26 Procedures in the Hybrid Operating Room
Transcatheter techniques are being used increasingly as an adjunct to, rather than a replacement for, cardiac surgery; the primary aim is to improve clinical outcomes by reducing the size and number of incisions and cardiopulmonary bypass (CPB) time, without compromising the long-term results offered by conventional cardiac surgery.1 Increasingly, it is only possible to perform these hybrid procedures in suites combining conventional cardiac operating room capability with standard cardiovascular imaging equipment, particularly because most existing cardiac operating rooms and catheterization laboratories do not meet the requirements for performing both surgery and interventional imaging.2 Hybrid operating rooms first emerged in vascular surgery, driven by lack of access to interventional radiology facilities at a time of expansion in endovascular techniques; more recently, an increase in the number of hybrid procedures has emphasized the need for suites specifically designed for this purpose. This chapter provides an overview of the rationale for building a hybrid cardiovascular suite and the planning, logistics, and design challenges that must be met to create and run it successfully. There is relatively little available on the design and logistics of hybrid operating rooms in the medical literature; the reference articles,3–5 including an excellent case study by Hirsch4 and detailed review by Nollert and Wich,5 were the primary source materials for this chapter and cover most of the aspects outlined here in more depth.
Rationale
Key aspects of building design depend on the intended use of the room. Given total costs of between $2 and $4 million, there may be a desire to ensure that the room is suitable for the full gamut of cardiovascular hybrid procedures (Table 26-1) to maximize use; and key stakeholders from adult and pediatric cardiac surgery, interventional cardiology, electrophysiology, vascular surgery, and anesthesiology should, therefore, be involved in planning at the earliest stages. It is vital to decide early in the process whether the aim is to build a cardiac catheterization laboratory that can be used for surgical procedures, a cardiac operating room that may be used for cardiovascular imaging, or a true hybrid suite meeting the specifications for cardiac surgery and catheterization and designed to allow state-of-the-art imaging, intervention, and surgery to take place at the same time.
TABLE 26-1 Procedures That Can Be Included in the Business Plan for a Hybrid Cardiovascular Suite
| Interventional Cardiology |
| Diagnostic and therapeutic cardiac catheterization, including percutaneous coronary intervention |
| Diagnostic and therapeutic electrophysiology procedures, including endocardial ablation, pacemaker and defibrillator device insertion and changes |
| Conventional Cardiac Surgery |
| All adult and pediatric cardiac surgery |
| Transplant, ventricular assist device, and extracorporeal membrane oxygenation |
| Trauma surgery |
| Fetal interventions |
| Endovascular Surgery |
| Abdominal aortic aneurysm stenting |
| Thoracic aortic aneurysm stenting |
| Carotid stenting |
| Hybrid Procedures |
| Pediatric |
| Hybrid stage I procedure for hypoplastic left-heart syndrome (modified Norwood) |
| Patent ductus arteriosus stenting with surgical Blalock-Taussig shunt |
| Pulmonary artery stenting |
| Percutaneous atrial septal defect with option to convert to on-bypass open procedure |
| Preventricular ventricular septal defect closure for muscular apical septal defects |
| Pulmonary valve replacement |
| Adult |
| Coronary artery bypass grafting in multivessel disease with either endoscopic, minithoracotomy or robotic mammary harvest, with direct or robotic left anterior descending coronary artery anastomosis, percutaneous intervention on other lesions, and operative angiography of bypass grafts |
| Transcatheter aortic valve implantation |
| Thoracoabdominal aneurysm stenting with surgical debranching or bypass |
Hybrid cardiovascular procedures
 Coronary Revascularization
Coronary Revascularization
Coronary artery surgery, which represents more than 90% of adult cardiac procedures nationally, offers some scope for a hybrid approach. The impact of graft failure after coronary artery bypass grafting (CABG) is well documented. In a recent prospective, multicenter study, the 1-year failure rate of saphenous vein grafts was reported to be more than 30%, that of the left internal mammary artery 8%, and the common end point of death or new myocardial infarction was 14% in these patients compared with 1% in patients with patent grafts.6 More recent data suggested saphenous vein failure rates of more than 40% at 12 to 18 months.7 Early graft failure, present in 5% to 20% of patients at discharge from the hospital, commonly is attributed to technical error and is the rationale for completion angiography with the option for percutaneous coronary intervention before leaving the operating room. In a recent series of 366 consecutive patients undergoing CABG surgery with completion angiography, 6% of all grafts required percutaneous coronary intervention to address technical problems compromising patency (including vein valves impeding flow [n = 9], left internal mammary artery dissection [n = 6], vein graft kinks [n = 7], and incorrect location or vessel [n = 8]). In an additional 49 cases (6.2% of grafts), angiography revealed problems that could be corrected either by minor adjustments such as removing a clip or adjustment of conduit lie or by traditional surgical revision8 (see Chapter 18).
 Transcatheter Valve Replacement
Transcatheter Valve Replacement
An emerging modality that will likely become a mainstay of hybrid operating rooms is transcatheter valve replacements.9 Aortic valve replacement is the treatment of choice for symptomatic severe aortic stenosis; medical management is associated with high mortality, and balloon valvuloplasty offers temporary symptomatic relief without any associated survival benefit. Despite the low operative mortality of isolated primary aortic valve replacement, up to 40% of patients with American Heart Association/American College of Cardiology Class I indications for aortic valve replacement are denied surgery. Reasons most commonly cited by clinicians include advanced patient age and morbidity, and this is a driving force behind the development of transcatheter aortic valve implantation. Transcatheter aortic valve replacement has been performed via either the transfemoral or transapical approach in several thousand patients in Europe, and as of 2010, the U.S. Food and Drug Administration approved the procedure in the United States (see Chapter 19).
The likelihood is that transfemoral aortic valve replacement will become the dominant treatment modality in high-risk patients requiring aortic valve replacement, greatly expanding the growing pool of eligible patients. Results have improved as both experience with the procedures and technology have developed, and currently mortality, associated stroke, major morbidity, and echocardiographic outcomes appear to offer very-high-risk and nonoperable patients a safe alternative to conventional surgery. Indications for transcatheter aortic valve implantation eventually may be expanded to lower-risk groups, based on outcomes of the large prospective clinical trials currently under way. Interventions for mitral and tricuspid valve repair are at a much earlier stage of development and are less likely to contribute significantly to the volume of hybrid procedures in the next decade.10
 Hybrid Thoracic Aortic Surgery
Hybrid Thoracic Aortic Surgery
Thoracoabdominal aortic disease increasingly is treated with hybrid procedures in which open repair, debranching, or bypasses are performed in conjunction with endovascular stenting. These procedures are particularly suited to hybrid suites capable of providing high-quality imaging, CPB capability, and optimal surgical conditions (see Chapter 21).
 Hybrid Congenital Cardiac Surgery
Hybrid Congenital Cardiac Surgery
Combined open and interventional approaches have been used successfully to treat multiple muscular ventricular septal defects, pulmonic stenosis, and hypoplastic left-heart syndrome. Hybrid techniques address the barriers to transcatheter approaches such as poor vascular access and hemodynamic compromise, as well as reduce the need for high-risk resternotomy and long CPB times (see Chapter 20).
 Cardiac Electrophysiology
Cardiac Electrophysiology
A hybrid suite would be the optimal location for totally endoscopic approaches to epicardial ablation combined with a modified transcatheter endocardial strategy, which may provide better long-term freedom from atrial fibrillation and stroke than patients treated using these methods in isolation, although data are currently limited to small, single-center series (see Chapter 4).
Planning
The process of building a hybrid operating room, from initial proposal to official opening, takes around 21 months (Table 26-2). All involved parties should establish a clear, early understanding of the primary role of the hybrid room, the statutory requirements, and site limitations that must be met.
| Time Required | Activity |
|---|---|
| Months 1–6 | Agree on planning group |
| Initial architectural plans and quotes produced | |
| Obtain vendor quotes and costs | |
| Produce business plan | |
| Administration approve business plan | |
| Month 7 | Formal presentation to institutional planning committee |
| Month 8 | Architectural plans finalized |
| Engineering plans finalized | |
| Information technology (IT) and audiovisual plans finalized | |
| Vendors selected | |
| Month 8 | Budget completed |
| Month 10 | Presentation to institutional capital expenditure committee |
| Month 11–18 | Construction of hybrid room |
| Month 18–20 | Hybrid room outfitted |
| Month 20 | Testing hybrid room equipment and setup |
| Month 21 | Hybrid room official opening |
 Construction
Construction
An operating room and an interventional catheterization laboratory are basically the same. In design and construction, many states enforce the 2006 Guidelines for Design and Construction of Health Care Facilities. This document has been revised and was published in 2010 (Box 26-1). In the guidelines, both rooms have virtually the same requirements for ventilation, cleanliness, and room finishes. These are 15 room-air changes per hour, relative humidity should be maintained between 30% and 60%, and temperature should be maintained at 68° C to 73° C in operating rooms and 70° C to 75° C in interventional catheterization laboratories. The major differences are in the suite support infrastructure; a surgical suite has requirements for support services that are not required in an interventional catheterization suite. These include:
BOX 26-1. EXCERPTS FROM 2010 GUIDELINES FOR DESIGN AND CONSTRUCTION OF HEALTH CARE FACILITIES
Excerpted from 2010 Guidelines for Design and Construction of Health Care Facilities. Dallas, TX: The Facility Guidelines Institute, 2010.
Surgical Suites
Layout
Operating and Procedure Rooms
General operating room(s)
Room(s) for cardiovascular, orthopedic, neurological, and other special procedures that require additional personnel and/or large equipment
Cardiac Catheterization Laboratory (Cardiology)
 Personnel
Personnel
To address the key needs of the interdisciplinary teams that will be using the hybrid suite, clinicians and technicians from adult and congenital cardiac surgery, interventional cardiology, anesthesiology, perfusion, vascular surgery, and interventional radiology should be involved from the earliest planning stages (Table 26-3). A multidisciplinary planning team should produce a list of requirements, as well as identify key constraints, such as the existing location of services. These include intensive care, cardiac operating room, and cardiac catheterization laboratories. Initial plans then are produced and refined in conjunction with specialist architects, working closely with equipment vendors. Visiting established hybrid rooms, reviewing plans with teams already familiar with the process and outcomes, and three-dimensional reconstructions are all essential parts of the design process because few hybrid rooms are identical, and it is usually difficult to visualize how a setup will function based purely on architectural drawings.
TABLE 26-3 Key Members of Planning Group for Hybrid Cardiovascular Suite
| Clinical Staff |
| Adult and pediatric cardiothoracic surgeons |
| Interventional cardiologist |
| Cardiac anesthesiologist |
| Vascular surgeon |
| Perfusionist |
| Microbiologist |
| Operating room nurse manager |
| Cardiac catheterization nurse manager |
| Business Administrators |
| Financial officer |
| Construction and Design |
| Specialist architect |
| Specialist interior designer |
| Hospital estates and facilities manager |
| Specialist construction manager |
| Electronic engineer |
| Information technology manager |
| Audiovisual specialist |
| Internal applications specialist |
Equipment
 Lights, Monitors
Lights, Monitors
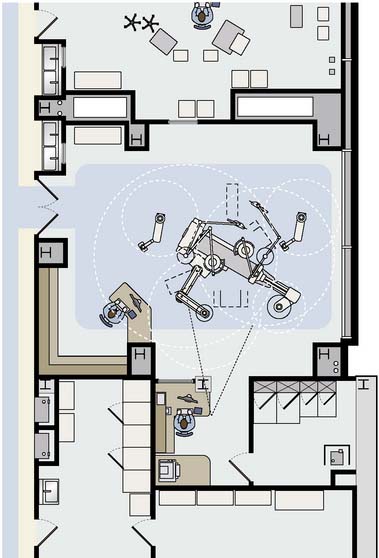
Surgical lights and banks of four to six imaging monitors (or, preferably, large flat-panel screens that can support multiple video inputs) are housed on overhead gantries. Optimally, monitors that display continuous invasive and noninvasive monitoring of vital signs should be placed in four quadrants of the room, in addition to the imaging monitors, which should be located opposite the surgeon, as well as in the control room. A specific plan should be created, including all ceiling-mounted components (lights, monitors, booms, air-conditioning, C-arms, etc.) to minimize collisions between devices (Figure 26-1). Conventional surgical lights are often mounted on very long gantries. It can be difficult to position the lights adequately and maintain sterility without assistance, without interfering with sight lines or other equipment, particularly the overhead monitors. A video camera integrated in the main overhead light enables nonsurgical personnel to obtain a view of the surgical field.
 Imaging Equipment
Imaging Equipment
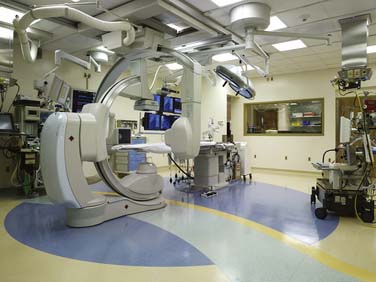
Fluoroscopy is the basic imaging mode provided by all angiography systems, and is the predominantly used modality during surgery because it exposes the patient to less radiation and provides high-resolution, real-time images. Biplanar rather than monoplanar imaging is essential for interventional cardiology-based procedures. Two kinds of C-arms are available: mobile and fixed. Mobile C-arms, which surgeons currently use routinely for screening for missing instruments and catheter placement, are not adequate for visualizing thin guidewires or fine stents or quantifying stenosis of small vessels. The technical specifications for power, frame rate, and heat storage capacity of mobile C-arms are well below those set by the regulatory bodies, and for a room to work as a true hybrid cardiovascular suite, a fixed C-arm should be installed (Figure 26-2). Where available space is lacking, a semimobile system with a fixed generator may be a reasonable compromise. Flat-panel detectors are preferable to image intensifiers because contrast resolution is higher; there is no edge distortion effect, and flat-panel detectors offer three-dimensional (3D) imaging capabilities (see Chapter 3).
1 King S.B.3rd. Who are interventionalists? What about surgeons? JACC Cardiovasc Interv. 2008;1:109-110.
2 Byrne J.G., Leacche M., Vaughan D.E., et al. Hybrid cardiovascular procedures. JACC Cardiovasc Interv. 2008;1:459-468.
3 Kpodonu J., Raney A. The cardiovascular hybrid room a key component for hybrid interventions and image guided surgery in the emerging specialty of cardiovascular hybrid surgery. Interact Cardiovasc Thorac Surg. 2009;9:688-692.
4 Hirsch R. The hybrid cardiac catheterization laboratory for congenital heart disease: From conception to completion. Catheter Cardiovasc Interv. 2008;71:418-428.
5 Nollert G., Wich S. Planning a cardiovascular hybrid operating room: The technical point of view. Heart Surg Forum. 2009;12:E125-E130.
6 Alexander J.H., Hafley G., Harrington R.A., et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A randomized controlled trial. JAMA. 2005;294:2446-2454.
7 Lopes R.D., Hafley G.E., Allen K.B., et al. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N Engl J Med. 2009;361:235-244.
8 Zhao D.X., Leacche M., Balaguer J.M., et al. Routine intraoperative completion angiography after coronary artery bypass grafting and 1-stop hybrid revascularization results from a fully integrated hybrid catheterization laboratory/operating room. J Am Coll Cardiol. 2009;53:232-241.
9 Chiam P.T., Ruiz C.E. Percutaneous transcatheter aortic valve implantation: Assessing results, judging outcomes, and planning trials: The interventionalist perspective. JACC Cardiovasc Interv. 2008;1:341-350.
10 Feldman T., Kar S., Rinaldi M., et al. Percutaneous mitral repair with the MitraClip system: Safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol. 2009;54:686-694.