Chapter 12 Purines and pyrimidines
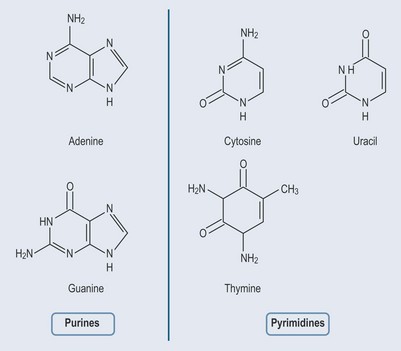
Purines and pyrimidines are known as nitrogenous bases. The two groups have different features (Figure 12.1):
These nitrogenous bases are crucial not only to the storage and transmission of genetic information, but also as a means of storing mobile energy. They can be synthesized in the body from basic components. The degraded bases can be salvaged and reused (see Chapter 37 ‘Metabolic disorders’, p. 295).
Nucleosides and Nucleotides
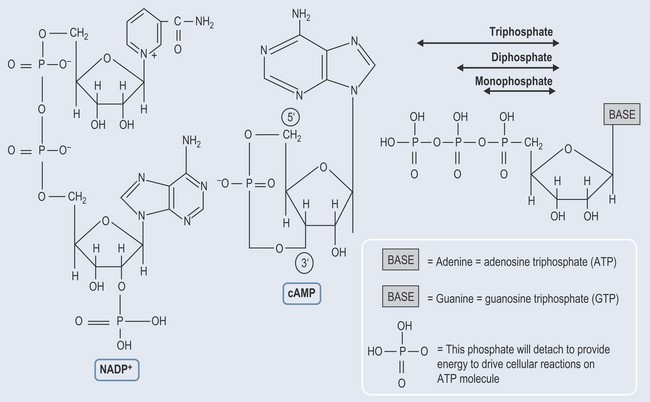
When a sugar is added to a nitrogenous base, a nucleoside is formed (Figure 12.2).
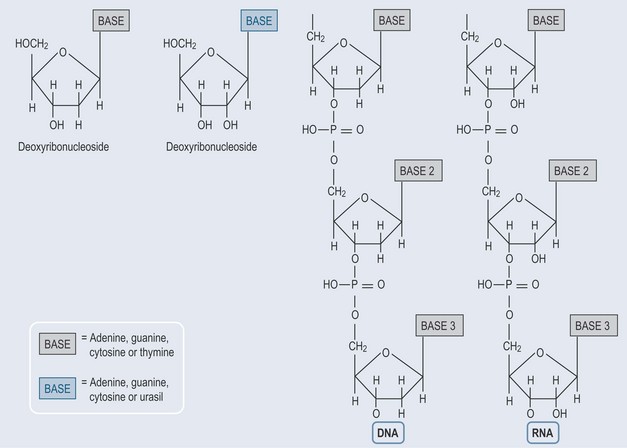
Figure 12.2 The basic structure of nucleosides and their inclusion in the nucleotide structures of RNA and DNA.
When phosphate groups attach to the nucleoside, a nucleotide is formed (Figure 12.2) with one phosphate (monophosphate), two phosphate (diphosphate) or three phosphate (triphosphate) groups attached.
Nucleotides
Nucleotides are very important chemicals. they:
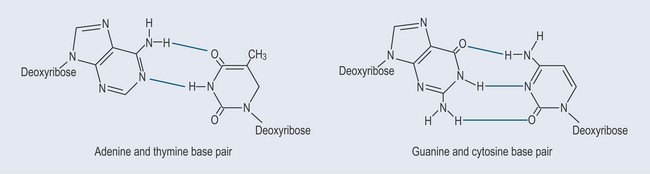
The Importance of Hydrogen Bonds in DNA (Figure 12.4)
The two strands of the DNA structure are held together by hydrogen bonds between the nitrogenous bases (see Chapter 3 ‘Bonds found in biological chemistry’, p. 18) (Figure 12.4). This allows the structure to be pulled apart and put back together in protein synthesis (see Figure 11.6, p. 85).
Metabolism of Purines and Pyrimidines
Those purines and pyrimidines that are not resynthesized are removed from the body and their breakdown results in the production of uric acid, primarily by the liver, which is excreted by the kidneys. A defect in this process will result in a clinical condition, as in the case of gout (see Chapter 37 ‘Metabolic disorders’, p. 295).
DNA and RNA
It is important to note that although there is a turnover of RNA within a cell, this does not occur with DNA. Instead, enzymes excise the damaged parts during a process of repair. If this process goes wrong then cell metabolism malfunctions. This is considered when treating viral infections and using chemotherapy to damage the viral or aberrant cell DNA (see Chapter 39 ‘Chemotherapy’, p. 309).