Chapter 2 Principles of Allergy Management
Core Concepts
In a strict sense, allergic load refers to the cumulative, clinically relevant, antigenic exposure affecting the patient at any one moment in time. Allergic load as a concept is important as it may directly influence the allergic response, both early and late phase inflammation, and expression of symptoms. Controlling allergic load therefore becomes an important tool in overall allergy management. Quantitative antigen exposure may vary from one point in time to another. For example, seasonal pollen counts may vary from one year to another and from day to day during any one year’s season. In addition, they are usually higher in the morning than in the evening, and will be reduced depending upon rainfall during the day. Dust mite exposure varies from time spent indoors versus outdoors, in humid versus arid climates, and as a function of elevation above sea level. Becoming familiar with the nature of antigens and antigen classes is essential in understanding and applying the concept of allergic load. This working knowledge directs history taking, the direction of diagnostic testing, and the formulation of a management action plan.
In addition to the importance of the allergic load, the concept of nasal priming further implies that the cumulative, clinically relevant antigenic exposure required to provoke symptoms tends to lessen as the patient is repeatedly challenged by antigen. From a basic immunologic standpoint, antigen-specific naïve lymphocytes may be primed when an antigen is presented to them in an immunogenic form (e.g., antigen incorporated in a presenting cell) which results in a differentiation into armed effector cells or memory cells capable of a second or subsequent immune response.1,2 Quantitatively, continued subsequent antigen exposures require less and less antigen to produce the response. Wachs et al describe nasal priming as “increased clinical response to daily nasal challenges.”3
Nasal priming is not antigen specific.4 Skoner et al discuss possible mechanisms for increased tissue responsiveness after antigen stimulation,5–10 and suggest how the priming effect and continued exposure to allergens would lead to prolonged and chronic inflammation with the promotion of chronic symptoms and comorbid conditions. With their work on Eustachian tube dysfunction, antigen exposure, and nasal priming, Skoner et al showed how inflammation continued for a period of time, up to weeks after cessation of pollen exposure, and theorized how this inflammation could possibly promote chronic Eustachian tube dysfunction and middle ear disease.4,5,11 In addition, only 23% of allergic rhinitic patients have pure seasonal rhinitis; 77% of allergic rhinitics, therefore, have perennial rhinitis, with or without seasonal flaring. Thus, seasonal antigens may add to chronic inflammation that already exists due to perennial antigenic exposure, which further amplifies the association with chronic, comorbid conditions such as middle ear disease, rhinosinusitis, and/or asthma.
While nasal priming was at one time thought to be specific only for antigenic triggering,12–14 other authors indicate this is not the case, for example citing histamine as a trigger of the phenomenon.5,15 Allergic and nonallergic triggering is therefore another essential concept in comprehending the additive nature of rhinitic and/or other respiratory inflammation. In assessing the history, seldom does the patient with allergic rhinitis solely have allergic triggers, and in some patients the rhinitis will be purely nonallergic. Vasomotor or idiopathic triggers may include, but are not limited to, temperature change (especially cold), exercise, barometric pressure change, gustatory stimulation, and chemical or irritant exposure. Infectious insults also augment inflammation in several ways. For example, allergic factors are suspected in the pathogenesis and expression of recurrent acute rhinosinusitis and acute exacerbations of chronic rhinosinusitis, which are often triggered by a viral respiratory infection. In addition to the direct inflammatory response to the viral particles, the allergic individual will reactively produce larger amounts of immunoglobulin during the acute illness. This effect is additive. Viral illness in the allergic individual versus the nonallergic tends to be more symptomatic, more morbid, and more likely to require an antibiotic and extended care for secondary bacterial sequellae. In addition, while Samter’s triad or aspirin (ASA) triad patients (nasal polyposis, asthma, and ASA and/or NSAID (nonsteroidal anti-inflammatory drugs) sensitivity) will usually not have allergic factors, a number of nasal polyp patients (including allergic fungal sinusitis (AFS) patients) may have concurrent allergic rhinitis. Previous studies lead the clinician to believe allergic management in these patients helps control the overall rhinitic impact, and improves the control of the hyperplastic membrane disease, approaching a level of control experienced with the nonallergic polyp patient. Other chronic diseases may trigger nonallergic rhinitis in the allergic patient, such as sarcoidosis, inflammatory bowel disease, granulomatous disease, and the neurogenic (trigeminal nerve/inferior salivary nucleus) mediated nasal congestion/lacrimation of some migraine/vascular headaches. Medication side effects may add to rhinitis symptoms. The Physicians’ Desk Reference cross-references side effects of sinusitis and rhinitis for 300 and 400 drugs respectively.16–18 In addition, nasal symptoms may have noninflammatory factors as well. For example, obstructive rhinitis or rhinosinusitis may have fixed mechanical component(s) such as a deviated nasal septum, paradoxical middle turbinate, concha bullosa, or some other anatomical form of adynamic obstruction. These adynamic factors act in concert with the more dynamic (mainly inflammatory) factors in producing the additive result of obstruction and other symptoms. In a more abstract sense, other medical comorbidities such as poorly controlled diabetes and immune deficiency, can amplify rhinitis symptoms.
Given the preceding discussion, some conclusions are suggested. In the broadest and most clinically relevant sense, the definition of allergic load now becomes the sum total of all environmentally and medically related factors which may impact the expression of allergic rhinitis and its sequellae in a given patient at any one point in time (Figure 2.1). Reducing the allergic load as a treatment modality therefore goes beyond the environmental control (EC) of allergens. It includes avoiding or controlling respiratory irritants and other vasomotor factors, managing respiratory infections, identifying and treating comorbid medical problems and other chronic inflammatory diseases, lessening medication side effects, and utilizing indicated airway operative procedures. An example in summary: the asthmatic allergic rhinitic with chronic rhinosinusitis, who smokes, has poorly controlled diabetes, and is on a beta blocker for poorly controlled migraine headache masquerading as a sinus headache, has a number of factors to deal with for optimum rhinitic outcome and quality of life.
Lastly, a discussion of the lower respiratory tract is necessary. Current models of allergic respiratory disease regard the respiratory tract not as divided upper and lower components, but as one unified system, responding in a homogeneous fashion to inflammation. The early conceptual work involved studies of allergic response. About 35% of patients with allergic rhinitis will develop asthma at some time in their life. Up to 95% of asthmatics have chronic rhinitis. Diagnosing asthma goes beyond bronchial hyperreactivity. There has to be an associated, reversible, obstructive functional change. That being said, most allergic rhinitics will demonstrate increased bronchial hyperreactivity during times of increased rhinitis and will be subject to priming and all of the conceptual nuances discussed thus far.5 As such with this unified airway concept in mind, the inflammatory problems of the respiratory tract to be examined in the bulk of the remaining chapters are rhinitis, rhinosinusitis, otitis, conjunctivitis, laryngitis, and asthma. With all of the previous comments noted, the last core concept to be discussed is the classification of antigens.
▪ Classification of Antigens
Inhalant allergens are categorized by phylogeny and to some extent by the times during the year in which the patient is exposed, i.e., perennial or seasonal (Box 2.1). The pollens are a broad group consisting of grasses, trees, and weeds. These antigens tend to be clinically significant on a seasonal basis. In temperate climates, grass pollination generally begins in the spring and extends into the summer. Tree pollination begins in the late winter, accelerates in the spring and declines in the summer. The weeds may begin in mid summer, accelerate in late summer and early fall, and decline in mid fall. Variations may occur on the basis of temperature, rainfall, and other environmental factors. For example a dry, cold, late winter–early spring, may delay pollen emergence or suppress quantity. However, in warmer climates, such as the southern USA, grass may pollinate almost on a perennial basis, or perhaps have two distinct seasons within a year’s time. For such locale-related inconsistencies in antigen description and other reasons, there is a current movement to describe allergic disease in terms of its chronicity as being intermittent versus persistent and its severity as being either mild, moderate, or severe. This description parallels current systems used in describing asthma. It is clear that clinicians need to be familiar with the antigens in their locale. Given the different routes of exposure through which allergens stimulate the patient, it is common to classify allergens by phylogeny and structure (e.g., pollens, dust components, fungi or molds, animal epithelials, insects, foods, venoms/drugs, and contactants). Some characteristics of these antigen classes will be discussed next. Additional characteristics will be presented in the section on environmental control (EC) and will be discussed then.
Plant Antigens (Pollen)
Pollen is the male germinal element of plant flora emitted during the reproductive phase. Such plants can be pollinated by the wind, known as anemophilous, or by insect transfer of heavier, stickier pollen, known as entomophilous. Most clinically significant pollens (mainly anemophilous) share certain characteristics that promote their clinical antigenicity, and are said to satisfy the elements of Thommen’s postulates listed in Box 2.2. Even though entomophilous plants do not produce light, buoyant, windborne pollen, some may still be clinically significant if the patient handles the pollen from the plant, or with a massive, direct exposure. Of the thousands of continental anemophilous plants, only a little over 100 appear to be clinically prominent. Due to climate, natural selection, and human management of the environment, numerous pollen and mold zones exist around the world. Up-to-date zonal pollen and mold information is available through the Internet, the printed literature, and through antigen extract and allergy testing device vendors. While university extension divisions can also be queried in narrowing the number of pollinating plant species of antigenic and clinical importance, this approach may be of limited utility. Box 2.3 demonstrates a collection of suggested antigens, including pollens by family that can be used for objective testing and screening purposes. Antigenic plants are generally classified as trees, grasses, or weeds.
BOX 2.2 Thommen’s postulates
The plant must be seed bearing, with pollen windborne
The pollen must be produced in large quantities
The pollen must be buoyant to be widely transported
The plant must be abundantly distributed, preferably close to human habitation
Trees
Tree pollination usually occurs most vigorously in the spring but varies according to latitude, longitude, and climatic influences. The seasons for each of the tree species tend to be short and well defined compared with the grasses. There may be cross-reactivity between closely related trees (genus), but as a rule, trees do not cross-react widely among different families. Therefore, antigen selection for testing and treatment may include a number of trees within the clinician’s area or other nonindigenous trees brought into the patient’s environment. Diameter of tree pollen is usually up to 40 μm. Trees are divided into two main groups, the larger flowering trees (angiosperms), and the cone-bearing trees (gymnosperms). Box 2.4 lists members of both of these classes with examples for each. Some of the members, marked in bold, may have implications as food sensitizers.
Grasses
Grass pollen is very potent, perhaps the most potent sensitizer among plant antigens. Only the fall weeds, specifically the ragweeds, sensitize more allergy patients seasonally. Of the thousands of grass species and their five major subfamilies of antigenic interest listed in Box 2.5, the three of most clinical significance are noted in bold with examples. There is a great deal of cross-reactivity within the subfamilies. As such, and with the high degree of potency and sensitizing capability, the number of grasses tested and treated should be limited to one member of each subfamily of importance within the patient’s environment. Testing and treating with multiple grasses in a more extensive fashion increases the risk of adverse reaction. The grass pollination season generally begins in the spring and continues into the summer. Seasons will vary depending upon latitude and climate. Shorter and better defined seasons may be found in temperate climates, as opposed to longer seasons for some species in warmer subtropical and tropical climates.
Weeds
The fall weed season has often been described using the term “hayfever” for the seasonal allergic rhinitis occurring at this time of year. Weeds usually pollinate in late summer into fall, but like the grasses, these seasons vary. They may overlap with grass, mold, and some tree seasons. Where prevalent, the ragweeds are the most significant pollen sensitizers. In most climates, the six families of most allergenic interest are listed in Box 2.6 with subfamilies and examples. There is some degree of cross-reactivity within subfamilies, thus there should be some economy in antigen selection for testing and treatment. In some cases, such as with the ragweeds, an extract of a ragweed mixture may be used. Some of the subfamily members are not readily windborne and are of lesser clinical importance. One such example cited is goldenrod. Minor weed subfamilies need to be considered, as there may be certain patients who will be exposed and sensitized to them.
Animal Antigens
Mammalian and avian antigens
Products of mammals and birds are also antigenic and may include epithelials such as feathers, hair, dander, pelt, saliva, or urine. In addition, even mold growing in aged feces can be antigenic. By far, cat seems to be the most significant sensitizer, of which dried saliva is of primary importance. In the atopic child, early exposure to mammalian antigen, especially cat, along with prematurity and exposure to wood burning appliances or cigarette smoke has been linked with the early emergence of allergic disease including asthma. Cat antigen is so ubiquitous in the environment and is such a potent allergen that it should always be screened for even if the patient does not come in known direct contact with the animal. This testing is especially important if the patient has reactive airways disease. Dog antigen may also be important, and antigenicity may vary from breed to breed. In some parts of the world, dog also may be a food allergen as well as inhalant allergen. When examining animal allergy, the patient history must extend beyond cat and dog. A thorough assessment of both the patient’s home and work environments is important, since patients in agriculture and laboratory settings may come into contact with mammalian species other than cat and dog.
Fungi – Yeast and Molds
The fungi may be antigenically important as inhalants, ingestants, or contactants. While fungi include yeasts and molds, they are referred to in this section globally as molds. Mold allergies may be perennial or seasonal, indoor or outdoor. In theory, molds may elicit a Gell-Coombs I (allergic) or IV (delayed) response. In the central USA, for example, mold activity is perennial outdoors, especially if the winter is warm. There are seasonal flares in the spring, with the return of warmer and rainy conditions; in mid to late summer with increased humidity and heat; and in the moderate warmth of fall, with the decay of foliage and the priming of the weed season. Indoors, mold is promoted by dampness, warmth, darkness, and any moist, organic material used as a source of nutrients. Although many tens of thousands of species are known, less than 100 are of allergenic significance. Clinically important molds are noted in Box 2.7. Four of the most common mold species found indoor/outdoor are Alternaria, Penicillium, Aspergillus, and Cladosporium. In addition, mold families that are clinically important in allergic fungal sinusitis (AFS) may differ from those involved in rhinitis. Box 2.8 demonstrates a list of molds often reported in cases of AFS. There is little cross-reactivity between mold families. The major molds in any one locale will be important in the evaluation of not only rhinitis, but also asthma. Buoyant mold spores, measuring up to 20 μm, are a common form of inhalant mold exposure, in addition to mycelial forms and yeasts, which may be contactants and/or ingestants as well. Mold spores may be transported over long distances by the wind, and are of higher concentration in the cool of the evening and during warm windy weather after precipitation. While 8–12 molds are often sufficient for testing, in the highly mold sensitive patient, the difficult to diagnose patient, or the patient who is not doing well under treatment due to a suspected unidentified mold, a more expanded mold test battery may be indicated. At times, it has been worthwhile setting out mold spore collection plates obtained from some extract vendors to identify the prevalent species in the difficult patient’s environment.
Ingestants
Fixed Food Allergy
Although virtually any protein with antigenic potential could cause an allergic reaction if ingested by a susceptible individual, ingestant allergy problems are usually foods or drugs. Only about 20–30% of adverse food reactions are felt to be IgE-mediated, and therefore allergic. The term adverse food reaction is inclusive of hypersensitivity reactions and the nonimmune food intolerance. To the patient, the two groups are undistinguishable. Most people feel that any food-related reaction is “allergic.” The hypersensitivity group, commonly called allergic, contains the true IgE-mediated reactions and other immune-related reactions which may or may not include an IgE participation. The overall incidence of food allergy has been estimated to be up to 8% in children and up to 2% in adults. The majority of documented food offenders in children include egg, peanut, soybean, fish, wheat, tree nuts, and milk. In adults the majority list would include tree nuts, peanut, fish, and shellfish. Members of food families often cross-react within specific families. True IgE-mediated clinical manifestations of food allergy would include urticaria and/or angioedema, respiratory symptoms including rhinosinusitis, laryngitis, Eustachian tube dysfunction, asthma, and conjunctival/scleral symptoms. Other IgE manifestations may include anaphylaxis, oral allergy syndrome (discussed later), atopic dermatitis, and eosinophilic GI disease. Patients with atopic dermatitis (eczema) may have IgE-mediated food sensitivities in an estimated 30–40% of cases. Only a portion of the eosinophilic GI patient’s symptoms may have IgE-mediated factors. Other suspected immune hypersensitivities include a few enteropathies and inflammatory bowel conditions described elsewhere. Food intolerance may include metabolic, toxic, or even functional/psychological conditions of nonhypersensitivity natures.19
Nonfixed food allergy
Food allergy may be rarely fixed, but is more commonly acquired in nature. Acquired food allergy is demonstrated in a susceptible individual, who begins to develop symptoms after repeated ingestion of a food. A current model for describing the conduct of this phenomenon is the cyclic nature of food allergy (Figure 2.2). In this model, after introduction and sensitization to the food, the patient begins to have symptoms with further ingestion of the food. If the ingestions are separated by intervals of time of several days or more, the symptoms may be easily identified with the ingestion. If the ingestions are very frequent, say daily, the symptoms may be more muted, diffuse and systemic, and more difficult to associate with the ingestion. Through elimination of the offending food, symptoms eventually improve/resolve, and in a period of months, the patient may become tolerant to the ingestion of the food. Tolerance may be maintained with infrequent ingestions, whereas the patient may cycle back to symptomatic expression if the interval of ingestion becomes more frequent. Fixed food allergy is always symptomatic and not affected by periods of abstinence from the food. Symptoms for peanut, seafood, and tree nuts generally do not improve with cessation and time.
Positive epicutaneous skin testing for food is often inaccurate, although negative epicutaneous tests may have better predictive ability. There is some controversy over the efficacy of in vitro methods for food testing, with IgG food testing only relevant for ingestion, not hypersensitivity. When feasible, any possible food allergen, identified by history or positive in vivo or in vitro test, should have its clinical significance determined by elimination over a period of 1–2 weeks resulting in improvement of symptoms, or the elimination for 5–7 days and the demonstration of symptoms with reintroduction over a 2-day period. Suspected fixed food allergies should not be challenged, and strict avoidance of those foods should be recommended. The other option, and the gold standard, for cyclic food allergy is the use of the double-blinded, placebo-controlled food challenge. This challenge also requires an elimination of the food to be tested for 1–2 weeks before the challenge.19
Drug Allergies
Most patient-reported “allergic reactions” to drugs are not allergic in nature. The range of real and perceived adverse reactions involving drugs is rather wide. Drug side effects, drug–drug interactions, dose related symptoms, and the like are not allergic in nature. Macy et al use the example of the non-IgE-mediated angioedema seen with ACE inhibitors as an idiosyncratic response.20 True IgE-mediated reactions involve an antigenic protein or hapten coupling to trigger the response. A common example of a true allergic reaction is that of penicillin sensitivity in which a penicillin metabolite couples with a protein to form a hapten which is immunogenic.20 Drugs may be involved in several Gell-Coombs types of reactions. Intravenous radiologic contrast sensitivity is an example of an anaphylactoid type response, i.e., a direct, nonallergic effect on mast cell release. IgE-mediated reactions have also been reported for a variety of hormonal proteins such as insulin. Skin testing, when prudent and possible, is still used to help with making a diagnosis. Unfortunately specific antigenic testing materials for drug testing are often not readily available. For example, not all of the determinants to be tested in penicillin allergy are made by commercial vendors. Cephalosporin minor determinant antigens are also not available for skin testing. In vitro testing is nonexistent or at best, limited. While avoidance of IgE-mediated drug allergens is the treatment of choice, there are times when graded challenges, provocation testing, and desensitization may be indicated for diagnosis and/or therapy.20 Lastly, drug reactions on occasion may be the subject of reactions to nonactive ingredients in the drug other than the active, named drug. These ingredients can include chemical additives or foods. Drug allergy is discussed in detail in Chapter 10.
Injectants
Allergic reaction by injection of allergenic material usually falls within the realm of drug allergy. Patient histories on occasion have indicated that oral ingestion of an allergen may not produce the reaction experienced with the injection of the drug. This observation is of limited clinical interest, since any true allergy to a drug by one route of exposure mandates avoidance of the use of that drug. Once again, some injectables have other nonactive ingredients which may be the provoking agent. Other injecting allergens may be via the puncture or sting of plants or animals. Reactions may be local, self-limited irritating responses, or delayed, more widespread immune responses, or an overwhelming systemic, IgE-mediated anaphylaxis. Allergic reactions to the stinging order of Hymenoptera (vespids and bees) are perhaps most important, followed by reactions to the fire ant. Distinction has to be made between a local reaction at the sting site versus a true allergic, more systemic or anaphylactic reaction. Desensitization in many cases is possible and indicated.21 Insect allergy is discussed in detail in Chapter 9.
Contactants
Boguniewicz and Beltrani22 estimate there are 85000 chemicals which will cause an irritant type of reaction to the skin upon contact. These irritant responses are nonimmunologic, usually cytotoxic. Approximately 2800 of these chemicals are capable of a delayed, Gell-Coombs type IV response, the mechanism of allergic contact dermatitis (ACD). Photocontact dermatitis is similar to ACD except that ultraviolet light is needed as part of the triggering mechanism. Irritants may include soaps and other cleaning agents, petroleum products, paint and printing chemicals, adhesives and synthetic resins, hair products, landscaping and pest control chemicals, building and insulating materials, and common allergens such as dust, plants, foods, and insects. Contact allergens include plants such as poison ivy or ragweed, metals such as nickel, plastic resins, organic dyes, preservatives, topical medications such as neomycin, and rubber chemicals including latex. These agents are often tested with patch testing, which is discussed later in this chapter. Identification and avoidance is the treatment of choice, augmented with topical and systemic medication.22
Cross-reactivity
Cross-reactivity between antigens occurs when an antibody directed against one specific antigen is successful in binding with another, different antigen. The two antigens in question have similar three-dimensional structural regions, known as epitopes, which allow the antibody for one antigen to recognize a second antigen as being structurally the same antigen. Cross-reactivity may be robust among antigens of similar phylogeny such as different types of oak trees or ragweeds. Seemingly different antigens within families may substantially cross-react, such as with timothy and rye grass, or there may be a weaker cross-reactivity, such as with timothy and Johnson grass. Foods may also cross-react with other foods such as within the families of grains. Foods may cross-react with inhalants as in the oral allergy syndrome described later. Other associations described have included ragweed and some melons or bananas for example.23 Patients may be more symptomatic during ragweed season when consuming cantaloupe, adding to their critical allergic load. This last example has been called concomitant food sensitivity. Latex represents an example of a collage of antigens to which some latex-sensitive individuals have reported a significant incidence of concomitant food sensitivity. Food cross-reactivities are not well understood and there has been a recent change in their emphasis. Knowledge of cross-reactivity allows for better management of allergic load, and the use of fewer antigens in objective testing and immunotherapy.
Testing Methods
▪ In Vivo Techniques
Skin testing is the primary technique used for the diagnosis of inhalant allergy. For at least 135 years physicians have been testing patients for specific antigen using the skin. Blackley described a first test, the precursor to the scratch test, for fall pollen in 1873.24 Noon and Cooke were among the early clinicians to use skin testing for immunotherapy.25,26 By the 1920s the scratch test, skin prick test (SPT), and fixed concentration intradermal test (FIT) were being used. Hansel described a 1 to 10 dilutional method of intradermal testing which was the precursor to skin (serial) endpoint titration (SET), a 1 to 5 quantitative, dilutional method described by Rinkel in the 1950s.27–30 More recently, hybrids of SPT and SET, mainly the modified quantitative test (MQT), have been introduced to improve patient and physician cost as well as patient convenience, while keeping the level of quality commensurate with SET.30 Because of the adaptation of intradermal testing beyond the methodology of SET, intradermal dilutional testing (IDT) is now the appropriate term for the use of varied but sequential dilutions of antigen concentrations in intradermal testing methods. Skin testing may be epicutaneous or intradermal. Epicutaneous tests include the scratch test, and the prick/puncture tests, for which physicians may use either single or multiple prick/puncture testing devices. Intradermal tests utilize either fixed concentration (FIT), or sequential concentration dilutions (IDT) for testing. These tests may be used in screening for allergy or in an expanded battery for a more comprehensive evaluation.
Scratch Testing
The scratch test, as its name implies, involves scratching the superficial, keratin layer of the skin with a sharp instrument. This scratch may be performed through a drop of concentrated antigen extract placed on the skin, or the antigen may be applied to the area after the scratch. The site is observed for 10–20 minutes and the wheal and flare of any reaction is subjectively graded. The results are compared with positive and negative controls. The positive control is usually histamine, and the negative control is usually saline or diluent. For a test to be considered truly positive, the skin must be reactive to a positive control, usually histamine. Unfortunately there is frequently a high incidence of false positive results with scratch testing. The trauma of the scratch on occasion can stimulate a histamine-related axonal reflex, resulting in an axonal driven wheal and flare at nearby test sites (false positives). In addition, there is a significant occurrence of insufficient antigen penetrating the subkeratin layer to elicit a positive response in a truly allergic patient (false negative).31–33 Thus, the scratch test has been considered unreliable in the diagnosis of allergy by the American Medical Association Council on Scientific Affairs due to its lack of sensitivity (false negatives) and specificity (false positives).34 This safe but unreliable qualitative test, once widely performed, is not in common use today.
Prick/Puncture Test
SPT may be performed in one of two ways. The “drop and puncture” method involves applying antigen extract to the skin first and then puncturing the skin through the droplet.31,33,35 The volar surface of the forearm is usually chosen as the site for the test, although the upper arm and the back can also be used for testing. With the SPT being performed initially on the arm, management or adverse systemic reactions may be facilitated by using a tourniquet on the arm above the site of the test. Therefore, the SPT is usually performed on the forearm and the FIT on the upper arm or back. The antigen used is usually a standardized concentrate or a nonstandardized concentrate of 1 : 10 or 1 : 20 w/v. The positive and negative controls are histamine and a diluent such as buffered, phenolated saline respectively. After alcohol cleansing and drying the volar surface of the forearm, areas at least 2 cm apart are marked. This distance between test sites will potentially avoid vigorous wheal and flare responses from running into each other as well as making an axonal response less likely. A drop of a different specific antigen, determined by the history, is placed at each mark. Using one of several single puncture devices available, the skin is punctured through the droplet to a superficial level without producing a show of blood (Figure 2.3). The device is discarded. As recommended by OSHA, the puncture and wipe technique should not be currently used because of the bodily fluid/pathogen contamination risk to the technician and the possibility of cross-contamination of antigens. There are numerous single puncture instruments to choose from. Some of these include: the Duotip-Test® and Duotip-Test®II (Lincoln Diagnostics, Decatur, IL), Sharp-Test™ (Panatrex, Inc., Placentia, CA), Morrow Brown® (Alkaline Corp., Oakhurst, NJ), Quintip® (Hollister-Steir Labs, Spokane, WA), GreerPick™ System (Greer Laboratories, Inc., Lenoir, NC), and AccuSet™ (ALK-Abelló, Round Rock, TX).
The “dip and puncture” method allows test site preparation in the same fashion as above.31,33,35 After marking the arm, the puncture device tip is dipped into a well with the concentrate of the specific antigen to be tested, and then the “loaded” device is applied to the test site. As with single prick testing, there are several multiple puncture devices available to apply more than one antigen simultaneously. Some of these devices and their manufacturers are: Multi-Test and Multi-Test®II (Lincoln Diagnostics, Decatur, IL), Quick-Test (Pantarex, Inc., Placentia, CA), Quin-Test® (Hollister-Steir, Spokane, WA), and Skintestor Omni™ (Greer Laboratories, Inc., Lenoir, NC). As an example, the Multi-Test®II device has two rows of four puncture heads in parallel (Figure 2.4). The plastic points of each head are 1.9 mm in length and hold the antigen after placement, until it is deposited into the epithelial/superficial dermal layers of the skin, for a total of eight tests per application. The firm but gentle pressure of the application, followed by a gentle rolling motion delivers a test comparable to a superficial intradermal test. Multiple puncture devices are safe, reproducible, and reliable, and may be used effectively as a screening test modality.31,36,37 Multiple puncture devices deliver more tests per unit time, and are cost-effective and well tolerated by patients.
Interpretation of SPT can utilize a subjective grading system or can involve precise measurement of wheal size. Tests are usually read in 15–20 minutes and area compared to positive and negative controls (Figure 2.5). Pseudopodia and irregular spreading are noted. Wheal sizes of 3 mm or greater than a negative control are generally considered positive. In another approach, SPT results can be given a score of 0 to 4+ based on size, amount of erythema, and presence of pseudopodia. While SPT testing is becoming more uniform, the scoring systems are still varied.31,33,35
A nuance in testing is the use of SPT in the evaluation of oral allergy syndrome (OAS). The technique has been called the prick + prick method, an attempt to use fresh, unprocessed antigen in the epicutaneous test format. Pastorello has described OAS as a complex of signs and symptoms exclusively involving the oral and pharyngeal mucosa subsequent to contact with a specific food.38–41 The foods involved are usually either a fresh fruit, nut, or fresh vegetable. There is almost always an association with an inhalant, most commonly a pollen. The presentation may be as minor as tingling in the mouth or as threatening as laryngopharyngeal edema with airway obstruction. The suspected relationship is a cross-reactivity between the food and the pollen. When the food is ingested, often during the pollen season, the syndrome is triggered.42 As an example, OAS has been demonstrated with silver birch and hazelnut.43
In evaluating this syndrome from an objective testing standpoint, there are problems with false negatives for both in vivo and in vitro techniques. It is suspected that there is a degree of physical lability of the functional immunogenic makeup of the antigens involved which makes detection difficult.44 For testing purposes, antigenic material has to be processed in some way. Antigens are processed to be made into allergenic extracts and they are processed to be attached to a substrate for an in vitro test. Even the food used for the double-blind, placebo-controlled food challenge has to be lyophilized, and there is a problem as well with gastric acid altering the processed food antigen(s).43,45 It is suspected that during the processing, the antigenic nature of the epitopes involved is changed enough such that the testing results in cases of truly positive patients are inconsistent. As such, some in vitro and in vivo testing methods using fresh, unprocessed antigenic material have shown some promise.36,46,47 One attempt is a prick + prick method. As an example, a fresh fruit being tested is punctured by the pricking device which hopefully loads the device with workable antigenic material. Immediately the skin of the patient is then pricked with the loaded device. While results using this method have shown somewhat better correlation of history with objective testing, less than optimal sensitivity still makes testing for OAS problematic. OAS represents a form of concomitant sensitivity and has been referred to as clustering of hypersensitivity.48
Single-dilution (Fixed-dilution) Intradermal Test
Epicutaneous tests such as SPT are often utilized as screening tests for allergy. They may be used in a screening battery, and in the traditional sense of allergy testing, as a safe first step in the testing sequence. If the SPT is negative for a particular antigen, and the history suggests presence of allergy to that antigen, it may be indicated to perform an intracutaneous or intradermal skin test for diagnosis. Intradermal tests are generally more sensitive than epicutaneous tests. They also have a higher risk of adverse reactions. It is therefore essential to demonstrate the absence of severe reactivity with a negative SPT first, or to use a dilutional intradermal method such as IDT, or a blended test such as modified quantitative test (MQT). In performing FIT, the concentration is usually 1 : 100 to 1 : 1000 of that concentration used for the negative SPT. This test should not be used for food testing because of the incidence of false positives and the risk of a systemic reaction in a highly allergic patient.35 The test is performed by injecting just enough test antigen (usually 0.02 mL) under the superficial epithelial layer of the skin (intradermal) to raise a 1- to 3-mm skin wheal. This is done with a tuberculin syringe, bevel down, at an approximately 45 degree angle. The test is read and scored in a similar manner as with SPT.35 With the traditional sequencing of SPT and FIT, highly sensitive (+SPT) and relatively lower sensitive (−SPT, +FIT) patients may be detected.
If a patient is going to be treated with environmental control (EC) and symptomatic medication, there has been some controversy as to the utility and accuracy of the single-dilution intradermal test.31 The use of SPT and FIT for diagnosis and the institution of immunotherapy, however, has been successfully practiced by allergists for many years. One specific issue of concern is diagnostically overlooking the patient with very low sensitivity to the antigen being tested. This failure to diagnosis low-level allergy may be a factor leading to less than optimal results with diagnosis and immunotherapy since the risk of undertreatment is higher. This issue led to the development of dilutional techniques such as IDT and MQT.
Intradermal Dilutional Test
Intradermal dilutional testing (IDT) is a skin testing method using a series of 1 to 6 dilutions of a test antigen concentrate, applied in sequence from dilute to more concentrated, until an endpoint determining diagnostic reactivity is obtained. This method is both qualitative and quantitative. IDT has been approved by the AMA Council on Scientific Affairs for the diagnosis of allergy and, when indicated, the initiation of immunotherapy.34 This method is safe, and does not require a preceding epicutaneous test. Other benefits of IDT include an improvement and standardization of test interpretation, reproducibility and standardization of testing procedure, and due to its functioning as a quantitative bioassay, a method for allowing safety with variations in antigen sourcing and single antigen extracts. The following explains the method. The reader is referred to other references for even more extensive details and nuances which are beyond the volume of this chapter.31,32
Antigen preparation for IDT testing is important. Extract concentrates formulated by the vendor in the weight/volume (w/v) format should be 1 : 20 w/v or converted to 1 : 20 if supplied as 1 : 10 w/v by diluting 1 to 2 with a buffered, phenolated saline diluent (diluent). Antigens supplied as standardized extracts are usually considered conceptually as 1 : 20 dilutions, although local practices for individual antigens may differ. The panel of six dilutions for each specific antigen needed for the testing will be made starting with six, 5-mL, sterile injection stoppered bottles filled with 4 mL of diluent. One milliliter of the concentrate is then injected and mixed with the diluent filled bottle labeled #1 dilution. This now becomes a 1 to 5 dilution of the concentrate designated for mixing the testing dilutions. The #2 dilution is then mixed in a similar manner taking 1 mL of the #1 dilution and mixing it with the 4 mL of the next diluent filled bottle, labeled #2 dilution (1/25 of the concentrate). This process is repeated until the #6 dilution (1/312 500 of the concentrate) is made. It is rare that dilutions weaker than a #6 dilution will be needed for testing or mixing antigen for immunotherapy. Extracts from the vendor will have glycerin in them to preserve potency. Glycerin, as an irritant, can be a nonallergenic trigger for a whealing response and will need to be used as one of the three controls for the testing method. The glycerin control results will be taken into consideration when evaluating IDT results for the more concentrate dilutions (higher glycerin content from the concentrate). The other two controls will be histamine and the phenolated saline diluent. These controls will help rule out a suppressed reactive state (anergy or medication suppression [e.g., antihistamine, tricyclic antidepressant] = negative histamine control) or a hyperreactive state (e.g., dermatographia = positive histamine, glycerin, and diluent control). Rarely a patient will react to the phenol in the diluent in which case the diluent control will be positive.31 With six-bottle series of dilutions for each antigen, the clinician is ready to perform IDT.
The upper outer, hairless surface of the arm is prepped with alcohol and dried, then marked horizontally at the arm’s top, left to right, 6 to 1, with 2 cm between marks (helps prevent axonal responses). Then, vertically down, below and left of the #6 mark, the arm is labeled for the antigens to be tested, again 2 cm spaced. After the controls are placed and a response indicating the patient’s appropriate state for testing established (refer to reference 31), specific antigen testing begins with an array of intradermal tests for the #6 dilution of all of the different antigens to be tested. This amounts to a vertically placed row of tests below the #6 mark and to the right of the respective antigen labeling (Figure 2.6). The technique for placing the test is as described before, except enough antigen volume is drawn into the testing syringe to produce a 4-mm skin wheal. Since this usually requires an injected volume of about 0.02 mL of antigen, accomplishing the 4-mm wheal comes from trial and error experience rather than trying to allocate 0.02 mL by a measured injection. The tests are observed for 10–20 minutes, after which the diameter of the wheal is measured, neglecting the erythema which may accompany it. A negative whealing response will be 5 mm, starting with the 4-mm bleb at the time of the intradermal injection, which grows to 5 mm due to nonallergic factors. If an initial test at the #6 dilution is negative, another test using the #5 dilution is placed in the appropriate column, and so on, until all the dilutions are shown to be negative or until a positive response occurs. A positive response will be a wheal size greater than the 5 mm. A positive allergic response with a defined endpoint will be the demonstration of an increase in whealing response starting with a negative test, and progressing over the next two dilutions, increasing 2 mm in whealing size per dilution. For example, a whealing pattern for a specific antigen demonstrating an allergic response might be 5 mm for the #5 dilution, followed by 7 mm for the #4 dilution, and 9 mm for the #3 dilution. This is a positive testing sequence indicating allergy for that antigen where the endpoint is defined as the first reacting dilution in the 2 mm per dilution succession, i.e., the #4 dilution. The confirming dilution is the dilution producing the second, successive 2-mm gain in whealing size, i.e., the #3 dilution in the example. There is no reason to test past the confirming test. In fact, there is an increased risk of provoking symptoms or an adverse reaction. In an allergy battery, the endpoints for the different positively tested antigens are usually varied, thus giving results quantitated to that patient’s degree of sensitivities. Compared to traditional methods, these various endpoints then provide the basis for tailored immunotherapy, which allows starting doses of therapy to be safely more concentrated, theoretically producing improvement in symptoms within an earlier timeframe. Testing from one dilution to the next in sequence across the dilutions from weaker to stronger is called horizontal testing, which is safe but can be time consuming and labor intense. When the initial test for a specific antigen is negative (#6 dilution), it is permissible to jump two dilutions and place the #4 dilution on next. Depending upon a positive result, the third test may have to be one dilution up or down to try to confirm an endpoint. If the second test (#4 dilution) is negative, the skipping process, i.e., go to a #2 dilution, is permissible. This process, especially when the testing sequence for other allergens in the battery can be performed in this manner at the same time, is called vertical testing.
There are a number of abnormal whealing patterns which may be encountered with IDT. A discussion of these patterns is beyond the scope of the current overview, but is more extensively detailed elsewhere.31,33
▪ Blended Techniques – Modified Quantitative Test
In recent years clinicians have tried to blend various in vivo and/or in vitro methods of objective allergy testing in an attempt to afford advantages in safety, patient cost and convenience, and in physician expense, while at the same time obtaining quantitative results without a sacrifice of efficiency and/or accuracy.49,50 One such blending of in vivo methods, modified quantitative testing (MQT), was introduced in 2002 and continues to be used with increasing frequency.51 MQT combines epicutaneous methodology as a screen and a first safe step in the testing sequence, followed by a single intradermal test, the concentration of which is based on IDT principles to permit a more sensitive and quantitative estimate of specific antigen testing results. For the epicutaneous phase of this method, a multiple-puncture device, such as the Multi-Test II device® (Lincoln Diagnostics, Decatur, IL), is used because of the demonstrated increased reproducibility and reliability of multiple-puncture devices over single prick-puncture instruments.30,52–54 The quantitative correlation between the Multi-Test II® and IDT is suggested to be approximately that of a 1 : 1500 w/v intracutaneous skin test (IDT dilutions #3 to #4).30,54 The method of MQT is outlined in the algorithm depicted in Figure 2.7.
The volar surface of the forearm and a hairless upper outer surface of the arm are used. The patient is interviewed and the arm prepped in the usual manner described above for multiple-puncture device and IDT testing. The multiple-puncture screen and/or expanded battery are applied as previously described. A positive test is defined as a 3 mm or greater wheal above the negative control. For each epicutaneous test, if the result is negative (<3 mm), a #2 dilution IDT is placed. For the IDT test, a wheal result = 6 mm is negative and thus the overall test for that antigen is negative. If the #2 IDT is = 7 mm, the test is positive and for that antigen, the overall result or endpoint (EP) is estimated to be the #3 dilution. If the initial epicutaneous test is 3–8 mm, then the #5 dilution is used for the IDT test. If this result is = 5 mm, the overall EP is the #4 dilution. If the #5 dilution IDT is 7–8 mm, the overall EP is the #5 dilution. If the #5 dilution IDT test is = 9 mm, the overall EP is the #6 dilution. Lastly, if the epicutaneous test is = 9 mm, the overall EP is assigned the #6 dilution without the need for an intradermal test. The key with this blending of methods is to obtain efficient and accurate results allowing any initiation of immunotherapy to be tailored and safe. For example, mixing antigen for immunotherapy based on a #6 EP via IDT formulating methods will conservatively produce a safe starting dose comparable to that of immunotherapy formulated for a positive multiple-puncture test. On the other hand, this method will identify a low reactor with a negative epicutaneous test and a = 7 mm IDT test given the MQT EP of a #3 dilution. This process allows a safe initiation of immunotherapy in this low reacting patient who otherwise might not have been identified at all if dependent upon the epicutaneous test result alone (increased sensitivity). Lastly the 3–8 mm epicutaneous test positive – less than 8 mm wheal IDT patient – will potentially benefit from immunotherapy initiated safely via IDT formulation and at doses higher than those which would have been formulated by epicutaneous results alone (potential clinical improvement earlier). For further comments and before using MQT, the reader is referred to a work of larger scope.30
▪ Other In Vivo Methods for Inhalant Allergy Testing
Various forms of nasal and bronchial provocation testing have been described and are mainly used for research.31 In clinical practice, these modalities could carry some risk of a severe or adverse reaction. In addition, these tests are qualitative for the most part, and what quantitative value may exist could be difficult to correlate into a meaningful scoring system for diagnosis or the institution of a tailored, quantitative immunotherapy. Other mucosal challenges, such as conjunctival or colonic testing, and other methods such as sublingual testing, as of yet only receiving a paucity of investigation in the USA, are beyond the scope of this chapter.
Patch Testing
As will be discussed later, there is no gold standard for the testing of inhalant allergy. However, the patch test is considered to be the gold standard for the diagnosis of allergic contact dermatitis (ACD), may be useful in the evaluation of irritant contact dermatitis (ICD), and perhaps can aid in the differentiation from other skin conditions. The history and/or the physical finding of recurrent or chronic atopic dermatitis (eczema) may be an indication for identifying contact sensitizers. The American Academy of Dermatology has set standards for the performance and interpretation of the patch test. The most commonly used product is the TRUE Test® (Allerderm Laboratories). This test has two panels of 12 standardized patch tests and evaluates 23 of the most common contact allergen sensitizers and one negative control.55 Standardized allergens are suspended in a hydrophilic gel, attached to a waterproof, adhesive backing. For the testing of compounded standardized allergens other than the 23 offered in the TRUE Test®, Finn Chambers (Allerderm Laboratories) may be used. These 8-mm chambers are most commonly available in tape-adhered sets of 10 in two rows of 5. The back is the usual site for testing. At the time of testing, the skin should be clear of dermatitis. The panels are applied in the manner per the manufacturer’s instructions and then removed in 48 hours. Although results may be viewed 30 minutes after the tapes are removed, the suggested interpretation is made in 72 and 96 hours. Test results may be affected by the concomitant use of oral corticosteroids, the equivalent of 20 mg of prednisone per day, or the use of high potency topical corticosteroids within 7 days of application. Oral and topical H-1 antihistamines do not appear to affect the patch test. The tests are graded from 0 = no reaction (no evidence of contact allergy), +/− = mild erythema (doubtful contact allergy), 1+ = 50% of test site showing erythema with edema (possible, or false positive for contact allergy), 2+ = 50% of site showing papular erythema (probable contact allergy), to 3+ = 50% of site showing vesicles or bulla (definite contact allergy).22,56,57 Adverse reactions to patch testing would include, but are not limited to, pruritus, erythema, burning, and hyperpigmentation. Other contact testing methods such as photopatch testing, or adjunctive tests aiding in the diagnosis or differential of ACD, such as skin biopsies, are described elsewhere.22,56
▪ In Vitro Techniques
In 1966 Ishizaka and Ishizaka identified immunoglobulin E (IgE) as the mediator of immediate sensitivity, formerly referred to as reagin.58 Concurrent work leading to IgE’s characterization was also done by Johansson and Bennich.59,60 Total IgE is found in very small quantities in the blood, roughly 10 000 fold less than IgG.61 In 1967 Johansson et al and Wilde et al introduced the radioallergosorbent test (RAST) as the first in vitro test for allergy.59,62 In the 1970s the Phadebas and Phadezym RAST (PhRAST; Pharmacia Diagnostics, Uppsala, Sweden) were early popular tests for measuring specific IgE. By 1979, Richard Fadal and Donald Nalebuff developed the modified RAST (mRAST) in response to PhRAST’s lack of sufficient sensitivity. In addition, they developed a quantitative scoring system for the results which was demonstrated to correlate with IDT results. Also developed in this era was a quantitative immunoassay, the enzyme-linked immunosorbent assay (ELISA), to which the Fadal/Nalebuff (F/N) scoring system was adapted. The newest generation of quantitative, antigen-specific in vitro tests includes the commonly used ImmunoCAP® system (PharmaciaDiagnostics, Kalamazoo, MI).60,63 All of these tests are variations of “sandwich” immunotechnology. A support or substrate with a fixed antigen and a tagged antibody to IgE (anti-IgE,  –IgE) are used to bind IgE. The tag is then counted. The supports or substrates vary from test to test and include paper, polystyrene plates, beads, or tubes, hydrophilic cellulose polymer, or a matrix of soluble polymers. The labels used to tag the final complexes include radioactive, colorimetric, fluorescent, and chemiluminescent vehicles.
–IgE) are used to bind IgE. The tag is then counted. The supports or substrates vary from test to test and include paper, polystyrene plates, beads, or tubes, hydrophilic cellulose polymer, or a matrix of soluble polymers. The labels used to tag the final complexes include radioactive, colorimetric, fluorescent, and chemiluminescent vehicles.
PRIST
Total IgE is of limited clinical value, especially when compared to specific IgE. As a predictor of the presence of allergy, total IgE level is not a reliable screen. Since the quantity of IgE is so small in the serum, a patient could be quite allergic to one or a few antigens, demonstrating elevated specific IgE levels, yet have a normal total IgE level. It is estimated that about one-half of allergic patients will have an elevated total IgE level with the usual allergic adult range being 300–600 ng/mL and the nonallergic adult 0–200 ng/mL.61 In clinical practice, norms are age-adjusted. The differential for an elevated total IgE would include allergy, parasitic infestations, atopic dermatitis, tobacco use, allergic fungal sinusitis (AFS), bronchopulmonary fungal disease, hyper-IgE syndrome (Job’s syndrome), Wiskott–Aldrich syndrome, and some malignancies. In parasite-free young children, an elevated total IgE level has been thought to predict the future emergence of allergic disease. In adults with very low total IgE levels, the chances of significant allergy is low. Changes in total IgE levels have been used to monitor therapeutic progress in both AFS and bronchopulmonary fungal disease.
Radioallergosorbent Test (RAST)
The term RAST refers to a radioimmunoassay sandwich technique in which a specific allergen is fixed to a paper disc substrate adhered to a testing well or tube. The patient’s serum is then mixed and incubated. After antigen-specific IgE in the serum has had a chance to complex with the fixed antigen (allergosorbent), the mixture is washed of excess proteins (including antigen-specific IgE not being tested), and is then mixed and incubated with radiolabeled anti-immunoglobulin E ( –IgE) forming another antigen/antibody complex (paper-specific antigen/specific antigen IgE/radiolabeled{nonspecific
–IgE) forming another antigen/antibody complex (paper-specific antigen/specific antigen IgE/radiolabeled{nonspecific  –IgE}). After washing, the final complexes are then counted for a quantitative result (Figure 2.8). Early RAST methods used for clinical diagnosis such as the PhRAST unfortunately had insufficient levels of sensitivity. As mentioned above, Fadal and Nalebuff modified the process by lowering the diagnostic cutoff point to increase sensitivity with only a slight lowering of specificity. In addition, time of incubation was increased. This modified RAST (mRAST) was noted to correlate with IDT, with endpoint dilutions paralleling with the Fadal/Nalebuff (F/N) scoring system.64–69 However, it is important for the clinician to realize that a quantitative in vitro test-based immunotherapy has to be validated for appropriateness and safety with an in vivo bioassay before immunotherapy is initiated. Hence the existence and function of the vial test.
–IgE}). After washing, the final complexes are then counted for a quantitative result (Figure 2.8). Early RAST methods used for clinical diagnosis such as the PhRAST unfortunately had insufficient levels of sensitivity. As mentioned above, Fadal and Nalebuff modified the process by lowering the diagnostic cutoff point to increase sensitivity with only a slight lowering of specificity. In addition, time of incubation was increased. This modified RAST (mRAST) was noted to correlate with IDT, with endpoint dilutions paralleling with the Fadal/Nalebuff (F/N) scoring system.64–69 However, it is important for the clinician to realize that a quantitative in vitro test-based immunotherapy has to be validated for appropriateness and safety with an in vivo bioassay before immunotherapy is initiated. Hence the existence and function of the vial test.
RAST and other forms of quantitative in vitro tests are attractive for clinical practice. They are preferentially indicated for certain situations, some of which are noted in Box 2.9.
BOX 2.9 Indications for RAST testing
For patient convenience and possibly economic advantage
At a time when mast cells may be transiently depleted, for example after an anaphylactic reaction
During pregnancy for diagnostic and nonimmunotherapeutic management reasons
In the patient with suspected seasonal allergy during the season of concern
Caution and judgment, aided by the perspective of the allergic history, must be exercised when interpreting in vivo and in vitro results and in correlating the degree of clinical sensitivity associated with the outcomes of the allergens tested. For example, an antigen may yield a F/N score of #1 or #2 in a mRAST (F/N scores 0–6, with 6 having the highest count range) or may produce an endpoint on a #1 or #2 dilution for an IDT test. Both cases seemingly indicate a low level of sensitivity in testing. In the former situation, the test indicates low counts of IgE complexes. In the latter, endpoint reactivity is demonstrated with the application of higher strength dilutions (less reactive), as opposed to low concentrations of antigen (more reactive). These results, however, may not clearly predict the degree of symptomatology among these patients. Despite this caution, higher mRAST scores, and the higher IDT endpoints, suggest greater clinical sensitivity and symptomatic expression.
ImmunoCAP
The ImmunoCAP® system (PharmaciaDiagnostics, Kalamazoo, MI) represents the newer generation of in vitro tests for specific antigen IgE. This test utilizes a fluoroenzyme immunoassay (FEIA), once again using the sandwich type technology. Specific antigen is bound to a three-dimensional hydrophilic cellulose polymer, which has the capability of binding significantly more allergen than older disk methods. This substrate theoretically allows for increased antigen binding and improved sensitivity. Patient serum is mixed with antigen-bound substrate, permitting antigen-specific patient IgE to complex. Next, the complexed substrate is mixed with an enzyme– –IgE conjugate forming a secondary immune complex ({patient serum complexed-substrate}/{enzyme–
–IgE conjugate forming a secondary immune complex ({patient serum complexed-substrate}/{enzyme– –IgE conjugate}). Subsequently a fluorogenic material is applied to the secondary immune complex, which results in the formation of a fluorescent secondary immune complex. Using a fluorometer, the fluorescence is read, the quantification of which correlates with the quantity of patient IgE. One scoring method for this test is known as the alternate scoring method (ASM), which reports the score for the test as a percentage of the sample measurement ratio using a 0.35 IU/mL calibration. Using this scoring method, the test correlates well with the mRAST, yet with evidence of better specificity.60 The test also has the advantage of taking only hours to perform. As will be further noted, there is no gold standard in inhalant allergy testing. Studies have indicated however that if clinical history and/or skin testing are held as the diagnostic standard, ImmunoCAP® demonstrates very high specificity, high efficiency, and 94% sensitivity.64,65,68,70 In addition to its use by allergy specialists, ImmunoCAP has also found growing use by primary care physicians as well as reference laboratories.
–IgE conjugate}). Subsequently a fluorogenic material is applied to the secondary immune complex, which results in the formation of a fluorescent secondary immune complex. Using a fluorometer, the fluorescence is read, the quantification of which correlates with the quantity of patient IgE. One scoring method for this test is known as the alternate scoring method (ASM), which reports the score for the test as a percentage of the sample measurement ratio using a 0.35 IU/mL calibration. Using this scoring method, the test correlates well with the mRAST, yet with evidence of better specificity.60 The test also has the advantage of taking only hours to perform. As will be further noted, there is no gold standard in inhalant allergy testing. Studies have indicated however that if clinical history and/or skin testing are held as the diagnostic standard, ImmunoCAP® demonstrates very high specificity, high efficiency, and 94% sensitivity.64,65,68,70 In addition to its use by allergy specialists, ImmunoCAP has also found growing use by primary care physicians as well as reference laboratories.
▪ Other In Vitro Tests
Several other quantitative specific IgE tests evolved and disappeared over the years. Some of these included the fluorescent allergosorbent tests (FAST), the Matrix, and the Magic-Lite.60,71 Two tests that were recently still available are the modified allergosorbent test (MAST) (MAST ImmunoSystems, Mountain View, CA) and the AlaStat Assay (DPC, Los Angeles, CA). With regard to the MAST, this test’s outcomes compare more with the PhRAST than the mRAST, suitable specificity yet less sensitive.60 AlaStat is an automated assay using matrices of soluble polymers, which at one time in studies indicated some inconsistencies, but continued on the market after some changes in the test.60,64
Qualitative tests have developed over the years to be used as screening methods. A positive test would perhaps direct a primary care physician to refer a patient for a more extensive battery of antigen-specific tests. Such multi-allergen tests would combine multiple allergens to a substrate. When mixed with patient serum, processed and read, a positive result would be realized if the serum had a quantity of one or more specific IgE antibodies corresponding to the antigens on the substrate. Further antigen-specific testing would have to be done to determine which allergens the patient is allergic to.64
A few comments on the basophil histamine release test (BHRT) are in order. This test tries to evaluate allergen-specific IgE. In vitro IgE-mediated histamine release is measured. This is at best a semiquantitative test that may be used as a screen. This is not a test to be used by itself alone for the initiation of a quantitative approach to immunotherapy. Any positive results from this test must be followed by an appropriate form of skin testing such as IDT or MQT if immunotherapy is to be started. One way this test is performed is for the application of a serum fraction containing the patient’s suspected, sensitized basophils into ELISA wells that are already coated with a known concentration of historically suspected antigens. The mix is then incubated to allow any antigen/antibody complex formation, the resultant cell destruction, and mediator release, i.e., histamine included. The amount of histamine released is then evaluated by a fluorometer or by other immunoassay techniques. The concentration of the antigen in the wells will help semiquantitate the results. The positive control to validate a negative test is a positive reaction to anti-IgE, since 5% of people will have basophils which will not release histamine in vitro. Furthermore, a positive response to anti-IgE will not confirm a positive test for allergy since most people will have some degree of circulating IgE.61 As of now, this test is of limited usefulness to the clinician, but may have some possible role in the evaluation of food and drug sensitivity.31,71
Lastly, there are a number of in vitro tests used primarily for the standardization and the production of allergen extracts. These tests would include RAST-inhibition, radial immunodiffusion (RID), crossed radioimmunoelectrophoresis (CRIE), and SDS-PAGE with a subsequent immunoblot test. They are of little value as primary clinical tools to help direct allergy diagnosis and therapy.71,72
▪ Sensitivity/Specificity of Testing Techniques
There is no gold standard for objective testing for inhalant allergy.64,73,74 There are some regarded gold standards in sensitivity testing. The double-blind placebo-controlled food challenge is the standard for diagnosing food sensitivity.75 The patch test remains the standard for separating acute contact dermatitis (atopic dermatitis) from other forms of dermatitis. The photopatch test is the standard of testing for photo-contact dermatitis.22 However, there is no gold standard for objective testing for inhalant allergy. No single test in the armamentarium is devoid of the possibility of a false positive or false negative while pursuing detection of the true positive or true negative. A positive or negative in vivo or in vitro test for inhalant allergy having to corroborate the allergy history and physical exam is the current model for allergy diagnosis. The reasons for provocation testing not being the standard are manifold. A few reasons would include: a disparity in antigen concentration presented to the nose or lung versus that occurring naturally, difficulty evaluating and standardizing results, and overcoming problems with variables in the provocation protocol.64,76 When correlated with the allergy history, both skin testing modalities and in vitro tests have been shown at times to produce positives or negatives which are false and not consistent with what is evident clinically from the patient.64,77–81
When comparing skin tests, the epicutaneous prick/puncture (SPT) is noted to be more specific but less sensitive than the intradermal test.64,77,79 The multiple prick/puncture SPT, notably the Multi-Test II®, is regarded to be more sensitive than the single prick/puncture method. The fixed intradermal test (FIT) is felt to be less sensitive than the intradermal dilutional test (IDT). Usually an increase in sensitivity may realize some, difficult to quantitate, decrease in specificity. The allergy history and the physician’s clinical judgment have to prevail in using test results. An examination of in vitro tests produces similar comparisons. As noted previously, the modified RAST (mRAST) was developed to be more sensitive, with little sacrifice of specificity, than the RAST (PhRAST). Also noted above, the ImmunoCAP® system, because of its increased binding capability, is predicted to be more sensitive. Other studies indicate the multiple allergosorbent test (MAST) to be comparable to the PhRAST with lower sensitivity and efficiency than the ImmunoCAP.64,70,82–84
Some other studies compare in vivo and in vitro techniques, sometimes using one method as a theoretical standard, others using the history as a reference point. As a qualitative and screening test, the multiple allergen in vitro Phadiatop® test, when compared with skin testing, was found to be 93% specific, 91% sensitive, and 92% efficient.64,85–87 With intradermal testing as the standard, Kam et al found ImmunoCAP to be 85% sensitive and 94% specific. Yet when ImmunoCAP was the standard, intradermal skin testing was calculated to be 88% sensitive and 93% specific. As a result, Poon et al then comment, it is not apparent as to which modality would be preferred between the two.64,70 Poon et al further note, when the clinical history is taken into consideration, other studies have indicated that the ImmunoCAP is comparable to SPT and IDT, or better than SPT in sensitivity, specificity, and efficiency.64,78,79
The 1998 “white paper” by Poon et al examining the clinical usefulness and the economic details of then current in vitro and skin testing methods is a “must read” for clinicians.64 This work clarified any previous assumption that the SPT is a gold standard for allergy diagnostics. It also empowers the clinician to have legitimate choices in testing modalities leading to meaningful management options. These choices allow the physician to tailor allergy investigation and management to the patient’s medical profile and his economic needs and limitations. It is the clinician’s responsibility to competently use these choices with the knowledge of the inherent pros and cons.
Therapeutic Methods
▪ Introduction
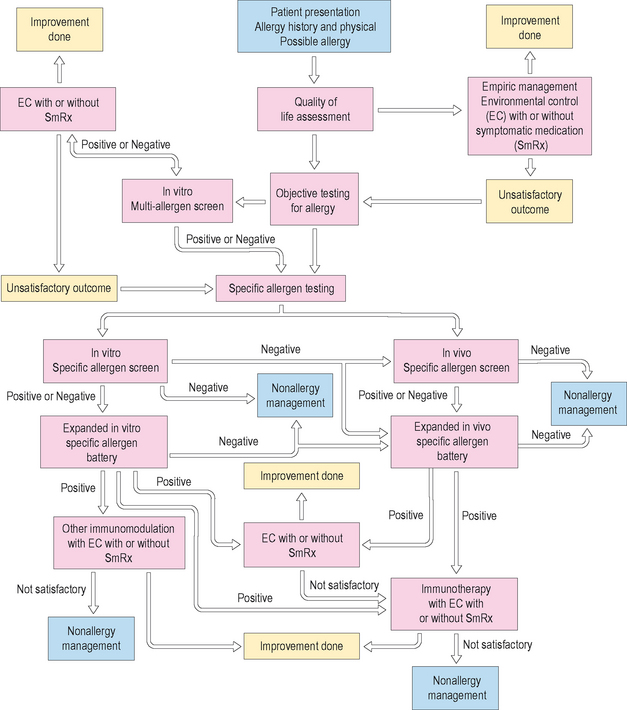
The management of allergic disease is predicated on three functional principles: environmental control (EC), symptomatic pharmacotherapy (Rx), and immunotherapy (IT). A fourth and very important principle is patient education (Ed). It is important to realize that the patient’s expectations are as important as, if not more important than, the physician’s hoped for symptomatic and physical exam improvements. Quality of life assessment, either formally with questionnaires or more informally with physician inquiry, is crucial. It is the responsibility of the clinician to make sure patient expectations are appropriate and realistic. Environmental control (EC) is about reducing the allergic load. While attention to the allergic load, as previously stated in the broadest sense, is the ultimate goal, attempts to eliminate or modify exposure to antigens and irritants in the environment is the primary priority. When EC is not enough, symptom-specific pharmacotherapeutic agent(s) may be used. Proactive versus and/or reactive venues, length of therapy, side effect profiles, compliance likelihood, over-the-counter (OTC) versus prescription medication, and cost/availability are some of the issues to be considered. When EC and Rx are not enough, not possible, or as an initial, proactive management decision, immunotherapy (IT) may be considered. Immunotherapy in a broader sense now considers other forms of immunomodulation as well. Recombinant anti-IgE (Xolair®) has been used for select allergic asthma as a current therapeutic modality. Other indications for this drug and other pipeline immunomodulator developments are yet to come. Patient education (Ed) is a must no matter how many or what combination of functional management principles are used. This may be accomplished with educational aids (including the Internet), face-to-face conversations with an allergy nurse/technician and/or the physician, along with other adjunctive medical or business professionals. Instilling a sense of self-inquiry in the patient is important, as is a routine of follow up, giving the physician regular opportunity to reconfirm and advance EC and Ed. Ed is an ongoing process that leads to both therapeutic and preventative measures.
▪ Environmental Control (EC)
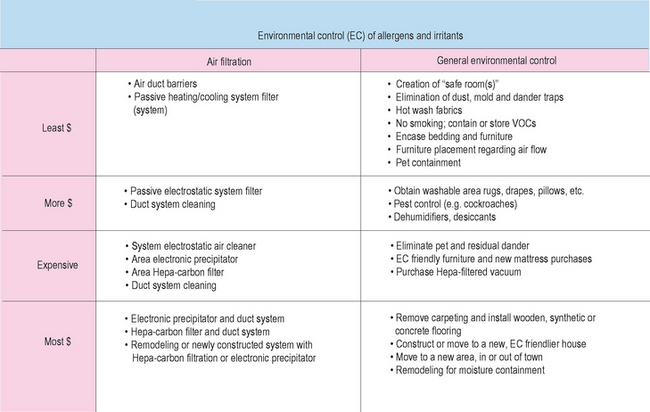
No matter how simple or complex the treatment regimen, no matter how mild or severe the patient’s symptoms, and given that varying degrees of effort may suffice, attention to EC is always a part of allergy management. From before, the all-inclusive definition of allergic load is the sum total of all environmentally and medically related factors which may impact the expression of allergic disease and its sequelae at any one point in time. To help reduce this allergic load, it may be necessary within reason to make sure other medical problems are being addressed. Remember that inflammation tends to be additive and that other conditions may affect the immune system, directly or indirectly. For example, the allergic diabetic who is not in good control of his/her diabetes is more predisposed to the additive inflammation of infection. Some forms of inflammatory bowel disease may have a rhinitic component. On occasion with a recalcitrant rhinitis, do not forget to look at drug side effect as a factor. Even allergy medication can have inflammatory side effects. Any form of immune deficiency or immunosuppression may be a factor and may be treatable. Proper sleep, exercise, dietary habits and psychological health are important. The list goes on and can be exhaustive. Orchestrating a total advancement of health for your allergy patients may be crucial. This may require patient education, the help of a primary care physician, specialist physician(s), and/or other health care and lay professionals. Detailing all of the possible scenarios is out of the scope of this chapter, however, the clinician should be more than competent to manage this concept. Attention will now be given primarily to allergen and irritant environmental control. EC considerations need to be for the home, vacation home, perhaps relatives’ homes; when possible, the workplace, or any other location the patient spends a significant amount of time. It seems easier to present comments through an antigen class format, however the author wishes to draw attention to a cost-related echelon of EC actions, since economic factors may be of great import for some patients (Box 2.9).
House Dust, Dust Mite, and Cockroach
These antigens are primarily perennial, indoor antigens. House dust as an entity is composed of at least 25 or more antigenic and irritant entities.88,89 These entities include, but not exclusively, dust mite, cockroach and other insect parts, some pollen, mammalian epithelials, food particles, mold, lysine sugars from organic degeneration,88 and residues of formaldehyde, tobacco smoke, various alcohols, and other hydrocarbons. If a seasonal flare could be attributed to these allergens, it would be whenever and for whatever length of time the weather is cold (or too hot), forcing more time spent indoors and therefore more exposure. For example, in the central Midwest, this would be approximately October through April (often noted as the reverse of baseball season). October–November is a particularly difficult inflammatory time in the central Midwest due primarily to priming and insult from the fall weed season, the beginning of the fall mold season, the start of the winter viral season, and the start of the seasonal flare of the perennial allergens.
The older the house dust is, the more antigenic it becomes mainly due to ongoing organic materials degeneration including carpets, upholsteries, bedding, air duct residue, and the list goes on. Therefore, when choosing a home, a more recently built home may be of benefit. Living in a 100-year-old farmhouse will be more antigenically challenging than a new home. Dust mites proliferate in higher humidity. Keeping the humidity between 40% and 50% is optimal. This discourages dust mite and mold proliferation and yet helps prevent mucosal drying often seen in the winter with the tendency for the humidity to drop below 35% due to the drying effect of heated air. The higher the altitude, potentially the dust mite population is lower. However, dust mite proliferation in the higher altitude dwelling may be enhanced due to the humidified, artificial environment designed for human comfort. Dust mites do not survive high temperatures. Professionally cleaning or regularly washing clothing and other cloth fabrics at a temperature >130–140°F (>50–60°C) will reduce the mite population and therefore the source of the antigenic feces. Choose hypoallergenic pillows or duvets which can be washed and tumble dried (140°F, 59°C), or easily replaced.90,91 Mattresses can be periodically flipped over and eventually replaced. Allergy product suppliers can furnish impermeable barriers for pillows and the mattress that are becoming more comfortable. Vacuuming carpets and cloth furniture or drapes on a regular basis is indicated. Some vacuums have extra vacuum power, closed dust receptacles to prevent recirculation, shampooing capabilities, and HEPA (High Efficiency Particulate Air) filters to more efficiently remove mites and feces. Consumer reports, the internet, and allergy supply vendors are among sources for the patient to seek specific models.89 Keep in mind that it will be impossible to fully eradicate all of the mites and all of the fecal material, yet allergic load reduction can be therapeutically relevant. Allergy supply vendors can also provide patients with carpet treatments designed to denature the fecal antigen or kill the mites, containing tannic acid or benzyl benzoate respectively. These will require periodic applications throughout the year, the frequency of which may be more dependent on how warm and humid the environment is throughout the year. Efforts to reduce “dust traps” such as excessive fabric items, shelved books and collectables, stuffed toys and animals, the presence of off-season clothing in the current closet, and plants (real or artificial) will help reduce dust exposure.88,90
Air filtration is of help in the control of house dust as well as other indoor antigens including mammalian epithelials, mold, pollen grains, and irritants such as tobacco smoke. There are basically four levels of filtration to consider. The least expensive and the least efficient is the passive pleated or fiberglass filter found in the average, forced air heating and air conditioning systems. In addition, allergy supply vendors can provide changeable, cloth or synthetic duct grill inserts to passively filter the air as it passes the grill. Keeping beds or chairs out of the direct airflow of forced air vents will be of help. Although it may be warm and comfortable to have a patient’s bed over the warm air vent during the winter months, an increased load of dust and mold antigen may give rise to added symptoms. It may also be of help to have the ducts professionally suction-cleaned. Professional clothing and home furnishing cleaners or building contractors will quite often advertise such services. Knowing who will do this in your community will be of help to your patients. The next level of filtration is the electrostatic filter. These filters are the first level of meaningful particulate filtration capable of filtering particles measuring 0.3 mm or less. There are passive, disposable electrostatic filters which need to be replaced usually three or four times a year. There are also permanent filters which fit into the same space as the regular fiberglass filter. The permanent model needs to be washed several times a year. Letting the filter stay dirty reduces the efficiency of the filter. These are available through your heating and cooling contractor, who can also advise you about a particular filter’s efficiency rating. The next level of filtration is the electrostatic precipitator. This highly efficient precipitation occurs when air is passed over electrically charged plates, attracting charged particles to the plates. The precipitators come in portable room-sized models or may be placed directly into the heating–air conditioning system. The latter may require alteration of the existing system and has to be easily accessible since, along with the portable model, it must be cleaned periodically. If cleaning is neglected, the charged particles will bypass the plates and glom onto walls and furnishings, leaving a greasy residue. The last, and probably the most efficient filtration system is the HEPA rated filter which can be installed into a central system or may be found in a portable, room-sized model, often with a carbon pre-filter. The carbon pre-filter is attractive for irritants such as tobacco smoke. If choosing a centrally placed HEPA filter, you have to consult a heating and cooling contractor since an additional duct system may have to be installed. It is obvious that the expense involved with the different filtration options varies with the choice. The HEPA filter will select out particles 0.3 μm in diameter. The vast majority of particles in room air are 1 μm or smaller. The largest particles may be dust or pollen at 100 μm, and the smallest may be dust or smoke at 0.01 μm. The patient should be advised of the potential significance of his/her sensitivity to indoor allergens such that he/she can make a decision about the extent of filtration he/she might desire. Collectively, the options sited above should be explored, to come up with the option that makes medical and economic sense.88
Cockroaches are controlled by professional extermination, the use of traps, over-the-counter preparations, and by making the environment less attractive to them. The American and German cockroaches are the most common types in the USA. Seeing one roach usually means there are many more. An estimated 20% of homes without visual evidence of cockroaches are cockroach infested.90 Moist, dusty, underventilated areas with food materials readily accessible, are of high risk. Cockroaches are often brought home from the grocery store in bagged vegetables or with other semi-open packaging, making the kitchen and food storage areas high risk places. Older homes, inner-city dwellings, and high humidity (semi- and tropical climes) are some other risk factors. Following EC measures for other indoor allergens will help decrease exposure to the feces and other allergenic breakdown products of the cockroach body.92
Mold
Areas in the home which deserve special attention include damp basements, crawl spaces, garages, bathrooms, refrigerators, leaking ceilings, underventilated closets, storage areas, feather pillows, old books, and so on. EC is directed at alleviating or controlling the sources of moisture, eradication of obvious mold, increasing ventilation, controlling excessive warmth and darkness. Dehumidifiers may be of use in the basement and the garage. Choosing to not place the patient in a bedroom over the garage or a damp crawlspace may be of help with mold, irritant, and dust exposure. Pre-packaged desiccants are available at grocery, hardware, and department stores for use in closets, bathroom cabinets, crawlspaces, and the like. Leaving a light on in high risk areas such as the basement or a closet may be of help. Monitoring humidity with a hygrometer may be necessary. Clean moldy surfaces with commercial mold/mildew cleaners or dilute bleach mixtures.88 Increase ventilation of crawl spaces, closets, basements, or storage areas. Consulting a building contractor may be necessary to fix the leaking basement, ceiling, or garage. Plants, animal droppings, leather products, and other organic substrates are of risk. Even house dust will be a substrate for mold such as Mucor. Outdoor landscaping can have an affect on the mold-sensitive patient. A heavily landscaped, shady yard with poor ventilation due to heavy vegetation or positioning of the house relative to the prevailing breeze will increase the mold envelope of the home. The proximity of bodies of water such as a lake, pond, creek, water runoff, or the like will be of significance. Any decaying vegetation such as a compost or landscape waste will be of risk. Provoked symptoms with the exposure to freshly cut grass are suggestive of mold, more so than pollen symptoms. Mold or pollen patients working outside may have to premedicate themselves and/or wear a mask. Upon coming in from gardening or landscaping, work clothing should be taken off and left in the laundry room for cleaning and isolation of mold and pollen from the rest of the house. Mold counts tend to be increased during the cool, evening hours and anytime moist, rainy weather is followed by warm windy days. Some patients may have to alter their outside activity accordingly. Patients may also depend upon pollen and/or mold counts available to them through the local media or as a service from a local allergist.
Animal Epidermals
This group of antigens includes mammalian and avian species. The allergenic sources include saliva and urine, in addition to the epidermal structures (feathers in birds). The particles typically range between 0.1 and 1.0 μm.88 Because cats meticulously groom themselves, dried cat saliva is a significant component of the cat-related antigens. In fact, cat is perhaps the single most important animal sensitizer. Mold in bird droppings, along with feather products, makes frequent cleaning of the bird cage a must for the sensitive patient. Eliminating a sensitizing animal will be the best solution for the seriously sensitive individual. This may not always be possible or needed depending upon the situation. Realize that it may take months of cleaning to get rid of all of the antigen products of a pet who has been taken out of the patient’s environment. This is especially true for cats. Animal isolation within the house or keeping animals outside will be of help. All allergens considered, the creation of a “safe room(s)” within the home is a must for the significantly sensitive patient. The most logical areas are the bedroom first, followed by the other room that the patient spends the most time in, usually a family or recreation room. EC efforts for all of the antigens and irritants discussed should be optimized for these rooms, especially when total home control is not going to be efficient. Washing and grooming of animals will help in most cases but may not always be easily accomplished, and in the case of washing, may not be healthy for the animal if overdone. Consulting the veterinarian will be of help on this issue and will be a source for other EC options. There are other pet options that are not mammal and avian species which may be more allergy friendly. Unfortunately, these species may or may not be desirable to the patient or rest of the family. Some patients may have to change career plans depending upon their level of sensitivity and the degree of difficulty in managing symptoms. Veterinary careers and farmhand jobs, among others, are high risk employment for some patients.
Pollen
Pollen EC can be difficult. Fully controlling exposure while outdoors is impossible. Time spent out of doors is usually the most desirable at the time of the year plants pollinate. However, knowing that daily pollination tends to be greatest in the morning hours, and that pollen counts temporarily fall after rain, may help a patient choose the time to be spent out of doors with less exposure. Windy, warm days will increase pollen and mold distribution. Removing clothing in the laundry or “dirty room” upon entering the home and/or taking a shower after a significant outdoors exposure may help prevent bringing pollen and mold spores into the indoor environment. Pollen grains will range from 10 to 100 μm88 in diameter and, as such, will be subject to the indoors filtration options as described previously. The concentration of pollinating plants to which the patient is sensitive, found in the neighborhood and in close proximity to the home, may be important both for pollen and mold exposure. Consultation with a landscaper or the local university botanist may help avoid choosing cross-reacting plants and will give direction as to plant placement with regard to the proximity of the house when planning the landscape. Many of the other EC details noted previously will help with pollen control indoors.
Other Considerations
The issues and controversies of bacterial environmental sensitivity are discussed elsewhere and are out of the range of this chapter.93 Unquestionably, bacteria may be found within the home; potentially anywhere skin/urine/respiratory/gastrointestinal soiled human or vector spreading might occur. Fomites then serve as an ongoing source for the life of the bacteria. Combinations of bioaerosols of which bacteria and, more importantly, mold are included have been implicated in the “sick building” syndrome. The term is used to describe a set of adverse health symptoms experienced within a constructed environment that lessen when the patient is out of the adverse environment. The respiratory tract and the skin are often symptomatic targets. In addition to any allergic contributions to the problem, bacterial and mold cell wall products may provide other nonspecific inflammatory effects. Molds which have been mentioned include Penicillium, Aspergillus, Stachybotrys, Fusarium, Trichothecium, Trichoderma, Myrothecium, and Cephalosporium to name a few.92–97 Other irritant effects may be added by volatile organic compounds (VOC) such as ketones, alcohols, and formaldehydes. These factors, along with other antigenic unfriendly conditions, including building dampness, high humidity, and poor ventilation, provide an environmental situation associated with this syndrome. Suspicion of the syndrome, identification of the contributing factors, and then remediation of the building environment has been shown to improve or resolve patient symptoms. The reader is referred to lengthier discussions on this topic of ongoing investigation, elsewhere.92
It is not uncommon for patients to announce that they will be moving, or more importantly, building a new home, and subsequently ask for advice on making the new abode more allergy friendly. A renewed and continued education of the patient on EC will be of immense help and will make some building decisions easier. A review of what, and to what degree, a patient is allergic is appropriate in this process. Contractors and other sources such as the Internet are essential in making environmentally friendly decisions. Cost is always a factor. Decisions about moisture barriers for the house, forced air versus hot water heating, a multiple storied house and basement versus one storey on a slab, the decision and choice on air filtration, landscaping design, wooden flooring versus carpeting, and the list goes on, are a few of many decisions to be made for a new home.
On occasion patients will consider moving to a new area or distant locale to improve their allergy related health. They may ask the clinician for advice. Great caution has to be taken in making this relatively rarely efficacious relocation. The patient must first realize that being allergic is a genetic predisposition and while they may experience some relief with a well-chosen move initially, the likelihood of developing new sensitivities in the new environment is high, the result of which may be disappointing to the patient and economically disastrous. Realistically, there is no locale which will be devoid of sensitizing exposures. If such a move is indicated, and with the risks of failure understood, a careful search using many sources needs to be undertaken before the move is finalized. Based on the patient’s allergic profile, such sources may include, not exclusively, pollen guides, climatic data, pollution levels, population figures, job and therefore workplace opportunities, home and building options, and so on. Such a decision made solely on EC criteria should come about with prudence and only after other management options have failed to give desired outcomes (Figure 2.9).
▪ Symptomatic Pharmacotherapy (Rx)
Antihistamines
Examples of older generation of antihistamines (OgenA) are listed in Box 2.10. Many of these agents are over-the-counter (OTC) today and some are in generic production. These agents are therefore less expensive and more readily available to the public than most prescription drugs. Diphenhydramine, for example, is still a market leader in sales. Using seasonal allergic rhinoconjunctivitis (SARC) as an example, these drugs effectively and potently treat itching, sneezing, rhinorrhea, and tearing. With their ability to cross the blood–brain barrier, sedation and potentially associated drowsiness or hampered performance is of concern, especially to the patient operating machinery, driving, or the like. This side effect may be additively enhanced if given concomitantly with other CNS depressing drugs or alcohol. Furthermore, such side effects may potentially worsen the disease effect in high risk patients such as the obstructive sleep apneic. The anticholinergic effect may make OgenAs difficult to use in asthmatics, bronchitics and sinusitic patients who need their mucoid discharge to flow easily rather than become more viscid, retentive, and obstructive. The anticholinergic effect may also promote bladder outlet obstruction in certain individuals, and is not to be used in patients with prostatic hypertrophy. Possible increased intraocular pressure is another anticholinergic effect, which precludes the use of OgenAs in patients with some types of glaucoma. Cardiac, gastrointestinal, and paradoxical stimulation side effects are also of primary concern.98–100
Examples of the current NgenAs are listed in Box 2.11. The first of these drugs was introduced in the 1980s, with the class designed for improved side effect profiles and more favorable frequencies of dosing. The potency/efficacy performances of the NgenAs are regarded as equal, not necessarily better or worse, to the OgenAs. The incidence of tachyphylaxis seen with these NgenAs is much less than that seen with some of the OgenAs. Most of these drugs are dosed once or twice daily, resulting in improved adherence. Some of the NgenAs have a low incidence of sedation, namely fexofenadine, loratadine, desloratadine, and cetrizine, and are considered nonsedating or minimally sedating as they do not readily cross the blood–brain barrier. In fact, fexofenadine, desloratadine, and loratadine are FAA approved for pilots. However, in doses higher than the recommended respiratory dosing, desloratadine, loratadine and cetrizine have increasing incidences of sedation. On the other hand, fexofenadine has been shown to be safe at doses considerably higher than the recommended dose. Recommended doses are to be followed for fexofenadine as the increased doses have not demonstrated therapeutic benefit over the recommended, and there is a limit in the amount of the higher doses studied. Two of the earliest NgenAs, terfenadine and astemizole, were taken off the market because of the incidence of life-threatening arrhythmias associated with increased serum levels mainly due to decreased liver clearance involving the P-450 CLYP3A4 cytochrome pathway. While other NgenAs have been scrutinized for potential problems of liver clearance relating to competition for the P-450 pathway with other concomitantly taken drugs, the clinician should be familiar with the antihistamine’s package insert (PI) and the cardiologist’s ever increasing list of drugs which may affect the cardiac QT interval. One other improvement in major adverse events is the lower incidence of anticholinergic effect. The incidence for some is low enough that clinicians are using these NgenAs for patients such as select allergic asthmatics in which the drying effect of other antihistamines would be problematic.
Azelastine is currently the only topical, intranasal antihistamine FDA approved for use in the USA. Olopatadine is currently in the process of being developed for intranasal use. Azelastine has activity in both the early and late phases of allergic inflammation. Efficacy is equal or better than some of the oral NgenAs, and even more improved with the concomitant use of an intranasal corticosteroid. As with all intranasal medications, possible sensory perception side effects, e.g. aftertaste and the willingness of the patient to use a nasal spray, are issues. There is an incidence of sedation with intranasal azelastine of about 11%. Topical ophthalmic NgenAs are regarded as the most efficacious for eye symptoms, however, oral, intranasal, and parenteral medications of numerous classes of drugs have efficacy for eye symptoms. As such, these ophthalmic preparations are of value as both first-line and as add-on choices for eye symptoms.101 The reader is referred to other sources for a more expansive and detailed account of topical antihistamines.98,100
Corticosteroids
For the appropriate patient, corticosteroids (steroids) have, and will, continue to play an important role in the management of atopic diseases such as eczema, asthma, and allergic rhinitis. Steroids intervene at a number of sites in both the early and late phases of the allergic reaction and inflammatory sequence. The route may be topical, oral, or parenteral. Drug-related short- and long-term adverse effects are of consideration in the management decision to choose a steroid, and limit the choice of potency, frequency, and duration of treatment. Clinician knowledge of steroid bioavailability, alternate treatment options, and risk–benefit ratios, along with a respect for the possible side effects are essential. For cases in which longer-term therapy using preparations of higher bioavailability are necessary, the clinician needs to have a plan for a routine surveillance of potential steroid-related sequellae. Such surveillance would include, but not be limited to, monitoring glucose tolerance, cataract formation, bone and joint complaints, and osteoporosis (e.g. Dexa Scanning). Recommendations for calcium/vitamin D supplements, exercise, and when appropriate, osteoporosis-limiting medications need to be appropriately made. Situations requiring long-term therapy may include some cases of asthma, recalcitrant eczema, or the nasal polyposis–sinusitis–asthma of the difficult Samter’s triad (aspirin triad) patients. The fact that idiosyncratic cases are reported of significant side effect with relatively short, average-dosed steroid courses, should only underscore the need for the clinician’s awareness of the potential risks, not to be overshadowed by the commonality of use of steroids in all aspects of medicine.
Intranasal corticosteroids are the most commonly used first line management for allergic rhinitis by otolaryngologists and allergists with primary care usage on the increase. The more commonly used molecules are listed in Box 2.12. All of these are FDA approved for seasonal and perennial allergic rhinitis, but not all are FDA approved for nonallergic rhinitis. Given the fact that the majority of patients with SAR activity have concomitant perennial and/or nonallergic rhinitis, that the anti-inflammatory action of the steroid is nonspecific, and that there is not a clear demonstration of drug-to-drug superiority, all of these molecules have been useful for allergic and nonallergic rhinitis. These molecules are currently available in a variety of carrier states, different delivery systems, with or without preservatives, with or without scent, and have evolved to compliance-friendly once- or twice-a-day dosage schedules. There are differences in bioavailability among the molecules, although significant effects of these current topical intranasal molecules on the consequences of hypothalamic–adrenal axis suppression, linear bone growth velocities, or osteoporosis are uncommon. Short-term studies have not demonstrated nonreversible HPA suppression or nonrecoverable linear bone growth suppression, although adverse 1-year effects on growth have been noted with beclometasone. By extrapolation of the HPA data, osteoporosis is not felt to be a likely issue.102 As new products of the current molecules or new molecules evolve, these concerns will need to be addressed as further studies on the current products will continue.103 Until such studies are completed, the prevailing thought is that these products, when used as instructed in the package insert, are safe for these steroid-related side effects. However, for longer-term management, the clinician should strive to attain the lowest dosage and frequency of use to control symptoms.
Although for some patients the usage of intranasal steroids may be not feasible due to a patient’s unwillingness to use an intranasal medication, there are willing patients who are handicapped in effectively using such products due to varying degrees of mechanical nasal obstruction preventing the appropriate distribution of the drug. In the case of severe swelling, intranasal steroid treatment may need to be augmented with the use of short-term oral or topical decongestants, or an initial systemic/oral steroid to allow the intranasal steroid better access and time to successfully take over the management. There are times when adynamic or fixed mechanical obstructions such as a deviated nasal septum, concha bullosa, or intranasal synechia are not affected by decongestion and need to be surgically altered for better medication efficacy and general airway improvement. The practice of injecting intranasal steroid is mainly limited to treating hyperplastic membrane disease of the turbinate. This modality, which carries a risk of adverse effects including blindness, is described elsewhere. The more common side effects of intranasal steroids include nasal stinging and sore throat, headache, and epistaxis, with nasal septal excoriation/perforation and nasal or oral candidiasis being possible but less common. Proper patient instructions on application of the drug are designed to maximize intranasal distribution while keeping nasal septal stimulation and possible bleeding to a minimum.
Leukotriene-modifying Drugs
In the USA, the leukotriene-receptor antagonist montelukast is available for use in the treatment of SAR, PAR, and asthma. Other leukotriene-receptor antagonists are also available for the treatment of asthma, including zafirlukast and pranlukast. Another drug with leukotriene-inhibitory effects, zileuton, is also available for the treatment of asthma, and works through the inhibition of the 5-OH-lipoxygenase enzymes in the conversion of arachadonic acid to the cysteinyl leukotrienes. The cysteinyl leukotrienes are proinflammatory products of the arachidonic acid metabolic cascade and affect allergic inflammation independent of histamine effect. Montelukast may be used as a first-line choice for treating SAR and PAR, as well as in the treatment of asthma. Nasal, eye, and total symptom scores are improved. The drug may also be useful as an add-on drug when other drugs such as an antihistamine and/or intranasal steroid have not given a desired control of symptoms. The belief in a decongestion potential for this drug makes it attractive for situations where some degree of decongestion is desirable but in which the use of a sympathomimetic drug is avoided due to the risk of side effects. Headache and sometimes insomnia are common, yet infrequent, side effects. The use of montelukast for migraine (another disorder with inflammatory potential) is also currently under evaluation. The reader is referred to other sources for further in-depth information on montelukast and the other molecules.104,105
Decongestants
Decongestants are α-adrenergic agonists which are sympathomimetic in nature and decrease vascular congestion in the nose, allowing less respiratory tract obstruction. These drugs may be used orally or as topical intranasal agents. The oral preparations may be used alone or in combination with other medications such as antihistamines or mucolytic agents. Of most concern with decongestants are their potential side effects. Cardiovascular side effects such as blood pressure elevation, and cardiac rate and/or rhythm modulation warrant clinician discretion in patient selection and length of time for therapy. In patients selected for such treatment, blood pressure should be monitored and patients warned to report any incidence of headache or palpitations. Anxiety, insomnia, headache, and bladder outlet obstruction are some of the more common complaints noted. While the CNS stimulatory effect may appear to negate the sedation effect of an older generation antihistamine, the use of a decongestant will not reverse effects on performance impairment caused by the OgenA.
Mast Cell Stabilizers
Mast cell stabilizers prevent the release of mast cell mediators such as histamine through stabilization of the mast cell membrane. These agents are most effective when used prior to antigen exposure. Sodium cromoglycate (cromolyn sodium) has now been available in an intranasal format OTC for almost 10 years. As such it is available as a first-line drug for management of mild allergic reactions and as an add-on choice to complement other regimens. The drug is relatively free of side effects, but has to be used for effect four times a day. Nedocromil is much more potent than cromolyn sodium but is only FDA approved in the USA as an inhaled preparation for asthma, and as a topical ophthalmic. Other ophthalmic mast cell stabilizers include cromolyn sodium, ketotifen, and pemirolast, and topical antihistamines such as olopatadine also demonstrate mast-cell stabilizing effects. Cromolyn sodium is also used as an oral preparation for the treatment of mastocytosis and has been used off-label in the management of food allergy. Other drugs of this class, such as lodoxamide, continue to be studied and/or await FDA approval.
Mucolytic Agents and Mucus Management
While water is the best mucolytic, guaifenesin is the most common drug on the market used for this purpose. For the adult, the dosage ranges up to 1200 mg bid of the long-acting preparations. There are preparations which are combined with other agents. There are also preparations for children. Most of the guaifenisin preparations are available over the counter. Side effects most commonly reported include GI symptoms such as nausea, and the sensation of dryness, especially if the patient does not drink enough water. Patients need to be told that adequate or increased water intake is needed for guaifenesin therapy to be successful. It is not clear as to whether guaifenesin actually lyses mucus or stimulates the production of a thinner, better quality mucus through a vagally mediated mechanism. Likewise, oropharyngeal rinsing and/or nebulized saline may be of benefit for the epilaryngeal and/or tracheobronchial tracts. Mucus condition and the adequacy of its transport are considerations for the clinician.
Monoclonal Antibodies and Other Immunomodulators
Omalizumab (Xolair®, Genentech) is the sole anti-IgE drug approved for human use, and only for severe asthma in adults. It is a recombinant humanized IgG1 monoclonal anti-IgE antibody that binds to nonspecific unbound IgE. Because of its structural/functional composition, it should not provoke anaphylaxis, although there have been a few reported cases.106 Patients have to satisfy certain elements in their asthmatic profile, have at least one positive skin test (usually to a perennial allergen), and demonstrate an elevated total IgE level. The dose, given subcutaneously monthly or twice a month in divided doses, is formulated on the basis of patient weight and the initial total IgE level. Although dramatically decreased levels in free IgE are seen after the initiation of omalizumab, clinical response may take a number of weeks. Omalizumab has an effect on both early- and late-phase response, a decrease in tissue, airway, and peripheral eosinophilia, and is associated with up to a 50% reduction in asthmatic exacerbations.2,107 The changes in eosinophilia are seen in the entire respiratory tract and skin as well.108,109 Interestingly changes in bronchial hyperreactivity to challenge with methachloline and to airflow obstruction are not as dramatic.110 Strunk and Bloomberg2 comment that the place for omalizumab in the armamentarium of management has not been fully defined, however they feel there are patients who may most likely benefit from such therapy. These patients would include perennial allergic asthmatics on high doses of inhaled steroids (at higher risk for side effects), unstable asthmatics with frequent flares of asthma, and those patients with severe disease and poor medication adherence. Although omalizumab is not approved for the treatment of allergic rhinitis, it has shown success in decreasing symptoms of rhinitis.111–115 Currently, management with omalizumab is expensive, and there have not as of yet been any well defined endpoints of therapy. In addition, the long-term side effect profiles, including the risk of neoplasm and parasitic disease, have not been clarified. As with any other form of therapy, the risks and benefits will have to be further evaluated.
Summary
It is apparent that there are a number of pharmacologic agents available to the clinician in the management of allergic disease. Such management will be presented in more detail in the appropriate problem-related chapters. The armamentarium will continue to grow as newer agents are developed. Choice of treatment method depends upon the medical profile, economic factors, availability, and the likelihood of patient adherence. Figure 2.10 demonstrates an algorithm to help the clinician through a general management process. It is clear that there are situations when environmental control of the allergic load (EC) and pharmacologic symptomatic medication (Rx) are not enough for the desired outcome and quality of life. In such situations, specific immunotherapy is indicated.
▪ Specific Allergen Immunotherapy (IT)
The literature for the world experience using multiple antigen sublingual drops was recently reviewed.116 This review demonstrated good efficacy with the use of SLIT for allergic rhinitis. At this writing several trials of SLIT are underway in the USA. The nuances of where in the allergy management algorithms these newer modalities of IT will be placed remains to be established.
Benefits and Indications – the “Why,” “When,” and “Who” of IT
The potential benefits of IT are many. First, maximal therapeutic outcomes may be attainable with the addition of IT. For some complex or difficult patients, the addition of IT may produce the best possible outcomes. Furthermore, specific antigen IT is the only proactive treatment that can lessen the patient’s sensitivity to specific antigens. The ultimate goal of allergy treatment would be the hope for a “clinical cure,” i.e., attaining an allergic state desensitized sufficiently to require little or no medication at all. Long-term effects of using anti-IgE with IT are not established at this writing, but may potentially yield an efficacious method for IgE immunomodulation and the possible “clinical cure” in select patients. The potential reduction in medication requirements is an attractive goal for many patients. Such a reduction can be economically beneficial, lessens the chances of medication nonadherence, reduces the incidence of drug-related side effects, and further improves quality of life by changing the regimented discipline of the drug schedule. Case-by-case the cost and regimen of IT has to be weighed with Rx. For someone who is comfortable with their established degree of EC and Rx without adverse effects or financial burden, there may be no real reason to consider IT. For many patients, the cost and time spent in a course of 3 to 5 years of IT is more attractive and cost-effective than the global cost of management of the allergic problem and its secondary sequelae, such as recurrent morbid infections, time lost from work/school, or poor performance. This economic benefit is highly dependent on the patient adhering with the IT program and the physician attaining high enough levels of delivered antigen over the appropriate time frame to effect desensitization.
It is an absolute contraindication to institute IT in a patient without proven and clinically relevant IgE-mediated disease. If not absolute, it is arguably a relative contraindication to perform in vivo testing or IT in patients on beta-blocking agents. Should an adverse reaction to testing or IT occur requiring epinephrine in the rescue, a possible unchecked alpha adrenergic effect of the epinephrine could lead to disastrous sequelae such as severe hypertension. Such reactions have been reported even with beta-blocking topical ophthalmics used in treating conditions like glaucoma. In addition, many clinicians regard beta-blocking agents as “pro-allergic,” often advising patients to seek other alternative medications when possible. The estimated impact of the beta-blocker on the allergic problem has to be weighed against the impact of its discontinuance on the medical problem for which it was prescribed. ACE inhibitors or ACE blockers concomitantly used in patients on IT can also be an area of concern to allergists. These two drugs (the inhibitors more so than the blockers) have been associated with an incidence of angioedema of the upper airway, at times causing potentially life-threatening airway obstruction. The mechanism is thought to be non-IgE-mediated, involving kinin metabolism. However, the incidence of angioedema appears to be increased in patients with IgE-mediated problems. The question as to whether treating patients with IT who are also concomitantly on an ACE-inhibitor or ACE-blocker will further predispose them to the risk of drug-related angioedema has not been fully answered as of this writing, although in clinical practice adverse events would appear rare. A corollary concern is whether patients with IgE-mediated disease should be on an ACE-inhibitor or ACE-blocker at all. These drugs have significant clinical value for serious medical problems quite often of great importance to the well being of the patient. The clinician(s) have to make decisions based on the risks and benefits involved.
There are age considerations in deciding who is a candidate for IT. As expected, these considerations pertain to the age extremes, the young and the elderly allergy patients. It is unusual for patients up to 2 or 3 years of age to be significantly allergic to inhalants. Typically the early expressions of sensitivity with any clinical significance begin with foods, then perennials, followed by seasonal inhalant allergens. Clinically relevant IgE-mediated inhalant sensitivity needs to be documented before injection IT is contemplated. Children from age 2 or 3 up to puberty are in the earliest range of consideration. The best approach, in general, is conservative, i.e. considering IT only when good effort of control of symptoms and quality of life improvement utilizing EC and Rx have failed in some compelling way. In such patients, however, remember while IT may improve outcomes and reduce sensitivity and allergic load for the present, it may subdue but not prevent the patient from forming sensitivity to other allergens as time and new exposures dictate. Just as some studies have indicated that empiric treatment of the atopic child with EC and Rx may have a delaying impact on the emergence of allergic conditions such as asthma, it may not be preventative. There are no long-term follow up studies to support the efficacy of early-age IT being preventative in the long term for the further evolution of allergic disease. IT in this age group should therefore be utilized for current symptomatic improvement, with the hope of curbing symptoms or other allergic disease emergence in the future. Quite often patients may experience a spontaneous short- or long-term improvement in symptoms during puberty through the teen or early adult years, with some patients experiencing even lifetime change in symptoms. This “growing out of allergy” period may end sometime in the late teen years, or early to late adulthood with a recrudescence of symptoms of varying degrees. Suspicion, testing, and the treatment of allergy in the senior population was thought to be foolish, considering the incidence of sensitivity generally decreases with age. While the development of new-onset allergic disease in the elderly is very uncommon, even the senescent immune system is capable of mounting IgE-mediated disease, and these patients may benefit from IT in the right setting. This observation is further supported by the fact that patients are living longer, healthier, and more active lives demanding a higher level quality of life with prevention or delay of the sequelae of chronic active allergic inflammation.
The mechanics of formulating, initiating, maintaining, trouble shooting, and discontinuing IT are not in the scope of this chapter and are well referenced elsewhere. There are widely accepted regimens for subcutaneous injection IT whether the formulating data is derived from epicutaneous prick/puncture (with/without fixed intradermal) skin testing, intradermal dilutional testing (IDT), modified quantitative testing (MQT), or in vitro tests such as the mRAST or ImmunoCAP. The use of sublingual IT, not yet widely studied or used in the USA, is also well referenced.117
Summary
The clinician must have an organized thought sequence for the diagnosis and management of the clinically relevant, allergy-suspicious patient. As an aid, Figure 2.10 is a flow algorithm giving options to the clinician for diagnosis and management. The following will give the clinician a general idea as to the flow of reasoning needed to make the algorithm user friendly and not overpowering, as it is a pretty complete diagram of options.
A patient presents with a history suggestive of allergy and a corroborating physical exam. In addition, a formal or informal quality of life assessment indicates an action is appropriate. Taking the medical and economic profile of the patient into consideration, the patient may be empirically treated or objective testing may be done. If empirically treated successfully, adequate symptomatic control may be obtained; if not, then objective testing is performed if the patient agrees. Objective testing may be a multiallergen in vitro screen or either an in vitro or in vivo specific allergen screen. If the multiallergen in vitro screen is positive, or if negative with a very compelling history, the patient may be treated with EC and/or SmRx. A negative multiallergen screen with an equivocal history may direct the clinician to management with nonallergic methods. Good results with allergy management may define a solution. A poor management outcome with a good history deserves specific allergy testing. The clinician may decide to do specific testing, on the basis of the history alone, on the basis of a positive multiallergen screen, or even a negative screen with a good history. A positive specific allergen in vitro or in vivo screen may trigger allergy management without IT or it may indicate a need for an expanded in vitro or in vivo test battery. A positive expanded battery would indicate allergy management which could be instituted at any or all of the desired levels, i.e., EC, SmRx, and/or IT if there are no contraindications. A negative expanded battery may indicate nonallergy or allergy management limited to positives on the screen. An elevated total IgE in the right type of asthmatic with a positive test for at least one specific allergen may indicate a setting for omalizumab management with/without IT. The options to the clinician may be numerous but should not be confusing if each patient is evaluated and managed on an individual basis. Remember that a sound history for allergy will usually justify more specific and/or expanded inquiry until negative results exceed the level of suspicion. Medical and economic profiles as well as patient willingness are definitely influential factors in testing and management.
Adelman D, Casale T, Corren J, editors. Manual of allergy and immunology, 4th edn., Philadelphia: Lippincott Williams & Wilkins, 2002.
Allen D. Systemic effects of intranasal steroids: an endocrinologist’s perspective. J Allergy Clin Immunol. 2000;106(suppl 4):S179-S190.
Derebery M, Osgulthorpe D, editors. Otolaryngic allergy. Otolaryngol Clin North Am. 36. 5. 2003.
King H, Mabry R, Mabry C, et al. Allergy in ENT practice, 2nd edn. New York: Thieme Medical Publishers, 2005.
Krouse J, Chadwick S, Gordon B, Derebery M, editors. Allergy and immunology – an otolaryngic approach. Philadelphia: Lippincott Williams & Wilkins, 2002.
Krouse J, Mabry R. Skin testing for inhalant allergy: current strategies. Otolaryngol Head Neck Surg. 2003;129(suppl 4):s33-49.
Leatherman B, Owen S, Parker M, Chadwick S. Sublingual immunotherapy – past, present, paradigm of the future? A review of the literature. Otolaryngol Head Neck Med Surg 2006. In press.
Lipworth B, Jackson C. Safety of inhaled and intranasal corticosteroids. Lessons for the new millennium. Curr Opin Drug Safety. 2000;1(23):11-33.
Poon A, Clifford S, Rubin R. In vitro and skin testing for allergy: comparable clinical utility and costs. Am J Managed Care. 1998;4:969-985.
White J, Bernstein D. Key pollen allergens in North America. Ann Allergy Asthma Immunol. 2003;91:425-435.
1 Janeway C, Travers P, Walport M, et al. Glossary in immunobiology, 5th edn., New York: Garland Publishing; 2001:701.
2 Strunk R, Bloomberg G. Omalizumab for asthma. N Engl J Med. 2006;354:2689-2695.
3 Wachs M, Proud D, Lichtenstein L, et al. Observations on the pathogenesis of nasal priming. J Allergy Clin Immunol. 1989;84(4, part 1):492-501.
4 Connell J, Sherman W. Quantitative intranasal pollen challenges. III. The priming effect in allergic rhinitis. J Allergy. 1969;43:33-44.
5 Skoner D, Doyle W, et al. Priming of the nose and eustachian tube during natural pollen exposure. Am J Rhinol. 1989;3(2):53-57.
6 Wachs M, Proud D, Lichtenstein L, et al. Nasal priming (abstract). J Allergy Clin Immunol. 1987;79:253.
7 Viegas M, Gomez E, Brooks J, et al. Effect of the pollen season on nasal mast cells. Br Med J. 1987;294:414.
8 Skoner D, Doyle W, Boehm S, et al. Late phase eustachian tube and nasal allergic responses associated with inflammatory mediator elaboration. Am J Rhinology. 1988;2:155.
9 Cockcroft D, Ruffin R, Dolovich J, et al. Allergen-induced increase in nonallergic bronchial reactivity. Clin Allergy. 1977;7:503.
10 Spiegelberg H, Simon R. Increase of lymphocytes with Fc receptors for IgE in patients with allergic rhinitis during the grass pollen season. J Clin Invest. 1981;68:845-852.
11 Friedman R, Doyle W, Casselbrant M, et al. Immunologic-mediated Eustachian tube obstruction, a double-blind crossover study. J Allergy Clin Immunol. 1983;71:442-447.
12 Bacos J, McLean J, Matthews K, et al. Priming of the nasal mucosa by ragweed extract or by an irritant (ammonia). J Allergy. 1981;67:111-116.
13 Konno A, Towawa K, Fujiwara T. The mechanisms involved in onset allergic manifestations. Eur J Respir Dis. 1983;64(suppl 128):155-166.
14 Borum P, Gronborg H, Brofeldt S, et al. Nasal reactivity in rhinitis. Eur J Respir Dis. 1983;64(suppl 128):65-71.
15 Skoner D, Doyle W, Fireman P. Eustachian tube obstruction (ETO) after histamine nasal provocation – a double blind dose response study. J Allergy Clin Immunol. 1987;79:27-31.
16 Chadwick S. Allergic rhinitis in the elderly. In: Calhoun K, Eibling D, Wax K, et al, editors. Geriatric otolaryngology. London: Taylor & Francis; 2006:213-224.
17 Side effects report. Citing for sinusitis. PDR® Electronic Library™ (Version 7.0.308a-2005.1.2) [CD-ROM] Montvale, NJ. Thomson PDR, pg 1–5.
18 Side effects report. Citing for rhinitis. PDR® Electronic Library™ (Version 7.0.308a-2005.1.2) [CD-ROM] Montvale, NJ. Thomson PDR, pg 1–6.
19 Burk W. Food allergy. In: Adelman D, Casale T, Corren J, editors. Manual of allergy and immunology. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2002:242-255.
20 Macy E, Melton M., Schatz, M., et al. Drug allergy. In: Adelman D, Casale T, Corren J, eds. Manual of allergy and immunology, 4th edn. Philadelphia: Lippincott Williams & Wilkins; 219–241.
21 Golden D. Insect allergy. In: Adelman D, Casale T, Corren J, eds. Manual of allergy and immunology, 4th edn. Philadelphia: Lippincott Williams & Wilkins; 208–218.
22 Boguniewicz M, Beltrani V. Atopic dermatitis and contact dermatitis. In: Adelman D, Casale T, Corren J, editors. Manual of allergy and immunology. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2002:165-186.
23 Anderson L, Dreyfuss E, Logan J, et al. Melon and banana sensitivity co-incident with ragweed pollinosis. J Allergy. 1970;45:310-319.
24 Blackley C. Experimental researches on the causes and nature of Cattarhus aestivus. London: Ballière, Tindall, & Cox, 1873.
25 Cooke R. The treatment of hayfever by active immunization. Laryngoscope. 1915;25:108-112.
26 Noon L. Prophylactic inoculation against hayfever. Lancet. 1911;1:1572-1573.
27 Keenan J. History of skin testing and evolution of skin endpoint titration. In: Mabry R, editor. Skin endpoint titration. New York: Thieme; 1992:12-16.
28 Rinkel H. The management of clinical allergy: III inhalant allergy therapy. Arch Otolaryngol. 1963;77:205-225.
29 Hansel F. Allergies of the nose and paranasal sinuses. St Louis: Mosby, 1936.
30 Krouse J, Mabry R. Skin testing for inhalant allergy 2003: Current strategies. Otolaryngol Head Neck Surg. 2003;129(suppl 4):s33-49.
31 King H, Mabry R, Mabry C, et al. Testing methods for inhalant allergy. In: King H, et al, editors. Allergy in the ENT practice. New York: Thieme; 2005:105-154.
32 Nelson H. Diagnostic procedures in allergy. I. Allergy skin testing. Ann Allergy. 1983;51:411-418.
33 Fornadley J. Skin testing in the diagnosis of inhalant allergy. In: Krouse J, Gordon B, Chadwick S, et al, editors. Allergy and immunology – an otolaryngic approach. Philadelphia: Lippincott, Williams & Wilkins; 2002:114-123.
34 American Medical Association Council on Scientific Affairs, Panel on Allergy. In vivo diagnostic testing and immunotherapy for allergy. JAMA. 1987;258:1363-1367.
35 Sanico A, et al. Skin testing methods. In: Adelman D, Casale T, Corren J, editors. Manual of allergy and immunology. 4th edn. Philadelphia: Lippincott, Williams & Wilkins; 2002:485. appendix II
36 Levine J, Mabry R, Mabry C. A comparison of multi-test skin testing and modified RAST results. Otolaryngol Head Neck Surg. 1998;118:797-799.
37 Knicker W. Multi-test skin testing in allergy: a review of published findings. Ann Allergy. 1993;71:485-491.
38 Pastorello E, et al. Mechanisms to adverse reactions to foods: the mouth and pharynx. Allergy. 1995;50:41-44.
39 Amlot PL, Kemeny DM, Zachary C, et al. Oral allergy syndrome (OAS): symptoms of IgE-mediated hypersensitivity to foods. Clin Allergy. 1987;17:33-42.
40 Ortolani C, Ispano M, Pastorello E, et al. The oral allergy syndrome. Ann Allergy. 1988;61:47-52.
41 Chadwick S. The pharynx and larynx. In: Krouse J, Gordon B, Chadwick S, et al, editors. Allergy and immunology – an otolaryngic approach. Philadelphia: Lippincott, Williams & Wilkins; 2002:249-269.
42 Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that cross-react, with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981;68:356-364.
43 Andersen K, Lowenstein H. An investigation of the possible immunologic relationship between allergen extracts from birch pollen, hazelnut, potato, and apple. Contact Dermatitis. 1978;4:73-79.
44 Björkstén F, Halmepuro L, Hannuksela M, Lahti A. Extraction and properties of apple antigens. Allergy. 1980;35:671-677.
45 Pastorello E, Ortolani C. Oral allergy syndrome. In: Metcalfe D, et al, editors. Food allergy: adverse reactions to foods and food additives. 2nd edn. Oxford: Blackwell Science; 1997:221-223.
46 Dreborg S, Foucard T. Allergy to apple, carrot, and potato in children with birch pollen allergy. J Allergy. 1983;38:167.
47 Lathi A, Hannuksela M. Hypersensitivity to apple and carrot can be reliably detected with fresh material. Allergy. 1978;33:143.
48 Eriksson N. Clustering of foodstuffs in food hypersensitivity and inquiry study in pollen-allergic patients. J Allergol Immunol Pathol. 1984;12:28.
49 Shah S, Emanuel I. Cost analysis of employing multi-test allergy screening to guide serial endpoint titration (SET) testing vs SET alone. Paper presented at the annual meeting of the American Academy of Otolaryngic Allergy Foundation, San Diego, California; September 18, 2002.
50 Mabry R. Blending skin endpoint titration and in vitro methods in clinical practice. Otolaryngol Clin North Am. 1992;25:61-70.
51 Krouse J. Modified quantitative testing (MQT) in clinical practice. Paper presented at the annual meeting of the American Academy of Otolaryngic Allergy Foundation, San Diego, California, September 18, 2002.
52 Mahan C, Spector S, Siegel S, et al. Validity and reproducibility of multi-test skin testing device. Ann Allergy. 1993;71:25-28.
53 Nelson H, Lahr J, Buchmeier A, et al. Evaluation of devices for skin prick testing. J Allergy Clin Immunol. 1998;101:153-156.
54 Murphee J, Knicker W. Correlation of immediate skin test responses to antigens introduced by multi-test and intracutaneous routes. Ann Allergy. 1979;43:279-285.
55 James W, Rosenthal L, Brancaccio R, et al. American Academy of Dermatology patch testing survey: use and effectiveness of this procedure. J Am Acad Dermatol. 1992;26:991-994.
56 TRUE TEST®. Reference Manual. Online. Available at: http://www.truetest.com.
57 Rietschel R, Fowler JJr. Fisher’s contact dermatitis, 4th edn., Baltimore: Williams & Wilkins; 1995:11-29.
58 Ishizaka K, Ishizaka T, Hornbrook M. Physiochemical properties of reaginic antibody: V. Correlation of reaginic activity with E-globulin antibody. J Immunol. 1966;97:840.
59 Johansson S, Bennich H. The clinical impact of the discovery of IgE. Ann Allergy. 1982;48:325.
60 Emanuel I. In vitro testing for allergies. In: Krouse J, Gordon B, Chadwick S, Derebery MJ, editors. Allergy and immunology – an otolaryngic approach. Philadelphia: Lippincott Williams & Wilkins; 2002:124-132.
61 Durham R, Church M. Principles of allergy diagnosis. In: Holgate S, Church M, Lichtenstein L, editors. Allergy. 2nd edn. London: Mosby; 2001:3-16.
62 Wide L, Bennich H, Johansson S. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967;2:1105-1107.
63 Ahlstedt S. Mediators in allergy. Clin Trends ACI Int. 1998;1012:37-41.
64 Poon A, Clifford S, Rubin R. In vitro and skin testing for allergy: comparable clinical utility and costs. Am J Managed Care. 1998;4(7):969-985.
65 Kelso J, Sodhi N, Gosselin V, et al. Diagnostic performance characteristics of the standard Phadebas RAST, modified RAST, and Pharmacia CAP system versus skin testing. Ann Allergy. 1991;67:511-514.
66 Perelmutter L. In vitro allergy testing – past, present, and future. Clin Rev Allergy. 1994;12:151-165.
67 Nalebuff D. In vitro testing methodologies. Evolution and current status. Otolaryngol Clin North Am. 1992;25:27-45.
68 Boccagni P, Favan F, Zanoni G, et al. Comparison of four in vitro assays for specific IgE detection. Int J Clin Lab Res. 1994;24:102-105.
69 Nalebuff D, Fadal R, Ali M. The study of IgE in the diagnosis of allergic disorders in an otolaryngology practice. Otolaryngol Head Neck Surg. 1979;87:351-358.
70 Kam K, Hsieh K. Comparison of three in vitro assays for serum IgE with skin testing in asthmatic children. Ann Allergy. 1994;73:329-336.
71 Fleisher T, Blessing J. Diagnostic immunology. In: Adelman D, Casale T, Corren J, editors. Manual of allergy and immunology. 4th edn. Lippincott: Philadelphia; 2002:441-456.
72 Mabry R. Standardized extracts. In: King H, Mabry R, Mabry S, et al, editors. Allergy in ENT practice. 2nd edn. New York: Thieme; 2005:268-279.
73 Blue Cross Blue Shield Technology Evaluation and Coverage. In vitro allergy tests for specific IgE. Newsletter. 1990;7:4.
74 Fisher L, Van Belle G. Biostatistics: a methodology for the health sciences. New York: John Wiley, 1993;206-209.
75 Sampson H. Immunologically mediated food allergy: the importance of food challenge procedures. Ann Allergy. 1988;60:262-269.
76 Smith T. Allergy testing in clinical practice. Ann Allergy. 1992;68:293-301.
77 Bernstein I, Storms W. Practice parameters for allergy diagnostic testing. Joint Task Force for the Diagnosis and Treatment of Asthma. The American Academy of Allergy, Asthma, and Immunology and the American College of Allergy, Asthma, and Immunology. Ann Allergy Asthma Immunol. 1995;75:543-625.
78 Williams P, Dolen W, Koeppke J, et al. Comparison of skin testing and three in vitro assays for specific IgE in the clinical evaluation of immediate hypersensitivity. Ann Allergy. 1992;68:35-45.
79 Pastorello E, Incorvavia C, Ortolani C, et al. Studies on the relationship between the level of specific IgE antibodies and the clinical expression of allergy: 1. Definition of levels distinguishing patients with symptomatic from patients with asymptomatic allergy to common aeroallergens. J Allergy Clin Immunol. 1995;96:580-587.
80 Anon J. Introduction to in vivo allergy testing. Otolaryngol Head Neck Surg. 1993;109:593-600.
81 Eriksson N. Diagnosis of IgE-mediated allergy in clinical practice. Allergol Immunopathol (Madr). 1994;22:139-151.
82 Mosbech H, Nielsen N, Dirksen A, et al. Comparison between specific IgE measured by RAST, two chemiluminescent assays and skin prick test. Allergol Immunopathol (Madr). 1992;20:220-224.
83 Ownby D, Bailey J. Comparison of MAST with radioallergosorbent and skin tests for diagnosis of allergy in children. Am J Dis Child. 1986;140:45-48.
84 Agata H, Yomo O, Hanashiro Y, et al. Comparison of the MAST chemiluminescent assay system with RAST and skin tests in allergic children. Ann Allergy. 1993;70:153-157.
85 Eriksson N. Allergy screening with Phadiatop and CAP Phadiatop in combination with a questionnaire in adults with asthma and rhinitis. Allergy. 1990;45:285-293.
86 Dekker F, Mulder D, Kramps J, et al. The Phadiatop in vitro test for allergy in general practice: is it useful? Fam Prac. 1997;7:144-148.
87 Wood R, Schuberth K, Sampson H. Value of a multiallergen radioallergosorbent test in diagnosing atopic disease in young children. J Pediatr. 1990;117:882-885.
88 Mabry R. Environmental control (avoidance). In: King H, Mabry R, Mabry C, et al, editors. Allergy in ENT practice. New York: Thieme; 2005:155-177.
89 NIAID. Dust Allergy. Publication No 83–490. Bethesda, MA: National Institute of Health; revised November 1982.
90 Gungor A, Ferguson B. Environmental management. In: Krouse J, Chadwick S, Gordon B, Derebery M, editors. Allergy and immunology – an otolaryngic approach. Philadelphia: Lippincott Williams & Wilkins; 2002:133-141.
91 Mason K, Riley G, et al. Hot tumble drying and mite survival in duvets. J Allergy Clin Immunol. 1999;104:499-500.
92 Chadwick S. Allergy and the contemporary laryngologist. Otolaryngol Clin North Am. 2003;36:957-988.
93 Krouse J, Gordon B, Parker M. Inhalant allergy. In: Krouse J, Chadwick S, Gordon B, Derebery M, editors. Allergy and immunology – an otolaryngic approach. Philadelphia: Lippincott Williams & Wilkins; 2002:35-49.
94 Berglund B, Gunnarson A. Relationships between occupant personality and the sick building explored. Indoor Air. 2000;10:152-169.
95 Ryan T, Whitehead L, Connor T, et al. Survey of Asp 1 allergen in office environments. Appl Occup Environ Hyg. 2001;16:679-684.
96 Gravesen S. Microbioligy of Indoor Air “99 – What is new and interesting? An overview of selected papers presented in Edinburgh, August, 1999. Indoor Air. 2000;10:74-80.
97 Mahmoudi M, Gershwin E. Sick building syndrome. III. Stahbotrys chariarium. J Asthma. 2000;37:191-198.
98 Krause H. Pharmacotherapy of otolaryngic allergy. In: Krouse J, Chadwick S, Gordon B, Derebery M, editors. Allergy and immunology – an otolaryngic approach. Philadelphia: Lippincott Williams & Wilkins; 2002:142-150.
99 Simons F. H1-receptor antagonists; safety issues. Ann Allergy Asthma Immunol. 1999;83:481-488.
100 King H. Pharmacotherapy of allergic rhinitis. In: King H, Mabry R, Mabry C, et al, editors. Allergy in ENT practice. New York: Thieme; 2005:178-204.
101 Leonardi A, Abelson M. Double-masked, randomized, placebo-controlled clinical study of the mast cell-stabilizing effects of treatment with olopatadine in the conjunctival allergen challenge model in humans. Curr Ther. 2003;25:2539-2552.
102 Wilson D. Systemic effects of intranasal steroids: an endocrinologist’s perspective. J Allergy Clin Immunol. 2000;106(suppl 4):S179-S190.
103 Lipworth B, Jackson C. Safety of inhaled and intranasal corticosteroids. Lessons for the new millennium. Curr Opin Drug Safety. 2000;1(23):11-33.
104 Van Adelsberg J, Philip G, LaForce C, et al. Randomized controlled trial evaluating the clinical benefit of Montelucast for treating spring seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2003;90:214.
105 Philip G, Malstrom K, Hampel F, et al. Montelucast for treating seasonal allergic rhinitis: a randomized double-blind, placebo-controlled trial performed in the spring. Clin Exp Allergy. 2002;32:1020-1028.
106 Food and Drug Administration, Center for Biologics Evaluation and Research. BLA STN 103976/0, review of clinical safety data: original BLS submitted on June 2, 2000 and response to complete review letter submitted on December 18, 2002. Rockville, MA: Department of Health and Human Services, 2003.
107 Fahy J. Anti-IgE: lessons learned from effects on airway inflammation and asthma exacerbation. J Allergy Clin Immunol. 2006;117:1230-1232.
108 Ong Y, Menzies-Gow A, Barkans J, et al. Anti-IgE (omalizumab) inhibits late phase reactions and inflammatory cells after repeat skin allergen challenge. J Allergy Clin Immunol. 2005;116:558-564.
109 Plewako H, Arvidsson M, Petruson K, et al. The effect of omalizumab on nasal allergic inflammation. J Allergy Clin Immunol. 2000;110:68-71.
110 Djukanovic R, Wilson S, Kraft M, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Resp Crit Care Med. 2004;170:583-593.
111 Lin H, Boesel K, Griffith V, et al. Omalizumab rapidly decreases nasal allergic response and Fc episilon RI on basophils. J Allergy Clin Immunol. 2004;113:297-302.
112 Corren J, Diaz-Sanchez D, Saxon A, et al. Effects of omalizumab, a humanized monoclonal anti-IgE antibody, on nasal reactivity to allergen, in local IgE synthesis. Ann Allergy Asthma Immunol. 2004;93:243-248.
113 Hanf G, Noga O, O’Connor A, et al. Omalizumab inhibits allergen challenged-induced nasal response. Eur Resp J. 2004;23:1414-1418.
114 Bez C, Schubert R, Kopp M, et al. Effect of anti-immunoglobulin E on nasal inflammation in patients with seasonal allergic rhinoconjunctivitis. Clin Exp Allergy. 2004;34:1079-1085.
115 Lee D. The role of omalizumab or rhuMAB-E 25 in the treatment of allergic rhinosinusitis. Eur Respir. 2004;24:330.
116 Leatherman B, Owen S, Parker M, et al. Sublingual immunotherapy – past, present, paradigm for the future? A review of the literature. Otolaryngol Head Neck Surg. In press.
117 Bousquet J. Sublingual immunotherapy: from proven prevention to putative rapid relief of allergic symptoms. Editorial. Allergy. 2005;60:1-3.

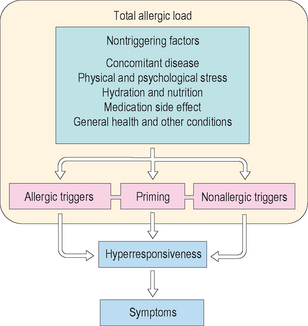
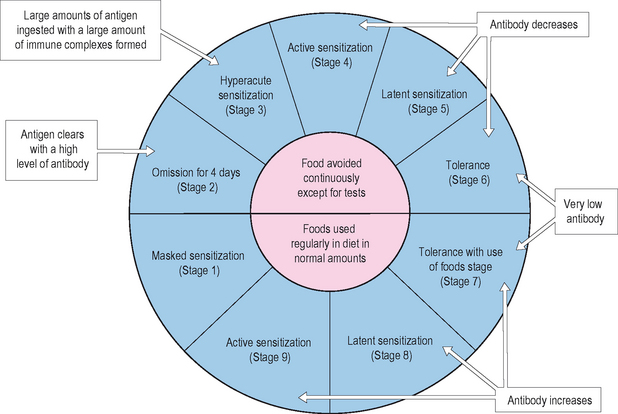
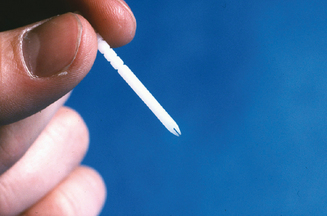

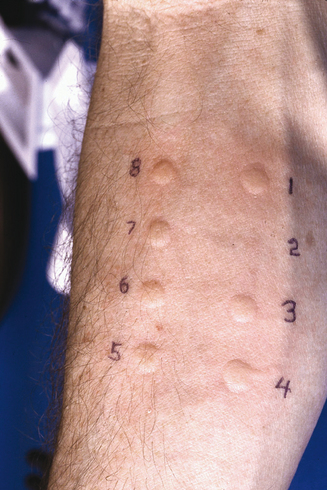
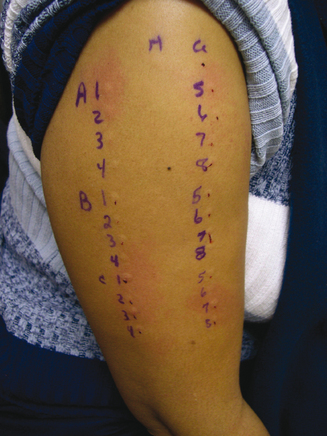
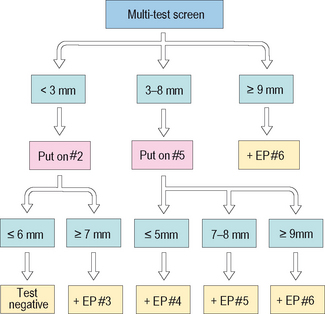
 –IgE is then mixed in the tube, now forming the final “sandwich,” a radioactively labeled, fixed
–IgE is then mixed in the tube, now forming the final “sandwich,” a radioactively labeled, fixed  –IgE/{IgE/anti-IgE}. The label is then counted, quantitating the bound, nonspecific IgE.
–IgE/{IgE/anti-IgE}. The label is then counted, quantitating the bound, nonspecific IgE.
 –IgE tag that produces a colormetric, fluorometric, or chemiluminescent reaction in the test mix that can be detected and quantified to assess levels of antigen-specific IgE. An F/N-like scoring system can be applied to this method. ELISA tests were designed to have better sensitivity/specificity ratios than tests such as the mRAST. ELISA methods, unfortunately, were never demonstrated to have the expected levels of sensitivity and specificity. With the emergence of the ImmunoCAP® system, ELISA techniques have reached a plateau in popularity.
–IgE tag that produces a colormetric, fluorometric, or chemiluminescent reaction in the test mix that can be detected and quantified to assess levels of antigen-specific IgE. An F/N-like scoring system can be applied to this method. ELISA tests were designed to have better sensitivity/specificity ratios than tests such as the mRAST. ELISA methods, unfortunately, were never demonstrated to have the expected levels of sensitivity and specificity. With the emergence of the ImmunoCAP® system, ELISA techniques have reached a plateau in popularity.