CHAPTER 67 Principles of Bone Fusion
Spinal fusion may be defined as a bony union between two vertebral bodies following surgical manipulation. Spinal fusion was first reported in 1911 for treatment of Pott disease. The mechanical stability provided by fusion was intended to inhibit progressive deformity and the spread of the tuberculous infection.1 Surgery to accomplish spinal fusion has now been extended to treat a variety of spinal conditions including scoliosis, kyphosis, fracture, dislocation, spondylolisthesis, and intervertebral disc disease.
Much has changed since the pioneering efforts of Albee1 and Hibbs2 in the early part of this century. Specialized techniques and surgical approaches have been developed for internal fixation and fusion of every part of the spine. Additionally, there have been significant advances in diagnostic techniques, intraoperative image guidance, intraoperative monitoring, minimally invasive surgical approaches, and bone graft materials. These advances have allowed for the aggressive correction of many severe spinal deformities with relative safety and predictability. Furthermore, the biologic principles on which these procedures are based have become increasingly better understood and used.
All fusion surgery involves preparation of bony surfaces at the site of the intended fusion. This usually involves the removal of soft tissues and decortication of bony surfaces. The stimulus for the bone healing response, commonly referred to as the “bone graft,” may be autologous or homologous bone (i.e., allograft bone) or one of an increasing number of synthetic materials or bioactive substances. As the graft is incorporated, bone tissue is formed by osteogenic cells. Union is accomplished when the newly synthesized bone matrix becomes mineralized and remodels with mature bone, having sufficient strength to bear physiologic loads without injury, thus becoming mechanically contiguous with the local host bone. Failure of bone formation, union, or effective remodeling results in pseudoarthrosis. The incidence of pseudoarthrosis ranges from 5% to 34% in large adult series.3–9
Fusions Site
Bone growth between vertebrae in a spinal fusion, as in all bone healing, is a cellular process, and unless cells are added to the fusion site, the tissues at the site are the only source of viable cells. Conventional autogenous bone grafts add osteogenic cells; however, it has long been recognized that only a small fraction of these cells survive.10–12 Consequently, preparation of the fusion site and handling of the tissue bed are of paramount importance for a successful arthrodesis. The components of the tissue bed that contribute most to the healing process are the local population of osteogenic stem cells and progenitor cells, local vascular tissues, the cells contributing to the inflammatory response, and the formation of a stable clot within the void spaces of the graft site. Bone, fat, and muscle have all been shown to contain osteogenic stem cells and progenitors that can contribute to new bone formation.13–20 These basic elements of the graft bed may be affected by local or systemic disease. However, the quality of these elements within the graft site is largely determined by surgical technique.
To preserve the local blood supply, the surgeon must attempt to minimize trauma to the host tissue bed imposed by the trauma of retraction, cautery, or desiccation. Any avascular, nonviable, or heavily traumatized tissues should be removed. The importance of the local blood supply cannot be overstated. The blood supply serves as (1) a source of oxygen and other nutrients to the healing tissue, as well as control of local pH; (2) a vehicle for endocrine stimulation; (3) a conduit for recruitment of inflammatory cells, which both produce paracrine factors, which may mediate the early proliferation of osteoblastic progenitors cells and serve to reduce the potential for infection; (4) a source of endothelial cells that produce paracrine factors, which may enhance osteoblastic differentiation21; and (5) a potential source of osteoblastic progenitors in the form of the vascular pericyte22 or circulating osteogenic cells.23
The effect of the postoperative hematoma on the success of fusion has been debated. It has been suggested that spinal fusion wounds should not be drained because the fibrin-rich local hematoma may provide an osteoconductive scaffold or matrix, which may assist some of the initial phases of bone healing. Additionally, the trapped platelets in the hematoma release platelet-derived growth factor (PDGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF or FGF-2), vascular endothelial growth factors (VEGF), transforming growth factor beta (TGF-β), and other growth factors that play a critical role during the repair process (see later).24 On the other hand, the presence of a large hematoma may displace some of the vascular tissue surrounding the graft site away from the graft, slowing the vascularization of the graft site. It may also increase the chance for displacement of the graft and the potential for nonunion or bone formation outside the intended site.
The inflammatory response in the wound site and the grafted bed represents a critical event in the healing process. This response will involve the removal of necrotic tissue debris, lysis of the local fibrin clot, the establishment and reestablishment of a vascular supply to the graft and host tissue, and synthesis of an early matrix rich in hyaluronic acid.12,25–28 After the surgical procedure, polymorphonuclear cells, lymphocytes, monocytes, and macrophages migrate to the fusion site and perform their various functions. Among these, and possibly the most important in terms of affecting vascular endothelial cells and osteoblastic progenitors in the graft site, is the local production of paracrine signals: cytokines, kinins, and prostaglandins. These messages act as chemotactic signals and growth factors, affecting the proliferation, migration, differentiation, and activity of a variety of cells, as well as modulation of local blood flow, vascular permeability, and angiogenic response of local endothelial cells. In this way, the inflammatory response establishes the local environment in which the early events of the bone healing response occur. It is not surprising, therefore, that agents that inhibit the inflammatory response have been shown to alter or inhibit bone healing.29–34
In addition to the influence of the local blood supply and the inflammatory response, the host bone surface itself is known to have a profound effect on the healing process in spinal fusions. When properly prepared by the surgeon, local bone will serve as a reservoir of osteogenic cells and osteoinductive signals. It also provides an osteoconductive surface for graft incorporation and serves as part of the local blood supply to the graft site. As such, the goal of surgical preparation of local bone is to minimize cellular and mechanical damage to the host bone, while maximizing the availability of osteoprogenitor cells and the osteoconductive and osteoinductive properties of this surface. This is generally achieved by subperiosteal dissection with or without decortication or roughening of the underlying bone to expose vascular osteonal or endosteal bone spaces. Decortication can be achieved with manual tools such as a rongeur or osteotome. Alternatively, a power bur may be used, provided caution is exercised to prevent thermal necrosis of the bone owing to the heat of friction at the site by using continuous irrigation and limiting periods of contact between the bur and bone at any one site.35
The surface area of cancellous bone exposed during decortication is another factor thought to affect the success of a spinal fusion. Increasing the available surface area also increases the number of osteogenic cells at the fusion site, which should have a positive effect on the amount of bone formed and the rate of graft incorporation. Additionally, an increase in the osteoconductive surface area available should increase in the area of contact between the osteogenic host bone and the graft material and potentially lead to greater mechanical strength of the subsequent bony union. This may account for the greater success of allografts in anterior fusions36 as compared with posterior fusions, which generally rely on a smaller area of decorticated bone per fusion segment. Similarly, this may also contribute to the lower fusion rates seen in myelomeningocele,37,38 where the laminae are not available as a surface for fusion.
In theory, any exposure of the local bone surface, osteonal spaces, or marrow spaces that does not excessively weaken the mechanical strength of local bone should increase the number of osteoblastic progenitors with access to the graft site.39
Bone Graft
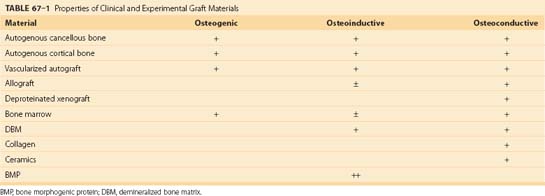
All bone grafting strategies involve either the transplantation or targeting of osteoblastic stem cells or progenitor cells. Bone grafts have been described as having osteogenic, osteoinductive, and osteoconductive properties. Grafts may also contribute to mechanical stability and vascularity at the graft site.40 Detailed reviews of the principles underlying these practical clinical concepts have been recently published.41–43
In contrast to osteoinduction, osteoconduction is the result of the structural and surface features of a graft matrix. Osteoconductivity refers to the capacity of a graft matrix to enhance the attachment, migration, proliferation, and differentiation of osteoblastic stem cells and progenitors, as well as other cells that contribute to the bone healing response. As a result, osteoconduction promotes the distribution of a bone healing response of the graft. The osteoconductivity of a matrix is a function of its macrostructure or architecture, the size and connection between pores on the material, and its surface chemistry and surface texture. In the case of degradable materials, the degradation properties of the material are also critical, specifically the degradation rate, the chemical species that are released by degradation, and their rate of clearance from the site.41
Autologous Cancellous Bone
Autologous cancellous bone has traditionally been considered to be the “gold standard” of graft materials. Autograft has maintained a track record as the most reliable and effective graft material, particularly in the challenging clinical setting of spinal fusion.44–47 This finding is reinforced by the recognition that an autogenous cancellous graft provides all three areas of functionality: osteogenic bone and marrow cells; an osteoconductive matrix of collagen, mineral, and matrix proteins; and a spectrum of osteoinductive proteins provided within the transplanted matrix of cells.
The principle disadvantage of autogenous cancellous bone graft relates to the process of graft harvest. Autograft harvest adds operative time, pain, and blood loss, and it carries an increased risk of infection, cutaneous nerve damage, and even local fracture. Autograft harvest leaves the patient with permanent scars and a risk of long-term pain at the graft site. Increased blood loss attributable to the graft harvest results in an increased potential exposure to blood products along with all the associated costs and risks of transfusion. The incidence of major complications associated with the harvest of iliac crest bone graft has been reported to be 5% to 10%.48–50 The time, effort, and complications associated with autograft harvest are also associated with tangible costs to the health care system, which have been estimated in the range of $700 to $2200, not including the pain and scars. A second disadvantage of autograft is that, in addition to its cost and morbidity, the amount of autogenous bone is limited and may be insufficient in many settings, particularly in children undergoing arthrodesis over multiple segments.
Finally, autogenous bone has a biologic limitation as a cellular graft. Although cancellous bone from the pelvis is the most abundant source of osteoblastic stem cells and progenitors, these cells represent only about 1 in 20,000 cells in normal bone marrow, which is packed with many other cells that do not necessarily contribute to bone healing. When autogenous cancellous bone is harvested, its diverse mixture of highly metabolic cells is dissociated from its blood supply and is then implanted under conditions where it must compete with all of the other cells in bone and marrow for the limited amount of oxygen and other nutrients that are available to diffuse into the graft site. The metabolic demand within the graft site far exceeds the capacity for nutrient diffusion. This results in profound hypoxia as one moves more deeply into the graft. As a result, only cells within 1 to 2 mm of the surface of an autograft are able to survive transplantation. This fact was recognized by Burwell from histologic assessment in the 1960s and can now be defined in more quantitative terms on the basis of chemical engineering principles.41 Necrosis within the graft then places an additional burden on the site. Necrotic debris must be removed before new bone formation can occur. In addition, cell debris and the cytokines that are released by dying cells escalate the local inflammatory response, bringing in additional cells that further increase local metabolic demand.
Autologous Cortical Bone
The only advantage of cortical bone versus cancellous bone and other graft materials is its superior mechanical strength and the availability of cortical segments of sufficient size to fill virtually any skeletal defect. The ability to provide immediate mechanical strength at the time of implantation is a critical advantage in many situations, particularly in anterior interbody fusions. However, the mechanical strength of a cortical graft is not constant over time. Allograft bone is remodeled by the process of creeping substitution, resulting in increased porosity and progressive loss of strength during the first 12 to 24 months after implantation before remodeling and new bone formation reconstitute the mechanical properties of the grafted segment.40,51,52 This is associated with increased risk of graft failure and collapse during the first 24 months after implantation.
Combined grafts consisting of intact cortical and cancellous bone from the iliac crest are common and readily available graft materials with good mechanical properties and biologic properties of incorporation. The mechanical strength of these grafts is variable, however. Grafts from the anterior crest exhibit greater mechanical compressive strength than grafts from the posterior crest.53
Vascularized Autologous Grafts
Vascularized grafts are used extensively in many centers for musculoskeletal reconstructive procedures. High rates of vascular patency can be achieved by experienced microsurgeons. Many studies have shown clear advantages to using vascularized grafts in a number of settings.54–57 In anterior spinal fusions, donor vessels are available to support the vascularized graft. Suitable grafts with good mechanical strength are available from the anterior iliac crest, posterior iliac crest,58 fibula,59 or rib.60 In addition, an iliac graft pedicle flap on quadratus lumborum has also been described.61 In intrathoracic procedures, a vascularized rib graft may be mobilized on its intercostal pedicle, with limited additional morbidity and in much less time than a free vascularized graft.62,63 However, a rib graft provides less mechanical strength when compared with the iliac crest or fibula64 and therefore must be mechanically supplemented by additional cortical bone or internal fixation.
Autologous Bone Marrow
Bone marrow is a valuable and easily accessible source of osteogenic cells that is probably underused in contemporary clinical practice. The osteogenic potential of transplanted bone marrow was first documented by Goujon in 1869,65 and later by Senn in 1889.66 Studies by Burwell in the 1960s concluded that the formation of new bone following autografting resulted from the differentiation of osteogenic precursor cells contained within the marrow, in addition to osteoblasts on the surface of the graft material itself.67 Burwell postulated that following transplantation, these reticular cells free themselves from the sinusoidal walls to become primitive migratory cells; they then differentiate into osteogenic cells when they are exposed to osteoinductive substances released from the necrotic portion of the graft,68 or perhaps from osteoinductive materials contained within or secreted by the marrow itself.
Many studies have demonstrated the ability of marrow cells to form bone intramuscularly,69,70 subcutaneously,71 interperitoneally,72–74 in the anterior chamber of eye,75 and orthotopically. Using a suspension of marrow cells in diffusion chambers, Friedenstein showed that hematopoietic cells die following transplantation, whereas fibroblasts and other stromal elements are more resistant to hypoxia and may proliferate close to the surface of the graft to produce immature bone, suggesting the presence of an undifferentiated precursor cell in postnatal marrow.71,74,76,77 It has been well established that the osteogenic cells that contribute to this response are not derived from one homogeneous population of cells, but rather appear to come from two or more compartments of stem cells or progenitor cells that are upstream from the osteoblastic population in bone.43,71,78,79 A more mature preosteoblastic cell appears to be localizable to the marrow space adjacent to trabecular or osteonal bone. One or more populations of less mature and potentially multipotent cells appear to be present in the more liquid phase of bone marrow, possibly including cells associated with perivascular tissue of vascular sinusoids or other marrow vessels.
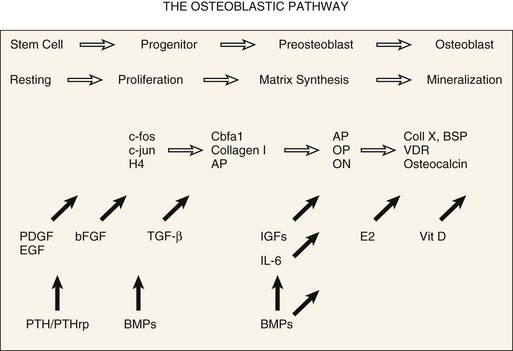
Osteoblastic differentiation proceeds in a series of steps, which can be conceptually divided into phases.80,81 An initial proliferative phase is characterized by expression of H4 histone, c-fos, and c-jun. A matrix synthesis phase is characterized by a reduction in proliferation and upregulation of gene products for type I collagen, osteopontin, osteonectin, and alkaline phosphatase. Finally, a matrix mineralization phase culminates in an osteoblastic phenotype characterized by expression of osteocalcin, bone sialoprotein, and responsiveness to 1,25-dihydroxyvitamin D and parathyroid hormone. A conceptual summary of the large body of literature related to osteoblastic differentiation is presented in Figure 67–1.43,82
The value of bone marrow as a bone graft, used alone or as a component in a composite bone graft material, has been supported by numerous studies in rats and rabbits.68,71,74–76,83–90 Lane and colleagues91 demonstrated the efficacy of autogenous bone marrow grafting in a 5-mm rat femoral defect and showed that the efficacy of bone marrow grafts were dependent on transplantation of viable cells. Yasko and colleagues92 also showed that bone marrow enhanced the performance of an effective synthetic BMP-2 material in rats, a finding consistent with prior reports on less pure BMP materials.88 However, in contrast to clinical practice, almost all of these studies in rodents have used bone marrow obtained by open harvesting of bone and/or irrigation of bone explants, rather than by aspiration.
Increasing evaluation of bone marrow grafting has been carried out in larger nonrodent models. Johnson and colleagues93 found canine bone marrow much less osteogenic than rabbit marrow when transplanted in diffusion chambers. Using a canine tibial model, Tiedeman and colleagues89 found that the percutaneous injection of marrow mixed with demineralized bone matrix powder produced overall results comparable with open cancellous grafting.
A few uncontrolled clinical series also imply that aspirated bone marrow can improve bone healing.68,94–97 Connolly reported successful treatment of 18 of 20 nonunions treated with casting or intramedullary nails plus percutaneous marrow injection. Healy and colleagues85 reported healing in five of eight delayed or nonunions of allograft host junction sites using marrow injection alone.
Recognizing the potential biologic values, many surgeons currently use bone marrow as an adjuvant to allograft bone grafts. This practice is currently supported primarily because the risk and morbidity of bone marrow aspiration from the iliac crest is low. The prospective trials needed to document the value or limitations of bone marrow grafting are only now being organized. However, these are informed by a significant volume of clinical information regarding methods and cellular yield of bone marrow aspiration98–100 and by a robust set of preclinical studies.101–105
The method of bone marrow aspiration has a significant effect on the concentration and prevalence of bone marrow–derived osteogenic cells. Muschler and colleagues,106 in a cohort of normal subjects undergoing elective orthopaedic procedures, showed that a mean of approximately 2100 osteoblastic progenitors (colony-forming units or CFU-Os) could be harvested in a 2-mL aspirate of human bone marrow from the iliac crest and that the mean prevalence of CFU-Os among nucleated marrow cells was approximately 1 in 37,000 cells. They further documented that the yield of CFU-Os harvested dropped rapidly as the volume of bone marrow aspirated was increased, owing to dilution with peripheral blood. On the basis of these findings, they recommended that aspiration of marrow be limited to 2 mL from each aspiration site in order to maximize the concentration of CFU-Os in the marrow graft. Further studies have demonstrated that the yield of osteoblastic stem cells and progenitors tends to decrease with age and that the prevalence of these cells may decrease more rapidly in women than in men.98,100,107–109 However, these data also show that there is marked variation from individual to individual in the cellularity of marrow and the prevalence of osteogenic cells that is not associated with age or gender.
McLain and colleagues110 obtained transpedicular aspirates from the vertebral bodies of 21 adults undergoing posterior lumbar arthrodesis and pedicle screw instrumentation. Aspirates were obtained from two depths within the vertebral body and were quantified relative to matched, bilateral aspirates from the iliac crest that were obtained from the same patient at the same time and served as a control. Aspirates of vertebral marrow demonstrated comparable or greater concentrations of progenitor cells compared with matched controls from the iliac crest. Progenitor cell concentrations were consistently higher than matched controls from the iliac crest (P = 0.05). The concentration of osteogenic progenitor cells was, on average, 71% higher in the vertebral aspirates than in the paired iliac crest samples (P = 0.05). With the numbers available, there were no significant differences relative to vertebral body level, the side aspirated, the depth of aspiration, or gender. An age-related decline in cellularity was suggested for the iliac crest aspirates. The authors concluded that the vertebral body is a suitable site for aspiration of bone marrow for graft augmentation during spinal arthrodesis.
One clinical grafting product, a collagen ceramic composite called Healos, has been released specifically for use as a delivery system for bone marrow–derived cells and has demonstrated strong clinical performance in terms of spinal fusion rates for both interbody and posterolateral fusion.111,112
Several authors have addressed the potential value of harvesting bone marrow by aspiration and then processing the cells that are collected to concentrate those that are most likely to be of value, thus eliminating those that are not. Connolly and colleagues83 described concentration of marrow-derived cells using centrifugation techniques and showed that a threefold to fourfold increase in the concentration of nucleated cells increased the amount of bone formation in a diffusion chamber in the rabbit. More recently, methods have been described that allow rapid intraoperative concentration and selection of osteoblastic stem cells and progenitors from bone marrow using an appropriately designed implantable allograft matrix as an affinity column to select osteoblastic cells on the basis of attachment behavior.113 This strategy has demonstrated increased bone formation, union rate, and mechanical performance in a validated canine posterior spinal fusion model. Performance of these concentrated grafts of marrow-derived cells appears comparable with that of autogenous cancellous bone, when cells are transplanted in an environment that includes a blood or marrow clot. The same strategy has been reported by Kadiyala and colleagues102,103 demonstrating union of a 5-cm canine femoral defect with both a rate and an outcome equal to autogenous cancellous bone. An early clinical cohort study applying bone marrow concentration strategies to lumbar interbody fusion has also reported a fusion rate of 85%, comparable with historical autograft controls.114
Muschler and colleagues41–43 have recently published three more detailed reviews of the biologic principles and practical strategies for harvest and use of stem cells and progenitor cells for bone healing applications. Other strategies such as the use of these cells as the delivery system or as the target cells for gene therapy applications are addressed in other chapters in this text.115–122
Structural Allografts and Cages
Use of allograft bone has been well characterized over the past 30 years.123 There are four principal advantages of allografts. First, they eliminate the morbidity associated with harvesting autologous bone. Second, and in contrast to autograft bone, the volume of available allograft is essentially unlimited. Third, because cortical allografts can be selected from any bone (not just the iliac crest or tibial hemicortex), they provide the surgeon with access to grafts that have mechanical strength and options for shaping that are superior to any autograft site. Fourth, allograft bone can be preprocessed into a wide range of specialized physical forms (e.g., blocks, threaded or nonthreaded dowels, sized rings and wedges, chips, fibers, powder) prepared from cortical and/or cancellous bone. These preprocessed grafts provide opportunity to customize and precertify the physical form and architectural properties (shape, size, mechanical strength, surface area, porosity) of a graft matrix to an individual site.
The method of sterilization and preparation of allograft tissue has a significant impact on osteoconductive, osteoinductive, and mechanical properties, as well as immunogenicity.124–127 Donor cells and cell fragments are the most immunogenic material in allogenic bone. Processing of allograft bone therefore includes steps that attempt to remove as many cells as possible from the graft. Immunogenicity is further reduced, although not eliminated, by freezing to –20° C.128–136 Freeze-drying is even more effective at reducing the immunogenicity of allogenic bone, but at the price of reducing mechanical strength by 50%.130,137
Using contemporary processing and storage techniques, clinical evidence of overt immunologic reaction against the graft is rare. Even so, histologic evidence of a low-grade inflammatory reaction can be found around essentially all allografts. This reaction probably slows the incorporation of many allografts and may contribute to the failure of some, as suggested by several canine studies that have documented improved biologic behavior in antigen-matched allografts.125,126,137,138 Antigen matching is not currently considered practical in the clinical setting, however. The relatively high current success rates for allografts makes the large cost of antigen matching not feasible and is probably unwarranted in general practice.139
Sterility of frozen allografts is ensured through expedient postmortem harvesting using sterile surgical technique and careful monitoring using surface cultures and recently polymerase chain reaction (PCR) screening for bacterial and viral genome fragments. The current risk of disease transmission via a musculoskeletal allograft is approximately 1 in 1,667,000.140,141 A variety of secondary sterilization procedures have been designed and may be used, depending on the source of the allograft. Ethylene oxide sterilization was evaluated by Cornell,142 who found a 70% decrease in bone induction by demineralized bone powder in rats. Other authors have reported variable changes in inductive capacity of ethylene oxide sterilized matrix.143–145 Heating or autoclaving bone tissue is generally avoided due to their disruption of matrix proteins. Some processing techniques such as high-dose irradiation compromise both the biologic potential of bone matrix, reducing bone formation and union rates,146,147 and also alter the mechanical properties of the graft.148–155 For example, irradiation to 2.5 megarads or freeze-dried processing may reduce the torsional strength of the cortical allograft by as much as 50%.137
Results from other clinical and experimental studies using allograft bone alone in spinal fusions have been mixed. Some investigators have found allograft to be significantly inferior to autogenous bone grafting when compared with other distinctive preparation methods,36,38,149,156–167 whereas others find little or no difference between them.36,62,168–182 Allograft bone appears to be particularly valuable in settings that require the graft to serve a significant mechanical function such as struts or ring allografts for anterior lumbar interbody fusions183–187 or as struts or bone-wire fixation constructs in the upper cervical spine.188–197 In these settings, allografts have essentially replaced the use of autografts from rib, fibula, tricortical iliac crest, and tibial hemicortex grafts, which are all associated with significant donor site morbidity.
In the past decade options for structural materials that can be incorporated into interbody fusion sites have expanded to include the use of structural cages composed of titanium or carbon fiber.197–205 These materials are capable of providing the structural function that is necessary to maintain the height and stability of the interbody site. These cages and wedges lack some of the osteoconductive capacity of allograft bone and the capacity of allograft bone to be biologically incorporated into the fusion mass and remodeled, remaining in the site essentially as a permanent foreign body. Metal cages also make radiographic assessment of the fusion site more difficult. However, in comparison with allograft, metal cages do offer the advantage of more consistent material properties, specifically strength and fracture resistance.
Regardless of material used for structural support, there is relatively general consensus that in order to achieve optimal rates of spinal fusion, the environment within and around these structural allografts or cages should be further supplemented with other osteogenic, osteoconductive, and/or osteoinductive graft materials. Autogenous cancellous bone, bone marrow aspirate,206 processed bone marrow–derived cells,114 processed nonstructural allograft materials, and BMPs3,207,208 are most commonly considered.
Demineralized Allograft Bone Matrix
The history of demineralization as a means to enhance allograft performance is richly linked to many of the recent biologic insights into bone biology and bone healing. It was more than a century ago, in 1889, that Senn reported the repair of long bone and cranial defects in patients with chronic osteomyelitis using hydrochloric acid-treated decalcified heterologous bone implants.66 Although his primary motive was to promote antisepsis within the bone cavities, Senn observed rapid substitution of the demineralized tissue with new bone formation invading from the perimeter of the defects. However, several of Senn’s contemporaries obtained equivocal results, and clinical efforts over the next 70 years were minimal.188,209–211
Reddi and Huggins212,213 revived this concept when they reported on their observation that matrix induced the bone induction phenomenon in rats. Urist went on to demonstrate bone induction using a variety of demineralized matrix preparations in muscular pouches of rabbits, rats, mice, and guinea pigs.214 Subsequently, matrix-induced heterotopic bone formation was documented at many soft tissue sites including muscle, tendon, and fascia,181,213,315-219 as well as in the thymus71 and soft connective tissue of visceral organs.220 Nathanson also observed the differentiation of neonatal embryonic skeletal tissue into cartilage when cultured on demineralized bone matrix substratum and suggested that the tissue transformation of bone induction was analogous to embryonic bone tissue differentiation.221,222
Reddi subsequently characterized the inductive phenomenon of bone matrix as a cascade of events parallel to those occurring in endochondral ossification and postulated that the process was the result of stimulation by a series of soluble matrix factors that potentiated events along the cascade.223–225 In this paradigm, bioactive factors in bone matrix stimulate activation and migration of osteogenic stem cells and progenitor cells. Mitogenic factors promote cell proliferation. Angiogenic factors promote local revascularization, and osteoinductive factors promote osteoblastic differentiation. Subsequently, bone matrix has been shown to contain a rich variety of growth factors and other bioactive molecules in concentrations that are bioactive.226,227
Sato and Urist228 showed that demineralized bone matrix was both inductive and was synergistic with bone marrow–derived cells in healing of rat femoral defects. He went on to provide a clinical outlet for these discoveries by developing a “chemosterilized, autolyzed, antigen-extracted allogenic bone (AAA),” prepared using chloroform-methanol extraction, 0.6 N hydrochloric acid extraction of soluble proteins with partial demineralization, and neutral phosphate autodigestion.215–217219 This preparation appeared to reduce the immunogenicity of the allograft matrix without loss of inductive properties. Using this preparation, Urist reported on 40 patients undergoing posterolateral lumbar spinal fusion with an 80% success rate and a pseudoarthrosis rate of 12%.229
The value of a variety of demineralized bone matrix preparations (chips, fibers, powders) has subsequently been described in a number of settings by a series of authors. Glowacki and colleagues230–232 were among the first to report successful repair of craniofacial defects. Tiedeman and colleagues89 and Wilkins and colleagues233 both reported clinical efficacy in long bone defects. Other reports have also shown efficacy for demineralized bone matrix in spine fusion models.234,235 Some studies have reported a benefit of adding demineralized bone matrix to autograft or ceramic matrices in animal spine fusion models.87,236–240 There is also recent evidence that some demineralized bone matrix preparations can be used as a substrate for selective attachment and concentration of bone marrow–derived osteogenic stem cells and progenitors.113,114
Of recent clinical concern is evidence that suggests that the biologic efficacy of commercially available preparations of demineralized bone matrix materials can vary significantly, depending on the method of processing, the individual batch of bone that is processed, or the donor of the bone that is used. This variation has raised questions about the possible value of implementing generalized standards for either in vitro or in vivo biologic assays for bone from each batch and donor in order to limit the potential for biologically deficient materials from compromising the performance of clinical grafting procedures.123,241,242 At present, without evidence that failed graft procedures cluster around individual donors or batches of demineralized bone, and that these failures can be predicted by any of the available assays, the clinical value and cost effectiveness of biologic assays is uncertain. Regardless, some providers of demineralized bone have elected to implement some form of bioassay and use these data to exclude some bone from use and to make claims of superior or more reliable performance in the marketplace.
Deproteinated Heterologous Bone
In contrast to allograft bone, heterologous bone (xenograft) fails to induce osseous repair due to its high level of antigenicity. Partially deproteinated and partially defatted heterologous bone (Kiel bone or Oswestry bone) does exhibit greatly reduced antigenicity and therefore evokes a minimal immune response.243 The denaturing process, however, also destroys osteoinductive matrix proteins. Accordingly, implantation of such materials in bone defects and muscular compartments has failed to generate bone formation.96
The impregnation of this material with cells capable of osteogenic activity, however, has been studied. Salama and colleagues96 and Plank and colleagues244 demonstrated that deproteinated xenograft bone supplemented with autologous marrow assisted osteogenesis in both experimental animals and humans. Deproteinated bone, in these experiments, served as an osteoconductive scaffolding, providing a stable mechanical environment for revascularization and proliferation and differentiation of osteogenic cells. Salama and Weissman97 reported satisfactory results in clinical attempts to use composite xenograft/autograft (Kiel bone/marrow) in a variety of bone defects. More recently, Rawlinson and colleagues245 reported poor results using bovine-derived Cloward grafts. Due to the wide availability of more effective allograft matrix materials in the United States at similar costs, xenograft materials are not currently used.
Synthetic Bone Graft Materials
Recent years have seen an explosion of new information about the cellular and molecular events involved in the bone healing response (Table 67–1). Purified human recombinant growth factors are now becoming available. Many are active in multiple events in the bone healing process and are therefore candidates as potential therapeutic agents. In addition, rapid developments in porous ceramic materials and bioerodible polymers of biologic and synthetic polymers enable the design of customized matrix materials that can be used both as osteoconductive scaffolds and as delivery systems for bioactive molecules. These converging events are now producing an army of first-generation and second-generation biosynthetic bone grafting materials. It is beyond the scope of this chapter to comprehensively review any one of these areas. Several recent reviews are available.41,124,246,247 The following is intended as an overview of some of the ongoing developments in this area, specifically the application of growth factors, collagen matrices, and ceramics in synthetic bone grafting materials.
Bone Morphogenic Proteins
A major advance occurred in 1978, when Urist and colleagues248 reported the isolation of a hydrophobic, low-molecular-weight protein fraction from insoluble bone matrix gelatin that was responsible for osteoinductive activity.218 Further characterization of this inductive factor, BMP, was made possible by quantitative extraction accomplished by differential precipitation in a buffer containing 4M guanidine hydrochloride.249 Lovell and Dawson went on to report the success of a partly purified BMP preparation on polylactic acid strips in a canine segmental spinal fusion model.250
After an extensive search for the protein responsible for the inductive activity of bone matrix extract, Wozney and colleagues,251 in 1988, identified and characterized three proteins isolated from a highly purified preparation from bovine bone, each capable of inducing bone formation in a rat subcutaneous bioassay. Human cDNA clones for each peptide were isolated and expressed as recombinant human proteins. Two of the encoded proteins were homologous and described as members of the TGF-β super gene family, whereas the third appeared to be a novel polypeptide (BMP-1). BMP-1 has turned out not to be a growth factor at all. Rather, this molecule has been characterized as a procollagen C-proteinase, which may have a biologic function in the activation of TGF-β-like molecules including the BMPs.252,253
The BMP story has developed even more rapidly in recent years. At the time of this writing the BMP family of growth factors currently includes 14 members. BMP-2 through BMP-14 are homologous proteins with molecular weight of 12 to 14 kD that are post-transcriptionally modified by glycosylation and are secreted as homodimers or heterodimers of 110 to 140 amino acid peptides linked by one disulfide bond (≈30 kD).251,254,255 In vivo, these proteins are secreted as soluble factors that have autocrine and paracrine effects. BMP-7 (OP-1) can also be found in systemic circulation and may also have positive hormonal effects on kidney function.256,257 BMPs are also embedded in bone matrix, both as homodimers and as heterodimers at a concentration of roughly 1 mg/kg of bone, where they are believed to play a role in bone remodeling and the coupling of osteoclastic and osteoblastic activity. Of interest is the finding that as much as 65% of the BMP in bone matrix is BMP-3, which is less active in bone formation and may, in fact, be a negative regulator of bone formation.258,259
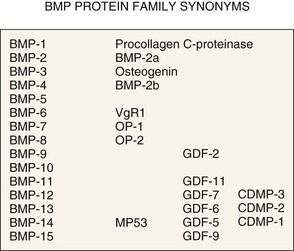
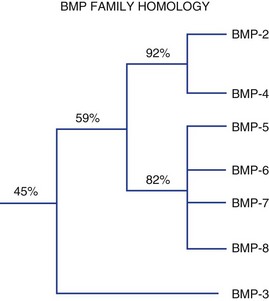
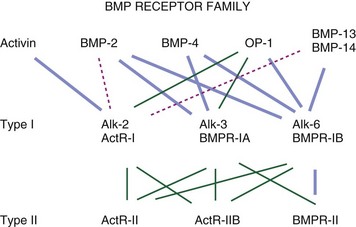
Figure 67–2 summarizes these proteins along with synonyms or alternative names that are now, or have been, used for some of these molecules. Figure 67–3 illustrates the percent RNA sequence homology within and between subgroups of the BMP protein family. Each of these proteins can interact with one or more of a family of cell surface receptors. Cells must express both type I and type II receptors (serine/threonine kinases) in order to be responsive to BMPs because a type I and type II receptor must interact in the presence of a BMP to mediate a cellular response. To date, three type I and three type II BMP receptors have been identified. BMPR-IA binds only BMPs. BMPR-IB binds BMPs and müllerian inhibitory substance (MIS). ALK-2 (also a type I receptor) binds BMPs and activin. BMPR-II binds only BMPs, and the activin type II receptors, ActR-IIA, and ActR-IIB bind both BMPs and activin.260 In vitro, BMPs demonstrate dose-responsive modulation in responsive cells, both primary osteogenic cells and in cell lines, in the range of 1 to 100 ng/mL, but each BMP demonstrates a unique and variable binding pattern for the individual receptors, as illustrated in Figure 67–4. A great deal of functional redundancy and promiscuity between these proteins and receptors appears to be present, though these genes appear to be independently regulated in both space and time in embryonic development and in bone healing settings, and expression of specific inhibitors of BMP function (e.g., noggin, chordin, connective tissue growth factor, follistatin) also plays a role.254,261–266
Among the BMP homodimers that are most active in bone induction in vivo are BMP-2, BMP-4, BMP-6, BMP-7 (i.e., Osteogenic Protein-1 or OP-1), and BMP-9. BMP-2 and BMP-7 have both been developed for clinical applications in bone grafting and skeletal reconstruction. BMP-13 (i.e., MP53 or GDF-5) and BMP-14 (i.e., GDF-6) are also under development.267–269 At present, both BMP-2 and OP-1 (BMP-7) have been fully evaluated and approved by the FDA for use in spinal fusion. This approval is specifically limited to the use of BMP-2 in a collagen carrier (INFUSE, Medtronic Sofamore Danek, Minneapolis) in the setting of anterior interbody fusion using a metallic cage. OP-1 Putty (Stryker, Kalamazoo, Mich.) can be used in patients who have failed a previous spinal fusion surgery and are not able to provide their own bone or bone marrow for grafting because of a condition such as osteoporosis, diabetes, or smoking.
A large number of animal studies have demonstrated the promise and relative safety for these proteins as powerful stimulants of a local bone healing response in rodents, sheep, canines, and nonhuman primates, using various carrier matrices and a dosage range of 100 to 10,000 µg/mL.255,270–295 Schimandle and colleagues293 reported 100% union in an uninstrumented posterolateral intertransverse fusion model in the rabbit using BMP-2 delivered in a collagen carrier, compared with only 42% fusion with autogenous corticocancellous iliac crest bone. Muschler and colleagues,296 using an instrumented posterior canine spinal fusion model, found that BMP-2 delivered in a degradable polymer carrier (PLGA) had comparable efficacy to autogenous cancellous bone. Cook and colleagues297 found similar results in a canine spine model using OP-1 (i.e., BMP-7). The use of rhBMP-6 stOPCs in a carrier of guanidine-extracted demineralized (gDBM) bone matrix significantly enhanced the rate and strength of single-level posterolateral spinal arthrodeses in the New Zealand white rabbit, compared with iliac crest bone graft, gDBM, and decortication alone.298 Using a rabbit model, Fu and colleagues demonstrated enhancement of posterolateral lumbar spine fusion using low-dose rhBMP-2 and cultured marrow stromal cells.
Several prospective clinical trials evaluating BMPs in the setting of spinal fusion have been performed, generally reporting performance that was comparable with autogenous cancellous bone, with fusion rates between 80% and 99%.299–305 These initial studies strongly support the clinical value of BMPs, particularly BMP-2, which has been most thoroughly studied in the clinical setting to date.
In a prospective, randomized, controlled, multicenter clinical pilot study, Vaccaro and colleagues306 demonstrated that the rates of radiographic fusion, clinical improvement, and overall success associated with the use of OP-1 Putty were at least comparable with that of the autograft controls for at least 48 months after surgery. Singh and colleagues307 performed a prospective, single-institution, clinical case-matched, radiographic cohort study involving 52 patients who underwent posterolateral lumbar arthrodesis with pedicle screw instrumentation. Using thin-slice computed tomography (CT) analysis, the authors demonstrated 97% fusion rates in the rhBMP-2 group compared with 77% in the iliac crest bone graft at 2 years. The authors concluded that the adjunctive use of rhBMP-2 and iliac crest bone graft seems to be safe and results in significantly larger and more consistent posterolateral fusion masses.307 In a human posterolateral lumbar spine trial, OP-1 reliably induced viable amounts of new bone formation, but the fusion success rate evaluated by surgical exploration was only 4 of 7.308 Lewandrowski and colleagues309 reported vertebral osteolysis with the use of rh-BMP-2 in posterior lumbar interbody fusions with 5 out of 68 patients developing osteolysis within 4 months of surgery. Violation of the endplate during decortication was thought to be a contributing factor. This often resolves spontaneously. Similar observations were made in the cervical spine by Vaidya and colleagues.310 In a prospective, consecutive patient enrollment with a minimum 24-month follow-up, 30 patients underwent anterior interbody allografts alone and 45 patients underwent anterior interbody allograft filled with rhBMP-2. All cases had posterior pedicle screw instrumentation. A total of 165 surgical levels (62 allograft alone; 103 allograft + BMP) were included. In the allograft rhBMP-2 group, fusion rates were 94%, 100%, and 100% at 6, 12, and 24 months, respectively, after surgery, while in the allograft-only group, fusion rates were 66%, 84%, and 89% at the same time intervals. Clinical outcomes were significantly improved in rhBMP-2 group when compared with the allograft group at 6 months. There were no revisions in the rhBMP-2 group and four revision fusion surgeries (13%) in the allograft group.311 In a randomized, controlled trial in patients older than 60 years of age, Glassman and colleagues312 concluded that RhBMP-2/INFUSE is a viable iliac crest bone graft replacement in older patients in terms of safety, clinical efficacy, and cost-effectiveness.
Recently, several investigators have evaluated the use of BMPs in cervical spine surgery.310,313,314 Anterior cervical discectomy and fusion performed with rhBMP-2 (0.9 mg BMP per level) allograft was found to be as effective as iliac bone graft in terms of patient outcomes and fusion rates. Safety concerns related to neck swelling and higher initial costs were associated with patients in the bone morphogenic protein group.313 Vaidya and colleagues compared 22 patients treated with rhBMP-2 and PEEK cages with 24 in whom allograft spacers and demineralized bone matrix was used. Radiographic examination following surgery revealed end plate resorption in all patients in whom rhBMP-2 was used. This was followed by a period of new bone formation commencing at 6 weeks. In contrast, allograft patients showed a progressive blurring of endplate-allograft junction. Dysphagia was a common complication and it was significantly more frequent and more severe in patients in whom rhBMP-2 was used. Postoperative swelling anterior to the vertebral body on lateral cervical spine radiograph was significantly larger in the rhBMP-2 group when measured from 1 to 6 weeks after which it was similar. There was no significant difference in the clinical outcome of patients in the two groups at 2 years. The authors concluded that despite providing consistently good fusion rates, they have abandoned using rhBMP-2 and PEEK cages for anterior cervical fusion, due to the side effects, high cost, and the availability of a suitable alternative.315 Shields and colleagues316 reviewed 151 patients who underwent either an anterior cervical discectomy and fusion (n = 138) or anterior cervical vertebrectomy and fusion (n = 13) augmented with high-dose INFUSE (BMP-2; Medtronic Sofamor Danek). They found a high morbidity rate with a total of 35 (23.2%) patients having complications that the authors thought were attributable to the use of high-dose INFUSE in the cervical spine. Fifteen patients were diagnosed with a hematoma including 11 on postoperative day 4 or 5, of whom 8 were surgically evacuated. Thirteen individuals had either a prolonged hospital stay (>48 hours) or hospital readmission because of swallowing/breathing difficulties or dramatic swelling without hematoma. The authors concluded that putative inflammatory effect that contributes to the effectiveness of INFUSE (Medtronic Sofamor Danek) in inducing fusion may spread to adjacent critical structures and lead to increased postoperative morbidity.316 On the basis of their experience with 69 patients who underwent anterior cervical fusion, Smucker and colleagues317 concluded that use of rhBMP-2 in the anterior cervical spine is associated with an increased rate of clinically relevant swelling events.
One of the principle requirements for optimal BMP activity is the presence of a local population of target cells, most likely osteogenic stem cells and progenitor cells that are responsive to the protein (i.e., they express appropriate receptors). In order for a BMP to be optimally effective, these target cells must be both available and activated in sufficient numbers to produce the desired result. If an optimal number of responsive cells is not present within the tissue volume that is exposed to the protein following implantation, the biologic response to the protein will inevitably be reduced and the implantation of a BMP may be completely ineffective. Variation in the concentration or biologic potential of target cell populations in bone, bone marrow, periosteum, and other tissues (e.g., muscle, fat) may explain much of the apparent variation in the magnitude and type of response seen to BMPs and other growth factors from site to site and individual to individual.99,100,318
Preclinical evaluation of BMPs in a series of animal models from rats to rabbits to dogs to nonhuman primates demonstrated the need for delivery of dramatically high concentrations of BMP to graft sites in higher animals.274 In fact, the formulations of BMP that are currently available deliver an amount of BMP that is roughly 50 times greater than the total amount of BMP that is present in an entire human skeleton. INFUSE (Medtronic Sofamor Danek) delivers BMP-2 in solution at a concentration of 1.5 mg/mL to be combined with a collagen carrier, resulting in a implanted concentration slightly less than 1 mg/mL. Similarly, the OP-1 device delivers 3.5 mg of OP-1 in a final volume of approximately 4 mL. The reason for this escalation of dose has not been clearly established, though several factors are likely to contribute. Species-specific differences in dose response may exist at the target cell level, though this has not been a consistent finding in in vitro culture of primary osteogenic cells. However, individual species do demonstrate significant differences in the concentration and prevalence of responsive target cells in local tissues and consistently lower numbers in higher animals. The geometry involved in mating active BMP with a responsive target population of cells in larger graft sites may also contribute. For both of these reasons, a huge dose may be necessary to provide a burst of BMP delivery that is sufficiently large that the BMP diffusing away from the graft site will penetrate into regional tissues to a sufficient depth at a sufficiently large concentration to activate enough stem cells and progenitors. As the graft sites become larger, activation of more target cells is required. This results in the need for a greater degree of penetration that can only be achieved with a larger dose. Similarly, if the concentration and prevalence of target cells in regional tissues decrease, as they do in higher animals, activation of a similar number of cells will require even deeper tissue penetration. These factors likely contribute to the seemingly exponential increase in dose that is necessary in larger animals.41 A second possible explanation for the massive dose relates to the issue of BMP retention at the graft site. A massive initial dose may also ensure that a sufficient, though perhaps small, quantity of BMP will remain at the site long enough to result in activation of target cells that may not enter the graft site until several days after implantation, with the associated inflammatory response or angiogenic response following the surgical trauma.
These issues in BMP delivery and function in a graft site suggest a number of options that could be used to increase the exposure of BMP to an appropriate target population of cells, thereby improving its performance. Direct delivery or supplementation of the target cell population in the graft site is one option. Evidence is provided by a number of studies that addition of a target population (e.g., bone marrow–derived cells) to a site of BMP implantation will significantly improve bone healing.92,318 Another possible method for enhancing the performance of BMPs is to refine the method and rate of BMP delivery into the graft site. The chemical surface of the matrix may influence the protein binding, its conformation, and stability. In some cases, BMP binding to matrix can actually enhance the biologic performance.319–321 The four major categories of BMP carrier materials are natural polymers such as collagen, hyaluronans, fibrin, chitosan, silk, alginate, and agarose; inorganic materials such as low- and high-temperature calcium orthophosphates (calcium phosphate cements and sintered ceramics) and calcium sulphate cements; poly(α-hydroxy acid) synthetic polymers such as PLA, polyglycolide (PLG); and their copolymers (poly[D,L-lactide-co-glycolide]) (PLGA).322 Furthermore, the carrier (collagen, ceramic, polymer) may have its own biologic effects associated with the release of ions or other degradation products.41 Currently both the OP-1/BMP-7 (Osteogenic Protein-1/BMP-7; Stryker) and INFUSE (Medtronic Sofamor Danek) use type I bovine collagen as the carrier. In the case of OP-1, the protein is lyophilized onto the surface of the collagen. With INFUSE, it is adsorbed onto the surface of the collagen out of an aqueous solution. Other options include release from degradable polymers, liposomes, and collagen-hydroxyapatite microsphere.323–325
Yet another option for enhancing the performance of BMPs is to prolong the period of time that effective concentrations of the protein are present in the graft site. Residence time of bioactive protein in a graft site is a complex function of the rate of delivery (e.g., release or solubilization); the rate of consumption within the graft site (e.g., degradation, inactivation, binding to inhibitors); and the rate of clearance from the graft site (e.g., diffusion, convection). With BMP preparations currently available, release kinetics are relatively rapid. Pharmacokinetic studies of residence time for OP-1 delivered as a lyophilized protein from a collagen carrier in a rabbit model indicate that the protein is released into the site and retained in measurable concentrations for approximately 7 days, with a maximal release rate in the first 24 hours. The implanted rhOP-1 diffuses out of the immediate graft site at low concentrations, entering systemic circulation, where it is rapidly cleared. Approximately 50% of the implanted dose is excreted in the urine.326 In the case of BMP-2 in the INFUSE Device, release kinetics are similar, with 50% of the rhBMP-2 cleared from the site in 48 hours and less than 1% remaining at 2 weeks.327
Longer residence time within the site is associated with improved efficacy at lower protein concentration.328 Residence time is influenced by the affinity of the protein to the carrier.329,330 Residence time can also be increased by modifying the BMP protein itself to reduce its solubility and therefore its rate of diffusion out of the implant.331 Prolonged residence time within the graft site may have two effects. It may allow the initial gradient of protein concentration around the site to be maintained for a longer period, which may positively influence chemotactic effects that may be mediated by the protein, drawing more activated progenitors and other cells into the defect. Prolonged residence may also serve to maintain a functional concentration of protein in the graft site for a longer time, providing the opportunity for additional stem cells and progenitors to migrate into the graft site where they may become activated.
Other Growth Factors
A large number of peptide growth factors and hormones are known to have important effects on the recruitment, proliferation, and differentiation of osteoblastic progenitors, which may have potential therapeutic importance. Only some of the examples of candidate growth factors can be listed.332 Epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) are both capable of inducing colony formation by osteoblastic progenitors in vitro,24 and local injection of PDGF has been shown to result in induction of new bone formation when applied close to a bone surface. Basic fibroblast growth factor (FGF; aka bFGF or FGF-2) will also increase proliferation of human osteoblastic progenitors and reversibly inhibit the expression of alkaline phosphatase and matrix synthesis, in addition to its known potent angiogenic effects. FGF has been evaluated in preclinical studies using a hyaluronic acid delivery system and has been shown to increase local bone formation and union rates.333
Vascular endothelial growth factors (VEGFs) are a family of proteins that function as dimers. They have a structure similar to PDGF and interact with transmembrane receptors Flt-1, Flk-1, and Flt-4 to activate a tyrosine kinase signaling cascade. VEGFs play several important roles in angiogenesis, osteoclast migration, and osteoblastic activity.334,335 VEGFs do not induce bone directly, but in addition to angiogenic effects, VEGF delivery has been shown to upregulate BMP activity in fracture healing and distraction osteogensis.336,337 When used in combination, VEGFs may enhance the performance of BMPs.338
Transforming growth factor beta (TGF-β) is another potent osteotropic factor. Of the five known isoforms of TGF-β, two—TGF-β1 and TGF-β2—are synthesized by bone cells. In fact, bone matrix deposited by osteoblasts is the largest source of TGF-β and is mostly present in a latent form that is released during bone remodeling.339,340 TGF-β has effects on bone formation and remodeling. It will induce new bone formation, but only when implanted or injected in close proximity to bone, suggesting that its bone formation effects are mediated primarily by trabecular or periosteal cells, a different or more limited target cell population than the BMPs.341 For example, Joyce and colleagues342 showed that subperiosteal injection of TGF-β can produce a marked periosteal response resulting in rapid formation of a cartilage tissue mass and bone formation via endochondral ossification. IGF-I and IGF-II potentiate a mature osteoblastic phenotype in culture.40,343–345 Both TGF-β and IGF-1 have been shown to promote spine fusion in a sheep model.346
Collagen
In contrast, fibrillar collagen (uncrosslinked collagen) is soluble and can be extracted from bone and skin. Fibrillar collagen can be engineered to produce a variety of matrices such as gels, sponges, and filaments. These are often secondarily crosslinked to stabilize their structure using a variety of chemical methods. Again, few of these engineered collagen matrices are effective by themselves in strongly promoting bone formation. Some formulations have appeared to actually compromise the efficacy of autograft in a graft site.105 However, in general collagen matrices have been effective as delivery systems for bone marrow–derived cells91 and for growth factors. Both of the two BMPs that are now available for clinical use utilize collagen I as their delivery vehicle. In addition, an interconnected porous mesh of purified bovine collagen (pore size ≈ 150 microns) with a thin coating of hydroxyapatite precipitated on its surface (Healos, DePuy Spine, Inc., Raynham, Mass.) has been specifically developed and marketed as a delivery system for bone marrow harvested by aspiration. Clinical assessments have demonstrated high fusion rates in both lumbar interbody fusions and posterolateral fusion sites.111
Noncollagenous Matrix Proteins
Bone matrix contains many proteins other than collagens and growth factors.98,227,347–349 These proteins may serve a role in organization of the collagenous matrix and other proteins into higher ordered structures. They may provide attachment sites for cells or binding sites for growth factors. They may serve as regulators of mineralization, as in the case of bone and dentin phosphoproteins, bone sialoprotein, osteonectin, and osteocalcin.350–354 They may also provide a source for release of locally active growth factors and other bioactive molecules during the process of matrix turnover and remodeling, as previously discussed.
Other than growth factors that are embedded in bone matrix, it is difficult to ascribe any one factor with exceptional function in the setting of bone grafting or a high potential for future clinical application. However, some may be relevant and deserve mention. For example, osteoblasts and osteoblastic cell lines appear to express integrins that bind selectively to both fibronectin and vitronectin355–358 and possibly osteopontin, bone sialoprotein, and laminin. In addition, osteocalcin appears to be chemotactic for osteoclasts and monocytes,359 critical elements of normal bone remodeling. Although these proteins are not likely to be exploited in terms of recombinant manufacturing processes used for BMPs, it is possible that these functions may be localized to specific functional domains of these proteins. This knowledge may be used to design specific low-molecular-weight surrogates that may be applied to tissue engineering constructs.
Ceramics
Calcium phosphate biomaterials fused at their crystal grain boundaries into polycrystalline ceramics by high temperature sintering confer stability to these minerals and reduce bioresorbability.360 A variety of ceramics are currently being evaluated, most of which are composed of either hydroxyapatite (HA) or tricalcium phosphate (TCP). Ceramics may be prepared as porous three-dimensional implants, dense block implants, granular particles (usually 0.5 to 3 mm in size), or thin surface coatings. Almost all calcium phosphate ceramics have a high degree of biocompatibility,236,361 and some have already been extensively used in dentistry and maxillofacial surgery.236,243,244,358–365
The minimal macropore size in porous ceramics needed for effective ingrowth of bone is approximately 100 µm.366 Most porous ceramics currently being manufactured contain interconnecting macropores ranging from 100 to 400 µm. The various calcium phosphate ceramics generally differ with regard to their bioresorbability characteristics. A number of investigators have reported that ceramic hydroxyapatite does not exhibit extensive bioresorption and is essentially inert.360,361,367 Conversely, there is unequivocal evidence that ceramic TCP undergoes biodegradation.236,360,361,367–369 In addition, implants with a large surface area will tend to exhibit more rapid degradation.236
Early studies of ceramics suggested that they may be capable of osteogenic stimulation.370 In fact, one can often find new bone formation in an HA ceramic implant placed at heterotopic sites in the absence of other stimuli. This occurs only after several months and would not be likely to contribute to the early success of a bone graft. The role of ceramics, therefore, is primarily that of osteoconduction. One possible mechanism for this apparent late osteoinductive property of HA ceramics is that an implanted HA implant will selectively bind proteins to its surface on the basis of their relative affinity to HA. This may result in the accumulation of some protein growth factors such as BMPs, TGF-βs, and insulin-like binding protein-5 (IGFBP-5), which have strong affinity to HA. Accumulation of these low-abundance proteins and their presentation on a stable surface may secondarily create a local growth factor environment on the ceramic surface that is capable of recruiting local osteoblastic progenitors and inducing bone formation. This affinity of many osteotropic growth factors for the highly charged surface of HA may also make HA ceramics an effective delivery system for growth factors as composite synthetic bone grafting materials are developed.
The stability of a bone-ceramic interface and preparation of local bone are also important. Cameron and colleagues371 demonstrated that ceramic implants placed against an unprepared bony cortex do not exhibit bone ingrowth and simply resorb over time. However, when placed subperiosteally and immobilized on a scarified cortex, bone ingrowth readily takes place. The sensitivity of these materials to micromotion likely results from magnification of the mechanical strain within the graft site at the interface between local tissues and the surface of a rigid ceramic block. Similar magnification of strain will occur in the regions of tissue between adjoining ceramic granules, inhibiting bone formation. As a result, the optimal settings for use of these materials may be limited to settings in which mechanical micromotion can be well controlled.
Another drawback of ceramic implants is that they are brittle and have low impact and fracture resistance.372 Furthermore, the limited solubility and remodeling capacity of highly crystalline HA ceramics may retard late stages of bone healing and remodeling, as well as compromise late mechanical properties of the bone formed in a fusion site.360 This concern has been reduced by the work of Ohgushi and colleagues,373 which showed that ceramic combined with bone marrow exhibited greater biomechanical properties following implantation with marrow cells as a result of new bone formation in the implant. In addition, Muschler and colleagues104,105 have performed a series of spinal fusion experiments evaluating composites of collagen and ceramic granules (60% hydroxyapatite, 40% TCP). Although these studies found that all composites tested had a significantly higher nonunion rate than autogenous cancellous bone graft, the mechanical properties of successful unions achieved with the collagen ceramic composites were comparable with the mechanical properties of unions resulting from autogenous bone graft, despite the presence of unresorbed granules in the fusion mass. Hing and colleagues374 compared the rate, quality, and extent of osseous healing in a standard rabbit defect model between dense calcium sulfate, ultraporous tricalcium phosphate, and porous silicated calcium phosphate. The authors concluded that in patients in whom bone regeneration may be compromised, the degradation observed with some resorbable bone grafts may contribute to the decoupling of bone regeneration and resorption within the graft site, which may ultimately lead to incomplete bone repair.
Ceramic blocks have been evaluated in a goat anterior cervical fusion model with a reported 50% to 70% fusion rate.375,376 Several injectable ceramic preparations that crystallize at body temperature have also been described. Resorption rates vary significantly, from weeks to months. These may provide means for improving the initial mechanical fixation for acute fractures, though they do not seem well conceived for achieving long-term fixation. These injectable setting ceramics may also have potential utility in providing extended local delivery for bioactive proteins.377 In a prospective, randomized study with 3-year follow-up, Dai and colleagues378 compared beta-tricalcium phosphate versus autograft in patients undergoing single-level instrumented posterolateral fusion of lumbar spine with beta-tricalcium phosphate versus autograft. The authors reported similar clinical outcomes and fusion rates and suggested that beta-TCP as bone graft substitute may eliminate the need for bone grafting harvesting from the ilium.378 In a different prospective study, Chen and colleagues379 placed autologous iliac crest bone graft in one posterolateral gutter, while on the other side, an equal quantity of autogenous laminectomy bone supplemented with calcium sulfate was placed. In a prospective, matched, and controlled study, Acharya and colleagues380 evaluated hydroxyapatite-bioactive glass ceramic composite as a stand-alone graft substitute for posterolateral fusion of lumbar spine by placing it in the left intertransverse bed. The autograft was placed in the right intertransverse bed. At the end of 1 year, excellent radiologic outcome was seen on the right side (autogenous graft) in all the cases, whereas 95% (21/22) of the cases had poor consolidation on the left side (hydroxyapatite composite). The authors reported fusion rates and fusion size to be similar between the two sides. Epstein381 found a 15% pseudarthrosis rate following multilevel laminectomy and one- to two-level noninstrumented posterolateral fusion using lamina autograft/B-TCP. Enriched bone-marrow–derived mesenchymal stem cells were combined with porous beta-tricalcium phosphate in 41 patients undergoing posterior spinal fusion.382 After 34.5 months, 95.1% cases had good spinal fusion results.
Systemic Factors Influencing Spinal Fusion
Many systemic factors have been shown to influence bone healing in the laboratory. Clinically, these factors are also likely to play an important role. A list of systemic factors and their relative effects on bone healing is shown in Table 67–2. Given the complexity of various factors in the clinical setting, it is difficult to demonstrate on a case-by-case basis or in a clinical series that each of these factors results in significant alterations of fracture healing or the success of spinal fusion procedures. Nevertheless, the surgeon should optimize each factor, whenever possible.
| Positive Factors | Negative Factors |
|---|---|
| Insulin | Corticosteroids |
| Insulin-growth factor and other somatomedins | Vitamin A intoxication |
| Testosterone | Vitamin D deficiency |
| Estrogen | Vitamin D intoxication |
| Growth hormone | Anemia; iron deficiency |
| Thyroxine | Negative nitrogen balance |
| Parathyroid hormone | Calcium deficiency |
| Calcitonin | Nonsteroidal anti-inflammatory drugs |
| Vitamin A | Adriamycin |
| Vitamin D | Methotrexate |
| Anabolic steroids | Rheumatoid arthritis |
| Vitamin C | Syndrome of inappropriate antidiuretic hormone |
| Castration | |
| Tobacco | |
| Sepsis |
Nutritional status has been shown to affect the clinical outcome of surgical procedures generally383 and on bone healing specifically.384 Identification of a nutritional deficit using anthropomorphic measurements, serum albumin levels, lymphocyte count, skin antigen testing, and nitrogen balance studies can be important in selected patients. Recent weight loss, anergy to skin testing, serum albumin levels less than 3.4 mg/dL, or a total lymphocyte count of less than 1500 are clinical red flags indicating the need for a careful nutritional evaluation and a possible need for nutritional support.385 Lenke and colleagues386 documented that patients undergoing multiple level spinal fusion procedures may take 6 to 12 weeks to recover from the perioperative nutritional insult and suggested more aggressive nutritional assessment in these patients.
Because most evidence suggests that the critical period in determining the success of a fusion attempt occurs in the first 3 to 7 days of healing, manipulation of systemic factors should be carefully controlled during this time period, especially the administration of radiation,387 chemotherapeutic agents,388 nonsteroidal anti-inflammatory drugs,29,31 and corticosteroids. Tobacco use, specifically nicotine, is a clinical factor that is both reversible and most strongly associated with negative results.294,389–394
Local Factors Influencing Spinal Fusion
Many local factors also influence bone healing, and a partial list of these is shown in Table 67–3. In some cases these factors are unavoidable. In other cases, rational methods can be employed to limit the negative effects. Osteoporosis is generally assumed to be an undesirable factor in fracture healing, but this is without direct clinical evidence. This is probably true but could relate to both mechanical and biologic factors. The quality of internal fixation is significantly affected by bone mass and is an important variable in the outcome of spinal fusions. Furthermore, it has been reported that the quality of the local bone marrow and other regional tissues in terms of the concentration, prevalence, and biologic potential of local osteogenic stem cells and progenitors may be reduced in the elderly patient.41,43,100 These age-related changes may or may not be directly related to the pathophysiology of osteoporosis but likely have a negative impact on the biology of the graft site for spinal arthrodesis. These effects may be partly reversed by strategies that allow concentration of osteogenic cells from bone marrow or other tissues.
| Positive Factors | Negative Factors |
|---|---|
| Increased surface area (bone and viable local tissue) | Osteoporosis |
| Local stem cell sources (e.g., bone marrow, periosteum) | Radiation scar |
| Osteoconductive scaffold (e.g., fibrin clot or other matrix material) | Radiation |
| Mechanical stability | Denervation |
| Mechanical loading | Tumor |
| Factors promoting recruitment, activation, and proliferation of osteoblastic stem cells (e.g., platelet degranulation products including PDGF, EGF) | Marrow-packing disorder |
| Osteoinductive factors (e.g., BMPs) | Infection |
| Factors promoting angiogenesis (e.g., FGF, EGF, VEGF) | Local bone disease |
| Mechanical motion | |
| Electrical stimulation | Bone wax (other materials inducing foreign body reaction) |
BMPs, bone morphogenic proteins; EGF, epidermal growth factor; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor and epidermal growth factor; VEGF, vascular endothelial growth factor.
The mechanical stability of the graft site is generally a factor that the surgeon can control. Solid internal fixation increases the chances of achieving a successful fusion. The anatomic site, patient’s weight, patient’s activity level, and use of external immobilization are additional variables. The generally higher union rates seen in patients with spinal muscular atrophy395 and Duchenne muscular dystrophy396,397 may be the result of decreased voluntary motion and improved local mechanics.
Local tumor invasion can replace normal marrow and weaken bone, and it may directly invade the fusion site. These problems may be partly overcome by the use of special fixation techniques398 and adjuvant radiation and chemotherapy, depending on the individual tumor. Use of autologous bone or bone marrow is desirable, but harvest must be performed in a separate surgical field to prevent tumor seeding in the donor site.
Radiation is an adverse factor for bone healing, especially when administered perioperatively. This may be a function of its direct cytotoxic effects on proliferating cells or the intense vasculitis induced by radiation injury. Long after the acute phase, radiation-induced osteonecrosis and the dense hypovascular scar left in the radiation bed may leave a poor environment for fusion. In some cases, therefore, it may be advantageous to use free vascularized grafts and donor vessels outside the area of previous radiation to enhance the vascular supply of local tissues and the likelihood of a successful fusion. Emery and colleagues399–401 have shown that the timing of radiation after a spine fusion procedure has a significant effect on outcome and that radiation has the least adverse effect if given at least 3 weeks after grafting. Radiation was best timed to be performed either preoperatively or in the late postoperative period, avoiding the early postoperative period when vascular invasion of the graft site and proliferating osteogenic progenitors would be most vulnerable. Settings of marrow replacement or regional scarring secondary to radiation or other causes are perhaps most likely to benefit from methods designed to supplement or replace the local population of osteogenic cells. However, their value is as yet unproven in clinical trials.
Electrical stimulation has been shown to be of benefit in the treatment of nonunions,402,403 failed arthrodeses,404,405 and congenital pseudarthroses.406 Evidence indicates that it may also be useful in spinal fusions in animal models.407,408 Several of these clinical studies have been small series often without a randomized control population.409–412 A recent double-blind clinical trial in 201 evaluable patients found a benefit of electrical stimulation following uninstrumented posterolateral lumbar fusions, but only in women.413 Another randomized trial in 179 patients with both instrumented and uninstrumented posterolateral fusions found a union score of 85% among treated patients compared with 65% in placebo controls.414
Future Considerations
Optimizing the use of current and future grafting materials will increasingly require a detailed understanding of the cell biology, materials science, and engineering principles upon which tissue engineering strategies are based.41–43,124,415 Central to this process is the recognition that stem cell and progenitor populations that are capable of proliferating and differentiating to form new tissues are the direct or indirect target cells for all implantable osteoconductive biomaterials, all bioactive or osteoinductive proteins, and all methods for biophysical physical intervention (i.e., mechanical or electrical stimulation). Application of extracorporeal shock wave treatment to enhance spinal fusion is another promising technique.416
Key Points
1 McLain RF, Fleming JE, Boehm CA, et al. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87:2655-2661.
2 Vaccaro AR, Whang PG, Patel T, et al. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft for posterolateral lumbar arthrodesis: Minimum 4-year follow-up of a pilot study. Spine J. 2008;8:457-465.
3 Shields LB, Rague GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542-547.
4 Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86:1541-1558.
5 Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
1 Albee FH. Transplantation of a portion of the tibia into the spine for Pott’s disease. JAMA. 1911;57:885-886.
2 Hibbs RA. A report of fifty-nine cases of scoliosis treated by the fusion operation. J Bone Joint Surg Am. 1924;6:3.
3 Boden SD: The Marshall Urist Lecture: Clinical Applications of BMPs for Spine Fusion. Proceeding of the Pittsburgh Bone Symposium, Pittsburgh, 2003:425-443.
4 DePalma AF, Rothman RH. The nature of pseudarthrosis. Clin Orthop. 1968;59:113-118.
5 Eie N, Solgaard T, Kleppe H. The knee—elbow position in lumbar disc surgery: a review of complications. Spine. 1983;8:897-900.
6 May VRJr, Mauck WR. Exploration of the spine for pseudarthrosis following spinal fusion in the treatment of scoliosis. Clin Orthop. 1967;53:115-122.
7 O’Beirne J, O’Neill D, Gallagher J, et al. Spinal fusion for back pain: a clinical and radiological review. J Spinal Disord. 1992;5:32-38.
8 Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop. 1992;284:80-90.
9 Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results. Spine. 1993;18:983-991.
10 Bos GD, Goldberg VM, Gordon NH, et al. The long-term fate of fresh and frozen orthotopic bone allografts in genetically defined rats. Clin Orthop. 1985:245-254.
11 Burwell RG. The fate of bone graft. In: Apley GA, editor. Recent Advances in Orthopaedics. London: Churchill; 1969:115-207.
12 Urist MR. Bone and bone transplants. In: Urist MR, editor. Fundamental and Clinical Physiology of Bone. Philadelphia: Saunders; 1980:131.
13 Beresford JN, Graves SE, Smoothy CA. Formation of mineralized nodules by bone derived cells in vitro: a model of bone formation? Am J Med Genet. 1993;45:163-178.
14 Bosch P, Musgrave DS, Lee JY, et al. Osteoprogenitor cells within skeletal muscle. J Orthop Res. 2000;18:933-944.
15 Halvorsen YC, Wilkison WO, Gimble JM. Adipose-derived stromal cells–their utility and potential in bone formation. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S41-S44.
16 Katzburg S, Lieberherr M, Ornoy A, et al. Isolation and hormonal responsiveness of primary cultures of human bone-derived cells: gender and age differences. Bone. 1999;25:667-673.
17 Lopez MJ, McIntosh K, Spencer ND, et al. Acceleration of spinal fusion using syngeneic and allogeneic adult adipose derived stem cells in a rat model. J Orthop Res. 2009;27:1-8.
18 Robey PG. Collagenase-treated trabecular bone fragments: a reproducible source of cells in the osteoblastic lineage. Calcif Tissue Int. 1995;56(Suppl 1):S11-S12.
19 Sheyn D, Pelled G, Zilberman Y, et al. Nonvirally engineered porcine adipose tissue-derived stem cells: use in posterior spinal fusion. Stem Cells. 2008;26:1056-1064.
20 Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
21 Villanueva JE, Nimni ME. Promotion of calvarial cell osteogenesis by endothelial cells. J Bone Miner Res. 1990;5:733-739.
22 Brighton CT, Lorich DG, Kupcha R, et al. The pericyte as a possible osteoblast progenitor cell. Clin Orthop. 1992;275:287-299.
23 Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477-488.
24 Gronthos S, Simmons PJ. The growth factor requirements of STRO-1-positive human bone marrow stromal precursors under serum-deprived conditions in vitro. Blood. 1995;85:929-940.
25 Simon SR, editor. Orthopaediac Basic Science, ed 2. Rosemont, IL: American Academy of Orthopaedic Surgeons. 1999:284-293.
26 Cruess RL. Healling of bone, tendon, and ligament. In: Rockwood CA, Green DP, editors. Fractures. Philadelphia, IL: JB Lippincott Co; 1984:153.
27 Prolo DJ, Rodrigo JJ. Contemporary bone graft physiology and surgery. Clin Orthop. 1985;200:322-342.
28 Simmons DJ. Fracture healing perspectives. Clin Orthop. 1985;200:100-113.
29 Glassman SD, Rose SM, Dimar JR, et al. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23:834-838.
30 Keller JC, Trancik TM, Young FA, et al. Effects of indomethacin on bone ingrowth. J Orthop Res. 1989;7:28-34.
31 Long J, Lewis S, Kuklo T, et al. The effect of cyclooxygenase-2 inhibitors on spinal fusion. J Bone Joint Surg Am. 2002;84-A:1763-1768.
32 McLaren AC. Prophylaxis with indomethacin for heterotopic bone. After open reduction of fractures of the acetabulum. J Bone Joint Surg Am. 1990;72:245-247.
33 Nilsson OS, Bauer HC, Brosjo O, et al. Influence of indomethacin on induced heterotopic bone formation in rats. Importance of length of treatment and of age. Clin Orthop. 1986;207:239-245.
34 Riew KD, Long J, Rhee J, et al. Time-dependent inhibitory effects of indomethacin on spinal fusion. J Bone Joint Surg Am. 2003;85-A:632-634.
35 Sucato DJ, Welch RD, Pierce B, et al. Thoracoscopic discectomy and fusion in an animal model: safe and effective when segmental blood vessels are spared. Spine. 2002;27:880-886.
36 Brown MD, Malinin TI, Davis PB. A roentgenographic evaluation of frozen allografts versus autografts in anterior cervical spine fusions. Clin Orthop. 1976;119:231-236.
37 Allen BLJr, Ferguson RL. The operative treatment of myelomeningocele spinal deformity—1979. Orthop Clin North Am. 1979;10:845-862.
38 Curtis BH. Orthopaedic management of muscular dystrophy and related disorders. Instr Course Lect. 1970;19:78-89.
39 Lim TH, Kwon H, Jeon CH, et al. Effect of endplate conditions and bone mineral density on the compressive strength of the graft-endplate interface in anterior cervical spine fusion. Spine. 2001;26:951-956.
40 Muschler G, Lane JM. Clinical applications of bone grafts in orthopaedic surgery. In: Habal MB, Reddi RH, editors. Bone Grafts and Bone Substitutes. Philadelphia: WB Saunders, 1992.
41 Muschler G, Nakamoto C, Griffith L. The engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86-A:1541-1558.
42 Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Ortho. 2002;395:66-80.
43 Muschler GF, Midura RJ, Nakamoto C. Practical modeling concepts for connective tissue stem cell and progenitor compartment kinetics. J Biomed Biotechnol. 2003;2003:170-193.
44 Heiple KG, Chase SW, Herndon CH. A comparative study of the healing process following different types of bone transplantation. J Bone Joint Surg Am. 1963;45-A:1592.
45 Oikarinen J, Korhonen LK. The bone inductive capacity of various bone transplanting materials used for treatment of experimental bone defects. Clin Orthop Relat Res. 1979;140:208-215.
46 Tuli SM. Bridging of bone defects by massive bone grafts in tumorous conditions and in osteomyelitis. Clin Orthop. 1972;87:60-73.
47 Wilson PD, Lance EM. Surgical reconstruction of the skeleton following segmental resection for bone tumors. J Bone Joint Surg Am. 1965;47:1629-1656.
48 Ackerman SJ, Mafilios MS, Polly DWJr. Economic evaluation of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion: an evidence-based modeling approach. Spine. 2002;27:S94-S99.
49 Hu RW, Bohlman HH. Fracture at the iliac bone graft harvest site after fusion of the spine. Clin Orthop Relat Res. 1994;309:208-213.
50 Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192-195.
51 Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983;174:28-42.
52 Enneking WF, Burchardt H, Puhl JJ, et al. Physical and biological aspects of repair in dog cortical-bone transplants. J Bone Joint Surg Am. 1975;57:237-252.
53 Takeda M. Experience in posterior lumbar interbody fusion: unicortical versus bicortical autologous grafts. Clin Orthop Relat Res. 1985;193:120-126.
54 Dell PC, Burchardt H, Glowczewskie FPJr. A roentgenographic, biomechanical, and histological evaluation of vascularized and non-vascularized segmental fibular canine autografts. J Bone Joint Surg Am. 1985;67:105-112.
55 Shaffer JW, Field GA, Goldberg VM, et al. Fate of vascularized and nonvascularized autografts. Clin Orthop Relat Res. 1985;197:32-43.
56 Weiland AJ, Moore JR, Daniel RK. Vascularized bone autografts. Experience with 41 cases. Clin Orthop Relat Res. 1983;174:87-95.
57 Weiland AJ, Phillips TW, Randolph MA. Bone grafts: a radiologic, histologic, and biomechanical model comparing autografts, allografts, and free vascularized bone grafts. Plast Reconstr Surg. 1984;74:368-379.
58 Hayashi A, Maruyama Y, Okajima Y, et al. Vascularized iliac bone graft based on a pedicle of upper lumbar vessels for anterior fusion of the thoraco-lumbar spine. Br J Plast Surg. 1994;47:425-430.
59 Hubbard LF, Herndon JH, Buonanno AR. Free vascularized fibula transfer for stabilization of the thoracolumbar spine. A case report. Spine. 1985;10:891-893.
60 Lascombes P, Grosdidier G, Olry R, et al. Anatomical basis of the anterior vertebral graft using a pediculated rib. Surg Radiol Anat. 1991;13:259-263.
61 Hartman JT, McCarron RF, Robertson WWJr. A pedicle bone grafting procedure for failed lumbosacral spinal fusion. Clin Orthop Relat Res. 1983;178:223-227.
62 McBride GG, Bradford DS. Vertebral body replacement with femoral neck allograft and vascularized rib strut graft. A technique for treating post-traumatic kyphosis with neurologic deficit. Spine. 1985;8:406-415.
63 Rose GK, Owen R, Sanderson JM. Transposition of rib with blood supply for the stabilization of spinal kyphosis. J Bone Joint Surg Am. 1975;57-B:112.
64 Bradford DS. Anterior vascular pedicle bone grafting for the treatment of kyphosis. Spine. 1980;5:318-323.
65 Goujon E. Researches experimentals sur les proprietes physiologiques de la moelle des os. Journal de l’Anatomie et de Physiologie Normales et Pathologiques de I’Homme et des Animaux. 1869;6:399.
66 Senn S. On the healing of aseptic cavities by implantation of antiseptic decalcified bone. Am J Med Sci. 1989;98:219.
67 Burwell RG. Studies in the transplantation of bone. VII. The composite homograft-autograft of cancellous bone. An analysis of factors leading to osteogenesis in marrow transplants and marrow containing bone graft. J Bone Joint Surg Am. 1964;46B:110.
68 Burwell RG. The function of bone marrow in the incorporation of a bone graft. Clin Orthop. 1985;200:125-141.
69 Nade S. Clinical implications of cell function in osteogenesis. A reappraisal of bone-graft surgery. Ann R Coll Surg Engl. 1979;61:189-194.
70 Nade S. Osteogenesis after bone and bone marrow transplantation. II. The initial cellular events following transplantation of decalcified allografts of cancellous bone. Acta Orthop Scand. 1977;48:572-579.
71 Friedenstein A. Determined and inducible osteogenic precursor cells. Ciba Found Sympos (new series). 1973:170-185.
72 Ashton BA, Allen TD, Howlett CR, et al. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980;151:294-307.
73 Budenz RW, Bernard GW. Osteogenesis and leukopoiesis within diffusion-chamber implants of isolated bone marrow subpopulations. Am J Anat. 1980;159:455-474.
74 Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331-340.
75 Pfeiffer CA. Development of bone from transplanted marrow in mice. Anat Res. 1948;102:225.
76 Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327-359.
77 Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247.
78 Owen M. The origin of bone cells in the postnatal organism. Arthritis Rheum. 1980;23:1073-1080.
79 Vaughan J. Osteogenesis and haematopoiesis. Lancet. 1981;2:133-136.
80 Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317-330.
81 Lian JB, Stein GS. Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med. 1992;3:269-305.
82 Aubin JE. Osteogenic cell differentiation. In: Davies JE, editor. Bone Engineering. Toronto: Em Squared Inc; 2000:19-30.
83 Connolly J, Guse R, Lippiello L, et al. Development of an osteogenic bone-marrow preparation. J Bone Joint Surg Am. 1989;71:684-691.
84 Connolly JF, Guse R, Tiedeman J, et al. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop. 1991;266:259-270.
85 Healey JH, Zimmerman PA, McDonnell JM, et al. Percutaneous bone marrow grafting of delayed union and nonunion in cancer patients. Clin Orthop. 1990;256:280-285.
86 Paley D, Young MC, Wiley AM, et al. Percutaneous bone marrow grafting of fractures and bony defects. An experimental study in rabbits. Clin Orthop. 1986;208:300-312.
87 Ragni P, Lindholm TS, Lindholm TC. Vertebral fusion dynamics in the thoracic and lumbar spine induced by allogenic demineralized bone matrix combined with autogenous bone marrow. An experimental study in rabbits. Ital J Orthop Traumatol. 1987;13:241-251.
88 Takagi K, Urist MR. The role of bone marrow in bone morphogenetic protein-induced repair of femoral massive diaphyseal defects. Clin Orthop. 1982;171:224-231.
89 Tiedeman JJ, Connolly JF, Strates BS, et al. Treatment of nonunion by percutaneous injection of bone marrow and demineralized bone matrix. An experimental study in dogs. Clin Orthop. 1991;268:294-302.
90 Tiedeman JJ, Huurman WW, Connolly JF, et al. Healing of a large nonossifying fibroma after grafting with bone matrix and marrow. A case report. Clin Orthop. 1991;265:302-305.
91 Lane JM, Muschler GF, Werntz J, et al. The use of composite bone graft materials in a segmental femoral defect model in the rat. Ortho Clin North Am. 1988;2:57-58.
92 Yasko AW, Lane JM, Fellinger EJ, et al. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992;74:659-670.
93 Johnson KA, Howlett CR, Bellenger CR, et al. Osteogenesis by canine and rabbit bone marrow in diffusion chambers. Calcif Tissue Int. 1988;42:113-118.
94 Garg NK, Gaur S. Percutaneous autogenous bone-marrow grafting in congenital tibial pseudarthrosis. J Bone Joint Surg Br. 1995;77:830-831.
95 Garg NK, Gaur S, Sharma S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop Scand. 1993;64:671-672.
96 Salama R, Burwell RD, Dickson IR. Recombined grafts of bone and marrow. The beneficial effect upon osteogenesis of impregnating xenograft (heterograft) bone with autologous red marrow. J Bone Joint Surg Br. 1973;55:402-417.
97 Salama R, Weissman SL. The clinical use of combined xenografts of bone and autologous red marrow. A preliminary report. J Bone Joint Surg Br. 1978;60:111-115.
98 Majors AK, Boehm CA, Nitto H, et al. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546-557.
99 Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699-1709.
100 Muschler GF, Nitto H, Boehm C, et al. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117-125.
101 Curylo LJ, Johnstone B, Petersilge CA, et al. Augmentation of spinal arthrodesis with autologous bone marrow in a rabbit posterolateral spine fusion model. Spine. 1999;24:434-438. discussion 8-9
102 Kadiyala S, Kraus KH, Attawia M, et al. Rapid bone regeneration in femoral defects by an autologous osteoplogenitor cell concentrate prepared using an intraoperative selective cell retention technique. The 49th Orthopaedic Research Society. New Orleans. 2003. p 317
103 Kadiyala S, Kraus KH, Attawia M, et al. Use of intra-operative selective cell retention technique to regenerate canine femoral segmental defects. The 5th International Meeting of the Tissue Engineering Society. Kobe, Japan. 2002.
104 Muschler GF, Huber B, Ullman T, et al. Evaluation of bone-grafting materials in a new canine segmental spinal fusion model. J Orthop Res. 1993;11:514-524.
105 Muschler GF, Negami S, Hyodo A, et al. Evaluation of collagen ceramic composite graft materials in a spinal fusion model. Clin Orthop. 1996;328:250-260.
106 Muschler G, Boehm C, Easley K. The harvest of osteoblastic progenitors from human bone marrow by aspiration. The influence of aspiration volume. J BoneJoint Surg. 1997;79A:1699-1709.
107 Erdmann J, Kogler C, Diel I, et al. Age-associated changes in the stimulatory effect of transforming growth factor beta on human osteogenic colony formation. Mech Ageing Dev. 1999;110:73-85.
108 Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305-311.
109 Nishida S, Endo N, Yamagiwa H, et al. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171-177.
110 McLain RF, Fleming JE, Boehm CA, et al. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87:2655-2661.
111 Grosse S, Argenson C, Tavna A, et al. Mineralized collagen aas a replacement for autogenous bone in posteolateral lumber spine fusion. Europian Spine Journal. 1998;8(Suppl. 1):S27.
112 Volenec FJ. Healos: Osteobiology and Clinical Experience. Proceeding of the Pittsburgh Bone Symposium, Pittsburgh. 2003:pp 713-721.
113 Muschler GF, Nitto H, Matsukura Y, et al. Spine Fusion Using Cell Matrix Composites Enriched in Bone Marrow-Derived Cells. Clin Orthop. 2003;407:102-118.
114 Youssef J, Brodke M, Haynesworth S, et al. Selective Cell Retention Technology in Spinal Fusion. Eighteenth Annual Meeting of North American Spine Society, San Diego. 2003.
115 Boden SD, Hair GA, Viggeswarapu M, et al. Gene therapy for spine fusion. Clin Orthop Relat Res. 2000;379(Suppl):S225-S233.
116 Boden SD, Titus L, Hair G, et al. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1). Spine. 1998;23:2486-2492.
117 Cui Q, Ming Xiao Z, Balian G, et al. Comparison of lumbar spine fusion using mixed and cloned marrow cells. Spine. 2001;26:2305-2310.
118 Kim HS, Viggeswarapu M, Boden SD, et al. Overcoming the immune response to permit ex vivo gene therapy for spine fusion with human type 5 adenoviral delivery of the LIM mineralization protein-1 cDNA. Spine. 2003;28:219-226.
119 Lieberman JR. Orthopaedic gene therapy. Fracture healing and other nongenetic problems of bone. Clin Orthop. 2000;379(Suppl):S156-S158.
120 Lieberman JR, Ghivizzani SC, Evans CH. Gene transfer approaches to the healing of bone and cartilage. Mol Ther. 2002;6:141-147.
121 Riew KD, Lou J, Wright NM, et al. Thoracoscopic intradiscal spine fusion using a minimally invasive gene-therapy technique. J Bone Joint Surg Am. 2003;85-A:866-871.
122 Wang JC, Kanim LE, Yoo S, et al. Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Bone Joint Surg Am. 2003;85-A:905-911.
123 Abjornson C, Lane JM. Bone allograft preparations: A critical review. Proceeding of the Pittsburgh Bone Symposium, Pittsburgh. 2003. 353-363
124 Bauer TW, Muschler GF. one graft materials. An overview of the basic science. Clin Orthop. 2000;371:10-27.
125 Stevenson S. The immune response to osteochondral allografts in dogs. J Bone Joint Surg Am. 1987;69:573-582.
126 Stevenson S, Hohn RB, Templeton JW. Effects of tissue antigen matching on the healing of fresh cancellous bone allografts in dogs. Am J Vet Res. 1983;44:201-206.
127 Tomford WW, Starkweather RJ, Goldman MH. A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Joint Surg Am. 1981;63:244-248.
128 Bos GD, Goldberg VM, Zika JM, et al. Immune responses of rats to frozen bone allografts. J Bone Joint Surg Am. 1983;65:239-246.
129 Chalmers J. Transplantation immunity in bone homografting. J Bone Joint Surg Am. 1959;41-B:160-179.
130 Friedlaender GE, Mankin HJ. Bone Banking: current methods and suggested guidelines. A.A.O.S. Instr Course Lect. 1981;30:36-55.
131 Friedlaender GE, Strong DM, Sell KW. Studies in antigenicity of bone. I. Freeze-dried and deep frozen allografts in rabbits. J Bone Joint Surg Am. 1976;58-A:854-858.
132 Friedlaender GE, Strong DM, Sell KW. Studies on the antigenicity of bone. II. Donor-specific anti-HLA antibodies in human recipients of freeze-dried allografts. J Bone Joint Surg Am. 1984;66:107-112.
133 Halloran PF, Lee EH, Ziv I, et al. Orthotopic bone transplantation in mice. II. Studies of the alloantibody response. Transplantation. 1979;27:420-426.
134 Langer F, Czitrom A, Pritzker KP, et al. The immunogenicity of fresh and frozen allogeneic bone. J Bone Joint Surg Am. 1975;57:216-220.
135 Lee EH, Langer F, Halloran P. The immunology of osteochondral and massive allografts. Trans Orthop Res Soc. 1979;4:61.
136 Muscolo DL, Kawai S, Ray RD. Cellular and humoral immune response analysis of bone-allografted rats. J Bone Joint Surg Am. 1976;58:826-832.
137 Pelker RR, Friedlaender GE, Markham TC. Biomechanical properties of bone allografts. Clin Orthop Relat Res. 1983;174:54-57.
138 Bos GD, Goldberg VM, Powell AE, et al. The effect of histocompatibility matching on canine frozen bone allografts. J Bone Joint Surg Am. 1983;65:89-96.
139 Muscolo DL, Caletti E, Schajowicz F, et al. Tissue-typing in human massive allografts of frozen bone. J Bone Joint Surg Am. 1987;69:583-595.
140 Fideler BM, Vangsness CTJr, Moore T, et al. Effects of gamma irradiation on the human immunodeficiency virus. A study in frozen human bone-patellar ligament-bone grafts obtained from infected cadavera. J Bone Joint Surg Am. 1994;76:1032-1035.
141 Swenson CL, Arnoczky SP. Demineralization for inactivation of infectious retrovirus in systemically infected cortical bone: in vitro and in vivo experimental studies. J Bone Joint Surg Am. 2003;85-A:323-332.
142 Cornell CN, Lane JM, Nottebeart M. The effect of ethylene oxide sterilization upon the bone inductive properties of demineralized bone matrix. Orth Transactions. 1987;30:1.
143 Doherty MJ, Mollan RA, Wilson DJ. Effect of ethylene oxide sterilization on human demineralized bone. Biomaterials. 1993;14:994-998.
144 Ijiri S, Yamamuro T, Nakamura T, et al. Effect of sterilization on bone morphogenetic protein. J Orthop Res. 1994;12:628-636.
145 Thoren K, Aspenberg P. Ethylene oxide sterilization impairs allograft incorporation in a conduction chamber. Clin Orthop Relat Res. 1995;318:259-264.
146 Dziedzic-Goclawska A, Ostrowski K, Stachowicz W, et al. Effect of radiation sterilization on the osteoinductive properties and the rate of remodeling of bone implants preserved by lyophilization and deep-freezing. Clin Orthop. 1991:30-37.
147 Zhang Q, Cornu O, Delloye C. Ethylene oxide does not extinguish the osteoinductive capacity of demineralized bone. A reappraisal in rats. Acta Orthop Scand. 1997;68:104-108.
148 Akkus O, Rimnac CM. Fracture resistance of gamma radiation sterilized cortical bone allografts. J Orthop Res. 2001;19:927-934.
149 Cornu O, Banse X, Docquier PL, et al. Effect of freeze-drying and gamma irradiation on the mechanical properties of human cancellous bone. J Orthop Res. 2000;18:426-431.
150 Fideler BM, Vangsness CTJr, Lu B, et al. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23:643-646.
151 Godette GA, Kopta JA, Egle DM. Biomechanical effects of gamma irradiation on fresh frozen allografts in vivo. Orthopedics. 1996;19:649-653.
152 Hamer AJ, Strachan JR, Black MM, et al. Biochemical properties of cortical allograft bone using a new method of bone strength measurement: A comparison of fresh, fresh-frozen and irradiated bone. J Bone Joint Surg Br. 1996;78:363-368.
153 Jinno T, Miric A, Feighan J, et al. The effects of processing and low dose irradiation on cortical bone grafts. Clin Orthop Relat Res. 2000;375:275-285.
154 Loty B, Courpied JP, Tomeno B, et al. Bone allografts sterilised by irradiation. Biological properties, procurement and results of 150 massive allografts. Int Orthop. 1990;14:237-242.
155 Sugimoto M, Takahashi S, Toguchida J, et al. Changes in bone after high-dose irradiation. Biomechanics and histomorphology. J Bone Joint Surg Br. 1991;73:492-497.
156 Bowen JR, Angus PD, Huxster RR, et al. Posterior spinal fusion without blood replacement in Jehovah’s Witnesses. Clin Orthop Relat Res. 1985;198:284-288.
157 Brantigan JW. Pseudarthrosis rate after allograft posterior lumbar interbody fusion with pedicle screw and plate fixation. Spine. 1994;19:1271-1279. discussion 80
158 Fernyhough JC, White JI, LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16:S561-S564.
159 Jorgenson SS, Lowe TG, France J, et al. A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient. A minimum of 1-year follow-up in 144 patients. Spine. 1994;19:2048-2053.
160 Kozak JA, Heilman AE, O’Brien JP. Anterior lumbar fusion options. Technique and graft materials. Clin Orthop Relat Res. 1994;300:45-51.
161 Nugent PJ, Dawson EG. Intertransverse process lumbar arthrodesis with allogeneic fresh-frozen bone graft. Clin Orthop Relat Res. 1993;287:107-111. 1993
162 Oikarinen J. Experimental spinal fusion with decalcified bone matrix and deep-frozen allogeneic bone in rabbits. Clin Orthop Relat Res. 1982;162:210-218.
163 Stabler CL, Eismont FJ, Brown MD, et al. Failure of posterior cervical fusions using cadaveric bone graft in children. J Bone Joint Surg Am. 1985;67:371-375.
164 Tenholder MJ, Kneisl JS, Harrow ME, et al. Biomechanical effects of processing bulk allograft bone with negative-pressure washing. Am J Orthop. 2003;32:289-297.
165 Wetzel FT, Hoffman MA, Arcieri RR. Freeze-dried fibular allograft in anterior spinal surgery: cervical and lumber applications. Yale J Biol Med. 1995;66:263-275.
166 Zdeblick TA, Cooke ME, Wilson D, et al. Anterior cervical discectomy, fusion, and plating. A comparative animal study. Spine. 1993;18:1974-1983.
167 Zdeblick TA, Ducker TB. The use of freeze-dried allograft bone for anterior cervical fusions. Spine. 1991;16:726-729.
168 Aurori BF, Weierman RJ, Lowell HA, et al. Pseudarthrosis after spinal fusion for scoliosis. A comparison of autogeneic and allogeneic bone grafts. Clin Orthop. 1985;199:153-158.
169 Bridwell KH, Lenke LG, McEnery KW, et al. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine. 1995;20:1410-1418.
170 Bridwell KH, O’Brien MF, Lenke LG, et al. Posterior spinal fusion supplemented with only allograft bone in paralytic scoliosis. Does it work? Spine. 1994;19:2658-2666.
171 Collis JS. Total disc replacement: a modified posterior lumbar interbody fusion. Report of 750 cases. Clin Orthop Relat Res. 1985;193:64-67.
172 Fabry G. Allograft versus autograft bone in idiopathic scoliosis surgery: a multivariate statistical analysis. J Pediatr Orthop. 1991;11:465-468.
173 Gepstein R, Nakamura K, Latta M. Posterior spinal fusion using preserved bone allografts. Trans Orthop Res Soc. 1986;8(Suppl. 1):73-76.
174 Grossman W, Peppelman WC, Baum JA, et al. The use of freeze-dried fibular allograft in anterior cervical fusion. Spine. 1992;17:565-569.
175 Malinin TI, Rosomoff HL, Sutton CH. Human cadaver femoral head homografts for anterior cervical spine fusions. Surg Neurol. 1977;7:249-251.
176 McCarthy RE, Peek RD, Morrissy RT, et al. Allograft bone in spinal fusion for paralytic scoliosis. J Bone Joint Surg Am. 1986;68:370-375.
177 Nasca RJ, Whelchel JD. Use of cryopreserved bone in spinal surgery. Spine. 1987;12:222-227.
178 Savolainen S, Usenius JP, Hernesniemi J. Iliac crest versus artificial bone grafts in 250 cervical fusions. Acta Neurochir (Wien). 1994;129:54-57.
179 Schneider JR, Bright RW. Anterior cervical fusion using preserved bone allografts. Transplant Proc. 1976;8:73-76.
180 Tiedeman JJ, Garvin KL, Kile TA, et al. The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics. 1995;18:1153-1158.
181 Whitehill R, Wilhelm CE, Moskal JT, et al. Posterior strut fusions to enhance immediate postoperative cervical stability. Spine. 1986;11:6-13.
182 Young WF, Rosenwasser RH. An early comparative analysis of the use of fibular allograft versus autologous iliac crest graft for interbody fusion after anterior cervical discectomy. Spine. 1993;18:1123-1124.
183 Cohen DB, Chotivichit A, Fujita T, et al. Pseudarthrosis repair. Autogenous iliac crest versus femoral ring allograft. Clin Orthop Relat Res. 2000;371:46-55.
184 Janssen ME, Nguyen C, Beckham R, et al. Biological cages. Eur Spine J. 2000;9(Suppl 1):S102-S109.
185 Kleinstueck FS, Hu SS, Bradford DS. Use of allograft femoral rings for spinal deformity in adults. Clin Orthop. 2002:84-91.
186 Liljenqvist U, O’Brien JP, Renton P. Simultaneous combined anterior and posterior lumbar fusion with femoral cortical allograft. Eur Spine J. 1998;7:125-131.
187 Siff TE, Kamaric E, Noble PC, et al. Femoral ring versus fibular strut allografts in anterior lumbar interbody arthrodesis. A biomechanical analysis. Spine. 1999;24:659-665.
188 Deaver JB. Secondary bone implantation by a modification of Senn’s method. Med News. 1889;55:714.
189 Kaiser MG, Haid RWJr, Subach BR, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50:229-236. discussion 36-38
190 Kummer FJ, Chen D, Spivak JM. Optimal selection and preparation of fresh frozen corticocancellous allografts for cervical interbody spinal fusion. Spine. 1998;23:2295-2298.
191 Lofgren H, Johannsson V, Olsson T, et al. Rigid fusion after cloward operation for cervical disc disease using autograft, allograft, or xenograft: a randomized study with radiostereometric and clinical follow-up assessment. Spine. 2000;25:1908-1916.
192 Martin GJJr, Haid RWJr, MacMillan M, et al. Anterior cervical discectomy with freeze-dried fibula allograft. Overview of 317 cases and literature review. Spine. 1999;24:852-858. discussion 8-9
193 McKoy BE, Wingate JK, Poletti SC, et al. Fibular allograft after anterior cervical corpectomy: long term follow-up. Iowa Orthop J. 2002;22:42-46.
194 McLaughlin MR, Purighalla V, Pizzi FJ. Cost advantages of two-level anterior cervical fusion with rigid internal fixation for radiculopathy and degenerative disease. Surg Neurol. 1997;48:560-565.
195 Parthiban JK, Singhania BK, Ramani PS. A radiological evaluation of allografts (ethylene oxide sterilized cadaver bone) and autografts in anterior cervical fusion. Neurol India. 2002;50:17-22.
196 Shapiro S, Bindal R. Femoral ring allograft for anterior cervical interbody fusion: technical note. Neurosurgery. 2000;47:1457-1459.
197 Vaccaro AR, Cirello J. The use of allograft bone and cages in fractures of the cervical, thoracic, and lumbar spine. Clin Orthop Relat Res. 2002;394:19-26.
198 Chen L, Tang T, Yang H. Complications associated with posterior lumbar interbody fusion using Bagby and Kuslich method for treatment of spondylolisthesis. Chin Med J (Engl). 2003;116:99-103.
199 DeBerard MS, Colledge AL, Masters KS, et al. Outcomes of posterolateral versus BAK titanium cage interbody lumbar fusion in injured workers: a retrospective cohort study. J South Orthop Assoc. 2002;11:157-166.
200 Hacker RJ, Cauthen JC, Gilbert TJ, et al. A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine. 2000;25:2646-2654. discussion 55
201 McAfee PC, Fedder IL, Saiedy S, et al. SB Charite disc replacement: report of 60 prospective randomized cases in a US center. J Spinal Disord Tech. 2003;16:424-433.
202 McAfee PC, Lee GA, Fedder IL, et al. Anterior BAK instrumentation and fusion: complete versus partial discectomy. Clin Orthop. 2002;394:55-63.
203 Merk H, Koch H, Liebau C, et al. [Implantation of a Harms titanium mesh cylinder for vertebral body replacement in spinal metastases]. Z Orthop Ihre Grenzgeb. 2000;138:169-173.
204 Togawa D, Bauer TW, Brantigan JW, et al. Bone graft incorporation in radiographically successful human intervertebral body fusion cages. Spine. 2001;26:2744-2750.
205 Togawa D, Bauer TW, Lieberman IH, et al. Histology of tissues within retrieved human titanium mesh cages. Spine. 2003;28:246-253. discussion 54
206 Espersen JO, Buhl M, Eriksen EF, et al. Treatment of cervical disc disease using Cloward’s technique. I. General results, effect of different operative methods and complications in 1,106 patients. Acta Neurochir (Wien). 1984;70:97-114.
207 Bostrom MP, Camacho NP. Potential role of bone morphogenetic proteins in fracture healing. Clin Orthop. 1998:S274-S282.
208 Sciadini MF, Johnson KD. Evaluation of recombinant human bone morphogenetic protein-2 as a bone-graft substitute in a canine segmental defect model. J Orthop Res. 2000;18:289-302.
209 Mackie W. Clinical observation of the healling of aseptic bone cavities by Senn’s method of implantation of antiseptic decalcified bone. Med News. 1890;57:202.
210 Miller AC. A case of bone grafting with decalcified bone chips. Lancet. 1890;2:618.
211 Weir RF. Antiseptic irrigation for synovitis of the bone: implantation of mucous membrane in traumatic structure of the urethra.Implantation of Bone. Med News. 1890;56:125.
212 Reddi AH. Bone matrix in the solid state geometric influence on differentiation of fibroblasts. In: Lawrence JH, Gotman JW, editors. Advances in Biological Medical Physics. New York: Academic Press; 1973:1.
213 Reddi AH, Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972;69:1601-1605.
214 Urist MR. Bone: formation by autoinduction. Science. 1965;150:893-899.
215 Urist MR, Hay PH, Dubuc F, et al. Osteogenetic competence. Clin Orthop Relat Res. 1969;64:194-220.
216 Urist MR, Iwata H, Ceccotti PL, et al. Bone morphogenesis in implants of insoluble bone gelatin. Proc Natl Acad Sci U S A. 1973;70:3511-3515.
217 Urist MR, Nakagawa M, Nakata N, et al. Experimental myositis ossificans: cartilage and bone formation in muscle in response to a diffusible bone matrix-derived morphogen. Arch Pathol Lab Med. 1978;102:312-316.
218 Urist MR, Silverman BF, Buring K, et al. The bone induction principle. Clin Orthop. 1967;53:243-283.
219 Van de Putte KA, Urist MR. Osteogenesis in the interior of intramuscular implants of decalcified bone matrix. Clin Ortho. 1966;43:257.
220 Chalmers J, Gray DH, Rush J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br. 1975;57:36-45.
221 Nathanson MA. Analysis of cartilage differentiation from skeletal muscle grown on bone matrix. III. Environmental regulation of glycosaminoglycan and proteoglycan synthesis. Dev Biol. 1983;96:46-62.
222 Nathanson MA, Hay ED. Analysis of cartilage differentiation from skeletal muscle grown on bone matrix. II. Chondroitin sulfate synthesis and reaction to exogenous glycosaminoglycans. Dev Biol. 1980;78:332-351.
223 Muthukumaran N, Reddi AH. Bone matrix-induced local bone induction. Clin Orthop. 1985;200:159-164.
224 Reddi AH. Cell biology and biochemistry of endochondral bone development. Coll Relat Res. 1981;1:209-226.
225 Reddi AH. Extracellular bone matrix dependent local induction of cartilage and bone. J Rheumatol Suppl. 1983;11:67-69.
226 Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone. 1996;19:1S-12S.
227 Mohan S, Baylink DJ. Bone growth factors. Clin Orthop Relat Res. 1991;263:30-48.
228 Sato K, Urist MR. Induced regeneration of calvaria by bone morphogenetic protein (BMP) in dogs. Clin Orthop Relat Res. 1985;197:301-311.
229 Urist MR, Dawson E. Intertransverse process fusion with the aid of chemosterilized autolyzed antigen-extracted allogeneic (AAA) bone. Clin Orthop. 1981;154:97-113.
230 Glowacki J, Altobelli D, Mulliken JB. Fate of mineralized and demineralized osseous implants in cranial defects. Calcif Tissue Int. 1981;33:71-76.
231 Glowacki J, Kaban LB, Murry JE. Application of the biological principle of induced osteogenesis for craniofacial defects. Lancet. 1981;93:959.
232 Kaban LB, Mulliken JB, Glowacki J. Treatment of jaw defects with demineralized bone implants. J Oral Maxillofac Surg. 1982;40:623.
233 Wilkins RM, Stringer EA. Demineralized bone powder. use in grafting space-occupying lesions of bone. Internat Orthop. 1994;2:71-78.
234 Martin GJJr, Boden SD, Titus L, et al. New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine. 1999;24:637-645.
235 Morone MA, Boden SD. Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel. Spine. 1998;23:159-167.
236 Flatley TJ, Lynch KL, Benson M. Tissue response to implants of calcium phosphate ceramic in the rabbit spine. Clin Orthop. 1983;179:246-252.
237 Frenkel SR, Moskovich R, Spivak J, et al. Demineralized bone matrix. Enhancement of spinal fusion. Spine. 1993;18:1634-1639.
238 Lindholm TS, Ragni P, Lindholm TC. Response of bone marrow stroma cells to demineralized cortical bone matrix in experimental spinal fusion in rabbits. Clin Orthop. 1988:296-302.
239 Lynch KL, LadWig DA, Skrade DA. Evaluation of collagen/ceramic bone graft substitutes in dogs with spinal fixation. Transactions of the Society for Biomaterials. 1990;13:196.
240 Zerwekh JE, Kourosh S, Scheinberg R, et al. Fibrillar collagen-biphasic calcium phosphate composite as a bone graft substitute for spinal fusion. J Orthop Res. 1992;10:562-572.
241 Boyce T, Edwards J, Scarborough N. Allograft bone. The influence of processing on safety and performance. Orthop Clin North Am. 1999;30:571-581.
242 Chakkalakal DA, Strates BS, Garvin KL, et al. Demineralized bone matrix as a biological scaffold for bone repair. Tissue Eng. 2001;7:161-177.
243 Elves MW, Salama R. A study of the development of cytotoxic antibodies produced in recipients of xenografts (heterografts) of iliac bone. J Bone Joint Surg Br. 1974;56:331-339.
244 Plank H, Hollman K, Wilfert KH. Experimental bridging of osseous defects in rats by the implantation of Kiel bone containing fresh autologous marrow. J Bone Joint Surg. 1972;54B:735.
245 Rawlinson JN. Morbidity after anterior cervical decompression and fusion. The influence of the donor site on recovery, and the results of a trial of surgibone compared to autologous bone. Acta Neurochir (Wien). 1994;131:106-118.
246 Costantino PD, Friedman CD. Synthetic bone graft substitutes. Otolaryngol Clin North Am. 1994;27:1037-1074.
247 Vacanti CA, Vacanti JP. Bone and cartilage reconstruction with tissue engineering approaches. Otolaryngol Clin North Am. 1994;27:263-276.
248 Urist MR, Mikulski A, Leitz A. Solubilized and insolubilized bone morphogenic protein. Proc Natl Acad Sci U S A. 1978;76:1828.
249 Urist MR, Lietze A, Mizutani H, et al. A bovine low molecular weight bone morphogenetic protein (BMP) fraction. Clin Orthop Relat Res. 1982;162:219-232.
250 Lovell TP, Dawson EG, Nilsson OS, Urist MR. Augmentation of spinal fusion with bone morphogenetic protein in dogs. Clin Orthop Relat Res. 1989;243:266-274.
251 Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528-1534.
252 Kessler E, Takahara K, Biniaminov L, et al. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 1996;271:360-362.
253 Reddi AH. BMP-1: resurrection as procollagen C-proteinase. Science. 1996;271:463.
254 Rosen V, Wozney JM. Bone morphogenetic protein. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2nd ed. San Diego: Academic Press; 2002:919-928.
255 Wozney JM. The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev. 1992;32:160-167.
256 Hruska KA, Guo G, Wozniak M, et al. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol. 2000;279:F130-F143.
257 Vukicevic S, Basic V, Rogic D, et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest. 1998;102:202-214.
258 Bahamonde ME, Lyons KM. BMP3: To Be or Not To Be a BMP. J Bone Joint Surg Am. 2001;83:S56-S62.
259 Daluiski A, Engstrand T, Bahamonde ME, et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27:84-88.
260 Miyazono K. Bone morphogenetic protein receptors and actions. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2nd ed. San Diego: Academic Press; 2002:929-942.
261 Abreu JG, Ketpura NI, Reversade B, et al. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599-604.
262 Bostrom MP, Saleh KJ, Einhorn TA. Osteoinductive growth factors in preclinical fracture and long bone defects models. Orthop Clin North Am. 1999;30:647-658.
263 Brunet LJ, McMahon JA, McMahon AP, et al. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455-1457.
264 Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A:1032-1044.
265 Merino R, Macias D, Ganan Y, et al. Expression and function of Gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Dev Biol. 1999;206:33-45.
266 Tsumaki N, Nakase T, Miyaji T, et al. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. J Bone Miner Res. 2002;17:898-906.
267 Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 2003;85-A:1544-1552.
268 Kuniyasu H, Hirose Y, Ochi M, et al. Bone augmentation using rhGDF-5-collagen composite. Clin Oral Implants Res. 2003;14:490-499.
269 Settle SHJr, Rountree RB, Sinha A, et al. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol. 2003;254:116-130.
270 Boden SD, Martin GJJr, Horton WC, et al. Laparoscopic anterior spinal arthrodesis with rhBMP-2 in a titanium interbody threaded cage. J Spinal Disord. 1998;11:95-101.
271 Boden SD, Martin GJJr, Morone M, et al. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine. 1999;24:320-327.
272 Boden SD, Martin GJJr, Morone MA, et al. Posterolateral lumbar intertransverse process spine arthrodesis with recombinant human bone morphogenetic protein 2/hydroxyapatite-tricalcium phosphate after laminectomy in the nonhuman primate. Spine. 1999;24:1179-1185.
273 Boden SD, Moskovitz PA, Morone MA, et al. Video-assisted lateral intertransverse process arthrodesis. Validation of a new minimally invasive lumbar spinal fusion technique in the rabbit and nonhuman primate (rhesus) models. Spine. 1996;21:2689-2697.
274 Boden SD, Schimandle JH, Hutton WC. 1995 Volvo Award in basic sciences. The use of an osteoinductive growth factor for lumbar spinal fusion. Part II: Study of dose, carrier, and species. Spine. 1995;20:2633-2644.
275 Boden SD, Schimandle JH, Hutton WC: Evaluation of a bovine-derived osteoinductive bone protein in a non-human primate model of lumbar spinal fusion. The 42nd Annual Meeting of the Orthopaedic Research Society, Atlanta 1996, p 118.
276 Boden SD, Schimandle JH, Hutton WC. Lumbar intertransverse-process spinal arthrodesis with use of a bovine bone-derived osteoinductive protein. A preliminary report. J Bone Joint Surg Am. 1995;77:1404-1417.
277 Boden SD, Schimandle JH, Hutton WC, et al. In vivo evaluation of a resorbable osteoinductive composite as a graft substitute for lumbar spinal fusion. J Spinal Disord. 1997;10:1-11.
278 Cook SD. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics. 1999;22:669-671.
279 Cunningham BW, Kanayama M, Parker LM, et al: Osteogenic protein versus autologous fusion in the sheep thoracic spine. A comparative endoscopic study using the BAK interbody fusion device. The 42nd Annual Meeting of the Orthopaedic Research Society. Atlanta, 1996, p 117.
280 Cunningham BW, Kanayama M, Parker LM, et al. Osteogenic protein versus autologous interbody arthrodesis in the sheep thoracic spine. A comparative endoscopic study using the Bagby and Kuslich interbody fusion device. Spine. 1999;24:509-518.
281 Damien CJ, Grob D, Boden SD, et al. Purified bovine BMP extract and collagen for spine arthrodesis: preclinical safety and efficacy. Spine. 2002;27:S50-S58.
282 David SM, Gruber HE, Meyer RAJr., et al. Lumbar spinal fusion using recombinant human bone morphogenetic protein in the canine. A comparison of three dosages and two carriers. Spine. 1999;24:1973-1979.
283 David SM, Gruber HE, Murakami T, et al. Lumbar spinal fusion using recombinant human bone morphogenetic protein (rhBMP-2): a randomized, blinded and controlled study. the 42nd Annual Meeting of the Orthopaedic Research Society, Atlanta. 1996. pp 119-120
284 Grauer JN, Patel TC, Erulkar JS, et al. 2000 Young Investigator Research Award winner. Evaluation of OP-1 as a graft substitute for intertransverse process lumbar fusion. Spine. 2001;26:127-133.
285 Holliger EH, Trawick RH, Boden SD, et al. Morphology of the lumbar intertransverse process fusion mass in the rabbit model: a comparison between two bone graft materials–rhBMP-2 and autograft. J Spinal Disord. 1996;9:125-128.
286 Magin MN, Delling G. Improved lumbar vertebral interbody fusion using rhOP-1: a comparison of autogenous bone graft, bovine hydroxylapatite (Bio-Oss), and BMP-7 (rhOP-1) in sheep. Spine. 2001;26:469-478.
287 Martin GJJr, Boden SD, Marone MA, et al. Posterolateral intertransverse process spinal arthrodesis with rhBMP-2 in a nonhuman primate: important lessons learned regarding dose, carrier, and safety. J Spinal Disord. 1999;12:179-186.
288 Martin GJJr, Boden SD, Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a nonsteroidal anti-inflammatory drug (NSAID), on posterolateral lumbar intertransverse process spine fusion. Spine. 1999;24:2188-2193. discussion 93-94
289 Meyer RAJr, Gruber HE, Howard BA, et al. Safety of recombinant human bone morphogenetic protein-2 after spinal laminectomy in the dog. Spine. 1999;24:747-754.
290 Paramore CG, Lauryssen C, Rauzzino MJ, et al. The safety of OP-1 for lumbar fusion with decompression—a canine study. Neurosurgery. 1999;44:1151-1155. discussion 5-6
291 Poynton AR, Lane JM. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine. 2002;27:S40-S48.
292 Sandhu HS, Kanim LEA, Kabo JM, et al. Effective doses of recombinant bone morphogenetic protein in experimental spinal fusion. the 42nd Annual Meeting of the Orthopaedic Research Society, Atlanta. 1996. p 116
293 Schimandle JH, Boden SD, Hutton WC. Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine. 1995;20:1326-1337.
294 Silcox DH3rd, Boden SD, Schimandle JH, et al. Reversing the inhibitory effect of nicotine on spinal fusion using an osteoinductive protein extract. Spine. 1998;23:291-296. discussion 7
295 Suh DY, Boden SD, Louis-Ugbo J, et al. Delivery of recombinant human bone morphogenetic protein-2 using a compression-resistant matrix in posterolateral spine fusion in the rabbit and in the non-human primate. Spine. 2002;27:353-360.
296 Muschler GF, Hyodo A, Manning T, et al. Evaluation of human bone morphogenetic protein 2 in a canine spinal fusion model. Clin Orthop. 1994;308:229-240.
297 Cook SD, Dalton JE, Tan EH, et al. In vivo evaluation of recombinant human osteogenic protein (rhOP-1) implants as a bone graft substitute for spinal fusions. Spine. 1994;19:1655-1663.
298 Valdes M, Moore DC, Palumbo M, et al. rhBMP-6 stimulated osteoprogenitor cells enhance posterolateral spinal fusion in the New Zealand white rabbit. Spine J. 2007;7:318-325.
299 Boden SD, Kang J, Sandhu H, et al. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial. Spine. 2002;27:2662-2673.
300 Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376-381.
301 Burkus JK, Gornet MF, Dickman CA, et al. Anterior Lumbar Interbody Fusion Using rhBMP-2 With Tapered Interbody Cages. J Spinal Disord Tech. 2002;15:337-349.
302 Laursen M, Hoy K, Hansen ES, et al. Recombinant bone morphogenetic protein-7 as an intracorporal bone growth stimulator in unstable thoracolumbar burst fractures in humans: preliminary results. Eur Spine J. 1999;8:485-490.
303 McKay B, Sandhu HS. Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine. 2002;27:S66-S85.
304 Sandhu HS, Khan SN. Recombinant human bone morphogenetic protein-2: use in spinal fusion applications. J Bone Joint Surg Am. 2003;85-A(Suppl 3):89-95.
305 Vaccaro AR, Anderson DG, Toth CA. Recombinant human osteogenic protein-1 (bone morphogenetic protein-7) as an osteoinductive agent in spinal fusion. Spine. 2002;27:S59-S65.
306 Vaccaro AR, Whang PG, Patel T, et al. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft for posterolateral lumbar arthrodesis: Minimum 4-year follow-up of a pilot study. Spine J. 2008;8:457-465.
307 Singh K, Smucker JD, Gill S, Boden SD. Use of recombinant human bone morphogenetic protein-2 as an adjunct in posterolateral lumbar spine fusion: A prospective CT-scan analysis at one and two years. J Spinal Disord Tech. 2006;19:416-423.
308 Kanayama M, Hashimoto T, Shigenobu K, et al. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 versus local autograft with ceramic bone substitute: Emphasis of surgical exploration and histologic assessment. Spine. 2006;31:1067-1074.
309 Lewandrowski KU, Nancon C, Calderon R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: a report of five cases. Spine J. 2007;7:609-614.
310 Vaidya R, Sethi A, Bartol S, et al. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21:557-562.
311 Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7:301-307.
312 Glassman SD, Carreon LY, Djurasovic M, et al. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: a randomized, controlled trial in patients over sixty years of age. Spine. 2008;15(33):2843-2849.
313 Buttermann. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8:426-435.
314 Tumialán LM, Pan J, Rodts GE, et al. The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenetic protein-2: a review of 200 patients. J Neurosurg Spine. 2008;8:529-535.
315 Vaidya R, Carp J, Sethi A, et al. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257-1265.
316 Shields LB, Raque GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542-547.
317 Smucker JD, Rhee JM, Singh K, et al. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31:2813-2819.
318 Takigami H, Latson L, Togawa D, et al. Bone Formation Following OP-1 Implantation is Improved by Autogenous Bone Marrow. American Society for Bone and Mineral Research 25th Annual Meeting, Minneapolis. 2003.
319 Arteaga-Solis E, Gayraud B, Lee SY, et al. Regulation of limb patterning by extracellular microfibrils. J Cell Biol. 2001;154:275-281.
320 Larrain J, Oelgeschlager M, Ketpura NI, et al. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128:4439-4447.
321 Ohkawara B, Iemura S, ten Dijke P, et al. Action range of BMP is defined by its N-terminal basic amino acid core. Curr Biol. 2002;12:205-209.
322 Issa JP, Bentley MV, Iyomasa MM, et al. Sustained release carriers used to delivery bone morphogenetic proteins in the bone healing process. Anat Histol Embryol. 2008;37:181-187.
323 Akamaru T, Suh D, Boden SD, et al. Simple carrier matrix modifications can enhance delivery of recombinant human bone morphogenetic protein-2 for posterolateral spine fusion. Spine. 2003;28:429-434.
324 Minamide A, Kawakami M, Hashizume H, et al. Evaluation of carriers of bone morphogenetic protein for spinal fusion. Spine. 2001;26:933-939.
325 Seeherman H, Wozney J, Li R. Bone morphogenetic protein delivery systems. Spine. 2002;27:S16-S23.
326 Takigami H, Kumagai K, Latson L, et al. Bone formation following OP-1 implantation is improved by addition of autogenous bone marrow cells in a canine femur defect model. J Orthop Res. 2007;25:1333-1342.
327 Louis-Ugbo J, Kim HS, Boden SD, et al. Retention of 125I-labeled recombinant human bone morphogenetic protein-2 by biphasic calcium phosphate or a composite sponge in a rabbit posterolateral spine arthrodesis model. J Orthop Res. 2002;20:1050-1059.
328 Uludag H, D’Agusta D, Golden J, et al. Implantation of human recombinant bone morphogenetic proteins with biomaterial carriers: A correlation between protein pharmacokinetics and osteoinduction in the rat ectopic model. J Biomed Mat Res. 2000;50:227-238.
329 Ruhe PQ, Hedberg EL, Padron NT, et al. rhBMP-2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am. 2003;85-A(Suppl 3):75-81.
330 Seeherman H, Li R, Wozney J. A review of preclinical program development for evaluating injectable carriers for osteogenic factors. J Bone Joint Surg Am. 2003;85-A(Suppl 3):96-108.
331 Brekke JH, Toth JM. Principles of tissue engineering applied to programmable osteogenesis. J Biomed Mater Res. 1998;43:380-398.
332 Rosen V. Growth Factors Involced in Bone Formation and Repair. Proceeding of the Pittsburgh Bone Symposium, Pittsburgh. 2003. pp 69-80
333 Radomsky ML, Aufdemorte TB, Swain LD, et al. Novel formulation of fibroblast growth factor-2 in a hyaluronan gel accelerates fracture healing in nonhuman primates. J Orthop Res. 1999;17:607-614.
334 Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757-1763.
335 Zelzer E, McLean W, Ng YS, et al. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893-1904.
336 Bouletreau PJ, Warren SM, Spector JA, et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg. 2002;109:2384-2397.
337 Eckardt H, Bundgaard KG, Christensen KS, et al. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. J Orthop Res. 2003;21:335-340.
338 Peng H, Wright V, Usas A, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751-759.
339 Bonewald LF. Transforming growth factor-beta. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2nd ed. San Diego: Academic Press; 2002:903-918.
340 Bonewald LF, Mundy GR. Role of transforming growth factor-beta in bone remodeling. Clin Orthop. 1990:261-276.
341 Pfeilschifter J, Wolf O, Naumann A, et al. Chemotactic response of osteoblastlike cells to transforming growth factor beta. J Bone Miner Res. 1990;5:825-830.
342 Joyce ME, Roberts AB, Sporn MB, et al. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110:2195-2207.
343 Baylink DJ, Finkelman RD, Mohan S. Growth factors to stimulate bone formation. J Bone Miner Res. 1993;8(Suppl 2):S565-S572.
344 Canalis E. The hormonal and local regulation of bone formation. Endocr Rev. 1983;42:62.
345 Delany AM, Pash JM, Canalis E. Cellular and clinical perspectives on skeletal insulin-like growth factor I. J Cell Biochem. 1994;55:328-333.
346 Kandziora F, Schmidmaier G, Schollmeier G, et al. IGF-I and TGF-beta1 application by a poly-(D,L-lactide)-coated cage promotes intervertebral bone matrix formation in the sheep cervical spine. Spine. 2002;27:1710-1723.
347 Lane JM, Sandhu HS. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18:213-225.
348 Majors AK, Ehrhart LA, Muschler GF: Basic fibroblast growth factor enhances proliferation and reversibly inhibits osteoblastic differentiation of human bone marrow stromal cell in culture. submitted.
349 Palecek SP, Loftus JC, Ginsberg MH, et al. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537-540.
350 Boyan-Salyers BD, Boskey AL. Relationship between proteolipids and calcium-phospholipid-phosphate complexes in Bacterionema matruchotii calcification. Calcif Tissue Int. 1980;30:167-174.
351 Nawrot CF, Campbell DJ, Schroeder JK, et al. Dental phosphoprotein-induced formation of hydroxylapatite during in vitro synthesis of amorphous calcium phosphate. Biochemistry. 1976;15:3445-3449.
352 Stanford CM, Jacobson PA, Eanes ED, et al. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP). J Biol Chem. 1995;270:9420-9428.
353 Termine JD, Kleinman HK, Whitson SW, et al. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99-105.
354 Veis A. The role of acidic proteins in biological mineralization. Ions in macromolecular and biological systems. In: Everett DH, Vicent B, editors. Colston Paper 29. Bristol: Society Technics; 1978:259-272.
355 Howlett CR, Evans MD, Walsh WR, et al. Mechanism of initial attachment of cells derived from human bone to commonly used prosthetic materials during cell culture. Biomaterials. 1994;15:213-222.
356 Hughes DE, Salter DM, Dedhar S, et al. Integrin expression in human bone. J Bone Miner Res. 1993;8:527-533.
357 Saito T, Albelda SM, Brighton CT. Identification of integrin receptors on cultured human bone cells. J Orthop Res. 1994;12:384-394.
358 Weiss RE, Reddi AH. Role of fibronectin in collagenous matrix-induced mesenchymal cell proliferation and differentiation in vivo. Exp Cell Res. 1981;133:247-254.
359 Mundy GR, Poser JW. Chemotactic activity of the gamma-carboxyglutamic acid containing protein in bone. Calcif Tissue Int. 1983;35:164-168.
360 Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop. 1981:259-278.
361 Jarcho M, Kay JF, Gumaer KI, et al. Tissue, cellular and subcellular events at a bone-ceramic hydroxylapatite interface. J Bioeng. 1977;1:79-92.
362 Canalis E. Effect of growth factors on bone cell replication and differentiation. Clin Orthop. 1985;193:246-263.
363 Dennison HW, de Groot K. Immediate dental root implants from synthetic dense calcium hydroxyapatite. J Prosthet Dent. 1979;42:511.
364 Dennison HW, de Groot K, Kakkas P. Animal and human studies of sintered hydroxyapatite as a material for tooth root implants (abstract). the First World Biomaterial Congress. Baden, Austria. 1980.
365 Kent J, James R, Finger I, et al. Augmentation of deficient edentulous alveolar ridges with dense polycrystalline hydroxyapatite (abstract). the First World Biomaterial Congress. Baden, Austria. 1980.
366 Klawitter JJ, Hulbert SF. Application of porous ceramics for the attachment of load bearing orthopaedic applications. J Biomed Mater Res. 1971;2:161.
367 Hoogendoorn HA, Renooij W, Akkermans LM, et al. Long-term study of large ceramic implants (porous hydroxyapatite) in dog femora. Clin Orthop Relat Res. 1984;187:281-288.
368 Grower MF, Haron M, Miller R, et al. Bone inductive potential of biodegradable ceramic in millipore filter chambers. J Dent Res. 1973;52:160.
369 Rejda BV, Peelen JG, de Groot K. Tri-calcium phosphate as a bone substitute. J Bioeng. 1977;1:93-97.
370 Ragni P, Lindholm TS. Interaction of allogeneic demineralized bone matrix and porous hydroxyapatite bioceramics in lumbar interbody fusion in rabbits. Clin Orthop. 1991:292-299.
371 Cameron HU, Macnab I, Pilliar RM. Evaluation of biodegradable ceramic. J Biomed Mater Res. 1977;11:179-186.
372 Bhaskar SN, Brady JM, Getter L. Biodegradable ceramic implants in bone. Oral Surg. 1980;32:294.
373 Ohgushi H, Goldberg VM, Caplan AI. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res. 1989;7:568-578.
374 Hing KA, Wilson LF, Buckland T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007;7:475-490.
375 Pintar FA, Maiman DJ, Hollowell JP, et al. Fusion rate and biomechanical stiffness of hydroxylapatite versus autogenous bone grafts for anterior discectomy. An in vivo animal study. Spine. 1994;19:2524-2528.
376 Zdeblick TA, Cooke ME, Kunz DN, et al. Anterior cervical discectomy and fusion using a porous hydroxyapatite bone graft substitute. Spine. 1994;19:2348-2357.
377 Constantz BR, Ison IC, Fulmer MT, et al. Skeletal repair by in situ formation of the mineral phase of bone. Science. 1995;267:1796-1799.
378 Dai LY, Jiang LS. Single-level instrumented posterolateral fusion of lumbar spine with beta-tricalcium phosphate versus autograft: a prospective, randomized study with 3-year follow-up. Spine. 2008;33:1299-1304.
379 Chen WJ, Tsai TT, Chen LH, et al. The fusion rate of calcium sulfate with local autograft bone compared with autologous iliac bone graft for instrumented short-segment spinal fusion. Spine. 2005;30:2293-2297.
380 Acharya NK, Kumar RJ, Varma HK, Menon VK. Hydroxyapatite-bioactive glass ceramic composite as stand-alone graft substitute for posterolateral fusion of lumbar spine: a prospective, matched, and controlled study. J Spinal Disord Tech. 2008;21:106-111.
381 Epstein NE. An analysis of noninstrumented posterolateral lumbar fusions performed in predominantly geriatric patients using lamina autograft and beta tricalcium phosphate. Spine J. 2008;8:882-887.
382 Gan Y, Dai K, Zhang P, et al. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29:3973-3982.
383 Einhorn TA, Bonnarens F, Burstein AH. The contributions of dietary protein and mineral to the healing of experimental fractures. A biomechanical study. J Bone Joint Surg Am. 1986;68:1389-1395.
384 Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66:71-75.
385 Jensen JE, Jensen TG, Smith TK, et al. Nutrition in orthopaedic surgery. J Bone Joint Surg Am. 1982;64:1263-1272.
386 Lenke LG, Bridwell KH, Blanke K, et al. Prospective analysis of nutritional status normalization after spinal reconstructive surgery. Spine. 1995;20:1359-1367.
387 Coventry MB, Scanlon PW. The use of radiation to discourage ectopic bone. A nine-year study in surgery about the hip. J Bone Joint Surg Am. 1981;63:201-208.
388 Nilsson OS, Bauer HC, Brostrom LA. Methotrexate effects on heterotopic bone in rats. Acta Orthop Scand. 1987;58:47-53.
389 Andersen T, Christensen FB, Laursen M, et al. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine. 2001;26:2623-2628.
390 Glassman SD, Anagnost SC, Parker A, et al. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 2000;25:2608-2615.
391 Patel TC, Erulkar JS, Grauer JN, et al. Osteogenic protein-1 overcomes the inhibitory effect of nicotine on posterolateral lumbar fusion. Spine. 2001;26:1656-1661.
392 Silcox DH3rd, Daftari T, Boden SD, et al. The effect of nicotine on spinal fusion. Spine. 1995;20:1549-1553.
393 Theiss SM, Boden SD, Hair G, et al. The effect of nicotine on gene expression during spine fusion. Spine. 2000;25:2588-2594.
394 Wing KJ, Fisher CG, O’Connell JX, et al. Stopping nicotine exposure before surgery. The effect on spinal fusion in a rabbit model. Spine. 2000;25:30-34.
395 Aprin H, Bowen JR, MacEwen GD, et al. Spine fusion in patients with spinal muscular atrophy. J Bone Joint Surg Am. 1982;64:1179-1187.
396 Bunch WH. Muscular dystrophy. In: Hardy JH, editor. Spinal Deformity in Neurological and Muscular Disorders. St. Louis, MO: C.V. Mosby Co.; 1974:92-110.
397 Swank SM, Brown JC, Perry RE. Spinal fusion in Duchenne’s muscular dystrophy. Spine. 1982;7:484-491.
398 Clark CR, Keggi KJ, Panjabi MM. Methylmethacrylate stabilization of the cervical spine. J Bone Joint Surg Am. 1984;66:40-46.
399 Bouchard JA, Koka A, Bensusan JS, et al. Effects of irradiation on posterior spinal fusions. A rabbit model. Spine. 1994;19:1836-1841.
400 Emery SE, Brazinski MS, Koka A, et al. The biological and biomechanical effects of irradiation on anterior spinal bone grafts in a canine model. J Bone Joint Surg Am. 1994;76:540-548.
401 Emery SE, Hughes SS, Junglas WA, et al. The fate of anterior vertebral bone grafts in patients irradiated for neoplasm. Clin Orthop. 1994;300:207-212.
402 Bassett CAL, Mitchell SN, Gaston SR. Treatment of ununited tibial diaphyseal fractures with pulsing electromagnetic fields. J Bone Joint Surg. 1981;63-A:511.
403 Paterson D. Treatment of nonunion with a constant direct current: a totally implantable system. Orthop Clin North Am. 1984;15:47-59.
404 Bassett CA. The development and application of pulsed electromagnetic fields (PEMFs) for ununited fractures and arthrodeses. Orthop Clin North Am. 1984;15:61-87.
405 Bassett CA, Mitchell SN, Gaston SR. Pulsing electromagnetic field treatment in ununited fractures and failed arthrodeses. Jama. 1982;247:623-628.
406 Bassett CA, Pilla AA, Pawluk RJ. A non-operative salvage of surgically-resistant pseudarthroses and non-unions by pulsing electromagnetic fields. A preliminary report. Clin Orthop. 1977:128-143.
407 Bozic KJ, Glazer PA, Zurakowski D, et al. In vivo evaluation of coralline hydroxyapatite and direct current electrical stimulation in lumbar spinal fusion. Spine. 1999;24:2127-2133.
408 Toth JM, Seim HB3rd, Schwardt JD, et al. Direct current electrical stimulation increases the fusion rate of spinal fusion cages. Spine. 2000;25:2580-2587.
409 Kahanovitz N, Arnoczky SP, Hulse D, et al. The effect of postoperative electromagnetic pulsing on canine posterior spinal fusions. Spine. 1984;9:273-279.
410 Kucharzyk DW. A controlled prospective outcome study of implantable electrical stimulation with spinal instrumentation in a high-risk spinal fusion population. Spine. 1999;24:465-468. discussion 9
411 Nerubay J, Marganit B, Bubis JJ, et al. Stimulation of bone formation by electrical current on spinal fusion. Spine. 1986;11:167-169.
412 Simmons JW. Treatment of failed posterior lumbar interbody fusion (PLIF) of the spine with pulsing electromagnetic fields. Clin Orthop. 1985:127-132.
413 Linovitz RJ, Pathria M, Bernhardt M, et al. Combined magnetic fields accelerate and increase spine fusion: a double-blind, randomized, placebo controlled study. Spine. 2002;27:1383-1389. discussion 9
414 Goodwin CB, Brighton CT, Guyer RD, et al. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine. 1999;24:1349-1356. discussion 57
415 Fleming JEJr, Cornell CN, Muschler GF. Bone cells and matrices in orthopedic tissue engineering. Orthop Clin North Am. 2000;31:357-374.
416 Lee TC, Huang HY, Yang YL, et al. Application of extracorporeal shock wave treatment to enhance spinal fusion: a rabbit experiment. Surg Neurol. 2008;70:129-134.