3 Principles of radiotherapy
Introduction
After surgery, radiotherapy is the most effective curative treatment for cancer, contributing up to 25–30% of cure. At least half of the patients with cancer require radiotherapy at some time in their illness of which about 60% are treated with curative intent, often in combination with surgery and chemotherapy. Radiotherapy involves use of various types of ionizing radiation, and X-ray is the commonest type of radiation used. Other forms of radiation include electrons, protons, neutrons and gamma radiations from radioactive isotopes. This chapter intends to review the principles of practical radiotherapy (Box 3.1) and a detailed discussion on radiobiology and mathematical modelling are beyond the scope of this chapter.
Methods of delivery of radiotherapy
External beam radiotherapy (EBRT)
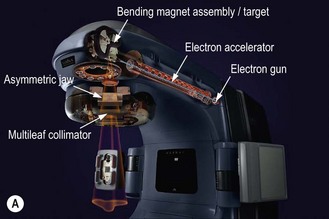
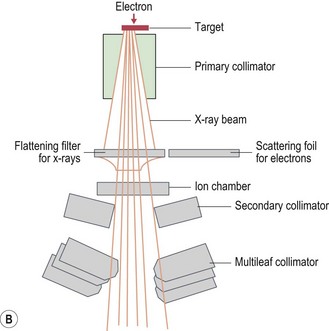
Modern radiotherapy is based on megavoltage X-rays (photons) and electrons. Megavoltage X-rays are produced by artificial acceleration of electrons through a vacuum to impact on a target in machines called linear accelerators (LINACs) (Box 3.2). The energy of the X-rays is proportional to the speed of electrons. Electrons are produced when the target in a linear accelerator is removed from the path of the electron beam (Box 3.2). Modern LINACs have facilities to produce both photons and electrons. Electrons have a predictable penetrability and hence are useful when it is important to limit the radiation dose to a deeper organ. Characteristics of photons that are useful in their clinical use are:
Box 3.3
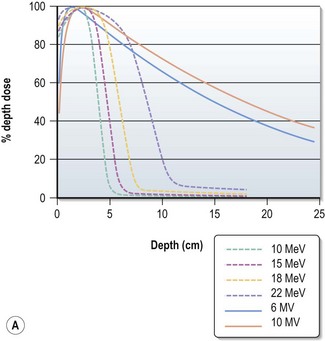
Choosing the energy (Figure 3.2)
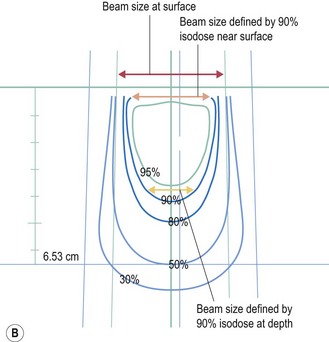
Electrons have a definite range (range in centimeters is approximately half of beam energy in MeV) and selection of electron is based on the depth needed to treat (effective treatment depth in centimeters, as defined by 90% isodose in centre, is approximately one-third of the beam energy in MeV ). With increasing energy, the surface dose decreases and hence if higher dose is needed at the surface, a bolus is used (p. 23). Bolus is also used to bring up high dose region to surface to avoid radiation to underlying critical structure. Higher energy electron beams exhibit a lateral constriction of 90% isodose line (Figure 3.2B) which need to be taken into consideration while deciding the field size at surface.
Brachytherapy
The previous problems of radiation safety for patients and staff, and prolonged treatment time, have become history with modern brachytherapy machines (Figure 3.3). Clinical applications of brachytherapy are as follows:
Radionuclide treatment
Systemic administration of radionuclides, which emit gamma-radiation, is a useful treatment for cancer. In well differentiated thyroid cancer, 131I is given to patients after thyroidectomy (p. 282). Strontium-89 and samarium-153 are used for palliation of bone metastases, particularly from prostate cancer.
Informed consent
Once a decision is made to treat with radiotherapy, this decision should be communicated to the patient. The patient should be informed of the intention of treatment, potential benefits and side effects, both short term and long term. It is also an obligation to explain to the patient the potentially serious short and long term consequences of treatment in order to obtain truly informed consent. Often patients would like to know details of how radiation works (Box 3.4), dose and length of treatment, rationale for a number of treatments (fractionation – the process of giving the total dose of radiation as small doses over a period of time) (Box 3.5) and side effects (p. 348). Side effects of radiotherapy depend on the area of treatment, total dose and dose per fraction. These can be acute (occurring during radiotherapy and within 3 months of completion of radiotherapy) or chronic (occurring 3 months after completion of radiotherapy).
Box 3.4
How does radiation work?
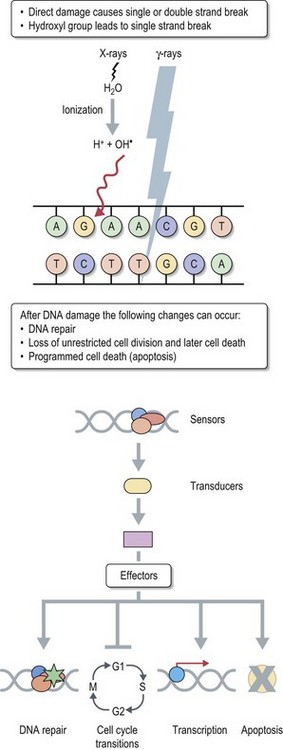
Therapeutic use of radiotherapy is born out of empiricism. Science caught up with empirical clinical use later to provide explanations for various patterns of response to treatment. The cancer cell has an unlimited capacity to multiply and radiotherapy is aimed to prevent this unlimited multiplication. The conventional explanation of radiation effect is based on radiation damage to DNA caused directly by photons and indirectly by free radicals which are produced when photons interact with water molecules in the tissue (see Figure 3.4). There are three different types of radiation damage to DNA:
Box 3.5
Rationale for fractionation
Radiotherapy planning and delivery
Position and immobilization
Radiotherapy aims to deliver a uniform (homogenous) therapeutic dose of radiation to the tumour and areas of microscopic spread with minimal possible dose to the adjacent organs. This necessitates accurate reproduction of treatment position on a daily basis. Patients are positioned and immobilized to ensure optimal beam delivery while maintaining a comfortable position. The common positions are either supine or prone.
Localization of tumour
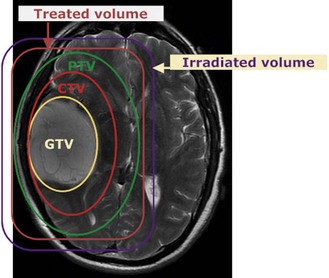
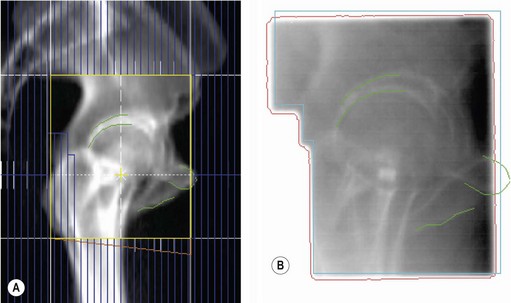
Localization of tumour is to define various treatment volumes (Box 3.6). Conventional localization (2-dimensional, 2D) is with orthogonal X-rays using special treatment planning machines called simulators. These are diagnostic X-ray machines, which can mimic all the positions and movements of a treatment machine (Figure 3.6A) and some of these machines have a CT scan facility. 2D localization is appropriate for palliative treatment. Radical radiotherapy utilizes a planning CT scan or advanced techniques to define the various tumour volumes in 3-dimensions. Modern computer software allows co-registration of the planning CT scan with an MRI or PET scan (Figure 3.6B), which improves the accuracy of GTV delineation. Planning CT scan is usually taken at a slice interval of 1.5–5 mm. A slice thickness of <3 mm helps to get better resolution of digitally reconstructed images (DRRs) which are used to check accuracy of treatment delivery (see later in Box 3.12).
Box 3.6
Volumes in radiotherapy (Figure 3.5)
The following are the volumes in radiotherapy:
Box 3.12
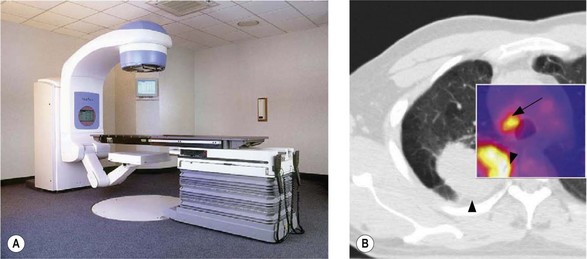
Verification of treatment (Figure 3.12)
DRR (Figure 3.12A) is used to verify treatment accuracy. This is commonly done by comparison of DRR with the treatment verification film (Figure 3.12B). The treatment set-up is checked by comparing the position of isocentre, bony landmarks and the positions of MLCs or beam shaping block for the same beams. If a systemic error is found during verification, it needs to be corrected before proceeding with further treatment and the corrected treatment plan should be verified for accuracy of treatment set-up.
Planning technique
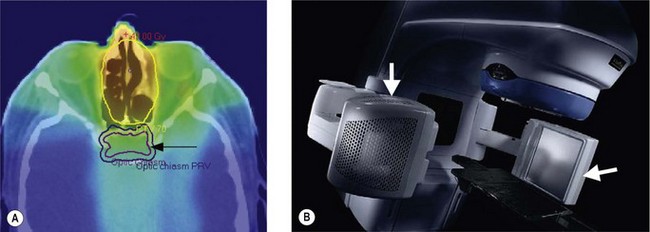
Image guided radiotherapy (IGRT) is a further advance in radiotherapy planning and delivery where the real time position of the tumour is taken into consideration (Figure 3.7). 3D, IMRT and IGRT use multiple beams to obtain the optimal treatment plan.
Treatment planning (Box 3.7)
Beam size and shape
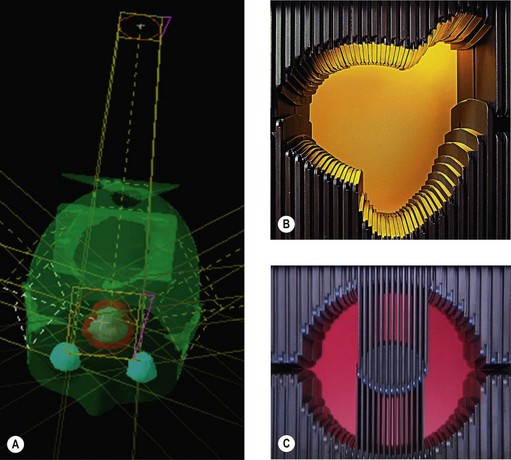
Conventional radiotherapy uses either rectangular or square fields with appropriate blocking of beams over critical organs (by shields, see below). Conformal radiotherapy shapes every beam according to the ‘beam’s eye view’ (BEV). The exact shaping of the beam used to be attained by shaped blocks. With the advent of multileaf collimators (MLC), shaping of beams becomes increasingly easy (Box 3.8).
Box 3.8
Beam shaping (Figure 3.9)
In the early 1950s radiotherapy beams used to be either square or rectangular. There was an option to shield part of the field with lead or equivalent material. When conformal radiotherapy started evolving, individual blocks were made based on the BEV of individual beams to shape the radiotherapy fields using cerrobend (an alloy of bismuth, lead, tin and cadmium with a melting point of 70°C and hence easy to make different shapes). Currently beam shaping is achieving with MLCs (see Figure 3.9).
Energy of the beam
For superficial lesions and lesions lying within a limited depth, electrons are used and energy of the electron beam depends on the depth which needs to be treated (Box 3.3). For deep seated tumours, megavoltage energy is used, usually 6 MV photons; in some patients a higher energy with high penetration is necessary to improve the dose homogeneity.
Evaluation of treatment plan
Once treatment is planned, it is necessary to evaluate the treatment plan before it is accepted for treatment. In difficult situations, such as when an OAR is lying near the PTV, the planning physicists come up with more than one plan for the oncologist to evaluate and choose one for treatment. Evaluation of the plan takes the following steps (Box 3.9):
Box 3.9
Evaluation of a treatment plan (Figure 3.10)
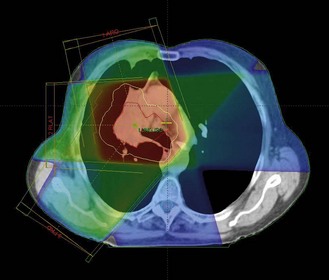
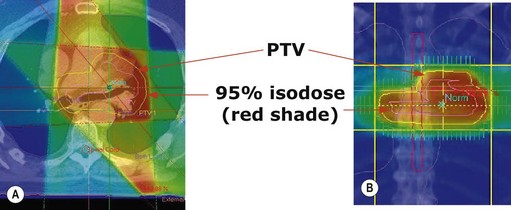
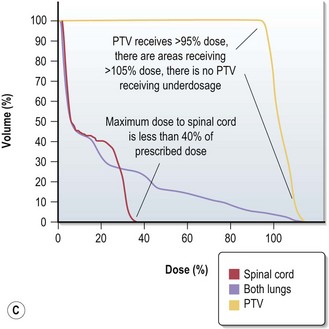
Treatment plan evaluation is to ensure that the PTV receives a homogenous dose and the doses to the OAR are within tolerance. Spatial distribution displays ensure that 95% isodose encloses the PTV (Figure 3.10A & B). Dose volume histogram (Figure 3.10C) is helpful to indicate underdosage to PTV as well as excessive dose to OAR.
Dose prescription for radiation treatment
For electrons, dose is prescribed at 100%; however, it is necessary that 90% isodose covers the PTV.
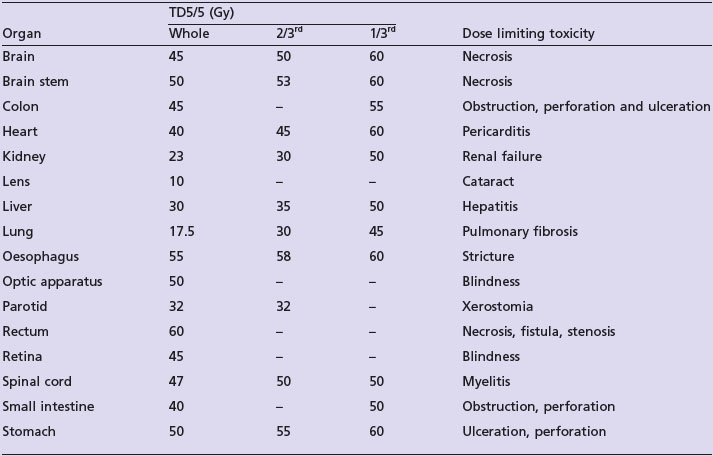
The prescription should indicate the total dose, number of fractions, dose per fraction, and duration of treatment (e.g. 50 Gy in 20 fractions over 4 weeks). Conventional fractionation is to deliver one radiotherapy fraction per day for 5 days a week. There are a number of alternate fractionation regimes (Box 3.10). The total dose of radiotherapy depends on the type of cancer, site and bulk of disease (Box 3.11). Gray (Gy) is the usual measure of radiotherapy which is defined as 1 joule of energy per 1 kg of material. The subunit is centigray (cGy) which is 1/100 of a Gray. Radiotherapy alone for cure needs a dose of >60–65 Gy as 1.8–2 Gy per day whereas adjuvant radiotherapy uses 50–60 Gy. Most studies indicate a direct relation between the dose and chances of control and cure.
Box 3.10
Fractionation schedules
Box 3.11
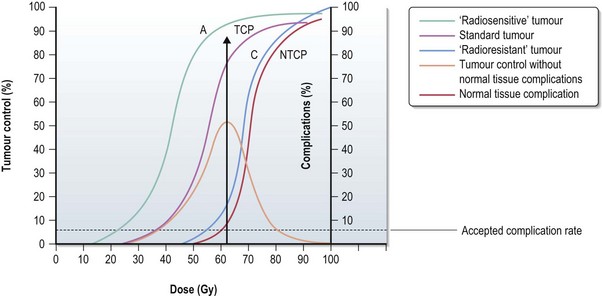
Tumour control probability (TCP), normal tissue complication probability (NTCP) and therapeutic ratio (Figure 3.11)
The probability of tumour control without normal tissue complications is highest in the therapeutic window. The further the NTCP curve to the right of the TCP (see Figure 3.11) cure it is easier to achieve a tumour control with minimal toxicity (A) and a larger therapeutic ratio. When NTCP and TCP (C) get closer the therapeutic ratio gets smaller.
In practical radiotherapy a better therapeutic ratio can be achieved by:
Treatment verification and corrections
Accurate position and immobilization during planning and treatment are important in accurate delivery of radiotherapy. In spite of this, treatment delivered can be different from the planned treatment. Hence it is important to ensure that treatment delivery is within the tolerance limit of accepted variation from the planning. The accepted tolerance generally depends on the immobilization method; e.g. a variation of <5 mm is acceptable with Perspex. The accuracy of planned treatment can be verified by:
During the process of verification two types of errors in treatment set up can occur:
Supportive care during radiotherapy
It is important to follow up patients regularly during radiotherapy to assess and manage radiotherapy toxicities, to monitor nutritional status and check blood counts. Studies have shown that during radical radiotherapy of head and neck, lung, oesophageal and cervical cancer, it is important to maintain a haemoglobin level of >12 gm/dl (a level lower than this may result in 10–12% less chance of disease control). The importance of level of haemoglobin is less clear in other cancers. However, symptomatic anaemia needs to be corrected. It is also important to make sure that the blood counts remains safe (i.e. platelet count of >100 × 109/dL and neutrophil count of >1.5 × 109/dL) during radiotherapy especially when it is given concurrently with chemotherapy or when a significant amount of bone marrow bearing area is being irradiated. Blood counts below the safe threshold necessitate either withholding chemotherapy or suspending chemotherapy until the blood count recovers.
Care after radiotherapy and survivorship
In the long term, there are a number of side effects needing special attention. Patients receiving radiotherapy to whole or part of the brain are at risk of cognitive impairment, cerebrovascular accidents, hormonal deficiency and second cancer (p. 58, late effects). Radiotherapy to the chest is associated with increased risk of second cancer and radiation damage to heart, particularly in patients receiving radiotherapy for left breast cancer. Pelvic radiotherapy often results in menopause and infertility in women and sexual dysfunction and infertility in men. Radiotherapy induced or promoted second cancer is an important survivorship issue. After cancer treatment, people often face an array of challenges including psycho-social functioning and economic well-being. As physicians, our triumph is not just in curing or controlling cancer, but also in helping cancer survivors to lead a high quality life in the society. For a detailed discussion on cancer survivorship, see p. 58.
Advances in radiotherapy
Based on experimental studies, there is a direct correlation between the radiation dose and chances of control of cancer, though not linear. However, various uncertainties in the radiotherapy planning process often lead to a large treatment volume (see Box 3.6). Advances in radiotherapy are aimed at understanding and controlling these uncertainties in radiotherapy planning. By doing so, we will be able to give a much higher dose to the tumour and areas of possible spread with minimal dose to the surrounding normal organs. The areas under research include advanced immobilization, improved radiotherapy with image guidance and incorporation of molecular imaging for tumour volume delineation.
Measures to improve efficacy of radiotherapy
Various measures are being studied to improve the efficacy of radiotherapy. These include alternative fractionations (Box 3.10), use of radiotherapy with concurrent chemotherapy, radiation sensitizers and recently, biological agents. The examples of alternative fractionation proven to improve tumour control include CHART for non-small cell lung cancer, 6 fractions per week in head and neck cancer and radiosurgery.