7 Psychological Care
After reading this chapter, you should be able to:
• implement appropriate evidence-based strategies to reduce patient anxiety
• describe the different instruments available to assess sedation needs in critically ill patients and discuss the benefits and limitations of each
• describe the three subtypes of delirium
• recognise risk factors for the development of delirium in the critically ill
• implement and evaluate delirium assessment screening instruments for the critically ill
• implement appropriate evidence-based strategies to manage patients’ sedative needs
• integrate best practice into pain assessment and management
• determine methods to promote rest and sleep for critically ill patients
Anxiety
Anxiety can occur both during and following a period of critical illness. Anxiety has been defined as an unpleasant emotional state or condition.1 Within that broad definition Spielberger recognises two related, but conceptually different constructs, specifically state and trait anxiety. Trait anxiety, a personality characteristic, refers to the relatively stable tendency of people to perceive stressful situations as stressful or anxiety-provoking.1 In contrast, and of more immediate concern during the care of critically ill patients, is state anxiety, an emotional state that exists at a given moment in time and is characterised by ‘subjective feelings of tension, apprehension, nervousness, and worry’.1 In addition, activation of the autonomic nervous system is present during state anxiety.
Factors that have been identified as precipitating anxiety include:2,3
• concern about current illness as well as any underlying chronic disease
• current experiences and feelings such as pain, sleeplessness, thirst, discomfort, immobility
• current care interventions including mechanical ventilation, indwelling tubes and catheters, repositioning and suctioning
Anxiety has been identified in approximately half of critically ill patients, with the majority of patients reporting moderate to severe anxiety in most cohorts.4–7 Further, the presence of anxiety in acute myocardial patients has been reported to be similar across multiple cultures.4
There are both physiological and psychological responses to anxiety, associated with feelings of apprehension, uneasiness and dread from a perceived threat. These responses reflect a stress response and incorporate avoidance behaviour, increased vigilance and arousal, activation of the sympathetic nervous system and release of cortisol from the adrenal glands.8 The humoral response, mediated by the hypothalamic-pituitary-adrenal (HPA) axis, regulates this activity. Physiological changes occur to multiple body systems, with the most relevant including inhibition of salivation and tearing, constriction of blood vessels, increased heart rate, relaxation of airways, increased secretion of epinephrine and norepinephrine as well as increased glucose production,8 which all contribute to the range of clinical indicators outlined in Table 7.1. These physiological manifestations illustrate the importance of early identification, active reduction and minimisation of anxiety in critically ill patients.
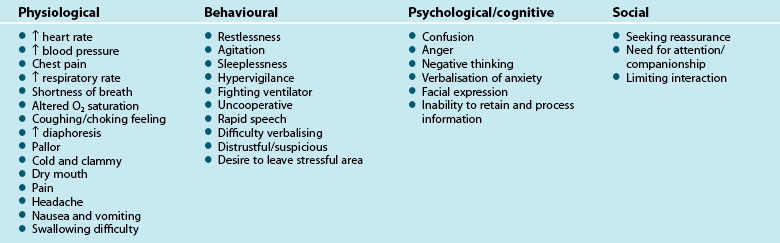
Clinical indicators of anxiety are broad and relate to four major categories including physiological, behavioural, psychological/cognitive and social (Table 7.1).9,10
Appropriate recognition of anxiety is important as there is beginning evidence that the physiological effects of anxiety can have important effects on outcomes for critical care patients. Many of the clinical signs listed in Table 7.1, for example, increased blood pressure and respiratory rate, are likely to lead to poorer outcomes for the critically ill patient. In addition, in acute myocardial infarction patients, in-hospital complications such as recurrent ischaemia, infarction and significant arrhythmias were significantly higher in patients with high levels of anxiety compared to those with low levels of anxiety.11
Anxiety Assessment
The importance of anxiety assessment with the aim of reducing or preventing the adverse effects it produces, is supported by the literature. However, recognition and interpretation of anxiety is complex, particularly when signs and symptoms are masked by critical illness, the effect of medications and/or mechanical ventilation. Further, alterations in levels of biochemical markers such as cortisol and catecholamines that are frequently associated with anxiety may also be attributed to physiological stress.12 Thus, anxiety rating scales are advocated and may offer benefits not found with unstructured clinical assessment.
The relationship between a patient’s self report of anxiety and clinician assessment of anxiety has been inconsistent. When chart reviews were undertaken to determine the relationship between clinicians’ routinely documented anxiety and patient self-report of anxiety, no relationship was found.5 In contrast, when clinicians were prompted to assess anxiety in intensive care patients their rating of the severity of anxiety did have moderate correlation with patients’ self report of anxiety.7
A number of self-reporting scales exist to measure anxiety (Table 7.2). These scales require cognitive interpretation and an ability to communicate responses, which presents challenges to many critically ill patients.13 In addition, some of these scales have up to 21 items, making them both time-consuming and unmanageable for regular use in the critical care setting. Patients with visual and auditory impairments will require additional assistance, such as larger print, hearing aids or glasses in order to complete the forms.
| Scale | Number of Items | Comments |
|---|---|---|
| Hospital Anxiety and Depression Scale (HADS)14 | 14 (including 7 anxiety items) | Easy and fast to complete |
| Extensively used and therefore international comparisons are available | ||
| Demonstrated validity15 | ||
| Depression Anxiety and Stress Scale 21 (DASS 21)16 | 21 (including 7 anxiety items) | Items measured on scale of 0 (did not apply to me at all) to 3 (applied to me very much or most of the time) |
| Demonstrated validity in clinical populations17 | ||
| Spielberger State Anxiety Inventory (SAI)1 | 20 items | Items measured on a scale of 1 (not at all) to 4 (very much so) |
| Validity demonstrated in various populations1 | ||
| Too long for routine clinical use, but may be useful in associated research | ||
| Visual Analogue Scale – Anxiety (VAS–A) | 1 item | 10 cm/100 mm line from ‘not at all anxious’ to ‘very anxious’ |
| Demonstrated validity18 | ||
| Faces Anxiety Scale19 | 1 item | 5 possible responses or ‘faces’ to reflect anxiety |
| Fast and easy to use | ||
| Validity has been demonstrated in a small number of ICU cohorts20,21 |
The visual analogue scale–anxiety (VAS–A) is fast and simple to complete as it is a single-item measure. It has been evaluated against a recognised anxiety scale (SAI) with 200 mechanically ventilated patients.13 The VAS–A comprises a 100-millimetre vertical line, with the bottom marker labelled ‘not anxious at all’ and the top marker labelled ‘the most anxious I have ever been’. Patients were able to successfully mark, or indicate, their present level of anxiety.
The Faces Anxiety Scale, another single-item scale that has recently been developed by a group of Australian researchers, has five possible responses to assess anxiety (see Figure 7.1).19 Initial testing with small numbers of critically ill patients indicates that the self-reporting single-item scale appears to accurately detect a patient’s anxiety.20,21
Anxiety Management
Critical care nurses recognise that anxiety is detrimental to patients and that anxiety management is important.22 Although pharmacological interventions such as anxiolytic and pain-relieving medication are well-recognised and often-used ways to reduce anxiety, non-pharmacological treatments are also useful, and can be divided into environmental and nurse-initiated interventions.
Non-pharmacological Treatments
An advantage of the non-pharmacological treatments is that they can be nurse-initiated or implemented when units are designed or refurbished (see Table 7.3). Although the benefits of non-pharmacological treatments may be widely accepted in the community, incorporation of complementary therapies is dependent on their acceptance within the clinical context and appropriate patient consent. Beneficial effects that have been reported include lowered blood pressure, heart rate and respiratory rate, improved sleep and reduced stress, anxiety and pain, although as with any therapy, each non-pharmacological treatment may have different effects on individual patients, consequently ongoing assessment is essential.23–25 In addition, the safety of these therapies within the critical care environment has not been well demonstrated, necessitating a high level of monitoring through administration.
| Nurse-initiated treatments | Environmental factors |
|---|---|
| Patient massage26 | Provision of natural light27,28 |
| Aromatherapy24,29 | Calming wall colours such as blue, green and violet27,28 |
| Music therapy2,30–32 | Noise reduction with consideration of alarms, paging systems, talking, etc. |
Other strategies to reduce anxiety include interpersonal interventions such as communication and information sharing by the healthcare team and inclusion of family members in care processes.22 The presence of a family member can provide additional reassurance and can facilitate communication between the health team and patients.
Pharmacological Treatment for Anxiety
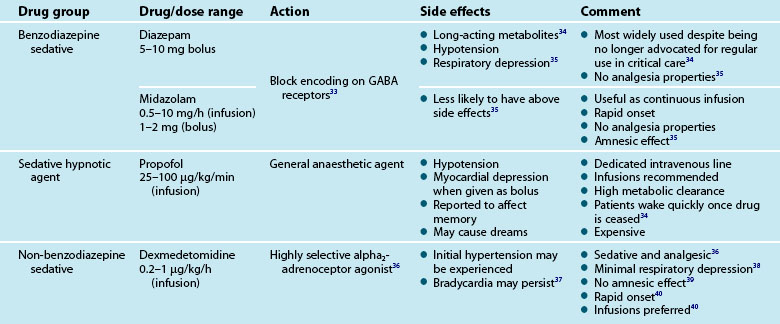
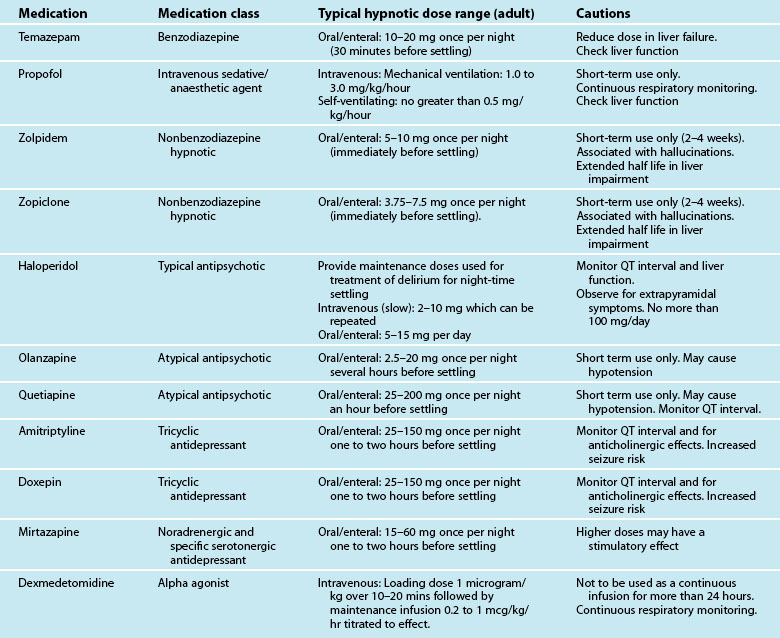
Treatment for pain and other reversible physiological causes of anxiety and agitation should be a priority. Should anxiety and agitation continue despite the incorporation of non-pharmacological interventions, pharmacological treatment with relevant agents may be initiated. Table 7.4 gives a brief overview of these medications in the treatment of unrelieved anxiety.
Delirium
Delirium is a significant concern for critically ill patients and the clinicians who care for them.41 It is a category of central nervous dysfunction42 where behaviours and physiological responses are not conducive to healing and recovery. Early detection and treatment of delirium is vital, as it is associated with adverse clinical outcomes such as prolonged duration of ventilation, length of ICU and hospital stay and higher rates of morbidity and mortality.43–48 Furthermore increased duration of delirium has been associated with long-term cognitive impairment.49 Arguably the condition has been under-recognised and under-treated50 and has only recently received the attention it deserves.46,51 Under-recognition is probably related to a number of factors including the high incidence of the hypoactive subtype as well as lack of use of formal screening instruments (without which exists a high degree of subjectivity when assessing delirium).
There are three subtypes of delirium: hypoactive, hyperactive or combined (a combination of both).52 A sudden reversible reduction in cognitive ability (e.g. inattention, reduced problem-solving ability and disorientation) and onset of perceptual disturbances (e.g. hallucinations) over hours or days are characteristic of all subtypes of delirium. This is in contrast to dementia in which cognitive decline occurs over months and years. Cognitive and perceptive ability often fluctuates through the day worsening at night. Sleep–wake cycle disturbance is also a feature of delirium.53 In addition there is a unique low voltage electroencephalography pattern present during delirium in which slow wave activity is evident even during wakefulness.54
Lethargy, slow quiet speech and reduced alertness are typical behaviours of hypoactive delirium.52 It is hypothesised that clinicians may not recognise the ‘quietly’ confused patient so the condition may be untreated55 or misdiagnosed as depression.56 Behaviours evident in hyperactive delirium such as hyperactivity and agitation52 cannot go unnoticed by clinicians and present overt risks of self harm such as unintentional extubation/decannulation and intravenous/arterial device removal. Combined delirium is characterised by fluctuations in activity and attention levels including the behaviours of both hyperactive and hypoactive subtypes.52
Reports in the healthcare literature about the prevalence of delirium in ICU vary widely from 15–70%;57,58 an unsurprising finding given that it is notoriously difficult to diagnose in patients who are unable to communicate verbally.59 Rates of delirium in Australian and New Zealand ICUs have fallen within this range, with 45% of the patients who were in the ICU for longer than 36 hours reported to have delirium,60 while 21% of 56 patients in a smaller study had delirium.61 The prevalence in other critical care areas such as emergency departments is thought to be lower.62
The exact pathophysiology of delirium is not yet fully understood, however, imbalances in brain cholinergic and dopaminergic neurotransmitter systems are thought to be responsible.42 Many predisposing and precipitating risk factors have been identified and current opinion suggests that there is an additive effect; patients with more than one predisposing factor will require less noxious precipitating factors to develop delirium than patients who have none. Predisposing factors include:
Precipitating risk factors occur during the course of critical illness and may be disease-related or iatrogenic. Increased severity of illness is a precipitant of delirium in ICU. Metabolic, fluid and electrolyte disturbances have also been implicated,65 particularly in the presence of infection (inflammatory response) or hypoxia. Acute injuries affecting the central nervous system (and especially those manifesting in coma) are predictive of developing delirium.44 Given the hypothesised mechanism underpinning delirium, medications that affect acetylcholine transmission such as atropine and fentanyl are potential precipitants. The risk associated with opioid, benzodiazepine and other psychoactive medication use is less clear-cut,63,66 although ‘emergence’ delirium, a rare complication during recovery from anaesthesia, is thought to be strongly related to the administration of benzodiazepines.67 Sudden cessation of benzodiazepines and tricyclic antidepressants and multiple medication administration may lead to delirium.51 Other iatrogenic factors such as pain, excessive noise levels, sleep deprivation and immobility have the most potential to be modifiable.68 Prevention and therapeutic management of risk factors is the mainstay of treatment for delirium.
Assessment of Delirium
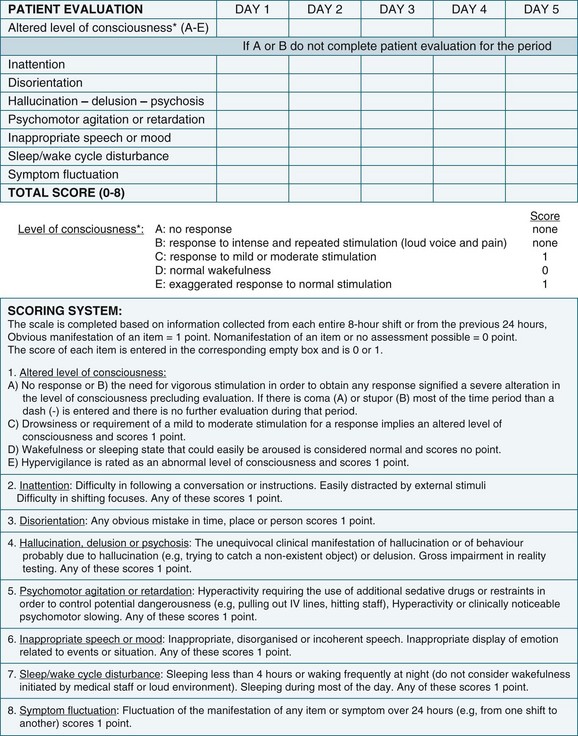
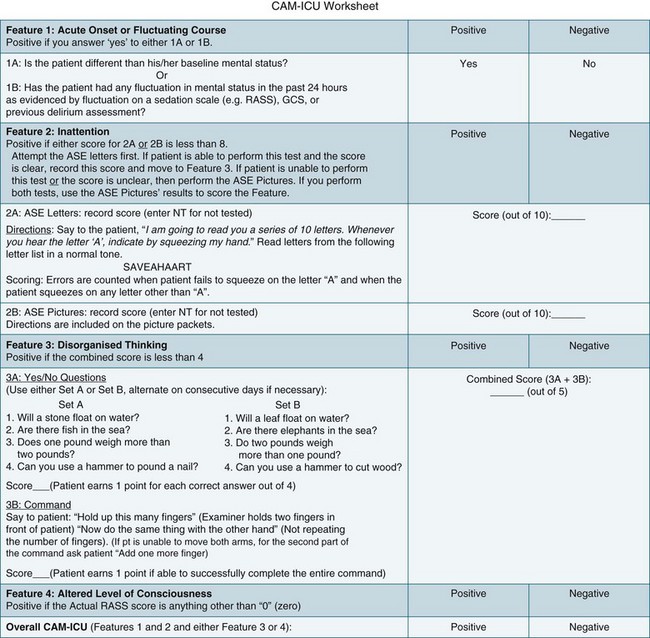
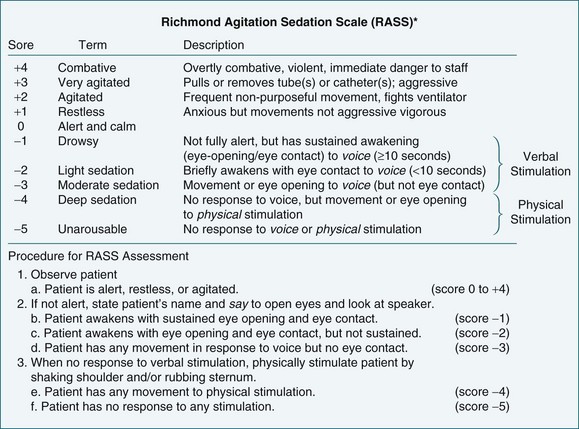
The higher morbidity and mortality associated with delirium and the relative ease of assessing its occurrence makes it imperative to incorporate relevant assessment in routine care. Delirium is diagnosed when both the features of acute onset of mental status changes or fluctuating course and inattention are present, together with either disorganised thinking or altered level of consciousness. A practical delirium assessment screening instrument for the critically ill cannot be reliant on patient–assessor verbal communication. Both the Intensive Care Delirium Screening Checklist (ICDSC)69 (Figure 7.2) and the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)58 (Figure 7.3) have been shown to fulfil these requirements.
The ICDSC contains eight items based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for delirium and was validated in a study conducted within ICU.69 It has been shown to be simple to use and easily integrated into existing patient documentation.60,69 All features of delirium are incorporated such as sleep pattern disturbances and hypo- or hyperactivity.69 The first step in using the ICDSC is an assessment of conscious level using a five point scale (A–E). Only patients who are adequately conscious, that is, responsive to moderate physical stimuli (C–E on the scale), are able to be assessed. The eight items of the ICDSC are rated present (1) or absent (0). A score of four or higher is considered to be indicative of delirium.
The CAM–ICU has also been shown to be valid for diagnosing delirium in the ICU population (see Further reading for more information).58 Acute onset of mental status changes or fluctuating course is assessed using neurological observations conducted over the previous 24 hours. Inattention is tested in patients who are unable to communicate verbally by using either a picture recognition or a random letter test. Disorganised thinking is assessed by listening to the patient’s speech and for patients who are unable to verbally communicate, a simple instruction is administered such as asking the patient to hold up some fingers. Any conscious level other than ‘alert’ is considered ‘altered’. Scores are not derived from the CAM-ICU; delirium is either present or absent.58
Prevention and Treatment of Delirium
• reassurance to reduce anxiety
• judicious use of sedative medications
• correction of the physiological effects of critical illness (for example hypoxia, hypotension and fluid and electrolyte imbalance)
Research into preventative interventions has not been conducted in ICU, however trials conducted in acute care with the elderly show that many risk factors are potentially modifiable. In one trial a multifaceted intervention which included: reorientation strategies, a non-pharmacological sleep regimen, frequent mobilisation, provision of hearing devices and glasses and early treatment of dehydration, led to a significant reduction in the incidence of delirium.70 The creation of environmental conditions that are conducive to rest and sleep, in particular noise reduction and adjusting light levels appropriate for the time of day, may also help.
In cases where non-pharmacological strategies have not succeeded medications such as haloperidol34 and atypical antipsychotics (e.g. Olanzapine)71 are recommended. However it should be noted that firm evidence of the efficacy of these medications is lacking, any medication designed to enhance cognition has the potential to make it worse and there are many unwanted side effects (e.g. Q-T interval prolongation). Therefore any psychoactive medication should be used judiciously in the critically ill.
Sedation
Assessment of Sedation
Assessment of the effect of all sedative treatments is essential. When pharmacological agents are used there is always a risk of over- or undersedation, and both can have significant negative effects on patients. Oversedation can lead to detrimental physiological effects including cardiac, renal and respiratory depression and can result in longer duration of mechanical ventilation, associated complications and recovery.72,73 Undersedation has the opposite effect on the cardiac system, with hypertension, tachycardia, dysrhythmias, ventilator dyssynchrony, agitation and distress, with the potential for incidents concerning patient safety.72,73 There is some evidence that heavy sedation is associated with psychological recovery, particularly in relation to delusional memories.74
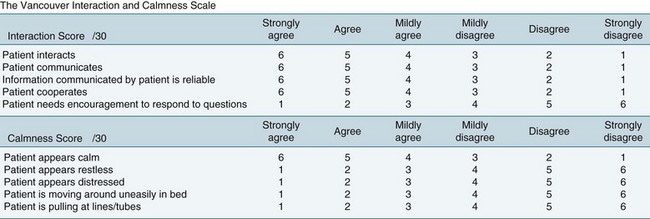
Objective sedation scales provide an effective method of assessing and monitoring a patient’s level of consciousness or arousal, as well as to evaluate parameters such as cognition, agitation and patient-ventilator synchrony (Figures 7.4 and 7.5). A number of different sedation scales have been developed for use in the intensive care environment (Table 7.5). Essential requirements of effective sedation scales include that it measures what is intended, is reliable and is easy to use.75
| Scale | Description | Comment |
|---|---|---|
| Ramsay sedation scale76 |
Bispectral index (BIS) monitoring is an assessment tool that provides an objective measure of sedation. It uses a self-adhesive pad secured to the patient’s forehead to continuously record cortical activity that is scored on a scale from 0 (absence of brain activity) to 100 (completely awake). There is not yet consensus on the most appropriate level of activity for intensive care patients or what role BIS might offer in their care.81,82 Continued studies to evaluate the efficacy of BIS are required.
Sedation Protocols
The sedation needs of patients are complex, with various reports of patients receiving sub-optimal care and inconsistent practice in this area.72,83 One of the responses to this gap in nursing practice has been the development of protocols.
• the sedation scale to be used, as well as frequency of assessment
• an algorithm-based process for selecting the most appropriate sedative agent
• the range of sedative agents that might be considered and associated administration guidelines
• when to commence, increase, decrease or cease use of sedative agents
Many sedation protocols will also incorporate an analgesia component.
Although sedation protocols have widespread support, there is mixed evidence regarding the benefits of implementation of such protocols. A number of studies have demonstrated the benefits associated with nurse-led sedation protocols, yet other studies do not demonstrate a benefit.84 Until further research is undertaken, sedation protocols should be implemented on a local basis where current practice conditions indicate potential benefit from standardisation of care. Appropriate evaluation of the impact of protocol implementation should be undertaken.
Pain
Pain is almost certainly a sensation widely experienced by critical care patients as it is one of the stressors most commonly reported by critically ill patients.85,86 Arguably pain management is often not afforded the same emphasis as more ‘life-threatening’ conditions such as haemodynamic instability in critical care. However its alleviation is an essential element of critical care nursing. Myths such as the possibility that patients may become addicted to analgesics and the very young and elderly having higher tolerance for pain and our cultural tendency to reward high pain tolerance may lead to inadequate pain management. This is evidenced by a study performed in post-coronary bypass surgery patients. Nurses administered only 47% of the patient’s prescribed analgesic medication, and yet these patients reported moderate to severe pain.87 In critical care, nurses assume a fairly autonomous role in titrating pain-relieving medication. With this increased autonomy comes a responsibility to be knowledgeable and aware of effective pain management and assessment of the ‘fifth vital sign’.
Pathophysiology of Pain
Pain is defined as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage …’.88, p. 250 Although unpleasant it has a role in protecting against further injury.89 There are three categories of pain receptors or nociceptors: mechanical nociceptors, that respond to damage such as cutting and crushing; thermal nociceptors, that respond to temperature; and polymodal nociceptors, that respond to all types of stimuli including chemicals released from injured tissue. Prostaglandins released from fatty acids in response to tissue damage reduce the threshold for activation of the nociceptors.89
Pain is transmitted to the central nervous system via one of two pathways. The fast pain pathway occurs where the stimuli are carried by small myelinated A-delta fibres, producing a sharp, prickling sensation that is easily localised. The slow pathway acts in response to polymodal nociceptors, is carried by small unmyelinated C fibres, and produces a dull, aching or burning sensation. It is difficult to locate, acts after fast pain, and is considered to be more unpleasant than fast pain.89
Perceptions of pain are thought to occur in the thalamus, whereas behavioural and emotional responses occur in the hypothalamus and limbic system.89 Perceptions of pain are influenced by prior experience, and by cultural and normative practices, and help to explain individual reactions to pain.89
There are negative physiological effects of pain that include a sympathetic response with increased cardiac work, thus potentially compromising cardiac stability.90 Respiratory function may be impaired in the critically ill undergoing surgical procedures where deep-breathing and coughing is limited by increased pain, thus reducing airway movement and increasing the retention of secretions and possibility of nosocomial pneumonia. Other known effects of unrelieved pain are nausea and vomiting.
Adverse psychological sequelae of poorly-treated pain include diminished feelings of control and self-efficacy and increased fear and anxiety. Inattention with an inability to engage in rehabilitation and health-promoting activities is not uncommon. Pain is commonly cited by patients as a significant negative memory of their ICU experience.85,86,91 The long-term effects of pain are not clearly understood but they almost certainly impact on recovery and may even lead to worsening chronic pain.92 When these unwanted outcomes are considered alongside the physiological effects of poorly treated pain, the vital importance of pain management is evident.
Pain Assessment
‘Pain is whatever the experiencing person says it is, existing whenever he says it does’.93, p. 26 The nebulous quality and subjective nature of the pain experience leads to considerable problems in assessing it. Compounding this is the challenge of assessment in the critically ill who often have insufficient cognitive acumen to articulate their needs and an inability to communicate verbally. A common language and process in which to assess pain is essential in ameliorating some of these challenges. Furthermore, accurate assessment and consistent recording are fundamental aspects of pain management. Without these vital components, it is impossible to evaluate interventions designed to reduce pain.94
Regardless of the patient’s communication capability, strategies to ensure consistent objective assessment and management should be implemented. Laminated cards displaying body diagrams, words to describe pain and pain intensity measures (including visual analogues and numerical scales) are useful instruments in meeting these requirements. Verbal numerical scale and visual analogue scales (VAS) are commonly used. These are outlined in Table 7.6. Visual analogue scales can be difficult to administer to critically ill patients however a combined VAS and numerical scale includes the benefit of a visual cue with the ability to quantify pain intensity.
| Scale | Description | Comments |
|---|---|---|
| Verbal numeric scale |
• Developed for cognitively impaired adults
• Based on the presence/absence of five non-verbal pain behaviours (one is non-verbal vocalisation, e.g. groaning) and verbal complaints
• Score 0 to 6 (score of 1 allocated for the presence of a pain behaviour/verbal complaint), higher scores indicate more pain
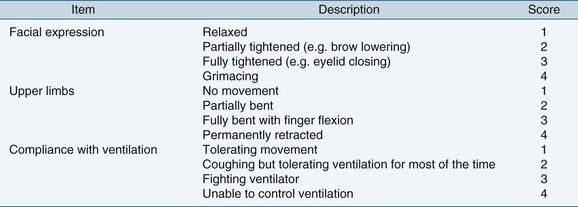
Other physiological and behavioural pain indicators may be used to assess pain in less responsive or unconscious patients.95 Research indicates that consistent assessment of a number of indicators together provides an adequate substitute for self-assessments.95,96 Several instruments have been developed and validated for use in the critically ill adult patient including the Behavioural Pain Scale (BPS) (see Figure 7.6),97 Checklist of Nonverbal Pain Indicators (CNPI)98 and the Critical Care Pain Observation Tool (CPOT)99 (see Table 7.6). Briefly, scores are assigned to categories such as altered body movements, restlessness and synchronisation with the ventilator, providing a global score for comparison after pain relief interventions. The BPS is one of the most widely used scales for use in patients unable to communicate verbally.97,100,101
Nurses are urged against solely relying on changes in physiological parameters, including cardiovascular (elevated blood pressure and heart rate) and respiratory recordings, as other pathophysiological or treatment related factors may be responsible.95 Classic reactions such as increased heart rate and blood pressure, to stressors, e.g. pain, do not always occur in ICU patients and are therefore unreliable methods of assessing pain in this patient group.104 A potential explanation is that autonomic tone may be dysfunctional in a large proportion of ICU patients.105 In haemodynamically-stable long-term critical patients, vital signs may be useful if used in conjunction with other forms of assessment.95
Pain Management
Although pain management is discussed here independently, in practice pain management is often combined with sedative administration to reduce anxiety. However pain management should always be the first goal for achieving overall patient comfort. Efforts to improve patient comfort for intubated patients favour the concurrent use of both forms of medication.94 This practice therefore makes it difficult to assess the single effect of each medication on the patient’s pain, and highlights its multidimensional properties. In addition to pharmacological treatment of pain, non-pharmacological strategies can prove effective as an adjunct to drug therapy or as an alternative.
Pain relief may be required for preexisting injuries or prior to specific procedures to prevent its occurrence. Being turned is often cited as the most painful procedure, however wounds, drain removal, tracheal suction, femoral catheter removal, placement of central-line catheter and non-burn wound dressings, and coughing may also cause considerable discomfort.90,103 Guidelines and written protocols for procedures such as femoral sheath removal and insertion of central-line catheter, can significantly reduce pain intensity as they often contain reminders to provide analgesia.106 Some procedures, such as insertion of a central-line catheter, require additional pain management considerations such as administration of local anaesthetic. This highlights the potential need for additional pain protocols linked to key standard procedures (e.g. patient turning) to reduce patients’ pain experience.
Epidural pain management requires additional evaluation, including sensory and functional assessment, due to the use of local anaesthetic agents in addition to opioid drugs. Sensory function should be regularly checked using a dermatome chart to gauge segments that are blocked by the local anaesthetic agent. In addition to sensory blockade, regular assessment for lower limb motor deficit is required to detect changes in motor response, which may impair ability to mobilise safely. Sudden or subtle changes may also indicate a complication such as epidural haematoma. The Bromage Assessment Scale is often used for assessing motor response. Regular checks of the catheter site are essential to identify complications such as bleeding, haematoma and infection early but also to ensure catheter patency. Intrathecal administration of analgesic medications has similar contraindications and complications to epidural analgesia and requires similar precautions. It is important to note that intrathecal (as compared to intravenous) administration does not eliminate all of the side effects of opioids (see Further reading).
Non-Pharmacological Treatment for Pain
Non-pharmacological strategies to reduce pain are linked to some key strategies to reduce stress. Excessive pain may lead to stress as the body attempts to maintain homeostasis and stress can exacerbate pain. Strategies to reduce stress and pain include both comfort measures and diversional interventions, which require the critical care nurse to individualise and adapt strategies to match the patient’s needs and preferences. Diversional methods may include strategies to distract the patient, and aim to refocus the patient’s thinking away from the pain and on to other more pleasant thoughts or activities. Table 7.7 lists some interventions that may prove effective.
| Comfort measures | Diversional measures |
|---|---|
Pharmacological Treatment for Pain
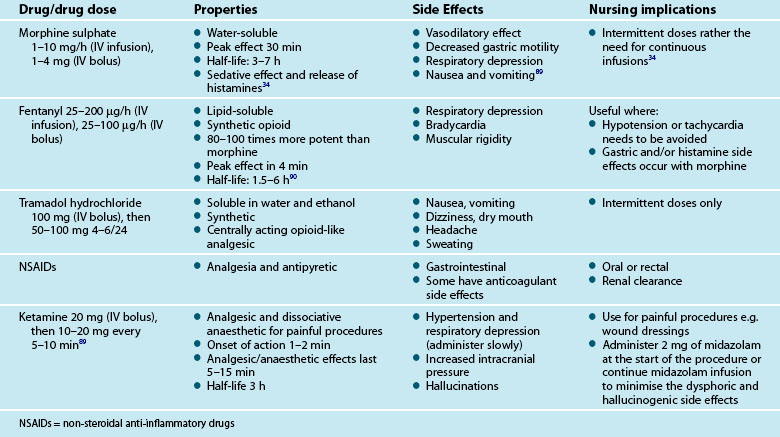
Pharmacological treatment for pain in critically ill patients centres on opioid drugs which act as opioid agonists binding to the µ-receptors in the brain, central nervous system and other tissues.88 Opioid drugs have a rapid action, are readily titrated and their metabolites, if present, are less likely to accumulate. Morphine sulphate and fentanyl are routinely used in critical care, and their properties, side effects and nursing implications are outlined in Table 7.8. For ischaemic chest pain, nitrates will be used together with morphine sulphate as first-line pain measures (see Chapter 10).
An alternative to opioid medication for procedural pain is ketamine.108,109 Single doses of the medication are effective in achieving analgesia during severely painful interventions such as deep wound care (for example, a burn injury). Ketamine is usually administered in conjunction with midazolam to reduce any potential emergent effects.
Pain relief is a primary goal for critical care nursing and requires regular assessment of pain intensity using reliable, objective, patient friendly instruments. No single medication is ideal for all patients, and clinicians need to carefully select, monitor and titrate the doses of any agent selected. In the case of, for example, cardiac surgery patients, patient-controlled analgesia may provide the most effective pain management strategy (see Chapter 12). Non-pharmacological strategies add to the relief of pain and come under the domain of nursing care. Without adequate pain management, patients will be unable to achieve adequate rest and sleep, both essential to healing processes and wellbeing.
Sleep
The function of sleep is not yet fully understood however it is considered to be required for many bodily functions.110 It is vital for wellbeing and sleep disruption or deprivation leads to psychological and physical ill health.111–113 Sleep is considered to be physically and psychologically restorative and essential for healing and recovery from illness. Arguably critically ill people are in greater need of undisrupted sleep but are more likely to experience poor quality sleep.
Evidence suggests that although critically ill patients may experience normal quantities of sleep, the quality is poor with very few experiencing deep or rapid eye movement sleep.114–116 Sleep is highly disrupted and distributed across 24 hours with roughly equal amounts occurring in the day and at night.117 These findings obtained using polysomnography (PSG) have been corroborated by patients’ self reports of their sleep in critical care.118 Patients consistently rate the overall quality of their sleep as poor and more specifically they report light sleep with frequent awakenings and considerable difficulty falling asleep and returning to sleep.119–121 Many factors are thought to affect the patient’s ability to sleep, including discomfort, treatment, medications, environmental noise and illness.117,122
Sleep in the healthy adult comprises one consolidated period of 6–8 hours (mean 7.5 hours) in each 24 hour period occurring at night according to natural circadian rhythms.123 There are two main sleep states; rapid eye movement sleep (REM) (approximately 25% of total sleep time [TST]) and non-rapid eye movement sleep (non-REM) (approximately 75% of TST). Non-REM sleep is comprised of 4 stages*; stages 1 and 2 or light sleep and stages 3 and 4 or slow wave sleep (SWS) or deep sleep, which must be completed in sequence in order to enter REM sleep. The consolidated sleep period consists of 4 to 6 sleep cycles; stages 1–4 followed by REM sleep, that lasts 60–90 minutes. Time spent awake during the sleep period is less than 5% of TST.123 All sleep stages are important to health and unfortunately critically ill patients commonly experience very little deep or REM sleep.
There are changes in sleep architecture over the adult lifespan which require consideration in the context of critical care nursing. TST and percentage of SWS decline (TST by 10 minutes and SWS by 2% per decade) and light sleep increases slightly (only by 5% between 20 and 70 years) with age.124 REM sleep remains fairly constant with an approximate 0.6% decline per decade until age 70 when REM increases with a simultaneous decrease in TST.125 Time spent awake after sleep onset increases with age by 10 minutes per decade after age 30.124
Sleep Assessment/Monitoring
Unfortunately, few objective methods of assessing sleep reliably in the critically ill are available. Polysomnography (PSG), a method of recording electroencephalography, electrooculography and electromyography, is the ‘gold standard’ for assessing sleep. PSG data are analysed according to Rechtschaffen and Kales’126 criteria and provide TST and sleep stage times. However a trained operator is required to ensure satisfactory signal quality, continuous recording and interpretation.123 This drawback precludes its routine use in clinical practice in critical care. Actigraphy is another method of recording sleep that has been attempted in the critically ill. Modern actigraphs are small wristwatch devices (they may also be located on the trunk or leg) containing accelerometers that detect motion in a single axis or multiple axes.127,128 Data obtained from actigraphy provides an over-estimation of sleep time (critically patients are typically immobile for long periods regardless of sleep state). The other objective method which has been attempted in critical care is BIS monitoring.129,130 At present, considerable algorithm development using comparisons with PSG data are required before it is a viable option to measure sleep accurately in any setting.
The most reliable option for the critical care clinician to assess sleep is a patient self-report (in any case the patient is best placed to judge the quantity and quality of their sleep if they are able). Two instruments have been specifically developed for use in critical care; the Richards-Campbell Sleep Questionnaire (RCSQ)131 and the Sleep in Intensive Care Questionnaire (SICQ).118 The RCSQ comprises five 100mm visual analogue scales (VAS): sleep depth, latency, awakenings, time awake and quality of sleep. It was pilot tested in a medical ICU (n = 9, 100% male)132 and validated in a more extensive investigation involving 70 male patients.133 There was a moderate correlation between total RCSQ score and PSG sleep efficiency index (SEI); r = 0.58, (p < 0.001).133 The SICQ was not validated against polysomnography. Therefore it is better suited for use when assessing a unit/organisation-wide change in practice rather than for individual patients (see Table 7.9).
| Instrument | Description | Comments |
|---|---|---|
| Richards Campbell Sleep Questionnaire131 |
Up to 50% of all patients treated in critical care may be unable to complete a self-assessment of their sleep; in which case the only remaining option is nurse assessment.119,134 The Nurses’ Observation Checklist (NOC)135 can be used to obtain the bedside nurses’ assessment of the quantity of the patient’s sleep. It is a relatively simple instrument to use. However, evidence from many studies suggests that nurses tend to overestimate sleep time, so sleep time derived from the NOC may be better used as a trend rather than a definitive report for an individual night’s sleep.134,136–138
Sleep Promotion and Maintenance
Comfort Measures
• Ensure pain relief is offered and administered if pain is suspected.
• Reduce anxiety by providing information and the opportunity to have questions answered. Anxiolytics such as benzodiazepines may also be required.
• Provide night time sedation as required (remember sedation is not natural sleep and patients may only appear to be asleep however it is possible to be sedated and asleep).
• Provide a light massage unless contraindicated.139
• Offer guided relaxation and imagery (audio guided relaxation and imagery sessions may be purchased).140
• Provide an extra cover for warmth (metabolic rate typically drops during sleep).
• Request the patient’s family to provide some of the patient’s own personal belongings such as pillows and toiletries.
• Ear plugs and eye covers may assist some patients, however it should be highlighted that studies have shown that neither provide protection from excessive noise and light levels.141 Patients provided with ear plugs and eye covers should have the ability to remove them without assistance if they wish.
Care Activities
• Attend to nursing care at the beginning of the night to reduce the likelihood of disturbing during the night for example:
• redress wounds and empty drainage bags
• wash, clean teeth and change gown and sheets
• reposition with suitable pressure support measures
• level the transducer at the phlebostatic axis to ensure accurate haemodynamic monitoring without the need to disturb the patient142
• ensure intravenous lines and drains are accessible
• Plan care activities to allow the patient 1.5–2 hour periods of undisturbed time during the night. (Negotiate with other health care personnel to allow these uninterrupted periods at night and during daytime rest times). ‘Cluster care’, for example, time medication administration and blood samples to coincide with pressure area care.
• Provide the daily bath to suit patient needs rather than organisational needs (either before settling for the night or during normal waking hours).
Environmental
• Reduce noise levels especially during rest times and at night (this may require a unit-wide change in practice) as several studies conducted in critical care have highlighted the association between noise levels and sleep disruption.114,143,144 Continuous noise levels in adult critical care areas consistently exceed hospital noise standards, for example, the Environmental Protection Agency (EPA) 35dB(A) at night and 45dB(A) during the day145 and the Australian Standard AS/NZS 2107/2000 minimum 40dB(A) and maximum 45dB(A).146–148
• Ensure lights are sufficiently dimmed and window blinds drawn during rest times and at night and that lighting is bright and blinds opened at all other times. It is known that critically ill patients’ melatonin metabolism is non-circadian so it is particularly important to attempt to use lighting that encourages normal circadian rhythm.149,150 Generally critical care areas contain fluorescent lights which may emit up to 600 lux.151 Light levels between 50 and 100 lux at night even for relatively brief periods are known to suppress melatonin production, a vital hormone in the promotion of sleep and maintenance of circadian rhythm.123 It is well known that artificial lights emit light with sufficient short wave content to affect melatonin secretion.
Treatments
• Discuss the need for alternative mechanical ventilation settings at night with the medical team. Hyperventilation caused by inappropriately high inspiratory pressure can cause hypocapnia which may lead to central apnoeas and sleep disturbance.152
• Many medications administered in critical care affect sleep architecture. Even vasoactive medications such as adrenaline have the capacity to affect the quality of sleep. Sedatives, especially benzodiazepines and opioids, reduce time in stage 3 and 4 and REM, thus reducing the amount and quality of sleep.153,154 However pain relief and anxiolysis may be essential for sleep to occur, but an awareness of potential adverse drug reactions is important in the prevention of escalating sleep disturbances.
• Specific sleep-promoting medications may be administered once non-pharmacological interventions have been attempted. Table 7.10 contains a summary of the commonly used medications for the general management of insomnia. It should be noted that investigations of the effectiveness of these medications have not been undertaken in the critically ill.
A Note on Melatonin
Melatonin is used for the short-term alleviation of insomnia. This naturally-occurring hormone is both sleep-promoting and maintaining. Despite its popularity in the treatment of primary insomnia, e.g. jet lag and shift work, the effectiveness of exogenous melatonin as a sleep medication is yet to be clearly elucidated.155,156 Investigations performed in ICU did not use polysomnography and were largely inconclusive.157–159 Difficulties occur in emulating the typical endogenous pulsatile secretion of the hormone160 together with its short half-life probably explain why many study results are inconclusive. The high doses required to achieve an adequate plasma level overnight when administered once at the beginning of the night are likely to persist in the body and may upset normal circadian rhythm. Some studies investigating the effect of melatonin on insomnia suggest that it may be more effective when administered to adults older than 55 years as there is an age-related decrease in endogenous melatonin.161 The typical dose is 2 mg once a day (1–2 hours before settling).
Summary
Case study
Treatment
• Regular assessments using the ICDSC were performed. It was agreed that a score of >6 (or behaviour likely to cause accidental self-harm) was the cut-off for ICU staff specialist review.
• The midazolam infusion was gradually discontinued.
• Small bolus doses of diazepam (2 mg) were administered when Brad was extremely anxious
• Quetiapine 100mg was administered twice a day
• Brad was moved into a quieter area of the intensive care unit.
• His wife and children were encouraged to provide constant reminders of the time and place, read to him and play music during the day.
• Photographs and personal items were placed around Brad’s bedspace. His wife brought his pillow from home.
• Extra attention was given to ensuring that the room lighting was appropriate for the time of day.
• A settling period was implemented in which a routine was established to encourage Brad to go to sleep by 2300hrs.
• Care was clustered (members of the ICU team consulted with the nurse to time their visit/treatment so that Brad had several 1.5–2 hour intervals in which he was not disturbed).
• The speech pathologist was involved to improve communication (both before and after the tracheostomy cuff was able to be deflated).
• During a period when Brad was not delirious, an assessment of his C spine was performed and the protective collar was removed.
Learning activities
Activities 1–2 relate to the clinical case study
1. Discuss possible strategies for assessing Brad’s pain levels, including considering various pain assessment instruments that may have been used. You should particularly consider the problems created by Brad’s variable response to the VAS being used to assess his pain.
2. Outline possible strategies that might be used to assist Brad to fill in the gaps in his memory that appear to be causing him some distress during his recovery after leaving ICU. Discuss the potential advantages and detrimental effects of each of these strategies.
3. The assessment of anxiety, sedation and pain intensity is integral to critical care nursing.
4. Critically ill patients who experience delirium require highly skilled and informed nursing. The following exercises may enhance your ability to manage delirium:
5. Compare and contrast the various sedation assessment instruments, and discuss the relative merits and disadvantages of using each of these instruments. Now repeat the exercise for each of the pain assessment instruments and the delirium assessment instruments.
6. Using the references provided in this chapter:
7. Think about the last time you experienced fragmented sleep or insufficient sleep and describe how you felt in terms of your:
Ballantyne J, Bonica JJ, Fishman S. Bonica’s management of pain. Philadelphia: Lippincott Williams & Wilkins; 2009.
Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864.
Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–2710.
Hardin KA. Sleep in the ICU: potential mechanisms and clinical implications. Chest. 2009;136:284–294.
1 Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologist Press; 1983.
2 Chlan L. A review of the evidence for music intervention to manage anxiety in critically ill patients receiving mechanical ventilatory support. Arch Psychiatr Nurs. 2009;23(2):177–179.
3 Sessler CN, Varney K. Patient-focused sedation and analgesia in the ICU. Crit Care Med. 2008;133:552–565.
4 De Jong MJ, Chung ML, Roser LP, Jensen LA, Kelso LA, et al. A five-country comparison of anxiety early after acute myocardial infarction. Eur J Cardiovasc Nurs. 2004;3(2):129–134.
5 Frazier SK, Moser DK, O’Brien JL, Garvin BJ, An K, Macko M. Management of anxiety after acute myocardial infarction. Heart Lung. 2002;31(6):411–420.
6 Nelson JE, Meier DE, Oei EJ, Nierman DM, Senzel RS, et al. Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med. 2001;29(2):277–282.
7 McKinley S, Stein-Parbury J, Chehelnabi A, Lovas J. Assessment of anxiety in intensive care patients by using the Faces Anxiety Scale. Am J Crit Care. 2004;13(2):146–152.
8 Bear M, Connors B, Paradiso M. Neuroscience, exploring the brain, 2nd edn. Baltimore, MD: Lippincott Williams & Wilkins; 2001.
9 Moser DK, Chung ML, McKinley S, Riegel B, An K, et al. Critical care nursing practice regarding patient anxiety assessment and management. Intensive Crit Care Nurs. 2003;19(5):276–288.
10 Porth C, Matfin G. Pathophysiology, Concepts of Altered Health States, 8th edn. Philadelphia, PA: Lippincott, Williams & Wilkins; 2009.
11 Moser DK, Dracup K. Is anxiety early after myocardial infarction associated with subsequent ischemic and arrhythmic events? Psychosom Med. 1996;58(5):395–401.
12 Schelling G. Effects of stress hormones on traumatic memory formation and the development of posttraumatic stress disorder in critically ill patients. Neurobiol Learn Mem. 2002;78(3):596–609.
13 Chlan LL. Relationship between two anxiety instruments in patients receiving mechanical ventilatory support. J Adv Nurs. 2004;48(5):493–499.
14 Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370.
15 Snaith RP, Taylor CM. Rating scales for depression and anxiety: a current perspective. Br J Clin Pharmacol. 1985;19(Suppl 1[1]):S17–S20.
16 Lovibond S, Lovibond P. Manual for the depression anxiety stress scales, 2nd edn. Sydney: Psychology Foundation; 1995.
17 Ng F, Trauer T, Dodd S, Callaly T, Campbell S, Berk M. The validity of the 21-item version of the Depression Anxiety Stress Scales as a routine clinical outcome measure. Acta Neuropsychiatrica. 2007;19:304–310.
18 Hornblow AR, Kidson MA. The visual analogue scale for anxiety: a validation study. Aust NZ J Psychiatry. 1976;10(4):339–341.
19 McKinley S, Coote K, Stein-Parbury J. Development and testing of a Faces Scale for the assessment of anxiety in critically ill patients. J Adv Nurs. 2003;41(1):73–79.
20 Gustad L, Chaboyer W, Wallis M. Performance of the faces anxiety scale in patients transferred from the ICU. Inten Crit Care Nurs. 2005;21(6):355–360.
21 McKinley S, Madronio C. Validity of the Faces Anxiety Scale for the assessment of state anxiety in intensive care patients not receiving mechanical ventilation. J Psychosom Res. 2008;64(5):503–507.
22 Frazier SK, Moser DK, Daley LK, McKinley S, Riegel B, et al. Critical care nurses’ beliefs about and reported management of anxiety. Am J Crit Care. 2003;12(1):19–27.
23 Chan MF, Chung YF, Chung SW, Lee OK. Investigating the physiological responses of patients listening to music in the intensive care unit. J Clin Nurs. 2009;18(9):1250–1257.
24 Halm MA. Essential oils for management of symptoms in critically ill patients. Am J Crit Care. 2008;17(2):160–163.
25 Richards K, Nagel C, Markie M, Elwell J, Barone C. Use of complementary and alternative therapies to promote sleep in critically ill patients. Crit Care Nurs Clin North Am. 2003;15(3):329–340.
26 Halcon LL, Chlan LL, Kreitzer MJ, Leonard BJ. Complementary therapies and healing practices: faculty/student beliefs and attitudes and the implications for nursing education. J Prof Nurs. 2003;19(6):387–397.
27 Fontaine DK, Briggs LP, Pope-Smith B. Designing humanistic critical care environments. Crit Care Nurs Q. 2001;24(3):21–34.
28 Stichler JF. Creating healing environments in critical care units. Crit Care Nurs Q. 2001;24(3):1–20. quiz following p. 97
29 Maddocks-Jennings W, Wilkinson JM. Aromatherapy practice in nursing: literature review. J Adv Nurs. 2004;48(1):93–103.
30 Chan MF. Effects of music on patients undergoing a C-clamp procedure after percutaneous coronary interventions: a randomized controlled trial. Heart Lung. 2007;36(6):431–439.
31 Twiss E, Seaver J, McCaffrey R. The effect of music listening on older adults undergoing cardiovascular surgery. Nurs Crit Care. 2006;11(5):224–231.
32 Hellstron A, Fagerstrom C, Willman A. Promoting sleep by nursing interventions in health care settings: A systematic review. Worldviews on Evidence-Based Nursing. 2010. In Press. DOI: 10.1111/j.741-6787.2010.000203.x
33 Liu LL, Gropper MA. Postoperative analgesia and sedation in the adult intensive care unit: a guide to drug selection. Drugs. 2003;63(8):755–767.
34 Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–141.
35 Young C, Knudsen N, Hilton A, Reves JG. Sedation in the intensive care unit. Crit Care Med. 2000;28(3):854–866.
36 Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101(3):787–790.
37 Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36(6):926–939.
38 Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4(5):302–308.
39 Grant SA, Breslin DS, MacLeod DB, Gleason D, Martin G. Dexmedetomidine infusion for sedation during fiberoptic intubation: a report of three cases. J Clin Anesth. 2004;16(2):124–126.
40 Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54(12):1136–1142.
41 McGuire BE, Basten CJ, Ryan CJ, Gallagher J. Intensive care unit syndrome: a dangerous misnomer. Arch Intern Med. 2000;160(7):906–909.
42 Trzepacz PT. Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord. 1999;10(5):330–334.
43 Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–2259.
44 Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73.
45 Han JH, Shintani A, Eden S, Morandi A, Solberg LM, et al. Delirium in the emergency department: an independent predictor of death within 6 months. Ann Emerg Med. 2010. Apr 2 XXX
46 Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Crit Care Med. 2007;132(2):624–636.
47 Ely EW, Gautam S, Margolin R, Francis J, May L, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900.
48 Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
49 Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520.
50 Spronk PE, Riekerk B, Hofhuis J, Rommes JH. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276–1280.
51 Arend E, Christensen M. Delirium in the intensive care unit: a review. Nurs Crit Care. 2009;14(3):145–154.
52 Meagher DJ, Trzepacz PT. Motoric subtypes of delirium. Semin Clin Neuropsychiatry. 2000;5(2):75–85.
53 American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV, 4th edn. Washington, DC: American Psychiatric Association; 1994.
54 Jacobson S, Jerrier H. EEG in delirium. Semin Clin Neuropsychiatry. 2000;5(2):86–92.
55 Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54(3):479–484.
56 Armstrong SC, Cozza KL, Watanabe KS. The misdiagnosis of delirium. Psychosomatics. 1997;38(5):433–439.
57 Easton C, MacKenzie F. Sensory-perceptual alterations: delirium in the intensive care unit. Heart Lung. 1988;17(3):229–237.
58 Ely EW, Margolin R, Francis J, May L, Truman B, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370–1379.
59 Bergeron N, Skrobik Y, Dubois MJ. Delirium in critically ill patients. Crit Care. 2002;6(3):181–182.
60 Roberts B, Rickard CM, Rajbhandari D, Turner G, Clarke J, et al. Multicentre study of delirium in ICU patients using a simple screening tool. Aust Crit Care. 2005;18(1):6. 8–9, 11–14 passim
61 Shehabi Y, Botha JA, Boyle MS, Ernest D, Freebairn RC, et al. Sedation and delirium in the intensive care unit: an Australian and New Zealand perspective. Anaesth Intensive Care. 2008;36(4):570–578.
62 Han JH, Zimmerman EE, Cutler N, Schnelle J, Morandi A, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193–200.
63 Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297–1304.
64 Ely EW, Girard TD, Shintani AK, Jackson JC, Gordon SM, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med. 2007;35(1):112–117.
65 Aldemir M, Ozen S, Kara IH, Sir A, Bac B. Predisposing factors for delirium in the surgical intensive care unit. Crit Care. 2001;5(5):265–270.
66 Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26.
67 Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96(6):747–753.
68 Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care. 2008;12(Suppl 3):S3.
69 Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–864.
70 Inouye SK, Bogardus ST, Jr., Charpentier PA, Leo-Summers L, Acampora D, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
71 American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(5 Suppl):1–20.
72 Jackson DL, Proudfoot CW, Cann KF, Walsh TS. The incidence of sub-optimal sedation in the ICU: a systematic review. Crit Care. 2009;13(6):R204.
73 Schweickert WD, Kress JP. Strategies to optimize analgesia and sedation. Crit Care. 2008;12(Suppl 3):S6.
74 Samuelson KA, Lundberg D, Fridlund B. Light vs. heavy sedation during mechanical ventilation after oesophagectomy – a pilot experimental study focusing on memory. Acta Anaesthesiol Scand. 2008;52(8):1116–1123.
75 Sessler CN, Grap MJ, Ramsay MA. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008;12(Suppl 3[3]):S2.
76 Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2(5920):656–659.
77 Sessler CN, Gosnell M, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care patients. Am J Resp Crit Care Med. 2002;166:1338–1344.
78 Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation–Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329.
79 Devlin JW, Boleski G, Mlynarek M, Nerenz DR, Peterson E, et al. Motor Activity Assessment Scale: a valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Critical Care Medicine. 1999;27(7):1271–1275.
80 de Lemos J, Tweeddale M, Chittock D. Measuring quality of sedation in adult mechanically ventilated critically ill patients. the Vancouver Interaction and Calmness Scale. Sedation Focus Group. J Clin Epidemiol. 2000;53(9):908–919.
81 Anderson J, Henry L, Hunt S, Ad N. Bispectral index monitoring to facilitate early extubation following cardiovascular surgery. Clin Nurse Spec. 2010;24(3):140–148.
82 Weatherburn C, Endacott R, Tynan P, Bailey M. The impact of bispectral index monitoring on sedation administration in mechanically ventilated patients. Anaesth Intensive Care. 2007;35(2):204–208.
83 Mehta S, McCullagh I, Burry L. Current sedation practices: lessons learned from international surveys. Crit Care Clin. 2009;25(3):471–488. vii–viii
84 Chaboyer W, Aitken L. Do nurse-led protocols reduce ICU stay? In: Chiche J, Moreno R, Putensen C, Rhodes A. Patient safety and quality of care in intensive care medicine. Berlin: Medizinisch Wissenschaftlicke Verlagsgesellschaft, 2009.
85 Roberts BL, Rickard CM, Rajbhandari D, Reynolds P. Factual memories of ICU: recall at two years post-discharge and comparison with delirium status during ICU admission – a multicentre cohort study. J Clin Nurs. 2007;16(9):1669–1677.
86 Rotondi AJ, Chelluri L, Sirio C, Mendelsohn A, Schulz R, et al. Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30(4):746–752.
87 Watt-Watson J, Stevens B, Garfinkel P, Streiner D, Gallop R. Relationship between nurses’ pain knowledge and pain management outcomes for their postoperative cardiac patients. J Adv Nurs. 2001;36(4):535–545.
88 Bonica JJ. The need of a taxonomy. Pain. 1979;6(3):247–248.
89 Ballantyne J, Bonica JJ, Fishman S. Bonica’s management of pain, 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
90 Milgrom LB, Brooks JA, Qi R, Bunnell K, Wuestfeld S, Beckman D. Pain levels experienced with activities after cardiac surgery. Am J Crit Care. 2004;13(2):116–125.
91 Adamson H, Murgo M, Boyle M, Kerr S, Crawford M, Elliott D. Memories of intensive care and experiences of survivors of a critical illness: an interview study. Intensive Crit Care Nurs. 2004;20(5):257–263.
92 Boyle M, Murgo M, Adamson H, Gill J, Elliott D, Crawford M. The effect of chronic pain on health related quality of life amongst intensive care survivors. Aust Crit Care. 2004;17(3):104–106. 108–13
93 McCaffery M. Understanding your patient’s pain. Nursing. 1980;10(9):26–31.
94 Gélinas C, Fortier M, Viens C, Fillion L, Puntillo K. Pain assessment and management in critically ill intubated patients: a retrospective study. Am J Crit Care. 2004;13(2):126–135.
95 Herr K, Coyne PJ, Key T, Manworren R, McCaffery M, et al. Pain assessment in the nonverbal patient: position statement with clinical practice recommendations. Pain Manag Nurs. 2006;7(2):44–52.
96 Puntillo KA, Miaskowski C, Kehrle K, Stannard D, Gleeson S, Nye P. Relationship between behavioral and physiological indicators of pain, critical care patients’ self-reports of pain, and opioid administration. Crit Care Med. 1997;25(7):1159–1166.
97 Payen JF, Bru O, Bosson JL, Lagrasta A, Novel E, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29(12):2258–2263.
98 Feldt KS. The checklist of nonverbal pain indicators (CNPI). Pain Manag Nurs. 2000;1(1):13–21.
99 Gélinas C, Fillion L, Puntillo KA, Viens C, Fortier M. Validation of the critical-care pain observation tool in adult patients. Am J Crit Care. 2006;15(4):420–427.
100 Young J, Siffleet J, Nikoletti S, Shaw T. Use of a Behavioural Pain Scale to assess pain in ventilated, unconscious and/or sedated patients. Intensive Crit Care Nurs. 2006;22(1):32–39.
101 Aissaoui Y, Zeggwagh AA, Zekraoui A, Abidi K, Abouqal R. Validation of a behavioral pain scale in critically ill, sedated, and mechanically ventilated patients. Anesth Analg. 2005;101(5):1470–1476.
102 Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197.
103 Puntillo KA, White C, Morris AB, Perdue ST, Stanik-Hutt J, et al. Patients’ perceptions and responses to procedural pain: results from Thunder Project II. Am J Crit Care. 2001;10(4):238–251.
104 Arbour C, Gelinas C. Are vital signs valid indicators for the assessment of pain in postoperative cardiac surgery ICU adults? Intensive Crit Care Nurs. 2010;26(2):83–90.
105 Frazier SK, Moser DK, Schlanger R, Widener J, Pender L, Stone KS. Autonomic tone in medical intensive care patients receiving mechanical ventilation and during a CPAP weaning trial. Biol Res Nurs. 2008;9(4):301–310.
106 Puntillo KA, Wild LR, Morris AB, Stanik-Hutt J, Thompson CL, White C. Practices and predictors of analgesic interventions for adults undergoing painful procedures. Am J Crit Care. 2002;11(5):415–429. quiz 430–31
107 Nilsson U. The anxiety- and pain-reducing effects of music interventions: a systematic review. AORN J. 2008;87(4):780–807.
108 MacPherson RD, Woods D, Penfold J. Ketamine and midazolam delivered by patient-controlled analgesia in relieving pain associated with burns dressings. Clin J Pain. 2008;24(7):568–571.
109 Zor F, Ozturk S, Bilgin F, Isik S, Cosar A. Pain relief during dressing changes of major adult burns: ideal analgesic combination with ketamine. Burns. 2010;36(4):501–505.
110 Siegel JM. Why we sleep. Scientific American. 2003;289(5):92–97.
111 Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol. 1991;71(3):1112–1118.
112 Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528.
113 Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, et al. A prospective study of change in sleep duration: assocications with mortality in the Whitehall II cohort. Sleep. 2007;30(12):1659–1666.
114 Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163(2):451–457.
115 Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping? J Trauma. 2007;63(6):1210–1214.
116 Hardin KA, Seyal M, Stewart T, Bonekat HW. Sleep in critically ill chemically paralyzed patients requiring mechanical ventilation. Crit Care Med. 2006;129(6):1468–1477.
117 Drouot X, Cabello B, d’Ortho M-P, Brochard L. Sleep in the intensive care unit. Sleep Medicine Reviews. 2008;12:391–403.
118 Freedman NS, Kotzer N, Schwab RJ. Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1155–1162.
119 Frisk U, Nordström G. Patients’ sleep in an intensive care unit – patients’ and nurses’ perception. Intensive Crit Care Nurs. 2003;19(6):342–349.
120 Knapp-Spooner C, Yarcheski A. Sleep patterns and stress in patients having coronary bypass. Heart Lung. 1992;21(4):342–349.
121 Nicolás A, Aizpitarte E, Iruarrizaga A, Vázquez M, Margall A, Asiain C. Perception of night-time sleep by surgical patients in an intensive care unit. Nurs Crit Care. 2008;13(1):25–33.
122 Simpson T, Lee ER, Cameron C. Relationships among sleep dimensions and factors that impair sleep after cardiac surgery. Res Nurs Health. 1996;19(3):213–223.
123 Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine, 4th edn. Philadelphia: Elsevier Saunders; 2005.
124 Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273.
125 Floyd JA, Janisse JJ, Jenuwine ES, Ager JW. Changes in REM-sleep in percentage over the adult lifespan. Sleep. 2007;30(7):829–836.
126 Rechtschaffen A, Kales A. A manual of standardized terminology: Techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968.
127 Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392.
128 Bourne RS, Minelli C, Mills GH, Kandler R. Clinical review: sleep measurements in critical care patients: research and clinical implications. Critical Care. 11(226), 2007.
129 Nieuwenhuijs D, Coleman EL, Douglas NJ, Drummond GB, Dahan A. Bispectral index values and spectral edge frequency at different stages of physiologic sleep. Anesth Analg. 2002;94(1):125–129.
130 Sleigh JW, Andrzejowski J, Steyn-Ross A, Steyn-Ross M. The Bispectral index: a measure of depth of sleep? Anesth Analg. 1999;88:659–661.
131 Richards KC, O’Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000;8(2):131–144.
132 Richards KC, Bairnsfather L. A description of night sleep patterns in the critical care unit. Heart Lung. 1988;17(1):35–42.
133 Richards KC, O’Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Measure. 2000;8(2):131–144.
134 Bourne RS, Minelli C, Mills GH, Kandler R. Clinical review: Sleep measurement in critical care patients: research and clinical implications. Crit Care. 2007;11(4):226.
135 Edwards GB, Schuring LM. Pilot study: validating staff nurses’ observations of sleep and wake states among critically ill patients, using polysomnography. Am J Crit Care. 1993;2(2):125–131.
136 Beecroft JM, Ward M, Younes M, Crombach S, Smith O, Hanly PJ. Sleep monitoring in the intensive care unit: comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Med. 2008;34(11):2076–2083.
137 Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. BMJ (Clin Res Ed). 1985;290(6474):1029–1032.
138 Richardson A, Crow W, Coghill E, Turnock C. A comparison of sleep assessment tools by nurses and patients in critical care. J Clin Nurs. 2007;16(9):1660–1668.
139 Richards KC. Effect of a back massage and relaxation intervention on sleep in critically ill patients. Am J Crit Care. 1998;7(4):288–299.
140 Richardson S. Effects of relaxation and imagery on the sleep of critically ill adults. Dimens Crit Care Nurs. 2003;22(4):182–190.
141 Richardson A, Allsop M, Coghill E, Turnock C. Earplugs and eye masks: do they improve critical care patients’ sleep? Nurs Crit Care. 2007;12(6):278–286.
142 Honkus VL. Sleep deprivation in critical care units. Crit Care Nurs Q. 2003;26(3):179–189.
143 Aaron JN, Carlisle CC, Carskadon MA, Meyer TJ, Hill NS, Millman RP. Environmental noise as a cause of sleep disruption in an intermediate respiratory care unit. Sleep. 1996;19(9):707–710.
144 Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167(5):708–715.
145 Environmental Protection Agency US. EPA identifies noise levels affecting health and welfare 1974. Cited Nov 2008. Available from: http://www.epa.gov/history/topics/noise/01.htm
146 Ryherd EE, Waye KP, Ljungkvist L. Characterizing noise and perceived work environment in a neurological intensive care unit. J Acoust Soc Am. 2008;123(2):747–756.
147 Tijunelis MA, Fitzsullivan E, Henderson SO. Noise in the ED. Am J Emerg Med. 2005;23(3):332–335.
148 Topf M, Davis JE. Critical care unit noise and rapid eye movement (REM) sleep. Heart Lung. 1993;22(3):252–258.
149 Frisk U, Olsson J, Nylén P, Hahn RG. Low melatonin excretion during mechanical ventilation in the intensive care unit. Clin Sci (Lond). 2004;107(1):47–53.
150 Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48(6):679–684.
151 Perras B, Meier M, Dodt C. Light and darkness fail to regulate melatonin release in critically ill humans. Intensive Care Med. 2007;33(11):1954–1958.
152 Cabello B, Thille AW, Drouot X, Galia F, Mancebo J, et al. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Crit Care Med. 2008;36(6):1749–1755.
153 Bourne RS, Mills GH. Sleep disruption in critically ill patients – pharmacological considerations. Anaesthesia. 2004;59(4):374–384.
154 Hardin KA. Sleep in the ICU: potential mechanisms and clinical implications. Crit Care Med. 2009;136(1):284–294.
155 Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2007;332(7538):385–393.
156 Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, et al. The efficacy and safety of exogenous melatonin for primary sleep disorders: a meta analysis. J Gen Intern Med. 2005;20(12):1151–1158.
157 Ibrahim MG, Bellomo R, Hart GK, Norman TR, Goldsmith D, et al. A double-blind placebo-controlled randomised pilot study of nocturnal melatonin in tracheostomised patients. Crit Care Resusc. 2006;8(3):187–191.
158 Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12(2):R52.
159 Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, et al. Effect of melatonin on sleep quality of COPD intensive care patients: a pilot study. Chronobiol Int. 2000;17(1):71–76.
160 Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9(1):11–24.
161 Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9(1):41–50.
162 Iber C, Ancoli-Israel S, Chesson A, Quan SF. AASM manual for the scoring of sleep and associated events: rules, terminology and technical specification. Westchester, IL: American Academy of Sleep Medicine; 2007.
* More recent sleep staging guidelines have combined stages 3 and 4 so that there are now only 3 stages of non-REM sleep.162 However the system has not yet been widely adopted and up to the date of publishing, few studies published on sleep in critical care have used the system.


 of the patients also received endotracheal suctioning, while a minority received turning alone or turning and hyperventilation. Further, the associated procedures of endotracheal suctioning or hyperventilation may have been more responsible for the changes in vital signs (particularly end-tidal CO2 and SpO2) than the turning and quantifying this influence is exacerbated by the inconsistent application of the procedure.
of the patients also received endotracheal suctioning, while a minority received turning alone or turning and hyperventilation. Further, the associated procedures of endotracheal suctioning or hyperventilation may have been more responsible for the changes in vital signs (particularly end-tidal CO2 and SpO2) than the turning and quantifying this influence is exacerbated by the inconsistent application of the procedure.