CHAPTER 100 Pulmonary Edema
Pulmonary edema is frequently classified as hydrostatic edema (e.g., cardiogenic pulmonary edema) or edema caused by increased capillary permeability (e.g., noncardiogenic pulmonary edema or capillary leak). Often, chest radiographs of patients with pulmonary edema are not as easily classified in such a dichotomous fashion. The following pulmonary edema classification has been proposed to better accommodate the histopathologic, physiologic, and radiographic findings of these patients1:
HYDROSTATIC PULMONARY EDEMA
Imaging Techniques and Findings
Radiography
The chest radiographic findings of hydrostatic pulmonary edema are detailed in Box 100-1. These findings are all more reliably distinguishable on posteroanterior (PA) and lateral chest radiographs than on portable radiographs, but commonly patients with the greatest likelihood of hydrostatic pulmonary edema will be imaged using an anteroposterior technique (AP). AP techniques can make the diagnosis of hydrostatic pulmonary edema difficult because heart magnification, resulting from the considerably shorter focus-film distance as well as projectional magnification, can render determination of cardiac size unreliable, particularly in patients with low lung volumes. Additionally, pulmonary vascular congestion is difficult to determine accurately in nonerect patients because upper lobe vessels frequently normally appear larger than lower lobe vessels for patients imaged in a supine or semierect position. Furthermore, azygos vein dilation, often considered an indicator of elevated right atrial pressure, is a common and potentially normal finding on supine radiographs. Finally, pleural effusions often appear only as hazy attenuation projecting over lungs in patients imaged in a supine or semierect position. However, this appearance is not specific for pleural effusion and can be seen with extensive posterior atelectasis because anterior aerated lung superimposes on the increased opacity of the posteriorly located atelectatic lung. This pattern is often seen in severely ill patients and patients in the intensive care unit, which is the same patient population at risk for hydrostatic pulmonary edema.
The first chest radiographic signs of pulmonary venous hypertension include pulmonary vascular redistribution, appearing as equalization of the size of the upper and lower lobe vessels, which progresses to the upper lobe vessels becoming larger than those in the lower lobes (a reversal of the normal situation). In the acute setting, this phenomenon is seen with left atrial (wedge) pressures in the range of 12 to 19 mm Hg.2 In chronically compensated patients, such as those with mitral stenosis, upper and lower lobe vascular equalization and reversal will occur with left atrial pressures between 15 to 25 mm Hg.2 As the physiologic derangements worsen, pulmonary venous hypertension progresses to frank interstitial pulmonary edema, with the development of interlobular septal thickening, often referred to as Kerley A and B lines (Fig. 100-1A), perihilar indistinctness and vascular haze (see Fig. 100-1B), peribronchial cuffing (see Fig. 100-1C), and subpleural edema (see later).2 Findings of interstitial edema usually are apparent on the chest radiograph, with left atrial pressures of 20 to 25 mm Hg in the acutely ill patient and 25 to 30 mm Hg in chronically compensated patients.2 At higher left atrial pressures, frank alveolar edema occurs, with spillage of fluid into the air spaces. In these patients, the chest radiograph will show an air space consolidation pattern (see Fig. 100-1D) and acinar nodules,2 often superimposed on findings of interstitial edema. The relationship between left atrial (pulmonary capillary wedge) pressure and radiographic findings is not precise, and the radiographic findings often lag behind the physiological changes. Furthermore, stepwise progression through these phases is not always seen, and some imaging findings of hydrostatic edema may be present in the absence of others.
CT
On thoracic CT, findings of hydrostatic pulmonary edema include those seen on chest radiographs, such as cardiomegaly, vascular engorgement, and pleural effusions. Findings of interstitial edema (see later), are also apparent and are more readily appreciable with thoracic CT, particularly high-resolution CT (HRCT), than with chest radiography. Bronchial wall thickening, smooth interlobular septal thickening (Fig. 100-2), and ground-glass opacity (often with hazy, poorly defined centrilobular nodules) are highly suggestive of hydrostatic pulmonary edema.
Classic Signs
Interstitial Edema
The findings of interstitial edema on chest radiography (see Box 100-1) are usually readily visible with CT scanning and, in addition to the findings noted, ground-glass opacity and centrilobular nodules are frequently present in patients with hydrostatic pulmonary edema.
Interlobular Septal Thickening (Kerley A and B lines)
Both Kerley A and B lines represent thickened interlobular septae. Kerley B lines are horizontal linear opacities, 1 to 2 cm in length, in contact with the pleural surface. Kerley B lines are most readily visible in the inferior and lateral aspects of the thorax, near the lateral costophrenic sulcus (see Fig. 100-1A). The regular appearance of Kerley B lines in the lung bases is the result of the regular organization of pulmonary lobules at the lung bases.
The interlobular septal thickening resulting from hydrostatic pulmonary edema is readily visualized on thoracic CT, especially HRCT. Interlobular septal thickening in patients with hydrostatic pulmonary edema is smooth and often most readily visualized in the lung bases and in the pulmonary apices (Fig. 100-2). Poorly defined, ground-glass attenuation centrilobular nodules or lobular ground-glass opacity often coexists.
Vascular Indistinctness and Perihilar Haze
Perihilar haze is a term occasionally used to refer to poor definition of and slightly increased opacity surrounding the perihilar vessels; it manifests as perihilar vascular indistinctness with a hazy or ground-glass appearance (see Fig. 100-1B). Although not always easily recognizable, perihilar haze can be a useful finding that suggests hydrostatic pulmonary edema in patients with lower lobe atelectasis.
Peribronchial Cuffing
Peribronchial cuffing represents bronchial wall thickening, possibly accompanied by a variable amount of peribronchial interstitial fluid. Peribronchial cuffing is most readily recognizable in the central and perihilar regions of lung, when bronchi are seen in cross section (see Fig. 100-1C). Peribronchial cuffing may also be seen as a tram-track appearance when bronchi are visualized in longitudinal section. Peribronchial cuffing may also appear as nonspecific thickening of the interstitium radiating outward from the hilum. On thoracic CT, peribronchial thickening or cuffing usually appears as bronchial wall thickening. Peribronchial cuffing may represent a finding of interstitial edema in the proper clinical setting, but is not specific for that diagnosis; peribronchial inflammation or tumor may also produce this finding.
Air Space (Alveolar) Edema
As factors producing interstitial edema progress, eventually interstitial fluid will begin to accumulate within the air spaces, producing air space edema. Because the edema fluid replaces air within the alveoli, air space edema represents a form of air space consolidation and appears as a confluent opacity (see Fig. 100-1D), which may be accompanied by air space or acinar nodules.
Distribution of Hydrostatic Edema
Hydrostatic edema may assume a number of different distributions, although a symmetric perihilar and basilar predominant distribution is most common. A distinct perihilar distribution of hydrostatic edema is often referred to as a bat wing or butterfly distribution, and is often seen when edema fluid accumulates rapidly. Uncommonly, hydrostatic edema will accumulate in a reverse bat wing distribution reminiscent of other causes of peripheral lung opacity. Other variations in the distribution of hydrostatic edema are frequently recognized (Box 100-2).2
PERMEABILITY EDEMA WITH DIFFUSE ALVEOLAR DAMAGE
Manifestations of Disease
Clinical Presentation
Adult Respiratory Distress Syndrome
The adult respiratory distress syndrome (ARDS) is a form of diffuse pulmonary parenchymal lung injury associated with noncardiogenic pulmonary edema that results in hypoxemic respiratory failure.3 Histopathologically, ARDS is characterized by DAD. ARDS represents a constellation of clinical and physiologic findings that are thought to represent a single common process. The diagnostic criteria based on the American-European Consensus Conference for ARDS4 is as follows:
Histopathologically, ARDS is characterized by DAD. The exact mechanism of injury that brings about DAD is not known, but undoubtedly free radicals and cellular infiltration with expression of proteolytic enzymes and cytokines play a prominent role in the resulting tissue damage. A wide variety of insults may result in ARDS (Box 100-3).
Histopathologic Abnormalities and Stages in Patients with Adult Respiratory Distress Syndrome
The histopathologic findings in patients with ARDS have been described in three overlapping stages (Box 100-4):
BOX 100-4
Stages of Adult Respiratory Distress Syndrome (ARDS)
Diagnostic criteria from the American-European Consensus Conference for ARDS
The histopathologic findings in these stages are similar regardless of the cause of ARDS. In the exudative stage, the predominant histopathologic pattern is DAD, with hyaline membrane formation associated with high protein content. Neutrophilic infiltration, hemorrhage, epithelial cell injury, and macrophage accumulation are also seen. This phase lasts for approximately 5 to 7 days. The exudative stage is sometimes subdivided into an early phase, which occurs hours after the insult, and in which endothelial cell edema, capillary congestion, interstitial edema, and hemorrhage occur. In the late exudative phase, which occurs several days following the inciting insult, DAD with hyaline membrane formation is seen, associated with type I pneumocyte necrosis and protein-rich alveolar edema fluid.3
Imaging Techniques and Findings
Radiography
Imaging Findings During Exudative Phase of Adult Respiratory Distress Syndrome
The HRCT findings of the various stages of ARDS have been described. In the exudative phase, patchy areas of ground-glass opacity (Fig. 100-3) and air space consolidation are common. These opacities initially tend to predominate in the dependent lung regions. Patchy multifocal opacities may rapidly evolve to diffuse opacities later in the exudative phase and as the proliferative phase progresses. Pleural effusions, if present, are small. Mild interlobular septal thickening may occur, but is less pronounced than in patients with hydrostatic pulmonary edema.
Imaging Findings During the Proliferative Phase of ARDS
After 1 week, air space consolidation begins to regress and linear and reticular opacities become more prominent (Fig. 100-4). Ground-glass opacity may persist. The imaging findings are not specific, but indicate development of a coarser, more organized appearance, which tends to reflect the underlying histopathologic changes. Traction bronchiectasis and bronchiolectasis and architectural distortion may appear. Patients with ARDS in the exudative or early proliferative phases who have more widespread findings of fibrosis, such as traction bronchiectasis, generally require prolonged ventilatory support, are at higher risk for ventilator-associated lung injury, and have higher mortality.
The chronic fibrotic phase of ARDS may begin as early as 8 days following the initial insult, but is more commonly encountered in patients who survive 1 month or longer. In patients who survive ARDS more than 30 days, many of these histopathologic abnormalities resolve, and areas of fibrosis, architectural distortion (Fig. 100-5), and even honeycombing are seen on imaging studies. Ground-glass opacity may persist, but is often associated with coarse reticulation architectural distortion, and reflects the presence of fibrosis rather than edema, hyaline membrane formation, and inflammatory cell infiltration, as in the exudate and proliferative phases. The HRCT findings may show a striking anterior lung involvement (see Fig. 100-5), perhaps because of sparing of the posterior atelectatic lung from the adverse effects of mechanical ventilation, such as high ventilatory pressures and high oxygen tension. If this hypothesis is correct, the prevalence of anterior lung fibrosis in survivors of ARDS may be altered in the future with the use of prone ventilation for the treatment of ARDS patients.
PERMEABILITY PULMONARY EDEMA WITHOUT DIFFUSE ALVEOLAR DAMAGE
Permeability pulmonary edema without DAD may be encountered in patients with reactions to various drugs (such as interleukin-2 treatment), transfusion reactions, infectious causes (e.g., hantavirus pulmonary syndrome), heroin overdose,1 or other insults that may result in ARDS, such as air emboli or toxic shock syndrome.
MIXED EDEMA
A combination of hydrostatic pulmonary edema and permeability edema, termed mixed edema, may be seen in the presence of conditions producing increased intravascular pressure and capillary endothelial injury. This pattern of edema may be seen in patients with re-expansion pulmonary edema, high-altitude pulmonary edema, and neurogenic pulmonary edema, among other less commonly encountered situations (Box 100-5).
NEUROGENIC PULMONARY EDEMA
Etiology and Pathophysiology
Neurogenic pulmonary edema occurs in patients with head trauma, intracranial hemorrhage, increased intracranial pressure, seizures, or other acute neurologic conditions producing intracranial hypertension.1 Drug overdoses, particularly opiates, barbiturates, and alcohol, may also produce neurogenic pulmonary edema. The mechanism of neurogenic pulmonary edema formation includes sympathetic discharge caused by CNS injury, resulting in systemic vasoconstriction, elevated systemic blood pressure and, subsequently, elevated left ventricular pressure and dysfunction. Because the alveolar edema fluid is protein-rich and pulmonary capillary wedge pressures are often normal, some element of capillary leak is also present. Therefore, neurogenic pulmonary edema shows a histopathologic pattern of both hydrostatic and permeability pulmonary edema, representing a mixed edema pattern.
RE-EXPANSION PULMONARY EDEMA
Imaging Techniques and Findings
Radiography and CT
Thoracic imaging studies will show the abnormality producing the pulmonary collapse, usually a large pleural effusion (Fig. 100-7) or pneumothorax, that subsequently undergoes drainage. The history will usually suggest that a large volume of fluid or air was removed, and that the pleural process has usually been present for a number of days. Within hours of the drainage procedure, increased opacity, usually ground-glass opacity or air space consolidation, develops within the expanded lung (see Fig. 100-7B). HRCT will also show ground-glass opacity (see Fig. 100-7C) and air space consolidation within the affected lung. The pathologic and radiographic findings associated with re-expansion pulmonary edema usually resolve within a few days to a week.
HIGH-ALTITUDE PULMONARY EDEMA
Etiology and Pathophysiology
Pulmonary edema may develop in some individuals after rapid ascent to high altitude, usually at altitudes higher than 10,000 feet. Pulmonary edema usually develops between 12 hours and 3 days after ascent,1 usually in the first day. Less commonly, high-altitude pulmonary edema develops in people with prolonged residence at a high altitude.
Manifestations of Disease
Clinical Presentation
Reduction in the partial pressure of oxygen in inspired air is responsible for the development of high-altitude pulmonary edema. The drop in partial pressure of oxygen that occurs at high altitude produces severe hypoxic vasoconstriction in the pulmonary arterial bed but, in susceptible individuals, this vasoconstriction is nonuniform. This inhomogeneous vasoconstriction response results in high-volume shunting of blood into pulmonary vessels unprotected by hypoxic vasoconstriction, producing severe elevations in pulmonary arterial pressure and resulting in pulmonary edema.1 Both hydrostatic and permeability pulmonary edema mechanisms are present in patients with high-altitude pulmonary edema.
Synopsis of Treatment Options
Supplemental oxygen administration or return to sea level results in resolution within 1 to 2 days,1 and radiographic findings clear rapidly with appropriate treatment.
Differential Diagnosis
From Imaging Findings
Differentiating among the various histopathologic patterns of edema on imaging studies can be challenging. Imaging features favoring one pattern over another are detailed in Box 100-6. Often, presumptive treatment for hydrostatic pulmonary edema with radiographic reassessment is useful—hydrostatic pulmonary edema will improve rapidly, whereas the time course to resolution for permeability edema with diffuse alveolar damage is prolonged.
BOX 100-6
Radiographic Differentiation of the Types of Pulmonary Edema
Permeability edema with diffuse alveolar damage (DAD)
 Radiographic appearance of opacities delayed for hours after clinical presentation; resolution prolonged
Radiographic appearance of opacities delayed for hours after clinical presentation; resolution prolongedKEY POINTS
 Pulmonary edema may be classified into four distinct categories: hydrostatic pulmonary edema, permeability edema associated with diffuse alveolar damage, permeability edema without associated diffuse alveolar damage, and mixed pulmonary edema patterns.
Pulmonary edema may be classified into four distinct categories: hydrostatic pulmonary edema, permeability edema associated with diffuse alveolar damage, permeability edema without associated diffuse alveolar damage, and mixed pulmonary edema patterns. Hydrostatic pulmonary edema is typically characterized on thoracic imaging studies by findings suggesting pulmonary venous hypertension and interstitial edema: peribronchial cuffing, perihilar indistinctness and vascular haze, subpleural edema and, in particular, interlobular septal thickening. Cardiomegaly and pleural effusions are often present.
Hydrostatic pulmonary edema is typically characterized on thoracic imaging studies by findings suggesting pulmonary venous hypertension and interstitial edema: peribronchial cuffing, perihilar indistinctness and vascular haze, subpleural edema and, in particular, interlobular septal thickening. Cardiomegaly and pleural effusions are often present.1 Gluecker T, Capasso P, Schnyder P, et al. Clinical and radiologic features of pulmonary edema. Radiographics. 1999;19:1507-1531.
2 Higgins CB. Essentials of Cardiac Radiology and Imaging. Philadelphia: JB Lippincott; 1992.
3 Donati SY, Papazian L. Role of open-lung biopsy in acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14:75-79.
4 Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818-824.





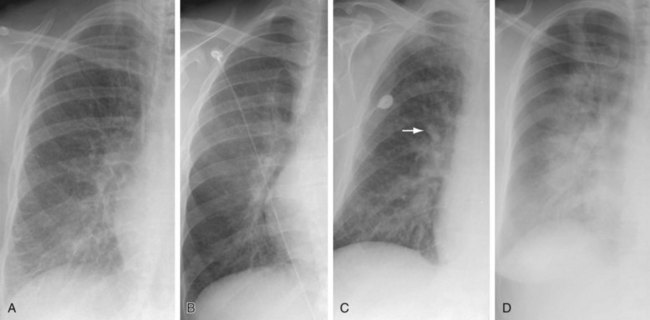
 FIGURE 100-1
FIGURE 100-1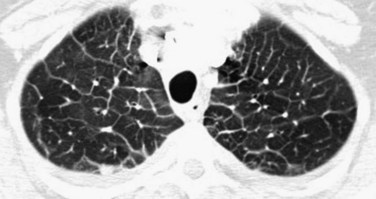
 FIGURE 100-2
FIGURE 100-2
































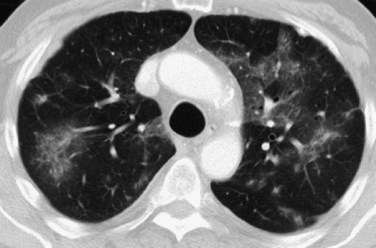
 FIGURE 100-3
FIGURE 100-3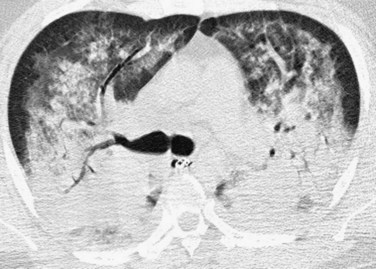
 FIGURE 100-4
FIGURE 100-4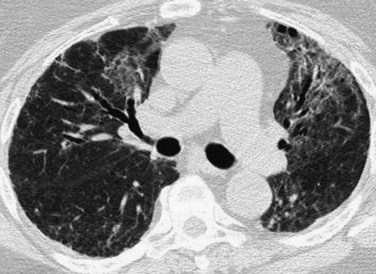
 FIGURE 100-5
FIGURE 100-5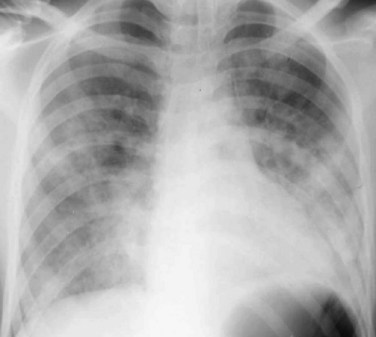
 FIGURE 100-6
FIGURE 100-6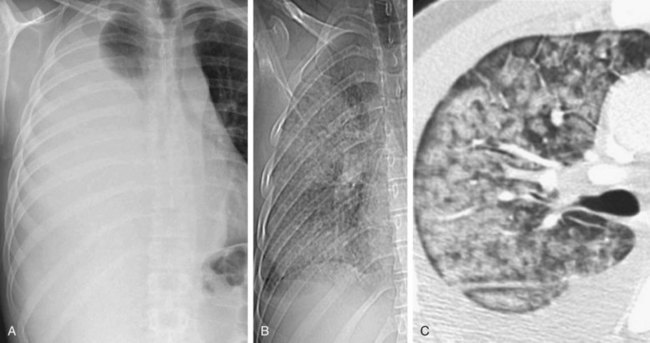
 FIGURE 100-7
FIGURE 100-7


















