Chapter 60 Rabies
Rabies has terrorized humanity since the dawn of civilization, and the menace continues. In industrialized nations where human rabies is rare, animal rabies abounds and humans are protected from infection only by vigorous animal vaccination programs, elimination of stray animals, and postexposure immunization. In developing countries, tens of thousands die each year, and over ten million endure agonizing anxiety following exposure to a possibly rabid animal.217 In the United States, approximately 23,000 persons receive postexposure prophylaxis each year.70,179 An encounter with this uniformly fatal infection, globally the most common form of viral encephalitis, leaves “a more indelible stamp of horror” than does any other disease.138
Current Status
Globally, rabies is the tenth most frequent cause of death from infectious disease.106 The actual number of deaths is unknown because reporting in the developing countries where this infection is common is unreliable. In Tanzania, the estimated incidence of human rabies mortality was 1499 a year (95% confidence interval 891 to 2238), but the average number of deaths officially recorded was 10.8.73 Most of these countries do not have laboratory facilities capable of establishing a dependable diagnosis.68 The World Health Organization (WHO) currently estimates the number of rabies deaths globally at 40,000 to 70,000 a year, although the median number of 55,000 deaths is widely accepted. That is an average of approximately one death every ten minutes.221
Some, perhaps many, human rabies infections are not diagnosed, even in nations with sophisticated medical systems. This problem was vividly dramatized in 2004 by the rabies deaths of four U.S. organ transplant recipients from a donor whose rabies infection had not been recognized.59,64 Only a few months later, three organ transplant recipients in Germany died of rabies and three others with liver and corneal transplants required postexposure prophylaxis.119 A review has suggested that rabies may be underdiagnosed in the United States because physicians see it so infrequently they do not include it in their differential diagnoses.217
In addition to being undiagnosed, rabies is probably incorrectly diagnosed with considerable frequency. Of 33,000 human rabies deaths reported worldwide in 1997, laboratory confirmation was available for less than 0.5%.68
The Rabies Virus
Rabies viruses belong to the group Rhabdoviridae, genus Lyssavirus. Seven genotypes are recognized, but genotype 1 is the only one of major significance. This genotype consists of multiple variants or lineages, each closely linked to a single mammal species such as raccoons, skunks, foxes, mongooses, or various bat species. In the 1980s these variants were distinguished with monoclonal antibodies. Subsequently, analysis of nucleotide substitutions in the rabies genome after reverse transcriptase—polymerase chain reaction (RT-PCR) amplification of the viral RNA has allowed identification of the primary reservoirs for each variant, mapping the geographic distribution of variants, and identification of virus spillover into animals and humans.68,191 In addition, for the past 30 years, the source of many human infections has been identifiable in the absence of a recognizable exposure to rabid animals.
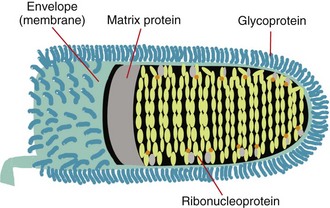
The rabies virus is bullet-shaped with one flat end and contains a single strand of RNA, which is made up of approximately 12,000 nucleotides and encodes five proteins. Three of these, the nucleoprotein (N), phosphoprotein (P, M1, or NS), and the large polymerase or transcriptase protein (L), make up the core of the virus. The other two, the matrix protein (M) and the transmembrane glycoprotein (G), form its coat.230
The external surface is studded with perpendicular aggregates of glycoprotein, the G protein that recognizes cell surface receptors and facilitates virus entry into cells.15 The 505-amino-acid G protein is composed of a 44-amino-acid internal or “cytoplasmic” portion, a 22-amino-acid hydrophobic transmembrane portion, and the large external “antigenic” portion.160,231 The complete amino acid sequences of these proteins have been determined for several fixed rabies strains.219
A lipid bilayer is closely associated with the matrix protein, and the two form a clearly defined shell for the virus. The M protein is the smallest of the rabies virus structural proteins, with only 202 amino acids,232 but makes up approximately 25% of the total virion protein. It is in close contact with the core and also interacts with the internal segment (cytoplasmic tail) of the surface G protein.
The core of the virus forms a tightly structured helix of 30 to 35 coils that extends end-to-end within the virion. The RNA genome is associated with about 1800 closely packed molecules of the 55 kDa nucleoprotein, which together are known as ribonucleoprotein (RNP). The N protein protects the genome from digestion and keeps it in a suitable configuration for transcription. Some 30 to 60 copies of the large (≈190 kDa) transcriptase-associated L protein and about 950 copies of the smaller (≈38 kDa) P or phosphoprotein are responsible for replication of the viral RNA232 (Figure 60-1).
Rabies in the United States
Incidence in Humans
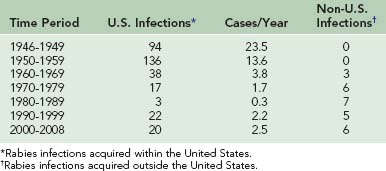
The incidence of human rabies in the United States fell dramatically from 23.5 infections per year in the late 1940s to 1.0 infection per year during the 1980s (Table 60-1). However, 27 infections were reported from 1990 through 1999, and 26 have been reported from 2000 to 2002. From 1980 to 1989, seven infections were acquired outside the United States. From 1990 to 1999, five infections were acquired outside the United States, and from 2000 to 2008, six infections were acquired in other countries.* Of the human rabies infections acquired in the United States, one originated from a skunk (1981) and two originated from dogs (1991 and 1994). The two dog infections were associated with the epizootic in coyotes that developed when rabies spread across the Rio Grande River from unvaccinated dogs in Mexico. The remaining 42 infections originated from bats. Of the 18 human rabies infections acquired outside the United States during these 28 years, 17 originated from dogs. Only an infection in 2008 in California was determined to have originated from a Mexican free-tailed bat34 (Table 60-2).
TABLE 60-2 Rabies Deaths in the United States Since 1980 From Infections Contracted Outside the Country
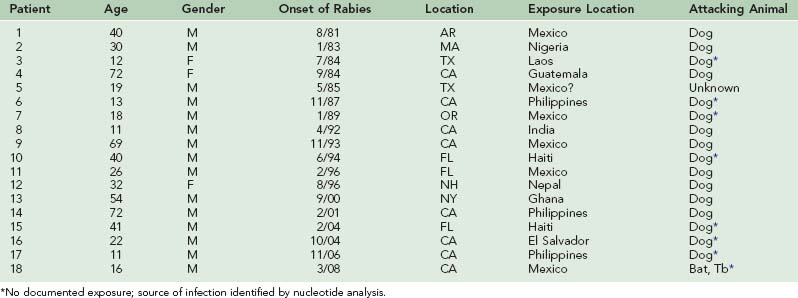
Although reliable rabies vaccines and antisera first became available during this 62-year period, extensive vaccination of domestic animals, particularly household pets, and elimination of unrestrained and stray animals are considered primarily responsible for the decline in the human infection rate.53,54,151 Such programs reduced the incidence of laboratory-confirmed rabies in dogs from 6949 in 1947 to 75 in 2008.30 The annual cost for these programs is over $300 million, most of which is borne by pet owners.192
Rabies in Wild Terrestrial Animals
In the United States and Canada, a vast reservoir of rabies persists in wild animals.192 During 2008, 49 states and Puerto Rico reported 6841 rabies infections in animals, approximately 93% in wild animals and 7% in domestic animals. However, some states accept only animals responsible for a human or domestic animal incident for rabies testing; others test all submitted specimens. Furthermore, the number of rabid wild animals that die without being detected is estimated to be more than 90% of the total, so the identified infections represent only a small fraction of wild animal rabies.125
Several major rabies epizootics are currently recognized. An epizootic of rabies started in Arctic foxes in northern Canada in the late 1940s and early 1950s and swept southward in the middle and late 1950s to involve red foxes in Alberta, Saskatchewan, Manitoba, Ontario, and Quebec. The epizootic crossed the St Lawrence River in 1961, where it involved red foxes in upper New York State, although currently it appears to be limited to the adjacent Canadian provinces, which are experiencing a lower incidence of fox rabies.11,28,127 The epizootic, which moved westward to involve arctic foxes in Alaska and the Northwest Territories,190 surrounds the North Pole and may cover the largest land area of any observed outbreak28 (Figure 60-2).
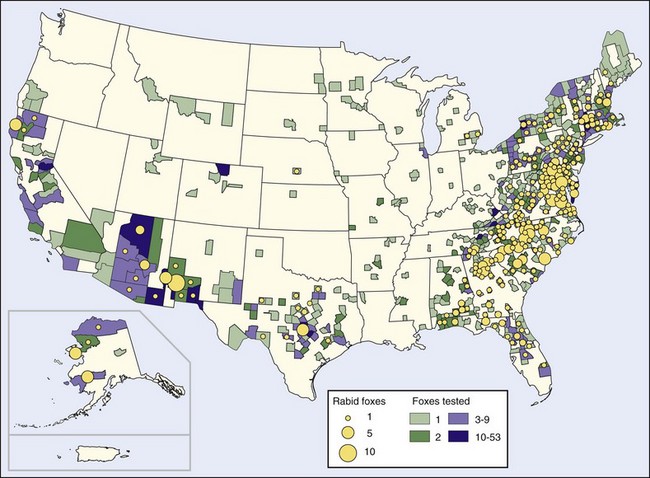
FIGURE 60-2 Rabid foxes reported in the United States in 2008.
(http://www.cdc.gov/rabies/resources/publications/2008-surveillance/foxes.html.)
An outbreak of raccoon rabies started in central Florida in the 1940s and spread at the rate of about 40 km (25 miles) per year, reaching Georgia in the early 1960s and Alabama and South Carolina in the 1970s. In the late 1970s a second outbreak appeared on the Virginia–West Virginia border. That epizootic has now spread north to all of New England; in 1999 it crossed into Canada. It has also spread south to join with the epizootic coming north from Florida in North Carolina.46,68,69 The second outbreak developed when raccoons were translocated from Florida for restocking for hunters. Although the animal suppliers held legal permits and health certificates, inclusion of some rabid animals among the more than 3500 transported raccoons has been documented.118,229
As of 2002 more than 50,000 rabies infections in raccoons had been reported in the United States since 1975. The land mass affected by this epidemic is approximately 1 million km2 (383,000 miles2) and includes the residences of 35% of the United States human population (Figure 60-3). The raccoon epizootic is considered particularly threatening because many raccoons live in densely populated urban and suburban areas.68 However, the only known human rabies infection resulting from this epizootic occurred in 2003.47 The spread of rabies from raccoons to humans appears to have been limited in part because raccoons are large animals and their bites are obvious. To some extent, well-vaccinated dogs and other pets form a barrier between wild animals and humans. Perhaps of greatest significance is the nonaggressive behavior of rabid raccoons. In 38 rabid raccoon incidents in Florida over a 5-year period, bites were inflicted only when humans or dogs tried to kill or capture raccoons that seemed tame.226
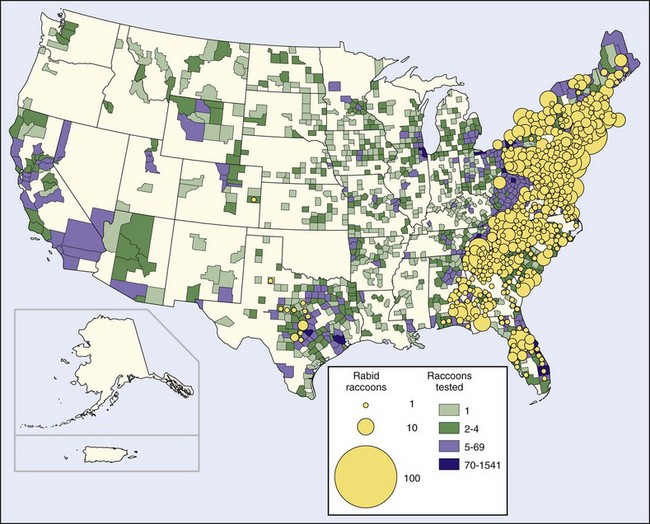
FIGURE 60-3 Rabid raccoons reported in the United States in 2008.
(http://www.cdc.gov/rabies/resources/publications/2008-surveillance/raccoons.html.)
Before the raccoon epizootic, most terrestrial rabies in the United States was in skunks. Human rabies resulting from exposure to a spotted skunk in California was reported in 1826.67 Four epizootics are recognized, one of which is in the province of Quebec, Canada, and New York state and is associated with the fox epizootic in that area. A larger epizootic started in Iowa in 1955. It has spread east to Ohio, west to Montana, north to the Canadian provinces of Manitoba (1959), Saskatchewan (1963), and Alberta (1971), and south to meet with a third epizootic that originated in Texas and has spread to surrounding South Central states, particularly Oklahoma and Arkansas. The fourth epizootic in skunks is located in northern California67 (Figure 60-4).
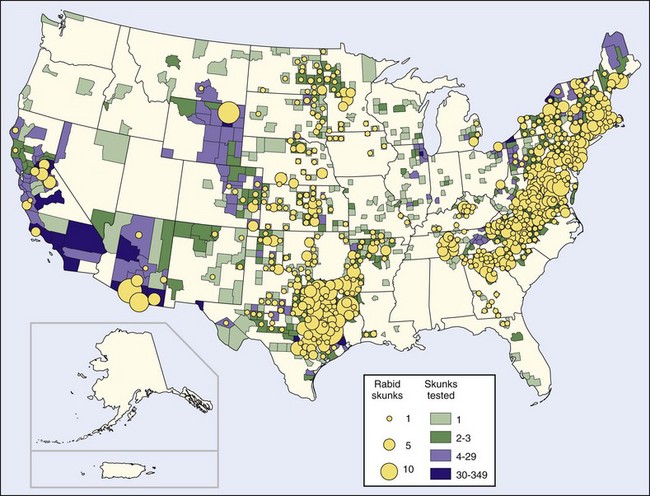
FIGURE 60-4 Rabid skunks reported in the United States in 2008.
(http://www.cdc.gov/rabies/resources/publications/2008-surveillance/skunks.html.)
An increase in the number of rabid skunks in the East Coast states has recently occurred, but analysis of these infections indicates they result from raccoon rabies spilling over into skunks and are not indicative of a separate skunk epizootic.103
Screening of rabies virus isolates from the epizootics has disclosed five distinctive patterns. Red foxes and skunks in New York and adjacent Canada present one pattern; raccoons from the Atlantic states present a second. The skunks in the south-central states present a third, and a fourth is represented only by a small outbreak in gray foxes in Arizona. However, the fifth pattern is found in skunks from the north-central states and from California, in dogs from the Mexico border states, and in a small rabies outbreak in gray foxes in Central Texas189 (Figure 60-5).
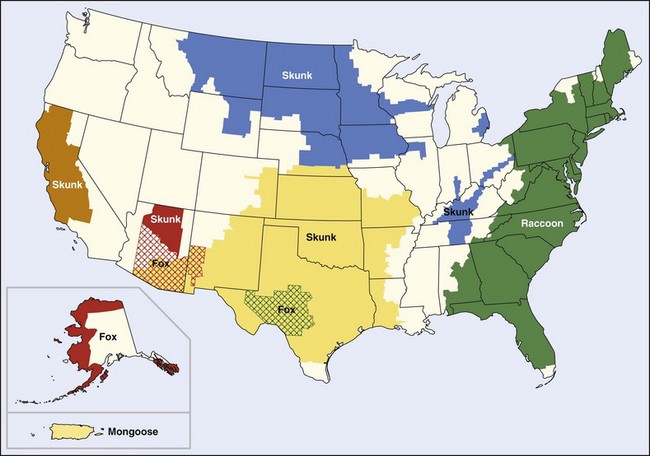
FIGURE 60-5 Terrestrial rabies reservoirs in the United States in 2008.
(http://www.cdc.gov/rabies/location/usa/surveillance/wild_animals.html.)
Rabies in rodents is an intriguing problem. Rodents are the animals of choice for rabies virus isolation in the laboratory; yet rabies in small free-living rodents is rare. One explanation is that rodents may usually be killed rather than simply infected by the bites of rabid animals, but rodents are carrion eaters and can be infected by that source as well. In recent years the largest number of rodent rabies infections has been in large rodents such as woodchucks that have been infected by rabid raccoons. Rabid beavers have attacked and bitten humans in North Carolina. However, no transmission of rabies to humans by rodents has been documented.200
Rabies in Bats
With the exception of Antarctica, rabies in bats is global. In Canada, the United States, parts of South America, Western Europe, and Australia (where rabies in carnivores, particularly dogs and foxes, has been controlled), bats are the most prominent source of human rabies.148
Rabies was diagnosed in insectivorous bats in Brazil in the 1920s and in frugivorous bats in Trinidad during the 1930s, although the principal subject of these studies was rabies in hematophagous (“vampire”) bats that was being transmitted to humans. The first definitive diagnosis of rabies in nonhematophagous bats was made in a frugivorous bat that flew into a “chemist’s” shop in Port of Spain, Trinidad, on September 10, 1931. However, the incident that drew widespread attention to bat rabies occurred in Tampa, Florida, on June 23, 1953. The 7-year-old son of a ranch hand was looking for a baseball near some shrubbery when a lactating female yellow bat suddenly flew out of the bushes and bit the boy on the chest, remaining firmly attached until knocked off by the boy’s mother. The ranch owner had heard of rabies in vampire bats in Mexico and insisted the bat be examined for infection. Negri bodies were found in smears of the brain, and the diagnosis was confirmed by mouse inoculation of brain tissue. The boy was given postexposure treatment and did not develop an infection.17,69,219
The publicity given this event led to many more bats being submitted for rabies examination. Subsequently, rabies has been found in bats in every state except Hawaii, as well as in eight Canadian provinces.17,165 In 2008, bat rabies was reported from all 48 of the continental states and the District of Columbia54 (Figure 60-6). The estimated incidence of rabies in bats in the United States is 0.5% to 1.0%; the incidence in bats that appear ill or injured is much higher, 7% to 50%.17,201
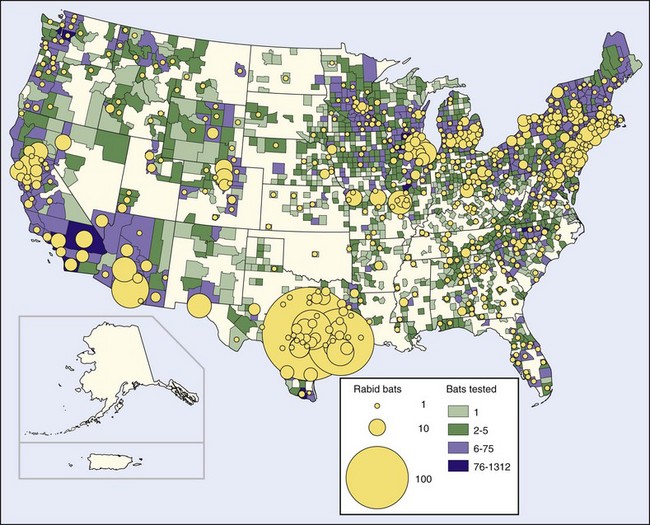
FIGURE 60-6 Rabid bats reported in the United States in 2008.
(http://www.cdc.gov/rabies/resources/publications/2008-surveillance/bats.html.)
Rabies virus variants from bats are species specific rather than geographically specific189 and are distinctly different from those of terrestrial animals in the same locations, including the major terrestrial epizootics. Clearly, little exchange of infection between bats and terrestrial animals takes place, although occasional animals infected with rabies virus strains typical of bats are found. Many large areas of the United States, particularly the Pacific Northwest and New England (prior to the raccoon epizootic), report rabies in bats but in no other species. Even though cats and foxes catch and eat bats, only 3 of 136 cat and fox rabies isolates over a 2-year period were antigenically similar to bat rabies strains.17,79,189
Approximately 70% of human rabies infections and 75% of cryptic rabies deaths in the United States have been caused by the variant associated with silver-haired and the eastern pipistrelle bats, which are reclusive animals rarely found around human habitation. Infections by variants associated with bats that frequent human dwellings are much less common. Infections in other animals by this variant are also disproportionately very high. These bats are small and their bites are difficult to detect. However, in comparison with other rabies virus variants, the variant associated with these two bats replicates better in fibroblasts and epithelial cells, and replicates better at the low temperature of 34° C (93.2° F). These features indicate this variant is better able to replicate in the peripheral tissues involved by most bites.140
Rabies in Domestic Animals
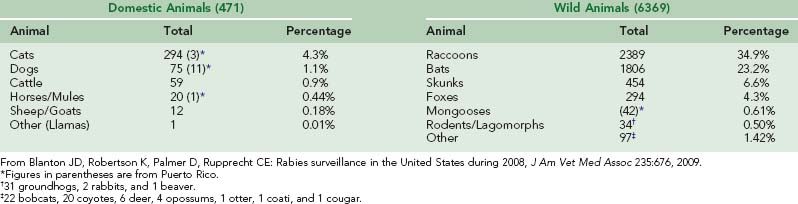
Since rabies in dogs has been controlled, rabies infections in cats have outnumbered infections in dogs (295 to 75 in 2008).30 A major problem in vaccinating cats is establishing ownership. Farmers value cats for rodent control but do not recognize them as property. Cats wander from farm to farm and contact wild animals with rabies. Capturing feral cats so they can be vaccinated is difficult.42 Rabies is not rare in other domestic animals, including cattle, horses, mules, sheep, and goats (Table 60-4).
Sources of Human Infection
In the late 1940s and 1950s most human rabies in the United States resulted from bites by dogs or cats. Of 146 infections in the years 1946 to 1961 for which a source of exposure could be identified, dogs were responsible for 120 and cats for 9 (88.4%). Foxes (7), skunks (5), and bats (5) were responsible for the rest.53 However, after rabies in domestic animals was controlled, human rabies resulting from bites by pets disappeared. Since 1966, all but 2 of the 19 human rabies infections resulting from exposures to rabid dogs were acquired outside of the United States.53,62,151
Before 1965, the CDC had recorded no human rabies occurring within the United States that had been acquired outside the country.151 However, since then the number of infections acquired outside the United States has been significant: 3 of 15 (20%) between 1965 and 1970, 6 of 23 (26%) in the 1970s, 7 of 10 (70%) in the 1980s, and 18 of the 63 cases (29%) since 1980. Lack of knowledge about the risk for rabies in developing countries has led some travelers to disregard animal encounters and not obtain rabies immunoprophylaxis, but some of these infections have been in children who did not inform their parents of the animal contact.
Until the 1980s, identifying the source of a number of human rabies infections in the United States was impossible. For many infected persons no animal exposure incident—even an opportunity for animal exposure—could be identified. An infectious source could not be found for 84 of 230 (35%) human rabies infections occurring in the United States between 1946 and 1961,151 or for 6 of 38 (16%) human infections between 1960 and 1970.52
Only since the 1980s has monoclonal antibody typing or RT-PCR nucleotide analysis allowed the source of human rabies infections to be determined when no animal exposure incident could be identified.17,79,189 However, such studies have made it unmistakably clear that bats are now the major source of human rabies in the United States.
Of the 45 human rabies infections acquired within the United States since 1980, 42 are attributed to bats. A study of human rabies of bat origin in the United States and Canada from 1950 through 2007 identified 56 infections, of which 22 (39%) reported a bite, 9 (16%) had a direct contact but no bite, 6 (11%) found one or more bats in their homes (two in the room in which they slept), and 19 (34%) had no history of a bat contact.76
How the infection is transmitted has been uncertain. In 1956 and 1959, two men died of rabies after exploring Frio Cave near Uvalde, Texas. The walls and ceiling of that cave hold astonishing numbers of bats—300 to 400 per square foot. Neither man had been bitten, and the infections were attributed to aerosol transmission of the rabies virus. Subsequently, when experimental animals of various species were placed in the cave in cages that only allowed the virus to be transmitted as an aerosol, a significant percentage developed rabies.74,75 Additionally, aerosol transmission of rabies to humans has occurred at least twice in laboratories.24,228 The CDC recommends rabies vaccination for spelunkers.56
However, nursery caves such as Frio Cave contain an astounding number of bats. Saliva and urine constantly rain down on anyone entering the cave, and the blanket of guano on the floor ranges from several inches to several feet in thickness. In Frio Cave air circulation is so poor that the bats warm the cave, the air is humidified by their respiration, and the concentration of ammonia from their urine is so high that the cave usually cannot be entered without respirators.74 Similar infections in other caves have not been reported.
Unrecognized bites appear to be the source of infection for most individuals who have had no recognized bat encounters. Bat teeth are so small and sharp that a bite may not be felt. Even the recognized bites are not particularly painful, although at least one of the individuals known to have been bitten was intoxicated with ethanol at the time.98 For centuries, South American vampire bats have been reported to bite sleeping victims without awakening them.
Limiting human rabies of bat origin is best addressed by informing the public of the risk.165 Reducing the bat population is not an acceptable approach. Significant population reduction would be difficult and, if achieved, would be an ecologic disaster because bats play such a major role in insect control (Table 60-3).
The CDC and other institutions now advocate the following measures:
Rabies in Other Countries
Epidemiology
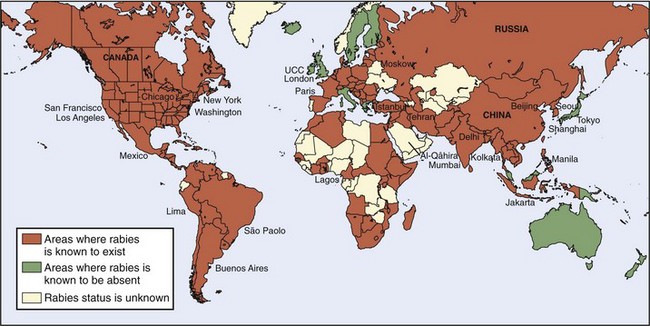
Rabies is found throughout the world, and although more common in tropical or temperate climates, is by no means limited to those areas. Arctic foxes with rabies have been found in Alaska, Northern Canada, Greenland, Norway’s Svalbard Islands, and much of Siberia. An epizootic in the Thule district of Greenland in 1958 and 1959 resulted in death from rabies of 50% of the sled dogs in that area. More than 1000 dogs in the Egedesminde district died in another epizootic in 1959 and 1960. For reasons that are not known, transmission of rabies to humans is rare in these areas, even though exposures are common.75 Perhaps the rabies variant is more infective for foxes and dogs.
Rabies is not found in a few areas of the world, all of which are landmasses or peninsulas isolated by water. Rabies does not occur in Hawaii (the only state in the United States in which rabies is not found), some of the Caribbean islands, Pacific Oceania (including Australia [although Australian bat lyssavirus is found there] and New Zealand), or Antartica.101,147 The two human genotype 1 rabies infections that have occurred in Australia are thought to have been acquired outside that country24,58,101 (Figure 60-7).
(Modified from http://www.nathnac.org/pro/factsjeets/rabies.htm, and http://www.ktl.fi/portal/sip,o/julkaisut/oppaat_ja_kirjat/matkailijan_terveysopas_old/4_matkailijoiden_rokotukset/1415.)
Great Britain had been free of rabies since an extensive dog confinement and vaccination program in 1903, although concern about reintroduction of rabies was raised by the Channel Tunnel and the reduction of border controls between members of the European Community.69,202 A 55-year-old Scotsman with a fatal infection in 2002 was the first locally acquired lyssavirus infection on that island in 100 years, but the virus was not of genotype 1.94
Rabies has been endemic in Japan since the 10th century. However, following World War II, members of the U.S. Army Veterinary Corps determined that no reservoir of rabies existed in the wild animal populations of Japan, Taiwan, and the Philippines—perhaps in part because wild animals were hunted for food during the war. Extensive campaigns to eliminate stray dogs (which in some areas of Japan reduced the canine population by 70% to 80%) and to vaccinate those remaining succeeded in eradicating the infection from Japan and Taiwan. Endogenously acquired rabies has not occurred in those islands since the late 1950s.5,191
The success of canine rabies eradication programs depends on the society in which the programs are initiated. Such programs achieve little success in nations that are predominantly Hindu or Buddhist, because the people do not support elimination of animals that have no apparent owner. They often put out food for stray dogs. In contrast, Malaysia, a peninsula that is predominantly Muslim, has been largely free of rabies since the early 1950s.25
Elimination of stray dogs must be combined with vaccination programs. Dogs that are eliminated because they cannot be associated with human ownership are quickly replaced. The annual turnover of the dog population in developing countries has been found to range between 30% and 40%.31
Vaccination of domestic animals for rabies is limited largely to industrialized nations. In many developing countries, vaccination of animals is considered unaffordable and rabies control resources are expended on postexposure immunoprophylaxis of humans. Even though rabies immunoprophylaxis is administered to 800 to 900 persons per million inhabitants annually in such countries, the human death rate from rabies is still high, an average of nearly five deaths for each 1 million population annually.32 In the United States that death rate would result in approximately 1500 rabies deaths a year.
In the 1990s the Institute’s postexposure rabies clinic treated about 18,000 patients with new animal bites each year—an average of almost 50 new patients a day! Furthermore, these patients were only 28% of the estimated 64,000 Thais who receive postexposure therapy annually, many of whom were residents of rural or remote portions of the country and were treated by local physicians.224 However, the number of human deaths from rabies in Thailand declined from about 400 a year in the 1970s to 70 in 1999, even though dog rabies has not been controlled.68
Other developing nations have similar rabies problems. WHO agencies have estimated that 87 countries and territories with a total population of about 2.4 billion people are afflicted with endemic canine rabies.32
Sources of Human Infection
Although domestic animals are rarely the sources of human rabies in the United States and other developed countries, in developing countries the vast majority of human rabies—99% by some estimates—is the result of exposure to rabid dogs.33,69,213,219 In Thailand, although rabies has been found in an array of exotic tropical animals, including tigers and leopards, between 1979 and 1985, 90.6% of human infections resulted from dog bites and an additional 6% followed unknown events. The remaining 3.6% followed cat attacks.181
Other animals, particularly bats, transmit rabies. Hematophagous, or “vampire,” bats are a major source of animal and human rabies in South and Central America, the only areas where such bats are found. Their range extends between northern Mexico and northern Argentina—basically between the tropics of Cancer and Capricorn—and fossils indicate vampire bats have inhabited those areas for 2.5 million years.93 These animals consume 20 to 25 mL of blood at a feeding, and although cattle are their preferred food source, a study in Colima, Mexico, found human blood in the stomachs of 15.7% of 70 vampire bats.13
Human rabies of vampire bat origin was first recognized in 1929 in Trinidad when Negri bodies were found in the brains of 17 individuals, mostly school-age children, who died with acute ascending paralysis. Subsequently, small epidemics have been recognized almost every year in that country. Interestingly, almost all of the rabies transmitted by vampire bats in Latin America is paralytic in type rather than furious.215
Human rabies resulting from vampire bat bites has been reported almost every year from Mexico, but was first reported from South America in 1953 when 9 of 43 diamond miners who slept outdoors died of a mysterious illness. Autopsies of five of the miners disclosed rabies. In an outbreak in two rural communities in the Amazon Jungle of Peru during the first four months of 1990, 29 of 636 residents (4.6%) died after a rapidly progressive illness characterized by hydrophobia, fever, and headache. Rabies virus was isolated from the brain of the only individual on whom necropsy was possible. Of the 29 victims, 96% had a history of bat bites, although bats also had bitten 22% of unaffected community members.6
Human infection is not the only major problem resulting from rabies transmitted by vampire bats in Central and South America. Migrating epizootics of vampire-bat–transmitted bovine paralytic rabies kill thousands of animals annually; the estimated cost in 1980 was $500 million.6,68 Efforts to control these epizootics have included vaccination of cattle and attempts to limit the vampire bat population by administering anticoagulants, usually warfarin.
It is interesting to note that meat is often consumed from cattle slaughtered at the first, virtually pathognomic sign of disease, paralysis of the hindquarters. Even normal appearing animals may have infected brains. Four of 1000 (0.4%) apparently healthy cattle selected at random at the Mexico City slaughterhouse of Ferrería were found to be infected by rabies when investigated with fluorescent antibody staining and animal inoculation of brain tissue. However, no cases of human rabies from this source have been reported.13 Human rabies has resulted from consumption of brain tissue from a rabid dog and a rabid cat in Vietnam.218
Mongooses are the major source of rabies in South Africa and in some Caribbean Islands such as Puerto Rico.14,88 The yellow mongoose is the main reservoir of rabies in South Africa.219 The small Indian mongoose, imported many years ago in an effort to control rodents,107 is an important reservoir and vector of rabies in Cuba, the Dominican Republic, Grenada, Haiti, and Puerto Rico.219 In Grenada, from 1968 to 1984, mongooses accounted for 787 (73%) of 1078 cases of animal rabies on the island. Of 208 human exposures requiring antirabies therapy, mongooses were responsible for 119 (57%). The possibility of eliminating the animals by hunting or trapping appears remote in view of the island’s topography and the animal’s skill at adapting to its surroundings. However, mongooses take oral baits, so oral vaccination appears feasible if an appropriate vaccine can be identified.133 About 20% to 40% of mongooses have naturally acquired antirabies antibodies, possibly from having survived infection.82
At the Canadian International Water and Energy Consultants (CIWEC) Clinic in Kathmandu, Nepal, 51 travelers required immunoprophylaxis following rabies exposure during a 2-year period. Although 36 of these encounters were with dogs, 10 were with monkeys at Swayambhunath, the “Monkey Temple,” a Buddhist shrine popular with tourists. The bites were inflicted when monkeys leaped for food carried by visitors.185
Human-to-human transmission of rabies is rare. Eight documented infections were in individuals who received corneal transplants (two from the same person) from individuals whose neuroparalytic disorder was not recognized as rabies.57,92,111 In 1996, Fekadu and colleagues reported two apparent instances of human-to-human rabies transmission in Ethiopia. A 41-year-old woman, who died of rabies 33 days after her 5-year-old son died of the same infection, had been bitten on a finger by her son. Another 5-year-old boy, who developed rabies 33 days after his mother died of that infection, had been repeatedly kissed on his mouth by his mother, apparently passing infected saliva to him. However, these infections were not confirmed by laboratory studies.90
The donor’s lungs were transplanted to a male who died of intraoperative complications. The liver and one kidney were transplanted to males, and one kidney was transplanted to a female, all of whom died of rabies 27, 37, and 39 days later. The fourth victim had a liver transplant from another donor, but a segment of iliac artery from the first (rabid) donor was inserted during the procedure. This recipient died of rabies approximately one month after the transplant.59,64
In the same year, three German individuals who had received a lung, a kidney, and a kidney/pancreas transplant from the same individual died of rabies. When the cause of their deaths was recognized, three additional transplant recipients who had received a liver and two corneas were given postexposure prophylaxis and survived. The corneas were removed. The donor had been scratched by a dog while visiting India and had not received postexposure therapy. The nature of her disease was not recognized until the three transplant recipients died.119
One case of human rabies appears attributable to transplacental infection. However, a number of mothers dying of rabies encephalitis have given birth to healthy babies, presumably because the virus travels through nerves—viremia has never been documented—and cannot reach the placenta or fetus.91,111
Features of Human Rabies
Mortality
Rabies in humans, once it has become clinically apparent, is uniformly fatal. No other infection is so lethal or progresses so rapidly. In the 1970s intensive support allowed three humans with clinical rabies to survive.61,105,159 Three rabies survivors have been reported subsequently. The first five infections were vaccination failures, and four of the five survivors had severe residual neurologic deficits, severe enough to be fatal 34 months later in one person.2,136 The rabies virus was not cultured from any of these patients and at least one may have had a reaction to neural-derived vaccine rather than an actual infection.117
Coma was induced with ketamine and midazolam, she was intubated and maintained on a ventilator, and received intravenous ribavirin and amantadine. Ketamine is a dissociative anesthetic, but it is also an N-methyl-D-aspartate (NMDA) antagonist, and the NMDA receptor has been speculated to be one of the rabies virus receptors. Later she also was given benzodiazepines and supplemental barbiturates. She recovered slowly, was removed from isolation on the 31st day, and was discharged from the hospital on the 76th day. Attempts to isolate the rabies virus, detect viral antigens, or identify rabies RNA in two skin biopsies and nine salivary specimens were uniformly unsuccessful. Five months after her initial hospitalization, she was alert and communicative, but had choreoathetosis, dysarthria, and an unsteady gait. Twenty-five months after hospitalization, she continued to have fluctuating dysarthria and gait difficulties, as well as an intermittent sensation of cold feet. She had no difficulties with activities of daily living, including driving. In high school, she took college-level courses in English, physics, and calculus, scored above average on a national college achievement test, graduated in 2007, and planned to attend a local college. She had no problems with peer relations or mood disorders.62,113,227
At the time, hope was held that this therapy might be a breakthrough in the treatment of clinical rabies. However, a number of individuals have subsequently been treated by the same protocol, and none survived.29,110,136
Testing for evidence of 30 other causative agents of encephalitis or aseptic meningitis was uniformly negative. Her illness has been classified as presumptive abortive human rabies.60 Other than the 15-year-old girl from Wisconsin and the 17-year-old from Texas who received only a single dose of vaccine, no person who has not been vaccinated has survived clinically evident rabies. (Subclinical human infections probably occur, as discussed later.) The clinical phase of rabies encephalitis rarely lasts more than a few days to a few weeks, and infected persons are severely incapacitated.3,92 The catastrophe of rabies is compounded by the young ages of many of its victims; 40% to 50% are 15 years old or younger.92
Incubation Period
For many years, some human rabies infections have been thought to follow prolonged incubation periods. In 1987, a 13-year-old boy who had immigrated from the Philippine Islands 6 years earlier died with rabies determined by nucleotide analysis to be of Philippine dog origin. He had not been out of the United States since he arrived.49 The second documented Australian rabies patient, a 10-year-old girl of Vietnamese origin, had experienced no identifiable animal contact since she had left North Vietnam 6 years and 4 months earlier. She had spent some time in Hong Kong, and the virus responsible for her death was of an immunotype found in China, although the brain tissue was partially decomposed and nucleotide sequencing was limited.24,101,120 Joshi and Regmi have reported an individual who had an apparent incubation period of 1100 days (3 years).123 The first documented patient with rabies reported in Australia, a 10-year-old boy who died in 1987, probably resulted from a monkey bite inflicted in northern India 16 months earlier.58 An 18-year-old Mexican man who died in Oregon in 1989 was infected with rabies virus of a strain to which he could not have been exposed for at least 10 months, although no history of any type of exposure could be obtained.52 Even longer incubation periods of 10 years and 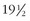 years have been reported, but these occurred in areas where rabies is endemic and a second exposure in the intervening period could not be ruled out.189
years have been reported, but these occurred in areas where rabies is endemic and a second exposure in the intervening period could not be ruled out.189
Confirmation of such prolonged incubation periods was achieved in three immigrants into the United States from the Philippines, Laos, and Mexico. Nucleotide analysis disclosed rabies viral amino acid compositions essentially identical to the patterns from rabies viruses isolated from dogs in their native countries and unlike rabies viruses found in the United States. These individuals had been in the United States for 6 years, 4 years, and 11 months before the onset of clinical disease.189,192
In 2004, a 22-year-old man from El Salvador who had been in Los Angeles for 15 months died of rabies that, by nucleotide analysis, was typical of rabies viruses found in dogs in El Salvador and unlike viruses found in the United States.126
Almost 99% of human rabies infections clinically manifest in less than 1 year, typically 2 to 12 weeks.15,24,92 The median incubation period in the United States for persons diagnosed between 1960 and 1990 was 24 days for those 15 years old and younger, but 43.5 days in people older than 15. Fixed (laboratory) strains of virus tend to produce shorter incubation periods than do wild or “street” strains. In 1960 in Brazil, 60 people were injected with vaccine that had been inadequately inactivated. Sixteen developed rabies, and the incubation periods ranged from 4 to 16 days.92
The size of the viral inoculum clearly influences the incubation period. Experimental animals injected with large numbers of viruses develop clinical infections significantly faster than do those receiving small inocula. Small inocula resulted in greater central nervous system histologic damage and more widespread infection outside the central nervous system, particularly in salivary glands.87
Pathogenesis of Central Nervous System Infection
Immediately after a bite (or investigational injection), rabies virus can be identified at the site with fluorescent antibodies and remains near the wound or injection site for hours to weeks, depending on the animal species. Viral antigen can be demonstrated in muscle, and viral particles budding into the sarcoplasmic reticulum and from the sarcolemma have been demonstrated by electron microscopy.67 The virus appears to enter both motor and sensory nerves, probably through motor endplates and neuromuscular spindles.12,177
Passage of the virus through peripheral nerves was demonstrated in 1887 when rats80 and rabbits81 were protected from rabies following injections of the virus in their hind legs by sectioning the sciatic nerve. After entry into peripheral nerves, the virus travels at a rate of about 5 to 10 mm per hour to neuronal cell bodies such as dorsal root ganglia.15,67 Replication can begin at this site, and prolonged ensconcement at this site has been suggested as one explanation for prolonged incubation periods.177
Upon reaching the CNS, the virus is widely disseminated with extreme rapidity, almost simultaneously with entry, but the manner in which the virus disseminates throughout the CNS is not known. Viremia has not been documented. Plasma membrane budding from infected to uninfected neurons and dissemination through intercellular spaces or cerebrospinal fluid have been suggested. Clusters of viral particles at neuromuscular junctions, reduction of viral infectivity by nicotinic acetylcholine receptor competitors, and other data suggest that the virus recognizes cholinergic binding sites and perhaps enters peripheral and central nerve fibers through those sites. The large numbers of muscle cholinergic binding sites in foxes, which are exquisitely sensitive to rabies, and the small number of such sites in opossums, which are highly resistant to rabies, support this hypothesis and possibly explain the mechanism of sensitivity or resistance.15 The glycoprotein that coats the viral particle is a major determinant of neuroinvasiveness, and alteration of this protein can markedly alter the kinetics of CNS viral spread.12
Viruses can be isolated from cerebrospinal fluid—a significant antibody concentration in this fluid is considered diagnostic of CNS rabies infection—and spread by this route could be quite fast.183 Additionally, rabies virions have been identified in intercellular spaces in the CNS by electron microscopy. Rabies antigen can be found in essentially all parts of the CNS and, although limited mostly to neurons, can also be found in oligodendrocytes.115
The rabies virus can infect a wide variety of cells in culture; no explanation for its localization to neurons in vivo has been found.124
After wide CNS involvement, the virus passes centrifugally through neural axoplasm to a wide variety of tissues, including salivary glands, corneas, and skin of the head and neck, sites at which identification of the virus may aid in the diagnosis of clinical illnesses. The route of spread to the periphery was demonstrated over 90 years ago, when Bartarelli sectioned nerves to salivary glands and found that the glands subsequently did not contain rabies virus.22 Even within the salivary gland, the virus appears to spread by neural networks and not between adjacent epithelial cells.67
An element of immunopathology is produced by disseminated rabies infection. Among persons exposed to rabies, those immunized with early vaccines who subsequently developed infections did so more rapidly than did unvaccinated individuals, a phenomenon termed “early death.” Experimental confirmation of this feature has been achieved by injecting mice with a lethal quantity of rabies virus and immunosuppressing a portion of them. The immunosuppressed animals survived 20% to 25% longer than unsuppressed animals, but their survivals were shortened to those of the control animals when they were injected with antirabies antibody. Additionally, cytolytic T cells appear to be a significant component of the protective response to rabies virus. Avirulent strains of rabies virus induce rabies specific cytolytic T cells, but virulent strains do not.145
Pathologic alterations in the CNS infected by rabies are surprisingly mild, unless supportive care has kept the patient alive for several weeks, which allows much more extensive, necrotic lesions to develop.165,177 Leptomeningeal congestion is the only grossly visible change typically found. Mild edema may be present if the patient has been hypoxic. Pressure grooves are rare. The meninges may be cloudy if severely inflamed.155
Typical histologic features are perivascular cuffing by mononuclear inflammatory cells, microscopic collections of reactive glial cells known as Babes nodules (named for the man who first described them in 18927), and areas of neuronal degeneration and neuronophagia. Some leptomeningeal inflammation is usually present. Spongiform degeneration similar to that found in prion diseases has been described in animals, particularly in skunks.67
Van Gehuchten and Nelis described a striking proliferation of the capsular cells surrounding ganglionic neurons that pushed these cells apart and separated them by a dense cellular layer. The neurons also contained degenerative changes. Subsequent studies have found the Van Gehuchten–Nelis changes to be present in almost everyone dying with rabies—and to be a much more consistent and reliable diagnostic feature than are viral inclusions.204,205
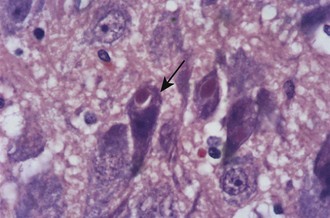
Negri bodies are the best-known histologic feature of rabies and were the first viral inclusions to be found (Figure 60-8). Negri described these cytoplasmic inclusions in 1903 and considered them parasites that caused the disease.146 Entirely independently, Bosc described identical inclusions in two separate papers published the same year, but he is rarely recognized.34,35 Negri bodies are eosinophilic cytoplasmic inclusions. Some contain a small, basophilic inner body, or innerkörperchen, and are known as lyssa bodies. They are found almost entirely within neurons; although most common in Ammon’s horn and Purkinje cells of the cerebellum, they may be seen in any part of the central nervous system, particularly in humans. Inclusions may be seen in other tissues, such as the salivary gland, skin, cornea, and pancreas, as well, but are not seen in the ependyma and choroid plexus.
The appearance of the inclusion bodies varies in different animal species, and uninfected animals such as cats commonly contain cytoplasmic inclusions that easily could be confused with rabies bodies.195
By electron microscopy, three types of bodies have been identified: one composed of a granular matrix of viral protein and typical virus particles, a second composed of matrix and tubular structures, and a third composed of matrix alone. Invaginations of cytoplasm into the inclusion give rise to the innerkörperchen, indicating that Negri bodies and lyssa bodies are both diagnostic of rabies.155
Clinical Features
“Although the clinical features of classical rabies are said to be too well known to require description, few clinicians practicing outside the tropical endemic zone have ever seen the disease, and the rare cases presenting in Europe and North America are often misdiagnosed.”215
Pain, paresthesias, or symptoms such as burning, itching, or numbness at the site of the bite or in the bitten limb are the most common early symptoms. Paresthesias may result from proliferation of rabies virus in the spinal cord at the level at which the nerves enter from the bite site.92 In Thailand, this initial symptom often takes the form of severe itching that can lead to frenzied scratching and extensive excoriations.215 This symptom is so well known among the Thai people, and animal bites are so common in that country, that any cause of itching or dysesthesia, even contact dermatitis, can lead to anxiety months to years after an animal exposure.224
Systemic symptoms usually develop later and are largely nonspecific. Local symptoms may not appear at all. One-third or less of all patients initially have symptoms that suggest the etiology of their infection to physicians who do not commonly encounter the disease. Complaints include malaise, chills, fever, or fatigue. Symptoms that suggest an upper respiratory infection are common and include sore throat, cough, and dyspnea. Gastrointestinal symptoms include anorexia, dysphagia, nausea and vomiting, abdominal pain, and diarrhea. Headache, vertigo, irritability, or anxiety and apprehension suggest CNS involvement. However, even advanced rabies often has nonspecific features.53,151 Hypoventilation and hypoxia are common during the prodrome and early acute neurologic phase, and the cause is not understood. Cardiac involvement is common and is manifested by tachycardia out of proportion to the fever; hypotension, congestive failure, or even cardiac arrest may ensue.92
Two clinical forms of human rabies are recognized. The furious, encephalitic, or agitated form that is associated with periodic episodes of hyperactivity, restlessness, or agitation is considered most typical. This form of rabies is characterized by periodic opisthotonic spasms or convulsions, particularly in response to tactile, auditory, visual, or olfactory stimuli (aerophobia and hydrophobia). Episodes of disorientation, sometimes with hallucinations or violent behavior, often alternate with periods of lucidity, which can be particularly horrifying because the patient recognizes the nature of his or her illness. The terror associated with hydrophobia has been labeled “powerful but indescribable.” Episodes of priapism, increased libido, insomnia, nightmares, and depression may suggest a psychiatric disorder. Patients maintained with supportive therapy progressively deteriorate, become comatose, lose peripheral nerve function, lose brainstem function, and die.92,215
Hydrophobia has been described in only 32% of recent U.S. patients,92 although one recent U.S. rabies victim, an 11-year-old boy, was so afraid of water he would not even take a bath.50 Experienced observers in Thailand have described hydrophobia as a violent, jerky contraction of the diaphragm and accessory muscles of inspiration that is triggered by attempts to swallow liquids and by other stimuli. Usually it is not associated with neck or throat pain or with laryngopharyngeal spasms. It has been likened to respiratory myoclonus (Leeuwenhoek’s disease). When patients lapse into coma, hydrophobia is typically converted into cluster breathing with long apneic periods.215
The variability of the clinical manifestations of rabies may result from heterogeneity in wild or “street” rabies viral populations (as contrasted with “fixed” viral strains maintained in laboratories), even in the viruses infecting a single animal. Rabies viruses isolated from a boy who died after being bitten by a fox and propagated by intracerebral inoculation of white mice produced three distinctly different forms of disease in the mice.102
The second clinical form of rabies, the paralytic, dumb, or Guillain-Barré–like form, is characterized by progressive paralysis without an initial furious phase. Even though the paralytic form of the disease does not appear to be as familiar to some health care providers, 20% to 30% of human rabies victims present in this manner.3,224 Other animals also have furious or dumb rabies presentations.148 Paralytic rabies is more common after rabid vampire bat bites, in persons injected with fixed virus strains, and in persons who have received postexposure vaccination.92 Distinction from Guillain-Barré syndrome may be difficult, although individuals with that disorder usually do not have urinary incontinence, which is common in rabies-infected persons.116
Individual case reports make clear that every patient is different. The most common misdiagnoses are psychiatric or laryngopharyngeal disorders.116 Physicians at QSMI, who have vast clinical experience, consider inspiratory spasms to be the most reliable clinical sign of rabies, particularly in comatose patients, regardless of whether the disease was initially furious or paralytic. Such respirations also have been described as rapid, irregular, or jerky, termed apneustic.
In addition, most of the QSMI patients have myoedema, particularly in the region of the chest, deltoid muscles, and thighs.219,224 However, this phenomenon, a brief, unpropagated, localized muscle contraction that appears in response to percussion with a tendon hammer, has not been confirmed as an important sign of paralytic rabies.116
Because the signs and symptoms are so nonspecific and often are rather mild at onset, many patients with rabies are not hospitalized the first time they are seen by a physician. (Two patients who died in the United States, one between 1960 and 1980 [the year of death was not stated] and the second in 2008, were never hospitalized.32) When admitted, most patients have a fever, which may be mild, but commonly is above 39.4° C (103° F). Of the 38 individuals who died of rabies in the United States between 1960 and 1980, 24 (63%) had difficulty swallowing, but only one-half of those had definite hydrophobia or aerophobia; 27 (72%) manifested excitement or agitation, 24 (63%) had paralysis or weakness, and 12 (32%) had hypersalivation. Dysesthesias at the exposure site were described by 19 (79%) of the 24 individuals who had an identifiable bite or similar exposure.3
Of the 28 patients diagnosed before death, 26 had a history of an animal exposure. Only four of eight patients diagnosed after death had this history. All 12 patients with hydrophobia were diagnosed before death.3
The duration of illness for patients not given supportive care averages 7.3 days and ranges from 2 to 23 days. For patients who are given supportive care, the average duration of illness is 25.3 days, with a range of 7 to 133 days.3,195
“The following [therapeutic] measures have … been tried in clinical rabies, but without any evidence of effectiveness: administration of vidarabine; multisite intradermal vaccination with cell-culture vaccine; administration of α-interferon and rabies immunoglobulin by intravenous as well as intrathecal routes; and administration of anti-thymocyte globulin, high doses of steroids, inosine pranobex, ribavirin and the antibody-binding fragment of immunoglobulin G.”219 In a recent review of the management of rabies in humans, a combination of some specific therapies was suggested. The authors point out that essentially no individuals with clinical rabies survive, and the best therapy often is palliative.117
Subclinical Rabies
Although clinical rabies in humans is a uniformly lethal infection with only six (perhaps seven) recognized exceptions, subclinical infections probably occur. Low titers of rabies virus neutralizing antibodies have been found in Canadian Inuit hunters and their wives, as well as in unimmunized students and faculty members of a veterinary medical school.116 In Nigeria, 28.6% of 158 healthy humans who had no history of exposure to rabies or of any antirabies prophylaxis were found to have serum-neutralizing antibodies against rabies. (Antibodies against Mokola virus [genotype 3] and Lagos bat virus [genotype 2] also were found in 7.5% and 2.5% of these individuals.) The investigators suggested that these individuals had been infected, but the infection had been halted before the virus had entered nerves. An attenuated strain of rabies virus also has been suggested as the cause of such antibodies, but an as-yet-unidentified virus of the Lyssa group, or even some other cross-reacting infectious agent, could be responsible.150 Among 48 family members of Peruvians who died following rabid vampire bat bites in 1990, seven had antirabies antibody levels that ranged from 0.14 to 0.66 international units. These antibodies could not be related to exposure to bats, exposure to other animals, or to other epidemiologic events.6
Some animals, including dogs, have significant amounts of nonspecific virus-neutralizing antibodies in their sera. Up to 20% of raccoons in Virginia and Florida180,229 and 20% to 40% of mongooses on Grenada have antirabies antibodies, which may be evidence of nonfatal infections.82 Up to 80% of bats in crowded nurseries may have rabies antibodies.17 The only finding considered diagnostic of prior rabies infection is antibodies in cerebrospinal fluid.85,86
Even clinical rabies is not always fatal in animals. Pasteur observed that some dogs recovered from early symptoms of rabies and subsequently could not be infected by rabies virus injections.153 Recoveries from infections that produced paralysis have been described in two dogs;89 recovery from clinical rabies has also been described in mice, donkeys, bats,13 and pigs.16
Undiagnosed Rabies
Clearly, many rabies infections are not diagnosed in developing countries. The possibility that in industrialized countries a number of human rabies infections are not diagnosed and the true incidence of this infection is higher than reported is suggested by several considerations. The clinical manifestations usually are nonspecific, and many infections are diagnosed late in the course of their illness as the result of testing for any identifiable cause of encephalitis, or subsequently by autopsy examination of the central nervous system. Of the 38 human rabies infections in the United States in the 1960s and 1970s, 8 (21%) were diagnosed after death. Subsequently, the percentage of infections diagnosed postmortem has been higher.52,217
In recent years in the United States, most individuals with rabies have no recognized exposure to potentially infective animals, yet an account of animal exposure often is the only stimulus for laboratory identification of rabies as the cause of the encephalitis.52
The significance of undiagnosed human rabies is uncertain. When the diagnosis is made, members of the patient’s family and all health care personnel who have had a significant exposure to the patient are given postexposure immunoprophylaxis. However, human-to-human transmission of rabies has been reported only following tissue or organ transplantation, except in two individuals in Ethiopia.90
Laboratory Diagnosis of Rabies
Currently available techniques for a definitive laboratory diagnosis of rabies usually are nondiagnostic in the early days of infection and become useful only a week or more after the onset of illness. The diagnosis of rabies should be confirmed as early as possible so that the number of persons exposed to the infection can be limited and therapy for those exposed can be initiated promptly. In industrialized nations, the number of persons exposed to a hospitalized rabies patient can number in the hundreds.163,200
If rabies is suspected, a complete set of samples should be collected for testing by all currently available diagnostic procedures. Consultation is available from the CDC 24 hours a day, seven days a week, and should be obtained (877-554-4625; http://www.cdc.gov/rabies/). However, samples taken antemortem cannot definitively rule out rabies. If infection is seriously suspected, repeated sampling is needed.200 Samples can be transported overnight to the CDC or to state laboratories.
Brain biopsies should not be routinely performed because human rabies is so rare in industrialized countries and no effective treatment is available. If a biopsy is performed to rule out another condition, it can also be examined for rabies.200
Rabies virus often can be isolated from body fluids, particularly saliva and cerebrospinal fluid. The murine neuroblastoma cells used for isolation of rabies are more susceptible to that virus than any other cell line tested, and culture on such cells can usually provide a diagnosis within 24 hours. Mouse inoculation may take 15 to 30 days, although the time can be shortened by sacrificing mice starting 5 days after inoculation and examining the brains with fluorescent antibodies.195,219 In industrialized nations, facilities for such studies can be found in local, state, and national public health laboratories. In developing countries, they often are unavailable.
Rabies in Attacking Animals
Before 1903, the diagnosis of rabies in attacking animals was based entirely on the clinical features of the disease or on the presence of unusual material in the stomach of an animal that evidenced bizarre behavior. The dog that attacked Joseph Meister, the first recipient of Pasteur’s antirabies vaccine, was diagnosed as rabid because it had hay, straw, and wood in its stomach.8 In that year, Negri and Bosch described the typical neuronal cytoplasmic inclusions, and for many years the laboratory diagnosis of rabies in animals depended on the detection of such bodies. However, only 60% to 80% of infected animals have identifiable inclusions.199 Typical inclusion bodies are scarce in Arctic foxes, for instance.75
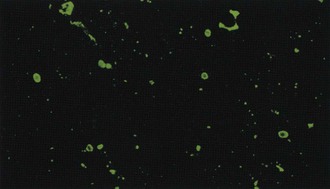
Immunofluorescent detection of rabies antigen in smears of cerebral tissues, which was introduced in 1958 and became widely used in the early 1960s,99 is far more reliable in experienced hands (Figure 60-9). Comprehensive analyses of the performance on survey examinations established that in major U.S. public health laboratories, the sensitivity of fluorescent antibody examination is nearly 100%. Fluorescent antibodies are used to identify rabies antigen in tissue culture (see later) because the rabies virus produces few cytopathogenic changes.
The major shortcoming of the procedure is its reduced sensitivity for rabies antigen in decomposed brain tissue. Some investigators have concluded that failure to identify rabies antigen with fluorescent antibodies in partially decomposed brain tissue cannot justify withholding postexposure therapy for exposed individuals.199
A procedure to confirm negative diagnoses is essential. Rabies diagnosis by intracerebral mouse inoculation was introduced in 1935 and demonstrated that only 85% to 95% of rabies infections could be identified by examination for inclusion bodies alone.193 Eventually, most laboratories, even those in developing countries, adopted adult or suckling mouse inoculation. Tissue-culture inoculation began with inoculation of chick embryo cells in 1942.195 Currently, tissue-culture isolation on mouse neuroblastoma cells is used, is far faster, and is also the most sensitive technique for confirming negative immunofluorescence examination results, at least in laboratories with suitable facilities and appropriate personnel.36,174,195 The rabies virus does not produce cytopathic changes in tissue culture, and fluorescent labeled antibodies must be used to diagnose the infection.
Postexposure Rabies Therapy
Postexposure treatment for humans consists of reducing the viral inoculum by cleansing the wound as thoroughly as possible, administering antiserum to help control viral reduplication and spread (passive immunization), and administering rabies vaccine to establish immunity to the virus before signs of infection appear (active immunization). “Rabies vaccination is a race between the active immunity induced by vaccination and the natural course of infection.”208
Identifying Exposure
Nonbite exposure consists of contamination of cutaneous wounds—including scratches, abrasions, and weeping skin rashes—with saliva, cerebrospinal fluid, or brain tissue from a rabid animal. If the material is dry, it is considered noninfectious.37 Contamination with urine from a rabid animal or person is not considered an exposure, even though rabies viruses have been isolated from kidneys and urine. A laboratory technician cut by a broken specimen container was given postexposure therapy.68
Exposure of medical personnel or family members caring for patients with rabies is a significant problem. High-risk contact is defined as a percutaneous (through needlestick or open wound) or mucous membrane contact with saliva, cerebrospinal fluid, or brain tissue. Such contact is considered essentially the same as bite and nonbite exposures to rabid animals. Individuals who have had a high-risk contact should receive postexposure immunoprophylaxis. However, routine infection isolation procedures, including respiratory precautions, minimize the risk for medical personnel caring for patients with rabies.37
In areas where canine rabies is not enzootic, which includes all of the United States except for the area along the border with Mexico (particularly southern Texas), a healthy domestic dog or cat that bites a person should be confined and observed for 10 days, particularly if the animal has been previously vaccinated. A veterinarian should evaluate any illness during confinement. If rabies is suspected, the animal should be humanely killed and its head should be shipped to a qualified laboratory.56 The head must be refrigerated during shipping. Examinations for rabies cannot be reliably performed on decomposed brain tissues.200
Such confinement and observation were judged safe for exposures to ferrets in 1998. Scientific evidence that the same quarantine period would be adequate for wolf hybrids does not exist, because studies of pathogenesis and virus shedding have not been performed. Hybrids that bite humans should be euthanized immediately, and their heads should be shipped to reliable laboratories.148
The significance of the laboratory’s qualifications was emphasized by the death from rabies of a U.S. citizen in 1981 after he was bitten by a dog in Mexico. The dog’s head was shipped to a Mexican laboratory, where it was examined with Sellers’ stain instead of a fluorescent antibody technique. Because no evidence of rabies was found with this less-sensitive technique, he was not given postexposure therapy.152 In a Thai investigation, 13 of 404 rabid animals diagnosed with fluorescent antibodies did not have Negri bodies identifiable with Sellers’ stain.187
Any stray or unwanted animal should be killed immediately and its head submitted for rabies examination. Euthanasia does not have to be delayed for further development of the infection in an attacking animal for a reliable diagnosis to be made.200
No one in the United States has died of rabies when the attacking dog or cat has been healthy after 10 days of observation.189 However, dogs injected with an Ethiopian strain of rabies virus excreted virus in the saliva up to 13 days before signs of disease were observed,88 and dogs that have recovered from experimental rabies excrete virus in saliva for as long as 6 months after recovery.91
If an attacking dog or cat is rabid or is suspected to be rabid, postexposure therapy should be initiated at once. However, a reliable determination of the presence or absence of rabies in animals can usually be completed in less than 1 day.200 If the dog or cat escapes and is not suspected to be rabid, the Advisory Committee on Immunization Practices (ACIP) of the U.S. Department of Health and Human Services recommends that local public health officials be consulted.56
In a study of postexposure therapy practices in the emergency departments of a number of university hospitals from all parts of the United States, the most frequent inappropriate administration of therapy was for animals that could be observed for 10 days. The most common failure to administer postexposure therapy was for animals that could not be observed. The investigators emphasized that physicians often failed to use the expert consultative services available 24 hours a day from their local health departments or the CDC.141
In the United States, any exposure to a skunk, raccoon, bat, fox, or other carnivore, including bears and cougars, should be considered a rabies exposure. Treatment should be initiated immediately, except when the exposure has occurred in a part of the continental United States known to be free of rabies and the results of immunofluorescent testing are to be available within 48 hours. If the animal is captured, it should be killed and its head shipped to a qualified laboratory immediately. The signs of rabies in wild animals, including wolf hybrids, are not consistent enough or sufficiently well known for a 10-day observation period to reliably determine whether the animal was rabid.56
Small rodents (squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, and mice) and rabbits are rarely found to be infected with rabies and have not been known to produce rabies in humans. ACIP recommends that local health officials be consulted following a bite by a rodent or rabbit.56
Initial Wound Management
Immediate and thorough cleansing of bite wounds with soapy water to reduce the viral inoculum is an essential and highly effective component of postexposure therapy. Fishbein, former Chief of the Viral and Rickettsial Zoonoses Branch of the CDC, has stated, “Local treatment is perhaps the single most effective means of preventing rabies.” He also has said, “In some groups exposed to a single rabid animal and many laboratory investigations, local wound care alone has been found to be as or more important than vaccine alone.”92 Vodopija has declared, “Washing the wound with soap and water or with other substances that are lethal to rabies virus may be crucial for survival, irrespective of subsequent immunization.”210 Fangtao, who has had extensive experience with rabies in China, asserts, “The protective effect of each (wound care, immune globulin, and vaccine) should be considered equivalent.”83 Neglect of the wound or inadequate wound care has resulted in rabies in individuals who received ideal immunotherapy.24,56
Many experimental studies have shown that the duration of the incubation period following rabies exposure is inversely proportional to the size of the viral inoculum—a large inoculum produces generalized infection in a much shorter time than does a small inoculum.67 If rabies vaccine is to induce immunity before infection appears, it must have sufficient time. Following a severe rabies exposure, such as a bite about the head or neck, or multiple bites, a major reduction in the number of virus organisms introduced may be essential to allow time for immunization.
In experimental studies, the best results have been obtained by thoroughly cleaning the wound with soap and water and then irrigating it with a virucidal agent such as povidone–iodine or a 1% solution of benzalkonium chloride (Zephiran). Soap neutralizes benzalkonium chloride and must be completely rinsed from the wound before it is irrigated with the detergent. With deep wounds, only virucidal substances, such as benzalkonium chloride or povidone–iodine, have been found to be effective. If neither of these agents is available, 70% alcohol (ethanol) or iodine, either tincture or aqueous solution, can be instilled in the wound. The best cleansing agent that can be obtained immediately must be used.37,219 If these measures are painful, which is often the case when they are carried out with appropriate vigor, the area can be anesthetized with a local anesthetic.
This vital procedure is often neglected, particularly in developing countries. In a study of 250 patients with rabies seen at the Sassoon Hospital in Pune, India (the eighth-largest city in India with a population over 3.5 million), the wound had been washed with soap and water for only nine patients.184
Appropriate measures to prevent bacterial infection, including antibiotics if indicated, and tetanus prophylaxis should also be instituted.37,173 The WHO Expert Committee and others recommend that bites not be sutured.37,43,56,219
Rabies Immune Globulin
Although proposed and investigated by Babes and colleagues over a century ago,9 the benefit of hyperimmune serum immediately after rabies exposure was established only about 55 years ago. The most dramatic study was initiated by Koprowski and carried out in Iran in 1954. Twenty-nine persons were bitten by a single rabid wolf. Thirteen individuals with bites about the head were treated with antiserum and neurally derived vaccine; only one died. Five individuals with similar bites received vaccine alone; three died.19,20,22
Similar results were demonstrated in China with a much more effective cell-culture vaccine by Lin and associates, who suggested that immune serum might be effective because it stops virus from entering susceptible cells (nerves).84 The need for immune serum with human diploid cell vaccine (HDCV) has been demonstrated by the death from rabies in a 29-year-old female U.S. citizen in Rwanda who received prompt therapy with HDCV but not with hyperimmune serum.77
Measurements of the antibody response to earlier rabies vaccines demonstrated that immune serum reacted with the vaccine and limited the antibody response in the first 7 to 10 days of administration. These data were considered evidence of passive immunity that was essential for early control of the rabies virus, particularly when the viral inoculum was large and the incubation period before the development of clinical rabies was likely to be short.19 However, with contemporary cell-culture vaccines that have a concentration of 2.5 international units/mL, such inhibition of the serum-neutralizing antibody response is not seen.210
The only rabies immune globulin licensed for use in the United States is human rabies immune globulin (HRIG), which is prepared by cold ethanol fractionation from plasma from hyperimmunized human donors and is virtually free of significant side effects.225
When immune serum is given, it is usually of equine origin, which costs only about 10% as much as does HRIG.223 However, contemporary highly purified equine rabies immune globulin (ERIG) is not associated with the 15% to 46% incidence of serum sickness typical of older equine antirabies serum.43 In 3575 individuals treated at the QSMI, ERIG from Pasteur Vaccins of France, Sclavo of Italy, and the Swiss Serum and Vaccine Institute produced allergic reactions in 0.87%, 3.58%, and 6.19% of the recipients, respectively. The reactions were uniformly mild and lasted less than a week with appropriate therapy. Only 9 of the 66 patients with reactions required steroid therapy; the remainder were satisfactorily treated with analgesics and antihistamines. ERIG is also produced in developing countries, but the purification procedures and potency criteria have not been published, and these products may be associated with a higher incidence of adverse reactions.
At QSMI, an initial intradermal injection of horse serum is administered, and individuals who present with sensitivity are treated with HRIG. One 17-year-old male did not react to the intradermal injection, but with subsequent injections he had an anaphylactic reaction that was successfully treated with epinephrine, hydrocortisone, and chlorpheniramine.223,225
Equine F(ab′)2 fragment rabies immunoglobulin is also used. In a study of 7660 patients in the Philippines, of 151 patients with laboratory-confirmed exposure, two died of rabies: a 4-year-old girl who had multiple deep lacerations on the nape, neck, and shoulders, and an 8-year-old boy who only received postexposure prophylaxis on the day of exposure. Local adverse reactions were documented in 35 individuals (0.46%); systemic adverse reactions were documented in 104 individuals (1.36%). Only two subjects had documented local adverse reactions within 30 minutes of immunization, and 11 individuals with systemic adverse reactions were documented to be having possible allergic reactions.162
Currently, all agencies recommend injection of as much immune globulin as possible around the bite site.219,225 For bites on the fingers and similar locations where only a limited amount of globulin can be injected locally, the remainder should be injected intramuscularly.37 For individuals with multiple bites, which is common with rabid dog attacks in developing countries, the immune globulin may have to be diluted with saline to achieve uniform injections. The syringe used to inject immune globulin must not be used to inject vaccine, which would be inactivated by residual antiserum.
Rabies Vaccines
Louis Pasteur revolutionized rabies therapy—and all of medicine—on July 7, 1885, when he began administering rabies vaccine to Joseph Meister, a 9-year-old boy who had been bitten 14 times by a dog that was killed and diagnosed as rabid because its stomach contained hay, straw, and fragments of wood.209,211 Although the rabies diagnosis in this animal may be suspect, and the boy may not have developed a clinical infection, Pasteur in 1886 observed only a single failure in a series of 688 treatments, many of whom must have been exposed to rabid animals and would have been expected to die without therapy.7 Meister’s only employment as an adult was as a security guard for the Pasteur Institute.193
Pasteur’s first rabies vaccine was produced from rabid rabbit spinal cords that were allowed to dry to attenuate the virus. After 15 days, the cords were noninfectious, so the first injections were made with 14-day-old spinal cords, and subsequent injections were made with cords that had been dried for shorter times.197,208 Roux and Callmette demonstrated that desiccated rabbit spinal cords retained appropriate virulence when preserved in glycerin, which greatly facilitated the production and storage of such vaccine.44,171 Although other vaccines were soon developed, the Pasteur rabbit spinal cord vaccine was administered at the Pasteur Institute of Paris as recently as 1953.208
Subsequently, similar vaccines have been prepared in brain tissues from various animals, particularly goats and sheep, containing virus killed with formaldehyde, phenol, or β-propiolactone.108,197 These preparations are collectively known as Semple or neural tissue vaccines, and Semple’s introduction of such vaccines in 1911 was a significant advance. In 1955, Fuenzalida and Palacios in Chile introduced the use of suckling mice less than 3 days old for the preparation of neurally derived vaccines. Such animals have little cerebral myelin and are thought to yield a product that contains less myelin basic protein. Currently, animals no older than 1 day are used. In Thailand, this vaccine has been found to be as poorly immunogenic and to be associated with as many side effects as are other similar vaccines,95,219,224 but other investigators have found the incidence of neural reactions to be lower.209 At the present time, neural tissue vaccines are still employed for a large percentage of all postexposure rabies therapy worldwide because they are inexpensive. Of 50 million doses of rabies vaccine administered worldwide each year, 20 million are still neural tissue vaccines.39
Such animal brain vaccines have been described as the crudest biologic material administered to man. These vaccines have low immunogenicity, and as many as fifteen 5-mL injections of a 5% to 10% suspension of brain tissue are required to produce satisfactory antibody titers. Intense local reactions are common, and many patients abscond from treatment.39 In Taiwan between 1952 and 1955, 55% of individuals who began rabies vaccination failed to complete treatment.214 In one study, 20 of 681 persons bitten by dogs proved to be rabid refused therapy because they had witnessed the side effects of vaccination in other villagers. Nine (45%) died of clinically diagnosed rabies. Another 62 persons received Semple vaccine, even though the dogs to which they were exposed were not rabid, presumably because the results of testing the dogs were not accepted as valid.187
The potency of these vaccines is highly variable. Some batches are known to have been ineffective. After 15 years of observations, the Pasteur Institute of Iran, a branch of the Pasteur Institute in Paris and a WHO Collaborating Center for Reference and Research in Rabies, concluded that rabies vaccines prepared in animal brains were totally ineffectual for preventing rabies in individuals bitten about the head or neck who would be expected to develop encephalitis within a short time after exposure.21 Pasteur observed that his treatment was not always successful, particularly after bites around the face.193 In Bangkok, 11% of a group of patients who died of rabies encephalitis had received complete courses of the vaccine starting soon after they were bitten.207 The vaccine appears to be beneficial for less severe exposures.206
Since the time of Pasteur, the failure of immunoprophylactically treated persons to develop clinical rabies has been attributed to the vaccine, and some of these individuals produced antirabies antibodies. However, many, perhaps most, individuals attacked by rabid animals do not develop clinical rabies. In fact, humans are considered to have a low susceptibility to rabies.85 Citing the 1912 studies of Babes, which now could not be repeated, Baer has emphasized that only about 15% of individuals bitten by rabid dogs and not vaccinated develop rabies10,177 (Table 60-5). The absence of rabies infection in many individuals treated with Semple vaccine undoubtedly is a reflection of failure to transmit the infection by attacking animals and not of vaccine effectiveness.
TABLE 60-5 Babes’ 1912 Estimates of Mortality Among Unvaccinated Persons Exposed to Animals Assumed to be Rabid
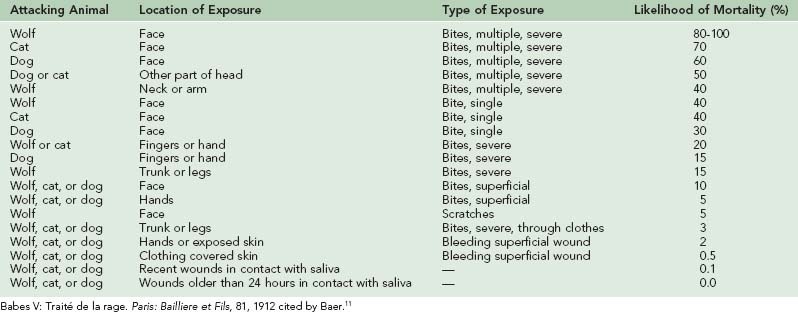
The neuroparalytic reactions associated with Semple rabies vaccines are the most serious neurologic complications that follow any vaccination109 and were reported only 4 years after Pasteur introduced his vaccine.21 Remlinger reported 26 cases between 1888 and 1905.164 Even dogs vaccinated with this product developed anorexia, as well as “depression, nervous symptoms, a morbid desire to bite,” or even paresis of the hind limbs.41 Of the patients receiving such vaccines, 15% develop electroencephalographic abnormalities, and 5% develop measurable antibodies against brain tissue. About one-fourth of the severe neurologic reactions are fatal, and an additional one-third cause permanent damage.14,19,147 An incidence of neuroparalytic reactions of approximately 1 in 1200 patients seems to be generally accepted, but much higher rates of 1 : 400, 1 : 220,95 1 : 142,187 or even ten times higher at 1 : 120 have been reported.196 Probably the most accurate evaluation of the frequency of such complications is that the incidence is not known. The countries that have a high incidence of rabies, which are the countries that use neural-derived vaccines for postexposure therapy, also have very poor or no programs for reporting complications.197
Recently, the specter of transmission to humans of spongiform encephalopathies caused by prions from infected sheep by neural tissue vaccines derived from sheep brains has been raised.39
Because so many problems were associated with Semple vaccines, a safer vaccine was developed with viruses grown in duck embryos. This vaccine was not as immunogenic as contemporary vaccines, and 14 to 23 injections were required to produce satisfactory antibody titers. In addition, this vaccine also had a significant incidence of adverse side effects. Reactions to duck embryo vaccine in one retrospective analysis of 424,000 persons consisted of 22 cases of anaphylaxis, 4 cases of transverse myelitis, 5 cases of cranial neuropathy, 2 cases of nonfatal encephalopathy, and 2 cases of fatal encephalopathy that may actually have been fatal rabies infections.172 Immunization with vaccine grown on duck embryos has failed to prevent rabies, but for many of the failures a prolonged interval separated the exposure and immunoprophylaxis.2
Human Vaccination
Postexposure prevention of human rabies by immunization is unique in the therapy of infectious diseases and is possible only because the moment of exposure can be vividly remembered and the incubation period is relatively long. For 30 years, the recommended procedure for producing active immunity in the United States has consisted of five vaccine injections into the deltoid muscle of 1.0 mL of vaccine on days 0, 3, 7, 14, and 28 after animal exposure. This schedule is known as the “Essen scheme” in recognition of the contributions of Ernst Kuwert of the Essen Institute of Medical Virology and Immunology.209 Immunoprophylaxis is most effective when begun within 24 hours of exposure, but the average delay following animal exposure in the United States and in Nepal has been 5 days.185 Since the incubation period for rabies may be prolonged, no upper limit can be set for the time after a potential rabies exposure at which postexposure vaccine therapy should be given.24,219
In 2010, a number of investigators and the ACIP, after a review of rabies virus pathogenesis, experimental animal models, human immunogenicity studies, prophylaxis effectiveness in humans, documented failures of prophylaxis in humans, and vaccine safety, recommended that postexposure vaccination be reduced to four injections given on days 0, 3, 7, and 14.65 A detailed review of the evidence supporting a 4-dose schedule for human PEP had been previously published.176
An alternative treatment schedule, termed the 2-1-1 regimen, was first developed in Yugoslavia. On day 0, two 1-mL doses of vaccine are injected into the left and right deltoid muscles. Additional 1-mL injections are given on days 7 and 21. This schedule was originally intended for use only after severe exposures when the incubation period could be expected to be short. However, in a number of countries it is used routinely because it produces antibody levels just as high as does the Essen scheme and only three clinic visits are required.210
The only rabies vaccines licensed for use in the United States are prepared from viruses grown in cell cultures and inactivated with β-propiolactone. HDCV, the first to be licensed and the gold standard against which other vaccines are compared, was made possible by the development of the WI 38 cell line of diploid human fibroblasts by Hayflick and Moorhead and by adaptation of the Pitman-Moore strain of rabies virus to growth in such cells by Wiktor, Plotkin, and Koprowski at the Wistar Institute in Philadelphia.193,209 The virus used to prepare this vaccine was originally isolated in 1882 by Pasteur from a cow bitten by a rabid dog and was maintained by serial passage in rabbit brain before it was adapted to cell culture.178,209 The HDCV available in the United States is produced in cultures of human diploid cells by Sanofi Pasteur. This vaccine was introduced in December 1974 and was licensed for general use in the United States on June 9, 1980.63 A single-dose vial containing lyophilized vaccine that is reconstituted in the vial to a final volume of 1.0 mL, Imovax Rabies is licensed for postexposure and preexposure intramuscular vaccination. The unreconstituted vial must be refrigerated; it cannot be carried into remote areas in first-aid kits.
Because HDCV is expensive and cannot be produced in sufficient quantities to supply worldwide demands, a number of cell-culture vaccines have been developed. These vaccines, when produced according to accepted standards, are all considered equally effective.37 These vaccines are far more immunogenic than are earlier products, and protective antibody levels are routinely achieved with only four or five injections.56,208 A protective level is defined as either an antibody concentration that will completely neutralize rabies viruses at a dilution of 1 : 5 as determined by the rapid fluorescent focus inhibition test, or 0.5 international units/mL. These vaccines are so effective that measurement of postvaccination antibody levels is recommended only for individuals who are immunodeficient as the result of disease or therapy.
The effectiveness of HDCV was dramatically demonstrated during 1975 and 1976 in Iran by complete prevention of rabies in 45 persons attacked by dogs or wolves subsequently proved to be rabid.19 Between 1980 and 1982, all 511 individuals bitten by rabid animals in the United States and treated with five doses of HDCV—and with HRIG—survived.
In the United States, two other cell-cultured vaccines have been licensed. Rabies Vaccine Absorbed (RVA) was prepared from viruses grown on fetal rhesus lung diploid cell cultures, concentrated by adsorption to aluminum phosphate, and produced in a liquid form. RVA was licensed on March 19, 1988. It has been used to vaccinate humans allergic to HDCV but has been unavailable for a number of years.179
At least in part because the raccoon rabies epizootic had increased consumption of rabies vaccine so dramatically, the Food and Drug Administration (FDA) licensed purified chick embryo vaccine (PCEV) in October 1997. This vaccine (RabAvert) has been widely used worldwide. It is thought to produce fewer allergic reactions because it does not contain human albumin altered by β-propiolactone. All three preparations are considered equally efficacious and safe for postexposure therapy and preexposure intramuscular vaccination.56
WHO has approved HDCV and PCEV, as well as purified Vero-cell rabies vaccine (PVRV or Verorab), which is widely used in developing countries. This vaccine is produced in continuous culture and is less expensive to produce, but has not been licensed for use in the United States or Canada.220,222
In its 1992 Eighth Report, the WHO Expert Committee on Rabies made the remarkable statement, “Prompt and thorough cleansing of the wound, and administration of purified equine or human rabies immunoglobulins and cell-culture rabies vaccine immediately after exposure virtually guarantee complete protection. … Pregnancy and infancy are never contraindications to post-exposure rabies vaccination.”219 Although some antigens, particularly surface glycoprotein antigens, vary widely in different viral strains, standard vaccines have provided effective protection against all wild, or “street,” rabies viruses. These vaccines also appear to be effective against other lyssaviruses, except Mokola and Lagos bat virus, although no alternative vaccines are available.217,219
Occasional failures with cell-culture vaccines have been reported, but in essentially every instance, treatment has not been administered correctly. Many of the individuals did not receive immune globulin.37,147,219 Some have had the vaccine injected into gluteal muscles, which is thought to be the cause of these failures.14,37,55,56,109 Hepatitis B vaccine gluteal injections have been found to be less consistently effective than injections in the deltoid.14 All injections of rabies vaccine must be made in the deltoid muscle, or in the quadriceps muscle for small children.
Approximately 18,000 persons receive preexposure vaccination and an additional 23,000 receive postexposure immunoprophylaxis each year in the United States, but reactions of any type are very uncommon.30 Reactions following HDCV administration during the first 46 months of use (and reported to the CDC) occurred in 108 individuals, a rate of 11 per 10,000 vaccinees. Few patients required hospitalization, and no fatal reactions were reported. Of these reactions, nine (1/10,000) were immediate hypersensitivity reactions that occurred within minutes to hours after injection and were characterized by bronchospasm, laryngeal edema, and generalized pruritic rash, urticaria, or angioedema. All nine reactions followed postexposure immunization of unvaccinated persons or preexposure vaccination.
Eighty-seven delayed reactions appeared 2 to 21 days after injection and were characterized by a generalized pruritic rash or urticaria. Some of the individuals also had arthralgias, arthritis, angioedema, nausea, vomiting, fever, and malaise. Twelve reactions were classified as indeterminate. Unlike the immediate reactions, 81 (93%) of the delayed reactions followed booster injections for individuals who had been vaccinated previously. Most patients improved in 2 to 3 days when treated with antihistamines, but a few required systemic corticosteroids and epinephrine.63
The reactions are attributed to β-propiolactone–altered albumen in the vaccine.170 HDCV vaccine that is free of β-propiolactone was developed by Connaught Laboratories but was not licensed in the United States.27 PCEV and RVA are not made with albumin. Further rabies booster immunizations are not recommended for individuals who have had this type of reaction, but exposure to rabies should be treated in the usual manner.63
Neurologic reactions following HDCV administration have been extremely rare. After millions of vaccinations worldwide, three Guillain-Barré type paralytic reactions have been described, and all three individuals recovered. Other neurologic disorders have occurred at the time of vaccination, but a definite causal relationship has not been established.27,170
Immunocompromised Individuals
Immunosuppression as the result of therapy such as corticosteroids, chemotherapy, antimalarials, or other agents, or as the result of illness, particularly human immunodeficiency virus (HIV) infection, may interfere with the immune response to vaccines. If the condition is transient, preexposure vaccination can be postponed until therapy has been completed. If delaying therapy is not feasible, a rabies antibody titer should be obtained after vaccination has been completed even though such determinations are not needed for individuals who are not immunocompromised. If antibody levels are not high enough to be protective, ACIP recommends that individuals who do not seroconvert should be managed in consultation with their physician and appropriate public health officials.137 Immunocompromised persons must be particularly careful to avoid rabies exposure, even avoiding travel to areas where rabies is endemic.
In one investigation of the response to vaccination, normal controls, asymptomatic individuals with HIV infection, and symptomatic persons with HIV infection were given a 5-dose simulated postexposure vaccination. Seroconversion occurred in 100% of the controls, 76% of the asymptomatic patients, and 57% of the symptomatic patients. However, rabies virus–neutralizing antibodies could not be detected in 40% of the symptomatic individuals with CD4+ T-cell counts below 400/µL.38
However, in another investigation, 18 individuals with CD4+ cell counts less than 200/µL and 9 individuals with CD4+ counts above 200/µL received eight 0.1-mL intradermal inoculations of PCEV each day on days 0, 3, 7, 14, and 30. By day 14, all patients in both groups had protective rabies-neutralizing antibody concentrations. No significant differences between the two groups developed in the following year.186
Thirty HIV-infected adults who had been treated for almost 4 years with highly active anti-retroviral therapy (HAART) and had median CD4+ T-cell counts of 537/µL received two rabies vaccinations within 3 months. The HIV-infected subjects had lower antibody levels than did normal controls, but the levels were protective. Five years after the vaccination, 63% of the HIV-infected individuals still had protective antibody levels.97
No documented failures of rabies postexposure vaccination in HIV-infected individuals have been reported.56
Preexposure Vaccination
Preexposure vaccination with 0.1 mL of HDCV injected intradermally on the same schedule was approved by the FDA in April 1987. However, intradermal vaccination does not result in antibody levels as high as those produced by intramuscular injections, and antibodies do not last as long. Some investigators have recommended that only intramuscular injections should be administered.39 In the United States, the question is moot because the raccoon epizootic on the East Coast has increased vaccine utilization so much that syringes with 0.1 mL of the vaccine are not being produced.
After a rabid animal bite, previously vaccinated individuals need only intramuscular injections of 1.0 mL of cell-culture vaccine on days 0 and 3. Immune serum is not needed, a major consideration for travelers in developing countries where immune serum may not be available. The additional two injections are essential. Fatal rabies encephalitis developed in a 23-year-old female Peace Corps Volunteer in Kenya who had previously been vaccinated for rabies, but did not report a bite by a pet puppy too young to have been vaccinated and did not receive postexposure therapy.151
Chloroquine interferes with the response to rabies vaccine; vaccination should be completed before malarial chemoprophylaxis with chloroquine is begun. Whether other agents used for malarial chemoprophylaxis interfere with the response to rabies vaccine has not been ascertained.39
Group three consists of veterinarians and animal control workers in areas of low rabies endemicity, travelers to foreign rabies-epizootic areas who are staying 30 days or more, and veterinary students, for whom vaccination but no subsequent serologic testing or boosters is advised. Vaccination is not recommended for the United States population at large.56
In 1990, investigators at the CIWEC Clinic in Nepal found the incidence of animal exposure requiring immunoprophylaxis in travelers to be quite low, approximately 1 in 123,000 days (337 years). They disagreed with the recommendation that travelers to foreign rabies epizootic areas staying 30 days or more be vaccinated, because the cost of immunization is high and because the incidence of allergic reactions to booster immunizations is significant.185 However, investigators from the QSMI in Bangkok subsequently surveyed 1882 departing English-speaking travelers in the Bangkok air terminal and determined that 1.2% had been bitten by a dog and 8.7% had been licked by a dog during their stay. These investigators recommended that all travelers be routinely vaccinated for rabies and that the vaccine be inoculated intramuscularly. The CIWEC Clinic now recommends that most travelers to Nepal be preimmunized.158
In a review of immunizations for travelers published in the New England Journal of Medicine, the authors state, “Optimal postexposure prophylaxis against rabies (including rabies immune globulin and tissue-culture–derived vaccines) is often unavailable in many developing countries. Vaccination against rabies before travel should be considered for long-term travelers to the developing world, those who will have unavoidable direct contact with animals, those who may be unable to receive timely postexposure prophylaxis, and those (such as young children) who may be unable to report possible exposure.”182 (emphasis added)
Several of the recent American victims of rabies acquired outside the United States, and the only genotype 1 rabies victims in Australia, were children who, perhaps in fear of punishment, did not tell their parents about bites they had received. The problem of protecting children, who run the highest risk for animal bites, may not be widely recognized.170 Children who cannot understand the hazard of rabies in developing countries probably should be routinely vaccinated; they must also be closely observed so that postexposure booster vaccination can be administered.
The effectiveness of preexposure vaccination is documented by the fact that no one residing in the United States who has received preexposure vaccination with a modern cell-culture vaccine has contracted rabies.37
Rabies Therapy in Developing Countries
Rabies therapy in developing countries must be modified by financial considerations. The cost for such therapy for a typical laborer could be devastating.187 At least four different measures have been taken to reduce the costs of postexposure rabies therapy and increase the availability of reliable cell-culture vaccines. The simplest has been the transfer of cell-culture vaccine manufacturing technology to developing countries, but in spite of support by WHO and other agencies, progress has been slow. Local manufacturers in Latin America and Asia are producing Vero-cell rabies vaccine “copies” and primary hamster kidney cell rabies vaccines. Because some of these vaccines do not adhere to the strict standards imposed by the FDA or the European Pharmacopeia, they are less expensive to produce.
The substitution of ERIG for HRIG, which results in a 90% cost savings, already has been discussed.
A third approach has been the use of small-volume intradermal vaccine injections instead of larger-volume intramuscular injections. The Warrells, Phanuphak, and their co-workers at QSMI pioneered the use of intradermal vaccination for postexposure immunoprophylaxis because a smaller volume of vaccine would be required. The regimen developed by the Warrells consisted of intradermal injections of 0.1 mL of HDCV at eight different sites on postbite day 0, injections at four sites on day 7, and injections at one site on days 28 and 90, which reduced the vaccine expense to 30% of the cost of five 1.0 mL intramuscular injections.216,224 Phanuphak intradermally injected 0.1 mL of PVRV at two sites on days 0, 3, and 14, and at one site on days 30 and 90. This regimen reduced the expense for PVRV, which is already much less expensive than HDCV, by an additional 68%.156,157
One hundred Thai patients, who had injuries severe enough to produce active bleeding inflicted by animals proved rabid by fluorescent antibody testing, were treated according to the schedule developed by Phanuphak and his colleagues in a prospective study. This procedure is now known as the Thai Red Cross intradermal postexposure rabies treatment schedule, or TRC-ID. All were uniformly protected from rabies. Since 1987, the TRC-ID regimen has been routinely used for postexposure therapy of over 1000 patients a month at QSMI, with only one failure, a 53-year-old alcoholic with cirrhosis who did not report for treatment until 6 days after his exposure.71
An anticipated problem with intradermal injections was the inadvertent subcutaneous injection of part or all of the vaccine. This has not been a problem at QSMI, but the staff of that institution is highly experienced.71 Nonetheless, deliberate subcutaneous injections have been found to produce antibody titers just as high as intradermal injections.157
The fourth measure has been development of reliable nonhuman cell-culture vaccines. Production of HDCV is characterized by “a demanding technology, low virus yield, and enormously high production costs per unit of vaccine.”210 It is not suitable for large-scale human rabies prevention. A variety of nonhuman cell cultures have been used to produce rabies vaccines: primary cell cultures (hamster kidney, dog kidney, or chick embryo fibroblast), diploid cell lines (human or rhesus monkey), and continuous cell lines (Vero and baby hamster kidney cells). Those made according to FDA standards are considered as effective as HDCV for postexposure immunoprophylaxis and for intramuscular preexposure vaccination.26,56,210
PVRV is produced in cultures of kidney cells from African green monkeys by Sanofi Pasteur in France and by other agencies and is extensively used at QSMI and in other institutions.71,104,224 This vaccine can be prepared in continuous cell lines cultured in suspension on microcarrier beads in biofermenters with a capacity of up to 1000 liters, which makes possible the production of vaccine on an industrial scale at greatly reduced cost.194,209,215
The nonhuman cell-culture vaccines are less satisfactory than HDCV in one respect. Approximately one-third of recipients reported pain at the injection site, and two-thirds of vaccinees given PVRV boosters developed local erythema. About 5% to 10% of recipients had systemic reactions such as fever, headache, malaise, or urticaria.209 In terms of safety and antigenicity, HDCV and the vaccines grown in other cell cultures are identical.
Rabies Considerations for Travelers
Rabies is a definite risk for travelers, as illustrated by the 32-year-old female American traveler who was bitten on the hand while petting a stray dog in Kathmandu, Nepal. She missed at least three opportunities to obtain treatment in Nepal, Thailand, and Australia, as well as any number of opportunities after returning to the United States, and died of rabies 75 days later.23 Of the 63 human rabies infections in the United States since 1980, 18 were acquired in other countries, although most of those infections were in immigrants. The United Kingdom, which has been free of rabies since 1919, encountered 23 rabies infections acquired outside the British Isles from 1946 to 2005. Sixteen originated in the Indian subcontinent. From 1981 to 2005, four of the nine rabies infections diagnosed in Germany were acquired elsewhere.119
Probably the major problem in preventing rabies in travelers is their uninformed status. In a study of 300 French travelers, only 6.7% knew that the risk for rabies was significant and 40.1% considered it moderate or low. The danger of dog bites appeared well known, but the risk for scratches (0.7%) and licks (10%) was not known. The danger of cat, fox, monkey, and bat bites was not well known. Only one-half of the travelers knew about preexposure vaccination, and 57.6% of the travelers going to rabies-endemic countries presented to travel clinics too close to their travel departure for vaccination to be completed. Immediate washing of bite wounds was described by only 3% of those questioned, although 21.3% mentioned disinfection with antiseptics.1
According to World Health Organization estimates, 99% of rabies infections are the result of dog bites.139 However, monkeys are a significant threat around some of the temples in parts of Asia, such as the Swayambhunath and Pashupati temples in Nepal. Most monkey bites result from attempts to snatch food carried or being eaten by travelers, which visitors to temples must avoid.72,181 Among foreign travelers in Nepal treated at the CIWEC Clinic, dog bites accounted for only about 75% of reported exposures, and monkeys inflicted 20% of the bites.185
In areas where canine rabies is endemic, which includes essentially all of Latin America and Africa and most of Asia, postexposure treatment should be initiated immediately following a bite by a dog or cat, but can be terminated if the animal remains healthy during a 10-day observation period. The vaccination status of the biting animal must be ignored. Vaccines used for animals in developing countries are not as reliable as those used in the United States, and fatal rabies has been reported in U.S. citizens and in others who were bitten by “vaccinated” dogs in developing countries and did not obtain prompt postexposure therapy.58,225 In the United States, rare rabies infections are found in dogs or cats that have been vaccinated.144
Immune Globulin
Even in areas where immune serum is available, travelers who receive postexposure therapy often are not given immune serum. Investigators in France found that of 261 travelers exposed to rabies, only 24% were given immune globulin. Of the travelers who did not receive immune globulin, 43% received a first dose of vaccine more than 7 days after return and before presenting to a clinic in their home country.96
Preexposure Vaccination
Investigators at the CIWEC clinic in Kathmandu, Nepal, have found that trekkers do not have a greater risk for being exposed to rabies. However, canine rabies exists in popular trekking areas, and trekkers and participants in similar activities are much farther from medical care than are individuals who remain in cities. For trekkers and climbers in the Mt Everest area of Nepal, it can take days to get to the site of the aircraft landing strip, and additional days elapse before good weather allows aircraft to land. Furthermore, aircraft landings and takeoffs from this site are usually filled to capacity, so an unscheduled traveler may have difficulty boarding a departing flight. WHO recommends that individuals who spend a lot of time outdoors, particularly in rural areas, receive preexposure vaccination.221
Children
Children are considered to be at higher risk for rabies exposure for several reasons: their small stature makes extensive bites more likely, bites occur higher on the trunk or on the face, children are attracted to animals, and they may be less likely to report an exposure for fear of punishment.181 Preverbal children cannot report such events. Children must be carefully monitored and not allowed to play with dogs (even puppies) or cats in rabies-endemic areas.
Obtaining Medical Care
In foreign countries, American embassies and consulates are reliable sources of help. The U.S. Department of State’s Bureau of Consular Affairs website states, “If an American citizen becomes seriously ill or injured abroad, a U.S. consular officer can assist in locating appropriate medical services and informing family or friends. If necessary, a consular officer can also assist in the transfer of funds from the United States.” The website provides important information in case of emergencies and crises abroad, including the telephone number to contact the State Department’s overseas American Citizens Services (from outside the United States, 202-501-4444).203
Other Lyssaviruses
With RT-PCR amplification and nucleotide analysis, seven rabies virus genotypes have been recognized. Genotypes other than genotype 1 cause very few human infections. (Genotype 2, Lagos bat virus, has not produced a recognized human infection.) However, these additional genotypes are a source of considerable concern, particularly in Europe where bats infected by genotypes 5 and 6 are common. Treatment of humans infected by viruses of these genotypes with the immune serum and vaccine used for genotype 1 appears to be effective, with the exceptions of genotypes 2 and 3. These two genotypes are so different from the others that specific antisera can be prepared for them; identification of the other genotypes is based almost entirely on nucleotide sequence analyses. True vaccine failure occurs when animals vaccinated with rabies vaccines are exposed to Mokola and Lagos bat viruses.68,94,116,188,217 Therefore genotypes 2 and 3 have been classified as phylogroup 2, and the other genotypes have been lumped into phylogroup 1.
No human infections by these viruses have been identified. Bats with antibodies to irkut, aravan, and khujand viruses, as well as the Australian bat lyssavirus (genotype 7) have been found in Thailand.135 These viruses are pathogenic for laboratory mice, hamsters, and bats by intracranial and intramuscular injection routes, except for WCBV, which is pathogenic for mice only by the intracranial route. WCBV has been found to be pathogenic for nonhuman primates by both routes. All produce acute progressive fatal encephalitis essentially identical to rabies. All produce intracytoplasmic inclusions (Negri bodies).When groups of nine Syrian hamsters were treated with various vaccines and challenged with injections of the different viruses, the results shown in Table 60-6 were obtained.131 Genome sequencing of the N, P, and G genes has indicated that the aravan, khujand, and irkut viruses are related to genotypes 4, 5, and 6, and these viruses manifest as a solid phylogroup of Old World bat lyssaviruses. The west Caucasian bat virus is more divergent than are any of the other genotypes, with only limited relatedness to genotypes 2 and 3.128,130
Current Rabies Developments
Rabies Eradication in Wild Animals
Attempts to destroy wild animals that provide a reservoir for rabies have been made, but these pursuits are expensive and largely ineffective, with the possible exception of a program to eliminate striped skunks in Alberta, Canada. Even if such attempts were cost-effective, the consequences are considered unacceptable—increased populations of animals that had been prey (e.g., rodents) for the destroyed animals.169,219 Eradication of rabies in wild animal populations by oral vaccination was first suggested by Baer, and a program using baits distributed by aircraft was initiated in Canada in the early 1980s. Foxes were the targets of the early studies because they are particularly susceptible to rabies, and an effective oral vaccine could be produced.
The most striking successes in vaccinating wild animal populations have been achieved in Western Europe and in Texas. In Europe, the only significant wild reservoir for rabies is the red fox. A wave of fox rabies began on the Polish-Russian border after World War II and swept over most of central Europe.28 The increased incidence of rabies in this species, and an economically distressing rise in the number of secondary rabies infections in livestock, principally sheep and cattle, led to efforts to control that source of infection.
After extensive trials to demonstrate its safety, particularly for humans, vaccination of foxes was achieved with an oral attenuated–live-virus vaccine, starting in 1978 in areas of Switzerland, and later in parts of Germany. The vaccine first was placed in capsules attached to chicken heads. Subsequently, a specially designed bait of fishmeal and fat, the “Tübingen fox bait,” was developed. The baits included tetracycline as a marker, and fluorescence microscopy of mandible or canine tooth sections from foxes that were subsequently killed or found dead was used to determine the number of animals that had been immunized. Because dentine is deposited daily, the date of bait uptake could be calculated by counting the increment (von Ebner) lines between the pulp cavity and the tetracycline deposit.121,122,212 Baits were uniformly distributed throughout the test areas by hand or by helicopter. Immunization campaigns were carried out in the spring and fall, the latter particularly to vaccinate young foxes that could not take the baits during the spring. Follow-up studies of tetracycline labeling indicated that 75% to 80% of the foxes had consumed the vaccine, an adequate number to interrupt the spread of infection within the fox population.
The attenuated live virus used for the initial vaccinations in Switzerland and Germany retains pathogenicity for rodents and can revert to virulence. In addition, this attenuated virus cannot be used in North America because it is pathogenic for striped skunks and ineffective for raccoons. Starting in 1990, a vaccinia-rabies recombinant vaccine that expressed the G glycoprotein was used in Belgium and France.40,219 In the areas where the vaccine has been distributed, genotype 1 rabies has largely been eradicated in all terrestrial animals, not just foxes.147 The incidence of rabies in livestock reported from the Belgium test area has fallen from more than 80 cases a year to none.40,226
An epizootic of rabies in raccoon dogs, which apparently had originated in the USSR, was detected in southern Finland, and in the autumn of 1988 a field trial of oral immunization of these dogs and foxes was initiated. Rabies was eliminated from these populations within 12 months.219
An epizootic of rabies in coyotes in southern Texas originated in unvaccinated Mexican dogs that crossed the Rio Grande River. The epizootic spread to domestic dogs and resulted in two human rabies infections and over 2000 postexposure treatments. Control by dropping baits containing oral rabies recombinant vaccine from aircraft has grown to the largest aerial vaccination program ever attempted (2.6 million vaccine/bait units over a 42,000 square mile region during 1997). The rabies baits contain markers such as tetracycline. In 1997, 87% of coyotes manifested tetracycline fluorescence, more than enough of the population to stop the epizootic, which has disappeared. In 2001, only one infection by the dog/coyote rabies variant was found; in 2002 none was encountered.127
Rare human vaccinia infections have been attributed to contact with the recombinant vaccine baits.175
Although such programs are expensive, they are considered less costly than the alternatives: treating humans, diagnosing animals, vaccinating domestic animals, compensating farmers for culling infected livestock, culling wild foxes, and paying the salaries of individuals who carry out these alternatives.40,147 Additionally, once rabies has been eliminated in a geographic area, it can be kept out by establishing buffer zones in which oral vaccination campaigns are carried out regularly. Vaccination campaigns for the entire area can be carried out less frequently or possibly eliminated entirely.219
In North America, control of rabies in wild animals is much more complex because this infection is enzootic in a variety of animals: skunks, raccoons, foxes, and bats. These animals vary considerably in their sensitivity to different strains of the rabies virus and vaccines prepared from those strains, they respond differently to the rabies virus sachet, and they have widely differing ranges (9.5, 2.5, 1.5, 0.8, and 0.037 km2 for coyotes, raccoons, red foxes, striped skunks, and mongooses). Furthermore, these animals have widely varying population densities, and they prefer different habitats.132 Even though North America contains vast sparsely populated areas, many of the animals live in urban areas. A 60-km2 (23.1-mile2) area of Toronto with a perimeter of 28 km (17.4 miles), in which 252,000 humans lived, also had a population of approximately 1540 skunks, 3510 raccoons, and 70 red foxes from 1987 to 1991.168 The entire city of Toronto is estimated to have a red fox population of approximately 1000 animals.167 A program to trap, vaccinate by injection, and release animals in this area was effective and cost $27,000 to $69,000 (Canadian) a year. That sum was considerably less than the $100,000 (Canadian) spent annually to treat humans exposed to rabid animals in Toronto.168
Although these programs control rabies in the target species, the ecologic effects of increasing the animal population by eliminating a significant cause of mortality are almost entirely unknown.28,212
Vaccine Developments
Subunit vaccines contain only one of the viral structural proteins. Since these vaccines contain only an antigenic portion of the virus and not the entire organism, they can induce immunity but are incapable of producing infection. The rabies-vaccinia recombinant vaccine that produces only the G surface protein of the rabies virus has proved to be noninfective, immunogenic, and very effective for vaccinating foxes in Europe.219 However, this recombinant vaccine cannot be used for humans because much of the world’s population has been vaccinated with vaccinia to prevent smallpox, and vaccinia can produce disseminated infections in humans.40
Some orthopoxviruses cannot completely replicate in mammalian cells and therefore cannot produce infections, but they can abortively replicate, so that proteins expressed by the virus are presented to the immune system. A recombinant with canarypox virus that expresses the G glycoprotein has been developed at Sanofi Pasteur; it has been found to be safe and effectively produces a neutralizing antibody response in a variety of animals and humans. This vaccine is currently licensed for vaccinating cats in the United States.39 Orthopox vectoring may permit incorporation of several antigens, such as measles, mumps, rubella, rabies, and pertussis, in a single vaccine, which would greatly facilitate and reduce the cost of human immunization.219
Adenoviruses replicate on mucosal surfaces and could be ideal vectors for oral and intranasal vaccines. Recombinant human adenovirus type 5, into which complementary DNA for glycoprotein (G protein) has been inserted, has been shown to elicit protective levels of neutralizing antibodies in skunks and foxes when administered orally. Deleted replication-defective recombinants have produced high titers of rabies virus–neutralizing antibody in dogs and have provided 100% protection against a lethal rabies challenge in mice. A vaccine containing an adenovirus recombinant that expressed G protein was protective for dogs when injected subcutaneously. These developments appear to hold promise for development of an oral vaccine for dogs.78,112,198 During August 2006 and 2007, baits containing this adenovirus recombinant were aerially distributed in Southwest Ontario, Canada, and successfully immunized raccoons and skunks.166
DNA vaccines based on plasmid vectors expressing the rabies G protein offer promise because they are easy to construct, manipulate, and produce. Such vaccines have produced high titers of virus-neutralizing antibody in mice, dogs, and nonhuman primates. They appear to be effective in much younger animals. In a field trial, a DNA vaccine injected into dogs’ ears with a jet injector was far more efficient for inducing long-lasting high titers of virus-neutralizing antibodies than was cell-culture vaccine.18 DNA vaccines have not proved as effective for postexposure therapy, because the antibody response to such vaccines is slow. Efforts to accelerate the antibody response appear to be effective.39,78
Incomplete rabies viruses that cannot replicate have been investigated as rabies vaccines. One of these experimental viruses lacks the P, or phosphoprotein, gene. (The P and L genes are responsible for viral replication.) The P gene–deficient virus is apathogenic in mice, even when inoculated intracranially. It induced a high titer of virus-neutralizing antibody and protected the mice from lethal challenges with rabies virus.142
The rabies matrix (M) protein plays an important role in assembly and budding of progeny viruses. M gene–deficient viruses failed to generate progeny viruses, and mice inoculated intracerebrally developed no signs of disease. Intramuscular injection of these viruses induced formation of neutralizing antibodies. Intranasal installation resulted in almost the same antibody response.114 In a comparison of M gene–deficient and P gene–deficient viruses as vaccines, the M gene–deficient virus induced a more rapid response and a four-fold higher virus-neutralizing antibody response in rhesus macaques than did a commercial vaccine. The authors concluded that the M gene–deleted vaccine has the potential for replacing current pre- and postexposure rabies vaccines.66
Transgenic maize has been employed to produce rabies G protein. The amount of G protein produced was approximately 1% of the total soluble plant protein. Transformed kernels were given orally to mice. When challenged 90 days later with a lethal dose of a vampire rabies virus, the edible vaccine had induced viral neutralizing antibodies that protected mice 100%.134 Transgenic tomatoes have been employed to produce full-length rabies nucleoprotein genes. When injected intraperitoneally or administered orally, a protein extract induced the production of antibodies. However, the intraperitoneal injections were only partially protective and the oral injections were not protective.154
Replacements for Rabies Immune Globulin
DNA recombinant technology has been used to express three human rabies virus–neutralizing monoclonal antibodies in a rhabdovirus vector. Growth of the recombinant in cell culture produced high yields of three monoclonal antibodies that differed in epitope recognition. A “cocktail” of these antibodies neutralized several fixed and street rabies viruses. Mice and hamsters treated with this cocktail after infection with a lethal dose of rabies virus were protected. The protection was comparable with that provided by human rabies immune globulin. Notably, such cocktails could help compensate for inadequate production of rabies immune globulin that now prevails in developing nations.143,161 A number of such products have been developed, and they have been used to treat humans.18,100 However, industrial production and widespread use of such products has yet to be initiated.
Simpler Laboratory Tests for Rabies
In countries where rabies infection is endemic, funds and infrastructure often are insufficient to allow employment of the direct fluorescent test, which is the “gold standard.” Therefore efforts are being made to develop simpler laboratory procedures for rabies testing. An example is the immunochromatographic test kit, which is both simple and rapid and does not need a cold chain for transportation or sophisticated training for personnel. The kit with two monoclonal antibodies has achieved a sensitivity of 93.2% but had a specificity of 100%.149
1 Altmann M, Parola P, Delmont J, et al. Knowledge, attitudes, and practices of French travelers from Marseille regarding rabies risk and prevention. J Trave Med. 2009;16:107.
2 Alvarez L, Fajardo R, Lopez E, et al. Partial recovery from rabies in a nine-year-old boy. Pediatr Infect Dis J. 1994;13:1154.
3 Anderson LJ, Nicholson KG, Tauxe RV, et al. Human rabies in the United States, 1960 to 1979: Epidemiology, diagnosis, and prevention. Ann Int Med. 1984;100:728.
4 Arai YT, Kuzmin IV, Kameoka Y, et al. New Lyssavirus genotype from the lesser mouse-eared bat (Myotis blythi), Kyrghyzstan. Emerg Infect Dis. 2003;9:333.
5 Arai YT, Ogata T, Oya A. Studies on Japanese-produced chick embryo cell culture rabies vaccines. Am J Trop Med Hyg. 1991;44:131.
6 Augusto LR, Percy MP, Edgar TV, et al. Outbreak of human rabies in the Peruvian jungle. Lancet. 1992;1:408.
7 Babes V. Sur certains caracteres des histologiques de la rage. Ann Inst Pasteur Paris. 1892;6:209. cited by Perl and Good
8 Babes V. Traité de la rage. Paris: Bailliere et Fils; 1921. p 81, cited by Baer
9 Babes V. Lepp: Recherches sur la vaccination antirabique. Ann Inst Pasteur. 1889;2:385. cited by Cabasso
10 Baer GM. Animal models in the pathogenesis and treatment of rabies. Rev Infect Dis. 1988;10:S739.
11 Baer GM. Oral rabies vaccination: An overview. Rev Infect Dis. 1988;10:S644.
12 Baer GM. Overview to Part II: Pathogenesis and pathology. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:103.
13 Baer GM. Vampire bat and bovine paralytic rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:389.
14 Baer GM, Fishbein DB. Rabies post-exposure prophylaxis (Editorial). New Eng J Med. 1987;316:1270.
15 Baer GM, Lentz TL. Rabies pathogenesis to the central nervous system. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:105.
16 Baer GM, Olson HR. Recovery of pigs from rabies. J Amer Vet Med Assoc. 1972;160:1127.
17 Baer GM, Smith JS. Rabies in nonhematophagous bats. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:341.
18 Bahloul C, Taieb D, Diouani MF, et al. Field trials of a very potent rabies DNA vaccine which induced long lasting virus neutralizing antibodies and protection in dogs in experimental conditions. Vaccine. 2006;24:1063.
19 Bahmanyar M, Fayaz A, Nour-Salehi S, et al. Successful protection of humans exposed to rabies infection: Postexposure treatment with the new human diploid cell rabies vaccine and antirabies serum. JAMA. 1976;236:2751.
20 Bakker ABH, Python C, Kissling CJ, et al. First administration to humans of a monoclonal antibody cocktail against rabies virus: Safety, tolerability, and neutralizing activity. Vaccine. 2008;26:5922.
21 Bareggi C. Su cinque casi di rabia paralitica (de laboratorio) nell’uomo. Gass Med Lombarda. 1889;48:217. cited by Swamy
22 Bartarelli E. Ueber die Wege, auf denen das Wutvirus zu den Speicheldrüsen des Hundes gelangt. Centrabl Bakt. 1908;37:213, [cited by Baer and Lentz.15.
23 Basgoz N, Frosch M. Case 21-1998: A 32-year-old woman with pharyngeal spasms and paresthesias after a dog bite. New Eng J Med. 1998;339:105.
24 Bek MD, Smith WT, Levy MH, et al. Rabies case in New South Wales, 1990: Public health aspects. Med J Aust. 1992;156:596.
25 Beran GW. Urban rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:427.
26 Berlin BS. Rabies vaccine adsorbed: Neutralizing antibody titers after three-dose pre-exposure vaccination. Amer J Pub Health. 1990;80:476.
27 Bernard KW, Smith PW, Kader FJ, et al. Neuroparalytic illness and human diploid cell rabies vaccine. JAMA. 1982;248:3136.
28 Blancou J, Aubert MFA, Artois M. Fox rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:257.
29 Blanton JD, Hanlon CA, Rupprecht CE. Rabies surveillance in the United States during 2006. J Am Vet Med Assoc. 2007;231:540.
30 Blanton JD, Robertson K, Palmer D, Rupprecht CE. Rabies surveillance in the United States during 2008. J Am Vet Med Assoc. 2009;235:676.
31 Bögel K. Control of Dog Rabies. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002:429.
32 Bögel K, Meslin F-X. Economics of human and canine rabies elimination: Guidelines for programme orientation. Bull WHO. 1990;68:281.
33 Bögel K, Motschwiller E. Incidence of rabies and post-exposure treatment in developing countries. Bull WHO. 1986;64:883.
34 Bosc FJ. Etude et signification des lesions de la rage, lesions du systeme nerveaus. C R Soc Biol. 1903;55:1284. cited by Perl and Good
35 Bosc FJ. Recherches sur l’etiologie de la rage. C R Soc Biol. 1903;55:1436. cited by Perl and Good
36 Bourhy H, Rollin PE, Vincent J, et al. Comparative field evaluation of the fluorescent-antibody test, viral isolation from tissue culture, and enzyme immunodiagnosis for rapid laboratory diagnosis of rabies. J Clin Microbiol. 1989;27:519.
37 Briggs DJ. Public health management of humans at risk. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002:401.
38 Briggs DJ. Rabies vaccination: Protecting vulnerable travelers. Infect Med. 2002;19:561.
39 Briggs DJ, Dreesen DW, Wunner WH. Vaccines. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002:371.
40 Brochier B, Kieny MP, Costy F, et al. Large-scale eradication of rabies using recombinant vaccinia-rabies vaccine. Nature. 1991;354:520.
41 Bunn TO. Canine and feline vaccines, past and present. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:415.
42 Bunn TO. Cat rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:379.
43 Cabasso VJ. Local wound treatment and passive immunization. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:551.
44 Calmette A. Notes sur la rage en Indochine et sur les vaccinations antirabiques practiques a Saigon du 14 avril au ler aout. Ann Inst Pasteur (Paris). 1891;5:633. cited by Vodopija and Clark
45 Centers for Disease Control and Prevention. Cases of rabies in human beings in the United States, by circumstances of exposure and rabies virus variant, 1990-2001. http://www.cdc.gov/ncidod/dvrd/rabies/Professional/publications/Surveillance/Surveillance-01/Table2-01.htm.
46 Centers for Disease Control and Prevention. Extension of the raccoon rabies epizootic—United States, 1992. MMWR Morb Mortal Wkly Rep. 1992;41:661.
47 Centers for Disease Control and Prevention. First human death associated with raccoon rabies—Virginia, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:1102.
48 Centers for Disease Control and Prevention. Human death associated with bat rabies—California, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:33.
49 Centers for Disease Control and Prevention. Human rabies: California, 1987. MMWR Morb Mortal Wkly Rep. 1988;37:305.
50 Centers for Disease Control and Prevention. Human rabies: California, 1992. MMWR Morb Mortal Wkly Rep. 1992;41:461.
51 Centers for Disease Control and Prevention. Human rabies: Iowa, 2002. MMWR Morb Mortal Wkly Rep. 2003;523:47.
52 Centers for Disease Control and Prevention. Human rabies: Oregon, 1989. MMWR Morb Mortal Wkly Rep. 1989;38:335.
53 Centers for Disease Control and Prevention. Human rabies: Texas, Arkansas, and Georgia, 1991. MMWR Morb Mortal Wkly Rep. 1991;44:765.
54 Centers for Disease Control and Prevention. Human rabies: Texas, 1990. MMWR Morb Mortal Wkly Rep. 1991;40:132.
55 Centers for Disease Control and Prevention. Human rabies despite treatment with rabies immune globulin and human diploid cell rabies vaccine: Thailand. MMWR Morb Mortal Wkly Rep. 1987;36:759.
56 Centers for Disease Control and Prevention. Human Rabies prevention: United States, 1999: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 1999;48:1.
57 Centers for Disease Control and Prevention. Human-to-human transmission of rabies via corneal transplant—Thailand. MMWR Morb Mortal Wkly Rep. 1981;30:473.
58 Centers for Disease Control and Prevention. Imported human rabies: Australia, 1987. MMWR Morb Mortal Wkly Rep. 1988;37:351.
59 Centers for Disease Control and Prevention. Investigation of rabies infections in organ donor and transplant recipients: Alabama, Arkansas, Oklahoma, and Texas, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:586.
60 Centers for Disease Control and Prevention. Presumptive Abortive Human Rabies: Texas, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:185.
61 Centers for Disease Control and Prevention. Rabies in a laboratory worker. MMWR Morb Mortal Wkly Rep. 1977;26:183.
62 Centers for Disease Control and Prevention. Recovery of a patient from clinical rabies: Wisconsin, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:1171.
63 Centers for Disease Control and Prevention. Systemic allergic reactions following immunization with human diploid cell rabies vaccine. MMWR Morb Mortal Wkly Rep. 1984;33:185.
64 Centers for Disease Control and Prevention. Update: Investigation of rabies infections in organ donor and transplant recipients: Alabama, Arkansas, Oklahoma, and Texas, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:615.
65 Centers for Disease Control and Prevention. Use of reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies. MMWR Morb Mortal Wkly Rep. 2010;59:1.
66 Cenna J, Hunter M, Tan GS, et al. Replication-deficient rabies virus-based vaccines are safe and immunogenic in mice and nonhuman primates. J Infect Dis. 2009;200:1251.
67 Charlton KM, Webster WA, Casey GA, et al. Skunk rabies. Rev Infect Dis. 1988;10:S626.
68 Childs JE. Epidemiology. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002:113.
69 Chomel BB. The modern epidemiological aspects of rabies in the world. Comp Immun Microbiol Infect Dis. 1993;16:11.
70 Christian KA, Blanton JD, Auslander M, et al. Epidemiology of rabies post-exposure prophylaxis—United States of America. 2006-2008. Vaccine. 2009;27:7156.
71 Chutivongse S, Wilde H, Supich C, et al. Postexposure prophylaxis for rabies with antiserum and intradermal vaccination. Lancet. 1990;1:896.
72 CIWEC Medical Clinic Travel Medicine Center. Rabies prevention in Nepal. http://www.ciwec-clinic.com/articles/rabies_prevention_in_nepal.php.
73 Cleaveland S, Fèvre EM, Kaare M, et al. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull World Health Organ. 2002;80:304.
74 Constantine DG. Rabies transmission by air in bat caves. Atlanta, Ga: USDHEW, CDC; 1967.
75 Constantine DG. Rabies transmission by nonbite route. Public Health Reports. 1962;77:287.
76 De Serres G, Dallaire F, Côte M, et al. Bat rabies in the United States and Canada from 1950 through 2007: Human cases with and without bat contact. Clin Infect Dis. 2008;46:1329.
77 Devriendt J, Staroukine M, Costy F, et al. Fatal encephalitis apparently due to rabies: Occurrence after treatment with human diploid cell vaccine but not rabies immune globulin. JAMA. 1982;248:2304.
78 Dietzschold B, Faber M, Schnell MJ. New approaches to the prevention and eradication of rabies. Expert Rev Vaccines. 2003;2:399.
79 Dietzschold B, Rupprecht CE, Tollis M, et al. Antigenic diversity of the glycoprotein and nucleocapsid proteins of rabies and rabies-related viruses: implications for epidemiology and control of rabies. Rev Infect Dis. 1988;10:S785.
80 DiVestea A, Zagari G. La transmission de la rage par voie nerveuse. Pasteur Ann. 1889;3:237. cited by Baer and Lentz
81 DiVestea A, Zagari G. Sulla transmissione rabbia per la via dei nervi. Ann Neurol. 1887;5:113. cited by Baer and Lentz
82 Everard COR, Everard JD. Mongoose rabies. Rev Infect Dis. 1988;10:S610.
83 Fangtao L. The protective effect of the large-scale use of PHKC rabies vaccine in humans in China. Bull WHO. 1990;68:449.
84 Fangtao L, Shubeng C, Yinzhon W, et al. Use of serum and vaccine in combination for prophylaxis following exposure to rabies. Rev Infect Dis. 1988;10:S766.
85 Fekadu M. Canine rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:367.
86 Fekadu M. Latency and aborted rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:191.
87 Fekadu M. Pathogenesis of rabies virus infection in dogs. Rev Infect Dis. 1988;10:S678.
88 Fekadu M. Rabies in Ethiopia. Amer J Epidemiol. 1982;115:266.
89 Fekadu M, Baer GM. Recovery from clinical rabies of two dogs inoculated with a rabies virus strain from Ethiopia. Am J Vet Res. 1980;41:1632.
90 Fekadu M, Endeshqw T, Alemu W, et al. Possible human-to-human transmission of rabies in Ethiopia. Ethiop Med J. 1996;34:123.
91 Fekadu M, Shaddock JH, Baer GM. Intermittent excretion of rabies virus in the saliva of a dog two and six months after it had recovered from experimental rabies. Am J Trop Med Hyg. 1981;30:1113.
92 Fishbein DB. Rabies, in humans. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:519.
93 Flores-Crespo R, Arellano-Sota C. Biology and control of the vampire bat. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:461.
94 Fooks AR, McElhinney LM, Pounder DJ, et al. Case report: Isolation of a European bat lyssavirus type 2a from a fatal human case of rabies encephalitis. J Med Virol. 2003;71:281.
95 Fuenzalida E, Palacios R. Rabies vaccine prepared from brains of infected suckling mice. Boletin Instituto Bacteriologico Chile. 1955;8:3. cited by Sureau
96 Gautret P, Shaw M, Gazin P, et al. Rabies postexposure prophylaxis in returned injured travelers from France, Australia, and New Zealand: A retrospective study. J Travel Med. 2008;15:25.
97 Gelinck LB, Jol-van derZijde CM, Jansen-Hoogendijk AM, et al. Restoration of the antibody response upon rabies vaccination in HIV-infected patients treated with HAART. Aids. 2009;23:2451.
98 Gibbons RV. Cryptogenic rabies, bats, and the question of aerosol transmission. Ann Emerg Med. 2002;39:528.
99 Goldwasser RA, Kissling R. Fluorescent antibody staining of street and fixed rabies virus antigens. Proc Soc Exp Biol Med. 1958;98:219.
100 Goudsmit J, Marissen WE, Weldon WC, et al. Comparison of an anti-rabies human monoclonal antibody combination with human polyclonal antirabies immune globulin. J Infect Dis. 2006;193:796.
101 Grattan-Smith PJ, O’Regan WJ, Ellis PS, et al. Rabies: A second Australian case, with a long incubation period. Med J Aust. 1992;156:651.
102 Gribencha SV, Gribanova LY, Malkov GB, et al. Population structure of some street rabies virus strains. Arch Virol. 1989;104:347.
103 Guerra MA, Curns AT, Rupprecht CE, et al. Skunk and raccoon rabies in the eastern United States: Temporal and spatial analysis. Emerg Infect Dis. 2003;9:1143.
104 Halstead SB. Tissue culture-based rabies vaccines: Vaccine production technology transfer. Rev Infect Dis. 1988;10:S764.
105 Hattwick MAW, Weis TT, Stechschulte CJ, et al. Recovery from rabies; a case report. Ann Int Med. 1972;76:931.
106 Haupt W. Rabies—Risk of exposure and current trends in prevention of human cases. Vaccine. 1999;17:1742.
107 Held JR, Escalante JA, Winkler WG. The international management of rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:505.
108 Hemachudha T, Griffin DE, Johnson RT, et al. Immunologic studies of patients with chronic encephalitis induced by post-exposure Semple rabies vaccine. Neurol. 1988;38:42.
109 Hemachudha T, Phanuphak P, Johnson RT, et al. Neurologic complications of Semple-type rabies vaccine: Clinical and immunologic studies. Neurol. 1987;37:550.
110 Hemachudha T, Sunsaneewitayakul B, Desudchit T, et al. Failure of therapeutic coma and ketamine for therapy of human rabies. J Neurovirol. 2006;12:407.
111 Houff SA, Burton RC, Wilson RW, et al. Human-to-human transmission of rabies virus by corneal transplant. New Eng J Med. 1979;300:603.
112 Hu R, Zhang S, Fooks AR, et al. Prevention of rabies virus infection in dogs by a recombinant canine adenovirus type-2 encoding the rabies virus glycoprotein. Microbes Infect. 2006;8:1090.
113 Hu WT, Willoughby REJr, Dhonau H, et al. Long-term follow-up after treatment of rabies by induction of coma. New Eng J Med. 2007;357:945.
114 Ito N, Sugiyama M, Yamada K, et al. Characterization of M gene-deficient rabies virus with advantages of effective immunization and safety as a vaccine strain. Microbiol Immunol. 2005;49:971.
115 Iwasaki Y. Spread of virus within the central nervous system. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:121.
116 Jackson AC. Human Disease. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002:219.
117 Jackson AC, Warrell MJ, Rupprecht CE, et al. Management of rabies in humans. Clin Infect Dis. 2003;36:60.
118 Jenkins SR, Perry BD, Winkler WG. Ecology and epidemiology of raccoon rabies. Rev Infect Dis. 1988;10:S620.
119 Johnson N, Brookes SM, Fooks AR, et al. Review of human rabies cases in the UK and in Germany. Veterinary Record. 2005;157:715.
120 Johnson N, Fooks A, McColl K. Human rabies case with long incubation. http://www.cdc.gov/EID/content/14/12/1950.htm.
121 Johnston DH, Tinline RR. Rabies control in wildlife. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002:445.
122 Johnston DH, Voight DR, MacInnes CD, et al. An aerial baiting system for the distribution of attenuated or recombinant rabies vaccines for foxes, raccoons, and skunks. Rev Infect Dis. 1988;10:S660.
123 Joshi DD, Regmi DN. Relation between site of bite, type of bite, type of animal bite, and incubation period of hydrophobia cases. J Inst Med (Nepal). 1983;5:135. cited by Shlim et al
124 Koprowski H. Glimpses into the future of rabies research. Rev Infect Dis. 1988;10:S810.
125 Krebs JW, Mandel EJ, Swerdlow DL, et al. Rabies surveillance in the United States during 2004. J Am Vet Med Assoc. 2005;227:1912.
126 Krebs JW, Mandel EJ, Swerdlow DL, et al. Rabies surveillance in the United States during 2003. J Amer Vet Med Assoc. 2004;225:1837.
127 Krebs JW, Wheeling JT, Childs JE. Rabies surveillance in the United States during 2002. J Am Vet Med Assoc. 2003;223:1736.
128 Kuzmin IV, Hughes GJ, Botvinkin AD, et al. Phylogenetic relationships of Irkut and West Caucasian bat viruses within the Lyssavirus genus and suggested quantitative criteria based on the N gene sequence for lyssavirus genotype definition. Virus Res. 2005;111:28.
129 Kuzmin IV, Niezgoda M, Franka R, et al. Possible emergence of West Caucasian bat virus in Africa. http://www.cdc.gov/EID/content/14/12/1887.htm.
130 Kuzman IV, Orciari LA, Arai YT, et al. Bat lyssa viruses (Aravan and Khujand) from Central Asia: Phylogenetic relationships according to N, P and G gene sequences. Viral Res. 2003;97:65.
131 Kuzmin IV, Tordo N. Application to International Committee on Taxonomy of Viruses. http://talk.ictvonline.org/files/ictv_official_taxonomy_updates_since_the_8th_report/m/vertebrate-2008/1208.aspx.
132 Linhart SB. Some factors affecting the oral rabies vaccination of free-ranging carnivores. Rev Sci Tech Off Int Epiz. 1993;12:109.
133 Linhart SB, Creekmore TE, Corn JL, et al. Evaluation of baits for oral rabies vaccination of mongooses: Pilot field trials in Antigua, West Indies. J Wildlife Dis. 1993;29:290.
134 Loza RE, Rojas E, Gomez L, et al. Development of an edible rabies vaccine in maize using the Vnukovo strain. Dev Biol (Basel). 2008;131:477.
135 Lumlertdacha B, Wacharapluesadee S, Chanhoome L, et al. Bat lyssavirus in Thailand. J Med Assoc Thai. 2005;88:1011.
136 Madhusudana SN, Nagaraj D, Uday M, et al. Partial recovery from rabies in a six-year-old girl. Int J Infect Dis. 2002;6:85.
137 McDermid RC, Saxinger L, Lee B, et al. Human rabies encephalitis following bat exposure: Failure of therapeutic coma. http://www.cmaj.ca/cgi/content/full/178/5/557.
138 Meara FS. The treatment of acute infectious diseases, ed 2 (revised). Macmillan; 1921. p 699, cited by Grattan-Smith et al
139 Meslin FX. Rabies as a traveler’s risk, especially in high-endemicity areas. J Travel Med. 2005;12:S30.
140 Messenger SL, Smith JS, Orciari LA, et al. Emerging pattern of rabies deaths and increased viral infectivity. Emerg Infect Dis. 2003;9:151.
141 Moran GJ, Talan DA, Mower W, et al. Appropriateness of rabies postexposure prophylaxis treatment for animal exposures. Emergency ID Net Study Group. JAMA. 2000;284:1001.
142 Morimoto K, Shoji Y, Inoue S. Characterization of P gene-deficient rabies virus: Propagation, pathogenicity and antigenicity. Virus Res. 2005;111:64.
143 Műller T, Dietzschold B, Ertl H, et al. Development of a mouse monoclonal antibody cocktail for post-exposure rabies prophylaxis in humans. http://www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0000542.
144 Murray KO, Holmes KC, Hanlon C. Rabies in vaccinated dogs and cats in the United States, 1997-2001. J Am Vet Med Assoc. 2009;235:691.
145 Nathanson N, Gonzalez-Scarano F. Immune response to rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:145.
146 Negri A. Beitrag zum studium der aetiologie der Tollwut. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1903;43:507. cited by Bourhy et al.
147 Nicholson KG. Modern vaccines: Rabies. Lancet. 1990;1:1201.
148 Niezgoda M, Hanlon CA, Rupprecht CE. Animal Rabies. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002:163.
149 Nishizono A, Khawplod P, Ahmed K, et al. A simple and rapid immunochromatographic test kit for rabies diagnosis. Microbiol Immunol. 2008;52:243.
150 Ogunkoya AB, Beran GW, Umoh JU, et al. Serologic evidence of infection of dogs and man in Nigeria by lyssaviruses (family Rhabdoviridae). Trans Roy Soc Trop Med Hyg. 1990;84:842.
151 Pacer RE, Fishbein DB, Baer GM, et al. Rabies in the United States and Canada, 1983. MMWR Morb Mortal Wkly Rep. 1985;34:11SS.
152 Parham GL. Rabies in the United States, 1981. MMWR Morb Mortal Wkly Rep. 1983;32:33SS.
153 Pasteur L, Chamberland CE, Roux M. Nouveaux faits pour servir a la connaissance de la rage. C R Acad Sci. 1882;95:1187. cited by Steele.
154 Perea AI, Loza RE, Rojas E, et al. Expression of the rabies virus nucleoprotein in plants at high-levels and evaluation of immune responses in mice. Plant Cell Rep. 2008;27:677.
155 Perl DP, Good PF. The pathology of rabies in the central nervous system. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:163.
156 Phanuphak P, et al. Humoral and cell-mediated immune responses to various economical regimens of purified Vero-cell rabies vaccine. Asian Pacific J Aller Immunol. 1987;5:33. cited by Phanuphak et al
157 Phanuphak P, Khaoplod P, Benjavongkulchai M, et al. What happens if intradermal injections of rabies vaccine are partially or entirely injected subcutaneously? Bull WHO. 1990;68:83.
158 Phanuphak P, Ubolyam S, Sirivichayakul S. Should travelers in rabies endemic areas receive pre-exposure rabies immunization? Ann Med Interne. 1944;145:409.
159 Porras C, Barboza JJ, Fuenzalida E, et al. Recovery from rabies in man. Ann Int Med. 1976;85:44.
160 Préhaud C, Takehara K, Flamand A, et al. Immunogenic and protective properties of rabies virus glycoprotein expressed by baculovirus vectors. Virology. 1989;173:390.
161 Prosniak M, Faber M, Hanlon CA, et al. Development of a cocktail of recombinant-expressed human rabies virus-neutralizing monoclonal antibodies for postexposure prophylaxis of rabies. J Infect Dis. 2003;187:53.
162 Quiambao BP, DyTioco HZ, Dizon RM, et al. Rabies post-exposure prophylaxis in the Philippines: Health status of patients having received purified equine F(ab’)2 fragment rabies immunoglobulin (Favirab). http://www.plosntds.org/article/info%3Adoi%2F10.1371%2Fjournal.pntd.0000542.
163 Remington PL, Shope T, Andrews J. A recommended approach to the evaluation of human rabies exposure in an acute-care hospital. JAMA. 1985;254:67.
164 Remlinger P. Accidents paralytiques au cours du tratement antirabique. Ann Inst Pasteur Paris. 1905;19:625. cited by Steele
165 Rosatte RC. Bat rabies in Canada: History, epidemiology and prevention. Can Vet J. 1987;28:754.
166 Rosatte RC, Donovan D, Davies JC, et al. Aerial distribution of Onrab® baits as a tactic to control rabies in raccoons and striped skunks in Ontario, Canada. J Wildlife Dis. 2009;45:363.
167 Rosatte RC, MacInnes CD, Power MJ, et al. Tactics for the control of wildlife rabies in Ontario (Canada). Rev Sci Tech Off Int Epiz. 1992;12:95.
168 Rosatte RC, Power MJ, MacInnes CD, et al. Trap-vaccinate-release and oral vaccination for rabies control in urban skunks, raccoons and foxes. J Wildlife Dis. 1992;28:562.
169 Rosatte RC, Pybus MJ, Gunson JR. Population reduction as a factor in the control of skunk rabies in Alberta. J Wildlife Dis. 1986;22:459.
170 Roumiantzeff M, Ajjan N, Vincent-Falquet JC. Experience with preexposure rabies vaccination. Rev Infect Dis. 1988;10:S751.
171 Roux E. Not sur un moyen de conserver les moelles rabiques avec leur virulence. Ann Inst Pasteur (Paris). 1987;1:87. cited by Vodopija and Clark
172 Rubin RH, Hattwick MAW, Jones S, et al. Adverse reactions to duck embryo rabies vaccine: Range and incidence. Ann Int Med. 1973;78:643.
173 Rubin RJ, Corey L. Preventing rabies in humans. South Med J. 1974;67:1472.
174 Rudd RT, Trimarchi CV. Development and evaluation of an in vitro virus isolation procedure as a replacement for the mouse inoculation test in rabies diagnosis. J Clin Microbiol. 1989;27:2522.
175 Rupprecht CE, Blass L, Smith K, et al. Brief Report: Human infection due to recombinant vaccinia-rabies glycoprotein virus. New Eng J Med. 2001;345:582.
176 Rupprecht CE, Briggs D, Brown CM, et al. Evidence for a 4-dose vaccine schedule for human rabies postexposure prophylaxis in previously non-vaccinated individuals. Vaccine. 2009;27:7140.
177 Rupprecht CE, Dietzschold B. Editorial: perspectives on rabies virus pathogenesis. Lab Invest. 1987;57:603.
178 Rupprecht CE, Dietzschold B, Wunner WH, et al. Antigenic relationships of Lyssaviruses. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:69.
179 Rupprecht CE, Gibbons RV. Prophylaxis against rabies. N Engl J Med. 2004;351:25.
180 Rupprecht CE, Hamir AN, Johnston DH, et al. Efficacy of a vaccinia-rabies glycoprotein recombinant virus vaccine in raccoons (Procyon lotor). Rev Infect Dis. 1988;10:S803.
181 Rupprecht CE, Shlim DR. Rabies. http://www.nc.cdc.gov/travel/yellowbook/2010/chapter-2/rabies.aspx.
182 Ryan ET, Kain KC. Health advice and immunizations for travelers. New Eng J Med. 2000;342:1716.
183 Schneider LG. Spread of virus from the central nervous system. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:133.
184 Shetty RA, Chaturvedi S, Singh Z. Profile of animal bite cases in Pune. J Commun Dis. 2005;37:66.
185 Shlim DR, Schwartz E, Houston R. Rabies immunoprophylaxis strategy in travelers. J Wilderness Med. 1991;2:15.
186 Sirkwin S, Likanonsakul S, Pattamadilok S, et al. Antibody response to an eight-site intradermal rabies vaccination in patients infected with Human Immunodeficiency Virus. Vaccine. 2009;27:4350.
187 Sitthi-Amorn C, Jiratanavattana V, Keoyoo J, et al. The diagnostic properties of laboratory tests for rabies. Inter J Epidemiol. 1987;16:602.
188 Smith JS. Molecular Epidemiology. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002:79.
189 Smith JS. Monoclonal antibody studies of rabies in insectivorous bats of the United States. Rev Infect Dis. 1988;10:S637.
190 Smith JS. Rabies virus epitopic variation: Use in ecologic studies. Adv Virus Res. 1989;36:215.
191 Smith JS, Orciari LA, Yager PA. Molecular epidemiology of rabies in the United States. Virology. 1995;6:387.
192 Smith JS, Seidel HD. Rabies: A new look at an old disease. Prog Med Virol. 1993;40:82.
193 Steele JH, Fernandez PJ. History of rabies and global aspects. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:1.
194 Sureau P. History of rabies: advances in research towards rabies prevention during the last 30 years. Rev Infect Dis. 1988;10:S581.
195 Sureau P, Ravisse P, Rollin PE. Rabies diagnosis by animal inoculation, identification of Negri bodies, or ELISA. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:203.
196 Swaddiwuthipong W, Weniger BG, Wattanasri S, et al. A high rate of neurological complications following Semple anti-rabies vaccine. Trans Roy Soc Trop Med Hyg. 1988;82:472.
197 Swamy HS, Shankar SK, Chandra PS, et al. Neurological complications due to beta-propiolactone (BPL)-inactivated antirabies vaccination. J Neurol Sci. 1984;63:111.
198 Tordo N, Foumier A, Jailet C, et al. Canine adenovirus based rabies vaccines. Dev Biol (Basel). 2008;131:467.
199 Trimarchi CV, Debbie JG. The fluorescent antibody in rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:219.
200 Trimarchi CV, Smith JS. Diagnostic Evaluation. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002:307.
201 Tuttle MD, Kern SJ. Bats and public health. Milwaukee Public Museum: Contributions in Biology and Geology No. 48. December 28, 1981.
202 Tyrell DAJ, Nicholson KG. Rabies in Britain. Brit Med J. 1990;300:137.
203 U.S. Department of State. Medical Information for Americans Abroad. http://travel.state.gov/travel/tips/brochures/brochures_1215.html.
204 Van Gehuchten A. Les lesions histologiques dans la rage humane. Sem Med. 1900;20:40. cited by Perl and Good
205 Van Gehuchten A, Nelis C. Les lesions histologiques de la rage chez les animaux et chez l’homme. Bull Acad R Med Belg. 1900;14:31. cited by Perl and Good
206 Veeraraghavan N, Subrahmanyan TP. The value of 5 per cent Semple vaccine prepared in distilled water in human treatment: comparative mortality among the treated and untreated. Ind J Med Res, 1958, 518. cited by Nicholson
207 Vibulbandhitkij S. Work report: Data from the rabies patients at Bamrasnaradura Hospital between 1971 and 1977. In: Thongcharoen P, editor. Rabies. Aksarasamai: Bangkok; 1980:235. cited by Warrell et al
208 Vodopija I. Overview to Part VI: Rabies and its prevention in man. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:515.
209 Vodopija I, Clark HF. Human vaccination against rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:571.
210 Vodopija I, Sureau P, Smerdel S, et al. Interaction of rabies vaccine with human rabies immunoglobulin and reliability of a 2-1-1 schedule application for post-exposure treatment. Vaccine. 1988;6:283.
211 Wallace CK. Opening remarks (International Symposium on Research Towards Rabies Prevention). Rev Infect Dis. 1988;10:S579.
212 Wandeler AI. Oral immunization of wildlife. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:485.
213 Wandeler AI, Budde A, Capt S, et al. Dog ecology and dog rabies control. Rev Infect Dis. 1988;10:S684.
214 Wang SP. Statistical studies of human rabies in Taiwan. J Formosa Med Assoc. 1956;55:548. cited by Vodopija and Clark
215 Warrell DA, Warrell MJ. Human rabies and its prevention: An overview. Rev Infect Dis. 1988;10:S726.
216 Warrell MJ, Nicholson KG, Warrell DA, et al. Economical multiple-site intradermal immunization with human diploid-cell-strain vaccine is effective for post-exposure rabies prophylaxis. Lancet. 1985;1:1059.
217 Warrell MJ, Warrell DA. Rabies and other lyssavirus diseases. Lancet. 2004;363:959.
218 Wertheim HFL, Nguyen TQ, Nguyen KAT, et al. Furious rabies after an atypical exposure. http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.1000044.
219 World Health Organization Expert Committee on Rabies. Eighth Report. Geneva: World Health Organization; 1992. (WHO Technical Report Series, No. 824)
220 World Health Organization. Expert Consultation on Rabies. First Report. Geneva: World Health Organization; 2004. (WHO Technical Report Series, No. 931) http://books.google.com/books?id=kMI0uDblIrQC&printsec=frontcover&source=gbs_v2_summary_r&cad=0#v=onepage&q=&f=false
221 World Health Organization. Media Center: Rabies. http://www.who.int/mediacentre/factsheets/fs099/en/.
222 World Health Organization. Rabies. http://www.who.int/rabies/PEP_prophylaxis_guidelines_June09.pdf.
223 Wilde H, Chutivongse S. Equine rabies immune globulin: A product with an undeserved poor reputation. Amer J Trop Med Hyg. 1990;42:175.
224 Wilhelm U, Schneider LG. Oral immunization of foxes against rabies: Practical experiences of a field trial in the Federal Republic of Germany. Bull WHO. 1990;68:87.
225 Wilkerson JA. Rabies: Epidemiology, diagnosis, prevention, and prospects for worldwide control. Wild Environ Med. 1995;6:48.
226 Wilkerson JA. Rabies update. Wild Environ Med. 2000;11:31.
227 Willoughby RE, Tieves KS, Hoffman GM, et al. Brief Report: Survival after treatment of rabies with induction of coma. New Eng J Med. 2005;352:2508.
228 Winkler WG, Fashinell TR, Leffingwell L, et al. Airborne rabies transmission in a laboratory worker. JAMA. 1973;226:1219.
229 Winkler WG, Jenkins SR. Raccoon rabies. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:325.
230 Wunner WH. Rabies Virus. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Academic Press; 2002:23.
231 Wunner WH. The chemical composition and molecular structure of rabies viruses. In: Baer GM, editor. The natural history of rabies. ed 2. Boca Raton, Fla: CRC Press; 1991:31.
232 Wunner WH, Larson JK, Dietzschold B, et al. The molecular biology of rabies viruses. Rev Infect Dis. 1988;10:S771.