Chapter 225 Principles of Antifungal Therapy
Due to advances in aggressive antineoplastic agents and organ transplantation, invasive fungal infections are a major cause of morbidity and mortality in children. Fortunately, the therapeutic armamentarium for invasive fungal infections has markedly increased since the turn of the century (![]() See Table 225-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com).
See Table 225-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com).
Azoles
Voriconazole
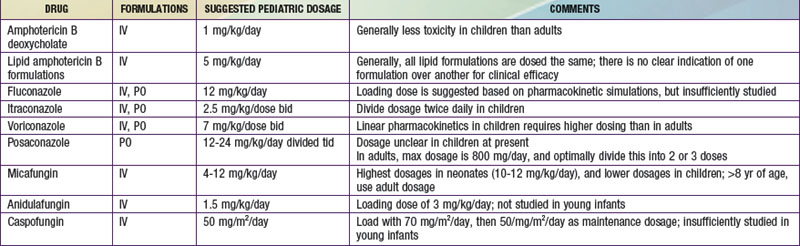
Voriconazole is available as an oral tablet, an oral suspension, and an intravenous solution. In adults, voriconazole exhibits nonlinear pharmacokinetics, has a variable half-life of approximately 6 hr with large interpatient variation in blood levels, and achieves good CSF penetration. In contrast to the situation in adults, elimination of voriconazole is linear in children. A multicenter safety, population pharmacokinetic study of intravenous voriconazole dosages in immunocompromised pediatric patients showed that body weight was more influential than age in accounting for the observed variability in voriconazole pharmacokinetics, and voriconazole needs to be dosed higher in pediatric patients than adult patients. Adult patients load with 6 mg/kg/dose and then transition to a maintenance dosage of 4 mg/kg/dose, but children should begin and continue with 7 mg/kg/dose (see Table 225-1). This need for an increased dosage in treating children is crucial to understand and is mandated by the fundamentally different pharmacokinetics of this drug in pediatric patients. Obtaining voriconazole serum levels is currently controversial, because it is unclear how to best interpret the results. Generally a trough level greater than the minimum inhibitory concentration (MIC) of the infecting organism is preferred, but much higher voriconazole levels have been associated with toxicity. The main side effects of voriconazole include reversible dosage-dependent visual disturbances (increased brightness, blurred vision) in as many as one third of treated patients, elevated hepatic transaminases with increasing dosages, and occasional skin reactions likely caused by photosensitization.
Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348-359.
Denning DW, Marr KA, Lau WM, et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect. 2006;53:337-349.
Hope WW, Smith PB, Arrieta A, et al. Population pharmacokinetics of micafungin in neonates and young infants. Antimicrob Agents Chemother. 2010;54:2633-2637.
Kaufman D, Boyle R, Hazen KC, et al. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660-1666.
Neely M, Jafri HS, Seibel N, et al. Pharmacokinetics and safety of caspofungin in older infants and toddlers. Antimicrob Agents Chemother. 2009;53:1450-1456.
Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503-535.
Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45:883-893.
Queiroz-Telles F, Berezin E, Leverger G, et al. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr Infect Dis J. 2008;27:820-826.
Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356:2472-2482.
Seibel NL, Schwartz C, Arrieta A, et al. Safety, tolerability, and pharmacokinetics of micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother. 2005;49:3317-3324.
Smith PB, Walsh TJ, Hope W, et al. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr Infect Dis J. 2009;28:412-415.
Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335-347.
Wade KC, Wu D, Kaufman DA, et al. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother. 2008;52:4043-4049.
Wermers RA, Cooper K, Razonable RR, et al. Fluoride excess and periostitis in transplant patients receiving long-term voriconazole therapy. CID. 2011;52:604-611.
Zaoutis TE, Jafri HS, Huang LM, et al. A prospective, multicenter study of caspofungin for the treatment of documented Candida or Aspergillus infections in pediatric patients. Pediatrics. 2009;123:877-884.
Zaoutis T, Lehrnbecher T, Groll AH, et al. Safety experience with caspofungin in pediatric patients. Pediatr Infect Dis J. 2009;28:1132-1136.