2 Principles of herbal pharmacology
Chapter contents
Defining our ground
Pharmacology can be defined as the study of the interaction of biologically active agents with living systems.1 The study of pharmacology is further divided into two main areas. Pharmacodynamics looks at the effects of an agent at active sites in the body. In contrast, pharmacokinetics is concerned with the effects the body has on the medicine, and specifically the concentrations that can be achieved at active sites. The approach used in this chapter, and to some extent in the therapeutic chapters, is to examine the pharmacology of key chemical groups in plants, the ‘archetypal plant constituents’, as much as individual herbs. (For detailed information on the pharmacology of selected herbs see Part Three.)
The chemical nature and classification of these archetypal plant constituents are studied within the discipline known as phytochemistry. Hence, any discourse on herbal pharmacology must be founded on a sound knowledge of phytochemistry. A misconception about ‘why medicinal plants work’ sometimes occurs in lay and even in professional circles. This is that the various nutrients such as vitamins, minerals and so on are largely responsible for the pharmacological activity of plants. (It is perhaps supported by the fact that most herbal products in the United States are regulated as ‘dietary supplements’.) Almost without exception, this is not the case. One notable exception is that certain plants, such as nettle leaf (Urtica species) and horsetail (Equisetum species), are rich sources of the trace element silicon. There is evidence that part of this silicon in horsetail is found intimately associated with cell wall polymers and can be released under mild extraction.2 Another interesting exception comes from the clinical use of a leaf concentrate from alfalfa (Medicago sativa) as an efficacious iron and folate supplement.3
• Bruneton J. Pharmacognosy, Phytochemistry, Medicinal Plants, 2nd ed. Paris: Lavoisier Publishing; 2008.
• Evans WC. Trease and Evans’ Pharmacognosy, 16th ed. London: Saunders Elsevier; 2009.
The field of pharmacognosy (from the Greek pharmacon for medicine and gnosis for knowledge) is the study of the definition, description and phytochemistry of natural drugs (typically medicinal plants or preparations derived from them).4 In fact, the term drug is derived from the old French word drogue, meaning to dry, referring originally to dried herbs. These days the application of pharmacognosy often extends to knowledge about the pharmacology of medicinal plants, and in some circles the term is used to specifically denote the study of herbal pharmacology, although this is technically incorrect.
There are also some primers in phytochemistry aimed at the herbal reader who does not have a strong background in natural products chemistry. In addition to this chapter, they represent a useful starting point for students of phytotherapy.5,6
Why should secondary metabolites have biological activity in animals? One suggestion put forward by Michael Baker is that enzymes in animals can share a common ancestry with enzymes or proteins in plants.7 This evolutionary kinship, when combined with structural similarities between plant and animal substrates for these enzymes, could explain the hormone-like or hormone-modulating effects of several archetypal plant constituents in humans. (See the licorice monograph for one such example.)
The role of secondary metabolites
However, there is still considerable debate on this complex issue. Even the line between primary and secondary metabolites can be arbitrary. They cannot be readily distinguished on the basis of precursor molecules, chemical structures or biosynthetic origins. For example, both primary and secondary metabolites are found among the diterpenes and triterpenes. In the diterpene series, both kaurenoic acid and abietic acid are formed by a similar sequence of related enzymatic reactions; the former is an essential intermediate in the synthesis of gibberellins. These are growth hormones found in all plants.8 The latter is a resin component. Similarly, the essential amino acid proline is classified as a primary metabolite, whereas the six-carbon ring analogue pipecolic acid is considered an alkaloid and hence a natural product. Even lignin, the essential structural polymer of wood and second only to cellulose as the most abundant organic substance in plants, is considered a natural product (secondary metabolite) rather than a primary metabolite.8
It has been suggested that, in the absence of a valid distinction based on chemical structure and biochemistry, a functional definition becomes the logical choice.8 Primary metabolites participate in nutrition and essential metabolic processes inside the plant, whereas secondary metabolites influence ecological interactions between the plant and its environment.
But even here, there is disagreement. Firn and Jones have recently argued that the terms primary and secondary metabolism as applied to plants are misleading and unsatisfactory.9 They suggested that important metabolites such as lipids, polysaccharides and carotenoids do not fit into either class, and proposed that they be classified as supportive metabolites (with the remainder of secondary metabolites classified as speculative metabolites).
By way of elaboration, lipids are essential for the short-term functioning of the cell and all cells contain a mix of lipids. But there are some individual lipids made by only a few species and, when their synthesis is inhibited, the cell suffers no short-term disadvantage.9
Since there is no consensus yet on this issue, the term ‘secondary metabolites’ will still be employed in this chapter. Be they speculative, supportive or secondary metabolites, their specialised functions include:10
• defence against herbivores (insects, vertebrates)
• defence against plant pathogens
• defence against other plants
• signal compounds to attract pollinating and seed dispersing animals
• signals for communication between plants and symbiotic microorganisms
• protection against ultraviolet light, oxidation and other physical stressors.
Based on their biosynthetic origins, plant secondary metabolites (the archetypal plant constituents) can be divided into three major groups: the terpenoids, the alkaloids, and the phenylpropanoid and allied phenolic compounds.8 All terpenoids, including the primary metabolites, are derived from the five-carbon precursor isopentenyl diphosphate. Alkaloids are biosynthesised principally from amino acids, and the phenolic compounds by either the shikimic acid pathway or the malonate/acetate pathway.8
According to Efferth and Koch, two large groups of secondary plant metabolites can be distinguished in terms of their biological/therapeutic activities:11
• A smaller group of highly active compounds possessing a high selectivity for cellular targets
• A larger group of moderately or weakly acting compounds that interact with a broad range of cellular targets (hence possessing molecular promiscuity).
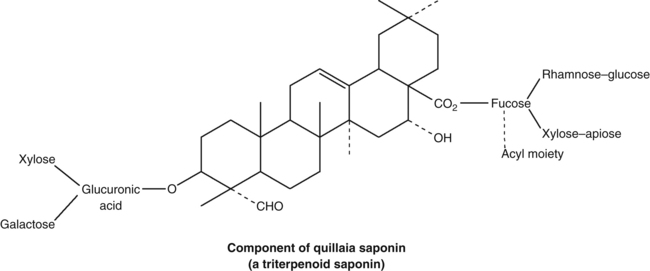
These authors reflect that medicinal plants with highly active phytochemicals represent only a minority of those commonly used (probably because of potential toxicity). However, plants with highly active phytochemicals are sought after in natural products research because they represent prime candidates for new drug discovery. In contrast, 90% of all thoroughly described medicinal plants contain broad-spectrum phytochemicals with weak or moderate bioactivity. Efferth and Koch suggest that plants have learned to cope with the problem of resistance development by producing combinations of pleiotropic multi-targeted phytochemical complexes (see also the concept of intelligent mixtures discussed later).11 They also note that those medicinal plants synthesising certain classes of highly active compounds might protect themselves by producing ‘prodrug’ phytochemicals that are activated only in predators or on damage to the plant.11 Many such molecules are glycosides (for example, see the later discussion of cyanogenic glycosides and glucosinolates).12,13
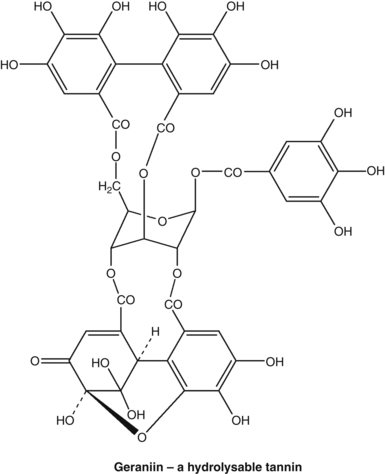
Alkaloids can play a defensive role in plants against herbivores and pathogens.8,14 Glucosinolates appear to have a role in protecting against insect attack.15 Tannins act to preserve the wood in living trees from microbial decomposition and insects.12 Several classes of secondary metabolites are induced by infection, wounding or grazing, which probably speaks to their defensive roles.16 Variation in the speed and extent of such induction may account, at least in part, for the differences between resistant and susceptible plant varieties.17 Both salicylic and jasmonic acids have been implicated as signals in such responses. Toxic chemicals formed in response to damage, especially from fungal attack, are called phytoalexins.12 In legumes, secondary metabolites are involved in interactions with beneficial micro-organisms (flavonoids as inducers of the Rhizobium symbiosis) and in defence against pathogens (isoflavonoid phytoalexins).8,18
As noted above, plants have also developed chemical defences against other plants, a phenomenon known as negative allelopathy. Many compounds are implicated, including phenolics19 and terpenoids.20,21 Positive biochemical interaction, or facilitation, among plants is also becoming increasingly recognised (positive allelopathy).22
Confusing the use of the term is the fact that ‘allelopathy’ can be applied in a broader context to denote the interactions between plants and animals. In other words, the word is sometimes used to describe the field of study of the broader ecological functions of secondary metabolites, as discussed above. A recent allelopathy textbook reflects this wider application of the term.21
How do herbs differ from conventional drugs?
Synergy and additive effects
‘The body is not a one-note melody, but a symphony of many interactive components functioning synergistically … the active ingredient model does not stem from a strength of the scientific method, as often supposed; rather, it stems from a weakness – from the inability of the reductionist method to deal with complex systems.’23
Synergy is an important hypothesis in herbal pharmacology in the context of the advantage of chemical complexity. It applies if the action of a chemical mixture is greater than the arithmetical sum of the actions of the mixture’s components: the whole is greater than the sum of the individual parts. In other words, there is a cooperative or facilitating effect between the components for a specific outcome. Another helpful definition of synergy is an effect seen by a combination of substances that is greater than would have been expected from a consideration of individual contributions.24 A well-known example of synergy is exploited in the use of insecticidal pyrethrins. A synergist known as piperonyl butoxide, which has little insecticidal activity of its own, interferes with the insect’s ability to break down the pyrethrins, thereby substantially increasing their toxicity. This example emphasises one important possible mechanism behind synergy as applied to medicinal plant components: increased or prolonged levels of key components at the active site. In other words, components of plants that are not active themselves can act to improve the stability, solubility, bioavailability or half-life of the active components. Hence a particular chemical might in pure form have only a fraction of the pharmacological activity that it has in its plant matrix. This key mechanism for synergy, therefore, has a pharmacokinetic basis.
Methods for assessing pharmacological synergy were proposed in an extensive paper by Berenbaum in 1989.25 Later Williamson26 and Houghton27 elaborated on these in the context of medicinal plants. Essentially, there are four mathematical techniques for demonstrating synergy proposed and discussed in these works, but only the isobole method is truly rigorous, although it is not always undertaken because of its complexity. Hence, several of the examples of herbal synergy discussed below need to be viewed in the context that they are only possible examples of synergy, since the isobole method was not performed.
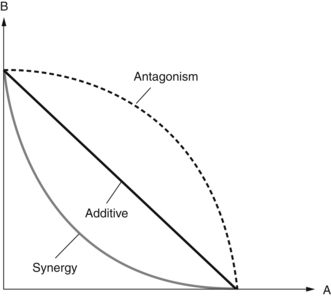
The isobole method has the advantage that it is independent of the mechanism of action involved. Although some explanations of the isobole method can be complex, a simple way to describe it follows. For a series of two-component mixtures, the concentration or dose of component A in each mixture required to give a defined effect (such as an ED50 or minimum inhibitory concentration, MIC) is plotted on the X axis against the concentration or dose of component B in the same mixture on the Y axis. The line joining all the points is then constructed. If there is no interaction between the effects of A and B, and their combination is merely additive, the line will be straight (Fig. 2.1). If there is a synergy between A and B, the line (the isobologram) will be concave. Alternatively, if antagonism exists the isobologram will be convex.
There are some published examples in the literature of the isobole method demonstrating synergy for mixtures of phytochemicals from the same herb. Williamson provides the example of ginkgolides A and B from ginkgo in terms of an anti-PAF (platelet activating factor) effect on platelets in vitro.26 In much earlier work in mice, a potentiating effect of different concentrations sennoside C on the purgative activity of sennoside A was observed.28 In this study an isobologram of ED50 values for laxative activity was not plotted, but one can be readily constructed from the data provided showing a concave line. Interestingly, a mixture of these compounds in the ratio 3:7 (which somewhat reflects the relative levels in senna leaf) gave the lowest ED50 value.
One example of where the combination of two components from the same plant has yielded a significantly greater effect than expected is a study on the upregulation of phase II detoxification enzymes in mice by the glucosinolate breakdown products from broccoli.29 An orally administered mixture of crambene and indole-3-carbinol caused a significantly greater induction of glutathione S-transferase and quinone reductase that could be expected from the activity of oral doses of the individual treatments. Another example is the study where the three major curcuminoids from turmeric (Curcuma longa) were investigated for their in vitro nematocidal activity against second stage larvae of Toxocara canis.30 Each of the three curcuminoids was ineffective on its own, whereas any combination of two, or all three together, was active. The combination of all three demonstrated the highest activity.
Another example identified in early research is the antibacterial activity of major components of lemongrass essential oil. While geranial and neral individually elicited antibacterial action, the third main component, myrcene, did not show any activity in vitro. However, when mixed with either of the other two main components, myrcene enhanced their activity.31 As pointed out in one review11 this example is technically not one of synergy, but rather exemplifies potentiation, where an inactive compound enhances the potency of a bioactive one.
In an interesting study, the impact on serum lipids of four active components of Chinese hawthorn (Crataegus pinnatifida), and their combination, was investigated in mice fed a high fat and cholesterol diet.32 The four compounds (quercetin, hyperoside, rutin and chlorogenic acid), and their combination at percentages reflected in the berry, were each fed orally to the mice at a dose of 2.85 mg/kg. The greatest reduction in total cholesterol and LDL-cholesterol was found for the combination. For LDL-cholesterol, only the combination yielded a significant reduction versus the control group (p<0.01).
Pharmacodynamic synergy has also been demonstrated for combinations of extracts or phytochemicals from different herbs. The in vitro antitumour activity for combinations of extracts of Corydalis and turmeric rhizomes (a traditional formulation) demonstrated a synergistic interplay, as determined using an isobologram.33 Berberine from Coptis chinensis and evodiamine from Evodia rutaecarpa demonstrated synergistic antitumour activity in vitro against a human hepatocellular carcinoma cell line.34 A pronounced convex curve was produced on an isobologram.
A recent review highlighted that two key developments are behind growing interest in synergy research in phytotherapy.35 The first is the new methods of analytical chemistry and molecular biology that have become available in the 21st century. The second is an unexpected change of paradigm in chemotherapy that has appeared without drawing much attention as to its radical nature. This is the transition in chemotherapy towards a multi-drug approach. Multi-drug therapy is already widely practised in the treatment of AIDS and other infectious diseases, hypertension, cancer and many other diseases.
In particular, the review notes this multi-drug concept in cancer chemotherapy is often aimed at being biomodulatory, not just at direct destruction of the tumour.35 Instead, suppression of different processes essential for the tumour’s survival are also targeted (such as angiogenesis, oncogene expression, anti-apoptotic mechanisms, immunological tolerance and inflammation). This concept of multi-targeted or polyvalent therapy is not new to phytotherapy and is discussed further below.
There are several examples in the herbal research literature where synergistic interactions are strongly implied, although they have not been absolutely demonstrated using the isobologram method. One example is the research cited by Williamson26 and several others where cannabis extract demonstrated higher antispastic activity in mice than the equivalent dose of pure tetrahydrocannabinol (THC). As pointed out by Houghton,27 this might not represent an example of true synergy. Rather other components in the cannabis extract might also have antispastic activity. In other words, there could be an additive effect between THC and other phytochemical components in the extract. However, the increased activity is so striking that synergy is a very plausible explanation. Testing a THC-free cannabis extract in the same model would have provided clear proof, one way or another.
Another example of a striking result that strongly implies a synergistic interaction is provided by Williamson.26 This is the research where the antiulcer activity of a fraction of ginger extract was found to be 66 times that calculated from the individual components of that fraction. More recent similar examples include:
• analysis of the in vitro butyrylcholinesterase inhibitory activities of essential oils of various Salvia species, which revealed that the activities of isolated major components could not account for the observed activity36
• tea tree oil (Melaleuca alternifolia), which exhibited pronounced in vitro antitumour activity against murine B16 melanoma cells.37 However, a ‘mock’ tea tree oil (containing its five major phytochemical components at the same concentrations found in the oil, and representing 80% of its total content) was only half as active. Of these five major oil components, only terpinen-4-ol (about 40% of the oil) was active in this model.
There are several examples of a pharmacokinetic basis for synergy, where chemical complexity results in enhanced solubility or bioavailability of key phytochemical components in plant extracts. The basic issues were discussed by Eder and Mehnert.38 The isoflavone glycoside daidzin given in the crude extract of Pueraria lobata achieves a much greater concentration in plasma than an equivalent dose of pure daidzin.39 Ascorbic acid in a citrus extract was more bioavailable than ascorbic acid alone.40 More recently, consumption of a preparation of fresh kiwi fruit resulted in up to five times more effective ascorbate delivery to tissues than when ascorbate was administered via the drinking water to vitamin C-deficient mice.41 The kava lactones appear to increase each other’s bioavailability, especially when given as the herbal extract. (For more details, see the kava monograph under Pharmacokinetics.)
Improved pharmacokinetic parameters can also apply to combinations of herbs, although this is not necessarily always the case. The traditional Chinese formulation Huangqin-Tang decoction, together with decoctions of its individual herbal components, were studied in rats after oral dosing.42 The constituents/metabolites baicalin, wogonoside, oroxylin-A-glucuronide, risidulin I and liquiritin demonstrated higher overall bioavailability from the compound formulation than from the relevant single herb decoctions, due largely to a longer residence time.
Often synergy is invoked to explain experimental results that can be readily explained by additive effects. Synergy is not always necessary to justify the use of complex mixtures, be they single herbal extracts or herb combinations. Additive effects can be just as favourable to enhanced therapeutic activity and are probably more common in phytotherapy. A team of German scientists headed by the late Dr Hilke Winterhoff discovered that the procyanidins in St John’s wort extract (Hypericum perforatum) significantly increased the in vivo antidepressant activity of hypericin and pseudohypericin, probably by a solubilising effect (these compounds otherwise have poor solubility).43 The same research team found that the flavonoids in St John’s wort have activity in an animal model of depression, with hyperoside and isoquercitrin showing significant activity.44 The flavonoid perspective was backed up by another in vivo study from Italy that found a flavonoid-enriched St John’s wort extract exhibited a more significant influence on central neurotransmitters.45 Later work from the German researchers demonstrated that a St John’s wort extract free of both hypericin and hyperforin (another phytochemical in the herb thought to play a role in the antidepressant activity) still exerted an antidepressant activity in rodent models.46 Hence, it seems that hypericin, procyanidins (indirectly), hyperforin and the flavonoids can all contribute to the clinically verified antidepressant activity of St John’s wort.
Additive effects also apply to herb combinations. The reduction of amphetamine-induced hypermotility was studied in mice after a single oral dose of passionflower extract (Passiflora incarnata, 250 mg/kg), kava extract (100 mg/kg) or the two combined (250 mg/kg+100 mg/kg, respectively).47 The combination yielded a much greater reduction in hypermotility over 2 hours than each individual herbal extract, which the authors attributed to a synergistic interaction. However, a careful analysis of the numbers suggests that the observed result is more likely to have been due to an additive effect (in terms of the differences observed from the control group). Additive benefits have also been observed at a clinical level. A combination of ginseng (Panax ginseng) and Ginkgo has been extensively investigated in volunteers for its acute and long-term impact on cognitive function. The effect of the combination appears to be significantly greater than either herb alone. This might be an example of synergy (which would be difficult to prove conclusively) but is at least an additive effect. (See the ginseng monograph for more details.)
Polyvalent or multifaceted activity
As touched on above, the concept of multi-agent medicines is a developing theme in modern drug therapy. However, it represents nothing new for phytotherapy. Due to their chemical complexity, even a single herbal extract is a nature-designed multi-agent medicine that can simultaneously target a range of desirable pharmacological effects. There are many examples of this provided in the herbal monographs in Part Three. This helps to explain why identifying the ‘active constituent’ in many herbal extracts has proved to be so difficult. For most if not all herbal extracts the ‘active constituent’ is the whole extract itself, as illustrated by the above discussion of the antidepressant activity of St John’s wort. The potential for chemical complexity to confer polyvalent activity or polypharmacology can also explain the apparent therapeutic versatility of herbal extracts. As an example there is the rather large and novel range of clinical uses for Ginkgo, all somewhat supported by evidence from randomised, controlled trials.
It has also been argued that polyvalence may be behind some effects that have been attributed to synergy (although ultimately such a distinction is probably academic, as both explanations argue in favour of chemical complexity).27 In whole organism or tissue studies, other compounds present in the mixture may be active against a range of targets, all acting to contribute to the observed outcome. Houghton goes on further to assert that:
Polyvalence can be defined as the range of biological activities that an extract may exhibit which contribute to the overall effect observed clinically or in vivo. It is often confused with synergism but the distinction lies in the fact that synergism is strictly concerned with only one pharmacological function, rather than a range of activities resulting in an overall effect.27
As outlined by Houghton, polyvalence can occur at three key levels:
1. Several types of phytochemicals are present that each exert a different biological effect.
2. Phytochemicals of one particular chemical type are present that have more than one biological effect relevant to treating the disease and/or improving the health of the patient.
3. Phytochemicals are present that do not affect the cause or symptoms of the disease itself, but instead modify the side effects, absorption, distribution, metabolism or excretion of active constituents.
In a recent review, Gertsch observed that herbal extracts might be ‘intelligent mixtures’ of secondary plant metabolites that have been shaped by evolutionary pressures.48 As such they could represent complex therapeutic mixtures possessing inherent synergy and polyvalence. Gertsch also notes that another important concept related to polyvalence is that of network pharmacology, as originally proposed by Hopkins.49 In the context of plant extracts (which for commonly used herbs typically contain hundreds of potentially bioactive natural products with only mild activity) it is possible that different proteins within the same signalling network are only weakly targeted. However, this is sufficient to shut down or activate a given process by network pharmacology.48 In other words, network pharmacology can explain how a number of weakly active plant secondary metabolites in an extract may be sufficient to exert a potent pharmacological effect without the presence of a highly bioactive compound.48 In the context of herbal pharmacology, Paul Ehrlich’s concept of the magic bullet is supplanted by one of a ‘green shotgun’, to paraphrase Gertsch and James Duke. However, the herbal research that can verify such theoretical constructs is only in its infancy.
The ‘-omic’ technologies (genomics, transcriptomics, proteomics and metabolomics) are recent developments in molecular biology that have the potential to open new perspectives for our understanding of the multi-faceted activities of complex plant mixtures. However, their meaningful application to medicinal plant research poses several challenges.50 The reviews of Wagner and colleagues and Efferth and Koch11 provide a more complete discussion of the application of these techniques to natural product research.
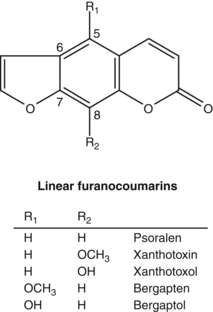
Intelligent mixtures
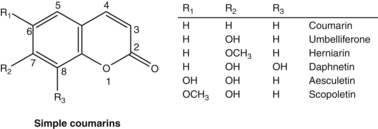
Whether it is a manifestation of synergy or by other means, the hypothesis that herbal extracts might act at a pharmacological level as intelligent mixtures is intriguing. One such example could be the root of Pelargonium sidoides. Its phytochemical content is unremarkable, including oligomeric procyanidins (OPCs) and highly oxygenated simple coumarins.51 Yet a liquid preparation of the herb has been shown to be effective in several clinical trials. A meta-analysis published in 2008 reviewed its efficacy for acute bronchitis.52 Six randomised trials were included, providing results for a total of 1647 patients (treatment and control groups): one trial compared Pelargonium against a non-antibiotic treatment (N-acetylcysteine); the other five trials tested Pelargonium against placebo. In two trials the ages of the participants were 6 to 12 years, and 6 to 18 years. Adults were enrolled in the other trials. Key findings were:52,53
• all trials reported clinical results suggesting Pelargonium was beneficial in the treatment of acute bronchitis
• meta-analysis of the four placebo-controlled trials involving adults indicated that Pelargonium root significantly reduced bronchitis symptom scores by day 7
• the duration of illness was significantly shorter for patients treated with Pelargonium compared to placebo (Pelargonium-treated patients were able to return to work nearly 2 days earlier than placebo-treated patients)
• patients noticed a treatment effect earlier under Pelargonium than under placebo.
Pharmacodynamics of the archetypal plant constituents
Simple phenols and glycosides
Phenols comprise the largest group of plant secondary metabolites. They range from simple structures with only one benzene ring to larger molecules such as tannins, anthraquinones, flavonoids and coumarins. Tannins and flavonoids are often referred to as polyphenolics. Phenols are defined as compounds that have at least one hydroxyl group attached to a benzene ring. They differ in their chemical properties from other tertiary alcohols because the presence of the benzene ring stabilises the phenolate ion; they are therefore more acidic and more reactive.
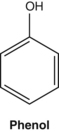
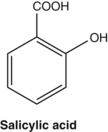
From a pharmacological perspective, the best known simple phenol is salicylic acid. Its precursors are found in willow (Salix species) and poplar (Populus species) barks and salicylic acid is subsequently formed on ingestion (see Pharmacokinetics later). Salicylic acid has recognised antipyretic and anti-inflammatory properties that underlie the use of willow bark for fever and arthritis. Acetylsalicylic acid (aspirin) is a synthetic derivative of salicylic acid that in addition has pronounced antiplatelet properties due to the presence of the acetyl group.54 Salicylic acid lacks this property and consequently willow bark is not suitable as a natural substitute for aspirin in cardiovascular patients. Simple phenols are also powerful antiseptics. Arbutin is a phenolic glycoside that confers bacteriostatic properties to urine (see the monograph on bearberry).
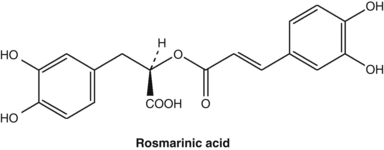
Several studies have shown that extracts of many members of the mint family (Lamiaceae or Labiatae) have considerable in vitro antioxidant activity due to the presence of phenolic acids such as rosmarinic acid. Highest antioxidant activity was found in Prunella vulgaris, which had a rosmarinic acid content of about 5%.55
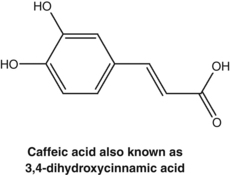
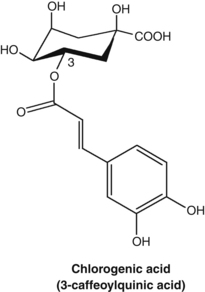
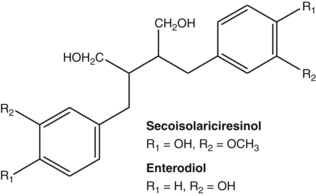
Caffeic acid, as well as its derivatives such as rosmarinic acid and chlorogenic acid, were found to exert antithyroid activity after oxidation. This activity may form the basis of the clinical use of Lycopus species for hyperthyroidism (see the bugleweed monograph).56 Similar oxidation products of caffeic acid inhibit protein biosynthesis in vitro and these compounds probably account for the clinically established antiviral activity of a topical preparation of Melissa officinalis (see also Chapter 8, p. 142).57
The simple phenol resveratrol is probably the most actively researched phytochemical worldwide, with many properties demonstrated in pharmacological models. There are more than 4000 studies on resveratrol. Resveratrol is a phytoalexin produced by several plants in response to fungal attack. However, well-known sources are grapes (Vitis vinifera) and the Chinese herb Polygonum cuspidatum. It has demonstrated an amazing array of (mainly in vitro) pharmacological activities including antioxidant, cardioprotective, antidiabetic, anticancer, antiviral, neuroprotective, antiplatelet, anti-inflammatory and modulation of fat metabolism.58–60 Resveratrol inhibits cancer development at all the three known phases of chemical carcinogenesis, namely initiation, promotion and progression.61 The development of other chronic diseases might also be reduced by resveratrol, based on the many laboratory studies. These diseases include cardiovascular disease, dementia, type 2 diabetes and osteoarthritis.62,63 In addition to its indirect effects on the ageing process via SIRT1 (hence possibly acting as a calorie restriction mimetic), this one simple molecule has the potential to prevent most of the chronic diseases associated with ageing.64 Resveratrol could well be the best example of the molecular promiscuity of common secondary metabolites: ‘Considering the structural simplicity of this stilbene, the intensity of interest is phenomenal’.61
One important pharmacological study was published in Nature in 2006. Rather than administering resveratrol to normal mice to see if it simulated calorie restriction, the effect of resveratrol on a high calorie diet was studied. Middle-aged (1-year-old) male mice on a high calorie diet (HCD) were given resveratrol and compared to untreated mice on the same diet or a standard diet.65 The administered doses of resveratrol were either 5.2 or 22.4 mg/kg/day for 6 months, but only results for the higher dose were reported.
One of the issues with resveratrol is that it is rapidly metabolised and has limited bioavailability as such. However, resveratrol metabolites (mainly phase II conjugates) might also be bioactive, or act as a reservoir of resveratrol at target tissues. One study found that to maximise plasma resveratrol levels it should be taken with a standard breakfast and not with a high fat meal.66 The high fat breakfast was observed to reduce its bioavailability by about 45%.
Cyanogenic glycosides
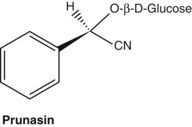
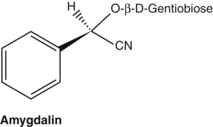
Cyanogenic glycosides are capable of generating hydrocyanic acid (prussic acid, cyanide). Structurally they are glycosides of 2-hydroxynitriles that can be hydrolysed by the enzyme beta-glucosidase into cyanohydrin. This is unstable and dissociates to hydrocyanic acid. Common cyanogenic glycosides include amygdalin found in bitter almonds and peach kernels (both used in Chinese medicine), and prunasin in wild cherry bark (Prunus serotina). The small quantities of cyanide generated from this bark are said to be responsible for its antitussive properties, although this has not been confirmed in modern pharmacological experiments. Both amygdalin and prunasin yield benzaldehyde on hydrolysis, which accounts for the characteristic almond-like aroma of wild cherry bark. The cyanogenic glycosides linustatin, neolinustatin and linamarin (trace) are found in linseeds (flax, Linum usitatissimum).67
Although hydrocyanic acid is a violent poison, oral intake of cyanogenic glycosides (for example via food, especially in primitive diets) is not necessarily toxic, particularly in the short-term. Hydrolysis of the glycosides in the digestive tract or by the liver leads to a slow release of hydrocyanic acid that is readily detoxified by the body. Addition of 10% apricot kernels to the diet of rats for 18 weeks showed only moderate toxic effects.68 Amygdalin given orally to humans at 500 mg three times a day produced no toxic effects and only moderately raised blood cyanide levels.69 However, co-administration of beta-glucosidase with amygdalin to rats substantially increased its toxicity.70 Acute poisoning has occurred in grazing animals.71
Considerable interest arose in the 1970s regarding the use of a synthetic cyanogenic glycoside, patented as Laetrile (mandelonitrile beta-glucuronide), as an alternative anticancer compound.72 However, most of what was subsequently sold as Laetrile was in fact amygdalin (which probably had similar properties on injection or ingestion).73 The theory proposed was that cancer tissues contain beta-glucosidase and circulating cyanogenic glycosides can therefore act as selective cytotoxic agents. However, amygdalin did not prove to be an effective anticancer agent either in animals74 or in humans,75 presumably because the beta-glucosidase activity of cancer cells is quite low.76 In an interesting development of the Laetrile theory, beta-glucosidase was conjugated to a tumour-associated antibody. When combined with amygdalin in vitro, the cytotoxicity of amygdalin to tumour cells was increased 36-fold.77
Longer periods of intake of subacute amounts of cyanogenic glycosides in daily food have led to chronic intoxication. Products of human detoxification processes, such as the formation of rhodanide and cyanocobalamine, lead to severe diseases, especially neurotoxic syndromes.67 This is thought to be involved in tropical ataxic neuropathy in Nigeria. In other African countries, consumption of cassava root (Manihot esculenta, also known as tapioca) together with a diet deficient in sulphur amino acids is apparently responsible for an endemic upper motor neuron disease known as konzo.67 The peeled root contains much lower levels of cyanogenic glycosides. Konzo typically occurs in epidemics in Mozambique, which may reflect inadequate processing of the root in times of drought or war.78
Mucilages
Mucilages are generally not chemically well defined. They are large, highly branched polymeric structures built from many different sugar and uronic acid units (uronic acids are carboxylic acids derived from sugars). They are very hydrophilic (water loving) and are capable of trapping water (and other molecules) in their cage-like structures to form a gel. Consequently, when a mucilage is mixed with water it swells to many times its original volume as it absorbs water. The saccharide linkages are in a beta configuration, which means that human digestive enzymes cannot break down mucilages. However, they can at least be partially decomposed by bowel flora into beneficial metabolites such as short-chain fatty acids (SCFA). This may explain the traditional use of slippery elm bark (Ulmus rubra) as a food for convalescents. Not only would the mucilage soothe a disturbed digestive tract, the SCFA formed in the colon would provide a source of readily absorbed and assimilated nourishment. There are some clinical and experimental studies that support the concept that mucilages can act as prebiotics, especially after their partial processing by the upper gastrointestinal tract.79–81
Mucilaginous remedies have been primarily used for their topical emollient and internal demulcent properties and their direct, if temporary, benefits in the management of inflammatory conditions of the digestive tract. This anti-inflammatory effect is probably more than just mechanical, although the protective benefits of a layer of mucilage on the digestive mucosa are obvious, especially as an extra barrier to gastric acid. The protective effect of mucilage isolated from Plantago major leaves against aspirin-induced gastric ulcer has been demonstrated in rats.82 Similar gastroprotective activity has been demonstrated for guar gum.83 It has also been shown that guar gum forms a layer closely associated with the intestinal mucosal surface when given to rats, providing a protective barrier.84
In order to assess the activity of mucilages on epithelia, a test system based on porcine buccal membranes was devised.85 While mucilages from marshmallow (Althea officinalis) and ribwort (Plantago lanceolata) showed moderate bioadhesion to epithelial tissue, polysaccharides from bladderwrack (Fucus vesiculosus) and Calendula exhibited strong adhesion. Histological studies of membranes indicated the presence of distinct polysaccharide layers on the apical membrane surface. In vitro investigations of an aqueous marshmallow root extract and its isolated mucilage on human epithelial KB cells (originating from the nasopharyngeal epithelia) and skin fibroblasts found a stimulating effect on the cell viability and proliferation of the former only.86 The marshmallow mucilage was internalised into epithelial cells, but not into fibroblasts, although it did form a bioadhesive layer on the latter. Microarray analysis indicated an upregulation of genes related to cell adhesion proteins, growth regulators, extracellular matrix, cytokine release and apoptosis. The authors concluded that their findings were consistent with the traditional use of marshmallow root for irritated mucous membranes.
Mucilages can also function as bulk laxatives and the most widespread use in this regard is ispaghula or psyllium husks, widely sold as a number of proprietary products. These are derived from the seeds of Plantago psyllium or P. ovata. However, the traditional use of mucilages such as linseed (flaxseed) and fenugreek (Trigonella foenum-graecum) as bulk laxatives often provides a valuable alternative,87 particularly where psyllium causes the characteristic side effects of bloating, abdominal pain and flatulence.88 Slippery elm powder (Ulmus rubra) can also be useful here. Mucilages can also be employed as weight loss agents and presumably act by creating a sensation of fullness.88 Since they are known to cause oesophageal obstruction, mucilages should be taken with plenty of water and not prescribed in tablet form.89 Even then, Health Canada issued a 2010 warning that glucomannan (a fibre very similar to mucilage) as a powder or capsule needs to be taken with copious amounts of water as otherwise it may cause serious choking.90
Mucilages are also used by phytotherapists to create reflex demulcency, especially to ease irritable and ticklish dry coughs. It is clear that there is no readily recognised pharmacological model for the transfer of demulcent properties directly to the bronchial mucosa: mucilages are too large to be absorbed and transported to this remote site. The emetic effect in reverse, that is the reflex effects on the tracheobronchial musculature of a soothing effect on the upper digestive tract, is instead postulated, mediated by the vagus nerve. Similar associations are used to justify the use of mucilages in painful conditions of the urinary tract.
Reflex demulcency does have experimental support. An extract of marshmallow root and its isolated mucilage demonstrated significant antitussive activity in an animal test. Doses were administered orally (50 and 100 mg/kg of mucilage) and cough from both laryngopharyngeal and tracheobronchial stimulation was depressed.91 The mucilage was as potent as some non-narcotic antitussive drugs. More recently, marshmallow mucilage was shown to be the most effective of a range of plant polysaccharides in terms of in vivo cough-suppressant activity.92 There was no negative impact on expectoration, unlike for codeine. A mechanistic study in guinea pigs demonstrated that marshmallow root mucilage (25 and 50 mg/kg, oral) produced an antitussive effect that was comparable to codeine (10 mg/kg, oral) in the model used.93 The effect was partially suppressed by a 5-HT2 receptor antagonist, suggesting an involvement of these serotonergic receptors thought to participate in the cough reflex.
There is a complex association now recognised between gastro-oesophageal reflux (GORD) and chronic cough and asthma.94–96 The link between reflux and asthma has long been suspected. In fact, there is quite long-standing evidence that reflux is an important cause of asthma in some asthmatics. Monitoring of oesophageal acidity revealed reflux in 7 out of 9 patients with persistent asthma.97 In another early study 61% of patients with asthma had reflux.98 Treatment of reflux by surgery or drugs can result in improvement or cure of asthma.98,99
While aspiration of gastric acid has been proposed as a possible cause, an effect from the reflux itself also has currency.100 One paper reported an investigation into the link between acid reflux into the oesophagus and coughing or wheezing fits in asthmatic patients.101 The scientists asked a question debated for some time among respiratory and gastrointestinal physicians: ‘Does cough cause reflux or does reflux cause cough?’ They studied more than 100 chronic adult asthmatics and found that half of all their coughs and wheezes occurred at the same time as when acid refluxed from the stomach into the oesophagus. But more importantly, they concluded that in the majority of cases it was the reflux that caused the coughing and not the other way around. The chronic cough that often follows a respiratory infection and seems to persist long after the infection is gone is probably due to acid reflux, which in turn is induced by the weakening of the oesophageal sphincter as a result of the violent coughing during the infection. Here the role of reflex demulcency is particularly valuable. Even further, if there is acid in the oesophagus, then the direct demulcent activity of the mucilage on the oesophageal wall will also soothe the irritation and thereby allay the very stimulus driving the cough or wheeze.
Animal models have confirmed that the association with GORD and cough is most likely a reflex mediated by the oesophageal afferent nerve fibres carried by the vagus nerve.102 The nocioceptive C-fibres are stimulated by the acid; however, multiple neural pathways are likely to be involved. In clinical studies, inhalation of a low concentration of a C-fibre stimulant leads to an irritating, itchy, urge-to-cough sensation that mimics the sensations associated with cough linked to respiratory tract infection, post-infection, GORD and asthma.103
Mucilages are also a class of viscous soluble fibre and in this context the properties of psyllium husks have been well studied. In particular, the mucilage from psyllium is effective at lowering blood cholesterol, as evidenced by reviews of the clinical data.104,105 Trial results suggest that it must be taken with food to be effective.106 The effective dose is around 10 g/day, with recent trials suggesting more modest effects of around a 7% reduction in LDL-cholesterol.
Viscous soluble fibre helps to retain glucose in the gut and reduce blood insulin levels after eating. Probably the main effect here is delayed gastric emptying. Psyllium seed was shown to have particular benefits in this regard, with a clear dose-related response on the effects of a glucose challenge.107 Results were not highly robust and obviously it needs to be taken with meals.105
Mucilages may compromise the absorption of nutrients and drugs (see Appendix C). However, the potential for psyllium to decrease the absorption of minerals is not proven.105
Essential oils
Phytochemistry
Essential oils are water-insoluble oily liquids that are usually colourless. Despite their being called oils, they are not related chemically to lipid oils (fixed oils) such as olive oil, corn oil and so on. Although often hydrocarbon in nature, they are unrelated to the hydrocarbon oils from the petrochemical industry. They will slowly evaporate if left in an open container, and placing a drop of oil on blotting paper can be used as a simple technique to test for adulteration with a fixed oil. If a fixed oil is present, an oily smear will remain on the paper a few days later.
Adulteration is an important issue in the trading of essential oils and many sophisticated techniques have been developed to imitate and extend essential oils. In some cases, such as oil of wintergreen, trade in the synthetic oil has completely supplanted the natural product. One survey found a large variability between the biological activities of different samples of oils and groups of oils under the same general name, for example lavender, eucalyptus or chamomile.108 This reflected on the blending, rectification and adulteration that occurs with commercial oils. Of course, this issue does not apply for oils prescribed as part of the whole plant extract, as used by phytotherapists.
Pharmacodynamics
Aromatherapy is a treatment system based on the use of essential oils. The oils may be inhaled, applied to the skin or orifices, added to baths or ingested. There is no doubt that ingested oils or those applied to the skin or added to baths are absorbed into the bloodstream in significant quantities.109 However, the use of essential oils by inhalation, mainly to influence mental function, is more controversial. In fact, in the German-speaking world, aromatherapy is typically defined as the therapeutic use of fragrances only by means of inhalation. Evidence is accumulating that this form of aromatherapy also has a pharmacological basis and is not placebo, nor is it a manipulation of emotions via the sense of smell. A recent review of the therapeutic properties of lavender essential oil exemplifies this, with 14 clinical studies demonstrating possible beneficial effects from its inhalation, including enhanced sleep, decreased anxiety and improved cognition.110
Most of the evidence for the antibacterial and antifungal activities of essential oils comes from in vitro tests, although case reports and clinical trials are scattered throughout the literature (see below). A review of the published in vitro work between 1976 and 1986 found that results were difficult to compare.111 Test methods used differed widely and important factors influencing results were frequently neglected. One of these factors was the composition of the essential oil being tested (given the existence of chemotypes and adulteration). This was highlighted in another study of commercial essential oils that found a wide variation in the antimicrobial activities of commercial samples of thyme oil, eucalyptus oil and geranium oil, among others.108
A review of more recent investigations summarised the antibacterial and antifungal activity data from around 50 studies.112 Most tests assessed either MICs or the concentration required to kill 50% of the test micro-organism culture (EC50). Many of the reviewed publications tested a range of oils and essential oil components. Wide variations in activity, depending on the test method, the essential oil tested and the test organism were tabulated.
Of 53 essential oils tested against four organisms, only a few oils exhibited remarkable activity, particularly thyme and origanum.113Pseudomonas aeruginosa was the least susceptible organism and Candida albicans the most susceptible. These oils stand out because they contain the highly active phenols thymol and carvacrol.114
Attempts have been made to identify the mechanisms behind the antifungal and antibacterial activities of essential oils and the key components responsible for this activity. Antimicrobial activity was said to parallel cytotoxic activity, suggesting a common mode of action, most probably exerted by membrane-associated reactions.115 In simple terms, it appears that the mobile and lipophilic nature of essential oil components, especially the monoterpenes, enables them to penetrate and disrupt cell membranes. Concentrations of tea tree oil that inhibit or decrease growth of Escherichia coli also inhibit glucose-dependent respiration and stimulate the leakage of intracellular potassium.116 According to a recent review, essential oils seem to have no specific cellular targets because of their great number of constituents.112 This suggests a very low risk of the development of microbial resistance against essential oils. Being lipophilic (fat-soluble) they pass through the cell wall and membranes, disrupting their structures. In bacteria, permeabilisation of the membranes is associated with the loss of ions and a reduction of membrane potential, collapse of the proton pump and depletion of the ATP pool. Essential oils can also coagulate the cytoplasm and damage lipids and proteins. Damage to the cell wall and membrane can lead to the leakage of macromolecules and eventually to lysis.
In eukaryotic cells, essential oils can provoke depolarisation of mitochondrial membranes by decreasing the membrane potential, impact ionic calcium cycling and other ionic channels and reducing the pH gradient, affecting the proton pump and the ATP pool. Chain reactions from the cell wall or membrane invade the whole cell, leading to widespread oxidative damage.112
Of five components tested for antibacterial activity, cinnamic aldehyde was the most active, followed by citral, geraniol, eugenol and menthol.117 Essential oils with high concentrations of thymol and carvacrol usually inhibit Gram-positive bacteria better than Gram-negative bacteria,118 although they still possess a good broad-spectrum activity. In another study, linalool was the most active antibacterial agent and citral and geraniol were the most effective antifungal agents.119 Essential oils with a high monoterpene hydrocarbon level were very active against bacteria but not against fungi, with the exception of dill.108 There was a negative correlation observed between cineole content and antifungal activity. In the case of tea tree oil, terpinen-4-ol was identified as the most important antimicrobial compound120,121 and cineole detracted from its antifungal activity.121
Recent research has highlighted the significant in vitro antiviral activity of essential oils.122 Most of the studies have been conducted against herpes simplex virus (HSV)-1 and HSV-2, with quite potent activities (at the parts-per-million level) being observed. A viral envelope is necessary, as growth of non-enveloped (naked) viruses is not affected by essential oils. They appear to act on enveloped viruses by affecting the viability of the free virus (virion), probably by interfering with the viral envelope (which like the cell membrane is lipid in nature).122
Clinical studies of the antimicrobial activity of essential oils have been published, with a focus on Australian tea tree oil used only topically. In an early double blind trial, 10% tea tree oil cream reduced symptoms of tinea pedis but was not effective in eradicating the fungus.123 However, a 5% tea tree oil gel was as effective as a 5% benzoyl peroxide lotion in the treatment of acne and patients experienced few side effects.124
A 2006 review of tea tree oil included published clinical trials.125 In addition to the trials summarised above, tea tree oil demonstrated efficacy as an antibacterial mouthwash or gel in three trials (with a residual effect on oral bacteria), reduced methicillin-resistant Staphylococcus aureus (MRSA) carriage, and successfully treated fungal infections of the nails, mouth and skin. Since then, topical tea tree oil has demonstrated clinical efficacy in another randomised, double blind trial assessing its value in acne patients126 and improved healing in recurrent herpes labialis after application of a 6% gel (although the results did not achieve statistical significance in this small trial).127 Tea tree oil as a topical application is also quite effective for head lice infestation.128,129
One review noted that other essential oils show bactericidal activity against oral and dental pathogenic micro-organisms and have been incorporated into mouth rinses.118
Essential oils certainly have a promising role in the management of intestinal pathogens, bacterial or otherwise. In an open label trial, oil of oregano was orally administered to 14 adult patients whose stools tested positive for the enteric parasites Blastocystis hominis, Entamoeba hartmanni and Endolimax nana.130 After 6 weeks of 600 mg/day of emulsified oil, there was a substantial reduction in detectable pathogens that correlated somewhat with an improvement in gastrointestinal symptoms. The clinical anthelmintic action of the (toxic) essential oil of wormseed (Chenopodium ambrosioides) is well documented, although this has been questioned.131
The spasmolytic activity of essential oils has been observed many times on isolated smooth muscle preparations and forms much of the basis of their use in functional gastrointestinal disorders.132 The effects of essential oils from 22 plants and some of their constituents on tracheal and ileal smooth muscle were investigated in one study.133 All of the oils had relaxant effects on tracheal smooth muscle, the most potent being angelica root, clove and elecampane root. Sixteen oils inhibited the phasic contractions of the ileal muscle preparation, the most potent being elecampane root, clove, thyme and lemon balm. Two oils (anise and fennel) increased the phasic contractions. Spasmolytic activity has also been confirmed clinically. For example, peppermint oil added to barium sulphate suspension significantly relieved colonic muscle spasm (p<0.001) during barium enema examination in a double blind, placebo-controlled study involving 141 patients.134 For more examples of the clinical spasmolytic activity of peppermint oil and its value in irritable bowel syndrome, see the peppermint monograph.
Carminatives relax sphincters and assist in the expulsion of intestinal gas. Their activity is somewhat related to spasmolytic activity. Certain essential oils or essential oil-containing herbs have been traditionally used as carminatives over many years. Oils of peppermint, sage and rosemary all relaxed Oddi’s sphincter, but peppermint was the most active.135 The carminative activity of cardamom and dill was confirmed in human studies.136 However, they were also shown to cause oesophageal reflux and should be used cautiously in susceptible patients.
Some essential oils are traditionally regarded as diuretics because they act as ‘kidney irritants’. The infusion and essential oil of juniper berries as well as terpinen-4-ol were tested for diuresis response in rats.137 On initial dosing, all three test substances exhibited an antidiuretic effect, but a significant diuretic effect was established on repeated doses, with the infusion having the strongest effect. The ‘irritant’ effect of juniper oil on the kidneys was also investigated, since there are concerns in the literature about its long-term use. No nephrotoxic effects were observed in an animal model and the authors suggested that provided high-quality oil is used (distilled from the ripe berries), concerns about the kidney irritant effects of juniper are unfounded.138 Essential oil terpenes used orally had shown some promise in dissolving small kidney stones in small, uncontrolled trials in adult patients, but these findings were not supported by controlled trials.139 However, a positive pilot trial in children suggests further investigations are desirable.
Certain essential oils (or the herbs that contain them) are used as expectorants. A proprietary product containing myrtle oil was popularly prescribed by doctors in Germany as an expectorant and mucolytic agent for acute and chronic bronchitis and sinusitis. The oil contains limonene, cineole and alpha-pinene. An expectorant activity for this oil was confirmed in a clinical trial in patients with chronic obstructive airways disease.140 Earlier animal experiments by Boyd suggested an expectorant activity for essential oils probably via influencing goblet cells to secrete more respiratory tract fluid (RTF) and mucus.141 In the 1940s, Boyd studied the effects of several essential oils in various experimental models.142,143 The most pronounced increase of RTF was seen after ingestion of oil of anise. Interestingly ingestion of oil of eucalyptus had a moderate effect that was not eliminated by cutting efferent gastric nerves. This finding supports the premise that essential oils do not act as reflex expectorants (see later).
One intriguing aspect of Boyd’s research is that he was able to demonstrate that inhaled essential oils also acted as expectorants. These results are summarised in Table 2.1.
Table 2.1 Expectorant effect of inhaled essential oils or their components
| Year of study | Expectorants studied | Results |
|---|---|---|
| 1968580 | Thuja oil | RTF markedly increased, as was the soluble mucin content of RTF. Effect was most marked in fall |
| Anise oil | No effect at normal doses | |
| Eucalyptus oil | No effect at normal doses | |
| 1969581 | Menthol | No change in amount of RTF, but its soluble mucin content was increased |
| Thymol | No effect at normal doses | |
| 1970582 | Lemon oil | RTF and its soluble mucin content increased |
| 1970583 | Nutmeg oil | RTF and its soluble mucin content moderately increased. Effect most pronounced in fall |
| 1970584 | Citral, geraniol | RTF and its soluble mucin content moderately increased |
RTF = respiratory tract fluid
The inhalation of essential oils into the lungs might exert a direct antimicrobial activity. A case report from Australia describes the successful use of inhaled eucalyptus oil to effect clinical improvement in a patient with tuberculosis.144 A 28-year-old woman presented with cough, shortness of breath, fever, night sweats, weight loss and malaise. She had been unwell for 12 months. A sputum sample was positive for Mycobacterium tuberculosis. X-ray of the chest confirmed the diagnosis of pulmonary tuberculosis.
A sedative activity following the ingestion of essential oils such as lavender is commonly recognised and was supported by early animal studies.145 More recently, a proprietary essential oil of lavender at 80 mg/day was found to be clinically effective in sub-threshold anxiety disorder. Three randomised, controlled, double blind clinical trials were identified in a review, demonstrating significant improvements in anxiety scores after 10 weeks of treatment with the lavender oil.146
Stimulant activity has also been attributed to some essential oils. Inhalation and oral doses of rosemary oil increased locomotor activity in mice.147 Infusions of essential oil-containing herbs are often taken as diaphoretics, especially during acute respiratory infections. In this context, it is interesting to note that a case report describes a patient who exhibited pronounced diaphoresis attributed to up to 10 cups a day of sassafras tea (not recommended because of potential carcinogenicity).148
Essential oils and essential oil components have been extensively studied in animal models for analgesic-like activity.149 Overall, 43 bioactive essential oil components were identified in one review, mainly monoterpenes. However, these findings are yet to translate into clinical applications, apart from topical use of peppermint oil or menthol (for example) in headache and joint pain (see the peppermint monograph). In contrast, the local anaesthetic activity of oil of cloves is well described and the local anaesthetic activity of lavender oil has been demonstrated in an in vivo model.150
Given their cytotoxic properties on bacterial and fungal cells, it is not surprising that essential oils have exhibited a wide range of antitumour activities against cancer cell lines.112,122 Essential oils exert cytotoxicity at levels considerably lower than their antibacterial activity (in the parts-per-million range). As in bacterial cells, the cell membrane is one of the sites of cytotoxic activity.122 This antitumour research is particularly well developed (at least at the in vitro level) for thymoquinone, a component of the essential oil from the seeds of the black cumin (Nigella sativa).151
For more information about the pharmacology of essential oils and associated anti-inflammatory, antiulcer, spasmolytic, oestrogenic, expectorant, antimicrobial and analgesic activities, see the monographs on chamomile, fennel, peppermint and thyme in Part Three.
Toxicology
Essential oils are highly concentrated from their original plant matrix and as a result can present specific safety issues when used as such. Their toxicology has already been comprehensively reviewed.152 Nevertheless, a few issues are worth noting here. Certain toxic essential oils, notably pennyroyal, tansy and parsley, have been used as abortifacients. However, as early as 1913 it was found that these oils have absolutely no specific or direct stimulating action on the uterine muscle.153 In fact, consistent with the known pharmacodynamics of essential oils, they were found to inhibit uterine contractions. Their abortifacient action was concluded to be due to general poisoning or gastrointestinal irritation, which makes their use not only uncertain, but also extremely dangerous.
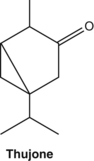
Thujone is a constituent of commonly used herbs such as wormwood, yarrow, thuja and sage. This compound is neurotoxic and its presence in liqueurs such as absinthe apparently caused widespread toxicity and abuse syndromes in the early 20th century.154 The first sign of toxicity is a headache. High and prolonged doses of the above herbs are hence best avoided, unless they are low-thujone varieties. It has been suggested that thujone intoxication may have played a part in Van Gogh’s style of painting.155 However, this has been recently challenged and it now appears that the syndrome known as absinthism is largely attributed to the toxic effects of ethanol.156 Safrole, a major component of sassafras oil, is carcinogenic and its use should be avoided.
Topical use of essential oils can cause contact dermatitis, as one review of tea tree oil cases noted.157 Oxidation, and hence the age of the oil, appears to increase the likelihood of a reaction.
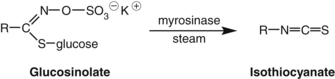
Glucosinolates and isothiocyanates
Pharmacodynamics
The main component of nasturtium oil (Tropaeolum majus) is benzyl isothiocyanate. This has potent antibacterial and antifungal activity. In Europe, enteric-coated capsules of the oil are used to treat bronchial and urinary tract infections.158 Horseradish preparations are used in the treatment of bronchial and sinus conditions. Presumably, the sulphur compounds confer a mucolytic effect. Recent research interest in benzyl isothiocyanate relates to its potential role in cancer.159
Isothiocyanates and some of their products (such as goitrin) are goitrogenic and interfere with the function of the thyroid gland.160 Goitrin inhibits iodine incorporation and the formation of thyroxin and its effects cannot be countered by iodine administration. Although this is a potentially toxic effect, it could possibly be exploited in cases of patients with hyperthyroidism.
The bulk of recent research attention on glucosinolates and their various transformation products (including sulforaphane, indole-3-carbinol and di-indoylmethane) has focused on their potential to prevent and modify cancer. This phenomenon has been known for more than 30 years. The anticarcinogenicity of these compounds, specifically in relation to brassica vegetables, has been recently reviewed for in vitro assays, animal experiments and various human studies.161–163 Alterations in phase I, and particularly phase II, detoxification enzymes are suggested as mechanisms by which these plant constituents might inhibit chemical carcinogenesis or alter the level of cancer promoting hormones such as oestrogen. On the other hand, possible mutagenicity, tumour promotion and carcinogenicity are also discussed, indicating that caution should be exercised if recommending long-term intake of these compounds at doses well in excess of optimum dietary levels.
The following brief review of the data, especially for broccoli sprouts, is provided as a good example of the progress in this field of research. Epidemiological evidence suggests that diets containing vegetables from the Brassica genus (including broccoli, cabbage, Brussels sprouts and cauliflower) are associated with a reduced cancer risk. After reviewing the results of 7 cohort studies and 87 case-control studies, it was concluded that the association appears to be most consistent for lung, stomach, colon and rectal cancer.164 A series of studies to find the active constituents and mechanisms of action began in the mid-1990s. As a result of this research, Brassica vegetables were found to be rich sources of inducers of phase II detoxification enzymes in vitro, with the isothiocyanate sulforaphane the main phase II inducer in broccoli.165
As noted above, glucosinolates in Brassica vegetables are broken down to form isothiocyanates (the aglycones) by the action of the enzyme myrosinase. This occurs when the plant is crushed, but also during cooking or in the human intestine by intestinal flora.166 Glucoraphanin is the glucosinolate precursor of sulforaphane. Broccoli sprouts contain about 15 times more glucoraphanin than the mature broccoli plant.167
Sulforaphane was found to be a potent in vitro inducer of the phase II enzymes quinone reductase (QR) and glutathione S-transferase (GST).168 Sulforaphane also induces phase II enzymes in vivo. In mice treated orally with sulforaphane, QR and GST were increased in many organs, including the liver.168 It is a potent inducer of the multiorgan protector pathway and hence initiates the cytoplasmic transcription factor Nrf2 to migrate into the cell nucleus, where it activates the antioxidant response element, upregulating the production of antioxidant and phase II enzymes.
The induction of phase II enzymes is one of the main mechanisms by which sulforaphane exerts its chemopreventative activity. Orally administered sulforaphane has been shown to be protective against carcinogen-induced cancer at a variety of sites.169 Taken together these findings suggest that sulforaphane can inhibit the development of cancer during the initiation and post-initiation stages.169
The chemopreventative activity of sulforaphane probably involves many mechanisms. These include:169
• inhibition of phase I enzymes
• induction of phase II enzymes
• antioxidant functions through increased tissue glutathione levels
• apoptosis-inducing properties
• induction of cell cycle arrest
A randomised, placebo-controlled trial found that sulforaphane exerted a phase II inducing effect in participants who achieved a good bioavailability (that is, who were able to convert glucoraphanin to sulforaphane in their intestine). Two hundred healthy volunteers from a region in China with a high risk for development of hepatocellular carcinoma were enrolled. Urinary levels of the markers of two environmental carcinogens were assessed. A strong, highly significant inverse association was observed when the marker levels were compared with levels of dithiocarbamate excretion (a measure of the metabolism of glucoraphanin).170 Oral administration of a broccoli sprout homogenate over 3 days induced mucosal phase II enzyme expression in the upper airway of human volunteers.171
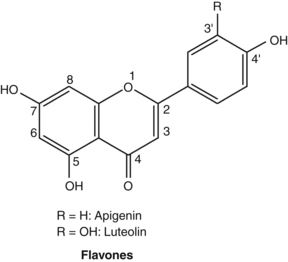
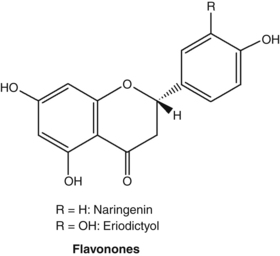
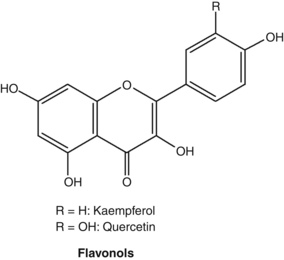
Flavonoids
Pharmacodynamics
Most of the studies conducted on flavonoids have used in vitro models, often employing isolated enzyme systems. The findings of these studies need to be interpreted with caution, since it is uncertain that oral doses of flavonoid glycosides or even their aglycones can reach sufficient concentrations in living organisms to reproduce these effects. This reservation applies even more for oral doses of herbs containing flavonoids, since the flavonoid dose will be commensurately lower in the plant matrix. The issue was well illustrated by one early clinical pharmacology study that found, while quercetin and apigenin inhibited platelet aggregation in vitro, no significant effect was found in human volunteers.172 Fortunately, clinical studies involving flavonoids (albeit at doses typically much higher than can be achieved by phytotherapy) and flavonoid-containing herbs are on the increase. (For a further discussion on the bioavailability of flavonoids, see the Pharmacokinetics section.) There are now tens of thousands of published studies on flavonoids. Hence, the brief section in this chapter serves only as a primer to this vast body of literature.
The original pharmacological interest in flavonoids arose during vitamin C research in the 1930s. Studies by Hungarian workers indicated that a number of vegetables and fruits (notably citrus) contained substances capable of correcting certain abnormalities associated with scurvy. In particular, this new factor, designated as vitamin P, corrected the capillary fragility associated with ascorbic acid deficiency. Vitamin P was subsequently found to be a mixture of flavonoids. Additional research disputed that vitamin P was essential to maintain human life and the term was dropped in the 1950s. However, research did confirm the therapeutic value of flavonoids for fragile capillaries (actually fragile connective tissue surrounding capillaries) and as extenders of vitamin C activity, possibly through improved absorption and protection from oxidation, and by partially substituting for some of its biological functions.173
A proprietary mixture of micronised flavonoids consisting of 90% diosmin and 10% hesperidin, usually administered at a dose of 1000 mg/day, has been investigated in several clinical trials. Such double blind, placebo-controlled trials have shown that this flavonoid combination improves venous tone in normal volunteers,174 enhances microcirculation in patients with venous insufficiency,175 assists healing of venous ulcers176 and relieves symptoms of acute haemorrhoids.177 A recent critical review confirmed the benefits of this combination in patients with advanced venous disease.178 A meta-analysis of clinical trials also supported its benefit in the treatment of haemorrhoids.179 Of course, this is more a ‘nutraceutical’ therapy and it is arguable whether these doses have any relevance to phytotherapy, as they could not be realistically achieved by the administration of plant extracts.
The in vitro antioxidant properties of flavonoids were the focus of much early research.180,181 The ability of flavonoids to complex pro-oxidant metallic ions such as iron probably augments their antioxidant effects in specific circumstances.182 Of particular interest is the capacity of flavonoids to inhibit macrophage-mediated oxidation of LDL and thereby attenuate atherogenesis.183 The antioxidant properties of flavonoids could also contribute to their observed anti-inflammatory and antiplatelet effects,184 and are related not only to their structural characteristics, but also to their ability to interact with and penetrate the lipid bilayers of the cell membrane.185 Flavonoids scavenge the nitric oxide radical,186 the superoxide anion187 and singlet oxygen.188 Like most other antioxidants, flavonoids can also act as pro-oxidants in particular circumstances.189
The molecular promiscuity conferred by the phenolic groups of flavonoids is well demonstrated by the range of enzymes inhibited by them in vitro. Enzyme activities inhibited to varying degrees by flavonoids in vitro include cyclo-oxygenase,190 lipoxygenase,190 lens aldose reductase,191 xanthine oxidase,192 cGMP phosphodiesterase,193 cAMP phosphodiesterase,194 angiotensin-converting enzyme,195 aromatase,196 thyroid peroxidase,197 hyaluronidase,180 phospholipases180 and protein phosphokinases.180 However, more research is needed to determine which of these activities might realistically translate into clinical effects. Since enzyme-inhibiting activity depends on the structure of the flavonoid, some compounds are more likely than others to be clinically active.
Using isolated cells or organs in vitro, the activities demonstrated for flavonoids include antiviral activity, especially for the 3-methoxylated flavones,181,195 anti-inflammatory activity,198 antimicrobial activity,195,199 inhibition of histamine release from mast cells,200,201 antiplatelet activity,195,202 tumour cell cytotoxicity, especially for highly methoxylated flavones,195 and spasmolytic activity.203,204 The flavonoid 8-isopentenylnaringenin (now 8-prenylnaringenin) isolated from the Thai herb Anaxagorea luzonensis was found to be an oestrogen agonist with an activity about 10 times greater than genistein.205 This flavonoid, which also occurs in hops (Humulus lupulus), has now been extensively studied as a phyto-oestrogen.206 It strongly activates oestrogen receptor alpha and at least partially accounts for the observed clinical value of hops in menopausal women experiencing mild vasomotor symptoms.207
Animal experiments (usually using high doses) have demonstrated a number of interesting pharmacological effects for flavonoids. One study found antioxidant effects in vivo for dietary flavonoids (quercetin and catechin), with reduced lipid peroxidation in rats.208 Flavonoids have also demonstrated hepatoprotective, antiulcer (gastroprotective)209 and analgesic activity in vivo.180 Several flavonoids tested showed anti-inflammatory effects in acute and chronic animal models.210,211 A favourable effect of quercitrin on experimentally induced diarrhoea was probably related to anti-inflammatory effects.212
Flavonoids have exhibited preventative activity against chemical carcinogens and tumour promotion in animal models,213,214 although most of the speculation over their cancer preventative role is based on in vitro studies.215 Anxiolytic properties have been demonstrated for some flavonoids (including chrysin and apigenin), which selectively bind with a high affinity to the central benzodiazepine receptor.216,217
The relationship between dietary flavonoid intake and cardiovascular disease was tested in several early epidemiological studies. The Zutphen Study in Holland found that flavonoid intake in elderly men (largely via tea, onions and apples) was significantly inversely associated with mortality and incidence of stroke.218 A Finnish study also found that people with very low intakes of flavonoids had a higher risk of coronary disease,219 but US studies found no significant association.220 These investigations are confounded by the difficulties in isolating effects due to just the flavonoids and the loose use of the term, as discussed above.221 This was well illustrated by another US study that found the observed non-significant inverse associations for broccoli, apples and tea on cardiovascular disease incidence in women were not mediated by flavonoids.222 The same researchers later detected no protection from dietary flavonoids on incidences of insulin resistance, type 2 diabetes223 and cancer,224 all from epidemiological investigations in women.
In another interesting Finnish study, the total dietary intakes of 10 054 men and women during the year preceding the baseline examination were determined with a dietary history method.225 Flavonoid intakes were estimated mainly on the basis of the flavonoid concentrations in Finnish foods. Participants with higher quercetin intakes exhibited lower mortality from ischaemic heart disease. The relative risk (RR) between the highest and lowest quartiles was 0.79 (95% CI: 0.63 to 0.99: p=0.02). The incidence of cerebrovascular disease was lower at higher kaempferol (0.70; p=0.003), naringenin (0.79; p=0.06) and hesperetin (0.80; p=0.008) intakes. Men with higher quercetin intakes had a lower lung cancer incidence (0.42; p=0.001), and men with higher myricetin intakes had a lower prostate cancer risk (0.43; p=0.002). Asthma incidence was lower at higher quercetin (0.76; p=0.005), naringenin (0.69; p=0.06) and hesperetin (0.64; p=0.03) intakes. A trend toward a reduction in risk of type 2 diabetes was associated with higher quercetin (0.81; p=0.07) and myricetin (0.79; p=0.07) ingestion.
Clinical studies of flavonoids (more as nutraceuticals than as herbal extracts) are yielding some interesting results. Citrus flavonoids have demonstrated promising results in patients with hypercholesterolaemia226 and in combination appear to exert significant anti-inflammatory activity in patients with osteoarthritis.227 Hesperidin, also from citrus, lowered diastolic blood pressure in a randomised, controlled trial.228 Flavopiridol, a flavonoid-derived drug, is being developed as a treatment for cancer and inflammation.229
The flavonoid most widely studied as a clinical nutraceutical is quercetin, and doses of around 500 to 1000 mg/day are typical. Generally, the clinical findings are modest, possibly reflecting on its low and variable bioavailability.230 Favourable effects were observed on endurance in a randomised, controlled trial,231 although other trials have not been positive.232–234 It did reduce blood pressure and oxidised LDL concentrations in overweight patients with a high cardiovascular disease risk phenotype at only 150 mg/day.235
Toxicology
Flavonoid aglycones, but not their glycosides, are mutagenic in various assay systems. Quercetin is probably the most mutagenic (and most widespread) flavonoid. When glycosides are incubated with beta-glucosidase or bacteria possessing this enzyme, they acquire mutagenic properties.236 However, the presence of methoxy groups markedly decreases the mutagenicity of the flavonoid.237 Concerns about the mutagenicity and carcinogenicity of quercetin first arose among Japanese researchers who were searching for the carcinogenic compounds in bracken fern. However, those compounds were later identified to be ptaquilosides.238 Dietary quercetin also enhanced pretumorous lesions in a model of rat pancreatic carcinogenesis, indicating possible promoting and progressing effects,239 but lacked tumour-promoting effects in a different model.240 On the other hand flavonoids, including quercetin, have shown antimutagenic effects and are widely considered to be antimutagens.241,242
With the initial discovery of the mutagenicity of quercetin and concerns about the carcinogenicity of bracken fern, tests were conducted to determine if quercetin was carcinogenic. While two studies were positive in rats, many other studies in rats, mice and golden hamsters failed to demonstrate carcinogenic activity.243 This is reassuring since quercetin is the most common flavonoid in the human diet. Proposed reasons for the lack of carcinogenicity included poor absorption and rapid microbial degradation, as already discussed.
However, the lack of carcinogenicity of a massive exposure to quercetin at 10% of diet suggests that more active mechanisms might be at work. This was confirmed in a study that demonstrated the rapid metabolic inactivation of quercetin by catechol-O-methyltransferase to form non-mutagenic methoxy groups on the flavonoid.243 This could be a major reason for the lack of carcinogenicity of other flavonoids in vivo. A more recent critical review of the data related to the safety of quercetin noted a lack of in vivo toxicity (including a lack of carcinogenic properties) and concluded that the weight of the available evidence supports the safety of this flavonoid.244
Tannins and oligomeric procyanidins
Unlike hydrolysable tannins, condensed tannins are polymeric flavans and are not readily hydrolysable. They often consist of molecules of catechin and epicatechin joined by carbon-carbon bonds. Hence catechin and epicatechin are referred to as monomers and molecules containing 2 to 4 monomers are referred to as oligomeric (a few) procyanidins (OPCs). Protein-binding capacity increases markedly with the degree of polymerisation, so dimers are much less astringent than hexamers, for example. It is, however, difficult to define the point at which OPCs end and true tannins start. From a pharmacological perspective, OPCs and their monomers behave much like flavonoids (they also resemble them chemically) and they are sometimes incorrectly classified with them. Condensed tannins are known as proanthocyanidins (procyanidins) because they release coloured cyanidin compounds on boiling with acid, hence the prefix ‘pro-‘ meaning ‘forming’.
The polyphenolics in green tea (Camellia sinensis) are a class of pseudotannins known as catechins. These are smaller molecules than tannins. While they do possess some astringency, they cannot tan hides, although they have been classified as condensed tannins in some publications. Green tea also contains condensed and hydrolysable tannins.245
Pharmacodynamics
Condensed tannins were able to bind to and inactivate the hypersecretory activity of cholera toxin.246 At present, there are attempts to develop new therapies for cholera, and one approach showing promise is the inhibition of transport proteins involved in cAMP-activated chloride channels. One of these is the cystic fibrosis transmembrane conductance regulator protein (CFTR). (Mutations in CFTR cause cystic fibrosis, hence its name.) A hydrolysable tannin from the Chinese gallnut inhibited CFTR in vitro. Intraluminal placement of this tannin (0.6 mg/kg) in mice reduced cholera-toxin-induced intestinal fluid secretion by 75%, with no effect on intestinal fluid absorption.247
Roots from the genus Potentilla typically contain hydrolysable and condensed tannins.248 In a randomised, double blind, placebo-controlled trial, an extract of tormentil root (Potentilla erecta) effectively treated rotavirus infection in children.249 The duration of diarrhoea in the herbal group was 3 days, compared with 5 days in the control group (p<0.0001).
Tannins in herbs such as meadowsweet were traditionally regarded as beneficial in mild peptic ulceration and inflammation. This application is analogous to the use of the synthetic antiulcer drug known as sulcrafate. Sulcrafate is an astringent aluminium-based compound (aluminium is highly astringent). It is said to bind selectively to the exposed proteins at the ulcer base. The barrier thus created protects the ulcer crater from gastric contents. Antiulcer activity has been demonstrated for black tea extract250 and for condensed tannins.251 Ellagic acid suppresses acid secretion252 and pomegranate prevented experimentally induced gastric ulcers.253
Tannins and pseudotannins can also affect bowel flora composition. For example, tea fed to chicks significantly changed the levels of particular microflora.254 This may explain why rhubarb and other tannin-containing herbs reduced levels of uraemic toxins in rats with renal failure (perhaps at least in part by inhibition of the bowel flora production of some of these compounds).255 Whatever the mechanism, tannin-containing herbs such as rhubarb have been used in China to treat renal failure.
Local use of tannins on bleeding surfaces renders a styptic or haemostatic effect due to localised vasoconstriction and possibly an increased rate of coagulation. Since the presence of tannins is widespread in higher plants, this would explain the folk use of so many different plants as ‘wound herbs’. An aqueous extract of a tannin-containing herb demonstrated haemostatic activity due to vasoconstriction and the formation of an ‘artificial clot’ (presumably resulting from a tannin-protein reaction), that tended to produce a mechanical plug to arrest bleeding from small blood vessels.256 This styptic effect can also be useful for mild internal bleeding.
The topical application of tannins can exert favourable effects on burns, weeping eczema and viral infections. In the early 20th century, tannin sprays were applied to severe burns as preferred therapy. The tannin-protein complex thus formed acted as an artificial semi-permeable membrane known as an eschar.257 This procedure was abandoned because toxic levels of tannic acid (the hydrolysable tannin used) were sometimes absorbed through the damaged skin. Tannic acid also damaged epithelial stem cells and caused excessive scar formation. However, the procedure is still practised in China using condensed tannins, which are less toxic and kinder to the regenerating epidermis.
One of the notable properties of tannins that has emerged in recent research is their antioxidant effects. Given their polyphenolic nature, this is not surprising. Hamamelitannin (from witchhazel) and gallic acid were more active than ascorbic acid in scavenging reactive oxygen species.258 Oral administration of geraniin was found to lower the level of lipid peroxide in the serum and liver of rats suffering liver injury.259 Most of the antioxidant research on tannins and pseudotannins has focused on green tea catechins. As well as impressive in vitro effects, it appears that an antioxidant activity can also be achieved in the human body, presumably brought about by the absorption of decomposition products as well as the catechins (see the Pharmacokinetics section).
A recent review classified the key antioxidant mechanisms of tannins as comprising free radical scavenging, chelation of transition metals and inhibition of pro-oxidative enzymes and lipid peroxidants.260 Conflicting results have been reported for the antioxidant activity of green tea in clinical studies. The results may have been confounded by how the tea was prepared (e.g. brewing time, concentration) and other dietary and lifestyle factors.261 A review of trials to 2000 found that antioxidant activity has been demonstrated, although a strong conclusion could not be drawn. In subsequent trials in healthy volunteers, green tea:
• decreased susceptibility of plasma and LDL to oxidation (using lag time of conjugated diene formation). SOD (superoxide dismutase) activity in plasma and serum antioxidant activity remained unchanged262
• was associated with a 37.4% reduction in the concentration of oxidised LDL, and a decrease in the levels of antioxidised LDL IgM antibodies263
• increased in plasma total antioxidant activity and decreased plasma peroxide levels and DNA oxidative damage in lymphocytes, compared to baseline values.264
There are many other clinical benefits demonstrated for green tea catechins.
Good clinical evidence exists for the role of cranberry juice and extracts in the prevention of urinary tract infections.265 It is believed that consumption of cranberry results in urine that discourages bacterial adherence to the bladder wall. The A-type proanthocyanidins (condensed tannins) are thought to be key here, although they might be too large to be excreted as such in the urine.266,267 Perhaps their decomposition products are active in urine?
As noted earlier, the pomegranate is a rich source of ellagitannins. Pomegranate extract and juice products standardised for their ellagic acid content are available (presumably the ellagic acid being measured is that contained in the ellagitannins). An initial uncontrolled clinical trial of pomegranate juice in patients with prostate cancer reported a significant prolongation of prostate specific antigen (PSA) doubling time. Anticancer effects in other types of cancers have been observed in vitro and in vivo.268,269 Pomegranate peel extract also promoted wound healing in rats after topical application, an effect certainly predicted by its tannin content.270
As alluded to previously, the many and impressive in vitro effects of tannins may not have clinical relevance because of their poor bioavailability. This is highlighted by a study that postulated hydrolysable tannins were responsible for the antiprostatic activity of Epilobium species via inhibition of 5-alpha-reductase.271 Sufficient quantities of almost any tannin will non-specifically inhibit almost any enzyme. Other effects of tannins unlikely to have clinical relevance include antiviral activity (except topically and in the gut lumen, see above),272 inhibition of elastase,273 cytotoxic effects,274 reverse transcriptase inhibition,259 antimutagenic activity,259 host-mediated antitumour activity259 and inhibition of lipoxygenase.259
The lower than expected rate of coronary artery disease in France has been termed the French paradox. Several reasons have been proposed for this effect, notably the high consumption of red wine rich in OPCs. Independently of this development, a French scientist named Masquelier researched the antioxidant and connective-tissue-stabilising benefits of OPCs over many years. This led to the development of two OPC products (containing predominantly B-type OPCs and their monomers), one from grapeseeds and the other from the bark of the maritime pine (Pinus pinaster). These products are prescribed for applications similar to flavonoids and early clinical research supported their use for varicose veins, capillary fragility and chronic venous insufficiency.275 An impressive array of outcomes from randomised, controlled trials has now accumulated, demonstrating the remarkable clinical diversity of these OPC-rich extracts. For example, a recent review of pine bark extract noted benefits in cardiovascular disorders, type 2 diabetes, cognition, asthma, osteoarthritis and glaucoma, among others.276 Given the postulated beneficial effects of OPCs in heart disease, it should come as no surprise that hawthorn, the most important herb for the heart in modern phytotherapy, is rich in these substances (for more details on the cardiovascular pharmacology of OPCs, see the hawthorn monograph).
Adverse reactions and toxicology
Chronic intake of tannins inhibits digestive enzymes, especially the membrane-bound enzymes on the small intestinal mucosa.277 Tannins complex metal ions and inhibit their absorption. One study found that as long as tea and iron are consumed separately, iron absorption is not affected.278 This iron-complexing property of tannins could be exploited in male patients with haemochromatosis, which is now recognised to be a relatively common disorder. (See Appendix C for more information on such potential interactions with tannin-containing herbs.) Tannins can also react with thiamine and decrease its absorption.279
Addition of tannic acid, a hydrolysable tannin, to the barium sulphate mixture used in barium enemas increases the yield and accuracy of the examination. The colonic mucosa stands out clearly and tumour visualisation is improved. However, the practice was banned in 1964 by the US FDA (Food and Drug Administration).280 Several deaths caused by acute hepatotoxicity, the majority in children, were attributed to this practice.281 In these cases, quantities of tannic acid sufficient to cause massive liver damage were absorbed directly into the bloodstream from the colon. This effect is highly unlikely to follow from use of tannin-containing herbs. Nonetheless, some unexplained cases of herbal hepatotoxicity have been recorded. It is therefore prudent to avoid the use of high doses of highly astringent herbs in patients with very damaged gastrointestinal tracts, other than in the circumstances outlined above. Green tea extract consumption has been linked to rare cases of idiosyncratic hepatotoxicity.282
Tannins are carcinogenic when injected subcutaneously283 and herbal teas containing tannins have been implicated in the possible development of oesophageal cancers.284,285 While these associations probably have little relevance to phytotherapy, they do suggest caution with the long-term oral and topical use (on damaged skin) of tannin-containing herbs.
Resins
Myrrh (Commiphora molmol) is an oleo-gum resin with astringent and antimicrobial properties. The former quality is probably entirely due to the resin and the latter is a combined effect from the resin and the essential oil. Tincture of myrrh is a potent antiseptic in the mouth and throat. There is a modern tradition that one of its effects is to provoke a local leucocytosis, to effectively stimulate defensive white blood cell responses and so involve the body in eliminating local infections. Clinical experience of long-standing improvement in recurrent throat and gum disease, for example, lends support to this view. In recent times, myrrh has also demonstrated clinical anthelmintic activity, possibly mediated by an effect on immune function. The resin could also play a role here (see the monograph in Part Three for more details).
Resins have also been applied to inflammatory conditions of the upper digestive tract with some benefit. This probably reflects on their astringent property. The oleoresin mastic (Pistacia lentiscus var. chia) is traditionally used for the relief of dyspepsia and peptic ulcers.286 Mastic demonstrated a duodenal ulcer healing effect at 1 g/day in a double blind, placebo-controlled clinical trial.286 Cytoprotective and mild antisecretory effects were demonstrated in a rat model of ulceration, consistent with astringent activity.287 Its effects on Helicobacter are controversial.288 Mastic improved dyspepsia symptoms in a randomised, double blind placebo-controlled trial.289 Boswellia (frankincense) is a resin with considerable anti-inflammatory activity in the digestive tract and elsewhere. (This is reviewed in its monograph in Part Three.)
Resins are contact allergens that can cause oral ulceration and contact dermatitis.290 Myrrh is particularly prone to this effect.
Bitters
Recent research has made considerable advances in our understanding of the bitter taste receptors. A family of approximately 30 such receptors (denoted TAS2R) has been identified in mammals.291 The TAS2Rs are broadly tuned to each detect multiple bitter substances, explaining how humans can recognise numerous bitter compounds with only a limited set of receptors. The TAS2Rs are expressed in a subset of taste receptor cells that are distinct from those mediating responses to other taste qualities. Cells with these receptors appear to be wired to elicit aversive behaviour, probably because many toxic chemicals are bitter in taste.291
One intriguing discovery is that bitter taste receptors are not restricted to the oral cavity.292 There are numerous reports of TAS2Rs expressed in the gut and in cell lines originating from gastrointestinal tissue, including the stomach. Insulin secretion can be stimulated from clonal pancreatic beta cells in vitro by a bitter molecule (denatonium). Gastric infusion of the same molecule (without it being tasted) delayed gastric emptying in rodents, presumably as a primordial defence against toxicity. There is also evidence of an elevated anion secretion within the large intestines of the rat and human induced by another bitter compound, 6-n-propyl-thiouracil (presumably against a defensive mechanism). An inactive variant of the TAS2R9 receptor has been associated with altered glucose homeostasis in a human family study.
Although direct humoral effects of bitter compounds in the gastrointestinal tract appear plausible, at least part of the bitter sensing mechanism seems to involve the activation of vagal nerve fibres. It was demonstrated that a mixture of bitter compounds administered intragastrically to mice led to an activation of brainstem neurons, the first relay station of taste information within the brain. This effect was abolished by cutting the vagus nerve, as well as inhibitors of cholecystokinin (CCK) and peptide YY (PYY) receptors. Since vagal nerve fibres contain receptors for these and terminate close to enteroendocrine cells, bitter compounds could stimulate the secretion of these peptide hormones.292 In other words, bitter taste receptors in the gastrointestinal tract appear to regulate metabolic and digestive functions.
Interesting as this may be, the story becomes even more fascinating with the discovery that TAS2Rs are also expressed on the human respiratory tract.293 It was demonstrated that bitter taste receptors are located in the nasal respiratory epithelium as well as in ciliated cells of lung epithelium, where they affect respiratory functions in response to noxious stimuli. Moreover, in the epithelium of the lower airways, a cell type of unknown function has been termed ‘brush cell’ because of an apical tuft of microvilli. Brush cells in the mouse trachea express bitter taste receptors and effect a reduction in respiration when stimulated with a bitter agent.294 Human airway smooth muscle cells also possess TAS2Rs, and their stimulation with bitter tastants effects a bronchodilation that is 3-fold greater than that elicited by beta-adrenergic receptor agonists.295 Inhaled bitter tastants decreased airway obstruction in a mouse model of asthma. Perhaps this provides a rational basis for the past use of asthma cigarettes containing bitter herbs?
The interaction of specific phytochemicals with specific human TAS2Rs (denoted as hTAS2Rs) is now reasonably well documented. For example, andrographolide interacts with hTAS2R50, strychnine with hTAS2R10 and the humulones from hops with hTAS2R10.296
Many cultures recognise the value of bitter substances in promoting digestive function and general health. In Holland, older people would celebrate the bitter hour in the early evening when they would partake of bitter food and drink to support their fading digestive powers. In India, it is said that those with liver problems seek bitter-tasting substances. In Africa, the medicinal value of bitter herbs, particularly as digestive stimulants, is commonly recognised in traditional medical systems.297 Bitter drinks taken before meals are still called aperitifs.
In the early 20th century, it was still widely accepted in medical and scientific circles that bitters promoted digestion. Even Pavlov was said to have acknowledged this connection.298 However, this was a time when such assumptions were being subjected to scientific scrutiny. In 1915, the American physiologist Carlson and co-workers published a study entitled ‘The Supposed Action of the Bitter Tonics on the Secretion of Gastric Juice in Man and Dog’.298 The group found that bitters either applied to the mouth or directly to the stomach, produced no change in the acidity and pepsin concentration of the gastric juice produced prior to food actually being in contact with the stomach. Despite the fact that this study had a number of methodological flaws, notably that gastric secretions were not tested under the stimulus of actual contact with food, it was largely interpreted as discrediting the concept of bitters as digestive stimulants.
However, work published also in 1915 by Moorhead, a colleague of Carlson, demonstrated a radically different activity profile for bitters.299 Moorhead found that a tincture of gentian (Gentiana lutea) given by mouth or directly into the stomach of cachectic dogs caused a marked increase in appetite. Also, only when gentian was given by mouth (tasted) did it cause a marked increase in gastric secretion and its acid and pepsin content. This effect only occurred after normal feeding and all the above effects were absent in normal animals.299
The conclusions that could be drawn from this early research are several:
• Bitters markedly increase appetite only if a cachectic, malnourished or debilitated state exists in the body.
• Similarly, bitters increase digestive power mainly when it is below optimum, as in a state of cachexia.
• Experiments with bitters should involve actual feeding; that is, the presence of food in the stomach is important for their activity.
• At normal doses, bitters act in the mouth; that is, tasting optimises their activity.
• an increase in gastric acid secretion
• an increase in pepsin secretion
Some more recent research supports this activity profile for bitters. Oral doses of liquid preparations of gentian and wormwood (Artemisia absinthium) were tested in human volunteers 5 minutes before a meal.300 Both bitter tonics stimulated gastric secretion. Gentian also stimulated bile release from the gallbladder and both herbs increased bile production by the liver. Another study found that oral doses of liquid wormwood caused a dramatic increase in the duodenal levels of pancreatic enzymes and bile.301 Studies have also shown that bitters increase the secretion of saliva. A lemon wedge saturated with Angostura bitters was also found to cure hiccups in 88% of subjects in an open trial.302
Some bitter herbs also appear to have a direct effect on the stomach, something that can now be reconciled with the discovery of bitter receptors further down the digestive tract. Wolf and Mack carried out an excellent study on the action of various bitters on the stomach of their famous patient, Tom, who had an occluded oesophagus and a gastric fistula.303 Bitters were administered by mouth and swallowed into the blind oesophagus; the resulting salivary volume and gastric secretion were compared with direct administration into the stomach. In the 96 experiments conducted, it was found that there was considerable variation in the effects of bitters. Surprisingly, golden seal (Hydrastis canadensis) was the most active herb and gentian was virtually inactive at the levels tested. The increase in salivation when the bitter was administered orally was usually comparable with the increase in gastric secretion after direct introduction into the stomach. The alcoholic content of the bitters was shown to evoke no response. It was concluded that bitters exerted a direct effect on the stomach, since no significant effect was observed in Tom’s stomach following their oral administration. These results therefore, contrast with the work of Moorhead, but the experimental design did not include a test meal, a common flaw in some of the early research.
A more recent publication also confirms that bitters do indeed exert a direct effect in the stomach.304 When isolated stomach cells were exposed to different levels of an extract of gentian root, a concentration-dependent rise in gastric acid production was observed. No stimulatory effect was exerted by globe artichoke extract (Cynara scolymus). Significant effects for gentian extract were observed at concentrations of 10 to 100 μg/mL. This concentration range can be readily achieved by normal doses of gentian. The author suggested that his results can explain why encapsulated gentian extracts also show therapeutic effects and downplayed the importance of the reflex effect to one of ‘supportive importance only’. While this is perhaps an over-extrapolation, the recent discovery of bitter receptors in the stomach makes sense of these findings.
Some support for this concept of direct activity in the stomach also comes from a multicentre, uncontrolled study of gentian capsules involving 205 patients.305 Patients took on average about five capsules per day, each containing 120 mg of a 5:1 dry extract of gentian root, and achieved rapid and dramatic relief of symptoms, including constipation, flatulence, appetite loss, vomiting, heartburn, abdominal pain and nausea.
Healthy upper digestive function is important for maintaining good health and preventing disease. Low acidity can lead to poor nutrient absorption and abnormal bowel flora. Patients with reduced gastric secretion are more susceptible to bacterial and parasitic enteric infections.306 The contribution of poor upper digestive function to the chronicity of intestinal dysbiosis is often overlooked by therapists.
Early studies associated low gastric acidity with a number of chronic diseases such as rosacea, gallbladder disease, eczema and asthma, and this has been reflected in writings that are more recent.307 The experimental method used in these early studies typically measured gastric pH following a test meal. This is now considered an invalid way to investigate a pathological hydrochloric acid deficiency (hypochlorhydria). Instead, a potent gastric cell stimulus such as pentagastrin is currently preferred to establish the diagnosis of hypochlorhydria.
As early as 1698, Floyer in his Treatise of the Asthma reflected that:
A modern study found that allergic asthma was associated with a reduction in histamine-stimulated peak acid output from the gastric mucosa (about 60% of normal). There is therefore a depression of gastric H2 histamine receptor function in asthma.308
Diabetics respond well to bitters, and some herbalists believe they can assist in normalising blood sugar levels in both reactive dysglycaemia and diabetes. A lack of insulin could impair the vagal stimulation of gastric acid secretion309 and oral doses of a bitter herb lowered blood sugar in healthy rats.310 Long-standing diabetics may have impaired upper digestive function secondary to vagal neuropathy.311 The finding that bitter receptors further down the digestive tract appear to be involved will hopefully encourage more research into the role of bitter herbs in insulin resistance.
It has been found that in some cases patients’ responses to herbal medicines depend on their upper digestive function.312 A Japanese study observed that the antioxidant properties of a herbal product could be increased by fermentation. A brewing process degraded high molecular weight polymers to smaller molecules that were more active. The unfermented herbal product was tried on patients with autoimmune diseases. Some responded to the product, others did not. Those who responded had a greater capacity to produce the small antioxidant molecules in their gastric secretions, which was correlated with their acid and pepsin secretion. It was concluded that one of the factors determining the patients’ response was the ability of their digestive system to produce low molecular weight compounds from the natural polymers.
Herbalists consider that bitters have a tonic effect on the body and the term ‘bitter tonic’ is often used. In addition to their use for poor upper digestive function, low appetite and hypochlorhydria and its consequences, bitters are used to treat anaemia. Bitters are valuable for food allergies and intolerances, since poorly digested proteins and other compounds probably contribute to this condition. Herbalists also believe that bitters stimulate immune function and a patient who is pale, lethargic and prone to infections is a prime candidate for bitters. Interestingly, the herb wormwood has been shown to be beneficial for Crohn’s disease, an autoimmune disease of the digestive tract, in two clinical trials.313,314 Whether this is a consequence of interaction with TAS2Rs remains to be investigated.
The famous herbalist Dr Weiss stressed that the action of bitters was most pronounced after continued use (probably because it is a conditioned reflex).315 He described how a physician in Vienna noted that dyspeptic patients liked wormwood tea and kept asking for it, despite the taste. Another Viennese paediatrician considered bitters to be an excellent remedy for anorexic children. Weiss claimed that bitters neutralised the negative influence of higher mental functions on digestion, which usually results from chronic stress. He claimed that bitters had a toning effect on the colon when applied over a long period. Interestingly, non-tasters with an inactive hTAS2R38 receptor were found to have an increased risk of colon cancer in one epidemiological study.316
A number of papers have been published on the subject of supertasters.317 Supertasters perceive the greatest bitterness and sweetness from many stimuli, as well as the greatest oral burn from alcohol and capsaicin (from Capsicum species). This is an inherited ability produced by a dominant allele. Women are more likely than men to be supertasters. This phenomenon needs to be kept in mind because some patients may be very sensitive to the taste (and possibly effects) of bitter and other strong-tasting herbs. These patients are also more likely to experience nausea if the dose of bitters used is too high.
In epidemiological studies, functional variants in bitter taste receptors have been linked to alcohol dependency,318 adiposity,319 eating behaviour disinhibition320 and body-mass index.321 Generally, people with lower bitter tasting sensitivity exhibited the poorer health measure.
Pungent constituents
Like bitters, pungency is a physiological classification rather than a phytochemical one. The three most commonly used hot spices are the cayenne pepper (Capsicum), the black pepper and ginger, and while their pungent components (respectively capsaicin, piperine and the gingerols and shogaols) are chemically distinct, it is now known that they act upon a common group of nerve cell receptors: the vanilloid receptors.322 Capsaicin and piperine are alkaloids based on homovanillic acid (hence vanilloid receptor), whereas the gingerols are substituted alkylphenols.
The transient potential receptor vanilloid 1 (TRPV1) receptor is a non-selective cation channel activated by capsaicin.323 TRPV1 receptors are predominantly expressed in primary afferent fibres, which are peptidergic sensory neurons, such as the unmyelinated C-fibres. TRPV1 is a pro-inflammatory receptor due to its key role in neuropathic pain, joint inflammation and inflammatory bowel disease. However, it appears to also play a protective role in sepsis and is involved in the physiological regulation of urinary bladder function, thermoregulation, neurogenesis323 and the cough reflex.324 Many of these functions are reflected in the traditional uses of cayenne and the modern applications of capsaicin (see below). TRPV1 is also strongly activated by piperine325 and the gingerols.326
Since capsaicin-sensitive sensory nerves (rich in TRPV1) are densely distributed in the cardiovascular and gastrointestinal system, activation of TRPV1 either by endogenous ligands or by exogenous agonists has been repeatedly reported to exert hypotensive activity or protective effects against cardiac or gastrointestinal injury. This is via stimulation of the synthesis and release of multiple neurotransmitters such as calcitonin gene-related peptide and substance P.327 Therefore, TRPV1 is not only a prime target for the pharmacological control of pain, but also a useful target for drug development to treat various diseases including cardiovascular and gastrointestinal diseases. However, considering the contribution of TRPV1 to the development of inflammation in the gastrointestinal tract (as touched on below), the potential side effects of TRPV1 agonists cannot be ignored.
Pharmacodynamics
Capsaicin has been the most commonly studied of the pungent compounds. As mentioned above, the C-fibre sensory neurons, which release inflammatory neuropeptides such as substance P, mediate a wide variety of responses including neurogenic inflammation, thermoregulation and chemical-initiated pain. Capsaicin functions to activate and then, at higher doses and over time, desensitise this class of neurons. This latter response, by a process known as tachyphylaxis, provides the basis for the therapeutic interest in capsaicin. Capsaicin stimulates C-fibres by interacting with TRPV1 receptors.328 The intense sensation of pain and heat which is experienced after eating a hot curry is testimony to this C-fibre activation. But, as experienced curry eaters will testify, they can tolerate hotter and hotter food over time due to tachyphylaxis.
The desensitisation of C-fibres has value for pain relief in a number of chronically painful disorders. Early controlled clinical trials of the topical use of capsaicin cream demonstrated symptom relief in osteoarthritis,329 neuropathy330 and postherpetic neuralgia.331 Topical capsaicin was effective for painful skin disorders such as psoriasis and pruritus and possibly useful for neural dysfunction in the form of cluster headaches and phantom limb pain.332,333 Vasomotor rhinitis could also be alleviated by topical capsaicin.334
However, Cochrane reviews have demonstrated mixed outcomes for the topical application of capsaicin, being negative for pruritus,335 mildly positive for neuropathic pain336 and stating insufficient evidence to support intranasal application in allergic rhinitis.337 Four weeks of a 0.0125% capsaicin gel was an effective treatment for mild to moderate knee osteoarthritis in a recent placebo-controlled trial involving 100 patients,338 but its role in this disorder is controversial,339 despite a positive meta-analysis (yielding moderate evidence of benefit).340
The higher fibrinolytic activity observed in Thai people has been attributed to daily intake of cayenne pepper.341 Cayenne also increases gastric acid output.342 Since gastric acid is a natural defence against gastrointestinal pathogens, it can be speculated that this could explain the preference for hot, spicy food in tropical countries. In the rat stomach there is clear evidence that capsaicin-sensitive sensory nerves are involved in a local defence mechanism against gastric ulcer. However, excessive capsaicin exposure caused tachyphylaxis and impaired this defensive mechanism.343 Perhaps the maxim of ‘too much of a good thing’ applies here.
Like capsaicin, piperine has attracted research interest, but for quite different reasons. Attention has focused on the capacity of piperine to enhance the bioavailability of other agents. These include aflatoxin B1 in rats344 and propanolol, theophylline,345 curcumin,346 vasicine and sparteine in humans.347 While there is good evidence that piperine inhibits drug metabolism by the intestine and liver,348 other possibilities include increased permeability of intestinal cells349 and even complexation with drugs.350 It strongly inhibits hepatic and intestinal aryl hydrocarbon hydroxylase and UDP-glucuronyl transferase.351 Hence, piperine also has the potential to induce herb-drug interactions.
In traditional Chinese medicine, a mixture of radish and pepper is used to treat epilepsy. Piperine and some synthetic derivatives have been shown to be anticonvulsant agents that can antagonise convulsions induced by physical and chemical methods.352 Antiepilepsirine, a derivative of piperine, is used as an antiepileptic drug in China.
Hot spices are used around the world for their general warming effects on the body, in modern terms stimulating circulatory activity. A mechanism for this universal experience is suggested. In a study that compared the effects of various pungent agents, an increase in catecholamine secretion, especially adrenaline, from the adrenal medulla was observed. Capsaicin was most active, but piperine and zingerone (from ginger) also showed activity, although allylisothiocyanate (from mustard) and diallyldisulphide (from garlic) did not have this effect. The effective principles were readily transported from the gut around the body.353 Capsaicin is known to increase energy expenditure in the body and boost basal metabolic rate, which has implications for its use in weight control.354
Epidemiological data reveal that the consumption of foods containing capsaicin is associated with a lower prevalence of obesity. Rural Thai people consume diets containing 0.014% capsaicin.355 Rodents fed a diet containing 0.014% capsaicin showed no change in caloric intake, but demonstrated a significant 24% reduction in visceral fat weight.
The above findings hark back to the use of cayenne promoted by Samuel Thomson, who regarded it as a life-promoting heating herb and a general metabolic stimulant. The physiomedicalists extended this concept and proposed that cayenne administered in conjunction with another herb would augment the particular stimulatory activity of that herb. Given what we now know about the bioavailability effects of piperine (and therefore possibly capsaicin), this apparently arcane dictum may have a rational basis.
A discussion of the pharmacology of the gingerols is included in the ginger monograph.
Toxicology
Investigations have been conducted to determine the potential mutagenic and carcinogenic activity of capsaicin and cayenne, but findings are contradictory.356 Results indicate that capsaicin also demonstrates chemoprotective activity against some chemical carcinogens and mutagens.357 Piperine appears to lack mutagenic activity.358 A review of the cayenne and capsaicin toxicity data did not find any reasons for concern.359
Saponins
Pharmacodynamics
A common misconception is that the haemolytic activity of saponins is related to their detergent-like characteristics. However, as early as the 1960s this was shown to be false. The mechanism of saponin-induced haemolysis was investigated by extracting the active haemolysing factor from ghost cells of saponin-haemolysed blood. The fact that only the corresponding aglycones could be extracted shows that hydrolysis of the glycosidic bond precedes haemolysis (RBC membranes possess a beta-glucosidase). The lack of haemolytic activity of a saponin was either due to the fact that it could not be hydrolysed by the beta-glucosidase or that it could not adsorb onto the RBC membrane.360 This is further supported by the observation that some sapogenins are also quite haemolytic (for example, glycyrrhetinic acid is actually more haemolytic than glycyrrhizin)360 and that haemolysis by saponins is inhibited by aldonolactones, which are glycosidase inhibitors.361
Saponins are more or less irritant to gastrointestinal mucous membranes (whether this is related to their detergent or haemolytic properties is not understood). This irritant property creates an acrid sensation in the throat when a saponin-containing herb is chewed. One resultant effect, like the emetics, may be by upper gastrointestinal irritation to induce a reflex expectoration. Certainly many of the traditional expectorant herbs such as soapbark, senega, primrose root and ivy leaf are rich in acrid saponins. This reflex expectorant effect, and its relationship to emesis, has been demonstrated in animal models.362 Presumably, it is mediated via the vagus nerve, as it was abolished when the afferent gastric nerves were cut.
However, this phenomenon for kava does not necessarily apply to other herbs. The influence of saponins on the water solubility of compounds having poor aqueous solubility was investigated with the model compounds digitoxin, rutin and aesculin.363 The saponins that were tested represented the most common structural types. Only slight effects on aqueous solubility were obtained for all the model compounds. These effects include both enhancement and reduction in aqueous solubility, depending on the saponin, the concentration of the saponin solution and the model compound. Hence, saponins generally should not be regarded as solubilisers.
Early research suggested that the incorporation of saponins into the cell membrane probably forms a structure that is more permeable than the original membrane.364 Saponins readily increase the permeability of the mammalian small intestine in vitro, leading to the increased uptake of otherwise poorly permeable substances and a loss of normal function.365 The disruptive effect of saponins on the architecture of the enterocyte cell membrane can lead to the impaired absorption of smaller nutrient molecules, which are otherwise rapidly absorbed via specific transporters. This appears to be the case for glucose and ethanol, based on in vitro models.366,367 Tablets containing an extract of the Ayurvedic herb Gymnema sylvestre are sold in Japan as a weight loss agent, which is attributed to reduced glucose absorption rates. Results from one study suggest that the saponin-rich herb Asparagus racemosus can act as a transdermal permeation enhancer for larger molecules.368
Sapogenins, both steroidal and triterpenoid, resemble cholesterol in their structure and may have a profound effect on cholesterol metabolism in the liver. Diosgenin is well studied in this regard. It interferes with the absorption of cholesterol of both dietary and endogenous origin; such interference is accompanied by increased rates of hepatic and intestinal cholesterol synthesis. Diosgenin also markedly enhanced cholesterol secretion into bile which, in conjunction with the unabsorbed cholesterol, resulted in increased faecal excretion of cholesterol (neutral sterols) without affecting excretion of bile acids.369 Higher levels of ingestion of saponins or sapogenins lead to cholestasis and jaundice associated with the presence of cholesterol-like crystals in hepatocytes. In grazing animals, this can in turn lead to photosensitivity due to the photosensitising effect of the high plasma levels of chlorophyll metabolites resulting from impaired biliary excretion (see the Tribulus monograph).
It is well established that saponin intake lowers plasma cholesterol levels in animal models.370 Various mechanisms have been put forward, such as increased faecal excretion of bile salts370 and loss of cholesterol via exfoliated mucosal cells,365 but the mechanism suggested by the above research on diosgenin seems most likely.369 In this context, it is pertinent to note that diosgenin (a sapogenin) inhibits cholesterol absorption, suggesting that the mechanism behind this phenomenon might have more to do with the haemolytic or steroid-like nature of the saponins rather than their detergent properties. As an interesting footnote, it has been suggested that the Masai, who consume 2000 mg of cholesterol per day yet maintain very low blood levels, might achieve this feat by their high consumption of saponins from both foods and medicines.371 To date this potential for saponin-containing herbs has not really been exploited clinically.
A recent review surveyed research of the effects of saponins on microbial populations and fermentation in ruminants.372 The primary effect of saponins in the rumen appears to be inhibition of protozoa (defaunation), which might increase the efficiency of microbial protein synthesis and protein flow to the duodenum. Furthermore, saponins may decrease methane production via defaunation and/or directly by decreasing the activities and numbers of methanogens. Saponins may also selectively affect specific rumen bacteria and fungi, which may alter the rumen metabolism. The effects of saponins on rumen fermentation have not been found to be consistent. These discrepancies appear to be related to the chemical structure and dosage of saponins, diet composition, microbial community and adaptation of microbiota to saponins. The clinical effects of saponins on human bowel flora have not yet been adequately explored.
One of the most interesting effects of saponins (or sapogenins) that follows from their ingestion is their capacity to interact with and influence steroid hormone metabolism. Much of this is covered in the monographs, but some of the principles behind this are worth highlighting here. The role of enzymes that metabolise steroid hormones in the regulation of the activity of these hormones has only been relatively recently appreciated. The most pertinent example for phytotherapy is 11-beta-hydroxysteroid dehydrogenase. This enzyme regulates glucocorticoid action by catalysing the interconversion of hydrocortisone (cortisol) and cortisone, an inactive steroid. In the kidney, hydrocortisone is oxidised to cortisone by the type 2 form of this enzyme, a reaction that prevents circulating hydrocortisone from occupying kidney mineralocorticoid receptors and stimulating a mineralocorticoid response. Aldosterone, being inert to 11-beta-hydroxy-steroid dehydrogenase (11beta-HSD), is then available to regulate mineralocorticoid responsive genes in the kidney on its own. Inhibition of 11beta-HSD in the kidney allows hydrocortisone to exert an additional aldosterone-like effect. This is exactly what licorice does.7
The influence of steroidal saponins on oestrogen metabolism may share similar aspects, but perhaps with some noteworthy differences. This is illustrated by research on Tribulus terrestris. Saponins from Tribulus appear to increase FSH (follicle-stimulating hormone) in premenopausal women, which in turn increases levels of oestradiol.373 They may do this by binding with, but only weakly stimulating, hypothalamic oestrogen receptors, which are part of the negative feedback mechanism of oestrogen control. The weak stimulus (as opposed to the strong stimulus of oestrogen) leads the body to interpret that oestrogen levels are lower than they really are. Consequently, the body increases oestrogen production via the negative feedback mechanism. In the postmenopausal woman, herbs such as Tribulus, wild yam (Dioscorea villosa) and false unicorn (Chamaelirium luteum) appear to alleviate symptoms of oestrogen withdrawal. It is possible that the binding of plant steroids to vacant receptors in the hypothalamus (in this low-oestrogen situation) is sufficient to convince the body that more oestrogen is present in the bloodstream than actually is, and calms the body’s response to oestrogen withdrawal.
Claims have arisen in the popular literature that the female body can manufacture progesterone from diosgenin, particularly if a wild yam cream is applied to the skin. This is despite the fact that wild yam contains dioscin and other steroidal saponins, not diosgenin, and there is no information about the dermal absorption of these compounds. Furthermore, no evidence exists for mammalian enzymes that are capable of effecting what is a difficult chemical conversion. The evidence that does exist strongly disputes the possibility of this conversion.374 Hopefully, the above discussion demonstrates that the interaction of saponins with steroid hormones is far more subtle than this. In fact, diosgenin appears to have oestrogenic properties in mice and lacks progesterogenic effects.375
A review of the in vitro and in vivo effects of triterpenoid saponins has documented pharmacological properties such as antiallergic, antiatherosclerotic and antiplatelet, antibacterial, anticomplementary, antidiabetic, contraceptive, antifungal, anti-inflammatory, antileishmanial, antimalarial/antiplasmodial, anti-obesity, anti-proliferative, antipsoriatic, antispasmodic, antisweet, antiviral, cytotoxic/antitumor, gastroprotective, hepatoprotective, immunomodulatory, anti-osteoporotic and insulin-like.376
Adverse reactions and toxicology
Saponins are toxic to fish and other cold-blooded animals and have been used to kill snails that harbour the bilharzia parasite.377 However, normal intake of the majority of saponins is not toxic to humans, as evidenced by the fact that saponin intake by vegetarians is in the range of 100 to 200/day.370 Saponins are permitted as food additives in many countries (for example, to give a satisfactory head to beer), although there are inconsistencies in regulations.378 Long-term feeding of saponins to animals did not demonstrate any signs of toxicity.378
As noted previously, grazing animals that consume large amounts of saponins can develop cholestatic liver damage. In South Africa, consumption of Tribulus by sheep contributes to a bilirubin-induced disorder known as geeldikkop (see the Tribulus monograph for a more comprehensive discussion).379 While it is unlikely that normal human doses would cause cholestasis, this phenomenon should be considered in unexplained cases of this disorder in patients taking herbs. More importantly, saponin-containing herbs are best kept to a moderate level in patients with pre-existing cholestasis.
Cardiac glycosides
Pharmacodynamics
The properties of cardiac glycosides are very well documented. They inhibit the sodium-potassium cellular pump leading to a rise in intracellular calcium levels that increases the contractile force and speed of the heart muscle (positive inotropy). In patients with compromised cardiac function, this positive isotropic effect translates into increased cardiac output and associated events that flow from this central effect. Heart rate is decreased (negative chronotropy) via the autonomic nervous system. Digitalis glycosides in particular also cause electrophysiological changes in the heart: conduction velocity at the atrioventricular node is slowed, together with an increase in its refractory period (negative dromotropy). This last feature underlies the use of digitalis glycosides in supraventricular rhythm abnormalities such as atrial fibrillation.
It is beyond the scope of this chapter to discuss the pros and cons of digitalis therapy or the various adverse reactions and toxicity considerations associated with its use. Its medical use certainly appears to be in decline, although there is continued evidence of its frequent clinical utility.380 Phytotherapists find digitalis to be too powerful for comfort and prefer to see it used, if necessary, under appropriate specialist care (generally the legal restrictions on its use render it available only on a medical prescription).
In some countries, herbalists and physicians practising phytotherapy find value in the use of milder herbs containing cardiac glycosides, in particular lily of the valley (Convallaria majalis). Its properties are similar to those of digitalis, but much less cumulative. The principal glycoside is convallatoxin, but the plant contains many minor cardenolides. Convallatoxin is poorly absorbed as a pure compound but the other components in the herb are said to aid its absorption.381 It is unsuitable for manifest congestive heart failure, but combines well with hawthorn in the management of milder forms of this condition, for digitalis hypersensitivity or for heart failure associated with a low pulse rate.381 All the usual cautions governing the use of cardiac glycosides should be observed.
Recent reviews have highlighted the novel therapeutic applications of cardiac glycosides, in particular the accumulating in vitro and in vivo studies reporting antitumour activity.382,383 The first generation of glycoside-based anticancer drugs are in clinical trials.
Anthraquinones
Pharmacodynamics
The laxative effect on the gut is largely a local one; systemic absorption is limited. Two distinct mechanisms are in force: a modification of intestinal motility and an accumulation of fluid in the intestinal lumen.384 Experiments in animals and humans have shown that the introduction of anthrones into the colon quickly induces vigorous peristaltic movements.384 Such a fast response is certainly not a secondary effect resulting from increased faecal water. This effect on motility is at least in part due to the release of prostaglandins, since its action is abolished by indomethacin and other cyclo-oxygenase inhibitors.385 However, other research shows that inhibition of intestinal tone and segmentation (and therefore reduced colonic transit time) could be the primary motility effect.384
Accumulation of fluid in the colonic lumen also leads to a laxative effect. While it has been suggested that this might be due to the inhibitory effect of anthrones on the sodium pump,384 these agents have instead been shown to stimulate active chloride secretion into the lumen, which is then balanced by an increase in sodium and water flow.386 Prostaglandins may be involved in this process387 but not PAF.388 Alteration of calcium transport may also play a role.384
Natural anthraquinones in the form of chrysarobin have also been used topically in the treatment of psoriasis. Chrysarobin, a mixture of substances including chrysophanol, is obtained from araroba or goa powder. Araroba is extracted from cavities in the trunk of the South American tree Andira araroba.389 Dithranol (or anthralin), a cheaper synthetic analogue of chrysarobin, has been the focus of the most studies.390 Chrysarobin is an effective topical agent for psoriasis, as was demonstrated in an open comparative study with coal tar and ultraviolet radiation.391 However, it has a number of drawbacks: it is only stable in a greasy base and it irritates and stains the skin.
The antiproliferative action of dithranol, and presumably chrysarobin, is thought to be either through effects on DNA, probably mitochondrial DNA, which reduces cell turnover, or through activity on various vital enzyme systems.392 A possible relationship with reduction-oxidation potential has also been observed.393 Other research on chrysarobin has been concerned with its tumour-promoting activity.394 Not surprisingly, this finding has further reduced its use in therapy.
Hypericin and pseudohypericin are dianthrones (structurally related to anthraquinones) with antiviral activity. Several anthraquinone aglycones including rhein, alizarin and emodin have also demonstrated antiviral activity against human cytomegalovirus.395 This may explain the traditional use of applying the leaves of Cassia species to viral skin conditions.389 It is unlikely that systemic antiviral effects would follow from the ingestion of anthraquinones, due to their low bioavailability.
Rhein is an anthraquinone aglycone found in rhubarb that inhibits the activity of cytokines in models of osteoarthritis.396 This observation led to the development of diacerhein (diacetylrhein), a synthetic derivative with better bioavailability. In early clinical studies, oral diacerhein at 100 mg/day improved symptoms in patients with osteoarthritis.396 Diarrhoea was a common side effect, as might be expected. A later Cochrane review of seven controlled clinical trials concluded that there is ‘gold’ level evidence that diacerhein (diacerein) has a small consistent benefit in the improvement of pain in osteoarthritis.397
Madder root (Rubia tinctorum) contains a characteristic spectrum of intensely coloured anthraquinone glycosides such as glycosides of lucidin and alizarin. As well as being used as a vegetable dye and natural food colouring, madder has traditionally been employed for the prevention and treatment of kidney stones. The anthraquinones in madder can function as chelating agents for some metal ions, such as calcium and magnesium. With oral doses of glycosides and aglycones, a pronounced calcium complexing effect and a significant reduction in the growth rate of kidney stones was observed in an animal model.389 A therapeutic oral dose of madder root will colour the urine slightly pink, which indicates that significant quantities of anthraquinones are excreted in the urine. Its regular use is said to slowly dissolve kidney stones. Given the concerns (below) over the carcinogenicity of madder, perhaps other anthraquinones sharing this property could be explored for potential value as oral chelation therapy in patients with atherosclerotic lesions.
Adverse reactions and toxicology
Madder has been withdrawn from the German market due to concerns over its mutagenicity and potential carcinogenicity. In particular, rats metabolise alizarin glycosides to alizarin and 1-hydroxyanthraquinone, a known rodent carcinogen.398 One long-term feeding of madder root to mice failed to produce neoplastic lesions.399 However, studies that are more recent do tend to confirm carcinogenic activity.400
Anthraquinone laxatives may cause mild abdominal complaints such as cramps and abdominal pain and should be used cautiously in patients with these symptoms. Other side effects include discoloration of the urine and haemorrhoid congestion. Overdosage can result in diarrhoea and, coupled with prolonged use, may cause excessive loss of electrolytes, particularly potassium.384
Abuse of anthraquinone laxatives has been stated to cause damage to the myenteric plexus. However, results from animal studies and a controlled study in humans have challenged this finding.401 Habituation can occur with laxative abuse, primarily due to hyperaldosteronism. The evidence is that anthraquinone laxatives used sensibly are unlikely to cause habituation. In fact, in several studies senna has been claimed to have a re-educative function and helped to restore normal bowel function.402 For example, out of 210 patients in a psychiatric hospital, 44% were taking laxatives at the outset, but 3 months’ treatment with senna lowered this to 8%.403
Cathartic colon coupled with hypokalaemia (low serum potassium) is a characteristic finding associated with chronic laxative abuse (not just for anthraquinones). The cathartic colon is characterised by the existence of a segment of intestine (typically the ascending colon) that has become largely non-functioning. Cathartic colon presents as chronic diarrhoea resistant to therapy. This is probably a rare disorder and is often associated with psychological abnormalities.404 Cathartic colon can be reversed; a case was described of a 78-year-old woman with cathartic colon who, after treatment was switched to psyllium, demonstrated complete reversal of the condition after 4 months.405
Contraindications for anthraquinone laxatives include ileus from whatever cause. Use in pregnancy and lactation is controversial. In traditional Chinese medicine anthraquinone- containing herbs are contraindicated in pregnancy because they promote a downward movement of energy. Apart from this consideration, they are unlikely to cause adverse effects since their action in the gut is largely topical and systemic absorption is limited (see Pharmacokinetics). Results from several clinical studies on senna indicate that its normal use does not involve any increased risk for the pregnancy or the fetus.406 Moreover, clinical observations of infants and analytical studies of breast milk both lead to the conclusion that the treatment of lactating mothers with senna does not carry a risk of producing a laxative effect in the infant.407
Long-term use of anthraquinone laxatives leads to a condition known as melanosis (or pseudomelanosis) coli. This is a brown discoloration of the intestinal mucosa beginning at the ileocolonic junction and may extend to the rectum. This harmless pigmentation is not due to staining by the anthraquinones, but is instead a lipofuscin-like substance within the macrophages of the colonic mucosa.384 (Lipofuscin is a pigmented, peroxidised fatty acid residue found in many organs with advancing age.) However, the intrinsic colour of anthraquinones may play some part in the development of this pigment. It is generally accepted that melanosis is a benign, reversible condition.408,409
The mutagenicity of isolated anthraquinones has been extensively studied and established in several models, particularly microbial assays such as the Ames test.410 On the other hand, some studies involving both in vitro and in vivo models found an absence of mutagenicity for senna, sennosides and rhein.411 The same research group concluded that senna does not represent a genotoxic risk to humans when used periodically at normal therapeutic doses.412
Nonetheless, their putative mutagenicity has led to the investigation of carcinogenic effects associated with the use of anthraquinone laxatives. One review examined the relationship between anthraquinone laxatives and colorectal cancer.413 Danthrone (two studies) and 1-hydroxyanthraquinone (one study) were carcinogenic in rodent models. Three clinical studies did not show an association of colorectal cancer with laxative abuse, but these studies also included bulk laxatives. When melanosis coli was taken as an indicator of anthraquinone laxative use, a retrospective study of 3049 patients undergoing endoscopic diagnosis for suspected colorectal cancer found an association between anthraquinone use and colorectal adenomas.413 A prospective study by the same research group found that 18.6% of patients with colorectal carcinoma had evidence of anthraquinone use.413 From their data, they suggested that a relative risk of 3.04 could be calculated for colorectal cancer due to anthraquinone laxative abuse. However, these studies did not differentiate between natural and synthetic (danthrone) laxative use. This is significant, because relatively higher doses of danthrone are required for a laxative effect and, since it is not a glycoside, its systemic absorption is greater. It was later asserted that epidemiological studies do not give conclusive evidence for any association between anthraquinone use and colorectal cancer.384 Since then, a comprehensive review of the pharmacological and human data concluded that (1) there is no convincing evidence that the chronic use of senna can induce a structural and/or functional alteration of the enteric nerves or the smooth intestinal muscle, (2) there is no relation between long-term administration of a senna extract and the appearance of gastrointestinal tumours (or any other type) in rats, (3) senna is not carcinogenic in rats even after a 2-year daily dose of up to 300 mg/kg/day, and (4) the current evidence does not show that there is a genotoxic risk for patients who take laxatives containing senna extracts or sennosides.414
Coumarins
Phytochemistry
Coumarins owe their name to ‘coumarin’ which was the common name for the tonka bean (Dipteryx odorata), from which the simple compound coumarin was first isolated in 1820. Coumarins are benzo-alpha-pyrones (lactones of o-hydroxycinnamic acid) formed via the shikimic acid pathway. Except for a few rare cases, including coumarin itself which is unsubstituted, all plant coumarins contain hydroxy or methoxy groups in position 7. These substituted simple coumarins, such as scopoletin, aesculetin and umbelliferone, are common and widespread in higher plants and often occur as glycosides.
Simple coumarin has a pleasant vanilla-like odour. It is probably not present in the intact plant, but is rather formed by enzymatic activity from a glycoside of o-hydroxycinnamic acid (such as melilotoside) after harvesting and drying. This accounts for the odour of newly-mown hay, not present in the undamaged plant. Coumarin is used to perfume pipe tobacco and can be sometimes found as an adulterant in commercial vanilla flavouring.415
The widespread nature of coumarins means that they are consumed in the human diet, for example carrots, celery and parsnip.416 Coumarins are fluorescent compounds and this property is widely utilised in a number of biochemical techniques. Simple substituted coumarins are also used as pigments in sunscreens.
Pharmacodynamics
Dicoumarol is a potent anticoagulant compound formed from coumarin by bacterial action in spoiled sweet clover hay (Melilotus species). Its discovery led to the development of modern anticoagulant drugs. Dicoumarol and related anticoagulants are hydroxylated in the 4 position. This is deemed an essential requirement (among others) for powerful anticoagulant activity. All of the common plant coumarins are not substituted at this position and therefore lack significant clinical anticoagulant activity, although many do possess very limited activity when given to animals in high doses.417 Coumarin has anti-oedema, anti-inflammatory, immune-enhancing and anticancer activities which are more fully described in the Melilotus monograph.
Simple substituted coumarins such as scopoletin and umbelliferone have exhibited a diverse range of pharmacological activities. The spasmolytic activity of scopoletin is probably a major reason for the use of Viburnum species such as cramp bark and black haw for hypertension and dysmenorrhoea. Scopoletin and aesculetin were identified in black haw as having significant spasmolytic activity on guinea pig small intestine.418 Another research team working at the same time also identified scopoletin as a component of the Viburnums that exhibited uterine spasmolytic activity.419 It has been suggested that the spasmolytic activity of scopoletin might be due to a blockade of autonomic neurotransmitters.420
Further studies are required to establish if scopoletin, umbelliferone and related compounds possess clinically relevant anti-inflammatory and analgesic activities, but such activities have been established in animal studies.421 Inhibition of cyclo-oxygenase and 5-lipoxygenase may play a role.422 Aesculetin and umbelliferone are also strong xanthine oxidase inhibitors in vitro.423
Of eight simple coumarins tested, aesculetin was the most potent at protecting cells in vitro against oxidative damage.424 The coumarins all inhibited xanthine oxidase in vitro.424 Aesculetin possessed hypouricaemic activity in rats (100 mg/kg, ip) and mice (150 mg/kg, ip), but was devoid of xanthine oxidase inhibitory activity at these doses.425 Scopoletin (50 to 200 mg/kg, ip) was also hypouricaemic after induced elevation of uric acid in mice, but did not affect the serum uric acid level in normal mice.426 It exhibited both xanthine oxidase inhibition and uricosuric activity. However, the high doses of such phytochemicals given by injection probably have little relevance to phytotherapy.
Like coumarin, substituted coumarins may have a role to play in cancer prevention and treatment. Umbelliferone and scopoletin are antimutagenic427,428 but were much less protective than coumarin in one model of chemical carcinogenesis.429 Aesculetin exhibited considerably higher cytotoxic activity than coumarin in vitro on two tumour cell lines, but scopoletin was inactive.430 Umbelliferone has similar cytotoxic activity to coumarin.431 Two reviews have outlined the potential of simple natural coumarins in cancer therapy, but most of the data to date are in vitro.432,433
Other in vivo properties of simple coumarins include gastroprotective activity for aesculin,434 and antiasthmatic activity for umbelliferone,435 all in mice, together with antithyroid activity for scopoletin in hyperthyroid rats436 and antidiabetic activity for umbelliferone in diabetic rats.437
Furanocoumarins have a long history of therapeutic use in humans. More than 3000 years ago, it was recorded in both Egyptian and Ayurvedic medicine that the ingestion of herbs containing psoralens followed by exposure to sunlight could assist in the treatment of vitiligo, a skin condition characterised by a loss of pigmentation.438 This traditional knowledge was developed into a modern therapy in the 1940s when xanthotoxin (8-methoxypsoralen, 8-MOP) plus sunlight exposure was introduced as a therapy for vitiligo.439 The treatment was not very successful, mainly due to phototoxic side effects, and it was only when ultraviolet A light sources (UVA) became available that significant advances were made. UVA radiation is less energetic and therefore less damaging than ultraviolet B (UVB) radiation. It was demonstrated that oral 8-MOP and UVA were highly effective in the control of psoriasis and the malignant skin condition mycosis fungoides.439 The treatment was called PUVA (psoralen plus UVA) and the new field of photochemotherapy was initiated.
Bergapten (5-methoxypsoralen, 5-MOP) is a psoralen with superior therapeutic characteristics in PUVA. Furanocoumarins in conjunction with ultraviolet radiation stimulate melanogenesis (tanning) and cause antiproliferative effects, but they also initiate phototoxic erythema (inflammation). For 8-MOP, the therapeutic dose is similar to the dose that causes erythema. With the use of oral 5-MOP it is possible to obtain melanogenic doses of drug-UVA or drug-sunlight combinations without the development of phototoxicity. This provides a wider margin of safety and permits the induction of a tan (known as a PUVA tan) that is more effective than a normal tan in attenuating the damaging effects of UV exposure. Psoralens are now the basis of yet another new field of therapy: photochemoprotection.439
The use of 5-MOP has also provided a more effective treatment for vitiligo and yields fewer side effects in PUVA therapy of psoriasis.440 As a further development in photochemoprotection, studies have shown that this furanocoumarin, in conjunction with UVB sunscreens, provides a faster tan (without burning) that is more protective against the harmful effects of ultraviolet radiation441 and even chemical irritation.442 It should be noted that certain citrus oils are rich sources of bergapten (5-MOP) and are used in these tanning products in preference to the synthetic chemical.
PUVA therapy is also used for psoriasis,443 although there are concerns over increased skin cancer risk (see below).444
Furanocoumarins in conjunction with UV light kill bacteria and inactivate viruses.445,446 In addition, they may be responsible for the enhanced bioavailability that grapefruit juice affords to several pharmaceuticals (see the Pharmacokinetics section).
The decoction of the fruits of Ammi visnaga has been used since ancient times in Egypt as a spasmolytic for kidney stones and in the treatment of angina pectoris. The pyranocoumarin visnadin was isolated and exhibited positive inotropic and marked coronary vasodilatory activities. Visnadin was developed as a drug treatment for angina, possibly acting as a calcium channel blocker.447Ammi visnaga also contains the spasmolytic furanochromone khellin, adopted for the treatment of angina and asthma. Its use was discontinued because of side effects such as drowsiness, headache and nausea.
As further evidence that the archetypal plant constituents still provide much of the inspiration for the development of modern drugs, sodium cromoglycate was discovered in an attempt to find a derivative of khellin which was devoid of its side effects. Unlike khellin, the antiasthmatic activity of this drug is believed to result from increased stability of mast cells.448
Toxicology
Plant coumarins are incorrectly implicated as anticoagulant agents. This is a fundamental misunderstanding of the relevant pharmacology (see Chapter 5 and the Melilotus monograph). Although a case of increased haemorrhagic tendency was attributed to coumarin intake from tonka beans, sweet clover and woodruff, intake was excessive and there were a number of confounding factors.449
As noted above, the furanocoumarins are potent photosensitising agents. Chance exposure to these compounds in the field can lead to severe photodermatitis and blistering. The giant hogweed is one example of a plant that consistently causes this problem. Celery pickers can develop photodermatitis after handling celery infected with fungus, which induces celery to produce high levels of psoralens.450
Furanocoumarins are powerful mutagens in the presence of UVA and psoralens are light-activated carcinogens.450 In the presence of UV light, psoralens are capable of binding to and cross-linking DNA, which causes genetic damage and mutations. Only linear furanocoumarins (psoralens) are able to bind two DNA bases to cause cross-links. Non-linear furanocoumarins (isopsoralens) can only bind to one base to form monoadducts. Although concerns were expressed that the development of isopsoralens as photochemotherapeutic agents may increase carcinogenic risk because monoadducts are subject to error-prone DNA repair mechanisms,450 this does not appear to be the case.451
The use of UV filters decreases the photomutagenic and photocarcinogenic effects of psoralens, but the employment of 5-MOP in sun tanning lotions remains controversial.452,453 Given the advantages of a PUVA tan, it is likely that the benefits will outweigh risks, provided that UV exposure is carefully controlled. This may only occur under clinical supervision.
Use of PUVA therapy in psoriasis is postulated to be associated with an increased risk of skin cancer, particularly squamous cell carcinoma.454 Risk of developing skin cancer was claimed to be proportional to the number of treatments and the degree of exposure to UVA.454 However, these associations have been disputed by at least one study, which proposed that prior exposure to arsenic treatments or X-rays largely explained the higher incidence of skin cancer in PUVA-treated psoriasis patients.455 Significant exposure of the unprotected human eyes to PUVA may accelerate cataract formation, but this is readily alleviated by protective eyeglasses.454
Phyto-oestrogens
Pharmacodynamics
Interest in the oestrogenic effects of plants first arose in the scientific world when clover disease was identified in Australia in the 1940s. It was observed that infertility developed in sheep after grazing on various species of clover (Trifolium species). Research interest at the time focused on understanding the factors that caused clover disease, which were identified as isoflavone glycosides. It was several decades later, when results from epidemiological studies suggested that the dietary consumption of soya products might have a protective role in breast cancer, that interest in the oestrogenic properties of isoflavones was rekindled.456
Early studies using various animal tissues demonstrated that, while isoflavones compete strongly with oestradiol at oestrogen receptors, their stimulation of these receptors is much weaker than oestradiol.457 In other words, they were thought to act as partial oestrogen agonists that could function as oestrogen agonists or antagonists depending on the hormonal milieu. The theory was that in a high-oestrogen environment (such as in the premenopausal woman), their displacement of endogenous oestrogens is postulated to have an antioestrogenic effect. In contrast, in a lower oestrogen environment, as in the postmenopausal woman, they were expected to provide a net oestrogenic effect. While this theory still has some relevance, the situation is now known to be more complex.
There are two isoforms of the oestrogen receptor, ERalpha and ERbeta. Their distribution and density varies, depending on the target tissue. The existence of an oestrogen receptor was first reported in the early 1960s. In 1996, an additional ER was discovered. This receptor was designated ERbeta and consequently the originally discovered receptor was renamed ERalpha.458 Both ERalpha and ERbeta may coexist in a tissue and relevant proportions often vary. ERalpha is the dominant receptor in the adult uterus. ERbeta is expressed in high levels in prostate, salivary glands, testis, ovary, vascular endothelium, bone, smooth muscle, certain neurons in the central and peripheral nervous system and the immune system.459 Both isoforms are present in the female breast and reproductive tissue.460
Each of the ERs may influence the function of the other. The effects of substances that interact with receptors when both forms are present are complex.460 It is hypothesised that ERbeta may modulate, or even antagonise, the actions of ERalpha.461 For example, in the breast ERalpha and ERbeta exhibit opposing functions in cell proliferation: ERalpha promotes epithelial proliferation, whereas ERbeta has a restraining influence.462 Other studies suggest several possible interactions for the two receptors: antagonistic, synergistic and sequential.463
The female sex hormones (the oestrogens) consist of 17beta-oestradiol, oestrone and oestriol. Oestradiol binds to ERalpha and ERbeta with equal affinity.464 The isoflavone phyto-oestrogen genistein has greater binding affinity for ERbeta than for ERalpha. This preferential binding of genistein to ERbeta indicates such isoflavones probably produce pharmacological and clinical effects quite distinct from oestrogens. In particular, they tend to exhibit antiproliferative activity.464 Other isoflavone phyto-oestrogens also bind more strongly to ERbeta.465
Furthermore, isoflavones bind to ERs with a much lower affinity than oestrogens and therefore produce less potent responses.466,467 However, they initiate greater gene transcription of ERalpha compared to ERbeta.467
As noted above, isoflavones and other phyto-oestrogens are thought to compete with oestradiol for binding and activation of ERs,460 potentially decreasing the effect of oestradiol in vivo in some circumstances. However, there have been some in vitro results to the contrary.468 The effect depends on the doses (of both phyto-oestrogens and oestradiol). In a pilot study, a soy protein isolate containing isoflavones (120 mg/day) taken for 6 months did not prevent oestradiol-induced endometrial hyperplasia in postmenopausal women.469 (But interestingly, there was also no additive effect.)
Research on selective oestrogen receptor modulators (SERMs) such as tamoxifen has provided more insights into how non-steroidal molecules can interact with the oestrogen receptor. A summary of the knowledge thus far is that once a SERM binds to the ER it causes a change in its shape. This allows recruitment of co-activators if it is destined to elicit an oestrogenic response, or co-repressors if its response is anti-oestrogenic. The binding of the coregulatory molecules leads to the activation of the promoter sequence of the oestrogenic responsive gene. Isoflavones have been proposed as natural SERMs.465
Studies have found that isoflavones have both agonistic and antagonistic effects, though they are strong ERbeta agonists and weak ERalpha agonists. The presence of a correctly positioned phenolic ring and also the distance between the two opposing phenolic oxygens in the isoflavones structure is similar to that of 17-beta-oestradiol. This similarity allows the isoflavones to bind to the ER, effectively displacing 17-beta-oestradiol. This action may help explain how phyto-oestrogens help protect against breast cancer, because ERbeta inhibits mammary cell growth as well as the stimulatory effects of ERalpha.465 As with the SERMs, studies have shown that the recruitment of coregulatory molecules may be important in determining the function of phyto-oestrogens. In particular, isoflavones appear to trigger selectively ERbeta transcriptional pathways, especially transcriptional repression.465
The in vitro and in vivo studies on phyto-oestrogens can be divided into three general categories: chemoprevention, treatment effects and lifetime exposure studies (in vivo only).465 This literature is so vast that a thorough review is beyond the scope of this primer. However, one aspect worth emphasising is that isoflavones have been shown to prevent cancer or inhibit cancer cell lines in a variety of models.470,471 Another is that isoflavones exert favourable effects in vivo and in vitro on parameters relevant to cardiovascular risk including insulin resistance, lipid peroxidation, haemostasis and endothelial function.472 Probably of greater relevance are the many human studies of the impact of phyto-oestrogens on various health outcomes or parameters. Some of the key findings of these studies are featured below, in a brief outline of the reviews.
A meta-analysis concluded that soya isoflavone consumption was associated with a reduced risk of breast cancer, but this protective effect has only been observed in studies in Asian populations.473 Soya isoflavone intake was also inversely associated with the risk of breast cancer recurrence. Moderate consumption of isoflavones in diet does not increase the risk of breast cancer recurrence in Western women who have survived breast cancer, and Asian breast cancer survivors exhibit better prognosis if they continue consuming a soya diet.474
Results from Asian epidemiological studies suggest a beneficial impact of isoflavones on bone health.475 However, clinical trials have yielded conflicting results on bone mineral density and turnover markers that may reflect on differences in study parameters, type and dose of phyto-oestrogen used and the variable metabolism of isoflavones, especially in terms of equol production.475
A systematic review and meta-analysis of soya isoflavones versus placebo in the treatment of menopausal vasomotor symptoms concluded that there was a significant tendency in favour of soya.476 Another meta-analysis found that consumption of 30 mg/day of soya isoflavones (or at least 15 mg genistein) reduced menopausal hot flushes by up to 50%.477 Individual responses in menopause could be determined by bioavailability and metabolism, especially to equol.478
The published effects of isoflavones on the circulating hormones in pre and postmenopausal women were the subject of a systematic review and meta-analysis.479 In all, 47 studies were included. In premenopausal women, meta-analysis suggested that isoflavone consumption did not affect levels of oestradiol, oestrone or sex hormone binding globulin (SHBG), but did significantly reduce FSH and luteinising hormone (LH) (by about 20%). In postmenopausal women there was no impact of isoflavones on any of these hormones, although there was a 14% non-significant increase in oestradiol.
Epidemiological studies indicate that soya isoflavone consumption is possibly protective against prostate cancer.480 There is also some suggestion from clinical trials that isoflavones can slow the disease progression.481
Isoflavone intake appears to confer cardiovascular benefits that may or may not be related to their phyto-oestrogenic properties. One systematic review identified improvement in arterial stiffness482 and another used meta-analysis to establish a small reduction in blood pressure.483 Soya isoflavone intake improved flow-mediated dilation (an indicator of cardiovascular health) based on a meta-analysis of randomised, controlled clinical trials.484
Intestinal metabolism of isoflavones to their aglycone form is crucial for ensuring bioavailability and therapeutic activity. In other words, the intestinal microflora is pivotal in the metabolism of oestrogenic isoflavones. Individual differences in intestinal microflora have been proposed as a possible reason why there is some inconsistency in the clinical effects of isoflavones.485 In particular, daidzein is further metabolised by gut microflora to produce equol, a more active oestrogenic compound. There are large individual variances in the capacity to produce equol and hence possibly a therapeutic effect. Dietary fat consumption is known to reduce this capacity.486 On the other hand, dietary supplementation with fructo- oligosaccharides such as inulin is known to increase equol.487
In contrast to the isoflavones, research on the oestrogenic lignans enterolactone and enterodiol has not been as extensive. Initial interest in these compounds resulted from the observation in the early 1980s that their urinary levels in menstruating women exhibited a cyclic pattern during the menstrual cycle, with maximum excretion in the luteal phase.488 The relatively high concentrations of these new lignans in urine, their cyclic pattern of excretion and their increased excretion in early pregnancy suggested that they were a new class of human hormone. It transpired what had been found was a plant chemical modified by bacteria in the human digestive tract.488 Selective antibiotic administration to humans suppressed oestrogenic lignan formation.488
Enterolactone, like isoflavones, inhibits oestradiol-stimulated breast cancer cell growth in vitro.489 Lignan ingestion has been associated with increased concentrations of SHBG, but this was not confirmed in clinical studies.489–491 Linseed intake by normal premenopausal women was consistently associated with longer luteal phase lengths and higher ratios of luteal phase progesterone to oestradiol.489 These findings may reflect favourably on breast cancer risk. An earlier animal study found that linseed supplementation reduced early risk markers for breast cancer.492 Dietary studies and assays of urinary lignans in postmenopausal women have found that lignan excretion is significantly lower in the urine of women with breast cancer,493 although the situation is not as clear from recent prospective cohort studies.494
The following is obviously not a comprehensive review of the clinical data for linseed and its lignans and focuses on hormonal effects. Linseed (25 g/day) altered oestrogen metabolism more than soya (25 g/day), and significantly shifted oestrogen metabolites to less biologically active forms (2-hydroxyoestrone) in postmenopausal women.495 This was confirmed in other clinical trials at 10 g/d, in pre- and postmenopausal women.496,497 At 10 g/day linseed decreased endogenous oestrogen levels and increased prolactin in postmenopausal women.491 Linseed supplementation does not appear to benefit menopausal symptoms, although more studies are necessary.498
Adverse reactions and toxicology
Controversy has arisen over the possible adverse effects of soya-based infant milk formulas. One earlier study found that infant exposure to isoflavones from these products was relatively much greater than levels shown to alter reproductive hormones in adults.499 It was suggested that further studies of possible developmental effects are highly desirable.
On the other hand, studies in males have found no indication of adverse effects. For example, a 2010 review found no evidence from rodent studies and nine clinical trials that isoflavone intake impacted oestrogen levels in males. Clinical evidence also indicates no impact on sperm and semen parameters in men.500 In addition, a meta-analysis that included 15 placebo-controlled clinical trials found that neither soya nor isoflavones altered testosterone parameters in men.501
Mammographic density may be a predictor of breast cancer risk. Women with a high percentage of dense tissue are at 4-fold greater risk of breast cancer. Mammographic parenchymal patterns assess the variation between radiological appearances: fat appears dark and epithelium and stroma appear light. Percentage density is the ratio of dense area to breast area. A meta-analysis of randomised, controlled clinical trials on soya and red clover (Trifolium pratense) isoflavones found no impact on mammographic breast density in postmenopausal women.502 However, isoflavones may cause a small increase in breast density in premenopausal women, which is of uncertain clinical relevance. An epidemiological study in 3315 Chinese women in Singapore found no significant impact of soya intake on breast density, with the suggestion of a possible reduction.503
The results of a case-control study involving nearly 800 participants conducted in New Jersey and published in 2009 suggest a reduction in endometrial cancer risk with intake of foods containing isoflavones (but not lignans) in lean women. Four case-control studies prior to this reported conflicting results for the role of dietary phyto-oestrogens in endometrial cancer risk, although there was a suggestion of a reduced risk for soya foods.504
A Cochrane meta-analysis of randomised, controlled, clinical trials published to March 2007 investigating relief of menopausal symptoms found no evidence that food or supplements containing phyto-oestrogens caused oestrogenic stimulation of the endometrium when used for up to 2 years. Trials of women who had breast cancer or a history of breast cancer were excluded.505 The dosage of isoflavones was 50 to 120 mg/day, with many of the soya products containing soya protein.
One concern is that isoflavones may adversely affect thyroid function and interfere with the absorption of synthetic thyroid hormone. A 2006 review evaluated the relevant literature describing the effects of soya on thyroid function.506 In total, 14 trials (thyroid function was not the primary health outcome in any trial) were identified where the effects of soya foods or isoflavones on at least one measure of thyroid function was assessed in presumably healthy people. Eight involved women only, four involved men, and two both men and women. With only one exception, no effects or only very modest changes, were noted in these trials. Collectively, the findings provide little evidence that soya foods or isoflavones adversely affect thyroid function in euthyroid, iodine-replete individuals. In contrast, some evidence suggests that soya, by inhibiting absorption, may increase the dose of thyroid hormone required by hypothyroid patients. In addition, there remains a theoretical concern based on in vitro and animal data that isoflavone intake by individuals with compromised thyroid function and/or marginal iodine intake may increase their risk of developing clinical hypothyroidism.
Alkaloids
Pharmacodynamics
Several alkaloid-containing herbs are used as mild analgesics and anxiolytics. California poppy (Eschscholtzia californica) is a traditional medicine used by the rural population of California for its analgesic and sedative properties. Animal studies have verified these actions and demonstrated low toxicity.507 A proprietary formula consisting of 80% California poppy and 20% Corydalis cava (both herbs are rich in isoquinoline alkaloids) has been the subject of clinical studies.508 Results from two clinical trials showed that disturbed sleep behaviour could be normalised by the combination, without evidence of carry-over effects or addiction.508 In vitro studies suggest that the two herbs co-operate in establishing an advantageous catecholamine status necessary for maintaining sedative and antidepressant effects.509
Various species of Ephedra contain the protoalkaloids ephedrine and pseudoephedrine.510 Ephedra is a commonly used Chinese herb possessing diaphoretic, antipyretic, antiallergic and antiasthmatic properties. It is also favoured in phytotherapy, particularly for the latter two activities. Ephedrine has been used as a conventional treatment for asthma.
Broom tops (Cytisus scoparius) contains the alkaloid sparteine and is an important herb for the treatment of cardiac arrhythmias.511 Sparteine acts as a potassium channel antagonist512 and delays systolic depolarisation. Normal sinus frequency is slightly reduced, the refractory period is prolonged and the threshold is raised, reducing the risk of fibrillation and extrasystoles. Indications for broom tops include sinus tachycardia, ventricular extrasystoles and arrhythmias following a heart attack.511 High doses of the herb are necessary to achieve therapeutic levels of sparteine and related alkaloids. Sparteine was once widely used as an oxytocic drug, but it fell out of favour because of the uterine spasm that occurred in women who were unable to metabolise it effectively. About 5% of male and female volunteers studied were unable to metabolise sparteine by N-oxidation513 and this defect appeared to have a genetic basis.514