 1979. ICRP Publication 30
14. 2007 Recommendations of the International Commission on Radiological Protection. Annals of the ICRP. New York: Elsevier, 2007. ICRP Publication 103
15. Cristy M. Development of mathematical pediatric phantoms for internal dose calculations: designs, limitations, and prospects. In: Watson E.E., Stelson A.T.S., Coffey J.L., Cloutier R.J., editors. Proceedings of the Third International Radiopharmaceutical Dosimetry Symposium (FDA 81-8166). Oak Ridge, TN: Oak Ridge Associated Universities; 1981:496-517.
16. Loevinger R., Budinger T., Watson E. MIRD Primer for Absorbed Dose Calculations. New York: Society of Nuclear Medicine, 1991.
17. Snyder W., Ford M., Warner G., Watson S. “S,” absorbed dose per unit cumulated activity for selected radionuclides and organs, MIRD Pamphlet No. 11. New York: Society of Nuclear Medicine, 1975.
18. Thompson R.C., Cullom S.J. Issues Regarding Radiation Dosage of Cardiac Nuclear and X-Ray Procedures. J Nucl Cardiol. 2006;13(1):19-23.
19. Cardiogen package insert http://www.nuclearonline.org/PI/Cardiogen.pdf
20. Mossman K.L., Goldman M., Masse F., Mills W.A., et al. Radiation Risk in Perspective. Health Physics Society Position Statement. McLean, VA: Health Physics Society, 2004.
21. Lazo T. The evolution of the international system of radiological protection: food for thought from the Nuclear Energy Agency Committee on Radiation Protection and Public Health. J Radiol Prot. 2003;23(3):241-246.
22. Mossman K.L. The linear no-threshold debate: where do we go from here? Med Phys. 1998;25(3):279-284.
23. National Council on Radiation Protection and Measurements. Exposure of the population in the United States and Canada from natural background radiation NCRP Report 94. Bethesda, MD: National Council on Radiation Protection and Measurements, 1988.
24. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR): Epidemiological studies of radiation and cancer, 2006. Paper presented at 54th Session of UNSCEAR; May 29-June 2, Vienna, Austria, 2006
25. Wackers F.J., Berman D.S., Maddahi J., et al. Technetium-99m hexakis 2-methoxyisobutyl isonitrile: human biodistribution, dosimetry, safety, and preliminary comparison to thallium-201 for myocardial perfusion imaging. J Nucl Med. 1989;30:301-311.
26. Tc-99m tetrofosmin package insert http://www.fda.gov/medwatch/SAFETY/2003/03NOV_PI/Myoview_PI.pdf
27. Berman D.S., Kiat H., Maddahi J. The new 99mTc myocardial perfusion imaging agents: 99mTc-sestamibi and 99mTc-teboroxime [review]. Circulation. 1991;84(3 Suppl):I7-121.
28. Thallium-201 Chloride package insert
29. Thomas SR, Stabin MG, Castronovo FP: Radiation-Absorbed Dose from 201Tl-Thallous Chloride. J Nucl Med 46:502–508
30. Siegel J.A. Guide for Diagnostic Nuclear Medicine and Radiopharmaceutical Therapy. Reston, VA: Society of Nuclear Medicine, 2004.
31. Stabin M.G., Breitz H.B. Breast milk excretion of radiopharmaceuticals: mechanisms, findings, and radiation dosimetry. J Nucl Med. 2000;41(5):863-873.
32. Stabin M.G. Health concerns related to radiation exposure of the female nuclear medicine patient. Environ Health Perspect. 1997;105(Suppl 6):1403-1409.
33. Di Carli M.F., Dorbala S., Meserve J., El Fakhri G., Sitek A., Moore S.C. Clinical myocardial perfusion PET/CT [review]. J Nucl Med. 2007;48(5):783-793.
34. Alvarez-Diez T.M., deKemp R., Beanlands R., Vincent J. Manufacture of strontium-82/rubidium-82 generators and quality control of rubidium-82 chloride for myocardial perfusion imaging in patients using positron emission tomography. Appl Radiat Isot. 1999;50(6):1015-1023.
35. Kearfott K.J. Radiation absorbed dose estimates for positron emission tomography (PET): K-38, Rb-81, Rb-82, and Cs-130. J Nucl Med. 1982;23(12):1128-1132.
36. Ryan J.W., Haiper P.V., Stark V.i., Peterson E.L., Lathrop K.A. Radiation absorbed dose estimate for rubidium-82 determined from in vivo measurements in human subjects. In: Schiafice-Stelson A.T., Watson E.E., editors. Fourth international radiopharmaceutical dosimetry symposium. Oak Ridge, TN: Oak Ridge Associated University Publishers; 1986:346-358.
37. Lodge M.A., Braess H., Mahmoud F., et al. Developments in nuclear cardiology: transition from single photon emission computed tomography to positron emission tomography-computed tomography [review]. J Invasive Cardiol. 2005;17(9):491-496.
38. Valentin J. Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53): ICRP publication 80 approved by the Commission in September 1997. Ann ICRP. 1998;28:1-126.
39. deKemp R.A., Beanlands R.S. A revised effective dose estimate for the PET perfusion tracer Rb-82. [abstract]. J Nucl Med. 2008;49(Suppl 1):183P.
40. Schelbert H.R. Blood flow and metabolism by PET. Cardiol Clin. 1994;12(2):303-315.
41. Hutchins G.D., Schwaiger M., Rosenspire K.C., Krivokapich J., Schelbert H., Kuhl D.E. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15(5):1032-1042.
42. Beller G.A. Assessment of myocardial perfusion and metabolism for assessment of myocardial viability. Q J Nucl Med. 1996;40(1):55-67.
43. Jones S.C., Alavi A., Christman D., Montanez I., Wolf A.P., Reivich M. The radiation dosimetry of 2-F-18 fluoro-2-deoxy-D-glucose in man. J Nucl Med. 1982;23:613-617.
44. Oak Ridge FDG data. Radiation Dose to Patients from Radiopharmaceuticals. Oxford, U.K.: Pergamon Press; 1999:76. ICRP Publication 80, Addendum 2 to ICRP Publication 53
45. Bixler A., Springer G., Lovas R. Practical aspects of radiation safety for using fluorine-18. J Nucl Med Technol. 1999;27(1):14-16.
46. Zanzonico P., Dauer L., St Germain J. Operational radiation safety for PET-CT, SPECT-CT, and cyclotron facilities. Health Phys. 2008;95(5):554-570.
47. Upton A.C., National Council on Radiation Protection and Measurements Scientific Committee 1–6. The state of the art in the 1990’s: NCRP Report No. 136 on the scientific bases for linearity in the dose-response relationship for ionizing radiation. Health Phys. 2003;85(1):15-22.
48. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: National Academy Press, 2005.
49. Brenner D., Elliston C., Hall E., Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176(2):289-296.
50. Einstein A.J., Henzlova M.J., Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317-323.
51. Sodickson A., Baeyens P.F., Andriole K.P., et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175-184.
52. Huang B., Law M.W., Khong P.L. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology. 2009;251(1):166-174. Epub 2009 Feb 27
53. United States Cancer Statistics: Denters for Disease Control, 2000. Atlanta, Georgia, 2000
54. Instruction Concerning Risks from Occupational Radiation Exposure. US Nuclear Regulatory Commission Regulatory Guide 8.29, Revision 1, February 1996
55. NCRP. National Council on Radiation Protection and Measurements. Statement No. 10. December 2004. Recent Applications of the NCRP Public Dose Limit Recommendation for Ionizing Radiation. http://www.ncrponline.org/NCRP%20Statement%20No.%2010.pdf.
56. Hall E.J., Brenner D.J., Worgul B., Smilenov L. Genetic susceptibility to radiation. Adv Space Res. 2005;35(2):249-253.
57. United States Nuclear Regulatory Commission: Standards for Protection against radiation, http://www.nrc.gov/reading-rm/doc-collections/cfr/part020/.
58. Schleipman A.R., Castronovo F.P.Jr, Di Carli M.F., Dorbala S. Occupational radiation dose associated with Rb-82 myocardial perfusion positron emission tomography imaging. J Nucl Cardiol. 2006;13(3):378-384.
59. Limacher, et al. Radiation safety in the practice of cardiology. J Am Coll Cardiol. 1998;31:892.
60. Gerber T.C., Carr J.J., Arai A.E., et al. Ionizing radiation in cardiac imaging. A science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119:1056-1065.
61. McCollough C.H., Bruesewitz M.R., Kofler J.M.Jr. CT dose reduction and dose management tools: overview of available options. Radiographics. 2006;26(2):503-512.
62. Amis E., et al. American College of Radiology white paper on Radiation Dose in Medicine. Radiat Prot Dosimetry. 2005;114:11-25.
63. American Society of Nuclear Cardiology. Information statement on variability in radiation dose estimates. 2008 by the American Society of Nuclear Cardiology. doi:10.1007/s12350-008-9026-0
64. Morin R.L., Gerber T.C., McCollough C.H. Radiation dose in computed tomography of the heart. Circulation. 2003;107(6):917-922.
65. Flohr T: Radiation exposure estimation and reduction approaches. In Ohnesorge, Becker, Flohr, Reiser. Multi-Slice CT in Cardiac Imaging, 2nd ed., Berlin: Springer Publishing, 2006.
66. McNitt-Gray M.F. Radiation dose in CT. AAPM/RSNA Physics Tutorial for Residents: Topics in CT. Radiographics. 2002;22:1541-1553.
67. Huda W., Ogden K.M., Khorasani M.R. Converting dose-length product to effective dose at CT. Radiology. 2008;248(3):995-1003.
68. Mahesh M., Cody D.D. Physics of cardiac imaging with multiple-row detector CT. Radiographics [review]. 2007;27(5):1495-1509.
69. Hausleiter J., Meyer T., Hermann F., et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500-507.
70. Kalra M.K., Naz N., Rizzo S.M., Blake M.A. Computed tomography radiation dose optimization: scanning protocols and clinical applications of automatic exposure control. Curr Probl Diagn Radiol. 2005;34(5):171-181.
71. Kalender W.A., Wolf H., Suess C. Dose reduction in CT by anatomically adapted tube current modulation. II. Phantom measurements. Med Phys. 1999;26:2248-2253.
72. Shuman W.P., Branch K.R., May J.M. et al: Prospective versus retrospective ECG gating for 64-detector CT of the coronary arteries: Comparison of image quality and patient radiation dose. Radiology.. Aug 2008;248(2):431-437.
73. Ertel D., Pflederer T., Achenbach S., Kalender W.A. Real-time determination of the optimal reconstruction phase to control ECG pulsing in spiral cardiac CT. Phys Med. Sep 2009;25(3):122-127.
74. Poll L.W., Cohnen M., Brachten S., et al. Dose reduction in multi-slice CT of the heart by use of ECG-controlled tube current modulation (“ECG pulsing”): phantom measurements. Rofo. 2002;174:1500-1505.
75. Parker M.S., Kelleher N.M., Hoots J.A., Chung J.K., Fatouros P.P., Benedict S.H. Absorbed radiation dose of the female breast during diagnostic multidetector chest CT and dose reduction with a tungsten-antimony composite breast shield: preliminary results. Clin Radiol. 2008;63(3):278-288.
76. Garcia M.J., Lessick J., Hoffmann M.H., CATSCAN Study Investigators. Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296(4):403-411.
77. Hunold P., Vogt F.M., Schmermund A., et al. Radiation exposure during cardiac CT: effective doses at multi-detector row CT and electron-beam CT. Radiology. 2003;226:145-152.
78. McCollough C.H. Patient dose in cardiac computed tomography. Herz. 2003;28:1-6.
79. Hacker M., Jakobs T., Matthiesen F., et al. Comparison of spiral multidetector CT angiography and myocardial perfusion imaging in the noninvasive detection of functionally relevant coronary artery lesions: first clinical experiences. J Nucl Med. 2005;46:1294-1300.
80. Coles D.R., Smail M.A., Negus I.S., et al. Comparison of radiation doses from multislice computed tomography coronary angiography and conventional diagnostic angiography. J Am Coll Cardiol. 2006;47:1840-1845.
81. Flohr T.G., Schoepf U.J., Kuettner A., et al. Advances in cardiac imaging with 16-section CT systems. Acad Radiol. 2003;10:386-401.
82. Hoffmann M.H., Shi H., Schmitz B.L., et al. Noninvasive coronary angiography with multislice computed tomography. JAMA. 2005;293:2471-2478.
83. Gerber T.C., Stratmann B.P., Kuzo R.S., Kantor B., Morin R.L. Effect of acquisition technique on radiation dose and image quality in multidetector row computed tomography coronary angiography with submillimeter collimation. Invest Radiol. 2005;40:556-563.
84. Nawfel R., Yoshizumi T. Update on radiation dose in CT. Am Assoc Physicists Med Newsl. 2005;30:12-13.
85. Sato Y., Matsumoto N., Ichikawa M., et al. Efficacy of multislice computed tomography for the detection of acute coronary syndrome in the emergency department. Circ J. 2005;69:1047-1051.
86. Trabold T., Buchgeister M., Kuttner A., et al. Estimation of radiation exposure in 16-detector row computed tomography of the heart with retrospective ECG-gating. Rofo. 2003;175:1051-1055.
87. Gaspar T., Halon D.A., Lewis B.S., et al. Diagnosis of coronary in-stent restenosis with multidetector row spiral computed tomography. J Am Coll Cardiol. 2005;46:1573-1579.
88. Caussin C., Larchez C., Ghostine S., et al. Comparison of coronary minimal lumen area quantification by sixty-four-slice computed tomography versus intravascular ultrasound for intermediate stenosis. Am J Cardiol. 2006;98:871-876.
89. Hausleiter J., Meyer T., Hadamitzky M., et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation. 2006;113:1305-1310.
90. Ghostine S., Caussin C., Daoud B., et al. Non-invasive detection of coronary artery disease in patients with left bundle branch block using 64-slice computed tomography. J Am Coll Cardiol. 2006;48:1929-1934.
91. Leber A.W., Knez A., von Ziegler F., et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147-154.
92. Mollet N.R., Cademartiri F., van Mieghem C.A., et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318-2323.
93. Muhlenbruch G., Seyfarth T., Soo C.S., Pregalathan N., Mahnken A.H. Diagnostic value of 64-slice multi-detector row cardiac CTA in symptomatic patients. Eur Radiol. 2007;17:603-609.
94. Nikolaou K., Knez A., Rist C., et al. Accuracy of 64-MDCT in the diagnosis of ischemic heart disease. AJR Am J Roentgenol. 2006;187:111-117.
95. Pugliese F., Mollet N.R., Runza G., et al. Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16:575-582.
96. Raff G.L., Gallagher M.J., O’Neill W.W., Goldstein J.A. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552-557.
97. Wu T.H., Huang Y.H., Lee J.J., et al. Radiation exposure during transmission measurements: comparison between CT- and germanium-based techniques with a current PET scanner. Eur J Nucl Med Mol Imaging. 2004;31(1):38-43. Epub 2003 Oct 8
98. Almeida P., Bendriem B., de Dreuille O., Peltier A., Perrot C., Brulon V. Dosimetry of transmission measurements in nuclear medicine: a study using anthropomorphic phantoms and thermoluminescent dosimeters. Eur J Nucl Med. 1998;25(10):1435-1441.
99. Fricke E., Fricke H., Weise R., et al. Attenuation correction of myocardial SPECT perfusion images with low-dose CT: evaluation of the method by comparison with perfusion PET. J Nucl Med. 2005;46(5):736-744.