Chapter 34 Pulmonary Rehabilitation
Principles
The most common form of lung disease in the United States is chronic obstructive pulmonary disease (COPD), primarily because of its link to smoking. In 2007 the American Cancer Society reported that 19.8% of adults 18 years of age or older were current smokers compared with 41.9% in 1965. Unfortunately, in 2006 it was reported that 20% of high school and 6.3% of middle school students smoked. COPD is very rare in nonsmokers, and the vast majority of the deaths from this disease can be attributed to cigarette smoking. Statistics regarding COPD are shown in Box 34-1.66
BOX 34-1 Facts Regarding Chronic Obstructive Pulmonary Disease
Pulmonary rehabilitation is defined as a multidisciplinary program that provides persons with the ability to adapt to their chronic lung disease.13 It includes physical conditioning, ongoing medical management, training in coping skills, and psychosocial support. Fear of dyspnea can lead to panic, which increases the work of breathing. Dyspnea also causes progressive inactivity, which further weakens the individual. Pulmonary rehabilitation addresses this fear and uses a greater tolerance of dyspnea to increase strength, endurance, and quality of life.
Treatment Options in Pulmonary Rehabilitation
General Medical Management
Pharmacologic therapy for COPD can include vaccination against influenza and pneumococcal pneumonia, inhaled quaternary anticholinergic and/or β2-adrenergic agonist bronchodilators, and inhaled corticosteroids. Oral theophylline can improve respiratory muscle endurance and provide ventilatory stimulation. Exposure to environmental and occupational pollution must be prevented. For those with emphysema resulting from α1-antitrypsin deficiency, α1-antitrypsin augmentation therapy is efficacious.56 In addition, several treatments prolong life or reduce progression in this disease.14 These include smoking cessation, noninvasive mechanical ventilation, LVRS, combined long-acting β-agonists with inhaled corticosteroids,16 and oxygen therapy.
Oxygen Therapy
Long-term oxygen therapy (LTOT), provided more than 15 hr/day, improves survival and quality of life in COPD if hypoxemia is present with arterial oxygen saturation (SaO2) less than 88%. It can also increase exercise tolerance and cognitive outcomes.14 LTOT is also needed if the patient’s SaO2 is less than 89% and there is evidence of pulmonary hypertension or peripheral edema, suggesting congestive cardiac failure, or polycythemia.29
Oxygen concentrators have become the most popular method of providing oxygen in the home. Portable models are readily available and can be used with an AC or a DC inverter. Some models are as light as 6 lb, with the average weight ranging between 6 and 20 lb. Most models have batteries that can be recharged in as little as 2 hours and have battery running times as long as 6 hours. Portable concentrators can easily be carried on a pull cart or on the body with a shoulder strap or waist harness. Most units are able to deliver 1 to 6 L/min. Some companies have produced models that provide up to 8 L/min of oxygen and have two flowmeters so that two persons can use oxygen from the same concentrator if the total use is no more than 8 L/min. Other systems incorporate a refill station as part of the concentrator, so that portable oxygen cylinders can be filled over a couple of hours. An oxygen-conserving regulator allows the oxygen to last 4 times as long. An oxygen cylinder can now be safely mounted on a motorized wheelchair if the motors and batteries are sealed and both are covered by a rigid housing.
Chest Physical Therapy
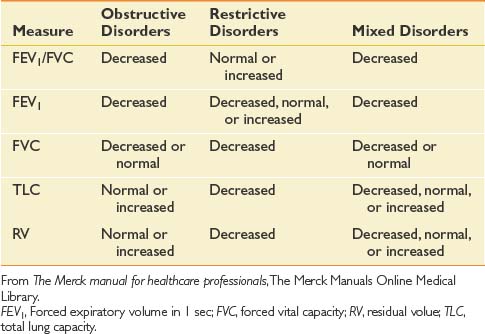
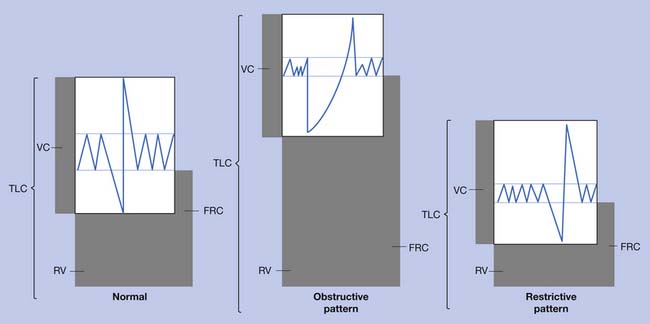
A good understanding of pulmonary function tests (Table 34-1 and Figure 34-1), as well as the mechanics and work of breathing in normal and diseased states, is essential in planning an effective therapy program for persons with pulmonary disease.32 Breathing exercises begin with relaxation techniques, which then become the foundation for breathing retraining. Retraining techniques for persons with COPD include pursed lip breathing, head down and bending forward postures, slow deep breathing, and localized expansion exercises or segmental breathing. These techniques maintain positive airway pressure during exhalation and help reduce overinflation. Although diaphragmatic breathing is widely taught, it has been shown to increase the work of breathing and dyspnea compared with the natural pattern of breathing in the patient with COPD.13 The other component occasionally used to reduce fatigue is respiratory muscle endurance training, which usually concentrates on inspiratory resistance training. Training of the expiratory muscles, however, has also been found to be of some value.43
Airway clearance strategies are indicated for persons with (1) abnormal cough mechanics (e.g., muscle weakness), (2) altered mucus rheology (e.g., cystic fibrosis), (3) structural airway defects (e.g., bronchiectasis), and (4) altered mucociliary clearance (e.g., primary ciliary dyskinesia).42 Clearance of secretions is mandatory to reduce the work of breathing, improve gas exchange, and limit infection and atelectasis. For chest physical therapy to be effective, mucoactive medications must be given.62 These include expectorants, mucolytics, bronchodilators, surfactants, and mucoregulatory agents that reduce the volume of mucus secretion. Antitussives must be used for uncontrolled coughing, which can precipitate dynamic airway collapse, bronchospasm, or syncope.
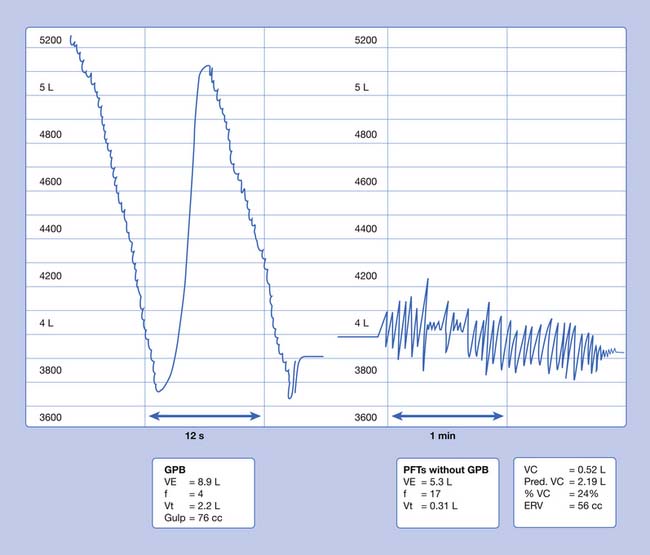
Techniques for clearing secretions include postural drainage, manual or device-induced chest percussion and vibration, device-induced airway oscillation, incentive spirometry, and other devices and measures that improve the ability to cough. Head-down tilt positions for postural drainage should be used with caution in persons with severe heart disease.47 In a manually assisted cough, the patient’s abdomen is compressed while the patient controls the depth of inspiration and the timing of opening and closing the upper airway. Noninvasive intermittent positive-pressure ventilation (NIPPV) with air stacking or glossopharyngeal breathing (GPB) is used to increase the depth of inspiration when inspiratory muscles are too weak to produce a deep breath. Air stacking is holding a portion of two or more breaths to fully inflate the lungs before exhalation. In GPB, the person uses the tongue to breathe. The technique is described more fully later in this chapter. When an upper motor neuron lesion above the midthoracic level has paralyzed the abdominal muscles, functional electrical stimulation of these muscles can produce a cough.34
Positive expiratory pressure mask therapy followed by “huff coughing” is a useful technique when other methods of mobilizing secretions are not tolerated.39 Autogenic drainage is a secretion clearance technique that combines variable tidal breathing at three distinct lung volume levels, controlled expiratory airflow, and huff coughing. The CoughAssist Mechanical In-Exsufflator cough machine (Figure 34-2), manufactured by the Philips HealthCare Respironics (Murraysville, PA) provides deep inspiration through a face mask or mouthpiece, or with an adapter, to a patient’s endotracheal or tracheostomy tube, followed rapidly by controlled suction. It has been shown to provide highly effective secretion removal.4 The device must be used with caution and is contraindicated in patients with bullous emphysema or a history of pneumothorax or pneumomediastinum in the recent past, especially if they are the result of barotrauma.
Exercise Conditioning: General Considerations
Aerobic exercise is the backbone of any pulmonary rehabilitation program. The inclusion criteria for exercise in pulmonary rehabilitation are straightforward. A candidate must demonstrate a decrease in functional exercise capacity as a result of pulmonary disease and be able to participate safely in a rigorous cardiorespiratory endurance training program. Cardiopulmonary exercise testing is necessary for the selection and evaluation of individuals in several circumstances before exercise conditioning. Indications for cardiopulmonary exercise testing have been adopted by the American Thoracic Society and the American College of Chest Physicians (Box 34-2).77 The pulmonary disease should be relatively stable. Medical comorbidities that contraindicate exercise should be absent. Patients should not have orthopedic or cognitive disabilities that prevent exercise. Patients must be motivated to exercise on a consistent basis. Finally, patients should abstain from tobacco products.
Exercise Prescription for Pulmonary Rehabilitation
Exercise prescription guidelines are largely based on programs in which the majority of participants are patients with COPD. These guidelines, however, appear to be appropriate for patients who have other pulmonary diseases as well. Exertional dyspnea is among the most frequently experienced symptoms of pulmonary diseases and leads to physical disability and functional impairment. Cardiorespiratory exercise training is often effective for decreasing exertional dyspnea. Provided the cardiovascular, respiratory, and neuromuscular systems have adequate reserve to undergo a program of progressive exercise, skeletal muscles can develop an increased ability to sustain physical activity. After training, muscle oxygenation might be improved, which lowers blood lactate levels at any given level of strenuous exercise. The confluence of increased oxygen extraction by the muscles and lower blood lactate lessens carbon dioxide production and the ventilatory requirement for a given workload.
The American Thoracic Society,48 the American College of Chest Physicians,60 and the American College of Sports Medicine78 have provided recommendations for pulmonary rehabilitation exercise. In general, pulmonary rehabilitation exercise programs should include cardiorespiratory endurance training of larger muscle groups. Over-ground or treadmill walking is generally the preferred method because walking is a functional activity. Leg-cycling is an acceptable alternative. Arm-cycling can also be incorporated. Many patients, however, might have difficulty tolerating arm-cycling because of an increased ventilatory drive that could worsen the patient’s dyspnea during the activity. Resistance and flexibility exercises might also improve functional capacity in patients with pulmonary diseases. The most specific exercise prescription guidelines have been provided by the American College of Sports Medicine (Table 34-2).
Table 34-2 Summary of ACSM Guidelines for Exercise Prescription
| Component | Cardiorespiratory Endurance Training |
| Activity | Dynamic exercise of large muscle groups |
| Mode | Over-ground or treadmill walking |
| Stationary leg-cycling or outdoor bicycling | |
| Stair climbing | |
| Frequency | 3-5 days/wk |
| Duration | 20-60 min/session |
| Intensity | 50%-85% heart rate reserve |
| 65%-90% maximum heart rate | |
| RPE = 12-16 (category scale) | |
| RPE = 4-8 (category-ratio scale)∗ | |
| Component | Strength and Muscle Endurance Training |
| Activity | Resistance training: low resistance with high repetition |
| Mode | Variable resistance or hydraulic weight machines |
| Isotonic weight machines | |
| Free weights | |
| Frequency | 2-3 days/wk |
| Duration | One set of 3-20 repetitions on 8-10 exercises that include all of the major muscle groups |
| Intensity | Volitional exhaustion on each set, or |
| Stop two to three reps before volitional exhaustion | |
| Component | Flexibility |
| Activity | Static stretching of all major muscle groups |
| Frequency | Minimum of 2-3 days/wk |
| Ideally 5-7 days/wk | |
| Duration | 15-30 s/exercise, 2-4 stretching exercise sets |
| Intensity | Stretch to tightness at the end of the range of motion but not to pain |
RPE, Rate of perceived exertion.
Modified from Whaley MH, Brubaker PH, Otto RM, editors: ACSM’s guidelines for exercise testing and prescription, ed 7, Philadelphia, 2006, Lippincott Williams & Wilkins.
Exercise in Chronic Obstructive Pulmonary Disease
All studies of exercise in COPD take into account the severity of the respiratory disability (Table 34-3).40 Numerous studies have been carried out on the effects of exercise on patients with COPD over the past quarter century (Box 34-3).∗ In patients with COPD, cardiorespiratory endurance exercise therapy has been shown to improve maximum or symptom-limited aerobic capacity, timed walk distance, and health-related quality of life. Adding resistance training to the rehabilitative regimen can provide additional benefits such as increased fat-free mass and muscle strength.
Table 34-3 Classification Scheme for Chronic Obstructive Pulmonary Disease Severity: National Heart, Lung, and Blood Institute–World Health Organization Global Initiative for Chronic Obstructive Lung Disease Criteria
| Stage | Criterion∗ |
|---|---|
| 0 | Normal lung function |
| 1 (Mild) | FEV1 ≥80% of predicted |
| 2 (Moderate) | 50%-79% of predicted |
| 3 (Severe) | 30%-49% of predicted |
| 4 (Very severe) | FEV1 <30% of predicted or presence of respiratory failure or clinical signs of right-sided heart failure |
∗ In the presence of FEV1/FVC ratio less than 70%.
BOX 34-3 Improvements Seen in Exercise Reconditioning in Moderate Chronic Obstructive Pulmonary Disease
Several adjunct modalities might reduce the extreme breathlessness and peripheral muscle fatigue that prevent patients with severe COPD from exercising at higher intensities.1 Continuous positive airway pressure and NIPPV during exercise might reduce the perception of dyspnea. Taking part of the work of breathing away from the respiratory muscles and reducing intrinsic positive end-expiratory pressure are considered two mechanisms by which these techniques relieve dyspnea. Nocturnal NIPPV in selected patients can improve their ability to exercise during the day. Adding electrical stimulation to strength exercises for peripheral muscles has been shown to further improve muscle strength in patients with COPD. Interval training with rest periods is capable of producing training effects in those who cannot tolerate a sustained period of exercise. Oxygen supplementation, even in patients who do not desaturate during exercise, allows for higher exercise intensities and produces a superior training effect. High-intensity physical group training in water can produce significant benefits as well.75
After an initial exercise rehabilitation program, patients must continue to participate in routine exercise to prevent the positive effects of exercise from being lost. Continuous outpatient exercise training, home-based or community-based exercise programs, or exercise training in groups of persons with COPD is necessary to sustain the benefits acquired during the initial rehabilitation program.67
Exercise in Asthma
Asthma severity guidelines published in 1997 and updated in 2002 by the National Heart, Lung, and Blood Institute are based on clinical symptoms during the day and at night, and on the results of pulmonary function testing.17 The two major categories of asthma patients are those who have it intermittently and those with persistent asthma. Bronchial biopsies in patients with intermittent asthma show evidence of ongoing airway inflammation. Studies have shown that aerobic exercise improves overall fitness and health of asthmatic patients.
A mouse model of asthma has been developed in which the effects of exercise have been studied.53 Exercise decreased the activation of the transcription factor nuclear factor-κB in the lungs of the mice. This factor regulates the expression of a variety of genes that encode inflammatory mediators. Moderate-intensity aerobic exercise training of the mice decreased leukocyte infiltration, cytokine production, adhesion molecule expression, and structural remodeling within the lungs. It is suggested that aerobic exercise in patients with asthma might reduce airway inflammation in a similar manner. On the other hand, exercise itself can induce bronchoconstriction in some asthmatic patients.
A study in patients with asthma found that airway vascular hyperpermeability, eosinophilic inflammation, and bronchial hyperactivity are independent factors predicting the severity of exercise-induced bronchoconstriction.52 Rundell et al.63 have described a laboratory method, eucapnic voluntary hyperpnea (EVH), that identifies 90% of athletes who have exercise-induced bronchoconstriction. The test is done by having athletes breathe 5% carbon dioxide and attempt to breathe for 6 minutes at a target ventilation equivalent to 30 times the baseline forced expiratory volume in 1 second (FEV1). If EVH is positive, there is a decrease in FEV1 of 10% to19%.
Because exercising in cold air is known to increase bronchial responsiveness compared with exercising in warm air, a study was done to determine whether facial cooling plays a role in asthmatic children.81 It was found that facial cooling, combined with either cold or warm air inhalation, caused greater exercise-induced bronchoconstriction than with cold air inhalation. This could indicate that vagal mechanisms play a role in exercise-induced asthma.
Barriero et al.5 have studied the perception of dyspnea in near-fatal asthma patients at both rest and the end point of various forms of exercise compared with other asthmatic patients. Exercise tolerance was similarly reduced in both groups, but perception of dyspnea both at rest and at peak exercise was significantly lower in the near-fatal asthma group.
Exercise in Cystic Fibrosis
Children with cystic fibrosis have reduced anaerobic performance and do not participate in activities requiring short bouts of high-energy expenditure to the same extent as healthy children. Klijn et al.35 recently carried out a study of anaerobic training on children with cystic fibrosis older than 12 years. No child had an FEV1 less than 55% of the predicted level. Subjects were trained 2 days/wk for 12 weeks, with sessions lasting 30 to 45 minutes. Each anaerobic activity lasted 20 to 30 seconds. Three months after the end of training, anaerobic performance and quality of life were significantly higher in the trained group. A measurable improvement in aerobic performance, however, was not sustained.
Children with cystic fibrosis are now surviving into adulthood, with the median survival in 2004 being approximately 32 years.80 For patients born in the 1990s, the median survival is predicted to be more than 40 years. The cornerstones of treatment that have produced this increase in survival are airway clearance, nutritional support, and antibiotic therapy.
Survival in cystic fibrosis is correlated with maximal oxygen uptake. Blau et al.7 have found that an intensive 4-week summer camp program for both children and young adults improved exercise tolerance and nutrition in patients with cystic fibrosis.
Exercise in Disorders of Chest Wall Function
Numerous causes of chest wall dysfunction exist with both mechanical and neuromuscular components. The chest wall can be viewed as encompassing the rib cage, spine, diaphragm, abdomen, shoulder girdles, and neck. Distortions of the trunk are reflected in distortions of the heart, lungs, airway, and abdominal contents as well. Many conditions negatively affect the chest wall, including ankylosing spondylitis, pectus excavatum, obesity, the sequelae of thoracoplasty or phrenic nerve crush for the treatment of pulmonary tuberculosis, neuromuscular diseases with weakness of the respiratory bellows mechanism and superimposed spinal curvatures, and Parkinson disease. Ventilatory muscle training in these disorders can reduce respiratory muscle fatigue. Persons with Parkinson disease have shown overall improvement with pulmonary rehabilitation.11 Patients with developmental disorders are a heterogeneous group in which severe scoliosis is commonly seen. Exercise and other ongoing rehabilitation techniques, such as positioning, and management of spasticity and dysphagia, are of value in their treatment.72
Exercise in Paradoxical Vocal Fold Dysfunction
Paradoxical vocal fold dysfunction (PVFD) is caused by the vocal folds coming together during inspiration, instead of opening normally. The diagnosis of PVFD is based on patient history and laryngoscopy. The disorder can occur from adolescence to old age. In one study by Patel,54 90% of the patients were female, 56% of them had asthma, 12% had laryngeal findings suggestive of gastroesophageal reflux disease, 12% had findings of chronic laryngitis, and 33% had additional findings of laryngomalacia, vocal fold motion impairment, sulcus vocalis, nodules, and subglottic stenosis. These additional findings were mostly seen in the exercise-induced group. If the patient is symptomatic during the examination, a flow-volume loop of the FVC shows a flattening of the inspiratory loop, indicative of the low flow associated with partial obstruction in this area during inspiration. Neurologically based dystonias are also a common cause. Throat tightness, dysphonia, and inspiratory difficulty can occur. Speech therapy can provide respiratory retraining. Acute exacerbations can be relieved by administration of helium–oxygen (70%:30%) with or without NIPPV. Direct vocal cord injection of botulinum toxin A is also used.74
Nutritional Issues
In moderate to severe COPD, weight loss is common and is related to decreased exercise capacity and health status and increased morbidity and mortality. To determine the exact cause of weight loss it is important to look at the underlying mechanism of weight loss and specific tissue loss. Weight loss could be a result of high insulin resistance, high catecholamine levels, and dyslipidemia.2 More specifically loss of fat mass is generally caused by energy imbalance, whereas fat-free mass loss could be a result of substrate and protein metabolism.24,64
Creutzberg et al.18 studied nutritional treatment of the severely underweight patient in an inpatient program. Nutritional supplementation therapy with two or three 200-mL oral liquid nutritional supplements daily in these nutritionally depleted patients with COPD produced increases in body weight, fat-free mass, maximal inspiratory mouth pressure, handgrip strength, and peak workload. Symptoms and impact scores on the St. George’s Respiratory Questionnaire were improved as well. Maintenance oral glucocorticosteroid treatment blunted the response with respect to maximal inspiratory mouth pressure and peak workload, as well as the symptom score.
Studies after Creutzberg’s work found that total daily energy expenditure in patients with COPD, whether they were underweight or not, has been shown to be no different than in healthy persons.70 Insufficient food intake was found to be the cause of the malnutrition. Patients had greater success gaining weight when their total energy intake was 1.3 times higher than their resting energy expenditure.57 It was also found that smaller doses (125 mL) of a dietary supplement led to greater improvements. The smaller doses caused less bloating and satiety, which was found to be less compromising to diaphragmatic movement, and patients were less likely to cut out meals.8
The energy cost of the exercise associated with a pulmonary rehabilitation program was estimated to be 191 kcal/day. Protein supplements with a high proportion of carbohydrates over fat are necessary when vigorous exercise training results in negative energy balance and weight loss.68 In selected well-nourished patients, it might actually increase the shuttle walking performance.
Diet counseling and self-management with changes in dietary behavior should start early in the course, when the patient with COPD is still under the care of a primary physician.9 Practical suggestions for shopping, food preparation, and eating smaller, more frequent meals should be offered. When fatigue, dyspnea, swallowing, or poor appetite interferes with eating, a dietitian can be helpful. Involuntary weight loss is best addressed preventively.
Psychosocial Support
Biopsychosocial considerations for persons with pulmonary dysfunction include the ongoing education of the patient and family, vocational counseling, and disability evaluation. Occupational therapy also can provide patients with severe respiratory disease counseling on energy conservation and techniques for performing the basic activities of daily living.38 Depression and anxiety are very common comorbidities associated with COPD because of the often drastic limitations in functional activities and the panic associated with severe dyspnea. Antidepressant and anxiolytic medications are often useful adjuncts to counseling.
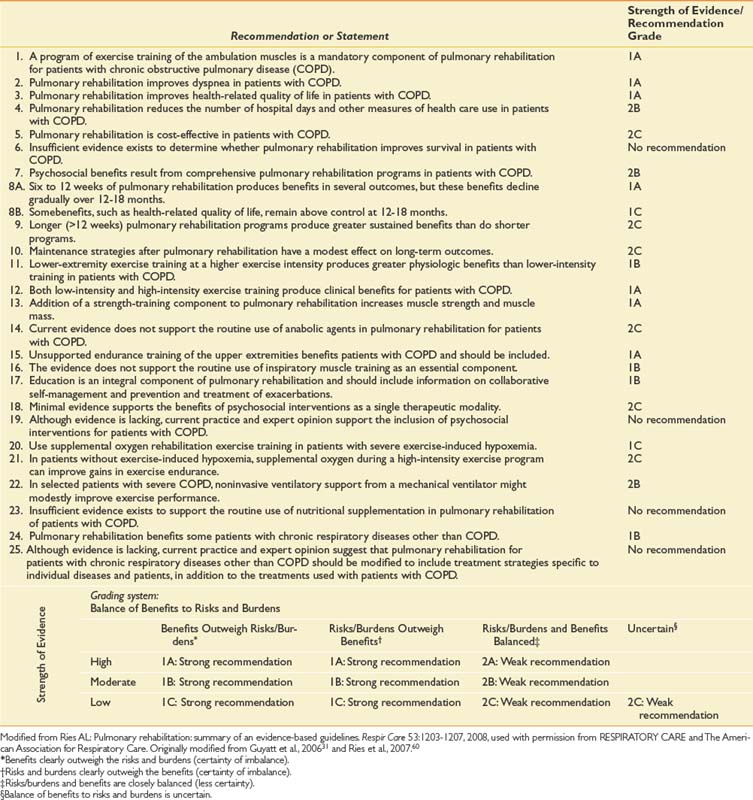
Table 34-4 presents a summary of recommendations, statements, and grades in the Evidence-Based Guidelines on Pulmonary Rehabilitation with Strength of Evidence/Balance of Benefits Grading System.
Management Options for Individuals With Severe Lung Disease
Mechanical Ventilation
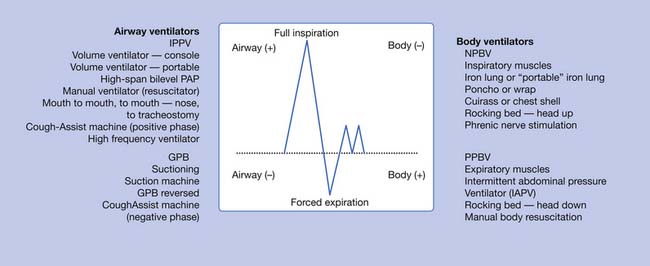
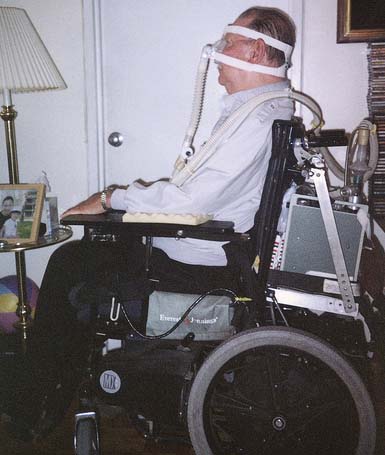
Within the past 25 years, there has been a dramatic reduction in the use of body ventilators (iron lungs). Their main function is to simulate the function of the inspiratory muscles. The intermittent abdominal pressure ventilator simulates the function of the expiratory muscles. Intermittent positive-pressure ventilation (IPPV) via noninvasive nasal–mouth interfaces or via tracheostomy is now the method of choice because the equipment is lightweight and portable, and powered by either AC or DC current. No contact is made with the individual other than the nasal–mouth interface or the attachment to the tracheostomy tube (Figures 34-3 through 34-6).
Portable ventilators are volume ventilators designed 25 years ago, high-span bilevel positive airway pressure units, and laptop volume ventilators (Figure 34-7). For further information on the wide range of noninvasive ventilator choices, the reader is referred to a recent publication.6
Air stacking (adding consecutive quantities of air into the lungs), maximal insufflations (passively inflating the patient’s lungs to maximal capacity), assisted coughing, and noninvasive ventilation are described as respiratory muscle aids by Bach.3 He has also developed an oximetry feedback respiratory aid protocol. In this protocol, the oximeter is used to provide patients and their families with feedback to maintain oxyhemoglobin saturation by pulse oximeter (SpO2) greater than 94%. This is accomplished by maintaining effective alveolar ventilation and eliminating airway secretion. If the SpO2 decreases to less than 95% in the patient with neuromuscular disease, it is due to one or more of three causes: (1) hypoventilation during which there will also be hypercapnia, (2) secretions, or (3) the development of intrinsic lung disease (usually gross atelectasis or pneumonia). Patients and families are taught to pursue airway clearance techniques conscientiously and to use NIPPV continuously. If these measures fail to bring the SpO2 back to normal or to baseline levels, patients must be evaluated by their clinician or in the emergency room. They should be admitted if necessary. Family members or primary care providers need to remain with the patient in the hospital to perform the ongoing routine of secretion removal. The routine itself is typically too time-consuming for hospital personnel to be able to remove secretions with the frequency that is necessary during an acute respiratory infection. When there is inadequate social support, hospital personnel usually find it necessary to intubate the patient for secretion removal and ventilation.
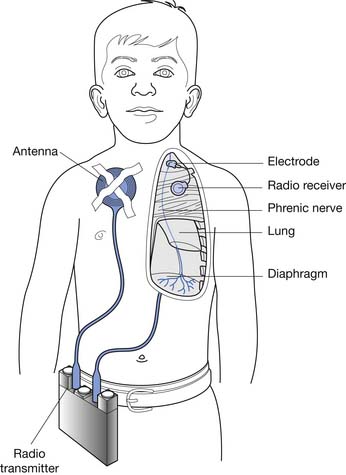
Diaphragmatic pacing (Figure 34-8) is an alternative method of ventilation that has been available for more than 35 years. The system attained a higher level of reliability and broader application in the early 1990s. Infection and component failure are now rare complications. The need for the patient to retain a tracheostomy because of obstructive sleep apnea remains common. Approximately 1500 phrenic nerve pacers have been implanted worldwide in persons of all ages. Many users have been successfully paced for more than 20 years.25 Diaphragmatic pacing is indicated in patients with congenital central hypoventilation syndrome (or Ondine’s curse), acquired central hypoventilation syndrome, and high SCI injury.22 Patients with inadequate social or financial support, poor motivation, or associated medical problems might not be good candidates. Although it is not common, the patient with a diaphragmatic pacer can require a mechanical ventilator as a backup during severe respiratory tract infections.
The pacing system consists of an external transmitter and antenna, and implanted electrodes and receiver. The working life expectancy of the receiver is for the lifetime of the patient, and batteries last for 2 to 3 weeks. A bipolar electrode is available for use in persons who already have demand cardiac pacers. Advanced warning is given of transmitter battery failure, with a gradual decrease in tidal volume over several days. The cervical implant is recommended in the older child and adult, with “customized” stimulation parameters that enable pacing with small numbers of residual fibers. Surgery in the supraclavicular region of the neck is relatively simple, and hospitalization is typical, although the procedure can be performed in an outpatient setting. Although thoracic phrenic nerve implants for infants and younger children have required thoracotomy in the past, thoracoscopic placement of the electrodes has now been reported in children as young as 5 years.65 Remote monitoring can be used to assess effectiveness and to diagnosis problems.
Krieger et al.36 reported performing 10 nerve transfers in six patients with SCI at the C3–C5 level, with axonal loss in the phrenic nerves to the extent that pacing was not possible. In their technique, the fourth intercostal nerve was attached to the distal segment of the phrenic nerve 5 cm above the diaphragm. The pacemaker was implanted on the phrenic nerve distal to the anastomosis. The average interval from surgery to diaphragmatic response to electrical stimulation was 9 months. All patients were able to tolerate diaphragmatic pacing as an alternative to IPPV.
Intramuscular diaphragm electrodes implanted via laparoscopic surgery were reported to successfully stimulate the phrenic nerves in one ventilator-dependent patient with tetraplegia.23 In patients having only a single phrenic nerve that can be paced, combined intercostal muscle and unilateral phrenic nerve stimulation has also been shown to maintain ventilatory support.65
Lung Volume Reduction Surgery
LVRS is used in patients with advanced emphysema. In LVRS, 20% to 30% of one or both lungs (usually at the apices) is removed. Decramer20 has reported the short-term results of six randomized studies, including the National Emphysema Treatment Trial. These studies have shown reduced hyperinflation (residual volume and total lung capacity decreased) and improved FEV1 and FVC. The 6-minute walk increased significantly, and quality of life improved. In general, inspiratory muscle function (maximal inspiratory pressure and maximal transdiaphragmatic pressure) also improved. Combining LVRS with exercise produces greater benefits than exercise alone.33
Not all patients will benefit from LVRS. Patients with an FEV1 less than 20% predicted and homogeneous disease, or a diffusion capacity less than 20% predicted, experience an increased mortality rate after LVRS. Additional studies suggest that patients getting the most benefit from LVRS are those with emphysema predominately in upper lobes who also present with low postrehabilitation exercise capacity.46 Conversely, patients with non–upper lobe involvement and high postrehabilitation measures of maximal work received little benefit from LVRS.46 The results of the National Emphysema Treatment Trial suggest that all LVRS candidates should first enroll in a pulmonary rehabilitation program because some patients can improve to the point that they might not qualify for the surgery.45 Furthermore, participation in a pulmonary rehabilitation program before LVRS helps to both identify appropriate surgical candidates61 and helps to ensure that candidates have appropriate levels of compliance and an understanding of their condition.69
Maxfield41 has discussed bronchoscopic LVRS as an alternative treatment option. Stapling and plication of the most emphysematous tissue are carried out. Atelectasis can also be induced in these areas by using endobronchial sealants, occluders, or valves. Extraanatomic tracts between the major airways and the emphysematous tissue are created and prevented from collapsing with stents to facilitate expiratory airflow.
Lung Transplant
Lung transplants are now performed worldwide. Living donor, lobar, and split-lung procedures are more common in children than in adults. Cystic fibrosis and primary pulmonary hypertension are the two main diseases of children for which lung transplant is performed. COPD is the main reason for lung transplant in adults, although pulmonary hypertension and pulmonary fibrosis are also common presenting diagnoses. Ongoing smoking is an absolute contraindication to lung transplant. Relative contraindications in most programs include previous cancer, psychiatric diagnosis, obesity, and correctable coronary artery disease.37 Fifty-eight percent of programs in North America that have been queried have a minimum requirement for exercise capacity to be considered for lung transplant. Covering a minimum of 600 feet on a 6-minute walk test is the most common requirement, although some programs require only 250 feet. If an individual can only transfer from bed to chair, 46% of lung transplant centers consider this an absolute contraindication to lung transplant, and 52% consider it a relative contraindication.
Special Considerations
Obesity-Related Pulmonary Dysfunction
Data from the 2003 to 2004 National Health and Nutrition Examination Survey indicate an estimated 66% of adults in the United States were overweight or obese (body mass index [BMI] > 25).50 Seventeen percent of U.S. children and adolescents are estimated to be overweight.50 All racial and ethnic groups and both genders are affected. Primary respiratory complications associated with obesity include obstructive sleep apnea, obesity-hypoventilation syndrome (OHS), and asthma.44 Chronic hypoxemia related to OHS can lead to polycythemia, pulmonary hypertension, cardiac dysrhythmias, and right heart failure. One study reported that three of four adults who presented to the emergency department with acute asthma were either overweight (BMI = 25 to 29.9; 30%) or obese (BMI > 30; 44%).71 Other conditions that can be associated with obesity include atelectasis, pneumonia, venous thrombosis, and pulmonary embolism.44 Daily physical activity is important in preventing and treating overweight and obesity because many of the aforementioned conditions are improved with weight loss.
Obesity increases the metabolic requirements of breathing and physical activity, resulting in decreased exercise tolerance. The weight relative maximal volume of oxygen consumption (VO2max) in obese persons can be significantly decreased compared with controls. Norman et al.49 studied severely obese adolescents (BMI >40) and found that although absolute VO2max (mL O2/min) was similar to controls, walk/run distance was approximately 60% of the control average. Although obese adults with COPD might have greater fatigue, functional impairment, and significantly lower 6-minute walk distance compared with normal-weight individuals with COPD, similar relative improvements in walk distance are seen after pulmonary rehabilitation.58
Spinal Cord Injury and Pulmonary Dysfunction
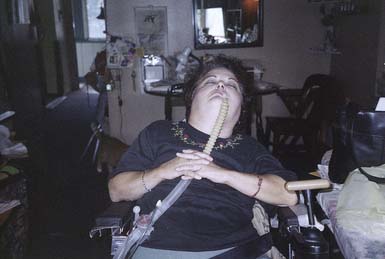
In SCI, there is diminished cardiopulmonary and circulatory function, as well as lower limb muscle atrophy and bone mass reduction. Patients with high cervical cord injuries, including children as young as 3 years, can learn neck breathing28 or GPB as forms of voluntary respiration. This allows some time off the ventilator. In GPB (or frog breathing), air is pumped into the lungs by the patient using the tongue as a piston (Figure 34-9). This technique is known as glottic press to speech pathologists. They teach it to patients who have had a laryngectomy, to pump air into the esophagus for esophageal speech. GPB can be used for a number of purposes in addition to being an alternative form of breathing. GPB can improve vocal volume and flow of speech to allow the patient to call for help, and to provide the deep breath needed for an assisted cough.
Long-Term Results of Pulmonary Rehabilitation
Long-Term Outcomes of Mechanical Ventilation
Many patients are now surviving significant illness only to become chronically ventilator-dependent. The number of long-term care hospitals in the United States has increased to more than 250 in the past 15 years to care for these patients. Acute lung injury survivors weaned from the ventilator have been studied 1 year after hospital discharge.19 Thirty percent still have generalized cognitive dysfunction, and 78% have decrements in attention, memory, concentration, and/or mental-processing speed. Many also have musculoskeletal complications such as weakness and numbness resulting from critical illness neuropathy and steroid-induced myopathy. Acute respiratory distress syndrome survivors weaned from the ventilator have been studied 1 year after hospital discharge.19 Emotional dysfunction included the presence of depression, anxiety, and posttraumatic stress disorder.
Long-term outcomes of diaphragm paralysis after acute SCI have been reported.51 Over a 16-year period, 107 patients required assisted ventilation in the acute phase of injury. Of this group, 31% (33 patients) with a level of injury between C1 and C4 (scale A in the American Spinal Injury Association impairment scale) had diaphragmatic paralysis at the time of respiratory failure. Twenty-one percentof the 33 patients were able to breathe independently after 4 to 14 months, with a vital capacity greater than 15 mL/kg. Another 15% (five patients) had some recovery that took more time and for whom the vital capacity remained less than 10 mL/kg.
Bach3 has described in detail the long-term outcomes of NIPPV in neuromuscular disease. No patient with DMD has been tracheostomized since 1983 at the Center for Ventilator Management Alternatives and Pulmonary Rehabilitation of the University Hospital in Newark, New Jersey.Some have been using 24-hour mechanical ventilation for up to 15 to 25 years. Cardiomyopathy has become the limiting factor for survival in this population as respiratory deaths are being avoided.
Wijkstra et al.79 reported the long-term outcomes of inpatients with chronic assisted ventilatory care over 15 years. Thirty-six percent of their 50 patients left the hospital to enter a more independent community-based environment. Better outcomes were seen among patients with SCI and neuromuscular disease than in patients with COPD and thoracic restriction.
Gonzalez et al.30 studied long-term NIPPV in kyphoscoliotic ventilatory insufficiency (thoracic restriction) over 3 years. Patients showed improved blood gas levels and respiratory muscle performance and reduced hypoventilation-based symptoms and number of hospital days.
Long-Term Results for Lung Volume Reduction Surgery
Naunheim et al.46 provided evidence that the distribution of emphysematic changes in lung parenchyma (measured by high-resolution computed tomography) and measures of exercise capacity after rehabilitation are important factors in predicting the long-term effects of LVRS. Patients who received LVRS and had emphysema predominantly in the upper lobes with lower levels of exercise capacity continued to have improved survival rates after 4 years, improved function after 3 years, and improved dyspnea after 5 years. Patients with upper lobe involvement and high levels of exercise capacity after rehabilitation continued to have symptomatic improvements when assessed after 4 years. Patients with non–upper lobe emphysema and low exercise capacity primarily experienced improvements in health-related quality of life that became insignificant after 3 years. Patients with non–upper lobe emphysema and high exercise capacity did not experience significant benefits from LVRS, and actually experienced a higher mortality rate during the first 3 years. Improvements in all groups were no longer significant after 5 years.
Long-Term Results in Lung Transplant
Survival rates for all lung transplants from 1994 through mid-2005 were 78% at 1 year, 50% at 5 years, and 26% at 10 years, with the highest mortality rate occurring during the first year.73 Although perioperative mortality rates are not significantly different for all age-groups, patients older than 50 years tend to experience a lower survival rate beyond the first year.73 Sepsis and bronchiolitis obliterans remain the two most common causes of death. These results, however, should be tempered with the knowledge that the mortality rate for those on the transplant wait list is also high. For example, the 3-year survival rate for those in the sickest category of the COPD BODE Index (Body mass index, Degree of airflow Obstruction, functional Dyspnea, Exercise capacity) is only 50%.15
1. Ambrosino N., Strambi S. New strategies to improve exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J. 2004;24:313-322.
2. Aniwidyaningsih W., Varraso R., Cano N., et al. Impact of nutritional status on body functioning in chronic obstructive pulmonary disease and how to intervene. Curr Opin Clin Nutr Metab Care. 2008;11:435-442.
3. Bach J.R. Management of patients with neuromuscular disease. Philadelphia: Hanley & Belfus; 2004.
4. Bach J.R., Smith W.H., Michaels J., et al. Airway secretion clearance by mechanical exsufflation for post-poliomyelitis ventilator-assisted individuals. Arch Phys Med Rehabil. 1993;74:170-177.
5. Barreiro E., Gea J., Sanjuás C., et al. Dyspnoea at rest and at the end of different exercises in patients with near-fatal asthma. Eur Respir J. 2004;24:219-225.
6. Benditt J.O. Full-time noninvasive ventilation: possible and desirable. Respir Care. 2006;51:1005-1012.
7. Blau H., Mussaffi-Georgy H., Fink G., et al. Effects of an intensive 4-week summer camp on cystic fibrosis: pulmonary function, exercise tolerance, and nutrition. Chest. 2002;121:1117-1122.
8. Broekhuizen R., Creutzberg E.C., Weling-Scheepers C.A.P.M., et al. Optimizing oral nutritional drink supplementation in patients with chronic obstructive pulmonary disease. Br J Nutr. 2005;93:965-971.
9. Brug J., Schols A., Mesters I. Dietary change, nutrition education and chronic obstructive pulmonary disease. Patient Educ Couns. 2004;52:249-257.
10. California Pulmonary Rehabilitation Collaborative Group. Effects of pulmonary rehabilitation on dyspnea, quality of life, and healthcare costs in California. J Cardiopulm Rehabil. 2004;24:52-62.
11. Casaburi R. Principles of exercise training. Chest. 1992;101:263S-267S.
12. Casaburi R., Patessio A., Ioli F., et al. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143:9-18.
13. Celli BR. Pulmonary rehabilitation in COPD. UpToDate. Online. Available at: http://www.uptodate.com. Accessed May 29, 2009.
14. Celli B.R. Update on the management of COPD. Chest. 2008;134:892.
15. Celli B.R., Cote C.G., Marin J.M., et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005-1012.
16. Celli B.R., Thomas N.E., Anderson J.A., et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332-338.
17. Colice G.L. The seduction of asthma severity categorization. Chest. 2003;124:2054-2056.
18. Creutzberg E.C., Wouters E.F.M., Mostert R., et al. Efficacy of nutritional supplementation therapy in depleted patients with chronic obstructive pulmonary disease. Nutrition. 2003;19:120-127.
19. Curtis J.R. The long-term outcomes of mechanical ventilation: What are they and how should they be used? Respir Care. 2002;47:496-505. discussion 505
20. Decramer M. Treatment of chronic respiratory failure: Lung volume reduction surgery versus rehabilitation. Eur Respir J. 2003;22:47S-56S.
21. Dekhuijzen P.N., Beek M.M., Folgering H.T., et al. Psychological changes during pulmonary rehabilitation and target-flow inspiratory muscle training in COPD patients with a ventilatory limitation during exercise. Int J Rehabil Res. 1990;13:109-117.
22. DiMarco A.F., Onders R.P., Ignagni A., et al. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest. 2005;127:671-678.
23. DiMarco A.F., Onders R.P., Kowalski K.E., et al. Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med. 2002;166:1604-1606.
24. Doucet M., Russell A.P., Léger B., et al. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:261-269.
25. Elefteriades J.A., Quin J.A., Hogan J.F., et al. Long-term follow-up of pacing of the conditioned diaphragm in quadriplegia. Pacing Clin Electrophysiol. 2002;25:897-906.
26. Fahy B.F. Pulmonary rehabilitation for chronic obstructive pulmonary disease: a scientific and political agenda. Respir Care. 2004;49:28-36. discussion 36
27. Garuti G., Cilione C., Dell’Orso D., et al. Impact of comprehensive pulmonary rehabilitation on anxiety and depression in hospitalized COPD patients. Monaldi Arch Chest Dis. 2003;59:56-61.
28. Gilgoff I.S., Barras D.M., Jones M.S., et al. Neck breathing: a form of voluntary respiration for the spine-injured ventilator-dependent quadriplegic child. Pediatrics. 1988;82:741-745.
29. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2008 Update. Online. Available at: http://www.goldcopd.com. Accessed May 29, 2009.
30. Gonzalez C., Ferris G., Diaz J., et al. Kyphoscoliotic ventilatory insufficiency: Effects of long-term intermittent positive-pressure ventilation. Chest. 2003;124:857-862.
31. Guyatt G., Gutterman D., Baumann M.H., et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129:174-181.
32. Hass F., Axen K., editors. Pulmonary therapy and rehabilitation: principles and practice, ed 2, Baltimore: Williams & Wilkins, 1991.
33. Hillerdal G., Löfdahl C.G., Ström K., et al. Comparison of lung volume reduction surgery and physical training on health status and physiologic outcomes: a randomized controlled clinical trial. Chest. 2005;128:3489-3499.
34. Jaeger R.J., Turba R.M., Yarkony G.M., et al. Cough in spinal cord injured patients: comparison of three methods to produce cough. Arch Phys Med Rehabil. 1993;74:1358-1361.
35. Klijn P.H.C., Oudshoorn A., Van Der Ent C.K., et al. Effects of anaerobic training in children with cystic fibrosis: a randomized controlled study. Chest. 2004;125:1299-1305.
36. Krieger L.M., Krieger A.J. The intercostal to phrenic nerve transfer: an effective means of reanimating the diaphragm in patients with high cervical spine injury. Plast Reconstr Surg. 2000;105:1255-1261.
37. Levine S.M. A survey of clinical practice of lung transplantation in North America. Chest. 2004;125:1224-1238.
38. Lorenzi C.M., Cilione C., Rizzardi R., et al. Occupational therapy and pulmonary rehabilitation of disabled COPD patients. Respiration. 2004;71:246-251.
39. Mahlmeister M.J., Fink J.B., Hoffman G.L., et al. Positive-expiratory-pressure mask therapy: theoretical and practical considerations and a review of the literature. Respir Care. 1991;36:1218-1230.
40. Man S.F.P., McAlister F.A., Anthonisen N.R., et al. Contemporary management of chronic obstructive pulmonary disease: clinical applications. JAMA. 2003;290:2313-2316.
41. Maxfield R.A. New and emerging minimally invasive techniques for lung volume reduction. Chest. 2004;125:777-783.
42. McCool F.D., Rosen M.J. Nonpharmacologic airway clearance therapies: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:250S-259S.
43. Mota S., Güell R., Barreiro E., et al. Clinical outcomes of expiratory muscle training in severe COPD patients. Respir Med. 2007;101:516-524.
44. Murugan A.T., Sharma G. Obesity and respiratory diseases. Chron Respir Dis. 2008;5:233-242.
45. National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059-2073.
46. Naunheim K.S., Wood D.E., Mohsenifar Z., et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg. 2006;82:431-443.
47. Naylor J.M., McLean A., Chow C.M., et al. A modified postural drainage position produces less cardiovascular stress than a head-down position in patients with severe heart disease: a quasi-experimental study. Aust J Physiother. 2006;52:201-209.
48. Nici L., Donner C., Wouters E., et al. American thoracic society/European respiratory society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390-1413.
49. Norman A.C., Drinkard B., McDuffie J.R., et al. Influence of excess adiposity on exercise fitness and performance in overweight children and adolescents. Pediatrics. 2005;115:e690-e696.
50. Ogden C.L., Carroll M.D., Curtin L.R., et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549-1555.
51. Oo T., Watt J., Soni B.M., et al. Delayed diaphragm recovery in 12 patients after high cervical spinal cord injury: a retrospective review of the diaphragm status of 107 patients ventilated after acute spinal cord injury. Spinal Cord. 1999;37:117-122.
52. Otani K., Kanazawa H., Fujiwara H., et al. Determinants of the severity of exercise-induced bronchoconstriction in patients with asthma. J Asthma. 2004;41:271-278.
53. Pastva A., Estell K., Schoeb T.R., et al. Aerobic exercise attenuates airway inflammatory responses in a mouse model of atopic asthma. J Immunol. 2004;172:4520-4526.
54. Patel N.J., Jorgensen C., Kuhn J., et al. Concurrent laryngeal abnormalities in patients with paradoxical vocal fold dysfunction. Otolaryngol Head Neck Surg. 2004;130:686-689.
55. Patessio A., Carone M., Ioli F., et al. Ventilatory and metabolic changes as a result of exercise training in COPD patients. Chest. 1992;101:274S-278S.
56. Pierson D.J. Translating new understanding into better care for the patient with chronic obstructive pulmonary disease. Respir Care. 2004;49:99-109.
57. Planas M., Álvarez J., García-Peris P.A., et al. Nutritional support and quality of life in stable chronic obstructive pulmonary disease (COPD) patients. Clin Nutr. 2005;24:433-441.
58. Ramachandran K., McCusker C., Connors M., et al. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis. 2008;5:205-209.
59. Ries A.L. Pulmonary rehabilitation: summary of an evidence-based guideline. Respir Care. 2008;53:1203-1207.
60. Ries A.L., Bauldoff G.S., Carlin B.W., et al. Pulmonary rehabilitation: Joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:4S-42S.
61. Ries A.L., Make B.J., Lee S.M., et al. The effects of pulmonary rehabilitation in the National Emphysema Treatment Trial. Chest. 2005;128:3799-3809.
62. Rubin B.K., Van Der Schans C.P., Kishioka C., et al. Mucus and mucoactive therapy in chronic bronchitis. Clin Pulm Med. 1998;5:1-14.
63. Rundell K.W., Anderson S.D., Spiering B.A., et al. Field exercise vs laboratory eucapnic voluntary hyperventilation to identify airway hyperresponsiveness in elite cold weather athletes. Chest. 2004;125:909-915.
64. Rutten E.P.A., Franssen F.M.E., Engelen M.P.K.J., et al. Greater whole-body myofibrillar protein breakdown in cachectic patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2006;83:829-834.
65. Shaul D.B., Danielson P.D., McComb J.G., et al. Thoracoscopic placement of phrenic nerve electrodes for diaphragmatic pacing in children. J Pediatr Surg. 2002;37:974-978.
66. Soriano J.B., Davis K.J., Coleman B., et al. The proportional venn diagram of obstructive lung disease: Two approximations from the United States and the United Kingdom. Chest. 2003;124:474-481.
67. Spruit M.A., Troosters T., Trappenburg J.C.A., et al. Exercise training during rehabilitation of patients with COPD: a current perspective. Patient Educ Couns. 2004;52:243-248.
68. Steiner M.C., Barton R.L., Singh S.J., et al. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2003;58:745-751.
69. Takaoka S.T., Weinacker A.B. The value of preoperative pulmonary rehabilitation. Thorac Surg Clin. 2005;15:203-211.
70. Tang N.L.S., Chung M.L., Elia M., et al. Total daily energy expenditure in wasted chronic obstructive pulmonary disease patients. Eur J Clin Nutr. 2002;56:282-287.
71. Thomson C.C., Clark S., Camargo C.A.Jr. Body mass index and asthma severity among adults presenting to the emergency department. Chest. 2003;124:795-802.
72. Toder D.S. Respiratory problems in the adolescent with developmental delay. Adolesc Med. 2000;11:617-631.
73. Trulock E.P., Christie J.D., Edwards L.B., et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-fourth official adult lung and heart-lung transplantation Report-2007. J Heart Lung Transplant. 2007;26:782-795.
74. Vlahakis N.E., Patel A.M., Maragos N.E., et al. Diagnosis of vocal cord dysfunction: the utility of spirometry and plethysmography. Chest. 2002;122:2246-2249.
75. Wadell K., Sundelin G., Henriksson-Larsén K., et al. High intensity physical group training in water: an effective training modality for patients with COPD. Respir Med. 2004;98:428-438.
76. Warburton D.E., Nicol C.W., Bredin S.S. Prescribing exercise as preventive therapy. CMAJ. 2006;174:961-974.
77. Weisman I.M., Marciniuk D., Martinez F.J., et al. II. Indications for cardiopulmonary exercise testing. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211-277.
78. Whaley M.H., Brubaker P.H., Otto R.M., editors. ACSM’s guidelines for exercise testing and prescription, ed 7, Philadelphia: Lippincott Williams & Wilkins, 2006.
79. Wijkstra P.J., Avendaño M.A., Goldstein R.S. Inpatient chronic assisted ventilatory care: a 15-year experience. Chest. 2003;124:850-856.
80. Yankaskas J.R., Marshall B.C., Sufian B., et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S.
81. Zeitoun M., Wilk B., Matsuzaka A., et al. Facial cooling enhances exercise-induced bronchoconstriction in asthmatic children. Med Sci Sports Exerc. 2004;36:767-771.
82. Zu Wallack R.L., Patel K., Reardon J.Z., et al. Predictors of improvement in the 12-minute walking distance following a six-week outpatient pulmonary rehabilitation program. Chest. 1991;99:805-808.