Chapter 41 Pulmonary Embolic Disease and Cardiac Tumors
Epidemiology and Risk Factors
PE results from the migration of thrombi formed in the systemic venous system, usually from the pelvis or lower extremities, into the pulmonary arterial system.1,2 These thrombi form as a result of abnormal veins, abnormal blood flow, or abnormalities of the coagulation system. Once formed, fragments of thrombi may become detached from their site of origin as a result of the ongoing coagulation cascade or due to local manipulation. These migrating fragments then become lodged in the pulmonary arterial tree and result in decreased oxygenation capacity in the lungs.
Thrombus formation in abnormal veins may be the result of trauma, iatrogenic manipulation such as catheter access, or venous compression or invasion by tumor.1,2 The presence of a catheter or other foreign body within the vein also predisposes to thrombus formation. Inflammation that involves the vein wall, thrombophlebitis, also produces thrombosis of the vein. The presence of a large thrombus in the vein can also incite an inflammatory reaction in the vein wall and surrounding soft tissues.
Decreased or turbulent flow within veins may instigate thrombus formation. This can be caused by extrinsic compression by a mass lesion or by prolonged positional obstruction of the veins such as in long-distance travel.1,2 In addition, prolonged immobility particularly of the lower extremities results in reduced extrinsic pump function that is normally performed by muscular movements. This results in decreased venous flow, edema, and potential thrombus formation in the affected extremity. Turbulent flow can also contribute to thrombus formation. This can be seen related to foreign bodies such as catheters that may cause thrombosis through chronic inflammation of the vein wall and direct triggering of the coagulation pathways along the surface of the catheter. Central venous catheters are a significant risk factor for thrombus formation with up to 4% of patients with central venous catheters developing catheter-related thrombus.3 The majority of upper extremity DVTs are associated with catheters.4
Abnormalities in the coagulation system are another etiology.1,2 These include various coagulation factor deficiencies or protein abnormalities such as protein C deficiency or Factor V (Leiden factor) abnormalities. Many drugs can alter the coagulation pathway in favor of thrombus formation. This is well known with use of oral contraceptives but also occurs with a variety of chemotherapeutic agents. Examples include methotrexate- and doxorubicin-based regimens as well as thalidomide or its relatives. In addition, hematopoietic stem cell–stimulating agents can also contribute to thrombus formation. Other abnormalities of the hematopoietic system—such as elevated erythrocyte or platelet formation—or abnormalities in the formation of either of these cell lines can also contribute to thrombus formation.
The presence of cancer alone is also associated with a hypercoagulable state. Venous thromboembolism rates are highest with patients on chemotherapy with an incidence as high as 10% in patients with ovarian cancer or lymphoma on therapy and up to 28% in patients with malignant gliomas.3 The highest rates of thrombus formations appear to be in patients with mucinous tumors. However, the frequency of presentation of venous thromboembolism is more closely related to the frequency of the tumor in the population. Most cases of venous thromboembolism in cancer patients are seen in patients with lung, colon, and prostate cancer.
Particular therapies are associated with thrombus formation. Combination therapies including antiangiogenic drugs such as thalidomide are particularly implicated. Thalidomide combination therapy in patients with myeloma has a venous thromboembolism rate approaching 30%, whereas in renal cell cancer, the venous thromboembolism rate is over 40% with this agent.3
Cancer patients are also at greater risk for poor outcomes after PE. For example, they are four to eight times more likely to die after a PE event.3 The 1-year survival for patients with cancer and venous thromboembolism is about one third that of patients with cancer without venous thromboembolism.
Anatomy
DVTs occur most often in the pelvic and lower extremity veins.1,2 Thrombi that form in the upper extremities are more likely to be related to prolonged catheter presence, catheter-related thrombophlebitis, or direct vein involvement by a tumor. Because they are formed within the lumen of vessels, the fragments that are detached are typically tubular in shape. Their size can vary from less than 1 mm in diameter up to 1 or 2 cm in diameter. The larger-diameter thrombi may tend to be longer as well and may have branching components.
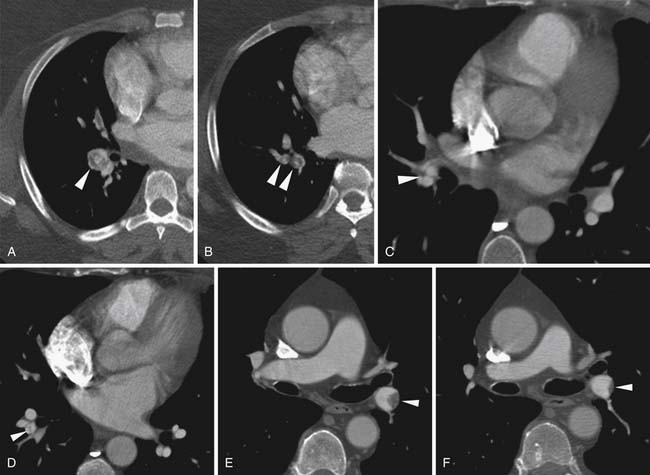
Once they are detached, emboli flow through the venous system until they encounter an impeding structure or reach a vessel that is too small to permit their passage. Most commonly, emboli reach and are captured in the pulmonary arterial system. Occasionally, emboli may be captured en route to the pulmonary arterial system, most commonly within the right atrium.5–7 This may occur as emboli become entangled in trabeculations in the right ventricle or right atrial appendage. A congenital Chiari network located at the base of the right atrium between the coronary sinus orifice and the tricuspid valve may entangle a flowing embolus.8 Emboli have also been identified passing through a patent foramen ovale. These “thrombi in transit” can lead to paradoxical emboli to systemic arterial structures including the brain causing embolic strokes.
The pulmonary arterial tree is a branching structure with progressively smaller branches. The main pulmonary artery bifurcates into the right and left pulmonary arteries. The right pulmonary artery has three lobar branches and the left has two lobar branches. Each of these branches into two or more segmental branches, and the branching patterns continue out to the periphery of the lung. Emboli that reach the lungs become lodged at arterial branch points or may reach an arteriole of lesser diameter than the embolus (Figure 41-1).
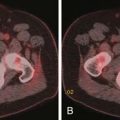
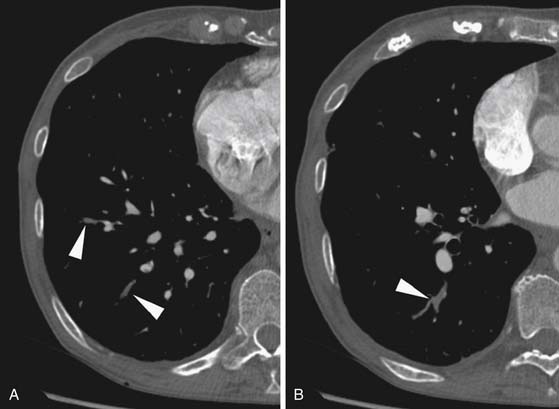
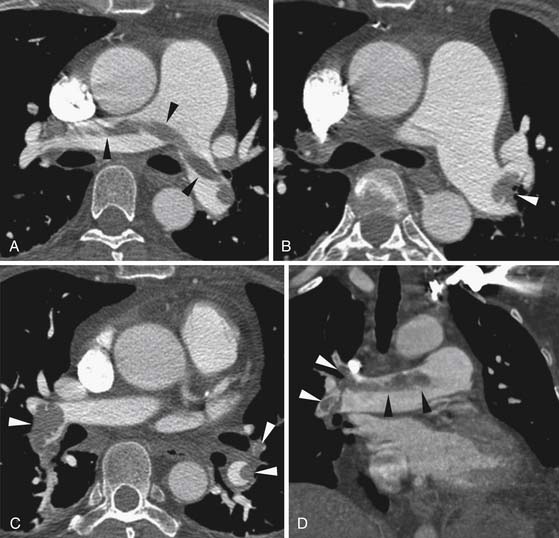
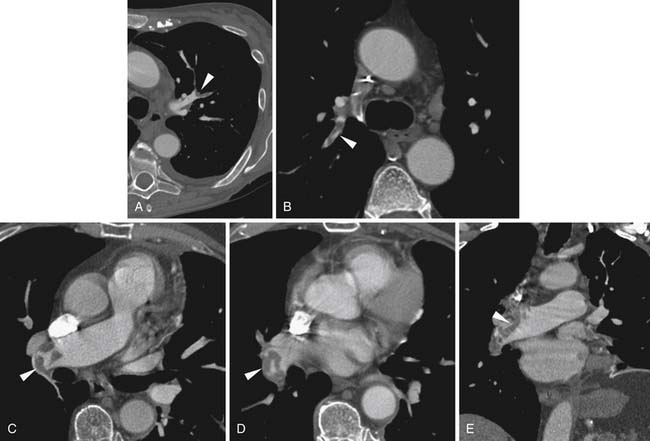
Larger and more elongated emboli will commonly be captured at branch points and may extend into multiple lobes and segments of the pulmonary arterial tree. The largest PEs may become lodged across the main pulmonary artery bifurcation and are called saddle emboli (Figure 41-2). Usually, there is a small bandlike component extending across the pulmonary artery bifurcation with larger components of thrombus extending into the right and left pulmonary arteries and lobar or segmental branches. Smaller emboli may become lodged across branch points at lobar, segmental, or subsegmental levels (Figure 41-3), and still smaller emboli become lodged as they reach subsegmental and smaller arterioles.
If the embolus completely occludes a segment of the pulmonary arterial system, it can result in infarction of the lung peripheral to that embolus.9 This is relatively uncommon because the lung has a parallel blood supply from the bronchial arteries. Pulmonary ischemia or infarction results in hemorrhage and edema within the affected portion of lung. The involved area typically extends out in a wedge-shaped pattern from the occluded arteriole and reaches the pleural surface (Figure 41-4). The hemorrhagic phase is followed by necrosis and coagulation of the underlying lung architecture. The infarct typically evolves over several days to weeks and may resolve completely or result in a small area of scarring.
Key Points Anatomy
• Emboli are typically elongated “casts” of the vein of origin.
• Emboli tend to become lodged at branch points or become wedged in smaller-caliber arteries.
• Emboli can become entrapped in transit through the right heart chambers or pass through a patent foramen causing systemic embolism.
• Wedge-shaped consolidation peripheral to an occluded vessel represents pulmonary ischemia or infarction and involutes over time.
Clinical Presentation
DVT may be completely asymptomatic. When symptomatic, DVT may present with a variety of nonspecific symptoms including asymmetrical lower extremity swelling and, rarely, an erythematous tender mass along the venous structure.10 Upper extremity thrombosis may also result in asymmetrical edema in the arm, neck, or face.
PEs themselves may be asymptomatic or cause nonspecific symptoms.10,11 The acute onset of dyspnea is the most suggestive symptom and may be accompanied by a sense of impending doom. Pleuritic chest pain also occurs. Larger emboli can result in right heart strain with its symptoms including systemic edema and abnormalities at cardiac auscultation.
Clinical history is helpful in suggesting PE.1,10,11 Any recent period of prolonged immobilization, such as during long travel by automobile or aircraft or after surgery, should suggest the possibility of DVT. A history of cancer or chemotherapy use, particularly those cancers and drugs most associated with thrombosis, also should raise a suspicion of PE in patients with new dyspnea.
Patients with PE often have nonspecific laboratory abnormalities.1,2,10,11 In particular, D-dimer levels are usually elevated. This is a very sensitive but nonspecific finding because cancer patients commonly have abnormalities of D-dimer levels related to their tumor. Coagulation measurements such as prothrombin time, partial thromboplastin time, and the International Normalized Ratio may be abnormal related to the cause of thrombus formation rather than a result of the thrombus or embolus itself. PEs and DVT are more commonly seen in patients with elevated erythrocyte counts and hemoglobin levels as well as in patients with platelet abnormalities of number or function. Arterial blood gas may show an increased difference between alveolar and arterial oxygen concentration called an increased A-a gradient. Pulse oximetry is also often abnormal. Most of these findings are nonspecific and indicate only an abnormality of pulmonary oxygenation, which might be due to pneumonia, embolism, or tumor, among other possibilities.
PEs may be detected on routine imaging of cancer patients without suspicion of PE.12–17 In patients without cancer, PEs are detected in 1% or fewer of the outpatient population and up to 3% of inpatients. Among patients with cancer, approximately 3% to 4% of outpatients and over 6% of inpatients will have unsuspected PE at routine CT scanning. These frequently go undetected because the embolism is not the focus of the examination. Up to 50% to 75% of these will not be seen at clinical interpretation.12,13,16 Even large emboli may be unobserved, particularly in patients with complex presentations of their underlying malignancy. If the PEs are small, subsegmental or smaller, their significance is unknown. Some studies have shown relatively low recurrence rates, particularly compared with the complication rates for therapy, in patients with isolated subsegmental PEs.12,18 These recurrence rates approach that of negative CT pulmonary angiography. They are much less than the recurrence and death rates for patients treated for PE (~8% and 2%, respectively).18 Other studies have shown that anticoagulation for unsuspected PE improves survival.19 Further study is needed to determine the appropriate treatment of isolated subsegmental PEs in particular and unsuspected, asymptomatic PEs in general.
Severity Assessment
Several scoring systems for the extent of pulmonary embolic disease have been proposed. They are generally derived from scoring systems initially proposed for catheter-directed pulmonary angiography.20 The scoring system by Mastora and coworkers21 counts the number of pulmonary arterial segments affected and also modifies the score for the degree of obstruction for each segment. A related scoring system by Qanadli and colleagues22 does not include the degree of obstruction. Both scoring systems correlate with short-term outcomes and the need for intensive care unit admissions.21–26 Assessment of the most proximal level of involvement has also been used as a rating for the severity of embolic disease. The simplified assessment of whether or not the embolism is a saddle embolism has some long-term prognostic value in the assessment of cancer patients.27 Despite the value of these assessments for the extent of PE involvement, the mere presence of emboli is predictive of future embolic events.1 This intermediate- to long-term prognosis is not greatly affected by the severity of the current episode of PE.
The risk of death from an acute episode of PE depends on the hemodynamic state of the patient. Patients with cardiac shock have a high risk of death, normotensive patients with right heart dysfunction have an intermediate risk, and patients without heart dysfunction do not have a high risk.28 Whereas cardiac shock is diagnosed clinically, right heart dysfunction may be evaluated with imaging.
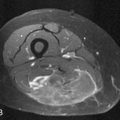
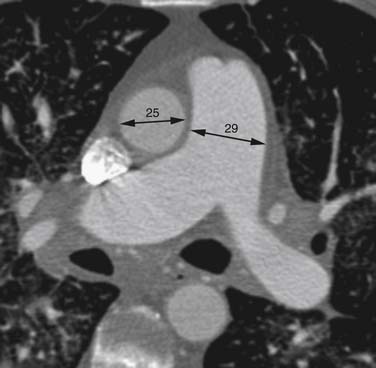
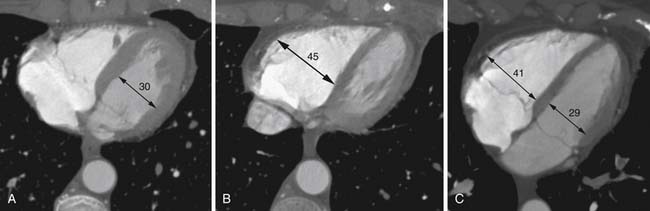
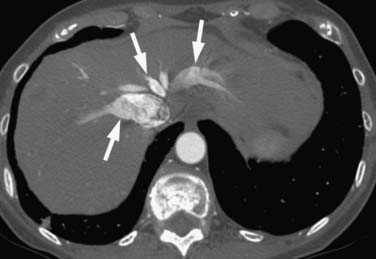
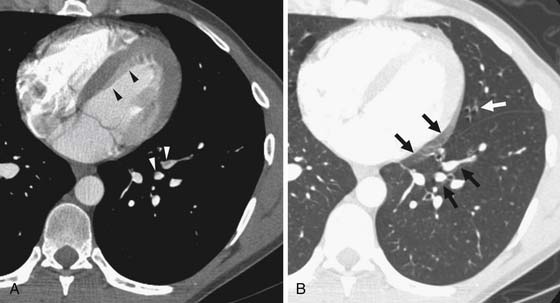
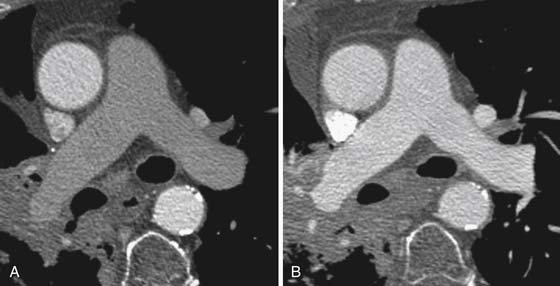
Right heart dysfunction as a result of PE is represented in the development of pulmonary hypertension, right heart strain, and right heart failure. Assessment of these parameters at echocardiography is predictive of survival to hospital discharge and the need for intensive care unit admission.1,2,29 Similar assessments can be done with CT and are also predictive of mortality.23,30–32 This may include simple measurements of the pulmonary artery diameter, which should be less than 33 mm33,34 and less than the aortic diameter (Figure 41-5), and also measurement of the ratio of the right ventricle to left ventricle diameter, which should be less than 1.5 in patients without right heart strain or right heart failure (Figure 41-6).35 However, although pulmonary artery measurements reflect pulmonary hypertension, their value as predictors of PE outcomes had varied.36 If cardiac-gated CT is used, these measurements can be obtained more accurately and additional measurements are also possible.37 Using gated CT, right ventricular ejection fraction compared with left ventricular ejection fraction and pulsatility index of the pulmonary artery can be used to identify decreased right heart function and pulmonary hypertension. However, these measurements have not been thoroughly assessed for their utility in guiding therapy or for their prognostic ability. Secondary signs of right heart strain and failure are less specific but include reflux of contrast material into the inferior vena cava (IVC) and hepatic veins and evidence of systemic edema including hepatic congestion and subcutaneous edema (Figure 41-7).38
Key Points Severity assessment
• Scoring systems reflect the number of lung segments involved by emboli and predict the need for intensive management.
• Right heart strain, reflected in right ventricle or pulmonary artery dilatation, also predicts survival and need for more intensive management.
• Identifying inciting factors is critical in long-term management.
Imaging
Traditionally, imaging for PE was previously done with chest x-ray and ventilation-perfusion (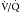 ) scanning. Unfortunately, these methods are nonspecific and often do not provide a final diagnosis. Imaging now focuses on CT pulmonary angiography, which allows direct and noninvasive imaging of the pulmonary arterial tree. Imaging for DVT is predominantly done with ultrasonography. Venous phase imaging in the lower extremity veins after CT pulmonary angiography has also been performed, but its radiation dose is high, especially relative to the nonionizing ultrasound examination.39
) scanning. Unfortunately, these methods are nonspecific and often do not provide a final diagnosis. Imaging now focuses on CT pulmonary angiography, which allows direct and noninvasive imaging of the pulmonary arterial tree. Imaging for DVT is predominantly done with ultrasonography. Venous phase imaging in the lower extremity veins after CT pulmonary angiography has also been performed, but its radiation dose is high, especially relative to the nonionizing ultrasound examination.39
Chest Radiography
Chest radiography usually demonstrates some abnormality, but normal examinations are not uncommon.11,40 The most common abnormal findings are focal pulmonary opacities and small pleural effusions, which can be seen with many etiologies of dyspnea.1 Other findings on chest x-ray are also nonspecific and less common. These include a Hampton hump, a peripheral pleural-based wedge-shaped opacity; the Fleischner sign, widening of the pulmonary artery on the side of the embolism; and the Westermark sign, oligemia in the distribution distal to the PE. The Hampton hump sign is relatively nonspecific and similar opacities may be seen in any cause of consolidation, such as atelectasis or pneumonia. When present in PE, it represents ischemia and infarction of the affected lung parenchyma. The Fleischner sign and the Westermark sign, which may occur together, are relatively poor predictors of PE because both are at least as common in patients without emboli as in those with emboli. In the case of large PEs that cause right heart strain, cardiac silhouette enlargement may occur.
Ventilation-Perfusion Scanning
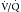 scanning was long the standard for the initial detection of pulmonary embolic disease.41 Because it images pulmonary perfusion and ventilation rather than directly imaging the emboli, it is highly sensitive but not very specific for the identification of emboli. However, several large trials established the value of
scanning was long the standard for the initial detection of pulmonary embolic disease.41 Because it images pulmonary perfusion and ventilation rather than directly imaging the emboli, it is highly sensitive but not very specific for the identification of emboli. However, several large trials established the value of 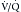 scanning in the identification of very high risk and very low risk patients, allowing their management without further imaging. Unfortunately, many patients fall into the intermediate or indeterminate probability group and should have further imaging performed.1 At the time of development of
scanning in the identification of very high risk and very low risk patients, allowing their management without further imaging. Unfortunately, many patients fall into the intermediate or indeterminate probability group and should have further imaging performed.1 At the time of development of 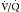 scanning, this further evaluation was conventional pulmonary arteriography. Because of perceived risks and availability issues, patients with intermediate or indeterminate probability of PE on
scanning, this further evaluation was conventional pulmonary arteriography. Because of perceived risks and availability issues, patients with intermediate or indeterminate probability of PE on 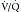 scanning were often managed without a final diagnostic determination of the presence of emboli. Currently, with the widespread use of CT pulmonary angiography for the evaluation of PE, the use of
scanning were often managed without a final diagnostic determination of the presence of emboli. Currently, with the widespread use of CT pulmonary angiography for the evaluation of PE, the use of 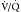 scanning has decreased considerably. However, it is an important alternative in some patients, particularly those with severe renal function compromise or significant contrast allergy history.
scanning has decreased considerably. However, it is an important alternative in some patients, particularly those with severe renal function compromise or significant contrast allergy history.
Interpretation of 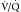 scans depends principally on the identification of perfusion defects without matched ventilation defects.41 Patients are categorized into the risk level for PE. High probability is indicated by two or more segments of
scans depends principally on the identification of perfusion defects without matched ventilation defects.41 Patients are categorized into the risk level for PE. High probability is indicated by two or more segments of 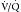 mismatch, which is a perfusion defect larger than the ventilation defect or no ventilation defect. Patients have very low probability for emboli if they have normal perfusion or nonsegmental perfusion abnormalities related to the heart, hila, and diaphragm; perfusion defects smaller than the radiographic or ventilation lesion; multiple matched
mismatch, which is a perfusion defect larger than the ventilation defect or no ventilation defect. Patients have very low probability for emboli if they have normal perfusion or nonsegmental perfusion abnormalities related to the heart, hila, and diaphragm; perfusion defects smaller than the radiographic or ventilation lesion; multiple matched 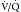 defects with a normal radiograph; one to three small segmental perfusion defects; the presence of a stripe of perfused tissue between the defect and the pleural surface; or a single matched defect with a matching chest radiograph abnormality in the mid or upper lung. All other findings are classified as indeterminate or low probability. Unfortunately, this later group can comprise at least one quarter of the studies that are performed. In addition, it does not provide an alternative diagnosis in those patients with very low probability or normal examinations.
defects with a normal radiograph; one to three small segmental perfusion defects; the presence of a stripe of perfused tissue between the defect and the pleural surface; or a single matched defect with a matching chest radiograph abnormality in the mid or upper lung. All other findings are classified as indeterminate or low probability. Unfortunately, this later group can comprise at least one quarter of the studies that are performed. In addition, it does not provide an alternative diagnosis in those patients with very low probability or normal examinations.
Computed Tomography
CT pulmonary angiography has emerged as the standard method of detection and identification of PE.1,2,42,43 On modern scanners of 16 or more detector rows, the scanning technique is easily accomplished in a short single breathhold. A limited number of artifacts can simulate PEs, but CT pulmonary angiography probably is equivalent to invasive catheter pulmonary angiography for the detection of PEs down to at least the subsegmental level. CT has the advantage of providing alternate diagnosis in cases that are negative for PE.
Scanning technique should maximize contrast enhancement of the pulmonary arteries and provide sufficient resolution to visualize pulmonary arteries down to approximately 1 mm in diameter.44,45 Short scan times are also important to allow adequate breathholding in dyspneic patients. Typically, scanning is performed at approximately 1-mm collimation and the scan acquired in as short a time as possible on the scanner. With 16-slice CT scanners, this should require less than 10 seconds. Larger detector scanners can perform the examination in less than 5 seconds. Radiation dose is a concern, and therefore, appropriate dose reduction strategies should be employed. These strategies include tube current modulation based on patient attenuation measurements and the use of anterior breast shields in premenopausal women.46 The use of newer iterative statistical reconstruction techniques47 and lower kilovolt techniques48 can also reduce dose.
To obtain optimal vascular enhancement, a high–iodine concentration contrast material is administered at a relatively high rate.43,49–51 Contrast material is typically 300 mg of iodine/mL or higher and administered at 4 mL/sec or faster. This generally requires a 20-gauge or larger peripheral intravenous access and a relatively large upper extremity vein. Alternatively, the injection may be performed through a power-injectable central venous catheter. A total of 100 to 150 mL of contrast media is injected and scanning begins, timed to complete within 2 to 3 seconds after the end of injection. The start time for scanning, therefore, must be adjusted based on the speed of the scanner being used. A larger-volume injection allows a longer scan delay. This increases the likelihood of maximum pulmonary arterial enhancement in a wider variety of patients despite differing cardiac outputs or collateral venous circulation due to central or upper extremity venous obstruction. When a power-injectable central venous catheter is used, a smaller total injection volume can be used. However, the injection should not complete until the end of scanning to prevent inflow of unopacified blood from the superior vena cava and IVC from reaching the pulmonary arterial system during the scan.
Scanning is frequently accomplished in a caudocranial direction.49,50 This is believed to reduce motion artifacts associated with suboptimal breathholding because the upper lungs are less affected by respiratory motion. Although still frequently used, its value is probably much less with 64–detector row or larger scanners because of the short scan duration.
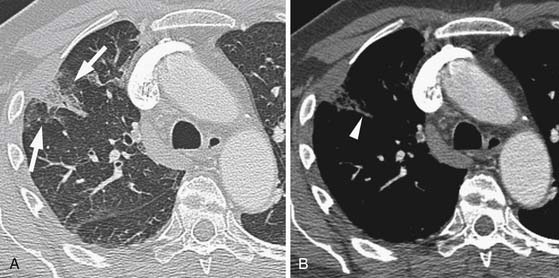
A variety of artifacts may degrade the images of the pulmonary arteries.49,50 The most significant is respiratory-related motion, which can usually be avoided with faster CT scanners (Figure 41-8). Both higher pitch and faster gantry rotation contribute to shorter breathhold requirements. Faster gantry rotations also improve the temporal resolution, which reduces the effect of any respiratory motion. Similarly, cardiac pulsations can cause motion artifacts in the adjacent parenchyma, seen most frequently in the left lower lobe (Figure 41-9). This can be minimized with faster gantry rotation times to minimize the amount of motion that occurs during a single image. Some have advocated the use of electrocardiographic gating of the CT acquisition.49 It is not yet clear that this significantly alters the detection of PEs, although it probably does reduce the extent of motion artifacts in small vessels adjacent to the heart.
Another significant source of artifact is that of contrast interruption (Figure 41-10) due to inflowing of unopacified blood from the IVC during the examination.52–54 The IVC inflow is accentuated during inspiration, whereas it is relatively stable during exhalation and during breathhold or Valsalva maneuver. This inflow during inspiration causes a short pulse of low-attenuation blood to be present in the pulmonary arteries a few seconds after the patient inhales. With faster scanners, this pulse of lower-attenuation blood can affect several centimeters of pulmonary arteries and simulate pulmonary arterial emboli. It may be recognized by its involvement of multiple vessels at the same level. This artifact appears to be increased by hyperventilation, taking multiple deep breaths prior to breathhold, so simple breathhold instructions may reduce its frequency and extent. When present, it may be necessary to repeat the examination. The repeat examination should be performed with a different breathhold timing to change the location of the apparent filling defect or performed without breathholding, particularly if a large portion of the central pulmonary system is affected on the initial scan.
PEs are identified as a filling defect in the pulmonary arterial tree with sharp margins with the contrast column.50,55,56 Acute PEs are typically located eccentrically and are rounded in contour or may fill a small vessel completely (see Figures 41-1 to 41-3). Larger central emboli may have elongated or lobular components and often extend into multiple smaller branches. The CT equivalent of Hampton’s hump may be demonstrated as a wedge-shaped peripheral focus of consolidation with the base at the pleural surface and the apex directed to the occluded pulmonary artery (see Figure 41-4).9
Over time, emboli may resolve or may become chronic and incorporate into the pulmonary artery wall. In that setting, the filling defect becomes crescentic in contour and occluded segments may recanalize, showing small foci of contrast within the thrombus.55 The filling defect may be reduced to a thin linear web within the vessel (Figure 41-11). Some emboli retain their acute appearance, but persistence over greater than 6 weeks indicates a chronic embolism.
Assessment of right heart strain should also be performed in patients who have PE. This includes the secondary signs of reflux of contrast material into the IVC and systemic edema as seen usually in the dependent subcutaneous tissue.36,38,57 The main pulmonary artery measured in its transverse diameter proximal to the bifurcation should be less than 33 mm.33,34 The ratio of pulmonary artery to aortic diameter is a more specific finding for developing pulmonary hypertension and should be less than 1.0 (see Figure 41-5). The ventricular diameter ratio is a ratio of the internal maximum short-axis diameter of the right ventricle divided by that of the left ventricle and indicates right ventricular dilatation if it is greater than 1.5 (see Figure 41-6).36,38,57 These diameters are typically not at the same level in axial scanning. Some reports indicate that the measurement is more consistently made on reconstructed horizontal long-axis images, but it is not certain that this adds significant predictive value.38 On those images, the right ventricle–to–left ventricle ratio should be less than 1.0.
Treatment
Treatment for PE and DVT depends on the hemodynamic status of the patient. This may range from therapy directed at removing the clot urgently through intensive monitoring and supportive care to long-term anticoagulation. Because there is a risk of fatality from any episode of PE, patients at a known high risk should undergo prophylactic treatment to prevent the development of thrombi and emboli. The appropriate therapeutic options depend on the overall clinical picture including any planned or ongoing therapies as well as the patient’s immediate clinical status.
Patients with severe hemodynamic compromise require urgent therapy directed at removing the clot. This can include surgical embolectomy or systemic thrombolytic therapy. Catheter-directed thrombolytic therapy has received limited investigation but so far has not shown improvement in outcomes compared with systemic therapy.2,58
Surgical embolectomy for PE became a successful operation after the report of its performance using cardiac bypass by Dr. Cooley and Dr. Beall in 1962.59 Since that time, it has been primarily reserved for the most severely compromised patients.60 European Society of Cardiology and British Thoracic Society guidelines suggest its use only for patients in cardiogenic shock or after failed thrombolytic therapy.1,2 However, more recent reports suggest that the procedure may be more effective in patients with right ventricular compromise but without cardiogenic shock.28 The procedure is typically performed on bypass without cardioplegia and an IVC filter is typically placed at the end of the procedure. The filter placement may account for the less frequent recurrence of PE in surgical patients compared with patients treated with thrombolytics and anticoagulation alone. Other indications for surgery in patients who have cardiac compromise are those for whom thrombolytic therapy is contraindicated, such as after stroke or recent surgery or with other active bleeding, and those patients with paradoxical emboli or patent foramen ovale and emboli within the right heart chambers. The presence of right heart dysfunction as a result of PE is a poor prognostic indicator for patient survival, but patients who recover right ventricular function, including after surgical therapy, have better outcomes.60 As a result of recent reports showing improved mortality in patients with less severe cardiac compromise, the indications for surgical treatment for acute massive PE are probably expanding.28
Thrombolytic therapy has predominantly been used in patients with evidence of cardiac dysfunction, particularly right heart dilatation or failure.61 However, there is little convincing evidence that thrombolytic therapy changes long-term outcomes if hemodynamically stable patients are included.60 The main benefit of thrombolytic therapy appears to be in patients who are hemodynamically unstable. Thrombolytic therapy is frequently accompanied by minor bleeding complications. Major bleeding complications occur in 15% to 20% of patients and intracranial hemorrhage or death occurs in fewer than 3% of patients.60
The use of thrombolytic therapy may not change the long-term outcome in patients surviving to hospital discharge.60 Those patients who develop recurrent emboli or worsening heart function after treatment have poor long-term outcomes. The use of thrombolytic therapy alone does not appear to affect the rate of recurrent embolism. In addition, there is concern for development of pulmonary arterial hypertension related to chronic emboli after thrombus fragmentation related to thrombolytic therapy and particularly catheter-directed fragmentation. These difficulties may explain the lack of effect of thrombolytic therapy on long-term outcomes.
Treatment of all acute PEs begins with early initiation of anticoagulation therapy. This may be done with unfractionated heparin using a loading dose followed by constant infusion or with low-molecular-weight heparin (LMWH) administered subcutaneously. In patients with symptomatic embolism, survival is improved by early institution of anticoagulation therapy, usually in the emergency room.62 A delay of 48 hours in achieving anticoagulation reduces overall survival. In the setting of urgent PE with evidence of right heart strain or right heart failure, unfractionated heparin is preferred for its rapid onset of effect. In addition, there is a lack of any clinical trials comparing effectiveness of unfractionated heparin and LMWH in patients with hemodynamic compromise. In this urgent setting, a typical loading dose is 80 IU/kg followed by infusion of 18 IU/kg/hr to achieve an activated partial thromboplastin time (aPTT) of 1.5 to 2.5 times control.63
Cancer patients have a higher risk of bleeding complications with long-term anticoagulation therapy, particularly with warfarin.2,63 Recent trials have shown better long-term results with use of LMWH injections rather than oral warfarin anticoagulation for the first 6 months after a PE event.63,64 The use of LMWH does not generally require the frequent laboratory monitoring that is needed with warfarin therapy. The CLOT (Randomized comparison of low-molecular-weight heparin versus oral anticoagulant therapy for the prevention of recurrent venous thromboembolism in patients with cancer) trial specifically addressed the use of LMWH compared with warfarin sodium (Coumadin) therapy in cancer patients with venous thromboembolism and found the LMWH superior in its risk profile and equivalent in its prevention of recurrent PE.65 Dalteparin, the LMWH used in that trial, was administered as 200 IU/kg daily for the first month followed by 150 IU/kg daily for the following 5 months. There are no data on the need to continue anticoagulation after 6 months or comparing the use of specific agents after that time. The principle advantages of LMWH over warfarin drugs are its reliable pharmacokinetics and lack of drug interactions. The use of warfarin drugs can complicate the administration of other pharmaceuticals that cancer patients are likely to require. In addition, dalteparin achieves satisfactory anticoagulation more quickly than warfarin and, therefore, allows for interruptions in therapy more easily.
IVC filters have been used in the acute treatment of PE. Their effectiveness in the long-term setting is less certain and there is no long-term mortality benefit for the use of IVC filters in addition to anticoagulation compared with the use of anticoagulation alone. The use of IVC filters decreases the incidence of recurrent PE but is associated with increased risk of recurrent lower extremity DVT. An additional complication in the setting of DVT is the development of postthrombotic syndrome including pain, swelling, and skin changes in the affected limb. These symptoms can be difficult to distinguish from recurrent DVT. The use of graduated compression stockings is recommended and has been shown to reduce postthrombotic syndrome by up to 50%.63
The best treatment of catheter-related thrombosis is less clear. The incidence of symptomatic PE after catheter-related thrombosis is less than 10%.4 There are no randomized, controlled trials to guide management of such catheters. It is not clear whether anticoagulation actually affects the resolution of catheter-associated thrombus. Catheter removal may have an effect on eventual thrombus resolution but it need not be immediate removal. New catheters placed at different sites after a catheter-related thrombosis event frequently also develop catheter-related thrombus. As such, in the absence of catheter-related infections, it is recommended that the catheter remain as long as it is needed and be removed once it is no longer necessary.4,63
Because of the significant mortality related to PE, prophylactic therapy is important. In postoperative patients, this includes compression devices and subcutaneous heparin in the immediate postoperative period.28 The appropriate role and method of thromboembolic disease prevention in cancer patients is more complicated and not well established. Anticoagulation is probably appropriate in patients receiving chemotherapy combinations known to carry a high risk of venous thrombosis, but the needed duration of such therapy is unknown. The thrombosis risk must be weighed against complicating factors such as platelet dysfunction and drug-related coagulopathies in determining the appropriate treatment for a particular patient.
Key Points Therapies
• Early anticoagulation is the mainstay of therapy for stable or unstable patients.
• Surgery is reserved for unstable patients, but it may be appropriate for stable patients with evidence of severe heart strain.
• Thrombolytic therapy is useful in unstable patients or those with heart strain, but it may not alter long-term outcomes.
• IVC filters reduce the recurrence risk but increase the risk of lower extremity DVT and may not provide a long-term mortality benefit over anticoagulation alone.
Conclusion
PE is a challenging complication in cancer patients. Cancer increases the risk of venous thromboembolism through local and humoral effects, and cancer treatments can contribute further. The presence of PE is most readily determined with CT pulmonary angiography, which provides information on the extent of embolic disease and heart strain. The development of PE is an indicator of poor prognosis in cancer patients, whether reflecting a later stage of disease or owing to the induced respiratory failure. Therefore, treatment and prevention of PE is important in the management of cancer patients.
1. Guidelines on diagnosis and management of acute pulmonary embolism. Task Force on Pulmonary Embolism, European Society of Cardiology. Eur Heart J. 2000;21:1301-1336.
2. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax. 2003;58:470-483.
3. Lee A.Y., Levine M.N. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23 Suppl 1):I17-I21.
4. Jones M.A., Lee D.Y., Segall J.A., et al. Characterizing resolution of catheter-associated upper extremity deep venous thrombosis. J Vasc Surg. 2010;51:108-113.
5. Torbicki A., Galie N., Covezzoli .A, et al. Right heart thrombi in pulmonary embolism: results from the International Cooperative Pulmonary Embolism Registry. J Am Coll Cardiol. 2003;41:2245-2251.
6. Chartier L., Bera J., Delomez M., et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation. 1999;99:2779-2783.
7. The European Cooperative Study on the clinical significance of right heart thrombi. European Working Group on Echocardiography. Eur Heart J. 1989;10:1046-1059.
8. Motovska Z., Widimsky P., Bilkova .D, et al. An embolus in the right atrium caught in the Chiari network and resistant to thrombolysis. J Thromb Thrombolysis. 2010;30:114-118.
9. Sinner W.N. Computed tomographic patterns of pulmonary thromboembolism and infarction. J Comput Assist Tomogr. 1978;2:395-399.
10. Bounameaux H., Perrier A., Righini M. Diagnosis of venous thromboembolism: an update. Vasc Med. 2010;15:399-406.
11. Stein P.D., Terrin M.L., Hales C.A, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100:598-603.
12. Engelke C., Rummeny E.J., Marten K. Pulmonary embolism at multi-detector row CT of chest: one-year survival of treated and untreated patients. Radiology. 2006;239:563-575.
13. Gladish G.W., Choe D.H., Marom E.M, et al. Incidental pulmonary emboli in oncology patients: prevalence, CT evaluation, and natural history. Radiology. 2006;240:246-255.
14. Hui G.C., Legasto A., Wittram C. The prevalence of symptomatic and coincidental pulmonary embolism on computed tomography. J Comput Assist Tomogr. 2008;32:783-787.
15. O’Connell C.L., Boswell W.D., Duddalwar V., et al. Unsuspected pulmonary emboli in cancer patients: clinical correlates and relevance. J Clin Oncol. 2006;24:4928-4932.
16. Ritchie G., McGurk S., McCreath C., et al. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax. 2007;62:536-540.
17. Storto M.L., Di Credico A., Guido F., et al. Incidental detection of pulmonary emboli on routine MDCT of the chest. AJR Am J Roentgenol. 2005;184:264-267.
18. Donato A.A., Khoche S., Santora J., et al. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb Res. 2010;126:e266-e270.
19. Sun J.M., Kim T.S., Lee J., et al. Unsuspected pulmonary emboli in lung cancer patients: the impact on survival and the significance of anticoagulation therapy. Lung Cancer. 2010;69:330-336.
20. Miller G.A., Sutton G.C., Kerr I.H, et al. Comparison of streptokinase and heparin in treatment of isolated acute massive pulmonary embolism. Br Med J. 1971;2:681-684.
21. Mastora I., Remy-Jardin M., Masson P., et al. Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol. 2003;13:29-35.
22. Qanadli S.D., El Hajjam M., Vieillard-Baron .A, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176:1415-1420.
23. van der Meer R.W., Pattynama P.M., van Struen M.J, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology. 2005;235:798-803.
24. Metafratzi Z.M., Vassiliou M.P., Maglaras G.C, et al. Acute pulmonary embolism: correlation of CT pulmonary artery obstruction index with blood gas values. AJR Am J Roentgenol. 2006;186:213-219.
25. Lu M.T., Cai T., Ersoy H., et al. Interval increase in right-left ventricular diameter ratios at CT as a predictor of 30-day mortality after acute pulmonary embolism: initial experience. Radiology. 2008;246:281-287.
26. Engelke C., Rummeny E.J., Marten K. Acute pulmonary embolism on MDCT of the chest: prediction of cor pulmonale and short-term patient survival from morphologic embolus burden. AJR Am J Roentgenol. 2006;186:1265-1271.
27. Yusuf S.W., Gladish G., Lenihan D.J, et al. Computerized tomographic finding of saddle pulmonary embolism is associated with high mortality in cancer patients. Intern Med J. 2010;40:293-299.
28. Carvalho E.M., Macedo F.I., Panos A.L, et al. Pulmonary embolectomy: recommendation for early surgical intervention. J Card Surg. 2010;25:261-266.
29. Sukhija R., Aronow W.S., Lee J., et al. Association of right ventricular dysfunction with in-hospital mortality in patients with acute pulmonary embolism and reduction in mortality in patients with right ventricular dysfunction by pulmonary embolectomy. Am J Cardiol. 2005;95:695-696.
30. Reid J.H., Murchison J.T. Acute right ventricular dilatation: a new helical CT sign of massive pulmonary embolism. Clin Radiol. 1998;53:694-698.
31. Schoepf U.J., Kucher N., Kipfmueller F., et al. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110:3276-3280.
32. Ocak I., Fuhrman C. CT angiography findings of the left atrium and right ventricle in patients with massive pulmonary embolism. AJR Am J Roentgenol. 2008;191:1072-1076.
33. Edwards P.D., Bull R.K., Coulden R. CT measurement of main pulmonary artery diameter. Br J Radiol. 1998;71:1018-1020.
34. Kuriyama K., Gamsu G., Stern R.G, et al. CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol. 1984;19:16-22.
35. Ng C.S., Wells A.U., Padley S.P. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging. 1999;14:270-278.
36. Ghaye B., Ghuysen A., Bruyere P.J, et al. Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics. 2006;26:23-39. discussion 39–40
37. Dogan H., Kroft L.J., Huisman M.V, et al. Right ventricular function in patients with acute pulmonary embolism: analysis with electrocardiography-synchronized multi-detector row CT. Radiology. 2007;242:78-84.
38. Ghuysen A., Ghaye B., Willems V., et al. Computed tomographic pulmonary angiography and prognostic significance in patients with acute pulmonary embolism. Thorax. 2005;60:956-961.
39. Goodman L.R., Stein P.D., Matta F., et al. CT venography and compression sonography are diagnostically equivalent: data from PIOPED II. AJR Am J Roentgenol. 2007;189:1071-1076.
40. Worsley D.F., Alavi A., Aronchick J.M, et al. Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED Study. Radiology. 1993;189:133-136.
41. Sostman H.D., Stein P.D., Gottschalk .A, et al. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology. 2008;246:941-946.
42. Remy-Jardin M., Pistolesi M., Goodman L.R, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology. 2007;245:315-329.
43. Remy-Jardin M., Remy J. Spiral CT angiography of the pulmonary circulation. Radiology. 1999;212:615-636.
44. Schoepf U.J., Holzknecht N., Helmberger T.K, et al. Subsegmental pulmonary emboli: improved detection with thin-collimation multi-detector row spiral CT. Radiology. 2002;222:483-490.
45. Patel S., Kazerooni E.A., Cascade P.N. Pulmonary embolism: optimization of small pulmonary artery visualization at multi-detector row CT. Radiology. 2003;227:455-460.
46. Kalra M.K., Rizzo S., Maher M.M, et al. Chest CT performed with z-axis modulation: scanning protocol and radiation dose. Radiology. 2005;237:303-308.
47. Prakash P., Kalra M.K., Digumarthy S.R, et al. Radiation dose reduction with chest computed tomography using adaptive statistical iterative reconstruction technique: initial experience. J Comput Assist Tomogr. 2010;34:40-45.
48. Heyer C.M., Mohr P.S., Lemburg S.P, et al. Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. Radiology. 2007;245:577-583.
49. Hartmann I.J., Wittenberg R., Schaefer-Prokop C. Imaging of acute pulmonary embolism using multi-detector CT angiography: an update on imaging technique and interpretation. Eur J Radiol. 2010;74:40-49.
50. Wittram C. How I do it: CT pulmonary angiography. AJR Am J Roentgenol. 2007;188:1255-1261.
51. Yankelevitz D.F., Shaham D., Shah .A, et al. Optimization of contrast delivery for pulmonary CT angiography. Clin Imaging. 1998;22:398-403.
52. Gosselin M.V., Rassner U.A., Thieszen S.L, et al. Contrast dynamics during CT pulmonary angiogram: analysis of an inspiration associated artifact. J Thorac Imaging. 2004;19:1-7.
53. Kuzo R.S., Pooley R.A., Crook J.E, et al. Measurement of caval blood flow with MRI during respiratory maneuvers: implications for vascular contrast opacification on pulmonary CT angiographic studies. AJR Am J Roentgenol. 2007;188:839-842.
54. Wittram C., Yoo A.J. Transient interruption of contrast on CT pulmonary angiography: proof of mechanism. J Thorac Imaging. 2007;22:125-129.
55. Wittram C., Maher M.M., Yoo A.J, et al. CT angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. Radiographics. 2004;24:1219-1238.
56. Remy-Jardin M., Remy J., Artaud D., et al. Spiral CT of pulmonary embolism: diagnostic approach, interpretive pitfalls and current indications. Eur Radiol. 1998;8:1376-1390.
57. Ghaye B., Ghuysen A., Willems V., et al. Severe pulmonary embolism:pulmonary artery clot load scores and cardiovascular parameters as predictors of mortality. Radiology. 2006;239:884-891.
58. Todoran T.M., Sobieszczyk P. Catheter-based therapies for massive pulmonary embolism. Prog Cardiovasc Dis. 2010;52:429-437.
59. Cooley D.A., Beall A.C.Jr. Surgical treatment of acute massive pulmonary embolism using temporary cardiopulmonary bypass. Dis Chest. 1962;41:102-104.
60. Samoukovic G., Malas T., deVarennes B. The role of pulmonary embolectomy in the treatment of acute pulmonary embolism: a literature review from 1968 to 2008. Interact Cardiovasc Thorac Surg. 2010;11:265-270.
61. Jenkins P.O., Sultanzadeh J., Bhagwat M., et al. Should thrombolysis have a greater role in the management of pulmonary embolism? Clin Med. 2009;9:431-435.
62. Smith S.B., Geske J.B., Maguire J.M, et al. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137:1382-1390.
63. Coleman R., MacCallum P. Treatment and secondary prevention of venous thromboembolism in cancer. Br J Cancer. 2010;102(Suppl 1):S17-S23.
64. Tran Q.N. Role of palliative low-molecular-weight heparin for treating venous thromboembolism in patients with advanced cancer. Am J Hosp Palliat Care. 2010;27:416-419.
65. Lee A.Y., Levine M.N., Baker R.I, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153.

 scanning does have the limitations indicated previously, it is still an important diagnostic tool in those patients for whom CT angiography is contraindicated. The large majority of patients will have a diagnostic result from perfusion scanning. This allows clinical management to proceed without further diagnostic testing for PE.
scanning does have the limitations indicated previously, it is still an important diagnostic tool in those patients for whom CT angiography is contraindicated. The large majority of patients will have a diagnostic result from perfusion scanning. This allows clinical management to proceed without further diagnostic testing for PE.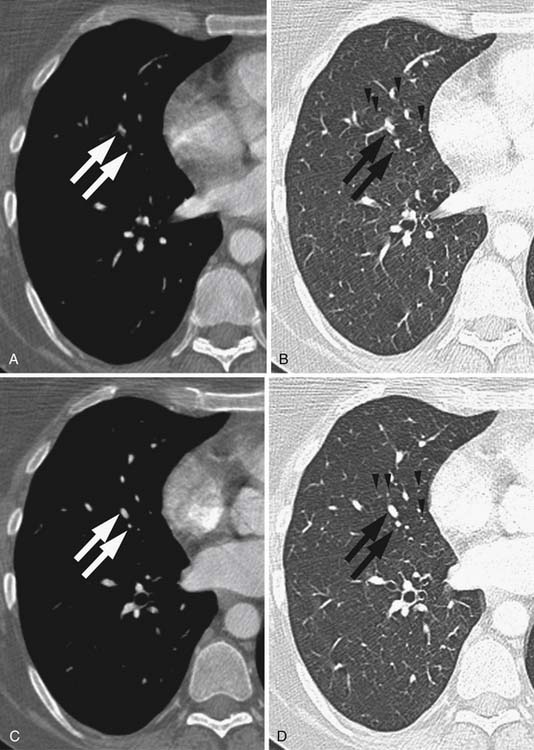
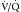 scanning and has essentially the same sensitivity and specificity as conventional catheter pulmonary angiography without the technical requirements and associated limited availability of that procedure.
scanning and has essentially the same sensitivity and specificity as conventional catheter pulmonary angiography without the technical requirements and associated limited availability of that procedure. 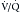 scanning still finds a role in patients for whom contrast administration is contraindicated.
scanning still finds a role in patients for whom contrast administration is contraindicated.