Advances in Anesthesia, Vol. 28, No. 1, 2010
ISSN: 0737-6146
doi: 10.1016/j.aan.2010.07.006
Postdural Puncture Headache
History and current relevance
As one of the earliest recognized complications of regional anesthesia, PDPH has a long and colorful history [1]. Dr August Bier noted this adverse effect in the first patient to undergo successful spinal anesthesia on August 16, 1898. Bier observed: “Two hours after the operation his back and left leg became painful and the patient vomited and complained of severe headache. The pain and vomiting soon ceased, but headache was still present the next day” (italics added) [2]. The following week, Bier and his assistant, Dr August Hildebrandt, performed experiments with cocainization of the spinal cord on themselves. In a description scarcely improved upon in an intervening century, Bier later reported first-hand his experience in the days to follow: “I had a feeling of very strong pressure on my skull and became rather dizzy when I stood up rapidly from my chair. All these symptoms vanished at once when I lay down flat, but returned when I stood up… I was forced to take to bed and remained there for nine days, because all the manifestations recurred as soon as I got up… The symptoms finally resolved nine days after the lumbar puncture” [2]. In medical history, few complications have come to be considered as closely linked to a specific technique as PDPH with spinal anesthesia.
Employing the methods of the early twentieth century, spinal anesthesia was frequently followed by severe and prolonged headache, casting a long shadow over the development and acceptance of this modality. Investigations into the cause of these troubling symptoms eventually led to the conclusion that they were due to persistent cerebrospinal fluid (CSF) loss through the rent created in the meninges. The most notable successful efforts to minimize the loss of CSF were through the use of smaller gauge and “noncutting” needles (as convincingly demonstrated by Vandam and Dripps [3] and Hart and Whitacre [4], respectively, in the 1950s). Despite these significant advances in prevention, PDPH remained a frustratingly common occurrence.
The extensive search for effective treatments for PDPH dates to Bier’s time. Yet efforts through the first half of the 20th century, while often intensive and creative, were questionably worthwhile. In a monograph intended to be a comprehensive review of PDPH from the 1800s through 1960, Dr Wallace Tourette and colleagues [5] cite dozens of separate and far-ranging treatment recommendations, including such interventions as intravenous ethanol, x-rays to the skull, sympathetic blocks, and manipulation of the spine. Unfortunately, before the introduction of the EBP there were no treatment measures that could be described as significant improvements over the simple passage of time. In his 1955 textbook, Complications of Regional Anesthesia, Dr Daniel Moore describes in detail a full 3-day treatment protocol for PDPH. He concludes by noting that 3 days is the usual duration of untreated mild to moderate headaches, but that “nevertheless, the patient feels an attempt to help his problem is being made” [6].
The EBP, a startlingly unique medical procedure, proved to be the breakthrough in the treatment of PDPH. The concept of using autologous blood to “patch” a hole in the meninges was introduced in late 1960 by Dr James Gormley, a general surgeon [7]. Yet Gormley’s brief report went largely unnoticed for nearly a decade because, to the practitioners of the day, an iatrogenic epidural hematoma raised serious concerns of scarring, infection, and nerve damage. The procedure was only later popularized in anesthesia circles, and performed as a true epidural injection, largely through the work of Drs Anthony DiGiovanni and Burdett Dunbar [8]. The EBP procedure was further refined through the 1970s as the volume of blood commonly used increased to 20 mL [9]. Today, the EBP is nearly universally employed as the cornerstone of treatment for severe PDPH [10].
PDPH remains a prominent clinical concern to the present day. Largely due to modifications in practice that followed the identification of risk factors, rates of PDPH following spinal anesthesia have steadily declined; from an incidence exceeding 50% in Bier’s time, to around 10% in the 1950s [3], until currently a rate of 1% or less can be reasonably expected. However, as perhaps the highest risk group, an unfortunate 1.7% of obstetric patients continue to experience PDPH after spinal anesthesia using 27-gauge Whitacre needles [11]. Since epidural techniques intend to avoid meningeal puncture, they are attractive alternatives to spinal anesthesia. Yet occasional ADP, with either the needle or catheter, is unavoidable (or may be unrecognized at the time in more than 25% of patients who eventually develop PDPH) [12]. In nonobstetric situations (eg, interlaminar epidural steroid injections), the rate of ADP should be considerably less than 0.5%. However, ADP is of greatest concern in the obstetric anesthesia setting, where the incidence of this adverse event is around 1.5% [11]. Over half of all patients who experience an ADP will eventually develop headache symptoms, and many studies in obstetric populations report PDPH rates exceeding 75%. In addition to anesthesia interventions, PDPH remains a too-common iatrogenic complication following myelography and diagnostic/therapeutic lumbar puncture (LP). In these situations, rates of PDPH around 10% are commonly cited, as practitioners often continue to use Quincke needles, and large-gauge needles are considered necessary because of the viscosity of contrast material as well as to facilitate the timely collection of CSF. Consequently, there is evidence to suggest that the majority of instances of PDPH now have a nonanesthesia origin [13].
The practical significance of PDPH is illustrated in being noted in the American Society of Anesthesiologists Closed Claims Project database as one of the most frequent claims for malpractice involving obstetric anesthesia [14], regional anesthesia [15], and chronic pain management [16]. Justifiably, headache is the most commonly disclosed risk when obtaining consent for spinal and epidural anesthesia [17]. The potentially serious nature of this complication necessitates inclusion in informed consent involving any procedure that may result in PDPH. As part of this discussion, patients should also be apprised of the normal delayed onset of symptoms and given clear instructions for the timely provision of advice or management should they experience adverse effects.
Pathophysiology
It has long been accepted that PDPH results from a disruption of normal CSF homeostasis. However, despite a great deal of research and observational data, the pathophysiology of PDPH remains incompletely understood [18].
CSF is produced primarily in the choroid plexus at a rate of approximately 0.35 mL/min and reabsorbed through the arachnoid villa. The total CSF volume in adults is maintained around 150 mL, of which approximately half is extracranial, and gives rise to normal lumbar opening pressures of 5 to 15 cm H2O in the horizontal position (40–50 cm H2O in the upright position). It has been shown experimentally that the loss of approximately 10% of total CSF volume predictably results in the development of typical PDPH symptoms, which resolve promptly with reconstitution of this deficit [19]. It is agreed that PDPH is caused by the loss of CSF through a persistent leak in the meninges. In this regard, it has been postulated that the cellular arachnoid mater (containing frequent tight junctions and occluding junctions) is perhaps more important than the more permeable and acellular dura mater in the genesis of PDPH [20]. This tenet has led some, including this author, to advocate the term “meningeal puncture headache” (MPH) as more accurate and descriptive than PDPH [21,22]. However, “postdural puncture headache” is used in this review as it is the more common terminology at the present time. Regardless of nomenclature, it is also important to acknowledge that references to “dural puncture” throughout the medical literature (including this article) actually describe puncture of the dura-arachnoid and are more correctly termed “meningeal puncture.” Furthermore, the apparent role of the arachnoid mater calls into question the significance of the many published studies concerning PDPH of isolated in vitro dura mater.
Multiple neural pathways are involved in generating the symptoms of PDPH. These pathways include the ophthalmic branch of the trigeminal nerve (CN V1) in frontal head pain, cranial nerves IX and X in occipital pain, and cervical nerves C1 to C3 in neck and shoulder pain [23]. Nausea is attributed to vagal stimulation (CN X). Auditory and vestibular symptoms are secondary to the direct communication between the CSF and the perilymph via the cochlear aqueduct, which results in decreased perilymphatic pressures in the inner ear and an imbalance between the endolymph and perilymph [24]. Significant visual disturbances may represent a transient palsy of the nerves supplying the extraocular muscles of the eye (CN III, IV, and, VI). Here, the lateral rectus muscle is most often involved, which is attributed to the long, vulnerable intracranial course of the abducens nerve (CN VI) [25]. Other, much less frequent cranial nerve palsies of the trigeminal (CN V), facial (CN VII), and auditory (CN VIII) nerves have also been reported [26].
Clinical presentation and characteristics
Onset
Onset of symptoms is generally delayed, with headache usually beginning 12 to 48 hours and rarely more than 5 days following meningeal puncture. In their landmark observational study, Vandam and Dripps reported onset of headache symptoms within 3 days of spinal anesthesia in 84.8% of patients for whom such data were available [3]. More recently, Lybecker and colleagues [27] performed a detailed analysis of 75 consecutive patients with PDPH following spinal anesthesia (primarily using 25-gauge cutting-point needles). Although none of their patients noted the onset of symptoms during the first hour following meningeal puncture, 65% experienced symptoms within 24 hours and 92% within 48 hours. An onset of symptoms within 1 hour of neuraxial procedures is suspicious for pneumocephalus, especially in the setting of an epidural loss-of-resistance technique using air [28]. Occasional reports of unusually delayed onset of PDPH highlight the importance of seeking a history of central neuraxial instrumentation whenever positional headaches are evaluated [29].
Presentation
The cardinal feature of PDPH is a postural nature, with headache symptoms worsening in the upright position and relieved, or at least improved, with recumbency. The International Classification of Headache Disorders further describes this positional quality as worsening within 15 minutes of sitting or standing, and improving within 15 minutes after lying [30]. Headache is always bilateral, with a distribution that is frontal (25%), occipital (27%), or both (45%) [27]. Headaches are typically described as “dull/aching”, “throbbing”, or “pressure-type”.
The severity of headache symptoms, a feature with important ramifications for treatment, varies considerably among patients. Although there is no widely accepted severity scale, one practical approach is to have patients simply rate their headache intensity using a 10-point analog scale, with 1 to 3 classified as “mild,” 4 to 6 “moderate,” and 7 to 10 “severe.” Lybecker and colleagues [27] further categorized patients according to restriction in physical activity, degree of confinement to bed, and presence of associated symptoms (Fig. 1). A prospective analysis of PDPH after spinal anesthesia using the Lybecker classification system demonstrated that 11% were mild, 23% moderate, and 67% severe.
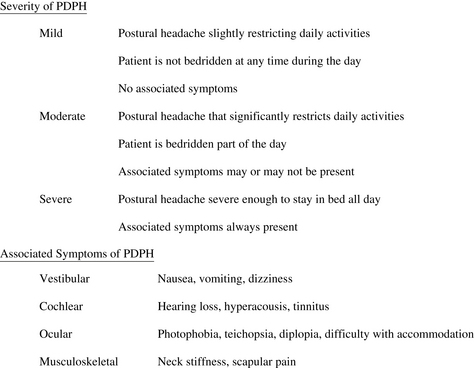
Fig. 1 Classification of severity of postdural puncture headache (PDPH).
(Adapted from Lybecker H, Djernes M, Schmidt JF. Postdural puncture headache (PDPH): onset, duration, severity, and associated symptoms. An analysis of 75 consecutive patients with PDPH. Acta Anaesthesiol Scand 1995;39:606; with permission.)
Associated Symptoms
If headaches are severe, they are more likely to be accompanied by a variety of other symptoms. Pain and stiffness in the neck and shoulders is common, and seen in nearly half of all patients experiencing PDPH [31]. With questioning, nausea may be reported by a majority of patients and can lead to vomiting [27].
Patients uncommonly may experience auditory or visual symptoms [24], and the risk for either appears to be directly related to needle size [25,32]. In the large study of PDPH by Vandam and Dripps [3], each was seen to a clinically apparent degree in 0.4% of patients. Auditory symptoms include hearing loss, tinnitus, and even hyperacousis, and can be unilateral. It is interesting to note that subclinical hearing loss, especially in the lower frequencies, has been found to be common following spinal anesthesia, even in the absence of PDPH [32]. Closely associated with auditory function, vestibular disturbances (dizziness or vertigo) may also occur. Visual problems include blurred vision, difficulties with accommodation, mild photophobia, and diplopia [25]. In contrast to headache complaints, which are consistently bilateral, nearly 80% of episodes of diplopia secondary to meningeal puncture involve unilateral cranial nerve palsies.
Risk factors
Patient Characteristics
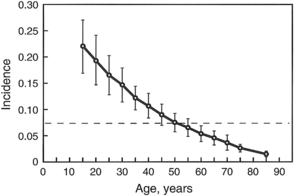
The patient characteristic having the greatest impact on risk of PDPH is age. Uncommonly reported in children younger than 10 years, PDPH has a peak incidence in the teens and early 20s [33]. The incidence then declines over time, becoming much less frequent in patients older than 50 years (Fig. 2). Females have long been recognized as being at increased risk for PDPH, and a systematic review of published studies found the odds of developing PDPH were significantly lower for male than age-matched nonpregnant female subjects (odds ratio = 0.55; 95% confidence interval, 0.44–0.67) [34]. The etiology behind this gender difference is not clear. Body mass index (BMI; calculated as the weight in kilograms divided by height in meters, squared) appears to be a mixed risk factor. Morbid obesity presents obvious technical difficulties for central neuraxial procedures, increasing the likelihood of multiple needle passes and ADP [35]. However, low BMI has been reported as an independent risk factor for PDPH [36] and high BMI (ie, obesity) may actually decrease risk, possibly due to a beneficial effect of increased intra-abdominal pressure [37].
Pregnancy has traditionally been regarded as a risk factor for PDPH [3], but this consideration partially reflects the young age as well as the high incidence of ADP in the gravid population. Although controversial, pushing during the second stage of labor, thought to promote the loss of CSF through a hole in the meninges, has been reported to influence the risk of PDPH following ADP. Angle and colleagues [38] noted that the cumulative duration of bearing down correlated with the risk of developing PDPH in patients who had experienced an ADP. These investigators also found that patients who avoided pushing altogether (proceeded to cesarean delivery before reaching second stage of labor) had a much lower incidence of PDPH (10%) than those who pushed (74%).
PDPHs appear to have an interesting association with other headaches. Patients who report having had a headache within the week prior to LP have been observed to have a higher incidence of PDPH [36]. On further analysis, only those with chronic bilateral tension-type headaches were found to be at increased risk [39]. A history of unilateral headache [39] or migraine [40] has not been linked to an increased risk of PDPH. Menstrual cycle, a factor in migraine headaches, did not influence the rate of PDPH in one small pilot study [41]. Patients with a history of previous PDPH, particularly women, appear to have an increased risk for new PDPH after spinal anesthesia [42]. With epidural procedures, patients with a history of ADP have been shown to be at slightly increased risk for another ADP (and subsequent PDPH) [43].
Procedural Details
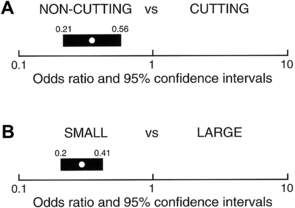
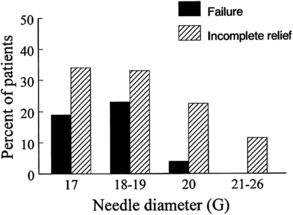
Needle size and tip design are the most important procedural factors related to PDPH (Fig. 3) [44]. Needle size is directly related to the risk of PDPH. Meningeal puncture with larger needles is associated with a higher incidence of PDPH [3], more severe headache and associated symptoms [44], a longer duration of symptoms [45], and a greater need for definitive treatment measures [46]. Needle tip design is also a major influence, with “noncutting” needles clearly associated with a reduced incidence of PDPH compared with “cutting” (usually Quincke) needles of the same gauge. In general, noncutting needles have an opening set back from a tapered (“pencil-point”) tip and include the Whitacre, Sprotte, European, Pencan, and Gertie Marx needles. Adding to this somewhat confusing terminology, noncutting needles are sometimes still incorrectly referred to as “atraumatic” needles, despite being shown with electron microscopy to produce a more traumatic rent in the dura than cutting needles (perhaps resulting in a better inflammatory healing response) [47]. The influence of needle size on risk of PDPH appears to be greatest for cutting needles (for example, the reduction seen in the incidence of PDPH between 22- and 26-gauge sizes is greater for cutting than noncutting needles). Insertion of cutting needles with the bevel parallel to the long axis of the spine significantly reduces the incidence of PDPH [48]. This observation was for many years attributed to a spreading rather than cutting of longitudinal-oriented dural fibers. However, scanning electron microscopy reveals the dura to be made of many layers of concentrically directed fibers [49], and the importance of needle bevel insertion is now thought to be related to longitudinal tension on the meninges, particularly in the upright position, and its influence on CSF leakage through holes having differing orientations.
Not surprisingly, a greater number of meningeal punctures have been shown to increase the rate of PDPH [50]. The degree of experience/comfort/skill of the operator is clearly associated with the incidence of ADP during epidural procedures, with higher ADP rates consistently reported when procedures are performed by residents [51,52]. The risk of ADP also appears to be higher for procedures done at night, strongly suggesting a significant contribution of operator fatigue [53].
Several procedural details do not appear to influence the rate of development of PDPH, including patient position at the time of meningeal puncture, “bloody tap” during spinal anesthesia, addition of opiates to spinal block, and volume of CSF removed (for diagnostic purposes) [1].
Prevention
Although prophylaxis is most simply thought of as preventing any symptoms of PDPH, in the clinical context this issue is deceptively complex. It is important to appreciate that significant “prevention” may encompass several other end points, such as a reduced incidence of severe PDPH, a shorter duration of symptoms, or decreased need for EBP. Unfortunately, despite the clear relevance of this issue, the overall quality of evidence for preventive measures is generally weak [54,55].
General Measures
Though only recently used for neuraxial techniques, the use of ultrasonography for regional anesthesia holds some promise in reducing the risk of PDPH. Ultrasonography can decrease the number of needle passes required for regional procedures and has been shown to accurately predict the depth of the epidural space [56]. Further study is ongoing to define this potential for ultrasonography to reduce the incidence of ADP and PDPH.
Pharmacologic measures, notably caffeine, continue to be widely used in hopes of decreasing the incidence of PDPH following meningeal puncture [10]. In support of this practice, one small study (n = 60) found that intravenous caffeine (500 mg caffeine sodium benzoate within 90 minutes after spinal anesthesia) significantly reduced the incidence of moderate to severe headache [57]. However, generalizing these results to other clinical settings is difficult, as this investigation involved the use of 22-gauge Quincke needles in a relatively young patient population. In another study, oral caffeine (75 or 125 mg) administered every 6 hours during the first 3 days following spinal anesthesia failed to influence the rate of PDPH [58]. A critical review of the available evidence fails to support the use of caffeine in prevention of PDPH [59]. More recently, a small pilot study raised the possibility of using the long-acting 5-HT receptor agonist frovatriptan (2.5 mg/d orally for 5 days) in the prevention of PDPH [60]. At present, however, there is no proven pharmacologic prophylaxis for PDPH.
A recent survey of United States anesthesiologists reported that bed rest and aggressive oral and intravenous hydration continue to be employed by a sizable majority as prophylactic measures against PDPH [10]. However, a systematic review of the literature regarding bed rest versus early mobilization after dural puncture failed to show any evidence of benefit from bed rest, and suggested that the risk of PDPH may actually be decreased by early mobilization [61]. It is notable that the practice of United States anesthesiologists regarding bed rest is in direct contrast to that seen in United Kingdom maternity units, where 75% encourage mobilization as early as possible following ADP as prophylaxis against PDPH [62]. Likewise, a randomized prospective trial of increased oral hydration following LP failed to decrease the incidence or duration of PDPH [61]. In summary, at this time there is no evidence to support the common recommendations of bed rest or aggressive hydration in the prevention of PDPH.
Spinal Technique
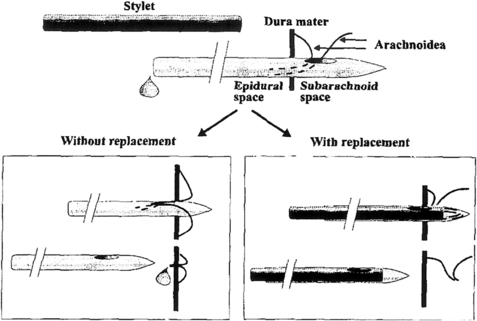
Replacing the stylet after CSF collection but before needle withdrawal is an effective means of lowering the incidence of PDPH after LP. This recommendation is based on a prospective, randomized study of 600 patients using 21-gauge Sprotte needles. In this setting, replacing the stylet reduced the incidence of PDPH from 16.3% to 5.0% (P <.005). This safe and simple maneuver is theorized to decrease the possibility of a wicking strand of arachnoid mater from extending across the dura (Fig. 4) [63].
Continuous spinal anesthesia (CSA) has been reported by some to be associated with surprisingly low incidences of PDPH compared with single-dose spinal techniques using similar gauge needles [64]. This observation has been attributed to reaction to the catheter, which may promote better sealing of a breach in the meninges. Continuous spinal anesthesia with small-gauge needles and catheters (“microcatheters”) is an appealing option when titration of spinal drug is desirable and duration of surgery is uncertain, but is currently unavailable in the United States, where the risk of PDPH with CSA remains concerning when using “macrocatheters” of approximately 20-gauge. For this reason, deliberate CSA may be underutilized and has been investigated almost exclusively in low-risk populations.
Epidural Technique
The issue of air versus liquid for identification of the epidural space with the loss-of-resistance technique has long been a source of controversy. Each method has acknowledged advantages and disadvantages, but neither has been convincingly shown to result in a lower risk of ADP [65]. In this case, operator preference and experience would be expected to strongly influence performance, and the overriding significance of this factor is illustrated in fewer instances of ADP noted when the medium is chosen at the anesthesiologist’s discretion [66].
Bevel orientation for epidural needle insertion remains a matter of debate. Norris and colleagues [67] found the incidence of moderate to severe PDPH after ADP was only 24% when the needle bevel was oriented parallel to the long axis of the spine (compared with 70% with perpendicular insertion). This orientation resulted in fewer therapeutic EBPs administered to patients in the parallel group (P<.05). However, this technique necessitates a controversial 90° rotation of the needle for catheter placement [68]. It seems that several factors regarding parallel needle insertion (lateral needle deviation, difficulties with catheter insertion, and dural trauma with needle rotation) are of greater concern to practitioners. Most respondents (71.3%) to a survey of United States anesthesiologists preferred to insert epidural needles with the bevel perpendicular to the long axis of the spine (consistent with the intended direction of catheter travel) [10].
Combined spinal-epidural (CSE) techniques have been reported to be associated with a low incidence of PDPH. While providing the advantages of a spinal anesthetic, CSE appears to have no increased incidence of PDPH or need for EBP when compared with plain epidural analgesia [69]. This observation may be due to several factors, including the ability to successfully use extremely small (eg, 27-gauge) noncutting spinal needles and tamponade provided by epidural infusions.
Measures to Reduce the Risk of PDPH after ADP
The risk-to-benefit ratio of prophylaxis should be most favorable in situations having the greatest likelihood of developing severe PDPH. Therefore, most efforts to reduce the risk of PDPH after ADP have been in the obstetric patient population. Several prophylactic measures, discussed below, are worthy of consideration and have been used alone or in combination [70]. However, because not all patients who experience an ADP will develop PDPH, and only a portion of those who do will require definitive treatment (ie, an EBP), a cautious approach in this regard is still generally warranted.
Stylet replacement
Although there have not been any studies to support the use of this technique in the setting of ADP, replacing the stylet is a simple and effective means of lowering the incidence of PDPH after LP [63]. Given the innocuous nature of this maneuver, if no other prophylactic measures are taken, there seems to be little reason not to replace the stylet before epidural needle removal in the event of ADP.
Subarachnoid saline
Limited evidence indicates that the subarachnoid injection of sterile preservative-free saline following ADP may be associated with a significant reduction in the incidence of PDPH and need for EBP. In one small study (n = 43), immediate injection of 10 mL saline through the epidural needle substantially reduced the incidence of PDPH (32%, compared with 62% in a matched control group) and resulted in a significant reduction in the need for EBP (P = .004) [71]. The injection of saline and the reinjection of CSF have been speculated as important in the prevention of PDPH by maintaining CSF volume [70]. However, given the relatively rapid rate of CSF regeneration, it may be that the benefit of fluid injection following ADP is actually in preventing a wicking strand of arachnoid (as proposed for stylet replacement). Further investigation into this issue is clearly needed.
Intrathecal catheters
Immediately placing an intrathecal catheter (ITC) after ADP has the advantages of being able to rapidly provide spinal analgesia as well as eliminate the possibility of another ADP under challenging clinical circumstances. However, the potential benefits of ITC use must be weighed against the readily appreciated risks involved (accidental use, misuse, and infection). Although evidence is extremely limited, ITC use has also been proposed to reduce the risk of PDPH after ADP [55]. Ayad and colleagues [72] placed and maintained an ITC for 24 hours following ADP. In their obstetric population, catheter placement resulted in a PDPH rate of only 6.2%, with an expected incidence of greater than 50% in this setting. A similar reduction in the development of PDPH with 24-hour ITC maintenance after ADP has been noted in orthopedic patients [73]. This impressive reduction in the incidence of PDPH has generally not been reported from studies where catheters have been left in place for less than 24 hours. It has been proposed that the mechanism of benefit from ITC maintenance may be due to reaction to the catheter, with inflammation or edema preventing further CSF loss after removal. There are also preliminary data to suggest that the incidence of PDPH may be further reduced by the injection of saline through an ITC immediately before removal [71]. With some accepted and other possible benefits, rates of ITC use following ADP have clearly increased during the past decade. Recent surveys of United States, United Kingdom, and Australian practice have noted rates of routine intrathecal catheterization following ADP in obstetric patients of 18%, 28%, and 35%, respectively [10,62,74].
Although ITC use has increased, reattempting an epidural at an adjacent interspace remains the preferred action following ADP [10]. Provided an epidural catheter can be successfully placed, several epidural approaches have been used in hope of reducing the incidence and severity of PDPH.
Epidural saline
Efforts have included both bolus (usually around 50 mL as a single or repeated injection) and continuous infusion techniques (commonly 600–1000 mL over 24 hours). As these measures are resource-intensive and may only serve to delay the inevitable onset of symptoms, they have generally not been continued beyond 36 hours. In one large analysis (n = 241), Stride and Cooper [75] reported a reduction in the incidence of PDPH from 86% in a conservatively treated control group to 70% with epidural saline infusion. Trivedi and colleagues [76] noted a similar reduction in PDPH (from 87% to 67%) in 30 patients who received a single prophylactic “saline patch” (40–60 mL) following completion of an obstetric procedure. Other studies of epidural saline have noted this modest decrease in the incidence of PDPH. Stride and Cooper also reported a lower incidence of severe headache (from 64% to 47%), but this effect has been inconsistently seen by other investigators, and there is no convincing evidence that epidural saline reduces the eventual need for EBP.
Epidural opiates
Epidural opiates (especially morphine), while long used for the treatment of PDPH, have been thought unlikely to influence the natural history of the disorder. However, recently revisiting the issue of opiates as prophylaxis after ADP, Al-metwalli found that 2 epidural injections of morphine (3 mg in 10 mL), compared with epidural injections of an equal volume of saline, resulted in fewer episodes of PDPH (P = .014) and decreased the need for EBP (P = .022) [77]. Because of the small number of patients involved (n = 25), further prospective investigation is warranted.
Prophylactic epidural blood patch
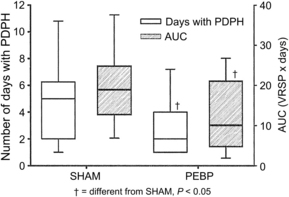
The impressive efficacy of the EBP when used as treatment for PDPH has fueled interest in the technique for prophylaxis. Research into the efficacy of the EBP for prophylaxis has yielded mixed results, and closer scrutiny indicates that optimism should be guarded. The strongest investigation to date has been by Scavone and colleagues [78], who performed a prospective, randomized, double-blind study in 64 parturients comparing the prophylactic EBP (PEBP) to a sham EBP. In this study, an identical 56% of patients in each group went on to develop PDPH. Although there was a trend toward fewer therapeutic EBPs recommended and performed in the prophylactic group, the difference was not statistically significant (P = .08). The primary benefit of the PEBP was a shorter total duration of symptoms (from a median of approximately 5 days to 2 days) and, consequently, the overall pain burden (Fig. 5). While conferring some benefit, the PEBP is not currently recommended as a routine measure based on available evidence [55,79]. Due to concerns of exposing patients to a potentially unnecessary and marginally beneficial procedure, prophylactic application of the EBP has declined substantially in recent years [10,62]. If used for prophylaxis, the EBP should be performed only after any spinal or epidural local anesthetic has worn off, as premature administration has been associated with excessive cephalad displacement of local anesthetic [80]. Residual epidural local anesthetic may also inhibit coagulation of blood, further decreasing the efficacy of the EBP [81].
Limiting and avoiding pushing
In the event of ADP, limiting the duration of the second stage of labor (usually to 30–60 minutes) and avoiding pushing may reduce the risk of PDPH. Whereas these measures are not uncommonly recommended in United Kingdom maternity units [62], such management is rare in United States practice [10].
Diagnostic evaluation
PDPH remains a diagnosis of exclusion. Although headache following meningeal puncture will naturally be suspected to be PDPH, it remains critical to rule out other etiologies (Fig. 6). Fortunately, a careful history with a brief consideration of other possible diagnoses is usually all that is necessary to differentiate PDPH from other causes of headache. Although numerous clinical variations have been reported, most cases of PDPH will have (a) a history of known or possible meningeal puncture, (b) delayed onset of symptoms (but within 48 hours), and (c) bilateral postural headache (possibly accompanied by associated symptoms if moderate or severe). Of importance, most nonmeningeal puncture headaches will not have a strong positional nature. Laboratory studies are usually not necessary for the diagnosis of PDPH and, if obtained, are generally unremarkable (most commonly, magnetic resonance imaging [MRI] may show meningeal enhancement and LP may reveal low opening pressures and increased CSF protein).
Physical examination plays a limited role in the diagnosis of PDPH. Vital signs (normal blood pressure and absence of fever) and a basic neurologic examination (gross motor and sensory function plus ocular and facial movements) should be documented. Firm bilateral jugular venous pressure, applied briefly (10–15 seconds), tends to worsen headaches secondary to intracranial hypotension [19]. Conversely, the “sitting epigastric pressure test” may result in transient relief of PDPH symptoms [82]. For this test, the patient is placed in a sitting position until headache symptoms become manifest. Firm, continuous abdominal pressure is applied with one hand while the other hand is secure against the patient’s back. In cases of PDPH, some improvement is usually noted within 15 to 30 seconds, with prompt return of symptoms on release of abdominal pressure.
It must be appreciated that benign headaches are frequently encountered in the perioperative setting, even in the absence of meningeal puncture, and have generally been noted to be less severe than PDPH (common causes include dehydration, hypoglycemia, anxiety, and caffeine withdrawal). With spinal anesthesia, the specific local anesthetic used, as well as the addition of dextrose or epinephrine, may influence the occurrence of nonspecific headache but do not affect the rate of true PDPH [83]. The majority of headaches following meningeal puncture will be benign nonspecific headaches. In a careful analysis of headache following spinal anesthesia using strict criteria for PDPH, Santanen and colleagues [84] found an incidence of nonmeningeal puncture headache of 18.5%, with an incidence of true PDPH of only 1.5%. Headaches and neck/shoulder pain are also common in the postpartum period [31]. In one study, 39% of postpartum patients were noted to be symptomatic, but more than 75% of these were determined to be primary headaches (migraine, tension-type, cervicogenic, and cluster) [52]. In this analysis, although 89% of patients received neuraxial anesthesia, only 4.7% of postpartum headaches were PDPH.
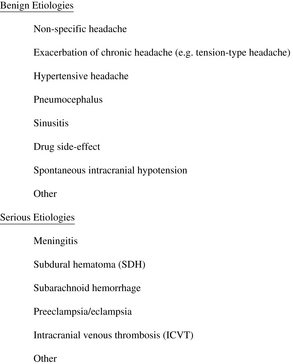
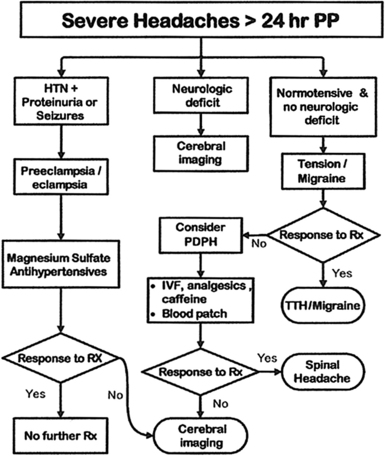
Benign headaches can often be differentiated from PDPH by characteristic features. Exacerbation of chronic headache (eg, tension-type, cluster, or migraine) is usually notable for a history of similar headaches. In the study cited above [52], a previous headache history was a significant risk factor for postpartum headache (adjusted odds ratio = 2.25, if >12 episodes per year). Significant hypertension may cause headaches and should be detected through routine vital sign assessment. Stella and colleagues [85] studied severe and unrelenting postpartum headaches with onset more than 24 hours from the time of delivery, and found that 39% were tension-type headaches, 24% were due to preeclampsia/eclampsia, and only 16% were PDPH (despite neuraxial anesthesia in 88% of patients). Based on this observation, they developed a treatment algorithm for severe postpartum headache that recommends treatment of tension/migraine headache before consideration of PDPH (Fig. 7). Pneumocephalus can produce a positional headache that can be difficult to distinguish from PDPH and does not respond to EBP, but is readily diagnosed with computed tomography (CT) [86]. Sinusitis may be associated with purulent nasal discharge and tenderness over the affected sinus, and is often improved with assuming an upright position. It should be kept in mind that headache is also a side-effect of some commonly used pharmacologic agents, such as ondansetron [87]. Although certainly unusual, classic PDPH symptoms may even conceivably represent a coincidental case of spontaneous intracranial hypotension. Several other benign etiologies are possible.
Serious causes of headache will be rare but must be excluded. It is important to remember that lateralizing neurologic signs, fever/chills, seizures, or changes in mental status are not consistent with a diagnosis of PDPH. Meningitis tends to be associated with fever, leukocytosis, changes in mental status, and meningeal signs (such as nuchal rigidity) [88]. Subdural hematoma (SDH) is a recognized complication of dural puncture, and is believed under these circumstances to be caused by intracranial hypotension resulting in excessive traction on cerebral vessels, leading to their disruption. Practitioners must maintain a high index of suspicion for SDH, which is often preceded by typical PDPH symptoms but progresses to lose its postural component, and may evolve to include disturbances in mentation and focal neurologic signs. It has been proposed that early definitive treatment of severe PDPH may serve to prevent SDH [89]. Subarachnoid hemorrhage, most commonly caused by rupture of a cerebral aneurysm or arteriovenous malformation, is usually associated with the sudden onset of excruciating headache followed by a decreased level of consciousness or coma [90]. Preeclampsia/eclampsia often presents with headache and may only become evident in the postpartum period [91]. Intracranial venous thrombosis (ICVT) is most often seen in the postpartum obstetric population, where headache symptoms are easily confused with PDPH but may progress to seizures, focal neurologic signs, and coma [92]. Predisposing factors for ICVT include hypercoagulability, dehydration, and inflammatory and infectious diseases. Reports of other intracranial pathology (intracranial tumor, intracerebral hemorrhage, and so forth) misdiagnosed as PDPH are rare, and such conditions will be detected only by a thorough neurologic evaluation [93].
Treatment
Once a diagnosis of PDPH has been made, patients should be provided a straightforward explanation of the presumed cause, anticipated natural course (factoring in the time from meningeal puncture), and a realistic assessment of treatment options (with consideration of needle gauge). A treatment algorithm, based primarily on the severity of symptoms, can serve as a useful guide for management (Fig. 8).
Time
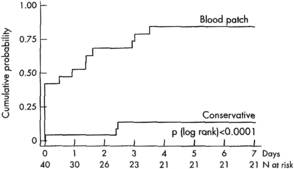
As PDPH is a complication that tends to resolve spontaneously, the simple passage of time plays an important role in the appropriate management of this disorder. Before the introduction of the EBP as definitive therapy, the natural history of PDPH was documented by Vandam and Dripps [3] as they followed 1011 episodes of PDPH after spinal anesthesia using cutting needles of various sizes. Although their analysis is flawed by a lack of information regarding duration in 9% of patients, if one considers their observed data, spontaneous resolution of PDPH was seen in 59% of cases within 4 days and 80% within 1 week. More recently, Lybecker and colleagues [27] closely followed 75 episodes of PDPH and, while providing an EBP to 40% of their patients (generally to those having the most severe symptoms), observed in the untreated patients a median duration of symptoms of 5 days with a range of 1 to 12 days. van Kooten and colleagues [94], in a small but prospective, randomized, blinded study of patients with moderate or severe PDPH, noted 18 of 21 patients (86%) in the control treatment group (24-hour bed rest, at least 2 L of fluids by mouth daily, and analgesics as needed) still having headache symptoms at 7 days, with over half of these still rating symptoms as moderate or severe (Fig. 9). These data serve to illustrate the unpredictable and occasionally prolonged duration of untreated PDPH. Indeed, Vandam and Dripps [3] reported 4% of patients still experiencing symptoms 7 to 12 months after spinal anesthesia. Given this reality, it is not surprising that there are several case reports of successful treatment of PDPH months and even years after known or occult meningeal puncture.
Largely due to the self-limited nature of PDPH, the optimal time course of treatment has not been well defined. Most practitioners currently advocate a trial, most commonly 24 to 48 hours, of conservative management [10]. However, the rationale behind this approach is questionable given the often severely disabling nature of symptoms, particularly in the postpartum period when newborn care may be significantly impaired. Clinically, the practical issue is how long definitive therapy (ie, the EBP) can appropriately be delayed, and is considered in comments regarding timing of the EBP.
Supportive Measures
Reassurance and measures directed toward minimizing symptoms, while not expected to alter the natural course of the disorder, are advised for all patients. By definition, the majority of patients with moderate to severe PDPH will naturally seek a recumbent position for symptomatic relief. Despite a lack of supportive evidence, aggressive hydration continues to be the most frequently recommended practice used in treatment of PDPH [10]. Although aggressive hydration does not appear to influence the duration of symptoms [61], patients should and often must be encouraged to avoid dehydration.
Analgesics (acetaminophen, NSAIDS, opiates, and so forth.) may be administered by several different routes and are commonly used, yet the relief obtained is often unimpressive, especially with severe headaches. Antiemetics and stool softeners should be prescribed when indicated. Abdominal binders can provide some degree of relief, but are uncomfortable and seldom used in modern practice. Acupuncture has been suggested as an alternative measure in the management of PDPH [95].
Pharmacologic Therapies
Several pharmacologic agents have been advocated as treatments for PDPH [96]. While appealing, these options have generally been poorly studied and are of questionable value due to the small numbers of patients treated, methodological flaws in published reports, and the self-limited nature of the disorder.
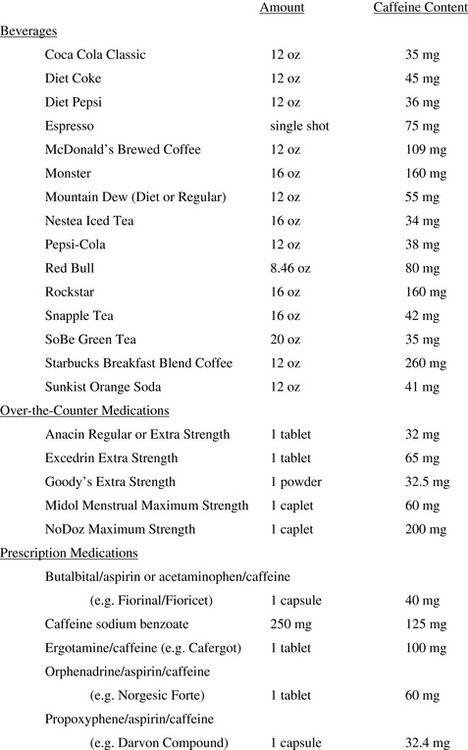
Methylxanthines are used for their cerebral vasoconstrictive effect and include aminophylline, theophylline, and most familiar, caffeine. Experimentally, caffeine has been used intravenously (usually 500 mg caffeine sodium benzoate, which contains 250 mg caffeine) and orally (eg, 300 mg). Published studies of caffeine for PDPH consistently demonstrate improvement at 1 to 4 hours in more than 70% of patients treated [96]. However, a single oral dose of 300 mg caffeine for treatment of PDPH is statistically no better than placebo at 24 hours [97]. With a terminal half-life of generally less than 6 hours, repeated doses of caffeine would seem necessary for treatment of PDPH, yet few studies have evaluated more than 2 doses for efficacy or safety (of particular concern in the nursing parturient). Furthermore, there is no convincing evidence that any pharmacologic agents reduce the eventual need for EBP. Overall, the use of caffeine for PDPH does not appear to be supported by the available literature [59]. Nevertheless, surveys indicate that it continues to be widely used in the treatment of PDPH [10,62]. Clinically, encouraging unmonitored caffeine intake is of extremely uncertain value, especially considering the general lack of awareness of caffeine content in readily available beverages and medications (Fig. 10). The temporary benefit often observed with caffeine would indicate that, if used, it is perhaps most appropriate for the treatment of PDPH of moderate (and possibly mild or severe) intensity while awaiting spontaneous resolution of the condition. Although the familiarity of caffeine for nonmedical purposes would argue for its general safety, practitioners should note that its use is contraindicated in patients with seizure disorders, pregnancy-induced hypertension, or a history of supraventricular tachyarrhythmias.
In addition to the methylxanthines, experiences with other pharmacologic approaches to PDPH continue to be reported. Sumatriptan, a serotonin type-1d receptor agonist that causes cerebral vasoconstriction, is commonly used for migraine headache and has been used to treat PDPH. Corticosteroidogenics (corticotropin and its synthetic form, cosyntropin/tetracosactin) have long been proposed as treatments for PDPH. Although the mechanism of action remains speculative, corticotropin is known to have multiple physiologic effects that could theoretically improve symptoms of PDPH [98]. However, neither sumatriptan [99] nor a synthetic corticotropin analogue [100] were found to be effective in small randomized, prospective studies for treatment of severe PDPH. Hydrocortisone (100 mg intravenously every 8 hours for 6 doses) has been reported to be effective in treatment of severe PDPH following spinal anesthesia using a 25-gauge Quincke needle [101]. A small, uncontrolled pilot study of methylergonovine (0.25 mg orally 3 times daily for 24–48 hours) indicated that this vasoconstrictive agent may hasten resolution of PDPH [102]. A case report of gabapentin (400 mg orally every 8 hours for 3 days) suggests that this agent may also be useful in the setting of severe PDPH [103]. Reports of the successful use of these and other pharmacologic agents are intriguing, but their proper place in the management of PDPH awaits further study. However, given the initial optimism but eventually disproved role for so many pharmacologic agents through the years, practitioners are advised to have guarded expectations in this regard, especially when dealing with severe PDPH.
Epidural Therapies
Epidural saline
Epidural saline, as bolus and infusion, has a long history of use for treatment of PDPH. Bolus injections of epidural saline (usually 20–30 mL, repeated as necessary if a catheter is present) have been reported to produce prompt and virtually universal relief of PDPH, yet the practice is plagued by an extremely high rate of headache recurrence. This transient effect is not surprising, as increases in epidural pressure following bolus administration of saline have been demonstrated to return to baseline within 10 minutes [104]. Favorable results achieved with this approach have been speculated to represent the mechanical reapproximation of a dural flap (the “tin-lid” phenomenon) [1]. However, bolus administration of saline for treatment of PDPH has been convincingly shown to be inferior to the EBP, especially when headaches are secondary to large-bore needle punctures [105]. Overall, epidural saline appears to be of limited value for established PDPH [55]. Nevertheless, the successful use of epidural saline, administered as bolus and/or infusion, continues to be reported occasionally under exceptional circumstances [106].
Epidural blood patch
During the past several decades, the EBP has emerged as the “gold standard” for treatment of PDPH [1]. A Cochrane review (a systematic assessment of the evidence) regarding the EBP recently concluded that the procedure now has proven benefit over more conservative treatment [79]. The mechanism of action of the EBP, while not entirely understood, appears to be related to the ability to stop further CSF loss by the formation of clot over the defect in the meninges as well as a tamponade effect with cephalad displacement of CSF (the “epidural pressure patch”) [107]. The appropriate role of the EBP in individual situations will depend on multiple factors, including the duration and severity of headache and associated symptoms, type and gauge of original needle used, and patient wishes. The EBP should be encouraged in patients experiencing ADP with an epidural needle and those whose symptoms are categorized as severe (ie, pain score > 6 on a 1–10 scale). Informed consent for the EBP should include a discussion with the patient regarding the common as well as serious risks involved, anticipated side effects, and true success rate. Finally, patients should be provided clear instructions for the provision of timely medical attention should they experience a recurrence of symptoms.
Several controversies surround the EBP, reflecting the scarcity of adequately powered, randomized trials. The procedure itself has been well described and consists of the sterile injection of fresh autologous blood near the previous dural puncture (Fig. 11). An MRI study of the EBP in 5 young patients (age 31–44 years) using 20 mL blood noted a spread of 4.6 ± 0.9 intervertebral spaces (mean ± SD), averaging 3.5 levels above and 1 level below the site of injection [107]. This and other observations of a preferential cephalad spread of blood in the lumbar epidural space has led to the common recommendation to perform the EBP “at or below” the meningeal puncture level. However, the influence on efficacy of the level of placement and use of an epidural catheter (often situated considerably cephalad to a meningeal puncture) for EBP have never been clinically evaluated.
The optimal timing of the EBP is a matter of debate. After diagnosis, most practitioners prefer to delay performing the EBP, possibly to further confirm the diagnosis as well as to allow an opportunity for spontaneous resolution. A 1996 survey of United Kingdom neurologic departments found that only 8% would consider the EBP before 72 hours had passed following LP [108]. A recent survey of United Kingdom maternity units reported that 71% would perform the EBP only “after the failure of conservative measures” [62]. Likewise, the majority of respondents in a recent survey of United States practice usually waited at least 24 hours from the onset of symptoms before performing the EBP [10]. Several studies suggest that the EBP procedure may become more effective with the passage of time [109,110]. Safa-Tisseront and colleagues [110] found a delay of less than 4 days from meningeal puncture before performing an EBP to be an independent risk factor for failure of the procedure. Yet these investigators were careful to state that failure of the EBP may be primarily related to the severity of the CSF leak (with larger, harder to treat situations demanding earlier attention) and that their study should not be grounds for delaying the EBP. Sandesc and colleagues [111] performed a prospective, randomized, double-blind study of the EBP versus conservative management (intravenous/oral fluids up to 3 L/d, nonsteroidal anti-inflammatory drugs, and caffeine sodium benzoate 500 mg intravenously every 6 hours) in 32 patients with severe PDPH symptoms (mean pain intensity = 8.1). At the time treatment was initiated, none of these patients had experienced symptoms for longer than 24 hours. Whereas all patients in the EBP group had satisfactory resolution of symptoms at 24-hour follow-up, the control group was essentially unchanged (mean pain intensity = 7.8). Of note, 14 of 16 patients in the conservatively treated group then elected for EBP treatment. The investigators concluded that there was no reason to delay the EBP for more than 24 hours after making a diagnosis of severe PDPH. This recommendation is further supported by a prospective analysis of 79 patients with PDPH, which determined that early EBP in those with moderate to severe symptoms minimized patient suffering [112].
The ideal volume of blood for EBP is unclear. Conceptually, the volume of blood used should be sufficient to form an organized clot over the meningeal defect as well as produce some degree of epidural tamponade [107]. When performing the EBP, anesthesiologists commonly inject as much blood as was drawn (usually around 20 mL), stopping when the patient complains of discomfort or fullness in the back, buttocks, or neck. There appear to be regional preferences regarding blood volume. The largest analysis of the EBP to date (n = 504) used a blood volume of 23 ± 5 mL (mean ± SD) [110]. Of importance, this French study found no significant difference in blood volumes between successful and failed EBP. The investigators noted “discomfort” in 78% of injections with 19 ± 5 mL and “pain” in 54% with 21 ± 5 mL, with the only independent risk factor for pain during EBP being age less than 35 years. A recent survey of United States anesthesiologists reported general unanimity for a smaller blood volume, with two-thirds (66.8%) most commonly using between 16 and 20 mL [10]. As previously mentioned, there may be some experimental support for using a blood volume of 15 to 20 mL, as early studies of CSF drainage in volunteers reported consistently producing positional headache symptoms with loss of 10% of total CSF volume (approximately 15 mL) [19]. Furthermore, the reduction in CSF pressures produced by this degree of fluid loss would be expected to reduce or eliminate transmeningeal driving pressure, resulting at that point in a relative CSF volume homeostasis (in the supine position). Formal studies designed to determine an ideal volume of blood for EBP in the treatment of PDPH have generally failed to achieve better results with volumes greater than 10 mL, and there are few data to encourage the use of volumes greater than 20 mL [113]. It is notable that although the value of the EBP in the treatment of spontaneous intracranial hypotension is uncertain [55], much larger blood volumes (up to 100 mL) are generally recommended for this indication [114]. However, a recent case report highlights some potential complications from large-volume EBP [115], and practitioners are generally encouraged to use the smallest effective volume.
To allow for clot organization and regeneration of CSF (approximately 0.35 mL/min), it is common practice to have patients remain recumbent for a period of time following the EBP. Although the optimal duration of bed rest immediately following an EBP remains unknown, one small study suggested that maintaining the decubitus position for at least 1 and preferably 2 hours may result in a more complete resolution of symptoms [116]. Patients are usually advised to avoid lifting, Valsalva maneuvers (eg, straining with bowel movement), and air travel for 24 to 48 hours after EBP to minimize the risk of patch disruption.
Contraindications to the EBP are similar to those of any epidural needle placement: coagulopathy, systemic sepsis, fever, infection at the site, and patient refusal. Modifications of usual EBP technique have been suggested to accommodate the special needs of Jehovah’s Witness patients [117]. Theoretical concerns have been expressed regarding the possibility of neoplastic seeding of the central nervous system in patients with cancer [118]. Although not free from concern and controversy, the EBP has been safely provided to patients with human immunodeficiency virus infection [119] and acute varicella [120]. The EBP may also be indicated and performed, with decreased volumes of blood, in the pediatric population (0.2–0.3 mL/kg has been associated with successful EBP in adolescents) [121] and at extralumbar sites (eg, cervical) [122].
Minor side effects are common following the EBP. Patients should be warned to expect aching in the back, buttocks, or legs (seen in approximately 25% of patients) [109]. While usually short-lived, backache was noted to be persistent in 16% of patients following EBP, lasting 3 to 100 days (with a mean duration in this subgroup of 27.7 days) [123]. Despite these lingering symptoms, patient satisfaction with the EBP is high. Other frequent but benign after-effects of the EBP include transient neck ache [123], bradycardia [124], and modest temperature elevation [123].
Largely through extensive clinical experience, the EBP has been sufficiently proven to be safe. The risks are essentially the same as with other epidural procedures (infection, bleeding, nerve damage, and ADP). On occasion, the aforementioned temporary back and lower extremity radicular pain has been reported to be severe. With proper technique, infectious complications are vanishingly rare. Although controversial, a previous EBP does not appear to significantly influence the success of future epidural interventions [125]. Serious complications secondary to the EBP do occur, but have usually consisted of isolated case reports and have often been associated with significant deviations from standard practice [1].
Other epidural therapies
For various reasons, several alternatives to blood have been promoted as patch materials. The most commonly proposed materials (dextran-40, hydroxyethyl starch, gelatin, and fibrin glue) have been adapted for a perceived ability to provide prolonged epidural tamponade and/or result in sealing of a meningeal rent. In a rat model, experimental support for a “blood-like” effect was best shown for fibrin glue [104]. However, clinical use of these alternatives is limited to case reports and small series, and their use is uncommon in the United States [10]. Though not necessarily without merit, these options remain poorly defined, and reports of their use should still be considered preliminary.
Persistent or recurrent PDPH
The EBP is associated with nearly immediate symptomatic relief in more than 90% of cases, but appropriate follow-up reveals many patients experiencing incomplete relief, failure, and recurrence. In an uncontrolled, prospective, observational study of 504 consecutive patients treated with EBP following meningeal puncture with needles of various sizes, Safa-Tisseront and colleagues [110] reported some relief of symptoms in 93%. Yet on closer analysis, complete relief of symptoms was seen in only 75% of patients, with 18% experiencing incomplete relief. These investigators also found that the EBP was more likely to fail if the original meningeal puncture was made with needles larger than 20-gauge (Fig. 12). For needles larger than 20-gauge, the unqualified success rate of the EBP was only 62%, with 17% of patients reporting incomplete relief of symptoms and 21% experiencing failure. Not surprisingly, the majority of these large needles were Tuohy epidural needles.
Expectations of success with the EBP must be further tempered in obstetric patients (all young and female) following ADP with epidural needles. Under these circumstances, Williams and colleagues [126] noted complete relief of symptoms with EBP in only 34% of patients, partial relief in 54%, and no relief in 7% (results unknown in 5%). If performed, a second EBP resulted in complete relief in 50%, partial relief in 36%, and no relief in 14%. In a similar patient population, Banks and colleagues [109], despite initially observing complete or partial relief with EBP in 95% of patients, reported the return of moderate to severe symptoms in 31%, with a mean time to development of recurrent headache of 31.8 hours (range 12–96 hours). The rates of repeat EBP for the Williams and Banks studies were 27% and 19%, respectively. These studies clearly demonstrate the reduced efficacy of the EBP following meningeal punctures made with large needles, which not uncommonly make it necessary to consider repeating the procedure. Overall, success rates of a second EBP appear to be approximately equal to that of a first. The ideal timing and blood volume for repeat EBP is even more uncertain than for a primary procedure. A majority of United States anesthesiologists would wait at least 24 hours after recurrence of PDPH before performing a second EBP [10]. If more than one EBP is performed within a short period of time, practitioners should remain cognizant of the cumulative amount of blood used, as excessive volumes under these circumstances have been implicated in adverse outcomes [115].
Insufficient evidence exists to guide management following a second failed EBP. Given the frequency of PDPH and significant failure rate of the EBP, instances of sequential EBP failure are not unheard of, especially following large-gauge meningeal punctures. In an analysis of outcomes following ADP with 18-gauge Tuohy needles in an obstetric unit, Sadashivaiah [127] reported 3 of 48 patients (6.25%) requiring a third EBP to relieve the headache. Each failure of the EBP obviously necessitates an even more critical reconsideration of the diagnosis. Although experiences with managing repeated EBP failure have been published [128], such sporadic case reports are insufficient to guide others. However, one frequently cited and logical recommendation regarding repeat EBP, and particularly a third EBP, is to use some form of radiologic guidance to ensure accurate epidural blood placement. Other measures under these difficult circumstances may include any of the aforementioned “treatments,” with open surgical repair usually constituting a last resort.
When to seek further consultation
As PDPH can be anticipated to resolve spontaneously, headaches that worsen over time and no longer have a positional nature should be strongly suspected to be secondary to SDH (especially if there are focal neurologic signs or decreases in mental status) [89]. Under these circumstances, a neurologic consultation should be obtained and diagnostic radiological studies performed.
Although headache and most associated symptoms, including auditory symptoms [32], resolve quickly following EBP, cranial nerve palsies generally resolve slowly (within 6 months), and may appropriately prompt a neurology consult for ongoing management and reassurance [25]. Although there are no accepted treatments for cranial nerve palsy associated with PDPH, it does not seem unreasonable to treat these conditions in a manner similar to idiopathic facial nerve (CN VII) palsy (Bell palsy). There is some evidence, for example, to suggest that corticosteroids administered early (within 72 hours of onset) may hasten resolution of symptoms from Bell palsy [129], and similar treatment has been suggested for cranial nerve palsy following meningeal puncture [26].
Summary
Unfortunately, the published literature concerning PDPH has generally been of poor quality [55,79,130]. Many questions remain regarding the optimal means of preventing and treating this troublesome complication. Even much of what is “known” to this point has not been confirmed in follow-up studies. It is anticipated that these issues will be resolved in the future through well-designed clinical investigations.
References
[7] J.B. Gormley. Treatment of postspinal headache. Anesthesiology. 1960;21:565-566.
[9] J.S. Crawford. Experiences with epidural blood patch. Anaesthesia. 1980;35:513-515.
[21] B.E. Harrington. Reply to Dr Colclough. Reg Anesth Pain Med. 2005;30:318.
[22] J.M. Neal, C. Bernards. Reply to Dr Colclough. Reg Anesth Pain Med. 2005;30:318.
[29] B.V. Reamy. Post-epidural headache: how late can it occur? J Am Board Fam Med. 2009;22:202-205.
[64] J.M. Moore. Continuous spinal anesthesia. Am J Ther. 2009;16:289-294.
[68] B. Duffy. Don’t turn the needle!. Anaesth Intensive Care. 1993;21:328-330.