Pterygium
Introduction
Pterygium (pleural: pterygia) is a prevalent ocular surface lesion, traditionally described as an encroachment of altered bulbar conjunctiva onto the cornea.1 Its name originates from the diminutive of πτερυζ, (Greek, small wing) referring to its characteristic wing-like growth pattern.2 Pterygium is an enigma and many theories have been proposed as to its pathogenesis. Historically, pterygia were considered to be degenerative lesions exemplified by degradation of Bowman’s layer and elastosis. The current view is that pterygium is an aberrant wound healing process, characterized by centripetal growth of a leading edge of altered limbal epithelial cells, followed by a squamous metaplastic epithelium with goblet cell hyperplasia and an underlying stroma of activated, proliferating fibroblasts, neovascularization, inflammatory cells, and extracellular matrix remodeling.3
Pterygia and other sun-related eye diseases (the ophthalmohelioses) pose a significant health problem to many communities. In Australia, with a population of approximately 22 million, it has been estimated that approximately 60 000 general practice visits annually include care for pterygia.4 The direct annual cost of pterygium in Australia is AU$8.3 million and this is likely to be an underestimation. Pterygium is often prevalent in developing countries with scarce health resources and in this setting can be a blinding disease.5,6 In biological terms, if conjunctival/limbal autografting is performed, 50% of the limbus and associated stem cells can be affected. Given the importance of stem cells in long-term corneal maintenance, pterygium is a condition of great significance.
Historically, the consequences of pterygium on the ocular surface have also been underestimated and the disease trivialized. Boxes 18.1 and 18.2 summarise associated vision loss and primary and secondary complications of pterygia. Systemic associations, such as polymorphous light eruption, porphyria cutanea tarda and skin malignancy7 have not been well recognized, yet pterygium is almost certainly a significant biomarker for substantial ultraviolet ocular and body surface insolation. Progressive pterygia can lead to complications, such as astigmatism and increased higher-order aberrations. These aberrations are decreased significantly at 2 weeks after surgery and remain relatively stable, yet they remain at levels much higher than in normal eyes.8 A possible cause may relate to the observation that invasion of the pterygium head deep to Bowman’s membrane, predicts postoperative corneal opacity/scarring.9 With ready availability of aberrometry and optical coherence tomography, a consideration is to operate before such changes are established. Given these findings, the era of refractive pterygium surgery may have arrived.
The association with dry eye is also well known. A twofold increased risk of dry eye symptoms exists in pterygium patients.10 While a direct mechanical mechanism is obvious, advances in our understanding of the inflammatory basis of dry eye syndrome11 suggest an indirect effect of inflammatory mediators in pterygium, resulting in a ‘pseudo-dry-eye state.’
Clinical Features
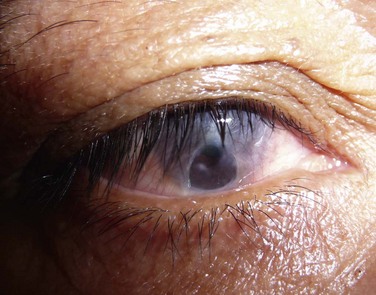
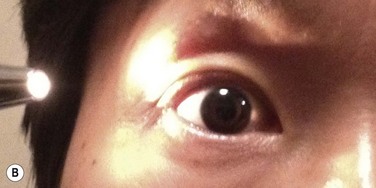
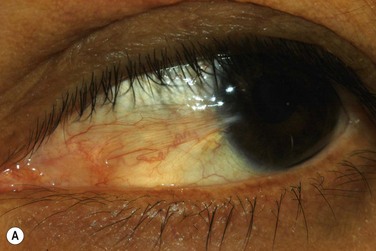
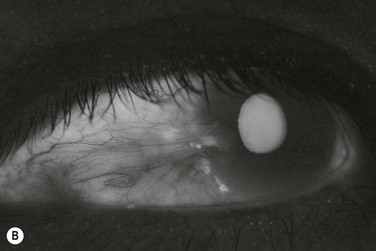
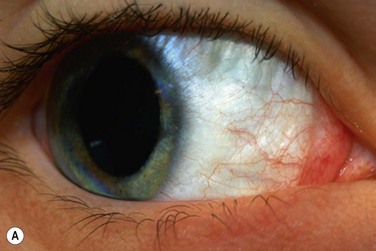
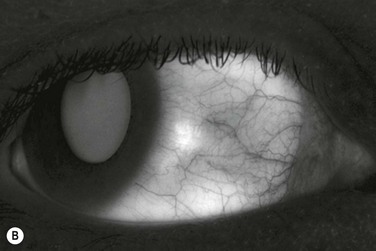
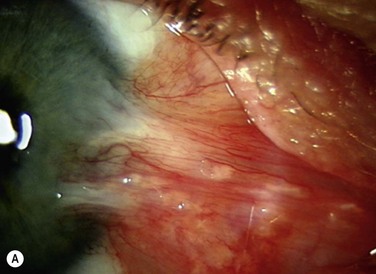
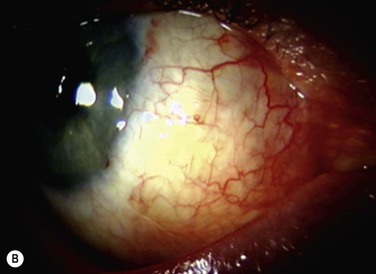
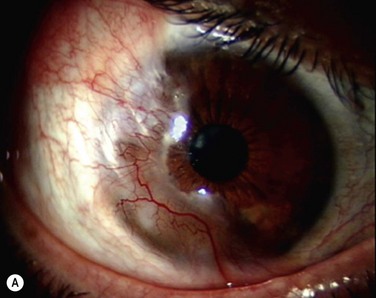
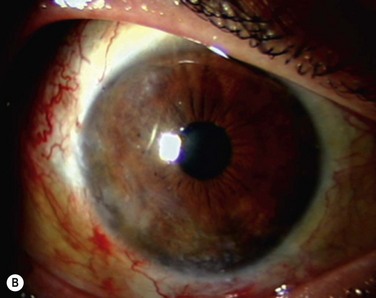
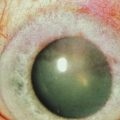
The classic appearance of a pterygium is that of a wing-shaped fibrovascular lesion extending from the bulbar conjunctiva onto the cornea (Fig. 18.1). Pterygia may be primary or recurrent, unilateral or bilateral, and present nasally or temporally in either or both eyes. They occur invariably in the palpebral fissure, predominantly at the nasal limbus, while temporal pterygia are less common and rarely found in isolation (Fig. 18.2).12 Anatomically, the pterygium may be divided into three parts. The ‘head’ consists of the apex enclosed by an avascular cap, the ‘neck’ refers to the region between the head and limbus overlying the cornea, and the ‘body’ is the portion overlying the sclera.13 When observed by slit lamp, gray ‘islets’ may be present within the cap at the advancing edge of the pterygium (Fig. 18.3). First described by Fuchs, these ‘flecks’ or Fuchs’ islets represent the most advanced parts of the pterygium, which coalesce over time with the migrating boundary, to form tongue-shaped extensions at the apex.14 Iron deposition in the corneal epithelium, just apical to the head of the pterygium, is known as Stocker’s line.15
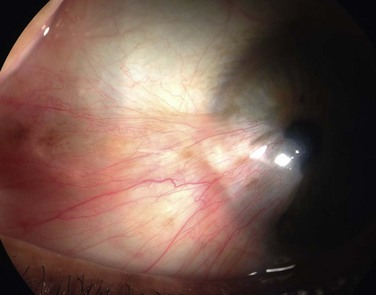
Figure 18.1 Primary pterygium.
Symptoms
Dry eye symptoms such as redness, irritation and blurred vision are common in pterygium.10 Reduced tear break-up time and abnormal tear-ferning have been reported with partial restoration of tear function once the pterygium is removed.16 Pterygium-associated visual disturbances are summarized in Box 18.1.
Histopathology
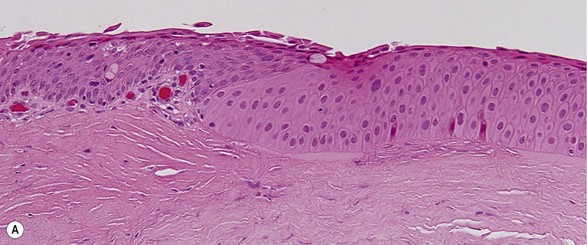
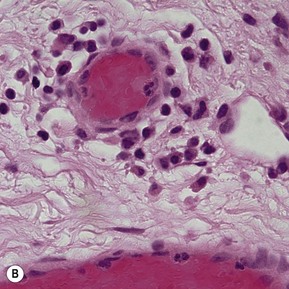
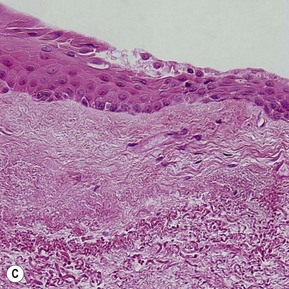
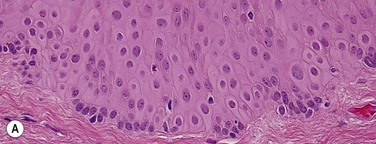
Topographic studies of the pterygium show that it is an epithelium-covered protuberance of connective tissue projecting over the ocular surface with an apex that extends in the direction of growth.17 The epithelium is characterized by centripetal growth of a leading edge of altered limbal epithelial cells,18 followed by abnormal conjunctival epithelium, with features of goblet cell hyperplasia and squamous metaplasia (Fig. 18.4A).19 The underlying connective tissue stroma consists of activated, proliferating fibroblasts and blood vessels.20 Chronic mixed inflammatory infiltrates may be present, comprising lymphocytes, plasma cells, mast cells, Langerhans cells, monocytes and macrophages, with neutrophils present in acutely inflamed lesions (Fig. 18.4B). Immunoglobulin (IgG and IgE) deposition along the basement membrane,21 aberrant expression of HLA-DR22 and adhesion molecules (ICAM-1 and VCAM-1)23 have also been reported. Extracellular matrix changes are prominent features of pterygium. These include dissolution of Bowman’s layer at the migrating head and accumulation of elastotic material within the stroma (Fig. 18.4C).
Differential Diagnosis
The differential diagnosis of pterygium includes pinguecula, pseudopterygium, and various conjunctival tumors. Although there are some similarities, these entities may be distinguished by morphology and clinical behavior. Of note, it is critical to consider limbal/conjunctival malignancy given that both pterygium and pinguecula may harbor epithelial dysplasia.24
Pinguecula
Pinguecula (pleural: pingueculae) appears as an elevated yellow white plaque in the conjunctiva adjacent to the nasal or temporal limbus. They are distinguished from pterygia by their lack of corneal invasion, possibly due to an intact limbal barrier. Although most pinguecula are slow growing with a benign course, it is recognized that some may evolve into pterygia.14 The histological changes of pinguecula are similar to those in pterygium and comprise predominantly subepithelial elastosis, hyaline degeneration, eosinophilic and basophilic concretions,25 squamous and metaplastic changes of the epithelium.26
Pseudopterygium and Other Inflammatory Conditions
Pseudopterygium originates from conjunctival adhesions to corneal defects as a result of trauma, previous surgery or inflammation. A key feature that differentiates pseudopterygia from true pterygia, is that the former may occur anywhere on the corneal circumference, whereas the latter are typically limited to the 3- and 9-o’clock position. The pseudopterygium has a broad, flat leading edge at its point of attachment, with the bulk of the lesion not adherent to the underlying cornea.27 Conditions that may be associated with pseudopterygium include Fuchs’ superficial marginal keratitis and Terrien’s marginal degeneration.28 Other inflammatory conditions to be considered include phlyctenular keratoconjunctivitis, nodular episcleritis and actinic granulomas.
Conjunctival Tumors
Benign and malignant tumors of the conjunctiva may be confused with pterygia. These include limbal dermoid, papilloma, amyloidosis, lymphoma of the conjunctiva, non-pigmented nevi, melanoma, conjunctival intraepithelial neoplasia and squamous cell carcinoma.29 Of special consideration are pre-neoplastic lesions, such as ocular surface squamous neoplasia30 and primary acquired melanosis,31 which may coexist with pterygia.32 A high index of suspicion should be maintained when managing pterygia with an atypical appearance. Given these lesions may not be clinically obvious, histological examination of all excised pterygia is recommended.
Epidemiology
Pterygium is present worldwide with prevalence rates depending on the age group and geographical location. In Cameron’s survey of the world’s distribution of pterygia,33 higher rates were observed in those living in peri-equatorial countries, including hot, dry and dusty climates. This is supported by an Australian study that showed a prevalence up to 15.2% in Aborigines living in the northern territories, compared to 4.5% in those living in the southern states. A positive correlation was seen with lifetime sun exposure.34 However, the high incidence of pterygia in Tibetans (14.5%)35 and Mongolians (17.8%)36 living in high altitudes but away from the equator, contradicts these pterygia epidemiology studies. The high incidence in Eskimos in Greenland (8.6%)37 and watermen in Chesapeake Bay (16.7%)38 results from reflected sunlight off snow or water. From these studies, it is clear that cumulative ultraviolet (UV) light exposure is a major risk factor for pterygia. Other risk factors include family history, increasing age, male gender, and rural residency, while wearing glasses or a hat have a protective effect.39,40 Certain groups, such as welders41, laborers and outdoor workers have increased incidence of pterygium, as a result of their occupational exposure.42 Pterygia are also more common in sawmill workers exposed to dusty environments, where chronic irritation and microtrauma is hypothesized to play a role in these cases.43 More recently, exposure to arsenic44 and petrochemicals45 have also been linked to pterygia.
Pathogenesis
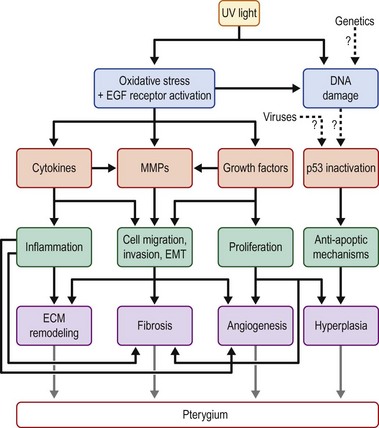
The pathogenesis of pterygium is poorly understood. The popular view of pterygium as a degenerative condition was based on histological evidence of the extracellular matrix degradation. However, this is contradicted by its invasive growth habit and propensity to recur when excised. In reality, both degenerative and hyperplastic processes are present in pterygium,1 with multiple mechanisms contributing to its formation (Fig. 18.5). These mechanisms may be divided into inherited factors, environmental triggers (UV light, viral infections) and factors that perpetuate its growth (cytokines, growth factors and matrix metalloproteinases). Cumulative DNA damage and activation of antiapoptotic factors may also contribute to the proliferative phenotype of pterygium, while the contribution of stem cells and neurogenic inflammation will also be discussed.
Ultraviolet Light
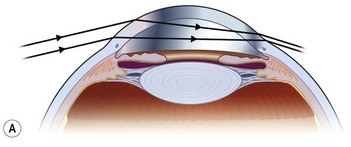
The role of chronic UV exposure in the pathogenesis of pterygium is well supported by epidemiological studies and its clinical associations with other UV-related conditions, such as photo-aged skin, cataracts, climatic droplet keratopathy, squamous cell and basal cell carcinomas.32 The curious growth habit of the pterygium and it predilection for the medial limbus is explained by our model of peripheral light focusing effect of the anterior chamber, which concentrates incidental light by 20× onto the medial limbus (Fig. 18.6).46 Chronic focal UV damage to this region is hypothesized to activate limbal stem cells, leading to formation of a pterygium.47 Also intriguing is autofluorescence of pterygia under UV light (300–400 nm) (Fig. 18.7),48 which may precede visible signs of an ocular surface lesion (Fig. 18.8).49 The cause for autofluorescence is unknown but we speculate that it may represent altered collagen or cellular activity as a result of solar damage.48 Additionally, pterygia share certain histological features with photo-aged skin including epidermal proliferation, inflammatory infiltrates, activated fibroblasts and extracellular matrix remodeling.3 To follow, we will discuss UV activated molecular mechanisms that are involved in the pathogenesis of pterygium.
Oxidative Stress and Growth Factor Receptor Signaling
UV-induced oxidative stress has been implicated in the pathogenesis of pterygium. Supportive evidence include the presence of 8-hydroxydeoxyguanosine (a DNA photo-oxidation product)50 and malondialdehyde (a product of lipid peroxidation),51 inducible nitric oxide synthase and nitric oxide (NO)52 in pterygium tissue. Reactive oxygen species (ROS), such as NO may act as a pro-angiogenic factor by mediating vascularization,53 endothelial proliferation and migration,54 MMP-2 activation,55 and potentially, MMP expression patterns in pterygia. Other ROS, such as hydrogen peroxide are known to activate epidermal growth factor receptors (EGFRs) and subsequent downstream signaling via the mitogen-activated protein kinase (MAPK) pathways, such as extracellular signal-regulated kinase (ERK), c-jun amino-terminal kinase (JNK) and p38.56 In pterygium cell cultures, UV activated JNK and p38 induces expression of pro-inflammatory cytokines,57 while activation of ERK is responsible for induction of MMP-1.58,59
Pro-inflammatory Cytokines and Immunological Mechanisms
Epidemiological studies have hinted that chronic inflammation from desiccation, chemical exposure and microtrauma may play a role in the pathogenesis of pterygia. Wong suggested a mechanism where inflammation at the junction of the conjunctival blood vessels and Bowman’s membrane degraded proteins which then act as angiogenic factors.60 The presence of mixed chronic inflammatory infiltrates including T lymphocytes, plasma cells, mast cells, Langerhans cells, monocytes, and macrophage supports the conclusion that immunological mechanisms21 might be involved. Although immune infiltrates may contribute to inflammation in pterygia, their presence are likely a consequence of cytokines and other pro-inflammatory mediators present in pterygium (Box 18.3).3 Interleukins (IL-1, IL-6, IL-8) and tumor necrosis factor-alpha (TNF-α) are known to be induced by UV light61 and NF-κB, signaling that is activated in pterygia.62,63 Their presence mediates influx of immune cells and induction of MMP expression in pterygia, while IL-4 may mediate fibrosis in recurrent lesions.64 Another UV-inducible enzyme that is overexpressed in pterygia is cycloxygenase-2 (COX-2), which converts arachidonic acid into prostaglandin.65 Its expression is associated with the anti-apoptotic factor survivin.66 COX-2 induces MMP-1 and MMP-9 in organ-cultured corneas,67 and may also contribute to elevated MMP expression in pterygia. S100 proteins are calcium-binding proteins that have roles in wound healing, inflammation and cancer,68 and have recently been described to be elevated in pterygium tissue69 and in the tears of patients with pterygia.70 The functional significance of S100 proteins in pterygia requires further study. However, their up-regulation may reflect induction by UV, cytokines or other environmental stressors.71,72 Stem cell factor (SCF) is also elevated in the plasma and ocular tissues of patients with pterygia.73,74 SCF attracts and induces maturation of mast cells,73 and their presence may promote fibrosis and neovascularization in pterygia.75 Langerhans cells have also been observed in pterygium tissue by immunohistochemical76 and in vivo confocal microscopy.77 It is speculated that a higher level of antigenic and mitogenic exposure,76 or the presence of cytokines in pterygia, might have aided in their recruitment and maturation.77 The role of Langerhans cells in pterygium pathogenesis requires further study but it was hypothesized that Langerhans cells may be involved in T-cell recruitment.78
It is interesting to note that while clinical evidence supports the notion that persistent inflammation may lead to postoperative recurrence,79 the quantity of infiltrating T cells in pterygia specimens did not correlate with clinical parameters, such as severity of inflammation or preoperative use of topical steroids or non-steroidal anti-inflammatory drugs.80 Additionally, recurrence was not predicted by histological appearance.81 This implies that inflammation does not act alone and that other factors may also contribute to recurrence of a pterygium.
Fibroangiogenic Growth Factors
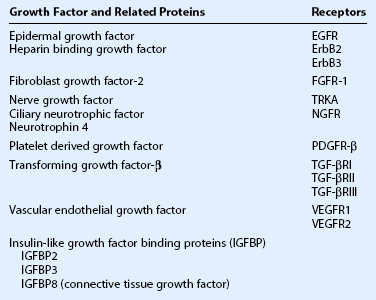
Growth factors and their receptors have been reported in pterygia (Table 18.1). They serve to induce proliferation and/or migration of epithelial cells, fibroblasts or vascular cells, which contribute to hyperplasia, fibrosis and angiogenesis in pterygium.3,81
Pro-fibrotic cytokines and growth factors expressed in pterygia include IL-1, TNF-α, CTGF, EGF family, FGF-2, PDGF, and TGF-β.82 Of these, TGF-β is particularly important since it induces myofibroblast differentiation, epithelial mesenchymal transition and alters synthesis of extracellular matrix components.83 Aberrant TGF-β signaling84,85 is thought to contribute to fibrosis in pterygia, and its suppression by amniotic membrane86 may explain the efficacy of this treatment.
Elevated pro-angiogenic factors (IL-8, TNF-α, FGF-2, HB-EGF and VEGF) combined with lack of angiogenic inhibitors (PEDF and thrombospondin-1) encourage prominent neovascularization in pterygia.82 VEGF, a major pro-angiogenic factor, is elevated in the tears, plasma and ocular tissues of patients with pterygia.74,87 VEGF expression in pterygia may be driven by multiple stimuli (UV, hypoxia, cytokines and growth factors) and probably represents a common pro-angiogenic pathway.3,82
More recently, neurotrophins and their receptors have been investigated in pterygia, where nerve growth factor (NGF), ciliary neurotrophic factor and neurotrophin-4/5 were reported to be elevated. In addition, the high- and low-affinity NGF receptors (TRKA and NGFR, respectively) have also been described in epithelial cells and blood vessels of pterygia.88–90 A pro-angiogenic role for neurotrophins is suggested by the correlation of NGF – TRKA staining with microvessel density in pterygia.89
Matrix Metalloproteinases and Extracellular Matrix Remodeling
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that degrade components of the extracellular matrix and cell surface molecules. These enzymes are counterbalanced by endogenous inhibitors, called tissue inhibitors of metalloproteinases (TIMPs). MMPs participate in ocular physiology, pathophysiology, and are key mediators of photo-aging, where they regulate proliferation, cell migration, inflammation and angiogenesis.91 Overexpression of MMPs relative to TIMPs is thought to contribute to the invasive phenotype of pterygia, where MMP expression may be induced by UV light, cytokines (IL-1 and TNF-α) and growth factors (EGF and TGF-α).3,82 Of interest is the association of MMP-2 and -9 with disease progression,92 suggesting that MMPs may be an attractive target for management of this disease.
Additional to extracellular matrix breakdown, altered synthesis of matrix components have been reported in pterygia, including tropoelastin,93 glycosaminoglycans,94 hyaluronic acid,95 periostin,64 fibulin-2 and fibulin-3.96 Altered matrix components may increase the bioavailability of heparin-binding growth factors and cytokines (e.g. IL-8, FGF-2, HB-EGF, VEGF, PDGF and TGF-β) which are normally sequestered by the extracellular matrix and released upon its degradation.97
Inherited Factors
Although environmental exposure plays a major role in the pathogenesis of pterygia, inherited factors may influence its development. Supporting this concept are families with pterygia where ocular disease often presents at a younger age and with an autosomal dominant inheritance pattern.98,99 Individuals with xeroderma pigmentosum,100 single nucleotide polymorphisms in 8-oxoguanine glycosylase 1 (hOGG1),101 X-ray repair cross-complementing-1 (XRCC1)102 or X-ray repair cross-complementing-6 (XRCC6),103 have been associated with pterygium development, suggesting defective DNA repair may contribute to the pathogenesis of this condition. Genetic variants in glutathione S-transferase M1 (GSTM1)104 and cytochrome P4501A1 (CYP1A1),105 enzymes that metabolize polycyclic aromatic hydrocarbons, are reported to be associated with pterygia. (See Box 18.4 for details on gene association studies in pterygia.) The CYP1A1 MspI polymorphism, in particular, is associated with accumulation of Benzo[a]pyrene 7,8 diol-9,10-epoxide (BPDE)-like DNA adducts in pterygium tissue,105 implying interactions between genetic and environmental factors in this condition.
Viral Infections
Based on Knudson’s hypothesis of cancer as a result of cumulative DNA mutations, Detorakis et al. proposed a role for oncogenic viruses in the pathogenesis of pterygia in their two-hit model.106 The ‘first hit’ is attributed to a genetic predisposition for pterygia or acquired DNA damage from UV exposure. The ‘second hit’ may be additional UV exposure or caused by infections, such as human papillomavirus (HPV) or herpes simplex virus (HSV).106 Evidence supporting HPV in the pathogenesis of pterygia include the detection of HPV subtypes 1, 2, 6, 11, 16, 18, 37, 52, 54 and HPV90 in pterygium tissue. However, the prevalence rate (0–100%) varies widely between studies.107 These findings might be explained by variable HPV infection rates between populations108 or methodological differences. A possible contributing mechanism might be the inactivation of p53 by viral oncoprotein (HPV 16/18 E6).109 Studies on HSV have been restricted to two centers with prevalence of 22% in Greece110 and 5% in Taiwan,111 and co-infection with HPV was associated with postoperative recurrence in one study.110 Given the limited and inconsistent data, viral infection is likely not an absolute requirement for pterygium formation.
Genetic and Epigenetic Changes
Some authors favor the idea that cumulative DNA damage106,112 might be involved in the pathogenesis of pterygia. Additional to the presence of 8-hydroxydeoxyguanosine (8-OHdG)50 and BPDE-like DNA adducts105 in pterygia of susceptible individuals, a number of genetic changes have been described including loss of heterozygosity, microsatellite instability,113 and mutations in Kirsten-ras114 and p53 genes.109,115,116 Of these, only p53 mutations have been studied extensively.
The tumor suppressor protein p53, is often referred to as the ‘guardian of the genome’. P53 is normally found in low levels due to its short half-life. In the presence of DNA damage, it stabilizes, translocates to the nucleus, inducing cell cycle arrest, DNA repair or apoptosis.117 Normal p53 function prevents accumulation of genetic aberrations and this gene is frequently mutated in cancers.118 Dushku and Reid proposed that inactivating mutations of p53 impairs programmed cell death and allows genetic mutations to accumulate, leading to the formation of pterygia.112 Using immunohistochemical methods, they observed elevated p53 in pterygia without concurrent apoptosis.112 Subsequently, others also reported elevated p53 staining in pterygia, relative to normal conjunctival tissues, but not all pterygia stained for p53,119 nor was p53 expression related to recurrence.120 DNA sequencing studies revealed four cases of monoallelic deletion116 and eight cases of point mutations.115 When DNA sequencing was paired with protein detection, it was observed that accumulation of p53 protein is not necessarily accompanied by p53 mutations,121 while deletion mutations may result in loss of p53 expression.115 Furthermore, viral oncoprotein inactivation of p53 has been suggested.109 Therefore, further studies are required to establish the true prevalence of p53 mutations and their role in the pathogenesis of pterygia.
Although little is known regarding the mechanisms involved, there is emerging evidence that epigenetic changes are present in pterygia. These include hypermethylation of the promoter of p16 (CDKN2A),122 E-cadherin (CDH1),123 transglutaminase 2 (TGM-2),124 and hypomethylation for MMP2 and CD24.124 The activity of DNA methyltransferases might be responsible. Chen et al. reported that 29% of pterygia expressed DNA methyltransferase 3b, a factor associated with hypermethylation of the p16 promoter and down-regulation of p16 protein.122 The role of aberrant methylation in pterygia requires further exploration.
Abnormal Proliferation and Anti-Apoptotic Mechanisms
Abnormal proliferation and anti-apoptotic factors have been documented in pterygia, which might explain their propensity for recurrence and the presence of a high proportion of dysplasia in some case series.24 Supporting evidence include elevated expression of anti-apoptotic proteins (BCL-2125 and survivin66) and cell cycle related molecules associated with proliferation (cyclin D1, Ki67 and proliferating cell nuclear antigen)126,127 and suppression of p27 (KIP1)128 in pterygium epithelial cells. Elevated DNA content is also reported in the fibrovascular layer,129 with pterygium fibroblasts exhibiting a transformed phenotype of reduced serum dependence and anchorage independent growth in culture,130 altered lipid metabolism131 and expression of insulin-like growth factor binding proteins, which are also known to be altered in cancers.132,133
Stem Cells
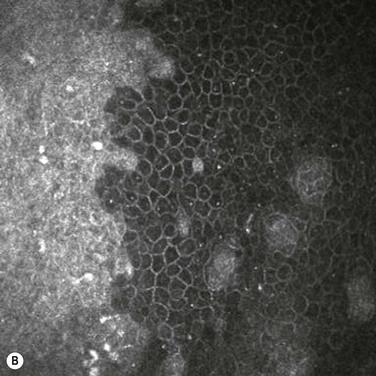
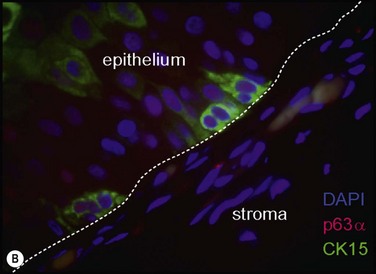
Chronic focal UV exposure was originally hypothesized to alter limbal stem cells, therefore, leading to an infiltrative phenotype that gave rise to a pterygium.18,47 Studies have now shown pterygium and limbal epithelium shared biomarkers, including cytokeratins,18 vimentin,18 telomerase,134 tumor protein p63,135 ATP-binding cassette, subfamily G, member 290 and nerve growth factor receptor.90 Our recent identification of stem-like cells corresponding to the clinical observation of Fuchs’ flecks at the advancing head of the pterygium (Fig. 18.9) lends further support to this theory.32 It is interesting to note that despite the presence of stem-like cells, pterygia also display features of focal limbal stem cell deficiency, including absent limbal palisades, conjunctival ingrowth and vascularization of the cornea. These changes respond to treatment by limbal autografts once the pterygium is excised.136,137 Therefore, one might speculate that pterygia primarily represent a dysfunction of limbal stem cells and the stem cell niche.
Epithelial Mesenchymal Transition
Epithelial mesenchymal transition (EMT), a process where epithelial cells take on characteristics of mesenchymal cells, has been described in pterygia. Specifically, two studies have shown pterygium epithelial cells concurrently expressed epithelial (cytokeratin 14) and mesenchymal markers (alpha-smooth muscle actin and vimentin), and signaling molecules associated with EMT (beta-catenin, snail and slug).138,139 This leads us to speculate that pterygium fibroblasts may have originated from limbal epithelium via EMT under the influences of TGF-β and FGF-2 or UV exposure.82 Alternatively, pterygium fibroblasts are hypothesized to be recruited from myofibroblasts in the periorbital fibroadipose tissue posterior to Tenon’s capsule.140
Bone Marrow-Derived Progenitor Cells
Bone marrow-derived progenitor cells expressing hematopoietic (CD34, AC133 or c-kit) or mesenchymal (STRO-1) markers have been reported in pterygium and are hypothesized to contribute to the fibrovascular stroma.141 Supporting this concept is the presence of bone marrow-derived cells in the normal corneal stoma,142 together with animal models showing recruitment of bone marrow-derived cells to areas of active fibrosis,143 and neovascularization.144 Ocular tissue hypoxia-induced cytokines are thought to act as chemoattractants to the eye.144 Lee et al. showed that patients with pterygia had elevated circulating CD34 and c-kit positive progenitor cells and plasma VEGF, stem cell factor and Substance P.74
Neurogenic Inflammation
The above theories do not explain some aspects of pterygia, such as its centripetal growth pattern, that gives rise to its characteristic wing-like appearance. To address this issue, a neurogenic model was proposed, whereby corneal nerves may influence the centripetal migration of corneal epithelium and pterygium cells.82,145 This model is based on several observations: (1) normal corneal epithelial turnover as summarized in the XYZ hypothesis;146 (2) the radial arrangement of corneal nerves and their close relationship with corneal epithelium and keratocytes;147 (3) involvement of sensory neurons in wound healing and inflammation;148 (4) the presence of myelinated and unmyelinated nerve fibers within the connective tissue mass of pterygia.149
The limbus is the focal point of chronic UV exposure, where limbal epithelium, stromal cells, blood vessels and the perilimbal nerve plexus may be damaged by UV light. The neuronal response to UV injury may also be an important trigger for the development of pterygia. UV exposure may induce release of sensory neuropeptides, such as substance P (SP) and calcitonin gene-related peptide (CGRP), which participate in neurogenic inflammation through their roles as vasodilators, immune cell chemoattractants, and by inducing cytokine production from resident corneal cells to amplify the inflammatory response.148 In pterygia, SP and its receptor (NK1R) are expressed by epithelial cells, fibroblasts and infiltrating immune cells, and have been shown to induce migration of pterygium fibroblasts and vascular endothelium, suggesting a fibroangiogenic role.145 The above evidence, together with the distribution of corneal nerves,147 suggests that corneal nerves might play a role in the development of pterygia.
Management
The ultimate aim of treating a pterygium is to restore the ocular surface anatomy, function, cosmesis, and alleviate all associated symptoms. This is not always achievable but having this goal may provide a therapeutic framework on which to base interventions. The aim should be for perfection, especially if surgery is contemplated. The eye has been termed the aesthetic center of the face and, in communicating, a great deal of attention is paid to ocular appearance and movement.150
Prevention
While prevention of ocular UV damage has remained an elusive goal, prevention of recurrence both short and long term remains a concern, particularly for high-risk groups, such as surfers. Advice about appropriate eye protection requires some consideration. It is unlikely that, in a brief consultation, advice about adequate wrap-around sunglasses and a brimmed hat is likely to change habits of a lifetime. We have found that demonstrating the peripheral light focusing effect (Fig. 18.10), especially while patients are wearing sunglasses, helps them understand the reasons for these recommendations. Specific advice about suppliers of appropriate sunglasses, particularly for patients with refractive error, is important since peripheral light can be difficult to block even with what might be considered an appropriate degree of side protection. Providing brief but pointed reading material is also helpful. In younger patients with early disease, demonstrating the lesions with anterior segment photography and fluorescence photography49 (see Fig. 18.8) has also been helpful. Serial photographs taken over many years can either reassure the patient that the condition is stable or help in making a decision to operate when growth is demonstrated.
Medical
There is little doubt that inflammation, acute and chronic, remains a crucial component for development of pterygia. The standard treatments of lubricants and topical anti-inflammatory drugs, such as nonsteroidal agents (NSAIDs) or steroids can be useful151 but often limited, particularly in advanced disease. While effective in the short term, NSAIDs in particular but also the newer low-dose formulation of dexamethasone (0.01%) can be effective,152 particularly in patients with recurrent disease in whom surgery is not desired. However, a more recent strategy is the use of topical cyclosporine 0.05%, which provides relatively safe medium- to long-term anti-inflammatory treatment and management of moderate to severe dry eye.153 Its use in the management of pterygium was proposed in 2006154 and it has since been used to prevent recurrences in the postoperative period.155,156 Given the setting of dry eye that appears common in patients with pterygia, it is safe and efficacious, although more formal studies are in process. Cyclosporine, apart from its anti-inflammatory effects, has multiple modes of action that may benefit treatment of pterygia. It is reassuring for some patients to undergo medical treatment before surgery is contemplated, particularly in patients with recurrent disease.
Surgical
A recent review of surgical treatment of pterygia,157 demonstrates that a wide variety of approaches exist, owing to the difficulty in curing this condition. This is hardly surprising given the relatively recent understanding of the pathophysiology (see Fig. 18.5) that involves stem cells and healing responses via complex pathways. Two key concepts in our approach are the use of reconstructive procedures and the control of the postoperative and any ongoing inflammatory response. Furthermore, there is a significant surgeon factor in outcomes that raises the issue of training, since the best chance of cure is at the first procedure and the risk of recurrence appears to increase with subsequent surgery. In our opinion, this should not be a procedure for the occasional surgeon.
The gold-standard technique is excision with autoconjunctival, reconstructive grafting, which provides low recurrence and complication rates, as well as excellent cosmesis158,160 but takes time and skill to perform. The procedure, first described in 1947161 and subsequently popularized,161 while seemingly straightforward, can be carried out with variations that could explain differences in reported recurrence rates. This procedure provides an excellent example of how reconstructive techniques are invariably superior to destructive surgical modalities.
Certain aspects of pterygium surgery deserve attention and are discussed below:
1. Transplantation of bulbar conjunctiva as opposed to limbal – conjunctival transplantation remains controversial. The general view is that it makes little difference if limbus is included and since there is the risk of limbal compromise at the donor site, many surgeons prefer to use conjunctival grafts. However, one study demonstrating that limbal transplantation was more effective than free conjunctival transplantation for treatment of recurrent pterygia.163 The long-standing concept that corneal epithelial stem cells reside mainly in the limbus has been challenged;164 it has been shown that basal cells of bulbar conjunctival epithelium share a similar expression pattern of stem cell-associated markers to the limbal epithelium.165 This may help explain why a limbal area appears to reform when conjunctival grafts are used.
2. Management of Tenon’s capsule also remains controversial. There is a long-held belief that Tenon’s capsule gives rise to recurrences and should therefore, be widely excised.159,160,166 Hirst described a technique known as, pterygium extended removal followed by extended conjunctival transplantation, which involves an extensive tenonectomy, followed by a large autoconjunctival graft. In his prospective series159,160 the recurrence rate for primary pterygium was 0.4% and there were no recurrences in the recurrent pterygium group. This was a single-surgeon series and intensive postoperative inflammatory treatment was used. Another approach for recurrent pterygia is to ‘seal the gap.’ After extensive excision of the pterygium and associated cicatrix, the medial fornix is reconstructed and the exposed areas are covered by conjunctival amniotic membrane grafts.167 A 6% recurrence rate was reported and one patient suffered from diplopia. An important part of these techniques is the adequate covering of underlying tissue by epithelial or membranous structures. This raises the possibility that the wound healing response is being modulated, in part by epithelial – stromal interactions168 and perhaps, with adequate graft cover, such extensive excisions are not necessary, particularly if the inflammatory response is controlled.
3. Inflammation is central to pterygium pathogenesis and surgical response, as well as the extent of signs, symptoms, growth and recurrence. In one study,169 inadequate use of postoperative topical steroids was associated with nearly triple the recurrence rate. Interestingly, in Hirst’s studies159,160 with low recurrence rates used topical steroids were used every 2 hours for the first 3 weeks with treatment extended to 9 weeks. More recently, topical cyclosporine has been used postoperatively following the high-recurrence rate bare sclera technique. This prospective, randomized study showed that topical cyclosporine 0.05% halved the recurrence rate from 44% in the control to 22% in the treatment group.156 It remains to be seen whether cyclosporine is as effective in the more sophisticated surgical techniques but it has a safer side effect profile.
4. Adjunctive antifibrotic treatment, such as mitomycin and beta irradiation, aims to modify the wound healing response by suppression of proliferation of tissues in the postoperative phase. By their nature, these are mostly destructive techniques, and all have had complications associated with their use. Unfortunately, serious complications, such as scleral necrosis can take more than a decade to manifest, yet it can take a generation for surgeons to change techniques. Accordingly, these techniques were the primary therapy to reduce pterygium recurrence for more than 50 years in Australia, yet it is only recently that practice patterns have changed after long-term studies reported a high rate of scleral necrosis and a moderately high recurrence rate.170,171 Since mitomycin is a ‘radiomimetic’ agent, there are concerns about its long-term safety, given the reports of serious complications with beta radiation use. While the short-term complications may be dose-dependent and doses have been adjusted downward following reports of blinding complications170 the long-term safety issue remains. Also, the potential role of damage to stem cells by adjunctive treatment is of interest, since these cells are critical to the long-term maintenance of ocular surface integrity.
Another aspect of the use of adjunctive treatments is the potential compromise of ocular tissue if future surgery is needed. Damaged scleral/episcleral vasculature can create problems, particularly for future conjunctival autografting techniques. Cameron noted that the aim of using irradiation was to destroy episcleral vessels, that could potentially provide nutrition for a recurrent pterygium.33 We have reported cases in which graft failure occurred following previous beta radiation or mitomycin used as adjunctive therapy in the initial surgical management for pterygia.172 This is of concern as adjunctive techniques can be associated with moderate recurrence rates. These observations resulted in our developing the use of adjuvant hyperbaric oxygen therapy to support autografts in the management of recurrent pterygium.173 This technique was associated with a very low recurrence rate and rapid healing of the graft (Fig. 18.11). It should be noted that hyperbaric oxygen is also useful in the management of scleral melts associated with pterygium procedures.174
A number of randomized clinical trials have examined recurrence rates after using amniotic membrane grafts to cover the excision defect, as compared to conjunctival autografting.175 These studies have all demonstrated a significantly lower pterygium recurrence rate in the conjunctival autograft group. Furthermore, amniotic membrane is not readily available in parts of the world where pterygia are common. The expense of amniotic membrane may be a problem in some parts of the world.
More recently, pterygium neovascularization, a process driven by elevated expression of multiple proangiogenic growth factors and cytokines, as well as a decrease in angiogenic inhibitors,82 has been proposed as a target in both primary and in particular, recurrent pterygia. An attractive therapeutic target is VEGF, a potent proangiogenic growth factor that induces corneal neovascularization in the absence of inflammation. VEGF antagonists, such as humanized monoclonal antibodies targeting VEGF (Bevacizumab and Ranibizumab), have become readily available. VEGF has been known to be present in pterygia for over a decade,52 being initially described in the epithelium of the head of the pterygium. A number of subsequent studies have confirmed this finding and have described VEGF in both basal and superficial epithelium, as well as in vascular endothelium and macrophages.3,57,176 VEGF levels were found to be significantly higher in recurrent, compared with primary pterygium.177 Since increased vascularity contributes to poor cosmesis and requests for early intervention, a logical tactic is to develop antiangiogenic/anti-VEGF therapy to induce regression of blood vessels and possibly retard pterygium progression.
Several small clinical studies have been conducted178,179 to treat primary and recurrent pterygia. Treatments have been delivered intra- or postoperatively with either topical therapy or intralesional injections. Results have been mixed, with some reports of an early but unsustained response.178 There is little effect on recurrence rate, according to published reports.179 Given the complexity of angiogenesis, it may be necessary to use combination angiostatic therapies to target multiple pathways.180 An indication that this might be necessary is the recent finding that COX-2 and VEGF are highly expressed in macrophages infiltrating human pterygia. This finding suggests a bridge between the immune process and the tumor-like characteristics of a pterygium because both COX-2 and VEGF are important in skin carcinogenesis, induced by UV light damage.176 Topical NSAIDs, inhibitors of COX, have been found to be useful in the management of pterygia151,181 and thus, could be used in combination with VEGF inhibitors.
Corticosteroids are another inhibitor of neovascularization. Preliminary data on anecortave acetate suggest an inhibitory effect on recurrent pterygium182 but it was not compared with other anti-inflammatory treatments.
Another approach has been to target another component of the pterygium pathogenesis cascade, matrix metalloproteinases, with an inhibitor such as doxycycline. This drug has been shown to inhibit, the growth of cultured pterygium epithelia cells183 but clinical data are not yet available.
5. A useful approach to the surgical time factor has been the advent of the ‘cut and paste technique,’ utilizing tissue glue to rapidly attach graft tissue during the procedure.184 A concern with these techniques is their safety with large grafts which in our view they provide excellent functional and cosmetic results. Of particular concern is the integrity of the limbal sutures, since if these break or dislodge, the graft can retract and compromise outcomes. A hybrid technique involving two limbal sutures and tissue gluing of the rest of the graft has been described (Ezra Maguen, personal communication) and may prove to be an excellent compromise for large grafts.
Recently, a promising technique that uses a simple method of ‘tissue welding’ wherein the graft is opposed to host conjunctiva with a tying forceps and a weld achieved by touching the forceps with a hand-held cautery has been described.185 Again, the strength of these welds, particularly in large grafts, must be assessed in larger multicenter studies.
6. Another aspect of pterygium surgery is the pain associated with surgery.186,187 The procedure can be one of the most painful ophthalmic procedures currently performed, hardly surprising given the extent of the ocular surface involved, particularly when an autograft is harvested. While the procedure is frequently carried out under topical anesthesia, we prefer peribulbar blocks, particularly for recurrent cases. Alternatively, long-acting topical anesthetics or gel preparations may be used.188
Another useful strategy is the use of a contact lens placed at the end of surgery and left in place during the acute healing phase.189 We routinely use a Balafilcon A extended-wear contact lens for a week after surgery and have found that this lens is less conducive to ocular surface cell adherence.190
7. Unsuspected neoplasia is present in ≈10–30% of specimens32 and it has been suggested that all pterygia specimens should be submitted for histopathologic examination.30,32 For cases in which neoplasia is detected, more frequent postoperative surveillance is recommended; however, such cases can be treated with topical interferon with or without retinoic acid, a very effective medical treatment for this condition.191,192
8. In very advanced cases, especially if there is bilateral disease (Fig. 18.12), there may be a relative shortage of conjunctival tissue that can be used in reconstruction. While other types of mucus or amniotic membrane can be used, reconstruction using a serum-free derived autologous cultivated conjunctival equivalent derived from ex vivo expansion of conjunctival epithelial cells on human amniotic membranes has been successfully utilized.193 While this technique can only be carried out in specialized units, improvements in stem cell transplantation holds promise for greater use of this technique. Potentially, it could obviate the need for creating autoconjunctival grafts.
Author’s Technique
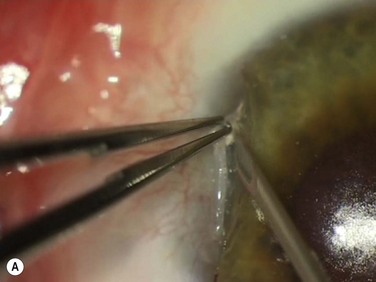
Following adequate anesthesia (typically a peribulbar block with long-acting anesthetic) a few drops of brimonidine are applied as a vasoconstrictor. Brimonidine does not dilate the pupil like adrenaline. Half-strength povidone is applied and careful lash-excluding draping is carried out. The head of the pterygium is excised using a 23-gauge needle-tip technique194 (Fig. 18.13A). This technique minimizes the amount of corneal tissue excised and even though a relatively rough stromal surface is left behind, epithelial smoothing results in an even ocular surface. This also allows complete removal of abnormal tissue from the cornea, since residual tissue left on the cornea is a cause of concern for many patients. For a right-handed surgeon operating on a left eye, the microscope can be moved so pterygium head dissection is carried out from the side.
The body of the pterygium is then excised, taking care to:
1. Excise tissue for about 1 mm above and below these sites where the pterygium crosses the limbus. This is because abnormal pterygium epithelial cells have been described in these locations18 and also because some recurrences appear to arise from these sites.
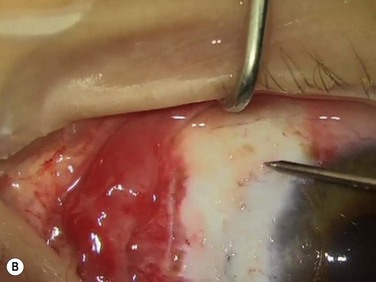
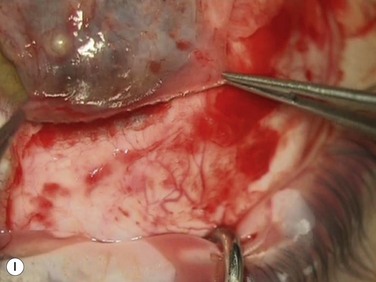
2. Excise inflamed tissue, sometimes as far as the caruncle (Fig. 18.13B). This is essential in obtaining good cosmesis. Care is taken to remain superficial and to avoid the check ligaments and capsule in a primary pterygium. Any excess Tenon’s capsule underlying this excised area is also trimmed but ‘fishing for Tenon’s’ by pulling on exposed connective tissue is avoided.
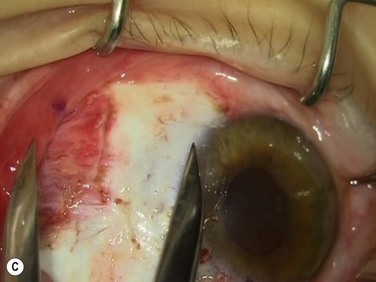
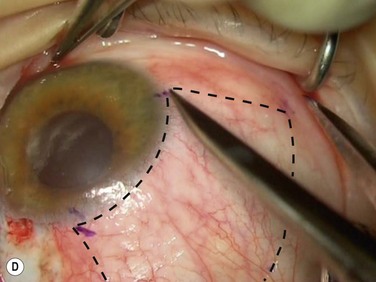
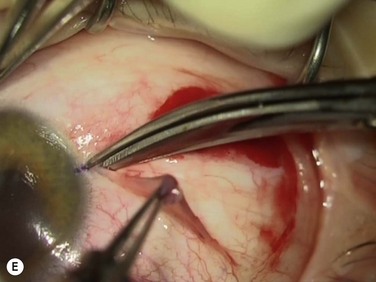
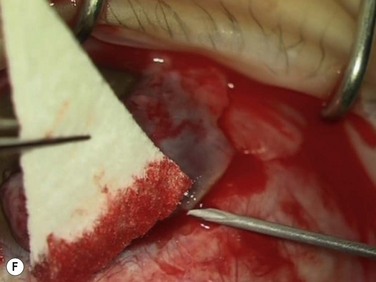
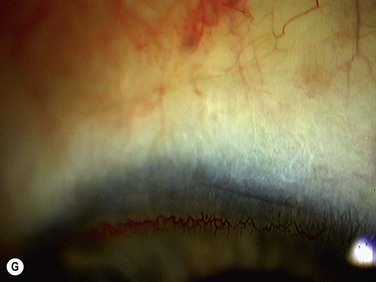
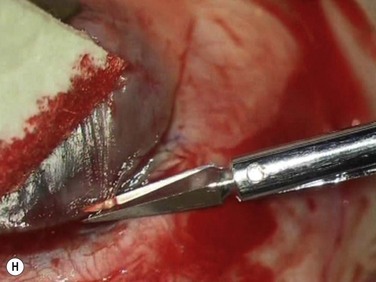
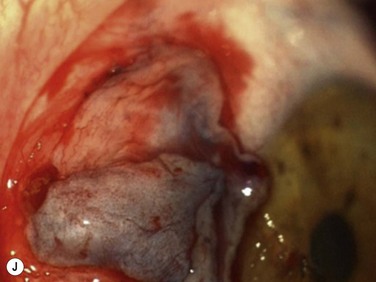
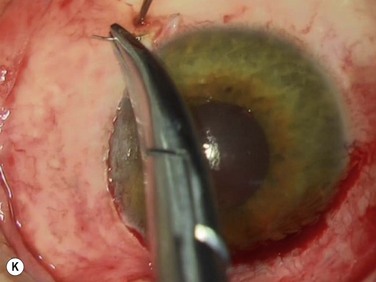
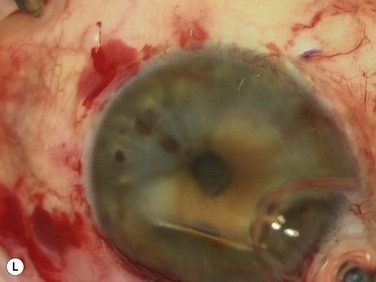
Bleeding is minimized with a minimalist use of an intraocular diathermy (Fig. 18.13B). Care is taken to check for bleeding in the cut edges of the conjunctiva, particularly in the region of the caruncle. The size of the defect is measured (Fig. 18.13C) and the graft size is marked (Fig. 18.13D). Not infrequently, the area to be dissected extends to the superior fornix. Using a squint hook to pull upwards on the superior blade of the speculum can enhance exposure in this area. Westcott-style spring scissors are used to create a thin flap (Fig. 18.13E). Such scissors can vary in size, tip configuration and blade sharpness, we favor those made by Martin (35-822-11), which are wrapped and sterilized separately from other instruments. The scissors are replaced if judged to be blunt. An assistant’s help in holding up the proximal aspect of the graft is useful, particularly for large grafts. The dissected graft is then folded down over the cornea (Fig. 18.13F). Excessive Tenon’s can be trimmed from this exposed under-surface of the graft, using a toothed forceps to engage the Tenon’s excess. Also, any buttonholes can be repaired, as the knot will be located on the under-surface of the graft. The short end of a Stroll wedge is used to pull the graft inferiorly and the limbus is dissected (Fig. 18.13F). There is often bleeding because of perforating vessels in this area and this is controlled with a second wedge. This maneuver allows a splitting of the limbus (Fig. 18.13G and H). The graft is then detached from the remaining limbus (Fig. 18.13I). The cut edges have a crenellated appearance, believed to be due to the morphology of the palisades of Vogt. The graft (Fig. 18.13J) is slid across a balanced salt-lubricated cornea, flipped over and placed in the defect created by the pterygium excision. Trypan blue can be used to check graft orientation, as this dye stains Tenon’s capsule preferentially to the epithelial surface (Fig. 18.13K). The graft is sutured in place commencing with limbal sutures (Fig. 18.13L). An extended-wear contact lens is placed at the end of the procedure. Care is taken to make sure that the contact lens covers the limbal aspect of the graft. The postoperative eye drop regimen consists of chloramphenicol, prednefrin forte and preservative-free diclofenac used four times daily and usually tapered after 1 week. Topical treatment is typically 4–6 weeks. If excessive inflammation occurs, topical treatment is prolonged and topical cyclosporine may be introduced.
For recurrent pterygia, a forced duction is used to help determine the extent of fibrosis of tissues between the recurrence site and medial rectus muscle. In severe cases the medial rectus muscle is isolated and appropriate dissection with excision of Tenon’s capsule carried out, forced duction tests being repeated to make sure that all fibrotic bands are cut. Such cases require extensive grafts. In cases of unilateral pterygium with inadequate superior bulbar conjunctiva, donor tissue can be taken from the other eye. This is discussed with the patient preoperatively. All such cases receive hyperbaric oxygen for a week after surgery.173 This technique has evolved and has a recurrence rate of ≈1%.
Training of Surgeons
A critical issue in the management of this prevalent disease is surgeon training. It is recognized that the widespread use of phacoemulsification has deprived generations of ophthalmologists of the ability to suture. Even with glue techniques, conjunctival graft suturing remains important, since the cost of glues makes this technique prohibitive in some settings and there are concerns about glue efficacy in very large grafts. For these reasons, we have developed a porcine eye model, for both teaching the creation and suturing of conjunctival grafts and for developing improved graft techniques.195
The Future
Advances in our understanding of the basic pathophysiology of a pterygium are likely to result in more sophisticated pharmacological interventions.82 It appears that the use of topical cyclosporine, with its multiple actions, is an early manifestation of this approach. There is a great need to develop animal models for this disease not only to improve our understanding of pathophysiology but also to better test surgical techniques. The current model,183 based on transplantation of cultured human pterygium epithelial cells into athymic nude mice, may not adequately represent human disease, and size may limit its use as a surgical model.196 Stem cell culture and/or graft techniques have the potential to replace the need for conjunctival graft dissection. Use of sophisticated laser techniques might allow faster, more accurate graft creation and better attachment techniques will also help to shorten surgical time.
Development and use of a grading scale for pterygium, perhaps using more advanced imaging techniques, such as optical coherence tomography,9,197 might aid in the evaluation of various techniques. Standardization and optimization of postoperative treatment regimes, will also allow better comparison of different techniques.
More sophisticated public health campaigns based on detection of early UV damage49 and perhaps self-monitoring using readily available cameras, may raise awareness of this disease and the need for adequate sun protection. The use of ultraviolet-blocking contact lenses, for the first time, has meant that the limbus can be protected from ultraviolet light.7 The inadequacy of current sunglass designs has been acknowledged in the new sunglass standards and provides a challenge for manufacturers to design better protection devices.7 The role of diet, known to confer protection against ultraviolet skin damage, as well as dry eye, requires investigation.198 There is also a need to overcome the trivialization of this disease, which in parts of the world, is a blinding condition and which even when managed adequately, has long-term consequences for the cornea.
References
1. Duke-Elder, S. Diseases of the outer eye. Part 1. In: Duke-Elder S, ed. System of Ophthalmology: volume 8 Diseases of the outer eye. London: Kimpton UK; 1965:569–585.
2. Murube, J. Pterygium: descriptive nomenclature of the past. Ocul Surf. 2008;6(3):104–107. [Epub 2008/09/11].
3. Di Girolamo, N, Chui, J, Coroneo, MT, et al. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23(2):195–228. [Epub 2004/04/20].
4. Wlodarczyk, J, Whyte, P, Cockrum, P, et al. Pterygium in Australia: a cost of illness study. Clin Exp Ophthalmol. 2001;29(6):370–375. [Epub 2002/01/10].
5. Sopoaga, F, Buckingham, K, Paul, C. Causes of excess hospitalizations among Pacific peoples in New Zealand: implications for primary care. J Prim Health Care. 2010;2(2):105–110. [Epub 2010/08/10].
6. Ramke, J, Brian, G, du Toit, R. Eye disease and care at hospital clinics in Cook Islands, Fiji, Samoa and Tonga. Clin Exp Ophthalmol. 2007;35(7):627–634. [Epub 2007/09/27].
7. Coroneo, M. Ultraviolet radiation and the anterior eye. Eye Contact Lens. 2011;37(4):214–224. [Epub 2011/06/15].
8. Shibata, N, Kitagawa, K, Sakamoto, Y, et al. Changes in corneal higher order aberration after pterygium excision. ARVO Meeting Abstracts. 2010;51(5):2399.
9. Ashizawa, J, Hori, Y, Saishin, Y, et al. Use of Fourier-domain optical coherence tomography to assess pterygium surgery. ARVO Meeting Abstracts. 2010;51(5):2408.
10. Lee, AJ, Lee, J, Saw, SM, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002;86(12):1347–13451. [Epub 2002/11/26].
11. Stern, ME, Pflugfelder, SC. Inflammation in dry eye. Ocul Surf. 2004;2(2):124–130. [Epub 2007/01/12].
12. Dolezalova, V. Is the occurrence of a temporal pterygium really so rare? Ophthalmologica. 1977;174(2):88–91. [Epub 1977/01/01].
13. Tan, DTH. Pterygium. In: Holland EJ, Mannis MJ, eds. Ocular surface disease: medical and surgical management. 1st ed. Springer-Verlag; 2002:65–89.
14. Fuchs, E. Ueber das Pterygium. Graefes Arch for Ophthalmol. 1892;38(2):1–89.
15. Hansen, A, Norn, M. Astigmatism and surface phenomena in pterygium. Acta Ophthalmol (Copenh). 1980;58(2):174–181. [Epub 1980/04/01].
16. Li, M, Zhang, M, Lin, Y, et al. Tear function and goblet cell density after pterygium excision. Eye. 2007;21(2):224–228. [Epub 2005/12/13].
17. Seifert, P, Eckert, J, Spitznas, M. Topological-histological investigation of the pterygium. Graefes Arch Clin Exp Ophthalmol. 2001;239(4):288–293. [Epub 2001/07/14].
18. Dushku, N, Reid, TW. Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr Eye Res. 1994;13(7):473–481. [Epub 1994/07/01].
19. Chan, CM, Liu, YP, Tan, DT. Ocular surface changes in pterygium. Cornea. 2002;21(1):38–42. [Epub 2002/01/24].
20. Seifert, P, Sekundo, W. Capillaries in the epithelium of pterygium. Br J Ophthalmol. 1998;82(1):77–81.
21. Pinkerton, OD, Hokama, Y, Shigemura, LA. Immunologic basis for the pathogenesis of pterygium. Am J Ophthalmol. 1984;98(2):225–228.
22. Ioachim-Velogianni, E, Tsironi, E, Agnantis, N, et al. HLA-DR antigen expression in pterygium epithelial cells and lymphocyte subpopulations: an immunohistochemistry study. Ger J Ophthalmol. 1995;4(2):123–129. [Epub 1995/03/01].
23. Tekelioglu, Y, Turk, A, Avunduk, AM, et al. Flow cytometrical analysis of adhesion molecules, T-lymphocyte subpopulations and inflammatory markers in pterygium. Ophthalmologica. 2006;220(6):372–378. [Epub 2006/11/11].
24. Clear, AS, Chirambo, MC, Hutt, MS. Solar keratosis, pterygium, and squamous cell carcinoma of the conjunctiva in Malawi. Br J Ophthalmol. 1979;63(2):102–109. [Epub 1979/02/01].
25. Hogan, MJ, Alvarado, J. Pterygium and pinguecula: electron microscopic study. Arch Ophthalmol. 1967;78(2):174–186. [Epub 1967/08/01].
26. Dong, N, Li, W, Lin, H, et al. Abnormal epithelial differentiation and tear film alteration in pinguecula. Invest Ophthalmol Vis Sci. 2009;50(6):2710–2715. [Epub 2009/02/03].
27. Soliman, W, Mohamed, TA. Spectral domain anterior segment optical coherence tomography assessment of pterygium and pinguecula. Acta Ophthalmol. 2012;90(5):461–465. [Epub 2010/11/03].
28. Keenan, JD, Mandel, MR, Margolis, TP. Peripheral ulcerative keratitis associated with vasculitis manifesting asymmetrically as fuchs superficial marginal keratitis and terrien marginal degeneration. Cornea. 2011;30(7):825–827. [Epub 2010/12/15].
29. Seitz, B, Fischer, M, Holbach, LM, et al. [Differential diagnosis and prognosis of 112 excised epibulbar epithelial tumors]. Klin Monbl Augenheilkd. 1995;207(4):239–246. [Epub 1995/10/01. Differential diagnose und Prognose bei 112 exzidierten epibulbaren epithelialen Tumoren].
30. Hirst, LW, Axelsen, RA, Schwab, I. Pterygium and associated ocular surface squamous neoplasia. Arch Ophthalmol. 2009;127(1):31–32. [Epub 2009/01/14].
31. Perra, MT, Colombari, R, Maxia, C, et al. Finding of conjunctival melanocytic pigmented lesions within pterygium. Histopathology. 2006;48(4):387–393. [Epub 2006/02/21].
32. Chui, J, Coroneo, MT, Tat, LT, et al. Ophthalmic pterygium a stem cell disorder with premalignant features. Am J Pathol. 2011;178(2):817–827. [Epub 2011/02/02].
33. Cameron, ME. Pterygium throughout the world. Springfield, Ill.: Thomas; 1965.
34. Moran, DJ, Hollows, FC. Pterygium and ultraviolet radiation: a positive correlation. Br J Ophthalmol. 1984;68(5):343–346. [Epub 1984/05/01].
35. Lu, P, Chen, X, Kang, Y, et al. Pterygium in Tibetans: a population-based study in China. Clin Exp Ophthalmol. 2007;35(9):828–833. [Epub 2008/01/05].
36. Lu, J, Wang, Z, Lu, P, et al. Pterygium in an aged Mongolian population: a population-based study in China. Eye. 2009;23(2):421–427. [Epub 2007/10/20].
37. Norn, MS. Spheroid degeneration of cornea and conjunctiva. Prevalence among Eskimos in Greenland and caucasians in Copenhagen. Acta Ophthalmol (Copenh). 1978;56(4):551–562. [Epub 1978/01/01].
38. Taylor, HR, West, SK, Rosenthal, FS, et al. Corneal changes associated with chronic UV irradiation. Arch Ophthalmol. 1989;107(10):1481–1484. [Epub 1989/10/01].
39. Threlfall, TJ, English, DR. Sun exposure and pterygium of the eye: a dose-response curve. Am J Ophthalmol. 1999;128(3):280–287. [Epub 1999/10/08].
40. McCarty, CA, Fu, CL, Taylor, HR. Epidemiology of pterygium in Victoria, Australia. Br J Ophthalmol. 2000;84(3):289–292. [Epub 2000/02/24].
41. Karai, I, Horiguchi, S. Pterygium in welders. Br J Ophthalmol. 1984;68:347–349. [Epub 1984/05/01].
42. Wong, TY, Foster, PJ, Johnson, GJ, et al. The prevalence and risk factors for pterygium in an adult Chinese population in Singapore: the Tanjong Pagar survey. Am J Ophthalmol. 2001;131(2):176–183. [Epub 2001/03/03].
43. Okoye, OI, Umeh, RE. Eye health of industrial workers in Southeastern Nigeria. West Afr J Med. 2002;21(2):132–137. [Epub 2002/10/31].
44. Lin, W, Wang, SL, Wu, HJ, et al. Associations between arsenic in drinking water and pterygium in southwestern Taiwan. Environ Health Perspect. 2008;116(7):952–955. [Epub 2008/07/17].
45. Omoti, AE, Waziri-Erameh, JM, Enock, ME. Ocular disorders in a petroleum industry in Nigeria. Eye. 2008;22(7):925–929. [Epub 2007/04/03].
46. Coroneo, MT, Müller-Stolzenburg, NW, Ho, A. Peripheral light focusing by the anterior eye and the ophthalmohelioses. Ophthalmic Surg. 1991;22:705–711.
47. Coroneo, MT. Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993;77(11):734–739. [Epub 1993/11/01].
48. Ooi, JL, Sharma, NS, Sharma, S, et al. Ultraviolet fluorescence photography: patterns in established pterygia. Am J Ophthalmol. 2007;143(1):97–101. [Epub 2006/11/23].
49. Ooi, JL, Sharma, NS, Papalkar, D, et al. Ultraviolet fluorescence photography to detect early sun damage in the eyes of school-aged children. Am J Ophthalmol. 2006;141(2):294–298. [Epub 2006/02/07].
50. Tsai, YY, Cheng, YW, Lee, H, et al. Oxidative DNA damage in pterygium. Mol Vis. 2005;11:71–75.
51. Lu, L, Wang, R, Song, X. [Pterygium and lipid peroxidation]. Zhonghua Yan Ke Za Zhi. 1996;32(3):227–229.
52. Lee, DH, Cho, HJ, Kim, JT, et al. Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea. 2001;20(7):738–742. [Epub 2001/10/06].
53. Kon, K, Fujii, S, Kosaka, H, et al. Nitric oxide synthase inhibition by N(G)-nitro-L-arginine methyl ester retards vascular sprouting in angiogenesis. Microvasc Res. 2003;65(1):2–8.
54. Ziche, M, Morbidelli, L, Masini, E, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94(5):2036–2044.
55. Brown, DJ, Lin, B, Chwa, M, et al. Elements of the nitric oxide pathway can degrade TIMP-1 and increase gelatinase activity. Mol Vis. 2004;10:281–288.
56. Peus, D, Vasa, RA, Meves, A, et al. H2O2 is an important mediator of UVB-induced EGF-receptor phosphorylation in cultured keratinocytes. J Invest Dermatol. 1998;110(6):966–971.
57. Di Girolamo, N, Wakefield, D, Coroneo, MT. UVB-mediated induction of cytokines and growth factors in pterygium epithelial cells involves cell surface receptors and intracellular signaling. Invest Ophthalmol Vis Sci. 2006;47(6):2430–2437. [Epub 2006/05/26].
58. Di Girolamo, N, Coroneo, MT, Wakefield, D. UVB-elicited induction of MMP-1 expression in human ocular surface epithelial cells is mediated through the ERK1/2 MAPK-dependent pathway. Invest Ophthalmol Vis Sci. 2003;44(11):4705–4714. [Epub 2003/10/28].
59. Di Girolamo, N, Coroneo, M, Wakefield, D. Epidermal growth factor receptor signaling is partially responsible for the increased matrix metalloproteinase-1 expression in ocular epithelial cells after UVB radiation. Am J Pathol. 2005;167(2):489–503. [Epub 2005/07/29].
60. Wong, WW. A hypothesis on the pathogenesis of pterygiums. Ann Ophthalmol. 1978;10(3):303–308. [Epub 1978/03/01].
61. Kennedy, M, Kim, KH, Harten, B, et al. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthalmol Vis Sci. 1997;38(12):2483–2491. [Epub 1997/12/31].
62. Siak, JJ, Ng, SL, Seet, LF, et al. The nuclear-factor kappaB pathway is activated in pterygium. Invest Ophthalmol Vis Sci. 2011;52(1):230–236. [Epub 2010/09/03].
63. Torres, J, Enriquez-de-Salamanca, A, Fernandez, I, et al. Activation of MAPK signaling pathway and NF-kappaB activation in pterygium and ipsilateral pterygium-free conjunctival specimens. Invest Ophthalmol Vis Sci. 2011;52(8):5842–5852. [Epub 2011/06/21].
64. Kuo, CH, Miyazaki, D, Yakura, K, et al. Role of periostin and interleukin-4 in recurrence of pterygia. Invest Ophthalmol Vis Sci. 2010;51(1):139–143. [Epub 2009/08/08].
65. Chiang, CC, Cheng, YW, Lin, CL, et al. Cyclooxygenase 2 expression in pterygium. Mol Vis. 2007;13:635–638. [Epub 2007/05/23].
66. Maxia, C, Perra, MT, Demurtas, P, et al. Relationship between the expression of cyclooxygenase-2 and survivin in primary pterygium. Mol Vis. 2009;15:458–463. [Epub 2009/02/28].
67. Ottino, P, Bazan, HE. Corneal stimulation of MMP-1, -9 and uPA by platelet-activating factor is mediated by cyclooxygenase-2 metabolites. Curr Eye Res. 2001;23(2):77–85.
68. Eckert, RL, Broome, AM, Ruse, M, et al. S100 proteins in the epidermis. J Invest Dermatol. 2004;123(1):23–33. [Epub 2004/06/12].
69. Riau, AK, Wong, TT, Beuerman, RW, et al. Calcium-binding S100 protein expression in pterygium. Mol Vis. 2009;15:335–342. [Epub 2009/02/19].
70. Zhou, L, Beuerman, RW, Ang, LP, et al. Elevation of human alpha-defensins and S100 calcium-binding proteins A8 and A9 in tear fluid of patients with pterygium. Invest Ophthalmol Vis Sci. 2009;50(5):2077–2086. [Epub 2009/01/27].
71. Lee, YM, Kim, YK, Eun, HC, et al. Changes in S100A8 expression in UV-irradiated and aged human skin in vivo. Arch Dermatol Res. 2009;301(7):523–529. [Epub 2009/05/26].
72. Lim, SY, Raftery, MJ, Goyette, J, et al. Oxidative modifications of S100 proteins: functional regulation by redox. J Leukoc Biol. 2009;86(3):577–587. [Epub 2009/02/25].
73. Nakagami, T, Watanabe, I, Murakami, A, et al. Expression of stem cell factor in pterygium. Jpn J Ophthalmol. 2000;44(3):193–197. [Epub 2000/07/29].
74. Lee, JK, Song, YS, Ha, HS, et al. Endothelial progenitor cells in pterygium pathogenesis. Eye. 2007;21(9):1186–1193. [Epub 2006/05/30].
75. Ribatti, D, Nico, B, Maxia, C, et al. Neovascularization and mast cells with tryptase activity increase simultaneously in human pterygium. J Cell Mol Med. 2007;11(3):585–589. [Epub 2007/07/20].
76. Chen, YT, Tseng, SH, Tsai, YY, et al. Distribution of vimentin-expressing cells in pterygium: an immunocytochemical study of impression cytology specimens. Cornea. 2009;28(5):547–552. [Epub 2009/05/08].
77. Zhivov, A, Beck, R, Guthoff, RF. Corneal and conjunctival findings after mitomycin C application in pterygium surgery: an in-vivo confocal microscopy study. Acta Ophthalmol. 2009;87(2):166–172. [Epub 2008/06/10].
78. John-Aryankalayil, M, Dushku, N, Jaworski, CJ, et al. Microarray and protein analysis of human pterygium. Mol Vis. 2006;12:55–64. [Epub 2006/02/01].
79. Kheirkhah, A, Casas, V, Sheha, H, et al. Role of conjunctival inflammation in surgical outcome after amniotic membrane transplantation with or without fibrin glue for pterygium. Cornea. 2008;27(1):56–63. [Epub 2008/02/05].
80. Awdeh, RM, DeStafeno, JJ, Blackmon, DM, et al. The presence of T-lymphocyte subpopulations (CD4 and CD8) in pterygia: evaluation of the inflammatory response. Adv Ther. 2008;25(5):479–487. [Epub 2008/05/17].
81. Rohrbach, IM, Starc, S, Knorr, M. [Predicting recurrent pterygium based on morphologic and immunohistologic parameters]. Ophthalmologe. 1995;92(4):463–468. [Epub 1995/08/01. Vorhersage von Pterygiumrezidiven aufgrund morphologischer und immunhistologischer Parameter].
82. Chui, J, Di Girolamo, N, Wakefield, D, et al. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf. 2008;6(1):24–43. [Epub 2008/02/12].
83. Saika, S, Yamanaka, O, Sumioka, T, et al. Fibrotic disorders in the eye: targets of gene therapy. Prog Retin Eye Res. 2008;27(2):177–196. [Epub 2008/02/05].
84. Kria, L, Ohira, A, Amemiya, T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 1996;98(2):195–201. [Epub 1996/04/01].
85. Kria, L, Ohira, A, Amemiya, T. Growth factors in cultured pterygium fibroblasts: immunohistochemical and ELISA analysis. Graefes Arch Clin Exp Ophthalmol. 1998;236(9):702–708. [Epub 1998/10/23].
86. Lee, SB, Li, DQ, Tan, DT, et al. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 2000;20(4):325–334. [Epub 2000/05/12].
87. Aspiotis, M, Tsanou, E, Gorezis, S, et al. Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye. 2007;21(8):1095–1101. [Epub 2006/07/11].
88. Hong, S, Choi, JY, Lee, HK, et al. Expression of neurotrophic factors in human primary pterygeal tissue and selective TNF-alpha-induced stimulation of ciliary neurotrophic factor in pterygeal fibroblasts. Exp Toxicol Pathol. 2008;60(6):513–520. [Epub 2008/07/01].
89. Ribatti, D, Nico, B, Perra, MT, et al. Correlation between NGF/TrkA and microvascular density in human pterygium. Int J Exp Pathol. 2009;90(6):615–620. [Epub 2009/09/18].
90. Di Girolamo, N, Sarris, M, Chui, J, et al. Localization of the low-affinity nerve growth factor receptor p75 in human limbal epithelial cells. J Cell Mol Med. 2008;12(6B):2799–2811. [Epub 2009/02/13].
91. Wong, TT, Sethi, C, Daniels, JT, et al. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol. 2002;47(3):239–256. [Epub 2002/06/08].
92. Yang, SF, Lin, CY, Yang, PY, et al. Increased expression of gelatinase (MMP-2 and MMP-9) in pterygia and pterygium fibroblasts with disease progression and activation of protein kinase C. Invest Ophthalmol Vis Sci. 2009;50(10):4588–4596. [Epub 2009/05/08].
93. Wang, IJ, Hu, FR, Chen, PJ, et al. Mechanism of abnormal elastin gene expression in the pinguecular part of pterygia. Am J Pathol. 2000;157(4):1269–1276. [Epub 2000/10/06].
94. Kaneko, M, Takaku, I, Katsura, N. Glycosaminoglycans in pterygium tissues and normal conjunctiva. Jpn J Ophthalmol. 1986;30(2):165–173. [Epub 1986/01/01].
95. Fitzsimmons, TD, Molander, N, Stenevi, U, et al. Endogenous hyaluronan in corneal disease. Invest Ophthalmol Vis Sci. 1994;35(6):2774–2782. [Epub 1994/05/01].
96. Perez-Rico, C, Pascual, G, Sotomayor, S, et al. Tropoelastin and fibulin overexpression in the subepithelial connective tissue of human pterygium. Am J Ophthalmol. 2011;151(1):44–52. [Epub 2010/11/26].
97. Tumova, S, Woods, A, Couchman, JR. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int J Biochem Cell Biol. 2000;32(3):269–288. [Epub 2000/03/15].
98. Zhang, JD. An investigation of aetiology and heredity of pterygium. Report of 11 cases in a family. Acta Ophthalmol (Copenh). 1987;65(4):413–416.
99. Hecht, F, Shoptaugh, MG. Winglets of the eye: dominant transmission of early adult pterygium of the conjunctiva. J Med Genet. 1990;27(6):392–394. [Epub 1990/06/01].
100. Goyal, JL, Rao, VA, Srinivasan, R, et al. Oculocutaneous manifestations in xeroderma pigmentosa. Br J Ophthalmol. 1994;78(4):295–297. [Epub 1994/04/01].
101. Kau, HC, Tsai, CC, Hsu, WM, et al. Genetic polymorphism of hOGG1 and risk of pterygium in Chinese. Eye. 2004;18(6):635–639. [Epub 2004/01/13].
102. Chen, PL, Yeh, KT, Tsai, YY, et al. XRCC1, but not APE1 and hOGG1 gene polymorphisms is a risk factor for pterygium. Mol Vis. 2010;16:991–996. [Epub 2010/06/26].
103. Tsai, YY, Bau, DT, Chiang, CC, et al. Pterygium and genetic polymorphism of DNA double strand break repair gene Ku70. Mol Vis. 2007;13:1436–1440. [Epub 2007/09/05].
104. Tsai, YY, Lee, H, Tseng, SH, et al. Null type of glutathione S-transferase M1 polymorphism is associated with early onset pterygium. Mol Vis. 2004;10:458–461. [Epub 2004/07/27].
105. Tung, JN, Wu, HH, Chiang, CC, et al. An association between BPDE-like DNA adduct levels and CYP1A1 and GSTM1 polymorphisma in pterygium. Mol Vis. 2010;16:623–629. [Epub 2010/08/12].
106. Detorakis, ET, Drakonaki, EE, Spandidos, DA. Molecular genetic alterations and viral presence in ophthalmic pterygium. Int J Mol Med. 2000;6(1):35–41. [Epub 2000/06/14].
107. Di Girolamo, N. Association of human papilloma virus with pterygia and ocular-surface squamous neoplasia. Eye (Lond). 2012;26(2):202–211. [Epub 2011/12/03].
108. Piras, F, Moore, PS, Ugalde, J, et al. Detection of human papillomavirus DNA in pterygia from different geographical regions. Br J Ophthalmol. 2003;87(7):864–866. [Epub 2003/06/19].
109. Tsai, YY, Chang, CC, Chiang, CC, et al. HPV infection and p53 inactivation in pterygium. Mol Vis. 2009;15:1092–1097. [Epub 2009/06/09].
110. Detorakis, ET, Sourvinos, G, Spandidos, DA. Detection of herpes simplex virus and human papilloma virus in ophthalmic pterygium. Cornea. 2001;20(2):164–167. [Epub 2001/03/15].
111. Chen, YF, Hsiao, CH, Ngan, KW, et al. Herpes simplex virus and pterygium in Taiwan. Cornea. 2008;27(3):311–313. [Epub 2008/03/26].
112. Dushku, N, Reid, TW. P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors. Curr Eye Res. 1997;16(12):1179–1192. [Epub 1998/01/14].
113. Spandidos, DA, Sourvinos, G, Kiaris, H, et al. Microsatellite instability and loss of heterozygosity in human pterygia. Br J Ophthalmol. 1997;81(6):493–496.
114. Detorakis, ET, Zafiropoulos, A, Arvanitis, DA, et al. Detection of point mutations at codon 12 of KI-ras in ophthalmic pterygia. Eye. 2005;19(2):210–214. [Epub 2004/07/03].
115. Tsai, YY, Cheng, YW, Lee, H, et al. P53 gene mutation spectrum and the relationship between gene mutation and protein levels in pterygium. Mol Vis. 2005;11:50–55.
116. Reisman, D, McFadden, JW, Lu, G. Loss of heterozygosity and p53 expression in Pterygium. Cancer Lett. 2004;206(1):77–83.
117. Lakin, ND, Jackson, SP. Regulation of p53 in response to DNA damage. Oncogene. 1999;18(53):7644–7655.
118. Drouin, R, Therrien, JP. UVB-induced cyclobutane pyrimidine dimer frequency correlates with skin cancer mutational hotspots in p53. Photochem Photobiol. 1997;66(5):719–726.
119. Onur, C, Orhan, D, Orhan, M, et al. Expression of p53 protein in pterygium. Eur J Ophthalmol. 1998;8(3):157–161.
120. Chowers, I, Pe’er, J, Zamir, E, et al. Proliferative activity and p53 expression in primary and recurrent pterygia. Ophthalmology. 2001;108(5):985–988.
121. Schneider, BG, John-Aryankalayil, M, Rowsey, JJ, et al. Accumulation of p53 protein in pterygia is not accompanied by TP53 gene mutation. Exp Eye Res. 2006;82(1):91–98. [Epub 2005/07/12].
122. Chen, PL, Cheng, YW, Chiang, CC, et al. Hypermethylation of the p16 gene promoter in pterygia and its association with the expression of DNA methyltransferase 3b. Mol Vis. 2006;12:1411–1416. [Epub 2006/12/07].
123. Young, CH, Chiu, YT, Shih, TS, et al. E-cadherin promoter hypermethylation may contribute to protein inactivation in pterygia. Mol Vis. 2010;16:1047–1053. [Epub 2010/07/03].
124. Riau, AK, Wong, TT, Lan, W, et al. Aberrant DNA methylation of matrix remodeling and cell adhesion related genes in pterygium. PLoS One. 2011;6(2):e14687. [Epub 2011/03/02].
125. Tan, DT, Tang, WY, Liu, YP, et al. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br J Ophthalmol. 2000;84(2):212–216. [Epub 2000/02/03].
126. Tsironi, S, Ioachim, E, Machera, M, et al. Presence and possible significance of immunohistochemically demonstrable metallothionein expression in pterygium versus pinguecula and normal conjunctiva. Eye. 2001;15(Pt 1):89–96. [Epub 2001/04/25].
127. Ueda, Y, Kanazawa, S, Kitaoka, T, et al. Immunohistochemical study of p53, p21 and PCNA in pterygium. Acta Histochem. 2001;103(2):159–165. [Epub 2001/05/23].
128. Kase, S, Takahashi, S, Sato, I, et al. Expression of p27(KIP1) and cyclin D1, and cell proliferation in human pterygium. Br J Ophthalmol. 2007;91(7):958–961. [Epub 2006/12/21].
129. Tan, DT, Liu, YP, Sun, L. Flow cytometry measurements of DNA content in primary and recurrent pterygia. Invest Ophthalmol Vis Sci. 2000;41(7):1684–1686. [Epub 2000/06/14].
130. Chen, JK, Tsai, RJ, Lin, SS. Fibroblasts isolated from human pterygia exhibit transformed cell characteristics. In Vitro Cell Dev Biol Anim. 1994;30A(4):243–248. [Epub 1994/04/01].
131. Peiretti, E, Dessi, S, Mulas, MF, et al. Fibroblasts isolated from human pterygia exhibit altered lipid metabolism characteristics. Exp Eye Res. 2006;83(3):536–542. [Epub 2006/05/16].
132. Wong, YW, Chew, J, Yang, H, et al. Expression of insulin-like growth factor binding protein-3 in pterygium tissue. Br J Ophthalmol. 2006;90(6):769–772. [Epub 2006/02/21].
133. Solomon, A, Grueterich, M, Li, DQ, et al. Overexpression of insulin-like growth factor-binding protein-2 in pterygium body fibroblasts. Invest Ophthalmol Vis Sci. 2003;44(2):573–580. [Epub 2003/01/31].
134. Shimmura, S, Ishioka, M, Hanada, K, et al. Telomerase activity and p53 expression in pterygia. Invest Ophthalmol Vis Sci. 2000;41(6):1364–1369. [Epub 2000/05/08].
135. Sakoonwatanyoo, P, Tan, DT, Smith, DR. Expression of p63 in pterygium and normal conjunctiva. Cornea. 2004;23(1):67–70. [Epub 2004/01/01].
136. Dekaris, I, Gabric, N, Karaman, Z, et al. Pterygium treatment with limbal-conjunctival autograft transplantation. Coll Antropol. 2001;25(Suppl):7–12. [Epub 2002/01/31].
137. Tan, D. Conjunctival grafting for ocular surface disease. Curr Opin Ophthalmol. 1999;10(4):277–281. [Epub 2000/01/06].
138. Kato, N, Shimmura, S, Kawakita, T, et al. Beta-catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. Invest Ophthalmol Vis Sci. 2007;48(4):1511–1517. [Epub 2007/03/29].
139. Kase, S, Osaki, M, Sato, I, et al. Immunolocalisation of E-cadherin and beta-catenin in human pterygium. Br J Ophthalmol. 2007;91(9):1209–1212. [Epub 2007/03/16].
140. Touhami, A, Di Pascuale, MA, Kawatika, T, et al. Characterisation of myofibroblasts in fibrovascular tissues of primary and recurrent pterygia. Br J Ophthalmol. 2005;89(3):269–274.
141. Ye, J, Song, YS, Kang, SH, et al. Involvement of bone marrow-derived stem and progenitor cells in the pathogenesis of pterygium. Eye. 2004;18(8):839–843. [Epub 2004/03/06].
142. Yamagami, S, Ebihara, N, Usui, T, et al. Bone marrow-derived cells in normal human corneal stroma. Arch Ophthalmol. 2006;124(1):62–69.
143. Ishii, G, Sangai, T, Sugiyama, K, et al. In vivo characterization of bone marrow-derived fibroblasts recruited into fibrotic lesions. Stem Cells. 2005;23(5):699–706.
144. Takahashi, T, Kalka, C, Masuda, H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438.
145. Chui, J, Di Girolamo, N, Coroneo, MT, et al. The role of substance P in the pathogenesis of pterygia. Invest Ophthalmol Vis Sci. 2007;48(10):4482–4489. [Epub 2007/09/28].
146. Thoft, RA, Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24(10):1442–1443.
147. Muller, LJ, Marfurt, CF, Kruse, F, et al. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542.
148. Benrath, J, Eschenfelder, C, Zimmerman, M, et al. Calcitonin gene-related peptide, substance P and nitric oxide are involved in cutaneous inflammation following ultraviolet irradiation. Eur J Pharmacol. 1995;293(1):87–96.
149. van der Zypen, F, van der Zypen, E, Daicker, B. [Ultrastructural studies on the pterygium. II. Connective tissue, vessels and nerves of the conjunctival part (author’s transl)]. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;193(3):177–187. [Epub 1975/01/01. Zur Ultrastruktur des Pterygium. II. Bindegewebe, Gefass- und Nervensystem des konjunktivalen Anteils].
150. Coroneo, MT, Rosenberg, ML, Cheung, LM. Ocular effects of cosmetic products and procedures. Ocul Surf. 2006;4(2):94–102. [Epub 2006/05/10].
151. Frucht-Pery, J, Siganos, CS, Solomon, A, et al. Topical indomethacin solution versus dexamethasone solution for treatment of inflamed pterygium and pinguecula: a prospective randomized clinical study. Am J Ophthalmol. 1999;127(2):148–152. [Epub 1999/02/25].
152. Jonisch, J, Steiner, A, Udell, IJ. Preservative-free low-dose dexamethasone for the treatment of chronic ocular surface disease refractory to standard therapy. Cornea. 2010;29(7):723–726. [Epub 2010/05/22].
153. Utine, CA, Stern, M, Akpek, EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010;18(5):352–361. [Epub 2010/08/26].
154. Schechter, BA. Efficacy of topical cyclosporine 0.05% in the prevention of ocular surface inflammation secondary to pterygia. Am Acad Ophthalmol Annual Meeting. 2006. [Poster #81].
155. Ozulken, K, Koc, M, Ayar, O, et al. Topical cyclosporine A administration after pterygium surgery. Eur J Ophthalmol. 2011. [Epub 2011/07/05].
156. Turan-Vural, E, Torun-Acar, B, Kivanc, SA, et al. The effect of topical 0.05% cyclosporine on recurrence following pterygium surgery. Clin Ophthalmol. 2011;5:881–885. [Epub 2011/07/16].
157. Hirst, LW. The treatment of pterygium. Surv Ophthalmol. 2003;48(2):145–180. [Epub 2003/04/11].
158. Starc, S, Knorr, M, Steuhl, KP, et al. Autologous conjunctiva-limbus transplantation in treatment of primary and recurrent pterygium. Ophthalmologe. 1996;93:219–223.
159. Hirst, LW. Prospective study of primary pterygium surgery using pterygium extended removal followed by extended conjunctival transplantation. Ophthalmology. 2008;115(10):1663–1672. [Epub 2008/06/17].
160. Hirst, LW. Recurrent pterygium surgery using pterygium extended removal followed by extended conjunctival transplant: recurrence rate and cosmesis. Ophthalmology. 2009;116(7):1278–1286. [Epub 2009/07/07].
161. Tagle, RB. A new operative technic of conjunctival grafting in the pterygium. Arch Ophthalmol. 1947;38(3):409.
162. Kenyon, KR, Wagoner, MD, Hettinger, ME. Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 1985;92(11):1461–1470. [Epub 1985/11/01].
163. Al Fayez, MF. Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 2002;109(9):1752–1755. [Epub 2002/09/05].
164. Sun, TT, Tseng, SC, Lavker, RM. Location of corneal epithelial stem cells. Nature. 2010;463(7284):E10–E11. [discussion E1. Epub 2010/02/26].
165. Qi, H, Zheng, X, Yuan, X, et al. Potential localization of putative stem/progenitor cells in human bulbar conjunctival epithelium. J Cell Physiol. 2010;225(1):180–185. [Epub 2010/05/12].
166. Barraquer, JI. Etiology, pathogenesis, and treatment of the pterygium. In: Transactions of the New Orleans Academy of Ophthalmology: Symposium on Medical and Surgical Diseases of the Cornea. St. Louis: Mosby; 1980:167–178.
167. Liu, J, Fu, Y, Xu, Y, et al. New grading system to improve the surgical outcome of multirecurrent pterygia. Arch Ophthalmol. 2012;130(1):39–49. [Epub 2012/01/11].
168. Notara, M, Shortt, AJ, Galatowicz, G, et al. IL6 and the human limbal stem cell niche: a mediator of epithelial–stromal interaction. Stem Cell Res. 2010;5(3):188–200. [Epub 2010/09/04].
169. Yaisawang, S, Piyapattanakorn, P. Role of post-operative topical corticosteroids in recurrence rate after pterygium excision with conjunctival autograft. J Med Assoc Thai. 2003;86(Suppl 2):S215–S223. [Epub 2003/08/22].
170. Hirst, LW. Mitomycin C in the treatment of pterygium. Clin Exp Ophthalmol. 2006;34(3):197–198. [Epub 2006/05/05].
171. Troutbeck, R, Hirst, L. Review of treatment of pterygium in Queensland: 10 years after a primary survey. Clin Exp Ophthalmol. 2001;29(5):286–290. [Epub 2001/11/27].
172. Assaad, NN, Coroneo, MT. Conjunctival autograft failure in eyes previously exposed to beta-radiation or mitomycin. Arch Ophthalmol. 2008;126(10):1460–1461. [Epub 2008/10/15].
173. Assaad, NN, Chong, R, Tat, LT, et al. Use of adjuvant hyperbaric oxygen therapy to support limbal conjunctival graft in the management of recurrent pterygium. Cornea. 2011;30(1):7–10. [Epub 2010/09/18].
174. Green, MO, Brannen, AL. Hyperbaric oxygen therapy for beta-radiation-induced scleral necrosis. Ophthalmology. 1995;102(7):1038–1041. [Epub 1995/07/01].
175. Ozer, A, Yildirim, N, Erol, N, et al. Long-term results of bare sclera, limbal-conjunctival autograft and amniotic membrane graft techniques in primary pterygium excisions. Ophthalmologica. 2009;223(4):269–273. [Epub 2009/04/03].
176. Park, CY, Choi, JS, Lee, SJ, et al. Cyclooxygenase-2-expressing macrophages in human pterygium co-express vascular endothelial growth factor. Mol Vis. 2011;17:3468–3480. [Epub 2012/01/06].
177. Detorakis, ET, Zaravinos, A, Spandidos, DA. Growth factor expression in ophthalmic pterygia and normal conjunctiva. Int J Mol Med. 2010;25(4):513–516. [Epub 2010/03/04].
178. Bahar, I, Kaiserman, I, McAllum, P, et al. Subconjunctival bevacizumab injection for corneal neovascularization in recurrent pterygium. Curr Eye Res. 2008;33(1):23–28. [Epub 2008/01/25].
179. Shenasi, A, Mousavi, F, Shoa-Ahari, S, et al. Subconjunctival bevacizumab immediately after excision of primary pterygium: the first clinical trial. Cornea. 2011;30(11):1219–1222. [Epub 2011/10/01].
180. Friedlander, M. Combination angiostatic therapies: targeting multiple angiogenic pathways. Retina. 2009;29(6 Suppl):S27–S29. [Epub 2009/07/09].
181. Frucht-Pery, J, Solomon, A, Siganos, CS, et al. Treatment of inflamed pterygium and pinguecula with topical indomethacin 0.1% solution. Cornea. 1997;16:42–47.
182. Santos, CI, Zeiter, JH, Speaker, MG. Efficacy and safety of topical 1.0% anecortave acetate (AL-3789) as anti-neovascular therapy for recurrent pterygium. Invest Ophthalmol Vis Sci. 1999;40(4):S1778.
183. Cox, CA, Amaral, J, Salloum, R, et al. Doxycycline’s effect on ocular angiogenesis: an in vivo analysis. Ophthalmology. 2010;117(9):1782–1791. [Epub 2010/07/08].
184. Koranyi, G, Seregard, S, Kopp, ED. The cut-and-paste method for primary pterygium surgery: long-term follow-up. Acta Ophthalmol Scand. 2005;83(3):298–301. [Epub 2005/06/14].
185. Xu, F, Li, M, Yan, Y, et al. A novel technique of sutureless and glue-less conjunctival autografting in pterygium surgery by electrocautery pen. Cornea. 2012. [Epub ahead of print 2012/05/15].
186. Wishaw, K, Billington, D, O’Brien, D, et al. The use of orbital morphine for postoperative analgesia in pterygium surgery. Anaesth Intensive Care. 2000;28(1):43–45. [Epub 2000/03/04].
187. Caccavale, A, Romanazzi, F, Imparato, M, et al. Ropivacaine for topical anesthesia in pterygium surgery with fibrin glue for conjunctival autograft. Cornea. 2010;29(4):375–376. [Epub 2010/02/19].
188. Young, AL, Leung, GY, Cheng, LL, et al. Randomised controlled trial on the effectiveness of lidocaine gel vs tetracaine drops as the sole topical anaesthetic agent for primary pterygium surgery. Eye (Lond). 2009;23(7):1518–1523. [Epub 2008/11/08].
189. Arenas, E, Garcia, S. A scleral soft contact lens designed for the postoperative management of pterygium surgery. Eye Contact Lens. 2007;33(1):9–12. [Epub 2007/01/17].
190. Di Girolamo, N, Chui, J, Wakefield, D, et al. Cultured human ocular surface epithelium on therapeutic contact lenses. Br J Ophthalmol. 2007;91(4):459–464. [Epub 2006/09/22].
191. Skippen, B, Tsang, HH, Assaad, NN, et al. Rapid response of refractory ocular surface dysplasia to combination treatment with topical all-trans retinoic acid and interferon alfa-2b. Arch Ophthalmol. 2010;128(10):1368–1369. [Epub 2010/10/13].
192. Krilis, M, Tsang, H, Coroneo, M. Treatment of conjunctival and corneal epithelial neoplasia with retinoic acid and topical interferon alfa-2b: Long term follow up. Ophthalmology. 2012. [Epub ahead of print 2012/06/19].
193. Ang, LP, Tan, DT, Cajucom-Uy, H, et al. Autologous cultivated conjunctival transplantation for pterygium surgery. Am J Ophthalmol. 2005;139(4):611–619. [Epub 2005/04/06].
194. Coroneo, MT. Beheading the pterygium. Ophthalmic Surg. 1992;23(10):691–692. [Epub 1992/10/01].
195. Kuo, M. Novel techniques for conjunctival autograft creation. [BSc (Med) Honours Thesis]: University of New South Wales; 2012.
196. Di Girolamo, N, Wakefield, D, Coroneo, MT. Doxycycline’s and ocular angiogenesis. Ophthalmology. 2011;118(4):789–790. [author reply 90-1. Epub 2011/04/05].
197. Kieval, JZ, Karp, CL, Shousha, MA, et al. Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterygia. Ophthalmology. 2011. [Epub 2011/12/14].
198. Coroneo, MT, Coroneo, H. Feast your eyes: the eye health cookbook. West Lakes, South Australia: Seaview Press; 2010.