Chapter 34 Prostate disease and cancer
Introduction
Clinicians encounter in clinical practice 3 common diseases of the prostate, namely, benign prostatic hypertrophy (BPH), prostate cancer and prostatitis (inflammation of the prostate).
Benign prostatic hypertrophy (BPH)
BPH is a common, often worrisome condition that affects ageing men.1 Approximately 60% of men in the Baltimore Longitudinal Study of Ageing2 demonstrated some degree of clinical BPH by age 60.3 Autopsy studies have confirmed microscopic evidence of BPH in 40% of men aged 50–60 years, and 90% of men aged 80 to 90 years.3 The profound impact of this condition spans all ethnic/racial groups, and African American and Latino men are affected to at least a similar degree as white European American men.3 BPH has been found to have an effect on quality of life (QOL) similar to that of other chronic diseases such as diabetes mellitus, hypertension, and heart disease.4–7 Also, depressive mood is more likely to occur in men with BPH.8 Despite the high prevalence of BPH, its specific cause is unknown, but the evidence suggests a multi-factorial process comparable to that of other chronic diseases, perhaps such as cardiovascular disease.9, 10, 11 The risk factors for BPH include high blood pressure, low levels of high-density lipoprotein cholesterol, increased weight gain (associated with waist-to-hip ratio increases), and peripheral arterial disease.10–15
Prostate cancer
Early estimates from autopsy data have reported that in Western countries, approximately 30–40% of all men will develop microscopic prostate cancer during their lifetime.16, 17 However, as the growth of most prostate cancers is slow, the risk of developing overt clinical disease in the US has been reported to be approximately 8–10% (lifetime risk), and the risk of actually dying from prostate cancer is approximately 3%, whereas the autopsy-based prevalence is 80% by the age of 80 years. Furthermore, epidemiological data from the US suggests that for a 50 year-old man with a life expectancy of a further 25 years, there is a 40–45% lifetime risk of having microscopic cancer.17, 18
In recent years, the veritable epidemic of prostate cancer has probably resulted from the widespread use of prostate-specific antigen (PSA) testing which allows the earlier diagnosis in men who have not yet developed symptoms. As an example, it is estimated that in 2005 in the USA there will be approximately 235 000 new diagnoses of prostate cancer and 29 000 deaths.17, 18, 19
In Australia, prostate cancer is the underlying cause of 4.3% of all male deaths registered in 2004.20 The median age of death from prostate cancer is 80.4 years. Prostate cancer is primarily a disease of men over the age of 50 years, and the trend towards an ageing worldwide population is likely to lead to an increased incidence of cases of prostate cancer. In 2004 in Australia there were 15, 759 new cases and 2,792 deaths from prostate cancer (∼18%).20 Approximately 11% of men in Australia will develop prostate cancer in their lifetime.21
Prostatitis
Prostatitis is the most common reason why men see a urologist with 1 in 2 men experiencing this condition in their lifetime. Prostatitis can be acute or chronic, bacterial, non-bacterial or inflammatory, plus non-inflammatory.22 More than 90% of symptomatic patients have chronic prostatitis and/or chronic pelvic pain syndrome. Very little is known about the aetiology of chronic abacterial prostatitis. It is possible that this form of prostatitis may be some form of autoimmune disease.
Bacterial prostatitis is treated using conventional approaches. The other forms of prostatitis are best managed using an integrative approach, as with other chronic medical problems.
Risk factors for prostate cancer
Alcohol
High alcohol consumers such as those that drink more than 10 drinks per day have triple the risk for development of prostate cancer.23 Another study reported that men who drank 4 glasses of red wine, 4 times weekly, had 50% reduction in relative risk of prostate cancer.24 This study reported that middle-aged men showed a relative risk reduction of 6% for each additional glass of red wine consumed per week.24 This study also demonstrated that consumption of alcohol above this level may increase risk of prostate cancer.
Meat and dairy
Studies have shown an association between circulating blood levels of some insulin-like growth factors (IGFs) and their binding proteins and the subsequent risk for prostate cancer.25 A recent study has investigated individual patient data from 12 prospective studies.26 This study investigated prostate cancer risk with relevance to IGFs, and their binding proteins. The study reported that men with high blood levels of IGF-1 were up to 40% more likely to develop prostate cancer than those with low levels. The conclusion was that high circulating IGF-I concentrations were associated with a moderately increased risk for prostate cancer.25 However, there were limitations with the study and these included that IGF-1 concentrations were measured in only 1 sample for each participant, and the laboratory methods to measure IGFs differed in each study. Not all patients had disease stage or grade information, and the diagnosis of prostate cancer could have varied among the studies.
Occupational exposure
Herbicides and pesticides are 2 common xenobiotics (chemicals and toxins that are foreign to the body) that can increase prostate cancer risk by causing DNA damage and altering hormone metabolism. Over 60 studies have investigated the relationship between farming and prostate cancer, and have generally shown an elevated risk for farmers.27
Endocrine disrupters — substances that mimic natural hormones — are xenobiotics that also increase prostate cancer risk, but also disrupt hormone metabolism. Endocrine disrupters include polychlorinated, biphenyls or PCBs (used to make plastic), ink, electrical and electronic equipment, and plasticizers (substances used to make plastic food-wrap more pliable). Dairy and beef products contain fat which can be contaminated with toxic pesticide and hormone residues which can act as endocrine disruptors.28, 29
Lifestyle
Benign prostatic hyperplasia and prostate cancer
Lifestyle modifications have received minimal attention in the medical literature, however, a limited number of studies seem to suggest a consistent beneficial impact of lifestyle interventions that can prevent BPH or cancer and that lifestyle interventions may diminish the progression of existing disease.30 The incidence of clinical prostate disease is around 30 times higher in Western men than in Eastern men of similar age.30 Recently, it was reported that excess Body Mass Index (BMI) has been associated with adverse outcomes in prostate cancer, and hyperinsulinaemia is a candidate mediator of increased risk.31 The study concluded that elevated BMI levels and a high plasma concentration of C-peptide both predisposed men who had been previously diagnosed with prostate cancer to an increased likelihood of dying of their disease.31 Furthermore, that in those patients with both factors, the survival outcome from the disease was with the lowest probability.
Prostate cancer
There is strong edpimiological evidence that links the development of prostate cancer to a Western type lifestyle.22 Furthermore, that there are specific components of the Western diet such as low fibre/high meat/high saturated fat consumptions that may have clinical significance in the late stages of tumour growth, promotion and disease progression.
A small study that investigated a plant based diet in combination with a mindfulness based stress reduction program reported significant reduction in the rate of PSA increase and slowed the rate of tumour progression in patients with biochemically proven recurrent prostate cancer.32 A recent and more robust study investigated lifestyle in patients with prostate cancer.33 Participation was in The Prostate Cancer Lifestyle Trial, a 1-year randomised controlled clinical trial that consisted of 93 patients with early-stage prostate cancer (Gleason score <7, PSA 4–10 ng/mL) who were undergoing active surveillance. The study reported that lifestyle and dietary changes could be important in delaying or even avoiding conventional treatment.
Mind–body medicine
Lifestyle changes and stress reduction
Psychosocial interventions can improve quality of life (QOL) and significantly improve survival in patients diagnosed with prostate cancer.34 A pilot study that enrolled prostate cancer patients into a mindfulness based stress reduction program reported enhanced QOL and decreased stress symptoms and also resulted in beneficial changes in hypothalamic-pituitary-adrenal (HPA) axis functioning.35 The study demonstrated that mental relaxation, stress management, ways to develop self-esteem and spirituality, imagery techniques and problem solving improved prognosis with the active group living twice as long as the men in the control group.35 The effects of comprehensive lifestyle changes from multiple Ornish research reports36–41 has demonstrated that patients with prostate cancer participating in the Prostate Cancer Lifestyle Trial gain significant quality of life benefits. A study investigating a reduction in PSA levels demonstrated inhibition of the human cell line–LNCaP cell growth, and fewer prostate cancer-related clinical events at the end of 1 year compared with control participants.36 The in vitro growth of the prostate cancer cells was inhibited 8 times more than in the control group using the serum from these patients. Another aspect of the Ornish study found that needle biopsy of prostate in the experimental group had significant changes in their prostate cancer genes and also increase in telomerase activity.36 Hence an enhanced understanding of the molecular response to comprehensive lifestyle changes by prostate cancer cells is further warranted.37, 38
The Ornish study of early stage prostate cancer patients,39 for instance, that randomised 93 men with prostate cancer (PSA 4–10ng/mL, cancer Gleeson scores < 7.0) to an intervention group versus a control group (no expectation of dietary or lifestyle changes), and observed over 1 year reported overall disease improvement. During the study time, the intervention group made vigorous lifestyle changes, namely, adhered to a vegan diet, daily moderate aerobic exercise and stress management techniques, weekly group support and daily supplementation of fish oil (3g/day), vitamin E (400IU/day), selenium (200mcg/day) and vitamin C (2g/day). The growth of prostate cancer cells were inhibited by 70% in the intervention group compared with 9% in the control. None of the patients in the intervention group required further conventional treatment and overall PSA dropped by 4%, compared with 6 patients in the control group requiring further conventional treatment and demonstrating an average increase of PSA of 6%. Diet and lifestyle changes may be the primary contributing factors, but it is hard to separate this benefit from the concomitant use of supplements.39 A further study with a plant-based diet, high in fibre and low in saturated fat, combined with a stress management technique (mindfulness-based stress reduction) was able to decrease the rate of PSA increase and may slow the rate of tumour progression in cases of biochemically recurrent prostate cancer.40 An additional recent study by this group further highlights the importance that plant based nutrition may have on slowing progression or preventing the development of prostate cancer.41 This study suggested that a very–low–fat vegan diet increased intake of protective dietary factors and that it simultaneously may decrease intake of pathogenic dietary factors.
Nutrients and risk of prostate cancer
A recent observational study has highlighted serious concerns with the high use of multivitamins in men, increasing the risk of advanced prostate cancer.42 The study enrolled 295 344 men aged 50–71 years who were cancer free over 1995–1996. Over a 5-year follow-up, 10 241 men were diagnosed with prostate cancer. The study demonstrated a 32% increase risk of advanced prostate cancer and a 98% increase risk of fatal prostate cancer among men ingesting multivitamins more than 7 times per week! This association was strongest in men with a family history of prostate cancer or those who took individual micronutrients such as selenium, beta-carotene or zinc. Unfortunately, they did not detail the exact quantities of multivitamins taken by those surveyed. The researchers acknowledge limitations of this study include its lack of information regarding duration of multivitamin use, information which may have helped to determine whether associations were limited to long-time users and that small case numbers limited their ability to investigate 3-way interactions among multivitamin use, single supplement use, and family history of prostate cancer. Furthermore, the authors speculate that the adverse effect of multivitamin supplements in combination with supplemental zinc on prostate cancer risk could be due to non-essential, potentially harmful trace elements contained in zinc supplements, such as cadmium, a known carcinogen. The authors did acknowledge that previous research demonstrated a positive association of multivitamin and mineral use amongst men (with PSA<3) leading to a marked reduction in prostate cancer risk, even up to 52% compared with placebo.
Physical activity/exercise
A recent study demonstrated that men who were more active had less lower urinary tract symptoms.15 More intense exercise, such as running and participating in sport activity, can significantly reduce the symptoms of BPH. In the Health Professionals Follow-up Study, after adjusting for age, race, ethnicity, alcohol intake, diet and smoking, it was found that men who were more active were also less likely to have BPH symptoms or surgery for the condition.15
Biologically plausible mechanisms exist to explain the protective effect of physical activity on the development of prostate cancer; the epidemiological data supporting this hypothesis is still incomplete.44 It is relevant to know whether exercise is indoors or outdoors, the latter providing an opportunity for increased vitamin D production through sun exposure.
Exercise can improve immunity, and reduce stress and depression, increase exposure to sunlight, all of which could increase protection against prostate cancer. Some evidence indicates that physical activity cannot only decrease the chance of prostate cancer, but may slow its progression.45 Resistance exercise in men receiving androgen deprivation therapy for prostate cancer reduces fatigue and improves QOL, and muscular fitness.46
Exercise is protective against BPH and prostate cancer. Men walking 2 to 3 hours per week have 25% lower risk of BPH. Exercise has been found to be protective and in 1 study in men younger than 60, found those that exercised were 4 times less likely to develop prostate cancer.47
Pelvic floor exercises for urinary incontinence following prostatectomy
The loss of urine should be evaluated by a clinician as it can have complicated sequelae.
Pelvic floor exercises and re-education of patients may help prevent and reduce the incidence of urinary incontinence after prostate surgery.48 A recent randomised prospective study investigating the benefit of implementing pelvic floor muscle exercises 30 days prior to surgery demonstrated efficacy.49 The study concluded that preoperative pelvic floor muscle exercises could improve early continence and quality of life outcomes in men following radical prostatectomy for prostate cancer.
Nutritional influences
Vegetables, legumes, soy and fruit
Diet plays an important role in the development of prostate disease.50 There is now a significant amount of research that strongly suggests that nutrition is closely linked with prostate health.30, 50–55 A diet rich in fruit, vegetables and fish and low in meat is likely to protect against BPH and prostate cancer.51–55 High meat, fat and possibly milk intake51, 52 and polyunsaturated fatty acids53–55 are also risk factors. According to a recent study of 6000 men over 30 years of age, high consumption of fatty fish, such as salmon, herring and mackerel was reported to be associated with a 50% reduced risk of prostate cancer.56 Men who ate no fish had a 2–3 fold higher risk of developing prostate cancer than men who ate moderate or high amounts. These benefits are further established in a recent epidemiological review that reported that the benefits were due to high omega-3 fatty acids contained in fatty fish57 which proved to inhibit the growth of prostate cancer cells in previous in vitro studies, albeit the exact mechanisms remain unclear.
In those countries where legumes and soy are the main protein source consumed in large amounts, BPH and the reported prostate cancer incidence is very low.58 It has been reported and hypothesised that soy is rich in isoflavones which actively concentrates in the prostate gland, regulates activity of androgens and oestrogens by acting on oestrogen receptors, and the isoflavonoids, genestein and diadzein are known to inhibit the enzyme 5-alpha-reductase.50, 58–60 This then prevents the development of BPH, and may explain the lower rates of prostate cancer in Asian countries. In BPH, there is an increase in dihydrotestosterone (DHT) and DHT receptors. The conversion of testosterone to DHT is mediated by 5-alpha-reductase. This enzyme inhibition can prevent the development of BPH.
Broccoli and cruciferous vegetables
Epidemiological studies suggest men who consume at least 1 portion of cruciferous vegetables (such as broccoli, cauliflower, cabbage and brussel sprouts) per week are at lower risk of the incidence of prostate cancer.52, 61 Diets rich in broccoli may reduce the risk of prostate cancer according to a 12-month study that randomised men to either a broccoli-rich or a pea-rich diet.62 The researchers quantified and interpreted changes in global gene expression patterns in the human prostate gland by biopsy before, during and after the 12-month diet and biopsies of the prostate were taken. Whilst the underlying mechanism for this observation is still not clear, the authors postulated that consuming broccoli interacts with GSTM1 genotype to result in complex changes to signalling pathways associated with inflammation and carcinogenesis in the prostate. It was proposed that these changes may be mediated through the chemical interaction of isothiocyanates with signalling peptides in the plasma. The report describes broccoli interacting with oncogenic signalling pathways in the prostate.62
Fish, omega-3 fatty acids
The type of fat in the diet plays a role in prostate cancer risk. Oily fish may help to reduce the risk of prostate cancer as omega-3 fatty acids appear to have inhibitory effects on tumour cells whereas omega-6 fatty acids (those contained in margarines rather than gamma-linolenic acid contained in evening primrose and borage seed oil) may have stimulatory effects. Omega-3 fatty acids can influence 5-alpha-reductase activity. Canola products increase the rate of prostate cancer and it is thought to be due to the instability with heat of alpha linolenic acid in canola. Also, omega-3 fatty acids found in fish are protective most probably due to their anti-inflammatory action through their influence on prostaglandins and leukotrienes.63
A small study examined the effects on men with dietary changes rich in plant-based foods and fish and its effect on QOL and PSA over 3 months.64 The intervention group significantly reduced the consumption of saturated fats, dairy foods and animal proteins, and increased consumption of vegetable proteins. The researchers concluded that men with an increasing PSA level after primary treatment were able to make a change to a prostate-healthy diet, accompanied by increases in QOL. No significant difference was found in the log PSA slope between the 2 groups; however, the PSA doubling time increased substantially in the intervention group compared with that in the controls.64 Further studies are required with larger samples.
Dairy products
A large study found the risk of BPH was significantly lower in men with a high intake of total vegetables, dark yellow vegetables, other vegetables, tofu and red meat and a greater risk with increasing intake of high-fat dairy products.65 It is thought that a high intake of calcium foods and dairy products is associated with an increased risk of prostate cancer by lowering concentrations of 1,25-dihydroxyvitamin D(3),66, 67 1,25(OH2D3), a hormone thought to protect against prostate cancer. The Physicians’ Health Study, a cohort of male US physicians in an 11-year follow-up study, documented 1012 incident cases of prostate cancer among 20 885 men. After adjustment for baseline age, BMI, smoking, exercise, and randomised treatment compared with men consuming less than or equal to 1–2/day servings of dairy products, those consuming >2.5 servings had an increase relative risk of prostate cancer by 34%.66 Men consuming >600mg/day of calcium had a 32% higher risk of prostate cancer. Of interest, at baseline, men who consumed >600mg Ca/day from skim milk had lower plasma 1,25(OH2D3) concentrations than did those consuming less than or equal to 150mg Ca/day, which explains the lack of protection towards developing prostate cancer. However, a recent large meta-analyses has concluded that the case-control analyses using calcium as the exposure of interest demonstrated no association with increased risk of prostate cancer in the review.68 Dietary intake of vitamin D also was not related to prostate cancer risk. Moreover, the data from observational studies did not support an association between dairy product use and an increased risk of prostate cancer.68
Soy milk
Drinking soy milk (several glasses per day) lowered risk of prostate cancer by 70% in 1 study.50, 69 Soy contains cancer-fighting substances called isoflavones, especially genistein which inhibits prostate cancer growth. Soy can inhibit oestrogen, cell growth, block activity of 5-alpha-reductase and tyrosine-specific protein kinase, plus reduce angiogenesis.
Phytoestrogens have been shown to cause prominent prostate cancer apoptosis in a human study.70 The major difference between Asian and US diets is the consumption of soy-based food stuffs. Soy protein powder decreases serum testosterone levels in healthy men and acts as an estrogen receptor (ER)-beta agonist, possibly inducing a biological effect to reduce the risk for prostate cancer development.71 This, however, needs to be carefully verified.
In general, there is support for the intake of legumes and yellow-orange plus cruciferous vegetables being inversely related to the risk of prostate cancer.72 Decreased prostate cancer risk has been found in Seventh-Day Adventists who have high intakes of lycopenes, lentils and peas.69 In general, multiple servings of fruit have also been shown to be inversely related to progression of prostate cancer.51
Pomegranate juice
Pomegranate juice is a major source of phytochemicals, and when consumed by men with a rising PSA after surgery or radiotherapy for their prostate cancer, it caused a statistically significant prolongation of PSA doubling with time.73 Approximately 2 x 30mls of pomegranate juice was consumed daily over the 2-year period of the trial. The serum of the men taking pomegranate also influenced in vitro prostate cancer cell proliferation and apoptosis, as well as oxidative stress.73
Special foods and teas
Green tea (Camellia sinensis)
Tea produced from the leaves of the plant Camellia sinensis along with water, is the most widely consumed beverage in the world.74 Alterations in the manufacturing process result in black, green, and oolong tea, which account for approximately 75%, 23%, and 2% of the global production, respectively. Even though each of these non-herbal teas is derived from the same source, different processing techniques render them chemically different from each other.74
Green tea has been reported in numerous recent reviews to be of benefit in preventing prostate diseases.75–80 According to a recent study79 with 404 men living in Southeast China, it was reported that compared to non-green tea drinkers, the risk of prostate cancer was 62% lower in all tea drinkers. Those who drank more than 3 cups daily reduced their risk by 63% and 88% for those who had consumed green tea for more than 40 years and 91% lower for those who consumed more than 1.5kg of tea leaves yearly.80 Green tea suppresses prostate cancer cells in vitro.
Lycopene
Based largely on the scientific evidence from epidemiologic, in vitro, animal and human clinical trials data, it is evident that lycopene, a non-provitamin A carotenoid, is a promising agent for the chemo-prevention of prostate cancer.81, 82 Lycopene is a natural plant compound found predominantly in tomatoes.
In the Health Professionals Follow-up study that followed just over 47 000 middle-aged male practitioners since 1986, a significant difference was reported in prostate cancer incidence in men with a high dietary intake of lycopene with a relative risk reduced to 0.84, even after adjusting for other risk factors such as smoking and other dietary factors.83 The main source of lycopene in the study was from tomato sauce. Men who ate the most sauce had the highest lycopene levels and lower prostate cancer rates. Another study found that men who consumed tomato sauce-based pasta dishes for 3 weeks before radical prostatectomy, had increased lycopene levels in blood and prostate, reduction in serum PSA levels and damage to prostate cancer cells.83 Recently the US Food and Drug Administration stated that lycopene was not associated with a reduced risk of prostate, lung, colorectal, breast, cervical or endometrial cancer.84 The role of lycopene in the area of prostate cancer prevention subsequent to this study was defended by an expert commentary.85
Garlic (Allium sativum)
Animal and in vitro studies provide evidence of an anti-carcinogenic effect of active ingredients in garlic.86, 87 A significant protective effect has also been noted in men with a consumption of >10g/day of allium vegetables.88 After adjusting for age and intake for other foods, high consumption of garlic and scallions were associated with the greatest protective effects.88
Pumpkin seeds (Curcurbita pepo)
A German multi centre study with 2245 patients diagnosed with BPH (Stage I to II) investigated the therapeutic use and safety of a pumpkin seed extract.89 Urinary symptoms were recorded by the International-Prostate-Symptom-Score according to the American Urological Association, the influence on quality of life has been recorded by a QOL index. Patients were treated for 12 weeks with 1–2 capsules per day containing 500mg of a pumpkin seed extract (15–25:1). The prostate symptom score decreased by 47.4% and QOL improved by 46.1% during therapy.
An early double-blind, placebo-controlled study of 3 months duration in 53 patients with BPH demonstrated that a daily intake of 4800mg of Cucurbita pepo (pumpkin seed) combined with the herb Serenoa repens (saw palmetto) showed significant improvement in objective and subjective parameters such as micturition time, frequency, dysuria, urinary flow (6.7 to 9.7 ml) as well as a reduction in residual volume by 32%. No side-effects were noted.90
A more recent double-blind, placebo-controlled study of 12 months duration in 47 patients with BPH demonstrated that a daily intake of 4800mg of Cucurbita pepo (pumpkin seed oil at a dose of 320mg/day) combined with the herb Serenoa repens (saw palmetto oil at a dose of 320mg/day) when compared with placebo or the 2 oils given singly. The study showed significant improvement in the international prostate symptom score (both oils singly or in combination), quality of life (both oils singly or in combination), serum prostate specific antigen (oil combination alone), prostate volume (no improvement) and maximal urinary flow rate improvement with both oils.91
Turmeric (Curcumin)
Curcumin is known to be a COX-2 inhibitor and can induce apoptosis of prostate cancer cells, as well as enhancing cytotoxicity of chemotherapeutic agents, and conferring radio-sensitising effects on prostate cancer cells.92 Curcumin can induce apoptosis in both androgen-dependent and androgen-independent prostate cancer cells by inhibiting tyrosine kinase activity of epidermal growth factor receptor, and depletes this protein.93 Curcumin may be a novel modality by which one can interfere with the signal transduction pathways of the prostate cancer cell and prevent it from progressing to its hormone refractory state.
Nutritional supplements
Prostate cancer
Vitamin E
Vitamin E, even in small doses, offers protection against prostate cancer.94, 95 In the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study, a large placebo-controlled trial involving 29 133 men (smokers aged 50–69 years), it was reported that there was a 32% decrease in prostate cancer incidence and mortality from prostate cancer by 41% among those males who received vitamin E (alpha-tocopherol at a 50mg dose per day).95, 96 Natural vitamin E (d-alpha-tocopherol) and mixed tocopherols (alpha, beta and gamma tocopherol) may confer additional protection. It is possible that vitamin E combined with selenium may offer more protection.96 Vitamin E inhibits human prostate cancer cell growth via modulating the cell cycle regulatory machinery.97 Sources of vitamin E include wheat germ, canola oil, nuts, spinach and egg yolk.
However, a recent long-term RCT trial study utilising individual supplements of 400IU of vitamin E every other day and 500mg of vitamin C daily has reported that neither vitamin E nor C supplementation reduced the risk of prostate or total cancer.98
A recent randomised placebo controlled trial of 35, 533 men from 427 participating sites in the United States, Canada, and Puerto Rico (Selenium and Vitamin E Cancer Prevention Trial [SELECT]) found that neither oral selenium (200 microgram/day from L-selenomethionine) nor vitamin E (400 IU/day of all rac-alpha-tocopheryl acetate) alone or in combination did not prevent prostate cancer in this population of relatively healthy men compared with placebo.99
Furthermore, it is best to avoid high doses of vitamin E greater than 400IU/day as some research suggests it may increase all-cause mortality, although there are mixed findings in a number of different studies and warrants further research.100, 101
Vitamin D
Experimental evidence suggests that vitamin D may reduce the risk of cancer through the regulation of cellular proliferation and differentiation as well as inhibition of angiogenesis.102 These anti-cancer properties have been attributed primarily to 1,25-dihydroxyvitamin D, the hormonal form of vitamin D.102, 103, 104 Moreover, numerous studies have reported the inverse association between ultraviolet solar exposure and mortality rates for prostate cancer.104 Analysis of the serum from 250 000 participants found that low vitamin D was a risk factor for prostate cancer.105 In vitro studies show that vitamin D can inhibit proliferation and also promotes differentiation in human prostate cancer cells.106 Receptors for vitamin D exist on human prostate cancer cells. Prostate cancer patients who have the highest levels of vitamin D mainly from sun exposure have a better prognosis.106
Selenium
The prevention of prostate cancer with nutritional supplements came to prominence in 1996 when the Nutritional Prevention of Cancer Trial reported a 65% reduction in prostate cancer incidence in men receiving selenium supplementation.107
Non-experimental epidemiological studies suggest that individuals with a higher selenium status are at decreased risk of cancer, and that includes cancer of the prostate.108–110 A US study reported that men with high selenium levels have a lower risk of prostate cancer.109 In a double-blind cancer prevention trial of 974 men, 400mcg of selenium per day reduced the incidence of prostate cancer by 63%.110 In a similar study involving over 10 000 people, selenium supplements of 200mcg/day reduced the risk of prostate cancer by two-thirds.111 In vitro experiments using human prostate cancer cells showed that selenium caused apoptosis and in animal experiments it retarded hormone refractory prostate cancer.112, 113
The National Cancer Institute in cooperation with the Southwest Oncology Group has begun one of the largest prostate cancer prevention studies, the Selenium and Vitamin E Chemo-prevention Trial (SELECT).114 In reviewing all of the available evidence comprehensively for the study, the authors concluded that there was promising evidence in support of these antioxidant compounds in the primary prevention of prostate cancer.114
Zinc
Despite theories that zinc may benefit the prostate by inhibiting 5 alpha-reductase,115 the Health Professionals Follow-up Study116 did not confirm this hypothesis. In this study, men who took low doses of zinc were compared to men who took more than 100mg of supplemental zinc a day and men who took supplemental zinc for more than 10 years; the latter 2 groups doubled their risk of prostate cancer. Chronic oversupply of zinc should be avoided as it could play a role in prostate carcinogenesis. Zinc obtained from food was not linked to an increased risk.117
Herbal medicine
Prostatitis
A study that investigated the safety and efficacy of saw palmetto versus finasteride in men with category III prostatitis or chronic pelvic pain syndrome in a 1 year prospective trial reported no efficacy for the herb.122 Long-term efficacy was reported for finasteride only. A multi-centre trial using saw palmetto (Serenoa repens) showed a positive response.123 A recent multi-centre trial reported that a mixture of Serenoa repens plus selenium and lycopene was effective in ameliorating the symptoms associated with chronic prostatitis/chronic pelvic pain.124 A placebo-controlled trial using quercetin found a response rate of 82%.125 Saw palmetto and quercetin have an anti-inflammatory action and this may explain their mechanism of action.126–129
Benign prostatic hypertrophy
Saw palmetto (Serenoa repens)
The plant saw palmetto has been used for hundreds of years in traditional medicine, particularly for male genitourinary conditions such as libido, impotence and problems of micturition. Clinical studies have confirmed its use in the treatment for BPH.129
The exact mechanism of action of saw palmetto is not known. Serenoa repens extracts are rich in fatty acids, especially its principle ingredient beta-sitosterol a weak inhibitor of 5-alpha-reductase and anti-inflammatory effects on the prostate. Beta-sitosterol is also found in soy products as well as other herbs that have been used to treat diseases of the prostate, including pygeum bark, stinging nettle root and pumpkin seed extract.129 It is a weak inhibitor of 5-alpha-reductase and may decrease DHT receptors as well as having an anti-inflammatory effect on the prostate. Finasteride is a more powerful inhibitor of 5-alpha-reductase. The mechanism of action of the sterols on the prostate remains unknown. Saw palmetto does not reduce the size of the gland.130
A systematic review of 18 clinical trials involved nearly 3000 men with mild to moderate BPH, and included 16 double-blind trials with a mean duration period study of 9 weeks.131 It was concluded that saw palmetto improved urinary flow and was well tolerated with minimal and infrequent adverse effects, notably upper digestive upset and minimal withdrawal rates of 9% when compared with 7% for placebo. Compared with placebo, saw palmetto improved urinary symptom scores, symptoms, and flow measures.
The Cochrane reviewers reported that the weighted mean difference (WMD) for the urinary symptom score was −1.41 points (scale range 0–19), (95% confidence interval [CI] = −2.52, −0.30, n = 1 study) and the risk ratio (RR) for self-rated improvement was 1.76 (95% CI = 1.21, 2.54, n = 6 studies). The WMD for nocturia was −0.76 times per evening (95% CI = −1.22, −0.32; n = 10 studies). The WMD for peak urine flow was 1.86 ml/sec (95%CI = 0.60, 3.12, n = 9 studies). Compared with finasteride, saw palmetto produced similar improvements in urinary symptom scores (WMD = 0.37 IPSS points [scale range 0–35], 95% CI = −0.45, 1.19, n = 2 studies) and peak urine flow (WMD = −0.74 ml/sec, 95% CI = −1.66, 0.18, n = 2 studies). Adverse effects due to saw palmetto were mild and infrequent. Withdrawal rates in men assigned to placebo, saw palmetto or finasteride were 7%, 9%, and 11%, respectively. The authors’ concluded the evidence suggests that Serenoa repens provides mild to moderate improvement in urinary symptoms and flow measures. Also, that Serenoa repens produced similar improvement in urinary symptoms and flow compared to finasteride and was associated with fewer adverse treatment events. The long-term effectiveness, safety and ability to prevent BPH complications are not as yet known.131
In a 6-month double-blind randomised control trial (RCT) 1098 men with mild to moderate BPH were randomised to saw palmetto extract 320mgs/day or finasteride 5mgs/day.132 The results concluded that saw palmetto and finasteride were equally effective in the management of BPH; both relieved symptoms in two-thirds of patients with less side-effects in the saw palmetto group. The study used the International Prostate Symptom Score (IPSS) as a primary end-point and found reduction by 39% in the finasteride group compared with 37% in the saw palmetto group. Quality of life improved by 41% in the finasteride compared with 38% in the saw palmetto group. Prostate volume reduced by 18% in finasteride compared with 6% in the saw palmetto group. PSA levels reduced by 41% in finasteride group with no changes in the saw palmetto group; an advantage as it allows for continued monitoring for prostate cancer. Sexual function, libido and potency were adversely affected in the finasteride group by 4.4% compared with the saw palmetto group by 1.1%.132
A large double-blind randomised study compared finasteride, tamsulosin and perimixon (a lipido-sterolic extract of Serenoa repens)133 evaluation of male sexual function in patients with lower urinary tract symptoms (LUTS) associated with BPH treated with a phytotherapeutic agent (Permixon). After 6-months, when compared to pre-treatment data, the study demonstrated that there was a slight negative impact on sexual disorders, especially ejaculation disorders in the Tamsulosin and Finasteride treated patients compared with a slight improvement on sexual function with Permixon therapy.133
A US study comparing saw palmetto and placebo found no statistical significance between the 2 groups in urinary flow rate, prostate size, residual volume after voiding, quality of life, or serum PSA levels during the 1-year study. The incidence of side-effects was also similar in the 2 groups.134
Curcumin
Numerous herbal medicine compounds (i.e. curcumin) for the chemo-prevention of prostate cancer have been investigated.135–138 Currently, there are no clinical trials investigating the efficacy of curcumin for the prevention or treatment of prostate diseases.
African plum tree (Pygeum africanum extract)
A recent updated Cochrane review of 18 RCTs involving 1562 men taking Pygeum africanum extract from African plum tree versus placebo found twice as likely improvements in overall urinary symptoms. Pygeum africanum extract may be a useful treatment option for men with lower urinary symptoms consistent with BPH.139,140 Additional placebo-controlled studies are warranted.
Stinging nettle (Urticae radix and diocia)
A recent review has documented the clinical evidence of effectiveness for stinging nettle in the treatment of BPH, based on many open studies and a small number of randomised controlled studies.141–144 The data indicate that a proprietary methanolic extract is effective in improving BPH complaints.144 Overall the studies have reported improvements of prostate size, nocturia, frequency of micturition, urine flow and residual urine.139 Moreover, the risk for adverse events during stinging nettle treatment was reported to be very low, as was its toxicity. However, pre-clinical safety data are yet to be completed.141, 142
In most studies, stinging nettle has been combined with saw palmetto or pygeum.140, 141, 142 A combination of 300mg of Urtica dioica root extract combined with 25mg of Pygeum africanum bark extract demonstrated improvement in QOL and reduced BPH symptoms.142 Results of studies using combinations with saw palmetto have demonstrated a response equal to finasteride, an alpha blocker.143–146 The mechanism action of stinging nettle is not clear but it is a potent antioxidant and also has anti-inflammatory activity.147, 148
Red clover (Trifolium pretense)
A small study of 20 men with BPH were treated with 60mg of red clover daily for 1 year. The study noted a statistically significant reduction of PSA by 33%.149 Also, the mean prostate volume had decreased slightly from 49.3 cm3 to 44.3 cm3 after 12 months (P < 0.097). Sexual hormone levels did not change throughout the study period. The authors also noted a significant increase in levels of all 3 liver transaminases after 3 months to the high normal range.149 This is a concern and raises the issue of potential herb–drug interactions as red clover affected the hepatic metabolism and may affect drugs metabolised by the liver (e.g. anaesthetics).
Prostate cancer
PC SPES and PC PLUS
PC stands for prostate cancer and SPES is Latin for hope. PC SPES is a product composed of 8 herbs but was removed from the market in the US because of impurities. PC SPES has been replaced by a new product with the same herbs without impurities, PC PLUS (called prostasol). PC SPES was shown to be useful in the treatment of both hormone-sensitive and hormone-insensitive prostate cancers. The herbs in this product have several actions, including inhibition of angiogeneses, stimulation of immunity, inducing an oestrogenic effect and inhibiting 5-alpha-reductase.150
Potential side-effects include fluid retention and thrombosis, as with oestrogen therapy.
Amazonian plant extract
An orally active Amazonian plant extract (BIRM) inhibits prostate cancer growth and metastasis.151 This study evaluated the anti-tumour activity of a simple Ecuadorian oral solution: an extract of an Amazonian plant extract, that was characterised in vitro and in vivo using established prostate cancer (CaP) cell lines and a tumour model in 4 human and 1 rat CaP cell lines. The plant extract appears to exert anti-tumour compounds with potent anti-proliferative activity in vitro and in vivo against prostate cancer cells.151 More research is warranted though.
Physical therapies
Acupuncture
Prostatitis
A meta- analysis from China152 has concluded that acupuncture therapy was significantly effective for the treatment of chronic prostatitis. This result was further reinforced in a recently clinical trial.153 This trial reported that acupuncture appeared to be safe and a potentially effective treatment in improving the symptoms and quality of life of men clinically diagnosed with chronic prostatitis and chronic pelvic pain syndrome.
Prostate cancer
A recent systematic review on the management of hot flushes in men diagnosed with prostate cancer has alluded to the possibility that acupuncture may assist in managing hot flushes.154 The review concluded though that recommendation was difficult given that there were no robust RCTs. An additional systematic review consisted of 6 studies. One study was an RCT clinical trial that compared the effects of manual acupuncture with that of acupuncture plus electro-acupuncture. The other 5 studies of this review were uncontrolled observational studies and hence the evidence was limited.155 The review concluded that the overall evidence was weak and acupuncture could not be recommended as an effective treatment for hot flushes in patients with prostate cancer.
Conclusion
Health care professionals should educate patients about the potential positive effects of simple lifestyle changes on BPH and prevention of prostate cancer. Some of the potential lifestyle and CAM factors that may positively affect the prostate are summarised in Table 34.1. Preliminary data continue to support the importance of lifestyle changes for men who are trying to prevent BPH and for men already diagnosed with the condition or for the prevention and treatment of prostate cancer. Clinicians must incorporate lifestyle modification advice (such as diet, exercise, sunshine exposure for vitamin D and stress management) into standard medical consultations, and future management guidelines should be adopted similar to what has been done for cardiovascular disease. This can improve educational efforts and help reduce the burden of prostate diseases.
Table 34.1 Levels of evidence for lifestyle and complementary medicines/therapies in the management of prostate cancer (a), benign prostatic hypertrophy (b), and prostatitis (c)
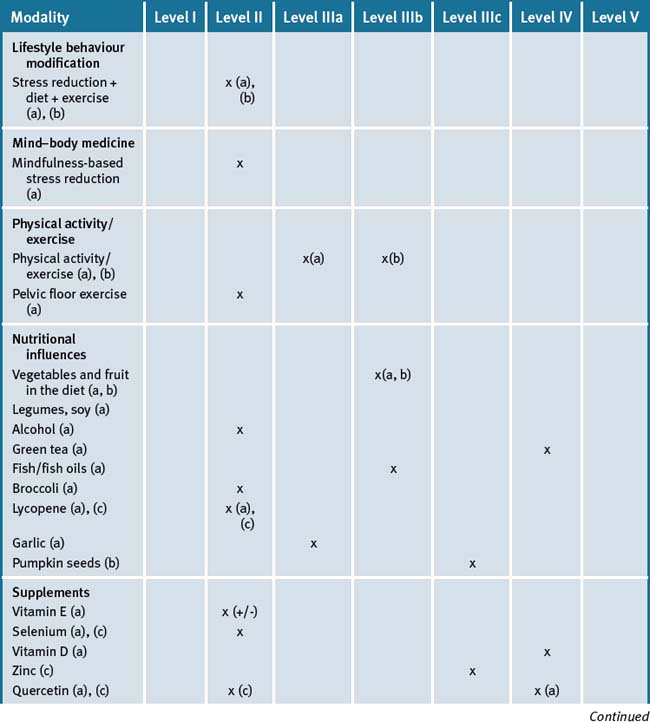
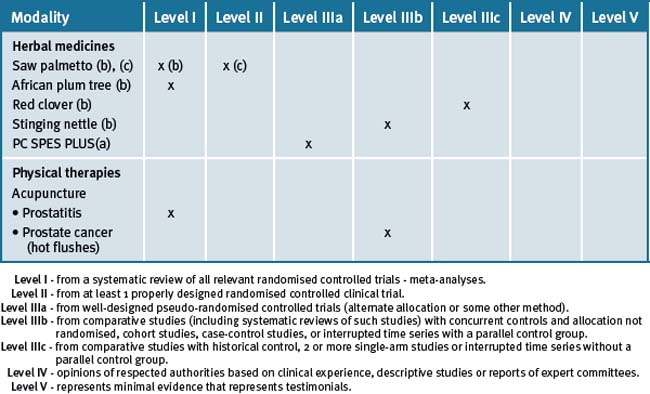
A great deal of the evidence relating to prostate cancer is at a preliminary stage. However, the Ornish studies36, 37, 38 are strongly suggestive that an integrative approach is likely to be beneficial. This is especially appropriate in those patients that are treated conservatively. The Ornish approach can be offered as an addition to established therapies.
Lifestyle changes go further than disease-specific concerns and can potentially improve overall health and wellbeing (both emotionally and physically), plus significantly reduce the risk for chronic disease development.
Clinical tips handout for patients — prostate disease
1 Lifestyle advice
Sunshine
2 Physical activity/exercise
3 Mind–body medicine
5 Dietary changes
7 Nutritional supplements
Vitamin E (natural gamma tocopherol)
Vitamin D3 (cholecalciferol)
Selenium (sodium selenite, organic selenium found in yeast)
Zinc sulfate or gluconate; elemental zinc
Herbal supplements
African plum tree (Pygeum africanum extract)
Red clover (Trifolium pretense)
Saw palmetto (Serenoa repens)
Stinging nettle (Urticae radix)
1 Nickel J.C. Inflammation and benign prostatic hyperplasia. Urol Clin North Am. 2008;35(1):109-115.
2 Arrighi H.M., Metter E.J., Guess H.A., et al. Natural history of benign prostatic hyperplasia and risk of prostatectomy: the Baltimore Longitudinal Aging Study. Urology. 1991;38(suppl 1):4-8.
3 Sarma A.V., Wei J.T., Jacobson D.J., et al. Comparison of lower urinary tract symptom severity and associated bother between community dwelling black and white men: the Olmsted County Study of Urinary Symptoms and Health Status and the Flint Men’s Health Study. Urology. 2003;61:1086-1091.
4 Berry S.J., Coffey D.S., Walsh P.C., et al. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474-479.
5 Kupelian V., Wei J.T., O’Leary M.P., et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381-2387.
6 Parsons J.K., Carter H.B., Partin A.W., et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91:2562-2568.
7 Michel M.C., Heemann U., Schumacher H., et al. Association of hypertension with symptoms of benign prostatic hyperplasia. J Urol. 2004;172:1390-1393.
8 Clifford G.M., Farmer R.D. Drug or symptom-induced depression in men treated with beta1-blockers for benign prostatic hyperplasia? A nested case control study. Pharmacoepidemiol Drug Saf. 2002;11:55-61.
9 Morton M.S., Turkes A., Denis L., et al. Can dietary factors influence prostatic disease? BJU Int. 1999;84:549-554.
10 Moyad M.A. Lifestyle changes to prevent BPH: Heart healthy prostate healthy. Urol Nurs. 2003;23:439-441.
11 Gibbons E.P., Colen J., Nelson J.B., et al. Correlation between risk factors for vascular disease and the American Urological Association Symptom Score. BJU Int. 2007;99:97-100.
12 Parsons J.K. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol. 2007;178:395-401.
13 Lee S., Min H.G., Choi S.H., et al. Central obesity as a risk factor for prostatic hyperplasia. Obesity (Silver Spring). 2006;14:172-179.
14 Dahle S.E., Chokkalingam A.P., Gao Y.T., et al. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J Urol. 2002;168:599-604.
15 Platz E.A., Kawachi I., Rimm E.B., et al. Physical activity and benign prostatic hyperplasia. Arch Intern Med. 1998;158:2349-2356.
16 Damber J.E., Aus G. Prostate cancer. Lancet. 2008;371:1710-1721.
17 Jemal A., Siegel R., Ward E., et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43-66.
18 Connolly D., Black A., Gavin A., et al. Baseline prostate-specific antigen level and risk of prostate cancer and prostate-specific mortality: diagnosis is dependent on the intensity of investigation. Cancer Epidem Biomarkers Prev. 2008;17(2):271-278.
19 Klotz L. Active surveillance for favorable risk prostate cancer: rationale, risks, and results. Urol Oncol. 2007;25(6):505-509.
20 Australian Institute of Health and Welfare (AIHW). Cancer survival and prevalence in Australia: cancers diagnosed from 1982 to 2004. Online. Available: www.aihw.gov.au/publications/can/cspia-cdf-82–;04/cspia-cdf-82–04.pdf (accessed March 2010).
21 Prostate Cancer Foundation of Australia. Online. Available: www.prostate.org.au/articleLive/pages/Prostate-Cancer-Statistics.html (accessed March 2010).
22 Krieger J.N., Nyberg L.Jr., Nickel J.C. NIH consensus definition and classification of prostatitis. JAMA. 1999;282(3):236-237.
23 Putnam S.D., Cerhan J.R., Parker A.S., et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann Epidemiol. 2000;10(6):361-369.
24 Schoonen W.M., Salinas C.A., Kiemeney L.A., et al. Alcohol consumption and risk of prostate cancer in middle-aged men. Int J Cancer. 2005;113(1):133-140.
25 Stattin P., Bylund A., Rinaldi S., et al. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. JNCI. 2000;92(23):1910-1917.
26 Roddam A.W., Allen N.E., Appleby P., et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149(7):461-471.
27 Parent M., Siemiatycki J. Occupation and prostate cancer. Epidemiological Reviews. 2001;23:138-143.
28 Simone C.B. Cancer and nutrition, a ten-point plan to reduce your risk of getting cancer. Garden City Park, NJ: Avery Publishing Group, Inc; 1994. 148
29 Robbins J. Diet for a new America, how your food choices affect your health, happiness and the future of life on Earth. Walpole, NH: Stillpoint Pubish; 1987. 315, 331, 343
30 Moyad M.A., Lowe F.C. Educating patients about lifestyle modifications for prostate health. Am J Med. 2008;121(8 Suppl. 2):S34-S42.
31 Ma J., Li H., Giovannucci E., et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9(11):1039-1047.
32 Saxe G.A., Hébert J.R., Carmody J.F., et al. Can diet in conjunction with stress reduction affect the rate of increase in prostate specific antigen after biochemical recurrence of prostate cancer? J Urol. 2001;166(6):2202-2207.
33 Frattaroli J., Weidner G., Dnistrian A.M., et al. Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology. 2008;72(6):1319-1323.
34 Ramachandra P., Booth S., Pieters T., et al. A brief self-administered psychological intervention to improve well-being in patients with cancer: results from a feasibility study. Psychooncology. 2009;18(12):1323-1326.
35 Carlson L.E., Speca M., Patel K.D., et al. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29(4):448-474.
36 Ornish D., Weidner G., Fair W.R., et al. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174:1065-1070.
37 Ornish D., Magbanua M.J., Weidner G., et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. PNAS. 2008;105(24):8369-8374.
38 Ornish D., Lin J., Daubenmier J., et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
39 Daubenmier J.J., Weidner G., Marlin R., et al. Lifestyle and health-related quality of life of men with prostate cancer managed with active surveillance. Urology. 2006;67(1):125-130.
40 Dewell A., Weidner G., Sumner M.D., et al. Relationship of dietary protein and soy isoflavones to serum IGF-1 and IGF binding proteins in the Prostate Cancer Lifestyle Trial. Nutr Cancer. 2007;58(1):35-42.
41 Dewell A., Weidner G., Sumner M.D., et al. A very-low-fat vegan diet increases intake of protective dietary factors and decreases intake of pathogenic dietary factors. J Am Diet Assoc. 2008;108(2):347-356.
42 Lawson K.A., et al. Multivitamin Use and Risk of Prostate Cancer in the National Institutes of Health-AARP Diet and Health Study. J National Cancer Inst. 2007;99:754-764.
43 McNaughton Collins M., Pontari M.A., O’Leary M.P., et al. Chronic Prostatitis Collaborative Research Network. Quality of life is impaired in men with chronic prostatitis: the Chronic Prostatitis Collaborative Research Network. J Gen Intern Med. 2001;16(10):656-662.
44 Lee I.M., Sesso H.D., Chen J.J., et al. Does physical activity play a role in the prevention of prostate cancer? Epidemiology Reviews. 2001;23:132-137.
45 Giovannucci E., Liu Y., Leitzmann M.F., et al. A prospective study of physical activity, and incident and fatal prostate cancer. Arc Int Med. 2005;165:1005-1010.
46 Segal R.J., Reid R.D., Courneya K.S., et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Cli Onc. 2003;21:1653-1659.
47 Lagiou A., Samoli E., Georgila C., et al. Occupational physical activity in relation with prostate cancer and benign prostatic hyperplasia. Eur J Cancer Prev. 2008;17(4):336-339.
48 Van Kampen M., De Weerdt W., Van Poppel H., et al. Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet. 2000;355:98-102.
49 Centemero A., Rigatti L., Giraudo D., et al. Preoperative Pelvic Floor Muscle Exercise for Early Continence After Radical Prostatectomy: A Randomised Controlled Study. Eur Urol. 2010 Mar 1.
50 Aldercreutz H., Mazur W. Phytoestrogens and Western diseases. Ann Med. 1997;29(2):95-120.
51 Kolonel L.N., Hankin J.H., Whittemore A.S., et al. Vegetables, fruits, legumes and prostate cancer: a multi-ethnic case-controlled study. Cancer Epidemiol Biomarkers Prev. 2000;9:795-804.
52 Cheung E., Wadhera P., Dorff T., et al. Diet and prostate cancer risk reduction. Expert Rev Anticancer Ther. 2008;8(1):43-50.
53 Stacewicz-Sapuntzakis M., Borthakur G., et al. Correlations of dietary patterns with prostate health. Mol Nutr Food Res. 2008;52(1):114-130.
54 Schulman C., Ekane S., ZlottaNutrition A. Prostate Cancer: Evidence or suspicion? Urology. 2001;58:318-334.
55 Key T.J., Fraser G.E., Thorogood M., et al. Mortality in vegetarians and non-vegetarians: a collaborative analysis of 8300 deaths among 76,000 men and women in five prospective studies. Public Health Nutr. 1998;1(1):33-41.
56 Terry P., Lichtenstein P., Feychting M., et al. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764-1766.
57 Terry P.D., Rohan T.E., Wolk A. Intakes of fish and marine fatty acids and the risks of cancers of the breast and prostate and of other hormone-related cancers: a review of the epidemiologic evidence. Am J Clin Nutr. 2003;77(3):532-543.
58 Sarkar F.H., Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21(5):744-757.
59 Morton M.S., Chan P.S., Cheng C., et al. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostrate. 1997;32:122-128.
60 Guy L., Védrine N., Urpi-Sarda M., et al. Orally administered isoflavones are present as glucuronides in the human prostate. Nutr Cancer. 2008;60(4):461-468.
61 Kirsh V.A., Peters U., Mayne S.T., et al. Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Prospective study of fruit and vegetable intake and risk of prostate cancer. JNCI. 2007;99(15):1200-1209.
62 Traka M., Gasper A.V., Melchini A., et al. Broccoli consumption interacts with GSTM1 to perturb oncogenic signaling pathways in the prostate. PLoS ONE. 2008;3(7):e2568.
63 Good for the heart but not for the prostate? The alpha-linolenic acid dilemma. Harv Mens Health Watch. 2002;6(6):1-3.
64 Carmody J., Olendzki B., Reed G., et al. A Dietary Intervention for Recurrent Prostate Cancer After Definitive Primary Treatment: Results of a Randomized Pilot Trial. Urology. 2008;72(6):1324-1328.
65 Ambrosini G.L., de Klerk N.H., Mackerras D., et al. Dietary patterns and surgically treated benign prostatic hyperplasia: a case control study in Western Australia. BJU Intern. 2008;101:853-860.
66 Giovannucci E., et al. Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res. 1998;58:442-447.
67 Chan J.M., Stampfer M.J., Ma J., et al. Dairy products, calcium, and prostate cancer risk in the Physicians’ Health Study. Am J Clin Nutr. 2002;76(2):490-491.
68 Huncharek M., Muscat J., Kupelnick B. Dairy products, dietary calcium and vitamin D intake as risk factors for prostate cancer: a meta-analysis of 26,769 cases from 45 observational studies. Nutr Cancer. 2008;60(4):421-441.
69 Jacobsen B.K., Nutsen S.F., Fraser G.E. Does high soy milk intake reduce prostate cancer incidence? The Adventist’s Health Study (United States). Cancer Causes Contr. 1998;9:553-557.
70 Stevens F.O. Phytoestrogens and prostate cancer: possible preventive role. Medical Journal of Australia. 1997;1671:38-40.
71 Goodin S., Shen F., Shih W.J., et al. Clinical and biological activity of soy protein powder supplementation in healthy male volunteers. Cancer Epidemiol Biomarkers Prev. 2007;16(4):829-833.
72 Mills P.K., et al. Cohort study of diet, lifestyle, and prostate cancer in Adventists men. Cancer. 1989;64:598-604.
73 Pantuck A.J., et al. Phase 2 study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018-4025.
74 Syed D.N., Suh Y., Afaq F., et al. Dietary agents for chemoprevention of prostate cancer. Cancer Lett. 2008;265(2):167-176.
75 Carlson J.R., Bauer Ba A., Vincent P., et al. Reading the tea leaves: anticarcinogenic properties of epigallocatechin-3-gallate. Mayo Clin Proc. 2007;82:725-732.
76 Adhami V.M., Mukhtar H. Anti-oxidants from green tea and pomegranate for chemoprevention of prostate cancer. Mol Biotechnol. 2007;37(1):52-57.
77 Fleshner N., Zlotta A.R. Prostate cancer prevention: past, present, and future. Cancer. 2007;110(9):1889-1899.
78 Adhami V.M., Mukhtar H. Polyphenols from green tea and pomegranate for prevention of prostate cancer. Free Radic Res. 2006;40(10):1095-1104.
79 Lee A.H., Fraser M.L., Meng X., et al. Protective effects of green tea against prostate cancer. Expert Rev Anticancer Ther. 2006;6(4):507-513.
80 Jian L., Ping Xie L., Lee A.H., et al. Protective effect of green tea against prostate cancer: A case-control study in southeast China. Inter J Cancer. 2004;108:130-135.
81 Dahan K., Fennal M., Kumar N.B. Lycopene in the prevention of prostate cancer. J Soc Integr Oncol. 2008;6(1):29-36.
82 Chen L., Stacewicz-Sapuntzakis M., Duncan C., et al. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entres as a whole-food intervention. JNCI. 2001;93:1872-1879.
83 Giovanucci E., Rimm E.B., Liu Y., et al. A prospective study of tomato products, lycopene, and prostate cancer risk. JNCI. 2002;94(5):391-398.
84 Peters U., Leitzmann M.F., Chatterjee N., et al. Serum lycopene, other carotenoids and prostate cancer risk: a nested case-control study in prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidem Biomark Prev. 2007;16:962-968.
85 GiovannucciEditorial E. Lycopene and Prostate. JNCI. 2007;99:1060-1062.
86 Fleischauer A.T., Arab L. Garlic and cancer: a critical review of the epidemiologic literature. J Nutr. 2001;131(3s):1032S-1040S.
87 Devrim E., Durak I. Is garlic a promising food for benign prostatic hyperplasia and prostate cancer? Mol Nutr Food Res. 2007;51(11):1319-1323.
88 Hsing A.W., Chokkalingam A.P., Gao Y.T., et al. Allium vegetables and risk of prostate cancer: a population-based study. JNCI. 2002;94:1648-1651.
89 Schiebel-Schlosser G., Friederich M. Phytotherapy of BPH with pumpkin seeds – A multicentric clinical trial. Zeitschrift fur Phytotherapie [Germany]. 1998;19(2):71-76.
90 Carbin B.E., Larsson B., Lindahl O. Treatment of benign prostatic hyperplasia with phytosterols. Br J Urol. 1990;66(6):639-641.
91 Hong H., Kim C.S., Maeng S. Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutr Res Pract. 2009 Winter;3(4):323-327.
92 Anand P., Sundaram C., Jhurani S., et al. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267(1):133-164.
93 Dorai T., Gehani N., Katz A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol Urol. 2000 Spring;4(1):1-6.
94 The Alpha-Tocopherol. Beta-Carotene Cancer Prevention Study Group. The effect of vitamin E and beta-carotene on the incidence of lung cancer and other cancers in male smokers. NEJM. 1994;330:1029-1035.
95 Heinonen O.P., Albanes D., Virtamo J., et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. JNCI. 1998;90:440-446.
96 Helzlsouer K.J., Huang H.Y., Alberg A.J., et al. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. JNCI. 2000;92:2018-2023.
97 Ni J., Chen M., Zhang Y., et al. Vitamin E succinate inhibits human prostate cancer cell growth via modulating cell cycle regulatory machinery. Bio Phys Res Commun. 2003;300:357-363.
98 Gaziano J.M., Glynn R.J., Christen W.G., et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301(1):52-62.
99 Lippman S.M., Klein E.A., Goodman P.J., et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). 2009;301(1):39-51.
100 Miller E.R.3rd, Pastor-Barriuso R., Dalal D., et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37-46.
101 Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., et al. Mortalitly in randomized trials of antioxidant supplements for primary and secondary prevention. Systematic review and meta-analysis. JAMA. 2007;297:842-857.
102 Ali M.M., Vaidya V. Vitamin D and cancer. J Cancer Res Ther. 2007;3(4):225-230.
103 Luscombe C.J., Fryer A.A., French M.E., et al. Exposure to ultraviolet radiation: association with susceptibility and age at presentation with prostate cancer. Lancet. 2001;358:641-642.
104 Donkena K.V., Karnes R.J., Young C.Y. Vitamins and prostate cancer risk. Molecules. 2010;15(3):1762-1783.
105 Corder E.H., Friedman G.D., Vogelman J.H., et al. Vitamin D and prostate cancer: a prediagnositic study with stored sera. Cancer Epidemiol Biomark Prev. 1993;2:467.
106 Robsahm T.E., Tretli S., Dahlback A., et al. Vitamin D3 from sunlight may improve the prognosis of breast, colon and prostate cancer. Cancer Causes Control. 2004;15:149-158.
107 Clark L.C., Combs G.F.Jr., Turnbull B.W., et al. Nutritional Prevention of Cancer Study Group. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. JAMA. 1996;276(24):1957-1963.
108 Platz E.A., Helzlsouer K.J. Selenium, zinc, and prostate cancer. Epidemiological Reviews. 2001;23:93-101.
109 Willett W.C., Polk B.F., Morris J.S., et al. Prediagnostic serum selenium and risk of cancer. Lancet. 1983;2:130-134.
110 Reid M.E., Duffield-Lillico A.J., Slate E., et al. The nutritional prevention of cancer: 400mcg per day selenium treatment. Nutr Cancer. 2008;60(2):155-163.
111 Clark L.C., Dalkin B., Krongrad A., et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81(5):730-734.
112 Ghosh J. Rapid induction of apoptosis in prostate cancer cells by selenium: reversal by metabolites of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun. 2004;315(3):624-635.
113 Corcoran N.M., Najdovska M., Costello A.J. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J Urol. 2004;171:907-910.
114 Pak R.W., Lanteri V.J., Scheuch J.R., et al. Review of vitamin E and selenium in the prevention of prostate cancer: implications of the selenium and vitamin E chemoprevention trial. Integr Cancer Ther. 2002 Dec;1(4):338-344.
115 Leake A., Chisholm G.D., Habib F.K. The effect of zinc on the 5 alpha-reduction of testosterone by the hyperplastic human prostate gland. J Steroid Bioch. 1984;20:651-655.
116 Leitzmann M.F., Stampfer M.J., Wu K., et al. Zinc supplement use and risk of prostate cancer. JNCI. 2003;95(13):1004-1007.
117 Gonzalez A., Peters U., Lampe J.W., et al. Zinc intake from supplements and diet and prostate cancer. Nutr Cancer. 2009;61(2):206-215.
118 Gómez Y., Arocha F., Espinoza F., et al. (Zinc levels in prostatic fluid of patients with prostate pathologies) Invest Clin. 2007;48(3):287-294.
119 Edorh A.P., Tachev K., Hadou T., et al. Magnesium content in seminal fluid as an indicator of chronic prostatitis. Noisy-le-grand: Cell Mol Biol; 2003. 49 Online Pub:OL419–23
120 Dutkiewicz S. Zinc and magnesium serum levels in patients with benign prostatic hyperplasia (BPH) before and after prazosin therapy. Mater Med Pol. 1995;27(1):15-17.
121 Sapota A., Daragó A., Taczalski J., et al. Disturbed homeostasis of zinc and other essential elements in the prostate gland dependent on the character of pathological lesions. Biometals. 2009 Jul 23.
122 Kaplan S.A., Volpe M.A., Te A.E. A prospective, 1-year trial using saw palmetto versus finasteride in the treatment of category III prostatitis/chronic pelvic pain syndrome. J Urol. 2004;171(1):284-288.
123 Lopatkin N.A., Apolikhin O.I., Sivkov A.V., et al. (Results of a multicenter trial of serenoa repens extract (permixon) in patients with chronic abacterial prostatitis) Urologiia. 2007;5:3-7.
124 Morgia G., Mucciardi G., Galì A., et al. Treatment of Chronic Prostatitis/Chronic Pelvic Pain Syndrome Category IIIA with Serenoa repens plus Selenium and Lycopene (Profluss(R)) versus S. repens Alone: An Italian Randomized Multicenter-Controlled Study. Urol Int. 2010 Mar 24.
125 Shoskes D.A., Zeitlin S.I., Shahed A., et al. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology. 1999;54(6):960-963.
126 Hsieh T.C., Wu J.M. Targeting CWR22Rv1 prostate cancer cell proliferation and gene expression by combinations of the phytochemicals EGCG, genistein and quercetin. Anticancer research. 2009;29(10):4025-4032.
127 Jung Y.H., Heo J., Lee Y.J., et al. Quercetin enhances TRAIL-induced apoptosis in prostate cancer cells via increased protein stability of death receptor 5. Life Sci. 2010;86(9–10):351-357.
128 Yuan H., Young C.Y., Tian Y., et al. Suppression of the androgen receptor function by quercetin through protein-protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells. Mol Cell Biochem. 2010 Feb 11.
129 Abe M., Ito Y., Oyunzul L., et al. Pharmacologically relevant receptor binding characteristics and 5alpha-reductase inhibitory activity of free Fatty acids contained in saw palmetto extract. Biol Pharm Bull. 2009;32(4):646-650.
130 Marks L.S., Hess D.L., Dorey F.J., et al. Tissue effects of saw palmetto and finasteride: use of biopsy cores for in situ quantification of prostatic androgens. Urology. 2001;57(5):999-1005.
131 Wilt T.J., Ishani A., Stark G., et al. Saw Palmetto extracts for treatment of Benign Prostrate Hyperplasia: a systematic review. JAMA. 1998;280(18):1604-1609.
132 Carraro J., Raynaud J., Koch G. Comparison of Phytotherapy (Permixon) with Finasteride in the Treatment of BPH: A Randomized International Study of 1,098 Patients. Prostate. 1996;29:231-240.
133 Zlotta A.R., Teillac P., Raynaud J.P., et al. Evaluation of male sexual function in patients with Lower Urinary Tract Symptoms (LUTS) associated with Benign Prostatic Hyperplasia (BPH) treated with a phytotherapeutic agent (Permixon), Tamsulosin or Finasteride. Eur Urol. 2005;48(2):269-276.
134 Bent S., Kane C., Shinohara K., et al. Saw palmetto for benign prostatic hyperplasia. NEJM. 2006;354(6):557-566.
135 Von Löw E.C., Perabo F.G., Siener R., et al. Review. Facts and fiction of phytotherapy for prostate cancer: a critical assessment of preclinical and clinical data. Vivo. 2007;21(2):189-204.
136 Hour T.C., Chen J., Huang C.Y., et al. Curcumin enhances cytotoxicity of chemotherapeutic agents in prostate cancer cells by inducing p21 (WAF1/CIPI) and C/EBPbeta expressions and suppressing NF-kappa B activation. Prostate. 2002;51:211-218.
137 Chendil D., Ranga R.S., Meigooni D., et al. Curcumin confers radiosensitising effect in prostate cancer cell lines PC-3. Oncogene. 2004;23:1599-1607.
138 Dorai T., Gehani N., Katz A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of the epidermal growth factor and depletes the protein. Mol Urol. 2000;4:1-6.
139 Wilt T., Ishani A., Mac Donald R., et al. Pygeum africanum for benign prostatic hyperplasia. Cochrane Database Systematic Review. (1):2002. CD001044
140 Levin R.M., Das A.K. A scientific basis for the therapeutic effects of Pygeum africanum and Serenoa repens. Urol Res. 2000;28(3):201-209.
141 Chrubasik J.E., Roufogalis B.D., Wagner H., et al. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7–8):568-579.
142 Krzeski T., Kazón M., Borkowski A., et al. Combined extracts of Urtica dioica and Pygeum africanum in the treatment of benign prostatic hyperplasia: double-blind comparison of two doses. Clin Ther. 1993;15(6):1011-1020.
143 Sökeland J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome. BJU Int. 2000;86(4):439-442.
144 Popa G., Hägele-Kaddour H., Walther C. (Benign prostate syndrome: urinary tract symptoms can be eased with phytotherapy) MMW Fortschr Med. 2005 Aug 18;147(33–34):42.
145 Schneider H.J., Honold E., Masuhr T. (Treatment of benign prostatic hyperplasia. Results of a treatment study with the phytogenic combination of Sabal extract WS 1473 and Urtica extract WS 1031 in urologic specialty practices) Fortschr Med. 1995;113(3):37-40.
146 Bondarenko B., Walther C., Funk P., et al. Long-term efficacy and safety of PRO 160/120 (a combination of sabal and urtica extract) in patients with lower urinary tract symptoms (LUTS). Phytomedicine. 2003;10 Suppl. 4:53-55..
147 Gülçin I., Küfrevioglu O.I., Oktay M., et al. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. 2004;90(2–3):205-215.
148 Riehemann K., Behnke B., Schulze-Osthoff K. Plant extracts from stinging nettle (Urtica dioica), an antirheumatic remedy, inhibit the proinflammatory transcription factor NF-kappaB. EBS Lett. 1999;442(1):89-94.
149 Engelhardt P.F., Riedl C.R. Effects of one-year treatment with isoflavone extract from red clover on prostate, liver function, sexual function, and quality of life in men with elevated PSA levels and negative prostate biopsy findings. Urology. 2008;71(2):185-190.
150 Hsieh T.C., Wu J.M. Mechanism of action of herbal supplement PC-SPES: elucidation of effects of individual herbs of PC-SPES on proliferation and prostate specific gene expression in androgen-dependent LNCaP cells. Int J Oncol. 2002;20(3):583-588.
151 Dandekar D.S., Lokeshwar V.B., Cevallos-Arellano E., et al. An orally active Amazonian plant extract (BIRM) inhibits prostate cancer growth and metastasis. Cancer Chemother Pharmacol. 2003;52(1):59-66.
152 Wang C.Y., Han R.F. [Acupuncture for chronic prostatitis: a meta-analysis] Zhonghua Nan Ke Xue. 2008;14(9):853-856.
153 Tugcu V., Tas S., Eren G., et al. Effectiveness of Acupuncture in Patients with Category IIIB Chronic Pelvic Pain Syndrome: A Report of 97 Patients. Pain Med. 2010 Jan 22.
154 Frisk J. Managing hot flushes in men after prostate cancer–a systematic review. Maturitas. 2010;65(1):15-22.
155 Lee M.S., Kim K.H., Shin B.C., et al. Acupuncture for treating hot flushes in men with prostate cancer: a systematic review. Support Care Cancer. 2009;17(7):763-770.