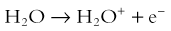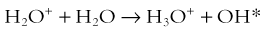Radiation Bioeffects, Risks, and Radiation Protection in Medical Imaging in Children
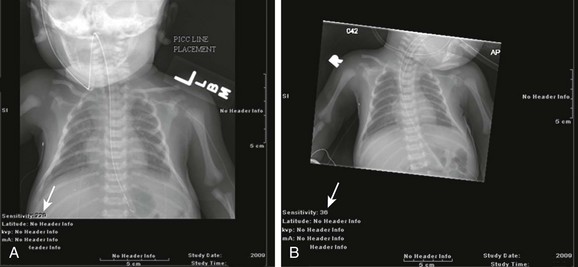
Diagnostic imaging has evolved from the single technique of radiography discussed in the first edition of Caffey’s Pediatric X-Ray Diagnosis in 1945 to a specialty with a choice of many modalities and techniques. Many of these modalities use ionizing radiation, and some entail relatively high doses of radiation, such as computed tomography (CT) and nuclear imaging, including positron emission tomography.1 For this reason, the imaging community (and our medical colleagues) must jointly adhere to two of the principles of radiation protection for our patients: justification (i.e., the examination is appropriate) and optimization (i.e., the appropriate technique is used). For example, we can substantially influence the radiation dose range within the radiographic arena depending on how we perform computed or direct digital radiographic examinations. Image processing for this digital technology can accommodate overexposures; that is, from a contrast and brightness standpoint, the image can be adjusted to appear as if it was obtained using standard techniques, whereas with screen film technology, the film image would be recognized as overexposed (dark) (Fig. 1-1). In addition, without accountability for displaying dose metrics (such as the exposure index available with digital radiography), it is difficult or impossible to account for patient exposures in clinical practice.2 In addition, uninformed and potentially irresponsible justification of medical imaging occurs when persons are unfamiliar with the methods of estimating an effective radiation dose during CT examinations in children.3,4 Increasing accountability is expected from the medical community with regard to the use of imaging modalities that expose children (as well as adults) to ionizing radiation, especially for the risk part of the risk/benefit ratio. Because of the need for such accountability, a basic understanding of radiation biology, including bioeffects, radiation doses of various types of imaging examinations, and risks of radiation, is essential for the pediatric imager. A glossary of terms and dose descriptors is found in the addendum at the end of this chapter.
Trends in Medical Radiation Exposure to Children
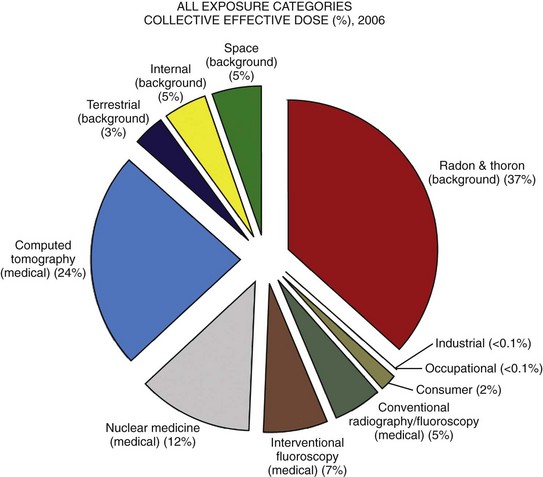
Approximately 4 billion imaging examinations using ionizing radiation (i.e., radiography, fluoroscopy/angiography, CT, and nuclear imaging) are performed annually worldwide.5 In the United States, medical imaging currently accounts for a significant percentage of the annual radiation exposure to the population (Fig. 1-2),6 and most sources demonstrate continued increased use during the past decade. For example, one study showed a fivefold increase in CT examinations in the pediatric acute care setting from 1995 to 2008.7 Natural or background sources account for about 50% of the annual radiation exposure in the United States, and diagnostic medical radiation accounts for most of the remainder, an approximately sixfold increase during the past 30 years. CT alone accounts for nearly 25% of all radiation exposure to the U.S. population.6 Many reasons exist for the increased use of diagnostic medical radiation, and much use of such radiation is based on sound medical decision making. However, other factors determine use as well, including defensive medicine.
Pathophysiology of Radiation Effects
Hall8 has written an excellent review of radiobiology for the radiologist. The biologic effects of radiation result primarily from damage to deoxyribonucleic acid (DNA)—the critical target. The first step in the absorption of x-rays is that the x-ray particle, the photon, gives up its energy to produce a fast recoil electron. The electron may damage DNA directly, but the electron also can interact with a molecule of water to produce a free radical (Fig. 1-3). A free radical is a highly reactive atom or molecule with an unpaired electron in the outer shell:
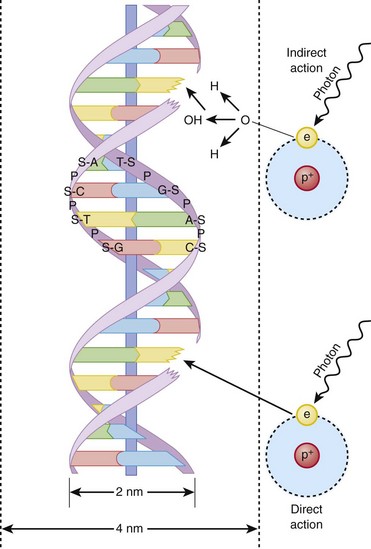
Figure 1-3 Direct and indirect action.
Indirect action (top part of figure), an electron damages the deoxyribonucleic acid (DNA). In indirect action, the secondary electron interacts with a water molecule to produce a hydroxyl radical that then damages DNA, in this case affecting a single strand. (From Hall EJ. Radiation biology for pediatric radiologists, Pediatr Radiol 39(1):S57-S64, 2009. Used with permission.)
where the asterisk indicates a free radical.
The hydroxyl radical (OH) can diffuse a short distance to cause DNA damage. The fact that two thirds of x-ray damage occurs via OH suggests that someday this component of radiation damage might be reduced through the use of chemical radioprotectants. The topic of radioprotectants was recently well reviewed.9
The biologic effects of radiation result primarily from damage to double-stranded DNA as opposed to single-strand injury (see Fig. 1-3). Single-strand breaks of DNA are readily repaired and are presumed to have a negligible effect. Breaks in both DNA strands that are opposite or separated by a few base pairs are much more difficult to repair. These double-strand breaks can cause important biologic effects, including genetic mutations, carcinogenesis, and cell death. Dicentric and fragmented breaks typically result in cell death, whereas nonlethal translocation repairs may cause impaired cellular function, including development of an oncogene.8
The biochemical and physiologic damage produced by radiation generally occurs within hours or days, but the impact of these changes, such as the induction of cancer, can take decades to manifest. This carcinogenesis process has several steps. Aberrations in chromosomes (e.g., deletions, translocations, or aneuploidy) are produced by DNA damage. Because these impaired cells survive, they become “stable aberrations” (some with neoplastic transformation), a morphologic change that is the first step of a multistep process to radiation-induced carcinogenesis. The second step of the process is cellular immortality. That is, most cancer cells are descendents of a single cell that originally underwent neoplastic transformation. The third step is tumorgenicity. The radiation exposure induces a cellular genomic instability that is transmitted to progeny, which Little10 described as “a persistent enhancement in the rate of which genetic changes arise in the descendents of the irradiated cells after many generations of replication … [this process] has been termed a nontargeted effect of radiation as genomic damage occurs in the cells that in themselves receive no direct radiation exposure.”
Most childhood tumors (about 85%) occur sporadically, but in 15% of the cases, a strong family association and genetic basis for radiation sensitivity are present. Persons with certain diseases are uniquely sensitive to radiation-induced cancers, although the exact mechanism is unclear (Box 1-1).
Types of Radiation Bioeffects
The two types of biologic effects from radiation are deterministic and stochastic (random). Deterministic bioeffects are characterized by a threshold dose, and the severity of the effect is dose dependent. For example, cataracts traditionally are reported not to occur with less than a 2.0 Gy exposure, although recent data suggest that this threshold is below 1.0 Gy.11 Table 1-1 shows some of the doses for deterministic effect. In general, deterministic effects from the doses used in diagnostic imaging are extremely rare. Recent exceptions with head perfusion CT scanning in adults have been reported.12 Deterministic effects such as skin ulcers and burns should never occur from diagnostic imaging, but they are seen with relatively lengthy interventional procedures.
Table 1-1
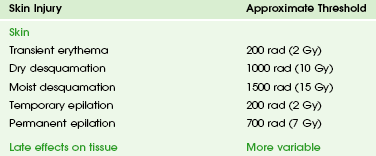
Modified from Hall EJ. Radiobiology for the radiologist, ed 5, Philadelphia: Lippincott Williams & Wilkins; 2000.
Fetuses and Children Have Greater Radiation Risks
From a public health perspective, all ionizing radiation from medical imaging is considered to be potentially harmful because we assume that no threshold exists below which radiation is safe (i.e., no harmful effects will occur). This “linear no threshold” model is applied to low-level radiation exposure.13,14
The effects of radiation are greatest on rapidly developing organisms—that is, fetuses, infants, and young children. In pregnancy, the major biologic effects of fetal demise, growth restriction, organ malformations, and cognitive deficits are seen only with doses far in excess of routine diagnostic imaging.15 The risk of developing cancer from exposure of a fetus to radiation is uncertain, as it is with exposure of a child to radiation; potential effects could be seen with uterine doses that occur as a result of relatively high direct exposures (e.g., a pelvis CT scan for possible appendicitis). No data in humans indicate that genetic effects result from diagnostic levels of radiation.
Compared with middle-aged adults, children have been reported to be from 2 to 15 times more sensitive to radiation-induced carcinogenesis.16,17 However, Shuryak et al18 recently noted that the cancer induction risk (greater at younger ages) must be balanced with the radiation-induced promotion of premalignant damage (greater in middle age), which may differ for certain types of cancer. Thus cancer risks may be higher in the adult population than traditionally believed.18
Low-dose effects have been described by Pierce and Preston,19 who studied the data from atomic bomb survivors reported by the Radiation Effects Research Foundation. Among persons who had received dosages of 0.005 to 0.2 sievert (500 mrad to 20 rad), 35,000 people survived, and 5000 cases of cancer developed. The authors made the following conclusions: first, the solid cancer risk persists for more than 50 years. Second, there is a 10% increase over the expected cancer rate.19 Low-dose relative risk factors are shown in Figure 1-4.
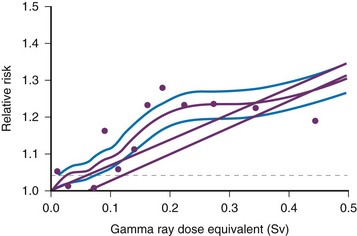
Figure 1-4 Estimated low-dose risks.
Age-specific cancer rates over the 1958 through 1994 follow-up period relative to the rates for unexposed people averaged over the follow-up for sex and for age at exposure. The dashed line represents ±1 standard deviation error for the smooth purple curve. The upper straight line is the linear risk estimate computed from the range 0 to 2 Sv. The second straight line beginning at 0.06 Sv is the upper 95% confidence limit for such a quantity. (From Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors, Radiat Res. 154:178-186, 2000.)
The overall risk of cancer for the entire population suggested by the International Commission on Radiological Protection is 5% per sievert for low doses and low-dose rate. However, this value is an average value. For adults in late middle age, the risk decreases to only 1% per sievert, whereas for children in the first decade of life, the risk may be as high as 16% per sievert for girls and 12% per sievert for boys. The female dose is higher because of the greater incidence of breast and thyroid cancers (Fig. 1-5). Radiation risks from diagnostic imaging low-level radiation were reviewed recently.20 Two excellent reviews also recently were published by Linet et al21,22 (Table 1-2). At this point, we have cautious uncertainty regarding cancer risk and low-level radiation. As Hricak et al23 state, “In brief, there is reasonable, though not definitive, epidemiologic evidence that organ doses in the range from 5 to 125 mSv result in a very small but statistically significant increase in cancer risk.”
Table 1-2
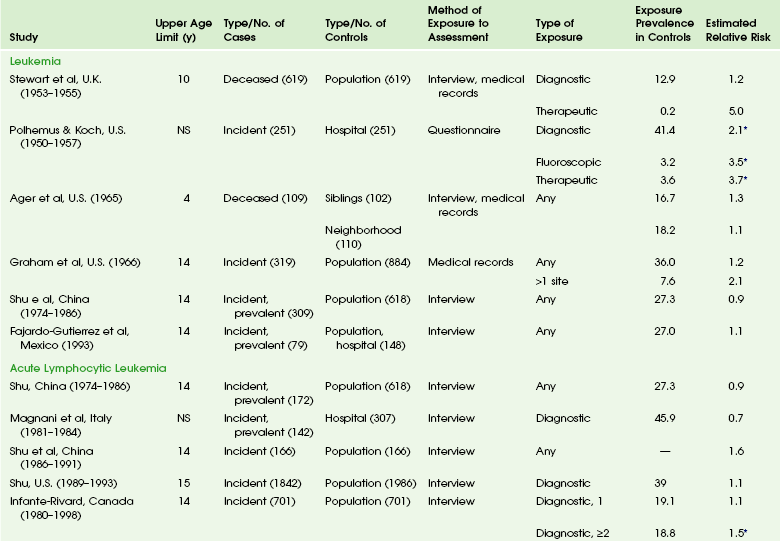
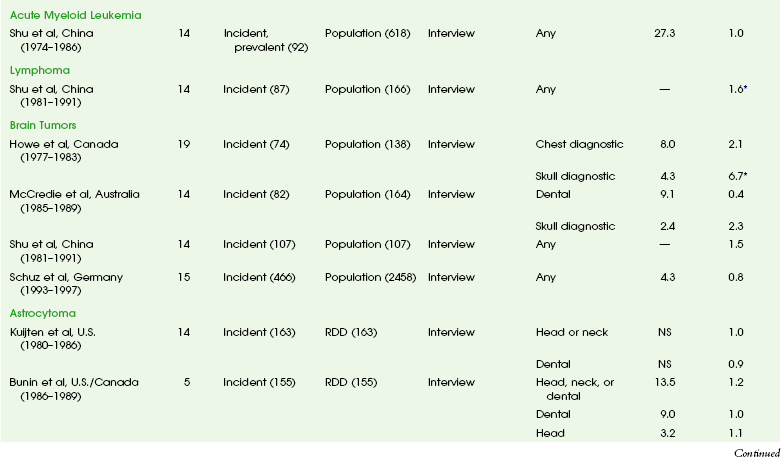
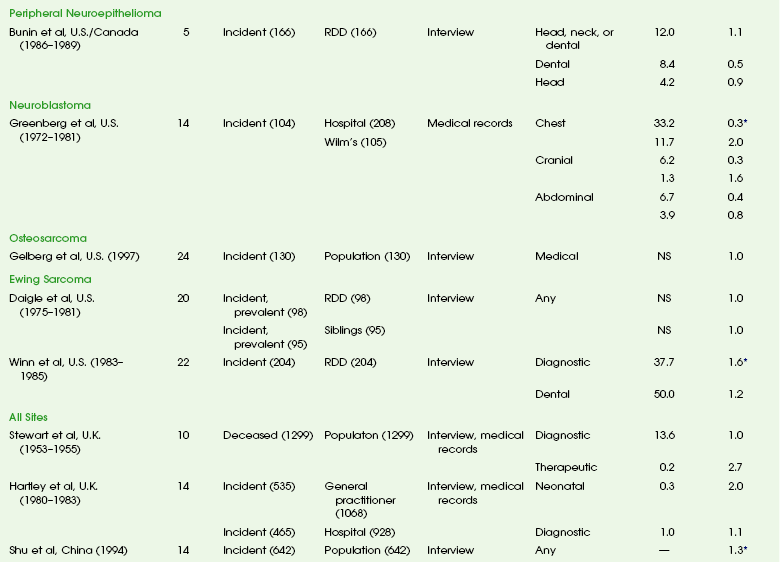
From Linet MS, Slovis TL, Miller DL, et al. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin 2012 [epub ahead of print].
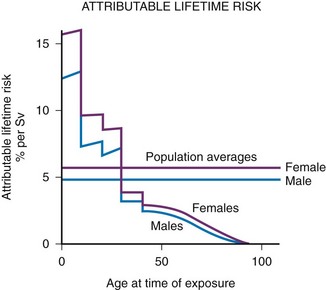
Figure 1-5 Lifetime risk of excess cancer per sievert as a function of age at the time of exposure.
Data from atomic bomb survivors. Although the average risk for a population is about 5% per sievert, the risk varies considerably with age; children are much more sensitive than adults. At early ages, girls are more sensitive than boys. (From Hall EJ: Introduction to session I. helical CT and cancer risk, Pediatr Radiol. 32:225-227, 2002.)
Radiation Exposures from Various Imaging Modalities
When discussing radiation dose, it is important to state clearly whether entrance dose, skin dose, exit dose, or organ (absorbed) dose is considered. For example, a vast difference can exist between skin dose and gonadal dose for the same incident radiation. (The terms for dose and quantitative comparisons are provided in the glossary, and dose metrics are provided in Box 1-2.) “Effective dose” is one measure of radiation that is widely used in discussions of medical radiation. It is commonly used because it is relatively easily derived and allows gross comparisons of dose estimations between examinations of different regions as well as different modalities. However, the application of effective dose in medical imaging is problematic.24,25
Published estimates for radiation doses in adults and children include those by Fahey et al.26 and Mettler et al.27 In summary, radiographic dose ranges from a fraction of a millisievert (for extremity evaluation) to somewhat larger doses of more than 1.0 mSv for more extensive examinations, such as a lumbar spine series. Fluoroscopic doses depend on technical parameters, especially fluoroscopy time and frame rate, and can vary widely from very low dose cystography to doses in tens of millisieverts for complex interventional procedures. CT is a modality that can deliver a relatively large dose of ionizing radiation. The number of these examinations has been increasing at a rapid rate, with children (birth to 15 years old) undergoing approximately 11% of all CT examinations.28 More recent information has shown that the dose (the CTDI; see glossary) from CT (conventional and spiral examinations) in adults is less than 1.0 mSv to as much as 40 mSv.26,27 Typical pediatric CT doses should be less than 10 mSv,26,29 and with new iterative reconstruction technologies, an increasing fraction of body examinations are approaching or being performed in the sub-millisievert range. Data on radiation doses from select pediatric imaging examinations are found in Table 1-3.
Table 1-3
Radiation Dose to Children by Age at Diagnostic Examination
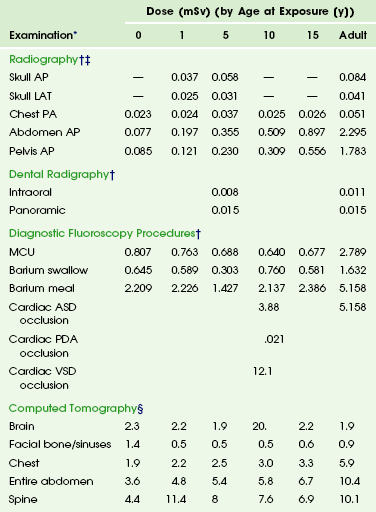
*Dosimetric quantities are all effective doses in millisieverts (mSv).
†From Hart D, Hillier MC. Dose to patients from medical x-ray examinations in the UK–2000 review. Chilton, UK: National Radiological Protection Board; 2007.
‡From Hart D, Hillier MC. Dose to patients from medical x-ray examinations in the UK—2002 review. Chilton, UK: National Radiological Protection Board; 2002.
§From Galanski M, Nagel HD, Stamm G. Paediatric CT exposure practice in the Federal Republic of Germany—results of a nation-wide survey in 2005/2006. Hannover, Germany, 2006, Hannover Medical School. Radiation doses are based on a German nationwide survey on multislice CT. The radiation dose in each age group category is the dose administered to pediatric patients who are newborn (the 0-year category), those ages >0-1 year (the 1-year category), those ages 2- 5 years (the 5-year category), those ages 6-10 years (the 10-year category), and those ages 11-15 years (the 15-year category).
Strategies for Optimizing Radiation Doses for Children
The fundamental principles for protecting children from radiation during imaging include justification and optimization. Justification for a study can be difficult to define, although summary recommendations and appropriateness criteria for medical imaging are available.30 The use of imaging modalities that do not depend on ionizing radiation, especially magnetic resonance imaging and sonography, is fundamental in justification considerations.
Patient preparation and examination planning are paramount for any examination. Planning includes communicating with the ordering clinician when necessary to clarify examination indications.31 Communication also is important both before and during fluoroscopic and angiographic examinations to minimize potentially nonuseful fluoroscopy. Use of appropriately trained staff and involvement with qualified medical physicists, along with licensing, certification, and accreditation considerations and routine reviews of equipment function and protocols as part of quality control and assessment, are becoming expected components of radiation protection in medicine.32
Strategies for computed radiography and direct radiography dose management in pediatric imaging include appropriate collimation, evaluation of the number of projections required, consideration of the source to film and patient to film distances, shielding, use of grids, filtering, consideration of exposure factors, and use of postprocessing techniques. General strategies for protecting patients from radiation during radiography can be found in International Atomic Energy Agency educational material.33
A number of radiation protection management strategies exist for fluoroscopy and interventional radiology, including those by the International Atomic Energy Agency and Alliance for Radiation Safety in Pediatric Imaging (Image Gently Campaign).34–36 These strategies include avoiding field overlap among different projection series, avoiding electronic magnification, placement of the image intensifier before fluoroscopic activation, use of appropriate grids and positioning (i.e., source to patient and patient to intensifier distances), collimation, and use of pediatric-specific filters. In addition, image hold, pulsed fluoroscopy, image store, video capture, and alerts are part of examination optimization for children. Video recording during studies can provide review without use of additional fluoroscopy.
Contemporary strategies and examination optimization for radiation dose management in pediatric CT, including protocol optimization (especially related to clinical indication), are available.37–40 Adjustment in the parameters that are primarily responsible for the dose delivered—tube current (milliamperes), gantry cycle time in seconds, peak kilovoltage, and pitch—should be based on the size of the child, examination indication, prior examinations, and region examined. Additional efforts should include minimizing multiphase examinations, excessive scan length, and overlapping regions of examination. Tube current modulation, organ-based dose modulation (in which the tube current is reduced in an arc to reduce the surface dose to anterior structures such as the breasts when the patient is supine), iterative reconstructive technologies, and dual-source/dual-energy technology also have provided real or potential opportunities for dose reduction, improved examination quality, or a combination of both. The use of shielding in the region of scanning, which usually is discussed for breast tissue, is debated.41,42 A more in-depth discussion of radiation protection in children undergoing diagnostic imaging is available.43
Educational efforts are extremely important, particularly given increased scrutiny and concern regarding medical radiation and the risk of cancer. To these ends, the Alliance for Radiation Safety in Pediatric Imaging, through the Image Gently campaigns,44,45 has been a successful education and awareness organization for pediatric imaging involving ionizing radiation.
Continued needs include more evidence-based research and decision support to improve utilization,46 better dose estimations,3,4 development of diagnostic reference levels for pediatric imaging, alerts and notifications on imaging equipment,47 and cumulative dose estimation reporting for all modalities as well as dose tracking.48
Fahey, FH, Treves, ST, Adelstein, SJ. Minimizing and communicating radiation risk in pediatric nuclear medicine. J Nucl Med. 2011;52:1240–1251.
Frush, DP. CT dose and risk estimates in children. Pediatr Radiol. 2011;41:483–487.
Hall, EJ. Radiation biology for pediatric radiologists. Pediatr Radiol. 2009;39(1):S57–S64.
Hricak, H, Brenner, DJ, Adelstein, SJ, et al. Managing radiation use in medical imaging: multifaceted challenge. Radiology. 2011;258:889–905.
Linet MS, Slovis TL, Miller DL, et al. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin. 2012 [epub ahead of print].
McCollough, CH, Schueler, BA, Atwell, TD, et al. Radiation exposure and pregnancy: when should we be concerned? Radiographics. 2007;27:909–917.
References
1. http://www.acr.org/~/media/ACR/Documents/AppCriteria/RRLInformation/pdf.
2. Don, S. Radiosensitivity of children: potential for overexposure in CR and DR and magnitude of doses in ordinary radiographic examinations. Pediatr Radiol. 2004;34(3):S167–S172.
3. Strauss, K. Radiation safety summit—method to estimate radiation dose to pediatric patients from CT scans. Pediatr Radiol. 2011;41:210–211.
4. http://www.aapm.org/pubs/reports/, 2012. [Accessed March 3].
5. International Atomic Energy Agency. Technical Meeting on Review of Radiation Protection Guidance for Medical Workers, Vienna, 17-19 November 2008 (website). http://rpop.iaea.org/RPOP/RPoP/Content/PastEvents/MeetingsRadiationprotectionmedicalworkersnov2008.htm. [Accessed March 3. 2012].
6. National Council on Radiation Protection & Measurements. Ionizing radiation exposure of the population of the United States (NCRP report No. 160. Bethesda, Md: National Council on Radiation Protection & Measurements; 2009.
7. Larson, DB, Johnson, LW, Schnell, LW, et al. Rising use of CT in child visits to the emergency department in the United States, 1995-2008. Radiology. 2011;259(3):793–801.
8. Hall, EJ. Radiation biology for pediatric radiologists. Pediatr Radiol. 2009;39(1):S57–S64.
9. Mettler, FA, Jr., Brenner, D, Coleman, CN, et al. Can radiation risks to patients be reduced without reducing radiation exposure? The status of chemical radioprotectants. AJR Am J Roentgenol. 2011;196(3):616–618.
10. Little, JB. Ionizing radiation. In Kufe DW, Pollock RE, Weichselbaum RR, et al, eds.: Holland-Frei cancer medicine, 6th ed, Ontario, BC: Decker, 2003.
11. Chodick, G, Bekiroglu, N, Hauptmann, M, et al. Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol. 2008;168:620–631.
12. U.S. Food and Drug Administration. Safety investigation of CT brain perfusion scans: update 11/9/2010. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm185898.htm, 2012. [Accessed July 24].
13. Little, MP, Wakeford, R, Tawn, EJ, et al. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology. 2009;251:6–12.
14. Tubiana, M, Feinendegen, LE, Yang, C, et al. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251:13–22.
15. McCollough, CH, Schueler, BA, Atwell, TD, et al. Radiation exposure and pregnancy: when should we be concerned? Radiographics. 2007;7:909–917.
16. Brenner, DJ, Elliston, CD, Hall, EJ, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. Am J Roentgenol. 2001;176:289–296.
17. Radiological Society of North America. Categorical course in physics: radiation dose and image quality for pediatric CT: clinical considerations. Oak Brook, IL: Radiological Society of North America; 2006.
18. Shuryak, I, Sachs, RK, Brenner, DJ. Cancer risks after radiation exposure in middle age. J Natl Cancer Inst. 2010;102:1–9.
19. Pierce, DA, Preston, DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000;154:178–186.
20. Frush, DP. CT dose and risk estimates in children. Pediatr Radiol. 2011;41:483–487.
21. Linet, M, Kim, K, Rajaraman, P. Children’s exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol. 2009;39(1):S4–S26.
22. Linet MS, Slovis TL, Miller DL, et al. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin. 2012 [epub ahead of print].
23. Hricak, H, Brenner, DJ, Adelstein, SJ, et al. Managing radiation use in medical imaging: a multifaceted challenge. Radiology. 2011;258:889–905.
24. McCollough, CH, Christner, JA, Kofler, JM. How effective is effective dose as a predictor of radiation risk? AJR Am J Roentgenol. 2010;194:890–896.
25. Martin, CJ. Effective dose: how should it be applied to medical exposures? Br J Radiol. 2007;80:639–647.
26. Fahey, FH, Treves, ST, Adelstein, SJ. Minimizing and communicating radiation risk in pediatric nuclear medicine. J Nucl Med. 2011;52:1240–1251.
27. Mettler, FA, Huda, W, Yoshizumi, TT, et al. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254–256.
28. Frush, DP, Applegate, K. Computed tomography and radiation: understanding the issues. J Am Coll Radiol. 2004;1:113–119.
29. Thomas, K, Wang, B. Age-specific effective doses for pediatric MSCT examinations at a large children’s hospital using DLP conversion coefficients: a simple estimation method. Pediatr Radiol. 2008;38:645–656.
30. Hendee, WR, Becker, GJ, Borgstede, JP, et al. Addressing overutilization in medical imaging. Radiology. 2010;257:240–245.
31. Malone, J, Guleria, R, Craven, C, et al. Justification of diagnostic medical exposures, some practical issues: report of an International Atomic Energy Agency Consultation. Br J Radiol. 2012;85(1013):523–538.
32. The Joint Commission. Sentinel Event Alert, Issue 47: Radiation risks of diagnostic imaging (website). http://www.jointcommission.org/sea_issue_47/. [Accessed July 24. 2012].
33. International Atomic Energy Agency. Be informed about the safe use of ionizing radiation in medicine (website). http://rpop.iaea.org, 2012. [Accessed July 24].
34. Miller, D, Balter, S, Schueler, B, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257(2):321–332.
35. Sidhu, M, Goske, MJ, Connolly, B, et al. Image Gently, Step Lightly: promoting radiation safety in pediatric interventional radiology. AJR Am J Roentgenol. 2010;195:299–301.
36. The Alliance for Radiation Safety in Pediatric Imaging. Welcome to Image Gently:a practice quality improvement (PQI) program in computed tomography (CT) scans in children (website). http://www.imagegentlypqi.org, 2012. [Accessed July 24].
37. Nievelstein, RAJ, van Dam, I, van der Molen, A. Multidetector CT in children: current concepts and dose reduction strategies. Pediatr Radiol. 2010;40:1324–1344.
38. Frush, DP. MDCT in children: scan techniques and contrast issues. In: Kalra MK, Saini S, Rubin GD, eds. MDCT: from protocols to practice. Milan, Italy: Springer, 2008.
39. Singh, S, Kalra, M, Moore, M, et al. Dose reduction and compliance with pediatric CT protocols adapted to patient size, clinical indication, and number of prior studies. Radiology. 2009;252(1):200–206.
40. Strauss, KJ, Goske, MJ, Kaste, SC, et al. Image Gently: ten steps you can take to optimize image quality and lower CT dose for pediatric patients. AJR Am J Roentgenol. 2010;194:868–873.
41. Kim, S, Frush, D, Yoshizumi, T. Bismuth shielding in CT: support for use in children. Pediatr Radiol. 2010;40:1739–1743.
42. Geleijns, J, Wang, J, McCollough, C. The use of breast shielding for dose reduction in pediatric CT: arguments against the proposition. Pediatr Radiol. 2010;40(11):1744–1747.
43. Frush, DP. Radiation protection in children undergoing medical imaging. In: Daldrup-Link HE, Gooding CA, eds. Essentials of pediatric radiology. Cambridge: Cambridge University Press, 2010.
44. Goske, MJ, Applegate, KE, Frush, DP, et al. The Image Gently campaign: working together to change practice. Am J Roentgenol. 2008;190(2):273–274.
45. Goske, MJ, Applegate, KE, Frush, DP, et al. Image Gently: progress and challenges in CT education and advocacy. Pediatr Radiol. 2011;41:461–466.
46. Sistrom, CL, Dang, PA, Weilburg, JB, et al. Effect of computerized order entry with integrated decision support on the growth of outpatient procedure volumes: seven-year time series. Radiology. 2009;251:147–155.
47. Hampton, T. Radiation oncology organization, FDA announces radiation safety initiatives. JAMA. 2010;303(13):1239–1240.
48. Rehani, M, Frush, DP. Tracking radiation exposure of patients. Lancet. 2010;376:754–755.
Addendum
As we probe more deeply into the effects of radiation, it is important to use precise definitions. Some of the most pertinent terms are provided in this glossary.*
Absolute risk: The risk of an adverse effect that is independent of other causes of that same health effect.
Absorbed dose (D): The energy imparted to matter per unit of mass by ionizing radiation at a specific point. The Systeme Internationale (SI) unit of absorbed dose is joules per kilogram (J/kg). The special name for this unit is the gray (Gy). The previously used special unit of absorbed dose, the rad, was defined as being an energy absorption dose of 100 erg/g; 1 Gy = 100 rad.
ALARA (as low as reasonably achievable): The principle of limiting the radiation dose administered to exposed individuals to levels as low as are reasonably achievable, taking into account economic and social factors.
Background radiation: The radiation in the natural environment, including cosmic rays and radiation from the naturally radioactive elements, found outside and inside the bodies of humans and animals. Background radiation also is called natural radiation. The term also may mean radiation that is unrelated to a specific experiment.
CTDI (computed tomography dose index): A measure of radiation dose obtained using dosimeters in a standard phantom (e.g., 32- or 16-cm acrylic cylinder).
Deterministic effect: An effect with a threshold, the severity of which increases as the dose increases.
Effective dose: The radiation dose, allowing for the fact that some types of radiation are more damaging than are others and some parts of the body are more sensitive to radiation than are others. It is defined as the sum, over specified tissues, of the products of the equivalent doses in the tissues and the weighting factors for the tissues.
Exposure: Exposure is used more often in its more general sense and not as the specially defined radiation quantity. It is a measure of the quantity of x-radiation or gamma radiation based on its ability to ionize air through which it passes. The previously used special unit of exposure, the roentgen (R), has been replaced with the SI unit of exposure, coulombs per kilogram (C/kg); 1 R = 2.58 × 10−4 C/kg (exactly). The physical quantity exposure may be replaced by the quantity air kerma in air, especially for calibration of monitoring instruments: 1 R = 10 mGy air kerma.
Free radical: A fragment of an atom or molecule that contains an unpaired electron in the outer shell and is very reactive.
Gamma rays: High-energy, short-wavelength electromagnetic radiation (γ). Gamma radiation frequently accompanies α and β emissions and always accompanies fission. Gamma rays are very penetrating and are best stopped or shielded against by dense materials, such as lead or depleted uranium. Gamma rays are indistinguishable from x-rays except for their source: gamma rays originate inside the nucleus, and x-rays originate from outside.
Gray (Gy): The special name for the SI unit of absorbed dose (kerma) and specific energy imparted equal to 1 J/kg. The previous unit of absorbed dose, the rad, has been replaced by the gray: 1 Gy = 100 rad.
Hereditary effects of radiation: Radiation effects that can be transferred from parent to offspring; any changes in the genetic material of sex cells caused by radiation.
Kerma (kinetic energy released per unit mass): The sum of the initial kinetic energies of all the charged ionizing particles liberated by uncharged ionizing particles per unit mass of a specified material. Kerma is measured in the same unit as absorbed dose. The SI unit of kerma is joules per kilogram (J/kg), and its special name is the gray (Gy). Kerma can be quoted for any specified material at a point in free space or in an absorbing medium.
Lifetime risk: The risk of dying of some particular cause over the entire course of a person’s life.
Linear no threshold (LNT): The theory that no level of radiation exposure can be assumed to be absolutely safe.
Rad: The old unit of absorbed dose, equivalent to an energy absorption of 100 erg/g. Superseded by the gray (see absorbed dose).
Relative risk: The situation in which the risk of a disease resulting from some injury is expressed as some percentage increase of the spontaneous rate of occurrence of that disease. Relative risk is in contrast to an absolute risk, in which the risk of a disease resulting from an injury does not depend on the normal rate of occurrence of that disease.
Rem: Old unit of equivalent or effective dose. It is the product of absorbed dose (in rads), the radiation weighting factor, and the tissue weighting factor; 1 rem = 0.01 Sv.
Roentgen (R): A unit of exposure to ionizing radiation named after Wilhelm Röntgen, the German scientist who discovered x-rays in 1895. It is that amount of γ rays or x-rays required to produce ions carrying one electrostatic unit of electric charge (either positive or negative) in 1 cm3 of dry air under standard conditions.
Sievert (Sv): Unit of equivalent dose or effective dose: 1 Sv = 100 rem.
Stochastic effect: The effect, the probability of which, rather than its severity, is a function of radiation dose without threshold. (More generally, stochastic means random in nature.)