CHAPTER 254 Proton Radiosurgery
Radiosurgery is a radiation therapy technique in which a high dose of radiation is delivered in a single sitting, thus simulating surgery as a defined procedure that is completed in one session. Because of the risk of injury to normal tissue with a single treatment consisting of high-dose radiation, precision of targeting is imperative, and the targets are typically small and radiographically discrete. Thus, radiosurgery has had both its origin and much of its current-day use in treating intracranial targets, where immobilization of the head is more easily achieved and relatively small targets are common. The majority of the radiosurgical experience has been with photon-based sources from linear accelerators (LINACs) or Gamma Knife units. Proton radiation offers unique physical characteristics that have advantages over photon methodologies when applied to radiosurgery.1 This chapter reviews the origin and development of the technology behind proton radiosurgery.
Historical Perspective on Radiosurgery
Radiosurgery was first proposed in 19512 and then clinically implemented at the University of California Berkeley cyclotron in 1954.3 The first patient was treated at the Uppsala University cyclotron facility in 1957.4 In comparison, the first Gamma Knife and LINAC radiosurgery treatments were delivered in 1967 and 1985, respectively.5 The majority of radiosurgery is currently performed with compact hospital-based LINACs or Gamma Knife units because of the significantly lower cost and complexity of these systems. Particle accelerators such as cyclotrons or synchrotrons have not been commonly adopted by hospital clinics because of the space required, cost, and necessary infrastructure to support such facilities. Today, radiosurgery programs of various modality involving the Gamma Knife (Elekta; U.S. office, Norcross, GA) number nearly 300; there are many more that use LINACs but less than a handful of proton therapy centers worldwide. Proton radiosurgery offers improved dose uniformity within large targeted volumes and reduced doses to nontarget normal tissues when compared with its photon counterparts. These benefits have created sufficient interest in the technology to motivate the construction and development of increasingly more clinical proton therapy centers.
Dose Principles: Photons and Protons
The clinical radiation dose is measured in the unit gray (Gy) and represents the energy deposited by ionization processes in material per unit mass of material (1 Gy = 1 J/kg). The energy absorbed causes damage at the molecular level in DNA and proteins that results in arrest of cell growth and death. The biologic effect of γ-rays or x-rays, which are both forms of photons, is essentially the same for given doses. Tissue responses vary with the cell type, as well as the radiation modality. The radiobiologic effectiveness (RBE) describes the equivalence between radiation modalities in terms of net biologic effects.6 Despite the complex dependence on many variables and the variation in RBE along the length of the proton path, a generic RBE of 1.1 for protons as compared with cobalt 60, the representative standard for photons, has been accepted in the radiation therapy community.7–9 Thus, a 10% reduction in proton physical dose is delivered to achieve the same biologic effect on the target if treatment were given by cobalt 60 or other photon sources.10 For ease of clinical use, the biologically equivalent dose to photons is expressed in standard fashion as units of Gy with RBE weighted absorbed dose Gy(RBE).10a
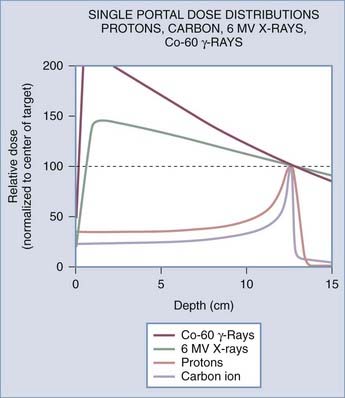
The difference in the biologic effects of radiation sources is a function of their physical properties. As a photon beam passes through material and is absorbed, the overall intensity of the beam is reduced. In contrast, particles such as protons and ions travel a finite distance, which is termed the range. They deposit a disproportionate amount of energy in the last few millimeters of their path. This large transfer of energy is known as the Bragg peak. The physical depth penetrated by the particles depends on tissue density and the beam’s energy. Figure 254-1 demonstrates the difference in dose deposition in tissue with various radiation sources. The dose deposited in the region of normal tissues leading to the target and extending beyond the target is termed the integral dose. The integral dose can be reduced by using multiple treatment beams from different directions such that a therapeutic dose is achieved at the intersection whereas the integral dose remains reasonably low. Proton radiosurgery typically uses 2 to 6 beams. Similarly, LINAC x-ray radiosurgery commonly uses 2 to 6 conformal beams or arcs of continuous radiation sweep. The Gamma Knife differs in that 201 narrow beams are used to deliver the treatment. Other modern conformal radiation therapies with photons, such as intensity-modulated radiation therapy (IMRT), achieve fairly high dose conformality at the expense of producing very large low-radiation dose baths to much of the surrounding normal tissue. Even though this dose bath may be below the clinical threshold for acute side effects, it introduces a potential risk for a significantly high rate of radiation-induced malignancies and late complications in normal tissue that are as yet poorly characterized. IMRT is widely used with fractionated radiation therapy for targets of variable size and shape. For small intracranial targets, it offers little advantage over stereotactic radiotherapy with fractionated treatment. IMRT is not typically used for stereotactic radiotherapy because of the unacceptably high surrounding dose bath to normal tissues.
The Original Approach: Proton Cross-Fire
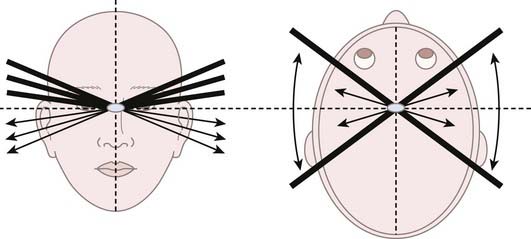
When Lawrence in 1954 and Leksell in 1957 pioneered radiosurgery, they used protons as their radiation source. Rather than using the effects of the Bragg peak, they relied on the flat portion of the proton depth dose profile that precedes the Bragg peak, termed the plateau. This technique was coined “strålkniven,” or ray knives, by Leksell. The plateau region of a 340-MeV proton beam provided a sharper lateral dose falloff than did the end of the Bragg peak or the x-ray beams that were available at the time. Typical 80% to 20% lateral dose falloffs, also known as the penumbra, for 340- and 185-MeV proton beams are 1.0 mm and 2.0 mm, respectively. These falloffs serve as much sharper lateral penumbras than the 5.0-mm falloff from the end of a Bragg peak. In addition, the end of the Bragg peak has inherent uncertainties when directed through heterogeneous tissue. Lawrence and colleagues at Berkeley treated pituitary targets with multiple proton cross-fire arcs from each side of the head, with the beams being oriented to avoid dose overlap in normal tissue but intersecting at the center of the target (Fig. 254-2).11 Bragg peaks occurred after exiting the patient on the contralateral side and were thus irrelevant in this early form of radiosurgery. Thirty patients were treated with protons at Berkeley from 1954 to 1957.12 Leksell used the same cross-fire principles with a 185-MeV proton beam and achieved superior targeting precision because of the use of a stereotactic fixation device.4 It also permitted treatment in a single session rather than the multiple sessions used at Berkeley. The lower proton energy generated by the Uppsala cyclotron in contrast to the Berkeley facility resulted in doses with a slightly less uniform depth. From 1957 to 1976, 73 patients were treated with protons at Uppsala in similar fashion.12,13 Subsequently, this technique was adopted by other groups with high-energy cyclotrons.14
The Second Approach: Proton Bragg Peak and Minimizing the Uncertainty
The team at the Harvard Cyclotron Laboratory (HCL) initiated its proton radiosurgery program in 1961.15,16 This facility was limited to a 160-MeV proton beam that had an insufficient beam range to use the cross-fire approach, the group was the first to implement the Bragg peak as the primary therapeutic source. The purpose of this new technique was to significantly reduce the dose to normal brain tissue. Unlike the cross-fire approach, beams aimed from the vertex of the head toward the feet could be used with no downstream dose to the thorax because of the finite range of protons, which were calculated to stop within the target. The technical challenge remained the ability to precisely stop the protons at the desired location in an era before computed tomography (CT). In comparison, the beam energies needed to achieve the desired depth of penetration are currently calculated readily from CT-derived tissue densities.
Delivery of the Bragg peak to the target was enabled by a system in which a variable amount of material was placed upstream in the proton beam path to adjust its depth in patients. A telescoping column of water was used to pull the Bragg peak back to the desired depth. Calibration of each treatment beam preceded positioning of the patient in the treatment unit. Three orthogonal plain films were taken to confirm alignment before treatment.16 A complex scattering and filtering system was used to prepare the beam for clinical use. Patients were placed in a positioner that rotated a maximum of 45 degrees to either side. Fine adjustments were made to the head while a nurse manually adjusted the positioner to ensure patient comfort and safety.
Aside from some technical improvements, the multistep treatment technique of using the Bragg peak as the primary therapeutic source remains the same today. The dose is delivered by first treating the most distal segment of the target with the Bragg peak. The second peak is delivered at a shallower depth by increasing the amount of water or equivalent material within the path of the proton beam before reaching the patient. The weight or relative dose delivered with the shallower peak is reduced in comparison to the first peak because it is additive to the dose of the first peak. This process, termed “lamination” planning, is repeated to cover the target width with 90% of the maximum dose. The combined peaks are called the spread-out Bragg peak (SOBP). The width of the SOBP 90% to 90% dose is known as the modulation. Corrections are required to account for the difference in density between air, bone, brain, and other tissues. To minimize uncertainty, ports are preferentially selected so that they penetrate bone perpendicular to the surface and avoid heterogeneous regions such as sinuses, mastoids, ears, and the base of the skull, which may introduce small errors in dosimetry calculations.17 The dose profiles of proton beams also vary as a factor of field size.17a A proportionally higher dose to the surface of tissues is delivered with smaller field sizes because of the reliance on more direct ionization as compared with the dose from scatter radiation.17 Therefore, lamination plans must be adjusted to account for the field size to generate a uniform SOBP.
In 1965, the first proton radiosurgery procedure was performed by Kjellberg for the treatment of an arteriovenous malformation (AVM). However, it was not until 1972 that the second and subsequent AVM patients would be treated by the Massachusetts General Hospital (MGH)/HCL radiosurgical group.18 The excellent visualization of AVMs with plain film angiography was well suited for designing noncircular collimation. In 1985, conformal collimation was fully implemented, with digitized collimation being transferred to an automated milling machine to create the fully conformal apertures. The extensive radiosurgical AVM experience from 1972 through 1979 allowed Kjellberg to create a model relating risk for complications to irradiated volume and dose.19,20 This program continued until 1991 and culminated in the treatment of 2928 radiosurgical cases. Roughly half of these treatments, 1441, were pituitary targets, whereas the remaining 1486 were AVMs.
Technologic Evolution: Stereotactic Alignment for Radiosurgery
In the 1980s, several advancements were made in the proton treatment system. A new patient positioner was designed to specifically accommodate the fixed horizontal beam line.21 This positioner, named STAR for stereotactic alignment for radiosurgery, was designed to be fully isocentric, defined as having the ability to be rotated or moved while maintaining the center of the target at a fixed point aligned with the proton beam. The new positioner enabled treatment of cranial targets from any direction in the superior hemisphere of the head. The STAR device incorporated the Brown-Roberts-Wells (BRW) coordinate system.22 Custom posts and pins were fabricated and adapted to the BRW frame to reduce the risk for flexure of the posts because of patient rotation load shifts. Local anesthesia was applied to the contact sites for placement of the fixation pins. Patients were secured with bolsters within the STAR system so that they could be rolled on their side as needed to treat with oblique fields.23 The primary limitation of the BRW frame was an inability to treat base of skull targets with inferior oblique fields because of interference by the hardware.
To improve the efficiency of treatment setup, a fiducial marker system was developed in which radiopaque, nonmagnetic surgical steel microspheres could be implanted in the outer table of the patient’s skull. These spheres are  inch in diameter (316SLVM) and are inserted in a 15-minute outpatient procedure.24 Typically, three fiducial markers are implanted to allow the acquisition of three-dimensional data and thereby ensure accuracy of patient positioning.
inch in diameter (316SLVM) and are inserted in a 15-minute outpatient procedure.24 Typically, three fiducial markers are implanted to allow the acquisition of three-dimensional data and thereby ensure accuracy of patient positioning.
Treatment-planning CT is performed with the patient immobilized in a custom alpha cradle, namely, a customized posterior occipital head support created from foam-like material that polymerizes to the molded form within minutes. Thin CT slices (1 mm) with a high-resolution field of view are used to provide optimal determination of the fiducial position in the CT coordinate system. Angiography with MR image fusion is used to assist in target definition.25 Once the target is defined, a treatment plan consisting of two to six conformal fields can be generated. Brass apertures are milled for each treatment portal. A patient alignment system was also designed and programmed to accommodate treatment positioning with the unique coordinate transformations required with STAR and the HCL beam line.26
A three-dimension treatment-planning algorithm and planning system was developed in the late 1980s.27,28 This planning system was modified from its original form to accommodate the water telescope principle, as well as the unique rotational properties of the STAR device. Similar to photon algorithms, in which CT Hounsfield units are converted to electron density to model the depth distribution, the CT numbers were converted to proton stopping power, which is a measure of the distance traveled by protons.29 A limitation of the early algorithm was an inability to account for scatter heterogeneities through tissue (e.g., bone, brain, air). Therefore, it was important that the beams be aimed through uniform paths to minimize uncertainty. The early planning system also did not recognize the increased surface dose inherent with small field sizes.
Hospital-Based Proton Therapy Facility
Loma Linda University Medical Center was the first hospital-based proton facility; it was opened in 1990 and is a center with proton radiosurgery capability.30 The Francis H. Burr Proton Therapy Center (FHBPTC), formerly known as the Northeast Proton Therapy Center when opened at the MGH in 2001 and then renamed in 2003, was the second hospital-based proton therapy center built in the United States. Of interest, of the subsequent new proton facilities opened in the United States, none are currently practicing radiosurgery. Locating proton therapy facilities on main hospital campuses provides far better access for patients and is particularly beneficial for coordination with other medical care. The FHBPTC is reviewed as an example of a modern hospital-based facility.
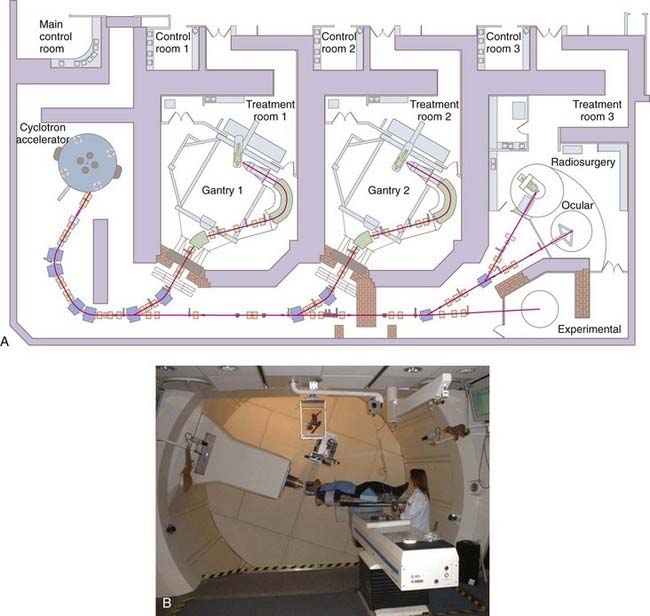
The FHBPTC facility includes a 230-MeV isosynchronous cyclotron to generate protons delivered to two gantry rooms and a third room with fixed horizontal beam lines (Fig. 254-3). Radiosurgery is performed with both the gantry units and the STAR system. The gantries are designed to accommodate large fields but can be adapted for radiosurgery, whereas the STAR system is a dedicated device for smaller intracranial targets and offers slightly sharper beam delivery. On the gantry, the combination of modulation wheels and dynamic adjustment of the beam current creates the desired flat SOBP from monoenergetic pristine Bragg peaks. The protons are guided with large bending magnets and refocused with quadrupole magnets as they are directed into the treatment rooms. Treatment is delivered with an isocentric gantry rather than a fixed horizontal beam line as used at the HCL. The gantry rooms use nonisocentric four-axis robotic patient positioners and amorphous silicon panels for digital imaging rather than film (Fig. 254-4). Final proton beam shaping is achieved with custom brass apertures for each treatment beam and Lucite compensators to create the distal shape of the beam.
The fixed beam line of the STAR system has fewer degrees of freedom of treatment positions but has the advantage of more conformal delivery of treatment. It has a maximum penetration of 19.5 cm, a maximum field diameter of 10 cm, and a maximum modulation of 9 cm. The range and modulation are controlled by a binary set of Lexan and lead absorbers rather than a modulation wheel (Fig. 254-5). The beam is transported from the cyclotron at 185 MeV and reduced to the necessary energy and depth with the appropriate combination of absorbers in the form of a single scattering system. The resulting lateral dose uniformity of ±2.5% at the isocenter depth produces a sharper lateral dose falloff than can be achieved with the gantry fields, which is particularly favorable for radiosurgery, where short dose gradients between target and nontarget tissues are desirable. Fields are delivered as a lamination sequence as described previously. Although the MGH has an active Linac radiosurgery program, 4,600 patients have been treated with proton radiosurgery between 1961 and 2010.
A noninvasive system for immobilization has been developed that incorporates the existing fiducial marker system that relied on patient anatomy and implanted hardware for alignment rather than an external frame. The Gill-Thomas-Cosman (GTC) bite block head immobilization system (Integra-Radionics, Inc., Burlington, MA), commonly used in LINAC stereotactic radiotherapy, was modified for proton radiosurgery. The modified GTC frame exchanged the original aluminum occipital support hardware for a thin carbon occipital head cup and headrest with low-density padding to allow full access of the beam to the head.31 It is applicable for both the gantry and STAR systems.
The first proton therapy planning system approved by the Food and Drug Administration was introduced in 1998. It uses a pencil beam dose algorithm to model scatter rather than using generic dose fits.32 Current treatment planning integrates a further developed algorithm combined with tools such as image fusion to enable easy use of MRI, positron emission tomography, and CT angiography data.33–35 Hounsfield corrections of CT contrast agents are made by using image fusion software to overlay the contrast-enhanced scans used for target delineation on the non–contrast-enhanced scans for treatment and dose planning.
Conclusion
Although clinical results documenting proton radiosurgery to have comparable effectiveness to other modalities have been published,36–40 randomized trials have never been conducted.
Boone ML, Lawrence JH, Connor WG, et al. Introduction to the use of protons and heavy ions in radiotherapy: historical perspective. Int J Radiat Oncol Biol Phys. 1985;3:65-69.
Bussière MR, Adams JA. Treatment planning for conformal proton radiation therapy. Technol Cancer Res Treat. 2003;2:389-399.
Butler WE, Ogilvy CS, Chapman PH, et al. Stereotactic alignment for Bragg peak radiosurgery. In: Steiner L, editor. Radiosurgery: Baseline and Trends. New York: Raven Press; 1992:85-91.
Chapman PH, Ogilvy CS, Butler WE. A new stereotactic alignment system for charged-particle radiosurgery at the Harvard Cyclotron Laboratory, Boston. In: Alexander E, Loeffler JS, Lunsford LD, editors. Stereotactic Radiosurgery. New York: McGraw-Hill; 1993:105-108.
Chen CC, Chapman PH, Bussiere M, et al. Stereotactic radiosurgery: basic principles, delivery platforms, and clinical applications. In: Newton HB, Jolesz FA, editors. Handbook of Neuro-Oncology Neuroimaging. Boston: Academic Press; 2007:192-214.
Colombo F, Benedetti A, Pozza F, et al. External stereotactic irradiation by linear accelerator. Neurosurgery. 1985;16:154-160.
De Salles AA, Asfora WT, Abe M, et al. Transposition of target information from the magnetic resonance and computed tomography scan images to conventional x-ray stereotactic space. Appl Neurophysiol. 1987;50:23-32.
Engelsman M, Mazel A, Jaffray D. Patient positioning and set-up verification for planning and treatment. In: DeLaney TF, Kooy HM, editors. Proton and Charged Particle Radiotherapy. Philadelphia: Lippincott Williams & Wilkins; 2008:57-69.
Gall K, Verhey L, Wagner M. Computer-assisted positioning of radiotherapy patients using implanted radiopaque fiducials. Med Phys. 1993;20:1153-1559.
Gerweck L, Paganetti H. Radiobiology of charged particles. In: DeLaney TF, Kooy HM, editors. Proton and Charged Particle Radiotherapy. Philadelphia: Lippincott Williams & Wilkins; 2008:8-18.
Goitein M, Abrams M. Multi-dimensional treatment planning: I. Delineation of anatomy. Int J Radiat Oncol Biol Phys. 1983;9:777-787.
Goitein M, Abrams M, Rowell D, et al. Multi-dimensional treatment planning: II. Beam’s eye-view, back projection, and projection through CT sections. Int J Radiat Oncol Biol Phys. 1983;9:789-797.
Graffman S, Brahme A, Larsson B. Proton radiotherapy with the Uppsala cyclotron: experience and plans. Strahlentherapie. 1985;161:764-770.
Harsh G, Loeffler JS, Thornton A, et al. Stereotactic proton radiosurgery. Neurosurg Clin N Am. 1999;10:243-256.
Hong L, Goitein M, Bucciolini M, et al. A pencil beam algorithm for proton dose calculations. Phys Med Biol. 1996;41:1305-1330.
, 1993 ICRU Report 49. Stopping Powers for Protons and Alpha Particles. Bethesda, MD: 1993.
Kjellberg RN. Isoeffective dose parameters for brain necrosis in relation to proton radiosurgical dosimetry. In: Szikla G, editor. Stereotactic Cerebral Irradiations. Amsterdam: Elsevier; 1979:157-166.
Kjellberg RN, Abe M. Stereotactic Bragg peak proton beam therapy. In: Lunsford LD, editor. Modern Stereotactic Neurosurgery. Boston: Martinus Nijhoff; 1988:463-470.
Kjellberg RN, Koehler AM, Preston WM, et al. Stereotaxic instrument for use with the Bragg peak of a proton beam. Confin Neurol. 1962;22:183-189.
Kjellberg RN, Sweet WH, Preston WM, et al. The Bragg peak of a proton beam in intracranial therapy of tumors. Trans Am Neurol Assoc. 1962;87:216-218.
Koehler AM, Dickinson J, Preston WM. The range of protons in human skull bone. Radiat Res. 1965;26:334-342.
Larsson B, Leksell L, Rexed B, et al. The high-energy proton beam as a neurosurgical tool. Nature. 1958;182:1222-1223.
Lawrence JH. Proton Irradiation of the pituitary. Cancer. 1957;10:795-798.
Lawrence JH, Tobias CA, Born JL, et al. Pituitary irradiation with high-energy proton beams: a preliminary report. Cancer Res. 1958;18:121-134.
Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
Linear energy transfer and relative biological effectiveness. In: Hall EJ, Giaccia AJ, editors. Radiobiology for the Radiologist. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:106-116.
Miller DW. A review of proton beam radiation therapy. Med Phys. 1995;22:1943-1954.
Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407-421.
Serago CF, Thornton AF, Urie MM, et al. Comparison of proton and x-ray conformal dose distributions for radiosurgery applications. Med Phys. 1995;22:2111-2116.
Shepard D, Yu C, Murphy M, et al. Treatment planning for stereotactic radiosurgery. In: Chin L, Regine W, editors. Principles and Practice of Stereotactic Radiosurgery. Philadelphia: Springer; 2008:69-90.
Shih HA, Chapman PH, Bussière MR, et al. Central nervous system. In: DeLaney TF, Kooy HM, editors. Proton and Charged Particle Radiotherapy. Philadelphia: Lippincott Williams & Wilkins; 2008:140-150.
Sisterson J. Worldwide charged particle patient totals. Proton Therapy Cooperative Group. 1988. Particles Newsletter
Tepper J, Verhey L, Goitein M, et al. In vivo determinations of RBE in a high energy modulated proton beam using normal tissue reactions and fractionated dose schedules. Int J Radiat Oncol Biol Phys. 1977;2:1115-1122.
Urano M, Verhey L, Goitein M, et al. Relative biological effectiveness of modulated proton beams in various murine tissues. Int J Radiat Oncol Biol Phys. 1984;10:509-514.
Wilson R. Harvard cyclotron laboratory—50th anniversary—a symposium celebrating 50 years of proton beams at the Harvard Cyclotron Laboratory (HCL. In: Wilson R, editor. A Brief History of the Harvard University Cyclotrons. Cambridge, MA: Harvard University Press; 2004:106-107.
1 Serago CF, Thornton AF, Urie MM, et al. Comparison of proton and x-ray conformal dose distributions for radiosurgery applications. Med Phys. 1995;22:2111-2116.
2 Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
3 Lawrence JH. Proton Irradiation of the pituitary. Cancer. 1957;10:795-798.
4 Larsson B, Leksell L, Rexed B, et al. The high-energy proton beam as a neurosurgical tool. Nature. 1958;182:1222-1223.
5 Colombo F, Benedetti A, Pozza F, et al. External stereotactic irradiation by linear accelerator. Neurosurgery. 1985;16:154-160.
6 Linear energy transfer and relative biological effectiveness. In: Hall EJ, Giaccia AJ, editors. Radiobiology for the Radiologist. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:106-116.
7 Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407-421.
8 Tepper J, Verhey L, Goitein M, et al. In vivo determinations of RBE in a high energy modulated proton beam using normal tissue reactions and fractionated dose schedules. Int J Radiat Oncol Biol Phys. 1977;2:1115-1122.
9 Urano M, Verhey L, Goitein M, et al. Relative biological effectiveness of modulated proton beams in various murine tissues. Int J Radiat Oncol Biol Phys. 1984;10:509-514.
10 Gerweck L, Paganetti H. Radiobiology of charged particles. In: DeLaney TF, Kooy HM, editors. Proton and Charged Particle Radiotherapy. Philadelphia: Lippincott Williams & Wilkins; 2008:8-18.
10a Prescribing, Recording and Reporting Proton-Beam Therapy, Journal of the ICRU; Vol. 7 No. 2 (2007) Report 78, Oxford University Press.
11 Lawrence JH, Tobias CA, Born JL, et al. Pituitary irradiation with high-energy proton beams: a preliminary report. Cancer Res. 1958;18:121-134.
12 Sisterson J. Worldwide charged particle patient totals. Proton Therapy Cooperative Group. 1988. Particles Newsletter
13 Graffman S, Brahme A, Larsson B. Proton radiotherapy with the Uppsala cyclotron: experience and plans. Strahlentherapie. 1985;161:764-770.
14 Boone ML, Lawrence JH, Connor WG, et al. Introduction to the use of protons and heavy ions in radiotherapy: historical perspective. Int J Radiat Oncol Biol Phys. 1985;3:65-69.
15 Kjellberg RN, Sweet WH, Preston WM, et al. The Bragg peak of a proton beam in intracranial therapy of tumors. Trans Am Neurol Assoc. 1962;87:216-218.
16 Kjellberg RN, Koehler AM, Preston WM, et al. Stereotaxic instrument for use with the Bragg peak of a proton beam. Confin Neurol. 1962;22:183-189.
17 Koehler AM, Dickinson J, Preston WM. The range of protons in human skull bone. Radiat Res. 1965;26:334-342.
17a Daartz J, Engelsman M, Paganetti H, et al. Field size dependence of the output factor in passively scattered proton therapy: influence of range, modulation, air gap and machine settings. Med Phys. 2009;36:3205-3210.
18 Wilson R. Harvard cyclotron laboratory—50th anniversary—a symposium celebrating 50 years of proton beams at the Harvard Cyclotron Laboratory (HCL). In: Wilson R, editor. A Brief History of the Harvard University Cyclotrons. Cambridge, MA: Harvard University Press; 2004:106-107.
19 Kjellberg RN. Isoeffective dose parameters for brain necrosis in relation to proton radiosurgical dosimetry. In: Szikla G, editor. Stereotactic Cerebral Irradiations. Amsterdam: Elsevier; 1979:157-166.
20 Kjellberg RN, Abe M. Stereotactic Bragg peak proton beam therapy. In: Lunsford LD, editor. Modern Stereotactic Neurosurgery. Boston: Martinus Nijhoff; 1988:463-470.
21 Chapman PH, Ogilvy CS, Butler WE. A new stereotactic alignment system for charged-particle radiosurgery at the Harvard Cyclotron Laboratory, Boston. In: Alexander E, Loeffler JS, Lunsford LD, editors. Stereotactic Radiosurgery. New York: McGraw-Hill; 1993:105-108.
22 Butler WE, Ogilvy CS, Chapman PH, et al. Stereotactic alignment for Bragg peak radiosurgery. In: Steiner L, editor. Radiosurgery: Baseline and Trends. New York: Raven Press; 1992:85-91.
23 Engelsman M, Mazel A, Jaffray D. Patient positioning and set-up verification for planning and treatment. In: DeLaney TF, Kooy HM, editors. Proton and Charged Particle Radiotherapy. Philadelphia: Lippincott Williams & Wilkins; 2008:57-69.
24 Harsh G, Loeffler JS, Thornton A, et al. Stereotactic proton radiosurgery. Neurosurg Clin N Am. 1999;10:243-256.
25 De Salles AA, Asfora WT, Abe M, et al. Transposition of target information from the magnetic resonance and computed tomography scan images to conventional x-ray stereotactic space. Appl Neurophysiol. 1987;50:23-32.
26 Gall K, Verhey L, Wagner M. Computer-assisted positioning of radiotherapy patients using implanted radiopaque fiducials. Med Phys. 1993;20:1153-1559.
27 Goitein M, Abrams M. Multi-dimensional treatment planning: I. Delineation of anatomy. Int J Radiat Oncol Biol Phys. 1983;9:777-787.
28 Goitein M, Abrams M, Rowell D, et al. Multi-dimensional treatment planning: II. Beam’s eye-view, back projection, and projection through CT sections. Int J Radiat Oncol Biol Phys. 1983;9:789-797.
29 ICRU Report 49. Stopping Powers for Protons and Alpha Particles. Bethesda, MD: 1993.
30 Miller DW. A review of proton beam radiation therapy. Med Phys. 1995;22:1943-1954.
31 Shih HA, Chapman PH, Bussière MR, et al. Central nervous system. In: DeLaney TF, Kooy HM, editors. Proton and Charged Particle Radiotherapy. Philadelphia: Lippincott Williams & Wilkins; 2008:140-150.
32 Hong L, Goitein M, Bucciolini M, et al. A pencil beam algorithm for proton dose calculations. Phys Med Biol. 1996;41:1305-1330.
33 Bussière MR, Adams JA. Treatment planning for conformal proton radiation therapy. Technol Cancer Res Treat. 2003;2:389-399.
34 Shepard D, Yu C, Murphy M, et al. Treatment planning for stereotactic radiosurgery. In: Chin L, Regine W, editors. Principles and Practice of Stereotactic Radiosurgery. Philadelphia: Springer; 2008:69-90.
35 Chen CC, Chapman PH, Bussiere M, et al. Stereotactic radiosurgery: basic principles, delivery platforms, and clinical applications. In: Newton HB, Jolesz FA, editors. Handbook of Neuro-Oncology Neuroimaging. Boston: Academic Press; 2007:192-214.
36 Halasz LM, Bussière MR, Dennis E, et al. Proton stereotactic radiosurgery for the treatment of benign meningiomas. Int J Radiat Oncol Biol Phys. 2010. (in press)
37 Petit JH, Biller BM, Yock TI, et al. Proton stereotactic radiotherapy for persistent ACTH-producing adenomas. J Clin Endocrinol Metabolism. 2008;93:393-399.
38 Petit JH, Biller BM, Coen JJ, et al. Proton stereotactic radiosurgery in management of persistent acromegaly. Endocrinol Pract. 2007;13:726-734.
39 Weber DC, Chan AW, Bussière MR, et al. Proton beam radiosurgery for vestibular schwannoma: tumor control and cranial nerve toxicity. Neurosurg. 2003;53:577-586.
40 Harsh GR, Thornton AF, Chapman PH, et al. Proton beam stereotactic radiosurgery of vestibular schwannomas. Int J Radiat Oncol Biol Phys. 2002;54:35-54.