Chapter 117 Procyanidolic Oligomers
 General Description
General Description
The proanthocyanidins (also referred to as “procyanidins”) are one of the most beneficial groups of plant flavonoids. The most active proanthocyanidins are those bound to other proanthocyanidins. Collectively, mixtures of proanthocyanidin dimers, trimers, tetramers, and larger molecules are referred to as procyanidolic oligomers (PCOs) or oligomeric proanthocyanidins (OPCs).1,2 Although PCOs exist in many plants as well as red wine, commercially available sources of PCOs include extracts from grape seed skin (Vitex vinifera) and the bark of the maritime (Landes) pine.1,2 This chapter reviews the benefits of PCOs from grape seeds and pine bark interchangeably due to their similar chemical composition and pharmacologic profiles.
 Chemical Composition
Chemical Composition
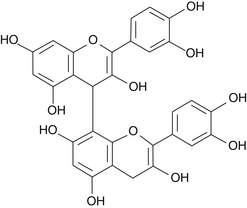
Grape seed and pine bark PCO extracts are well defined chemically. Grape seed extracts are available that contain 92% to 95% PCOs, whereas the PCO content of pine bark extracts varies from 80% to 85%. Proanthocyanidin B2 is shown in Figure 117-1.
 History and Folk Use
History and Folk Use
Masquelier termed the active components of the pine bark pycnogenols.1,3 This term was used to describe an entire complex of proanthocyanidin complexes found in a variety of plants, including pine bark, grape seeds, lemon tree bark, peanuts, cranberries, and citrus peels. The term pycnogenol has been replaced in the scientific community by the terms proanthocyanidins, OPC, and PCO. In the United States, Pycnogenol is a registered trademark of Horphag Ltd of Guernsey, United Kingdom, and refers to the PCO extracted from the bark of the French maritime pine.
 Pharmacology
Pharmacology
Extracts of PCOs demonstrate a wide range of activity as listed in Box 117-1. Pharmacokinetic studies showed that proanthocyanidins and other breakdown products of PCOs from either grape seed extract or maritime pine are detectable in the serum after oral intake.4,5 Pharmacokinetic studies in humans and animals given C14-labelled PCOs also showed efficient oral absorption.6,7
BOX 117-1 Basic Pharmacologic Activity of Proanthocyanidins
• Increase intracellular vitamin C levels
• Decrease capillary permeability and fragility
• Scavenge oxidants and free radicals
Data from references 1 and 2.
Antioxidant and Free Radical Scavenging Activity
Perhaps the most celebrated effects of PCOs in the United States are their potent antioxidant and free radical scavenging activities. The antioxidant and free radical scavenging effects of PCOs were discovered by Masquelier in 1986.1
One study evaluated the free radical scavenging activity of PCOs and determined their inhibitory effects on xanthine oxidase (a primary generator of oxygen-derived free radicals) and the lysosomal enzyme system (which governs the release of enzymes that can damage the connective tissue framework acting as a protective sheath around capillary walls).8 This research, summarized in Box 117-2, provides a detailed explanation of the vascular protective action of PCOs and a strong rationale for their use in vascular disease.
BOX 117-2 Antioxidant and Free Radical Scavenging Activities of Procyanidolic Oligomers
• Trap lipid peroxides and free radicals
• Markedly delay the onset of lipid peroxidation
• Chelate free iron molecules, thereby preventing iron-induced lipid peroxidation
• Inhibit production of free radicals by noncompetitively inhibiting xanthine oxidase
• Inhibit the damaging effects of the enzymes (e.g., hyaluronidase, elastase, collagenase) that can degrade connective tissue structures
In experimental models, the antioxidant activity of PCOs is much greater (approximately 50 times) than that of vitamin C and vitamin E. PCOs reduce lipid peroxidation9 and oxidation of low-density lipoprotein (LDL),10 increase basal levels of α-tocopherol in endothelial cells, protect endothelium from oxidative stress induced by reactive nitrogen species,11,12 and protect endothelial cells from activated macrophage-induced glutathione depletion.13 From a cellular perspective, one of the most advantageous features of PCOs’ free radical scavenging activity is that, because of their chemical structure, they are incorporated into cell membranes. This physical characteristic, along with their ability to protect against both water- and fat-soluble free radicals, provides significant cellular protection against damage by free radicals.8
Protection of Collagen
Collagen, the most abundant protein of the body, is responsible for maintaining the integrity of ground substance as well as the integrity of tendons, ligaments, and cartilage. Collagen is also the support structure of the dermis and blood vessels. PCOs are remarkable in their effects of supporting collagen structures and preventing collagen destruction. They affect collagen metabolism in several ways. They have the unique ability to cross-link collagen fibers, resulting in reinforcement of the natural cross-linking of collagen that forms the so-called collagen matrix of connective tissue.14,15 They also protect against free radical damage with their potent antioxidant and free radical scavenging action, and they inhibit enzymatic cleavage of collagen by enzymes secreted by leukocytes during inflammation and microbes during infection.8,16 PCOs also prevent the release and synthesis of compounds that promote inflammation and allergies, such as histamine, serine proteases, prostaglandins, and leukotrienes.1,2,17,18
Antiinflammatory Activity
In vitro and in vivo studies showed PCOs significantly inhibit cyclooxygenase (COX-1) and COX-2 enzymes as well as the formation of proinflammatory cytokines, interleukin-1β, and tumor necrosis factor-β, as well as histamine release from mast cells.19–22 When 10 volunteers received a single dose of 300 mg Pycnogenol, within 30 minutes serum samples showed statistically significant inhibition of both COX-1 and COX-2 (P <0.002). Blood samples before and after 5 days administration of 200 mg Pycnogenol to five healthy humans showed similar inhibition.
Cardiovascular Protection
In addition to protecting against oxidative damage to LDL and endothelial cells, PCOs increase erythrocyte membrane fluidity; reduce platelet aggregation; enhance endothelial nitric oxide synthase (eNOS) activity to increase nitric oxide levels; and inhibit angiotensin-I–converting enzyme (ACE).23–27 PCO extracts were shown to lower blood cholesterol levels and to shrink the size of cholesterol deposits in arteries in animal and human studies.1,28,29
PCOs also significantly lower C-reactive protein (CRP), a major risk factor for cardiovascular disease. In a double-blind study, 29 subjects in the Pycnogenol group and 26 patients in the placebo group had CRP levels higher than 3 mg/L at baseline.21 After 3-month treatment with Pycnogenol, at a dose of 100 mg/day, CRP significantly decreased plasma free radicals to 70.1% of baseline values. Specifically, plasma CRP levels decreased from 3.9 mg/L at baseline to 1.1 mg/L in the Pycnogenol group, whereas the control group had initial values of 3.9 mg/L that decreased to 3.6 mg/L. Fibrinogen levels were found to be lowered to 62.8% of initial values in the Pycnogenol group.
Anticancer Activity
PCOs exert a number of anticancer effects beyond their general antioxidant actions. PCOs were shown to selectively induce apoptosis (programmed cell death) in human breast cancer cells, inhibit cell proliferation and induce differentiation and apoptosis in leukemia cells, and inhibit nitrosamine activation in lung microsomes.30–33
 Clinical Applications
Clinical Applications
The primary clinical applications of PCOs are in the treatment of the following conditions:
• Atherosclerosis, hypertension, metabolic syndrome, and type 2 diabetes
Antioxidant Therapy
PCOs exert broad-spectrum antioxidant activity and are clinically useful in many health conditions because of this action alone. In healthy human subjects, supplementation with PCOs for 6 weeks was shown to considerably improve the plasma oxygen radical absorbance capacity score (ORAC).28 In one double-blind study, the effect of a standardized formulation of a PCO bound to phosphatidylcholine (Leucoselect-Phytosome) was determined on susceptibility of LDL to oxidation in a group of heavy smokers.34 Among oxidative indexes, the concentration of thiobarbituric acid reactive substances was significantly reduced in subjects taking the PCO extract, and the lag phase was prolonged compared with the values in subjects taking placebo, as well as with baseline values.
In a double-blind, placebo-controlled study in 101 elderly participants (60 to 85 years), consuming a daily dose of 150 mg of Pycnogenol for 3 months produced significant improvements in several memory-based cognitive assessments and lipid peroxidation products (F2-isoprostanes) relative to the control group.35
Asthma
PCOs appear to have an affinity for the lungs, indicating a possible benefit in lung disorders, including asthma. In a double-blind study involving 60 subjects (aged 6 to 18 years old) with mild-to-moderate asthma, patients were given either Pycnogenol (1 mg/lb body weight per day) or placebo for 3 months. Subjects were instructed to record their peak expiratory flow with an Assess Peak Flow Meter each evening. Compared with subjects taking placebo, the group who took Pycnogenol had significantly more improvement in pulmonary function and asthma symptoms. The Pycnogenol group was able to reduce or discontinue their use of rescue inhalers more often than the placebo group. There was also a significant reduction of urinary leukotrienes in the Pycnogenol group. The results of this study demonstrate the efficacy of Pycnogenol as an adjunct in the management of mild-to-moderate childhood asthma. Given the safety of PCOs, higher dosages (e.g., 2 to 3 mg/lb body weight) may produce better results than the lower dosage used in the clinical trial.36
Atherosclerosis, Hypertension, Metabolic Syndrome, and Type 2 Diabetes
PCO extracts, although in a supplement form, should be regarded as a necessary food in the prevention and treatment of atherosclerosis, metabolic syndrome, and type 2 diabetes. PCOs are similar to red wine components that are so protective against heart disease.37 The active components in the wine are thought to be proanthocyanidins. Flavonoid consumption, in general, has an inverse correlation with death due to heart attack.38 Both Pycnogenol and grape seed extract showed considerable ability to reduce cardiovascular risk factors, especially in patients with type 2 diabetes.39,40 However, even healthy subjects can take advantage of the beneficial effects of PCOs against cardiovascular risk factors. In one study, when 25 healthy subjects received Pycnogenol (150 mg/day) for 6 weeks, a significant increase in ORAC in plasma was observed throughout the supplementation period (returning to baseline after a 4-week washout period).28 Pycnogenol also significantly reduced LDL cholesterol levels and increased high-density lipoprotein (HDL) cholesterol levels in plasma of two thirds of the subjects. Although the LDL changes reversed during washout, the HDL increase did not. Like other flavonoid sources, PCO extracts appear to be very helpful in improving endothelial function. Again, this effect may be particularly important in patients with hypertension, metabolic syndrome, and diabetes. In one double-blind study in 58 patients with hypertension who received supplementation with 100 mg PCO over 12 weeks, endothelin-1 concentrations were significantly lower, and concentrations of 6-keto prostaglandin F1a in plasma were significantly higher in the PCO group than in the placebo group. Additionally, the PCOs helped reduce the effective dose of the calcium antagonist nifedipine in a statistically significant manner.41 In another double-blind study, PCOs from grape seed extract were shown to produce a mild hypotensive effect (−11 mm Hg for both systolic and diastolic readings) in patients with the metabolic syndrome at dosages of 150 and 300 mg/day.42
Kidney function improved in both groups as judged by a significant reduction of protein detected in collected urine. With ramipril alone, urinary protein decreased by 22%, and with the addition of Pycnogenol, it decreased by 52.7%. Further, the group taking Pycnogenol had a lower fasting blood glucose level, which was reduced from a high average value of 135.6 mg/dL at baseline to an essentially healthy reference value of 102.3 mg/dL after 6 months of treatment. Pycnogenol also led to a remarkable improvement of blood flow velocity of the kidney arteries. Blood flow velocity in the kidneys significantly increased with ramipril from 17.2 to 23.8 cm/s for systolic and 4.2 to 2.0 cm/s for diastolic; the addition of Pycnogenol was more effective, improving blood flow from 18.2 to 27.2 cm/s for systolic and 4.1 to 9.8 cm/s for diastolic.
Other studies showed that PCOs might lower fasting glucose levels. In a study in type 2 diabetes, patients received, in succession, 50, 100, 200, and 300 mg Pycnogenol in intervals of 3 weeks.43 Every 3 weeks, fasting and postprandial glucose, endothelin-1, glycosylated hemoglobin (HbA1c), and insulin were analyzed. Fasting blood glucose was lowered dose dependently until a dose of 200 mg Pycnogenol was administered. Increasing the dose from 200 to 300 mg did not further decrease blood glucose. Compared with baseline, 100 to 300 mg of Pycnogenol lowered fasting glucose significantly from 8.64 to 7.54 mmol/L (155 to 135 mg/dL). The maximum decrease of postprandial glucose was observed with 200 mg of Pycnogenol (0.07 ± 2.69 mmol/dL [180 mg/dL]). HbA1c levels decreased continuously from 8.02% to 7.37%. Endothelin-1 decreased significantly after 100 to 300 mg Pycnogenol, from 104 to 91 pg/mL. Stimulation of insulin secretion was excluded as a cause for lower glucose levels because insulin levels were not affected.
These effects were confirmed in a double-blind, placebo-controlled study with 77 patients with type 2 diabetes. After 8 weeks of treatment, the median drop in fasting blood glucose levels in the Pycnogenol group was 1.96 mmol/L (35.28 mg/dL).44
PCOs are particularly useful in addressing the microvascular pathology of diabetes. In one study in patients with severe diabetic microangiopathy, 30 patients received oral Pycnogenol (50 mg capsules, 3 times daily, for a total of 150 mg/day for 4 weeks), whereas 30 comparable patients were observed as controls.45 After 4 weeks, microcirculatory and clinical evaluations showed a progressive decrease in skin flux at rest in the foot (indicating an improvement in the level of microangiopathy), a significant decrease in capillary filtration, and a significant improvement in the venoarteriolar response in all treated subjects. There were no visible effects in controls, except a slight reduction in skin flux at rest in the foot.
PCOs are also very much indicated in the treatment of diabetic retinopathy. Early intervention in diabetic retinopathy led to significant improvements in microcirculation, retinal edema, and visual acuity.46
Attention Deficit Disorder
Increased oxidative damage is believed to be a central factor in attention deficit hyperactivity disorder (ADHD), indicating a possible application for PCO therapy. To date, four studies have been conducted in this patient population. In one study, children with ADHD supplementation with PCO (1 mg/kg body weight per day) had an improved reduced glutathione to oxidized glutathione ratio (an indication of improved antioxidant status).47 A second study confirmed this effect and also showed a reduced urinary catecholamine concentration, leading to less hyperactivity.48 Another study showed that PCOs reduced the level of oxidized purines represented by 8-oxo-7,8-dihydroguanine in the urine and also increased total antioxidant status.49 Finally, 61 children with ADHD supplemented with 1 mg/kg per day Pycnogenol or placebo over 4 weeks showed that 1-month Pycnogenol administration caused a significant reduction of hyperactivity, improved attention and visual-motoric coordination, and improved concentration of children with ADHD.50 No positive effects were found in the placebo group. Standard questionnaires, the Child Attention Problems teacher rating scale, Conner’s Teacher Rating Scale, the Conner’s Parent Rating Scale, and a modified Wechsler Intelligence Scale for children were used as assessments. These results point to an option to use Pycnogenol as a nutritional adjunct in children with ADHD.
Male Infertility
Pycnogenol alone51 and in combination with L-arginine52 was shown to be helpful in improving sperm quality and function (see Chapter 180, Male Infertility, for more information).
Periodontal Disease
Given the importance of supporting periodontal collagen structures in periodontal disease, PCOs are an important clinical consideration (see Chapter 199 on Periodontal Disease for more information).
Venous Insufficiency and Capillary Fragility
• Increase the integrity of the venous wall
• Inhibit the breakdown of the compounds composing the ground substance
Numerous double-blind studies with Pycnogenol validated the effectiveness of PCOs in chronic venous insufficiency, including studies showing an ability to decrease venous ulcer size,53,54 reduce the edema and thrombosis associated with airline travel,55,56 reduce nighttime claudication, and reduce other signs and symptoms of chronic venous insufficiency and microangiopathy.57–59
Visual Function, Retinopathy, and Macular Degeneration
Increased intake of PCO is likely to benefit almost everyone. This suggestion is perhaps best illustrated by research evaluating the effects of grape seed PCOs extract on visual function in healthy subjects.60,61 In one of the studies, 100 normal volunteers with no retinal disorder received either 200 mg/day of PCOs or placebo for 5 or 6 weeks. The group receiving PCOs demonstrated significant improvement in visual performance in dark and after-glare tests compared with the placebo group. PCO extracts were also shown to be of benefit in treating retinopathies, especially diabetic retinopathy. In one study, 40 patients with diabetes, atherosclerosis, and other vascular diseases involving the retina were randomly treated with placebo or PCO extract (50 mg 3 times daily for 2 months). The results demonstrated a beneficial effect of PCOs on the progression of retinopathy. In patients without any treatment (placebo group), the retinopathy progressively worsened during the trial, and the visual acuity significantly decreased. In contrast, the PCO-treated patients showed no deterioration of retinal function, and a significant recovery of visual acuity was also obtained. Fluorangiography showed an improvement of retinal vascularization and reduced endothelial permeability and leakage in the PCO group, but not in the placebo-treated patients. Ophthalmoscopy and electroretinography also confirmed the beneficial effects of PCOs. In addition to the PCOs exerting free radical scavenging, anti-inflammatory, and capillary protective activities, it was suggested that PCOs might bind to the blood vessel wall proteins and mucopolysaccharides and produce a capillary “sealing” effect, leading to reductions in capillary permeability and edema formation.62
 Dosage
Dosage
As antioxidant support, a daily dose of 50 to 100 mg of either the grape seed or pine bark extract is suitable. For comparison, it is now estimated that the average daily intake of total flavonoids in the United States is about 25 mg. An intake greater than 30 mg offers a significant reduction in risk for cardiovascular mortality.38
1. Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40:158–168.
2. Schwitters B., Masquelier J. OPC in practice: biflavanols and their application. Rome: Alfa Omega; 1993.
3. Masquelier J. [Procyanidolic oligomers.]. J Parfums Cosmetiques Aromes. 1990;95:89–97. [French]
4. Grimm T., Skrabala R., Chovanova Z., et al. Single and multiple dose pharmacokinetics of maritime pine bark extract (pycnogenol) after oral administration to healthy volunteers. BMC Clin Pharmacol, 2006;6:4. http://dx.doi.org/10.1186/1472-6904-6-4.
5. Sano A., Yamakoshi J., Tokutake S., et al. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci Biotechnol Biochem. 2003;67:1140–1143.
6. Benakis A., Schopfer C., Ritschard J. Disposition of 14C-labelled ENDOTELON in humans. Eur J Drug Metab Pharmacokinet. 2008;33:37–43.
7. Tsang C., Auger C., Mullen W., et al. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br J Nutr. 2005;94:170–181.
8. Maffei Facino R., Carini M., Aldini G., et al. Free radicals scavenging action and anti-enzyme activities of procyanidines from Vitis vinifera. A mechanism for their capillary protective action. Arzneimitteforschung. 1994;44:592–601.
9. Virgili F., Kim D., Packer L. Procyanidins extracted from pine bark protect alpha-tocopherol in ECV 304 endothelial cells challenged by activated RAW 264.7 macrophages: role of nitric oxide and peroxynitrite. FEBS Lett. 1998;431:315–318.
10. Virgili F., Kobuchi H., Packer L. Procyanidins extracted from Pinus maritima (Pycnogenol): scavengers of free radical species and modulators of nitrogen monoxide metabolism in activated murine RAW 264.7 macrophages. Free Radic Biol Med. 1998;24:1120–1129.
11. Rimbach G., Virgili F., Park Y.C., et al. Effect of procyanidins from Pinus maritima on glutathione levels in endothelial cells challenged by 3-morpholinosydnonimine or activated macrophages. Redox Rep. 1999;4:171–177.
12. Devaraj S., Vega-Lopez S., Kaul N., et al. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids. 2002;37:931–934.
13. Dvorakova M., Sivonova M., Trebaticka J., et al. The effect of polyphenolic extract from pine bark, Pycnogenol on the level of glutathione in children suffering from attention deficit hyperactivity disorder (ADHD). Redox Rep. 2006;11:163–172.
14. Masquelier J., Dumont M.C., Dumas J. Stabilization of collagen by procyanidolic oligomers. Acta Therap. 1981;7:101–105. [French]
15. Tixier J.M., Godeau G., Robert A.M. Evidence by in vivo and in vitro studies that binding of pycnogenols to elastin affects its rate of degradation by elastases. Biochem Pharmacol. 1984;33:3933–3939.
16. Meunier M.T., Duroux E., Bastide P. Free-radical scavenger activity of procyanidolic oligomers and anthocyanosides with respect to superoxide anion and lipid peroxidation. Plant Med Phytother. 1989;4:267–274.
17. Sharma S.C., Sharma S., Gulati O.P. Pycnogenol inhibits the release of histamine from mast cells. Phytother Res. 2003;17:66–69.
18. Cho K.J., Yun C.H., Packer L., et al. Inhibition mechanisms of bioflavonoids extracted from the bark of Pinus maritima on the expression of proinflammatory cytokines. Ann N Y Acad Sci. 2001;928:141–156.
19. Schäfer A., Chovanová Z., Muchová J., et al. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol). Biomed Pharmacother. 2006;60:5–9.
20. Blazso G., Gabor M., Rohdewald P. Antiinflammatory activities of procyanidin-containing extracts from Pinus pinaster Ait. after oral and cutaneous application. Pharmazie. 1997;52:380–382.
21. Belcaro G., Cesarone M.R., Errichi S., et al. Variations in C-reactive protein, plasma free radicals and fibrinogen values in patients with osteoarthritis treated with Pycnogenol. Redox Rep. 2008;13:271–276.
22. Sharma S.C., Sharma S., Gulati O.P. Pycnogenol inhibits the release of histamine from mast cells. Phytother Res. 2003;17:66–69.
23. Chang W.C., Hsu F.L. Inhibition of platelet aggregation and arachidonate metabolism in platelets by procyanidins. Prostagland Leukot Essent Fatty Acids. 1989;38:181–188.
24. Meunier M.T., Villie F., Jonadet M. Inhibition of angiotensin I converting enzyme by flavanolic compounds: in vitro and in vivo studies. Planta Med. 1987;54:12–15.
25. Sivonova M., Waczulikova I., Kilanczyk E., et al. The effect of Pycnogenol on the erythrocyte membrane fluidity. Gen Physiol Biophys. 2004;23:39–51.
26. Golanski J., Muchova J., Golanski R., et al. Does pycnogenol intensify the efficacy of acetylsalicylic acid in the inhibition of platelet function? In vitro experience. Postepy Hig Med Dosw (Online). 2006;60:316–321.
27. Fitzpatrick D.F., Bing B., Rohdewald P. Endothelium-dependent vascular effects of Pycnogenol. J Cardiovasc Pharmacol. 1998;32:509–515.
28. Devaraj S., Vega-Lopez S., Kaul N., et al. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids. 2002;37:931–934.
29. Wegrowski J., Robert A.M., Moczar M. The effect of procyanidolic oligomers on the composition of normal and hypercholesterolemic rabbit aortas. Biochem Pharmacol. 1984;33:3491–3497.
30. Huynh H.T., Teel R.W. Selective induction of apoptosis in human mammary cancer cells (MCF-7) by pycnogenol. Anticancer Res. 2000;20:2417–2420.
31. Huang W.W., Yang J.S., Lin C.F., et al. Pycnogenol induces differentiation and apoptosis in human promyeloid leukemia HL-60 cells. Leuk Res. 2005;29:685–692.
32. Huynh H.T., Teel R.W. Effects of pycnogenol on the microsomal metabolism of the tobacco-specific nitrosamine NNK as a function of age. Cancer Lett. 1998;132:135–139.
33. Huynh H.T., Teel R.W. Effects of intragastrically administered Pycnogenol on NNK metabolism in F344 rats. Anticancer Res. 1999;19(3A):2095–2099.
34. Vigna G.B., Costantini F., Aldini G., et al. Effect of a standardized grape seed extract on low-density lipoprotein susceptibility to oxidation in heavy smokers. Metabolism. 2003;52:1250–1257.
35. Ryan J., Croft K., Mori T., et al. An examination of the effects of the antioxidant Pycnogenol on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J Psychopharmacol. 2008;22:553–562.
36. Lau B.H., Riesen S.K., Truong K.P., et al. Pycnogenol as an adjunct in the management of childhood asthma. J Asthma. 2004;41:825–832.
37. Frankel E.N., Kanner J., German J.B., et al. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–457.
38. Hertog M.G., Feskens E.J., Hollman P.C., et al. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011.
39. Zibadi S., Rohdewald P.J., Park D., et al. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr Res. 2008;28:315–320.
40. Kar P., Laight D., Rooprai H.K., et al. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: a double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet Med. 2009;26:526–531.
41. Belcaro G., Cesarone M.R., Ricci A., et al. Control of edema in hypertensive subjects treated with calcium antagonist (nifedipine) or angiotensin-converting enzyme inhibitors with Pycnogenol. Clin Appl Thromb Hemost. 2006;12:440–444.
42. Sivaprakasapillai B., Edirisinghe I., Randolph J., et al. Effect of grape seed extract on blood pressure in subjects with the metabolic syndrome. Metabolism. 2009;58:1743–1746.
43. Liu X., Zhou H.J., Rohdewald P. French maritime pine bark extract Pycnogenol dose-dependently lowers glucose in type 2 diabetic patients. Diabetes Care. 2004;27:839.
44. Cesarone M.R., Belcaro G., Rohdewald P., et al. Improvement of diabetic microangiopathy with pycnogenol: A prospective, controlled study. Angiology. 2006;57:431–436.
45. Liu X., Wei J., Tan F., et al. Antidiabetic effect of Pycnogenol French maritime pine bark extract in patients with diabetes type II. Life Sci. 2004;75:2505–2513.
46. Steigerwalt R., Belcaro G., Cesarone M.R., et al. Pycnogenol improves microcirculation, retinal edema, and visual acuity in early diabetic retinopathy. J Ocul Pharmacol Ther. 2009;25:537–540.
47. Dvoráková M., Sivonová M., Trebatická J., et al. The effect of polyphenolic extract from pine bark, Pycnogenol on the level of glutathione in children suffering from attention deficit hyperactivity disorder (ADHD). Redox Rep. 2006;11:163–172.
48. Dvoráková M., Jezová D., Blazícek P., et al. Urinary catecholamines in children with attention deficit hyperactivity disorder (ADHD): modulation by a polyphenolic extract from pine bark (pycnogenol). Nutr Neurosci. 2007;10:151–157.
49. Chovanová Z., Muchová J., Sivonová M., et al. Effect of polyphenolic extract, Pycnogenol, on the level of 8-oxoguanine in children suffering from attention deficit/hyperactivity disorder. Free Radic Res. 2006;40:1003–1010.
50. Trebatická J., Kopasová S., Hradecná Z., et al. Treatment of ADHD with French maritime pine bark extract. Pycnogenol. Eur Child Adolesc Psychiatry. 2006;15:329–335.
51. Roseff S.J. Improvement in sperm quality and function with French maritime pine tree bark extract. J Reprod Med. 2002;47:821–824.
52. Stanislavov R., Nikolova V., Rohdewald P. Improvement of seminal parameters with Prelox: a randomized, double-blind, placebo-controlled, cross-over trial. Phytother Res. 2009;23:297–302.
53. Belcaro G., Cesarone M.R., Errichi B.M., et al. Venous ulcers: microcirculatory improvement and faster healing with local use of Pycnogenol. Angiology. 2005;56:699–705.
54. Cesarone M.R., Belcaro G., Rohdewald P., et al. Comparison of Pycnogenol and Daflon in treating chronic venous insufficiency: a prospective, controlled study. Clin Appl Thromb Hemost. 2006 Apr;12(2):205–212.
55. Belcaro G., Cesarone M.R., Rohdewald P., et al. Prevention of venous thrombosis and thrombophlebitis in long-haul flights with pycnogenol. Clin Appl Thromb Hemost. 2004;10:373–377.
56. Cesarone M.R., Belcaro G., Nicolaides A.N., et al. Prevention of venous thrombosis in long-haul flights with Flite Tabs: the LONFLIT-FLITE randomized, controlled trial. Angiology. 2003;54:531–539.
57. Cesarone M.R., Belcaro G., Rohdewald P., et al. Improvement of signs and symptoms of chronic venous insufficiency and microangiopathy with Pycnogenol: a prospective, controlled study. Phytomedicine. 2010;17:835–839.
58. Cesarone M.R., Belcaro G., Rohdewald P., et al. Rapid relief of signs/symptoms in chronic venous microangiopathy with pycnogenol: a prospective, controlled study. Angiology. 2006;57:569–576. erratum Angiology 2008;59:385
59. Nuzum D.S., Gebru T.T., Kouzi S.A. Pycnogenol for chronic venous insufficiency. Am J Health Syst Pharm. 2011 Sep 1;68(17):1589–1590. 1599-1601
60. Corbe C., Boissin J.P., Siou A. Light vision and chorioretinal circulation: study of the effect of procyanidolic oligomers Endotelon. J Fr Ophtalmol. 1988;11:453–460. [French]
61. Boissin J.P., Corbe C., Siou A. Chorioretinal circulation and dazzling: use of procyanidol oligomers. Bull Soc Ophtalmol Fr. 1988;88:173–174. 177-179
62. Spadea L., Balestrazzi E. Treatment of vascular retinopathies with Pycnogenol. Phytother Res. 2001;15:219–223.