Chapter 22 Pulmonary Host Defenses
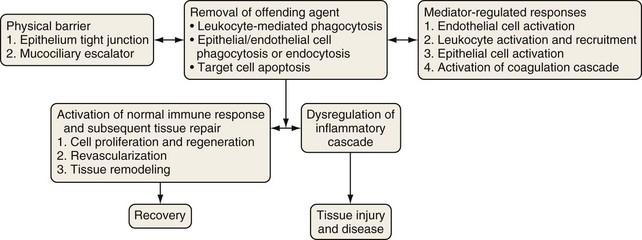
The epithelial surface of the lung is continuously exposed to a variety of potentially pathogenic microorganisms, allergens, particulate pollutants, and other noxious agents. An intricate defense system has evolved over time to protect the lungs from these potentially harmful entities while preserving homeostasis and lung function (Figure 22-1). This system of defense mechanisms has two components: an innate (nonspecific) response and an adaptive or acquired (specific) response.
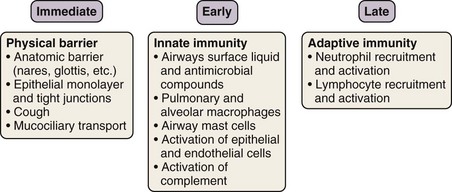
The innate immune response is evolutionarily conserved to provide immediate (occurring over seconds to minutes) host defense in a broad, nonspecific manner. Only vertebrates have an additional, adaptive immune system, which is directed at specific pathogens or molecules. Although the two systems work in concert to protect the host, each has several distinctive features. With rare exceptions, the innate component of the immune system depends on proteins and signaling pathways that exist in a fully functional form, does not require priming, and is not strengthened with subsequent exposures. By contrast, the adaptive immune response requires additional time (days to weeks) (see Figures 22-2 and 22-3) to ramp up to full capacity, is specific to the pathogen (and even to specific molecular determinants of the pathogen), and has memory to provide for stronger responses with subsequent attacks (“anamnestic response”).
Structural Defenses
With a surface area in adults of 70 m2, which comes into contact with roughly 10,000 L of air a day, the lung is confronted with constant threats from microbes. In addition to inhaled pathogens, high bacterial concentrations are present in oropharyngeal secretions (108/mL), and aspiration of these secretions also may pose a serious risk for invasive infection (aspiration pneumonia). Historically, the lung has been considered to be a sterile environment; recent developments in molecular analysis have provided evidence for the presence of a microbial flora of considerable diversity in the lung, which moreover is altered in disease states. Accordingly, the respiratory tract has developed a series of structural barriers that are designed both to minimize the number of pathogenic microbes entering the lungs and to hasten their clearance before an infection can be established (Table 22-1).
| Structure | Functions |
|---|---|
| Nose |
Particle size is an important factor determining the degree of penetration into the airways (Table 22-2). Very large particles are filtered by vibrissae (nasal hairs). Particles approximately 30 µm in diameter are removed in the nasal passages, where turbulent airflow results in prolonged air-mucosa contact, with subsequent particle impaction. Most particles between 10 and 30 µm in diameter also will be deposited on the turbinates and nasal septum, carina, or within the larger bronchi. The branching nature of the airways provides two additional mechanisms of protection: (1) the secondary, tertiary, and quaternary carinae force particles to embed in the airway mucosa, thereby preventing further penetration into the lung, and (2) reduced airflow with increased airway branching allows gravity to sediment most particles larger than 2 µm. Particles less than 0.2 to 0.5 µm across tend to stay suspended as aerosols and are exhaled. Much smaller particles (less than 0.1 µm) may be deposited as a result of brownian motion (bombardment with gas molecules).
Table 22-2 Effect of Particle Size on Penetration into Airways
| Particle Size | Fate |
|---|---|
| >>>30 µm | Filtered by vibrissae |
| >30 µm | Nasopharyngeal impaction |
| 10 to 30 µm | Nasopharyngeal, tracheal, and large bronchial impaction |
| 2 to 10 µm | Sedimentation in airways |
| 0.2 to 2 µm | Reach alveoli |
| 0.2 to 0.5 µm | Exhaled |
| <0.2 µm | Exhaled or deposited (brownian motion) |
Cough/Sneeze
Mechanical or chemical stimulation of receptors in the nose, larynx, or trachea or elsewhere in the respiratory tree may produce bronchoconstriction to prevent deeper penetration of irritants and also may trigger the cough or sneeze reflex to expel particles deposited in the airways (Table 22-3). The cough reflex aids mucociliary transport to remove trapped particles. Mucus usually is conveyed to the carina by the cilia and then expelled by coughing from this location. Disruption of the cough reflex (e.g., in smokers or patients with vocal cord palsy or stroke) results in a predisposition to pneumonias.
| Component | Event(s) |
|---|---|
| 1. Inspiratory phase | Deep inspiration, usually 1 to 2 times tidal volume |
| 2. Compression phase | Begins with closure of the glottis and contraction of respiratory muscles, resulting in the generation of high intrathoracic pressure (up to 100 to 200 cm H2O in adults) |
| 3. Expressive phase | Glottal opening, with airflow at rates as high as 25,000 cm/sec (partly helped by compression of airway cross section) |
| 4. Relaxation phase | Relaxation of respiratory muscles with temporary bronchodilatation |
Respiratory Epithelium
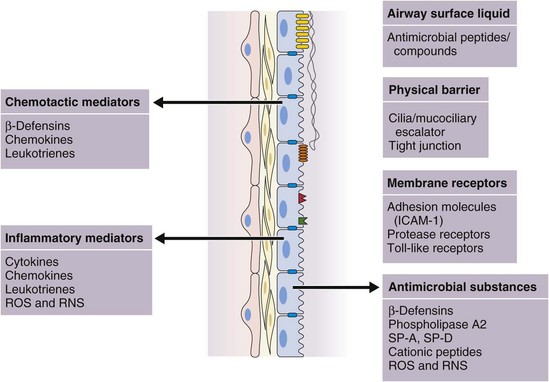
In addition to this important physical barrier function, epithelial cells, including ciliated cells, goblet cells, serous cells, basal cells, and Clara cells, are integral to the normal function of the mucociliary escalator, the production of a variety of antimicrobial molecules, and the initiation and regulation of inflammatory responses (see Figure 22-4).
Mucociliary Escalator
The airway epithelium is lined from the trachea to the respiratory bronchioles by the airway surface liquid (ASL), a 5- to 25-µm-thick surface film the primary function of which is to trap and facilitate the physical removal of foreign particles, as well as to provide an environment conducive to the activity of antimicrobial molecules (Table 22-4).
| Structure | Function(s) | Dysfunction in Disease |
|---|---|---|
ASL is a critical component of the mucociliary escalator and is the result of secretion by glands and serous cells and of plasma transudation (Table 22-5). The inner low-viscosity periciliary sol facilitates the coordinated beating action of the cilia that propels the outer viscous mucous blanket toward the glottis, thereby facilitating the removal of trapped pathogens or particles by expectoration or ingestion. The viscous mucus is composed of mucopolysaccharides, produced predominantly by submucous glands in the larger airways, with increasing contributions from goblet cells and Clara cells with successively larger airway generations.
Table 22-5 Properties of Airway Surface Liquid (ASL)
| Composition | Function | Dysfunction in Disease |
|---|---|---|
| Secretions from glands and goblet cells Plasma transudation |
Antimicrobial properties due to low pH and secreted antimicrobial compounds | Cystic fibrosis Asthma COPD |
COPD, chronic obstructive pulmonary disease.
Specific Cell Responses
Diverse cell populations contribute to the host defense system of the lung. In addition to the cells of the respiratory epithelium, these include other structural cells of the lung, the pulmonary vascular endothelium and fibroblasts, resident leukocytes, and, at later stages of immune responses, recruited immune cells (Table 22-6), as reviewed next.
Table 22-6 Cells Mediating Pulmonary Host Defense and the Inflammatory Response
| Timing | Immune Cells | Nonimmune Cells |
|---|---|---|
| Early phase |
Epithelial Cells
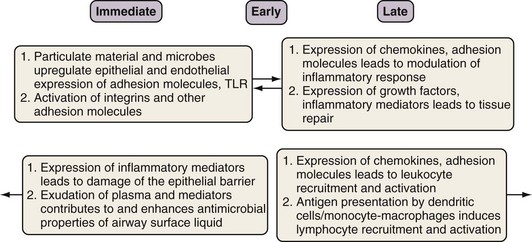
The contribution of the respiratory epithelium is not limited to its roles as a structural barrier and facilitator of mucociliary clearance (Box 22-1). Respiratory epithelial cells actively participate in the regulation of inflammation and are capable of mounting an immune response by internalization of organisms and secretion of cytotoxic and antimicrobial peptides. Inhaled microbial pathogens including bacteria and viruses and other antigens can trigger activation of pathogen recognition receptors (such as Toll-like receptors [TLRs], discussed later in some detail under “Innate Immune Receptors”) expressed by epithelial cells. Epithelial cells are induced by bacterial components, such as LPS, and by cytokines such as tumor necrosis factor (TNF)-α and interleukin-1β (IL-1β) to express various gene products (by the NFκB signaling pathway, discussed later on) that modulate the inflammatory response (Figure 22-4). Such inducers include the following:
• Cytokines such as TNF-α, IL-1β, and thymic stromal lymphopoietin (TSLP)
• Chemokines that include macrophage inflammatory protein (MIP)-2, CXC chemokines, monocyte chemoattractant protein (MCP)-1, IL-7, IL-8, IL-15
• Nitric oxide (NO) and reactive nitrogen species (ONOO−)
• Adhesion molecules such as β-integrins and intercellular adhesion molecule-1 (ICAM-1)
• TLRs such as TLR-2 and TLR-4
• TNF-α receptors, TNFR1 and TNFR2
• Growth factor receptors such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR)
Endothelial Cells
• Reactive nitrogen and oxygen species, including nitric oxide (NO•), peroxynitrite (ONOO−), superoxide (O2−), and hydrogen peroxide (H2O2), which are cytotoxic to microorganisms and cells
• Inflammatory cytokines such as TNF-α, IL-1β, and IL-6, as well as chemokines such as IL-8, RANTES (regulated on activation, normal T cell–expressed and secreted), and MIP-1, which activate and recruit leukocytes
• Cytokine and chemokine receptors such as TNFR1 and IL-1R
• Adhesion molecules, including ICAM-1, ICAM-2, platelet endothelial cell adhesion molecule (PECAM), vascular cell adhesion molecule-1 (VCAM-1), E-selectin, and P-selectin, which have differential binding specificities for specific leukocyte populations
• Procoagulants and protease-activated receptors
• Leukotrienes and prostaglandins
• Growth factors such as vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)-β
• Alterations in the surface adhesion molecules, cytoskeleton, and intercellular (junctional) proteins to allow for leukocyte adhesion transmigration and to regulate changes in vascular permeability
Immune Cells
Several leukocyte populations play distinct and vital roles in host defense in the lung and can be broadly classified as those that are normal residents of the lung (monocyte-macrophages, mast cells, dendritic cells) and those recruited in response to infection in injury (neutrophils and lymphocytes) (Table 22-7). Studies in animal models and in humans have demonstrated the increased risk of lung infection associated with defects in or absence of these cell types.
| Immune Cell | Primary Role(s) | Primary or Unique Inflammatory Mediators |
|---|---|---|
| Neutrophils |
ICAM-1, intercellular adhesion molecule-1; IL-1, interleukin-1 [etc.]; MCP-1, monocyte chemoattractant protein-1; MIP-1, macrophage inflammatory protein-1; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; PAF, platelet-activating factor; TLRs, Toll-like receptors; VEGF, vascular endothelial growth factor.
Monocyte-Macrophages
Mast Cells
• Pre-formed mediators that are granule-associated (such as histamine)
• Mediators synthesized de novo (such as leukotriene C4, platelet-activating factor, and prostaglandin D2)
• A vast array of cytokines and chemokines, including IL-1, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-16, TNF-α, VEGF, TGF-β, MIP-1α, and MCP-1
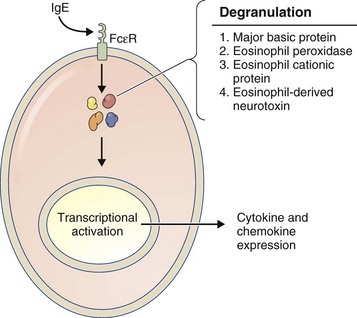
Eosinophils
Eosinophils (Figure 22-5) are considered to be effector cells of allergic responses and of parasite elimination. These bone marrow–derived cells contain four distinct granule cationic proteins: (1) major basic protein; (2) eosinophil peroxidase; (3) eosinophil cationic protein; and (4) eosinophil-derived neurotoxin.
Polymorphonuclear Neutrophils
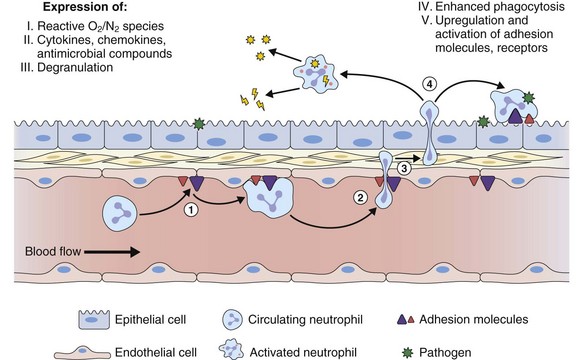
The primary function of neutrophils in the innate immune response is to contain and kill invading microbial pathogens. Neutrophils merit particular mention in pulmonary host defense as the pulmonary microvascular endothelium preferentially recruits this leukocyte population in response to infection and inflammation (see Figure 22-6) by the expression of adhesion molecules and chemokines that target neutrophils. Recently it has been recognized that the TH17 pathway involving the production of IL-17 and IL-23 can lead to neutrophil recruitment. Different stimuli including allergen sensitization, mast cell activation, various infections and TNF production, can promote a TH17 response and thereby recruit neutrophils to the lung.
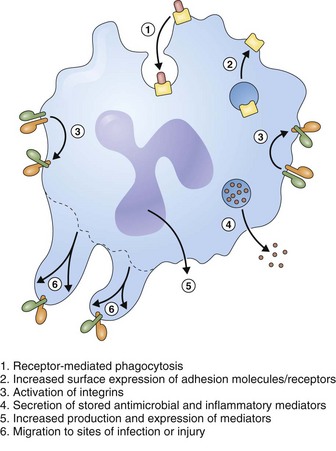
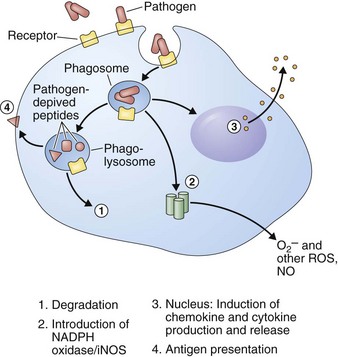
Neutrophils achieve their antimicrobial function through a series of rapid and coordinated responses that culminate in phagocytosis and destruction of the pathogens (Figure 22-7). Neutrophils have a potent antimicrobial arsenal that includes the following roles:
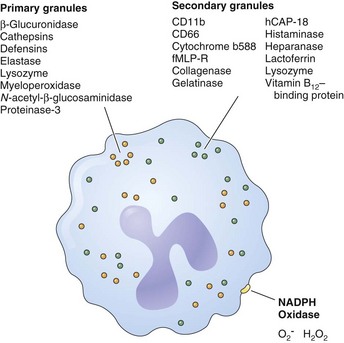
Oxidants such as O2− and H2O2 are produced by a multicomponent enzyme termed the phagocyte NADPH oxidase (NOX2). Granules within the cytoplasm of polymorphonuclear neutrophils (i.e., leukocytes) (PMNs) contain potent proteinases and cationic proteins that can digest a variety of microbial substrates. These compounds are released directly into the phagosome, compartmentalizing both the pathogen and the cytotoxic products (Figure 22-8). Conversely, neutrophil serine proteinases can be externalized in a weblike fashion together with nucleosomes to function as a trap for pathogens. These neutrophil extracellular traps (NETs) have been described in the vasculature and in the airways. NETs have an important antimicrobial function independent of phagocytosis by bringing and immobilizing microbial pathogens in close proximity to proteinases. NETs also provide a physical barrier to microbial spread. Of interest, some evidence suggests that eosinophils also produce NET-like structures in the lung.
Antimicrobial Molecules
Antimicrobial molecules (Table 22-8) are expressed by multiple cell types and play important roles in destruction and removal of pathogens. An overview of the important factors that have been shown to impede microbial growth and infection in the context of host defense of the lung is presented next.
| Factor | Role(s)/Function(s) |
|---|---|
| Lactoferrin |
LPS, lipopolysaccharide; PMN, polymorphonuclear neutrophil [leukocyte].
Antimicrobial Components in the Airway Surface Liquid
Lactoferrin
• Enhancement of neutrophil functions—motility, adherence, and superoxide production
• Inhibition of biofilm formation by Pseudomonas aeruginosa
• The ability to injure bacterial membranes with resultant release of LPS
• Modulation of LPS activity by competitively binding LPS, thus preventing LPS-binding protein from binding and promoting an LPS-CD14 interaction, which would result in cell activation
Complement
C5a: potent anaphylatoxin and chemoattractant that recruits neutrophils into the lung and enhances neutrophil-mediated microbial killing
C3a: another potent anaphylatoxin that also stimulates mucus production from goblet cells and enhanced LPS-induced synthesis of TNF and IL-1 by adherent phagocytes while decreasing synthesis in nonadherent cells
C5a-C9 complex: also known as the membrane attack complex (MAC), which creates pores in cell membranes, effectively killing bacteria
Multiple complement components can function as opsonins aiding phagocytosis of foreign particles (Figure 22-9). Of importance, hereditary deficiency of specific components of the complement pathway results in recurrent respiratory tract infections.
Antimicrobial Peptides
Defensins
• Enhanced bacterial binding and killing and cytokine production by airway epithelial cells
• Chemotaxis for monocytes and immature dendritic cells
• Direct binding to CCR6 receptors on dendritic cells, thus linking the innate immune system to adaptive immunity (β-defensins)
• Promotion of phagocytosis in macrophages and neutrophils
• Downregulation of the inflammatory response by increasing SLPI release from airway epithelial cells, inhibiting the complement cascade, and reducing macrophage cytokine release and neutrophil oxidant production
Cathelicidins
Innate Immune Receptors
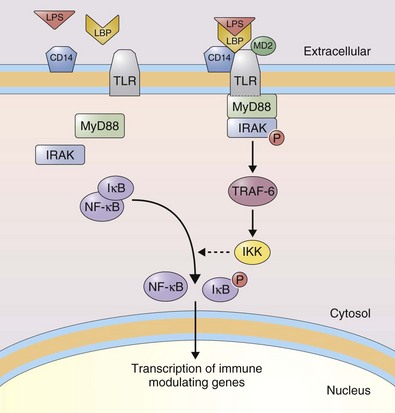
As highlighted earlier, the respiratory epithelium and other structural cells of the lung express innate immune receptors. These receptors function primarily as pattern recognition receptors to recognize conserved molecular patterns in microbial pathogens, called pathogen-associated molecular patterns (PAMPs), which are not normally found in the host (Table 22-9). This “pre-recognition” allows the host to respond to specific microbial products in the absence of an adaptive response; thus, the response does not require priming and is always present. Innate immune receptors include the membrane bound Toll-like receptors (TLRs) and additional cytosolic receptors. The 10 human TLRs share a protein structure characterized by an extracellular domain with a number of leucine-rich repeats (LRRs) and a cytoplasmic domain containing a Toll/IL1 receptor homology domain (TIR). They are highly expressed by leukocytes and in tissues in contact with the external environment, such as the lung. Cellular expression of TLRs is modulated by microbial stimuli. TLRs are found as homodimers with the exception of TLR-1 and TLR-6, which form heterodimers with TLR-2. TLR activation after binding of its cognate ligand triggers signal transduction pathways that ultimately lead to altered gene expression. The signaling pathway after recruitment of MyD88 is conserved for all TLRs (see Figure 22-10) with the exception of TLR-3, which utilizes TRIF. In addition, specific LPS chemotypes can activate TLR-4 in an MyD88-independent manner. Binding of MyD88 to the activated TLR leads to recruitment of IL-1 receptor–associated kinase (IRAK) and IRAK autophosphorylation. TNF receptor–associated factor-6 (TRAF-6) links with this complex and activates IκB kinase (IKK). IKK phosphorylates IκB, leading to its dissociation from nuclear factor κB (NFκB). NFκB then translocates to the nucleus to activate gene transcription. Alternatively, TRIF leads to activation of IRF3 (interferon regulatory factor 3) that can translocate to the nucleus and lead to the transcription of interferon-α and -β. This pathway also activates NFκB.
| Receptor | Reported Ligands |
|---|---|
| TLR-1 |
Peptidoglycan and lipoteichoic acid (components of gram-positive bacteria)
Bacterial lipoproteins (components of Borrelia burgdorferi)
Components of mycobacterial cell walls
Mannuronic acid polymers (components of Pseudomonas aeruginosa)
Glycosylphosphatidylinositol lipid (components of Trypanosoma cruzi)
Phenol-soluble modulin (from Staphylococcus epidermidis)
Soluble tuberculosis factor (19-kDa lipoprotein secreted by Mycobacterium tuberculosis)
CpG, cytosine-phosphate-guanine [DNA sequence]; dsRNA, double-stranded RNA; LPS, lipopolysaccharide; RSV, respiratory syncytial virus; ssRNA, single-stranded RNA.
Inflammatory Mediators
Cytokines
Cytokines (Table 22-10) are soluble, low-molecular-weight proteins that play important roles in host defense by regulating the inflammatory response and are expressed not only by leukocytes but also by endothelial cells, epithelial cells, and fibroblasts. Expression of cytokines is transcriptionally regulated, and secretion can be quickly enhanced after cell stimulation.
| Cytokine | Function | Other Clinical Roles |
|---|---|---|
| TNF-α |
ARDS, acute respiratory distress syndrome; GM-CSF, granulocyte-macrophage colony-stimulating factor; ICAM-1, intercellular adhesion factor-1; IL, interleukin; NK1R, neurokinin 1 receptor; TNF, tumor necrosis factor; PAF, platelet-activating factor.
Chemokines
Chemokines (Table 22-11) are 8- to 10-kDa glycoproteins that, although structurally related to cytokines, are distinct from them in their ability to bind and signal via G protein–coupled receptors. Chemokines are both chemotactic and cellular activating factors for leukocytes and can be classified into four groups on the basis of their amino acid structure. The two primary groups that play an important role in host defense are the CC chemokines (e.g., MCP-1, MIP-1α, and RANTES), which are chemotactic for monocytes, lymphocytes, basophils, and eosinophils and the CXC chemokines (e.g., IL-8, GRO-α [growth-related oncogene α], and ENA-78 [epithelial cell–derived neutrophil-activating peptide-78]), which act primarily on neutrophils.
| Family | Member(s) | Function(s) |
|---|---|---|
| CXC |
Promotes adhesion
ENA-78, epithelial cell–derived neutrophil-activating peptide-78; GRO-α, growth-related oncogene; IL-8, interleukin-8; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; NAP-2, neutrophil-activating protein-2.
Gurish MF, Boyce JA. Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell. J Allergy Clin Immunol. 2006;117:1285–1291.
Hiemstra PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp Lung Res. 2007;33:537–542.
Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–1879.
Lambrecht BN. Lung dendritic cells: targets for therapy in allergic disease. Curr Mol Med. 2008;8:393–400.
Moraes TJ, Zurawska JH, Downey GP. Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol. 2006;13:21–27.
Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201.
Rohmann K, Tschernig T, Pabst R, et al. Innate immunity in the human lung: pathogen recognition and lung disease. Cell Tissue Res. 2011;343:167–174.