62 Pulmonary Embolism
Venous thromboembolism (VTE), deep venous thrombosis (DVT), pulmonary embolism (PE), or all three can complicate the course of sick hospitalized patients but may also affect ambulant and otherwise apparently healthy individuals.1–3 Pulmonary embolism remains the most common preventable cause of hospital death and is responsible for approximately 150,000 to 200,000 deaths per year in the United States. Most patients who die from PE succumb suddenly or within 2 hours of the acute event before therapy can be initiated or can take effect.4 Effective prophylaxis against VTE is now available for most high-risk patients.5,6 Prophylaxis is more effective in preventing death and morbidity from VTE than is treatment of the established disease.
 Pathophysiology
Pathophysiology
Acquired and inherited risk factors for VTE have been identified and are shown in Table 62-1. The risk of VTE increases when more than one predisposing factor is present.7,8
TABLE 62-1 Factors Predisposing to Development of Venous Thromboembolism
| Clinical Risk Factors |
Activated protein C resistance is the most common hereditary abnormality predisposing to VTE. The defect results from substitution of glutamine for arginine at residue 506 in the factor V molecule, making factor V resistant to proteolysis by activated protein C. The gene mutation is commonly designated factor V Leiden and follows autosomal dominant inheritance. Patients who are homozygous for the factor V Leiden mutation have a markedly increased risk of thromboembolism and present with clinical thromboembolism at a younger age (median 31 years) than those who are heterozygous (median age 46 years).7,9 Factor V Leiden is present in approximately 5% of the normal Caucasian population, 16% of patients with a first episode of DVT, and up to 35% of patients with idiopathic DVT.7,9,10
Prothrombin G20210A is another gene mutation that predisposes to VTE. It is present in approximately 2% to 3% of apparently healthy individuals and in 7% of those with DVT.9 An inherited abnormality cannot be detected in up to 40% to 60% of patients with idiopathic DVT, suggesting that other gene mutations are present and have an etiologic role.
Pulmonary embolism originates from thrombi in the deep veins of the leg in 90% or more of patients.11–13 Other less common sources of PE include the deep pelvic veins, renal veins, inferior vena cava, right ventricle, and axillary veins. Most clinically important PE arise from thrombi in the popliteal or more proximal deep veins of the leg. Pulmonary embolism occurs in 50% of patients with objectively documented proximal vein thrombosis; many of these emboli are asymptomatic.11 Usually only part of the thrombus embolizes, and 50% to 70% of patients with angiographically documented PE have detectable DVT of the legs at the time of presentation.12 The clinical significance of PE depends on the size of the embolus and the cardiorespiratory reserve of the patient.
 Clinical Features
Clinical Features
The clinical features of DVT include leg pain, tenderness and swelling, a palpable cord, discoloration, venous distention, prominence of the superficial veins, and cyanosis. The clinical diagnosis of DVT is highly nonspecific because none of the symptoms or signs is unique, and each may be caused by nonthrombotic disorders. Patients with relatively minor symptoms and signs may have extensive DVT, whereas those with florid leg pain and swelling, suggesting extensive DVT, may have negative results on objective testing. Thus, objective testing is mandatory to confirm or exclude a diagnosis of DVT.14–16
The location of the initial DVT has an impact on the incidence of recurrence; thus the presence of an ilial femoral vein thrombosis was shown to have a higher rate of recurrent VTE compared with popliteal vein thrombosis.17 Also, there is a high correlation between venographic results as measured by the Marder Score and recurrence of VTE.18
The prognosis for long-term survival and recurrent VTE may be worse for patients presenting with PE as opposed to DVT. This may be a reason to treat patients presenting with PE more aggressively in the future, but at the present time, anticoagulant management for each entity is identical. Various studies have attempted to identify risk factors for recurrent VTE, including fatal PE, in patients presenting initially with PE. Factors contributing to recurrent VTE include length of initial hospitalization, presence of cancer, older age, hospitalization for multiple injuries, and surgery within 3 months.19,20 Risk factors for an adverse outcome include factors such as older than age 70, hypotension, congestive heart failure, chronic obstructive pulmonary disease (COPD), cancer, presence of a DVT, and right ventricular hypokinesis on echocardiography. Using a standardized Pulmonary Embolism Severity Index21 and measurement of troponin and beta-type natriuretic peptides are useful in the initial diagnosis and in estimating prognosis in patients presenting with PE.22–27
 Etiology and Pathogenesis
Etiology and Pathogenesis
Pulmonary embolism occurs in at least 50% of patients with objectively documented proximal vein thrombosis.1 Many of these emboli are asymptomatic. The clinical importance of PE depends on the size of the embolus and the patient’s cardiorespiratory reserve. Usually only part of the thrombus embolizes, and 30% to 70% of patients with PE detected by angiography also have identifiable DVT of the legs.11,12 Deep vein thrombosis and PE are not separate disorders but a continuous syndrome of VTE in which the initial clinical presentation may be symptoms of either DVT or PE. Therefore, strategies for diagnosis of VTE include both tests for detection of PE (lung scanning, computed tomography [CT], or pulmonary angiography)8–10 and tests for DVT of the legs (ultrasound or venography)11–13
 Prevention of Venous Thromboembolism
Prevention of Venous Thromboembolism
Over the years, numerous clinical trials have been carried out for the prevention of VTE, particularly in patients undergoing orthopedic surgery and in hospitalized medical patients. Agents tested include heparin, low-molecular-weight heparin, fondaparinux, warfarin, and more recently, specific inhibitors of activated factor X or thrombin. In addition, medical devices and, in particular, intermittent pneumatic compression alone or in addition to pharmacologic agents have been studied. Effective prophylaxis against VTE is now available for most high-risk patients; prophylaxis is more effective for preventing death and morbidity and more cost-effective than treatment of the established disease. Evidence-based recommendations for the prevention of VTE are available.5,6
 Assessment of Clinical Probability
Assessment of Clinical Probability
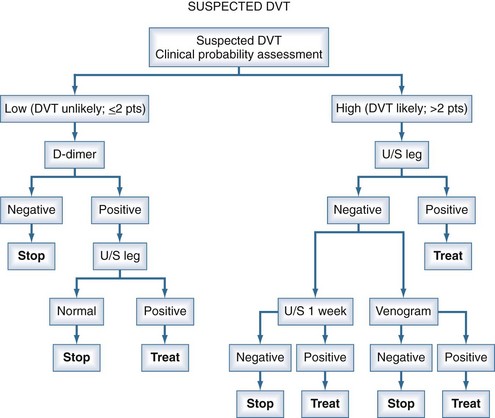
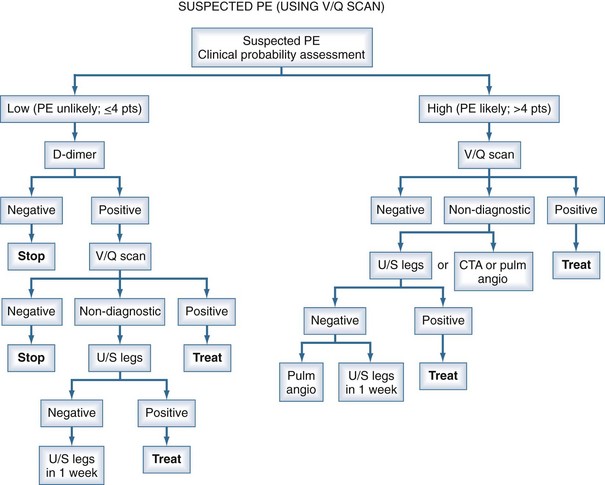
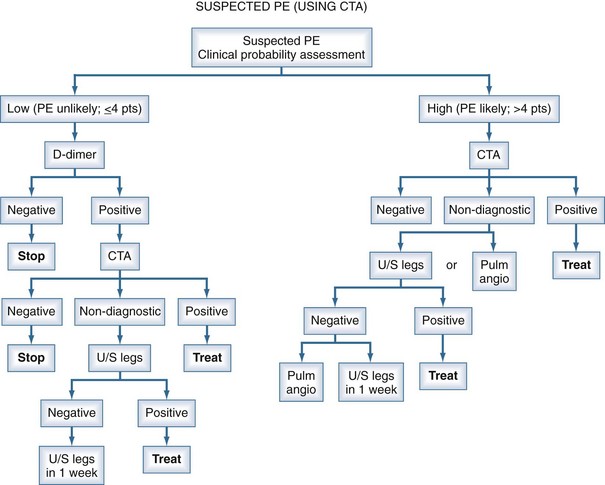
Management studies over the past 2 decades have demonstrated that patients can be assigned categories of pretest probability using decision rules such as the Geneva Score or the approach of Wells.28–34 With the shift of the burden of thromboembolic disease to the out-of-hospital population, these clinical probability guidelines have proven to be extremely useful in stratifying patients into low, moderate, or high risk for the diagnosis of PE. However, the prevalence of PE in these categories is not sufficiently low or high to withhold further investigations altogether based on the clinical probability assessment. The measurement of a D-dimer or performance of an objective diagnostic test is mandatory to exclude or confirm the presence of PE in many patients. The assessment of pretest probability and measurement of the D-dimer have now been integrated into diagnostic algorithms for PE (using either CT angiography [CTA] or ventilation/perfusion [V/Q] scanning) and for DVT (using ultrasonography to objectively confirm the diagnosis28–34 (Figures 62-1, 62-2, and 62-3).
D-Dimer Assay
Measurement of the plasma D-dimer has been extensively studied for the exclusion of patients with suspected PE.35–39 Numerous assays for the D-dimer exist, but the most extensively studied have been enzyme-linked immunosorbent assay (ELISA) and quantitative rapid ELISA, which have high sensitivity and negative likelihood ratios equal to a normal perfusion lung scan. A positive D-dimer result is not useful for the exclusion of PE. Numerous management studies have demonstrated that PE can be excluded without performing imaging studies in patients with a low clinical probability35–39 (see Figures 62-2 and 62-3). Patients with a high clinical probability (i.e., PE likely) should not undergo D-dimer testing but go directly to objective diagnostic tests.
 Differential Diagnosis
Differential Diagnosis
The differential diagnosis in patients with suspected PE includes cardiopulmonary disorders for each of the modes of presentation (see Clinical Features). For the presentation of dyspnea and tachypnea, they include atelectasis, pneumonia, pneumothorax, acute pulmonary edema, bronchitis, bronchiolitis, and acute bronchial obstruction. For pulmonary infarction exhibited by pleuritic chest pain or hemoptysis, they include pneumonia, pneumothorax, pericarditis, pulmonary or bronchial neoplasm, bronchiectasis, acute bronchitis, tuberculosis, diaphragmatic inflammation, myositis, muscle strain, and rib fracture. For the clinical presentation of right-sided heart failure, they include myocardial infarction, myocarditis, and cardiac tamponade. For cardiovascular collapse, they include myocardial infarction, acute massive hemorrhage, gram-negative septicemia, cardiac tamponade, and spontaneous pneumothorax.
 Diagnostic Imaging
Diagnostic Imaging
Computed Tomography and Computed Tomography Angiography
Spiral CT imaging has gained an increasingly important role in the diagnosis of PE in recent years and is now the primary imaging test in most centers. Single-detector spiral CT is highly sensitive for large emboli (segmental or larger arteries) but much less sensitive for emboli in subsegmental pulmonary arteries40; such emboli may be clinically important in patients with severely impaired cardiorespiratory reserve. Therefore, a negative result by single-detector spiral CT should not be used alone to exclude the diagnosis of PE. A filling defect of a segmental or larger artery on single-detector spiral CT is associated with a high probability (>90%) of PE.40
The development of multidetector row CT, together with the use of contrast enhancement, has further improved the utility of CT for the diagnosis of PE.41–44 Contrast-enhanced CTA has the advantage of providing clear results (positive or negative) with a relatively low rate of non-diagnostic test results, good characterization of nonvascular structures for alternate or associated diagnoses, and the ability to simultaneously evaluate the deep venous system of the legs (CT venography [CTV]).
The accuracy and clinical utility of multidetector CTA and combined CTA-CTV was evaluated in the PIOPED II Study.42 Among 824 patients with a reference diagnosis and a completed CT study, CTA was inconclusive in 51 (6%) because of poor image quality. Sensitivity of CTA was 83%, and specificity was 96%. CTA and CTV were inconclusive in 87 (11%) of 824 patients because the image quality of either CTA or CTV was poor. Multidetector CTA-CTV had a higher sensitivity (90%) than CTA alone (83%), with similar specificity (about 95%) for both testing techniques. Positive results on CTA in combination with a high or intermediate probability of PE by the clinical assessment, or normal findings on CTA with a low clinical probability, had a predictive value (positive or negative) of 92% to 96%.32 Such values are consistent with those generally considered adequate to confirm or rule out the diagnosis of PE. Additional testing is necessary when clinical probability is discordant with CTA or CTA-CTV imaging results.42
Radionuclide Lung Scanning
Radionuclide V/Q scanning continues to have a role in the diagnosis of suspected PE. A normal perfusion lung scan excludes the diagnosis of clinically important PE.45,46 A normal perfusion lung scan is found in approximately 10% of patients with suspected PE seen at academic health centers or tertiary referral centers. A high-probability V/Q scan result (i.e., large perfusion defects with ventilation mismatch) has a positive predictive value for PE of 85% and provides a diagnostic endpoint to give antithrombotic treatment in most patients.45–47 A high-probability V/Q scan is found in approximately 10% to 15% of symptomatic patients. For patients with a history of PE, careful comparison of the lung scan results to the most recent lung scan is required to ensure the perfusion defects are new. Further diagnostic testing is indicated for patients with a high-probability V/Q scan who have a “low” pretest clinical suspicion, and in those who are at high risk for major bleeding, to reduce the likelihood of a false-positive diagnosis.
The major limitation of V/Q scanning is that the results are inconclusive in most patients, even when considered together with the pretest clinical probability.45 The nondiagnostic V/Q scan patterns are found in about 70% of patients with suspected PE.12,45,47 These lung scan results have historically been called “low-probability” (matching ventilation/perfusion abnormalities or small perfusion defects), “intermediate probability,” or indeterminate (because the perfusion defects correspond to an area of abnormality on chest x-ray film). Further diagnostic testing is required in most of these patients because regardless of the pretest clinical suspicion, the posttest probabilities of PE associated with these lung scan results are neither sufficiently high to give antithrombotic treatment nor sufficiently low to withhold therapy. The uncommon exception is the patient with a low clinical suspicion and a so-called low-probability V/Q scan result. However, even in these patients, objective testing for DVT with ultrasound may provide added diagnostic value. A randomized trial has established that CTA is not inferior to using V/Q scanning for excluding the diagnosis of PE when either test is used in an algorithm together with venous ultrasonography of the legs.43
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) appears to be a promising diagnostic approach for PE. However, clinically important interobserver variation exists in the sensitivity for PE, ranging from 70% to 100%.48,49 Further studies are required to determine the clinical role of MRI in the diagnosis of patients with suspected PE.
Pulmonary Angiography
Pulmonary angiography using selective catheterization of the pulmonary arteries is a relatively safe technique for patients who do not have pulmonary hypertension or cardiac failure.45,46 If the expertise is available, pulmonary angiography should be used when other approaches are inconclusive and when definitive knowledge about the presence or absence of PE is required.
Objective Testing For Deep Vein Thrombosis
Objective testing for DVT is useful in patients with suspected PE, particularly those with nondiagnostic lung scan results47 or inconclusive CT results.42 Detection of proximal vein thrombosis by objective testing provides an indication for anticoagulant treatment regardless of the presence or absence of PE and prevents the need for further testing. However, a negative result by objective testing for DVT does not exclude the presence of PE.12
Currently, the primary role for using ultrasound testing of the legs is for those centers that do not have the capability for combined CTA-CTV, or if the results of such imaging are inconclusive. If the patient has adequate cardiorespiratory reserve, serial ultrasound testing for proximal vein thrombosis can be used as an alternative to pulmonary angiography in patients with non-diagnostic lung scan or CT results, and withholding anticoagulant therapy is safe if repeated ultrasound testing of the legs is negative.50–53 The rationale is that the clinical objective in such patients is to prevent recurrent PE, which is unlikely in the absence of proximal vein thrombosis. Selective pulmonary angiography should be done among patients with features suggesting a possible source of embolism other than proximal DVT of the leg (e.g., upper-extremity thrombosis, renal vein thrombosis, pelvic vein thrombosis, or right-heart thrombus).
 Integrated Strategies for Diagnosis of Pulmonary Embolism
Integrated Strategies for Diagnosis of Pulmonary Embolism
Figure 62-3 summarizes the approach to diagnosis of suspected PE using CTA or CTA-CTV as the primary imaging test. Figure 62-2 summarizes the approach to diagnosis using V/Q scanning for settings in which CTA capabilities are not available. Figure 62-3 summarizes the approach to the diagnosis of DVT using ultrasonography. The specific approach used will depend on the local availability of technology, expertise with the different diagnostic techniques, and individual patient circumstances.
An appropriately validated assay for plasma D-dimer, if available, provides a simple and rapid first-line exclusion test in patients with low, intermediate, or unlikely clinical probability. The appropriate use of D-dimer can reduce the need for more expensive imaging tests without compromising patient safety. If a validated D-dimer test is not available or the patient has high clinical probability for PE, diagnostic imaging should be employed. If capability for combined CTA-CTV exists, that is the preferred approach for most patients because it provides a definitive basis to give or withhold antithrombotic therapy in about 90% of patients. Lung scanning may be indicated as the first-line imaging test in women of reproductive age, because the radiation exposure to the breast is significantly less than with CTA.53
Echocardiography
Echocardiography provides a number of independent parameters related to pulmonary hemodynamics and, in addition to measurement of troponin and beta-type natriuretic peptide levels, can identify patients with non-massive PE who are at risk of dying or are candidates for thrombolytic therapy.22–27 Transthoracic echocardiography is particularly useful for patients in the intensive care unit (ICU) and can further identify patients who are candidates for thrombolysis or catheter fragmentation or who may progress to chronic thromboembolic pulmonary hypertension.23,24
 Clinical Course of Venous Thromboembolism
Clinical Course of Venous Thromboembolism
Proximal DVT is a serious and potentially lethal condition. Untreated proximal vein thrombosis is associated with a 10% rate of fatal PE. Inadequately treated proximal vein thrombosis results in a 20% to 50% risk of recurrent VTE events.54,47,55 Prospective studies of patients with clinically suspected DVT or PE indicate that new venous thromboembolic events on follow-up are rare (≤2%) among patients in whom proximal vein thrombosis is absent by objective testing.50–52 The aggregate data from diagnostic and treatment studies indicate that the presence of proximal DVT is the key prognostic marker for recurrent VTE.
Thrombosis that remains confined to the calf veins is associated with low risk (≤1%) of clinically important PE. Extension of thrombosis into the popliteal vein or more proximally occurs in 15% to 25% of patients with untreated calf vein thrombosis.11 Patients with documented calf vein thrombosis should receive either anticoagulant treatment to prevent extension or undergo monitoring for proximal extension using serial noninvasive tests.
The postthrombotic syndrome is a frequent complication of DVT.56,57 Patients with postthrombotic syndrome complain of pain, heaviness, swelling, cramps, and itching or tingling of the affected leg. Ulceration may occur. The symptoms usually are aggravated by standing or walking and improve with rest and elevation of the leg. A prospective study documented a 25% incidence of moderate to severe postthrombotic symptoms 2 years after the initial diagnosis of proximal DVT in patients who were treated with initial heparin and oral anticoagulants for 3 months.56 The study also demonstrated that ipsilateral recurrent DVT is strongly associated with subsequent development of moderate or severe postthrombotic symptoms. Thus prevention of ipsilateral recurrent DVT likely reduces the incidence of the postthrombotic syndrome. Application of a properly fitted graded compression stocking, as soon after diagnosis as the patient’s symptoms will allow and continued for at least 2 years, is effective in reducing the incidence of postthrombotic symptoms, including moderate to severe symptoms.58
Chronic thromboembolic pulmonary hypertension is a serious complication of PE. Historically, thromboembolic pulmonary hypertension was believed to be relatively rare and occur only several years after the diagnosis of PE. A prospective cohort study provides important information on the incidence and timing of thromboembolic pulmonary hypertension.59–61 The results indicate that thromboembolic pulmonary hypertension is more common and occurs earlier than previously thought. On prospective follow-up of 223 patients with documented PE, the cumulative incidence of chronic thromboembolic pulmonary hypertension was 3.8% at 2 years after diagnosis despite state-of-the-art treatment for PE. The strongest independent risk factors were a history of PE (odds ratio 19) and idiopathic PE at presentation (odds ratio 5.7).59 Further clinical studies on identification and prevention of chronic thromboembolic pulmonary hypertension are needed.
 Objectives and Principles of Antithrombotic Treatment
Objectives and Principles of Antithrombotic Treatment
Recommendations for treatment of established VTE are linked to the strength of the evidence from clinical trials using the approach for grading evidence of the American College of Chest Physicians (ACCP) guideline committee.55 Recommendations classified as 1A are supported by evidence from scientifically valid randomized clinical trials (grade A evidence), and the results provide a clear risk-to-benefit conclusion (grade 1). Such recommendations should be implemented for most patients. Grade 2A recommendations also are supported by definitive clinical trial evidence (grade A), but the results indicate a less clear risk-to-benefit conclusion (grade 2); therefore, such recommendations may or may not be appropriate for the individual patient. The remaining grades of recommendation are based on nondefinitive evidence (grade B or C) and are less strong.
 Anticoagulant Therapy
Anticoagulant Therapy
Heparin Therapy
Unfractionated Heparin Therapy
Unfractionated heparin (UFH) has been used extensively to prevent and treat VTE, but more recently, low-molecular-weight heparin (LMWH) has replaced UFH for the treatment of VTE in most cases, either entirely or predominantly in the out-of-hospital setting. However, there are patients in whom UFH by continuous infusion continues to be used primarily because the anticoagulant effect can be reversed by stopping the intravenous (IV) infusion and/or administering protamine sulphate.62 Such patients include critically ill patients in the ICU or cardiovascular unit, patients who may be candidates for interventions requiring interruption of anticoagulant therapy (e.g., surgical procedures, thrombolysis), or patients with severe renal failure.62 In some countries, UFH is the anticoagulant of choice for patients suffering PE who are hemodynamically unstable.
The anticoagulant activity of UFH depends upon a unique pentasaccharide that binds to antithrombin (AT) and potentiates the inhibition of thrombin and activated factor X (Xa) by ATIII.62–64 About one-third of all heparin molecules contain the unique pentasaccharide sequence.62–64 It is the pentasaccharide sequence that confers the molecular high affinity for AT.62–64 In addition, heparin catalyses the inactivation of thrombin by another plasma cofactor, cofactor II, which acts independently of AT.62
Heparin has a number of effects other than inhibition of thrombin and activated factor X.63 These include the release of tissue factor pathway inhibitor; suppression of platelet function; increase in vascular permeability, and binding to numerous plasma and platelet proteins, endothelial cells, and leucocytes. The anticoagulant response to a standard dose of UFH varies widely between patients. This makes it necessary to monitor the anticoagulant effects of UFH, using either the activated partial thromboplastin time (APTT) or heparin levels, and to titrate the dose to the individual patient.62
The simultaneous use of initial UFH and warfarin has become clinical practice for all patients with VTE who are medically stable.62,65 Exceptions include patients who require immediate medical or surgical intervention, such as in thrombolysis or insertion of a vena cava filter, or patients at very high risk of bleeding. Heparin is continued until the International Normalized Ratio (INR) has been within the therapeutic range (2 to 3) for 2 consecutive days.62
It has been established from experimental studies and clinical trials that the efficacy of UFH therapy depends upon achieving a critical therapeutic level of UFH within the first 24 hours of treatment.66–68 Data from double blind clinical trials indicate that failure to achieve the therapeutic APTT threshold by 24 hours was associated with a 23.3% subsequent recurrent VTE rate, compared with a rate of 4% to 6% for the patient group who were therapeutic at 24 hours.67,68 Recurrences occurred throughout the 3-month follow-up period and could not be attributed to inadequate oral anticoagulant therapy.67 The critical therapeutic level of UFH, as measured by the APTT, is 1.5 times the mean of the control value or the upper limit of the normal APTT range.66–68 This corresponds to a UFH blood level of 0.2 to 0.4 U/mL by the protamine sulphate titration assay, and 0.35 to 0.70 by the antifactor Xa assay. It is vital for each laboratory to establish the minimal therapeutic level of UFH, as measured by the APTT, that will provide a UFH blood level of at least 0.35 U/mL by the antifactor Xa assay for each batch of thromboplastin reagent being used, particularly if a new batch of reagent is provided by a different manufacturer.62
Numerous audits of UFH therapy indicate that administration of IV UFH is fraught with difficulty, and that the clinical practice of using an ad hoc approach to UFH dose titration frequently results in inadequate therapy. Use of a prescriptive approach or protocol for administering IV UFH therapy has been evaluated in two prospective studies in patients with VTE.66,68 Both protocols were shown to achieve therapeutic UFH levels in the vast majority of patients. Using the weight-based nomogram, there were fewer episodes of recurrent VTE as compared to standard care. Continued use of the weight-based nomogram has been shown to be similarly effective.69
Adjusted-dose subcutaneous UFH has been used in initial treatment of VTE.70 Four randomized clinical trials compared the efficacy of subcutaneous UFH with subcutaneous LMWH in patients with proven VTE.71–74 Nomograms have been developed for subcutaneous UFH. The importance of achieving the therapeutic range by 24 hours was reaffirmed.75 The largest of these trials compared subcutaneous UFH dose adjusted with the use of APTT by means of a weight-adjusted algorithm with fixed-dose LMWH for the initial treatment of patients with VTE, 16% of who presented with PE.74 Subcutaneous UFH was shown to be similar to fixed-dose LMWH in terms of efficacy and safety.74
Complications of Unfractionated Heparin Therapy
Management of bleeding on heparin will depend on the location and severity of bleeding, risk of recurrent VTE, and APTT; in these instances, heparin should be discontinued temporarily or permanently. Patients with recent VTE may be candidates for insertion of an inferior vena cava filter. If urgent reversal of heparin effect is required, protamine sulphate can be administered.62
Heparin-induced thrombocytopenia is a well-recognized complication of UFH therapy, usually occurring within 5 to 10 days after heparin treatment has started.76,77 Approximately 1% to 2% of patients receiving UFH will experience a fall in platelet count to less than the normal range or a 50% fall in the platelet count within the normal range. In the majority of cases, this mild to moderate thrombocytopenia appears to be a direct effect of heparin on platelets and is of no consequence. However, patients receiving UFH may develop an immune thrombocytopenia mediated by immunoglobulin G (IgG) antibody directed against a complex of PF4 and heparin.29 In some cases, neutrophil acting peptide 2 (NAP-2) and interleukin 8 (IL-8) also play a role in pathogenesis.
The incidence of heparin-induced thrombocytopenia (HIT) is lower with the use of LMWH76,78–82; however, the clinical manifestations may be as or more severe than those seen with UFH. Furthermore, the nadir of the platelet count, onset, and duration of thrombocytopenia have been shown to be somewhat different.80 Recently, delayed onset of HIT has been described, with the onset being as long as several weeks after the end of exposure to heparin, thus making this syndrome sometimes more difficult to diagnose. Furthermore, the incidence and severity of HIT varies among different patient populations, being more prevalent in patients having cardiac or orthopedic procedures than for medical patients.83 The development of thrombocytopenia may be accompanied by arterial or DVT which may lead to serious consequences such as death or limb amputation.76,83
When a clinical diagnosis of HIT is made, heparin in all forms must be stopped immediately.77,84 In most centers, the confirmatory laboratory test is an ELISA for the PF4-heparin complex but where possible, this should be confirmed with a functional assay such as the serotonin release assay.83 In those patients requiring ongoing anticoagulation, an alternative form of anticoagulation must be undertaken immediately because of the high incidence of thrombosis when heparin is stopped.85 Some authorities recommend the use of alternative anticoagulants in all patients once a diagnosis is made. The most common alternative agents are the specific antithrombin, argatroban,77,86,87 or the direct thrombin inhibitor, lepirudin.88–91 Both agents are given by IV infusion. Lepirudin, which is renally excreted, has the advantage that it can be given to patients with hepatic insufficiency,77,85 but it has the disadvantage that with prolonged use, antibodies develop, and some of these can have series deleterious effects, including anaphylaxis.92 Argatroban is only partially excreted by the kidney, so it can be used in persons with renal failure, but it cannot be used in patients with significant hepatic insufficiency.77,85 Both agents can be used in conjunction with vitamin K antagonists, but it should be noted that argatroban by itself increases the INR beyond that observed with warfarin alone, and this must be taken into account in controlling the vitamin K antagonist.87 The alternative antithrombotic agents should be continued until the platelet count is at least back to 100 × 109/L and/or the INR is therapeutic for 2 consecutive days.77 The pentasaccharide, fondaparinux, has been used as an alternative antithrombotic agent in HIT patients, and it has the advantage that it is given by a once-daily subcutaneous injection.93,94 Insertion of an inferior vena cava filter is seldom indicated.
Osteoporosis has been reported in patients receiving UFH in dosages of 20,000 U/day (or more) for more than 6 months.62 Demineralization can progress to the fracture of vertebral bodies or long bones, and the defect may not be entirely reversible.62 Laboratory and clinical studies indicate that the incidence of osteoporosis with use of long-term LMWH is low.62
Low-Molecular-Weight Heparin for Initial Treatment of VTE
Heparin currently in use clinically is polydispersed unmodified heparin with a mean molecular weight ranging from 10 to 16 kD. Low-molecular-weight derivatives of commercial heparin have been prepared that have a mean molecular weight of 4 to 5 kD.62,95,96
The LMWHs commercially available are made by different processes (e.g., nitrous acid, alkaline, or enzymatic depolymerization) and they differ chemically and pharmacokinetically.95,96 The clinical significance of these differences, however, is unclear, and there have been very few studies comparing different LMWHs with respect to clinical outcomes.96 The doses of the different LMWHs have been established empirically and are not necessarily interchangeable. Therefore, at this time, effectiveness and safety of each of the LMWHs must be tested separately.96
The LMWHs differ from UFH in numerous ways. Of particular importance are increased bioavailability (>90% after subcutaneous injection); prolonged half-life and predictable clearance, enabling once- or twice-daily injection; and predictable antithrombotic response based on body weight, permitting treatment without laboratory monitoring.62,95,96 Other possible advantages are their ability to inactivate platelet-bound factor Xa, resistance to inhibition by platelet factor 4, and their decreased effect on platelet function and vascular permeability (possibly accounting for fewer hemorrhagic effects at comparable antithrombotic doses).
Subcutaneous unmonitored LMWH has been compared with continuous IV heparin in a number of clinical trials for the treatment of proximal DVT or PE using long-term follow-up as an outcome measure.97–105 These studies have shown that LMWH is at least as effective and safe as unfractionated heparin in the treatment of proximal venous thrombosis. Pooling of the most methodologically sound studies indicates a significant advantage for LMWH in the reduction of major bleeding and mortality.106 LMWH used predominantly out of hospital was as effective and safe as IV UFH given in hospital.101–103 Economic analysis of treatment with LMWH versus IV UFH demonstrated that LMWH was cost-effective for treatment in hospital as well as out of hospital.101,102 As these agents become more widely available for treatment, they have replaced IV UFH in the initial management of most patients with VTE. LMWH is now the recommended agent for initial treatment of VTE.55
There has been a hope that the LMWHs will have fewer serious complications such as bleeding,69 heparin-induced thrombocytopenia,80,81,107 and osteoporosis108 when compared with unfractionated heparin. Evidence is accumulating that these complications are indeed less serious and less frequent with the use of LMWH.
Recent reviews suggest the absolute risk for heparin-induced thrombocytopenia with LMWH was 0.2%, compared with 2.6% with UFH. Accordingly, there is an advantage in this regard to using LMWH.108
In obese patients, the clinician should review the pharmacopeia recommendations for the particular LMWH agent being used concerning dosage guidelines.62 For patients with significant renal impairment, the clinician should also review the pharmacopeia guidelines for dosage modifications for the individual LMWH agent. In patients with severe renal failure, it may be preferable to use UFH.55,62
Long-Term Low-Molecular-Weight Heparin
The use of LMWH for the long-term treatment of acute VTE has been evaluated in randomized clinical trials.109–111 Taken together, these studies110–111 indicate that long-term treatment with subcutaneous LMWH for 3 to 6 months is more effective in cancer patients with VTE than adjusted doses of oral vitamin K antagonist therapy (INR 2.0–3.0) for preventing recurrent VTE. The ACCP recommendation states: “For most patients with DVT and cancer, we recommend treatment with LMWH for at least the first 3 to 6 months of long-term treatment.”55
Oral Vitamin K–Antagonist Therapy (Warfarin)
The anticoagulant effect of warfarin is mediated by inhibition of the vitamin K–dependent γ-carboxylation of coagulation factors II, VII, IX, and X.112,113 This results in the synthesis of immunologically detectable but biologically inactive forms of these coagulation proteins. Warfarin also inhibits the vitamin K–dependent γ-carboxylation of proteins C and S. Protein C circulates as a proenzyme that is activated on endothelial cells by the thrombin-thrombomodulin complex to form activated protein C. Activated protein C in the presence of protein S inhibits activated factor VIII and activated factor V activity.112,113 Therefore, vitamin K antagonists such as warfarin create a biochemical paradox by producing an anticoagulant effect due to the inhibition of procoagulants (factors II, VII, IX, and X) and a potentially thrombogenic effect by impairing the synthesis of naturally occurring inhibitors of coagulation (proteins C and S). Heparin and warfarin treatment should overlap by 4 or 5 days when warfarin treatment is initiated in patients with thrombotic disease.
The anticoagulant effect of warfarin is delayed until the normal clotting factors are cleared from the circulation, and the peak effect does not occur until 36 to 72 hours after drug administration.114,115 During the first few days of warfarin therapy, the prothrombin time (PT) reflects mainly the depression of factor VII, which has a half-life of 5 to 7 hours.114 Equilibrium levels of factors II, IX, and X are not reached until about 1 week after the initiation of therapy. The use of small initial daily doses (e.g., 5–10 mg) is the preferred approach for initiating warfarin treatment.116,119
The dose-response relationship to warfarin therapy varies widely between individuals, so dosage must be carefully monitored to prevent overdosing or underdosing. A number of drugs interact with warfarin.113 Critical appraisal of the literature reporting such interactions indicates that the evidence substantiating many of the claims is limited.118 Nonetheless, patients must be warned against taking any new drugs without the knowledge of their attending physician.
Laboratory Monitoring and Therapeutic Range
The laboratory test most commonly used to measure the effects of warfarin is the one-stage PT test. The PT is sensitive to reduced activity of factors II, VII, and X but is insensitive to reduced activity of factor IX. Confusion about the appropriate therapeutic range has occurred because the different tissue thromboplastins used for measuring the PT vary considerably in sensitivity to the vitamin K–dependent clotting factors and in response to warfarin.119,120
To promote standardization of the PT for monitoring oral anticoagulant therapy, the World Health Organization (WHO) developed an international reference thromboplastin from human brain tissue and recommended that the PT ratio be expressed as the International Normalized Ratio, or INR.62 The INR is the PT ratio obtained by testing a given sample using the WHO reference thromboplastin. For practical clinical purposes, the INR for a given plasma sample is equivalent to the PT ratio obtained using a standardized human brain thromboplastin known as the Manchester Comparative Reagent, which has been widely used in the United Kingdom.55
Warfarin is administered in an initial dose of 5 to 10 mg per day for the first 2 days.116,117 The daily dose is then adjusted according to the INR. UFH or LMWH therapy is discontinued on the fourth or fifth day following initiation of warfarin therapy, provided the INR is prolonged into the recommended therapeutic range (INR 2 to 3).55 Because some individuals are either fast or slow metabolizers of the drug, selection of the correct dosage of warfarin must be individualized. Therefore, frequent INR determinations are required initially to establish therapeutic anticoagulation.
Once the anticoagulant effect and patient’s warfarin dose requirements are stable, the INR should be monitored at regular intervals throughout the course of warfarin therapy for VTE. However, if there are factors that may produce an unpredictable response to warfarin (e.g., concomitant drug therapy), the INR should be monitored frequently to minimize the risk of complications due to poor anticoagulant control.55 Several warfarin nomograms and computer software programs are now available to assist healthcare givers in the control of warfarin therapy. Also, there is increasing interest in the use of self-testing with portable INR monitors and, in selected cases, self-management of oral anticoagulant therapy.
Adverse Effects of Oral Anticoagulants
Bleeding
The major side effect of warfarin therapy is bleeding.113,119,120 A number of risk factors have been identified that predispose to bleeding on oral anticoagulants.113,121,122 The most important factor influencing bleeding risk is the intensity of the INR.121,122 Other factors include a history of bleeding, previous history of stroke or myocardial infarction, hypertension, renal failure, diabetes, and decreased hematocrit.121 Efforts have been made to quantify the bleeding risk according to these underlying clinical factors.121,122 Introduction of a multicomponent intervention combining patient education and alternative approaches to the maintenance of INR resulted in a reduced frequency of major bleeding in the patients in this group.121 Furthermore, patients in the intervention group were within the therapeutic INR a significantly greater amount of time than were patients in the standard care group. In a retrospective cohort study of patients with an INR greater than 6.0, it was shown that a prolonged delay in the return of the INR to the therapeutic range was seen in patients who had an INR over 4.0 after two doses of warfarin were withheld, patients with an extreme elevation of the INR, and older age patients, particularly those with decompensated congestive heart failure or active cancer.122,123 Numerous randomized clinical trials have demonstrated that clinically important bleeding is lower when the targeted INR is 2.0 to 3.0, and that bleeding increases exponentially when the INR increases above 4.5 or 5.0.121,122 There is a strong negative relationship between the percentage of time patients are within the targeted range for INR and both bleeding and recurrent thrombosis.
Warfarin therapy in elderly patients can present problems.124,125 Many of these patients require long-term anticoagulants because of underlying clinical conditions that increase with age, while they are more likely to have underlying causes for bleeding, including the development of cancer, intestinal polyps, renal failure, and stroke; and they are more prone to having frequent falls. The daily requirements for warfarin to maintain the therapeutic INR also decrease with age, presumably due to decreased clearance of the drug. Therefore, before initiating oral anticoagulant treatment in elderly patients, the risk/benefit ratio of treatment must be considered. If they are placed on oral anticoagulant therapy, careful attention to the INR is required.
Patients with cancer are more likely to bleed on warfarin treatment.126 Compared with patients on oral anticoagulants who do not have cancer, patients with cancer have a higher incidence of both major and minor bleeding, and anticoagulant withdrawal is more frequently due to bleeding. Patients with cancer have a higher thrombotic complication rate and a higher bleeding rate regardless of the INR, whereas bleeding in non-cancer patients was seen only when the INR was greater than 4.5. Safer and more effective anticoagulant therapy is required for the treatment of VTE in patients with cancer.126
Management of Over-Anticoagulation
The approach to the patient with an elevated INR depends on the degree of elevation of the INR and the clinical circumstances.113,127,128 Options available to the physician include temporary discontinuation of warfarin treatment, administration of vitamin K, administration of blood products such as fresh frozen plasma or prothrombin concentrate to replace the vitamin K–dependent clotting factors, or administration of activated factor VII. If the increase is mild and the patient is not bleeding, no specific treatment is necessary other than reduction in the warfarin dose. The INR can be expected to decrease during the next 24 hours with this approach. With more marked increase of the INR in patients who are not bleeding, treatment with small doses of vitamin K (e.g., 1 mg) given either orally or by subcutaneous injection should be considered.127,128 With very marked increase of the INR, particularly in a patient who is either actively bleeding or at risk for bleeding, the coagulation defect should be corrected. Vitamin K can be given IV slowly or by the subcutaneous or oral routes.113,127 Where possible, the oral route is preferred. If ongoing anticoagulation with warfarin is planned, repeated small doses of vitamin K should be given so there is no problem with warfarin resistance.113,127
Reported side effects of vitamin K include flushing, dizziness, tachycardia, hypotension, dyspnea, and sweating.113 Intravenous administration of vitamin K1 should be performed with caution to avoid inducing an anaphylactoid reaction; risk of anaphylactoid reaction can be reduced by slow administration. In most patients, IV administration of vitamin K produces a demonstrable effect on the INR within 6 to 8 hours and corrects the increased INR within 12 to 24 hours. Because the half-life of vitamin K is less than that of warfarin sodium, a repeat course of vitamin K may be necessary. If bleeding is very severe and life threatening, vitamin K therapy can be supplemented with concentrates of factors II, VII, IX, and X.
Long-Term Treatment of Venous Thromboembolism Using Vitamin K Antagonists
Patients with established DVT or PE require long-term anticoagulant therapy to prevent recurrent disease.55,113 Warfarin therapy is highly effective54,55 and is preferred in most but not all patients. Adjusted-dose subcutaneous heparin or unmonitored LMWHs have been used for the long-term treatment of patients in whom oral anticoagulant therapy proves to be very difficult to control,109 and LMWH is the preferred treatment in patients with DVT and cancer.109–111
The preferred intensity of the anticoagulant effect of treatment with warfarin has been confirmed by the results of randomized trials.55,113,136,137 The results of two recent randomized trials136,137 indicate that although low-intensity warfarin therapy is more effective than placebo, it is less effective than standard-intensity therapy (INR 2-3), and does not reduce the incidence of bleeding complications. Additional important evidence regarding the intensity of anticoagulant therapy with warfarin is provided by a recent randomized trial138 that compared standard-intensity warfarin therapy (INR 2-3) with high-intensity warfarin therapy (INR 3.1-4.0) for the prevention of recurrent thromboembolism in patients with persistently positive antiphospholipid antibodies and a history of thromboembolism (venous or arterial). High-intensity warfarin therapy (INR 3.1-4.0) did not provide improved antithrombotic protection. The high-intensity regimen has been previously shown to be associated with a high risk (20%) of clinically important bleeding in a series of randomized trials138–141 in patients with DVT. The evidence outlined above provides the basis for recommending an INR of 2.0 to 3.0 as the preferred intensity of anticoagulant treatment with warfarin.
 Duration of Anticoagulant Therapy and Recurrent Venous Thromboembolism
Duration of Anticoagulant Therapy and Recurrent Venous Thromboembolism
The appropriate duration of warfarin treatment for VTE has been evaluated by multiple randomized clinical trials.55,142–148 Treatment should be continued for at least 3 months in patients with a first episode of proximal DVT or PE secondary to a transient (reversible) risk factor (grade 1A). Stopping treatment at 4 to 6 weeks resulted in an increased incidence of recurrent VTE during the following 6 to 12 months (absolute risk increase 8%). In contrast, treatment for 3 to 6 months resulted in a low rate of recurrent VTE during the following 1 to 2 years (annual incidence 3%).
Patients with a first episode of idiopathic VTE should be treated for 3 to 6 months55 (grade 1A) and considered for indefinite anticoagulant therapy. This decision should be individualized, taking into consideration the estimated risk of recurrent VTE, risk of bleeding, and patient compliance and preference. Indefinite therapy is recommended for patients in whom risk factors for bleeding are absent and in whom good anticoagulant control can be achieved (grade 1A).55 If indefinite anticoagulant treatment is given, the risk-benefit of continuing such treatment should be reassessed at periodic intervals.
Numerous attempts have been made to identify patients who are at particularly high risk for recurrent VTE when anticoagulant therapy is discontinued.149–155 Measurement of the D-dimer either before anticoagulants are stopped or 1 month after discontinuation can help predict patients at risk of recurrent VTE if the D-dimer is elevated.152,153,155 In a recent study, measurement of the D-dimer assay prior to discontinuing anticoagulants, combined with assessment of signs of postthrombotic syndrome, in consideration of age or those who are obese, can help identify patients at high or low risk for recurrent VTE. Similarly, assessment of residual proximal venous thrombosis based on non-compressibility of the previously involved segment of the vein can predict patients who are at higher risk for recurrent VTE.149–151
Warfarin treatment should be given indefinitely for most patients with a second episode of unprovoked VTE55,113 (grade 1A), because stopping treatment at 3 to 6 months in these patients results in a high incidence (21%) of recurrent VTE during the following 4 years. The risk of recurrent VTE during 4-year follow up was reduced by 87% (from 21% to 3%) by continuing anticoagulant treatment; this benefit is partially offset by an increase in the cumulative incidence of major bleeding (from 3% to 9%).55
Use of LMWH for long-term treatment of VTE has been evaluated in clinical trials.109–111 The studies indicate that long-term treatment with subcutaneous LMWH for 3 to 6 months is at least as effective as (and in cancer patients more effective than) warfarin adjusted to maintain the INR between 2.0 and 3.0. Therefore, patients with VTE and cancer should be treated with LMWH for the first 3 to 6 months of long-term treatment (grade 1A).55 The patient then should receive anticoagulation indefinitely or until the cancer resolves. The regimens of LMWH that are established as effective for long-term treatment are dalteparin, 200 U/kg once daily for 1 month, followed by 150 U/kg daily thereafter; or tinzaparin, 175 U/kg once daily.
 Fondaparinux and Related Compounds
Fondaparinux and Related Compounds
Fondaparinux, a synthetic indirect inhibitor of factor Xa has been studied in a wide variety of patients for the prevention and treatment of VTE. Based on the results of such clinical trials, fondaparinux has been approved as a substitute for UFH or LMWH for the initial treatment of VTE.156,157 Idraparinux, a derivative of fondaparinux, has a high affinity for antithrombin, and this high affinity prolongs the plasma half-life to 80 hours. Because of this long half-life, idraparinux can be given subcutaneously on a once-weekly basis. In a clinical trial in a treatment of DVT, idraparinux was given in a once-weekly subcutaneous injection and compared with either LMWH or UFH followed by warfarin for a 3-month period.158 Idraparinux was similar in efficacy in terms of recurrent VTE, but clinically relevant bleeding was less common with idraparinux than with conventional therapy. However, in patients presenting with PE, idraparinux given by weekly subcutaneous injection for 3 months was less effective than conventional therapy with similar bleeding rates.113 In a long-term study, idraparinux was compared with placebo in patients with DVT or PE who had had an initial 6-month treatment with standard therapy.159 There was a significant reduction in the risk of recurrent VTE with idraparinux, but there was an increase in major bleeding, including three fatal intracranial hemorrhages. Given these results and the fact that the anticoagulant effect of idraparinux could not be blocked, this agent has not been further developed. However, a biotinylated form of idraparinux has been developed that provides the opportunity of removing the long-acting compound by administering an antibody to the biotin molecule. This agent is under investigation for the treatment of DVT and PE.
 New Oral Anticoagulants
New Oral Anticoagulants
There has been much interest in developing new oral antithrombotic agents that may be able to replace warfarin. The most advanced agents are specific inhibitors of factor Xa or thrombin (factor 2). Advantages of these agents are that they can be given by the oral route once or twice daily, they require no laboratory monitoring, and in most cases, the same dose is taken by all patients. In clinical trials, all these agents are compared with enoxaparin, either 40 mg once daily beginning 12 hours prior to surgery, or 30 mg twice daily beginning 12 to 24 hours postoperatively in patients undergoing total hip or total knee replacement surgery.160–165 These procedures carry a high risk for VTE, and because of the nature of the procedure, there is a significant risk of bleeding. Therefore, agents which can be shown to be effective and safe in this clinical situation show promise for prevention and treatment of VTE in other settings. To date, there has been publication of clinical trials in patients undergoing total hip or total knee replacement with the factor Xa inhibitors, rivaroxaban (Bayer Health Care) and apixaban (BMS-Pfizer), and the antithrombin agent, dabigatran (Boehringer-Ingelheim). Results have varied somewhat depending on the dosage of the comparative agent, enoxaparin, and the dose and timing of the investigative agent. Rivaroxaban and dabigatran have been approved by a number of agencies for thromboprophylaxis in hip and knee arthroplasty patients and are used in a number of countries, but neither has been approved by the U.S. Food and Drug Administration (FDA) at this time.
All three agents are being investigated for initial and/or extended treatment of VTE. In the RECOVER study, dabigatran etexilate, 150 mg twice daily, was compared with standard warfarin therapy, with an INR target of 2 to 3 in patients presenting with VTE who had an initial course of parenteral therapy, usually with LMWH for 8 to 11 days.166 Treatment continued for 6 months, and there was a follow-up of 30 days. Dabigatran was shown to be non-inferior to standard therapy in the prevention of recurrent VTE or VTE-related death, and the incidence of major bleeding was comparable. However, the incidence of combined major and non-major clinically relevant bleeding was significantly less with dabigatran.
 Thrombolytic Therapy
Thrombolytic Therapy
Thrombolytic therapy is indicated for patients with PE who present with evidence of vascular collapse (hypotension and/or syncope) and for selected patients with PE who have clinical findings of right ventricular failure or echocardiographic evidence of right ventricular hypokinesia.55,167–169 Thrombolytic therapy provides more rapid lysis of PE and more rapid restoration of right ventricular function and pulmonary perfusion than anticoagulant treatment.167–170
 Inferior Vena Cava Filter
Inferior Vena Cava Filter
Insertion of an inferior vena cava filter is indicated for patients with acute VTE and an absolute contraindication to anticoagulant therapy and for those rare patients who have objectively documented recurrent VTE during adequate anticoagulant therapy.55,100,170
Insertion of a vena cava filter is effective for preventing important PE. However, use of a permanent filter results in an increased incidence of recurrent DVT 1 to 2 years after insertion (increase in cumulative incidence at 2 years increases from 12% to 21%).100 Therefore, if the indication for filter placement is transient, such as a contraindication to anticoagulation due to a temporary high risk of bleeding, a retrievable vena cava filter should be used.171,172 A retrievable filter can then be removed after several weeks to months, once the filter is no longer required. If a permanent filter is placed, long-term anticoagulant treatment should be given as soon as safely possible to prevent morbidity from recurrent DVT.
 Conclusions
Conclusions
Based on a large number of clinical trials, the accepted medical treatment for acute PE has been established. Historically this consisted of UFH given by continuous IV infusion, with warfarin starting on days 1 or 2 and continued for 3 months, with a targeted INR of 2.0 to 3.0. A number of LMWHs have been shown to be at least as effective as UFH in decreasing recurrent VTE and in fact are associated with less major bleeding. Low-molecular-weight heparin has become the treatment of choice for both in-hospital and out-of-hospital treatment of DVT and, more recently, submassive PE as well. Long-term LMWH is the therapy of choice in patients with VTE and cancer. Although warfarin has been used for years for the long-term treatment of patients suffering VTE, the optimal duration of treatment after a first episode or recurrent episodes of venous thrombosis remains uncertain. Patients with a first episode of idiopathic DVT require at least 3 to 6 months of anticoagulant treatment, and patients who have a first recurrence require at least 1 to 2 years of anticoagulant treatment. In all cases, the duration of therapy should be reviewed periodically. Because the risk of recurrent VTE continues even after these extended periods of treatment, recommendations have been made for longer periods of treatment, particularly if additional risk factors are present.55 Indeed, current guidelines suggest considering indefinite anticoagulation in appropriate patients.55 The advent of new oral anticoagulants which do not require laboratory monitoring will simplify long-term therapy.
Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133(6 Suppl):381S-453S.
The most recent guidelines from the ACCP for prevention of venous thromboembolism.
Mookadam F, Jiamsripong P, Goel R, Warsame TA, Emani UR, Khandheria BK. Critical appraisal on the utility of echocardiography in the management of acute pulmonary embolism. Cardiol Rev. 2010;18(1):29-37.
A critical review of the utility of echocardiography in the management of acute pulmonary embolism.
Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010, Feb 9. [Epub ahead of print]
Stein P, Hull RD, Patel K, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism. A systematic review. Ann Intern Med. 2004;140(8):589-602.
Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II Investigators. Am J Med. 2006;119(12):1048-1055.
Recommended diagnostic pathways for acute PE from the PIOPED II Investigators.
Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133(6 Suppl):454S-545S.
The most recent ACCP Guidelines for the treatment of VTE.
Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133(6 Suppl):141S-159S.
Warkentin TE, Greinacher A, Koster A, et al. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133(6 Suppl):340S-380S.
Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med. 1999;130(10):800-809.
Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133(6 Suppl):160S-198S.
Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133(6 Suppl):299S-339S.
Young Young T, Tang H, Aukes J, et al. Vena caval filters for the prevention of pulmonary embolism. Cochrane Database Syst Rev 2007;(4):CD0006212.
A Cochrane review of the role of vena caval filters for the prevention of PE.
1 Dismuke SE, Wagner EH. Pulmonary embolism as a cause of death. The changing mortality in hospitalized patient. JAMA. 1986;255(15):2039-2042.
2 Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975;17:259.
3 Anderson FA, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. Arch Intern Med. 1991;151:933.
4 Donaldson GA, Williams C, Scanell J, et al. A reappraisal of the application of the Trendelenburg operation to massive fatal embolism. N Engl J Med. 1963;268:171.
5 Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. (8th ed). Chest. 2008;133(6 suppl):381S.
6 Pineo GF. Prevention of venous thromboembolic disease, Part 1 surgical patients and Part 2 medical patients. In: UpToDate, Basow DS, editor, UpToDate, Waltham, MA, 2010.
7 Rosendaal FR. Risk factors for venous thrombosis: prevalence, risk and interaction. Semin Haematol. 1997;34:171.
8 Heit JA, O’Fallon WM, Peterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162:1245.
9 Bezemer ID, Bare LA, Doggen CJ, et al. Gene variants associated with deep vein thrombosis. JAMA. 2008;299:1306.
10 Simioni P, Prandoni P, Lensing AWA, et al. The risk of recurrent venous thromboembolism in patients with an Arg506-Gln mutation in the gene for factor V (factor V Leiden). N Engl J Med. 1997;336:399.
11 Moser KM, LeMoine JR. Is embolic risk conditioned by location of deep venous thrombosis? Ann Intern Med. 1981;94:439.
12 Hull RD, Hirsh J, Carter CJ, et al. Pulmonary angiography, ventilation lung scanning, and venography for clinically suspected pulmonary embolism with abnormal perfusion lung scan. Ann Intern Med. 1983;98:891.
13 Huisman MV, Buller HR, ten Cate JW, et al. Serial impedance plethysmography for suspected deep-vein thrombosis in outpatients. The Amsterdam general practitioner study. N Engl J Med. 1986;314:823.
14 Nicolaides AN, Kakkar VV, Field ES, et al. The origin of deep vein thrombosis: a venographic study. Br J Radiol. 1971;44:653.
15 Hull RD, Hirsh J, Sackett DL, et al. Clinical validity of a negative venogram in patients with clinically suspected venous thrombosis. Circulation. 1981;64:622.
16 Rabinov K, Paulin S. Roentgen diagnosis of venous thrombosis in the leg. Arch Surg. 1972;104:134.
17 Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med. 2001 May;110(7):515.
18 Hull RD, Marder VJ, Mah AF, et al. Quantitative assessment of thrombus burden predicts the outcome of treatment for venous thrombosis: a systematic review. Am J Med. 2005 May;118(5):456.
19 White RH, Dager WE, Zhou H, et al. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb Haemost. 2006 Sep;96(3):267.
20 Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two chorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004 Jul 1;117(1):19.
21 Moores L, Aujesky D, Jimenez D, et al. Pulmonary Embolism Severity Index and troponin testing for the selection of low-risk patients with acute symptomatic pulmonary embolism. Thromb Haemost. 2010;8:517.
22 Mookadam F, Jiamsripong P, Goel R, et al. Critical appraisal on the utility of echocardiography in the management of acute pulmonary embolism. Cardiol Rev. 2010 Jan-Feb;18(1):29.
23 Kline JA, Steuerwald MT, Marchick MR, et al. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest. 2009 Nov;136(5):1202. [Epub 2009 Jun 19]
24 Stawicki SP, Seamon MJ, Kim PK, et al. Transthoracic echocardiography for pulmonary embolism in the ICU: finding the “right” findings. J Am Coll Surg. 2008;206(1):42. [Epub 2007]
25 Jimenez D, Uresandi F, Otero R, et al. Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism: systematic review and meta-analysis. Chest. 2009;136(4):974. [Epub 2009]
26 Janata KM, Leitner JM, Holzer-Richling N, et al. Troponin T predicts in-hospital and 1-year mortality in patients with pulmonary embolism. Eur Respir J. 2009;34(6):1357. [Epub 2009]
27 Lega JC, Lacasse Y, Lakhal L, et al. Natriuretic peptides and toponins in pulmonary embolism: a meta-analysis. Thorax. 2009;64(10):869. [Epub 2009]
28 Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295(2):199.
29 Stein PD, Sostman HD, Bounameaux H, et al. Challenges in the diagnosis of acute pulmonary embolism. Am J Med. 2008;121(7):565.
30 Perrier A, Roy PM, Aujesky D, et al. Diagnosing pulmonary embolism in outpatients with clinical assessment, D-dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study. Am J Med. 2004;116(5):291.
31 Ceriani E, Combescure C, Le Gal G, et al. Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost. 2010, Feb 9. [Epub ahead of print]
32 Donze J, Le Gal G, Fine MJ, et al. Prospective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolism. Thromb Haemost. 2008 Nov;100(5):943.
33 Klok FA, Mos IC, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008 Oct 27;168(19):2131.
34 Le Gal G, Kovacs MJ, Carrier M, et al. Validation of a diagnostic approach to exclude recurrent venous thromboembolism. J Thromb Haemost. 2009 May;7(5):752. [Epub 2009 Feb 18]
35 Verhovsek M, Douketis D, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med. 2008;149(k7):481. W94
36 Stein P, Hull RD, Patel K, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism. A systematic review. Ann Intern Med. 2004;140:589.
37 Bernardi E, Prandoni P, Lensing AW, et al. D-dimer testing as an adjunct to ultrasonography in patients with clinically suspected deep-vein thrombosis: prospective cohort study. BMJ. 1998;317:1037.
38 Ten Cate-Hoek AJ, Prins MH. Management studies using a combination of D-dimer test result and clinical probability to rule out venous thromboembolism: a systematic review. J Thromb Haemost. 2005;3(11):2465.
39 Kearon C, Ginsberg J, Hirsh J. The role of venous ultrasonography in the diagnosis of suspected deep vein thrombosis and pulmonary embolism. Ann Intern Med. 1998;129:1044.
40 Rathbun S, Whitsett T, Raskob G. Negative D-dimer to exclude recurrent deep-vein thrombosis in symptomatic patients. Ann Intern Med. 2004;141:839.
41 Perrier A, Roy PM, Sanchez O, et al. Multi-detector row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352(17):1760.
42 Stein PD, Fowler SE, Goodman LR, et al. Multi-detector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354(22):2317.
43 Anderson DR, et al. Computed tomographic pulmonary angiography versus ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298:2743.
44 Ragni MV. Pulmonary embolism and spiral computerized tomographic scans. Curr Opin Pulm Med. 2009;15(5):430.
45 PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism: Results of the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED). JAMA. 1990;263:2753.
46 Hull RD, Raskob GE, Coates G, et al. Clinical validity of a normal perfusion lung scan inpatients with suspected pulmonary embolism. Chest. 1990;97:23.
47 Hull RD, Raskob GE, Ginsberg JS, et al. A noninvasive strategy for the treatment of patients with suspected pulmonary embolism. Arch Intern Med. 1993;154:289.
48 Meaney JFM, Weg JG, Chenevert TL, et al. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med. 1997;336:1422.
49 Sampson FC, Goodacre SW, Thomas SM, et al. The accuracy of MRI in diagnosis of suspected deep vein thrombosis: systematic review and meta-analysis. Eur Radiol. 2007;17(1):175-181. [Epub 2006]
50 Hull RD, Raskob GE, Coates G, et al. A new non-invasive management strategy for patients with suspected acute pulmonary embolism. Arch Intern Med. 1989;149:2549.
51 Turkstra F, Kuijer P, van Beck EJ, et al. Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med. 1997;126:775.
52 Van Strijen M, de Monye W, Schiereck J, et al. Single-detector helical computed tomography as the primary diagnostic test in suspected pulmonary embolism: A multicenter clinical management study of 510 patients. Ann Intern Med. 2003;138:307.
53 Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II Investigators. Am J Med. 2006;119(12):1048.
54 Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med. 1979;301:855.
55 Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians Evidence-based Clinical Practice Guidelines. (8th ed). Chest. 2008;133(6 suppl):454S.
56 Prandoni P, Kahn S. Post-thrombotic syndrome: prevalence, prognostication and need for progress. Br J Haematol. 2009;145(3):286.
57 Kahn SR. Post-thrombotic syndrome after deep venous thrombosis: risk factors, prevention, and therapeutic options. Clin Adv Hematol Oncol. 2009 Jul;7(7):433.
58 Prandoni P, Lensing AWA, Prins MH, et al. Below knee elastic compression stockings to prevent the post-thrombotic syndrome; a randomized controlled trial. Ann Intern Med. 2004;141(4):249.
59 Pengo V, Lensing A, Prins M, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257.
60 Poli D, Grifoni E, Antonucci E, et al. Incidence of recurrent venous thromboembolism and of chronic thromboembolic pulmonary hypertension in patients after a first episode of pulmonary embolism. J Thromb Thrombolysis. 2010 Feb 16. [Epub ahead of print]
61 Lang I. Advances in understanding the pathogenesis of chronic thromboembolic pulmonary hypertension. Br J Haematol. 2010 Mar 16. [Epub ahead of print]
62 Hirsh J, Bauer KA, Donati MB, et al. Parenteral Anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. (8th ed). Chest. 2008;133(6)):141S-159S.
63 Lane DA. Heparin binding and neutralizing protein. In: Lane DA, Lindahl U, editors. Heparin, Chemical and Biological Properties, Clinical Applications. London: Edward Arnold, 1989. 189, 363
64 Rosenberg RD, Lam L. Correlation between structure and function of heparin. Proc Natl Acad Sci U S A. 1979;76:1218.
65 Hull RD, Raskob GE, Rosenbloom D, et al. Heparin for 5 days as compared with 10 days in the initial treatment of proximal venous thrombosis. N Engl J Med. 1990;322:1260.
66 Hull RD, Raskob GE, Rosenbloom D, et al. Optimal therapeutic level of heparin therapy in patients with venous thrombosis. Arch Intern Med. 1992;152:1589.
67 Hull RD, Raskob GE, Brant RF, et al. The relation between the time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep-vein thrombosis. Arch Intern Med. 1997;157:1562.
68 Raschke RA, Reilly BM, Guidry JR, et al. The weight based heparin dosing nomogram compared with a “standard care” nomogram. Ann Intern Med. 1993;119:874.
69 Raschke R, Hirsh J, Guidry JR. Suboptimal monitoring and dosing of unfractionated heparin in comparative studies with low-molecular-weight heparin. Ann Int Med. 2003;138:720.
70 Van Den Belt AG, Prins MH, Lensing AW, et al. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2004;(4):CD001100.
71 Faivre R, Neuhart Y, Kieffer Y, et al. Un nouveau traitement des thromboses vein euses profondes: les fractions d’heparine de bas poids moleculaire. Etude randomisee. Presse Medicale. 1988;17:197.
72 Lopaciuk S, Meissner AJ, Filipecki S, et al. Subcutaneous low molecular weight heparin versus subcutaneous UFH in the treatment of DVT: a Polish multicenter trial. Thromb Haemost. 1992;68:14.
73 Belcaro G, Nicolaides AN, Cesarone MR, et al. Comparison of low-molecular-weight heparin, administered primarily at home, with UFH, administered in hospital, and subcutaneous heparin, administered at home for deep-vein thrombosis. Angiology. 1999;50:781.
74 Writing Committee for the Galilei Investigators. Subcutaneous adjusted-dose UFH vs fixed-dose low-molecular-weight heparin in the initial treatment of venous thromboembolism. Arch Intern Med. 2004;164:1077.
75 Prandoni P, Bagatella P, Bernardi E, et al. Use of an algorithm for administering subcutaneous heparin in the treatment of deep vein thrombosis. Ann Intern Med. 1998;129:299.
76 Warkentin TE. Review. Heparin-Induced thrombocytopenia: Pathogenesis and management. Br J Haematol. 2003;121:535.
77 Warkentin TE, Greinacher A, Koster A, et al. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. (8th ed). Chest. 2008;133(6 Suppl):340S-380S.
78 Amiral J, Peynaud-Debayle E, Wolf M, Bridley F, et al. Generation of antibodies to heparin-PF4 complexes without thrombocytopenia in patients treated with unfractionated or low-molecular-weight heparin. Am J Hematol. 1996;52:90.
79 Ahmad S, Untch B, Haas S, et al. Differential prevalence of anti-heparin-PF4 immunoglobulin subtypes in patients treated with Clivarine and heparin: implications in the HIT pathogenesis. Mol Cell Biochem. 2004;258:163.
80 Gruel Y, Pouplard C, Nguyen P, et al. and the French heparin-induced thrombocytopenia study group. Biological and clinical features of low-molecular-weight heparin-induced thrombocytopenia. Br J Haematol. 2003;121:786.
81 Girolami B, Prandoni P, Stefani PM, et al. The incidence of heparin-induced thrombocytopenia in medical patients treated with low molecular weight heparin: a prospective cohort study. Blood. 2003;101:2955.
82 Rice L, Attisha WK, Drexler A, et al. Delayed-onset heparin-induced thrombocytopenia. Ann Intern Med. 2002;136:210.
83 Warkentin TE. An overview of the heparin-induced thrombocytopenia syndrome. Semin Thromb Hemost. 2004;30:273.
84 Wirth SM, Macaulay TE, Armitstead JA, et al. Evaluation of a clinical scoring scale to direct early appropriate therapy in heparin-induced thrombocytopenia. J Oncol Pharm Pract. 2009. [Epub]
85 Hirsh J, Heddie N, Kelton JG. Treatment of heparin-induced thrombocytopenia: a critical review. Arch Intern Med. 2004;164:361.
86 Lewis BE, Wallis DE, Leya F, et al. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Arch Intern Med. 2003;163:1849.
87 Matthai WHJr, Hursting MJ, Lewis BE, et al. Argatroban anticoagulation in patients with a history of heparin-induced thrombocytopenia. Thromb Res. 2005;116:121.
88 Greinacher A, Eichler P, Lubenow N, et al. Heparin-induced thrombocytopenia with thromboembolic complications: meta-analysis of two prospective trials to assess the value of parenteral treatment with lepirudin and its therapeutic aPTT range. Blood. 2000;96:846.
89 Call JT, Deliargyris EN, Sane DC. Direct thrombin inhibitors in the treatment of immune-mediated heparin-induced thrombocytopenia. Semin Thromb Hemost. 2004;30:297.
90 Greinacher A, Eichler P, Albrecht D, et al. Antihirudin antibodies following low-dose subcutaneous treatment with desirudin for thrombosis prophylaxis after hip-replacement surgery: incidence and clinical relevance. Blood. 2003;101:2617.
91 Greinacher A, Lubenow N, Eichler P. Anaphylactic and anaphylactoid reactions associated with lepirudin in patients with heparin-induced thrombocytopenia. Circulation. 2003;108:2062.
92 Harenberg J, Jorg I, Fenyvesi T, et al. Treatment of patients with a history of heparin-induced thrombocytopenia and anti-lepirudin antibodies with argatroban. J Thromb Thrombolysis. 2005;19:65.
93 Savi P, Chong BH, Greinacher A, et al. Effect of fondaparinux on platelet activation in the presence of heparin-independent antibodies: a blinded comparative multicenter study with unfractionated heparin. Blood. 2005;105:139.
94 Kuo KHM, Kovacs MJ. Successful treatment of heparin induced thrombocytopenia (HIT) with fondaparinux. Thromb Haemost. 2005;93:999.
95 Barrowcliffe TW, Curtis AD, Johnson EA, et al. An international standard for low molecular weight heparin. Thromb Haemost. 1988;60:1.
96 Weitz JI. Low molecular weight heparins. N Engl Med. 1997;337:688.
97 Hull RD, Raskob GE, Pineo GF. Subcutaneous low molecular weight heparin compared with continuous intravenous heparin in the treatment of proximal vein thrombosis. N Engl J Med. 1992;326:975.
98 Prandoni P, Lensing AW, Buller HR, et al. Comparison of subcutaneous low molecular weight heparin with intravenous standard heparin in proximal deep vein thrombosis. Lancet. 1992;339:441.
99 Simonneau G, Charbonnier B, Decousus H, et al. Subcutaneous low molecular weight heparin compared with continuous intravenous unfractionated heparin in the treatment of proximal deep vein thrombosis. Arch Intern Med. 1993;153:1541.
100 Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N Engl J Med. 1998;338:409.
101 Levine M, Gent M, Hirsh J, et al. A comparison of low molecular weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep vein thrombosis. N Engl J Med. 1996;334:677.
102 Koopman MMW, Prandoni P, Piovella F, et al. Treatment of venous thrombosis with intravenous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low molecular weight heparin administered at home. N Engl J Med. 1996;334:682.
103 The Columbus Investigators. Low-molecular weight heparin in the treatment of patients with venous thromboembolism. N Engl J Med. 1997;337:657.
104 Simonneau G, Sors H, Charbonnier B, et al. A comparison of low molecular weight heparin with unfractionated heparin for acute pulmonary embolism. N Engl J Med. 1997;337:663.
105 Hull RD, Raskob GE, Brant RF, et al. Low-molecular-weight heparin versus heparin in the treatment of patients with pulmonary embolism. American-Canadian Thrombosis Study Group. Arch Intern Med. 2000;160:229.
106 Gould MK, Dembitzer AD, Doyle RL, et al. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med. 1999;130:800.
107 Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710.
108 Shaghnessy SG, Young E, Deschamps P, et al. The effects of low molecular weight and standard heparin on calcium loss from fetal rat calvaria. Blood. 1995;86:1368.
109 Van Der Heijden JF, Hutten BA, Buller HR, et al. Vitamin K1 antagonists or low-molecular-weight heparin for the long term treatment of symptomatic venous thromboembolism. Cochrane Database Syst Rev 2000;(4):CD002001.
110 Lee A, Levine M, Baker R, et al. Low-molecular-weight heparin versus Coumadin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146.
111 Hull R, Pineo G, Brant R, et al. Long-term low-molecular weight heparin versus usual care in proximal vein thrombosis patients with cancer. Am J Med. 2006;119:1062.
112 Freedman MD. Oral anticoagulants: pharmacodynamics, clinical indication and adverse effects. J Clin Pharmacol. 1992;32:196.
113 Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. (8th ed). Chest. 2008;133(6 Suppl):160S-198S.
114 Wessler A, Gitel SN. Warfarin: From bench to bedside. N Engl J Med. 1984;311:645.
115 Zivelin A, Rao LV, Rapaport SI. Mechanism of the anticoagulant effect of warfarin as evaluated in rabbits by selective depression of individual procoagulant vitamin K-dependent clotting factors. J Clin Invest. 1993;92:2131.
116 Crowther MA, Ginsberg J, Kearon C, et al. A randomized trial comparing 5 mg and 10 mg warfarin loading doses. Arch Intern Med. 1999;159:46.
117 Wells PS, LeGal G, Tierney S, et al. Practical application of the 10 mg warfarin initiation nomogram. Blood Coagul Fibrinolysis. 2009;20(6):403.
118 Holbrook AM, Pereira JA, Labiris R, et al. systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095.
119 Poller L, Taberner DA. Dosage and control of oral anticoagulants: an international collaborative survey. Br J Haematol. 1982;51:479.
120 Hull RD, Hirsh J, Jay R, et al. Different intensities of oral anticoagulant therapy in the treatment of proximal vein thrombosis. N Engl J Med. 1982;307:1676.
121 Beyth RJ, Quinn L, Landefeld CS. A multicomponent intervention to prevent major bleeding complications in older patients receiving warfarin. A randomized, controlled trial. Ann Intern Med. 2000;133:687.
122 Hylek EM, Regan S, Go AS, Hughes RA, Singer DE, Skates SJ. Clinical predictors of prolonged delay in return of the international normalized ratio to within the therapeutic range after excessive anticoagulation with warfarin. Ann Intern Med. 2001;135:393.
123 Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333:11.
124 Fihn SD, McDonnell M, Martin D, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann Intern Med. 1993;118:511.
125 Henderson MC, White RH. Anticoagulation in the elderly. Curr Opin Pulm Med. 2001;7:365.
126 Palareti G, Legnani C, Lee A, et al. A comparison of the safety and efficacy of oral anticoagulation for the treatment of venous thromboembolic disease in patients with or without malignancy. Thromb Haemost. 2000;84:805.
127 Crowther MA, Julian J, McCarty D, et al. Treatment of warfarin-associated coagulopathy with oral vitamin K: a randomized controlled trial. Lancet. 2000;356:1551.
128 Ageno W, Crowther M, Steidl L, et al. Low dose oral vitamin K to reverse acenocoumarol-induced coagulopathy: a randomized controlled trial. Thromb Haemost. 2002;88(1):48.
129 Kovacs MJ, Kearon C, Rodger M. Single-arm study of bridging therapy with low-molecular-weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin. Circulation. 2004;110:1658.
130 Spyropoulos AC, Turpie AG, Dunn AS, et al. Clinical outcomes with unfractionated heparin or low-molecular-weight heparin as bridging therapy in patients on long-term oral anticoagulants: the REGIMEN registry. J Thromb Haemost. 2006;4(6):1246.
131 Dunn AS, Turpie AGG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med. 2003;163:901.
132 Garcia DA, Regan S, Henault LE, et al. risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med. 2008;168(1):63.
133 Bajkin BV, Popovic SL, Selakovic SD. Randomized, prospective trial comparing bridging therapy using low-molecular-weight heparin with maintenance of oral anticoagulation during extraction of teeth. Oral Maxillofac Surg. 2009;67(5):990.
134 Ahmed I, Gertner E, Melson WB, et al. Continuing warfarin therapy is superior to interrupting warfarin with or without bridging anticoagulation therapy in patients undergoing pacemaker and defibrillator implantation. Heart Rhythm. 2010. [Epub ahead of print]
135 Douketis JD, Berger PB, Dunn AS, et al. The Perioperative Management of Antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. (8th ed). Chest. 2008;133(6):299S.
136 Ridker P, Goldhaber SZ, Danielson E, et al. Long-term low-intensity warfarin therapy with conventional-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348:1425.
137 Kearon C, Ginsberg JS, Kovacs MH, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349:631.
138 Crowther MA, Ginsberg JS, Kovacs MH, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med. 2003;349:1133.
139 Hull RD, Delmore TJ, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med. 1979;301:855.
140 Hull RD, Delmore TJ, Carter C, et al. Adjusted subcutaneous heparin versus warfarin sodium in the long-term treatment of venous thrombosis. N Engl J Med. 1982;306:189.
141 O’Donnell M, Hirsh J. Establishing an optimum therapeutic range for coumarins, filling in the gaps. Arch Intern Med. 2004;164:588.
142 Research Committee of the British Thoracic Society. Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Lancet. 1992;340:873.
143 Schulman S, Rhedin A-S, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. N Engl J Med. 1995;332:1661.
144 Levine M, Hirsh J, Gent M, et al. Optimal duration of oral anticoagulant therapy: a randomized trial comparing four weeks with three months of warfarin in patients with proximal deep-vein thrombosis. Thromb Haemost. 1995;74:606.
145 Schulman S, Granqvist S, Holmström M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. N Engl J Med. 1997;336:393.
146 Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first-episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901.
147 Agnelli G, Prandoni P, Santamaria M, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep-venous thrombosis. N Engl J Med. 2001;345:165.
148 Campbell IA, Bentley DP, Prescott RJ, et al. Anticoagulation for three versus six months in patients with deep vein thrombosis or pulmonary embolism, or both: randomized trial. BMJ. 2007;334(7595):674. [Epub 2007]
149 Prandoni P, Lensing A, Prins M, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med. 2002;137:955.
150 Young L, Ockelford P, Milne D, et al. Post-treatment residual thrombus increases the risk of recurrent deep vein thrombosis and mortality. J Thromb Haemost. 2006;4(9):1919.
151 Siragusa S, Malato A, Anastasio R, et al. Residual vein thrombosis to establish duration of anticoagulation after a first episode of deep vein thrombosis: the Duration of Anticoagulation based on Compression UltraSonography (DACUS) study. Blood. 2008;179(5):417.
152 Palareti G, Cosmi B, Vigano D’Angelo S, et al. D-dimer testing to determine the duration of anticoagulant therapy. N Engl J Med. 2006;355:1780.
153 Cosmi B, Legnani C, Lorio A, et al. Residual venous obstruction, alone and in combination with D-dimer, as a risk factor for recurrence after anticoagulation withdrawal following a first idiopathic deep vein thrombosis in the prolong study. Eur J Vasc Endovasc Surg. 2010 Mar;39(3):356. [Epub 2010 Jan 19]
154 Kyrle P, Minar E, Bialonczyk, et al. The risk of recurrent venous thromboembolism in men and women. N Engl J Med. 2004;350:2558.
155 Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. 2008;179(5):417.
156 Büller HR, Davidson BL, Decousus H, et al. Fondaparinux or Enoxaparin for the Initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004;140(11):867.
157 Büller HR, Davidson BL, Decousus H, et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349(18):1695.
158 The van Gogh Investigators. Idraparinux versus standard therapy for venous thromboembolic disease. N Engl J Med. 2007;357:1094.
159 The van Gogh Investigators. Extended prophylaxis of venous thromboembolism with idraparinux. N Engl J Med. 2007;357:1105.
160 Turpie AG, Lassen MR, Davidson Bl, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomized trial. Lancet. 2009;373(9676):1673. [Epub 2007]
161 Lassen MR, Ageno W, Borris LC, et al. Rovaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26):2776.
162 Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765.
163 Eriksson BI, Friedman R. dabigatran Etexilate: Pivotal trails for venous thromboembolism prophylaxis after hip or knee arthroplasty. Clin Appl Thromb Haemost. 2009. [Epub 2007]
164 Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(18):594.
165 Lassen MR, RaAskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomized double-blind trial. Lancet. 2010;375(9717):779.
166 Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342.
167 Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: Randomized trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993;341:507.
168 Dong BR, Hao Q, Yue J, et al. Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev 2009;(3):CD004437.
169 Kuo WT, Gould MK, Louie JD, et al. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20(11):1431.
170 Mismetti P, Rivron-Guillot K, Quenet S, et al. A prospective long-term study o 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism. Chest. 2007;131(1):223.
171 Young T, Tang H, Aukes J, et al. Vena caval filters for the prevention of pulmonary embolism; Cochrane Database Syst Rev 2007;(4):CD0006212.
172 Tschoe, Kim HS, Brotman DJ, et al. Retrievable vena cava filters: a clinical review. J Hosp Med. 2009;4(7):441.