Chapter 13 Problems in the injured patient
13.1 Introduction
Care of the injured patient begins at the scene of injury and should optimally follow a continuum of integrated care from soon after the moment of injury to definitive care in hospital and subsequent rehabilitation. Initial assessment and resuscitation should occur simultaneously to identify and manage life-threatening conditions. Once stable the patient can be assessed for definitive care that can occur in the primary hospital, but if the resources are not adequate then transfer to another hospital should be arranged.
Should the doctor fortuitously be the first at the scene where a person has been injured then they should assume leadership and establish order, delegate others to contact emergency services and protect the injured from further trauma by keeping the scene clear of bystanders and traffic, while simultaneously performing initial assessment and resuscitation. The injured patient may face environmental hazards at the scene: fire and explosion, electrocution, continuing civil or military violence, as well as inappropriate intervention by bystanders. The doctor first on the scene must be prepared to attend to non-medical priorities, like dousing fire, diverting traffic and arranging to move the injured patient rapidly to a safer environment. These first aid principles are summarised by the pneumonic ‘DRABC’ (Box 13.1) — where the initial priority at the accident scene is to identify potential Dangers (to the patient and bystanders) and to assess patient Response (conscious or unconscious).
13.2 Managing the injured patient
Initial assessment
Initial assessment of the injured patient is time critical; management received during the first hour directly influences patient outcomes in the longer term. In this ‘golden hour’ the main objectives are to identify life-threatening injuries and to institute early management and resuscitative measures. An ordered protocol is required and has a stabilising influence on the emotional concerns of both the injured person(s) and onlookers. Throughout this process good communication and comprehensive documentation are vital.
Primary survey and resuscitation
Airway management and cervical spine protection
Circulation with control of haemorrhage
Disability and neurological assessment
Exposure and environmental control
A Airway and cervical spine
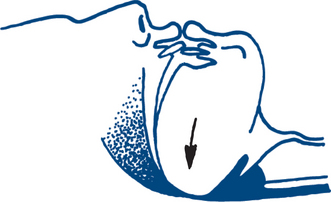
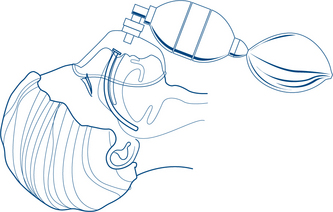
A compromised airway may occur secondary to maxillofacial or neck trauma, foreign body obstruction or simply from anatomical narrowing of the airway in the flexed neck (Fig 13.1a). In assessing the airway it is essential to simultaneously protect the cervical spine and spinal cord by avoiding excessive movement or rotation and by using an immobilising device such as a cervical collar. If the patient needs to be moved, it is important to stabilise the cervical spine with manual in-line immobilisation, which should be the sole focus of one member of the trauma team. The cervical collar should remain in place until radiological clearance has been obtained.
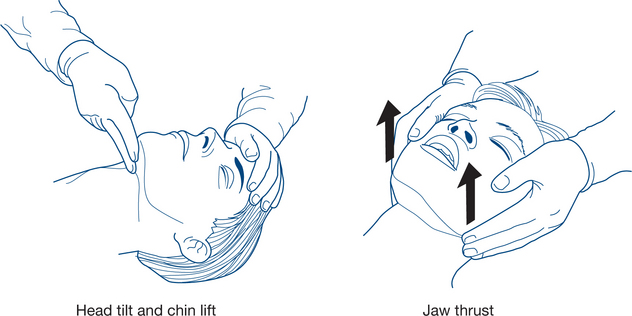
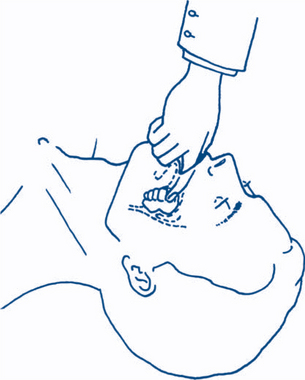
Simple measures to obtain a patent airway include the head tilt, chin lift and jaw thrust (protraction by lifting the angles of the mandible forwards) (Fig 13.2a). The mouth and pharynx are cleared manually of blood, vomitus or other foreign bodies (e.g. false teeth) if necessary (Fig 13.1b). An oropharyngeal (Guedel) or nasopharyngeal airway may be inserted.
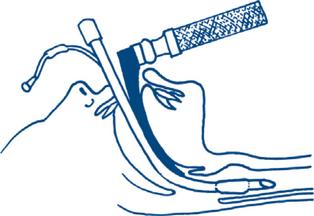
In the unconscious patient, a definitive airway is required. This is achieved with tracheal intubation using an inflatable cuffed tube (Fig 13.2d). If the means of intubation are not available in the presence of an obstructed airway, surgical cricothyroidotomy may be required to secure a definitive airway. The procedure is not without hazard (especially in the very young, where the brachiocephalic vein may be inadvertently damaged) and requires a careful technique.
B Breathing and ventilation
Once airway patency and cervical spine protection have been confirmed the patient’s chest should be assessed. Adequate exposure will facilitate inspection, palpation, auscultation and percussion. Thoracic injuries that may compromise ventilation include open pneumothorax, tension pneumothorax, fractured ribs or flail chest, pulmonary contusion or massive haemothorax. The clinical signs and emergency management of these conditions are indicated in Table 13.2.
Table 13.2 Recognition and initial management of life-threatening thoracic injuries
| Condition | Clinical signs | Initial management |
|---|---|---|
Tension pneumothorax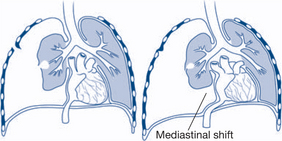 |
↓ chest wall excursion, neck vein distension/cyanosis, tracheal deviation to opposite side, unilateral absent breath sounds, hyperresonant percussion note | The diagnosis is clinical — there is no time for a chest X-ray Insert large-bore (12–14G) needle into second intercostal space in the mid-clavicular line |
Open pneumothorax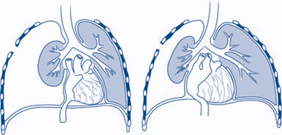 |
‘Sucking chest wound’, decreased breath sounds, hyperresonant percussion note | Close the defect in the chest wall with occlusive dressing that is taped only on three sides (to create a one-way valve) |
Flail chest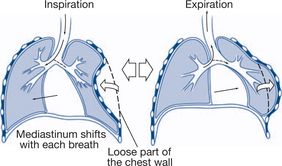 |
Paradoxical/asymmetrical movement of chest wall Crepitus over ribs/cartilage |
Analgesia Meticulous fluid balance May need to consider intubation and ventilation |
Massive haemothorax |
↓ chest wall excursion, tracheal deviation to opposite side, decreased breath sounds, stony dull percussion note | Insert 28 or 32G intercostal catheter |
Cardiac tamponade |
Beck’s triad (↓ arterial pressure, distended neck veins from ↑ venous pressure, muffled heart sounds) Kussmaul’s sign (↑ venous pressure with inspiration) |
Pericardiocentesis via subxyphoid approach |
Based on www.netterimages.com and www.beliefnet.com
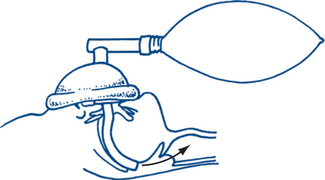
Oxygenation
Supplemental oxygen should be administered to all trauma patients. The conscious patient with a nasopharyngeal or oropharyngeal airway should receive oxygen via a bag-valve mask. The use of an oxygen reservoir device maximises oxygen delivery to the patient (Fig 13.2b and c) and is therefore strongly recommended.
C Circulation and control of haemorrhage
Although the body’s physiological response to hypovolaemia is predictable, this statement needs qualification. At extremes of age (i.e. the elderly and the young child or toddler) tachycardia may be absent in the setting of hypovolaemia. One possible reason for this is polypharmacy; it is not uncommon to find elderly patients taking beta-blockers for cardiovascular disease. Other contributing factors include chronic illness and age-related blunting of the sympathetic response to hypovolaemia. By virtue of their large physiological reserves, children (and athletes) are well able to compensate for significant reductions in blood volume. Clinical signs of hypovolaemia may be initially absent. If these patients are severely injured, decompensation is often precipitous. In caring for such patients, one cannot afford to be solely comforted by the presence of a ‘normal’ heart rate or blood pressure. Frequent reassessment during resuscitation is mandatory.
External bleeding
| Patient assessment | Management |
|---|---|
| Speaking | Unlikely to have immediate airway compromise. Reassess patient. |
| Conscious, but possibility of deterioration in airway | Nasopharyngeal airway (better tolerated than oropharyngeal airway in conscious patients). Reassess with a view to securing a definitive airway (intubation). |
| Unconscious Glasgow coma scale ≥8 No gag reflex/bleeding (risk of aspiration) Severe facial trauma (risk of obstruction) |
Definitive airway required immediately via: endotracheal intubation or nasotracheal intubation or surgical cricothyroidotomy. |
dressing pads and bandaging, together with elevation of the part, will control most limb haemorrhage. Tourniquets are not advised as they can exacerbate bleeding if too loose and imperil limb viability if too tight. Massive limb bleeding may require additional temporary proximal compression of the brachial artery against the humerus in the axilla or the femoral artery against the femoral head in the groin. The use of artery forceps to control bleeding is not recommended; their application in the uncontrolled, poorly lit environment of the trauma scene may lead to unnecessary tissue damage (nerves, veins, muscle), as well as being time consuming.
Fluid resuscitation
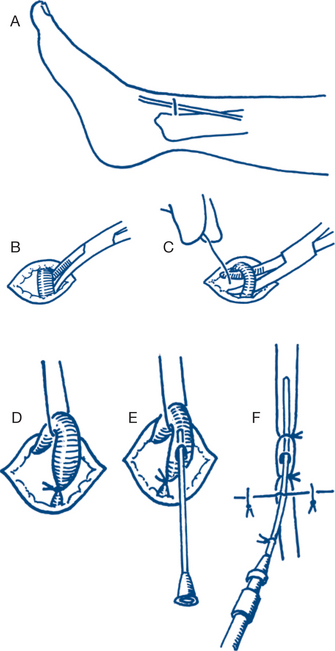
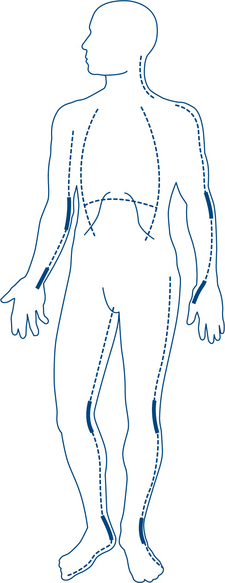
When percutaneous access is difficult, a venous cutdown technique should be considered. The best sites are the long saphenous vein on the medial side of the ankle or any vein in the cubital fossa (Fig 13.3). Intra-osseous access in children over the upper tibia using an intra-osseous needle is very effective.
When the rapid infusion of large volumes of fluid is anticipated the percutaneous insertion of a large bore cannula (8G) in the femoral vein in the groin is indicated. The insertion of a central venous catheter in the subclavian or internal jugular vein is more useful in monitoring the response to fluid resuscitation than in gaining access for the rapid infusion of fluids.
D Disability and neurological assessment
The cooperative, conscious patient may be asked to identify the site of the injuries, whereas the drowsy, head-injured patient who only responds to painful stimuli warrants reassessment of airway patency and consideration of intubation. This is seen particularly in patients with extradural haematomas, where approximately one-third will have the classic ‘lucid interval’ (see Ch 13.5).
The GCS is more commonly used during the secondary survey and will be discussed later.
E Exposure and environmental control
Complete removal of the patient’s garments is necessary to facilitate a thorough inspection and examination. Equally as important is the conservation of core body temperature. As such, once a thorough examination has been conducted, it is important to use a warming blanket to prevent the loss of body heat. Exposure to the cool of the ground, the wind or rain prior to retrieval — combined with blood loss and polytransfusion — lead to core body temperatures well below normal. The surgeon dealing with ongoing ooze during emergency laparotomy knows only too well the effect of hypothermia on the coagulation cascade. The use of pre-warmed intravenous fluids for resuscitation should be standard procedure. Patient temperature should be monitored and documented, alongside blood pressure and heart rate; it should be regarded as an important part of the vital statistics.
Investigations and procedures following the primary survey and resuscitation
During the primary survey the medical officer attempts to identify and treat life-threatening injuries in a logical, sequential manner. There are a number of useful investigations and procedures that enhance initial management of the injured patient (Table 13.3).
Table 13.3 Important investigations and procedures following the primary survey and resuscitation
| Investigation/procedure | Clinical value |
|---|---|
| ECG monitoring All trauma patients Simple, noninvasive |
Detection of: |
Contraindicated in suspected urethral injury (i.e. blood at urethral meatus, perineal ecchymoses, scrotal haematoma, high-riding/impalpable prostate)
(Naso/orogastric tube insertion)
In suspected fracture of the cribriform plate, a nasogastric tube should not be inserted (orogastric should be used instead)
Note: The presence of a gastric tube does not completely remove the risk of aspiration
Note: Pulse oximetry is not a measure of PaO2 or ventilation
Each hospital emergency department should have a ‘trauma series protocol’
Secondary survey
In assessing the trauma patient, some injuries are obvious while others may be subtle or concealed. The sucking chest wound will draw the immediate attention of the doctor, whereas the slow leak of cerebrospinal fluid (CSF) rhinorrhoea or the perineal bruise may not be discovered for some time. The secondary survey is designed to address this issue through history-taking and a comprehensive head-to-toe examination of the injured patient, including a neurological assessment.
History
Past medical history/pregnancy
Events/environment related to injury
The patient will be delivered to the emergency department by an ambulance officer or paramedic who has already performed a thorough initial assessment. Respect their role in the management of the injured patient and obtain a handover of the events surrounding the injury, if you have not done so already before the primary survey. An understanding of the mechanism of trauma is always helpful in predicting the pattern of injuries sustained. Useful questions to ask an ambulance officer specifically relating to the events or environment surrounding the injury are shown in Box 13.2.
Examination
Perineum and genitalia: The perineum should be inspected for bruising, swelling or extravasated blood at the urethra. Rectal examination should also be performed, assessing sphincter tone (spinal injury), position of the prostate in males (high-riding prostate in pelvic fractures), bony discontinuity and bleeding. The genitalia should be inspected for the presence of blood (e.g. in the vaginal vault) or external trauma (e.g. penile laceration or degloving injury).
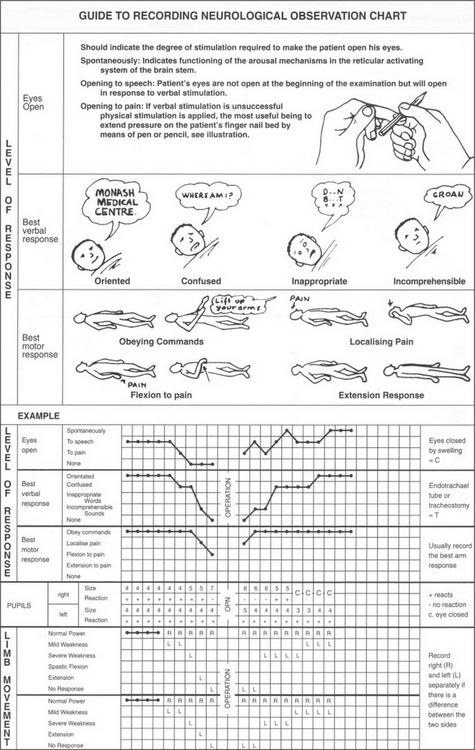
Neurological: Glasgow coma scale (GCS)
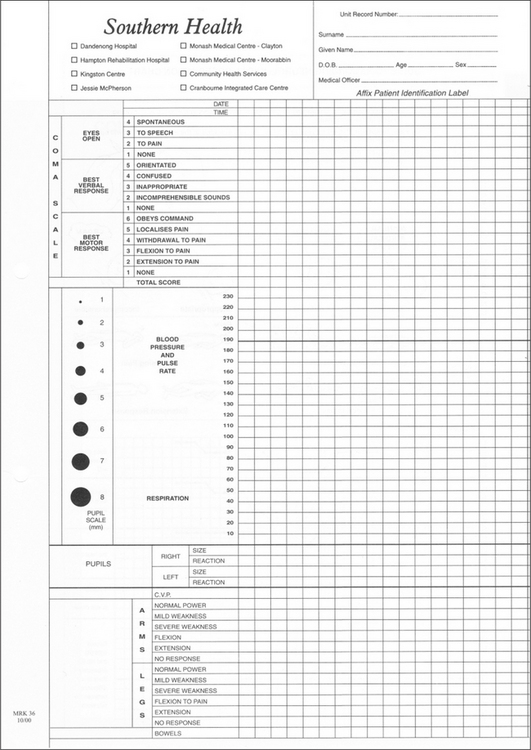
The GCS (Figs 13.13 and 13.14) is a widely used scoring system designed to assess neurological function in three areas: eye opening (‘open your eyes’), verbal response (‘what’s your name?’) and motor function (‘squeeze my hand’). A patient who responds appropriately to these instructions has a GCS of 15 and is alert and conscious. The unconscious patient with GCS ≥8 usually requires a definitive airway.
Definitive care and transfer
Interhospital transfer
Medical records and documentation
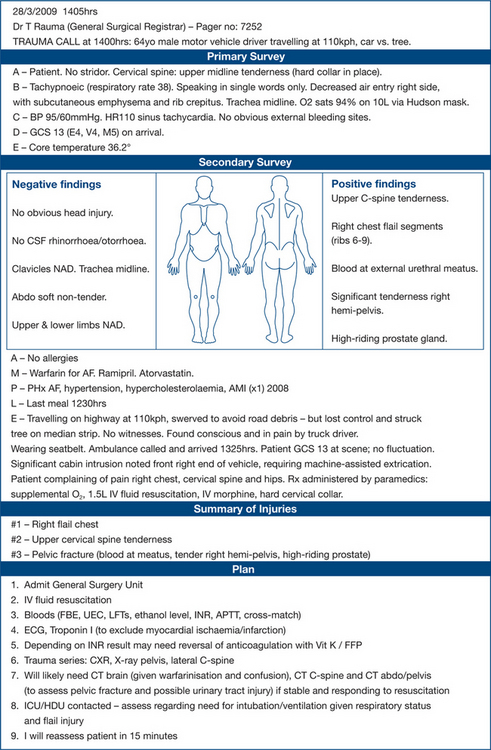
Two important reasons for keeping meticulous records are: the patient receives a higher standard of care and detailed records are essential whenever medicolegal problems arise. The best examples of documentation describe the clinical history, findings and management in a systematic manner, reflecting the components of the initial assessment described above. Figure 13.4 is one example.
Shock
Diagnostic and treatment plan
Non-haemorrhagic shock may arise from a number of conditions, including cardiac tamponade, pulmonary embolism, tension pneumothorax, sepsis or neurogenic mechanisms. A large pulmonary embolus causes pulmonary arterial obstruction, with hypotension and increased right ventricular pressure. Cardiac tamponade interferes with cardiac filling and decreases cardiac output, resulting in hypotension. In tension pneumothorax mediastinal shift causes a reduction in venous return that decreases cardiac output. Chest X-ray, central venous pressure monitoring, electrocardiogram, blood gas analysis and pH are important guides to diagnosis and treatment. Noninvasive monitoring with transoesophageal echocardiography (TOE) may also provide useful information regarding cardiac function. Continuing refractory shock due to severe systemic sepsis can occur later after injury and is often caused by a continuing septic focus (necrotic tissue or pus) and demands initially an appropriate antibiotic regimen and cardiovascular support and timely surgical exploration and drainage. Surgical control of the septic focus is essential because the patient will not improve until the causative focus is removed. Neurogenic shock results from a loss of sympathetic tone, leading to bradycardia, vasodilation and hypotension.
13.3 Soft tissue injury and wound care
Classification of wounds
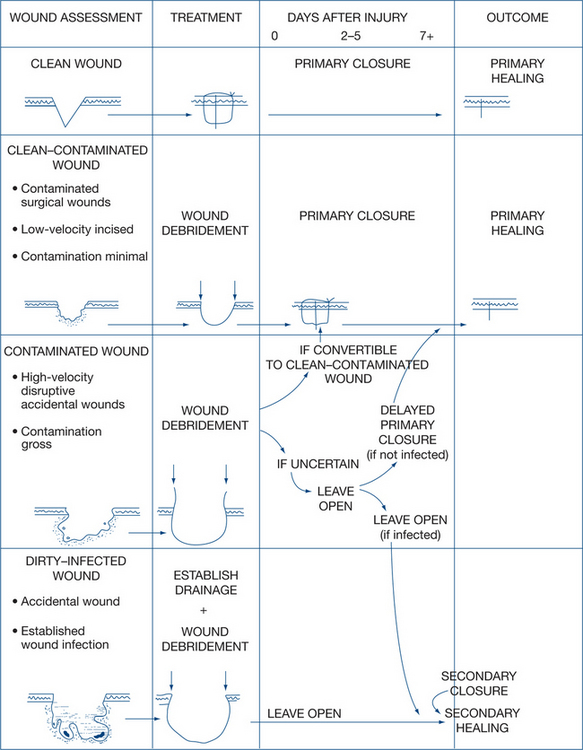
These classifications determine a spectrum of severity and of potential complications and markedly influence early wound management. A convenient classification is specified by the Centers for Disease Control and Prevention (CDC) is detailed below (see also Table 13.4).
 |
Principles of wound healing
Phases in wound healing
There are three major phases in wound healing: haemostasis and inflammation (two to five days); proliferation (beginning from about three days); and maturation (extending over many months). Haemostasis occurs via platelet aggregation and activation of the clotting cascade. Following this, polymorphonuclear leucocytes (PMN) and macrophages migrate to the area of injury, followed by lymphocytes (inflammation). Proliferation involves fibroblastic proliferation and angiogenesis, with resultant collagen formation. During the final stage of wound healing, the collagen and extracellular matrix are remodeled (maturation). There is overlap in these phases and wound strength progressively increases. Epithelialisation over the defect begins from the first day after injury; in clean surgical wounds treated by primary closure this is completed by the second day. In large wounds left to heal by second intention the process is hastened by wound contraction but will nevertheless take significantly longer.
Factors adversely affecting wound healing
Local factors
Local factors are the most common causes of failure of wound healing. Bacterial contamination, particularly when combined with a nidus facilitating bacterial growth, is followed by infection. Common factors acting as niduses are areas of dead or dying tissue, foreign bodies (including suture materials) and collections of blood, serum and lymph. Other local tissue factors — such as the adequacy of the arterial and venous circulation and lymphatic drainage and previous irradiation damage — are extremely important. Excessive tension during repair of wounded tissues inevitably leads to local tissue ischaemia, subsequent necrosis and wound breakdown (Box 13.3).
General factors
Age, diabetes, malignancy. Advanced age is associated with impaired or delayed wound healing. This may be due to a higher prevalence of other adverse factors such as vascular insufficiency, metabolic disease, malnutrition, cancer and drugs. Diabetes may cause neuropathy and microvascular or macrovascular disease leading to tissue ischaemia. Defects in angiogenesis, granulocyte function and wound matrix formation have also been described. Patients with cancer may be malnourished and immunocompromised from treatment (chemoradiotherapy) or disease progression (Box 13.4)
Definitive care
Wound care — debridement
The wound must be thoroughly explored and should be enlarged on either side as far as is required to determine the extent of deeper damage. Excision of necrotic tissue proceeds in depth. Skin edges usually need only a narrow margin of excision. Partially avulsed and bruised skin flaps require complete defatting and excision of the apex of the flap back to the point of dermal bleeding. Subcutaneous fat is freely excised back to pristine bleeding fat. Deep fascia is split widely to expose underlying structures and damaged muscle is radically excised back to healthy tissue (which bleeds and contracts when cut). Free bone fragments devoid of periosteum are removed and foreign material and debris must be removed from bone ends and marrow cavities. Subsequent treatment of fractures and injuries to other deep tissues (major tendons, nerves and vessels) depends on the degree of damage and contamination.
Timing of wound closure
Delayed primary closure. The first few days of wound healing are phagocytic and preparative rather than fibroblastic and reparative — the continuing biological debridement complements the surgical procedure. Because of this, closure can be delayed for a few days without prejudice to the end result or to the speed of healing. If closure is performed within a few days, the tissues are still soft, with little fibroblastic activity, and the wound can readily be closed. Delayed closure is thus best done between the second and fifth days after wounding. The end result is similar to that of primary healing. If closure is deferred for more than five days, granulation tissue will have developed on the exposed wound surface. The tissues are stiffer and do not approximate well and the procedure then becomes one of secondary wound closure.
Wound closure techniques and materials
Local flaps (Figs 13.5 and 13.6). Advancement describes the situation where one edge of a flap is simply advanced to cover the defect. Undermining the skin edges around a wound before suturing it effectively forms two advancement flaps. With larger defects, local flaps may be moved by rotation or transposition as illustrated. Rotation flaps rotate in the arc of a circle of which the primary defect is a segment; skin closure redistributes tension over a much more extensive suture line. Transposition flaps are pivoted to fill the gap, leaving a secondary defect that may require grafting or may be closed directly. Combined rotation–transposition flaps are common. A Z-plasty (Fig 13.6) redistributes wound tension by transposing two triangular flaps, bringing in tissue from the sides to lengthen the wound and break up tension across it. It is more commonly used as a secondary procedure to relieve contractures than in primary wound closure. It can be helpful in wounds with unequal sides and may be used to advantage to break up tension across the defect left after excision of a skin lesion. Particularly when wounds cross skin lines or cleavage lines, a Z-plasty may be used to stagger the line of the scar and place a portion of it in the crease line.
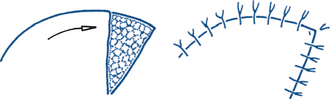
Figure 13.5 Local wound flaps
In random flaps without an axial blood supply the ratio of length to width should not exceed 1:1.
Distant flaps are used if local tissue rearrangement is impossible or inappropriate. They may be directly applied to the defect without an appreciable pedicle or they may require a long carrier vascular pedicle. The vascular pedicle may be tubed or may be buried or tunneled to insert the flap as an island into the defect. The anatomy of flaps and their vascularity enable distinctions to be made between randomly or axially supplied flaps that determine their safe limits. A transverse rectus abdominis myocutaneous (TRAM) flap is one type of distant flap (Fig 13.7).
Closed soft tissue (sporting) injuries
Soft tissue (including sporting) injuries can be classified into three groups:
Ice application for 20–30 minutes every few hours
Compression bandaging for 48 hours after the injury
Later treatment during the phase of fibrous repair of the muscle or ligament requires maintenance of muscle mobility as repair proceeds. Graduated increase in use of the injured part is combined with passive and active exercises. Attention must also be given to strengthening adjacent muscle groups, particularly those that act as joint stabilisers. Early physiotherapy involvement is essential to prevent long-term limitation of movement.
13.4 Burns
Pathophysiology of burns
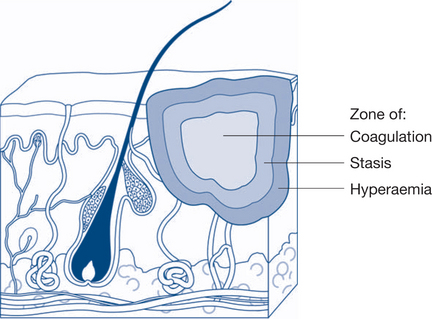
Local response to burns: Jackson’s burn zones
In 1947 DM Jackson described the three zones of a burn and this still forms the foundation of our understanding today (Fig 13.8). The zone of coagulation is the area where the most severe thermal injury occurs: cells are necrotic and irreversible tissue loss necessitates debridement. Surrounding this area is the zone of stasis, which is characterised by decreased tissue perfusion due to changes in the microcirculation. The viability of tissue in this zone may be preserved by restoring perfusion through appropriate resuscitation. Suboptimal fluid resuscitation or subsequent infection may, however, convert the zone of stasis into one of coagulation and necrosis, resulting in a larger and deeper burn. The outermost area is the zone of hyperaemia, where tissue perfusion is increased secondary to the release of inflammatory mediators from viable cells. This area usually recovers fully in burns.
Systemic response to burns: SIRS and MODS
The release of inflammatory mediators causes an increased capillary permeability with significant fluid shifts from the intravascular to the extravascular space. A reduction in the plasma volume contributes to hypovolaemia, which leads to shock and a reduction in end-organ perfusion. Other organ systems may be affected; bronchoconstriction and a reduction in splanchnic perfusion may exist. The resultant systemic inflammatory response syndrome (SIRS) is defined as two or more of: patient temperature >38°C or <36°C; heart rate >90 beats/minute; respiratory rate >20/min or PaCO2 <32 mmHg or leucocyte count >12,000/mm3; <4,000/mm3 or >10% immature (band) cells. Multiple organ dysfunction syndrome (MODS) exists when there is organ dysfunction in an acutely ill patient, such that homeostasis cannot be maintained without intervention.
Initial assessment
Extent of burns
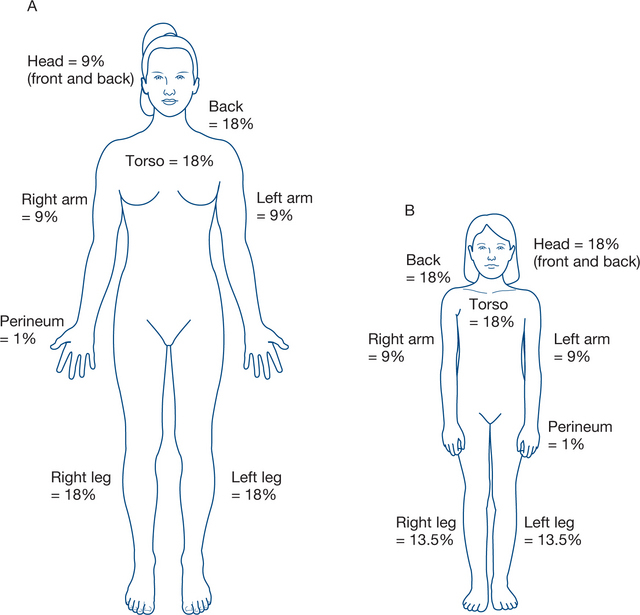
The burnt area is established as a percentage of body surface area (BSA), using the ‘rule of nines’ (Fig 13.9). A cross-check is always done by estimating the area not burnt. It is important to recognise that children have different proportions to adults and paediatric charts displaying body surface area estimations are useful in more accurately estimating burn extent.
Classification of burn depth
Burns are most helpfully considered as either shallow or deep. Shallow burns are further classified as epidermal (first-degree) or superficial partial-thickness (second-degree). Deep burns comprise deep partial-thickness (second-degree) and full thickness (third-degree). In areas where the skin is thick (e.g. palm, sole, back, buttock) burns are more likely to be partial thickness. Where skin is thin (dorsum of hands and feet, skin around the eyes), deep burns are more likely.
Deep partial-thickness burns (second-degree). Involvement of the deeper (reticular) layers of the dermis may also cause blistering of the skin but the underlying tissue has a mottled pinkish-white appearance. This reflects variable damage to the deeper dermis, with blood supply to some areas still viable, interspersed among islands of non-viable tissue. Left
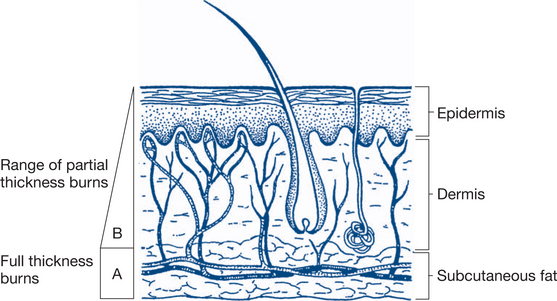
Figure 13.10 Depth of burns
A: full thickness (deep) burn; B: partial thickness (superficial) burn
Based on Williamson & Waxman, 1998
untreated, these wounds may heal spontaneously (over three months) by surface re-epithelialisation from surviving hair follicle, sebaceous and sweat gland remnants but with a considerable degree of subepidermal scarring. Surgical excision combined with conventional skin-grafting methods will achieve a better cosmetic result.
Subsequent assessment and definitive care
General management
Antibiotics. Antibiotic prophylaxis is not indicated, their use reserved until required for management of an established infection.
volume replacement for the first 24 hours
Monitoring
Fluid replacement is monitored by the following observations.
Other biochemical analyses. Frequent arterial blood gas analyses and serum electrolyte (particularly serum K+) and creatinine levels are required for patients with severe burns over 30%. Carboxyhaemoglobin and random blood glucose should also be performed.
Burn wound management
The patient is admitted to hospital (burn unit) or treated as an outpatient.
Escharotomy and fasciotomy. Escharotomy (Fig 13.11) involves an incision through burnt skin (eschar), to release any constriction that may compromise circulation. It is urgently indicated in burns that encircle the body wall (leading to abdominal compartment syndrome or restriction of respiration) or limb (threatening neurovascular supply). Electrical burns causing injury to deeper tissues may lead to significant swelling and increased compartment pressures and may require fasciotomy.
Later care. The patient with a severe burn requires prolonged follow-up to prevent or treat late deformities and to aid rehabilitation. Skin destroyed by burns, even when excised early and replaced by a split skin graft, may never regain full function and convalescence is often slowed by painful and hypertrophic scars. A multidisciplinary approach to burn management is critical in improving patient outcomes and is the core strategy of burn units throughout the world. Physiotherapists and occupational therapists with training in burns physical therapy form an integral part of the team.
13.5 Head injury
Classification and definitions
Traumatic head injury may affect the scalp, brain or skull. Most head injuries are closed and follow blunt injury. Scalp wounds are common and mainly of importance as a source of significant haemorrhage or infection. Traumatic injury to the brain is either classified as primary or secondary. Primarily injury refers to damage sustained at the time of impact. This occurs from compression, stretching and shearing stresses or by collision with the skull or dural structures such as the falx or tentorium. The damage may be directly under the site of impact (coup injury) or diagonally opposite the site of injury (contrecoup) because of the to and fro movement of the brain (Fig 13.12a). Primary injuries can be further classified into ‘focal’ (contusions, lacerations, skull fractures, intracranial haemorrhage or haematoma) or ‘diffuse’ (diffuse axonal injury or concussion). Secondary brain injury usually develops hours to days after the initial impact (primary injury) and may be due to cerebral oedema, raised intracranial pressure, hydrocephalus or brain herniation. A significant goal of managing the head-injured patient is preventing secondary brain injury.
Primary injury: focal lesions
Skull fractures significantly increase the likelihood of underlying brain injury and may be classified according to whether they are open or closed, by morphology (linear vs stellate vs depressed) or by site (cranial vault vs base of skull). Depressed skull fractures imply a significant impact, with the potential for the fractured fragment to cause underlying dural penetration or brain laceration. Such injuries may increase the risk of infection (meningitis) by providing a portal of entry for bacteria. Basilar (base of skull) fractures usually involve the temporal bone and are characterised by the following clinical features: CSF otorrhoea or rhinorrhoea (from dural trauma); periorbital ecchymosis (raccoon eyes); ecchymosis around the mastoid process (Battle’s sign); haemotympanum (disruption of the temporal bone that houses the middle ear); and cranial nerve deficits (nerves III, IV and VI — from disruption of the cavernous sinus).
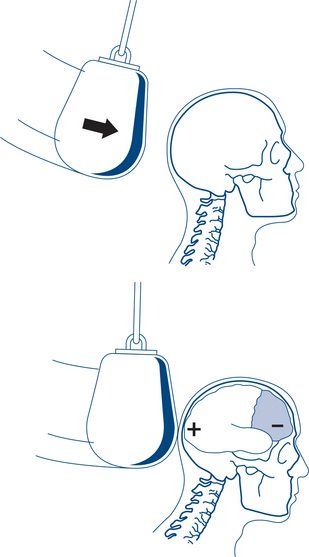
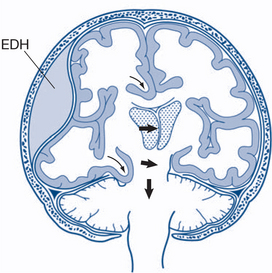
Intracranial haemorrhage may be extradural, subdural or intracerebral. Extradural haematomas (EDH) result, in most cases, from laceration of the posterior branch of the middle meningeal artery adjacent to a skull fracture. This leads to a unilateral increase in intracranial pressure. Only about one-third of patients have the classic ‘lucid interval’. There may be no initial loss of consciousness or minimal concussion with a rapid recovery of normal brain function. Prodromal features include increasing headache and restlessness, and oedema and bruising of the scalp over the fracture. With increasing size of the haematoma the temporal lobe is compressed, CN 3 palsy develops, increasing hemiparesis of the opposite side and eventually transtentorial herniation of the mid-brain occurs (Fig 13.12b). CT scanning typically reveals a biconvex, hyperdense, lenticular lesion that does not extend beyond the suture lines (sites of dural attachment).
Primary injury: diffuse lesions
Secondary injury
Cerebral oedema occurs when there is excess water within the brain parenchyma. Two main forms are described: vasogenic cerebral oedema (due to disruption of the tight endothelial junctions of the blood brain–barrier) and cytotoxic cerebral oedema (sodium–potassium cell membrane pump failure). The resultant increase in the volume of the brain can cause a precipitous rise in ICP once compensatory mechanisms are exhausted.
Initial assessment
Secondary survey
Abnormal neurological signs
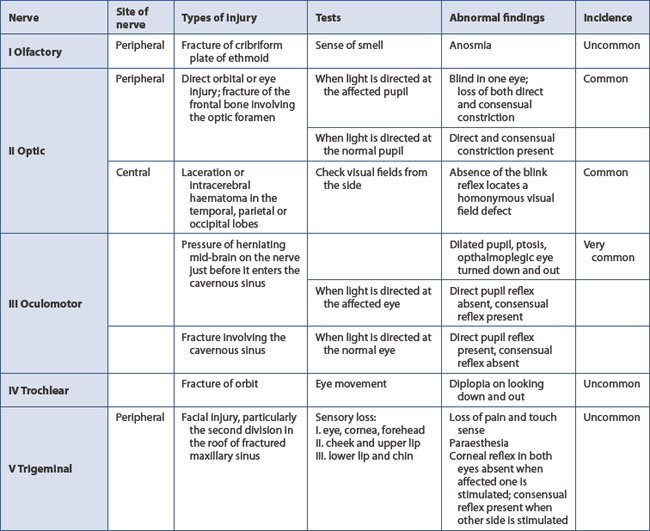
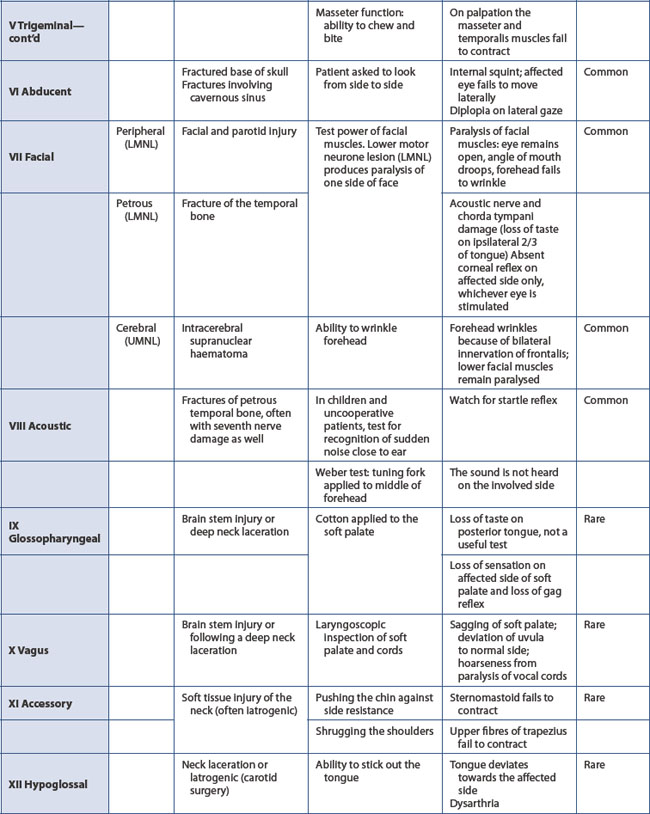
A baseline assessment of the neurological state (GCS) is essential (if not already performed in the primary survey). Pupil dilatation is the best guide to the side of a developing space-occupying lesion. Constriction in response to light indicates function of both the optic nerve and the oculomotor nerve, the latter conducting the parasympathetic constricting fibres. A baseline observation of equal and reacting pupils is required before a dilated pupil can be unequivocally attributed to ipsilateral extradural haemorrhage. A fixed dilated pupil may be present from the time of injury due to optic nerve damage. Paralysis of pupillary constrictor fibres in the occulomotor cranial nerve often denotes unilateral cerebral compression. The other cranial nerves are also tested (Table 13.5).
Vital functions are also recorded. These are of most value in monitoring the state of other systems but they may give warning of cerebral compression. Typically this is signified first by slowing of the pulse rate, then a progressive rise in systolic blood pressure and finally slowing of the respiratory rate. By the time respiratory rate slows the process is advanced and recovery less likely.
Re-evaluation
A standard head injury observations chart is required so that progress of the patient’s consciousness, eye signs, pain response, motor function and vital signs is recorded. Any deviations in trends or changes in neurological status can thus be recognised and treated promptly (Figs 13.13 and 13.14). Clinical deterioration is best detected by serial examination.
Definitive care
General principles of management
Intravenous fluid resuscitation. The treatment of hypotension in head injury should be with intravenous infusions of normal saline or Hartmann’s solution. Hypotonic solutions such as 5% dextrose should not be used; hyponatraemia may lead to cerebral oedema and subsequent coma or death.
Management of specific complications
The treatment of head injuries can lead to a number of potentially dangerous complications that clinicians must be aware of (Box 13.5).
Compound or depressed fracture of the skull. The main aim in treating open fractures (in the absence of significant underlying brain injury) is to prevent infection. Debridement and closure of scalp wounds should be performed as soon as practicable. The amount of skin excised is determined by skin mobility, as well as the extent of damage to skin, but wounds of the scalp heal well, so tissue can usually be preserved. Debridement of imbedded foreign material and identification of underlying skull and cerebral damage is very important in minimising complications. In addition to infection risk, depressed fractures are also associated with a higher risk of early posttraumatic seizures; anticonvulsant therapy may therefore be indicated in the emergency department.
CSF leak, meningitis and intracranial abscess (Fig 13.15). In most cases with CSF rhinorrhoea or otorrhoea, the leak will close spontaneously. CSF rhinorrhoea is detected by noting a thin nasal discharge, which must be distinguished from the discharge of allergic rhinitis. Beta-2-transferrin is found almost only in CSF and its presence in fluid assay confirms the diagnosis of CSF leak. Blowing the nose or sitting up should be avoided so that chances of retrograde CSF flow are minimised. There is no current evidence to support the routine use of prophylactic antibiotics. Rhinorrhoea is more likely to cease spontaneously than otorrhoea.
Post-concussion syndrome (PCS). The duration of symptoms in concussion have been arbitrarily defined as less than six hours. When symptoms following the initial concussion persist for weeks, months or even years later, this is termed PCS. Headaches and dizziness are the most common symptoms experienced. Emotional disturbance and cognitive deficits may also be associated with PCS.
13.6 Facial injury
Facial skeletal injuries may be arbitrarily divided into three different zones for ease of description: upper, middle and lower thirds (Table 13.6).
| Potential skeletal injury | Anatomical structures at risk | |
|---|---|---|
Upper third |
Frontal bones, supraorbital rim | Frontal sinus, frontal lobe of brain, supraorbital nerve, supratrochlear nerve, cribriform plate (olfactory function) orbit (ocular function), lacrimal apparatus |
Middle third |
Zygoma orbital bones (below supraorbital rim), nasal and maxillary bones | Blowout fracture, globe injury, infraorbital nerve, maxillary sinus, naso-orbital-ethmoid fractures, upper teeth, parotid gland and duct |
Lower third |
Mandible | Temporomandibular joint (TMJ), condylar fractures, inferior alveolar nerve injury, lower teeth, malocclusion |
Source: Department of Radiology, University of Washington
Initial assessment
Testing for sensory deficits will provide clues to underlying injury, for instance, loss of sensation in the distribution of the infraorbital nerve suggests a fracture of the inferior orbital rim. In the cooperative, stable patient a full cranial nerve examination should be performed. This will include a formal assessment of eye movement, visual fields and acuity. Blowout fracture of the orbit is frequently associated with diplopia on upward gaze because of entrapment of the inferior rectus muscle in the fracture site (Fig 13.16). The patient’s bite should be assessed — maxillary and mandibular fractures frequently result in malocclusion, premature molar contact or sensory deficits in the distribution of the inferior alveolar nerve.
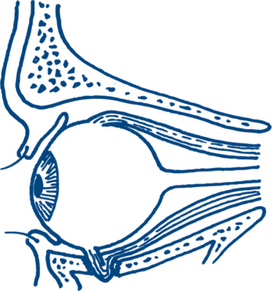
Figure 13.16 Blowout fracture of the orbital floor with an intact orbital rim
The entrapped inferior rectus muscle produces diplopia on upward gaze.
Le Fort classification
René Le Fort was a French army surgeon whose experiments with facial fractures in cadaveric skulls were published in 1901, giving rise to the Le Fort classification of midface fractures. Although some consider this classification to be an oversimplification, it is still used widely today (Table 13.6 and Fig 13.17). All Le Fort fractures involve a fracture of the pterygoid plates. Combinations of fractures frequently occur.
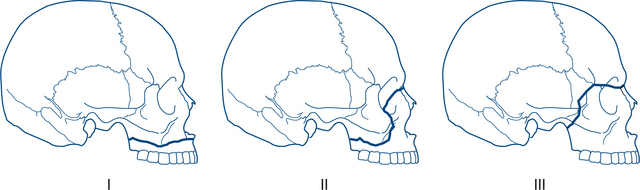
Figure 13.17 Le Fort classification of fractures of the maxilla
Source: Department of Radiology, University of Washington
Le Fort I: horizontal fracture of the maxilla immediately above the teeth and hard palate.
13.7 Eye and orbital injury
Introduction
Ocular injuries may occur in isolation or in association with major trauma, particularly with injuries involving the face. Common causes of ocular injury are blunt force trauma by either a direct blow from a fist or large projectiles such as squash or tennis balls. Smaller projectiles travelling at low velocity are likely to cause superficial trauma, whereas high-velocity projectiles often cause serious penetrating injury to the globe or orbit. Ultraviolet radiation and chemicals in both dry and liquid form are also important causes of ocular injury, with acids and alkalis the most serious. Despite improvements in occupational health and safety, workplace injuries remain a common source of referral. A significant proportion of injuries also occur at home, particularly during activities such as gardening.
Examination
One of the most important goals in the history-taking and initial assessment of the eye is to determine whether the patient has a penetrating injury or rupture of the globe. Visualisation of the eye may be severely hampered by periorbital bruising and blepharospasm (i.e. involuntary lid closure with inability or difficulty in opening the eye). If the globe is ruptured then further manipulation of the eye or eyelids may result in extrusion of intraocular contents through the wound. Pressure on the eye or periorbital tissues, removing intraocular foreign objects or the use of eye drops or ointment must be avoided. If an open injury is suspected then no further attempt at examination should be made until the patient is in the operating theatre.
Corneal flash burns
Ultraviolet (UV) radiation can damage the surface of the eye in the same way as it causes sunburn to the skin. Common sources of UV radiation include welding, tanning lamps, sunlight reflected from snow and lamps used for sterilisation. Patients are usually asymptomatic at the time of the exposure and therefore unaware of the danger. The onset of symptoms is typically delayed several hours so that patients frequently present late in the evening. Symptoms include blurred vision, photophobia, pain, foreign body sensation, profuse tearing and blepharospasm.
Perforating globe injuries
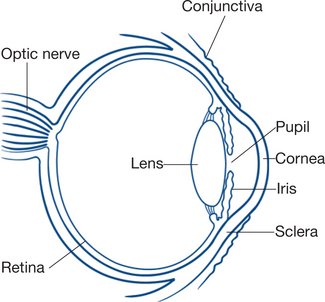
The anatomy of the globe is shown in Figure 13.18.
Clinical assessment
As mentioned previously, an attempt should be made to test and record visual acuity in each eye, even with severe injuries. A Snellen chart designed for use at three metres is usually the best option, particularly for patients examined in a bed or on a trolley. If this is not practical due to pain, severe blepharospasm or altered conscious state, simply asking the patient to detect a penlight beam or hand motions is still useful clinical information.
Superficial foreign body injury
Clinical assessment
Ask about the possible mechanism or injury and details of safety measures in place. If a penetrating injury is suspected then proceed as detailed in the previous section. Test and record vision and make an initial inspection of the eye using a slit lamp. If you are confident that no penetration has occurred then instill a drop of single-use, non-preserved topical anaesthetic into the eye. This will facilitate your examination and allow vision to be tested if the patient has difficulty in opening their eye. Inspect the corneal and conjunctival surfaces of the eye, including the conjunctival surfaces of the upper and lower eyelids. The upper eyelid can be everted by first asking the patient to look down, grasping the upper eyelashes and applying gentle counter pressure to the upper eyelid crease. Practice is necessary to accomplish this manoeuvre reliably with minimal distress to the patient. Your examination will be enhanced by staining the tear film with fluorescein and examining the surfaces using the cobalt blue light. Fine linear abrasions or larger areas of epithelial loss on the corneal and conjunctival surfaces suggest a subtarsal foreign body until proven otherwise.
Injury to the orbit
Blunt trauma to the eye or orbit often results in the development of a periorbital haematoma. The subcutaneous blood is confined to the eyelids and tissues anterior to the orbital septum. This type of haematoma should be distinguished from a true orbital haematoma where the blood lies within the orbital cavity, either outside or within the extraocular muscle cone or beneath the periosteum (periorbita). A large periorbital haematoma may result in complete closure of the eyelids making examination of the globe difficult, but not threatening the eye. If a glimpse of the globe can be obtained then you will notice that ocular motility is normal and there is no blood or fluid beneath the conjunctiva.
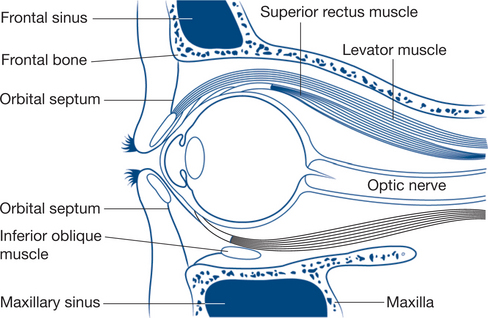
The anatomy of the orbit is shown in Figure 13.19.
Diagnostic plan
A CT scan performed with fine axial and coronal sections through the orbits will demonstrate the presence of an orbital fracture, any prolapse of orbital tissue and the likelihood of extraocular muscle entrapment. The latter should be suspected when the muscle is seen in close proximity to the fracture site. Blood in the maxillary sinus is a strong indicator of orbital floor fracture, even if the bony landmarks do not appear displaced. Associated fractures of the zygoma, maxilla and nasal bones may also be evident.
Injury to the eyelids
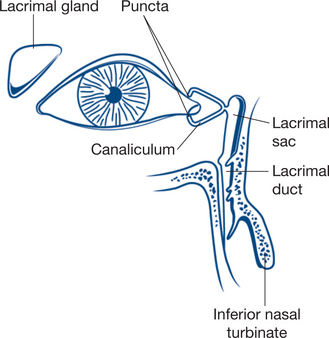
The eyelid is composed of skin, orbicularis muscle, tarsal plate and conjunctiva (Fig 13.20). There is a lacrimal punctum on the posterior aspect of the upper and lower eye lid margins, about 3–5 mm from the inner canthus. The lacrimal puncta are the openings to the lacrimal canaliculi that drain tears into the lacrimal duct and then into the nose. A full-thickness laceration of the eyelid margin medial to the lacrimal punctum may involve the lacrimal canaliculus. Full-thickness lacerations of the upper eyelid above the tarsal plate may involve the levator palpebrae superioris muscle or its tendon sheath.
13.8 Chest injury
In civilian practice the majority of chest injuries are closed and follow blunt trauma in motor vehicle accidents. Associated injuries, particularly head injuries, are common. Upper respiratory obstruction can occur at any time from the moment of injury to arrival at hospital. Airway obstruction may lead to death in an otherwise salvageable patient. The unconscious patient with chest injury is prone to airway obstruction not only from aspiration of vomitus and falling back of the tongue but also from haemoptysis. In most cases open thoracotomy is not necessary and there is time for careful assessment, immediate nonoperative treatment, chest X-ray and CT scan.
Life-threatening chest injuries
Definitive care
General management
The patient with major thoracic trauma should be managed at a facility that offers cardiothoracic surgical services. Immediate measures to establish a clear airway, assist ventilation and treat circulatory failure are instituted. Endotracheal intubation and ventilation are indicated in most patients with severe chest injuries, associated head injury and altered consciousness, airway obstruction because of facial or neck injuries, sucking chest wounds, flail chest and associated severe shock. Failure to respond to adequate resuscitation also suggests the possibility of massive haemothorax, tension pneumothorax, cardiac injury or cardiac tamponade. Immediate thoracotomy is indicated in patients with a penetrating chest wound that has possibly involved the heart (traversing the mediastinum) and is associated with cardiopulmonary arrest or with shock that has not responded rapidly to blood transfusion. Immediate thoracotomy is rarely required or of benefit in blunt chest injuries. Thoracotomy may be indicated at a somewhat later stage for significant and continuing haemothorax or for large air leaks preventing re-expansion of the lung. An intrapleural chest tube (intercostal catheter) is required for flail chest and in patients with haemothorax or pneumothorax or tension pneumothorax.
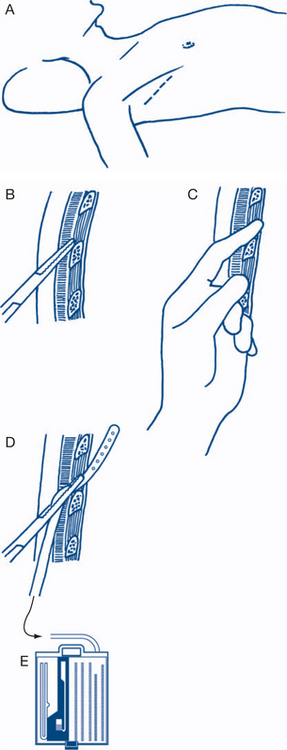
Insertion of an intercostal catheter (ICC)
The successful insertion of an ICC relies on two factors: understanding the anatomy of the intercostal space and utilising a safe method of insertion. The intercostal space is bounded by the ribs (one above and one below). It is important to appreciate that the neurovascular bundle lies under the cover of the rib above. This comprises the vein, artery and nerve (V-A-N) from superior to inferior, in the plane between the internal intercostal and transversus group of muscles. The chest tube is preferably inserted through the fifth intercostal space anterior to the mid-axillary line (Fig 13.21) and ‘just above the rib below’ to avoid the neurovascular structures. In obese patients or in women with large breasts, the anterior mid-clavicular second intercostal space approach may be preferred. The pleura should be entered with the spreading tips of a forceps rather than with uncontrolled thrusting of a trochar. The intercostal catheter (size 30Fr) is passed posterosuperiorly (towards apex) for evacuation of air in pneumothorax or inferiorly, to facilitate dependent drainage of a haemothorax.
Management of specific types of chest injury
Tension pneumothorax. Sometimes tension pneumothorax occurs immediately because a rib fracture has produced a one-way flap valve leak from the pleural surface. It may also be secondary to positive pressure ventilation in patients in whom an initially simple pneumothorax has not been recognised. Thus it is prudent to insert an ICC in all patients with posttraumatic pneumothorax or subcutaneous emphysema who need early endotracheal intubation and positive pressure ventilation. A suspected tension pneumothorax should be relieved immediately by inserting a wide-bore needle into the second intercostal space of the affected side. The diagnosis is made on clinical grounds — there is no time for a chest X-ray. The needle is subsequently replaced by a formal ICC with underwater drainage. A cardiothoracic referral should be made and the patient considered for VATS (video-assisted thoracoscopic surgery) pleurodesis at a later stage.
Blunt cardiac injury. The mechanism of the thoracic injury is important. Patients not wearing seatbelts and with clinical evidence of contact with the steering column are most likely to have a cardiac injury. Blunt cardiac injury may result in myocardial contusion, valvular disruption or rupture of the atria or ventricles. Patients often present with a fractured sternum and the following management is necessary: initial ECG followed by continuous cardiac monitoring (telemetry) for at least 24 hours (to exclude myocardial ischaemia/infarction and arrhythmias); serial cardiac enzyme estimations (biochemical evidence of myocardial injury); and echocardiography (to exclude pericardial tamponade or other cardiac injury).