Fig. 9.1
Kaplan–Meier renal survival from renal biopsy (a) and disease onset (b) in 1,155 patients with IgAN. ESRD end-stage of renal disease
For patients with proteinuria >1.0 g/day at biopsy, the 10-, 15- and 20-year cumulative renal survival rates from renal biopsy were 71 %, 63 %, and 54 %, respectively, and the 10-, 15- and 20-year cumulative renal survival rates from renal disease onset were 83 %, 72 %, and 67 %, respectively (Fig. 9.1).
The 20-year renal survival rate after renal biopsy in this cohort of patients (64 %) was highly similar to those of patients from Japan and Korea. A study of 1,012 patients in Japan reported that the 20-year cumulative survival rate from renal biopsy to ESRD was 66.6 % [6], and a study of 1,364 patients in Korea reported that the 20-year cumulative renal survival rate was 66.9 % [7]. The variability in renal outcome of IgAN in different studies may account for the difference in indications of kidney biopsy and health screening practice to some extent. Patients with selective biopsy indications would have lower renal survival rates. In this study, the indication of renal biopsy was relatively less selective, as suggested by the lower incidence of renal insufficiency (21.3 %), proteinuria >1.0 g/day (43.4 %), and hypertension (29.7 %) at the time of biopsy.
9.2.1 Long-Term Renal Outcome in IgA Nephropathy Patients with Recurrent Macro-Hematuria
Macro- and microscopic hematuria are frequent in patients with IgAN. However, the prognostic value of hematuria is highly complex in patients with IgAN. The persistent microscopic hematuria is associated with an increased incidence of renal failure, whereas episodic recurrent macro-hematuria (MH) is associated with relative benign renal outcomes [1, 8, 9].
Patients with recurrent hematuria (MH) (RMH, episodes of MH≥2 times, episode intervals >1 months) are a distinct subtype of IgAN [5]. Of the 1,155 patients with IgAN, 158 (14 %) of them presented with a history of RMH at biopsy. Compared with patients without a history of MH (NMH), patients with RMH were younger and more likely to be female and had lower proteinuria, a lower incidence of hypertension, a higher eGFR, a lower level of mesangial hypercellularity and segmental glomerulosclerosis, and a lower level of tubular atrophy. More importantly, as shown in Fig. 9.2, the long-term renal prognosis in RMH patients is dramatically better than that in NMH patients. The renal prognosis of patients with RMH was also better than those with isolated MH (IMH, one episode of MH) [10]. During a median follow-up of 7.9 years, the 10- and 20-year cumulative renal survival rates after biopsy, as calculated by K–M methods, were 91 % and 91 % in the RMH group, 89 % and 64 % in the IMH group, and 79 % and 57 % in the NMH group, respectively.
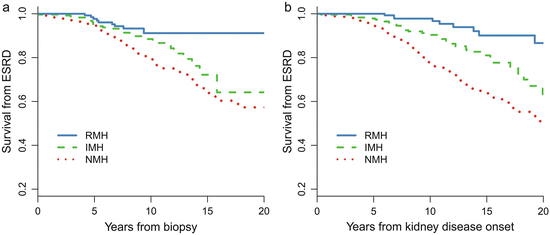

Fig. 9.2
Kaplan–Meier renal survival curve in IgAN patients with different MH patterns. NMH no history of macro-hematuria, IMH isolated onset of macro-hematuria, and RMH recurrent onset of macro-hematuria
Why patients with recurrent episodes of MH had relative benign renal outcomes remains unknown. It has been hypothesized that a kidney injury mechanism, mainly triggered by an upper respiratory infection, is more evident but discontinuous in patients with RMH, whereas in those without RMH, the injury mechanism is less potent but persists and could induce more severe and progressive kidney damage over time [1]. Moreover, little is known about the molecular mechanisms that cause episodic MH in IgAN [11].
9.2.2 Long-Term Renal Outcome in IgA Nephropathy Patients with Minimal Change Disease
There is another special entity of IgAN called IgAN with minimal change disease (MCD-IgAN), which was first reported in 1986 [12]. Those patients clinically present with nephrotic syndrome and a lack or mild degree of microscopic hematuria and have good response to corticosteroid therapy; meanwhile, pathologically, they have mild glomerular lesions under light microscopy, diffuse IgA-dominant depositions in mesangial region, and diffuse effacement of podocyte foot process and mesangial electron-dense deposition under electron microscopy. MCD-IgAN accounted for 2.6 % of the total biopsy-proven IgAN in our center. However, the clinicopathological characteristics and long-term renal outcome of MCD-IgAN in large cohorts have not yet been well described.
A retrospective review of 247 cases of biopsy-proven MCD-IgAN from the Glomerulonephritis Registry in Jinling Hospital was performed to characterize its clinicopathological features, treatment response, and long-term prognosis. In this cohort of patients, there was a male predominance (67.7 %), with an average age of 27.1 ± 11.0 years at biopsy. Pathological data revealed that 34 patients (13.7 %) had moderate to severe acute tubulointerstitial lesions and that 11 cases (4.5 %) had mild chronic tubulointerstitial lesions. After an 8-week course of corticosteroid therapy, a complete remission of proteinuria was observed in 216 patients (87.4 %), partial remission was observed in 21 patients (8.5 %), and no remission was observed in 10 patients (4.0 %). No patients with MCD-IgAN progressed to ESRD. MCD-IgAN patients had significantly better renal outcome compared with the cohort of 1,155 IgAN patients reported previously (Fig. 9.3).
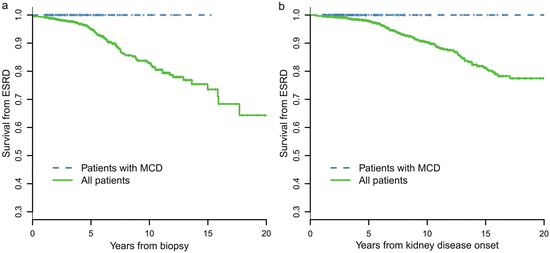

Fig. 9.3
Comparison of cumulative probabilities of renal survival between MCD-IgAN and non-MCD-IgAN patients
9.3 Prognostic Indicators in IgA Nephropathy
9.3.1 Clinical Prognostic Indicators
It is important to predict which patients are at high risk of progression and should be treated. Using the 1,155-patient follow-up data, a univariate Cox regression model found that eGFR, hypertension, proteinuria, hypoproteinemia, hyperuricemia, hypercholesterolemia, hypertriglyceridemia, and body mass index (BMI) were associated with a combined renal outcome (ESRD or 50 % drop in eGFR) during follow-up. Multivariate Cox model confirmed that eGFR, proteinuria, hypoproteinemia, hypertension at presentation, and hyperuricemia were the five most important clinical predictors independently associated with renal outcome at the time of biopsy.
9.3.2 Prognostic Indicators During Follow-Up
The effects of clinical parameters during follow-up on the outcome were also examined in the 1,155 patients with IgAN. The summary measures (time-weighted averages) for proteinuria, blood pressure (MAP), and microscopic hematuria were calculated for each patient from the area under the curve of serial measurements standardized by the duration of follow-up. For instance, the time-average MAP (TA-MAP) was defined as the ratio of the area under the curve of MAP during follow-up to the duration of follow-up. The time-average blood pressure (TA-BP) was calculated with the same method as TA-MAP. Similarly, for each patient, the time-average proteinuria (TA-P) represents the area under the curve of proteinuria during follow-up divided by the years of total follow-up. As microscopic hematuria was highly skewed during the follow-up, logarithmic transformation was used to stabilize variance of the microscopic hematuria. After log transformation of microscopic hematuria, we also calculated the time-average microscopic hematuria (TA-RBC) in a similar manner. The TA-P, TA-RBC, and TA-MAP were all associated with renal outcome in both univariate and multivariate Cox models [5].
The predictive value of proteinuria and blood pressure is well defined in previous studies, whereas the predictive value of the extent of microscopic hematuria, measured as the count of urinary erythrocytes, remains uncertain; microscopic hematuria is extremely variable over time in the same patient. We did not find an association between renal outcome and the extent of microscopic hematuria at the time of renal biopsy. However, TA-RBC was associated with renal outcome when TA-RBC was used to quantify the extent of microscopic hematuria during follow-up. The multivariate Cox model showed that the risk for a combined event increased by 210 % for every ten-fold increase in microscopic hematuria.
9.3.2.1 Target Levels of Proteinuria During the Treatment of IgA Nephropathy
Proteinuria has been proven to be the most important predictor of renal failure in IgAN. However, the threshold of proteinuria above which the risk develops, and the goal of a safe proteinuria level during follow-up, remains the subject of debate. Evidence remains lacking that the long-term renal outcomes differ between patients with a proteinuria at 0.5–1.0 g/day and those at <0.5 g/day. We used the TA-P to quantify the mean exposure of proteinuria during the follow-up in patients with IgAN, and a ROC of TA-P was drawn to determine the optimal cutoffs predicting a worse renal outcome (ESRD or 50 % reduction in eGFR) [5]. The area under ROC (AUC) was 0.9 for TA-P, indicating that TA-P had high diagnostic accuracy for an unfavorable renal outcome. The optimal cutoff of TA-P was 0.97 g/day (sensitivity 83 %, specificity 84 %) in this cohort, which was approximated to 1.0 g/day to facilitate clinical practice, indicating that the relationship between TA-P and renal outcome is dramatically altered down to levels as low as 1.0 g/day. Patients with TA-P >1.0 g/day were associated with a 14-fold higher risk of the combined events than those with TA-P <1.0 g/day (p < 0.001) [2].
In addition, we found that patients with TA-P <0.5 g/day had a significantly more favorable renal outcome than those with TA-P between 0.5 and 1.0 g/day. As shown in Fig. 9.4, the cumulative renal survival from ESRD, as well as from the combined renal events in patients with TA-P 0.5–1.0, was significantly lower than those with TA-P <0.5 g/day. The multivariate Cox analysis, adjusted with TA-MAP, TA-RBC, and eGFR at biopsy, showed that patients with TA-P >1.0 g/day and patients with TA-P 0.5–0.1.0 g/day were associated with 76.5-fold (p < 0.001) and 13.2-fold (p = 0.01), respectively, increased risks of ESRD compared with those with TA-P <0.5 g/day (Fig. 9.5a). The multivariate Cox analysis also showed that patients with TA-P >1.0 g/day and patients with TA-P 0.5–0.1.0 g/ day were associated with a 46.5-fold (p < 0.001) and 9.1-fold (p < 0.01), respectively, increased risk of ESRD or 50 % drop in eGFR during follow-up than those with TA-P <0.5 g/day (Fig. 9.5b).
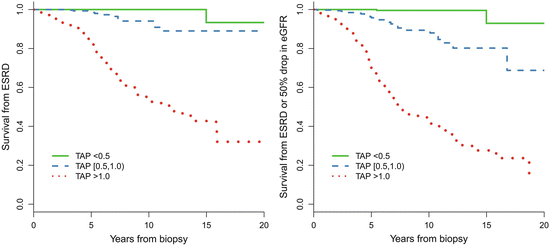
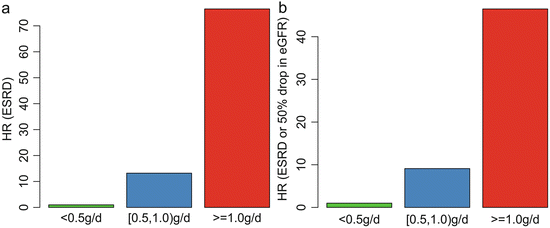

Fig. 9.4
Predictive value in renal survival of time-averaged proteinuria

Fig. 9.5
Associations between the TA-P and hazard ratio of ESRD (a) and the combined renal outcome (b). The results were adjusted with TA-MAP, TA-RBC, and eGFR at biopsy in the multivariate Cox analysis
These results suggest that the basic goal of anti-proteinuric therapy for Chinese patients is to lower proteinuria to <1.0 g/day, whereas the optimal goal is to lower proteinuria to <0.5 g/day.
9.3.3 Pathological Prognostic Indicators
9.3.3.1 Validation of the Oxford Classification in China
The Oxford classification identified four definitive histologic lesions with high reproducibility, low collinearity, and independence of clinical factors. These lesions were mesangial hypercellularity (M), endocapillary proliferation (E), segmental sclerosis or adhesion (S), and tubular atrophy/interstitial fibrosis (T) [13, 14]. Several validation studies were performed after the Oxford classification of IgAN was published and were reviewed by Roberts [15].
A multicenter validation study of the Oxford classification was conducted in a cohort of 1,026 adult patients with IgAN from China [16]. The reproducibility (ICCs) of pathologic lesions was good in this cohort. Compared with the Oxford cohort, the Chinese cohort had a lower proportion of patients with M (43 %) and E (11 %), a higher proportion with S (83 %) and necrosis (15 %), and a similar proportion with crescents (48 %) and T (moderate, 24 %; severe, 3.3 %). The lesions M, S, E, and T, as well as crescents and necrosis, were associated strongly with proteinuria at biopsy. The lesion E, crescents, and necrosis were also strongly associated with subsequent immunosuppressive treatment. During a median follow-up of 53 months, 159 (15.5 %) patients reached the combined event (ESRD or a 50 % reduction in eGFR). Patients with M1 were associated with a 2.0-fold (95 % CI, 1.5–2.8; p < 0.001) risk of the combined event than patients with M0. Patients with T1/2 versus T0 were associated with a 3.7-fold (95 % CI, 2.6–5.1; p < 0.001) and 15.1-fold (95 % CI, 9.5–24.2; p < 0.001) higher risk of the combined event, respectively. The prognostic value of E, crescents, and necrosis was not associated with renal outcome in this study. Another validation study, from a single center in China, showed that the lesions S and T were independent predictive factors of ESRD [17].
We also assessed the validity of the Oxford classification of IgAN in a multicenter cohort of pediatric patients from China [18]. The clinical characteristics in this cohort were very similar to pediatric patients in the original Oxford cohort. Our study shows that tubular interstitial fibrosis was the only pathological feature independently associated with renal outcomes in Chinese pediatric patients with IgAN.
9.3.3.2 Evidence from Repeat Renal Biopsy
The active lesions (lesion E, crescents, and necrosis) are strongly associated with proteinuria at biopsy; however, these lesions were not associated with renal outcome in our validation studies of Oxford classification and in most other validation studies [15]. Recently, we evaluated histological changes in 60 patients with IgAN who received repeat biopsies after immunosuppressive treatment and found that these active lesions are reversible after immunosuppressive treatment [19]. The active lesions were markedly decreased at the second biopsy after immunosuppressive therapy (36.7 % vs. 8.3 %, p < 0.001; 85.0 % vs. 25.0 %, p < 0.001; and 51.7 % vs. 3.3 %, p < 0.001). However, the resolution or reduction of T was not observed in those patients. Tubular atrophy continued to progress, regardless of treatment. Reversal of the active lesions was accompanied with the clinical improvement. Patients with reversal of the active lesions exhibited significant decreases in proteinuria and hematuria after immunosuppressive treatment. The reversal of these lesions during the disease process may explain the lack of significant correlation of these lesions with clinical outcomes in this study, as well as in the patient cohort on which the original Oxford classification was based, and a number of validation studies of this classification [19].
9.4 Treatment of IgA Nephropathy in China
Despite a better understanding of the pathogenic mechanisms, there is no disease-specific treatment for patients with IgAN. To date, the treatment of IgAN has mainly been based on clinical risk factors, such as proteinuria. However, the renal pathological characteristics of IgAN may provide helpful information for decision making regarding the treatment.
9.4.1 IgA Nephropathy Patients with Minimal Change Disease (MCD-IgAN)
To evaluate the efficacy of corticosteroids for MCD-IgA patients, we conducted a single-center study of MCD-IgAN patients [20]. A total of 27 biopsy-proven adult MCD-IgAN patients were enrolled and given prednisone at a daily dose of 1 mg/kg/day (maximum 60 mg/day) prednisone until complete remission (CR), followed by gradually decreasing dosage. The cumulative CR (proteinuria <0.4 g/24 h) rates were 3.70 %, 48.1 %, 92.6 %, and 100 % after 1, 2, 4, and 8 weeks of treatment, respectively. Proteinuria at each time point after treatment was markedly decreased during follow-up. Infection, alanine aminotransferase elevation (>2-fold), fasting blood glucose (FBG) elevation (>6.2 mmol/L), and hypokalemia (<3.5 mmol/L) occurred in 2, 5, 2, and 5 cases, respectively, but were eliminated after treatment. These results suggest that prednisone is an effective and safe therapy for IgAN patients with MCD lesions.
9.4.2 IgA Nephropathy Patients with Glomerular Active Proliferative Lesions
Patients with glomerular proliferative lesions are considered active forms of IgAN; immunosuppressive therapy may provide an additional benefit for these patients. We launched a multicenter, randomized, controlled trial to compare the efficiency and safety of the regimen of mycophenolate mofetil (MMF) plus prednisone in IgAN patients with active proliferative lesions. IgAN patients with active proliferative lesions (one of the following lesions of glomeruli: cellular and fibrocellular crescents; endocapillary hypercellularity; or necrosis) and proteinuria at 1 g/24 h were eligible. Eligible patients were randomly assigned to either MMF with prednisone (MMF 1.5 g/day for 6 months and prednisone 0.6 mg/kg/day for the first 2 months and then reduced by 0.1 mg/kg/day per month for the next 4 months, MMF group) or prednisone monotherapy (prednisone 1.0 mg/kg/day for 2 months and then reduced by 0.2 mg/kg/day per month for the next 4 months, PRED group), and both were followed for another 6 months.
The results showed that 32/86 patients (37.2 %) in the MMF group and 33/88 patients (37.5 %) in the PRED group went into complete remission, and no difference between the two groups was observed (p > 0.05). The overall response rate was 61/86 (70.9 %) in the MMF group and 64/88 (72.7 %) in the PRED group (p = 0.79). The median times to complete remission were 8.7 and 8.5 months (p = 0.58) for the MMF and PRED groups, respectively. The repeat renal biopsy data showed that active proliferative lesions were significantly ameliorated in both groups; the adverse event rate did not differ between the two groups. However, the incidences of newly diagnosed diabetes (13.6 % vs. 1.1 %, p = 0.002) and Cushing’s syndrome (47.7 % vs. 18.4 %, p < 0.001) were higher in the PRED group than in the MMF group.
Both MMF combined with prednisone and prednisone monotherapy benefit IgAN patients with active proliferative lesions by reducing proteinuria and ameliorating active proliferative lesions. The adverse event rate was similar in the two groups, but patients administered the MMF regimen were more tolerant.
9.4.3 IgA Nephropathy Patients with Crescentic Glomerulonephritis
Crescentic IgAN is defined as IgAN with crescents in more than 50 % of glomeruli in the renal biopsy with rapidly progressive renal deterioration. In our center, the proportion of crescentic IgAN is 1.1 % in the all biopsied-proven IgAN but 16.4 % in the total crescentic glomerulonephritis [21]. Patients with crescentic IgAN have extremely poor prognosis. In 25 crescentic IgAN patients from our center, 88 % had rapidly progressive glomerulonephritis associated with a high level of serum creatinine. In 15 patients followed up for more than 6 months, all of whom were treated with immunosuppression, five were dialysis dependent, ten maintained sufficient renal function to avoid renal replacement therapy,four had a normal range of serum creatinine (<124 μmol/l) [21]. Recently, a study enrolled 113 crescentic IgAN patients from multiple centers in China, and the renal survival rates at 1, 3, and 5 years after renal biopsy were 57.4 %, 45.8 %, and 30.4 %, respectively [22]. Initial renal function is the only independent risk factor for ESRD, whereas the percentage of crescents was not independently associated with ESRD.
There is no RCT in the treatment of crescentic IgAN. The multicenter cohort study of crescentic IgAN from China suggested that immunosuppressive therapy reduced the risk of ESRD in patients with cellular or fibrocellular crescents by >50 % but not in those with high chronic tubular lesions (interstitial fibrosis >50 %) [22].
9.5 Conclusion
The 10- and 20-year cumulative renal survival rates after renal biopsy were 83 % and 64 %, respectively, in Chinese patients with IgAN. The most important clinical risk factors are proteinuria, hypertension, impaired renal function, hypoproteinemia, and hyperuricemia; histologic risk factors are mesangial hypercellularity and tubular atrophy in Chinese patients with IgAN. The repeat renal biopsy-based observations indicated that the active proliferative glomerular lesions (endocapillary hypercellularity, crescent, or necrosis) are reversible following immunosuppressive treatment. The renal pathological lesion-based guidance for treatment provides new tools for individual therapy for IgAN patients.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
1.
2.
Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66(3):920–3. doi:10.1111/j.1523-1755.2004.00837.x.CrossRefPubMed
3.
Barbour SJ, Cattran DC, Kim SJ, Levin A, Wald R, Hladunewich MA, et al. Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int. 2013;84(5):1017–24. doi:10.1038/ki.2013.210.CrossRefPubMed
4.
Coppo R, D’Amico G. Factors predicting progression of IgA nephropathies. J Nephrol. 2005;18(5):503–12.PubMed
5.
Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27(4):1479–85. doi:10.1093/ndt/gfr527.CrossRefPubMed
6.
Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9(3):e91756. doi:10.1371/journal.pone.0091756.CrossRefPubMedPubMedCentral
7.
Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, et al. Mortality of IgA nephropathy patients: a single center experience over 30 years. PLoS One. 2012;7(12):e51225. doi:10.1371/journal.pone.0051225.CrossRefPubMedPubMedCentral
8.
Gutierrez E, Zamora I, Ballarin JA, Arce Y, Jimenez S, Quereda C, et al. Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J Am Soc Nephrol. 2012;23(10):1753–60. doi:10.1681/ASN.2012010063.CrossRefPubMedPubMedCentral
9.
Moreno JA, Martin-Cleary C, Gutierrez E, Rubio-Navarro A, Ortiz A, Praga M, et al. Haematuria: the forgotten CKD factor? Nephrol Dial Transplant. 2012;27(1):28–34. doi:10.1093/ndt/gfr749.CrossRefPubMed
10.
Le W, Liang S, Chen H, Wang S, Zhang W, Wang X, et al. Long-term outcome of IgA nephropathy patients with recurrent macroscopic hematuria. Am J Nephrol. 2014;40(1):43–50. doi:10.1159/000364954.CrossRefPubMed
11.
Eitner F, Floege J. In search of a better understanding of IgA nephropathy-associated hematuria. Kidney Int. 2012;82(5):513–5. doi:10.1038/ki.2012.160.CrossRefPubMed
12.
Lai KN, Lai FM, Ho CP, Chan KW. Corticosteroid therapy in IgA nephropathy with nephrotic syndrome: a long-term controlled trial. Clin Nephrol. 1986;26(4):174–80.PubMed
13.
Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76(5):546–56. doi:10.1038/ki.2009.168.CrossRefPubMed
14.
Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76(5):534–45. doi:10.1038/ki.2009.243.CrossRefPubMed
15.
Roberts IS. Oxford classification of immunoglobulin A nephropathy: an update. Curr Opin Nephrol Hypertens. 2013. doi:10.1097/MNH.0b013e32835fe65c.PubMed
16.
Zeng CH, Le W, Ni Z, Zhang M, Miao L, Luo P, et al. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult Chinese patients. Am J Kidney Dis. 2012;60(5):812–20. doi:10.1053/j.ajkd.2012.06.011.CrossRefPubMed
17.
Shi SF, Wang SX, Jiang L, Lv JC, Liu LJ, Chen YQ, et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol. 2011;6(9):2175–84. doi:10.2215/CJN.11521210.CrossRefPubMedPubMedCentral
18.
Le W, Zeng CH, Liu Z, Liu D, Yang Q, Lin RX, et al. Validation of the Oxford classification of IgA nephropathy for pediatric patients from China. BMC Nephrol. 2012;13:158. doi:10.1186/1471-2369-13-158.CrossRefPubMedPubMedCentral
19.
Shen XH, Liang SS, Chen HM, Le WB, Jiang S, Zeng CH, et al. Reversal of active glomerular lesions after immunosuppressive therapy in patients with IgA nephropathy: a repeat-biopsy based observation. J Nephrol. 2015. doi:10.1007/s40620-014-0165-x.PubMed
20.
Wang J, Juan C, Huang Q, Zeng C, Liu Z. Corticosteroid therapy in IgA nephropathy with minimal change-like lesions: a single-centre cohort study. Nephrol Dial Transplant. 2013;28(9):2339–45. doi:10.1093/ndt/gft211.CrossRefPubMed
21.
22.
Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo Z, et al. Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J Am Soc Nephrol. 2013;24(12):2118–25. doi:10.1681/ASN.2012101017.CrossRefPubMedPubMedCentral