Chapter 47 Protection From Blood-Feeding Arthropods
Of all the hazards, large and small, that may befall the outdoor enthusiast, perhaps the most vexatious come from the smallest perils—blood-feeding arthropods. Mosquitoes, flies, fleas, mites, midges, chiggers, and ticks all readily bite humans. The bites are, at best, a minor annoyance; at worst, arthropod bites transmit to humans multiple bacterial, viral, protozoan, parasitic, and rickettsial infections (Box 47-1). Mosquito-transmitted diseases alone will be responsible for the deaths of 1 out of every 17 people currently alive.160 This chapter reviews the arthropod species that bite humans, suggests ways to avoid them, and discusses various options for personal protection against these nefarious organisms.
Mosquitoes (Family Culicidae)
Mosquitoes are responsible for more arthropod bites than any other blood-feeding organism. They occur in all major terrestrial regions except Antarctica. These two-winged insects belong to the order Diptera, the flies. There are 170 species of mosquitoes in North America, and more than 3000 species worldwide. Anopheline, or malaria-transmitting, mosquitoes can be distinguished by their resting position on the skin—their bodies are characteristically raised high, as if they are standing on their heads. Most other species, in contrast, alight with their backs parallel to the skin surface (Figure 47-1, A).

(A1 from http://commons.wikimedia.org/wiki/File:Anopheles_albimanus_mosquito; A2 from http://commons.wikimedia.org/wiki/File:CulexNil; B from http://www.flickr.com/photos/rich_parker/3686158791/courtesy Richard Parker; C from http://www.ars.usda.gov/is/graphics/photos/jul99/k8488-1.htm; D from http://commons.wikimedia.org/wiki/File:Nied_fg02; E from http://commons.wikimedia.org/wiki/File:Phlebotomus_pappatasi_bloodmeal_begin; F courtesy David Bygott; G from http://commons.wikimedia.org/wiki/File:Fly_November_2007-14; H from http://en.wikipedia.org/wiki/File:Rhodnius_prolixus70-300; I courtesy Rui Andrade, http://www.flickr.com/photos/ruiamandrade/2734079506/; J courtesy Anthony Gould, http://www.flickr.com/photos/8463947@N08/765513406/; K from http://phil.cdc.gov/phil/details.asp, search: 7663; L from http://pathmicro.med.sc.edu/parasitology/soft%20tick.)
Mosquitoes vector more diseases to humans than any other blood-feeding arthropod. They transmit malaria to 300 to 500 million people each year, resulting in as many as 3 million deaths per year.148 In addition, about 30,000 international travelers visiting malaria-endemic countries contract the disease yearly.74 Mosquitoes vector multiple arboviruses to humans, including several forms of encephalitis, epidemic polyarthritis, yellow fever, and dengue fever (see Chapter 48). Mosquitoes also transmit the larval form of the nematode that causes lymphatic filariasis.
Mosquitoes rely on visual, thermal, and olfactory stimuli to help them locate a blood meal.* For mosquitoes that feed during the daytime, host movement and dark-colored clothing may initiate orientation toward an individual. Visual stimuli appear to be important for in-flight orientation, particularly over long ranges, whereas olfactory stimuli become more important as a mosquito nears its host. Carbon dioxide and lactic acid are the best-studied attractants. Carbon dioxide serves as a long-range attractant, luring mosquitoes at distances of up to 36 m (118.1 feet).56,57,152At close range, skin warmth and moisture serve as attractants.15,29 Volatile compounds, derived from sebum, eccrine and apocrine sweat, and/or the cutaneous microflora bacterial action on these secretions, may also act as chemoattractants.65,87,103,143,170 Floral fragrances found in perfumes, lotions, soaps, and hair-care products can also lure mosquitoes.48 One study has shown that alcohol ingestion increases the likelihood of being bitten by mosquitoes.149 Mosquitoes are more attracted to individuals infected with transmittable malaria than to uninfected people or to treated individuals who are no longer infected.89
There can be significant variability in the attractiveness of different individuals to the same or different species of mosquitoes22,32,81—a point that travelers should keep in mind when visiting new areas. In some studies, men have been bitten more than women, and adults more than children.81,113 Other studies have shown that repellents based on N,N-diethyl-3-methylbenzamide (DEET) (previously called N,N-diethyl-m-toluamide) may protect women more poorly than they do men.60 Heavyset people are more likely to attract mosquitoes, perhaps due to their greater relative heat or carbon dioxide output.128 Of note is that some studies have failed to confirm these findings.22
Mosquito bites commonly produce small local wheal reactions and associated itching. More dramatic reactions, including generalized urticaria, angioedema, and anaphylaxis have been reported in highly sensitive individuals. Rare “skeeter syndrome” has been documented in five young children who had dramatic localized redness, swelling, warmth and induration develop within hours of a mosquito bite. This entity mimics cellulitis but resolves spontaneously and is associated with significantly elevated serum levels of IgE and IgG to mosquito salivary gland antigens.151 Antihistamines may be useful when taken prophylactically to reduce the intensity of mosquito bite reactions: Compared with placebo, levocetirizine was shown in one study to reduce the size of the 24-hour bite papules by 71% and the accompanying pruritus by 56%.75
Blackflies (Family Simuliidae)
At 2 to 5 mm (0.1 to 0.2 inch) in length, blackflies20,39,59,79,104 (Figure 47-1, B) are smaller than mosquitoes. They have short antennae, stout humpbacked bodies, and broad wings. Blackflies are found globally wherever there are fast-running, clear rivers or streams, which they require for larval development. In temperate regions, adults are most prevalent in late spring and early summer and are most likely encountered near larval habitats, where they are difficult to avoid. However, unlike most mosquitoes, blackflies tend to bite during the daytime. They primarily use visual cues to locate a host. Dark moving objects are particularly attractive, but carbon dioxide and body warmth also serve as attractants. Only the female bites, taking up to 5 minutes to feed. Blackflies may be present at high densities, inflicting numerous bites on their victims.
Biting Midges (Family Ceratopogonidae)
Also known as no-see-ums, sand gnats, sand fleas, and sand flies, biting midges20,39,59,79,104 are small, slender flies (<2 mm [0.1 inch] body length) with narrow wings (Figure 47-1, C). Their small size makes them difficult to see, and they can pass readily through common window screens. Biting midges occur from low to high latitudes worldwide. They breed most commonly in salt marshes, but larvae also develop in moist organic matter associated with freshwater wetlands and irrigated pastures. Despite their inconspicuous size, female midges are aggressive biters, frequently attacking in swarms and inflicting multiple painful and pruritic bites within minutes. Midges often crawl into the hair before biting. Depending on the species, midges may bite during the day or at nighttime. Their activity is greatest during calm weather, declining as wind speed increases. They may be avoided by moving to open areas with greater airflow or into shelters with no-see-um netting. Biting midges are not known to transmit diseases to humans in temperate regions but vector filarial and microbial parasites in tropical regions.
Tabanids (Family Tabanidae)
The family Tabanidae (Figure 47-1, D) includes horseflies, deerflies, greenheads, and yellow flies.20,39,59,79,104 These insects are relatively large (10 to 20 mm [0.4 to 0.8 inch]) robust fliers, with numerous species worldwide. Tabanids breed in aquatic or semiaquatic environments, with a life cycle of over 1 year. They are able to fly for miles and rely primarily on vision to locate a host by movement. Dark-colored clothing may increase the likelihood of being bitten. These flies are most active on warm, overcast days. Only the females bite, using scissor-like mouthparts to create within the skin a bleeding slash, which is slow to heal. Despite their size, these flies usually bite painlessly, but the resulting reaction can include intense itching, secondary infection, and, rarely, systemic reactions, such as urticaria or anaphylaxis. Because the adult fly usually lives only about 1 month, and only one generation emerges per year, the potential season for being bitten is relatively short in higher latitudes and altitudes. In the United States, deerflies have been shown to be capable of transmitting tularemia to humans; in Africa, the deerfly may vector the filarial parasitic worm, Loa loa.
Sand Flies (Family Psychodidae)
Sand flies* are tiny (2 to 3 mm [0.1 inch]), hairy, and long-legged flies, with multisegmented antennae and a characteristic V-shape to the wings when at rest. (Figure 47-1, E ). Only female sand flies are blood feeders, feeding mostly during calm, windless nights and resting during the day in animal burrows, tree holes, or caves, which should be avoided. Larvae develop in moist organic matter within such habitats. Most sand fly bites occur on the face and neck, but all exposed skin may be attacked.
Tsetse Flies (Family Glossinidae)
Tsetse flies20,39,59,79,104 are found only in tropical Africa. They are 7 to 14 mm (0.3 to 0.6 inch) long, yellowish-brown, with wings that fold over their backs, giving them the appearance of honeybees at rest (Figure 47-1, F ). Both sexes bite, feeding in daytime on a wide variety of mammals, including humans. Light-colored, thickly-woven, loose-fitting clothing may deter biting. Tsetse flies seem to rely primarily on vision and movement to identify their hosts. Their bites are painful and may cause petechiae or pruritic wheals. Tsetse flies vector African trypanosomiasis (sleeping sickness).
Stable Flies (Family Muscidae)
Stable flies59,79 resemble common houseflies, and are most often encountered in coastal areas. Unlike a housefly, which rests with its body parallel to the surface, a stable fly rests with its head held higher than its posterior (Figure 47-1, G). Both male and female stable flies are vicious daytime biters, requiring a blood meal every 48 hours in order to survive. If disturbed, they will attempt to feed multiple times, preferring to bite the lower extremities. Horses and cattle are the preferred hosts, but hungry stable flies readily bite humans. These flies have knife-like mouthparts that they use to puncture flesh before pumping up the blood. Stable flies breed in decaying vegetation (e.g., wet hay bales, beach seaweed) and herbivore manure and are frequently found congregated on sunny walls. Moving away from these breeding habitats will reduce the likelihood of being bitten. Stable fly bites are generally self-limited. They are not known to transmit disease to humans.
Kissing Bugs (Family Reduviidae)
Kissing or Chagas bugs* (triatomine or assassin bugs) are large (10 to 30 mm [0.4 to 1.2 inches] adult length) insects with cone-shaped heads, overlapping wings, and an alternating pattern of dull orange and dark brown stripes on the lateral abdomen (Figure 47-1, H ). Kissing bugs get their name from a tendency to bite around the human mouth, but they also bite other parts of the body. Both male and female kissing bugs bite, requiring a blood meal in order to mature through each of five nymphal growth stages. They are nocturnal feeders, attracted to their hosts by warmth, carbon dioxide, and odor. During the day, they rest in trees or indoors in crevices of house walls and ceilings. Kissing bug bites are initially painless, but frequent exposure to the bites can produce erythema, edema, and pruritus at the bite sites. Kissing bugs are the vector for Trypanosoma cruzi, the causative agent of Chagas’ disease, which has been reported in Central and South America, as well as in the southwestern United States. Chagas’ disease symptoms are more severe with long-term repeated inoculations and are also more harmful in South America than farther north. Kissing bugs often feed on rodents that may live within thatch roofing material. Avoiding sleeping under thatch roofing in Chagas-endemic areas may reduce the probability of infection.
Fleas (Family Pulicidae)
Adult fleas20,39,59,79,104 are small (2 to 6 mm [0.1 to 0.2 inch]), wingless insects with powerful legs that enable adults to jump distances of up to 30 cm (11.9 inches) (Figure 47-1, I ). Hungry adult fleas of both sexes feed on humans and other warm-blooded mammals and birds. Different flea species are associated with specific hosts, and they may be especially abundant around rodent and bird nests, which serve as both adult and larval habitats and should be avoided. Fleas usually move actively on the host, probing and biting several times, resulting in grouped lesions of pruritic papules. Fleas are capable of transmitting sylvatic plague and murine typhus.
Chigger Mites (Family Trombiculidae)
Trombiculid mites20,39,59,79,104 (Figure 47-1, J ) may be found worldwide. Commonly known as chiggers, redbugs, or harvest mites, these reddish-yellow insects are readily encountered in damp, grassy, and wooded areas, especially along the margins of forests, where they may number in the thousands. Only the tiny (less than 0.2 mm [0.1 inch]) larval stages are parasitic, feeding on mammals, birds, reptiles, and amphibians. Chiggers are most active in the summer and early autumn. They usually infest humans by crawling up the shoes and legs, preferring to attach to skin at areas where the clothing fits tightly, such as at the tops of socks or around the elastic edges of underwear. Chiggers do not burrow into the skin or actively suck blood. Rather, they pierce skin with their mouthparts and secrete a proteolytic salivary fluid that dissolves host tissue, which they in turn suck up. If undisturbed, chiggers may feed for several days, before dropping off. In humans, this rarely occurs, because the larvae usually cause enough irritation that they are dislodged by scratching. The host response to chigger bites is brisk, often leading to intensely pruritic, bright-red 1- to 2-cm (0.4- to 0.8-inch) nodules.
Ticks (Families Ixodidae and Argasidae)
Ticks,* like mites, are arachnids rather than insects. There are hard ticks (family Ixodidae) and soft ticks (family Argasidae) (Figure 47-1, K and L). Hard ticks are so named because of the presence of a sclerotized plate, or scutum, that covers part of the body. Both types of ticks may be found worldwide, but hard ticks are more commonly encountered in North America. Hard ticks are usually found in weedy or shrubby areas, along trails, and at forest boundaries, where mammalian hosts, such as deer, are plentiful. Soft ticks are more resistant to desiccation than are hard ticks. Soft ticks thrive in hot and dry climates and are commonly found in animal burrows or caves.
People in suspected tick habitats should check clothing frequently for the presence of ticks. If multiple ticks are seen on clothing, they are most easily removed by permanently trapping them on a piece of cellophane duct tape or by rolling a sticky tape type of lint remover across them; hundreds of small ticks can be easily removed by this method. Tape can be stuck onto the inner thigh area of pants for storage between episodes of tick removal. Laundering infested garments cannot be relied on to kill nymphs, unless the clothing is subjected to the hot cycle of the dryer.21
Attached ticks are more difficult to remove. Tick mouthparts are barbed, and some species of tick also secrete a cement that firmly anchors the tick into the skin. Erythema, pruritus, and edema are commonly seen at the site of a tick bite. Improper partial removal of the mouthparts may initiate a long-lasting foreign body reaction, leading to secondarily infected lesions that are slow to heal, or granuloma formation that may persist for months. (For a discussion of the best method for tick removal, see Chapter 51.)
After the tick is removed, the bite site should be cleansed with soap and water, or an antiseptic, and hands should be washed. It may be prudent to save the tick, in case later identification becomes necessary. Laboratory studies of Borrelia burgdorferi (Lyme disease)–infected ticks show that duration of attachment is directly correlated with the risk for transmission of the spirochete.* Prompt removal of attached ticks (ideally within 24 hours of attachment) will greatly reduce the likelihood of disease transmission.125
In the United States, soft ticks of the single genus Ornithodoros are capable of transmitting to humans the Borrelia spirochete that causes relapsing fever. Three genera of hard ticks transmit disease to man: Ixodes (which vectors Lyme disease, babesiosis, tick paralysis, and Russian spring–summer encephalitis), Dermacentor (vectors tularemia, Rocky Mountain spotted fever, ehrlichiosis, Colorado tick fever, and tick paralysis), and Amblyomma (vectors tularemia, ehrlichiosis, and tick paralysis).107,155 Larval, nymph, and adult ticks may all transmit disease during feeding. Transovarial transmission also enables female ticks to directly infect their offspring.
Personal Protection
Physical Protection
Physical barriers can be extremely effective in preventing insect bites, by blocking arthropods’ access to the skin. Long-sleeved shirts, socks, long pants, and a hat will protect all but the face, neck, and hands. Tucking pants into the socks or boots makes it much more difficult for ticks or chigger mites to gain access to the skin. Rubber boots are commonly worn, particularly in tropical areas, to reduce chigger and tick contact while walking and hiking. Light-colored clothing is preferable, because it makes it easier to spot ticks and is less attractive to mosquitoes and biting flies. Ticks will find it more difficult to cling to smooth, closely woven fabrics (e.g., nylon).146 Loose-fitting clothing, made out of tightly woven fabric, with a tucked-in T-shirt undergarment is particularly effective at reducing bites on the upper body. A light-colored, full-brimmed hat will protect the head and neck. Deerflies tend to land on the hat instead of the head; blackflies and biting midges are less likely to crawl to the shaded skin beneath a hat brim.
Mesh overgarments, or garments made of tightly woven material, are available to protect against insect bites. Head nets, hooded jackets, pants, and mittens are available from a number of manufacturers, in a wide range of sizes and styles (Box 47-2). Mesh garments are usually made of either polyester or nylon and, depending on the manufacturer, are available in either white or dark colors. With a mesh size of less than 0.3 mm (0.01 inch), many of these garments are woven tightly enough to exclude even biting midges and immature ticks. As with any clothing, bending or crouching may still pull the garments close enough to the skin surface to enable insects to bite through. Shannon Outdoors addresses this potential problem with a double-layered mesh that reportedly prevents mosquito penetration. Similarly, Outdoor Research manufactures head nets with a lightweight spring-steel hoop positioned to prevent the netting from collapsing against the face. Although mesh garments are effective barriers against insects, some people may find them uncomfortable during vigorous activity or in hot weather.
BOX 47-2 Manufacturers of Protective Clothing, Protective Shelters, and Insect Nets
Lightweight insect nets and mesh shelters are available to protect travelers, sleeping indoors or in the wilderness (Figure 47-2). The effectiveness of insect nets or shelters may be enhanced by treating them with a permethrin-based contact insecticide (discussed below), which can provide weeks of efficacy following a single application.
Repellents
For many people, applying a topical insect repellent may be the most effective and easiest way to prevent arthropod bites. The quest to develop the “perfect” insect repellent has been an ongoing scientific goal for years and has yet to be achieved. The ideal agent would repel multiple species of biting arthropods, remain effective for at least 8 hours, cause no irritation to skin or mucous membranes, possess no systemic toxicity, be resistant to abrasion and washing off, and be greaseless and odorless. No presently available insect repellent meets all of these criteria. Efforts to find such a compound have been hampered by the multiplicity of variables that affect the inherent repellency of any chemical. Repellents do not all share a single mode of action, and different species of insects may react differently to the same repellent.133
Many chemicals that are effective repellents evaporate or absorb into the skin too quickly to be of great usefulness. To be effective as an insect repellent, a chemical must be volatile enough to maintain an effective repellent vapor concentration at the skin surface but not evaporate so rapidly that it quickly loses its effectiveness. Multiple factors play a role in effectiveness, including concentration, frequency and uniformity of application, the user’s activity level and overall attractiveness to blood-sucking arthropods, interaction between the individual user and the repellent formulation, and the number and species of the organisms trying to bite. The effectiveness of any repellent is reduced by abrasion from clothing; evaporation and absorption from the skin surface; being washed off by sweat, rain, or water; physical activity; and a windy environment.* Each 10° C (18° F) increase in ambient temperature can lead to as much as 50% reduction in protection time.83 Recent formulation developments improve the duration of effectiveness and the cosmetic properties of repellent active ingredients, such that a well-formulated mediocre active ingredient may outperform a poorly formulated stronger active ingredient. Insect repellents do not “cloak” the user in a chemical veil of protection; any untreated exposed skin can be readily bitten.102 Individuals not wearing insect repellent may be bitten with increased frequency when standing next to someone who is wearing insect repellent.111
Chemical Repellents
DEET
DEET has been the gold standard of insect repellents for many decades. Only in the past 5 years have other repellents come to market that show comparable broad-spectrum efficacy (discussed later). DEET has been registered for use by the general public since 1957. It is effective against many species of crawling and flying insects, including mosquitoes, biting flies, midges, chiggers, fleas, and ticks. The U.S. Environmental Protection Agency (EPA) estimates that about 30% of the U.S. population uses a DEET-based product every year; worldwide use exceeds 200 million people annually.167,168 Decades of empirical testing of more than 20,000 other compounds has not yet led to the release of a clearly superior repellent.* The mechanism of action of DEET is only partially understood. One recent study demonstrated that DEET blocks a specific olfactory sensory neuron in mosquitoes, effectively masking odors that would normally attract the mosquito to a host.40 Another study, in contrast, found that a single olfactory neuron in mosquitoes responds to DEET and induces avoidance behaviors.159
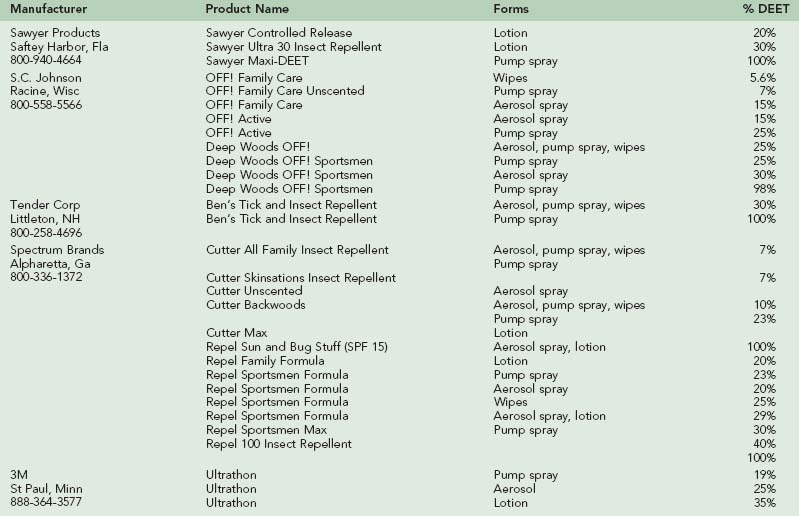
In the United States, DEET is sold in concentrations from 5% to 100%, in various formulations, including lotions, solutions, sprays, and impregnated wipes (Table 47-1). As a general rule, higher concentrations of DEET will provide longer-lasting protection. Duration of efficacy of DEET increases only slowly above 35% concentrations, and the longest-lasting (extended duration) formulations all use concentrations no higher than 35%. Higher-concentration and extended-duration DEET formulations are probably best reserved for circumstances in which the wearer will be in an environment with a very high density of insects (e.g., tundra in early summer, or salt marsh), where there is a high risk for disease transmission from insect bites, or under circumstances where there may be rapid loss of repellent from the skin surface, such as under conditions of high temperature and humidity or rain. Under these circumstances, reapplication of the repellent will likely be necessary to maintain its effectiveness.
For most uses, however, there is no need to use the highest concentrations of DEET. Most manufacturers, responding to consumer demand, offer a large variety of low-concentration DEET products. Persons averse to applying DEET directly to their skin may get long-lasting repellency by applying DEET only to their clothing. DEET-treated garments, stored in a plastic bag between wearings, maintain their repellency for several weeks.32
Sequential application of a DEET-based repellent and a sunscreen can reduce the efficacy of the sunscreen. In a study of 14 patients who applied a 33% DEET repellent, followed by a sun protection factor (SPF) 15 sunscreen, the sunscreen’s SPF was decreased by a mean of 33%.109 One study showed that the efficacy of a polymer-based DEET repellent was not decreased by the subsequent application of sunscreen 5 minutes later.115 Another study, however, showed that reapplication of sunscreen 2 hours after DEET repellent was applied significantly decreased the protection time of the DEET repellent.172 There has been some concern that DEET and the sunscreen oxybenzone act synergistically to enhance the percutaneous absorption of the other chemical.77 Combination sunscreen-DEET products are available and will deliver the SPF as stated on the label. However, these products are generally not the best choice, because it is rare that the need for reapplication of sunscreen and repellent is exactly the same. For these reasons, in 2000 Health Canada decided to discontinue approval of combination sunscreen and insect repellent products, pending further safety data. Likewise, the EPA in 2007 also issued a request for information regarding proper regulation of combination products, given the conflicting issues regarding proper application frequency.165
3M and Sawyer Products currently manufacturer extended-release formulations of DEET that make it possible to deliver long-lasting protection without relying on high concentrations of DEET. 3M’s product, Ultrathon, was originally developed for the U.S. military but is also available to the general public. This acrylate polymer 35% DEET formulation, when tested under multiple different environmental/climatic field conditions, was as effective as 75% DEET in ethanol, providing up to 12 hours of greater than 95% protection against mosquito bites.* Sawyer Products’ controlled-release 20% DEET lotion traps the chemical in a protein particle that slowly releases it to the skin surface, providing repellency equivalent to a standard 50% DEET preparation, lasting about 5 hours.46 Sixty percent less of this encapsulated DEET is absorbed when compared with a 20% ethanol-based preparation of DEET.45 In vitro experiments confirm that microencapsulation of DEET can reduce its cutaneous absorption.78 Sawyer Products also markets a controlled-release 30% DEET lotion in which the chemical is slowly released from a lipid sphere (liposome). This nongreasy formulation has low odor and can provide more than 10 hours of protection. Liposomal preparations of DEET can help to retain potency of the repellent on the skin surface, while minimizing percutaneous absorption.134
Given its use by millions of people worldwide for 50 years, DEET continues to show a remarkable safety profile. In 1980, as part of the EPA registration standard for DEET,168 over 30 additional animal studies were conducted to assess acute, chronic, and subchronic toxicities; mutagenicity; oncogenicity; and developmental, reproductive, and neurologic toxicities. The results of these studies neither led to any product changes to comply with current EPA safety standards nor indicated any new toxicities under normal usage. The EPA’s reregistration eligibility decision,167 released in 1998, confirmed that the agency believes that “normal use of DEET does not present a health concern to the general U.S. population.”
Case reports of potential DEET toxicity have been extensively reviewed.8,50,119,157,169 Fewer than 50 cases of significant toxicity from DEET exposure have been documented in the medical literature over the last four decades; over three-quarters of these resolved without sequelae. Many of these cases involved long-term, excessive, or inappropriate use of DEET repellents; the details of exposure were frequently poorly documented, making causal relationships difficult to establish. These cases have not shown a correlation between concentration of the DEET product used and the risk for toxicity.
The reports of DEET toxicity that raise the greatest concern involve 18 cases of encephalopathy, 14 in children under age 8 years.* Three of these children died, one of whom had ornithine carbamoyl transferase deficiency, which might have predisposed her to DEET-induced toxicity.69,70 The 11 surviving children recovered without sequelae. The EPA’s analyses of these cases concluded that they “do not support a direct link between exposure to DEET and seizure incidence.”167 Animal studies in rats and mice show that DEET is not a selective neurotoxin.117,136,168 Even if a link between DEET use and seizures does exist, the observed risk, based on DEET use patterns, would be less than 1 per 100 million users.167 Other studies have confirmed that children are not at greater risk for developing adverse effects from DEET when compared with older individuals.3,8,88,169
A review of adverse events reported to the DEET Registry from 1995 to 2001 concluded that individuals with underlying neurologic disorders were not predisposed to a greater risk for DEET toxicity. There was no evidence that using higher concentrations of DEET increases the risk for adverse events.119
Very limited studies have investigated DEET safety during pregnancy. One followed 450 Thai women who used 20% DEET daily during the second and third trimesters of pregnancy to reduce the risk for contracting malaria.106 Of these women, 4% had detectable levels of DEET in umbilical cord blood at the time of delivery. However, no differences in survival, growth, or neurologic development could be detected in the infants born to mothers who used DEET when compared with an equal number of mothers treated with a daily placebo cream during their pregnancies.
The EPA has issued guidelines to ensure safe use of DEET-based repellents167 (Box 47-3). Careful product choice and common-sense application will greatly reduce the possibility of toxicity. Current recommendations of the American Academy of Pediatrics are that children over the age of 2 months can safely use up to 30% DEET.173 When required, reapplication of a low-strength repellent can compensate for their inherent shorter duration of protection.
BOX 47-3
Guidelines for Safe and Effective Use of DEET Insect Repellents
Modified from U.S. Environmental Protection Agency, Office of Pesticide Programs, Prevention, Pesticides and Toxic Substances Division: Reregistration Eligibility Decision (RED): DEET, (EPA-738-F-95-010), Washington, DC, 1998, EPA.
Questions regarding the safety of DEET may be addressed to the EPA-sponsored National Pesticide Information Center, available every day from 6:30 AM to 4:30 PM PST at 800-858-7378 or via their website at http://npic.orst.edu/.
Picaridin
Picaridin is a synthetic repellent developed by the Bayer Corporation using molecular modeling techniques. From more than 800 screened substances, KBR 3023 showed the best repellent efficacy against a variety of arthropods,16 along with a very favorable safety profile, low percutaneous absorption, and good aesthetic qualities.135
In 2005, the first picaridin-based repellent was brought to the market in the United States. It is currently available in concentrations ranging from 5% to 20% (Table 47-2). This nearly odorless, nongreasy repellent is effective against mosquitoes, biting flies, and ticks. The 7% repellent provides protection against mosquito bites for up to 4 hours. At 20%, picaridin repellents offers comparable efficacy with DEET against mosquitoes, giving up to 8 or more hours of protection.* Picaridin is superior to DEET as a tick repellent.12 The chemical is aesthetically pleasant and, unlike DEET, shows no detrimental effects on contact with plastics. The EPA found picaridin to have a very low toxicity risk, with low dermal absorption. In 2005, the Centers for Disease Control and Prevention (CDC) released a statement adding picaridin to the list of approved repellents that could be used effectively to prevent mosquito-borne diseases.
IR3535 (ethyl-butylacetylaminoproprionate)
IR3535 is an analog of the amino acid β-alanine and has been sold in Europe as an insect repellent for 20 years. In the United States, this compound is classified by the EPA as a biopesticide, effective against mosquitoes, ticks, and flies. IR3535 was brought to the U.S. market in 1999 and is sold by Avon Products, Inc and Sawyer Products in concentrations from 7.5% to 20%, with and without sunscreen (see Table 47-2). Depending on the species of mosquito and the testing methodology, this repellent has demonstrated widely variable effectiveness, with complete protection times ranging from 23 minutes to over 10 hours.6,7,23,27,51 IR3535 can provide up to 12 hours of protection against black-legged ticks.23 Higher concentrations of IR3535 give longer protection times. In general, IR3535 does not match the efficacy of high-concentration DEET.6,7,51,52 IR3535 is most effective at 20% concentrations. It is nongreasy, nearly odorless, will not dissolve plastics, and has a very good safety profile. In 2008, the CDC released a statement adding IR3535 to the list of approved repellents that could be used effectively to prevent mosquito-borne diseases.
Botanical Repellents
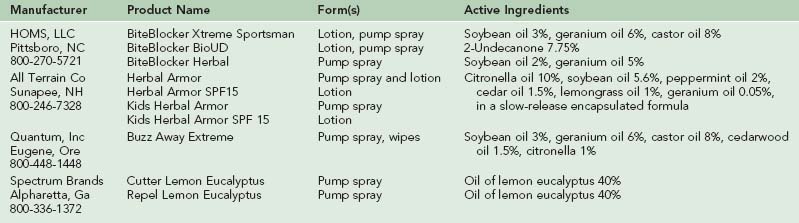
Thousands of plants have been tested as sources of insect repellents. Although none of the plant-derived chemicals tested to date demonstrates the broad effectiveness and duration of DEET, many show repellent activity and some may be superior against certain important vectors such as anopheline mosquitoes. Plants with essential oils that have been reported to possess repellent activity include citronella, eucalyptus, neem, cedar, verbena, pennyroyal, geranium, catnip, lavender, pine, cajeput, cinnamon, vanilla, rosemary, basil, thyme, allspice, garlic, and peppermint.* Unlike DEET-based repellents, botanical repellents have been relatively poorly studied. When tested, most of these essential oils tended to show short-lasting protection, lasting minutes to 2 hours, after which insufficient material remains on the skin to be repellent. A summary of readily available plant-derived insect repellents is shown in Table 47-3.
Citronella
Conflicting data exist on the efficacy of citronella-based products, varying greatly depending on the study methodology, location, and species of biting insect tested. One citronella-based repellent was found to provide no repellency when tested in the laboratory against Aedes aegypti mosquitoes.26 Another study of the same product, however, conducted in the field, showed an average 88% repellency over a 2-hour exposure. The product’s effectiveness was greatest within the first 40 minutes after application and then decreased with time over the remainder of the test period.158 All Terrain Company produces citronella-based lotion in which the essential oil has been encapsulated into a beeswax matrix, which slowly releases it to the skin surface, prolonging its efficacy. In laboratory testing against A. aegypti, this product provided complete protection for the first 2 hours and 77% protection 4 hours after application.67
Many citronella repellents on the market incorporate geranium oil and/or soybean oil to increase the repellent effect of the product. In general, studies show that citronella-based repellents are less effective than DEET repellents. Citronella provides a shorter protection time, which may be partially overcome by frequent reapplication of the repellent. In 1997, after analyzing available data on the repellent effect of citronella, the EPA concluded that citronella-based insect repellents must contain the following statement on their labels: “for maximum repellent effectiveness of this product, repeat applications at one-hour intervals.”166
Citronella candles have been promoted as an effective way to repel mosquitoes from one’s local environment. One study compared the efficacy of commercially available 3% citronella candles, 5% citronella incense, and plain candles to prevent bites by Aedes species mosquitoes under field conditions.97 Subjects near the citronella candles had 42% fewer bites than did controls who had no protection (a statistically significant difference). However, burning ordinary candles reduced the number of bites by 23%. There was no difference in efficacy between citronella incense and plain candles. The ability of plain candles to decrease biting may be due to their serving as a “decoy” source of warmth and carbon dioxide. In contrast, in a field study, candles with 5% geraniol placed 1 m from volunteers reduced the mosquito biting pressure by an average of 56% and the sand fly pressure by 62%, compared with paraffin control candles.114 These efficacies are, unfortunately, far too low to offer useful protection from mosquito-borne diseases.
The citrosa plant (Pelargonium citrosum “Van Leenii”) has been marketed as being able to repel mosquitoes through the continuous release of citronella oils. Unfortunately, when tested, these plants offer no protection against bites.28,105 In experimental hut trials in Africa, potted live plants of Ocimum americanum, Lantana camara, and Lippia uckambensis repelled an average of 40%, 32%, and 33% of mosquitoes, respectively.147
BiteBlocker
BiteBlocker is a “natural” repellent that was released to the United States market in 1997, after being sold in Europe for several years.163 BiteBlocker’s present formulation combines derivatives of soybean oil, geranium oil, and castor oil. Studies conducted at the University of Guelph showed that this product was capable of providing over 97% protection against Aedes species mosquitoes under field conditions, even 3.5 hours after application. During the same time period, a 6.65% DEET-based spray afforded 86% protection, whereas Avon’s Skin-So-Soft citronella-based repellent gave only 40% protection.94 A second study showed that BiteBlocker provided a mean of 200 ± 30 (SD) minutes of complete protection from mosquito bites.95 A laboratory study using three different species of mosquitoes showed that BiteBlocker provided an average protection time of about 7 hours.7 Another study showed that BiteBlocker could give about 10 hours of protection against biting black flies; in the same test, 20% DEET only protected for about 6.5 hours.96
BioUD (2-Undecanone)
HOMS is the sole distributor in the United States of another repellent, BioUD (2-Undecanone). This repellent was derived from the wild tomato plant and registered by the EPA in 2007 as a biopesticide for use against mosquitoes and ticks. In field studies against mosquitoes, 7.75% BioUD provided repellency comparable with 25% DEET.13 BioUD repelled the American dog tick Dermacentor variabilis from human skin for over 2.5 hours and was still effective 8 days after application to cotton fabric.13 Laboratory testing demonstrated that BioUD was two to four times more effective than 98% DEET at repelling Amblyomma americanum, D. variabilis, and Ixodes scapularis.11 BioUD was significantly better than either IR3535 or PMD (see below) at repelling A. americanum.14
Lemon Eucalyptus
A derivative (p-menthane-3,8-diol, or PMD) isolated from oil of the lemon eucalyptus (or pine) plant has a strong lemony scent and has shown promise as an effective “natural” repellent.33 PMD evaporates more slowly from the skin surface than do molecularly similar botanical insect repellents like citronella, and it offers longer-lasting protection. PMD has been very popular in China for years and is currently sold in Europe as Mosi-Guard and in the United States as Repel Lemon Eucalyptus Insect Repellent and Cutter Lemon Eucalyptus Insect Repellent. Tests of this repellent have shown mean complete protection times ranging from 4 to 8 hours, depending on the mosquito species and testing methodology used.* Several studies suggest that PMD-based repellents are superior to DEET against malaria-vectoring Anopheles mosquitoes.22 PMD-based repellents can cause significant ocular irritation, so care must be taken to keep them away from the eyes and that they are not used in children younger than 3 years. In 2005, the CDC added this repellent to the approved list of products that can be effectively used to prevent mosquito-borne disease.
Efficacy of DEET Versus Botanical Repellents
Studies comparing “natural” repellents to low-strength DEET products, conducted under carefully controlled laboratory conditions with caged mosquitoes, typically demonstrate dramatic differences in effectiveness between currently marketed insect repellents.7,51 Citronella-based insect repellents usually provide the shortest-duration protection, often lasting only a few minutes. Low-concentration DEET lotions (under 7%) typically prove to be more effective than citronella-based repellents in their ability to prevent mosquito bites and can generally be expected to provide about 1.5 to 2 hours of complete protection.51,68 Reapplication of these low-concentration DEET products can compensate for their shorter durations of action. Because DEET repellents show a clear dose–response relationship, higher concentrations of DEET can be used to provide proportionately longer complete protection times—up to 6 to 8 hours after a single application. BiteBlocker and oil of eucalyptus repellents offer the best protection of the “botanical” repellents, but some consumers may object to their odor; the aesthetic qualities of IR3535 and picaridin may be better choices for those sensitive individuals. BioUD also shows promise as an effective alternative to DEET in repelling both mosquitoes and ticks.
Alternative Repellents
There has always been great interest in finding an oral insect repellent. Oral repellents would be convenient and would eliminate the need to apply creams to the skin or put on protective clothing. Unfortunately, no effective oral repellent has been discovered. For decades, lay literature has made the claim that Vitamin B1 (thiamine) works as a systemic mosquito repellent. When subjected to scientific scrutiny, however, thiamine has unanimously been found not to have a repellent effect on mosquitoes.84,174 The U.S. Food and Drug Administration (FDA), prompted by misleading consumer advertising, issued the following statement in 1983: “There is a lack of adequate data to establish the effectiveness of thiamine or any other ingredient for OTC [over the counter] internal use as an insect repellent. Labeling claims for OTC orally administered insect repellent drug products are either false, misleading, or unsupported by scientific data.”47 Tests of over 100 ingested drugs, including other vitamins, failed to reveal any that worked well against mosquitoes.156 Ingested garlic has also never proved to be an effective insect deterrent.131
Insecticides
Permethrin
Pyrethrum is a powerful, rapidly acting insecticide, originally derived from the crushed dried flowers of the daisy Chrysanthemum cinerariifolium.25 Permethrin is a synthetic pyrethroid. It does not repel insects but instead works as a contact insecticide, causing nervous system toxicity, leading to death, or “knockdown,” of the insect. The chemical is effective against mosquitoes, flies, ticks, fleas, lice, and chiggers. Permethrin has low mammalian toxicity, is poorly absorbed by the skin, and is rapidly metabolized by skin and blood esterases.71,176
Permethrin should be applied directly to clothing or to other fabrics (tent walls138 or mosquito nets98), not to skin. Permethrins are nonstaining, nearly odorless, and resistant to degradation by heat or sun, and they will maintain their effectiveness for at least 2 weeks, through several launderings.140,145
The combination of permethrin-treated clothing and skin application of a DEET-based repellent creates a formidable barrier against biting insects.64,86,150 In an Alaskan field trial against mosquitoes, subjects wearing permethrin-treated uniforms and a polymer-based 35% DEET product had greater than 99.9% protection (one bite per hour) over 8 hours; unprotected subjects sustained an average of 1188 bites per hour.93
Permethrin-sprayed clothing also proved very effective against ticks.12 One hundred percent of Dermacentor occidentalis ticks (which carry Rocky Mountain spotted fever) died within 3 hours of touching permethrin-treated cloth.90 Permethrin-sprayed pants and jackets also provided 100% protection from all three life stages of Ixodes dammini ticks, the vector of Lyme disease.146 In contrast, DEET alone (applied to the skin), provided 85% repellency at the time of application; this protection deteriorated to 55% repellency at 6 hours, when tested against the lone star tick A. americanum.153 Ixodes scapularis ticks, which may transmit Lyme disease, also seem to be less sensitive to the repellent effect of DEET.141
Permethrin-based insecticides available in the United States are listed in Table 47-4. To apply to clothing, spray each side of the fabric (outdoors) for 30 to 45 seconds, just enough to moisten it. Allow to dry for 2 to 4 hours before wearing it. Permethrin solution is also available for soak-treating large items, such as mesh bed nets, or for treating multiple garments simultaneously.
Reducing Local Mosquito Populations
Consumers may still find advertisements for small ultrasonic electronic devices that are meant to be carried on the body and claim to repulse mosquitoes by emitting “repellent” sounds, such as that of a dragonfly (claimed to be the “natural enemy” of the mosquito), male mosquito, or bat. Multiple studies, conducted both in the field and laboratory, show that these devices do not work.9,31,49,73,92 Although most studies have shown that DEET-impregnated wristbands offered no protection against mosquito bites,51,73 in one study wearing impregnated anklets, wristbands, shoulder strips, and pocket strips provided up to 5 hours of complete protection.76
Likewise, mass-marketed backyard bug “zappers,” which use ultraviolet light to lure and electrocute insects, are also ineffective: mosquitoes continue to be more attracted to humans than to the devices.116 One backyard study showed that of the insects killed by these devices, only 0.13% were female (biting) mosquitoes.54An estimated 71 to 350 billion beneficial insects may be killed annually in the United States by these devices.54 Newer technology, using more specific bait, such as a warm, moist plume of carbon dioxide, as well as other known chemical attractants (e.g., octenol), may prove to be a more successful way to lure and selectively kill biting insects. Although the manufacturers of these machines accurately claim that these machines can lure and kill thousands of mosquitoes, it remains to be proven that an individual unit can actually kill enough mosquitoes to reduce the local biting pressures. Pyrethrin-containing “yard foggers” set off before an outdoor event can temporarily reduce the number of biting arthropods in a local environment. These products should be applied before any food is brought outside and should be kept away from animals or fish ponds. Burning coils that contain natural pyrethrins or synthetic pyrethroids (such as D-allethrin or D–trans allethrin) can also temporarily reduce local populations of biting insects.73,100,175 Concerns have been raised about the long-term cumulative safety of using these coils in an indoor environment.1,121
Wood smoke from campfires can also reduce the likelihood of being bitten by mosquitoes. The smoke’s ability to repel insects may vary depending on the type of wood or vegetation burned.120,171
Integrated Approach to Personal Protection
Persons traveling to parts of the world where insect-borne disease is a potential threat will be best able to protect themselves if they learn about indigenous insects and the diseases they might transmit. Protective clothing, mesh insect tents or bedding, insect repellent, and permethrin spray should be carried. Travelers should check the most current CDC recommendations about traveling to countries where immunizations (e.g., against yellow fever) or antibiotic prophylaxis (e.g., against malaria) should be undertaken before departure. The CDC maintains these recommendations on its website at http://www.cdc.gov/travel/index.htm, or by telephone at 888-232-3228. An excellent summary of information on issues relating to travel health can also be found at http://www.tripprep.com. This website culls its information daily from the CDC, the Morbidity and Mortality Weekly Report, World Health Organization, and the U.S. State Department.
1 Achmadi UF, Pauluhn J. Household insecticides: Evaluation and assessment of inhalation toxicity: A workshop summary. Exp Toxicol Pathol. 1998;50:67.
2 Annis B. Comparison of the effectiveness of two formulations of DEET against Anopheles flavirostris. J Am Mosq Control Assoc. 1990;6:430.
3 Antwi FB, Shama LM, Peterson RK. Risk assessments for the insect repellents DEET and picaridin. Regul Toxicol Pharmacol. 2008;51:31.
4 Badolo A, Ilboudo-Sanogo E, Ouedraogo AP, et al. Evaluation of the sensitivity of Aedes aegypti and Anopheles gambiae complex mosquitoes to two insect repellents: DEET and KBR 3023. Trop Med Int Health. 2004;9:330.
5 Barnard DR. Repellency of essential oils to mosquito (Diptera: Culicidae). J Med Entomol. 1999;36:625.
6 Barnard DR, Bernier UR, Posey KH, et al. Repellency of IR3535, KBR3023, para-menthane-3,8-diol, and DEET to black salt marsh mosquitoes (Diptera: Culicidae) in the Everglades National Park. J Med Entomol. 2002;39:895.
7 Barnard DR, Xue RD. Laboratory evaluation of mosquito repellents against Aedes albopictus, Culex nigripalpus, and Ochlerotatus triseriatus (Diptera: Culicidae). J Med Entomol. 2004;41:726.
8 Bell JW, Veltri JC, Page BC. Human exposures to N,N-diethyl-m-toluamide insect repellents reported to the American Association of Poison Control Centers 1993-1997. Int J Toxicol. 2002;21:347.
9 Belton P. An acoustic evaluation of electronic mosquito repellers. Mosq News. 1981;41:751.
10 Berger BW, Johnson RC, Kodner C, et al. Cultivation of Borellia burgdorferi from human tick bite sites: A guide to the risk of infection. J Am Acad Dermatol. 1995;32:184.
11 Bissinger BW, Apperson CS, Sonenshine DE, et al. Efficacy of the new repellent BioUD against three species of ixodid ticks. Exp Appl Acarol. 2009;48:239.
12 Bissinger BW, Roe RM. Tick repellents: Past, present, and future. Pestic Biochem Physiol. 2010;96:63.
13 Bissinger BW, Stumpf CF, Donohue KV, et al. Novel arthropod repellent, BioUD, is an efficacious alternative to Deet. J Med Entomol. 2008;45:891.
14 Bissinger BW, Zhu J, Apperson CS, et al. Comparative efficiency of BioUD to other commercially available arthropod repellents against ticks Amblyomma americanum and Dermacentor variabilis on cotton cloth. Am J Trop Med Hyg. 2009;81:685.
15 Bock GR, Cardew G, editors. Olfaction in mosquito-host interactions. New York: J Wiley, 1996.
16 Boeckh J, Breer H, Geier M, et al. Acrylated 1,3-aminopropanols as repellents against bloodsucking arthropods. Pest Sci. 1996;48:359.
17 Bowen MF. The sensory physiology of host-seeking behavior in mosquitoes. Annu Rev Entomol. 1991;36:139.
18 Briassoulis G, Narlioglou M, Hatzis T. Toxic encephalopathy associated with use of DEET insect repellents: A case analysis of its toxicity in children. Hum Exp Toxicol. 2001;20:8.
19 Brown M, Hebert AA. Insect repellents: An overview. J Am Acad Dermatol. 1997;36:243.
20 Busvine JR. Disease transmission by insects. New York: Springer-Verlag; 1993.
21 Carroll JF. A cautionary note: Survival of nymphs of two species of ticks (Acari: Ixodidae) among clothes laundered in an automatic washer. J Med Entomol. 2003;40:732.
22 Carroll SP. Evaluation of topical insect repellents and factors that affect their performance. In: Debboun M, Frances SP, Strickman D, editors. Insect repellents: Principles, methods and uses. Boca Raton, Fla: CRC Press; 2007:245-259.
23 Carroll SP. Prolonged efficacy of IR3535 repellents against mosquitoes and blacklegged ticks in North America. J Med Entomol. 2008;45:706.
24 Carroll SP, Loye J. PMD, a regsitered botanical mosquito repellent with DEET-like efficacy. J Am Mosq Control Assoc. 2006;22:507.
25 Casida JE, Quistad GB. Pyrethrum flowers: Production, chemistry, toxicology and uses. Oxford, UK: Oxford University Press; 1995.
26 Chou JT, Rossignol PA, Ayres JW. Evaluation of commercial insect repellents on human skin against Aedes aegypti (Diptera: Culicidae). J Med Entomol. 1997;34:624.
27 Cilek J, Petersen J, Hallmon C. Comparative efficacy of IR3535 and DEET as repellents against adult Aedes aegypti and Culex quinquefasciatus. J Am Mosq Control Assoc. 2004;20:299.
28 Cilek JE, Schreiber ET. Failure of the “mosquito plant,” Pelargonium x Citrosum “Van Leenii,” to repel adult Aedes albopictus and Culex quinquefasciatus in Florida. J Am Mosq Control Assoc. 1994;10:473.
29 Clements AN. The physiology of mosquitoes. Oxford, UK: Pergamon Press; 1963.
30 Costantini C, Badolo A, Ilboudo-Sanogo E. Field evaluation of the efficacy and persistence of insect repellents DEET, IR3535 and KBR3023 against Anopheles gambiae complex and other Afrotropical vector mosquitoes. Trans R Soc Trop Med Hyg. 2004;98:644.
31 Curtis CF. High frequency radio keeps mosquitoes at bay. Lancet. 1998;352:992.
32 Curtis CF, Lines JD, Ijumba J, et al. The relative efficacy of repellents against mosquito vectors of disease. Med Vet Entomol. 1987;1:109.
33 Curtis CF, Lines JD, Lu Baolin L, et al. Natural and synthetic repellents. In: Curtis CF, editor. Appropriate technology in vector control. Boca Raton, Fla: CRC Press; 1990:76-89.
34 Davis EE. Insect repellents: Concepts of their mode of action relative to potential sensory mechanisms in mosquitoes (Diptera: Culicidae). J Med Entomol. 1985;22:237.
35 Davis EE, Bowen MF. Sensory physiological basis for attaction in mosquitoes. J Am Mosq Control Assoc. 1994;10:316.
36 Debboun M, Strickman D, Klein K, et al. Laboratory evaluation of AI3-37220, AI3-35765, CIC-4, and DEET repellents against three species of mosquitoes. J Am Mosq Control Assoc. 1999;15:342.
37 Debboun M, Strickman D, Solberg VB, et al. Field evaluation of DEET and a piperidine repellent against Aedes communis (Diptera: Culicidae) and Simulium venustum (Diptera: Simuliidae) in the Adirondack Mountains of New York. J Med Entomol. 2000;37:919.
38 deGarbino JP, Laborde A. Toxicity of an insect repellent: N,N-diethyltoluamide. Vet Hum Toxicol. 1983;25:422.
39 Despommier DD, Gwadz RW, Hotez PJ, et al. Parasitic diseases, ed 5. New York: Springer Verlag; 2005.
40 Ditzen M, Pellegrino M, Vosshall LB. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 2008;319:1838.
41 Duke J. USDA-Agricultural Research Service phytochemical and ethnobotanical databases. http://www.ars-grin.gov/duke/.
42 Edwards DL, Johnson E. Insect repellent induced toxic encephalopathy in a child. Clin Pharmacol. 1987;6:496.
43 Elston DM. What’s eating you? Sand flies. (Diptera: Psychodidae, Phlebotominae: Lutzomyia, Phlebotomus). Cutis. 1998;62:164.
44 Elston DM, Stockwell S. What’s eating you? Triatome reduviids. Cutis. 1999:63.
45 Feller L: Personal communication, July 20, 1999, unpublished report, Absorption rate of microencapulated DEET.
46 Feller L: Personal communication, July 20, 1999, unpublished report, Insect repellent test report, Nomad Traveller’s Store.
47 Food and Drug Administration. Drug products containing active ingredients offered over-the-counter (OTC) for oral use as insect repellents. Fed Reg. 1983;48:26987.
48 Foster WA, Hancock RG. Nectar-related olfactory and visual attractants for mosquitoes. J Am Mosq Control Assoc. 1994;10:288.
49 Foster WA, Lutes KI. Tests of ultrasonic emissions on mosquito attraction to hosts in a flight chamber. J Am Mosq Control Assoc. 1985;1:199.
50 Fradin MS. Mosquitoes and mosquito repellents: A clinician’s guide. Ann Intern Med. 1998;128:931.
51 Fradin MS, Day JF. Comparative efficacy of insect repellents. N Engl J Med. 2002;347:13.
52 Frances SP, MacKenzie DO, Rowcliffe KL, et al. Comparative field evaluation of repellent formulations containig DEET and IR3535 against mosquitoes in Queensland, Australia. J Am Mosq Control Assoc. 2009;25:511.
53 Frances SP, Van Dung N, Beebe NW, et al. Field evaluation of repellent formulations against daytime and nighttime biting mosquitoes in a tropical rainforest in northern Australia. J Med Entomol. 2002;39:547.
54 Frick TB, Tallamy DW. Density and diversity of non-target insects killed by suburban electric insect traps. Ent News. 1996;2:77.
55 Gabel ML, Spencer TS, Akers WA. Evaporation rates and protection times of mosquito repellents. Mosq News. 1976;36:147.
56 Gillies MT. The role of carbon dioxide in host-finding by mosquitoes (Diptera:Culicidae): A review. Bull Entomol Res. 1980;70:525.
57 Gillies MT, Wilkes TJ. The range of attraction of animal baits and carbon dioxide for mosquitoes: Studies in a freshwater area of West Africa. Bull Entomol Res. 1972;61:389.
58 Gjullin CM. Effect of clothing color on the rate of attack of Aedes mosquitoes. J Econ Entomol. 1947;40:326.
59 Goddard J. Physician’s guide to arthropods of medical importance, ed 4. Boca Raton, Fla: CRC Press; 2002.
60 Golenda CF, Solberg VB, Burge R, et al. Gender-related efficacy difference to an extended duration formulation of topical N,N-diethyl-m-toluamide (DEET). Am J Trop Med Hyg. 1999;60:654.
61 Govere J, Durrheim DN, Baker L, et al. Efficacy of three insect repellents against the malaria vector Anopheles arabiensis. Med Vet Entomol. 2000;14:447.
62 Grainger J, Moore C. Natural insect repellents for pets, people and plants. Austin, Tex: The Herb Bar; 1991.
63 Gupta RK, Rutledge LC. Laboratory evaluation of controlled-release repellent formulations on human volunteers under three climatic regimens. J Am Mosq Control Assoc. 1989;5:52.
64 Gupta RK, Sweeney AW, Rutledge LC, et al. Effectiveness of controlled-release personal-use arthropod repellents and permethrin-impregnated clothing in the field. J Am Mosq Control Assoc. 1987;3:556.
65 Hallem EA, Fox AN, Zwiebel LJ, et al. Olfaction: Mosquito receptor for human-sweat odorant. Nature. 2004;427:212.
66 Hampers LC, Oker E, Leikin JB. Topical use of DEET insect repellent as a cause of severe encephalopathy in a healthy adult male. Acad Emerg Med. 1999;6:1295.
67 Heal JD, Surgeoner GA. Laboratory evaluation of the efficacy of All Terrain, an essential oil-based product, to repel Aedes aegypti mosquitoes. Department of Environmental Biology. University of Guelph: sponsored by All Terrain Company; 1998. November 6
68 Health Canada. Personal insect repellents containing DEET (N,N-diethyl-m-toluamide and related compounds), http://www.hc-sc.gc.ca/pmra-arla/english/pdf/rrd/rrd2002-01-e.pdf, 2002.
69 Heick HM, Peterson RG, Dalpe-Scott M, et al. Insect repellent, N,N-diethyl-toluamide, effect on ammonia metabolism. Pediatrics. 1988;82:373.
70 Heick HM, Shipman RT, Norman MG, et al. Reye-like syndrome associated with use of insect repellent in a presumed heterozygote for ornithine carbamoyl transferase deficiency. J Pediatr. 1980;97:471.
71 Insect repellents. Med Lett Drug Ther. 1989;31:45.
72 Jacobson M, editor. Glossary of plant-derived insect deterrents. Boca Raton, Fla: CRC Press, 1990.
73 Jensen T, Lampman R, Slamecka MC, et al. Field efficacy of commercial antimosquito products in Illinois. J Am Mosq Control Assoc. 2000;16:148.
74 Kain KC, Keystone JS. Malaria in travelers. Infect Dis Clin North Am. 1998;12:267.
75 Karppinen A, Brummer-Korvenkontio H, Petman L, et al. Levocetirizine for treatment of immediate and delayed mosquito bites. Acta Derm Venereol (Stockh). 2006;86:329.
76 Karunamoorthi K, Sabesan S. Field trials on the efficacy of DEET-impregnated anklets, wristbands, shoulder, and pocket strips against vectors of disease. Parasitol Res. 2009;105:647.
77 Kasichayanula S, House JD, Wang T, et al. Percutaneous characterization of the insect repellent DET and sunscreen oxybenzone from topical skin application. Toxicol Appl Pharmacol. 2007;223:187.
78 Kasting GB, Bhatt VD, Speaker TJ. Microencapsulation decreases the skin absorption of N,N-diethyltoluamide (DEET). Toxicol in Vitro. 2008;22:548.
79 Kettle DS, editor. Medical and veterinary entomology. New York: John Wiley & Sons, 1982.
80 Keystone JS. Of bites and body odour. Lancet. 1996;347:1423.
81 Khan AA. Mosquito attractants and repellents. In: Shorey HH, McKelvey JJ, editors. Chemical control of insect behavior. New York: J Wiley; 1977:305-325.
82 Khan AA, Maibach H, Skidmore D. Addition of vanillin to mosquito repellents to increase protection time. Mosq News. 1975;35:223.
83 Khan AA, Maibach HI, Skidmore DL. A study of insect repellents: Effect of temperature on protection time. J Econ Entomol. 1972;66:437.
84 Khan AA, Maibach HI, Strauss WG, et al. Vitamin B1 is not a systemic mosquito repellent in man. Trans St Johns Hosp Dermatol Soc. 1969;55:99.
85 King WV. Chemicals evaluated as insecticides and repellents at Orlando, Fla. USDA Agricutural Handbook. 1954;69:1.
86 Kline DL, Schreck CE. Personal protection afforded by controlled-release topical repellents and permethrin-treated clothing against natural populations of Aedes taeniorhynchus. J Am Mosq Control Assoc. 1989;5:77.
87 Knols BG, de Jong R, Takken W. Trapping system for testing olfactory responses of the malarial mosquito Anopheles gambia in a wind tunnel. Med Vet Entomol. 1994;8:386.
88 Koren G, Matsui D, Bailey B. DEET-based insect repellents: Safety implications for children and pregnant and lactating women. CMAJ. 2003;169:209.
89 Lacroix R, Mukabana WR, Gouagna LC, et al. Malaria infection increases attractiveness of humans to mosquitoes. PLoS. 2005;3:1.
90 Lane RS, Anderson JR. Efficacy of permethrin as a repellent and toxicant for personal protection against the Pacific Coast tick and the pajaroello tick (Acari: Ixodidae and Argasidae). J Med Entomol. 1984;21:692.
91 Leo RJ, del Regno PA, Gregory C, et al. Insect repellent toxicity associated with psychosis. Psychosomatics. 2001;42:78.
92 Lewis DJ, Fairchild WL, Leprince DJ. Evaluation of an electronic mosquito repeller. Can Entomol. 1982;114:699.
93 Lillie TH, Schreck CE, Rahe AJ. Effectiveness of personal protection against mosquitoes in Alaska. J Med Entomol. 1988;25:475.
94 Lindsay RL, Heal JD, Surgeoner GA. Comparative evaluation of the efficacy of BiteBlocker, Off! Skintastic, and Avon Skin-So-Soft to protect against Aedes species mosquitoes in Ontario. Guelph, Ontario: Department of Environmental Biology, University of Guelph; August 1996.
95 Lindsay RL, Heal JD, Surgeoner GA. Evaluation of BiteBlocker as a repellent against spring Aedes spp. mosquitoes. Guelph, Ontario: Department of Environmental Biology, University of Guelph; July 1996.
96 Lindsay RL, Surgeoner GA, Heal JD. Comparative evaluation of the efficacy of BiteBlocker and 20% DEET to repel black flies in Ontario, Canada. Guelph, Ontario: Department of Environmental Biology, University of Guelph; July 1996.
97 Lindsay RL, Surgeoner GA, Heal JD, et al. Evaluation of the efficacy of 3% citronella candles and 5% citronella incense for protection against field populations of Aedes mosquitoes. J Am Mosq Control Assoc. 1996;12:293.
98 Lines JD, Myamba J, Curtis CF. Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania. Med Vet Entomol. 1987;1:37.
99 Lipscomb JW, Kramer JE, Leikin JB. Seizure following brief exposure to the insect repellent N,N-diethyl-m-toluamide. Ann Emerg Med. 1992;21:315.
100 Lukwa N, Chandiwana SK. Efficacy of mosquito coils containing 0.3% and 0.4% pyrethrins against An gambiae sensu lata mosquitoes. Cent Afr J Med. 1998;44:104.
101 Maibach HI, Akers WA, Johnson HL, et al. Insects: Topical insect repellents. Clin Pharmacol Ther. 1974;16:970.
102 Maibach HI, Khan AA, Akers WA. Use of insect repellents for maximum efficacy. Arch Dermatol. 1974;109:32.
103 Maibach HI, Skinner WA, Strauss WG, et al. Factors that attract and repel mosquitoes in human skin. JAMA. 1966;196:263.
104 Marquardt WC, editor. The biology of disease vectors, ed 2, San Diego: Elsevier Academic Press, 2005.
105 Matsuda BM, Surgeoner GA, Heal JD, et al. Essential oil analysis and field evaluation of the citrosa plant “Pelargonium citrosum” as a repellent against populations of Aedes mosquitoes. J Am Mosq Control Assoc. 1996;12:69.
106 McGready R, Hamilton KA, Simpson JA, et al. Safety of the insect repellent N,N-dietyyl-m-toluamide (DEET) in pregnancy. Am J Trop Med Hyg. 2001;65:285.
107 McHugh CP. Arthropods: Vectors of disease agents. Lab Med. 1994;25:429.
108 Mehr ZA, Rutledge LC, Morales EL, et al. Laboratory evaluation of controlled-release insect repellent formulations. J Am Mosq Control Assoc. 1985;1:143.
109 Montemarano AD, Gupta RK, Burge JR, et al. Insect repellents and the efficacy of sunscreens. Lancet. 1997;349:1670.
110 Moore SJ, Darling ST, Sihuincha M, et al. A low-cost repellent for malaria vectors in the Americas: Results of two field trials in Guatemala and Peru. Malaria J. 2007;6:101.
111 Moore SJ, Davies CR, Hill N, et al. Are mosquitoes diverted from repellent-using individuals to non users? Results of a field study in Bolivia. Trop Med Int Health. 2007;12:532.
112 Moore SJ, Lenglet A, Hill N. Field evaluation of three plant-based insect repellents against malaria vectors in Vaca Diez Province, the Bolivian Amazon. J Am Mosq Control Assoc. 2002;18:107.
113 Muirhead-Thomson RC. The distribution of anopheline mosquito bites among different age groups: A new factor in malaria epidemiology. Br Med J. 1951;1:1114.
114 Muller G, Junnila A, Kravchenko V, et al. Ability of essential oil candles to repel biting insects in high and low biting pressure environments. J Am Mosq Control Assoc. 2008;24:154.
115 Murphy ME, Montemarano AD, Debboun M, et al. The effect of sunscreen on the efficacy of insect repellent: A clinical trial. J Am Acad Dermatol. 2000;43:219.
116 Nasci RS, Harris CW, Porter CK. Failure of an insect electrocuting device to reduce mosquito biting. Mosq News. 1983;43:180.
117 Osimitz TG, Grothaus RH. The present safety assessment of DEET. J Am Mosq Control Assoc. 1995;11:274.
118 Osimitz TG, Murphy JV. Neurological effects associated with use of the insect repellent N,N-diethyl-m-toluamide (DEET). J Toxicol Clin Toxicol. 1997;35:435.
119 Osimitz TG, Murphy JV, Fell LA, et al. Adverse events associated with the use of insect repellents containing N,N-diethyl-m-toluamide (DEET). Regul Toxicol Pharmacol. 2010;56:93.
120 Paru R, Hii J, Lewis D, et al. Relative repellency of woodsmoke and topical applications of plant products against mosquitoes. P N G Med J. 1995;38:215.
121 Pauluhn J. Hazard identification and risk assessment of pyrethroids in the indoor environment. Toxicol Lett. 1999;107:193.
122 Peterson C, Coats J. Insect repellents: past, present, and future. Pesticide Outlook. 2001;12:154.
123 Piesman J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J Infect Dis. 1993;167:1082.
124 Piesman J. Dispersal of the Lyme disease spirochete Borrelia burgdorferi to salivary glands of feeding nymphal Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 1995;32:519.
125 Piesman J, Dolan MC. Protection against Lyme disease spirochete transmission provided by prompt removal of nymphal Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 2002;39:509.
126 Piesman J, Mather TN, Sinsky RJ, et al. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557.
127 Piesman J, Maupin GO, Campos EG, et al. Duration of adult female Ixodes dammini attachment and transmission of Borrelia burgdorferi, with description of a needle aspiration isolation method. J Infect Dis. 1991;163:8957.
128 Port GR, Boreham PFL. The relationship of host size to feeding by mosquitoes of the Anopheles gambiae Giles complex (Diptera:Culicidae). Bull Entomol Res. 1980;70:133.
129 Quarles W. Botanical mosquito repellents. Common Sense Pest Control. 1996;12:12.
130 Quarles W. Lighted and baited mosquito traps. Common Sense Pest Control. 1996;12:5.
131 Rajan T, Hein M, Porte P, et al. A double-blind, placebo-controlled trial of garlic as a mosquito repellent: A preliminary study. Med Vet Entomol. 2005;19:84.
132 Rueda LM, Rutledge LC, Gupta RK. Effect of skin abrasions on the efficacy of the repellent DEET against Aedes aegypti. J Am Mosq Control Assoc. 1998;14:178.
133 Rutledge LC, Collister DM, Meixsell VE, et al. Comparative sensitivity of representative mosquitoes (Diptera: Culicidae) to repellents. J Med Entomol. 1983;20:506.
134 Salafsky B, Shibuya T, He YX, et al. Lipodeet: An improved formulation for a safe, long-lasting repellent. In: Debboun M, Frances SP, Strickman D, editors. Insect repellents: Principles, methods, and uses. Boca Raton, Fla: CRC Press, 2007.
135 Sangha GK. Toxicology and safety evaluation of the new insect repellent picaridin (saltidin). In: Kreiger R, editor. Hayes handbook of pesticide technology. New York: Academic Press, 2010.
136 Schoenig GP, Hartnagel REJr, Osimitz TG, et al. Absorption, distribution, metabolism, and excretion of N,N-diethyl-m-toluamide in the rat. Drug Metab Dispos. 1996;24:156.
137 Schofield S, Tepper M, Gadawski R. Laboratory and field evaluation of the impact of exercise and the performance of regular and polymer-based DEET repellents. J Med Entomol. 2007;44:1026.
138 Schreck CE. Permethrin and dimethyl phthalate as tent fabric treatments against Aedes aegypti. J Am Mosq Control Assoc. 1991;7:533.
139 Schreck CE. Protection from blood-feeding arthropods. In: Auerbach PS, editor. Wilderness medicine: Management of wilderness and environmental emergencies. ed 3. St Louis: Mosby; 1995:813-830.
140 Schreck CE, Carlson DA, Weidhass DE, et al. Wear and aging tests with permethrin-treated cotton-polyester fabric. J Econ Entomol. 1980;73:451.
141 Schreck CE, Fish D, McGovern TP. Activity of repellents applied to skin for protection against Amblyomma americanum and Ixodes scapularis ticks (Acari: Ixodidae). J Am Mosq Control Assoc. 1995;11:136.
142 Schreck CE, Kline DL. Repellency of two controlled-release formulations of DEET against Anopheles quadrimaculatus and Aedes taeniorhynchus mosquitoes. J Am Mosq Control Assoc. 1989;5:91.
143 Schreck CE, Kline DL, Carlson DA. Mosquito attraction to substances from the skin of different humans. J Am Mosq Control Assoc. 1990;6:406.
144 Schreck CE, McGovern TP. Repellents and other personal protection strategies against Aedes albopictus. J Am Mosq Control Assoc. 1989;5:247.
145 Schreck CE, Posey K, Smith D. Durability of permethrin as a potential clothing treatment to protect against blood-feeding arthropods. J Econ Entomol. 1978;71:397.
146 Schreck CE, Snoddy EL, Spielman A. Pressurized sprays of permethrin or DEET on military clothing for personal protection against Ixodes dammini (Acari: Ixodidae). J Med Entomol. 1986;23:396.
147 Seyoum A, Kabiru EW, Lwande WL, et al. Repellency of live potted plants against Anopheles gambiae from human baits in semi-field experimental huts. Am J Trop Med Hyg. 2002;67:191.
148 Shell ER. Resurgence of a deadly disease. Atlantic Monthly. 1997;280:45.
149 Shirai O, Tsuda T, Kitagawa S, et al. Alcohol ingestion stimulates mosquito attraction. J Am Mosq Control Assoc. 2002;18:91.
150 Sholdt LL, Schreck CE, Qureshi A, et al. Field bioassays of permethrin-treated uniforms and a new extended duration repellent against mosquitoes in Pakistan. J Am Mosq Control Assoc. 1988;4:233.
151 Simons FE, Peng Z. Skeeter syndrome. J Clin Immunol. 1999;104:705.
152 Snow WF. The effect of a reduction in expired carbon dioxide on the attractiveness of human subjects to mosquitoes. Bull Entomol Res. 1970;60:43.
153 Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (A13-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870.
154 Sood SK, Salzman MB, Johnson BJ, et al. Duration of tick attachment as a predictor of the risk of Lyme disease in an area in which Lyme disease is endemic. J Infect Dis. 1997;175:996.
155 Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936.
156 Strauss WG, Maibach HI, Khan AA. Drugs and disease as mosquito repellents in man. Am J Trop Med Hyg. 1968;17:461.
157 Sudakin DL, Trevathan WR. DEET: A review and update of safety and risk in the general population. Clin Toxicol. 2003;41:831.
158 Surgeoner GA. Efficacy of Buzz Away Oil against spring Aedes spp. mosquitoes. Guelph, Ontario: Department of Environmental Biology, University of Guelph; 1995.
159 Syed Z, Leal WS. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci. 2008;105:13598.
160 Taubes G. A mosquito bites back. New York Times Magazine. 1997:40. Aug 24
161 Tawatsin A, Wratten SD, Scott RR, et al. Repellency of volatile oils from plants against three mosquito vectors. J Vector Ecol. 2001;26:76.
162 Trongtokit Y, Rongsriyam Y, Komalamisra N, et al. Comparative repellency of 38 essential oils against mosquito bites. Phytother Res. 2005;19:303.
163 University of California at Berkeley. Finally, a safer insect repellent. Wellness Letter. 1997;13:2.
164 U.S. Department of Agriculture. Materials evaluated as insecticides, repellents, and chemosterilants at Orlando and Gainesville, Fla., 1952-1964. USDA Agricultural Handbook. 1967;340:1.
165 U.S. Environmental Protction Agency. Insect repellent-sunscreen combination products; request for information and comments. Environ Prot. 2007;72:7941.
166 U.S. Environmental Protection Agency. Office of Pesticide Programs, Prevention, Pesticides and Toxic Substances Division: Reregistration eligibility decision (RED) for oil of citronella (EPA-738-F-97-002). Washington, DC: EPA; 1997.
167 U.S. Environmental Protection Agency. Office of Pesticide Programs, Prevention, Pesticides and Toxic Substances Division: Reregistration eligibility decision (RED): DEET (EPA-738-F-95-010). Washington, DC: EPA; 1998.
168 U.S. Environmental Protection Agency. Offices of Pesticides and Toxic Substances, Special Pesticide Review Division: N,N-diethyl-m-toluamide (DEET) pesticide registration standard (EPA-540/RS-81-004). Washington, DC: EPA; 1980.
169 Veltri JC, Osimitz TG, Bradford DC, et al. Retrospective analysis of calls to poison control centers resulting from exposure to the insect repellent N,N-diethyl-m-toluamide (DEET) from 1985-1989. J Toxicol Clin Toxicol. 1994;32:1.
170 Verhulst NO, Beijleveald H, Knols BG, et al. Cultured skin microbiota attracts malaria mosquitoes. Malaria J. 2009;17:302.
171 Vernede R, van Meer M, Alpers MP. Smoke as a form of personal protection against mosquitoes, a study in Papua New Guinea. Southeast Asian J Trop Med Public Health. 1994;25:771.
172 Webb CE, Russell RC. Insect repellents and sunscreen: Implications for personal protection strategies against mosquito-borne disease. Aust N Z J Public Health. 2009;33:485.
173 Weil WB. New information leads to changes in DEET recommendations. AAP News. 2001;19:52.
174 Wilson CW, Mathieson DR, Jachowski LA. Ingested thiamine chloride as a mosquito repellent. Science. 1944;100:147.
175 Yap HH, Tan HT, Yahaya AM, et al. Field efficacy of mosquito coil formulations containing D-allethrin and D-transallethrin against indoor mosquitoes especially Cules quinquefasciatus Say. Southeast Asian J Trop Med Public Health. 1990;21:558.
176 Young D, Evans S. Safety and efficacy of DEET and permethrin in the prevention of arthropod attack. Mil Med. 1998;5:324.
177 Zadikoff CM. Toxic encephalopathy associated with use of insect repellent. J Pediatr. 1979;95:140.
* References 15, 17, 29, 35, 58, 80.
* References 20, 39, 43, 59, 79, 104.
* References 20, 39, 44, 59, 79, 104.
* References 20, 39, 59, 79, 104, 155.
* References 10, 123, 124, 126, 127, 154, 155.
* References 55, 81, 83, 101, 102, 132, 137.
* References 34, 72, 85, 130, 139, 164.
* References 2, 63, 86, 108, 142, 144.
* References 18, 38, 42, 50, 66, 69, 70, 91, 99, 117, 118, 177.
* References 4, 30, 36, 37, 53, 153.