Chapter 21 Proximal Radial Fractures
Introduction
Radial head fractures are very common. They comprise of up to 5% of all fractures and up to one-third of elbow fractures. Radial head and neck fractures have been estimated to occur in nearly 20% of all elbow trauma.1 The incidence of radial head and neck fractures in the entire population has been shown to be 2.5 per 10 000 per year and they account for 0.2% of all visits to accident and emergency departments.2
Radial head fractures occur more often than radial neck fractures in a ratio of 7 : 1. The average age of patients at the time of fracture is 45 years old. Although male : female ratio is approximately equal, in general men are older at the time of fracture (48 vs 41 years old) and sustain more severe types of injury.3
Clinical Pearl 21.1
Radial head fractures are not always simple fractures and approximately one-third of radial head fractures are complicated by associated injuries to the elbow or ipsilateral extremity. In more severe, comminuted fractures the percentage of clinically important associated lesions can increase to 80%.3
Because of the high incidence of proximal radial fractures it is important for any clinician treating these injuries to have a good knowledge of its function. The role of the proximal radius and its relationship and interaction with other structures at both the elbow and forearm has become increasingly clear in recent years. Anatomically, the proximal radius is formed by the radial neck and radial head. The radial neck is about 13 mm long4 and assures both the position of the radial head onto the capitellum and aligns the radiohumeral joint during forearm rotation. The radial neck is not straight on the radial shaft, but forms an average angle of 16.8°. This angle is variable, ranging from 6° to 28°.4 This plays a role in forearm rotation, as the rotation axis passes through the radial neck and radial head.5 The radial head articulates with the capitellum on the distal humerus, and also with the sigmoid notch (radial notch) on the proximal ulna, forming the radiohumeral and proximal radio-ulnar joints respectively. The radial head is therefore an important anatomical structure for both flexion and extension of the elbow as well as pronation and supination of the forearm. There is also some longitudinal motion between the radius and the ulna during rotation of the forearm. The radius moves about 1 mm proximally relative to the ulna when the forearm is rotated from supination to pronation.5
Besides motion, the proximal radius has biomechanical functions. The radial head plays a key role in load transfer between the forearm and the humerus. It is assumed that up to 60% of the load on the forearm is transferred to the humerus via the radiohumeral joint.6 Both the outer shape of the radial head and the articulating inner dish are elliptical in shape;7 this is an important feature for efficient transfer of forces as different areas of the dish will be in contact with different positions of the elbow and forearm. Load through the proximal radius depends on the position of the elbow and forearm and is greater with the elbow in extension and the forearm pronated.8 Varus or valgus stress also alters the load transfer between the forearm and the humerus; varus stress can decrease the portion of the load through the radiohumeral joint to less than 10%.9
Besides playing a role in mobility and load transfer, the proximal radius also acts as a stabilizing structure. The stabilizing role of the radial head has been studied extensively. The radial head has been shown to be a secondary stabilizer to valgus stress, with the medial collateral ligament as the primary stabilizing structure.10 Together with the lateral ulnar collateral ligament (LUCL) and the coronoid, the radial head has recently also been shown to be important in posterolateral rotatory (in) stability (PLRI).11
Background/aetiology
Proximal radial fractures typically occur from a fall on the outstretched hand, with the forearm in pronation. The impact is transferred through the radiocapitellar joint and the radial head and/or neck when it impacts onto the capitellum. There is also palmar translation of the radius in pronation. This is why most commonly the radial head fracture fragment is located on the anterolateral side of the radial head. Concomitant displaced capitellar fractures only occur in about 2%;3 however, smaller osteochondral lesions of the capitellum are ten times more common and capitellar bone bruising occurs in almost all patients.12 The radial head has been shown to fracture with the elbow flexed in a range between 0° and 80°.13 Finally, direct impact to the elbow may also cause the proximal radius to fracture, although this occurs much less frequently.
Associated lesions frequently complicate proximal radius fractures. The likelihood of associated lesions increases with the severity of the fracture. This increases from about 10% of patients with a non-displaced fracture, to 50% of patients with a displaced fracture and up to as much as 75% of patients with a comminuted fracture. Generally, approximately one-third of patients have one or more additional lesions adjacent to the proximal radius fracture. Nearly 25% of patients have ligamentous rupture or one or more additional fractures to the elbow. Clinically significant ligamentous lesions that require some form of treatment occur in 10% of fractures, where either the lateral collateral ligament (LCL), medial collateral ligament (MCL) or both are ruptured.3 If an MRI investigation is performed after the fracture, ligamentous injuries are found much more frequently: up to 80% of cases.12 Fortunately only a small percentage of these will need to be addressed surgically.
Associated articular elbow fractures are found in 15% of cases, with coronoid fractures being the most common (10%). In addition, between 15% and 20% of all radial head fractures are associated with a dislocation of the elbow.3,14 Many patients in this group need secondary treatment and will develop chronic problems such as stiffness or residual instability and require further treatment.
Distal radio-ulnar joint dissociation (Essex-Lopresti injury) is diagnosed acutely in only 0.3% of patients following a radial head fracture.3 This may, however, be an underestimation of the number of patients that have an Essex-Lopresti injury, as longitudinal instability can easily be missed at the initial presentation. Despite the small number of patients, it is extremely important to be vigilant for this type of injury as it can result in severe long-term disability if undiagnosed. Nine out of ten patients operated for longitudinal radio-ulnar dissociation at the Mayo Clinic between 1997 and 2002 were treated for chronic lesions as a consequence of failure of previous treatment.3
Radial head fractures are historically classified according to the Mason classification.15 Recently, it has become increasingly clear that the outcome of the treatment of radial head fractures does not depend solely on the type of fracture but also on associated lesions. As was stated above, clinically significant associated lesions occur in a large percentage of patients, and this does depend on the type of fracture. Several changes have therefore been made to the original Mason classification; the most recent adaptation was based on a demographic study of over 300 radial head fractures and their associated lesions.1,16
The proximal radial fracture itself is classified according to the original Mason classification, from types I to III. A type I fracture is non-displaced. The fragments are displaced more than 2 mm in a type II fracture, and a comminuted, non-reconstructible radial head fracture constitutes a type III. The Mayo extended classification then adds a suffix to the fracture type to show any associated lesions.1 A ‘c’ is added for coronoid fractures and an ‘o’ for olecranon fractures. Ligamentous lesions are represented by ‘m’ for medial collateral ligament (MCL) lesions, ‘l’ for the lateral collateral ligament (LCL) complex and ‘d’ for longitudinal distal radio-ulnar joint (DRUJ) dissociation (Fig. 21.1). This classification, as well as guiding treatment, was extended to provide information after treatment. The classification is adapted post surgery depending on the structures that needed surgical care. Capital letters are used if surgery was necessary. For example, ‘type IImL’ means that both the medial and lateral ligaments were ruptured in a displaced fracture, but that only the LCL was repaired. Finally, all options for treatment of the radial head fracture have been included as well; ‘X’ (excision), ‘F’ (fixed), ‘P’ (prosthesis). Results of treatment of radial head fractures can be compared more easily if associated lesions are taken into account using this classification system.
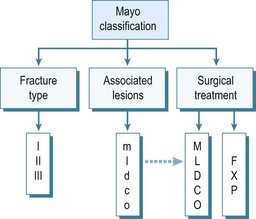
Figure 21.1 van Riet and Morrey,16 Mayo Classification1 of radial head fractures. Type I fractures are non- or minimally displaced fractures that can be treated conservatively. Type II fractures are displaced fractures that probably need surgical reduction and fixation. Type III fractures are non-reconstructible, comminuted radial head fractures. These are best treated with radial head resection or prosthetic replacement. Associated lesions are classified by adding a suffix to the description of the fracture type. m: MCL; l: LCL; d: DRUJ; c: coronoid process; o: olecranon. Upper case is used if the associated lesion needed treatment. A second suffix is added illustrating radial head treatment. X: excision; F: fixation; P: prosthesis.
Adapted with permission from: van Riet RP, Van Glabbeek F, Morrey BF. Radial head fracture. In: Morrey BF, ed. The elbow and its disorders. 4th edn. Philadelphia, PA: Saunders Elsevier; 2009:359–381.
Presentation, investigation and treatment options
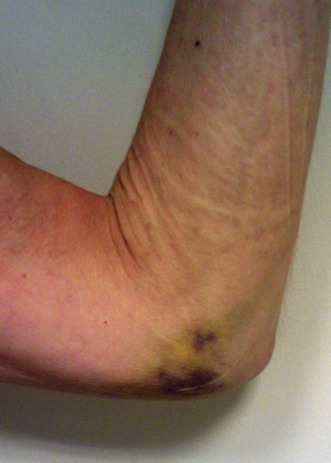
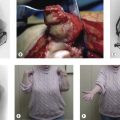
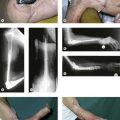
Patients with a proximal radial fracture present at the accident and emergency department with a painful elbow. The history will usually reveal a fall on the outstretched hand, but other mechanisms such as direct trauma are possible. The elbow is inspected for bruising or obvious deformity, indeed dislocation of the elbow. A haematoma on the medial side may be a sign of an MCL rupture (Fig. 21.2). A visible and palpable swelling over the lateral ‘soft spot’ is indicative of a haemarthrosis. The elbow capsule can contain nearly 25 mL of fluid.17 Flexion and extension motion decreases nearly 2° per millilitre of intracapsular fluid.18
Rotation of the forearm may be nearly normal in non-displaced fractures, being limited and painful only when the fragments are displaced. Crepitus may also be heard or palpated when the elbow or forearm is moved. The patient may be guarding the elbow. Intra-articular pressure is least at about 70° of elbow flexion17 and, as pressure of the capsule is one of the most important mediators of pain in these patients, this will therefore be the least painful position for the patient.
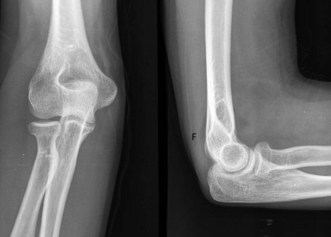
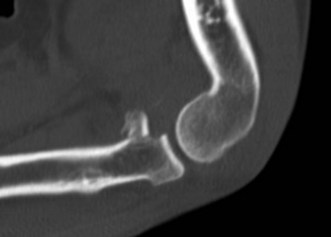
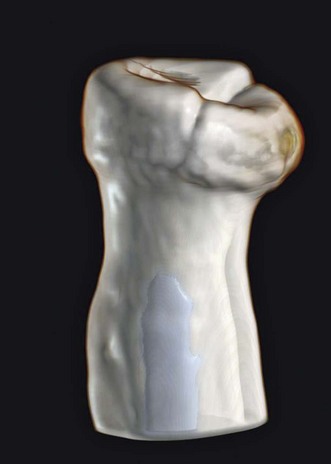
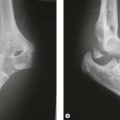
Plain anteroposterior (AP) and lateral radiographs of the elbow are taken and analysed. Specific radial head views may reveal a fracture that is not visible in other views. Care should be taken not to miss subtle associated injuries, such as a small coronoid fragment. Non-displaced radial head fractures may not be visible on plain radiographs. In this case a posterior fat pad sign (Fig. 21.3) is almost pathognomonic for a radial head fracture and treatment should be directed as such. There should be a low threshold for a CT scan of the elbow in cases of minimally displaced fractures, as this may show associated lesions (Fig. 21.4) and displacement of the fracture can be more easily assessed (Fig. 21.5). Fragments can be displaced more than one would suspect from the plain radiographs only. Both associated lesions and a correct measurement of the displacement may change the course of treatment.
Unless there is a contraindication for surgery or an acute infection of the elbow, displaced Mayo type II fractures are treated by surgical reduction and fixation of the fracture fragments. Small displaced fragments that do not block motion may be treated conservatively depending on the patient’s needs. However, it has been shown that it is probably advantageous to fix smaller radial head fragments as well.19
Reduction and fixation of radial head fractures may be undertaken arthroscopically20 or open.21 Fixation can be undertaken using different types of screws. It is important to place these screws in the non-articulating portion of the radial head or to use headless screws that can be buried beneath the articulating cartilage. Bioabsorbable pins or screws have also been shown to be effective, seemingly without any adverse effects from the material.22 The fixation of radial neck fractures is evolving as well. Plate fixation has been the treatment of choice for a long time and still is for specific types of radial neck fracture. Low-profile plates have been developed in response to the bulk of earlier, bigger plates which resulted in problems, especially rotation of the forearm. Other low-profile techniques have been described and more recently screw fixation of the radial head onto the neck has been shown to achieve reasonably stable fixation.23
Mayo type III fractures are, by definition, comminuted fractures and treatment is somewhat controversial. Reduction and fixation of type III proximal radius fractures can be technically challenging and results reported in clinical follow-up studies show that it may not be in the best interest of the patient to do everything possible to fix these fractures, if there are more than three fragments.24,25 As a consequence, it may be necessary to alter treatment during surgery from reduction and internal fixation to resection or radial head replacement.
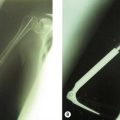
Resection has been the gold standard for the treatment of comminuted radial head and neck fractures in the past. In recent years, however, prosthetic replacement of the radial head has become the treatment of choice for many surgeons treating these injuries. This is for several reasons. First, some of the complications following radial head resection are extremely difficult to treat. A small degree of cubitus valgus and up to 2 mm of proximal migration of the radius are usually not symptomatic. It has been postulated that this is a physiological phenomenon.26 However, symptomatic proximal migration of the radius relative to the ulna following resection of the radial head has been shown to occur in up to 25% of patients, when the radial head was excised without consideration of associated lesions.27 Patients mainly complain of wrist pain and decreased range of motion. Ulnar shortening and Sauvé–Kapandji procedures can be indicated for wrist pain, but some patients may even end up needing a so-called ‘one-boned forearm’ procedure, with reduced function. Results of delayed placement of a radial head prosthesis following resection of the radial head in these patients are generally poor when compared to acute placement. Delayed placement has also resulted in erosion of the capitellum as a result of an inability to reduce the forearm once proximal migration has occurred.28 The second reason why the use of radial head prostheses to treat comminuted fractures of the proximal radius has become increasingly more common is the evolution of prosthetic design. Increased knowledge of the anatomy of the proximal radius and biomechanics of the elbow has led to changes in radial head prosthesis design. The first radial head prosthesis widely used was the silastic radial head developed by Swanson. Because of material problems such as foreign body synovitis and breakage of the prostheses, this type of prosthesis is no longer used. Secondary to these complications, and perhaps more importantly, the biomechanical and material properties of the silicone radial head prostheses were not able to reproduce the normal stability and kinematics of the native radial head. Metal radial head prostheses, on the other hand, are better equipped to improve stability. Metal radial head prostheses also replicate the normal biomechanics more closely as they are able to withstand higher loads29 than silicone prostheses. In addition, these modern radial head prostheses are also designed to replicate the normal anatomy of the elbow. Most designs are modular and allow for the shaft and the head to be placed in several different combinations. In this way the size of the radial head can be adapted to the actual size of the fractured head, while the stem fits separately into the radial intramedullary canal. The head and stem components are usually assembled outside the patient, and the prosthesis is then placed in the injured elbow. Another feature of the native radial head that has been addressed in prosthetic design is the shape of the radial head itself. As was mentioned earlier, the radial head is not round but has an elliptical shape.7 Recently this shape has also been incorporated into radial head prosthetic design in order to improve load transfer and radiocapitellar alignment.
While bipolar radial head prostheses have been shown not to be as stable as a rigid prosthetic design in optimal laboratory circumstances, they are more stable than radial head resection in the MCL-deficient elbow30 and will intuitively be better than a subluxed rigid prosthesis.
Surgical techniques and rehabilitation
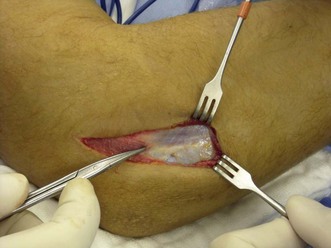
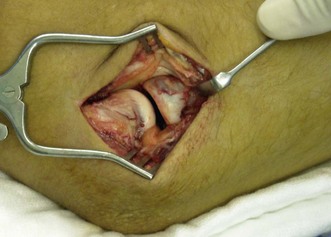
For the open approach, the patient is usually positioned supine on the operating table, although lateral decubitus is an option. Depending on the approach used, the arm can be placed on a hand table or positioned over the chest of the patient when a posterior incision is used. A posterior incision is used if medial structures, such as a coronoid fracture, need to be surgically addressed. The treatment of associated fractures will not be discussed further in this chapter. The incision is centred over the olecranon but may be slightly lateral to this to avoid subsequent wound problems. Full-thickness flaps are developed laterally and medially, allowing standard approaches to be performed. The lateral Kocher approach will be described later. A lateral or posterolateral incision can be used for simple radial head fractures. In this case the arm of the patient can again be supported on a hand table, but also pulled over the chest of the patient. The incision is centred over the lateral epicondyle. Kocher’s interval is indicated by a ‘fatty streak’ laterally (Fig. 21.6). The interval is entered by incising the fascia between the extensor carpi ulnaris and the anconeus muscle. Alternatively, Kaplan’s interval between the extensor carpi radialis and extensor digitorum communis may be used. The annular ligament is then divided longitudinally (Fig. 21.7). Stay sutures can be placed on both sides of the incision, so that the ligament can be closed anatomically at the end of the procedure. Care should be taken not to injure the LCL complex, including the LUCL. Sometimes, however, the LCL complex is torn, together with the common extensor tendon, and the approach to the radial head is then directed by the tear in the tendon and ligaments (Fig. 21.8). The proximal radius fracture is then visualized and the fracture site debrided.
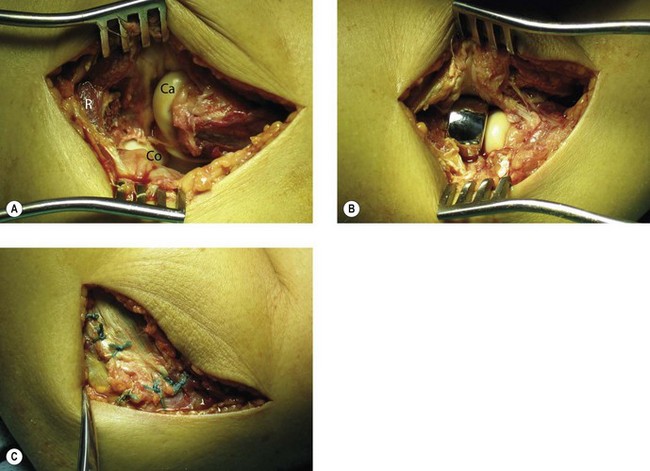
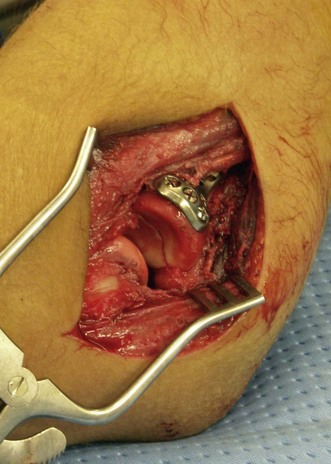
Once the fracture site is debrided, any haematoma is evacuated and the area is irrigated thoroughly. Thereafter, radial head and neck fixation is carried out using the necessary hardware. Pins or various types of screws can be used to fix radial head fractures. Radial neck fractures are traditionally fixed using plates and screws (Fig. 21.9), but stable fixation can be achieved by screws alone.23 In complex radial head and neck fractures it may be useful to reconstruct the head on the operating table before fixing it onto the neck. Rotation of the forearm after fixation of the radial head and neck may result in impingement of the hardware into the lesser sigmoid notch. To avoid this complication any hardware should be placed under the surface of the articular cartilage and preferably in the non-articulating portion of the radial head.31
If it is not possible to reduce and fix the fragments, or if there are more than three fragments,24 the radial head is excised. Stability of the elbow and forearm is then examined. Valgus instability, PLRI or longitudinal instability are clear indications for a radial head replacement (Fig. 21.8). Fluoroscopy can be used to check for medial gapping of the ulnohumeral joint, while a valgus load is applied to the elbow. Longitudinal stability can be tested using the ‘radial pull test’, described by Smith et al.32 Proximal translation of the radius of more than 3 mm is indicative of an interosseous membrane lesion. Fluoroscopic imaging of the wrist during stress testing may also reveal instability, indicative of acute longitudinal radio-ulnar dissociation. If the elbow and forearm remain stable following resection of the radial head fracture fragments, radial head resection alone should be considered as the definitive treatment. The majority of type III radial head fractures are, however, complex injuries with associated bone or ligamentous lesions, and in these patients radial head replacement will be the treatment of choice.
The native radial head is elliptical in shape,7 which influences the load transfer through the proximal radius.8,33 If an elliptical prosthesis is to be used, care should be taken to orient the radial head properly.
Clinical Pearl 21.7
The orientation of an elliptical radial head prosthesis should be such that the longer axis of the radial head is placed perpendicular to the lesser sigmoid notch when the forearm is in neutral rotation.7
A second important technical issue in replacing the radial head with a prosthesis is the correct length of the reconstruction.21,34,35 Overstuffing has been linked to loss of motion36,37 and capitellar wear.28 Conversely, if the reconstructed radius is too short it does not provide stability to the elbow. Anatomical guidelines are now available to make sure the length is reconstructed properly.
Clinical Pearl 21.8
The correct length of the proximal radius can be judged from the ulna. In the uninjured elbow, when the forearm is in neutral rotation the top of the radial head is level to the top of the lesser sigmoid notch.38 The lesser sigmoid notch therefore dictates the length of a radial head replacement.
After the radial head is fixed, resected or replaced, the lateral soft tissues are repaired. It may be necessary to repair the LCL complex or reattach this to the lateral epicondyle with an anchor. The annular ligament is sutured. Sometimes, however, it is not possible to completely close the annular ligament owing to the bulk of the hardware used. The annular ligament can be lengthened and approximated in these cases to provide stability to the proximal radio-ulnar joint. Kocher’s interval is closed tightly, and the common extensor tendon is reattached if necessary (Fig. 21.8). Stability is tested again following closure of the lateral soft tissues and a decision is made to repair the MCL if necessary. Unless there is clear instability, the MCL is usually not repaired or reconstructed, as this may lead to excessive stiffness of the elbow. Depending on the strength of fixation of other associated injuries such as a distal humerus fracture, or if repair of the soft tissues needs to be protected temporarily, a hinged external fixator or brace can be used to protect the repair/fixation. A dynamic external fixator is only rarely necessary following the surgical treatment of simple radial head fractures.
After surgery a protective and compressive bandage is placed on the elbow and forearm to reduce swelling. Range-of-motion exercises are typically started on the first day after surgery, with the elbow in a hinged brace if necessary. Alternatively, the arm can be placed in a long arm cast for 3–4 days. This provides some pain relief and will probably not influence the final outcome in terms of stiffness.39
Outcome including literature review
Good long-term results can be expected from conservative treatment of Mayo type I radial head fractures.40 Range of motion exercises should be initiated as soon as pain allows. A delay of a few days does not negatively influence early outcome.39 Although good results have been published from conservative treatment of selected fractures with moderate displacement (2–5 mm),41 surgical reduction and internal fixation seem to yield better results. Residual pain was found in 42% of conservatively treated patients, compared to 32% of patients that were treated surgically. Good to excellent Mayo Elbow Performance Scores were found in 52% and 88% respectively.42
Results of open reduction and internal fixation (ORIF) of radial head fractures depend significantly on associated lesions and type of fracture.24,25 Several reports have shown that the results of osteosynthesis for comminuted fractures can be disappointing.24,25 Up to 93% good to excellent results are reported with Mayo type II fractures.24 However, direct comparison of radial head replacement to ORIF in type III fractures reveals that the results for replacement are superior, with 12.5% good or excellent results in the ORIF group, compared to 93% in the radial head replacement group.43
Radial head resection is somewhat controversial as results of resection are typically good in the long term.44,45 However, in the short term and in small patient cohorts, resection has been shown to be inferior to osteosynthesis46,47 or metal radial head replacement.48 Furthermore, and as was stated previously, some of the complications of radial head resection may be disastrous for the patient.27
The results of radial head prosthesis have been summarized by Morrey. In general, about 80% of patients are satisfied after a mean follow-up of nearly 4 years. This increases to a little over 90% if only acute fractures are considered.49 Although good results have been reported in chronic conditions,50,51 in general satisfactory results are only found in approximately 50% for delayed radial head replacement.49
Complications of treatment
The most common complication of treatment of radial head fractures is degeneration of the radiohumeral articulation. This has been reported following conservative treatment, osteosynthesis, radial head resection and prosthetic replacement.40,51–53 Another common complication of the treatment of a type I radial head fracture is loss of extension of the elbow.15 This is more commonly seen following long periods of immobilization but can still occur following immediate mobilization. Non-displaced fractures are less prone to an extension deficit than mildly displaced fractures.40,53 Range of motion can increase for a long time after injury and conservative treatment should be continued as long as there is improvement. Physiotherapy, including both passive stretches and active range-of-motion exercises, are indicated early in patients in whom range of motion does not increase soon after the fracture. Static and dynamic bracing may also increase range of motion. Other potential complications of Mayo type I radial head fractures include non-union and secondary displacement of the fracture. Non-union is rare and usually asymptomatic.54 Secondary displacement is also very infrequent. If it does occur, it will happen early and should be anticipated. Radiographs should therefore be repeated at 2 weeks following the fracture. If repeat radiographs show secondary displacement, a CT scan should be ordered to show the extent of displacement. A type I radial head fracture could become a type II and should then be treated accordingly.
As in conservative treatment of radial head fractures, some limitation of range of motion can be expected following ORIF of the radial head, with reported motion from an average of 10° extension to 130° of flexion in type II radial head fractures.24 Loss of reduction and failure of hardware can also complicate the treatment of type II radial head fractures. Especially when plate fixation is performed, hardware removal is sometimes indicated in an attempt to improve range of motion.
Limited data are available on complications of radial head replacement. Range of motion following radial head replacement is often decreased37,51,52 and has been shown to improve following removal of the prosthesis.37,52 Radiolucent lines are frequently seen with intentionally loose radial head prostheses but this does not seem to correlate with functional results or pain.55 Temporary nerve dysfunction has been described following radial head replacement surgery of the ulnar, radial and posterior interosseous nerves.52 This usually resolves spontaneously. The modularity of modern radial head replacements has introduced a unique complication, in that they can dissociate.56 Heterotopic ossification57 and capitellar erosion28 have also been described as case reports or as part of a larger series. Pain, stiffness and instability are the most common clinical causes of failure of a radial head replacement, necessitating removal or revision of the implant. Loosening, over-lengthening and radial head subluxation are common radiographic findings in these patients.58
Conclusions/personal view
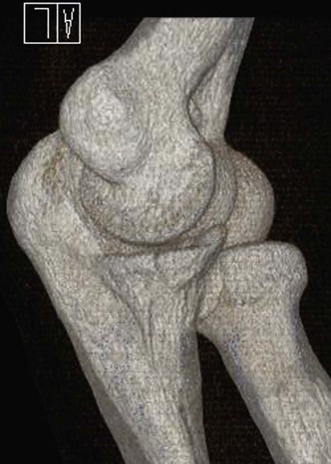
Unless there is no displacement whatsoever we routinely obtain CT scans of the elbow, with three-dimensional rendering of the images (Fig. 21.10). The decision to operate is based on these X-ray and CT findings, taking the patient’s age and comorbidity into consideration. The size of the fragment is almost of secondary importance, as small fragments can have large biomechanical consequences. Arthroscopic reduction is performed in acute fractures (<1 week), where there is one large fragment with little or no impaction. The majority of displaced fractures are treated by open reduction. The radial head is typically fixed with one or two headless cannulated screws. Radial neck fractures are fixed using screws with the low-profile method, or with a specifically designed radial head plate and screws.
1 van Riet RP, Van Glabbeek F, Morrey BF. Radial head fracture. In: Morrey BF, editor. The elbow and its disorders. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:359-381.
2 Kaas L, van Riet RP, Vroemen JP, et al. The incidence of associated fractures of the upper limb in fractures of the radial head. Strategies Trauma Limb Reconstr. 2008;3(2):71-74.
3 van Riet RP, Morrey BF, O’Driscoll SW, et al. Associated injuries complicating radial head fractures: a demographic study. Clin Orthop Relat Res. 2005;441:351-355.
4 van Riet RP, Van Glabbeek F, Neale PG, et al. Anatomical considerations of the radius. Clin Anat. 2004;17(7):564-569.
5 Tay SC, van Riet R, Kazunari T, et al. A method for in-vivo kinematic analysis of the forearm. J Biomech. 2008;41(1):56-62.
6 Halls A, Travill A. Transmission of pressures across the elbow joint. Anat Rec. 1964;150:243-248.
7 van Riet RP, Van Glabbeek F, Neale PG, et al. The noncircular shape of the radial head. J Hand Surg (Am). 2003;28(6):972-978.
8 van Riet RP, Van Glabbeek F, Baumfeld JA, et al. The effect of the orientation of the radial head on the kinematics of the ulnohumeral joint and force transmission through the radiocapitellar joint. Clin Biomech. 2006;21(6):554-559.
9 Markolf KL, Lamey D, Yang S, et al. Radioulnar load-sharing in the forearm: a study in cadavera. J Bone Joint Surg Am. 1998;80(6):879-888.
10 Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315-319.
11 Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86-A(5):975-982.
12 Itamura J, Roidis N, Mirzayan R, et al. Radial head fractures: MRI evaluation of associated injuries. J Shoulder Elbow Surg. 2005;14(4):421-424.
13 Amis AA, Miller JH. The mechanisms of elbow fractures: an investigation using impact tests in vitro. Injury. 1995;26(3):163-168.
14 Frankle MA, Koval KJ, Sanders RW, et al. Radial head fractures associated with elbow dislocations treated by immediate stabilization and early motion. J Shoulder Elbow Surg. 1999;8(4):355-360.
15 Mason ML. Some observations on fractures of the head of the radius with a review of hundred cases. Br J Surg. 1954;42:123-132.
16 van Riet RP, Morrey BF. Documentation of associated injuries occurring with radial head fracture. Clin Orthop Relat Res. 2008;466(1):130-134.
17 O’Driscoll SW, Morrey BF, An KN. Intraarticular pressure and capacity of the elbow. Arthroscopy. 1990;6(2):100-103.
18 McGuigan FX, Bookout CB. Intra-articular fluid volume and restricted motion in the elbow. J Shoulder Elbow Surg. 2003;12(5):462-465.
19 Beingessner DM, Dunning CE, Beingessner CJ, et al. The effect of radial head fracture size on radiocapitellar joint stability. Clin Biomech. 2003;18(7):677-681.
20 Michels F, Pouliart N, Handelberg F. Arthroscopic management of Mason type 2 radial head fractures. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1244-1250.
21 Van Glabbeek F, van Riet R, Verstreken J. Current concepts in the treatment of radial head fractures in the adult: a clinical and biomechanical approach. Acta Orthop Belg. 2001;67(5):430-441.
22 Givissis PK, Symeonidis PD, Ditsios KT, et al. Late results of absorbable pin fixation in the treatment of radial head fractures. Clin Orthop Relat Res. 2008;466(5):1217-1224.
23 Smith AM, Morrey BF, Steinmann SP. Low profile fixation of radial head and neck fractures: surgical technique and clinical experience. J Orthop Trauma. 2007;21(10):718-724.
24 Ring D, Quintero J, Jupiter JB. Open reduction and internal fixation of fractures of the radial head. J Bone Joint Surg Am. 2002;84(10):1811-1815.
25 King GJ, Evans DC, Kellam JF. Open reduction and internal fixation of radial head fractures. J Orthop Trauma. 1991;5(1):21-28.
26 Morrey BF, Chao EY, Hui FC. Biomechanical study of the elbow following excision of the radial head. J Bone Joint Surg Am. 1979;61(1):63-68.
27 Mikic ZD, Vukadinovic SM. Late results in fractures of the radial head treated by excision. Clin Orthop. 1983;181:220-228.
28 van Riet RP, Van Glabbeek F, Verborgt O, et al. Capitellar erosion caused by a metal radial head prosthesis: a case report. J Bone Joint Surg Am. 2004;86-A(5):1061-1064.
29 van Riet RP, van Glabbeek F. History of radial head prosthesis in traumatology. Acta Orthop Belg. 2007;73(1):12-20.
30 Pomianowski S, Morrey BF, Neale PG, et al. Contribution of monoblock and bipolar radial head prostheses to valgus stability of the elbow. J Bone Joint Surg Am. 2001;83-A(12):1829-1834.
31 Smith GR, Hotchkiss RN. Radial head and neck fractures: anatomic guidelines for proper placement of internal fixation. J Shoulder Elbow Surg. 1996;5(2 Pt 1):113-117.
32 Smith AM, Urbanosky LR, Castle JA, et al. Radius pull test: predictor of longitudinal forearm instability. J Bone Joint Surg Am. 2002;84-A(11):1970-1976.
33 van Riet RP, Van Glabbeek F, Baumfeld JA, et al. The effect of the orientation of the noncircular radial head on elbow kinematics. Clin Biomech. 2004;19(6):595-599.
34 Van Glabbeek F, van Riet RP, Baumfeld JA, et al. Detrimental effects of overstuffing or understuffing with a radial head replacement in the medial collateral-ligament deficient elbow. J Bone Joint Surg Am. 2004;86-A(12):2629-2635.
35 Van Glabbeek F, van Riet RP, Baumfeld JA, et al. The kinematic importance of radial neck length in radial head replacement. Med Eng Phys. 2005;27(4):336-342.
36 Birkedal JP, Deal DN, Ruch DS. Loss of flexion after radial head replacement. J Shoulder Elbow Surg. 2004;13:208-213.
37 Wretenberg P, Ericson A, Stark A. Radial head prosthesis after fracture of radial head with associated elbow instability. Arch Orthop Trauma Surg. 2006;126(3):145-149.
38 van Riet RP, van Glabbeek F, de Weerdt W, et al. Validation of the lesser sigmoid notch of the ulna as a reference point for accurate placement of a prosthesis for the head of the radius: a cadaver study. J Bone Joint Surg Br. 2007;89(3):413-416.
39 Liow RYL, Cregan A, Nanda R, et al. Early mobilisation for minimally displaced radial head fractures is desirable: a prospective randomised study of two protocols. Injury. 2002;33:801-806.
40 Herbertsson P, Josefsson PO, Hasserius R, et al. Displaced Mason type I fractures of the radial head and neck in adults: a fifteen- to thirty-three-year follow-up study. J Shoulder Elbow Surg. 2005;14(1):73-77.
41 Akesson T, Herbertsson P, Josefsson PO, et al. Primary nonoperative treatment of moderately displaced two-part fractures of the radial head. J Bone Joint Surg Am. 2006;88(9):1909-1914.
42 Struijs PA, Smit G, Steller EP. Radial head fractures: effectiveness of conservative treatment versus surgical intervention: a systematic review. Arch Orthop Trauma Surg. 2007;127(2):125-130.
43 Ruan HJ, Fan CY, Liu JJ, et al. A comparative study of internal fixation and prosthesis replacement for radial head fractures of Mason type III. Int Orthop. 2009;33(1):249-253.
44 Herbertsson P, Josefsson PO, Hasserius R, et al. Fractures of the radial head and neck treated with radial head excision. J Bone Joint Surg Am. 2004;86-A(9):1925-1930.
45 Wallenbock E, Potsch F. Resection of the radial head: an alternative to use of a prosthesis? J Trauma. 1997;43(6):959-961.
46 Rochwerger A, Bataille JF, Kelberine F, et al. Analyse retrospective d’une serie de 78 fractures de la tete radiale operees. Acta Orthop Belg. 1996;62(Suppl. 1):87-92.
47 Ikeda M, Sugiyama K, Kang C, et al. Comminuted fractures of the radial head. Comparison of resection and internal fixation. J Bone Joint Surg Am. 2005;87(1):76-84.
48 Obert L, Lepage D, Huot D, et al. Unreconstructible radial head fracture: resection, implant of Swanson or prosthesis? Retrospective comparative study (in French). Chir Main. 2005;24(1):17-23.
49 Morrey BF. Prosthetic radial head replacement. In: Morrey BF, editor. The elbow and its disorders. 4th ed. Philadelphia, PA: Saunders Elsevier; 2009:381-388.
50 Brinkman JM, Rahusen FT, de Vos MJ, et al. Treatment of sequelae of radial head fractures with a bipolar radial head prosthesis: good outcome after 1–4 years follow-up in 11 patients. Acta Orthop. 2005;76(6):867-872.
51 Shore BJ, Mozzon JB, MacDermid JC, et al. Chronic posttraumatic elbow disorders treated with metallic radial head arthroplasty. J Bone Joint Surg Am. 2008;90(2):271-280.
52 Smets S, Govaers K, Jansen N, et al. The floating radial head prosthesis for comminuted radial head fractures: a multicentric study. Acta Orthop Belg. 2000;66(4):353-358.
53 Herbertsson P, Josefsson PO, Hasserius R, et al. Uncomplicated Mason type-II and III fractures of the radial head and neck in adults: a long-term follow-up study. J Bone Joint Surg Am. 2004;86-A(3):569-574.
54 Ring D, Psychoyios VN, Chin KR, et al. Nonunion of nonoperatively treated fractures of the radial head. Clin Orthop. 2002;398:235-238.
55 Fehringer EV, Burns EM, Knierim A, et al. Radiolucencies surrounding a smooth-stemmed radial head component may not correlate with forearm pain or poor elbow function. J Shoulder Elbow Surg. 2009;18(2):275-278.
56 O’Driscoll SW, Herald J. Symptomatic failure of snap-on bipolar radial head prosthesis. J Shoulder Elbow Surg. 2009;18(5):e7-11.
57 Bimmel R, van Riet RP, Sys J. Heterotopic ossification causing proximal radioulnar synostosis after insertion of a radial head prosthesis. J Hand Surg (Br). 2006;31(4):383-384.
58 van Riet RP, Sanchez-Sotelo J, Morrey BF. Failure of metal radial head arthroplasty. J Bone Joint Surg Br. 2010;92(5):661-667.