Pulmonary Embolism and Deep Vein Thrombosis
Perspective
Pathophysiology of Thrombosis
VTE forms as a result of excessive fibrin production from the action of thrombin on fibrinogen. Factors that enhance fibrinogen synthesis and promote its catalysis to fibrin include systemic inflammation, traumatic or immune-related vascular trauma, inherited thrombophilias and hemoglobinopathies, cancer, pregnancy, and sluggish blood flow. Venous injury, slow blood flow, and hypercoagulability are the cardinal inciting mechanisms for VTE, and most clinical decision rules for VTE incorporate these factors. In addition, each year of life independently increases the likelihood of imbalanced clot formation.2
Deep Vein Thrombosis
Clinical Presentation
The initial symptoms of DVT can be as subtle and nonspecific as a mild cramping sensation or sense of fullness in the calf, without objective swelling, and may be difficult to differentiate clinically from myriad other, unrelated disorders (Box 88-1). Many patients use the term charley horse to describe the sensation of an early DVT. It is precisely at this early stage, however, that DVT can be treated most effectively to minimize the potential morbidity and mortality associated with VTE. Because the left iliac vein is vulnerable to compression by the left iliac artery, leg DVT occurs with a slightly higher frequency in the left leg compared with the right; bilateral leg DVT is found in fewer than 10% of ED patients diagnosed with DVT. Likewise, the clinical signs of DVT vary and may include unilateral swelling, edema, erythema, and warmth of the affected extremity; tenderness to palpation along the distribution of the deep venous system; dilation of superficial collateral veins; and a palpable venous “cord.” The classic Homan’s sign (pain felt in the calf or posterior aspect of the knee on passive dorsiflexion of the foot while the knee is extended) is insensitive and nonspecific for DVT and has no role in clinical assessment of the patient. Upper extremity DVT refers to a thrombosis in the axillary vein and causes arm swelling on the same side as an indwelling catheter or recent intravenous infusion site. In the absence of a catheter, the most frequent location of arm DVT is on the dominant hand side. Patients frequently note that their rings become tight as an early sign of DVT.
Diagnosis
Diagnosis of DVT and PE starts with an estimation of the pretest probability (PTP). This estimation may be accomplished either by the clinical gestalt of an experienced practitioner or in conjunction with a clinical decision tool, such as that derived and validated by Wells and colleagues (Table 88-1). Patients with a low PTP can have DVT excluded with a normal quantitative D-dimer.
Table 88-1
Clinical Model for Estimating the Pretest Probability of Deep Vein Thrombosis
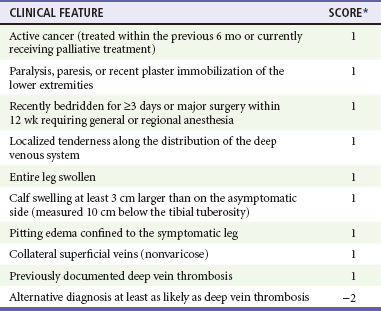
*A score of <2 indicates that the probability of deep vein thrombosis is low.
Adapted from Wells PS, Anderson D, Bormanis J: Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 350:1795, 1997.
Radiographic Evaluation
DVT evaluation is by a combination of D-dimer testing and duplex venous ultrasonography.
Venous duplex ultrasonography, performed by a certified sonographer and interpreted by a board-certified radiologist or similarly credentialed expert, has a sensitivity and specificity of approximately 95% for proximal DVT and is the diagnostic test of choice in most centers. It is important for emergency physicians to know the technique of the examination. Most radiology departments use the three point sequence (common and superficial femoral veins and popliteal vein, excluding the calf and saphenous veins). Although management of calf vein and saphenous clots remains controversial, the diagnostic sensitivity of a single venous ultrasound for the exclusion of a clot at risk of progressing to a proximal DVT is increased significantly by including the calf and saphenous veins. A patient at low risk may have the diagnosis of DVT effectively excluded by a negative three-point venous duplex ultrasound. However, for patients at higher than low risk, a single negative three-point ultrasound is inadequate as a sole method to exclude DVT, whereas a single normal whole-leg ultrasound (including normal calf and saphenous veins) is sufficient to exclude DVT with any PTP.1 A negative three-point ultrasound together with a negative quantitative D-dimer is sufficient to exclude DVT with any PTP. In a patient with a high PTP in whom the D-dimer is elevated (or not performed), a negative three-point ultrasound at the index visit should be followed by a repeat ultrasound in 2 to 7 days, which if negative is sufficient to exclude PE. An expertly performed and interpreted positive ultrasound is sufficient to confirm the diagnosis of DVT. Ultrasound cannot be used to rule out iliac or pelvic vein thrombosis.
Many emergency physicians use bedside ED ultrasound in their daily practice, but at present the data are conflicting as to whether emergency physician–performed ultrasound (EPPU) for lower-extremity DVT has adequate diagnostic accuracy. Follow-up with formal diagnostic duplex ultrasound seems reasonable.2
Indirect computed tomography (CT) venography (CTV) is not a primary imaging modality for DVT but may be performed in conjunction with CT pulmonary angiography (CTPA) of the chest during the evaluation of suggested PE. Adding CTV to CTPA provides an incremental increase in the sensitivity for VTE, identifying DVT in approximately 2% of patients in whom the CTPA is read as negative for PE but at the expense of significant additional radiation exposure to the pelvis and lower extremities.3 Interobserver agreement among radiologists interpreting CTV appears to be less than that for the CTPA portion of the study, possibly because of poor venous opacification in many cases.4 At this time, routine use of CTV is not necessary when CTPA is performed.11
Treatment
Superficial Leg Thrombophlebitis
Based on the results of a large randomized controlled trial, patients with a clot in the greater saphenous vein that extends above the knee are at risk for progression to DVT via the saphenous-femoral vein junction and may require an abbreviated course of anticoagulation.5 The published evidence suggests that saphenous vein thrombophlebitis can adequately be treated with nonsteroidal anti-inflammatory drugs, heat, and graded compression stockings (fitted to exert 30-40 mm Hg of pressure at the ankle) followed by a mandatory repeat ultrasound in 2 to 5 days. If the clot is extending, then anticoagulation is indicated. The duration of anticoagulation treatment remains uncertain, but full-dose low molecular weight heparin or fondaparinux for 10 days followed by repeat ultrasound seems reasonable.
Isolated Calf Vein Thrombosis
The optimal management strategy for thromboses of the tibial or peroneal veins remains controversial.7 Approximately 25% of isolated calf vein thromboses propagate proximally, prompting recommendations for treatment with anticoagulation as for proximal leg DVT.8 However, most of these data were from hospitalized or postoperative patients with a higher risk of propagation than ambulatory patients.9 For tibial or peroneal vein thrombosis in an otherwise healthy, ambulatory patient with no other indication for anticoagulation, I recommend antiplatelet therapy with aspirin (325 mg of enteric-coated acetylsalicylic acid per day) and close follow-up with repeat duplex ultrasound scan at 2 to 5 days to evaluate for clot propagation.
Upper Extremity Venous Thromboses
DVTs of the upper extremity have become more common in association with increased use of indwelling venous catheters and wires for electronic cardiac devices. Upper extremity DVT can cause PE, and all patients with DVT above the elbow require definitive treatment.10–12
Complications
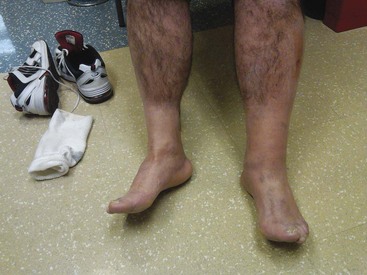
Although the most feared complication of DVT is fatal PE, DVT damages venous valves, causing venous insufficiency. Venous insufficiency, in turn, manifests as a spectrum ranging from painless varicosities to severe postphlebitic syndrome, which can cause unremitting pain and swelling, varicose veins, skin changes, and nonhealing ulcers. Figure 88-1 shows the leg of a construction worker with a femoral DVT that produces swelling on the job, impairing his ability to work.
Pulmonary Embolism
PE results from a clot that formed hours, days, or weeks earlier in the deep veins and dislodged, traveled through the venous system, and traversed the right ventricle into the pulmonary vasculature. What the patient experiences during this process varies widely, ranging from no symptom to cardiovascular collapse. No one knows exactly how many patients pass through the ED with PE because there is no reliable way of identifying missed cases. Assuming that ED populations have a risk for PE somewhere between that of hospitalized patients (who are at high risk for PE) and outpatients (who are at lower risk), approximately 1 in every 500 to 1000 ED patients has PE. About 8% of ED patients with PE die within 30 days, even when PE is promptly diagnosed and treated.10
Pathophysiology of Pulmonary Vascular Occlusion
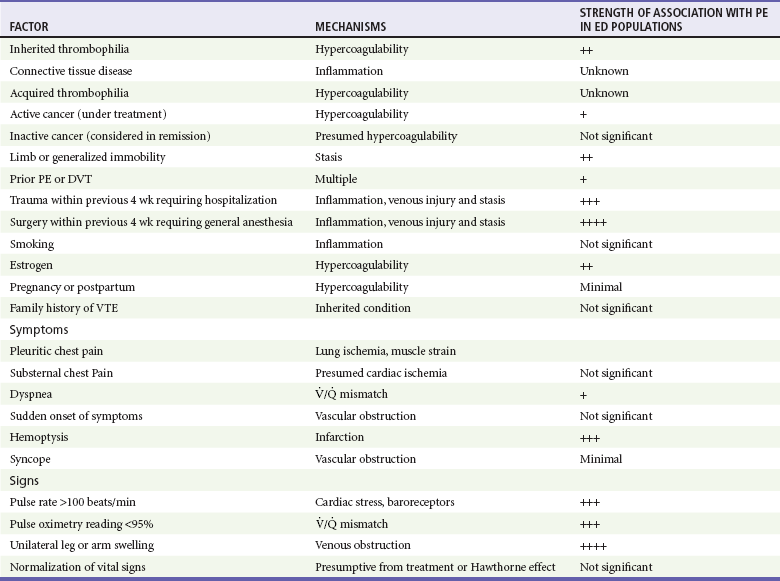
In contrast, if asked in a detailed and structured way, about 90% of patients with noninfarcting emboli admit to the sensation of dyspnea. The dyspnea may be constant and oppressive or may be intermittent and perceived only with exertion, possibly because of an exercise-induced increase in pulmonary vascular resistance. Rest dyspnea seems to be the clinical manifestation of distorted and irregular blood flow within the lung, referred to as ventilation-perfusion inequality. With each breath a patient with PE wastes ventilation because of increased alveolar dead space (alveoli that are ventilated but not perfused). A lodged clot can redistribute blood flow to areas of the lung with already high perfusion relative to ventilation and therefore cause more blue blood to pass through the lung without being fully oxygenated. This venous admixture is probably the primary cause of hypoxemia with PE and the increased alveolar-arterial oxygen difference (the A-a gradient). About 15% of patients with PE have a normal A-a gradient of oxygen (with normal defined as age in years/4 + 4), however, and the A-a gradient is abnormally high in most patients who are evaluated for PE but ultimately found to not have PE. In a multicenter registry of 348 patients with PE, 37 (10.6%) had a pulse oximetry reading of 100% at the time of arrival to the ED, while breathing room air. Despite its shortcomings as a single diagnostic step, the presence of hypoxemia (pulse oximetry <95%, breathing room air) that cannot be explained by a known disease process increases the probability of PE. Conversely, a normal oxygen saturation can be used only when considered together with multiple other clinical features and should not alone or independently be used to forego testing for PE (Box 88-2).18,19 In addition, when PE is diagnosed, the severity of hypoxemia represents a powerful independent predictor of patient outcome.
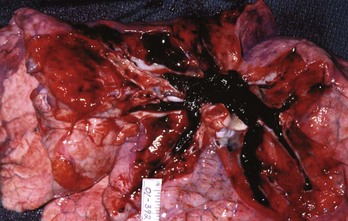
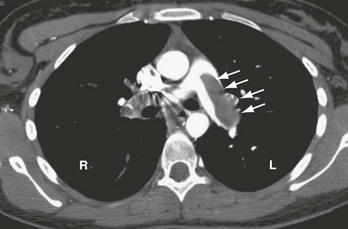
PE also causes highly variable effects on vital signs. In the ED, about half of all patients with PE have a heart rate greater than 100 beats/min.11 Tachycardia from PE probably results from impaired left ventricular filling, leading to a pathophysiologic process that parallels that of hemorrhagic shock. In one study, the probability of PE was not reduced in patients who normalized any vital sign while in the ED.12 When PE obstructs more than 50% of the vasculature, it usually causes an acute increase in right ventricular pressure. In contrast to the left ventricle, the right ventricle does not show an elastic response to acutely increased afterload; it quickly dilates, showing echocardiographic hypokinesis early in the course. In about 40% of cases, the right ventricular damage persists for at least 6 months and probably longer. Arterial hypotension represents an ominous hemodynamic consequence of PE; it occurs in only about 10% of patients but signifies a fourfold increase in risk of death compared with normotensive patients.20 In its most extreme form, PE can obstruct the right ventricular outflow entirely, either by casting the entire pulmonary vascular tree (Fig. 88-2) or by acutely occluding the main pulmonary artery. Pulseless electrical activity (PEA) is the most common electrocardiogram (ECG) result from obstructive PE. The survival rate for cardiac arrest from PE is abysmally low, even if the arrest is witnessed and heroic treatment is initiated.
Clinical Presentation
Table 88-2 presents a listing of factors that significantly increase the probability of PE in the ED population.11 As is the case for cardiac risk factors in the evaluation of acute chest pain, variables that increase the probability of PE in epidemiologic studies are not useful for individual ED patients with signs and symptoms suggesting PE. From an epidemiologic standpoint, people who smoke are at a significantly higher risk for venous clots than are people who do not smoke. However, in the ED, smoking by a given patient does not seem to increase that person’s risk for PE over that of a nonsmoker with an otherwise identical clinical presentation. It is possible that smokers are simply more likely to have other lung problems that manifest a clinical presentation similar to PE. As many as 50% of patients diagnosed with PE have no apparent clinical risk factors for VTE, but testing for genetic thrombophilia has no value in the ED setting.13
Virtually any ED visit related to weakness, shortness of breath, dizziness or syncope, pain, extremity discomfort, or nonspecific malaise or functional deterioration could represent a potential PE; however, this does not mean that every patient with one of these symptoms should be worked up for PE, and these symptoms should be considered in the context of the entire clinical picture. A patient with PE typically presents with 2 to 3 days of shortness of breath, now worsened enough to seek care. The chest pain usually is vaguely described. A few patients have focal pleuritic chest pain, but many say nonspecifically that their chest hurts with breathing, usually on the lateral aspects. Purely substernal chest pain is a rare presentation for PE and in general suggests a cardiac or other origin. The presence or absence of sudden onset of symptoms neither increases nor decreases the probability of PE.11 When the antemortem histories of patients who die suddenly and unexpectedly from PE are reconstructed by interviewing family and examining medical records, most have complained of nagging symptoms for weeks before collapse, and 40% already had seen a physician for care.14 PE with lung infarction can result in a clinical picture that is similar to lobar pneumonia, including focal chest pain, fever, and unilateral rales on auscultation. However, a temperature greater than 101.5° F suggests infection rather than infarction. An occasional clue to pulmonary infarction is the onset of pain and blood-red hemoptysis on the same day, whereas lobar pneumonia usually causes productive cough for a few days before rust-tinged sputum.
Diagnosis
Likewise, a 12-lead ECG provides more information about the presence of alternative diagnoses, such as pericarditis or cardiac ischemia, than the presence of PE. When PE causes ECG changes, this is usually a result of acute or subacute pulmonary hypertension. The most common effects of pulmonary hypertension on ECG are rapid heart rate, symmetrical T-wave inversion in the anterior leads (V1-V4), the McGinn-White S1Q3T3 pattern, and incomplete or complete right bundle branch block (Fig. 88-3); any one of these findings approximately doubles the probability of PE in a symptomatic patient.15
In some cases PE can be excluded with reasonable certainty based on data that are available at the bedside, gathered only via the medical history and physical examination. Multicenter studies of urban academic EDs have suggested that emergency physicians currently evaluate about 1 to 2% of all patients for PE.16 Each year more than 16 million patients come to the ED with chest pain or dyspnea. Although numerous cases of PE are probably still missed, overtesting for PE can also be harmful. Specific risks include exposure to the ionizing radiation and intravenous contrast necessary for CTPA and CTV and the risk of false-positive interpretation, which may occur in as many as 10% of scans read as positive for PE.17 The appropriate use of D-dimer testing decreases the need for imaging in all but patients at high risk.
One approach to the workup for PE is to compare the PTP with the “test threshold” for PE. The test threshold represents the point above which some type of workup should be initiated and below which the clinician can justify not starting the workup. For PE the test threshold is 1 to 2.5%.18 Patients with a PTP less than 2.5% are more likely to be harmed than benefited by a workup and vice versa for patients with a PTP greater than 2.5%.
The question becomes how to quantify the PTP accurately.19 Clinical judgment alone is subject to cognitive error inherent to medical diagnosis, including framing heuristic and conditions in the ED at the time (e.g., is one less likely to pursue a relatively low-likelihood diagnosis when the department is very busy), conditions of the clinician (e.g., fatigue, dysphoria, subjective feelings about the patient), and the availability of diagnostic studies (e.g., daytime vs. nighttime, weekday vs. weekend). In addition, there is variability among clinicians who may not agree that a 19-year-old with cough and pleuritic chest pain, a normal chest radiograph, and no other risk factors has less than a 2% probability of PE.
Decision rules help to deal with these problems because they are structured and more transparent. Several rules have been derived and validated for the risk stratification of patients with possible PE; however, difficulty with spontaneous recall and a preference for gestalt reasoning by clinicians may limit their use in clinical practice.20 Fortunately, clinical reasoning appears to be comparable to at least two of the validated decision rules. In a large single-center study of 2603 ED patients evaluated for PE, the treating clinician’s unstructured estimate of PTP was equivalent to that provided by the Wells score and the Charlotte rule. This finding was independent of training level.21
Although gestalt reasoning and clinical decision rules may provide adequate stratification to guide the workup (i.e., D-dimer vs. pulmonary vascular imaging), they have not been able to reproducibly identify the “very low-risk” population whose PTP lies below the 2% test threshold. In one validation study, patients with Wells score less than 2 had a 1.3% probability of PE,30 but this finding has not been repeated. To identify the very low-risk group in whom PE could be safely excluded at the bedside with no diagnostic testing, the PE rule-out criteria (the PERC rule) were derived and prospectively validated in 8138 patients in 13 different hospitals across the United States and in New Zealand (see Box 88-2).16
PE also is less likely when some other disease process can explain the patient’s complaints and findings (e.g., asthma causing bronchospasm proven by a low peak expiratory flow rate, or findings of wheezing and prolonged expiration on auscultation) in a patient otherwise not believed to be at high risk for PE. A tangible alternative diagnosis reduces the probability of coincident PE. The flow algorithms provided here are predicated on the following goals: (1) to identify as many ED patients as possible who have PE; (2) to avoid mislabeling (and administering anticoagulants to) patients who do not have PE; and (3) to use available resources efficiently and effectively (Fig. 88-4).
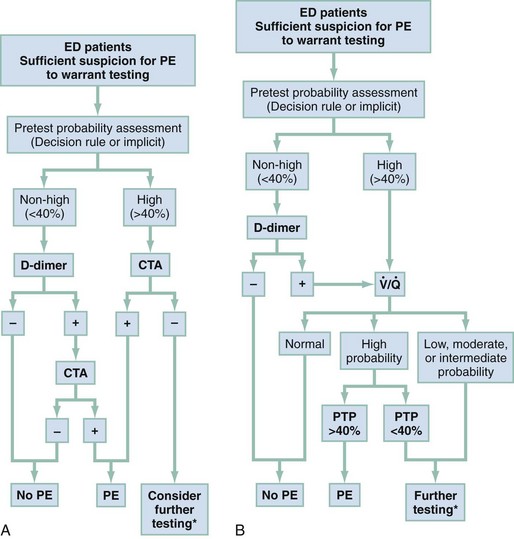
Figure 88-4 Suggested algorithms to evaluate for pulmonary embolism (PE) in the emergency department (ED). These algorithms include the use of pretest probability (PTP), enzyme-linked immunosorbent assay (ELISA), or immunoturbidimetric quantitative D-dimer assay, and pulmonary vascular imaging (computed tomography angiography [CTA]). A, Algorithm incorporating contrast-enhanced CTA. A negative D-dimer is an immunoturbidimetric assay or ELISA that returns a concentration less than 500 fibrinogen equivalent units (FEUs) (ng) per milliliter. B, Algorithm incorporating ventilation-perfusion (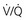 ) scanning.
) scanning.
*May require patient admission. Additional testing—Option 1: “Crossover” to perform either scanning or computed tomography for algorithms A and B. Option 2: Perform lower extremity venous ultrasound, and if initial venous ultrasound is negative, repeat the lower extremity venous ultrasound in 1 week. Option 3: Perform formal pulmonary angiography.
For a patient for whom suspicion is implicit or with explicit score suggesting a PTP over 40%, clinicians should skip the D-dimer, order pulmonary vascular imaging, and strongly consider initiating anticoagulation in the absence of contraindications. Because the half-life of circulating D-dimer is less than 8 hours, the sensitivity of the D-dimer may decrease if the patient’s symptoms have been present for longer than 3 days. False-negative D-dimer measurements may also be seen with ongoing warfarin therapy and in the subset of patients with pulmonary infarction. As was discussed in the DVT section, quantitative D-dimer assays are more reliable for the exclusion of PE than are qualitative assays.32–35
Most academic centers now use CTPA as the primary method of evaluating for PE. The Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) II study evaluated the test characteristics of CTPA for the diagnosis of PE in a prospective, multicenter study of 824 patients imaged primarily with four-row multidetector scanners.3 CTPA images were deemed adequate for interpretation in 773 (94%), and the sensitivity in these patients was 83% (95% CI 76-92%) with a specificity of 96% (95% CI 93-97%).
The importance of isolated subsegmental pulmonary embolus either missed or detected by CTPA can arise because of a radiologist’s statement that “subsegmental clot cannot be excluded by CTPA.” More recently, increased detection of isolated subsegmental filling defects has occurred, with more thinly collimated images acquired with a higher number multidetector scanner.17 No firm evidence yet exists to guide these circumstances. When two radiologists independently evaluate CTPA, their agreement on the presence of isolated subsegmental filling defects is poor.17 The same lack of agreement with regard to subsegmental clots holds for formal pulmonary angiography. It is reasonable that if the patient has no evidence of DVT, no signs of cardiopulmonary stress, and no ongoing major risk for thrombosis (e.g., active malignancy), isolated subsegmental findings are the equivalent of no findings, and anticoagulation is not indicated. If a patient with negative CTPA has signs of pulmonary hypertension or hypoxemia without an apparent alternative cause, or has a known thrombophilia, further testing is advised. Subsequent testing after negative CTPA is individualized by institution in consultation with radiology. Most commonly, if venous studies were not performed as part of the CTPA, duplex ultrasonography of both lower extremities should be undertaken. Positive sonographic evidence of DVT is considered confirmation of the presence of PE. A negative sonogram does not exclude the diagnosis, however, and the procedure should be repeated in 2 to 7 days.
CTPA can provide additional information to enhance its usefulness in the ED. Although the scan can be extended to include the leg veins, this is not recommended as a routine (see earlier discussion.) CTPA often provides information about alternative processes that might explain the patient’s symptoms (Box 88-3). Pneumonia is the most common alternative diagnosis found in ED patients. In about 10% of ED patients evaluated for PE, CT as a single test could (1) show absence of PE; (2) provide evidence of alternative disease, which can be used with other evidence to reduce the probability of PE to a reasonably low level to stop the workup; and (3) facilitate treatment for the alternative disease.
The scintillation scan remains a good diagnostic option for patients with contraindications to iodinated intravenous contrast. The accuracy and precision of the scan were shown in the PIOPED study, which compared the results of scanning with the most accurate criterion standard test available at the time—formal pulmonary angiography (Table 88-3).22 This multicenter study showed that a high-probability scan can be used to diagnose PE, and a normal scan (i.e., no perfusion defect) excludes the diagnosis of PE with an acceptable degree of certainty. A moderate probability or indeterminate scan requires additional formal pulmonary angiography or a CTPA. Even in patients with a low PTP, a low-probability scan usually indicates a need for additional testing, such as either CTPA or venous duplex ultrasonography of the legs. The latter should be repeated at least once, 2 to 7 days later, if negative at the initial presentation. In patients with a chest radiograph that shows airspace disease, the specificity of scanning can be expected to decrease, and the relative diagnostic utility of CTPA can be expected to increase.
Table 88-3
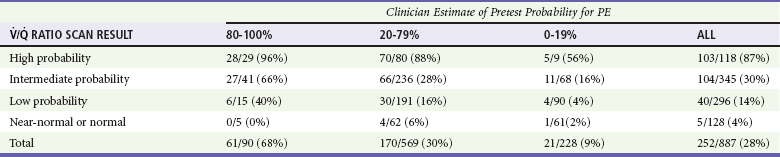
PE, pulmonary embolism;  , ventilation-perfusion ratio.
, ventilation-perfusion ratio.
Adapted from the PIOPED Investigators: Value of the ventilation/perfusion scan in acute pulmonary embolism. JAMA 263:2753, 1990.
Management
A large registry found that emergency physicians begin heparin in approximately 10% of patients who are diagnosed with PE, before knowing the results of pulmonary vascular imaging (“empirical heparin”). Only 15% of patients who died from PE received empirical heparin, but this fact in the absence of other published data can be used to assert that empirical heparin administration can improve outcomes from PE.23 In consideration of its risks and benefits, empirical treatment probably confers more benefit than harm in a hemodynamically stable patient when the PTP of PE exceeds 30%, the patient has no major contraindication to anticoagulation, and the clinical circumstances predict that imaging will not be available for several hours.24 All patients with a high PTP, no contraindication to anticoagulation, and evidence of hemodynamic instability, including recent syncope, any hypotension, hypoxemia, or clinical evidence of right-sided heart strain (criteria defined in Table 88-4 as more severe moderate PE or high-risk PE), should receive immediate empirical heparin.
Table 88-4
Risk-Stratification and Associated Treatment Recommendations for Acute Pulmonary Embolism (PE)
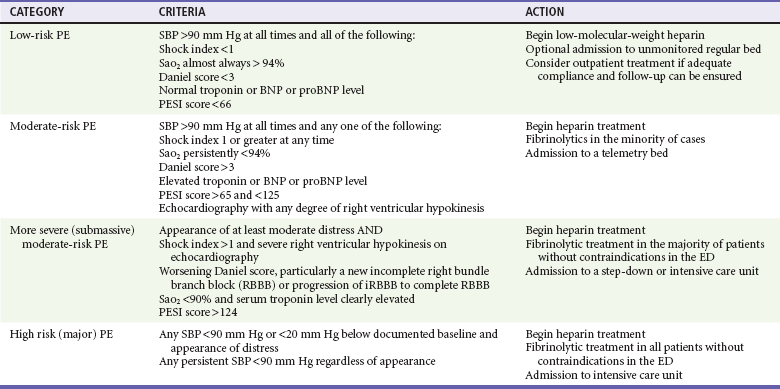
Recent evidence has indicated that up to 50% of outpatients diagnosed with PE may be stable enough to be treated as outpatients.25–27 A multicenter trial that randomized patients with low-risk PE (see Table 88-4 for definition) found equivalent outcomes for patients who were discharged from the ED compared with hospital admission.25 It should be noted that more than 95% of patients in that study were enrolled from Europe, where the public health resources for initiation and maintenance of high-quality anticoagulation generally exceed those of the United States. In settings where good follow-up can be obtained, the patient can be taught to self-administer low-molecular-weight heparin, and the patient can access an anticoagulation clinic within 48 hours, a low-risk patient with PE can be discharged from the ED.
Most patients with PE look and feel better the day after starting heparin anticoagulation, and more than half go on to a nearly full recovery of pre-PE health status. The in-hospital mortality rate of patients diagnosed with PE who remain hemodynamically stable while in the ED has been thought to be 10%, but a recent large, multicenter United States–based registry of 1880 patients diagnosed with PE in the ED found an in-hospital mortality rate directly attributable to PE of 1.1% and an all-cause mortality rate of 5.4%.23 Approximately 10 to 20% of patients report persistent dyspnea and exercise intolerance that permanently degrades their quality of life. Systolic hypotension (<90 mm Hg) represents a highly specific and moderately sensitive indicator of severe PE and increases the mortality rate dramatically. In the absence of hypotension, several parameters available at the bedside can help with prognosis. A heart rate that is persistently above the systolic blood pressure indicates more severe clot, as does a pulse oximetry reading less than 95%. Presence of prior congestive heart failure or advanced chronic obstructive pulmonary disease serves to magnify the severity of PE. An elevated serum troponin measurement or an elevated brain natriuretic peptide or pro-brain natriuretic peptide concentration portend a worse outcome. Echocardiography demonstrating right ventricular hypokinesis or dilation also increases the probability of death from PE. Table 88-4 summarizes the criteria that can be used to risk stratify patients with PE into four groups. Table 88-4 also shows how consideration of these criteria may help guide the decision to place the patient in an ICU versus an intermediate or regular inpatient bed, and whether to administer heparin only or consider escalated therapy.
Thrombolytic Therapy
Thrombolytic therapy in PE is controversial. Administration of alteplase to patients with PE results in more rapid symptomatic improvement than standard antithrombotic therapy alone and causes more rapid and complete normalization of right ventricular function.28 Alteplase also increases the risk of hemorrhage, especially minor hemorrhage. It is not known with certainty how many patient lives would be saved, or definitively improved, by the addition of thrombolytic treatment to heparin therapy versus the number of patients who would experience a fatal or life-threatening bleeding event as a result of thrombolytic treatment. On balance, the benefit-risk analysis suggests that fibrinolysis is of greatest value in the subset of patients with proven, massive PE. Massive PE is defined by hypotension with a systolic blood pressure below 90 mm Hg for more than 15 minutes. In patients with preexisting hypertension, the threshold is adjusted to below 100 mm Hg or a reduction in the baseline systolic blood pressure of more than 60 mm Hg. In the absence of contraindications, patients with proven massive PE probably benefit from fibrinolysis. There is insufficient evidence to recommend initiation of empirical fibrinolysis in the absence of confirmatory pulmonary vascular imaging or the presence of otherwise unexplained shock in a patient with known DVT and high clinical suspicion for PE.
A subset of patients with submassive PE may benefit from fibrinolysis, including those with moderate-to-severe respiratory distress and hypoxia (oxygen saturation less than 95%) and those with echocardiographic evidence of right ventricular dysfunction. When echocardiography is unavailable in a timely manner, surrogate laboratory markers of right ventricular dysfunction may be useful, including an elevated troponin level or a brain natriuretic peptide level greater than 90 pg/mL (see Table 88-4).29 Consultation with cardiology or cardiac surgery should be obtained before administration of fibrinolytic therapy for patients with PE who are not in extremis. The FDA-approved regimens for thrombolysis are shown in Table 88-5.
Table 88-5
| Streptokinase | 1 million units infused over 24 hr |
| Urokinase | 1 million–unit bolus followed by 24-hr infusion at 300,000 units/hr |
| Alteplase | 15-mg bolus followed by 2-hr infusion of 85 mg; discontinue heparin during infusion |
Tenecteplase (TNKase) is a recombinant plasminogen-activating enzyme with several pharmacologic properties that may favor its use for fibrinolysis of acute PE; it has been studied in one randomized controlled trial.30 Tenecteplase does not have FDA approval for PE treatment. Tenecteplase differs from alteplase because it has a longer half-life, resistance to plasminogen activator inhibitor–1, and increased fibrin specificity, which results in less fibrinogenolysis and less coagulopathy.
The clinical course of patients with obstructive PE can be unpredictable. Many patients with massive PE remain stable in the ED. Other patients are stable on arrival but progressively deteriorate over hours as right ventricular function declines. Three percent of ED patients without hypotension while in the ED experience cardiac arrest and die within 24 hours.23 A patient can be stable and then hypotensive within minutes because of the highly variable effect of the clot on right ventricular outflow obstruction, especially when it is perched in the main pulmonary artery (Fig. 88-5). Additional mechanisms of rapid instability include new embolization of clot material, release of mediators of pulmonary vasospasm, sudden bradycardiac or asystolic arrhythmias, and respiratory failure. Clues to oncoming cardiopulmonary decompensation include worsening respiratory distress and worsening hypoxemia, a rising shock index (the heart rate divided by the systolic blood pressure), systolic arterial blood pressure less than 90 mm Hg, and syncope or a seizure-like convulsive episode while in the ED. A particularly ominous finding is the evolution on ECG from a narrow-complex tachycardia to an incomplete right bundle branch block to a complete right bundle branch block (Fig. 88-6) as evidence of life-threatening pulmonary hypertension and incipient cardiac arrest.
Surgical Embolectomy
For patients with known floating thrombi in the right side of the heart or for patients with severe refractory hypotension, surgery is the most likely intervention to save the patient’s life. Surgical embolectomy requires extracorporeal cardiopulmonary bypass and an experienced cardiothoracic surgeon. Surgical embolectomy may be the best option for patients who have severe PE with a contraindication to fibrinolysis; however, extracorporeal perfusion requires intensive heparin anticoagulation, and the patient’s mental status cannot be monitored during surgery—a key concern in patients with high risk of intracranial hemorrhage. Catheter-directed thrombectomy also may be lifesaving but requires that the patient be sent to the relatively uncontrolled environment of the interventional radiology suite. No data support the use of catheter intervention for treatment of PE.31
Box 88-4 details questions that commonly arise regarding the diagnosis and treatment of PE in the ED.
References
1. Johnson, SA, et al. Risk of deep vein thrombosis following a single negative whole-leg compression ultrasound: A systematic review and meta-analysis. JAMA. 2010;303:438–445.
2. Kline, JA, O’Malley, PM, Tayal, VS, Snead, GR, Mitchell, AM. Emergency clinician–performed compression ultrasonography for deep venous thrombosis of the lower extremity. Ann Emerg Med. 2008;52:437–445.
3. Stein, PD, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–2327.
4. Burnside, PR, Green, E, Kline, JA. Indirect computed tomography venography: A report of vascular opacification. Emerg Radiol. 2010;17:195–201.
5. Decousus, H, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med. 2010;363:1222–1232.
6. Schwarz, T, et al. Therapy of isolated calf muscle vein thrombosis: A randomized, controlled study. J Vasc Surg. 2010;52:1246–1250.
7. Righini, M. Is it worth diagnosing and treating distal deep vein thrombosis? No. J Thromb Haemost. 2007;5:55–59.
8. Meissner, MH, Caps, MT, Bergelin, RO, Manzo, RA, Strandness, DE. Early outcome after isolated calf vein thrombosis. J Vasc Surg. 1997;26:749–756.
9. Righini, M, et al. Clinical relevance of distal deep vein thrombosis. Review of literature data. Thromb Haemost. 2006;95:56–64.
10. Laporte, S, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: Findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117:1711–1716.
11. Courtney, DM, et al. Clinical features from the history and physical examination that predict the presence or absence of pulmonary embolism in symptomatic emergency department patients: Results of a prospective, multicenter study. Ann Emerg Med. 2010;55:305–315.
12. Kline, JA, Corredor, DM, Hogg, MM, Hernandez, J, Jones, AE. Normalization of vital signs does not reduce the probability of acute pulmonary embolism in symptomatic emergency department patients. Acad Emerg Med. 2012;19:11–17.
13. Kruse, L, Mitchell, AM, Camargo, CA, Jr., Hernandez, J, Kline, JA. Frequency of thrombophilia-related genetic variations in patients with idiopathic pulmonary embolism in an urban emergency department. Clin Chem. 2006;52:1026–1032.
14. Courtney, DM, Kline, JA. Identification of prearrest clinical factors associated with outpatient fatal pulmonary embolism. Acad Emerg Med. 2001;8:1136–1142.
15. Marchick, MR, et al. 12-Lead ECG findings of pulmonary hypertension occur more frequently in emergency department patients with pulmonary embolism than in patients without pulmonary embolism. Ann Emerg Med. 2009;55:331–335.
16. Kline, JA, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6:772–780.
17. Courtney, DM, et al. Prospective multi-center assessment of interobserver agreement for radiologist interpretation of multidetector CT angiography for pulmonary embolism. J Thromb Haemost. 2010;8:533–540.
18. Lessler, AL, Isserman, JA, Agarwal, R, Palevsky, HI, Pines, JM. Testing low-risk patients for suspected pulmonary embolism: A decision analysis. Ann Emerg Med. 2010;55:316–326.
19. Lucassen, W, et al. Clinical decision rules for excluding pulmonary embolism: A meta-analysis. Ann Intern Med. 2011;155:448–460.
20. Runyon, MS, Richman, PB, Kline, JA. Emergency medicine practitioner knowledge and use of decision rules for the evaluation of patients with suspected pulmonary embolism: Variations by practice setting and training level. Acad Emerg Med. 2007;14:53–57.
21. Runyon, MS, Webb, WB, Jones, AE, Kline, JA. Comparison of the unstructured clinician estimate of low clinical probability for pulmonary embolism to the Canadian score or the Charlotte rule. Acad Emerg Med. 2005;12:587–593.
22. Zagorski, J, Marchick, MR, Kline, JA. Rapid clearance of circulating haptoglobin from plasma during acute pulmonary embolism in rats results in HMOX1 up-regulation in peripheral blood leukocytes. J Thromb Haemost. 2010;8:289–296.
23. Pollack, CV, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: Initial Report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). J Am Coll Cardiol. 2011;57:700–706.
24. Hogg, KE, Brown, MD, Kline, JA. Estimating the pretest probability to justify the empiric administration of heparin prior to pulmonary vascular imaging for pulmonary embolism. Thromb Res. 2006;118:547–553.
25. Aujesky, D, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: An international, open-label, randomised, non-inferiority trial. Lancet. 2011;378:41–48.
26. Erkens, PM, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost. 2010;8:2412–2417.
27. Baglin, T. Fifty per cent of patients with pulmonary embolism can be treated as outpatients. J Thromb Haemost. 2010;8:2404–2405.
28. Kline, JA, Steuerwald, MT, Marchick, MR, Hernandez-Nino, J, Rose, GA. Prospective evaluation of right ventricular function and functional status six months after acute submassive pulmonary embolism: Frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest. 2009;136:1202–1210.
29. Kline, JA, Hernandez, J, Rose, G, Norton, HJ, Camargo, CA, Jr. Surrogate markers for adverse outcomes in normotensive patients with pulmonary embolism. Crit Care Med. 2006;34:2773–2780.
30. Becattini, C, et al. Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb Res. 2010;125:e82–e86.
31. Verstraete, M, et al. Intravenous and intrapulmonary recombinant tissue-type plasminogen activator in the treatment of acute massive pulmonary embolism. Circulation. 1988;77:353–360.
32. Kline, JA, Hambleton, GW, Hernandez, J. D-dimer concentrations in normal pregnancy: New diagnostic thresholds are needed. Clin Chem. 2005;51:825–829.
33. Schuster, ME, et al. Pulmonary embolism in pregnant patients: A survey of practices and policies for CT pulmonary angiography. AJR Am J Roentgenol. 2003;181:1495–1498.
34. Shahir, K, Goodman, LR, Tali, A, Thorsen, KM, Hellman, RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol. 2010;195:W214–W220.
35. Cahill, AG, Stout, MJ, Macones, GA, Bhalla, S. Diagnosing pulmonary embolism in pregnancy using computed-tomographic angiography or ventilation-perfusion. Obstet Gynecol. 2009;114:124–129.
36. Ridge, CA, et al. Pulmonary embolism in pregnancy: Comparison of pulmonary CT angiography and lung scintigraphy. AJR Am J Roentgenol. 2009;193:1223–1227.
37. Gilet, AG, Dunkin, JM, Fernandez, TJ, Button, TM, Budorick, NE. Fetal radiation dose during gestation estimated on an anthropomorphic phantom for three generations of CT scanners. AJR Am J Roentgenol. 2011;196:1133–1137.
38. Winer-Muram, HT, et al. Pulmonary embolism in pregnant patients: Fetal radiation dose with helical CT. Radiology. 2002;224:487–492.
39. Parker, MS, et al. Female breast radiation exposure during CT pulmonary angiography. AJR Am J Roentgenol. 2005;185:1228–1233.
40. Chatterson, LC, Leswick, DA, Fladeland, DA, Hunt, MM, Webster, ST. Lead versus bismuth-antimony shield for fetal dose reduction at different gestational ages at CT pulmonary angiography. Radiology. 2011;260:560–567.

 , ventilation-perfusion ratio; VTE, venous thromboembolism.
, ventilation-perfusion ratio; VTE, venous thromboembolism.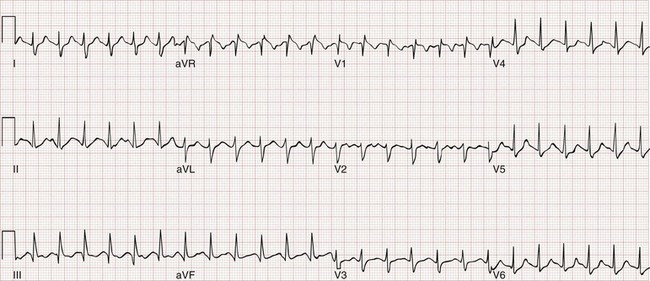
 scanning) in their choice for imaging for PE in pregnancy.
scanning) in their choice for imaging for PE in pregnancy. scanning with those after CTPA scanning in pregnancy.
scanning with those after CTPA scanning in pregnancy.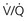 scanning, but the dose is approximately equal between CTPA and perfusion-only “
scanning, but the dose is approximately equal between CTPA and perfusion-only “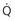 ” scanning.
” scanning.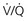 and CTPA, but for the subset of pregnant patients with a plain film chest radiograph that is normal, the indeterminate rate is lower for
and CTPA, but for the subset of pregnant patients with a plain film chest radiograph that is normal, the indeterminate rate is lower for 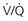 scanning than for CTPA scanning.
scanning than for CTPA scanning.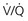 scanning does not, although one study showed that most alternative diagnoses were pneumonia, which were also seen on plain film chest radiography.
scanning does not, although one study showed that most alternative diagnoses were pneumonia, which were also seen on plain film chest radiography.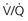 scanning is not believed to confer this risk.
scanning is not believed to confer this risk.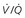 , ventilation-perfusion ratio.
, ventilation-perfusion ratio.






