CHAPTER 98 Prosthetic Management of Head and Neck Defects
Dental Diagnoses and Oral Surgical Management Preceding Radiation Therapy
Extractions are indicated for teeth with poor prognoses meeting the criteria listed in Box 98-1.1–13 Although the assessment is accomplished in the interest of preventing osteoradionecrosis, it is also done considering the potential for any prosthetic rehabilitation that may become necessary. Because this assessment requires consideration of primary tumor size and location, as well as regional lymph node status, diagnostic information gleaned through participation in multidisciplinary head and neck tumor board conferences supplements decisions made in the dental clinical setting where medical imaging is limited and endoscopy is not typically available.
Box 98-1 Clinical Presentations Leading to the Assignment of Questionable or Poor Lifelong Prognoses
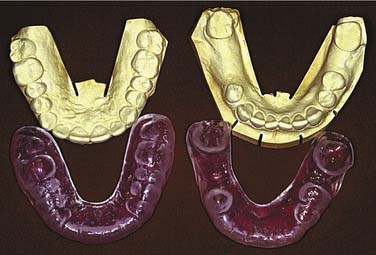
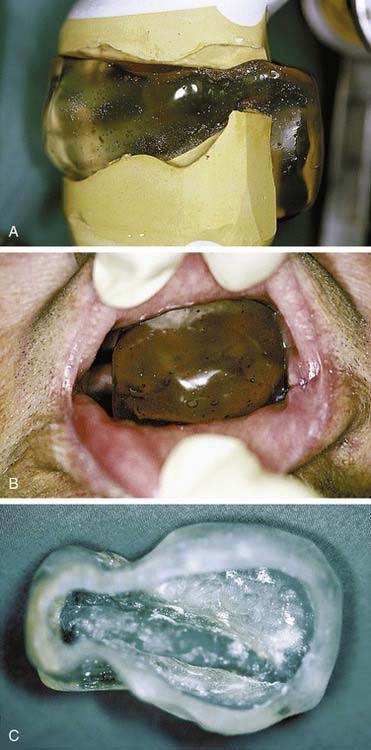
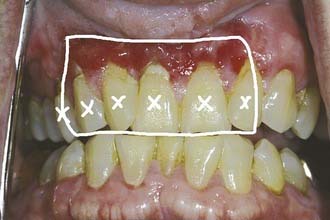
For patients retaining a full or partial natural dentition whose head and neck radiation therapy will induce xerostomia, it is important to institute a daily and lifelong regimen of topical fluoride gel use to concur with the initiation of radiation therapy.1–7,9,12,14–18 This is true for patients whose retained dentition is or is not encompassed by radiation therapy fields. Dental casts generated from clinical impressions recorded before radiation therapy but subsequent to any oral surgical procedures in preparation for radiation therapy should be used to fashion thermoplastic vinyl fluoride gel carriers (Fig. 98-1) into which topical fluoride gel can be applied. After routine brushing and flossing, patients should be instructed to place pharmacy-prescribed 1.1% neutral sodium fluoride or 0.4% stannous fluoride gel into the dental aspects of the fluoride gel carriers for 5 to 10 minutes every day indefinitely before applying them to their dentition. The topical fluoride gel application should be followed by expectoration of excess gel and a period of 30 minutes during which the patient is encouraged not to rinse, eat, or drink.
Prosthodontic Support of the Radiation Oncologist
The maxillofacial prosthodontist may be called on to lend support to the radiation oncology team in their efforts to manage dosimetry by way of patient posturing,19–22 anatomic displacement or shielding,3,6,19,23,24 fabrication of brachytherapy appliances,3,25 or construction of devices capable of mimicking normal tissues.3,19,24 Although the devices should be constructed following any oral surgical procedures that are done in preparation for radiation therapy, they must be manufactured before radiation therapy simulation appointments in that they must be positioned at the time of simulation.
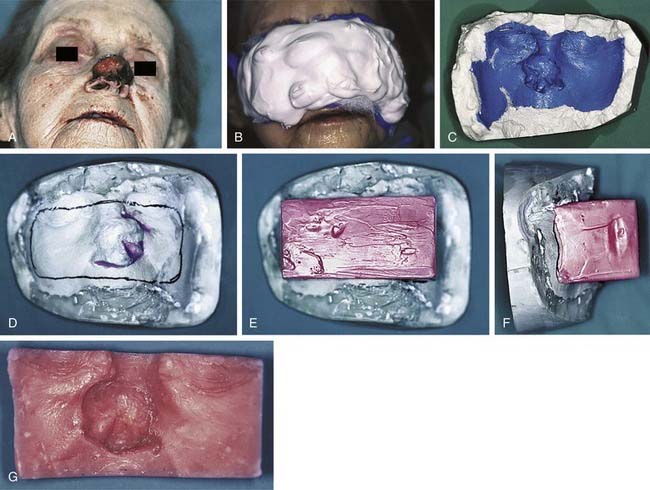
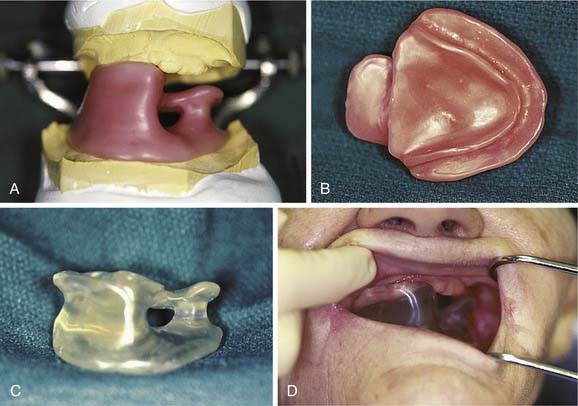
The undulating topography of head and neck cutaneous structures makes the uniform delivery of radiation difficult at best without the use of boluses constructed of materials considered more or less to be tissue equivalent in terms of their relative resistance to photon or electron penetrance. Consequently, the radiation oncologist may call on the maxillofacial prosthodontic team to assist with facial moulage recording and bolus fabrication when evaluating the physics associated with treating malignant head and neck skin lesions. A facial moulage cast with a gypseous dental stone can aid the radiation oncology team’s delineation of a proposed treatment field and prescription of an appropriate bolus thickness before a bolus is constructed of dental baseplate wax or polymethylmethacrylate. Boluses can be particularly useful for providing more even dose distributions for nasal (Fig. 98-2) or orbital malignancies.
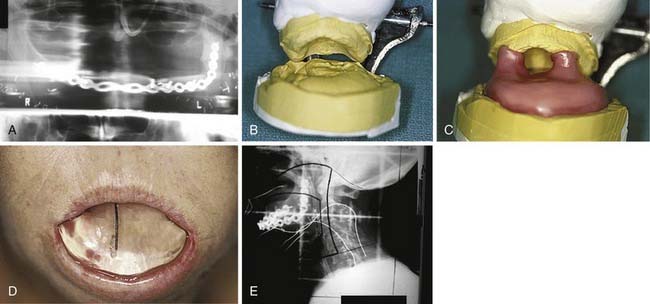
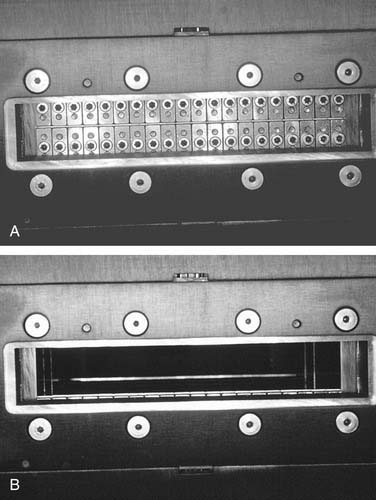
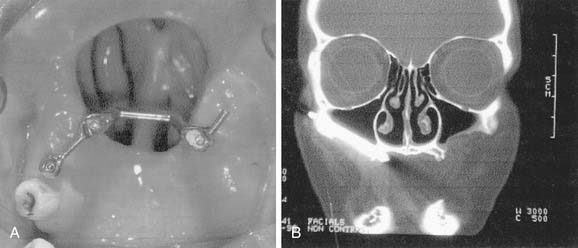
Although Aquaplast masks are routinely used to immobilize patients throughout the course of head and neck radiation therapy,19 customized intraoral devices can be used to provide a stable maxillomandibular relationship when attempts are made to precisely localize an external photon or electron beam relative to oral cavity anatomy. The use of intraoral positioning devices is particularly useful when there is a desire to shield all or part of the maxilla or the mandible from radiation when the opposing jaw is to be included in an external beam field. By positioning the mandible in a prescribed open position with the use of a polymethylmethacrylate appliance that prevents deviation from an arranged spatial relationship to the maxilla, the radiation oncology team can make shielding possible by way of replicable daily positioning of patients from the time of simulation to the completion of radiation therapy. This strategy is helpful when mandibular structures are to be spared from sinonasal or maxillary radiation or when maxillary structures are to be excluded from fields involving the floor of mouth, oral tongue, or other sites encompassing the mandible. In addition to stabilizing the maxillomandibular relationship, the incorporation of radiopaque materials such as orthodontic wire, ball bearings, or gutta percha into such devices can lend support to the radiation oncology team’s efforts to identify structures for inclusion in proposed radiation fields at the time of simulation (Fig. 98-3).
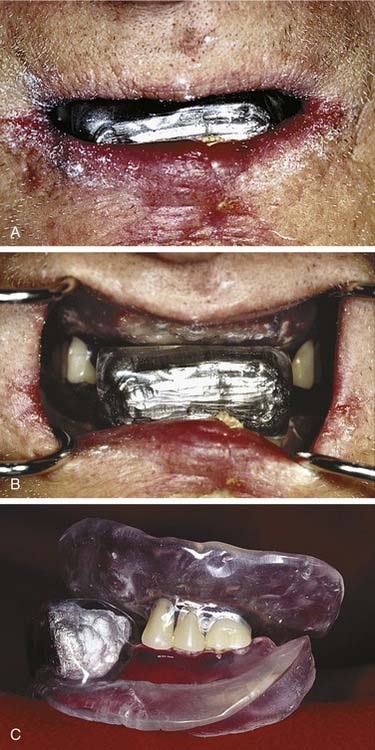
Furthermore, intraoral polymethylmethacrylate appliances can be constructed that harbor Lipowitz’s alloy.19,21,26 Being the same metal used by radiation oncology teams to create the portals and shields suspended from frames situated between patients and linear accelerator collimators that fashion conventional three-dimensional (3D) conformal radiation therapy fields, Lipowitz’s alloy, a low-fusing combination of lead, bismuth, tin, and cadmium, can suitably shield intraoral anatomy when electron beam therapy is indicated. These devices typically serve the dual purposes of shielding and maxillomandibular positioning because their interocclusal construction also stabilizes the maxillomandibular relationship. These alloy-containing intraoral appliances are often fabricated for lip (Fig. 98-4) and ipsilateral parotid gland (Fig. 98-5) fields. The protective intent of these appliances is supplemented when they are constructed in such a fashion as to physically displace soft tissues away from the source of an electron beam. When parotid gland shielding devices are used, patients are instructed to position the tongue anteriorly during treatment. Doing this while occluding on the interocclusal appliance forces the bulk of the tongue into a contralateral position where less radiation is encountered. When parotid gland malignancies are treated with a combination of photons and electrons, radiation oncologists sometimes find it helpful to use two appliances, one with Lipowitz’s alloy and one lacking metal. The use of the appliance devoid of alloy during the delivery of photons offers the advantages of consistent day-to-day maxillomandibular positioning and soft tissue displacement without the electron backscatter potentially associated with an appliance that harbors a large volume of metal.
The radiation oncologist’s desire for tissue equivalence is not limited to the application of surface boluses because postoperative tissue voids can give rise to an uneven distribution of radiation resulting in suboptimal dosing or overdosing of tissue peripheral to cavernous surgical defects such as those resulting from a maxillectomy. Unfortunately, however, the rigidity of wax and polymethylmethacrylate does not solely allow the dependable use of these materials as intracavitary tissue mimicking materials by virtue of soft tissue and bony undercuts usually associated with these defects. Consequently, filling an intracavitary void with tissue-equivalent material in the interest of improving radiation therapy dose distribution often requires the use of pliable materials that assist daily insertion and removal without unduly traumatizing surrounding tissue. The combination of an inflexible customized intraoral device that stabilizes the maxillomandibular relationship with a pliable material that fills an intracavitary void can, however, overcome the limitations posed by the complex nature of a maxillectomy defect. This combination can be achieved by constructing an intraoral positioning device that incorporates a shelf of polymethylmethacrylate immediately inferior to the maxillectomy defect. The device is capable of supporting a balloon that can be inflated with a tissue-equivalent mixture of radiopaque liquid and water before radiation therapy simulation and each fraction (Fig. 98-6).
Although the maxillofacial prosthodontist is not likely to lend assistance in the clinical application of interstitial brachytherapy, the management of intracavitary brachytherapy often requires the combined efforts of the radiation oncology and maxillofacial prosthetic teams. The orbit and nasopharynx comprise the most common intracavitary head and neck brachytherapy sites, and the stents used for these sites are typically constructed of polymethylmethacrylate that surrounds catheters into which radioisotopic seeds are inserted. The radiation oncologist or physicist, in accord with an intended dose distribution specific for the radioisotope proposed for use, prescribes the catheter positions in consultation with the maxillofacial prosthodontic team after the generation of a master cast from an impression of the site to be treated. When the stents are positioned, they are retained for the duration of treatment by anatomic soft tissue undercuts (Fig. 98-7) or dentoalveolar structures.
Dental Management after Radiation Therapy
Follow-up examinations should be arranged every 4 to 8 weeks after the completion of radiation therapy with patients returning to a standard interval thereafter as long as they comply with hygiene and daily topical fluoride gel regimens. Patients can be considered routine patients in terms of their candidacy for restorative dental care after head and neck radiation therapy; however, they must understand that they should not undertake bone-exposing oral surgery within anatomic areas that received more than 5000 cGy of radiation. Such exposures could prompt the development of osteoradionecrosis that could precipitate a pathologic fracture or necessitate a bony resection.2,6,7,9,12,27–34 Therefore when patients complete their radiation therapy, they should be asked to inform the maxillofacial prosthodontist of any future oral treatment proposal to include a tooth extraction, the surgical placement of an endosseous titanium implant, or periodontal or endodontic flap surgery in advance so that an estimation of dosimetry can be provided to the surgeon. When these estimates indicate that a bone-exposing violation of mucosa could occur in an area that received more than 5000 cGy of radiation, the Marx hyperbaric oxygen protocol should be considered as an adjunct to the surgical procedure.3,6,7,9,12,27,28,32 Marx’s hyperbaric oxygen regimen involves 90-minute dives at a barometric pressure of 2.4 atmospheres while breathing 100% oxygen. Twenty dives are undertaken preoperatively, and 10 dives are completed postoperatively in the interest of stimulating an angioneogenesis that partially and irreversibly32 counteracts the hypovascularity caused by radiation therapy that is responsible for the increased risk of osteoradionecrosis.
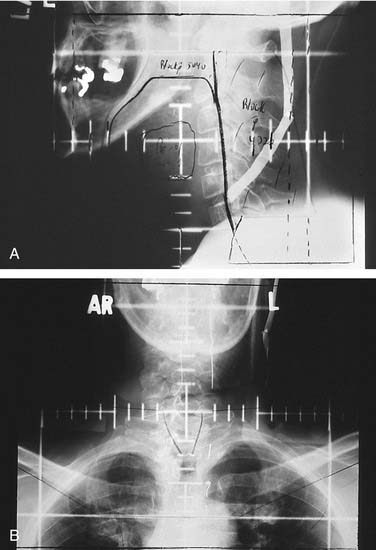
Oral surgical planning after conventional 3D conformal radiation therapy first involves the acquisition of simulation and portal radiographs, as well as a complete description of the radiation oncologist’s dosimetric and clinical assessments.3,6,19 Although the possession of simulation and portal radiographs provides the dental team a mere 2D perception of the fields prescribed by the radiation oncologist, their value in determining whether dentoalveolar structures of interest lie within or without treatment fields allows for surgical treatment planning often without the need for further conference with radiation oncology personnel. When parallel opposed field simulation and portal radiographs are shown to encompass dentoalveolar structures, the dental team must take for granted the fact that an invasive procedure within that field will place the patient at risk for osteoradionecrosis if the prescribed dose to that field surpassed 5000 cGy. However, as is frequently the case in head and neck radiation oncology, the exclusion of dentoalveolar structures anterior, superior, or inferior to a field boundary allows surgical intervention in the excluded area without risking osteoradionecrosis (Fig. 98-8) because osteoradionecrosis by definition comprises a volume of necrotic bone within a radiation therapy field.30 Uncertainty can arise, however, when treatment field borders bisect or tangentially appose dentoalveolar structures to be included in a proposed surgical intervention. Although dosages at the borders of conventional 3D conformal radiation therapy fields approximate only 50% of the prescribed dose, patient positioning can vary by as much as 5 mm in any given dimension despite exceptional efforts on the part of radiation oncology teams to reproducibly orient their patients on a daily basis. Considering the possibility of positioning errors during the course of daily radiation therapy sessions, such scenarios warrant erring on the side of caution with the assumption that affected field border areas could become osteoradionecrotic after a surgical insult.
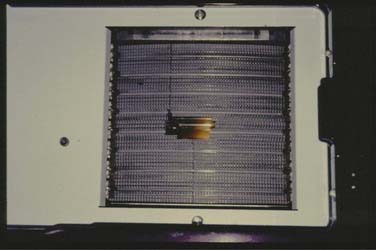
In contrast to conventional 3D conformal radiation therapy, in which gantry positions are limited and static, intensity-modulated radiation therapy can be delivered by way of a mobile collimator. The collimator arcs across several swaths of parallel appositional fields while two rows of tungsten leaves within the collimator (Fig. 98-9) concentrically open and close to strategically deliver radiation to targeted tissues while sparing normal structures of the highest radiation burdens.22,35–38 In another technique that has come to be known as step and shoot intensity-modulated radiation therapy, radiation is directed from multiple stationary gantry positions that are each uniquely and statically collimated through the use of multiple layers of tungsten leaves capable of converging from the periphery of the linear accelerator’s nonadjustable port to fashion customized portal shapes in accord with the radiation oncologist’s or physicist’s treatment prescription (Fig. 98-10). In either scenario, intensity-modulated radiation therapy simulation is based on an interface between computed tomography imaging and dosimetric treatment planning software such as the CORVUS system. As such, intensity-modulated radiation therapy simulation and portal radiographs offer limited or no value for establishing surgically induced osteoradionecrosis risks after radiation therapy, and intensity-modulated radiation therapy dosimetry must be evaluated using software that is at this time almost universally unavailable to any dental team. This software, however, offers an evaluation of each computer-generated tomographic slice that is so detailed in its resolution that anatomic structures as small as individual tooth roots can be identified and dosages can be estimated at the pinpoint level of a computer mouse cursor superimposed over the area of dosimetric interest. Because CORVUS and other intensity-modulated radiation therapy software firms offer axial, sagittal, and coronal tomography sections, a comprehensive 3D depiction of dosimetry can be achieved by means of a review of computer records. This can be done without the need for assessing multiple simulation and portal radiographs that may be geometrically confusing, tattered from age, untidily drawn, of poor radiographic quality, or entirely lost or discarded.
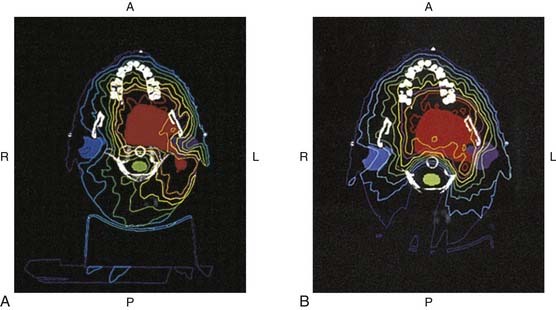
Although intensity-modulated radiation therapy dosimetric evaluations performed in the interest of establishing the relative safety of proposed dental interventions are readily achieved by way of the dental team’s access to computer records when maxillofacial prosthodontic support is found in the facility in which a patient’s therapy was delivered, communicative problems can arise between dental and radiation oncology personnel when they are separated geographically. Radiation oncology personnel are not always familiar with dental anatomy and terminology or oral surgical procedures, and dental personnel are not generally knowledgeable of intensity-modulated radiation therapy computer simulation packages or the general delivery of intensity-modulated radiation therapy. It can thus benefit dental practitioners routinely involved in invasive oral procedures to personally meet a radiation oncology team involved in the delivery of intensity-modulated radiation therapy before encountering their first head and neck intensity-modulated radiation therapy patient. Conversely, dental personnel can share an oral surgical treatment plan with a patient’s radiation oncology team face-to-face when they first encounter a patient treated by way of intensity-modulated radiation therapy. Either way, an initial interdisciplinary conference at the intensity-modulated radiation therapy facility could lend clarification to each team’s needs and concerns when future patients are shared and fiscal, travel, and time constraints make it impractical or impossible to convene personally. Once dental and intensity-modulated radiation therapy providers possess a working knowledge of one another’s methodology and concerns, distant communication can be assisted. The most convenient means by which to request dosimetric data from a remote intensity-modulated radiation therapy center involves obtaining printed colorized serial computed tomograms displaying isodose information that is superimposed over imaged anatomy (Fig. 98-11). These images are generated by all intensity-modulated radiation therapy computer packages and offer a reasonable representation of dosages delivered in three dimensions. A common impediment to dental teams’ treatment planning efforts involves their receipt of black-and-white photocopied computerized tomography isodose images that are essentially illegible by virtue of the fact that intensity-modulated radiation therapy computer packages routinely rely on the use of color for the assignment of dosimetric values.
Overview of Maxillofacial Prosthodontic and Radiation Oncology Interactions
Intraoral Rehabilitation of Tumor-Ablative Surgical Defects
The incidence of oral cancer approaches about 5% of all new cancers diagnosed in the general population of the United States.21 A significant number of head and neck oncology patients are treated for neoplasms of the lip, tongue, oropharynx, mandible, maxilla, soft palate, larynx, external ear, orbit, and external nose. The highest incidence of oncologic disease afflicts those persons with significant risk factors such as an excessive use of alcohol and tobacco or exposure to ultraviolet light and human papillomaviruses. To successfully eradicate disease, these tumors are treated with multimodal therapy including tumor-ablative surgery, radiotherapy, and chemotherapy. Patients treated for tumor-ablative surgery of the oral and pharyngeal areas may have significant anatomic deficits compromising speech, swallowing, and mastication.
Prosthetic Maxillary Obturation
The maxilla may require resection for tumor control that may create a host of problems related to speech, swallowing, and aesthetics. Traditional resection of the maxilla involves an infrastructure, medial, or total maxillectomy to include the infraorbital rim. The maxillectomy procedure used to control incipient disease of the oral cavity has been classified by Aramany39 on the basis of frequency of occurrence. As with other anatomic defects, tumor surveillance dictates keeping these areas clearly visible to detect recurrences, should they arise. Obviously, the more teeth, bone, and soft tissue available, the more easily prosthetic rehabilitation can be achieved. However, edentulous and some partially dentate patients requiring a maxillectomy may have significant difficulty in obtaining stability with prostheses, and in these cases a consideration for the use of dental implants may be warranted.
Surgical Obturation
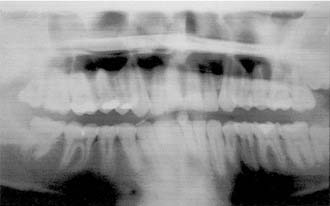
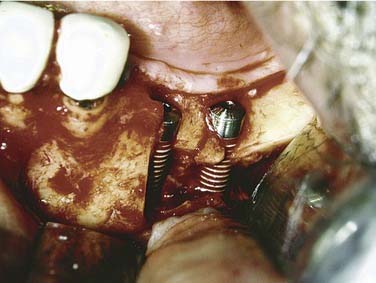
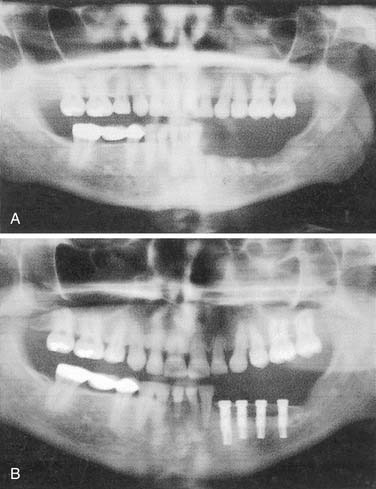
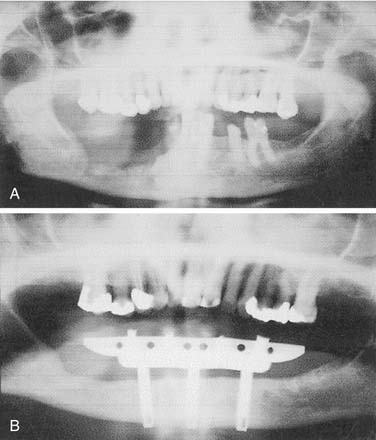
Resection of maxillary tumors that involve the oral cavity is dedicated to total eradication of disease. Similarly, preservation of anatomy conducive to prosthetic rehabilitation should be considered. Preoperative assessment of the patient by the maxillofacial prosthodontist is integral to overall treatment success by the head and neck treatment team (Fig. 98-12). Clinical examination with panoramic radiography may assist with decisions to extract diseased teeth at the time of surgical resection (Fig. 98-13). In this way, if adjunctive radiotherapy is necessary, a 4- to 6-week healing time offers a distinct advantage (Fig. 98-14). Preoperative assessment also aids to plan osteotomy planes through the existing tooth sockets in dentate patients to preserve the support of adjacent teeth.40 These teeth, also called abutments, may be strategic for retention of a prosthesis. In fact, the more arch curvature formed by remaining teeth, the more favorable retention may be for a prosthesis of this type.
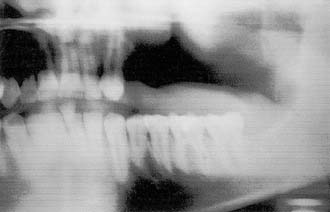
Figure 98-14. Postoperative panoramic radiograph of patient in Figure 98-13. Note removal of maxillary segment and impacted lower third molar with ample healing time before radiation therapy.
Arches planned for maxillectomy should be approached with the thought of disease eradication. As a secondary thought, preservation of structures vital for support should be kept in mind for maximum potential of successful rehabilitation. The first of these preserves as much of the hard palate as possible, which is the primary stabilizing structure of a maxillary prosthesis. Second is grafting of the cheek flap with epidermis or split-thickness skin (discussed further later). Third comes removal of the inferior conchae, which, if not eliminated, frequently obstruct proper obturation. This is most evident with posterior and some lateral resections that involve the oral cavity. Finally, the sinus walls should be grafted, if possible. Again, split-thickness skin will limit polypoid tissue formation and keep mucus formation to a minimum.41 The roof of a sinus grafted with skin will allow its use as a supporting wall for the prosthesis.
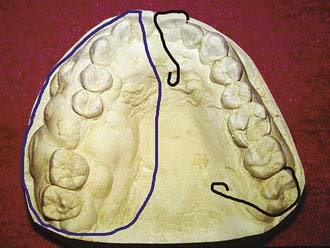
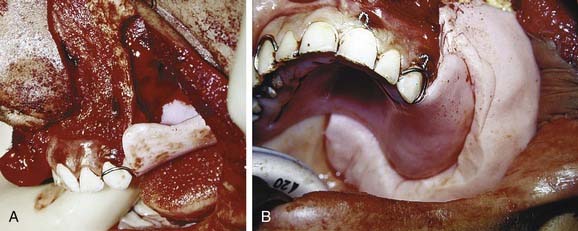
Impressions can be made before surgery to provide diagnostic casts (Fig. 98-15). These casts will aid in fabrication of a prosthesis to be inserted at the time of surgery. These prostheses are usually made from polymethylmethacrylate resin and can be made with or without prosthetic teeth. The advantage of having no functioning dental components to these prostheses is that occlusal forces are left off the wound to allow for efficient healing. A surgical prosthesis offers advantages relating to speech, swallowing, and psychological well-being. Also, the prosthesis is helpful in securing a surgical bolster and aids the patient in acclimating to wearing a removable prosthesis. Because respiratory epithelium may remain within a sinus defect, prosthetic loading of this area can be problematic. The ability to clean the defect also becomes a concern, with hardened mucus being a particular obstacle for placement of a prosthesis. Grafting of the defect with split-thickness skin can create a more comfortable surface with fewer mucus secretions. As with any skin graft, positive pressure with a surgical bolster can aid with providing this type of surface. Perforating the graft with dispersed small incisions can minimize the likelihood of hematoma formation, thus improving take of the skin graft. In partially dentate patients, securing the prosthesis can be accomplished by incorporating stainless steel wrought wires in the prosthesis that encircle the most terminal teeth (also called abutments) (Fig. 98-16). In addition, the prosthesis can be wired to the abutment tooth closest to the defect using 25-gauge stainless steel wires. In edentulous patients, the prosthesis can be held in place using sutures, circum-zygomatic wiring, or several titanium screws (2.7, 10 mm) into the remaining hard palate. Care should be taken to ensure that the screws are placed in an angled fashion so as to allow screwdriver insertion at pack removal. In most cases the surgical prosthesis should be kept in place between 5 and 7 days.
After this time, removal of the packing material and prosthesis should ideally be undertaken by the surgeon and prosthodontist. Removal of the packing material can precipitate a hemorrhage, and preparations with oxidized cellulose, gelatin foam, or microfibrillar collagen can be invaluable. At this time, transitioning the prosthesis by addition of tissue-conditioning material assists patients in becoming accustomed to placement and removal (Fig. 98-17). The patient is also instructed on how to cleanse the defect twice daily with a dilute solution of baking soda and salt. This can best be accomplished using an irrigation syringe and a dispersion attachment head. The patient returns weekly during the following month for changing of the material on the prosthesis as the defect continues to change shape.
Definitive Obturation
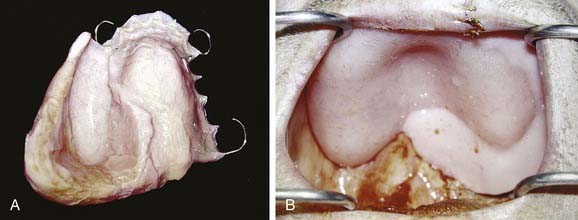
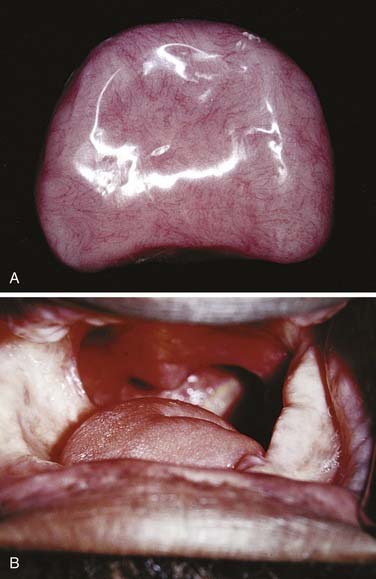
After an extended period of healing (i.e., 6 weeks) following surgery and/or radiation therapy fabrication of a definitive prosthesis may take place. For dentate patients, this prosthesis can be made of either acrylic resin and stainless steel wrought wire clasps or a cast chromium framework that supports a bulb extension made of polymethylmethacrylate (Fig. 98-18). Specific recommendations for design have been well published.26 Prostheses with masses of less than 45 g may remain solid, but prostheses of greater masses should be hollowed.16,42 Edentulous patients are treated with prostheses made entirely of acrylic resin, the bulbs of which can be hollowed similarly. Teeth placed in the area of the defect serve as aesthetic replacements and prevent opposing tooth supraeruption but are of limited functional value.
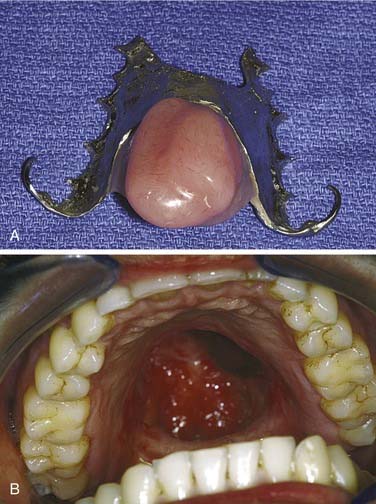
Figure 98-18. Definitive obturator made with cast chrome base (A) for resection defect of pleomorphic adenoma (B).
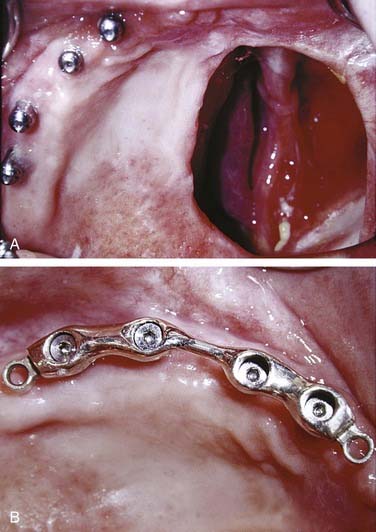
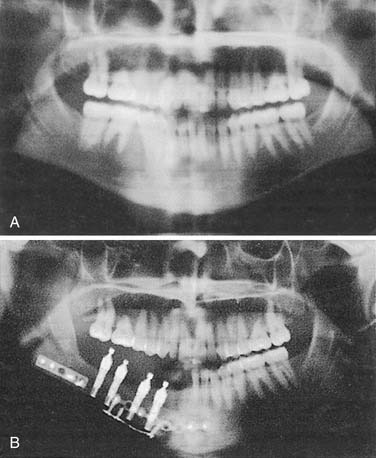
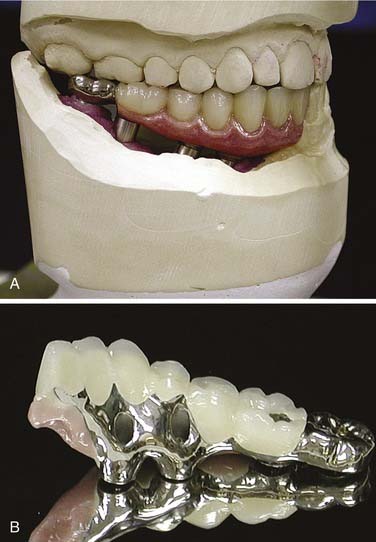
Many edentulous or partially edentulous patients have difficulty with retention and, in some cases, difficulty with support as well. In these cases, osseointegrated implants can anchor and support the obturator prosthesis. Although long-term follow-up studies of patients possessing implants in irradiated bone warrants their cautious use, implants remain potentially advantageous.43 Because maxillary sinuses may be extensively pneumatized following tooth extractions, installation of implants into this area can be problematic unless bone is grafted into the sinuses. This use of sinus augmentation has been well documented and deemed to be successful with the use of implants.44 This technique can be used on a nondefect side where a unilateral or posterolateral defect of the opposite side is present. Splinting of approximately four to five implants with a stress-breaking bar is generally suggested and provides the patient with a retentive, stable prosthesis that may offer improved support as well (Fig. 98-19). Recently, the use of zygomatic implants (extended length) has been suggested as an alternative to sinus augmentation.20,45 The implant protocol for zygomatic implants mandates bilateral placement, and preservation of the infraorbital rim on the defect side may improve surgical stability. Both techniques require a screw-retained bar attachment to be made with the obturator (Fig. 98-20).46 Remote anchorage sites have been suggested as a means of stabilizing prostheses rehabilitating maxillary defects.46,47
Mandibular Resection Prostheses
If a marginal mandibulectomy is performed, a general guideline is to surgically preserve about 1 cm of vertical mandibular height for integrity. Subsequently, the remaining mandible can be rehabilitated with a split-thickness skin graft and removable tissue-borne prostheses, or alternatively it can be reconstructed with osseointegrated dental implants. In the dentate patient, preservation of the inferior alveolar nerve in the posterior mandible may preclude placement of implants if there is minimal bone available above the canal position to stabilize implants. In these cases, either nerve transposition48 (Fig. 98-21) or onlay bone grafting can serve to provide an osseointegrated rehabilitation (Fig. 98-22).
In edentulous patients, placement of either conventional prosthetics or implant supported/retained prosthetics may be desirable. Often, prosthetics can rely on select placement of implants in the anterior mandible with specified posterior occlusal cantilevering (Fig. 98-23). This procedure is well documented, with recent evidence indicating immediate functionality when implants are placed in the native mandible.49,50
Discontinuity Defects
If a discontinuity defect is created by composite resection, it may be feasible to collapse the defect and not reconstruct mandibular continuity. This becomes more feasible if the patient is edentulous because dentate patients will have significant occlusal discrepancies that make postoperative rehabilitation difficult to impossible. In these cases a significant cosmetic deformity may result because the lower third of the face is asymmetric (Fig. 98-24). Also, disability of the tongue usually results because portions of the tongue may be used to close the wound, making swallowing problems inevitable. Additional consideration is given to the fate of the proximal resection segment of the mandible after the resection. Reconstruction bars may create additional backscatter radiation if adjunctive radiation is used for tumor control (Fig. 98-25).
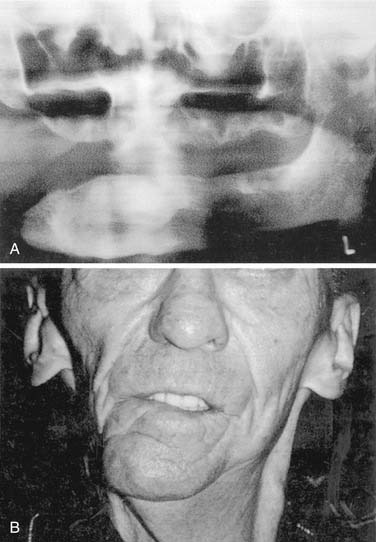
Figure 98-24. A, Mandibular composite resection without reconstruction resulting in, B, significant mandibular deviation.
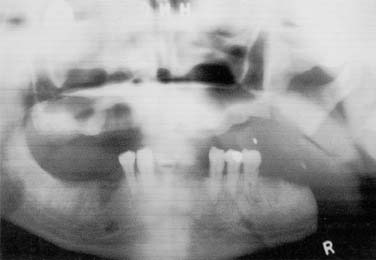
Figure 98-25. Proximal segment without reconstruction results in deviation and displacement against maxillary posterior ridge.
The key to functioning in these cases lies with preservation of the ramus, for which attachment of the muscles of mastication is preserved. Through removal of the ramus, less function is evident without balance of contralateral pull of pterygoid, temporal, and masseter muscles. If more of the ramus is retained and not maintained in its preoperative position, it may collapse on the maxillary buccal vestibule and obstruct maxillary dental hygiene procedures or the fabrication of a maxillary prosthesis. Furthermore, the ramal segment could be sequestered into the oral cavity. Reconstruction in these cases is the wiser choice and does not advocate joining the plate to small condylar remnants.51 If mandibular continuity is not preserved after resection, it may be desirable to reconstruct the area with an autogenous or alloplastic graft. Location of the resection may be indicative of the relative success of the reconstruction. Anterior reconstructions that cross the midline with a plate system alone may be less successful than those with an osseous reconstruction as well.52 Cosmetic and functional deformities are also more of a consideration in the anterior mandibular areas. This is perhaps due to the influence of the suprahyoid musculature on swallowing and respiration. Concurrently, it is considerably difficult to conform an osseous graft to an arch form in this area. Posterior or lateral resections appear to be less debilitating and problematic because a linear replacement of bone is frequently sufficient to reconstruct this area. A reconstruction with pedicled soft tissue may serve to deviate the remaining mandibular segments to the affected side. Osteomyocutaneous flaps are superior in reconstructing mandibular discontinuity to avoid this deviation and preserve maxillomandibular relationships vital to rehabilitation.
In some cases, autologous grafts offer a greater volume of viable bone with progenitor cells capable of creating a more favorable environment for osseointegration.36 Nonvascularized or vascularized osteomyocutaneous flaps can also be used for reconstruction. It may be preferable to use vascularized flaps in previous operative beds or in areas that have been or will be irradiated, because the provision of a blood supply may offer a more predictable opportunity for the graft to remain viable. Vascularized iliac crest has been used with some degree of success for mandibular defects and some maxillary defects. Introduced by Hidalgo,53,54 the use of vascularized fibular grafts has also shown a promising degree of success in the reconstruction of these complex mandibular defects.55 Being a non-weight-bearing bone, the fibula is of reasonable dimension to functionally and cosmetically reconstruct the mandible. Additional phases of rehabilitation with titanium implants have been demonstrated to be uniquely applicable to these cases. Bicortical stability is typically well obtained at surgical installation, and long-term success has been favorable (Fig. 98-26). The choice of using either a sectional overdenture design or a screw-retained fixed prosthesis may be predicated on the amount of tissue missing, the function of the tongue, perioral scarring, and adjacent/opposing occlusion (Fig. 98-27). Frequently, the restoration to implant height ratio is seen to be greater than 1 : 1. Passive splinting of these implants is crucial to their long-term success, and close attention should be paid to the development of the occlusal scheme. Occasionally, it may be necessary to perform soft tissue revision procedures if the skin pedicle is thick or if a greater vestibular depth is necessary. This ensures soft tissue health and visibility for hygiene procedures.
Tongue Resection
The competency of the lips is mandatory for the oral phase to function effectively because a lip seal will create a boundary for bolus transportation. Mastication is an independent function of the tongue for tougher-consistency foods, and efficiency is also dependent on dental status of the patient. The time taken for the bolus to be transported to the posterior oral cavity past the anterior faucial arch demarcates the oral transit time. In healthy adults, the oral transit time can be about 1 to 2 seconds. After tumor-ablative surgery of the tongue or mandible, the range of motion needed for swallowing and speech production may not be sufficient. In these cases the height of the palatal vault may not allow appositional contact of the remaining tongue tissue to propel the bolus of food or liquid into the pharyngeal area where the involuntary phase of the swallowing reflex is initiated. Consequently, lowering of the palatal surface prosthetically can aid in providing a surface that approximates the extent of the tongue’s range of motion postsurgically. This prosthesis, known as a palatal augmentation prosthesis, can be used in dentate or edentulous patients (Fig. 98-28).56,57 In patients with dysphagia, palatal augmentation prostheses have been shown to decrease oral transit times, thereby increasing swallowing efficiency.58
Articulatory speech debility is dependent on the area(s) of the tongue that are resected. For instance, resection of the anterior or tip of the tongue may not allow articulation of linguoalveolar sounds such as /t/, /d/, /n/, or /s/. In other cases the base or sides of the tongue may be resected to create deficiencies in /g/, /ng/, /k/, or /c/. In these cases a prosthesis may serve to better aid production of these sounds. Because the shape needed for swallowing may be markedly different from that which is necessary for speech production, a prosthesis with interchangeable surfaces being incorporated into a palatal augmentation or a mandibular “tongue” prosthesis has been advocated. Each respective surface is used specifically for speech or swallowing.51,59 Regardless of the type of prosthesis being constructed, involvement of a speech pathologist can be quite instructive to patients and the maxillofacial prosthodontist.
Soft Palate Resection
As a component of Waldeyer’s ring, the soft palate is occasionally included in resection of tumors of squamous cell origin. Because of its dynamic nature, the soft palate, like the tongue, is integral to swallowing and speech. The anterior faucial arch serves as a landmark for the involuntary phase of swallowing and, if included in resection, can predispose the patient to aspiration. An anatomic deficit of the soft palate can also create velopharyngeal insufficiency, which may result in nasal regurgitation of swallowed matter and nasal speech production. Replacement of velar function with pharyngeal flaps is reserved for conservative soft palatal defects that may be acquired or congenital. A prosthesis (known as a speech aid) replacing soft palate function should obturate the pharyngeal recess at the level of the first cervical vertebra or Passavant’s pad, frequently seen as a conglomeration of muscular tissue in the superior pharyngeal constrictor (Fig. 98-29). At this level, the extension of the prosthesis should serve to partially fill the pharyngeal recess while providing room for the dynamic nature of the pharyngeal and tongue musculature. A stable reference for the prosthesis starts with assessment of the oral cavity. Anchorage for the prosthesis can be tooth borne or partially or completely tissue borne. For tooth-borne prostheses, anchorage with molar teeth becomes advantageous because anchorage closer to the vectors of dislodging forces discourages premature loosening with function.
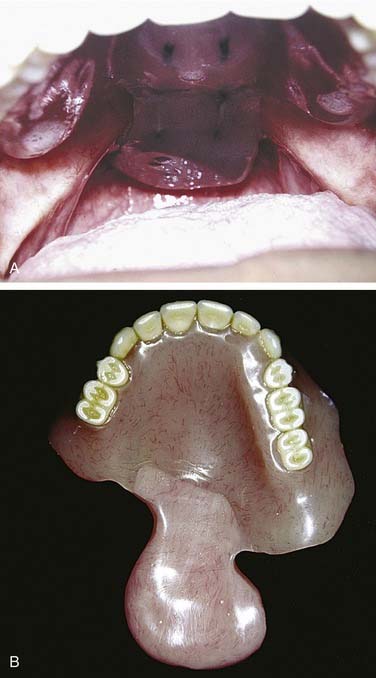
Figure 98-29. Patient with soft palate resection restored with speech aid (A) or pharyngeal obturator prosthesis (B).
Soft palate function can also be affected by cerebrovascular accidents, demyelination diseases, or surgical procedures. The anatomic structures, in these cases, may be incompetent in performing velopharyngeal closure. A palatal lift prosthesis may serve to provide the patient also with better speech production and improved swallowing patterns and it may stimulate the musculature to perform at increased functional levels.60–62 For the palatal lift prosthesis to be effective, some pharyngeal muscular movement must be present. To effectively evaluate the closure of the soft palate, a nasal endoscope can aid in directly identifying deficiencies.
Prosthetic Management of Acquired Facial Defects
Prosthetic management of facial defects has its disadvantages as well. Although excellent contour and color are attainable, the prosthetic materials used have fairly limited life spans—usually 18 to 24 months. The prosthesis may have to be retinted during that time, depending on its environment. Craniofacial implants or adhesives must be used to retain the prosthesis, and it should be removed for a few hours regularly each day to maintain the underlying tissues in a healthy state. Various masking techniques such as large-framed glasses or cosmetics are often used to hide margins. Some patients develop chronic mechanical tissue irritation from wearing prostheses, and allergic responses, though not common, may affect prosthesis use. Despite this, excellent cosmetic results and patient acceptance are obtainable in the vast majority of cases (Table 98-1).
Nasal Defects
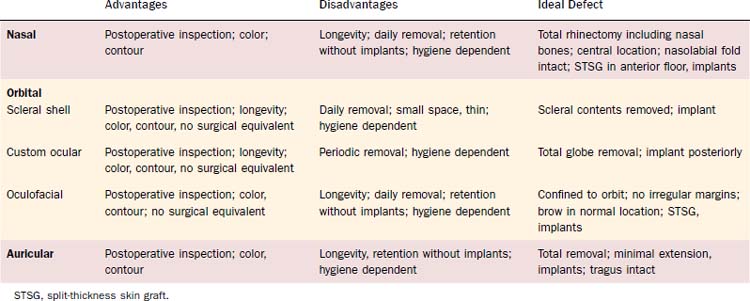
Many variations of acquired defects are caused by ablative surgery involving the nose. Where there is a relatively small, isolated defect, a split- or full-thickness skin graft can be used if primary closure is not feasible. In these cases, prosthetic management need not be considered unless the surgeon wishes to observe the defect for an extended period of time. Defects involving greater areas including not only skin but bone, subcutaneous tissues, and mucosal lining can be reconstructed using various flap procedures with or without autogenous support. If more than half the nose is resected, a complete rhinectomy should be considered. Alar tabs or unsupported tissue surrounding the defect do nothing for prosthetic support and, in fact, hinder the fabrication of a cosmetic prosthesis. Although it may be possible for the patient to wear a prosthesis, more margins will be visible, thus compromising the aesthetic value. Centrally located defects, leaving the nasolabial folds intact with surrounding supported tissue, provide the best base for restoration (Fig. 98-30). Those defects that extend beyond the nose laterally or toward the upper lip are more difficult to restore due to greater tissue mobility in these areas; they therefore require greater exposure of margins. Placement of split-thickness skin grafts, particularly at those margins extending toward the lip, will be beneficial in preventing scar contraction of the lip and will provide a firm, immobile base for the inferior margin of the prosthesis. Craniofacial implants are useful in the retention of a nasal prosthesis and should be placed in the anterior floor of the nose where there is sufficient bony support. They are best used in conjunction with split-thickness skin grafts, and their position should be such that the abutments will extend into the greatest thickness of the subsequent nasal prosthesis (Fig. 98-31).
Auricular Defects
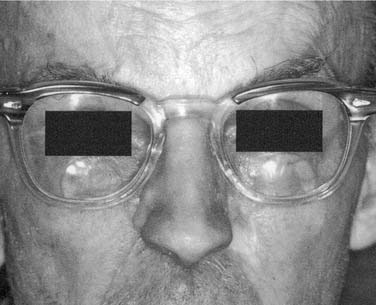
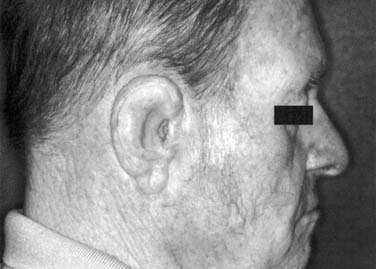
As with nasal defects, flexible remnants of the ear such as the lobe and helix should be removed unless surgical reconstruction of the ear is anticipated. The tragus, however, should be retained because part of the anterior margin of the prosthesis can be hidden behind it. A defect that results from a partial resection is much more difficult to restore prosthetically than that of a total auriculectomy. If an attempt at surgical reconstruction has been made and has failed, the tissue should be completely removed (Fig. 98-32). A split-thickness skin graft will provide a firm, immobile tissue bed for the prosthesis. Full-thickness or hair-bearing skin grafts should not be used. It should be remembered that prosthesis margins that must overlay the condylar area can loosen during function if they rely on adhesive for retention. If implants can be placed in a position such that they will be in the thickest part of the auricular prosthesis, usually close to the helix, they will provide maximum retention and patient confidence.
The auricular prosthesis has two major advantages over the nasal or midface prosthesis; the viewer cannot focus on both ears at the same time because they are located laterally, and the patient’s hair can be used to hide a portion of the margin (Fig. 98-33).
Ocular Defects
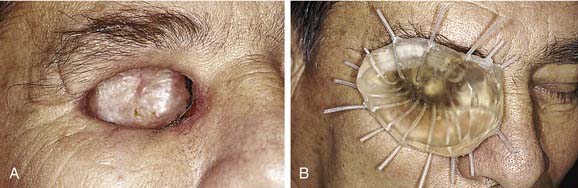
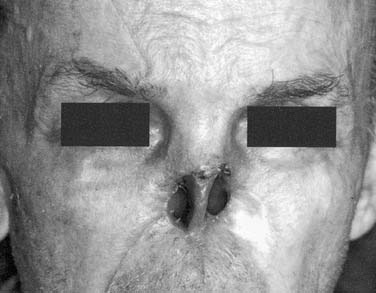
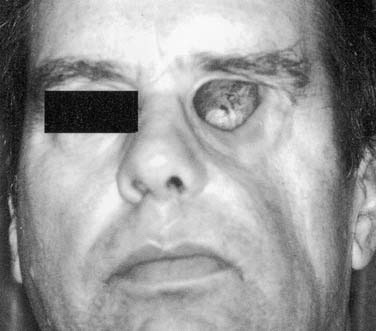
An ideal defect is one that is within the orbital boundaries with no marginal irregularities, lined with a split-thickness skin graft, and having sufficient depth to recreate normal anatomy with the oculo-facial prosthesis (Fig. 98-34). Without the skin graft, the tissues are much more sensitive and difficult to manage hygienically. If the eyebrow cannot be kept in normal position, it is best to remove it and include it in the area of the skin graft. Where possible, placement of implants in the lateral or superior orbital rims is a great advantage for retention of the prosthesis. As with placement for other types of facial prostheses, location of the implants and their angulation should be such that they will be in the thickest portion of the prosthesis. If these requirements can be met without compromising the patient’s cure, it will be possible to provide a defect that the patient can manage hygienically and one for which the most aesthetically pleasing and retentive prosthesis can be made (Fig. 98-35).
Beumer J, Curtis TA, Marunick MT, editors. Maxillofacial Rehabilitation: Prosthodontic and Surgical Considerations. St. Louis: Ishiyaku EuroAmerica, 1996.
Block MS, Kent JN, editors. Endosseous Implants for Maxillofacial Reconstruction. Philadelphia: WB Saunders Company, 1995.
Branemark PI, de Oliveira MF, editors. Craniofacial Prostheses: Anaplastology and Osseointegration. Chicago: Quintessence Publishing Company, 1997.
McKinstry RE, editor. Cleft Palate Dentistry. Arlington: ABI Professional Publications, 1998.
McKinstry RE, editor. Fundamentals of Facial Prosthetics. Arlington: ABI Professional Publications, 1995.
Taylor TD, editor. Clinical Maxillofacial Prosthetics. Chicago: Quintessence Publishing Company, 2000.
Thomas KF. Prosthetic Rehabilitation. London: Quintessence Publishing Company; 1994.
1. Beumer J, Curtis T, Harrison RE. Radiation therapy of the oral cavity: sequelae and management, part 1. Head Neck Surg. 1979;1:301-312.
2. Beumer J, Curtis T, Harrison RE. Radiation therapy of the oral cavity: sequelae and management, part 2. Head Neck Surg. 1979;1:392-408.
3. Beumer J, Curtis TA, Nishimura R. Radiation therapy of head and neck tumors: oral effects, dental manifestations, and dental treatment. In: Beumer J, Curtis TA, Marunick MT, editors. Maxillofacial Rehabilitation: Prosthodontic and Surgical Considerations. St. Louis: Ishiyaku EuroAmerica, Inc, 1996.
4. Beumer JIII, Harrison R, Sanders B, et al. Preradiation dental extractions and the incidence of bone necrosis. Head Neck Surg. 1983;5:514-521.
5. Jansma J, Vissink A, Spijkervet FK, et al. Protocol for the prevention and treatment of oral sequelae resulting head and neck radiation therapy. Cancer. 1992;70:2171-2180.
6. Kramer DC. The radiation therapy patient: treatment planning and posttreatment care. In: Taylor TD, editor. Clinical Maxillofacial Prosthetics. Chicago: Quintessence Publishing Company, 2000.
7. Little JW, Falace DA, Miller CS, et al. Dental Management of the Medically Compromised Patient, ed 6. St. Louis: Mosby; 2002.
8. Lockhart PB, Clark J. Pretherapy dental status of patients with malignant conditions of the head and neck. Oral Surg Oral Med Oral Pathol. 1994;77:236-241.
9. Markt JC. Pre-irradiation dental evaluation. In: Hoffman HT, et al, editors. Iowa Head and Neck Protocols: Surgery, Nursing, and Speech Pathology. San Diego: Singular Publishing Group, 2000.
10. Maxymiw WG, Rothney LM, Sutcliffe SB. Reduction in the incidence of postradiation dental complications in cancer patients by continuous quality improvement techniques. Can J Oncol. 1994;4:233-237.
11. Rothwell BR. Prevention and treatment of the orofacial complications of radiotherapy. JADA. 1987;114:316-322.
12. Sandow PL, Baughman RA. Dental and oral care for the head and neck cancer patient. In Million RR, Cassisi NJ, editors: Management of Head and Neck Cancer: A Multidisciplinary Approach, ed 2, Philadelphia: JB Lippincott Company, 1994.
13. Singh N, Scully C, Joyston-Bechal S. Oral complications of cancer therapies: prevention and management. Clin Oncol. 1996;8:15-24.
14. Fleming TJ. Oral tissue changes of radiation-oncology and their management. Dent Clin North Am. 1990;34:223-237.
15. Katz S. The use of fluoride and chlorhexidine for the prevention of radiation caries. JADA. 1982;104:164-170.
16. King GE. Obturator framework design, continuing education. Presented at the MD Anderson Cancer Center Symposium on Maxillofacial Prosthetic and Dental Oncology, March 1998.
17. Liu RP, Fleming TJ, Toth BB, et al. Salivary flow rates in patients with head and neck cancer 0.5 to 25 years after radiotherapy. Oral Surg Oral Med Oral Pathol. 1990;70:724-729.
18. Toljanic JA, Saunders VW. Radiation therapy and management of the irradiated patient. J Prosthet Dent. 1984;52:852-858.
19. Ang KK, Kaanders JHAM, Peters LJ. Radiotherapy for Head and Neck Cancers: Indications and Techniques. Philadelphia: Lea & Febiger; 1994.
20. Bedrossian E, Stumpel L, Beckely ML, Indresano T. The zygomatic implant: preliminary data on treatment of severely resorbed maxillae: a clinical report. 3rd. Int J Oral Maxillofac Implants. 2002;17:861-865.
21. Canto MT, Devesa SS. Oral cavity and pharynx cancers incidence rates in the United States, 1975-1998. Oral Oncol. 2002;38:610-617.
22. Chao KS, Low DA, Perez CA, et al. Intensity-modulated radiation therapy in head and neck cancers: the Mallinckrodt experience. Int J Cancer (Radiat Oncol Invest). 2000;90:92-103.
23. Fleming TJ, Rambach SC. A tongue-shielding radiation stent. J Prosthet Dent. 1983;49:389-392.
24. Kaanders JHAM, Fleming TJ, Ang KK, et al. Devices valuable in head and neck radiotherapy. Int J Radiat Oncol Biol Phys. 1992;23:639-645.
25. Van Derhei PM, Lim WC, Timmerman RD, et al. Use of computed tomography for the fabrication of a custom brachytherapy carrier: a clinical report. J Prosthet Dent. 2003;89:15-18.
26. Beumer J, Curtis TA, Marunick M. Maxillofacial rehabilitation: prosthodontic considerations, ed 2. St. Louis: Ishiyaku Euroamerica; 1997.
27. Granstrom G, Jacobsson M, Tjellstrom A. Titanium implants in irradiated tissue: benefits from hyperbaric oxygen. Int J Oral Maxillofac Implants. 1992;7:15-25.
28. Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41:351-357.
29. Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283-288.
30. Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol. 1987;64:379-390.
31. Marx RE, Johnson RP, Kline SN. Prevention of osteoradionecrosis: a randomized prospective clinical trial of hyperbaric oxygen versus penicillin. JADA. 1985;111:49-54.
32. Marx RE, Stern D. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Chicago: Quintessence Publishing Company, Inc; 2003.
33. Silverman S. Complications of treatment. In Silverman S, editor: Oral Cancer, ed 4, Hamilton, Ontario: BC Decker, 1998.
34. Widmark G, Sagne S, Heikel P. Osteoradionecrosis of the jaws. Int J Oral Maxillofac Surg. 1989;18:302-306.
35. Curran B. Where goest the peacock? Med Dosim. 2001;26:3-9.
36. Dierks EJ, Karakourtis MH. Segmental resection of the anterior mandibular arch with fibular microvascular reconstruction. Atlas Oral Maxillofac Surg Clin North Am. 1997;5:55-73.
37. Eisbruch A, Dawson LA, Kim HM, et al. Conformal and intensity modulated irradiation of head and neck cancer: the potential for improved target irradiation, salivary gland function, and quality of life. Acta Oto-Rhino-Laryngologica Belg. 1999;53:271-275.
38. Grant W, Woo SY. Clinical and financial issues for intensity-modulated radiation therapy delivery. Semin Radiat Oncol. 1999;9:99-107.
39. Aramany MA. Basic principles of obturator design for partially edentulous patients: part I classification. J Prosthet Dent. 1978;40:554-557.
40. Rahn AO, Goldman BM, Parr GR. Prosthodontic principles in surgical planning for maxillary and mandibular resection patients. J Prosthet Dent. 1979;42:429-433.
41. Jacob RFK. Clinical management of the edentulous maxillectomy patient. In: Taylor TD, editor. Clinical Maxillofacial Prosthetics. Chicago: Quintessence; 2000:85-102.
42. Schwartzmann B, Caputo AA, Beumer J. Gravity-induced stresses by an obturator prosthesis. J Prosthet Dent. 1990;64:466-468.
43. Roumanas ED, Nishimura RD, Davis BK, et al. Clinical evaluation of implants retaining edentulous maxillary obturator prostheses. J Prosthet Dent. 1997;77:184-190.
44. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38:613-616.
45. Boyes-Varley JG, Howes DG, Lownie JF. The zygomaticus implant protocol in the treatment of the severely resorbed maxilla. SADJ. 2003;58:106-109.
46. Nakai H, Okazaki Y, Ueda M. Clinical application of zygomatic implants for rehabilitation of the severely resorbed maxilla: a clinical report. Int J Oral Maxillofac Implants. 2003;18:566-570.
47. Parel SM, Brånemark PI, Ohrnell LO, et al. Remote implant anchorage for the rehabilitation of maxillary defects. J Prosthet Dent. 2001;86:377-381.
48. Louis PJ. Inferior alveolar nerve repositioning. Atlas Oral Maxillofac Surg Clin North Am. 2001;9:93-128.
49. Brånemark PI, Engstrand P, Ohrnell LO, et al. Branemark novum: a new treatment concept for rehabilitation of the edentulous mandible. Preliminary results from a prospective clinical follow-up study. Clin Implant Dent Relat Res. 1999;1:2-16.
50. Schnitman PA, Wohrle PS, Rubenstein JE, et al. Ten year results for Branemark implants immediately loaded with fixed prostheses at implant replacement. Int J Oral Maxillofac Implants. 1997;12:495-503.
51. Carlson ER. Disarticulation resections of the mandible: a prospective review of 16 cases. J Oral Maxillofac Surg. 2002;60:176-181.
52. Boyd JB, Mulholland RS, Davidson J, et al. The free flap and plate in oromandibular reconstruction: long-term review and indications. Plast Reconstr Surg. 1995;95:1018-1028.
53. Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84:71-79.
54. Zlotolow IM, Huryn JM, Piro JD, et al. Osseointegrated implants and functional prosthetic rehabilitation in microvascular fibula free flap reconstructed mandibles. Am J Surg. 1992;164:677-681.
55. Hidalgo DA, Pusic AL. Free-flap mandibular reconstruction: a 10-year follow-up study. Plast Reconstr Surg. 2002;110:438-451.
56. Aramany MA, Downs JA, Beery QC, et al. Prosthodontic rehabilitation for glossectomy patients. J Prosthet Dent. 1982;48:78-81.
57. Robbins KT, Bowman JB, Jacob RF. Postglossectomy deglutitory and articulatory rehabilitation with palatal augmentation prostheses. Arch Otolaryngol Head Neck Surg. 1987;113:1214-1218.
58. Wheeler RL, Logemann JA, Rosen MS. Maxillary reshaping prostheses: effectiveness in improving speech and swallowing of postsurgical oral cancer patients. J Prosthet Dent. 1980;43:313-319.
59. McKinstry RE, Aramany MA, Beery QC, et al. Speech considerations in prosthodontic rehabilitation of the glossectomy patient. J Prosthet Dent. 1985;53:384-387.
60. Esposito SJ, Mitsumoto H, Shanks M. Use of palatal lift and palatal augmentation prostheses to improve dysarthria in patients with amyotrophic lateral sclerosis: a case series. J Prosthet Dent. 2000;83:90-98.
61. Mazaheri M, Mazaheri EH. Prosthodontic aspects of palatal elevation and palatopharyngeal stimulation. J Prosthet Dent. 1976;35:319-326.
62. Turner GE, Williams WN. Fluoroscopy and nasoendoscopy in designing palatal lift prostheses. J Prosthet Dent. 1991;66:63-71.