Chapter 204 Psoriasis
 Diagnostic Summary
Diagnostic Summary
• Circumscribed red, thickened plaques overlying silvery-white scale.
• Characteristically involves the scalp; the extensor surfaces of the wrists, elbows, knees, buttocks, and ankles; and sites of repeated trauma.
• Positive family history in 30% of cases.
• Nail involvement results in characteristic pitting. Other nail changes include onycholysis, discoloration, thickening, and dystrophy.
 General Considerations
General Considerations
Psoriasis is a common skin disorder. Its rate of occurrence in the United States is between 2% and 4% of the population. Psoriasis affects few black people in tropical zones but is more common among those in temperate zones, though still lower than for whites. It appears commonly among Japanese but is rare in American Indians. Psoriasis occurs in men and women equally, and the mean onset is at 33 years of age,1 although 2% of patients show onset by 2 years of age.
Psoriasis is a classic example of a hyperproliferative skin disorder. The rate of keratinocyte proliferation, assessed by proliferating cell nuclear antigen staining, is highest in verrucae (72.1%) and psoriasis (45%), followed by squamous cell carcinoma (28%), actinic keratosis (23%), and basal cell carcinoma (21.4%). The apoptosis inhibitor survivin is a recently described member of the “inhibitor of apoptosis” family expressed in most human cancers and also known to be a regulator of mitosis. Survivin has been detected in 88% of patients with psoriasis. It is likely that other factors are also involved in the development of a hyperplastic epidermis.2
The pathogenesis of psoriasis is incompletely understood; it is a complex, multifactorial disease with a genetic predisposition. The etiopathogenicity of psoriasis can be examined from several different viewpoints. It is a polygenic disorder. Oxidative stress has been linked to the inflammation in psoriasis.3 It is an immune dysfunction. Angiogenesis plays a critical role. Neuropeptides are involved.4 Research into each of these factors reveals increasing levels of sophisticated studies. At this time, it does not seem possible to combine them all under one unifying theory.
HLA-Cw6, which lies in the major histocompatibility region on chromosome 6, is currently considered the major genetic determinant of psoriasis. Variants in or near genes that encode subunits of cytokines (IL12B, IL23A) or cytokine receptors (IL23R) are of interest. The gene product of IL12B, p40, is the target of a recently approved monoclonal antibody therapy for psoriasis (ustekinumab). Association with psoriasis has also been found in genes coding for tumor necrosis factor-alpha (TNF-α) and other cytokines. Many of these genetic variations are also associated with immune disorders considered psoriatic comorbidities, including Crohn’s disease and diabetes.5
Although in the past psoriasis was considered a disorder of keratinocytes, it is now generally accepted that it is primarily an immune-mediated disorder. The cells of the skin consist of cytokine-synthesizing keratinocytes, antigen presenting cells, epidermotrophic and circulating T lymphocytes, dermal capillary endothelial cells, mast cells, tissue macrophages, granulocytes, fibroblasts, and non-Langerhan cells. These cells all communicate by means of cytokine secretion and respond accordingly via stimulation by bacteria, chemical, ultraviolet light, and other irritating factors. The primary cytokine released in response to antigen presentation is TNF-α. Ordinarily this is a controlled process unless the insult to the skin is prolonged, in which case an imbalanced cytokine production in a susceptible host leads to a pathologic state such as psoriasis. Studies have shown that activated keratinocytes in psoriatic lesions not only undergo accelerated turnover but also are a major source of proangiogenic cytokines, such as vascular endothelial growth factor (VEGF), endothelial-cell stimulating angiogenesis factor, TNF-α, and platelet-derived growth factor (PDGF).6 Other angiogenic mediators—such as hypoxia-inducible factors, angiopoietins, interleukin-8 (IL-8), and IL-17—are upregulated in psoriasis.7 Patients with psoriasis receiving anti-VEGF treatment for cancer showed complete remission of their cutaneous symptoms.8
Initially, immature dendritic cells in the epidermis stimulate T cells from lymph nodes in response to antigen stimulation. The lymphocytic infiltrate in psoriasis comprises predominately CD4 and CD8 T cells. Adhesion molecules that promote leukocyte adherence are highly expressed in psoriatic lesions.9 After T cells receive primary stimulation and activation, a resulting synthesis of mRNA for IL-2 occurs, resulting in a subsequent increase in IL-2 receptors. The increased IL-2 from activated T cells and IL-12 from Langerhans cells ultimately regulate genes that code for the transcription of cytokines, such as IFN-gamma, TNF- α, and IL-2, which are responsible for the differentiation, maturation, and proliferation of T cells into memory effector cells. Ultimately, T cells migrate to the skin, where they accumulate around dermal blood vessels, producing proangiogenic cytokines and other angiogenic factors as described above. These are the first in a series of immunologic changes that result in the formation of acute psoriatic lesions.10 The above-described immune response is a somewhat normal response to antigen stimulation. It remains unclear why the T-cell activation that occurs, followed by subsequent migration of leukocytes into the epidermis and dermis, creates accelerated cellular proliferation. One factor that contributes to this is fibronectin (FN), a cell adhesion molecule that anchors cells to collagen or proteoglycan substrates and is involved in many cellular processes, including tissue repair, embryogenesis, blood clotting, and cell migration/adhesion. Transforming growth factor beta (TGF-β) stimulates extra domain A-FN (EDA-FN), a form of FN with an additional domain domain. EDA-FN in turn stimulates keratinocyte hyperproliferation, and through stimulation of Toll-like receptor (TLR) 4, produces proinflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-12. These FN loops contribute to the maintenance and progression of psoriatic lesions. Although the association between psoriasis and cardiovascular disease remains somewhat unclear, one plausible link may be the promotion of atherosclerosis and thrombotic heart disease by EDA-FN.11
It is known that psoriasis develops in bone marrow transplant recipients from donors with psoriasis, clears in recipients from donors without psoriasis, and that immunosuppressive drugs are effective in reducing psoriasis.12,13 Given the genetic predisposition to this disease, what can be done to reduce the phenotypic expression besides resorting to immunosuppressive and biological therapies with their attendant side-effect profiles?
It is interesting that researchers have recognized “unidentified antigens” as ostensibly the source of the psoriatic cascade. A clear relationship of psoriasis with conditions like celiac disease14 and Crohn’s disease15 has been reported. Furthermore, the bowel mucosa of psoriatic patients without bowel symptoms have shown microscopic lesions and greater intestinal permeability, even when the mucosa appeared macroscopically normal.16,17 Given information regarding bowel toxemias, food allergies, low proteolytic and bile enzymes, as well as suboptimal liver function and food allergies (see the following sections), a plausible naturopathic suggestion here would be to consider that factors leading to poor intestinal function most likely encourage greater intestinal permeability and inflammation, which ultimately allow these antigenic and endotoxic compounds to leave the intestinal confines, travel through the bloodstream, and initiate activated immune cascades in susceptible tissues.
 Therapeutic Considerations
Therapeutic Considerations
Gastrointestinal Function
Incomplete Protein Digestion
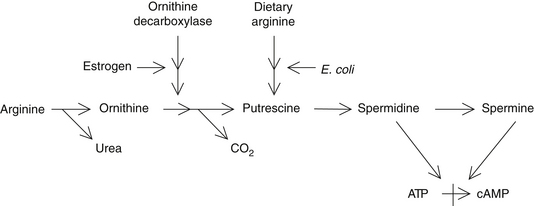
Incomplete protein digestion or poor intestinal absorption of protein breakdown products can result in elevations of amino acids and polypeptides in the bowel. These are metabolized by bowel bacteria into several toxic compounds. The toxic metabolites of the amino acids arginine and ornithine are known as polyamines (e.g., putrescine, spermidine, and cadaverine) and have been shown to be higher in individuals with psoriasis. Polyamines inhibit the formation of cyclic adenosine monophosphate and therefore contribute to the excessive rate of cell proliferation (Figure 204-1).18–20 Lowered skin and urinary levels of polyamines are associated with clinical improvement in psoriasis.18
A number of natural compounds can inhibit the formation of polyamines and may be of benefit in the treatment of psoriasis. For example, vitamin A and the alkaloids of Hydrastis canadensis (goldenseal) such as berberine inhibit bacterial decarboxylase, the enzyme that converts amino acids into polyamines.21,22 However, the best way to prevent the excessive formation of polyamines is to evaluate digestive function with the aid of Heidelberg Gastric Analysis and/or a comprehensive digestive stool analysis and then take the action necessary (e.g., hydrochloric acid supplementation) to ensure complete protein digestion and absorption (these assessment methods can be found in Chapters 18 and 27).
Bowel Toxemia
A number of gut-derived toxins are implicated in the development of psoriasis, including endotoxins (cell-wall components of gram-negative bacteria), streptococcal products, Candida albicans, yeast compounds, and immunoglobulin (Ig) E and IgA immune complexes.23–25 Endotoxins have been found in high levels in the blood of psoriatic patients,26 and these compounds lead to increases in cyclic guanosine monophosphate (GMP) levels within skin cells, thereby increasing the rate of proliferation dramatically. Overgrowth of C. albicans in the intestines (chronic candidiasis) may play a role in some patients with psoriasis.
A diet low in dietary fiber is associated with increased levels of gut-derived toxins.23 Dietary fiber is critical to maintaining a healthy colon. Many fiber components bind bowel toxins and promote their excretion in the feces. It is therefore essential that the diet of an individual with psoriasis be rich in beans, fruits, and vegetables. Natural compounds that bind endotoxins and promote their excretion may also be used. For example, an aqueous extract of the herb Smilax sarsaparilla was found in a 1942 study to be effective in psoriasis, particularly the more chronic, large plaque–forming variety.27 In this controlled study of 92 patients, S. sarsaparilla greatly improved the psoriasis in 62% of the patients and resulted in complete clearance in another 18% (i.e., 80% of the subjects experienced significant benefits). This benefit is apparently due to S. sarsaparilla’s components’ binding to and promoting the excretion of bacterial endotoxins.
Liver Function
The correction of abnormal liver function may be of benefit in the treatment of psoriasis.28 The connection between the liver and psoriasis relates to one of the liver’s basic tasks—filtering and detoxifying the blood returning through the portal circulation from the bowels. Structurally, the hepatic architecture may already be altered in psoriatic patients.29 As mentioned previously, psoriasis has been linked to the presence of several microbial by-products in the blood. If hepatic function is compromised by excessive levels of these toxins from the bowel or if there is a decrease in the liver’s detoxification ability, the systemic toxin level rises and the psoriasis worsens.
Alcohol consumption is known to significantly worsen psoriasis.30 Alcohol has this effect because it both increases the absorption of toxins from the gut (by damaging the gut mucosa) and impairs liver function. Alcohol intake must be restricted in individuals with psoriasis.
Silymarin, the flavonoid component of Silybum marianum, has been reported to be of value in the treatment of psoriasis.28 Presumably this is a result of its ability to improve liver function, inhibit inflammation, and reduce excessive cellular proliferation.31,32
Bile Deficiencies
In the psoriatic patient, endotoxins are able to translocate from the intestine into the bloodstream.26 Bile acids normally present in the intestines act to detoxify bacterial endotoxins. In the absence of sufficient amounts of bile acids, endotoxins translocating into the bloodstream can produce pathologic conditions that vary in severity depending on their amount, including the release of inflammatory cytokines known to play a role in psoriasis.
A fascinating Hungarian study of 800 psoriatic patients was conducted in which 551 were treated with oral bile acid (dehydrocholic acid) supplementation for 1 to 6 weeks or 3 to 8 weeks for acute or chronic cases, respectively. Conventional therapies were administered to 249 patients as a comparison group. Both groups were advised to eat a diet high in vegetables and fruits and were instructed to avoid hot spices, alcohol, raw onion, garlic, and carbonated soft drinks. Of the 551 patients receiving bile acid, 434 (78.8%) became asymptomatic, whereas only 62 (24.9%) of the 249 patients receiving conventional therapies demonstrated clinical recovery during this treatment period. Additionally, the curative effect of bile acid supplementation was more pronounced in the acute form of psoriasis; 95.1% of the patients in this group became asymptomatic. In follow-up assessments 2 years later, 319 of the 551 patients with acute and chronic psoriasis who had been treated with bile acid (57.9%) were asymptomatic, compared with only 15 of the 249 patients (6%) who had received the conventional treatment.26 The bile acid supplements used in the preceding study were either 2 or 3 dehydrocholate sodium (Suprachol) sugar-coated pills once a day or dehydrocholic acid powder (acidum dehydrocholicum pulvis) at two to three doses of 0.25 g/day.
Because there is a theoretical risk of malignant tumors in patients with sluggish intestinal function given long-term bile acid therapy, the investigators in this study recommended that their subjects not continue ingesting bile acids on a regular basis but to supplement with them only after a fatty meal once the initial treatment period had been completed. Two separate evaluations of bile acid effects on the proliferation of colonic mucosa report conflicting results.33,34 Two other studies actually found a cancer-protective effect of ursodeoxycholic acid. One was an observational study of 114 patients with primary biliary cirrhosis in whom a reduction in the risk for colon adenoma was discovered. The prevalence of colorectal adenomas was 13% in the treated group versus 24% in the untreated group. Additionally, the colon epithelial cell proliferation index was significantly lower in treated patients than in untreated patients.35 A second randomized controlled clinical trial of 52 subjects found significant declines in the risk for developing colorectal dysplasia or cancer in patients with ulcerative colitis and primary sclerosing cholangitis.36 More clinical research is needed to examine the different varieties of bile acids and their efficacy as well as their safety profile. Nevertheless, given the preceding information, it seems reasonable to consider the use of bile acids for the short-term treatment of psoriasis and to monitor the colon before and after treatment in patients at high risk for colon cancer.
Nutrition
Omega-3 Fatty Acids
The manipulation of dietary oils is extremely important in the management of psoriasis because serum levels of free fatty acids are typically abnormal in affected patients.37 Of particular benefit are the omega-3 fatty acids. Most of the clinical research has utilized fish oils rich in eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Several double-blind clinical studies have demonstrated that supplementing the diet with 10 to 12 g/day of fish oils (providing 1.8 g EPA and 1.2 g DHA) results in significant improvement.38–40 This amount of EPA and DHA would be equivalent to the amount of EPA in about 150 g of salmon, mackerel, or herring.
The improvement in psoriasis from EPA is partly due to the competition of EPA for arachidonic acid binding sites, producing leukotriene B5 (LTB5), which is only one tenth as potent as the inflammatory mediator LTB4. Levels of LTB4 have been shown to be elevated in psoriatic plaques and to demonstrate chemotactic properties necessary for the infiltration of leukocytes and the proliferation of keratinocytes.41 In the skin of individuals with psoriasis, the production of inflammatory leukotrienes from arachidonic acid is many times greater than normal.42 Leukotrienes are potent inflammatory agents and promoters of guanylate cyclase activity.
In the psoriatic epidermis, the cellular contents of free arachidonic acid and 12-hydroxyeicosatetraenoic acid (12-HETE; a product of lipoxygenase metabolism of arachidonic acid) are 250 and 810 times greater, respectively, than in uninvolved epidermal tissue.42
Immunomodulatory effects of omega-3 fatty acids include the suppression of lymphocyte proliferation, CD4 cells, antigen presentation, adhesion molecule presentation, Th1 and Th2 responses, and proinflammatory cytokine production (such as IL-1, TNF-α, and PDGF), which prevents vascularization within the psoriatic plaque.43
As might be expected, cyclooxygenase inhibitors (e.g., aspirin and most other nonsteroidal antiinflammatory agents) may exacerbate psoriasis, whereas lipoxygenase inhibitors (e.g., benoxaprofen) may bring improvement.44 Naturally occurring substances such as quercetin (the ubiquitous plant flavonoid), vitamin E, onion, and garlic are known to inhibit lipoxygenase and therefore may be of benefit. However, it is improbable that selective inhibition of one component or enzyme (e.g., a 5-lipoxygenase inhibitor) would do more than create an imbalance in this closely integrated network of mediators, which may not necessarily be beneficial.45
Diet, Fasting, and Food Allergy Control
An evaluation of 316 patients with psoriasis and 366 control subjects, both groups being in the age range of 16 to 65 years, found that psoriasis was positively associated with body mass index and inversely related to the intake of carrots, tomatoes, fresh fruits, and the index of beta-carotene intake.46 Research at a Swedish hospital studying the effects of fasting and vegetarian regimens on chronic inflammatory disease found that such diets helped psoriatic patients.47 The improvement was probably due to decreased levels of gut-derived toxins and polyamines. Patients have also benefited from gluten-free and elimination diets.48,49 Several herbs used as seasonings—including turmeric, red pepper, cloves, ginger, cumin, anise, fennel, basil, rosemary, garlic and pomegranate—can block the activation of nuclear factor-kappa B of inflammatory cytokines.50
Individual Nutrients
Decreased levels of vitamin A and zinc are common in patients with psoriasis.51–53 Given the critical roles of these nutrients in the health of the skin, supplementation might be warranted even without this association.
Chromium supplementation may be indicated to increase the sensitivity of insulin receptors, because psoriatic patients typically have increased serum levels of both insulin and glucose and carry an increased risk for type 2 diabetes mellitus and metabolic syndrome.54
Substantial evidence indicates that psoriasis is an independent risk factor for cardiovascular disease.55 It remains difficult to conclude whether risk factors are caused by psoriasis or share a common pathogenesis. Inflammation, characterized by the presence of proinflammatory cytokines and endothelial activation, is a common theme underlying these conditions. Dyslipidemia, coronary calcification, increased highly sensitive C-reactive protein, decreased folate, and hyperhomocysteinemia are found significantly more often in psoriatic patients.56
The inflammatory processes underlying psoriasis suggest the possibility of omega-3 fatty acid, folate, and vitamin B12 deficiencies, which are also found in cardiovascular disease.57 High homocysteine and decreased folate levels correlate with the psoriasis area and severity index (PASI). The rapid keratinocyte turnover rate in psoriasis may result in folate utilization and subsequent deficiency.58 The authors of one study conclude that “Dietary supplementation of folic acid, B6, and B12 appears reasonable in psoriasis patients, particularly those with elevated homocysteine, low folate and additional cardiovascular risk factors.”59 In supplementing with folic acid or its active form 5-methyltetrahydrofolate, the recommended dose is 1 to 5 mg/day.
Glutathione peroxidase (GP) levels are low in psoriatic patients, possibly because of such factors as alcohol abuse, malnutrition, and the excessive loss of skin due to the hyperproliferative disease. The depressed levels of GP normalize with oral selenium and vitamin E therapy.60 One investigation found that patients with longer-term psoriasis (3 years or more) demonstrated low plasma selenium status.61 Another study comparing 113 patients with moderate to severe psoriasis and 104 healthy controls found that male psoriatic patients between 20 and 49 years of age and women with the disease of longer than 20 years’ duration had particularly low selenium concentrations. The lowest whole-blood selenium values were found in the subgroup of male patients with widespread disease of long duration who also required treatment with methotrexate and retinoids.62
It has been established that patients with disseminated psoriasis have significantly decreased serum levels of the biologically active form of vitamin D, 1,25-dihydroxycholecalciferol (calcitriol) compared with age- and gender-matched controls and also compared with patients with moderate psoriasis.63 Whether this is a contributing factor to psoriasis or a result of the disorder has not been elucidated.
Keratinocytes in the epidermis convert 7-dehydrocholesterol to vitamin D3 in the presence of ultraviolet B. Sunlight, UVB phototherapy, oral calcitriol, and topical vitamin D and its analogs are effective therapy for psoriasis owing to vitamin D’s antiproliferative and prodifferentiating actions on keratinocytes.64 Vitamin D also encourages a shift toward type 2 helper T-cell cytokine expression, with an increase in IL-10 and a decrease in IL-8, which may be responsible for the improvements seen in psoriasis.65
Vitamin D analogs like calcipotriol have also been shown to mediate the expression proinflammatory antimicrobial peptides such as β-defensin and to decrease IL-17A, IL-17F, and IL-8 in lesional psoriatic skin.66 Topical calcitriol has also been shown to reduce the number of dendritic cells in the skin.67
Calcitriol-binding to vitamin D receptors (VDRs) in the skin modulates the expression of a large number of genes including cell-cycle regulators, growth factors, and their receptors. Polymorphisms of the VDR gene are associated with psoriasis and may predispose to the development of psoriasis and resistance to calcipotriol therapy while also contributing to liver dysfunction in patients with psoriasis.68
Given the importance of vitamin D in psoriasis, cancer, inflammatory diseases, and other conditions, it has been suggested by some investigators that recommendations for sun protection and skin cancer prevention may need to be reevaluated to allow for sufficient vitamin D status. However, studies conducted in Honolulu, Miami, and southern Arizona showed that abundant sun exposure did not necessary ensure vitamin D adequacy, which points to the need for vitamin D supplementation to achieve optimal blood levels while also protecting the skin from sun damage and skin cancer.69
It has been demonstrated that oral vitamin D can be safely taken in daily doses of up to 5000 IU/day, with some experts recommending up to 10,000 IU/day to correct a deficiency.70
Fumaric Acid
Over the past three decades, fumaric acid therapy has become increasingly popular in western Europe for psoriasis. Therapy consists of the oral intake of dimethylfumaric acid (240 mg/day) or monoethylfumaric acid (720 mg/day) and the topical application of 1% to 3% of monoethylfumaric acid. Clinical studies have shown that it is useful in many patients with psoriasis,71 but side effects such as flushing of the skin, nausea, diarrhea, general malaise, gastric pain, and mild liver and kidney disturbances can occur.72 We recommend using fumaric acid therapy only after other natural therapies have proved ineffective.
Psychological Aspects
Research work concerning the role of stress in psoriatic exacerbations is mixed. One investigation revealed that a large proportion (39%) of patients with psoriasis report the occurrence of a specific stressful event within 1 month before their initial episode. Such patients have a better prognosis.73 Yet other findings show a limited relationship between stress and vulnerability to psoriasis, whereby correlation seemed to occur mostly in patients with repeated stressors occurring four times or more in 1 year.74 Stress management can benefit individuals with psoriasis.75 As judged by two independent dermatologists, subjects who listened to a guided meditation tape while undergoing phototherapy cleared four times as quickly as those who received phototherapy only. Psoriasis status was assessed in three ways: direct inspection by unblinded clinic nurses, direct inspection by physicians blinded to the patient’s study condition (tape or no-tape), and blinded physician evaluation of photographs of psoriatic lesions. Four sequential indicators of skin status were monitored during the study: a First Response Point, a Turning Point, a Halfway Point, and a Clearing Point. Subjects in the tape groups reached the Halfway Point (P = 0.013) and the Clearing Point (P = 0.033) significantly more rapidly than those in the no-tape condition for both UVB and PUVA treatments. Finally, psychotherapy can be an essential adjunct for individuals with persistent unresolved psychological issues such as anxiety, depression, and the psychosocial stress of this chronic skin disease. A few case histories have been reported that document the successful treatment of psoriasis with hypnosis and biofeedback alone.76
Physical Therapeutics
Sunlight and Ultraviolet Light
Sunlight (which contains ultraviolet light) is extremely beneficial for individuals with psoriasis.77,78 Outdoor 4-week heliotherapy was shown to promote significant clearance of psoriatic symptoms in 84% of 373 subjects.79 Studies employing nonprescription commercial tanning beds have shown that a majority of patients find them helpful80; they also objectively facilitate improvements in both psoriasis severity and health-related quality of life.81 In addition, an open-label retrospective trial studied the combined use of the retinoid acitretin (a vitamin A derivative) along with a 4- to 5-day-per-week tanning regimen; 83% of 23 subjects experienced complete or near-complete recovery.82
Specific ultraviolet exposure may also be of benefit owing to its induction of vitamin D synthesis in the skin. The standard ultraviolet medical treatment of psoriasis typically involves the use of the drug psoralen and ultraviolet A (PUVA therapy; 320 to 340 nm). Ultraviolet B (UVB; 280 to 320 nm) exposure alone also leads to inhibition of cell proliferation; in certain studies, it has been shown to be as effective as PUVA therapy, with fewer side effects.87 At the Dead Sea, where 80% to 85% of psoriatic conditions clear in 4 weeks, UVB wavelengths are known to be the dominant light.83,84 In one investigation of 28 patients, UVB had a clear advantage over PUVA therapy.18 Conversely, another report comparing UVB with PUVA in 100 patients demonstrated clearance of psoriasis in a significantly greater proportion of patients treated with PUVA (84%) than with UVB (63%), and with significantly fewer treatments.85 Other studies have found both to be effective, with similar untoward side effects.86,87 It is clear that more study is needed to clarify both the risks and benefits of these therapies and whether certain presentations of psoriasis may respond best to a specific ultraviolet therapy. As noted above, ultraviolet light deactivates vitamin D topical agents, Therefore, if they are used in conjunction, the topical agent should be applied only after ultraviolet treatment. Furthermore, any light therapy should be monitored carefully, especially in patients at risk for skin cancers.
Other Physical Therapeutics
The induction of localized elevation of temperature (42°C to 45°C) to the affected area by ultrasound and heating pads has been shown to be an effective treatment of psoriasis.88,89 Italian researchers have conducted small clinical trials using a hypotonic sulfate water (Leopoldine water) in a balneotherapeutic manner. These treatments have yielded favorable immunohistologic profiles of the affected tissues with significant decreases in the numbers of T lymphocytes, Langerhans cells, and markers of keratinocyte inflammatory expression.90
Topical Treatments
Aloe vera
A very exciting, double-blind, placebo-controlled study found that topical application of an A. vera extract in a hydrophilic cream was highly effective in psoriasis vulgaris. Sixty patients with slight to moderate chronic plaque-type psoriasis and the PASI scores between 4.8 and 16.7 (mean 9.3) whose mean duration of disease averaged 8.5 years (range 1 to 21 years) applied either the aloe or a placebo gel three times a day. The treatment was well tolerated by all the patients, with no adverse drug-related symptoms and no dropouts. By the end of the study (4 to 12 months of treatment), the A. vera extract cream had cured 25 of 30 patients (83.3%) compared with the placebo cure rate of only 2 of 30 (6.6%), resulting in significant clearing of the psoriatic plaques (328 of 396 [82.8%] vs. placebo 28 of 366 [7.7%]) and a decrease in PASI score to a mean of 2.2.91a
Curcumin
Curcumin gel yielded 90% resolution of plaques in 50% of patients within 2 to 6 weeks; the remainder of the study subjects showed 50% to 85% improvement.91 Curcumin was found to be twice as effective as calcipotriene cream (which generally takes 3 months to exert its full effect). Curcumin acts as a selective phosphorylase kinase inhibitor, thereby reducing inflammation through the inhibition of NFκB.
Mahonia aquifolium
Several open and placebo-controlled trials have supported the effectiveness of Mahonia aquifolium topical cream in the treatment of psoriasis. On the basis of physician and patient assessments, 71% to 81% of patients improved in these trials.92 A randomized double-blind placebo-controlled trial of 200 patients also demonstrated statistically significant improvement when this agent was applied twice daily for 12 weeks.93
Topical Vitamin D
Topical corticosteroids are the most common treatment for psoriasis; however, their long-term use is associated with a potential risk for side effects. Topical vitamin D modulators have been developed as an option for use in place of or in addition to, topical corticosteroids. Topically, vitamin D inhibits keratinocyte proliferation, normalizes differentiation, and modulates immune cell activity with minimal effect on serum calcium.94 Calcipotriene is the most widely used topical vitamin D. Although evidence suggests that in the long term it is approximately as effective as low- to medium-potency corticosteroids (response is not obtained as quickly as with corticosteroids), it is associated with cutaneous irritation, especially when used on sensitive skin. Calcitriol ointment was recently approved and contains the naturally occurring active form of vitamin D3 and is associated with a low rate of cutaneous and systemic adverse effects.
By inhibiting proinflammatory cytokines such as IL-12, IL-23, and TNF-α, topical nicotinamide has been shown to enhance the efficacy of calcipotriene therapy when used in combination.95
Emollients
The scaliness of psoriasis benefits from the use of emollients. Intercellular lipids such as ceramides play an important role in regulating the homeostasis of the skin water barrier and the skin’s water-holding capacity, and it has been shown that ceramides are decreased in the psoriatic epidermis. Newer ceramide-containing emollients (e.g., CeraVe, Mimyx, Aveeno Eczema Care) have shown benefit in psoriasis and may improve skin barrier function and decrease water loss.96
 Therapeutic Approach
Therapeutic Approach
Supplements
• High-potency multivitamin/multimineral formula.
• Vitamin D: 5000 to 10,000 IU/day based on serum 25(OH)D level.
• Consider vitamin E 400 IU/day, chromium 400 mcg/day, selenium 200 mcg/day, zinc 30 mg/day.
• Consider digestive enzymes and/or bile acids with meals.
• Water-soluble fiber (psyllium, pectin, guar gum, etc.): 5 g at bedtime.
Botanical Medicines
Consider the following if indicated by impaired digestion or liver function:
• The dose should be based on berberine content, because there is a wide range of quality in goldenseal preparations, standardized extracts are preferred three times a day.
• Dried root or as infusion (tea): 2 to 4 g three times a day.
• Fluid extract (1:1): 2 to 4 mL (0.5 to 1 tsp) three times a day.
• Solid (powdered dry) extract (4:1 or 8% to 12% alkaloid content): 250 to 500 mg three times a day.
1. Nevitt G.J., Hutchinson P.E. Psoriasis in the community: prevalence, severity and patients belief and attitudes towards the disease. Br J Dermatol. 1996;135:533–537.
2. Bowen A.R., Hanks A.N., Murphy K.J., et al. Proliferation, apoptosis, and Survivin expression in keratinocyte neoplasms and hyperplasias. Am J Dermatopathol. 2004 June;26(3):177–181.
3. Coaccioli S., Panaccione A., Biondi R., et al. Evaluation of oxidative stress in rheumatoid and psoriatic arthritis and psoriasis. Clin Ter. 2009 Nov-Dec;160(6):467–472.
4. Nigam R., El-Nour H., Amatva B., et al. GABA and GABA(A) receptor expression on immune cells in psoriasis: a pathophysiological role. Arch Dermatol Res. 2010 Sept;302(7):507–515.
5. Duffin K.C., Woodcock J., Kruegger G.G. Genetic variations associated with psoriasis and psoriatic arthritis found by genome-wide association. Dermatol Ther. 2010 Mar;23(2):1011–1113.
6. Micali G., Lacarrubba F., Musumeci M.L., et al. Cutaneous vascular patterns in psoriasis. Int J Dermatol. 2010 Mar;49(3):249–256.
7. Heidenreich R., Rocken M., Choreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol. 90(3), 2009 Jun. 323-248
8. Canavese M., Altruda F., Ruzicka T., et al. Vascular endothelial growth factor (VEGF) in the pathogenesis of psoriasis: a possible target for novel therapies? J Dermatol Sci. 2010 Jun;58(3):171–176.
9. Robert C., Kupper T.S. Inflammatory skin diseases, T cells and immune surveillane. N Engl J Med. 1999;341:1817–1878.
10. Traub M., Marshall K. Psoriasis: pathophysiology, conventional, and alternative approaches to treatment. Alt Med Rev. 2007;4(12):319–330.
11. McFadden J.P., Baker B.S., Powles A.V., et al. Psoriasis and extra domain A fibronectin loops. Br J Dermatol. 2010 July;163(1):5–11.
12. Wahie S., Alexandroff A., Reynolds N.J., et al. Psoriasis occurring after myeloablative therapy and autologous stem cell transplantation. Br J Dermatol. 2006;154:194–195.
13. Eedy D.J., Burrows D., Bridges J.M., et al. Clearance of severe psoriasis after allogenic bone marrow transplantation. BMJ. 1990;300:908.
14. Ojetti V., Aguilar Sanchez J., Guerriero C., et al. High prevalence of celiac disease in psoriasis. Am J Gastroenterol. 2003;98:2574–2575.
15. Najarian D.J., Gottlieb A.B. Connections between psoriasis and Crohn’s disease. J Am Acad Dermatol. 2003;48:805–821.
16. Scarpa R., Manguso F., D’Arienzo A., et al. Microscopic inflammatory changes in colon of patients with both active psoriasis and psoriatic arthritis without bowel symptoms. J Rheumatol. 2000;27:1241–1246.
17. Humbert P., Bidet A., Treffel P., et al. Intestinal permeability in patients with psoriasis. J Dermatol Sci. 1991;2:324–326.
18. Proctor M., Wilkenson D., Orenberg E., et al. Lowered cutaneous and urinary levels of polyamines with clinical improvement in treated psoriasis. Arch Dermatol. 1979;115:945–949.
19. Voorhees J.J. Polyamines and psoriasis. Arch Dermatol. 1979;115:943–944.
20. McDonald C.J. Polyamines in psoriasis. J Invest Dermatol. 1983;81:385–387.
21. Haddox M., Scott K.F., Russel D. Retinol inhibition of ornithine decarboxylase induction and G1 progression in Chinese hamster ovary cells. Cancer Res. 1979;39:4930–4938.
22. Kuwano S., Yamauchi K. Effect of berberine on tyrosine decarboxylase activity of Streptococcus faecalis. Chem Pharm Bull. 1960;8:491–496.
23. Rosenberg E., Belew P. Microbial factors in psoriasis. Arch Dermatol. 1982;118:1434–1444.
24. Rao M., Field M. Enterotoxins and ion transport. Biochem Soc Trans. 1984;12:177–180.
25. Juhlin L., Vahlquist C. The influence of treatment and fibrin microclot generation in psoriasis. Br J Dermatol. 1983;108:33–37.
26. Gyurcsovics K., Bertók L. Pathophysiology of psoriasis: coping endotoxins with bile acid therapy. Pathophysiology. 2003;10:57–61.
27. Thurmon F.M. The treatment of psoriasis with sarsaparilla compound. N Engl J Med. 1942;337:128–133.
28. Weber G., Galle K. The liver, a therapeutic target in dermatoses. Med Welt. 1983;34:108–111.
29. Pietrzak A., Lecewicz-Torun B., Kadziela-Wypyska G. Changes in the digestive system in patients suffering from psoriasis. Ann Univ Mariae Curie Sklodowska [Med]. 1998;53:187–194.
30. Monk B.E., Neill S.M. Alcohol consumption and psoriasis. Dermatologica. 1986;173:57–60.
31. Hikino H., Kiso Y., Wagner H., et al. Antihepatotoxic actions of flavonolignans from Silybum marianum fruits. Planta Medica. 1984;50:248–250.
32. Adzet T. Polyphenolic compounds with biological and pharmacological activity. Herbs Spices Medicinal Plants. 1986;1:167–184.
33. Ochsenkuhn T., Marsteller I., Hay U., et al. Does ursodeoxycholic acid change the proliferation of the colorectal mucosa? A randomized, placebo-controlled study. Digestion. 2003;68:209–216.
34. Ochsenkuhn T., Bayerdorffer E., Meining A., et al. Colonic mucosal proliferation is related to serum deoxycholic acid levels. Cancer. 1999;85:1664–1669.
35. Serfaty L., De Leusse A., Rosmorduc O., et al. Ursodeoxycholic acid therapy and the risk of colorectal adenoma in patients with primary biliary cirrhosis: an observational study. Hepatology. 2003;38:203–209.
36. Pardi D.S., Loftus E.V., Jr., Kremers W.K., et al. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–893.
37. Zlatkov N.B., Ticholov J.J., Dourmishev A.L. Free fatty acids in the blood serum of psoriatics. Acta Derm Venereol. 1984;64:22–25.
38. Bittiner S.B., Tucker W.F., Cartwright I., et al. A double-blind, randomized, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378–380.
39. Grimmunger F., Mayser P., Papavassilis C. A double-blind, randomized, placebo-controlled n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis. Rapid improvement of clinical manifestations and changes in neutrophil leukotriene profile. Clin Invest. 1993;71:634–643.
40. Maurice P.D., Allen B.R., Barkley A.S., et al. The effects of dietary supplementation with fish oil in patients with psoriasis. Br J Dermatol. 1987;1117:599–606.
41. Mayser P., Grimm H., Grimminger F. n-3 fatty acids in psoriasis. Br J Nutr. 2002;87:S77–S82.
42. Voorhees J.J. Leukotrienes and other lipoxygenase products in the pathogenesis and therapy of psoriasis and other dermatoses. Arch Dermatol. 1983;119:541–547.
43. Calder P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:S1505–S1519.
44. Kragballe K., Herlin M.D. Benoxaphren improves psoriasis: a double-blind study. Arch Dermatol. 1983;119:548–552.
45. Greaves M.W., Camp R.D. Prostaglandins, leukotrienes, phospholipase, platelet activating factor, and cytokines: an integrated approach to inflammation of the skin. Arch Dermatol Res. 1988;280(suppl):S33–S41.
46. Naldi L., Parazzini F., Peli L., et al. Dietary factors and the risk of psoriasis: results of an Italian case-controlled study. Br J Dermatol. 1996;134:101–106.
47. Lithell H., Bruce A., Gustafsson I.B., et al. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm Venerool. 1983;63:397–403.
48. Bazex A. Diet without gluten and psoriasis. Ann Derm Symp. 1976;103:648.
49. Douglas J.M. Psoriasis and diet. Calif Med. 1980;133:450.
50. Aggarwal B.B., Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann NY Acad Sci. 2004;1030:434–441.
51. Majewski S., Janik P., Langer A., et al. Decreased levels of vitamin A in serum of patients with psoriasis. Arch Dermatol Res. 1989;280:499–501.
52. Hinks L.J., Young S., Clayton B. Trace element status in eczema and psoriasis. Clin Exp Dermatol. 1987;12:93–97.
53. Donadini A., Dazzaglia A., Desirello G. Plasma levels of Zn, Cu and Ni in healthy controls and in psoriatic patients: possible correlations with vitamins. Acta Vitamin Enzymol. 1980;1:9–16.
54. Fratino P., Pelfini C., Jucci A., et al. Glucose and insulin in psoriasis: the role of obesity and genetic history. Panminerva Medica. 1979;21:167–172.
55. Tobin A.M., Veale D.J., Fitzgerald O., et al. Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. J Rheumatol. 2010 July;37(7):1386–1394.
56. Rocha-Pereira P., Santos-Silva A., Rebelo I., et al. Dyslipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clin Chim Acta. 2001;303:33–39.
57. Ludwig R.J., Herzog C., Rostock A., et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271–276.
58. Vanizor Kural B., Orem A., Cimsit G., et al. Plasma homocysteine and its relationship with atherothrombotic markers in psoriatic patients. Clin Chim Acta. 2003;332:23–30.
59. Malerba M., Gisondi P., Radaeli A., et al. Plasma homocysteine and folate levels in patients with chronic plaque psoriasis. Br J Dermatol. 2006;155:1165–1169.
60. Juhlin L., Edqvist L.E., Ekman L.G., et al. Blood glutathione-peroxidase levels in skin diseases: effect of selenium and vitamin E treatment. Acta Derm Venereol. 1982;62:211–214.
61. Serwin A.B., Wasowicz W., Gromadzinska J., et al. Selenium status in psoriasis and its relations to the duration and severity of the disease. Nutrition. 2003;19:301–304.
62. Michaelsson G., Berne B., Calmark B., et al. Selenium in whole blood and plasma is decreased in patients with moderate and severe psoriasis. Acta Derm Venereol. 1989;69:29–34.
63. Staberg B., Oxholm A., Klemp P. Abnormal vitamin D metabolism in patients with psoriasis. Acta Derm Venereol. 1987;67:65–68.
64. Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618–625.
65. Mendonça C.O., Burden A.D. Current concepts in psoriasis and its treatment. Pharmacol Ther. 2003;99:133–147.
66. Peric M., Koglin S., Dombrowski Y., et al. Vitamin D analogs differentially control antimicrobial peptide/”alarmin” expression in psoriasis. PLoS One. 4(7), 2009 Jul 22. e6340
67. Gorman S., Judge M.A., Hart P.H. Immune-modifying properties of topical vitamin D: Focus on dendritic cells and T cells. J Steroid Biochem Mol Biol. 2010 Jul;121(1-2):247–249.
68. Okita H., Ohtsuka T., Yamakage A., et al. Polymorphism of the vitamin D(3) receptor in patients with psoriasis. Arch Dermatol Res. 2002;294:159–162.
69. Jacobs E.T., Alberts D.S., Foote J.A., et al. Vitamin D insufficiency in southern Arizona. Am J Clin Nutr, March 2008(87):608–613.
70. Grant W.B., Holick M.F. Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev. 2005;10:94–111.
71. Altmeyer P.J., Matthes U., Pawlak F., et al. Antipsoriatic effect of fumaric acid derivatives: results of a multicenter double-blind study in 100 patients. J Am Acad Dermatol. 1994;30:977–981.
72. Nieboer C., de Hoop D., van Loenen A.C., et al. Systemic therapy with fumaric acid derivates: new possibilities in the treatment of psoriasis. J Am Acad Dermatol. 1989;20:601–608.
73. Seville R.H. Psoriasis and stress. Br J Dermatol. 1977;97:297–302.
74. Picardi A., Pasquini P., Cattaruzza M.S., et al. Only limited support for a role of psychosomatic factors in psoriasis: results from a case-control study. J Psychosom Res. 2003;55:189–196.
75. Kabat-Zinn J., Wheeler E., Light T., et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA). Psychosomatic Medicine. 1998;60(5):625–632.
76. Winchell S.A., Watts R.A. Relaxation therapies in the treatment of psoriasis and possible pathophysiologic mechanisms. J Am Acad Dermatol. 1988;18:101–104.
77. Kazandjieva J., Grozdev I., Darlenski R., et al. . Climatotherapy of psoriasis. Clin Dermatol. 2008;269(5):477–485.
78. Ben-Amitai D., David M. Climatotherapy at the Dead Sea for pediatric-onset psoriasis vulgaris. Pediatr Dermatol. 2009;26(1):103–104.
79. Snellman E., Lauharanta J., Reunanen A., et al. Effect of heliotherapy on skin and joint symptoms in psoriasis: a 6-month follow-up study. Br J Dermatol. 1993;128:172–177.
80. Fleischer A.B., Jr., Feldman S.R., Rapp S.R., et al. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216–220.
81. Fleischer A.B., Jr., Clark A.R., Rapp S.R., et al. Commercial tanning bed treatment is an effective psoriasis treatment: results from an uncontrolled clinical trial. J Invest Dermatol. 1997;109:170–174.
82. Carlin C.S., Callis K.P., Krueger G.G. Efficacy of acitretin and commercial tanning bed therapy for psoriasis. Arch Dermatol. 2003;139:436–442.
83. Kudish A.I., Abels D., Harari M. Ultraviolet radiation properties as applied to photoclimatherapy at the Dead Sea. Int J Dermatol. 2003;42:359–365.
84. Kushelevsky A.P., Harari M., Kudish A.I., et al. Safety of solar phototherapy at the Dead Sea. J Am Acad Dermatol. 1998;38:447–452.
85. Gordon P.M., Diffey B.L., Matthews J.N., et al. A randomized comparison of narrow-band TL-01 phototherapy and PUVA photochemotherapy for psoriasis. J Am Acad Dermatol. 1999;41:728–732.
86. Markham T., Rogers S., Collins P., Narrowband UV- B. (TL-01) phototherapy vs oral 8-methoxypsoralen psoralen-UV-A for the treatment of chronic plaque psoriasis. Arch Dermatol. 2003;139:325–328.
87. Das S., Lloyd J.J., Walshaw D., et al. Response of psoriasis to sunbed treatment: comparison of conventional ultraviolet A lamps with new higher ultraviolet B-emitting lamps. Br J Dermatol. 2002;147:966–972.
88. Urabe H., Nishitani K., Kohda H. Hyperthermia in the treatment of psoriasis. Arch Dermatol. 1981;117:770–774.
89. Orenberg E.K., Deneau D.G., Farber E.M. Response of chronic psoriatic plaques to localized heating induced by ultrasound. Arch Dermatol. 1980;116:893–897.
90. Tsoureli-Nikita E., Menchini G., Ghersetich I., et al. Alternative treatment of psoriasis with balneotherapy using Leopoldine spa water. J Eur Acad Dermatol Venereol. 2002;16:260–262.
91. Heng M.C., Song M.K., Harker J., et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000 Nov;143(5):937–949.
91a. Syed T.A., Ahmad S.A., Holt A.H., et al. Management of psoriasis with aloe vera extract in a hydrophilic cream: a placebo-controlled, double-blind study. Trop Med Int Health. 1996 Aug;1(4):505–509.
92. Gulliver W.P., Donsky H.J. A report on three recent clinical trials using Mahonia aquifolium 10% topical cream and a review of the worldwide clinical experience with Mahonia aquifolium for the treatment of plaque psoriasis. Am J Ther. 2005 Sep-Oct;12(5):398–406.
93. Bernstein S., Donsky H., Gulliver W., et al. Treatment of mild to moderate psoriasis with Relieva – a Mahonia aquifolium extract: a double blind, placebo-controlled study. Am J Ther. 2006;13:121–126.
94. Tanghetti E.A. The role of topical vitamin D modulators in psoriasis therapy. J Drugs Dermatol. 2009 Aug;8(suppl 8):S4–S8.
95. Levine D., Even-Chen Z., Lipets I. Pritulo OA Pilot, multicenter, double-blind, randomized placebo-controlled bilateral comparative study of a combination of calcipotriene and nicotinamide for the treatment of psoriasis. J Am Acad Dermatol. 2010 Nov;63(5):775–781.
96. Lew B.L., Cho Y., Kim J., et al. Ceramides and cell signaling molecules in psoriatic epidermis: reduced levels of ceramides, PKC-alpha, and JNK. J Korean Med Sci. 2006 Feb;21(1):95–99.