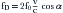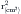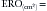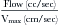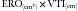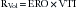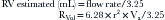16 Proximal Isovelocity Surface Area and Flow Convergence Methods
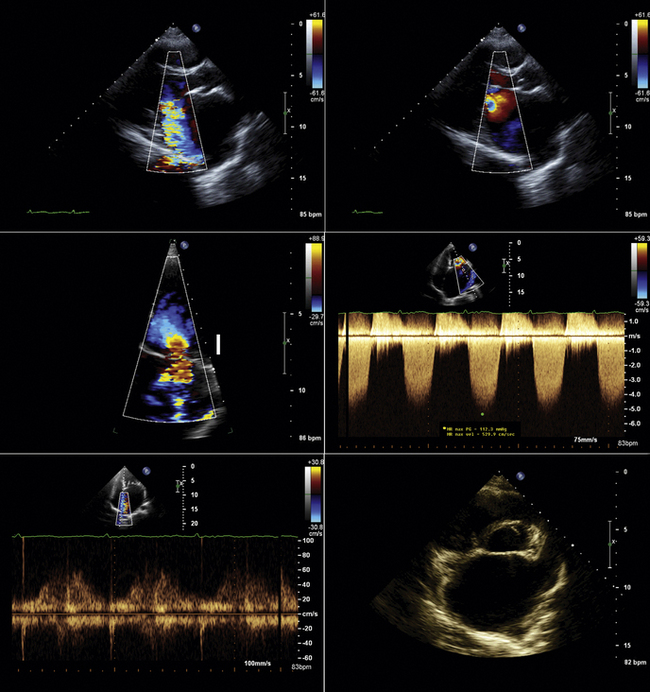
The proximal isovelocity surface area (PISA)/flow convergence technique is an accepted quantitative measure of both valvular regurgitation and stenosis. Although it can be applied to any valve, subvalvular lesion,1 valve prosthesis,2 or any other structure with an orifice (e.g., a ventriculoseptal defect3), the PISA technique is used principally to assist in determining the severity of mitral regurgitation (MR), mitral stenosis (MS), and aortic insufficiency (AI) when other methods are less concordant and appear less sound.
The shortcomings of color Doppler flow mapping to determine the severity of valvular insufficiency are numerous and have been repeatedly characterized.4–6 Although MR color Doppler jet size (area and length) predict angiographic grade, they exhibit a weak correlation with regurgitant volume (RVol) and do not predict hemodynamic dysfunction.7 In some lesions, such as functional/ischemic MR, color Doppler flow mapping tends to systematically overestimate the severity of mitral insufficiency; in fact, most jets larger than 8 cm2 do not correspond to severe MR, advancing the concept of the need for quantitative determination of the severity of mitral insufficiency.8 Eccentric jets of MR correlate much less well with severity of MR9 due to complex spatial redistribution and loss from frictional forces.10 The effect of general anesthesia on the severity of mitral insufficiency is profound: more than half (51%) of patients with moderate to severe MR improved by at least one severity grade when assessed by transesophageal echocardiography under general anesthesia.11 In the postoperative state, PISA determination of grade of MR correlates far better with angiographic grade of MR (r = 0.89 and 0.92, P < 0.001) than does color Doppler flow mapping determination of severity (r = 0.44, P < 0.1).12 Given essentially perfect specificity (100%, positive predictive value: 100%),13 the finding of upper vein pulmonary venous flow reversal is the single most useful parameter to determine that MR is severe, but is limited by imperfect transthoracic sampling (reducing sensitivity: 82%),13,14 and occasionally by the effect of highly eccentric jets or massive atrial compliance. The single most common scenario in which the PISA technique is applied is in describing the severity of MR when color flow mapping is confounded by severe jet eccentricity and the pulmonary venous spectral tracings are confounded by poor quality.
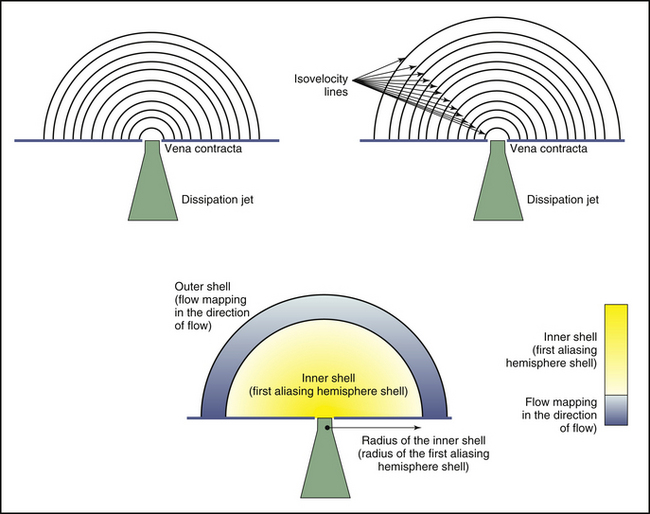
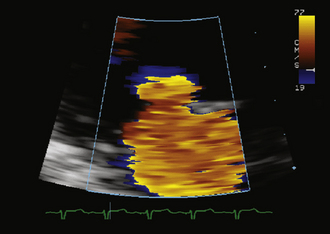
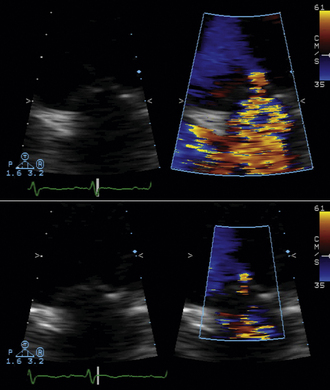
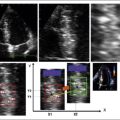
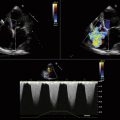
Flow acceleration occurs within a hemisphere before the orifice, largely independently of the shape of the orifice, which eliminates one of the most common variables encountered in valve disease. The greater the flow volume, the larger the hemisphere of flow acceleration and the greater dimension of the concentric isovelocity rings. Hence, the dimension of the isovelocity rings depicts the flow rate: a large PISA is consistent with a large flow rate. Optimal hemispheric depiction by color Doppler occurs when the contour velocity is approximately 5% to 10% of the orifice velocity.15
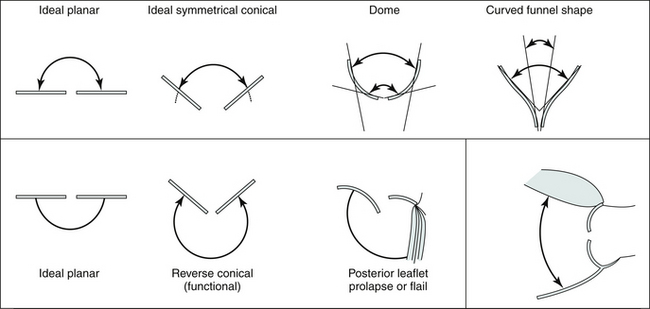
In many cases, the full hemisphere of flow acceleration cannot form because physical structures are so close to the orifice that they deny (“constrain”) the formation of a geometric hemisphere. Isovelocity mapping constraint occurs commonly: in organic MR, as with mitral valve prolapse and flail leaflets; in mitral stenosis, should the diastolic shape of the valve leaflets yield a cone, as invariably happens when subvalvar disease predominates; or in aortic stenosis, as the walls of the left ventricular outflow tract confine the isovelocity rings. In such cases, applying the usual PISA method yields less accurate or inaccurate results. “Angle correction” has been proposed as a remedy for cone-shaped orifices, which are common in mitral stenosis (the orifice area calculation is multiplied by the oblique angle of the orifice [in degrees] divided by 180). The correction often is feasible for MR and mitral stenosis, but less so for aortic stenosis. Without angle correction, in the presence of constraining walls, there is significant overestimation of flow when a hemispheric model is used.16
Proximal Isovelocity Surface Area Scanning Parameters
Color Doppler Measurements
Proximal Isovelocity Surface Area Hemisphere
The following technical issues may arise:
 For the PISA radius measurement, one should attempt to measure the radius in line with the angle of insonation.
For the PISA radius measurement, one should attempt to measure the radius in line with the angle of insonation.
 Use the largest zoom of the flow at the mitral tips.
Use the largest zoom of the flow at the mitral tips.
 Move the color aliasing baseline between 20 and 40 cm/sec. However
Move the color aliasing baseline between 20 and 40 cm/sec. However
 Use of lower aliasing velocities may engender greater variation in the delineation of the outside of the shell.
Use of lower aliasing velocities may engender greater variation in the delineation of the outside of the shell.
 Measure the radius of the internal isovelocity hemisphere (the first aliased hemisphere)—this is invariably encoded as the color opposite the direction of flow.
Measure the radius of the internal isovelocity hemisphere (the first aliased hemisphere)—this is invariably encoded as the color opposite the direction of flow.
 Measure at the average distance of the color boundary, not the maximal distance.
Measure at the average distance of the color boundary, not the maximal distance.
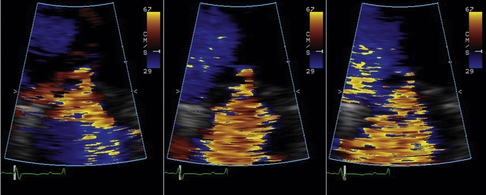
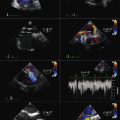
Color Doppler Aliasing Velocity
The actual velocity is assigned to the first aliasing velocity (usually the interface of blue to red). If looking at mitral regurgitation from the apical four-chamber view, the aliasing baseline is shifted in the direction of the jet, to between 20 and 40 cm/sec, allowing for a better defined visual demarcation of the PISA hemisphere. This allows for a more accurate measurement of the radius of the flow, as the PISA is larger and less prone to measurement error.
Radius of the Proximal Isovelocity Surface Area Hemisphere
The following technical issues may arise:
 The outer margin of the hemisphere may or may not be uniform, and may or may not lend itself to measurement.
The outer margin of the hemisphere may or may not be uniform, and may or may not lend itself to measurement.
 The average outer margin, rather than the greatest outer margin, probably should be used.
The average outer margin, rather than the greatest outer margin, probably should be used.
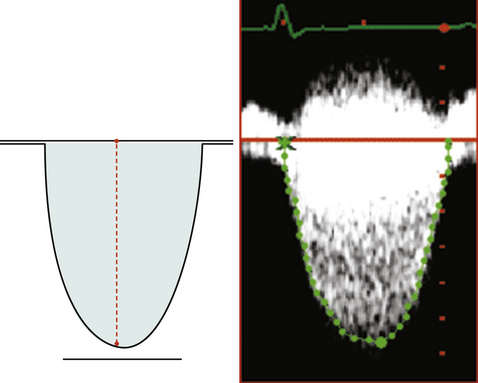
 The ideal measurement of the innermost aspect of the hemisphere is made to the base of the waist of the apparent vena contracta.
The ideal measurement of the innermost aspect of the hemisphere is made to the base of the waist of the apparent vena contracta.
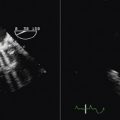
Vena Contracta
The following technical issues may arise:
 The vena contract remains a more conceptual/flow chamber notion than a reliable clinical imaging finding. Until the advent of anti-aliasing filters it is likely that it often will remain ambiguously depicted.
The vena contract remains a more conceptual/flow chamber notion than a reliable clinical imaging finding. Until the advent of anti-aliasing filters it is likely that it often will remain ambiguously depicted.
 The proximal end of the vena contracta, as depicted by color Doppler flow mapping, does not always co-register with the level of the orifice as seen on two-dimensional grayscale imaging.
The proximal end of the vena contracta, as depicted by color Doppler flow mapping, does not always co-register with the level of the orifice as seen on two-dimensional grayscale imaging.
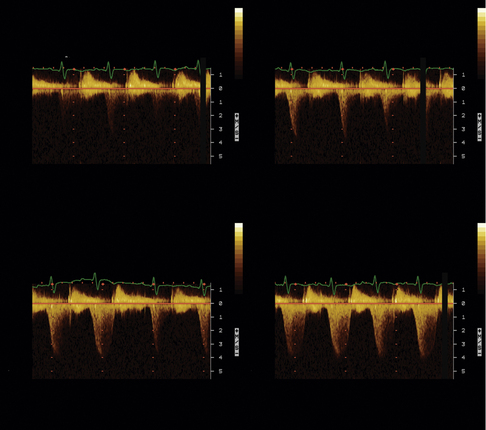
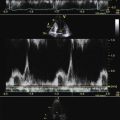
Continuous Wave Doppler Measurements
Incomplete spectral profiles are a common problem for mild and moderate grades of insufficiency.
Proximal Isovelocity Surface Area Equations
Derivations of the Proximal Isovelocity Surface Area
Aliasing velocity is calclulated in the direction of flow at the radial distance r:
 VFR, or simply flow (cc/sec, mL/sec) = 2π ×
VFR, or simply flow (cc/sec, mL/sec) = 2π ×
× Va(cm/sec)
where Va = distance from the orifice level or vena contracta; Vmax = peak velocity of the regurgitant jet; VTI = velocity time integral of the mitral regurgitant jet; VFR = volumetric flow rate; ERO = effective regurgitant orifice; and RVol = regurgitant volume. (When measuring peak mitral regurgitation velocity in m/sec, convert it to cm/sec.)
where ERO is in cm2 and VTI is in cm.
Transesophageal echocardiography often provides optimal depiction of flow convergence of mitral stenosis and insufficiency jets. However, due to the limited means of transesophageal echocardiography to align sampling with the flow, sampling may be suboptimal or inadequate; this may apply to all left-sided flow disturbances (MR, mitral stenosis, aortic insufficiency, and aortic stenosis). Heavily calcified mitral leaflets may diminish the depiction of MR flow convergence patterns on the far side of the mitral leaflets due to shadowing.
Simplified Methods
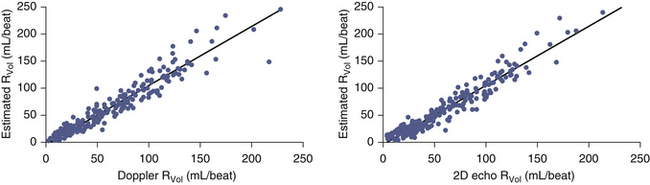
Adapted/simplified methods have been proposed and validated for use in situations where some needed parameters, such as the peak velocity and VTI of the CW spectral display, are poorly determined due to either insufficient signal or sampling malalignment. The technique of Rossi et al.17 uses the generally tight relationship of the peak velocity and VTI, which they observed among 272 patients, to average 3.25 (mean ± SD, 3.25 ± 0.47), allowing an estimated flow rate calculation:
This technique was demonstrated to have excellent correlation with Doppler and volumetric reference standards for estimation of RVol (r = 0.96 and 0.97, SEE = 11 mL [for both]; P < 0.001). This technique was noted to overestimate very large RVol and to be less suitable for MR due to mitral valve prolapse.17
Technical points on proximal isovelocity surface area method
By convention, although not without recognized limitations, PISA radius is measured at mid-systole. The convention is based on the rationale that consistently making all measurements (i.e., radius, peak velocity) at mid-systole provides the best correlation for instantaneous assessment. In most pathologies, regurgitant flow increases rapidly in early systole and is maximal by mid-systole; therefore, maximal flow is identified. However, given the differing pathologies responsible for regurgitation, some of which provide dynamic orifices, mid-systolic flow rate may or may not be maximal and representative of average flow. For example, in mitral valve prolapse, mid-systole may not actually be the time of peak regurgitation—progressive prolapse through systole may increase the ERO area progressively until it is maximal in late systole. Similarly, functional MR, as may happen with cardiomyopathy, may lessen progressively through systole as the ventricular volume diminishes, allowing better approximation of the mitral leaflets and a smaller regurgitant orifice. From the point of view of orifice stability, the prototypic lesions of MR are rheumatic MR and MR due to perforations, where the orifice is essentially constant throughout systole. However, in many adult patient populations, functional MR and MVP greatly outnumber the rheumatic and endocarditic cases.18,19 With holosystolic and central jet MR the PISA technique is appropriate, whereas with late systolic and eccentric jet PISA is problematic.
Ideally, the PISA would be sampled at numerous times through the flow interval to account for differing flow rates that may occur.19 The flow convergence radius should be measured on three different cardiac cycles.
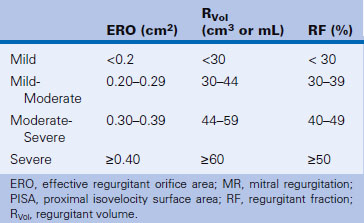
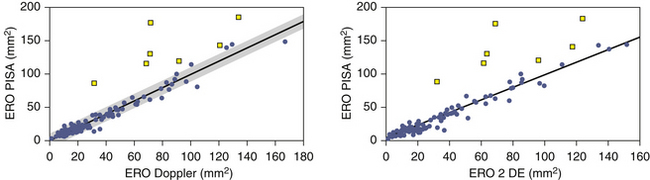
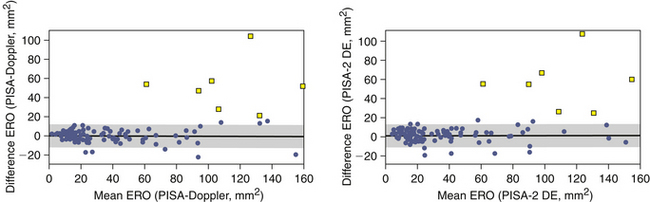
The PISA technique tends to overestimate ERO area, especially when it is large.20 Other factors that may incite error are, not surprisingly, poor-quality PISA hemispheres, which greatly lessen the accuracy of the calculations. MVP often affords a dynamic orifice that may reduce the accuracy of calculations, as mid-systolic flow rate may not be the maximal flow rate. Among patients with optimal depiction of flow convergence, the correlation of PISA determination of ERO area with those obtained by qualitative Doppler and quantitative two-dimensional methods is excellent.20 Table 16-1 presents a grading scheme for judging the severity of valvular insufficiency.
Proximal Isovelocity Surface Area: a Summary
 The PISA technique enables quantification of regurgitant or stenotic flow rate, orifice area, and RVol.
The PISA technique enables quantification of regurgitant or stenotic flow rate, orifice area, and RVol.
 Scrupulous technique is critical to optimize acquisition of the variables needed to make calculations:
Scrupulous technique is critical to optimize acquisition of the variables needed to make calculations:
 Several assumptions are implicit in the use of the technique, such as hemispheric flow acceleration across a planar orifice.
Several assumptions are implicit in the use of the technique, such as hemispheric flow acceleration across a planar orifice.
 Overestimation of ERO and RVol by the PISA technique may occur for several reasons:
Overestimation of ERO and RVol by the PISA technique may occur for several reasons:
 Constraint by nearby structures devalidates the assumption of hemispheric flow model, and confers a “constraint angle” that should prompt the use of angle correction. Complex constraint geometry renders application of the technique unwise.
Constraint by nearby structures devalidates the assumption of hemispheric flow model, and confers a “constraint angle” that should prompt the use of angle correction. Complex constraint geometry renders application of the technique unwise.
 In the setting of multiple regurgitant orifices, assessments of the multiple PISAs may add up to the cumulative effect, but provide a tiresome challenge.
In the setting of multiple regurgitant orifices, assessments of the multiple PISAs may add up to the cumulative effect, but provide a tiresome challenge.
 Highly eccentric MR may afford the parasternal long-axis view the means by which to assess both aliasing radius and peak velocity.
Highly eccentric MR may afford the parasternal long-axis view the means by which to assess both aliasing radius and peak velocity.
 Atrial fibrillation, common in mitral valve disease, renders PISA determinations of MR volume less accurate. In atrial fibrillation, more than 5 cardiac cycles (7–10) are needed to ascertain average MR.
Atrial fibrillation, common in mitral valve disease, renders PISA determinations of MR volume less accurate. In atrial fibrillation, more than 5 cardiac cycles (7–10) are needed to ascertain average MR.
 Simplified versions of the equations may be used:
Simplified versions of the equations may be used:
 The PISA technique has contributed to the understanding of the variable and often complex nature of some orifices (Table 16-2).
The PISA technique has contributed to the understanding of the variable and often complex nature of some orifices (Table 16-2).
TABLE 16-2 Proximal Intervelocity Surface Area Method Summary: Pros and Cons
| Pros | Cons | |
|---|---|---|
| Mitral Regurgitation |
• The principal application of PISA techniques is in the resolution of severity of mitral insufficiency (especially distinguishing2 moderate from severe), particularly when color Doppler flow mapping techniques are unreliable (e.g., eccentric wall impacting jets or multilobulated jets, and pulmonary venous sampling is poor quality). • Uncertainty about MR severity is common, and PISA is a useful adjunctive method. |
• Technical factors may render the technique inapplicable.
• The PISA technique for MR severity determination suffers from
• Technically poor color Doppler depiction of converging hemispheres
• Difficulty in assessing multiple regurgitant orifices
• Very oblique orifices (which are common in myxomatous disease—flail and prolapse lesions). Even if the PISA hemisphere is well depicted, CW interrogation may be significantly misaligned, leading to undersampling of true peak velocity and VTI.
• Aliasing velocities of <20 cm/sec are less reliable, and would require use of regression equations.23
• There is a systematic tendency to overestimate regurgitant flow when it is more severe.22
• Standard techniques to determine MVA are relatively robust, and TTE and TEE calculations of mitral valve gradient are very good, leaving a lesser role for adjunctive methods.
• Oblique orientation of the leaflets and the orifice may diminish feasibility.
• Cone-like orifices are a problem, as the standard PISA equation assumes a planar orifice. “Angle correction” should be considered (orifice angle/180 degrees × ERO), although not all studies demonstrated its benefit.28
• Most MS orifices are long and slit-like, and are less suitable for the PISA method.
• Standard techniques to determine AVA are relatively robust, and TTE calculations of aortic valve gradient are very good, leaving a lesser role for adjunctive methods.
• PISA hemispheres within the LVOT generally become confined/constrained by the walls of the LVOT and distorted into nonhemispheres, devalidating the use of hemispheric models.
• Concurrent subvalvar, LVOT, or intracavitary gradients will contaminate the isovelocity maps.
AVA, aortic valve area; CW, continuous wave (Doppler); ERO, effective regurgitant orifice; LVOT, left ventricular outflow tract; MR, mitral regurgitation; MVA, mitral valve area; PISA, proximal isovelocity surface area; SI, stroke index; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; VTI, velocity time integral.
1. Goodkin G.M., Tunick P.A., Kronzon I. Proximal isovelocity surface area (PISA) in the evaluation of fixed membranous subaortic stenosis. Echocardiography. 2002;19:157-159.
2. Tunick P.A., Kronzon I. Homograft pulmonic stenosis after the Ross procedure: evaluation of the stenotic valve area by proximal isovelocity surface area (PISA). J Am Soc Echocardiogr. 2001;14:67-69.
3. Kosecik M., Sagin-Saylam G., Unal N., et al. Noninvasive assessment of left-to-right shunting in ventricular septal defects by the proximal isovelocity surface area method on Doppler colour flow mapping. Can J Cardiol. 2007;23:1049-1053.
4. Chaliki H.P., Nishimura R.A., Enriquez-Sarano M., Reeder G.S. A simplified, practical approach to assessment of severity of mitral regurgitation by Doppler color flow imaging with proximal convergence: validation with concomitant cardiac catheterization. Mayo Clin Proc. 1998;73:929-935.
5. Blumlein S., Bouchard A., Schiller N.B., et al. Quantitation of mitral regurgitation by Doppler echocardiography. Circulation. 1986;74:306-314.
6. Enriquez-Sarano M., Tajik A.J., Bailey K.R., Seward J.B. Color flow imaging compared with quantitative Doppler assessment of severity of mitral regurgitation: influence of eccentricity of jet and mechanism of regurgitation. J Am Coll Cardiol. 1993;21:1211-1219.
7. Spain M.G., Smith M.D., Grayburn P.A., et al. Quantitative assessment of mitral regurgitation by Doppler color flow imaging: angiographic and hemodynamic correlations. J Am Coll Cardiol. 1989;13(3):585-590.
8. McCully R.B., Enriquez-Sarano M., Tajik A.J., Seward J.B. Overestimation of severity of ischemic/functional mitral regurgitation by color Doppler jet area. Am J Cardiol. 1994;74:790-793.
9. Shiota T., Jones M., Teien D., et al. Color Doppler regurgitant jet area for evaluating eccentric mitral regurgitation: an animal study with quantified mitral regurgitation. J Am Coll Cardiol. 1994;24:813-819.
10. Cape E.G., Yoganathan A.P., Weyman A.E., Levine R.A. Adjacent solid boundaries alter the size of regurgitant jets on Doppler color flow maps. J Am Coll Cardiol. 1991;17:1094-1102.
11. Grewal K.S., Malkowski M.J., Piracha A.R., et al. Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am J Cardiol. 2000;85:199-203.
12. Kolev N., Brase R., Wolner E., Zimpfer M. Quantification of mitral regurgitant flow using proximal isovelocity surface area method: a transesophageal echocardiography perioperative study. J Cardiothorac Vasc Anesth. 1998;12:22-26.
13. Kamp O., Huitink H., van Eenige M.J., et al. Value of pulmonary venous flow characteristics in the assessment of severity of native mitral valve regurgitation: an angiographic correlated study. J Am Soc Echocardiogr. 1992;5:239-246.
14. Enriquez-Sarano M., Dujardin K.S., Tribouilloy C.M., et al. Determinants of pulmonary venous flow reversal in mitral regurgitation and its usefulness in determining the severity of regurgitation. Am J Cardiol. 1999;83:535-541.
15. Pu M., Vandervoort P.M., Griffin B.P., et al. Quantification of mitral regurgitation by the proximal convergence method using transesophageal echocardiography. Clinical validation of a geometric correction for proximal flow constraint. Circulation. 1995;92:2169-2177.
16. Pu M., Vandervoort P.M., Greenberg N.L., et al. Impact of wall constraint on velocity distribution in proximal flow convergence zone. Implications for color Doppler quantification of mitral regurgitation. J Am Coll Cardiol. 1996;27:706-713.
17. Rossi A., Dujardin K.S., Bailey K.R., et al. Rapid estimation of regurgitant volume by the proximal isovelocity surface area method in mitral regurgitation: can continuous-wave Doppler echocardiography be omitted? J Am Soc Echocardiogr. 1998;11:138-148.
18. Schwammenthal E., Chen C., Benning F., et al. Dynamics of mitral regurgitant flow and orifice area. Physiologic application of the proximal flow convergence method: clinical data and experimental testing. Circulation. 1994;90:307-322.
19. Enriquez-Sarano M., Sinak L.J., Tajik A.J., et al. Changes in effective regurgitant orifice throughout systole in patients with mitral valve prolapse. A clinical study using the proximal isovelocity surface area method. Circulation. 1995;92:2951-2958.
20. Enriquez-Sarano M., Miller F.A.Jr., Hayes S.N., et al. Effective mitral regurgitant orifice area: clinical use and pitfalls of the proximal isovelocity surface area method. J Am Coll Cardiol. 1995;25:703-709.
21. Vandervoort P.M., Rivera J.M., Mele D., et al. Application of color oppler flow mapping to calculate effective regurgitant orifice area. An in vitr study and initial clinical observations. Circulation. 1993;88:1150-1156.
22 Chen C., Koschyk D., Brockhoff C., et al. Noninvasive estimation of regurgitant flow rate and volume in patients with mitral regurgitation by Doppler color mapping of accelerating flow field. J Am Coll Cardiol. 1993;21:374-383.
23. Tokushima T., Reid C.L., Hata A., Gardin J.M. Simple method for estimating regurgitant volume with use of a single radius for measuring proximal isovelocity surface area: an in vitro study of simulated mitral regurgitation. J Am Soc Echocardiogr. 2001;14:104-113.
24. Rodriguez L., Thomas J.D., Monterroso V., et al. Validation of the proximal flow convergence method. Calculation of orifice area in patients with mitral stenosis. Circulation. 1993;88:1157-1165.
25. Rifkin R.D., Harper K., Tighe D. Comparison of proximal isovelocity surface area method with pressure half-time and planimetry in evaluation of mitral stenosis. J Am Coll Cardiol. 1995;26:458-465.
26. Shiota T., Jones M., Valdes-Cruz L.M., et al. Color flow Doppler determination of transmitral flow and orifice area in mitral stenosis: experimental evaluation of the proximal flow-convergence method. Am Heart J. 1995;129:114-123.
27. Ikawa H., Enya E., Hirano Y., et al. Can the proximal isovelocity surface area method calculate stenotic mitral valve area in patients with associated moderate to severe aortic regurgitation? Analysis using low aliasing velocity of 10% of the peak transmitral velocity. Echocardiography. 2001;18:89-95.
28. Bargiggia G.S., Scopelliti P., Bertucci C., et al. Doppler estimation of the stenotic mitral valve area. Direct application of the continuity equation to the flow convergence region. G Ital Cardiol. 1991;21:815-823.
29. Oku K., Utsunomiya T., Mori H., et al. Calculation of mitral valve area in mitral stenosis using the proximal isovelocity surface area method. Comparison with two-dimensional planimetry and Doppler pressure half time method. Jpn Heart J. 1997;38:811-819.
30. Tribouilloy C.M., Enriquez-Sarano M., Fett S.L., et al. Application of the proximal flow convergence method to calculate the effective regurgitant orifice area in aortic regurgitation. J Am Coll Cardiol. 1998;32:1032-1039.