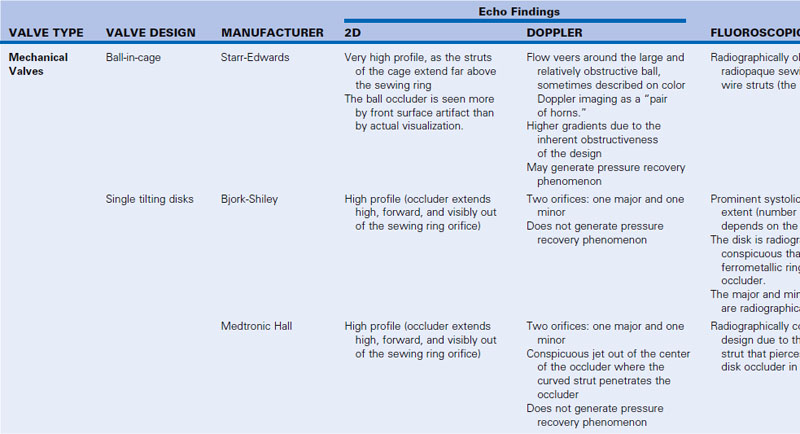
8 Prosthetic Valves
Scanning Issues
Required Parameters to Obtain from Scanning
 Motion of valve ring and (bioprosthetic) leaflet or (mechanical) occluder(s)
Motion of valve ring and (bioprosthetic) leaflet or (mechanical) occluder(s)
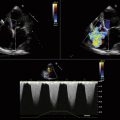
 Identification and characterization of insufficiency, if present
Identification and characterization of insufficiency, if present
Notes
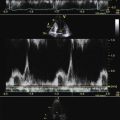
 Recall that the correlation of pressure half time (PHT) to mitral valve effective oriface area is poor for all forms of mechanical mitral valve replacement (MVR), and that the PHT method should not be emphasized for the assessment of mechanical MVRs.
Recall that the correlation of pressure half time (PHT) to mitral valve effective oriface area is poor for all forms of mechanical mitral valve replacement (MVR), and that the PHT method should not be emphasized for the assessment of mechanical MVRs.
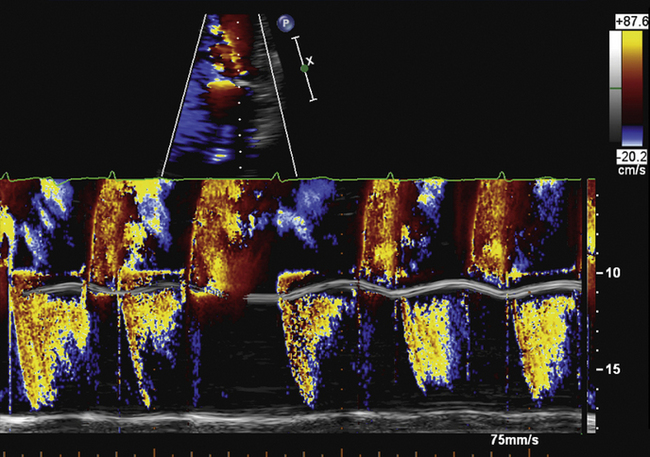
 Diastolic turbulent flow into the left ventricular outflow tract in the presence of a mechanical MVR suggests mechanical MVR obstruction.
Diastolic turbulent flow into the left ventricular outflow tract in the presence of a mechanical MVR suggests mechanical MVR obstruction.
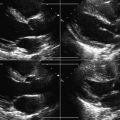
 The presence of a bileaflet occluder mechanical prosthesis requires the attempt to visualize the motion of both occluders. Prosthesis thrombosis usually occurs with one leaflet more frozen and one leaflet less so—so do not extrapolate the impression of one occluder to the other. Visualize both.
The presence of a bileaflet occluder mechanical prosthesis requires the attempt to visualize the motion of both occluders. Prosthesis thrombosis usually occurs with one leaflet more frozen and one leaflet less so—so do not extrapolate the impression of one occluder to the other. Visualize both.
Reporting Issues
Know (and record) the type, model, size, and year of the valve you are scanning.
 The effect of valve type (P = 0.0003)1 and size (P = 0.01)1 on gradient and area is considerable; therefore, it is necessary to know the type and size of the prosthesis. This will facilitate interpretation and imaging recognition of abnormalities.
The effect of valve type (P = 0.0003)1 and size (P = 0.01)1 on gradient and area is considerable; therefore, it is necessary to know the type and size of the prosthesis. This will facilitate interpretation and imaging recognition of abnormalities.
 Clearly state whether the recorded gradient is expected or greater than expected for that type of prosthesis.
Clearly state whether the recorded gradient is expected or greater than expected for that type of prosthesis.
 Clearly state whether any insufficiency present is within the expected amount or of either pathologic quantity or pathologic origin (paravalvar is always pathologic).
Clearly state whether any insufficiency present is within the expected amount or of either pathologic quantity or pathologic origin (paravalvar is always pathologic).
Terminology
 If there is a severe gradient across a mechanical MVR, use the term obstruction.
If there is a severe gradient across a mechanical MVR, use the term obstruction.
 Leakage across the prosthesis is transvalvar.
Leakage across the prosthesis is transvalvar.
 Leakage beside the prosthesis is paravalvar.
Leakage beside the prosthesis is paravalvar.
 If there is ≥3+ paravalvar insufficiency, and rocking, use the term dehiscence.
If there is ≥3+ paravalvar insufficiency, and rocking, use the term dehiscence.
 The moving mechanical elements of prostheses are occluders or hemi-disk occluders, depending on their geometry.
The moving mechanical elements of prostheses are occluders or hemi-disk occluders, depending on their geometry.
 The implanted ring is the sewing ring.
The implanted ring is the sewing ring.
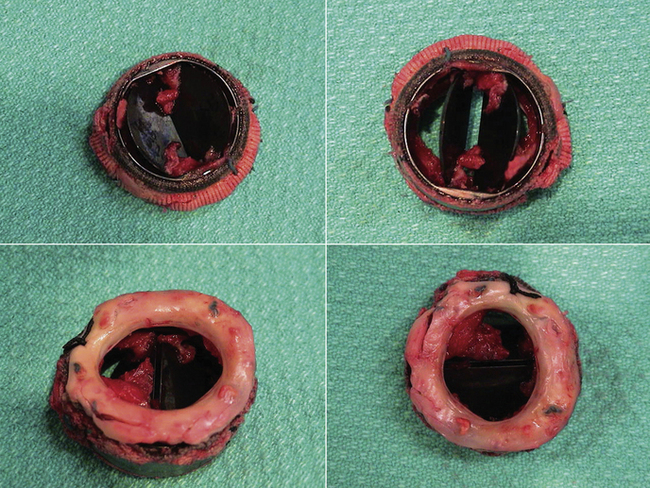
 Soft tissue on the sewing ring may be thrombus, pannus, or vegetations—or a combination. There is little ability case-by-case to accurately resolve (by echocardiography alone) which it is, or whether multiple causes are present.
Soft tissue on the sewing ring may be thrombus, pannus, or vegetations—or a combination. There is little ability case-by-case to accurately resolve (by echocardiography alone) which it is, or whether multiple causes are present.
 Flail bioprosthetic leaflets may be reasonably referred to as torn.
Flail bioprosthetic leaflets may be reasonably referred to as torn.
 Use of the term retroverted leaflet is questionable, because it is difficult to be certain, short of surgical inspection.
Use of the term retroverted leaflet is questionable, because it is difficult to be certain, short of surgical inspection.
 Do not state “normal appearance” for a mechanical prosthesis, because mechanical prostheses are so poorly visualized that such a phrase says little, especially for bileaflet occluders, and may easily be wrong. Most of the “appearance” of mechanical prostheses is artifact.
Do not state “normal appearance” for a mechanical prosthesis, because mechanical prostheses are so poorly visualized that such a phrase says little, especially for bileaflet occluders, and may easily be wrong. Most of the “appearance” of mechanical prostheses is artifact.
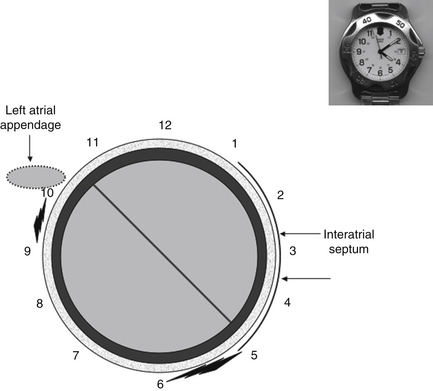
 When describing by transesophageal echocardiography (TEE) the position(s) of paravalvar leaks, use a clock-face numeric system, with 12 o’clock at the top, 6 o’clock at the bottom, and the left atrial appendage at about 10 o’clock.
When describing by transesophageal echocardiography (TEE) the position(s) of paravalvar leaks, use a clock-face numeric system, with 12 o’clock at the top, 6 o’clock at the bottom, and the left atrial appendage at about 10 o’clock.
 Describe the location of all paravalvar leaks, not only the largest. If the patient is submitted to surgical repair, all leaks will need to be addressed.
Describe the location of all paravalvar leaks, not only the largest. If the patient is submitted to surgical repair, all leaks will need to be addressed.
Gradient Issues
Important issues to understand include the following:
Correlation of mean gradient is generally good for simultaneous comparisons of the following:
 Bioprosthesis: r = 0.93; standard error of estimate (SEE) = 3 mm Hg2
Bioprosthesis: r = 0.93; standard error of estimate (SEE) = 3 mm Hg2
 Mechanical: r = 0.93; SEE = 3 mm Hg2 or better2,3
Mechanical: r = 0.93; SEE = 3 mm Hg2 or better2,3
However, overestimation may occur while the correlation is good, especially for the following:
Nonsimultaneous correlation of mean gradient is less, as would be expected:
Mechanical prosthetic valve gradients are flow dependent. In the case of very small prostheses in the aortic position, gradients rise very rapidly and conspicuously as flow increases beyond resting state flow (90 mm Hg peak for SJM and Hancock valves).3 Therefore, be attentive to anything likely to be causing increased cardiac output (e.g., anemia, fever, pregnancy, thyrotoxicosis).
Recall the significant 5-, 10-, and 15-year incidence of structural valve failure for older designs of bioprostheses (Table 8-2).4
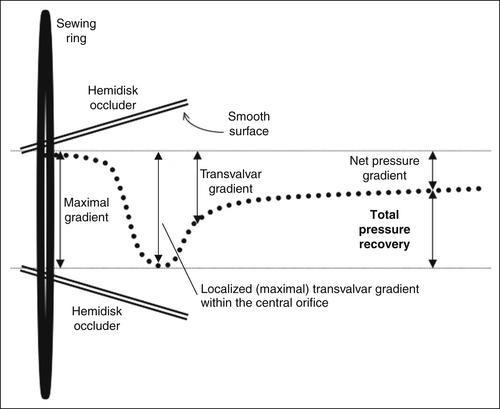
Pressure Recovery Phenomenon
 The pressure recovery phenomenon is a fascinating occurrence of hydrodynamics in which a localized gradient develops within a smooth-walled restrictive orifice. Bileaflet occluders are the most common cause of localized gradients and pressure recovery.
The pressure recovery phenomenon is a fascinating occurrence of hydrodynamics in which a localized gradient develops within a smooth-walled restrictive orifice. Bileaflet occluders are the most common cause of localized gradients and pressure recovery.
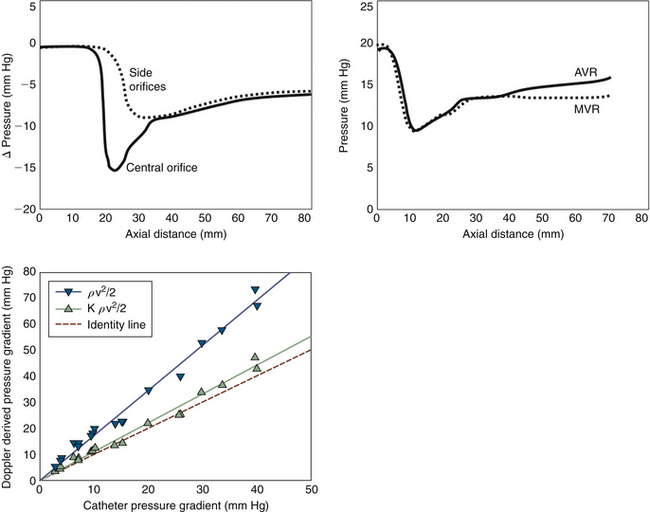
 The pressure recovery phenomenon is well described for bileaflet occluder prostheses in the aortic position, and for the ball-in-cage Starr-Edwards prosthesis. The phenomenon is described in vitro, but probably does account for some patient discrepancies with catheterization. The pressure recovery is greatest for the central orifice. The pressure loss coefficient for the continuity relation has been shown in vitro to be K = 0.64. As much as 40% of the initial pressure loss is recovered by the end of the leaflets in the central orifice, and another 20% within another 5 cm. The pressure recovery of the side orifices is less (30%), and occurs further downstream. Side orifice velocities are 85 ± 4% of the center orifice velocity.5
The pressure recovery phenomenon is well described for bileaflet occluder prostheses in the aortic position, and for the ball-in-cage Starr-Edwards prosthesis. The phenomenon is described in vitro, but probably does account for some patient discrepancies with catheterization. The pressure recovery is greatest for the central orifice. The pressure loss coefficient for the continuity relation has been shown in vitro to be K = 0.64. As much as 40% of the initial pressure loss is recovered by the end of the leaflets in the central orifice, and another 20% within another 5 cm. The pressure recovery of the side orifices is less (30%), and occurs further downstream. Side orifice velocities are 85 ± 4% of the center orifice velocity.5
 Overestimation of peak and mean trans–aortic valve replacement (AVR) gradient is described for the St. Jude prostheses and for the Starr-Edwards prosthesis (with gradient differences as great as 44 mm Hg), but not for the Medtronic Hall tilting disk prosthesis.3
Overestimation of peak and mean trans–aortic valve replacement (AVR) gradient is described for the St. Jude prostheses and for the Starr-Edwards prosthesis (with gradient differences as great as 44 mm Hg), but not for the Medtronic Hall tilting disk prosthesis.3
 The pressure recovery phenomenon also may occur for mitral prostheses.5
The pressure recovery phenomenon also may occur for mitral prostheses.5
A smooth-walled flaring restrictive orifice may establish pressure recovery: some of the kinetic energy recovers to potential energy (pressure).5 The pressure recovery phenomenon is greater for small valve prostheses (26-mm valve: 167 ± 52% vs. 31-mm valve: 123 ± 41%), and for the centerline gradients than the side orifice gradients (13 ± 12 mm Hg vs. 6 ± 4 mm Hg).5 Although there is good correlation of valve gradient by Doppler and catheterization, the Doppler estimates experimentally are significantly higher. A total pressure loss coefficient2 for bileaflet occluder devices of 0.64 (±0.04) can be used.5
Within a nonplanar, smooth-walled orifice such as the central (minor) orifice of a bileaflet occluder valve prosthesis, a localized gradient can occur within the length of the smooth-walled orifice (“early” or “valvular” recovery). There is a small amount of subsequent recovery of pressure (“late” or “post-valvular” recovery). Within a few centimeters of the tip of the occluders, the total pressure recovery has occurred.
Patient–Prosthesis Mismatch
When a prosthesis is so small (i.e., its EOA is so small) that it is conferring a large gradient that may fall within the severe range, patient prosthesis mismatch (PPM) is said to exist. PPM is likely to occur with an EOA of <0.9 cm2/m2.6 Surgeons insert the largest prosthesis than can be fitted into the annulus, to minimize the frequency of this complication, but ultimately, the annulus size may be small (mismatched) for the size of the patient.
Smaller prostheses produce higher gradients3,6 in the resting state, and especially with exercise or any context of increased flow. With exercise gradients not only rise, but rise very steeply.3 For example a small St. Jude prosthesis in the aortic position may produce a 90-mm Hg peak gradient with exercise levels of flow.3 Therefore, when interrogating a prosthesis, it is imperative to know the size of the prosthesis and type, and to anticipate its gradients. For smaller prostheses, where there are symptoms and a somewhat elevated gradient, mild exercise may bring out the degree of gradient and pulmonary hypertension in such states.
Assumptions Inherent in the Modified Bernoulli Equation
 That a blood viscosity effect is negligible (as boundary layer formation is negligible other than against the walls to flow)
That a blood viscosity effect is negligible (as boundary layer formation is negligible other than against the walls to flow)
 That inertial issues are negligible for an orifice restrictive to flow (as the amount of weight of blood involved is very small)
That inertial issues are negligible for an orifice restrictive to flow (as the amount of weight of blood involved is very small)
 That most energy proximal to the orifice is potential, and can be omitted
That most energy proximal to the orifice is potential, and can be omitted
 That there is no pressure recovery (i.e., all kinetic energy developed is lost to friction, heat, and vortices).5
That there is no pressure recovery (i.e., all kinetic energy developed is lost to friction, heat, and vortices).5
A subvalvar gradient may be present in some cases of (native valve) AS cases7; therefore, the omission of V1 from the modified Bernouilli equation may be a relevant problem in a clinically meaningful number of cases.
Discordance of Gradient Assessment with Catheterization
Area Issues
General Issues of Correlation
When comparing to Gorlin-derived areas, the factors most commonly influencing Doppler EOA and Gorlin area are difference in cardiac output and difference in AVG.8 It has been shown that there is flow dependence and pressure dependence of the Gorlin and continuity equations.9
Flow and Pressure Dependence of Gorlin and Continuity
 In a pulse duplicator system, Gorlin overall yields slightly higher bioprosthetic valve areas (1–2%) for AVRs and moderately greater areas for MVRs (12–13%).9
In a pulse duplicator system, Gorlin overall yields slightly higher bioprosthetic valve areas (1–2%) for AVRs and moderately greater areas for MVRs (12–13%).9
 For any given size and type of bioprosthesis, areas calculated by both formulas increase with increasing flow: up to 20% for bioprosthetic AVRs and 35% for bioMVRs.9
For any given size and type of bioprosthesis, areas calculated by both formulas increase with increasing flow: up to 20% for bioprosthetic AVRs and 35% for bioMVRs.9
Bioprosthetic Mitral Valve Replacements
 The PHT method for mitral valve area (MVA) determination for bioprosthetic MVR correlates poorly with in vitro (r = 0.15; P > 0.3) and continuity methods (r = 0.23; P > 0.2), and yields an MVA above predicted in 70% of cases.10 Therefore, its use should be avoided. In most hearts, PHT represents LV filling characteristics more than transmitral flow characteristics, unless the transmitral flow is markedly abnormal.
The PHT method for mitral valve area (MVA) determination for bioprosthetic MVR correlates poorly with in vitro (r = 0.15; P > 0.3) and continuity methods (r = 0.23; P > 0.2), and yields an MVA above predicted in 70% of cases.10 Therefore, its use should be avoided. In most hearts, PHT represents LV filling characteristics more than transmitral flow characteristics, unless the transmitral flow is markedly abnormal.
 Bioprosthetic MVR area by continuity correlates well (r = 0.82; SEE = 0.1) with in vitro areas.10 For any given size and type of bioprosthesis, areas calculated by both formulas increase with increasing flow: up to 35% for bioprosthetic MVRs.9
Bioprosthetic MVR area by continuity correlates well (r = 0.82; SEE = 0.1) with in vitro areas.10 For any given size and type of bioprosthesis, areas calculated by both formulas increase with increasing flow: up to 35% for bioprosthetic MVRs.9
 MVR area by continuity is only feasible if there is no MR and no aortic insufficiency (or if amounts of MR and aortic insufficiency are equal).10
MVR area by continuity is only feasible if there is no MR and no aortic insufficiency (or if amounts of MR and aortic insufficiency are equal).10
Mechanical Mitral Valve Replacements
Bioprosthetic Aortic Valve Replacements
 The continuity relation is accurate for bioprosthetic AVRs, as is well shown for the porcine Hancock bioprostheses.3
The continuity relation is accurate for bioprosthetic AVRs, as is well shown for the porcine Hancock bioprostheses.3
 The Gorlin equation is accurate for assessment of bioprosthetic AVRs. One study demonstrated with a pulse duplicator system that Gorlin overall yields only slightly higher bioprosthetic valve areas (1–2%) for AVRs9; whereas another study determined that for Hancock and Bjork-Shiley AVRs, the mean AVA error by Gorlin is 0.36 ± 0.32 cm2. Gorlin overestimated AVA by >0.25 cm2 in 32% of cases, and underestimated by >0.25 cm2 in 21%.12
The Gorlin equation is accurate for assessment of bioprosthetic AVRs. One study demonstrated with a pulse duplicator system that Gorlin overall yields only slightly higher bioprosthetic valve areas (1–2%) for AVRs9; whereas another study determined that for Hancock and Bjork-Shiley AVRs, the mean AVA error by Gorlin is 0.36 ± 0.32 cm2. Gorlin overestimated AVA by >0.25 cm2 in 32% of cases, and underestimated by >0.25 cm2 in 21%.12
 For any given size and type of bioprosthetic AVR, areas calculated by both formulas increase (up to 20%) with increasing flow.9
For any given size and type of bioprosthetic AVR, areas calculated by both formulas increase (up to 20%) with increasing flow.9
Prosthetic Valve Dysfunction
The ACC/AHA recommendations regarding prosthetic valve thrombosis are presented in Box 8-3.
Summary
 The diverse engineering of prosthetic heart valves, and the many problems that they are susceptible to, render assessment of prosthetic valves by echocardiography a challenge.
The diverse engineering of prosthetic heart valves, and the many problems that they are susceptible to, render assessment of prosthetic valves by echocardiography a challenge.
 The different hydrodynamic properties of different valves confer differing transvalvar flow patterns, and different gradients.
The different hydrodynamic properties of different valves confer differing transvalvar flow patterns, and different gradients.
 Shadowing by the sewing ring and/or occluders greatly compromises echocardiographic evaluation of both anatomic and functional consequences of prosthesis dysfunction.
Shadowing by the sewing ring and/or occluders greatly compromises echocardiographic evaluation of both anatomic and functional consequences of prosthesis dysfunction.
 The pressure recovery phenomenon is an issue for the Doppler assessment of AVRs in a small aorta.
The pressure recovery phenomenon is an issue for the Doppler assessment of AVRs in a small aorta.
From ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 2006;48(3):e1–e148.
BOX 8-2 Selection of a Mitral Valve Prosthesis: ACC/AHA 2006 Recommendations
Class IIa
1. A mechanical prosthesis is reasonable for MV replacement in patients under 65 years of age with long-standing atrial fibrillation. (Level of evidence: C)
2. A bioprosthesis is reasonable for MV replacement in patients 65 years of age or older. (Level of evidence: C)
3. A bioprosthesis is reasonable for MV replacement in patients under 65 years of age in sinus rhythm who elect to receive this valve for lifestyle considerations after detailed discussions of the risks of anticoagulation versus the likelihood that a second MV replacement may be necessary in the future. (Level of evidence: C)
From ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 2006;48(3):e1–e148.
BOX 8-3 Thrombosis of Prosthetic Heart Valves: ACC/AHA 2006 Recommendations
Class I
1. Transthoracic and Doppler echocardiography are indicated in patients with suspected prosthetic valve thrombosis to assess hemodynamic severity. (Level of evidence: B)
2. Transesophageal echocardiography and/or fluoroscopy are indicated in patients with suspected valve thrombosis to assess valve motion and clot burden. (Level of evidence: B)
Class IIa
1. Emergency operation is reasonable for patients with thrombosis of a left-sided prosthetic valve and New York Heart Association (NYHA) functional class III–IV symptoms. (Level of evidence: C)
2. Emergency operation is reasonable for patients with thrombosis of a left-sided prosthetic valve and a large clot burden. (Level of evidence: C)
3. Fibrinolytic therapy is reasonable for thrombosis of right-sided prosthetic heart valves with NYHA class III–IV symptoms or a large clot burden. (Level of evidence: C)
Class IIb
1. Fibrinolytic therapy may be considered as a first-line therapy for patients with thrombosis of a left-sided prosthetic valve, NYHA functional class I–II symptoms, and a small clot burden. (Level of evidence: B)
2. Fibrinolytic therapy may be considered as a first-line therapy for patients with thrombosis of a left-sided prosthetic valve, NYHA functional class III–IV symptoms, and a small clot burden if surgery is high risk or unavailable. (Level of evidence: B)
3. Fibrinolytic therapy may be considered for patients with an obstructed, thrombosed left-sided prosthetic valve who have NYHA functional class II–IV symptoms and a large clot burden if emergency surgery is high risk or unavailable. (Level of evidence: C)
4. Intravenous unfractionated heparin (UFH) as an alternative to fibrinolytic therapy may be considered for patients with thrombosis of a valve who are in NYHA functional class I–II and have a small clot burden. (Level of evidence: C)
From ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 2006;48(3):e1–e148.
Transthoracic Echocardiography
ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography14
Prosthetic Valves with TTE
 Initial postoperative evaluation of prosthetic valve for establishment of baseline
Initial postoperative evaluation of prosthetic valve for establishment of baseline
 Routine surveillance (<3 yr after valve implantation) of prosthetic valve if no known or suspected valve dysfunction
Routine surveillance (<3 yr after valve implantation) of prosthetic valve if no known or suspected valve dysfunction
 Routine surveillance (≥3 yr after valve implantation) of prosthetic valve if no known or suspected valve dysfunction
Routine surveillance (≥3 yr after valve implantation) of prosthetic valve if no known or suspected valve dysfunction
 Evaluation of prosthetic valve with suspected dysfunction or a change in clinical status or cardiac examination
Evaluation of prosthetic valve with suspected dysfunction or a change in clinical status or cardiac examination
 Re-evaluation of known prosthetic valve dysfunction when it would change management or guide therapy
Re-evaluation of known prosthetic valve dysfunction when it would change management or guide therapy
ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography
Recommendations for Echocardiography in Interventions for Valvular Heart Disease and Prosthetic Valves
ACC/AHA 2006 Recommendations for Follow-Up Visits15
ACC/AHA 1997 Guidelines for the Clinical Application of Echocardiography16
Indications for Echocardiography in Interventions for Valvular Heart Disease and Prosthetic Valves
ACC/AHA 2006 Guidelines for the Management of Patients with Valvular Heart Disease17
Follow-Up Visits
Transesophageal Echocardiography
Cardiac Magnetic Resonance
ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 Appropriateness Criteria for Cardiac Magnetic Resonance Imaging20
Nuclear
ACCF/ASNC/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging22
Evaluation of LV Function
 Assessment of LV function with radionuclide angiography (ERNA or FP RNA)
Assessment of LV function with radionuclide angiography (ERNA or FP RNA)
In absence of recent reliable diagnostic information regarding ventricular function obtained with another imaging modality
Appropriateness criteria: A, appropriate; I, inappropriate; U, uncertain.
LV, left ventricular; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
TABLE 8-3 Utility of Different Imaging Modalities and Cardiac Catheterization in the Assessment of Valve Prostheses
• For MVR (especially for mechanical MVRs),
• TEE is the single best test to identify
• TEE is useful to identify restricted mechanical MVR occluder motion, but this is subjective.
• By TEE, the characteristic patterns of expected transvalvar insufficiency of mechanical prostheses (unique to the type of prosthesis) can be recognized.
• The metallic components of prosthetic sewing rings produce prominent streaking, blooming, and partial volume artifacts that require optimal filtering to enable visualization of soft tissue lesions such as vegetations and pannus.
• Atrial fibrillation and other irregular heart rhythms are a technical problem
• Higher heart rates (>75 bpm) are often still a technical problem.
• Enables identification of most prostheses as long as
• The lateral view is by far the most useful to identify and recognize prostheses.
• Can depict the presence of left heart failure from left heart valve prostheses dysfunction
AI, aortic insufficiency; AVR, aortic valve replacement; bpm, beats per minute; CMR, cardiac magnetic resonance; LV, left ventricle; MR, mitral regurgitation; MVR, mitral valve replacement; PR, pulmonary regurgitation; RV, right ventricle; SSFP, steady-state free precession; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
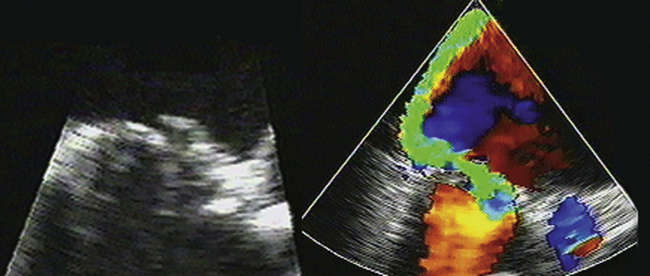
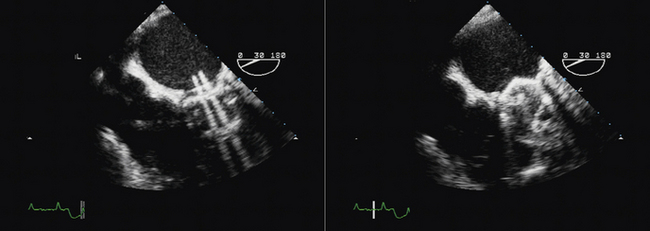
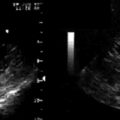
Figure 8-4 Torn leaflet of a mitral bioprosthesis, resulting in a very eccentrically directed jet or MR.
1. Stewart S.F., Nast E.P., Arabia F.A., et al. Errors in pressure gradient measurement by continuous wave Doppler ultrasound: type, size and age effects in bioprosthetic aortic valves. J Am Coll Cardiol. 1991;18(3):769-779.
2. Burstow D.J., Nishimura R.A., Bailey K.R., et al. Continuous wave Doppler echocardiographic measurement of prosthetic valve gradients. A simultaneous Doppler-catheter correlative study. Circulation. 1989;80(3):504-514.
3. Baumgartner H., Khan S., DeRobertis M., et al. Effect of prosthetic aortic valve design on the Doppler-catheter gradient correlation: an in vitro study of normal St. Jude, Medtronic-Hall, Starr-Edwards and Hancock valves. J Am Coll Cardiol. 1992;19(2):324-332.
4. Sundt T.M. Current options for replacing the aortic valve in adults. ACC Current Journal Review Jan/Feb. 2002:78-83.
5. Vandervoort P.M., Greenberg N.L., Powell K.A., et al. Pressure recovery in bileaflet heart valve prostheses. Localized high velocities and gradients in central and side orifices with implications for Doppler-catheter gradient relation in aortic and mitral position. Circulation. 1995;92(12):3464-3472.
6. Pibarot P., Dumesnil J.G. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol. 2000;36(4):1131-1141.
7. Laskey W.K., Kussmaul W.G. Subvalvular gradients in patients with valvular aortic stenosis: prevalence, magnitude, and physiological importance. Circulation. 2001;104(9):1019-1022.
8. Burwash I.G., Dickinson A., Teskey R.J., et al. Aortic valve area discrepancy by Gorlin equation and Doppler echocardiography continuity equation: relationship to flow in patients with valvular aortic stenosis. Can J Cardiol. 2000;16(8):985-992.
9. Dumesnil J.G., Yoganathan A.P. Theoretical and practical differences between the Gorlin formula and the continuity equation for calculating aortic and mitral valve areas. Am J Cardiol. 1991;67(15):1268-1272.
10. Dumesnil J.G., Honos G.N., Lemieux M., Beauchemin J. Validation and applications of mitral prosthetic valvular areas calculated by Doppler echocardiography. Am J Cardiol. 1990;65(22):1443-1448.
11. Fernandes V., Olmos L., Nagueh S.F., et al. Peak early diastolic velocity rather than pressure half-time is the best index of mechanical prosthetic mitral valve function. Am J Cardiol. 2002;89(6):704-710.
12. Cannon S.R., Richards K.L., Crawford M.H., et al. Inadequacy of the Gorlin formula for predicting prosthetic valve area. Am J Cardiol. 1988;62(1):113-116.
13. Nishimura R.A., Carabello B.A., Faxon D.P., et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:676-685.
14. Douglas P.S., Garcia M.J., Haines D.E., et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57(9):1126-1166.
15. Bonow R.O., Carabello B.A., Chatterjee K., et al. A report of the ACC/AHA on the 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 48(3), 2006. e1–e148
16. Cheitlin M.D., Chair J.S., Alpert J.S., et al. ACC/AHA guidelines for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Circulation. 1997;95:1686-1744.
17. Bonow R.O., Blase A.C., Chatterjee K., et al. ACC/AHA 2006 guidelines for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2006;114:e84-e231.
18. Cheitlin M.D., Armstrong W.F., Aurigemma G.P., et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. 2003;108(9):1146-1162.
19. Taylor A.J., Cerqueira M., Hodgson J.M., et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. J Am Coll Cardiol. 2010;56(22):1864-1894.
20. Hendel R.C., Manesh P.R., Kramer C.M., Poon M. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol. 2006;48(7):1475-1497.
21. Pennell D.J., Sechtem U.P., Higgins C.B., et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. J Cardiovasc Magn Reson. 2004;6(4):727-765.
22. Hendel R.C., Berman D.S., Di Carli M.F., et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging. J Am Coll Cardiol. 2009;53(23):2201-2229.